Abstract
Background
Severe reactions may develop during cow's milk (CM) oral immunotherapy (OIT). We investigated the safety and efficacy of low‐dose OIT with heated milk (HM) or unheated milk (UM) in children with anaphylaxis.
Methods
Children with symptom onset after ingestion of 3‐mL HM on a double‐blind, placebo‐controlled food challenge were randomly assigned to the HM (n = 17) or UM (n = 16) group. HM group ingested milk powder heated at 125°C for 30 seconds, whereas the UM group used UM. Patients were hospitalized for 5 days; the HM or UM was gradually increased to 3 mL/day; 3‐mL/day ingestion was continued at home. One year later, the patients underwent 2‐day consecutive 3‐ and 25‐mL HM‐oral food challenges (OFCs) after 2‐week avoidance.
Results
At baseline, milk‐ and casein‐specific immunoglobulin E (IgE) levels were 56.0 and 51.4 kUA/L in the HM group, and 55.2 and 65.6 kUA/L in the UM group, respectively. One year later, 35% and 18% in the HM group and 50% and 31% in UM group passed the 3 and 25 mL OFCs, respectively. Rates of moderate or severe symptoms and respiratory symptoms per home dose were significantly lower in the HM than in the UM group (0.7% and 1.2% vs 1.4% and 2.6%, respectively, P < .001). β‐lactoglobulin‐specific IgG4 levels significantly increased from baseline only in the UM group, whereas casein‐specific IgG4 levels significantly increased from baseline in both groups.
Conclusions
HM‐OIT induced immunological changes more safely than the UM‐OIT. The possibility of lower treatment efficacy with HM‐OIT needs to be evaluated in larger studies.
Keywords: anaphylaxis, casein, cow's milk allergy, desensitization, heated, milk, oral immunotherapy, randomized controlled trial, unheated, β‐lactoglobulin
1. INTRODUCTION
Cow's milk (CM) allergy is one of the main causes of food allergy, with an estimated prevalence of 2%‐3%. 1 , 2 In Japan, CM allergy is the second commonest cause of anaphylaxis. 3 Approximately 80% of children with CM allergy acquire tolerance, but school‐aged children with a high level of specific IgE (sIgE) and a history of anaphylaxis face difficulty in acquiring tolerance. 4 , 5 In a Spanish survey, 40% of children diagnosed with CM allergy reacted on accidental exposure to CM during a 12‐month period. 6 Therefore, patients with severe CM allergy are at risk of reactions following accidental exposure. Oral immunotherapy (OIT) has been reported for food allergy, but adverse reactions, including anaphylaxis, may occur during OIT. 7 , 8 It is more challenging to acquire tolerance with CM‐OIT, and there is a greater likelihood of inducing symptoms with CM‐OIT than with other food antigens. 8 , 9 Moreover, there are only a few studies on OIT among patients with CM anaphylaxis. 7 , 10
The heating process induces conformational changes of the CM epitope—in particular, whey proteins such as β‐lactoglobulin, which trigger reactions in some patients with CM allergy. 11 To improve safety of OIT, some reports have assessed OIT by using baked or heated milk (HM). 12 , 13 , 14 In 2017, a randomized controlled trial (RCT) showed that safety did not differ with the use of baked milk, although the protocol and baseline characteristics were different for the baked and raw milk groups. 14 Furthermore, a comparison of the safety and efficacy of HM and unheated milk (UM) with the same protocol has not been reported thus far.
We hypothesized that OIT with HM would progress more safely than with UM. We conducted this study to investigate the safety and efficacy of OIT by using HM vs UM in children with anaphylactic‐type CM allergy.
2. METHODS
2.1. Study design
This RCT was conducted at the Sagamihara National Hospital (Kanagawa, Japan) between July 2016 and March 2018. Participants were randomized 1:1 to the HM‐OIT or UM‐OIT group, by using a random number generator. Randomization was stratified by a threshold of double‐blind, placebo‐controlled food challenge (DBPCFC; ≤0.75 or >0.75 mL).
2.2. Eligibility criteria
Participants were 5 years old or older and had a history of milk anaphylaxis. 15 , 16 The inclusion criterion was the development of symptoms during DBPCFC with 3‐mL HM. The exclusion criteria were negative DBPCFCs, uncontrolled atopic dermatitis, bronchial asthma, or another ongoing immunotherapy.
2.3. Materials
For the HM group of children, we used milk powder, prepared by heating CM at 125°C for 30 seconds and spray‐drying for 3 seconds. For the UM group of children, we used unheated CM, which is sterilized at 125°C for 2 seconds according to food safety regulations, which is considered to be ultra‐high‐temperature instantaneous sterilization.
2.4. DBPCFC
The DBPCFC was undertaken during two separate days of hospitalization. We used cocoa cake containing 3‐mL CM (102 mg CM protein) or a placebo with a cocoa cake without CM. The cocoa cake was heated to 90°C for 90 seconds in a 1000‐W microwave. One quarter of OFC food was provided initially, and the remaining three quarters were provided 60 minutes later, as previously reported. 17 Treatment was provided appropriately based on the severity of reaction, according to the Japanese anaphylaxis guidelines (Table S1). 3 The threshold dose was defined as the accumulated dose which patients had ingested at the time of symptom onset.
2.5. OIT protocol
Patients were hospitalized for 5 days, and DBPCFC was conducted on the first and second day; if positive, patients were randomized, and OIT was started from the third day, together with a premedication of 10 mg loratadine, and children consumed HM or UM at half the threshold of the baseline DBPCFC (Figure 1). The OIT comprised eight steps, from 0.1 to 3 mL (Table S2). If symptoms were absent or mild, the OIT dose for the next day remained the same. If moderate or severe symptoms developed, the dose for the next day was reduced by 1 or 2 steps, respectively. After discharge, the starting dose was continued at home every day for 1 month. After 1 month, if patients were able to asymptomatically ingest HM or UM for five consecutive days, the dose was increased at the patients' home by 1 step up to 3 mL per day. We offered direct telephone support for OIT patients 24 hours a day, 365 days a year. If children were able to asymptomatically ingest 3 mL of the material for 1 month, loratadine treatment was terminated; this was defined as “desensitization to 3 mL.” After 12 months from the start of OIT, a 2‐day consecutive 3‐ and 25‐mL HM‐OFC was conducted for both groups after 2 weeks of OIT cessation.
FIGURE 1.
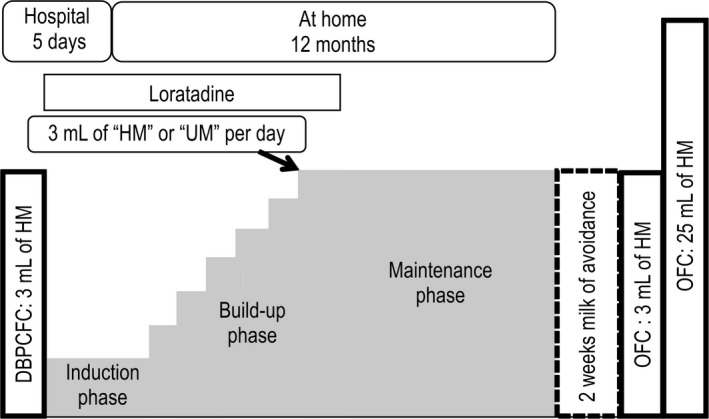
Oral immunotherapy protocol. Children received 3‐ and 25‐mL oral food challenge after 2‐wk avoidance at 12 mo. DBPCFC, double‐blind, placebo‐controlled food challenge; HM, heated milk; OFC, oral food challenge; UM, unheated milk
2.6. Outcomes
The primary endpoint was the rate of total number of symptoms per total number of ingestions at home during the 12‐month study. The secondary endpoints were symptom severity, symptoms by organ, the proportion of desensitization to 3 mL, passing the 3‐ and 25‐mL OFC, and immunological changes.
2.7. Immunological parameters
The sIgE to milk, casein, α‐lactalbumin and β‐lactoglobulin; specific IgG (sIgG); and specific IgG4 (sIgG4) to casein and β‐lactoglobulin were measured using the ImmunoCAP assay system (Thermo Fisher Scientific/Phadia AB, Uppsala, Sweden) at baseline and after 1, 3, 6, and 12 months in both groups.
2.8. Statistical analysis
We hypothesized that the primary outcome, which was that the rate of symptoms would be reduced by 15% with HM, would be 17% and 20% in the HM and UM groups, respectively, with a power of 80%. Therefore, we estimated that 30 participants with a ratio of 1:1 would be sufficient to detect this difference. Values are expressed as the median and range. Differences in categorical data were evaluated with Fisher's exact test. Continuous variables were evaluated with Wilcoxon rank‐sum tests. A P‐value of <.05 was considered statistically significant. Statistical analyses were conducted in SPSS version 24.0 software (IBM Corp., Armonk, NY, USA).
2.9. Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Sagamihara National Hospital Ethics Committee (no.: 2016‐003). This trial was registered at the University Hospital Medical Information Network Clinical Trials Registry (no: UMIN000011202). Written informed consent was obtained from the guardians following an explanation of the study design and risk of symptoms. We anonymized all data before analysis.
3. RESULTS
3.1. Study population
During screening, 35 children underwent the baseline DBPCFC, but two passed the test and were excluded. Thirty‐three children with CM anaphylaxis were randomized to the HM or UM group (n = 17 or 16, respectively; Figure 2). Median milk‐ and casein‐specific IgE levels were 56.0 and 51.4 kUA/L vs 55.2 and 65.6 kUA/L in the HM and UM groups, respectively (Table 1).
FIGURE 2.
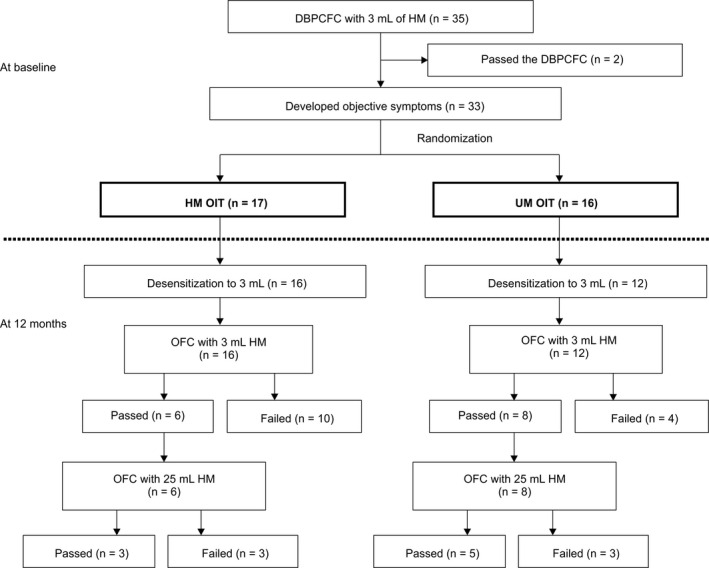
Patient inclusion flowchart. One patient in the UM group discontinued OIT because of eosinophilic esophagitis at 1 mo. One patient in HM group and three patients in UM group did not reach desensitization because of adverse reactions (one due to mucosal symptom in HM group, two due to respiratory symptom, and one due to mucosal symptoms in the UM group) at home. For patients who passed the 3‐mL OFC, we allowed ingestion of 10 g butter. DBPCFC, double‐blind, placebo‐controlled food challenge; HM, heated milk; OFC, oral food challenge; OIT, oral immunotherapy; UM, unheated milk
TABLE 1.
Patient characteristics in the HM and UM groups
| HM group (n = 17) | UM group (n = 16) | P‐value | |
|---|---|---|---|
| Age (y) | 7.6 (5.2‐11.2) | 6.1 (5.3‐10.8) | .052 |
| Male, n (%) | 14 (82.4%) | 11 (68.8%) | .43 |
| Current complications | |||
| BA, n (%) | 9 (53%) | 8 (50%) | >.99 |
| AD, n (%) | 10 (59%) | 10 (63%) | .82 |
| AR, n (%) | 4 (24%) | 4 (25%) | >.99 |
| History of anaphylaxis to milk a | 2 (1‐3) | 2 (1‐5) | .17 |
| Most recent anaphylaxis to milk | |||
| Period until entry (mo) | 13 (3‐60) | 9.5 (4‐62) | .51 |
| HM caused anaphylaxis, n (%) | 16 (94%) | 14 (88%) | .87 |
| DBPCFC | |||
| Threshold to induce symptoms, n (%) | ≤0.75 mL: 10 (59%) | ≤0.75 mL: 10 (63%) | >.99 |
| >0.75 mL: 7 (41%) | >0.75 mL: 6 (38%) | ||
| Severity of symptoms | Mild 4, Moderate 13 | Mild 2, Moderate 13, Severe 1 | .65 |
| Total IgE (IU/mL) | 1140 (146‐11 000) | 648 (69‐6770) | .20 |
| Specific IgE (kUA/L) | |||
| Milk | 56.0 (4.3‐2630) | 55.2 (12.5‐745) | >.99 |
| Casein | 51.4 (2.9‐2950) | 65.6 (8.5‐645) | .91 |
| α‐lactalbumin | 8.8 (0.05‐46.3) | 9.7 (0.11‐49.7) | .62 |
| β‐lactoglobulin | 1.9 (0.05‐298) | 7.5 (0.05‐68.2) | .27 |
| Specific IgG (mgA/L) | |||
| Casein | 7.4 (3.4‐29.1) | 7.7 (2.7‐30.9) | .99 |
| β‐lactoglobulin | 3.0 (1.0‐8.2) | 2.9 (1.0‐10.8) | .73 |
| Specific IgG4 (mgA/L) | |||
| Casein | 0.52 (0.14‐7.09) | 0.84 (0.13‐7.52) | .61 |
| β‐lactoglobulin | 0.08 (0.03‐0.38) | 0.11 (0.03‐1.03) | .42 |
All patients' BA, AD, and AR were well controlled during OIT protocol.
If symptoms occurred during DBPCFC, intake was discontinued, and the accumulated dose was calculated.
Abbreviations: DBPCFC, double‐blind, placebo‐controlled food challenge; HM, heated milk; IgE, immunoglobulin E; UM, unheated milk.
3.2. Efficacy outcomes
One patient in the UM group discontinued OIT because of eosinophilic esophagitis at 1 month. All other patients completed the protocol in both groups and were included in the final analysis dataset.
Although the sample size is insufficient to compare efficacy, one year later, 94% and 75% of patients achieved desensitization to 3‐mL CM in the HM and UM groups (P = .17), respectively, whereas 35% and 18% in the HM group, and 50% and 31% in the UM group, passed the 3‐ and 25‐mL OFC (P = .34, P = .43; Figure 2), respectively.
3.3. Safety
The only adverse symptoms recorded were mild adverse symptoms during hospitalization; rates of total adverse symptoms were 20.6% and 32.4% in the HM and UM groups, respectively, without any significant intergroup difference (P = .20; Table S3).
In the home‐dosing phase, the total adverse symptom rate per dose was 8.1% and 9.6% in the HM and UM groups, respectively (P = .01). Rates of moderate/severe symptoms and respiratory symptoms were 0.7% and 1.2% vs 1.4% and 2.6% in the HM and UM groups (P = .0002, P < .0001), respectively. In the HM and UM groups, respectively, 0.1% and 0.4% symptoms necessitated corticosteroid therapy (P < .0001).
The rate of gastrointestinal symptoms, mostly mild, in the HM group was significantly higher than in the UM group (Table 2). In the UM group, one patient developed diarrhea and hematochezia; esophagogastroduodenoscopy revealed eosinophilic gastroenteritis.
TABLE 2.
Adverse symptoms and treatment at home
| HM group (n = 17) | UM group (n = 16) | P‐value | |
|---|---|---|---|
| Number of intakes of OIT | 4916 | 4383 | |
| Number of adverse symptoms, n (%) | 396 (8.1%) | 419 (9.6%) | .01 |
| Severity of symptoms | |||
| Mild | 363 (7.4%) | 357 (8.1%) | .17 |
| Moderate | 32 (0.7%) | 62 (1.4%) | .0002 |
| Severe | 1 (0.02%) | 0 (0.0%) | ‐ |
| Organ system of symptoms | |||
| Skin | 134 (2.7%) | 127 (2.4%) | .61 |
| Mucosal | 285 (5.8%) | 215 (4.9%) | .06 |
| Respiratory | 59 (1.2%) | 105 (2.6%) | <.0001 |
| Gastrointestinal | 111 (2.3%) | 56 (1.3%) | .0003 |
| Cardiovascular | 0 (0.0%) | 1 (0.02%) | ‐ |
| Anaphylaxis | 1 (0.02%) | 2 (0.05%) | ‐ |
| Total number of symptoms requiring any treatments | 107 (2.2%) | 103 (2.3%) | .57 |
| Antihistamines | 86 (1.7%) | 90 (2.1%) | .28 |
| Corticosteroids | 7 (0.1%) | 28 (0.4%) | <.0001 |
| β2‐stimulant inhalation | 30 (0.6%) | 42 (1.0%) | .06 |
| Adrenaline | 1 (0.02%) | 1 (0.02%) | ‐ |
The patients' guardians kept a daily record of ingestion, symptoms, and treatment requirements. Patients visited the hospital at 1, 3, 6, 9, and 12 mo from the initiation of OIT. At the hospital visit, we checked the diary and recorded adverse symptoms. If moderate or severe symptoms developed, the patients' guardians reported these to the investigators via telephone.
Abbreviations: HM, heated milk; OIT, oral immunotherapy; UM, unheated milk.
There were no significant between‐group differences in the per‐patient rates of adverse symptoms (Table S4). Table S5 showed the adverse symptom rate over the treatment period, and rates from OIT initiation to 3 months; rates from 3 to 6 months in the HM group were significantly lower than those in the UM group (P = .002, P < .0001, respectively).
3.4. Laboratory data
Median milk‐ and casein‐specific IgE levels significantly decreased from baseline after 3 months and further decreased to 25.8 and 23.7 vs 24.1 and 22.7 kUA/L in the HM and UM groups at 12 months, respectively (Figure 3); α‐lactalbumin‐ and β‐lactoglobulin‐sIgE significantly decreased from the baseline after 12 months (6.0 and 1.1 vs 6.7 and 4.6 kUA/L in the HM and UM groups, respectively; Figure S1).
FIGURE 3.
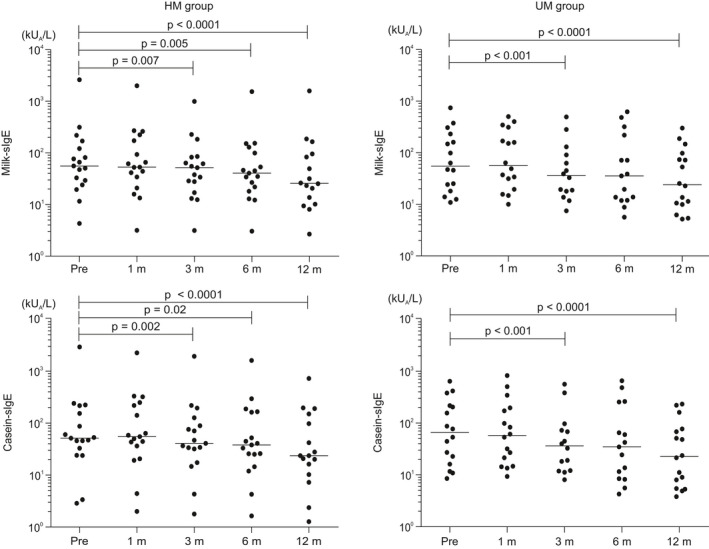
Milk‐ and casein‐specific immunoglobulin E (IgE) changes in the HM and UM groups; the x‐axis represents milk‐ and casein‐specific IgE, and the y‐axis represents time from start of oral immunotherapy. Milk‐ and casein‐specific IgE levels significantly decreased from baseline to 12 mo in both groups. The rates of reduction of milk‐, casein‐, α‐lactalbumin‐, and β‐lactoglobulin‐sIgE levels from baseline to 12 months did not significantly differ between the HM and UM groups. HM, heated milk; sIgE, specific IgE; UM, unheated milk
Furthermore, the casein‐sIgG and casein‐sIgG4 levels increased significantly from baseline after 1 month in both groups (11.4 and 0.7 vs 12.8 and 1.5 mgA/L in the HM and UM groups, respectively). In addition, β‐lactoglobulin‐sIgG and β‐lactoglobulin‐sIgG4 levels increased significantly from the baseline after 1 month only in the UM group (2.8 and 0.1 vs 3.5 and 0.2 mgA/L in the HM and UM groups, respectively). In the HM group, the β‐lactoglobulin‐sIgG and β‐lactoglobulin‐sIgG4 levels did not significantly change during the course of the OIT protocol (Figure 4, Figure S2).
FIGURE 4.
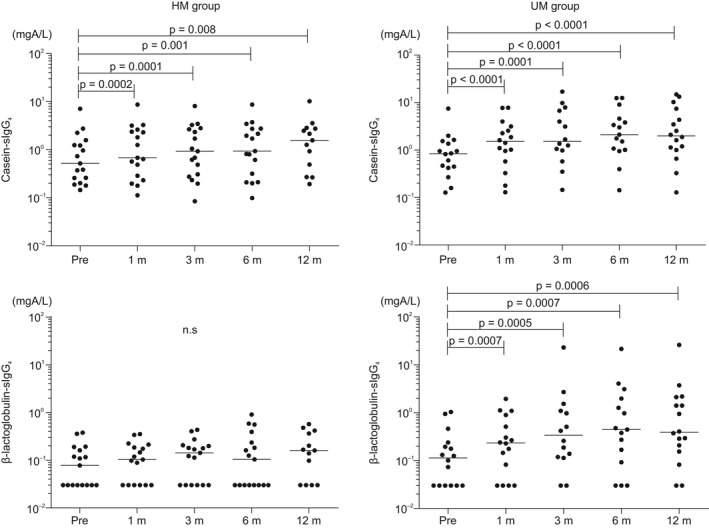
Casein‐ and β‐lactoglobulin‐specific immunoglobulin G4 changes in the HM and UM groups; the x‐axis represents casein‐ and β‐lactoglobulin‐sIgG4, and the y‐axis represents time from start of OIT. β‐lactoglobulin‐sIgG4 levels significantly increased only in the UM group. HM, heated milk; sIgG4, specific IgG4; UM, unheated milk
4. DISCUSSION
We conducted an RCT using the same protocol, except for the food formulation, to investigate the safety and efficacy of HM‐OIT or UM‐OIT in children with CM anaphylaxis. We found that the rates of total adverse symptoms, moderate or severe symptoms, and respiratory symptoms in the HM group were significantly lower than those in the UM group. Despite a sample size was insufficient to assess efficacy, HM‐OIT induced immunological changes and desensitization with 3 mL in the majority of patients, and some participants even passed the 25‐mL OFC. However, in the HM‐OIT, the proportion of patients who passed the 3‐ and/or 25‐mL OFC tended to be lower than that of participants with UM‐OIT; moreover, β‐lactoglobulin‐sIgG and β‐lactoglobulin‐sIgG4 levels did not significantly change, whereas β‐lactoglobulin‐sIgG and β‐lactoglobulin‐sIgG4 levels significantly increased in UM‐OIT. Heating reduces the allergenicity of conformational epitopes of CM protein, especially whey proteins that include β‐lactoglobulin. 11 The effects of heating could have contributed to the results of this study.
Regarding safety, the rates of total, moderate/severe, and respiratory symptoms per home dose in the HM group were significantly lower than those in the UM group; especially, these differences were seen in the first 6 months. Reducing adverse symptom rate during first 6 months, including up‐dosing phase, would be important for safely conducting OIT. 18 Previous reports showed that safety did not differ when using raw or baked milk, although the OIT protocol and patient age were different between the baked milk and raw milk groups. 14 The present study used the same protocol, with the exception of food material, and showed similar baseline profiles between the HM and UM groups. Therefore, this study design appeared appropriate for establishing the investigational safety of OIT. Although the gastrointestinal symptom rate in the HM group was higher than in the UM group, most of symptoms involved mild pruritus of the throat or oral cavity. This was possibly because the HM group used powder, and the actual consumed doses were higher; therefore, the increased amount of contact in the mouth may have led to the increased rate of oral symptoms. 19 Furthermore, the threshold dose varies with heating, particularly in patients with severe CM allergy. 20 In the present study, allergy‐related characteristics among study participants were severe (high sIgE levels, and all patients had a history of anaphylaxis); therefore, the HM group can be considered to have more safely undergone OIT than the UM group.
Regarding efficacy, during low‐dose OIT with a target dose of 3 mL of HM, 38% and 19% of participants passed the 3‐ and 25 mL‐OFC after 2 weeks of avoidance, respectively. These rates are lower than those with high‐dose OIT but similar to the results of previous low‐dose OIT with a target dose of 3‐mL. 8 , 10 , 21 In addition, these rates in the HM group were not significantly different than those in the UM group. Some reports have shown that HM ingestion accelerates tolerance acquisition in patients with CM allergy. 22 , 23 The results of the present RCT suggest that HM‐OIT is an effective strategy in patients with severe CM allergy. However, when comparing the rates of passing the 25‐mL OFC, the 17% reported for the HM group is lower than the 31% for the UM group. In a larger‐sample study, treatment efficacy could be significantly lower in the HM group.
Some previous OITs with a target dose of 200‐mL or more showed a decrease in sIgE levels and an elevation in sIgG4 levels. 24 , 25 In the present study, we showed that sIgE to milk, casein, α‐lactalbumin, and β‐lactoglobulin were significantly reduced, and the sIgG and sIgG4 to casein were significantly increased during low‐dose OIT with a target dose of 3‐mL. These findings are similar to those of previous low‐dose OITs. 17 , 26 , 27 , 28 Interestingly, β‐lactoglobulin‐sIgG and β‐lactoglobulin‐sIgG4 levels significantly increased only in the UM group. β‐lactoglobulin has a conformational structure that is denatured by heating, whereas casein is less denatured on heating. Therefore, β‐lactoglobulin‐sIgG and β‐lactoglobulin‐sIgG4 would not change in the HM group. Increase in the levels of β‐lactoglobulin‐sIgG and β‐lactoglobulin‐sIgG4 may require exposure to unheated β‐lactoglobulin. We theorized that these immunological results may be related to differences in treatment efficacy: higher tendency of the rates of passing the 3‐ and 25‐mL OFCs in UM group. The reason for the unaltered β‐lactoglobulin‐sIgG4 despite significant decreases in β‐lactoglobulin‐sIgE might be that increases in sIgG4 seem to require a high‐dose antigen. 21 However, the sample size was insufficient to draw definitive conclusions about these immunological changes, and therefore, larger studies are needed.
One limitation of our study was that we did not conduct raw milk OFC. The participants developed symptoms against 3‐mL HM and had a history of anaphylaxis; thus, they had severe CM allergy. Therefore, we did not undertake raw milk OFC due to the risk of inducing severe symptoms. We instructed patients who passed the 25‐mL HM‐OFC to ingest processed food containing 25‐mL HM at home; we subsequently considered raw milk OFC after the second year.
Second limitation was that the UM group did not use completely raw CM, as most of the commercially available milk in Japan is sterilized at 125°C for 2 seconds due to food‐related regulations.
Third limitation was that adverse symptom rates per patients were not significantly lower in the HM group than those in the UM group, despite significantly lower rates per intake number. However, the efficacy data seem to suggest that UM may be advantageous compared with HM. Larger studies are desired to assess this.
In conclusion, HM‐OIT is apparently safer than UM‐OIT in patients with CM anaphylaxis. Despite the slightly lower treatment efficacy in the HM group, the number of participants was insufficient to compare efficacy. Larger studies are necessary to confirm efficacy between the HM‐OIT and UM‐OIT protocols.
CONFLICT OF INTEREST
Motohiro Ebisawa serves on the clinical medical advisory board of DBV Technologies. Sato Sakura and Motohiro Ebisawa have received speaker honoraria from Mylan EPD. The other authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTION
Ken‐ichi Nagakura: Conceptualization (equal); data curation (lead); formal analysis (lead); funding acquisition (equal); investigation (lead); methodology (lead); project administration (lead); resources (lead); software (equal); supervision (equal); validation (supporting); visualization (supporting); writing – original draft (lead); writing – review and editing (equal). Sakura Sato: Conceptualization (lead); data curation (supporting); formal analysis (supporting); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (lead); validation (lead); visualization (lead); writing – original draft (equal); writing – review and editing (lead). Yoko Miura: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); investigation (equal); methodology (supporting); project administration (supporting); resources (supporting); software (supporting); supervision (supporting); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Makoto Nishino: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); investigation (equal); methodology (supporting); project administration (supporting); resources (supporting); software (supporting); supervision (supporting); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (equal). Kyohei Takahashi: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); investigation (equal); methodology (supporting); project administration (supporting); resources (supporting); software (lead); supervision (supporting); validation (lead); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Tomoyuki Asaumi: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); investigation (equal); methodology (supporting); project administration (supporting); resources (supporting); software (supporting); supervision (supporting); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Kiyotake Ogura: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); investigation (equal); methodology (supporting); project administration (supporting); resources (supporting); software (lead); supervision (supporting); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Motohiro Ebisawa: Conceptualization (lead); data curation (supporting); formal analysis (supporting); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (lead); validation (lead); visualization (equal); writing – original draft (equal); writing – review and editing (lead). Noriyuki Yanagida: Conceptualization (lead); data curation (supporting); formal analysis (supporting); funding acquisition (lead); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (lead); validation (lead); visualization (lead); writing – original draft (equal); writing – review and editing (lead).
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13352.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all pediatricians, nutritionists, and nurses who supported this trial at Sagamihara National Hospital.
Nagakura K‐I, Sato S, Miura Y, et al. A randomized trial of oral immunotherapy for pediatric cow's milk‐induced anaphylaxis: Heated vs unheated milk. Pediatr Allergy Immunol. 2021;32:161–169. 10.1111/pai.13352
Funding information
Key Message.
DATA AVAILABILITY STATEMENT
If reasonable request, the datasets used to support the findings of this trial are available from the corresponding author.
REFERENCES
- 1. Savage J, Sicherer S, Wood R. The natural history of food allergy. J Allergy Clin Immunol Pract. 2016;4(2):196‐203; quiz 4. [DOI] [PubMed] [Google Scholar]
- 2. Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol Pract. 2011;127(3):594‐602. [DOI] [PubMed] [Google Scholar]
- 3. Ebisawa M, Ito K, Fujisawa T; Committee for Japanese Pediatric Guideline for Food Allergy TJSoPA , Clinical Immunology TJSoA . Japanese guidelines for food allergy 2017. Allergol Int. 2017;66(2):248‐264. [DOI] [PubMed] [Google Scholar]
- 4. Wood RA, Sicherer SH, Vickery BP, et al. The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol Pract. 2013;131(3):805‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koike Y, Sato S, Yanagida N, et al. Predictors of persistent milk allergy in children: a retrospective cohort study. Int Arch Allergy Immunol. 2018;175(3):177‐180. [DOI] [PubMed] [Google Scholar]
- 6. Boyano‐Martinez T, Garcia‐Ara C, Pedrosa M, Diaz‐Pena JM, Quirce S. Accidental allergic reactions in children allergic to cow's milk proteins. J Allergy Clin Immunol. 2009;123(4):883‐888. [DOI] [PubMed] [Google Scholar]
- 7. Longo G, Barbi E, Berti I, et al. Specific oral tolerance induction in children with very severe cow's milk‐induced reactions. J Allergy Clin Immunol. 2008;121(2):343‐347. [DOI] [PubMed] [Google Scholar]
- 8. Nagakura KI, Sato S, Yanagida N, Ebisawa M. Novel immunotherapy and treatment modality for severe food allergies. Curr Opin Allergy Clin Immunol. 2017;17(3):212‐219. [DOI] [PubMed] [Google Scholar]
- 9. Sato S, Yanagida N, Ogura K, et al. Clinical studies in oral allergen‐specific immunotherapy: differences among allergens. Int Arch Allergy Immunol. 2014;164(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 10. Yanagida N, Sato S, Asaumi T, Okada Y, Ogura K, Ebisawa M. A single‐center, case‐control study of low‐dose‐induction oral immunotherapy with cow's milk. Int Arch Allergy Immunol. 2015;168(2):131‐137. [DOI] [PubMed] [Google Scholar]
- 11. Nowak‐Wegrzyn A, Fiocchi A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr Opin Allergy Clin Immunol. 2009;9(3):234‐237. [DOI] [PubMed] [Google Scholar]
- 12. Goldberg MR, Nachshon L, Appel MY, et al. Efficacy of baked milk oral immunotherapy in baked milk‐reactive allergic patients. J Allergy Clin Immunol. 2015;136(6):1601‐1606. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi M, Taniuchi S, Soejima K, Hatano Y, Yamanouchi S, Kaneko K. Two‐weeks‐sustained unresponsiveness by oral immunotherapy using microwave heated cow's milk for children with cow's milk allergy. Allergy Asthma Clin Immunol. 2016;12(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amat F, Kouche C, Gaspard W, et al. Is a slow‐progression baked milk protocol of oral immunotherapy always a safe option for children with cow's milk allergy? A randomized controlled trial. Clin Exp Allergy. 2017;47(11):1491‐1496. [DOI] [PubMed] [Google Scholar]
- 15. Simons FE. World Allergy Organization survey on global availability of essentials for the assessment and management of anaphylaxis by allergy‐immunology specialists in health care settings. Ann Allergy Asthma Immunol. 2010;104(5):405‐412. [DOI] [PubMed] [Google Scholar]
- 16. Simons FE, Ardusso LR, Bilo MB, et al. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4(2):13‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yanagida N, Okada Y, Sato S, Ebisawa M. New approach for food allergy management using low‐dose oral food challenges and low‐dose oral immunotherapies. Allergol Int. 2016;65(2):135‐140. [DOI] [PubMed] [Google Scholar]
- 18. Pajno GB, Fernandez‐Rivas M, Arasi S, et al. EAACI Guidelines on allergen immunotherapy: IgE‐mediated food allergy. Allergy. 2018;73(4):799‐815. [DOI] [PubMed] [Google Scholar]
- 19. Fauquert JL, Michaud E, Pereira B, et al. Peanut gastrointestinal delivery oral immunotherapy in adolescents: results of the build‐up phase of a randomized, double‐blind, placebo‐controlled trial (PITA study). Clin Exp Allergy. 2018;48(7):862‐874. [DOI] [PubMed] [Google Scholar]
- 20. Remington BC, Westerhout J, Campbell DE, Turner PJ. Minimal impact of extensive heating of hen's egg and cow's milk in a food matrix on threshold dose‐distribution curves. Allergy. 2017;72(11):1816‐1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yanagida N, Sato S, Asaumi T, Ebisawa M. Comparisons of outcomes with food immunotherapy strategies: efficacy, dosing, adverse effects, and tolerance. Curr Opin Allergy Clin Immunol. 2016;16(4):396‐403. [DOI] [PubMed] [Google Scholar]
- 22. Nowak‐Wegrzyn A, Lawson K, Masilamani M, Kattan J, Bahnson HT, Sampson HA. Increased tolerance to less extensively heat‐denatured (baked) milk products in milk‐allergic children. J Allergy Clin Immunol Pract. 2018;6(2):486‐495.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okada Y, Yanagida N, Sato S, Ebisawa M. Better management of cow's milk allergy using a very low dose food challenge test: a retrospective study. Allergol Int. 2015;64(3):272‐276. [DOI] [PubMed] [Google Scholar]
- 24. Pajno GB, Caminiti L, Salzano G, et al. Comparison between two maintenance feeding regimens after successful cow's milk oral desensitization. Pediatr Allergy Immunol. 2013;24(4):376‐381. [DOI] [PubMed] [Google Scholar]
- 25. Perezabad L, Reche M, Valbuena T, Lopez‐Fandino R, Molina E, Lopez‐Exposito I. Oral food desensitization in children with IgE‐mediated cow's milk allergy: immunological changes underlying desensitization. Allergy Asthma Immunol Res. 2017;9(1):35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagakura KI, Yanagida N, Sato S, et al. Low‐dose oral immunotherapy for children with anaphylactic peanut allergy in Japan. Pediatr Allergy Immunol. 2018;29(5):512‐518. [DOI] [PubMed] [Google Scholar]
- 27. Yanagida N, Sato S, Asaumi T, Nagakura K, Ogura K, Ebisawa M. Safety and efficacy of low‐dose oral immunotherapy for hen's egg allergy in children. Int Arch Allergy Immunol. 2016;171(3–4):265‐268. [DOI] [PubMed] [Google Scholar]
- 28. Nagakura KI, Yanagida N, Sato S, et al. Low‐dose oral immunotherapy for children with wheat‐induced anaphylaxis. Pediatr Allergy Immunol. 2020;31(4):371‐379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
If reasonable request, the datasets used to support the findings of this trial are available from the corresponding author.


