Abstract
Some of the earliest success in de novo tissue generation was in bone tissue, and advances, facilitated by the use of endogenous and exogenous progenitor cells, continue unabated. The concept of one health promotes shared discoveries among medical disciplines to overcome health challenges that afflict numerous species. Carefully selected animal models are vital to development and translation of targeted therapies that improve the health and well‐being of humans and animals alike. While inherent differences among species limit direct translation of scientific knowledge between them, rapid progress in ex vivo and in vivo de novo tissue generation is propelling revolutionary innovation to reality among all musculoskeletal specialties. This review contains a comparison of bone deposition among species and descriptions of animal models of bone restoration designed to replicate a multitude of bone injuries and pathology, including impaired osteogenic capacity.
Keywords: animal models, bone regeneration, critical size bone defects
1. INTRODUCTION
The goal and focus of innumerable scientific efforts throughout recorded history was to decipher and harness the power and unlimited potential of the cell. Discovery, isolation, and culture of cells that can assume characteristics of numerous lineages, including those from distinct embryonic layers, ignited a virtual explosion of discovery in the vast arena of cell therapies over the last two to three decades. A natural trajectory of the therapeutic momentum is to replace musculoskeletal tissue compromised by trauma, disease, or malformation with healthy tissue via de novo tissue generation. Broad approaches include in vitro generation of viable, implantable tissue, and application of exogenous cells and materials to recruit and direct endogenous cells. Carriers for cell delivery are composed of materials that facilitate tissue formation by progenitor cells, and they are routinely customized at the macro‐, micro‐, and ultra‐structural levels to replicate tissue matrix, including organic and inorganic components. Tremendous advances in de novo tissue generation provide unlimited opportunities to restore musculoskeletal tissue and impact the health and wellbeing of global community members at any stage of life.
The process of moving innovative de novo musculoskeletal tissue generation from concept to clinical reality is incremental and iterative. Key elements of successful translation from bench to bedside are reproducible animal models that recapitulate targeted musculoskeletal pathology. Models vary widely among joints and limbs and between traumatic, degenerative, and developmental conditions. Many are induced by surgical or chemical means, and test therapies are applied immediately or after a period of time following the initial injury. Numerous considerations are associated with selection of an animal model. There are specific factors related to the scientific questions or techniques to be tested and practical considerations like animal cost, availability, and regulation‐compliant surgical and housing facilities. Published substantiation of, and investigator experience with a model in addition to validated outcome assessment assays, including proteomic and genomic panels, also guide selection. Customized genetic makeup and immunodeficiency are found primarily in rodents. Findings from such highly tailored models require testing in larger mammal models before clinical translation and implementation.
Orthotopic evaluation of bone healing in a large animal model is frequently part of the final preclinical testing stages. In addition to anatomy and magnitude of load bearing, 1 bone formation and microstructure are critical assessments of an animal model (Table 1). It is also important to remember that, while bone composition is relatively highly conserved, it is not identical among species 2 ; canine and porcine are relatively close in composition and density to human, while rat has few similarities. Additionally, bone regeneration declines and morphology 3 changes differently with age among distinct life spans. 4 , 5 This is especially relevant to defining critical size defect (CSD) 6 sizes at various maturity levels in animals (Table 2). The following sections provide an overview of animal bone regeneration models beginning with a general comparison of bone turnover rates.
Table 1.
Animal model long bone characteristics
| Small mammal | Large mammal | NHP and human | |
|---|---|---|---|
| Sexual maturity age | Murine: 6–8 weeks 7 | Canine: 7–21 months 8 | Human: ~17 years 9 |
| Rat: 6 weeks 10 | Ovine: 7–8 months 11 | NHP: 4–6 years 12 | |
| Lapin: 10–12 weeks 13 | Porcine: 5–6 months 11 | ||
| Equine: 7–14 months 10 | |||
| Skeletal maturity age | Murine: 16–24 weeks | Canine: 10–11 months | Human: ~25 years |
| (Growth plate closure age/life expectancy age x 100) 14 | (13.9–27.8) 7 | (4.3–6.9) 8 | (16.7–25) 15 |
| Rat: 24–32 weeks | Ovine: ~40 months | NHP: 7.2‐10 years | |
| (22–35) 10 | (9.4) 16 | (11.2–17.5) 17 , 18 | |
| Lapin: 28–30 weeks | Porcine: 18–22 months 11 , 19 , 20 | ||
| (5.5–8.1) 13 | Bovine: 12‐37 months | ||
| (6.7–20.1) 14 | |||
| Equine: ~3 years | |||
| (5.8–6.3) 21 | |||
| Fractional area of secondary bone (FASB) | Rat: minimal 22 | Ovine: 2%–91% 23 | Human: ≈48% 24 |
| Lapin: minimal 25 | Bovine: ≈11% 24 | NHP: 61%–74% 26 | |
| Equine: 5%–75% 23 , 27 | |||
| Bone remodeling period | Murine: ~2 weeks 7 | Canine: ~2 months 28 , 29 | Human: 6–9 months 7 |
| Rat: ~6 days 30 | Ovine: ~80 days 31 | NHP: 8–24 months 32 | |
| Lapin: 70 days 33 | Porcine: 1–5 months 32 | ||
| Bone formation rate/bone volume (BFR/BV) at skeletal maturity (bone type) | Murinea: ≈1900% (cancellous) 34 , 35 , 36 | Canine: 0.5%–6.4% (cortical) 28 , 37 , 38 | Human: 3%–4% (cortical) 39 , 40 , 41 |
| Rat: ≈19% (cortical) 42 | 20%–50% (cancellous) 38 | ≈26.3% (cancellous) 43 | |
| ≈1158% (cancellous) 44 | Ovine: 55%–72% (cancellous) 45 | NHP: 13%–38% (cancellous) 46 , 47 | |
| Lapin: ≈20.7% (cortical) 33 | Porcine: ≈53% (cancellous) 32 | ||
| Equine: ≈10% (cortical) 48 | |||
| Pelvic limb axial force | Lapin: 201% BW 49 | Caprine: ≈100% BW 50 , 51 | Human: 470% BW 52 |
| Ovine: 48% BW 53 |
Abbreviations: BW, body weight; NHP, nonhuman primate.
keletally immature.
Table 2.
Critical defect size and fixation among bones and species
| Bone | Species | Defect size (mm) | Fixation | Potential advantages |
|---|---|---|---|---|
| Calvarium | Murine | >Ø 2 54 , 55 | ||
| Rat | >Ø 5 56 , 57 | |||
| Guinea Pig | 10 58 | |||
| Lapin | >Ø 6 59 , 60 | |||
| Canine | 20 61 , 62 | |||
| Ovine | >30 63 , 64 , 65 | |||
| Porcine | >Ø 10 66 , 67 | Bone composition similar to human 2 | ||
| Rib | Canine | >50 68 , 69 | Thoracic wall kinetics similar to human 70 , 71 | |
| Ovine | 40 72 | Plate | ||
| Porcine | 100 73 | |||
| Ilium | Lapin | >Ø 5 74 , 75 | ||
| Caprine | >Ø 8 76 , 77 , 78 | |||
| Humerus | Lapin | >7 79 , 80 , 81 | Plate, intramedullary rod | |
| Canine | >Ø 5 82 , 83 , 84 , 85 , 86 | |||
| Ovine | >Ø 6 87 , 88 , 89 | |||
| Radius | Rat | >5 90 , 91 | ||
| Lapin | >14 92 , 93 , 94 | Segmental defect without fixation possible | ||
| Established radiographic and histologic scoring system 95 | ||||
| >10 96 , 97 , 98 | Plate | |||
| Femur | Rat | >4 99 , 100 , 101 , 102 , 103 | Plate, external fixator, Intramedullary rod | Highly standardized fixation systems |
| Macrostructurally similar to human 1 | ||||
| Canine | >21 104 , 105 , 106 , 107 | Plate, Intramedullary Rod | Macrostructurally similar to human 1 | |
| Bone composition similar to human 2 | ||||
| Caprine | Ø 8 76 | |||
| Tibia | Lapin | 15 108 | Plate | |
| Ovine | >30 4 , 109 , 110 , 111 , 112 | Plate, external fixator | Defect strain similar to human | |
| Vertebrae | Rat | >Ø 3 113 , 114 , 115 | ||
| Caprine | Ø 5 76 | |||
| Ovine | >Ø 6 116 , 117 , 118 , 119 , 120 , 121 , 122 | |||
| Mandible | Rat | >Ø 3 123 , 124 , 125 , 126 , 127 , 128 | ||
| Porcine | >17 129 , 130 , 131 | Macrostructure and microstructure and masticatory force similar to human 132 , 133 |
Note: Ø, cortical defect diameter.
2. BONE FORMATION DURING NORMAL HOMEOSTASIS
2.1. Bone remodeling
For comparisons among species, the rate of natural bone formation during normal homeostasis should be considered (Table 1, Figure 1). Two measures of bone activity are the extent of remodeling and rate of remodeling. Both vary with species, age, bone, and bone region. The fractional area of secondary bone (FASB), area comprised of secondary osteons in cortical bone, represents the amount of remodeling present. It is defined as the percentage of the total area of secondary bone relative to the total area of interstitial bone and secondary bone together. 134 The higher the FASB, the greater the extent of bone remodeling. In general, the amount of remodeled bone increases with age. Additionally, bone regions under compressive stress have the highest extent of remodeling and therefore secondary osteons while those under tensile stress retain more primary bone. 23 , 135 , 136 , 137 Due to anatomical differences among species, there are regional differences within bones. As an example, the human femur experiences largely compressive stresses, 138 , 139 while most quadruped femurs are subjected to both tensile and compressive stress; this leads to important species‐specific characteristics in regional bone remodeling. 140
Figure 1.
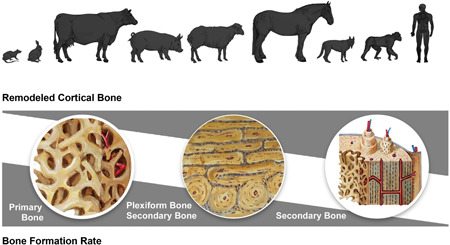
Animal and human remodeled cortical bone (fractional area of secondary bone) and bone formation rate (bone formation rate per unit of bone volume) with wedge area representing relative amounts and rates [Color figure can be viewed at wileyonlinelibrary.com]
Bone maturity is an important consideration in animal models. Broadly speaking, humans and nonhuman primates have highly remodeled cortical bone at maturity followed by large mammals; small mammals like lapin, rat, and murine have minimal remodeled bone as adults. 24 , 25 , 26 , 141 Among large mammals, the canine and equine FASB are closest to that of human. 23 , 27 , 142 , 143 Another distinct difference between animal and human bone is the prominent proportion of plexiform bone, a form of primary bone present during bone growth in rapidly growing mammals, that can result in a relatively low FASB (Figure 2). 23 , 24 , 144 Large mammals typically develop secondary bone near the endosteum while plexiform bone remains adjacent to the periosteum. 22 Additionally, the relative size of the osteonal resorption and Haversian canal areas within secondary osteons is positively correlated with body mass; the higher the body mass, the greater the area of each. Animals close in size to human counterparts may have similar secondary osteon structures. 145
Figure 2.
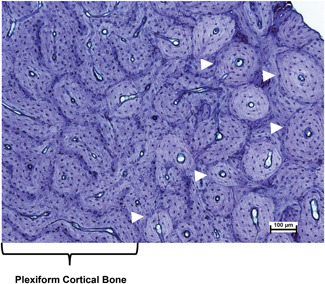
Photomicrograph of an undecalcified section of ovine endosteal cortical bone from the radius. Plexiform cortical bone is on the left. Active remodeling is indicated by the presence of secondary osteons (white arrow heads) on the right. Toluidine blue stain. Scale bar = 100 µm. (Photo courtesy of Dr. Clifford Les) [Color figure can be viewed at wileyonlinelibrary.com]
2.2. Bone formation rate
Distinct from the amount of remodeled bone is the rate of trabecular or cortical bone turnover, often measured as the bone formation rate per unit of bone volume (BFR/BV). 146 In humans, the BRF/BV is a well established measure 147 , 148 that is affected by age, 149 use, 42 , 150 and comorbidities. 39 , 151 , 152 In animal models, it is used to assess both the rate of bone remodeling and bone healing. 153 In general, BFR/BV is higher in cancellous bone than cortical bone, and tends to be higher in small versus large mammals and lowest in human cortical and cancellous bone. 28 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 Age has a large impact on BFR/BV in animal models. In rats, the BFR/BV of the proximal tibial metaphysis varies from 290.9% and 335.2% at 1 and 3 months of age, to 61.9% and 80.1% at 6 and 14 months of age, respectively 154 ; in dogs, the BFR/BV of the femoral mid‐diaphysis is 72% in immature and 1%–6.4% in mature animals. 28 , 37 The process of bone remodeling during normal homeostasis is somewhat demonstrative of, but not identical to, bone healing capacity. 155 Additionally, the FASB and BFR/BV permit some relative comparisons among species, but they are only two representative measures of normal bone remodeling (Figure 1). Any number of measures may be used or combined to monitor inherent bone forming capability, 146 an important consideration when utilizing animal models to test bone regeneration strategies.
3. MODELS OF BONE REGENERATION
3.1. Flat Bone
Common flat bone models include the calvarium, costae, and ilium. These non‐load bearing bones permit use of multiple CSDs without fixation, and intramembranous ossification is highly conserved among species. 156 , 157 Among the three, full‐thickness (bicortical), round defects in the rodent and lapin calvarium are the most popular for initial in vivo, orthotopic, and non‐orthotopic testing (Figure 3). 56 , 57 , 59 , 60 Notably, the dura mater is reported to be a source of bone morphogenetic protein 2 (BMP‐2) in young animals that seems to diminish with age. 58 , 158 The surgical procedure for calvarial defect creation is relatively simple, and the thin murine calvarium permits in vivo cell imaging with multi‐photon microscopy to investigate spatiotemporal coordination of cells that contribute to bone healing. 159 As an example, two‐photon microscopic imaging was used to confirm that exogenous bone marrow derived multipotent stromal cells on collagen/hydroxyapatite scaffolds were primarily responsible for new bone formation in murine calvarial defects while host cells participated most in periosteum regeneration. 160 Both circular defects and craniectomies are reported in large mammals like canine, ovine, and porcine in which ostectomies recapitulate craniectomies for congenital malformation, trauma, and neoplasia. 61 , 62 , 63 , 64 , 161 Instrumented transport osteogenesis models are reported as well as self‐retaining materials that facilitate detailed magnetic resonance imaging. 61 , 62 , 63 , 64 Collectively, calvarial defect models in both small and large mammals are valuable models for proof‐of‐concept testing in non‐load bearing bone.
Figure 3.
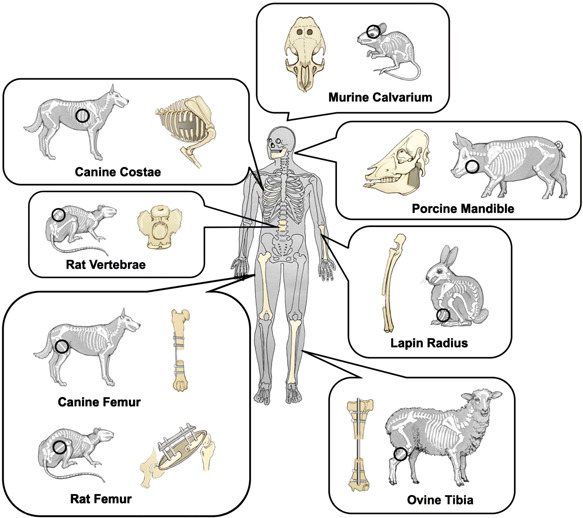
Schematic representation of common animal bone defect models [Color figure can be viewed at wileyonlinelibrary.com]
Costal bone is a common harvest site for autologous bone and costochondral grafts, and thoracic surgery or trauma can necessitate rib resection. 162 , 163 , 164 Rib ostectomy models to test rib regeneration options are designed to address pain, instability, and cosmesis associated with large defects. 72 , 73 , 165 , 166 In part, due similarity in size to human, ovine models of rib resection are common. 72 , 167 , 168 Thoracic wall reconstruction models are typically in species that share human thoracic cavity dynamics like canine and lapin. 68 , 69 , 70 , 71 , 169 A concavoconvex costovertebral joint 170 in cursory mammals like humans facilitates thoracic cavity expansion by intercostal and diaphragmatic musculature. 171 , 172 Non‐cursory mammals like caprine and ovine species have a flat costovertebral joint that relies on diaphragmatic musculature for thoracic expansion. 170
The iliac crest is another non‐load bearing bone used for materials testing with the important distinction of healing by endochondral ossification. Caprine and ovine models of circular unicortical or bicortical defects along the iliac crest are popular because microstructural cancellous bone volume and connectivity are similar to human 173 , 174 and ovine models of osteoporosis are well established. 175 , 176 The ilium is one of the most common sites of autologous cancellous and corticocancellous bone graft harvest. 163 , 177 Vascularized iliac bone block resections for treatment of avascular bone lesions or multiple corticocancellous bone harvests for staged surgical reconstructions drive efforts to enhance iliac bone regeneration. 178 , 179 , 180 For large defects and iliac bone blocks, the ovine is particularly advantageous due to anatomical properties that are close to that of human. 181 The ovine ilium has only a slightly longer iliac shaft and smaller wing than the human female.
3.2. Long Bone
Many animal models of long bone generation correspond to the most prevalent long bone fractures in humans. 76 , 92 , 182 , 183 , 184 Multiple, round, unicortical or bicortical, CSDs and non CSDs are used for orthotopic testing in virtually every bone of large and small animals. 87 , 185 Defect creation typically requires minimal soft tissue trauma and internal fixation is not required. Models that incorporate ostectomy or osteotomy require internal or external stabilization. 58 , 186 , 187 , 188 , 189 , 190 Large animal models have advantages of large defects and use of standard surgical tools and devices that are not possible in small animals. 191 As a general rule, long bone diaphyseal CSDs correspond to approximately 2–2.5 times the diaphyseal diameter, about 3–5 cm in ovine 109 , 192 , 193 , 194 or 3 cm in porcine 195 adult tibiae. Ostectomies are typically used to represent comminuted or unstable fractures while osteotomies represent minimally displaced fractures with limited comminution. 196
As indicated above, load bearing varies between quadrupeds and bipeds, especially in the forelimb equivalent of human arms. Based on bone mineral density changes in astronauts, bone deposition in the human pelvic limb is especially responsive to frequency and magnitude of loading, while the thoracic limb is less so. 135 , 197 , 198 , 199 , 200 , 201 The relative physiologic load is comparably higher in the forelimbs and lower in the hind limbs of most animal models. Large mammals bear about 60% of body weight on the forelimbs while rodents and lapin bear approximately 55%. 202 , 203 In terms of tibial loading at a walk, the lapin 49 is reportedly closest to human, 52 201% and 470% of body weight, respectively, while caprine is about 100% of body weight. 50 These important distinctions must be carefully considered when selecting animal models and comparing results among species. Specific information about popular long bone models is provided below (Figure 3).
3.2.1. Thoracic Limb
There are a number of animal models to assess proximal humeral epi‐ and metaphyseal bone regeneration. 82 , 83 , 84 , 87 , 88 , 89 , 204 In part due to anatomical congruity between the human and canine humerus, canine cylindrical defect models are commonly employed in young to aged adult dogs. 82 , 83 , 84 , 85 , 204 Additionally, proximal humeral osteosarcoma occurs naturally in adolescent to young adult dogs, 18–24 months, somewhat analogous to human adolescents. 205 These points support the value of canine models to optimize osteogenesis in the proximal humerus. A typical critical size cylindrical defect in the canine proximal humerus is about 5 mm wide and 4 mm deep in middle‐size dogs (25–35 kg). 206 A valuable stage to assess treatment effects in the model is reportedly during the fibrous to lamellar bone transition between 4 and 6 weeks postoperatively. 82 , 84 , 85 , 86 In addition to histologic and histomorphometric analyses, electron probe microanalysis can be used to determine regenerated bone maturity based on chemical composition, typically the calcium/phosphorus ratio. 82 , 83 , 84 , 85 , 204 , 207 Using the outcomes above and a proximal metaphyseal cylindrical defect model in young (1–2 years) and senior (10–12 years) adult dogs, transforming growth factor‐β2 on titanium cylinders increased bone volume to tissue volume by three‐fold compared with implant alone, though regenerated trabeculae were thinner and unmineralized osteoid higher in senior animals. 84
A popular segmental defect model in the humerus is in the lapin mid‐diaphysis. 79 , 80 , 81 CSDs in skeletally mature rabbits are around 7 mm long and stabilized with an intramedullary rod or bicortical plates. 79 , 80 , 81 In vivo monitoring is typically via radiography and nuclear scintigraphy, and postmortem histology is standard. 79 , 80 , 81 Determination of torsional strength and stiffness via mechanical testing is well established and consistent with predominant physiologic stresses. 81 , 208 , 209 Complete healing of segmental defects can be achieved as early as 6 weeks, but there is a high rate of non‐union up to 8 months after injury, 43%–100%, reportedly a result of poor healing capacity. 79 , 80 , 81 This makes the model appealing for developing treatments to overcome similar complications in human humeral fractures. 210 , 211 , 212 In one report, titanium mesh implants with BMP‐2 in polymer gel had 100% complete bony bridging of 15 mm humeral defects 6 weeks after implantation, while none of the defects without implants achieved bridging. 80 This and other reports help establish that the lapin humeral segmental defect model is amenable to testing therapies for suboptimal healing capacity. 79 , 81
Most lapin and rat species have a radio‐ulnar synostosis. 90 , 91 , 92 , 93 , 94 , 96 , 97 Though load bearing is shared between the bones, radial ostectomies are stable and do not require internal fixation. 90 , 91 , 93 , 94 At lapin skeletal maturity, segmental radial defects range from 10 to 14 mm, though 14 mm is recommended for a CSD; the segmental radial CSD in a skeletally mature rat is greater than 5 mm. 90 , 91 , 93 , 94 , 96 , 97 Radiography and microcomputed tomography (µ‐CT) as well as histology outcome measures are standard, 90 , 91 , 93 , 94 and the Lane–Sandhu scoring system for both radiograph and histologic quantification of bone healing 95 facilitates comparisons among studies. 90 , 91 , 94 Serum biomarkers are also possible outcome measures; prolonged healing in aged rats is associated with significantly lower levels of bone biomarkers like osteocalcin and alkaline phosphatase. 213 Evidence of rat and lapin radiographic bony bridging typically coincides with full recovery of mechanical strength in compression and bending. 90 , 91 , 96 Nanoindentation of thin sections (~ 100 μm) to measure modulus and hardness of new bone has also been reported in the lapin model. 98 Although less common, segmental radial ostectomies are reported in Yucatan miniature swine which also have a radio‐ulnar synosthosis. 214 Previously, 25–30 mm long defects filled with polymeric membrane in one‐year‐old animals were bridged radiographically by 8 weeks. 214 The miniature swine model has unique advantages of a large size without the need for internal or external fixation.
3.2.2. Pelvic Limb
The most common femoral segmental defect models are rat and murine. Anatomically, the rat femur resembles that of human, and femoral neck and greater trochanter ossification centers do not coalesce in either species. Closure of the rat and murine femoral and tibial physes relative to lifespan are comparable to humans and later than other mammals. 14 Immunocompromised rodent strains permit testing of xenogeneic cells and biomaterials. Recently, human adipose stromal vascular fraction cells on ceramic scaffolds that enhanced bone formation in immunocompromised rats in preclinical testing also promoted proximal femoral fracture healing in a clinical trial. 99 Detection of human cells in immunocompromised animals can be accomplished by standard methods including identification of human genetic sequences and antigens by in situ hybridization and immunolabeling, respectively. 99 Commercially available fixation systems for rodent femoral stabilization range from radiolucent plates 99 and interlocking nails 100 to external fixators. 99 , 101 Segmental defects greater than 4 mm in adult rats require about 8 weeks for complete bridging, though study end points typically range from 4 to 12 weeks, and bone formation is monitored similarly to the rodent forelimb. 99 , 100 , 101 , 102 Evaluation of bone mineral density in regenerated rat bone with scanning electron microscope‐based quantitative backscattered electron imaging has been reported. 101 , 215 Mechanical tests are frequently designed to assess torsional properties, though mechanical testing varies widely. 99 , 103 A potential disadvantage of the rodent femoral defect model is the well‐recognized robust healing capacity with and without fixation that can necessitate outcome validation in larger mammals. 216
Similarities between human and canine femoral anatomy contribute to the value of the canine femoral segmental defect model despite thinner cortices in the canine bone. 1 , 217 , 218 Intramedullary rods or cortical plates are used to stabilize CSDs of at least 21 mm in skeletally mature, middle‐ to large‐size dogs (12–55 kg). 104 , 105 , 106 , 107 Outcome assessments are similar to other species, bony bridging typically occurs around 12 weeks, and remodeling has been monitored for extended periods, 24 weeks or more, postoperatively. 104 , 105 , 106 , 107 Unlike small mammals, however, recovery of mechanical strength does not always coincide with radiographic healing; this is likely a consequence of extensive remodeling associated with canine bone healing, similar to human bone. 107 , 219 Use of gait kinetics to quantify limb use are fairly common in canine studies. 220 , 221 Ground reaction forces measured with a force platform are positively correlated with bone healing and have a strong association with callus mineralization and defect stiffness. 222 A recent study showed that addition of human osteogenic protein‐1 to cortical allograft strips in canine femoral defects improved limb use over allograft alone 10 weeks after surgery. 104 Wide use of canine gait kinetic measures permits comparisons among a multitude of orthopedic studies, including those with a focus on accelerated bone formation. 220 , 221
Among long bones, tibial ostectomies are frequently used to model traumatic bone loss. 223 As mentioned above, lapin tibial loading is closer to human than other small mammals, so the lapin tibial mid‐diaphyseal segmental defect model, typically stabilized with a bone plate, may have the strongest translational value. 49 , 52 , 53 , 108 In a large animal model, ovine tibial defects of 30 mm or more in the mid‐diaphysis are often treated with plates or external fixators and monitored by standard means up to 3 to 12 months followed by histology and mechanical testing. 4 , 109 , 110 , 111 , 112 , 224 The ovine tibial diaphysis has a relatively simple cylindrical macrostructure and loading mechanism compared to more structurally complex bones. 225 , 226 , 227 This, in addition to similarity in weight to adult humans, lends itself to testing of three‐dimensional printed grafts with varied microstructure and composition. 109 , 110 , 111 , 112
3.3. Vertebrae
Animal models of de novo vertebral bone synthesis are largely divided into two types, vertebral body defects and spinal fusion. In small mammals, vertebral body defects are typically spherical 113 , 114 , 115 , 228 , 229 as in an osteoporotic rat model that showed increased bone formation and improved stiffness of new bone with platelet‐rich plasma combined with a gelatin/β‐tricalcium phosphate (TCP) sponge. 230 Both kyphoplasty materials and novel implants are frequently tested in ovine. 116 , 117 , 118 , 119 , 231 , 232 Defect models often replicate highly prevalent lumbar (L) 2–5 vertebral body compression fractures. Midbody defects up to 6 mm in diameter and burst fractures created by manual compression are reported in skeletally mature animals with 12–36 weeks postoperative follow up. 116 , 117 , 118 , 119 , 120 , 121 , 122 , 233 As with all bones, there are important anatomical differences between human and animal vertebrae, however, the immature domestic porcine (55–65 kg) vertebral macrostructure resembles the human in pedicle dimensions, 234 vertebral body height, and end‐plate and spinal canal shape. 235 Polymethylmethacrylate cement containing magnets injected into porcine thoracic vertebrae to mimic kyphoplasty attracted systemically administered magnetic nanoparticles. 236 Lumber posterolateral spinal fusion is modeled in large and small animals, among which the rodent and lapin are commonly used to evaluate de novo bone formation and remodeling in a non‐instrumented model (Figure 4). 237 , 238 , 239 , 240 Bilateral decortication of the L4–L5 or L5–L6 transverse processes, with or without decortication of the spinous processes and lamina, produces stable fracture beds to which materials are applied topically. As illustrated by a rat spinal fusion study that showed syngeneic adipose tissue‐derived multipotent stromal cells (ASCs) on β‐TCP/collagen type I matrix enhanced bone formation over matrix with allogeneic ASCs or matrix alone, 240 bone formation is readily assessed with radiographs, μ‐CT and routine histology. 237 , 238 , 239
Figure 4.
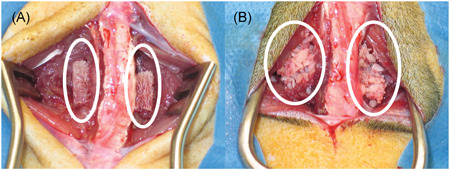
Demineralized bone matrix (A, white circles) and corticocancellous bone (B, white circles) during implantation in a rat model of lumbar spinal fusion. The lumbar spine is evident between the circles in each image [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Facial Bone
The mandible, orbital, zygoma, maxilla, and frontal bones are frequently sites of congenital malformation and trauma. 241 , 242 Facial bone regeneration is somewhat distinct in that minimal soft tissue coverage and esthetics require close replication of the original structure. In the rat model, CSDs in the mandible beneath the pterygomasseteric sling are used to test materials. 123 , 124 , 125 , 126 , 127 , 128 A distinct feature of the rat mandible is absence of a bony symphysis between hemimandibles that reduces load transfer and allows asynchronous motion between them. 243 , 244 , 245 Asymmetric masticatory function and dominant and nondominant hemimandibles are described in rats. 246 As such random assignment of treatment among hemimandibles or bilateral defects may be more important in rats than other models. Segmental defects in large animal models are frequently reported in the mandible, 129 , 130 , 247 , 248 , 249 , 250 , 251 hard palate, 252 , 253 , 254 and zygoma. 131 Unicortical and bicortical defects consisting of partial and full thickness bone resections are reported throughout the mandible in multiple large animal species including canine, ovine and porcine. Unicortical, partial bone thickness alveolar bone “saddle back” defects are frequently used to test bone regeneration in the unique bone‐tooth interface. 249 , 255 Stabilization varies with defect size and configuration as does time required for bone healing with 12 weeks typical for canine and ovine and porcine requiring slightly longer. 129 , 130 , 131 , 247 , 248 , 249 , 250 , 251 As in long bones, the best models are in those bones with proportionately similar loading and comparable anatomy to human. In terms of bone volume, trabecular thickness, and trabecular spacing, ovine and porcine mandible are among the closest to human. 132 Additionally, the porcine temporomandibular micro‐ and macrostructure resembles that of the human, and the joints in both species experience similar masticatory forces. 133 This makes the porcine mandibular condylectomy model well aligned with condyle and ramus regeneration studies. 130
4. FUTURE DIRECTIONS
Numerous novel interventions tested in animal models are the foundation on which current standard bone regeneration therapies are based. The future is bright as humanized animal models make it possible to more closely align outcomes between human and nonhuman species. Further advances may include larger species with bone size, shape and stresses that are similar to human. Continued efforts to identify shared conditions that occur naturally in animals and humans may increase parallel clinical trials, especially for age‐related tissue changes. Three‐dimensional printing with organic and inorganic materials has limitless possibilities for treatment customization, not only for optimal bone size and shape, but composition and therapeutics. Cellular therapies will be enhanced by mechanisms to control cell migration and measure cell longevity in vivo. These are among the innumerable other ambitious goals that are the basis for discovery efforts that will change the future of health care options.
5. CONCLUSIONS
The information above provides a limited glimpse of the burgeoning scientific efforts focused on bone restoration. It also highlights that shared goals to improve treatment options benefit all members of our global community. The importance of carefully selected animal models contributes to advances in de novo bone formation daily. This drives development and translation of targeted therapies that improve the health and well‐being of humans and animals alike. The concept of one health has gained renewed attention recently. In a nutshell, the message supports the benefits of sharing discoveries to address medical challenges afflicting numerous species among medical disciplines that attend to them. Naturally, it is vital to both recognize and respect inherent differences among species that limit direct translation of scientific knowledge. Nonetheless, the rapid progress of ex vivo and in vivo de novo bone generation is clearing propelling a wealth of revolutionary innovation to reality among scientific and clinical specialists.
AUTHOR CONTRIBUTIONS
Mandi J. Lopez conceived the original idea. Takashi Taguchi and Mandi J. Lopez performed the literature search and wrote the manuscript. All authors read and approved the final submitted manuscript.
ACKNOWLEDGMENTS
This study is funded in part by the Louisiana State University Equine Health Studies Program and the Tynewald Foundation. The authors thank Dr. Clifford Les for the image in Figure 2.
Taguchi T, Lopez MJ. An overview of de novo bone generation in animal models. J Orthop Res. 2021;39:7–21. 10.1002/jor.24852
Scientific editing by Clare Yellowley.
REFERENCES
- 1. Bagi CM, Berryman E, Moalli MR. Comparative bone anatomy of commonly used laboratory animals: implications for drug discovery. Comp Med. 2011;61:76‐85. [PMC free article] [PubMed] [Google Scholar]
- 2. Aerssens J, Boonen S, Lowet G, et al. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139:663‐670. [DOI] [PubMed] [Google Scholar]
- 3. McNeil CJ, Raymer GH, Doherty TJ, et al. Geometry of a weight‐bearing and non‐weight‐bearing bone in the legs of young, old, and very old men. Calcif Tissue Int. 2009;85:22‐30. [DOI] [PubMed] [Google Scholar]
- 4. Lammens J, Marechal M, Geris L, et al. Warning about the use of critical‐size defects for the translational study of bone repair: analysis of a sheep tibial model. Tissue Eng Part C Methods. 2017;23:694‐699. [DOI] [PubMed] [Google Scholar]
- 5. Malhotra A, Pelletier MH, Yu Y, et al. A sheep model for cancellous bone healing. Front Surg. 2014;1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vajgel A, Mardas N, Farias BC, et al. A systematic review on the critical size defect model. Clin Oral Implants Res. 2014;25:879‐893. [DOI] [PubMed] [Google Scholar]
- 7. Jilka RL. The relevance of mouse models for investigating age‐related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68:1209‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geiger M, Gendron K, Willmitzer F, et al. Unaltered sequence of dental, skeletal, and sexual maturity in domestic dogs compared to the wolf. Zoological Lett. 2016;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beunen GP, Rogol AD, Malina RM. Indicators of biological maturation and secular changes in biological maturation. Food Nutr Bull. 2006;27:S244‐256. [DOI] [PubMed] [Google Scholar]
- 10. Sengupta P. The laboratory rat: relating its age with human's. Int J Prev Med. 2013;4:624‐630. [PMC free article] [PubMed] [Google Scholar]
- 11. Reiland S. Growth and skeletal development of the pig. Acta Radiol Suppl. 1978;358:15‐22. [PubMed] [Google Scholar]
- 12. Drevon‐Gaillot E, Perron‐Lepage MF, Clement C, et al. A review of background findings in cynomolgus monkeys (Macaca fascicularis) from three different geographical origins. Exp Toxicol Pathol. 2006;58:77‐88. [DOI] [PubMed] [Google Scholar]
- 13. Masoud I, Shapiro F, Kent R, et al. A longitudinal study of the growth of the New Zealand white rabbit: cumulative and biweekly incremental growth rates for body length, body weight, femoral length, and tibial length. J Orthop Res. 1986;4:221‐231. [DOI] [PubMed] [Google Scholar]
- 14. Kilborn SH, Trudel G, Uhthoff H. Review of growth plate closure compared with age at sexual maturity and lifespan in laboratory animals. Contemp Top Lab Anim Sci. 2002;41:21‐26. [PubMed] [Google Scholar]
- 15. Cech DJ, Martin ST. Skeletal system changes In: Cech DJ, Martin ST, eds. Functional Movement Development Across the Life Span. Saint Louis: W.B. Saunders; 2012:105‐128. [Google Scholar]
- 16. Moran NC, O'Connor TP. Age attribution in domestic sheep by skeletal and dental maturation: a pilot study of available sources. Intl J Osteoarchaeol. 1994;4:267‐285. [Google Scholar]
- 17. Colman RJ, Lane MA, Binkley N, et al. Skeletal effects of aging in male rhesus monkeys. Bone. 1999;24:17‐23. [DOI] [PubMed] [Google Scholar]
- 18. Silverman S, Morgan JP, Ferron R, et al. Radiographic evaluation of appendicular skeletal maturation in the rhesus‐monkey. Vet Radiol. 1983;24:25‐34. [Google Scholar]
- 19. Pfeifer CG, Fisher MB, Saxena V, et al. Age‐dependent subchondral bone remodeling and cartilage repair in a minipig defect model. Tissue Eng Part C Methods. 2017;23:745‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bode G, Clausing P, Gervais F, et al. The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods. 2010;62:196‐220. [DOI] [PubMed] [Google Scholar]
- 21. Strand E, Braathen LC, Hellsten MC, et al. Radiographic closure time of appendicular growth plates in the Icelandic horse. Acta Vet Scand. 2007;49:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hillier ML, Bell LS. Differentiating human bone from animal bone: a review of histological methods. J Forensic Sci. 2007;52:249‐263. [DOI] [PubMed] [Google Scholar]
- 23. Skedros JG, Keenan KE, Williams TJ, et al. Secondary osteon size and collagen/lamellar organization ("osteon morphotypes") are not coupled, but potentially adapt independently for local strain mode or magnitude. J Struct Biol. 2013;181:95‐107. [DOI] [PubMed] [Google Scholar]
- 24. Saha S, Hayes WC. Relations between tensile impact properties and microstructure of compact bone. Calcif Tissue Res. 1977;24:65‐72. [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Mabrey JD, Agrawal CM. An interspecies comparison of bone fracture properties. Biomed Mater Eng. 1998;8:1‐9. [PubMed] [Google Scholar]
- 26. Skedros JG, Kiser CJ, Keenan KE, et al. Analysis of osteon morphotype scoring schemes for interpreting load history: evaluation in the chimpanzee femur. J Anat. 2011;218:480‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mason MW, Skedros JG, Bloebaum RD. Evidence of strain‐mode‐related cortical adaptation in the diaphysis of the horse radius. Bone. 1995;17:229‐237. [DOI] [PubMed] [Google Scholar]
- 28. Vajda EG, Kneissel M, Muggenburg B, et al. Increased intracortical bone remodeling during lactation in beagle dogs. Biol Reprod. 1999;61:1439‐1444. [DOI] [PubMed] [Google Scholar]
- 29. Dannucci GA, Martin RB, Patterson‐Buckendahl P. Ovariectomy and trabecular bone remodeling in the dog. Calcif Tissue Int. 1987;40:194‐199. [DOI] [PubMed] [Google Scholar]
- 30. Vignery A, Baron R. Dynamic histomorphometry of alveolar bone remodeling in the adult rat. Anat Rec. 1980;196:191‐200. [DOI] [PubMed] [Google Scholar]
- 31. Delmas PD, Vergnaud P, Arlot ME, et al. The anabolic effect of human PTH (1‐34) on bone formation is blunted when bone resorption is inhibited by the bisphosphonate tiludronate—is activated resorption a prerequisite for the in vivo effect of PTH on formation in a remodeling system? Bone. 1995;16:603‐610. [DOI] [PubMed] [Google Scholar]
- 32. McAnulty PA, Dayan AD, Ganderup N‐C, et al. The Minipig in Biomedical Research. CRC Press; 2011. [Google Scholar]
- 33. Mashiba T, Burr DB, Turner CH, et al. Effects of human parathyroid hormone (1‐34), LY333334, on bone mass, remodeling, and mechanical properties of cortical bone during the first remodeling cycle in rabbits. Bone. 2001;28:538‐547. [DOI] [PubMed] [Google Scholar]
- 34. Motyl KJ, Dick‐de‐Paula I, Maloney AE, et al. Trabecular bone loss after administration of the second‐generation antipsychotic risperidone is independent of weight gain. Bone. 2012;50:490‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng S, Aghajanian P, Pourteymoor S, et al. Prolyl hydroxylase domain‐containing protein 2 (Phd2) regulates chondrocyte differentiation and secondary ossification in mice. Sci Rep. 2016;6:35748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nallamshetty S, Wang H, Rhee EJ, et al. Deficiency of retinaldehyde dehydrogenase 1 induces BMP2 and increases bone mass in vivo. PLOS One. 2013;8:e71307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huja SS, Fernandez SA, Hill KJ, et al. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1243‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forwood MR, Burr DB, Takano Y, et al. Risedronate treatment does not increase microdamage in the canine femoral neck. Bone. 1995;16:643‐650. [DOI] [PubMed] [Google Scholar]
- 39. Tanizawa T, Itoh A, Uchiyama T, et al. Changes in cortical width with bone turnover in the three different endosteal envelopes of the ilium in postmenopausal osteoporosis. Bone. 1999;25:493‐499. [DOI] [PubMed] [Google Scholar]
- 40. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131‐S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115‐137. [DOI] [PubMed] [Google Scholar]
- 42. Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low‐magnitude mechanical stimuli. FASEB J. 2001;15:2225‐2229. [DOI] [PubMed] [Google Scholar]
- 43. Recker RR, Kimmel DB, Parfitt AM, et al. Static and tetracycline‐based bone histomorphometric data from 34 normal postmenopausal females. J Bone Miner Res. 1988;3:133‐144. [DOI] [PubMed] [Google Scholar]
- 44. Shahnazari M, Burr DB, Lee WH, et al. Cross‐calibration of 45calcium kinetics against dynamic histomorphometry in a rat model to determine bone turnover. Bone. 2010;46:1238‐1243. [DOI] [PubMed] [Google Scholar]
- 45. Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone. 1995;16:277S‐284S. [DOI] [PubMed] [Google Scholar]
- 46. Balena R, Toolan BC, Shea M, et al. The effects of 2‐year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J Clin Invest. 1993;92:2577‐2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lees CJ, Ramsay H. Histomorphometry and bone biomarkers in cynomolgus females: a study in young, mature, and old monkeys. Bone. 1999;24:25‐28. [DOI] [PubMed] [Google Scholar]
- 48. Da Costa Gomez TM, Barrett JG, Sample SJ, et al. Up‐regulation of site‐specific remodeling without accumulation of microcracking and loss of osteocytes. Bone. 2005;37:16‐24. [DOI] [PubMed] [Google Scholar]
- 49. Reifenrath J, Gottschalk D, Angrisani N, et al. Axial forces and bending moments in the loaded rabbit tibia in vivo. Acta Vet Scand. 2012;54:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weinans H, Blankevoort L. Reconstruction of bone loading conditions from in vivo strain measurements. J Biomech. 1995;28:739‐744. [DOI] [PubMed] [Google Scholar]
- 51. Shahar R, Banks‐Sills L. Biomechanical analysis of the canine hind limb: calculation of forces during three‐legged stance. Vet J. 2002;163:240‐250. [DOI] [PubMed] [Google Scholar]
- 52. Wehner T, Claes L, Simon U. Internal loads in the human tibia during gait. Clin Biomech. 2009;24:299‐302. [DOI] [PubMed] [Google Scholar]
- 53. Taylor WR, Ehrig RM, Heller MO, et al. Tibio‐femoral joint contact forces in sheep. J Biomech. 2006;39:791‐798. [DOI] [PubMed] [Google Scholar]
- 54. Im JY, Min WK, You C, et al. Bone regeneration of mouse critical‐sized calvarial defects with human mesenchymal stem cells in scaffold. Lab Anim Res. 2013;29:196‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Y, Wang L, Deng F, et al. Determination of a critical size calvarial defect in senile osteoporotic mice model based on in vivo micro‐computed tomography and histological evaluation. Arch Gerontol Geriatr. 2015;61:44‐55. [DOI] [PubMed] [Google Scholar]
- 56. Suenaga H, Furukawa KS, Suzuki Y, et al. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow‐derived mesenchymal stromal cell spheroids. J Mater Sci Mater Med. 2015;26:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu R, Qiao W, Huang B, et al. Fluorination enhances the osteogenic capacity of Porcine hydroxyapatite. Tissue Eng Part A. 2018;24:1207‐1217. [DOI] [PubMed] [Google Scholar]
- 58. Hobar PC, Schreiber JS, McCarthy JG, et al. The role of the dura in cranial bone regeneration in the immature animal. Plast Reconstr Surg. 1993;92:405‐410. [DOI] [PubMed] [Google Scholar]
- 59. Salamanca E, Hsu CC, Huang HM, et al. Bone regeneration using a porcine bone substitute collagen composite in vitro and in vivo. Sci Rep. 2018;8:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fujioka‐Kobayashi M, Kobayashi E, Schaller B, et al. Effect of recombinant human bone morphogenic protein 9 (rhBMP9) loaded onto bone grafts versus barrier membranes on new bone formation in a rabbit calvarial defect model. J Biomed Mater Res A. 2017;105:2655‐2661. [DOI] [PubMed] [Google Scholar]
- 61. Umeda H, Kanemaru S, Yamashita M, et al. Bone regeneration of canine skull using bone marrow‐derived stromal cells and beta‐tricalcium phosphate. Laryngoscope. 2007;117:997‐1003. [DOI] [PubMed] [Google Scholar]
- 62. Liu G, Zhang Y, Liu B, et al. Bone regeneration in a canine cranial model using allogeneic adipose derived stem cells and coral scaffold. Biomaterials. 2013;34:2655‐2664. [DOI] [PubMed] [Google Scholar]
- 63. Gerety PA, Wink JD, Sherif RD, et al. Treatment of large calvarial defects with bone transport osteogenesis: a preclinical sheep model. J Craniofacial Surg. 2014;25:1917‐1922. [DOI] [PubMed] [Google Scholar]
- 64. Kramer FJ, Mueller M, Rahmstorf M, et al. Ortho‐ and heterotopic bone grafts in bifocal transport osteogenesis for craniofacial reconstruction—an experimental study in sheep. Int J Oral Maxillofac Surg. 2004;33:575‐583. [DOI] [PubMed] [Google Scholar]
- 65. Blaszczyk B, Kaspera W, Ficek K, et al. Effects of polylactide copolymer implants and platelet‐rich plasma on bone regeneration within a large calvarial defect in sheep. Biomed Res Int. 2018;2018:4120471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cao Y, Xiong J, Mei S, et al. Aspirin promotes bone marrow mesenchymal stem cell‐based calvarial bone regeneration in mini swine. Stem Cell Res Ther. 2015;6:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rolfing JH, Jensen J, Jensen JN, et al. A single topical dose of erythropoietin applied on a collagen carrier enhances calvarial bone healing in pigs. Acta Orthop. 2014;85:201‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hoshino M, Egi T, Terai H, et al. Regenerative repair of long intercalated rib defects using porous cylinders of beta‐tricalcium phosphate: an experimental study in a canine model. Plast Reconstr Surg. 2007;119:1431‐1439. [DOI] [PubMed] [Google Scholar]
- 69. Tang H, Xu Z, Qin X, et al. Chest wall reconstruction in a canine model using polydioxanone mesh, demineralized bone matrix and bone marrow stromal cells. Biomaterials. 2009;30:3224‐3233. [DOI] [PubMed] [Google Scholar]
- 70. Wilson TA, Rehder K, Krayer S, et al. Geometry and respiratory displacement of human ribs. J Appl Physiol. 1985;62:1872‐1877. [DOI] [PubMed] [Google Scholar]
- 71. Margulies SS, Rodarte JR, Hoffman EA. Geometry and kinematics of dog ribs. J Appl Physiol. 1985;67:707‐712. [DOI] [PubMed] [Google Scholar]
- 72. Chang DW, Satterfield WC, Son D, et al. Use of vascularized periosteum or bone to improve healing of segmental allografts after tumor resection: an ovine rib model. Plast Reconstr Surg. 2009;123:71‐78. [DOI] [PubMed] [Google Scholar]
- 73. Zhang R, Magel L, Jonigk D, et al. Biosynthetic nanostructured cellulose patch for chest wall reconstruction: five‐month follow‐up in a Porcine model. J Invest Surg. 2017;30:297‐302. [DOI] [PubMed] [Google Scholar]
- 74. Huri PY, Huri G, Yasar U, et al. A biomimetic growth factor delivery strategy for enhanced regeneration of iliac crest defects. Biomed Mater. 2013;8:045009. [DOI] [PubMed] [Google Scholar]
- 75. Tian XF, Heng BC, Ge Z, et al. Comparison of osteogenesis of human embryonic stem cells within 2D and 3D culture systems. Scand J Clin Lab Invest. 2008;68:58‐67. [DOI] [PubMed] [Google Scholar]
- 76. Alt V, Cheung WH, Chow SK, et al. Bone formation and degradation behavior of nanocrystalline hydroxyapatite with or without collagen‐type 1 in osteoporotic bone defects—an experimental study in osteoporotic goats. Injury. 2016;47(suppl 2):S58‐65. [DOI] [PubMed] [Google Scholar]
- 77. Kruyt MC, Dhert WJ, Yuan H, et al. Bone tissue engineering in a critical size defect compared to ectopic implantations in the goat. J Orthop Res. 2004;22:544‐551. [DOI] [PubMed] [Google Scholar]
- 78. Anderson ML, Dhert WJ, Bruijn JD, et al. Critical size defect in the goat's os ilium. A model to evaluate bone grafts and substitutes. Clin Orthop Relat Res. 1999:231‐239. [DOI] [PubMed] [Google Scholar]
- 79. Kaempfen A, Todorov A, Guven S, et al. Engraftment of prevascularized, tissue engineered constructs in a novel rabbit segmental bone defect model. Int J Mol Sci. 2015;16:12616‐12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Murakami N, Saito N, Horiuchi H, et al. Repair of segmental defects in rabbit humeri with titanium fiber mesh cylinders containing recombinant human bone morphogenetic protein‐2 (rhBMP‐2) and a synthetic polymer. J Biomed Mater Res. 2002;62:169‐174. [DOI] [PubMed] [Google Scholar]
- 81. Kohler P, Ehrnberg A, Kreicbergs A. Osteogenic enhancement of diaphyseal reconstruction: comparison of bone grafts in the rabbit. Acta Orthop Scand. 1990;61:42‐45. [DOI] [PubMed] [Google Scholar]
- 82. Rhodes NP, Hunt JA, Longinotti C, et al. In vivo characterization of Hyalonect, a novel biodegradable surgical mesh. J Surg Res. 2011;168:e31‐38. [DOI] [PubMed] [Google Scholar]
- 83. Faria PE, Carvalho AL, Felipucci DN, et al. Bone formation following implantation of titanium sponge rods into humeral osteotomies in dogs: a histological and histometrical study. Clin Implant Dent Relat Res. 2010;12:72‐79. [DOI] [PubMed] [Google Scholar]
- 84. Sumner DR, Turner TM, Cohen M, et al. Aging does not lessen the effectiveness of TGFbeta2‐enhanced bone regeneration. J Bone Miner Res. 2003;18:730‐736. [DOI] [PubMed] [Google Scholar]
- 85. Luneva SN, Talashova IA, Osipova EV, et al. Effects of composition of biocomposite materials implanted into hole defects of the metaphysis on the reparative regeneration and mineralization of bone tissue. Bull Exp Biol Med. 2013;156:285‐289. [DOI] [PubMed] [Google Scholar]
- 86. Turner TM, Urban RM, Hall DJ, et al. Local and systemic levels of tobramycin delivered from calcium sulfate bone graft substitute pellets. Clin Orthop Relat Res. 2005:97‐104. [DOI] [PubMed] [Google Scholar]
- 87. Pobloth AM, Johnson KA, Schell H, et al. Establishment of a preclinical ovine screening model for the investigation of bone tissue engineering strategies in cancellous and cortical bone defects. BMC Musculoskelet Disord. 2016;17:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Uebersax L, Apfel T, Nuss KM, et al. Biocompatibility and osteoconduction of macroporous silk fibroin implants in cortical defects in sheep. Eur J Pharm Biopharm. 2013;85:107‐118. [DOI] [PubMed] [Google Scholar]
- 89. Nuss KM, Auer JA, Boos A, et al. An animal model in sheep for biocompatibility testing of biomaterials in cancellous bones. BMC Musculoskelet Disord. 2006;7:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Meimandi‐Parizi A, Oryan A, Bigham‐Sadegh A, et al. Effects of chitosan scaffold along with royal jelly or bee venom in regeneration of critical sized radial bone defect in rat. Iran J Vet Res. 2018;19:246‐254. [PMC free article] [PubMed] [Google Scholar]
- 91. Oryan A, Alidadi S, Bigham‐Sadegh A, et al. Healing potentials of polymethylmethacrylate bone cement combined with platelet gel in the critical‐sized radial bone defect of rats. PLOS One. 2018;13:e0194751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang ZX, Chen C, Zhou Q, et al. The treatment efficacy of bone tissue engineering strategy for repairing segmental bone defects under osteoporotic conditions. Tissue Eng Part A. 2015;21:2346‐2355. [DOI] [PubMed] [Google Scholar]
- 93. Zhao MD, Huang JS, Zhang XC, et al. Construction of radial defect models in rabbits to determine the critical size defects. PLOS One. 2016;11:e0146301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhao L, Zhao J, Wang S, et al. Comparative study between tissue‐engineered periosteum and structural allograft in rabbit critical‐sized radial defect model. J Biomed Mater Res B Appl Biomater. 2011;97:1‐9. [DOI] [PubMed] [Google Scholar]
- 95. Lane JM, Sandhu HS. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18:213‐225. [PubMed] [Google Scholar]
- 96. Sharifi D, Khoushkerdar HR, Abedi G, et al. Mechanical properties of radial bone defects treated with autogenous graft covered with hydroxyapatite in rabbit. Acta Cir Bras. 2012;27:256‐259. [DOI] [PubMed] [Google Scholar]
- 97. Yilmaz O, Ozmeric A, Alemdaroglu KB, et al. Effects of concentrated growth factors (CGF) on the quality of the induced membrane in Masquelet's technique—an experimental study in rabbits. Injury. 2018;49:1497‐1503. [DOI] [PubMed] [Google Scholar]
- 98. Witek L, Alifarag AM, Tovar N, et al. Repair of critical‐sized long bone defects using dipyridamole‐augmented 3D‐printed bioactive ceramic scaffolds. J Orthop Res. 2019;37:2499‐2507. [DOI] [PubMed] [Google Scholar]
- 99. Saxer F, Scherberich A, Todorov A, et al. Implantation of stromal vascular fraction progenitors at bone fracture sites: from a rat model to a first‐in‐man study. Stem Cells. 2016;34:2956‐2966. [DOI] [PubMed] [Google Scholar]
- 100. Montijo HE, Kellam JF, Gettys FK, et al. Utilization of the AO LockingRatNail in a novel rat femur critical defect model. J Invest Surg. 2012;25:381‐386. [DOI] [PubMed] [Google Scholar]
- 101. Angle SR, Sena K, Sumner DR, et al. Healing of rat femoral segmental defect with bone morphogenetic protein‐2: a dose response study. J Musculoskelet Neuronal Interact. 2012;12:28‐37. [PubMed] [Google Scholar]
- 102. Jager M, Sager M, Lensing‐Hohn S, et al. The critical size bony defect in a small animal for bone healing studies (II): implant evolution and surgical technique on a rat's femur. Biomed Tech. 2005;50:137‐142. [DOI] [PubMed] [Google Scholar]
- 103. Shen HC, Peng H, Usas A, et al. Structural and functional healing of critical‐size segmental bone defects by transduced muscle‐derived cells expressing BMP4. J Gene Med. 2004;6:984‐991. [DOI] [PubMed] [Google Scholar]
- 104. Fukuroku J, Inoue N, Rafiee B, et al. Extracortical bone‐bridging fixation with use of cortical allograft and recombinant human osteogenic protein‐1. J Bone Joint Surg Am. 2007;89:1486‐1496. [DOI] [PubMed] [Google Scholar]
- 105. Johnson AL, Shokry MM, Stein LE. Preliminary study of ethylene oxide sterilization of full‐thickness cortical allografts used in segmental femoral fracture repair. Am J Vet Res. 1985;46:1050‐1056. [PubMed] [Google Scholar]
- 106. Zabka AG, Pluhar GE, Edwards RB, et al. Histomorphometric description of allograft bone remodeling and union in a canine segmental femoral defect model: a comparison of rhBMP‐2, cancellous bone graft, and absorbable collagen sponge. J Orthop Res. 2001;19:318‐327. [DOI] [PubMed] [Google Scholar]
- 107. Kraus KH, Kadiyala S, Wotton H, et al. Critically sized osteo‐periosteal femoral defects: a dog model. J Invest Surg. 1999;12:115‐124. [DOI] [PubMed] [Google Scholar]
- 108. Nather A, David V, Teng JW, et al. Effect of autologous mesenchymal stem cells on biological healing of allografts in critical‐sized tibial defects simulated in adult rabbits. Ann Acad Med Singapore. 2010;39:599‐606. [PubMed] [Google Scholar]
- 109. Pobloth AM, Schell H, Petersen A, et al. Tubular open‐porous beta‐tricalcium phosphate polycaprolactone scaffolds as guiding structure for segmental bone defect regeneration in a novel sheep model. J Tissue Eng Regen Med. 2018;12:897‐911. [DOI] [PubMed] [Google Scholar]
- 110. Ben‐David D, Fishman B, Rubin G, et al. Autologous cell‐coated particles for the treatment of segmental bone defects‐a new cell therapy approach. J Orthop Surg Res. 2019;14:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Li JJ, Dunstan CR, Entezari A, et al. A novel bone substitute with high bioactivity, strength, and porosity for repairing large and load‐bearing bone defects. Adv Healthc Mater. 2019;8:e1801298. [DOI] [PubMed] [Google Scholar]
- 112. Berner A, Henkel J, Woodruff MA, et al. Delayed minimally invasive injection of allogenic bone marrow stromal cell sheets regenerates large bone defects in an ovine preclinical animal model. Stem Cells Transl Med. 2015;4:503‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liang H, Wang K, Shimer AL, et al. Use of a bioactive scaffold for the repair of bone defects in a novel reproducible vertebral body defect model. Bone. 2010;47:197‐204. [DOI] [PubMed] [Google Scholar]
- 114. Shen GY, Ren H, Tang JJ, et al. Effect of osteoporosis induced by ovariectomy on vertebral bone defect/fracture in rat. Oncotarget. 2017;8:73559‐73567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sakata M, Tonomura H, Itsuji T, et al. Osteoporotic effect on bone repair in lumbar vertebral body defects in a rat model. J Orthop Surg. 2018;26:2309499018770349. [DOI] [PubMed] [Google Scholar]
- 116. James AW, Chiang M, Asatrian G, et al. Vertebral implantation of NELL‐1 enhances bone formation in an osteoporotic sheep model. Tissue Eng Part A. 2016;22:840‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yang HL, Zhu XS, Chen L, et al. Bone healing response to a synthetic calcium sulfate/beta‐tricalcium phosphate graft material in a sheep vertebral body defect model. J Biomed Mater Res B Appl Biomater. 2012;100:1911‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kobayashi H, Fujishiro T, Belkoff SM, et al. Long‐term evaluation of a calcium phosphate bone cement with carboxymethyl cellulose in a vertebral defect model. J Biomed Mater Res A. 2009;88:880‐888. [DOI] [PubMed] [Google Scholar]
- 119. Fujishiro T, Bauer TW, Kobayashi N, et al. Histological evaluation of an impacted bone graft substitute composed of a combination of mineralized and demineralized allograft in a sheep vertebral bone defect. J Biomed Mater Res A. 2007;82:538‐544. [DOI] [PubMed] [Google Scholar]
- 120. Zhu X, Chen X, Chen C, et al. Evaluation of calcium phosphate and calcium sulfate as injectable bone cements in sheep vertebrae. J Spinal Disord Tech. 2012;25:333‐337. [DOI] [PubMed] [Google Scholar]
- 121. Zhu XS, Zhang ZM, Mao HQ, et al. A novel sheep vertebral bone defect model for injectable bioactive vertebral augmentation materials. J Mater Sci Mater Med. 2011;22:159‐164. [DOI] [PubMed] [Google Scholar]
- 122. Wang Z, Lu B, Chen L, et al. Evaluation of an osteostimulative putty in the sheep spine. J Mater Sci Mater Med. 2011;22:185‐191. [DOI] [PubMed] [Google Scholar]
- 123. Miller MQ, McColl LF, Arul MR, et al. Assessment of Hedgehog signaling pathway activation for craniofacial bone regeneration in a critical‐sized rat mandibular defect. JAMA Facial Plast Surg. 2019;21:110‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Baskin JZ, Soenjaya Y, McMasters J, et al. Nanophase bone substitute for craniofacial load bearing application: Pilot study in the rodent. J Biomed Mater Res B Appl Biomater. 2018;106:520‐532. [DOI] [PubMed] [Google Scholar]
- 125. Jorge RS, Jorge J Jr., Luz JG. Reconstruction of a mandibular critical‐sized defect using iliac graft in rats. Implant Dent. 2006;15:282‐289. [DOI] [PubMed] [Google Scholar]
- 126. Kowalczewski CJ, Tombyln S, Wasnick DC, et al. Reduction of ectopic bone growth in critically‐sized rat mandible defects by delivery of rhBMP‐2 from kerateine biomaterials. Biomaterials. 2014;35:3220‐3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Saadeh PB, Khosla RK, Mehrara BJ, et al. Repair of a critical size defect in the rat mandible using allogenic type I collagen. J Craniofac Surg. 2001;12:573‐579. [DOI] [PubMed] [Google Scholar]
- 128. Bhattarai G, Kook SH, Kim JH, et al. COMP‐Ang1 prevents periodontitic damages and enhances mandible bone growth in an experimental animal model. Bone. 2016;92:168‐179. [DOI] [PubMed] [Google Scholar]
- 129. Zhang W, Abukawa H, Troulis MJ, et al. Tissue engineered hybrid tooth‐bone constructs. Methods. 2009;47:122‐128. [DOI] [PubMed] [Google Scholar]
- 130. Bhumiratana S, Bernhard JC, Alfi DM, et al. Tissue‐engineered autologous grafts for facial bone reconstruction. Sci Transl Med. 2016;8:343ra383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mulloy C, Guidry RF, Sharma S, et al. Experimental model of zygomatic and mandibular defects to support the development of custom three‐dimensional—printed bone scaffolds. J Craniofac Surg. 2020;31(5):1488‐1491. [DOI] [PubMed] [Google Scholar]
- 132. Watson PJ, Fitton LC, Meloro C, et al. Mechanical adaptation of trabecular bone morphology in the mammalian mandible. Sci Rep. 2018;8:7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Herring SW. TMJ anatomy and animal models. J Musculoskelet Neuronal Interact. 2003;3(4):391‐394. [PMC free article] [PubMed] [Google Scholar]
- 134. Skedros JG, Su SC, Bloebaum RD. Biomechanical implications of mineral content and microstructural variations in cortical bone of horse, elk, and sheep calcanei. Anat Rec. 1997;249:297‐316. [DOI] [PubMed] [Google Scholar]
- 135. Christen P, Ito K, Ellouz R, et al. Bone remodelling in humans is load‐driven but not lazy. Nat Commun. 2014;5:4855. [DOI] [PubMed] [Google Scholar]
- 136. Hart NH, Nimphius S, Rantalainen T, et al. Mechanical basis of bone strength: influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact. 2017;17:114‐139. [PMC free article] [PubMed] [Google Scholar]
- 137. Skedros JG, Sorenson SM, Jenson NH. Are distributions of secondary osteon variants useful for interpreting load history in mammalian bones? Cells Tissues Organs. 2007;185:285‐307. [DOI] [PubMed] [Google Scholar]
- 138. Taylor ME, Tanner KE, Freeman MAR, et al. Stress and strain distribution within the intact femur: compression or bending. Med Eng Phys. 1996;18:122‐131. [DOI] [PubMed] [Google Scholar]
- 139. Duda GN, Schneider E, Chao EY. Internal forces and moments in the femur during walking. J Biomech. 1997;30:933‐941. [DOI] [PubMed] [Google Scholar]
- 140. Shahar R, Banks‐Sills L, Eliasy R. Stress and strain distribution in the intact canine femur: finite element analysis. Med Eng Phys. 2003;25:387‐395. [DOI] [PubMed] [Google Scholar]
- 141. Simmons T, Goodburn B, Singhrao SK. Decision tree analysis as a supplementary tool to enhance histomorphological differentiation when distinguishing human from non‐human cranial bone in both burnt and unburnt states: a feasibility study. Med Sci Law. 2016;56:36‐45. [DOI] [PubMed] [Google Scholar]
- 142. Nganvongpanit K, Pradit W, Pitakarnnop T, et al. Differences in osteon structure histomorphometry between puppyhood and adult stages in the golden retriever. Anat Sci Int. 2017;92:483‐492. [DOI] [PubMed] [Google Scholar]
- 143. Stover SM, Pool RR, Martin RB, et al. Histological features of the dorsal cortex of the third metacarpal bone mid‐diaphysis during postnatal growth in thoroughbred horses. J Anat. 1992;181(pt 3):455‐469. [PMC free article] [PubMed] [Google Scholar]
- 144. Mori R, Kodaka T, Sano T, et al. Comparative histology of the laminar bone between young calves and foals. Cells Tissues Organs. 2003;175:43‐50. [DOI] [PubMed] [Google Scholar]
- 145. Felder AA, Phillips C, Cornish H, et al. Secondary osteons scale allometrically in mammalian humerus and femur. R Soc Open Sci. 2017;4:170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Recker RR, Ste‐Marie LG, Langdahl B, et al. Effects of intermittent intravenous ibandronate injections on bone quality and micro‐architecture in women with postmenopausal osteoporosis: the DIVA study. Bone. 2010;46:660‐665. [DOI] [PubMed] [Google Scholar]
- 148. Essen HW, Holzmann PJ, Blankenstein MA, et al. Effect of raloxifene treatment on osteocyte apoptosis in postmenopausal women. Calcif Tissue Int. 2007;81:183‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Rehman MT, Hoyland JA, Denton J, et al. Age related histomorphometric changes in bone in normal British men and women. J Clin Pathol. 1994;47:529‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mori T, Okimoto N, Sakai A, et al. Climbing exercise increases bone mass and trabecular bone turnover through transient regulation of marrow osteogenic and osteoclastogenic potentials in mice. J Bone Miner Res. 2003;18:2002‐2009. [DOI] [PubMed] [Google Scholar]
- 151. Chen S, Liu D, He S, et al. Differential effects of type 1 diabetes mellitus and subsequent osteoblastic beta‐catenin activation on trabecular and cortical bone in a mouse model. Exp Mol Med. 2018;50:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Slatopolsky E, Cozzolino M, Lu Y, et al. Efficacy of 19‐Nor‐1,25‐(OH)2D2 in the prevention and treatment of hyperparathyroid bone disease in experimental uremia. Kidney Int. 2003;63:2020‐2027. [DOI] [PubMed] [Google Scholar]
- 153. Xu J, Wang B, Sun Y, et al. Human fetal mesenchymal stem cell secretome enhances bone consolidation in distraction osteogenesis. Stem Cell Res Ther. 2016;7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lu H, Cui L, Zuo C, et al. Evaluation of morphological parameters of bone formation in Sprague‐Dawley rats of different ages by in vivo fluorochrome labeling. Italian J Zoo. 2015;82:33‐40. [Google Scholar]
- 155. Kondo E, Yasoda A, Fujii T, et al. Increased bone turnover and possible accelerated fracture healing in a Murine model with an increased circulating C‐type natriuretic peptide. Endocrinology. 2015;156:2518‐2529. [DOI] [PubMed] [Google Scholar]
- 156. Connolly JF, Hahn H, Davy D. Fracture healing in weight‐bearing and nonweight‐bearing bones. J Trauma. 1978;18:766‐770. [DOI] [PubMed] [Google Scholar]
- 157. Lim J, Lee J, Yun H‐S, et al. Comparison of bone regeneration rate in flat and long bone defects: Calvarial and tibial bone. Tissue Eng Regen Med. 2013;10:336‐340. [Google Scholar]
- 158. Levi B, Nelson ER, Li S, et al. Dura mater stimulates human adipose‐derived stromal cells to undergo bone formation in mouse calvarial defects. Stem Cells. 2011;29:1241‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mizuno H, Kikuta J, Ishii M. In vivo live imaging of bone cells. Histochem Cell Biol. 2018;149:417‐422. [DOI] [PubMed] [Google Scholar]
- 160. Villa MM, Wang L, Huang J, et al. Visualizing osteogenesis in vivo within a cell‐scaffold construct for bone tissue engineering using two‐photon microscopy. Tissue Eng Part C Methods. 2013;19:839‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Jensen J, Tvedesøe C, Rölfing JHD, et al. Dental pulp‐derived stromal cells exhibit a higher osteogenic potency than bone marrow‐derived stromal cells in vitro and in a porcine critical‐size bone defect model. SICOT‐J. 2016;2:16‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Vila PM, Jeanpierre LM, Rizzi CJ, et al. Comparison of autologous vs homologous costal cartilage grafts in dorsal augmentation rhinoplasty: a systematic review and meta‐analysis. JAMA Otolaryngol Head Neck Surg. 2020;146(4):347‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Brown JS, Lowe D, Kanatas A, et al. Mandibular reconstruction with vascularised bone flaps: a systematic review over 25 years. Br J Oral Maxillofac Surg. 2017;55:113‐126. [DOI] [PubMed] [Google Scholar]
- 164. Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg. 2006;81:279‐285. [DOI] [PubMed] [Google Scholar]
- 165. Liu F, Chen K, Hou L, et al. Determining the critical size of a rabbit rib segmental bone defect model. Regenerative biomaterials. 2016;3:323‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ishihara A, Zekas LJ, Weisbrode SE, et al. Comparative efficacy of dermal fibroblast‐mediated and direct adenoviral bone morphogenetic protein‐2 gene therapy for bone regeneration in an equine rib model. Gene Ther. 2010;17:733‐744. [DOI] [PubMed] [Google Scholar]
- 167. Tatara AM, Shah SR, Demian N, et al. Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors. Acta Biomater. 2016;45:72‐84. [DOI] [PubMed] [Google Scholar]
- 168. Tatara AM, Koons GL, Watson E, et al. Biomaterials‐aided mandibular reconstruction using in vivo bioreactors. Proc Natl Acad Sci USA. 2019;116:6954‐6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Liu FZ, Wang DW, Zhang YJ, et al. Comparison of rabbit rib defect regeneration with and without graft. J Mater Sci Mater Med. 2017;28:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Callison WE, Holowka NB, Lieberman DE. Thoracic adaptations for ventilation during locomotion in humans and other mammals. J Exp Biol. 2019;222:jeb189357. [DOI] [PubMed] [Google Scholar]
- 171. De Troyer A, Wilson TA. Action of the isolated canine diaphragm on the lower ribs at high lung volumes. J Physiol. 2014;592:4481‐4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. De Troyer A, Legrand A, Wilson TA. Respiratory mechanical advantage of the canine external and internal intercostal muscles. J Pysiol. 1999;518:283‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Thomsen JS, Ebbesen EN, Mosekilde L. Static histomorphometry of human iliac crest and vertebral trabecular bone: a comparative study. Bone. 2002;30:267‐274. [DOI] [PubMed] [Google Scholar]
- 174. Giavaresi G, Fini M, Martini L, et al. Histomorphometric characterization of cancellous and cortical bone in an ovariectomized sheep model. J Appl Animal Res. 2001;20:221‐232. [Google Scholar]
- 175. Gogolewski S, Gorna K, Turner AS. Regeneration of bicortical defects in the iliac crest of estrogen‐deficient sheep, using new biodegradable polyurethane bone graft substitutes. J Biomed Mater Res A. 2006;77:802‐810. [DOI] [PubMed] [Google Scholar]
- 176. Egermann M, Goldhahn J, Holz R, et al. A sheep model for fracture treatment in osteoporosis: benefits of the model versus animal welfare. Lab Anim. 2008;42:453‐464. [DOI] [PubMed] [Google Scholar]
- 177. Engelstad ME, Morse T. Anterior iliac crest, posterior iliac crest, and proximal tibia donor sites: a comparison of cancellous bone volumes in fresh cadavers. J Oral Maxillofac Surg. 2010;68:3015‐3021. [DOI] [PubMed] [Google Scholar]
- 178. Boone DW. Complications of iliac crest graft and bone grafting alternatives in foot and ankle surgery. Foot Ankle Clin. 2003;8:1‐14. [DOI] [PubMed] [Google Scholar]
- 179. Pohlmeyer K, Schmelzeisen R, Botel C. Anatomical basis for transplantation of microsurgical anastomosed autologous and allogenic bone transplantants for reconstruction of the mandible in animal experiments (Gottingen‐Minipig). Anat Anz. 1990;171:221‐226. [PubMed] [Google Scholar]
- 180. Lei P, Du W, Liu H, et al. Free vascularized iliac bone flap based on deep circumflex iliac vessels graft for the treatment of osteonecrosis of femoral head. J Orthop Surg Res. 2019;14:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Urbankova I, Vdoviakova K, Rynkevic R, et al. Comparative anatomy of the ovine and female pelvis. Gynecol Obstet Invest. 2017;82:582‐591. [DOI] [PubMed] [Google Scholar]
- 182. Meling T, Harboe K, Soreide K. Incidence of traumatic long‐bone fractures requiring in‐hospital management: a prospective age‐ and gender‐specific analysis of 4890 fractures. Injury. 2009;40:1212‐1219. [DOI] [PubMed] [Google Scholar]
- 183. Joeris A, Lutz N, Wicki B, et al. An epidemiological evaluation of pediatric long bone fractures—a retrospective cohort study of 2716 patients from two Swiss tertiary pediatric hospitals. BMC Pediatr. 2014;14:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Scholes S, Panesar S, Shelton NJ, et al. Epidemiology of lifetime fracture prevalence in England: a population study of adults aged 55 years and over. Age Ageing. 2014;43:234‐240. [DOI] [PubMed] [Google Scholar]
- 185. Iwasashi M, Funayama T, Watanabe A, et al. Bone regeneration and remodeling within a unidirectional porous hydroxyapatite bone substitute at a cortical bone defect site: histological analysis at one and two years after implantation. Materials. 2015;8:4884‐4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Huffer WE, Benedict JJ, Turner AS, et al. Repair of sheep long bone cortical defects filled with COLLOSS, COLLOSS E, OSSAPLAST, and fresh iliac crest autograft. J Biomed Mater Res B Appl Biomater. 2007;82:460‐470. [DOI] [PubMed] [Google Scholar]
- 187. Meadows TH, Bronk JT, Chao YS, et al. Effect of weight‐bearing on healing of cortical defects in the canine tibia. J Bone Joint Surg Am. 1990;72:1074‐1080. [PubMed] [Google Scholar]
- 188. Herten M, Zilkens C, Thorey F, et al. Biomechanical stability and osteogenesis in a tibial bone defect treated by autologous ovine cord blood cells‐a pilot study. Molecules. 2019;24(2):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Street J, Bao M, de Guzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656‐9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zhang H, Shi X, Wang L, et al. Intramembranous ossification and endochondral ossification are impaired differently between glucocorticoid‐induced osteoporosis and estrogen deficiency‐induced osteoporosis. Sci Rep. 2018;8:3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Arens D, Wilke M, Calabro L, et al. A rabbit humerus model of plating and nailing osteosynthesis with and without Staphylococcus aureus osteomyelitis. Eur Cell Mater. 2015;30:148‐161. [DOI] [PubMed] [Google Scholar]
- 192. Fernandes MB, Guimaraes JA, Casado PL, et al. The effect of bone allografts combined with bone marrow stromal cells on the healing of segmental bone defects in a sheep model. BMC Vet Res. 2014;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Pobloth AM, Checa S, Razi H, et al. Mechanobiologically optimized 3D titanium‐mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci Transl Med. 2018:828. [DOI] [PubMed] [Google Scholar]
- 194. Christou C, Oliver RA, Pelletier MH, et al. Ovine model for critical‐size tibial segmental defects. Comp Med. 2014;64:377‐385. [PMC free article] [PubMed] [Google Scholar]
- 195. Runyan CM, Vu AT, Rumburg A, et al. Repair of a critical porcine tibial defect by means of allograft revitalization. Plast Reconstr Surg. 2015;136:461e‐473e. [DOI] [PubMed] [Google Scholar]
- 196. Southam BR, Archdeacon MT. “Iatrogenic” segmental defect: how I debride high‐energy open tibial fractures. J Orthop Trauma. 2017;31(suppl 5):S9‐S15. [DOI] [PubMed] [Google Scholar]
- 197. Liebschner MA. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials. 2004;25:1697‐1714. [DOI] [PubMed] [Google Scholar]
- 198. Liu C, Cabahug‐Zuckerman P, Stubbs C, et al. Mechanical loading promotes the expansion of primitive osteoprogenitors and organizes matrix and vascular morphology in long bone defects. J Bone Miner Res. 2019;34:896‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Liu C, Carrera R, Flamini V, et al. Effects of mechanical loading on cortical defect repair using a novel mechanobiological model of bone healing. Bone. 2018;108:145‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Collet P, Uebelhart D, Vico L, et al. Effects of 1‐ and 6‐month spaceflight on bone mass and biochemistry in two humans. Bone. 1997;20:547‐551. [DOI] [PubMed] [Google Scholar]
- 201. Vico L, Collet P, Guignandon A, et al. Effects of long‐term microgravity exposure on cancellous and cortical weight‐bearing bones of cosmonauts. Lancet. 2000;355:1607‐1611. [DOI] [PubMed] [Google Scholar]
- 202. Giszter SF, Davies MR, Graziani V. Coordination strategies for limb forces during weight‐bearing locomotion in normal rats, and in rats spinalized as neonates. Exp Brain Res. 2008;190:53‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Gushue DL, Houck J, Lerner AL. Rabbit knee joint biomechanics: motion analysis and modeling of forces during hopping. J Orthop Res. 2005;23:735‐742. [DOI] [PubMed] [Google Scholar]
- 204. Martin RB, Chapman MW, Sharkey NA, et al. Bone ingrowth and mechanical properties of coralline hydroxyapatite 1 yr after implantation. Biomaterials. 1993;14:341‐348. [DOI] [PubMed] [Google Scholar]
- 205. Fenger JM, London CA, Kisseberth WC. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J. 2014;55:69‐85. [DOI] [PubMed] [Google Scholar]
- 206. Saulsman B, Oxnard CE, Franklin D. Long bone morphometrics for human from non‐human discrimination. Forensic Sci Int. 2010;202(110):e111‐115. [DOI] [PubMed] [Google Scholar]
- 207. Landis WJ, Glimcher MJ. Electron diffraction and electron probe microanalysis of the mineral phase of bone tissue prepared by anhydrous techniques. J Ultrastruct Res. 1978;63:188‐223. [DOI] [PubMed] [Google Scholar]
- 208. Carrera EF, Nicolao FA, Netto NA, et al. A mechanical comparison between conventional and modified angular plates for proximal humeral fractures. J Shoulder Elbow Surg. 2008;17:631‐636. [DOI] [PubMed] [Google Scholar]
- 209. Lind PM, Lind L, Larsson S, et al. Torsional testing and peripheral quantitative computed tomography in rat humerus. Bone. 2001;29:265‐270. [DOI] [PubMed] [Google Scholar]
- 210. Zura R, Xiong Z, Einhorn T, et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 2016;151:e162775. [DOI] [PubMed] [Google Scholar]
- 211. Tzioupis C, Giannoudis PV. Prevalence of long‐bone non‐unions. Injury. 2007;38:S3‐S9. [DOI] [PubMed] [Google Scholar]
- 212. Ekegren CL, Edwards ER, Steiger R, et al. Incidence, costs, and predictors of non‐union, delayed union and Mal‐union following long bone fracture. Int J Environ Res Public Health. 2018;15:2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Netto CC, Vieira VC, Marinheiro LP, et al. Are skeletally mature female rats a suitable model to study osteoporosis? Arq Bras Endocrinol Metabol. 2012;56:259‐264. [DOI] [PubMed] [Google Scholar]
- 214. Meinig RP, Buesing CM, Helm J, et al. Regeneration of diaphyseal bone defects using resorbable poly(L/DL‐lactide) and poly(D‐lactide) membranes in the Yucatan pig model. J Orthop Trauma. 1997;11:551‐558. [DOI] [PubMed] [Google Scholar]
- 215. Roschger P, Fratzl P, Eschberger J, et al. Validation of quantitative backscattered electron imaging for the measurement of mineral density distribution in human bone biopsies. Bone. 1998;23:319‐326. [DOI] [PubMed] [Google Scholar]
- 216. Garcia P, Histing T, Holstein JH, et al. Rodent animal models of delayed bone healing and non‐union formation: a comprehensive review. Eur Cell Mater. 2013;26:1‐12. [DOI] [PubMed] [Google Scholar]
- 217. Bloebaum RD, Ota DT, Skedros JG, et al. Comparison of human and canine external femoral morphologies in the context of total hip replacement. J Biomed Mater Res. 1993;27:1149‐1159. [DOI] [PubMed] [Google Scholar]
- 218. Sumner DR Jr., Devlin TC, Winkelman D, et al. The geometry of the adult canine proximal femur. J Orthop Res. 1990;8:671‐677. [DOI] [PubMed] [Google Scholar]
- 219. Claes LE, Cunningham JL. Monitoring the mechanical properties of healing bone. Clin Orthop Relat Res. 2009;467:1964‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Taguchi T, Koh R, Takawira C, et al. Agmatine for pain management in dogs with coxofemoral joint osteoarthritis: a pilot study. Front Vet Sci. 2018;5:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Lopez MJ, Quinn MM, Markel MD. Evaluation of gait kinetics in puppies with coxofemoral joint laxity. Am J Vet Res. 2006;67:236‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Seebeck P, Thompson MS, Parwani A, et al. Gait evaluation: a tool to monitor bone healing? Clin Biomech. 2005;20:883‐891. [DOI] [PubMed] [Google Scholar]
- 223. Kironde E, Sekimpi P, Kajja I, et al. Prevalence and patterns of traumatic bone loss following open long bone fractures at Mulago Hospital. OTA Intl. 2019;2:e015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Fourie PD, Kirton AH, Jury KE. Growth and development of sheep: 2. Effect of breed and sex on growth and carcass composition of southdown and Romney and their cross. New Zealand J Agric Res. 1970;13:753. [Google Scholar]
- 225. Gautier E, Perren SM, Cordey J. Strain distribution in plated and unplated sheep tibia an in vivo experiment. Injury. 2000;31(suppl 3):C37‐44. [DOI] [PubMed] [Google Scholar]
- 226. Herring SW, Mucci RJ. In vivo strain in cranial sutures: the zygomatic arch. J Morphol. 1991;207:225‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Herring SW, Rafferty KL, Liu ZJ, et al. Jaw muscles and the skull in mammals: the biomechanics of mastication. Comp Biochem Physiol A Mol Integr Physiol. 2001;131:207‐219. [DOI] [PubMed] [Google Scholar]
- 228. Renaud M, Farkasdi S, Pons C, et al. A new rat model for translational research in bone regeneration. Tissue Eng Part C Methods. 2016;22:125‐131. [DOI] [PubMed] [Google Scholar]
- 229. Vanecek V, Klima K, Kohout A, et al. The combination of mesenchymal stem cells and a bone scaffold in the treatment of vertebral body defects. Eur Spine J. 2013;22:2777‐2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Sakata M, Tonomura H, Itsuji T, et al. Bone regeneration of osteoporotic vertebral body defects using platelet‐rich plasma and gelatin beta‐tricalcium phosphate sponges. Tissue Eng Part A. 2018;24:1001‐1010. [DOI] [PubMed] [Google Scholar]
- 231. Gunnella F, Kunisch E, Maenz S, et al. The GDF5 mutant BB‐1 enhances the bone formation induced by an injectable, poly(l‐lactide‐co‐glycolide) acid (PLGA) fiber‐reinforced, brushite‐forming cement in a sheep defect model of lumbar osteopenia. Spine J. 2018;18:357‐369. [DOI] [PubMed] [Google Scholar]
- 232. Eschler A, Roepenack P, Roesner J, et al. Cementless titanium mesh fixation of osteoporotic burst fractures of the lumbar spine leads to bony healing: results of an experimental sheep model. Biomed Res Int. 2016;2016:4094161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Eschler A, Ropenack P, Herlyn PK, et al. The standardized creation of a lumbar spine vertebral compression fracture in a sheep osteoporosis model induced by ovariectomy, corticosteroid therapy and calcium/phosphorus/vitamin D‐deficient diet. Injury. 2015;46(suppl 4):S17‐23. [DOI] [PubMed] [Google Scholar]
- 234. McLain RF, Yerby SA, Moseley TA. Comparative morphometry of L4 vertebrae: comparison of large animal models for the human lumbar spine. Spine. 2002;27:E200‐206. [DOI] [PubMed] [Google Scholar]
- 235. Busscher I, Ploegmakers JJ, Verkerke GJ, et al. Comparative anatomical dimensions of the complete human and porcine spine. Eur Spine J. 2010;19:1104‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Denyer S, Bhimani AD, Papastefan S, et al. Magnetic kyphoplasty: A novel drug delivery system for the spinal column. PLOS One. 2018;13:e0201402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ando K, Imagama S, Kobayashi K, et al. Feasibility and effects of a self‐assembling peptide as a scaffold in bone healing: an in vivo study in rabbit lumbar posterolateral fusion and tibial intramedullary models. J Orthop Res. 2018;36:3285‐3293. [DOI] [PubMed] [Google Scholar]
- 238. Glazer PA, Spencer UM, Alkalay RN, et al. In vivo evaluation of calcium sulfate as a bone graft substitute for lumbar spinal fusion. Spine J. 2001;1:395‐401. [DOI] [PubMed] [Google Scholar]
- 239. Kai T, Shao‐qing G, Geng‐ting D. In vivo evaluation of bone marrow stromal‐derived osteoblasts‐porous calcium phosphate ceramic composites as bone graft substitute for lumbar intervertebral spinal fusion. Spine. 2003;28:1653‐1658. [DOI] [PubMed] [Google Scholar]
- 240. Lopez MJ, McIntosh KR, Spencer ND, et al. Acceleration of spinal fusion using syngeneic and allogeneic adult adipose derived stem cells in a rat model. J Orthop Res. 2009;27:366‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Hwang K, You SH. Analysis of facial bone fractures: an 11‐year study of 2094 patients. Indian J Plast Surg. 2010;43:42‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Eppley BL, Aalst JA, Robey A, et al. The spectrum of orofacial clefting. Plast Reconstr Surg. 2005;115:101e‐114e. [DOI] [PubMed] [Google Scholar]
- 243. Weijs WA. Mandibular movements of the albino rat during feeding. J Morphol. 1975;145:107‐124. [DOI] [PubMed] [Google Scholar]
- 244. Lieberman DE, Crompton AW. Why fuse the mandibular symphysis? A comparative analysis. Am J Phys Anthropol. 2000;112:517‐540. [DOI] [PubMed] [Google Scholar]
- 245. Alsagheer A, Kline LW, Doschak MR, et al. A novel experimental model for studying transverse orthodontic tooth movement in the rat mandible. Angle Orthod. 2013;83:774‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kimura S, Kitagawa T. Some asymmetries in the weight, area and linear dimensions of the mandible of rats. J Nihon Univ Sch Dent. 1977;19:151‐158. [DOI] [PubMed] [Google Scholar]
- 247. Khojasteh A, Hosseinpour S, Dehghan MM, et al. Antibody‐mediated osseous regeneration for bone tissue engineering in canine segmental defects. Biomed Res Int. 2018;2018:9508721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Hussein KA, Zakhary IE, Elawady AR, et al. Difference in soft tissue response between immediate and delayed delivery suggests a new mechanism for recombinant human bone morphogenetic protein 2 action in large segmental bone defects. Tissue Eng Part A. 2012;18:665‐675. [DOI] [PubMed] [Google Scholar]
- 249. De Kok IJ, Peter SJ, Archambault M, et al. Investigation of allogeneic mesenchymal stem cell‐based alveolar bone formation: preliminary findings. Clin Oral Implants Res. 2003;14:481‐489. [DOI] [PubMed] [Google Scholar]
- 250. Abu‐Serriah M, Kontaxis A, Ayoub A, et al. Mechanical evaluation of mandibular defects reconstructed using osteogenic protein‐1 (rhOP‐1) in a sheep model: a critical analysis. Int J Oral Maxillofac Surg. 2005;34:287‐293. [DOI] [PubMed] [Google Scholar]
- 251. Abu‐Serriah MM, Ayoub AF, Odell E, et al. A minimally invasive novel design for a vascular‐pedicled bone segment for experimental studies of reconstruction of mandibular defects. Br J Oral Maxillofac Surg. 2004;42:236‐240. [DOI] [PubMed] [Google Scholar]
- 252. Aoki A, Kawamoto T, Aoki K, et al. Amount of bone lengthening affects blood flow recovery and bone mineralization after distraction osteogenesis in a canine cleft palate model. Cleft Palate Craniofac J. 2010;47:303‐313. [DOI] [PubMed] [Google Scholar]
- 253. Wang Y, Shi B, Li Y, et al. Comparative study of maxillary growth and occlusal outcome after autogenous rib grafting in complete cleft palate defect. J Craniofac Surg. 2006;17:68‐79. [DOI] [PubMed] [Google Scholar]
- 254. Caballero M, Jones DC, Shan Z, et al. Tissue engineering strategies to improve osteogenesis in the juvenile swine alveolar cleft model. Tissue Eng Part C Methods. 2017;23:889‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Sato A, Yanagi T, Yamaguchi Y, et al. Effect of DNA/protamine complex paste on bone augmentation of the mandible: a pilot study on dogs. Arch Oral Biol. 2020;115:104729. [DOI] [PubMed] [Google Scholar]


