Abstract
Recently, our group used exosomes from mesenchymal stromal/stem cells (MSCs) to simulate an M2 macrophage phenotype, that is, exosome‐educated macrophages (EEMs). These EEMs, when delivered in vivo, accelerated healing in a mouse Achilles tendon injury model. For the current study, we first tested the ability of EEMs to reproduce the beneficial healing effects in a different rodent model, that is, a rat medial collateral ligament (MCL) injury model. We hypothesized that treatment with EEMs would reduce inflammation and accelerate ligament healing, similar to our previous tendon results. Second, because of the translational advantages of a cell‐free therapy, exosomes alone were also examined to promote MCL healing. We hypothesized that MSC‐derived exosomes could also alter ligament healing to reduce scar formation. Similar to our previous Achilles tendon results, EEMs improved mechanical properties in the healing ligament and reduced inflammation, as indicated via a decreased endogenous M1/M2 macrophage ratio. We also showed that exosomes improved ligament remodeling as indicated by changes in collagen production and organization, and reduced scar formation but without improved mechanical behavior in healing tissue. Overall, our findings suggest EEMs and MSC‐derived exosomes improve healing but via different mechanisms. EEMs and exosomes each have attractive characteristics as therapeutics. EEMs as a cell therapy are terminally differentiated and will not proliferate or differentiate. Alternatively, exosome therapy can be used as a cell free, shelf‐stable therapeutic to deliver biologically active components. Results herein further support using EEMs and/or exosomes to improve ligament healing by modulating inflammation and promoting more advantageous tissue remodeling.
Keywords: exosomes, ligament healing, macrophages, mesenchymal stromal cells
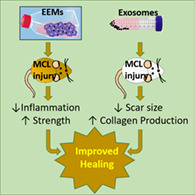
Exosome‐educated macrophages (EEMs) and exosomes differentially improve ligament healing. EEM treatment to an injured rat medial collateral ligament resulted in reduced inflammation and improved ligament strength. In contrast, exosome treatment reduced scar size, and increased collagen organization and production. Despite different outcomes, both treatments were uniquely effective in accelerating healing.
Significance statement.
This study demonstrates that exosomes and exosome‐educated macrophages (EEMs) improve ligament healing in a normally scar‐forming tissue. Although both treatments were effective in improving healing, the biological effects were different, with EEMs reducing inflammation and improving ligament strength, and exosomes decreasing scar size, and increasing collagen organization and production. These results have potential clinical relevance in that EEMs are advantageous as a cell therapy as they are terminally differentiated and will not proliferate or differentiate to undesirable cell types, whereas exosomes can be used as an alternative cell‐free therapy to modulate the remodeling process.
1. INTRODUCTION
Despite surgical and physical therapy advancements designed to improve ligament healing, none eliminate scar formation, thereby making them prone to further injury. Recently, we delivered exosome‐educated macrophages (EEMs) to simulate an M2‐mediated anti‐inflammatory response within a tendon wound. 1 EEMs were generated by exposing CD14+ macrophages to mesenchymal stromal cell (MSC) derived exosomes. 1 , 2 In vitro tests showed EEMs exhibit M2‐like behavior as indicated by an increase in M2 cell surface markers: CD206, PD‐L1, and PD‐L2. 2 , 3 When tested in vivo, treatment of mouse Achilles tendon injuries with EEMs reduced inflammation and improved strength of the healing tendon, supporting the use of EEMs to promote healing. 1 Indeed, results were superior when compared to treatment with MSCs. Further development of a more predictably beneficial therapy would be clinically significant.
Exosomes are small (40‐200 nm) lipid membrane‐bound vesicles that participate in cell‐to‐cell communication by transferring specific host cell‐derived nucleic acid/protein to targeted recipient cells and reprogramming cell behavior, making the clinical potential of exosomes significant. 4 , 5 Previous in vivo studies indicated that exposure of healing Achilles tendon to exosomes reduced the M1/M2 macrophage ratio and increased the number of endothelial cells 2 weeks after healing. 1 However, the functional/mechanical benefit of exosomes was not as obvious as the EEMs in that mouse Achilles tendon model. The goals of the current study were twofold. First, to reproduce the healing effects of EEMs in a different rodent model, that is, a rat medial collateral ligament (MCL). We hypothesized treatment with EEMs would reduce inflammation and accelerate ligament healing, similar to our previous tendon results. Second, to study the effects of exosome therapy in the rat MCL injury given the cell‐free translational advantage of this therapy. We hypothesized that MSC‐derived exosomes could similarly improve ligament healing and reduce scar formation.
2. MATERIALS AND METHODS
2.1. Cell culture
Human cell use was approved by the Health Sciences Institutional Review Board, University of Wisconsin (UW)‐Madison, School of Medicine and Public Health (Protocol number: 2016‐0298). MSCs were isolated from bone marrow of normal healthy donors as previously reported. 1 , 6 Identity of MSCs was confirmed via cell adherence and flow cytometry. 7 , 8 Passage 4‐6 MSCs were used to isolate exosomes via differential ultracentrifugation. Exosome protein and RNA was characterized with a NanoDrop spectrophotometer (Thermo‐Fisher, Waltham, Massachusetts). Particle concentration and diameter were measured using IZON qNano Nanoparticle (Zen‐Bio, Inc, Research Triangle Park, North Carolina) analysis.
Mononuclear cells were obtained from granulocyte‐colony stimulating factor‐mobilized peripheral blood of healthy donors, using Ficoll Paque Plus density gradient separation (GE Healthcare Bio‐Sciences, Piscataway, New Jersey). 1 , 9 Red blood cells were lysed. Monocytes were isolated by via autoMACS Pro Separator (Miltenyi Biotech, Auburn, California) using anti‐human CD14+ microbeads (Miltenyi Biotech). CD14+ monocytes were cultured 7 days for macrophage differentiation (Figure 1A). To generate EEMs, CD14+ macrophages were exposed to 3 × 109 exosomes for 3 days.
FIGURE 1.
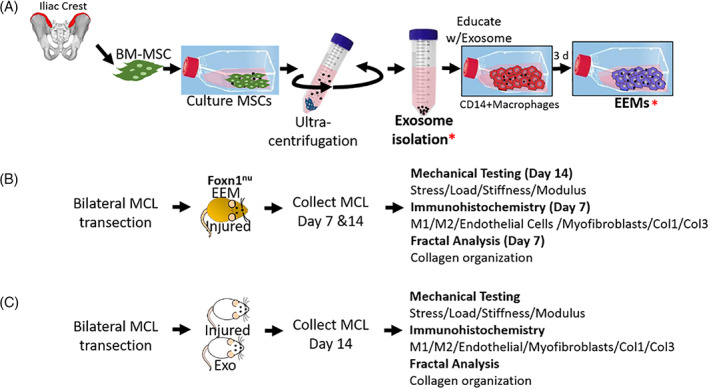
Fabrication of exosome‐educated macrophages (EEMs) and experimental design for EEM and exosome studies. A, Bone marrow mesenchymal stromal cells (MSCs) were expanded. Exosomes (Exo) were then isolated from the MSCs via ultracentrifugation. CD14+ monocytes were isolated from human peripheral blood. Monocytes were cultured 7 days, activated to CD14+ macrophages, and then educated with Exo for 3 days, producing EEMs. Two experiments were performed. B, For the first study, Foxn1 nu (nude) rats were subjected to bilateral MCL transection. Immediately after injury, ligaments were treated with EEMs (ipsilateral) or PBS (contralateral). At 7 and 14 days postinjury, MCLs were collected and used for mechanical testing (day 14) or immunohistochemistry (day 7 and day 14). C, For the second study, Wistar rats were subjected to bilateral MCL transection. Rats were treated with either Exo or PBS (serving as the control). At 14 days postinjury, MCLs were collected and used for mechanical testing and immunohistochemistry.* indicates the stage when sample was used for in vivo work
2.2. MCL healing model
This study was approved by the UW Institutional Animal Care and Use Committee (Protocol number: M005785). Fourteen female (n = 28 MCLs) Foxn1 −/− “nude” rats (175‐200 g) were used as an animal model for ligament healing. 10 , 11 To avoid xenograft‐induced immune rejection, Foxn1 −/− rats were chosen as human EEMs would be administered. Rats were anesthetized via isoflurane and subjected to bilateral MCL transection. A 1 cm incision was made over the medial aspect of each stifle. The MCL was exposed, and transected at its mid‐point. EEMs (20 μL of 1 × 106) were administered directly to the injured MCL (Figure 1B). The contralateral injured MCL received 20 μL of PBS, serving as the vehicle control. Dexon suture (4‐0) was used for skin closure. Based on previous studies, ligaments were collected at 7‐ (granulation tissue formation) and 14‐days (early scar formation) postsurgery and used for immunohistochemistry (IHC; day 7; n = 4/treatment) or mechanical testing (day 14; n = 10/treatment). As the tissue was too friable at day 7, mechanical testing was only performed on day 14. 1
In a second study, 10 male Wistar (300‐350 g) rats underwent similar bilateral MCL transection (Figure 1). Immunocompetent rats were selected for this study as exosomes are acellular and considered nonimmunogenic. 12 MCLs were administered 1 × 109 exosomes (Exo; n = 5) or PBS (control; n = 5). At 14 days postinjury, MCLs were collected and used for IHC (ipsilateral) and mechanical testing (contralateral). As functional improvement during ligament healing is a key parameter to determine improved healing, day 14 was chosen as the collection time.
2.3. IHC/histology and image quantification
In order to examine treatment effects on endogenous cells, IHC was performed. 10 , 11 , 13 Briefly, cryosections were exposed to rabbit or mouse primary antibodies CD68, CD163, CD31, and α‐smooth muscle actin to identify M1 macrophages, M2 macrophages, endothelial cells, and myofibroblasts (Biorad, Hercules, California), respectively as well as type I (Biorad) and type III collagen (Sigma Aldrich, St. Louis, Missouri). Samples were exposed to secondary antibodies, mouse‐on‐rat HRP polymer or rabbit‐on‐rodent HRP polymer (Biocare Medical, Pacheco, California). Bound antibody complex was visualized using diaminobenzidine. H&E staining was performed and used for fractal analysis (to quantify collagen organization) and wound area measurements. 14 , 15 Stained sections were imaged via camera‐assisted microscope (Nikon Eclipse, model E6000 with Olympus camera, model DP79). Images (3‐5 sections/animal) were captured within and outside of the granulation/scar tissue. The border of the wound was defined as the interface between the organized and disorganized collagen. Staining and wound size were quantified using ImageJ (NIH, Bethesda, Maryland). Scar formation was estimated at day 14 by quantifying type I and III collagen IHC staining, performing fractal analysis to measure collagen organization, and measuring wound size of H&E images.
2.4. Mechanical testing
Pull‐to‐failure testing was performed. 16 Each MCL was removed with both femoral and tibial insertion sites intact. The surrounding tissue was excised. The femur‐MCL‐tibia complex was mounted in a custom testing bath and mechanical testing machine. A pre‐load of 0.1 N was applied, cross‐section was measured, and each MCL was preconditioned (cyclically loaded to approximately 1% strain for 10 cycles). The ligament was pulled to failure at a rate of 10% strain per second. Failure force, failure stress, ligament stiffness, and Young's modulus were measured/computed to determine posttreatment MCL mechanical behavior.
2.5. Statistical analysis
A one‐way analysis of variance (ANOVA) examined treatment differences of all data. If the overall P‐value for the F‐test in ANOVA was significant (P ≤ .05), Fisher's LSD post hoc comparisons were performed. Data were presented as mean ± SD. All analyses were performed using KaleidaGraph, version 4.03 (Synergy Software, Inc, Reading, Pennsylvania).
3. RESULTS
3.1. EEM study
3.1.1. Mechanical testing
Compared to the control, EEM treatment significantly increased failure load (P = .03; Figure 2A) and max stress (P = .01; Figure 2B). However, treatment differences in stiffness (P = .64; Figure 2C) and Young's modulus (P = .41; Figure 2D) were not significant. Collectively, these results support the concept that EEM treatment improves the mechanical properties of the healing MCL.
FIGURE 2.
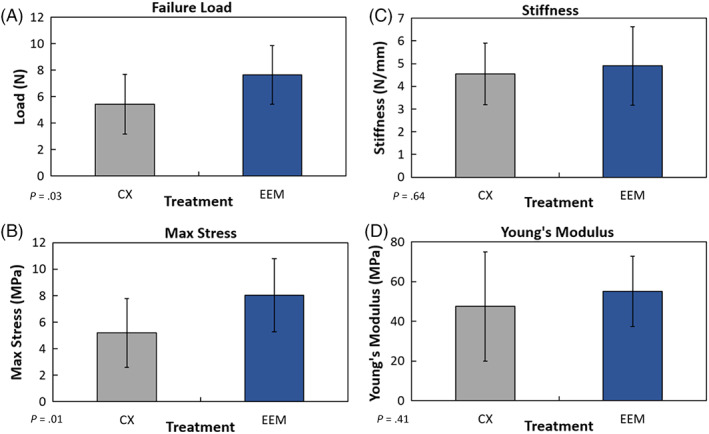
Mechanical results of the healing medial collateral ligament (MCL) after exosome‐educated macrophages (EEM) treatment. Treatment of the injured MCL with EEMs significantly increased (A) failure load and (B) maximum stress, 14 days postinjury compared to the PBS control (CX). No treatment differences were noted in (C) stiffness or (D) Young's modulus. Data are results of Fisher's LSD post hoc pairwise analysis from 10 MCLs/treatment, (P ≤ .05). Results are expressed as mean ± SD
3.1.2. IHC of cellular factors
Macrophage immunophenotypes indicated that the number of day 7 endogenous M1 macrophages was significantly reduced after EEM treatment (P < .01) compared to the control (Figure 3A). In contrast, M2 macrophages were significantly increased by EEMs (Figure 3B). These changes in macrophage phenotypes by EEMs significantly reduced the M1/M2 macrophage ratio (Figure 3C,D; P = .01). No changes were noted in the number of endothelial cells or myofibroblasts (P > .05; Figure 3E).
FIGURE 3.
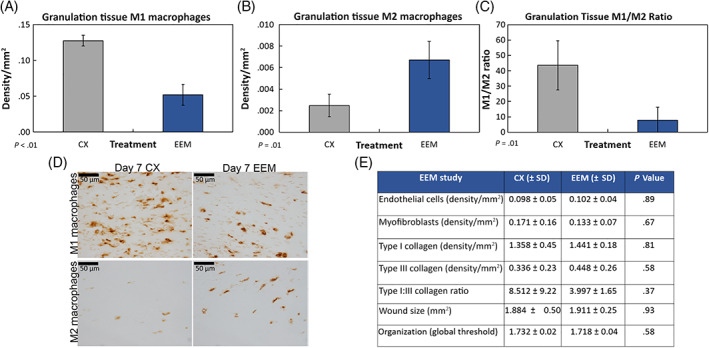
Macrophage immunohistochemistry results of the day 7 healing medial collateral ligament (MCL) after treatment with exosome‐educated macrophages (EEMs). Treatment with EEM significantly (A) reduced the number of M1 macrophages, (B) increased the M2 macrophages, and (C) reduced the overall M1/M2 macrophage ratio localized within the granulation tissue compared to the PBS control (CX). Representative images of the (D) M1 macrophages (top) and M2 macrophages (bottom) by the CX (left) and EEM (right) treated MCLs collected 7 days postinjury. E, Table showing results of tested factors that were not significantly different after EEM treatment. Data are considered significantly different (P ≤ .05) based on Fisher's LSD post hoc pairwise analysis from four MCLs/treatment. Values are expressed as mean density/mm2 ± SD. Scale bars = 50 μm
3.1.3. Scar size, collagen production, and organization
No significant changes were noted in wound size, IHC quantified type I and type III collagen production, and fractal analysis of collagen organization (P > .05; Figure 3E).
3.2. Exosome study
3.2.1. Mechanical testing and IHC of cellular factors
At the concentration tested, treatment with exosomes did not significantly improve mechanical function (Figure 1C), nor did they elicit changes in endogenous M1 and M2 macrophages (Figure 4A‐D), endothelial cells, and myofibroblasts (Figure 4L).
FIGURE 4.
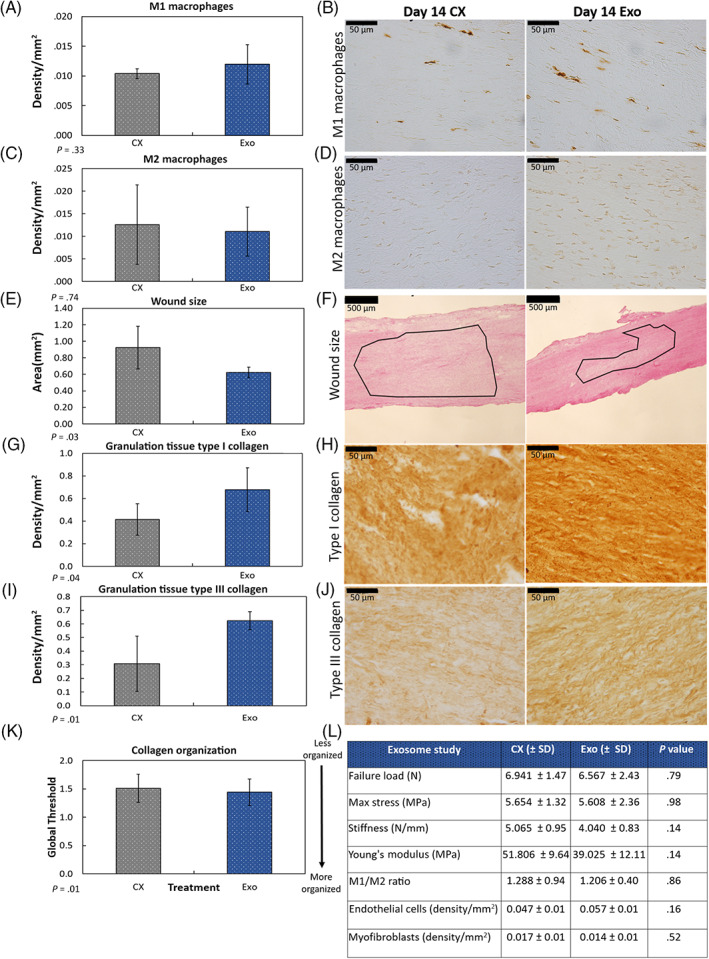
Immunohistochemistry and histology results by the day 14 healing medial collateral ligament (MCL) after exosome treatment. Treatment of the injured MCL with exosomes (Exo) had no significant effect on the number of (A,B) M1 macrophages, or (C,D) M2 macrophages 14 days postinjury. E,F, In contrast, Exo treatment significantly (E) reduced size of scar tissue compared to the control. F, Representative H&E stained images indicate the reduction in scar size. Outlined region (ie, border between the organized and disorganized collagen fibers) indicates wound area. Exo treatment also significantly increased (G,H) type I and (I,J) type III collagen production within the granulation tissue. K, Exo treatment significantly improved collagen organization. L, Table showing results of tested factors that were not significantly different after Exo treatment. Data are considered significantly different (P < .05) based on Fisher's LSD post hoc pairwise analysis from five MCLs/treatment. Values are expressed as mean area or density/mm2 ± SD
3.2.2. Scar size, collagen production, and organization
Unlike EEMs, treatment with exosomes significantly reduced scar formation 14 days postinjury compared to the control (Figure 4E,F). Exosome treatment also increased type I and type III collagen production within the granulation tissue (Figure 4G‐J). Collagen organization, determined via fractal analysis, was also significantly improved (Figure 4K).
4. DISCUSSION
To our knowledge, this is the first study to test the effects of EEMs on ligament healing. Similar to our previous Achilles tendon results, EEMs improved the rat MCL's mechanical properties and reduced the endogenous M1/M2 macrophage ratio indicating less inflammation, 1 whereas exosomes improved ligament remodeling through changes in collagen production, improved organization, and reduced scar without improvements in mechanical function.
Few studies have reported the effects of direct macrophage application on the healing process. 17 , 18 , 19 , 20 , 21 Activated macrophages delivered to a cutaneous guinea pig wound, reduced wound size. 17 Likewise, treatment of human ulcers with macrophages were reported to stimulate wound repair. 19 , 20 More recently, a phase I in‐human trial was conducted to evaluate the safety and feasibility of autologous macrophage delivery in patients with liver fibrosis. 21 One year posttreatment, patients were alive and remained transplant free. In our study, direct delivery of EEMs to the transected MCL was able to suppress/prevent inflammation normally present after injury, by increasing the presence of endogenous M2 macrophages and reducing the M1 macrophages. Our earlier experiments tracking EEMs in vivo, indicated that the majority of exogenously administered EEMs are gone from the wound by day 7, thereby suggesting the effects are likely via paracrine or chemotactic action. 1 Altogether, these studies support the concept that exogenous administration of M2‐like macrophages are able to accelerate healing in various healing models and thus hold a much broader orthopedic promise.
Similar to our Achilles tendon results, EEMs improved strength of the MCL without increased production of type I collagen or improved collagen organization. 1 The known mechanism for improved strength after EEM treatment extends beyond the scope of this manuscript but may involve changes in collagen fibril size, molecular cross‐linking, and/or MMP/TIMP activity. 22 , 23 For instance, one study demonstrated that collagen fibril formation was markedly reduced and cardiac rupture increased following M2 macrophages depletion after injury. 22 Additional research is underway to elucidate the molecular mechanisms of EEM therapy on ligament healing.
In our study, exosomes elicited a significant biological response, indicated by an increase in type I and III collagen, improved collagen organization, and a reduction in scar size 14 days after injury. The ability for exosomes to reduce scar formation and upregulate collagen type I and III expression has been documented in tendon healing models, but this is the first study to our knowledge to report this in ligaments. 1 , 24 We hypothesize that the postinjury timing of exosome delivery is important. At the time of injury and exosome administration, few macrophages are present, thereby reducing the likelihood for exosomes to target macrophages and control inflammation. Instead, exosomes would target fibroblasts and/or tissue stem cells to affect ECM production and remodeling. 25 Future studies will include treatment of injuries with both EEMs and exosomes in a temporal manner where EEMs would reduce inflammation and exosomes would stimulate remodeling.
5. CONCLUSION
We demonstrate that EEMs as well as MSC‐derived exosomes have the potential to improve ligament healing via different mechanisms. EEMs and exosomes each have attractive characteristics as therapeutics. As a cell therapy EEMs are terminally differentiated and will not proliferate or differentiate to undesirable cell types, which remain a concern for many stem cell therapies. Moreover, EEMs could be generated from a patient's monocytes using off‐the‐shelf exosomes, resulting in a faster and more facile process compared to autologous MCS. Alternatively, exosome therapy could be a cell free, shelf‐stable therapeutic to deliver biologically active components. Altogether, results herein support the use of EEMs and/or exosomes to improve ligament healing by modulating inflammation and tissue remodeling.
CONFLICT OF INTEREST
C.S.C. declared employment/leadership position and stock ownership in Dianomi Therapeutics. A.M.S. declared consultant/advisory role with Stryker Endoscopy. M.A.H. declared research funding from OREF Grant Funding. R.V. declared patent holder for EEM technology filed by Wisconsin Alumni Research Foundation. The other authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
C.S.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; J.A.K.: conception and design, collection and/or assembly of data, provision of study material or patients; L.A.W.: collection and/or assembly of data, manuscript writing; M.M., K.H.: collection and/or assembly of data; A.M.S.: revision and final approval of manuscript; M.A.H.: financial support, revision and final approval of manuscript; P.H.: conception and design, provision of study material or patients, final approval of manuscript; R.V.: conception and design, provision of study material or patients, final approval of manuscript.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the Orthopedic Research and Education Foundation (OREF) Award numbers MSN180250 and MSN197479. The content is solely the responsibility of the authors and does not necessarily represent the official views of the OREF.
Chamberlain CS, Kink JA, Wildenauer LA, et al. Exosome‐educated macrophages and exosomes differentially improve ligament healing. Stem Cells. 2021;39:55–61. 10.1002/stem.3291
Funding information Orthopaedic Research and Education Foundation, Grant/Award Numbers: MSN180250, MSN197479
Contributor Information
Peiman Hematti, Email: pxh@medicine.wisc.edu.
Ray Vanderby, Email: vanderby@ortho.wisc.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chamberlain CS, Clements AEB, Kink JA, et al. Extracellular vesicle‐educated macrophages promote early Achilles tendon healing. Stem Cells. 2019;37:652‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kink JA, Forsberg MH, Reshetylo S, et al. Macrophages educated with exosomes from primed mesenchymal stem cells treat acute radiation syndrome by promoting hematopoietic recovery. Biol Blood Marrow Transplant. 2019;25:2124‐2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eslani M, Putra I, Shen X, et al. Cornea‐derived mesenchymal stromal cells therapeutically modulate macrophage immunophenotype and angiogenic function. Stem Cells. 2018;36:775‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto‐enzymes in the form of micro‐vesicles. Biochim Biophys Acta. 1981;645:63‐70. [DOI] [PubMed] [Google Scholar]
- 5. Elahi FM, Farwell DG, Nolta JA, Anderson JD. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells. 2020;38:15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim J, Hematti P. Mesenchymal stem cell‐educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315‐317. [DOI] [PubMed] [Google Scholar]
- 8. Trivedi P, Hematti P. Simultaneous generation of CD34+ primitive hematopoietic cells and CD73+ mesenchymal stem cells from human embryonic stem cells cocultured with murine OP9 stromal cells. Exp Hematol. 2007;35:146‐154. [DOI] [PubMed] [Google Scholar]
- 9. Bouchlaka MN, Moffitt AB, Kim J, et al. Human mesenchymal stem cell‐educated macrophages are a distinct high IL‐6‐producing subset that confer protection in graft‐versus‐host‐disease and radiation injury models. Biol Blood Marrow Transplant. 2017;23:897‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain CS, Brounts SH, Sterken DG, Rolnick KI, Baer GS, Vanderby R. Gene profiling of the rat medial collateral ligament during early healing using microarray analysis. J Appl Physiol. 2011;111:552‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chamberlain CS, Crowley E, Vanderby R. The spatio‐temporal dynamics of ligament healing. Wound Repair Regen. 2009;17:206‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu XQ, Yan TZ, Wang ZW, Wu X, Cao GH, Zhang C. BM‐MSCs‐derived microvesicles promote allogeneic kidney graft survival through enhancing micro‐146a expression of dendritic cells. Immunol Lett. 2017;191:55‐62. [DOI] [PubMed] [Google Scholar]
- 13. Chamberlain CS, Crowley EM, Kobayashi H, Eliceiri KW, Vanderby R. Quantification of collagen organization and extracellular matrix factors within the healing ligament. Microsc Microanal. 2011;17:779‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frisch KE, Duenwald‐Kuehl SE, Kobayashi H, Chamberlain CS, Lakes RS, Vanderby R. Quantification of collagen organization using fractal dimensions and Fourier transforms. Acta Histochem. 2012;114:140‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frisch KE, Marcu D, Baer GS, Thelen DG, Vanderby R. The influence of partial and full thickness tears on infraspinatus tendon strain patterns. J Biomech Eng. 2014;136:051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Provenzano PP, Heisey D, Hayashi K, Lakes R, Vanderby R Jr. Subfailure damage in ligament: a structural and cellular evaluation. J Appl Physiol (1985). 2002;92:362‐371. [DOI] [PubMed] [Google Scholar]
- 17. Dachir S, Cohen M, Sahar R, et al. Beneficial effects of activated macrophages on sulfur mustard‐induced cutaneous burns, an in vivo experience. Cutan Ocul Toxicol. 2014;33:317‐326. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki T, Arumugam P, Sakagami T, et al. Pulmonary macrophage transplantation therapy. Nature. 2014;514:450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Danon D, Madjar J, Edinov E, et al. Treatment of human ulcers by application of macrophages prepared from a blood unit. Exp Gerontol. 1997;32:633‐641. [DOI] [PubMed] [Google Scholar]
- 20. Zuloff‐Shani A, Kachel E, Frenkel O, Orenstein A, Shinar E, Danon D. Macrophage suspensions prepared from a blood unit for treatment of refractory human ulcers. Transfus Apher Sci. 2004;30:163‐167. [DOI] [PubMed] [Google Scholar]
- 21. Moroni F, Dwyer BJ, Graham C, et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat Med. 2019;25:1560‐1565. [DOI] [PubMed] [Google Scholar]
- 22. Shiraishi M, Shintani Y, Shintani Y, et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest. 2016;126:2151‐2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma Y, Halade GV, Zhang J, et al. Matrix metalloproteinase‐28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res. 2013;112:675‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen H, Yoneda S, Abu‐Amer Y, Guilak F, Gelberman RH. Stem cell‐derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J Orthop Res. 2020;38:117‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu H, Cheng J, Shi W, et al. Bone marrow mesenchymal stem cell‐derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020;106:328‐341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


