ABSTRACT
Tropical Africa is home to an astonishing biodiversity occurring in a variety of ecosystems. Past climatic change and geological events have impacted the evolution and diversification of this biodiversity. During the last two decades, around 90 dated molecular phylogenies of different clades across animals and plants have been published leading to an increased understanding of the diversification and speciation processes generating tropical African biodiversity. In parallel, extended geological and palaeoclimatic records together with detailed numerical simulations have refined our understanding of past geological and climatic changes in Africa. To date, these important advances have not been reviewed within a common framework. Here, we critically review and synthesize African climate, tectonics and terrestrial biodiversity evolution throughout the Cenozoic to the mid‐Pleistocene, drawing on recent advances in Earth and life sciences. We first review six major geo‐climatic periods defining tropical African biodiversity diversification by synthesizing 89 dated molecular phylogeny studies. Two major geo‐climatic factors impacting the diversification of the sub‐Saharan biota are highlighted. First, Africa underwent numerous climatic fluctuations at ancient and more recent timescales, with tectonic, greenhouse gas, and orbital forcing stimulating diversification. Second, increased aridification since the Late Eocene led to important extinction events, but also provided unique diversification opportunities shaping the current tropical African biodiversity landscape. We then review diversification studies of tropical terrestrial animal and plant clades and discuss three major models of speciation: (i) geographic speciation via vicariance (allopatry); (ii) ecological speciation impacted by climate and geological changes, and (iii) genomic speciation via genome duplication. Geographic speciation has been the most widely documented to date and is a common speciation model across tropical Africa. We conclude with four important challenges faced by tropical African biodiversity research: (i) to increase knowledge by gathering basic and fundamental biodiversity information; (ii) to improve modelling of African geophysical evolution throughout the Cenozoic via better constraints and downscaling approaches; (iii) to increase the precision of phylogenetic reconstruction and molecular dating of tropical African clades by using next generation sequencing approaches together with better fossil calibrations; (iv) finally, as done here, to integrate data better from Earth and life sciences by focusing on the interdisciplinary study of the evolution of tropical African biodiversity in a wider geodiversity context.
Keywords: tropical Africa, dated molecular phylogenies, palaeoclimate models, speciation models, fossils, African geology, Cenozoic
I. INTRODUCTION
The African continent is a land of biological contrasts (Linder, 2001). Africa hosts the largest desert in the world, the Sahara, together with some of the most endemic‐rich (e.g. Cape Flora; Linder, 2003) and species‐rich (e.g. African rain forests; Linder et al., 2012) ecosystems worldwide. In this review, we focus on tropical Africa, loosely defined as the region below the Sahara and excluding southern (austral) Africa and Madagascar (Fig. 1). A central focus is the tropical rain forests as they contain the highest levels of species diversity and endemicity for both plants (Linder et al., 2012; Droissart et al., 2018) and animals (Jenkins, Pimm, & Joppa, 2013) across the continent. Tropical rain forests are distributed from West Africa into the Congo Basin, Guineo‐Congolia, and in smaller patches along the East African coast and Eastern Arc Mountains (Tanzania–Kenya). African rain forests are, however, overall less species rich than tropical rain forests in other tropical regions such as the Neotropics (Richards, 1973; reviewed in Couvreur, 2015).
Fig 1.
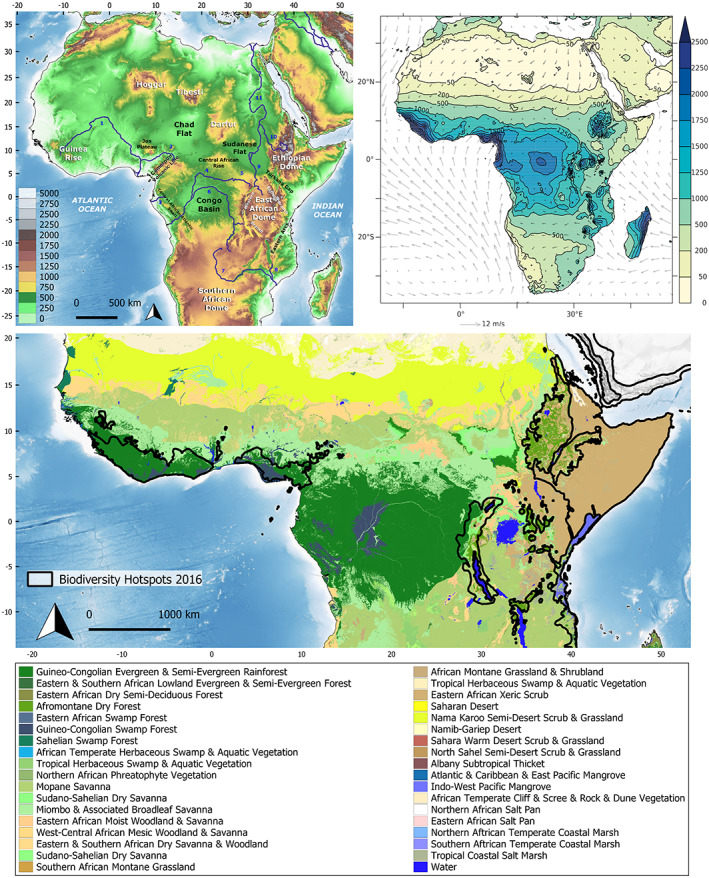
The modern geophysical, climatic and vegetation setting of tropical Africa. (A) Topography of tropical Africa, modified from Guillocheau et al. (2018). Topographic and bathymetric data taken from the GEBCO 2020 Grid (doi: 10.5285/a29c5465‐b138‐234de053‐6c86abc040b9). Scale on bottom left is altitude in meters. Numbers refer to major rivers: 1, Niger; 2, Benue; 3, Ogooué; 4, Ubangi; 5, Uele; 6, Congo; 7, Zambezi; 8, Shire; 9, White Nile; 10, Blue Nile; 11, Nile. (B) Summed annual rainfall amount (colour‐shading, in millimetres) and averaged surface wind velocity (vectors, in m/s); rainfall data retrieved from the 1961–1990 climatology from the Climate Research Unit data set, wind velocities are averages from the 1989–2010 ERA‐Interim reanalyses [data from New et al. (2002) and Dee et al. (2011)]. (C) Major vegetation types across Tropical Africa following Sayre et al. (2013). Major divisions are shown according to Sayre et al. (2013). Delimitation of biodiversity hotspots taken from https://zenodo.org/record/3261807#.Xvu69lVKiUk (doi: 10.5281/zenodo.3261807).
Besides tropical rain forest, numerous other biomes have been identified but their limits and characteristics depend on the biota studied, the data and the approach used (White, 1983; Linder, 2001; Klerk et al., 2002; Linder et al., 2005, 2012; Lévêque et al., 2007; Droissart et al., 2018). East Africa is particularly diverse with substantially more bioregions identified than in West or Central Africa, reflecting higher topographic and climatic diversity (Linder, 2017; Droissart et al., 2018). Remarkably, bioregions defined using different groups (e.g. plants, animals) show broad general congruence (Linder et al., 2012). Finally, using a slightly different concept to that of a biome (which is solely based on species composition), Linder (2014) identified six different groups of clades or ‘floras’ for Africa, which shared similar geographical distributions, extra‐African geographical affinities, diversification histories, and maximum ages.
Africa contains eight of the now 36 recognized global biodiversity hotspots (Fig. 1B; Mittermeier et al., 2011). Additional hotspots defined in terms of species richness have been identified in the coastal regions of Cameroon, Gabon, the Republic of Congo, and Mozambique (Küper et al., 2004; Sosef et al., 2017). Noteworthy are the East Afromontane hotspots which contain the second highest total number of endemic vertebrate genera on Earth (Mittermeier et al., 2011). The Eastern Arc Mountain hotspot, as originally defined but now comprising two separate hotpots (Mittermeier et al., 2011), was estimated to have the highest concentration of endemic plants (number of endemics per 100 km2) of all hotspots (Myers et al., 2000). Overall, African biodiversity is vulnerable with a high risk of extinction by the end of this century for both plants (McClean et al., 2005; Blach‐Overgaard et al., 2015; Stévart et al., 2019) and animals (Thuiller et al., 2006; Tolley et al., 2016), and Africa is expected to host more than half of global population growth by 2050 (Gerland et al., 2014).
Understanding the evolutionary history of regions and how clades originated and diversified are important facets of biodiversity conservation (Erwin, 1991). Indeed, molecular dating and subsequent biogeographic and diversification analyses of reconstructed phylogenetic trees have become routine in many studies on the evolution of biodiversity (Sauquet, 2013; Morlon, 2014; Sanmartín & Meseguer, 2016; Silvestro et al., 2018). However, as for all methods, these approaches have potential limits (e.g. Carruthers & Scotland, 2020; Louca & Pennell, 2020) which are important to keep in mind when interpreting their outcome. The latest review on the evolution of tropical African flora and fauna, mainly focused on the tropical rain forest biome, is now 15 years old (Plana, 2004), and concluded that “The small number of species‐level phylogenies for African rainforest plants hinders a more incisive and detailed study into the historical assembly of these continental forests” (p. 1585).
To date, around 90 dated molecular phylogenies have been published documenting the diversification of tropical African animals and plants (see online Supporting Information, Appendix S1). Most clades diversified within tropical Africa and this will be the focus of our review. Biogeographic analyses of pantropical plant clades tend to support the idea that Africa has been an important source of tropical diversity (the ‘out of Africa’ hypothesis), with numerous major tropical families inferred to have originated in Africa (e.g. Muellner et al., 2006; Zhou et al., 2012; Couvreur, 2015). In animals, the origin of major groups is less clear with studies disagreeing on the geographical origin of groups such as Mammalia (Springer et al., 2011; O'Leary et al., 2013).
In parallel, knowledge of the geophysical settings of Africa has improved. Information from both modelling and fieldwork has improved our understanding of the topographic history of the continent, and numerical climate simulations have begun to clarify how these changes influenced the climate of Africa. This new wealth of information provides a unique opportunity to improve our understanding of the diversification of tropical African biodiversity.
Here, we first review African geodiversity and climate events throughout the Cenozoic and link these to diversification processes in tropical African terrestrial plant and animal clades. Finally, using dated molecular phylogenetic and diversification studies, we synthesize the different speciation models and mechanisms proposed for tropical Africa.
II. THE PHYSICAL CONTEXT
Climatically, tropical Africa is bounded by three regions receiving less than 200 mm of precipitation per year (Fig. 1B): the Sahara Desert to the north; the Kalahari and Namib deserts to the south; and the Ogaden desert in the Horn of Africa to the northeast. The rainfall regime in tropical Africa also varies longitudinally, with the western African monsoon region and the western Congo Basin being far wetter (Fig. 1B; >2000 mm/year) than the margin of the continent east of the East African and Ethiopian Domes. Rainfall over the Congo Basin is considered to follow a bimodal regime, with the rainiest seasons (precipitation ~200 mm/month) occurring during so‐called ‘transition seasons’, from March to May and September to November. During these two seasons, convective activity is at its peak and as a result, the Congo Basin climate has considerable influence over atmospheric dynamics at the planetary scale (Washington et al., 2013). The transition seasons are separated by two dry seasons from June to August and December to February. While the western and southern regions of the Congo Basin exhibit this precipitation regime, the dry season is less pronounced to the east of the Basin, along the western flank of the East African Dome. Further north and south of the Congo Basin, the bimodal rainfall regime subsides, and a single rainy season occurs.
Rainfall patterns in the East African Dome region also display a bimodal distribution, although less pronounced than in the Congo Basin. Precipitation is highest over the topographical highs, enhanced by orographic lift and the convergence of the Atlantic and Indian air masses. To the east, surface winds over coastal areas are controlled by the Asian monsoon circulation over the Indian Ocean. The dry season occurs during boreal summer, when moisture from the Indian Ocean is transported north‐eastward toward the Indian continent. Conversely, during boreal winter wet air masses blowing from the tropical Indian Ocean enter coastal East Africa and trigger rainfalls. To the north, the region of the Horn of Africa is arid and marked by repeated events of severe inland droughts (Viste, Korecha, & Sorteberg, 2013), and even hyper‐arid with deserts near the coast (Somali–Chelbi deserts). Conversely, the Ethiopian highlands (i.e. Ethiopian Dome) capture moisture from multiple sources (Viste & Sorteberg, 2013) and are characterized by high rates of orographic precipitation.
Finally, the climate of western tropical Africa is characterized by a monsoonal regime, the so‐called West African monsoon. Thermal contrasts between sea‐surface temperatures in the Gulf of Guinea and the surface temperature in the Sahelian region drive the seasonal reversal of surface winds, bringing moisture inland. West African monsoon progression inland is characterized by a ‘jump’ between a first regime of high rainfall along the Guinean coast in May to July and a second period of less‐intense precipitation over the Sahel from July to September (Im & Eltahir, 2018). During boreal winter, the Sahelian region is dry, with the tropospheric dynamics driven by north‐easterlies channelled by the topographic features at the border of the Chad Basin, namely Hoggar, Tibesti and Darfur reliefs.
The modern topography of Africa (Fig. 1A; Guillocheau et al., 2018) is characterized by a set of heterogeneously elevated plateaus that strongly influence temperature and rainfall patterns at the continental scale. The largest, the southern African (or Kalahari) Plateau, extends from 1500 to 2000 km longitudinally, and 2500 km latitudinally, with an elevation ranging between 1000 and 1500 m. In contrast to other major tropical regions such as South America and Southeast Asia, Africa is defined by passive rather than active continental margins (Goudie, 2005). The distribution of elevation in Africa is bimodal, an ancient feature probably inherited from the upper Palaeozoic (Doucouré & de Wit, 2003), with one peak around 300–400 m above sea level (asl) in central and west Africa and one ranging from 900 to 1100 m asl in southern and East Africa (Guillocheau et al., 2018). The highest elevations correspond to the Kalahari Plateau, and the East African and Ethiopian Domes, but also to the Cameroon Highlands, Darfur, Tibesti, Hoggar and the Guinea Rise (Fig. 1A). The lowest elevations correspond to the Sahara and the Congo Basin (Fig. 1A).
Overall, the present‐day topography of Central Africa is mostly a post‐Eocene product of so‐called very long (1000–2000 km) wavelength deformations that result from mantle dynamics. The study of planations surfaces (i.e. large‐scale mainly flat surfaces) recording these deformations shows that the growth of the Cameroon Dome and East African Dome initiated 34 million years ago (Ma), the Angola mountains at 15–12 Ma, and that the low‐elevation Congo Basin was uplifted between 10 and 3 Ma (Guillocheau et al., 2018). However, understanding of the precise timing of topographic changes in Africa remains limited at the regional scale.
The relief in the East African Dome (East African Plateau, Fig. 1A) results both from large‐scale doming [deformation of the crust due to mantle dynamics (plume, convection cell) and characterized by a long horizontal wavelength (500–1000 km) and some uplift of the Earth's surface (0.1–2 km)] and from rifting [stretching and thinning of the lithosphere leading to the formation of a single or several central linear depressions bounded by normal faulting and, in the case of a single depression, by rift‐flank uplifts] propagation within the East African Rift System (EARS) during the Late Miocene and the Pliocene (Macgregor, 2015). The EARS is divided into two major branches: the eastern branch, running from northern Ethiopia to northern Tanzania, and the western branch from Uganda to central Mozambique (Fig. 1A). Active rifting started during the Oligocene (30–24 Ma) along the northern East branch (Afar and Ethiopian plateau) progressing southwards raising the East African plateau (Chorowicz, 2005; but see Roberts et al., 2012). Rifting in the western branch remains controversial (Roberts et al., 2012) and is suggested to have initiated either during the middle Late Miocene, around 12 Ma (Chorowicz, 2005) or synchronously with the East branch around 25 Ma (Roberts et al., 2012). Nevertheless, the Middle Miocene was an important period of tectonic activity and major uplift phases of the rift shoulders (Chorowicz, 2005; Ring, Albrecht, & Schrenk, 2018). Dynamic topography modelling suggests that the Kenyan dome uplifted from 500 m to 1000 m asl between 15 and 10 Ma (Wichura et al., 2015).
Finally, the Eastern Arc Mountains consist of a series of 13 isolated fault‐bounded mountain blocks that stretch from southern Kenya to eastern Tanzania (Burgess et al., 2007) independent from the EARS (Fig. 1A). Geologically, these reliefs belong to the Mozambique Orogenic Belt, a major suture zone along which eastern and western Gondwana collided to form the Gondwana continent (Muhongo & Lenoir, 1994; Johnson et al., 2003). The Eastern Arc Mountains were mainly formed by block faulting, which results from tensional forces in the Earth's crust causing large bodies of rock to uprise. The origin of this geological relief is possibly the result of thickening of the continental crust (due to magmatic underplating) ca. 640 Ma that subsequently exhumed in response to the continental collision that led to the formation of Gondwana at ca. 550 Ma (Muhongo, Kröner, & Nemchin, 2001; Johnson et al., 2003). Faulting was suggested to have occurred between 290 and 180 Ma during the Karroo period (Griffiths, 1993; Newmark, 2002). Since then, the Eastern Arc Mountains have gone through repeated cycles of erosion and uplifting, with the latest uplift suggested to have occurred during the last 7 million years (Myr) coinciding with the development of the EARS (Griffiths, 1993; Newmark, 2002). Thus the Eastern Arc Mountains are geologically very old (>100 Ma), with their modern topography the result of more recent activity occurring in the region.
Understanding how these topographic changes altered the environment and biota during the Neogene is still challenging, as it requires (i) a rare combination of fine topographic reconstruction in space and time with climate simulations, and (ii) deciphering signals from larger climate changes induced by variations in atmospheric carbon dioxide concentration (CO2 partial pressure, pCO2) and/or insolation.
III. SIX MAJOR ‘GEO‐CLIMATIC’ PERIODS IMPACTING TROPICAL AFRICAN BIODIVERSITY
Understanding climate change in tropical Africa requires the consideration of multiple drivers, including greenhouse gas‐induced global cooling/warming, oceanic upwellings, continental drift, tectonic uplift, rifting, and insolation variations. Knowledge of African climatic evolution over the Cenozoic is incomplete because of (i) under‐sampling compared to other continents, (ii) the relative rarity of fossilization in humid environments that was prevalent through the Cenozoic and, (iii) the weak sedimentation rates that affect most of the continent with the exception of East Africa.
The opening of the Equatorial Atlantic Ocean during the Albian (ca. 100 Ma) isolated the African continent from other landmasses which lasted until the closure of the east‐Tethys seaway during the Middle Miocene Climatic Transition at ca. 14 Ma [see Hamon et al. (2013) for a review]. This ~84–65 Myr isolation contributed to the radiation of the Afrotheria, a unique group of mammals found only in Africa (Meredith et al., 2011; O'Leary et al., 2013). It was suggested as an important reason for the absence or low diversity of several major tropical plant clades in Africa compared with other tropical regions (e.g. Chloranthaceae, Elaeocarpaceae, Lauraceae, Winteraceae; Morley, 2000). Long‐distance dispersal from Africa to other regions has been inferred for at least one plant family before the Cenozoic (Baker & Couvreur, 2013). Nevertheless, Late Cretaceous land connections between Gondwana landmasses might still have been possible. Such land connections have been suggested to explain distribution patterns within the Gondwanan salt‐intolerant frogs Microhylidae and Natatanura which diverged during the Late Cretaceous (Van Bocxlaer et al., 2006). The African continent has drifted northward by ~15° and rotated counter clockwise since the Early Cenozoic (Figs 2, 3). This drift and the latitudinal palaeo‐position of the African continent were likely crucial in determining the location of moisture advection and convection, and associated palaeo‐temperature and rainfall patterns, as well as oceanic currents (Walker, 1990). Yet, among the numerous detailed accounts of the African fossil record for plants and animals throughout the Cenozoic, few have considered the influence of this drift and palaeo‐position on biodiversity (Morley, 2000; Murray, 2000; Werdelin & Sanders, 2010; Gardner & Rage, 2016).
Fig 2.
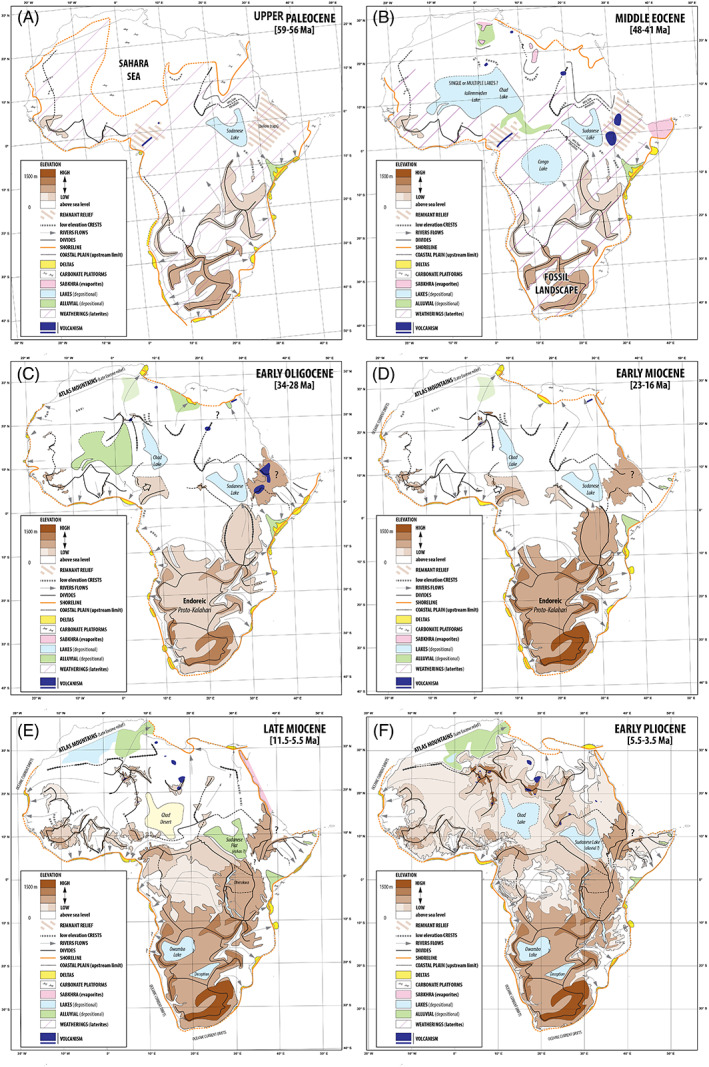
Geological evolution of Africa during the Cenozoic. The maps depict the geological setting for six periods of the Cenozoic: (A) Late Paleocene (59–56 Ma), (B) Middle Eocene (48–41 Ma), (C) Early Oligocene (34–28 Ma), (D) Early Miocene (23–16 Ma), (E) Late Miocene (11.5–5.5 Ma) and (F) Early Pliocene (5.5–3.5 Ma). These maps characterize the palaeotopography and the palaeohydrography (drainage divides, catchment areas and paths of the main rivers) of Africa. They also include data such as shorelines, deltas, depositional alluvial plains and lakes. Reconstruction of the palaeotopography was based on the restoration of the stepped planation surfaces constituting the plateaus (Guillocheau et al., 2018). These planation surfaces, mainly pediments and pediplains associated with weathering processes of laterite type, result from uplifts sometimes enhanced by climate (precipitation) changes. See Guillocheau et al. (2018) for details. The highest surfaces are the oldest (from Late Cretaceous to Middle Eocene) and the lowest are the youngest (Pliocene).
Fig 3.
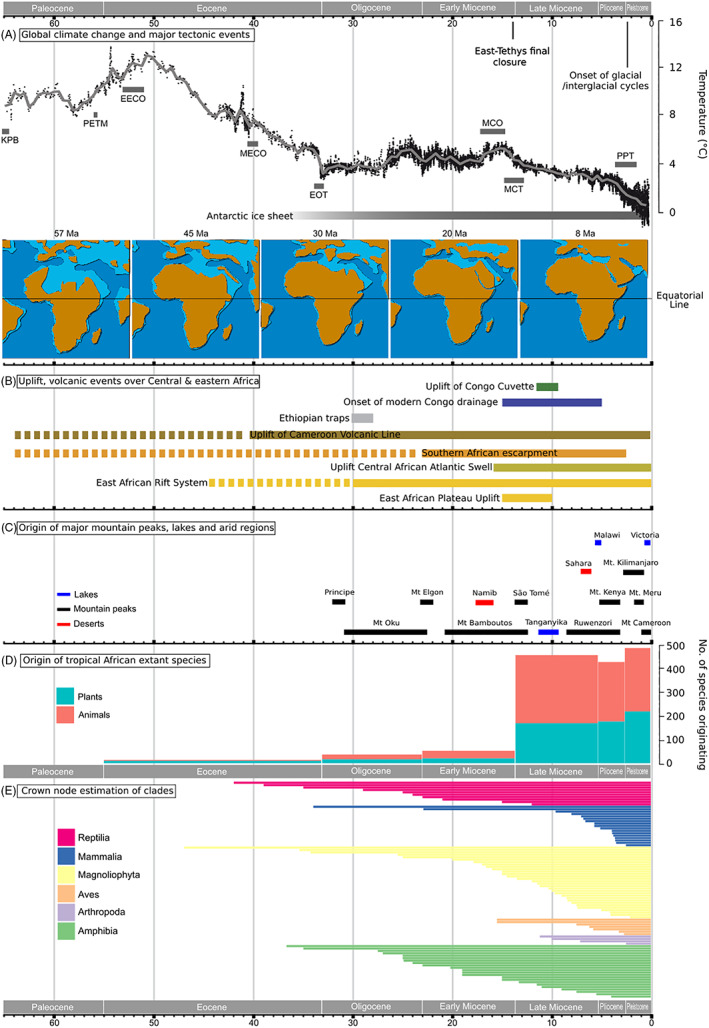
Geo‐climate evolution and biological diversification of tropical African biodiversity. (A) Global temperature change during the Cenozoic (Hansen et al., 2008) and major climate and tectonic events across Africa. KPB, Cretaceous–Paleogene Boundary; PETM, Paleocene–Eocene Thermal Maximum; EECO, Early Eocene Climatic Optimum; MECO, Mid‐Eocene Climatic Optimum; EOT, Eocene–Oligocene transition; MCO, Miocene Climatic Optimum; MCT, Miocene Climate Transition; PPT, Pliocene–Pleistocene Transition. (B) Temporal representation of major uplift and volcanic events in central and eastern Africa (Sepulchre et al., 2006; Guillocheau et al., 2015, 2018). (C) Origin of major mountain peaks, lakes and arid regions in Africa (Marzoli et al., 2000; Gehrke & Linder, 2014; Zhang et al., 2014). (D) Origin of extant species of plants and animals based on time‐calibrated molecular phylogenies (see Appendix S1). (E) Crown node mean age estimates of plant and animal genera based on time‐calibrated molecular phylogenies (see Appendix S1).
In this section, we review the climatic, geological and fossil history of Africa during the Cenozoic by focusing on six defining periods suggested to have impacted the diversification of tropical African biodiversity above the species level. We synthesize the latest data from Earth sciences – namely from geological fieldwork, palaeoclimate and palaeovegetation modelling – and life sciences (mainly dated molecular phylogenies). We do not review how these changes affected the evolution of hominoids which is covered elsewhere (e.g. Joordens et al., 2019). Finally, we do not review in detail climate evolution during the Pleistocene (Trauth, Larrasoana, & Mudelsee, 2009; see Hoag & Svenning, 2017).
(1). Mass extinction? The Cretaceous–Paleogene boundary (~66 Ma)
Although global climate exhibited a long‐term cooling trend at the end of the Cretaceous (i.e. the late Maastrichtian), deposits of black shales in Egypt indicate a hot and humid climate in northern Africa at that time (Fathy et al., 2018). However, both marine and continental records indicate a highly perturbed climate system in the 100000 years preceding the Cretaceous–Paleogene boundary (KPB) (Barnet et al., 2018; Huber et al., 2018). The KPB is marked by the last recorded mass extinction, triggered by global‐scale environmental perturbations driven by both the massive volcanic eruptions of the Deccan Traps (India) (Courtillot & Fluteau, 2014; Schoene et al., 2015; Zhang et al., 2018) and the Chicxulub bolide impact (Schulte et al., 2010).
Our understanding of how these climatic fluctuations of variable length altered tropical African biodiversity remains limited because of the near absence of studied KPB fossils (Nichols & Johnson, 2008; Schulte et al., 2010; Spicer & Collinson, 2014; Vajda & Bercovici, 2014). This is mirrored by few dated molecular phylogenies stretching back to the KPB (e.g. Koenen et al., 2020). Based on these few data, extinction events are inferred at the KPB across the tropical African flora and fauna (Coetzee, 1993; Morley, 2000; Pan et al., 2006; Schulte et al., 2010). However, there is mounting evidence that the KPB did not lead to a large‐scale taxonomic disruption in plants globally in contrast to marine biodiversity (McElwain & Punyasena, 2007;Cascales‐Miñana & Cleal, 2014; Silvestro et al., 2015). To a certain extent, this is also visible for the western African palm fossil record where most fossil genera span the boundary, going extinct during the Paleocene rather than at the KPB (Morley, 2000; Pan et al., 2006). The study of west to central African palaeofloras by Salard‐Cheboldaeff (1990) also documents a continuous transition in fossil taxa throughout the boundary, with many forms common to the Late Cretaceous and Early Cenozoic. Globally, diversification analyses of vascular plant fossils suggested little extinction rate variation across the KPB (Cascales‐Miñana & Cleal, 2014; Silvestro et al., 2015). Dated molecular phylogenies also inferred little or no diversification rate changes across the KPB for several key pantropical lineages which originated during the Cretaceous (e.g. Arecaceae; Couvreur, Forest, & Baker, 2011a), although these should be interpreted with caution given the few data points available during that time period. Rather the KPB initiated an increase in speciation leading to a rapid increase in generic diversity (Cascales‐Miñana & Cleal, 2014). Overall, the KPB also provided more ecological opportunities for increased global diversification of major animal groups such as mammals (Meredith et al., 2011), frogs (Feng et al., 2017; Portik et al., 2019), birds (Feduccia, 2014; Jarvis et al., 2014) and certain plant groups such as Leguminosae (or Fabaceae), one of the most dominant plant families in African biomes (Koenen et al., 2020). Thus, the meteorite impact and the Deccan volcanism could have led to short‐lived ecosystem traumas and extinction, with plant and animal clades quickly recovering (Spicer & Collinson, 2014), especially in tropical ecosystems (Johnson & Ellis, 2002). Overall, the KPB was the start of a second large‐scale flowering plant and animal diversification burst (O'Leary et al., 2013; Silvestro et al., 2015; Feng et al., 2017; Koenen et al., 2020), which initiated the diversification of tropical African biota (Linder, 2014).
(2). Extreme conditions: the Paleocene–Eocene climatic optimum (66–51 Ma)
The Paleocene and Eocene were the warmest intervals of the Cenozoic, dominated by ‘greenhouse’ climates, characterized by the absence of polar ice caps (Foster et al., 2018). The Paleocene ended with the short‐lived Paleocene–Eocene Thermal Maximum (PETM) (ca. 56 Ma, Fig. 3A), a ‘hyperthermal’ period characterized by 5–7°C global warming (Turner, 2018). The early Eocene was marked by the longest and warmest interval of the Cenozoic (Zachos, Dickens, & Zeebe, 2008), the Early Eocene Climatic Optimum (EECO; 53–51 Ma, Fig. 3A). A final climatic optimum occurred during the Mid‐Eocene Climatic Optimum (MECO; ~40 Ma, Fig. 3A), followed by a cooling trend that culminated with the Eocene–Oligocene transition (EOT; 34.1–33.6 Ma, Fig. 3A). During the Paleocene, Africa had a lower elevation than at present and most of the northern part of the continent was submerged by the large Sahara Sea (Fig. 2A). Exceptions include southern Africa which inherited the Late Cretaceous uplift of the South African Plateau (Flowers & Schoene, 2010) and the Guinea Rise in western Africa (Fig. 1A), a remnant of the early Cretaceous rift shoulders of the Equatorial Atlantic Ocean Rift (J. Ye et al., 2017a). Other reliefs were likely present in Ethiopia and Cameroon, but are quite difficult to map in detail because of active magmatism. Volcanic activity was maintained in the Cameroon Volcanic Line over the last 42 Ma (Marzoli et al., 2000). Continental palaeoclimate data is almost non‐existent for the Paleocene–Eocene in Africa, and large uncertainties remain especially regarding precipitation. Results from cores in Tanzania suggest “overall hot and arid conditions punctuated by intense, perhaps seasonal, precipitation events” in East tropical Africa during the PETM (Handley et al., 2012, p. 10), but do not document pre‐ and post‐PETM climate states. Climate models of the early Eocene in Africa simulate temperatures warmer than present‐day by 4°C to 18°C, depending on the prescribed pCO2 and the region considered (Lunt et al., 2012). Precipitation responses in tropical Africa to Eocene conditions are highly variable, ranging from less than 1000 mm/year to more than 3300 mm/year, depending on the model used (Huber & Caballero, 2011; Lunt et al., 2012; Carmichael et al., 2016).
Few fossil sites are recorded for the Paleocene and Eocene for both plants (Bonnefille, 2010; Jacobs, Pan, & Scotese, 2010) and animals (Mayr, 2009; Werdelin & Sanders, 2010; Gardner & Rage, 2016) across tropical Africa, leading to a poor understanding of vegetation distribution and biodiversity at this time (Mayr, 2009; Jacobs et al., 2010; Gardner & Rage, 2016). Nevertheless, the favourable warm and humid Paleocene–Eocene climate is suggested to have led to an important period of diversification in plants and animals, ultimately defining tropical Africa's current biodiversity (Plana, 2004; Morley, 2007; Tolley, Townsend, & Vences, 2013; Koenen et al., 2020).
(a). A pan‐African rain forest?
During the Paleocene and Eocene a pan‐African rain forest is suggested to have extended continuously from western to East Africa linked to the favourable climatic conditions (Axelrod & Raven, 1978; Coetzee, 1993; Lovett, 1993; Morley, 2000, 2007; Willis & McElwain, 2014). Its existence plays a central role in explaining present‐day faunal and floral biogeographic patterns across tropical Africa (Moreau, 1966; Hamilton & Faden, 1974; White, 1979). The repeated fragmentation of this pan‐African rain forest into western/central and East or West and Central blocks (Morley, 2000), during drier periods of the Late Oligocene, mid‐Miocene and Pliocene, is invoked to explain the origin of major trans‐African disjunct distributions (Hamilton & Faden, 1974; Loader et al., 2007; Couvreur et al., 2008; Zimkus, Rödel, & Hillers, 2010; Pokorny et al., 2015).
The existence of a continuous Eocene coast‐to‐coast rain forest, however, has been called into question (Bonnefille, 2010; Linder, 2017). Fossil evidence suggesting the presence of a humid closed‐canopy type vegetation during these times is clearly documented, especially in the west (Salard‐Cheboldaeff, 1990; Morley, 2000). Fossil taxa belonging to characteristic rain forest plant families, such as Annonaceae, Arecaceae, Meliaceae and Myristicaceae were recovered from the Paleocene and Eocene (Morley, 2000; Jacobs et al., 2010). These conditions were also suggested to be favourable for animal taxa, with, for example, dated molecular phylogenies documenting the radiation of modern chameleon genera during the Eocene, ancestrally inferred to be arboreal in closed‐canopy forests (Tolley et al., 2013). However, there is very little direct fossil evidence for rain forest vegetation in East Africa during the Paleocene and Eocene. This is not surprising given the few fossil sites available in that region (Jacobs & Herendeen, 2004; Jacobs et al., 2010; Linder, 2017). The Middle Eocene Mahenge site from north‐central Tanzania in East Africa documents a woodland resembling present‐day miombo rather than rain forest vegetation (Jacobs & Herendeen, 2004). However, during the Eocene, Africa was located some 10° south of its present location and Arabia was still connected to the continent (Figs 2B, 3A). Climate simulations of the Eocene suggest a hot climate and a strong hydrological cycle in the tropics, but also show reduced precipitation south of 20°S (Sagoo et al., 2013, see Fig. S1). Thus, it is likely that during the Paleocene–early Eocene the Tanzanian region, including Mahenge, was too far south (about 15°S) to permit the development of rain forest vegetation. In contrast to Mahenge, the Kaninah Formation, a Middle Eocene fossil site in Yemen located near the palaeo‐equator during that time, documents the presence of rain forest‐type vegetation, with fossils linked to, for example, Annonaceae (As‐Saruri, Whybrow, & Collinson, 1999). Additionally, evidence of rain forests from the Paleocene was found along the Red Sea in Egypt (Boureau et al., 1983). Thus, Paleocene and Eocene rain forest vegetation on the east coast of Africa is not undocumented, but was probably located further north than its current location (Bonnefille, 2010).
Biogeographic studies based on dated molecular phylogenies of clades restricted to rain forests also support the existence of a once‐continuous pan‐African rain forest during the Cenozoic. If the fragmentation of this pan‐African forest was responsible for the observed disjunct patterns between East and West/Central blocks we expect vicariant events to be synchronous with periods of increased African aridity (Loader et al., 2007; Couvreur et al., 2008). In addition, we would expect these events to be temporally concordant between different rain forest clades. Interestingly, independently inferred vicariant events have been dated to around the EOT (~33 Mya) in at least two major plant (Annonaceae; Couvreur et al., 2008) and animal clades (chameleons; Tolley et al., 2013). These were suggested to be the result of the break up of the pan‐African forest, leading to the isolation and speciation of lineages in western/central and East Africa. Unfortunately, there are only two studies to date that uncover this pattern for the Eocene, as most extant clades diversified after the Eocene (Fig. 3D, E). Nevertheless, the concordance in the recovered dating of these vicariant events between clades is quite striking, favouring a common response between these groups, rather than relying on random processes such as long‐distance dispersal (Linder, 2017).
The history of forest fragmentation between the West (or upper Guinea) and Central (or lower Guinea) Africa, which are separated by the ca. 200 km wide drier ‘Dahomey gap’ corridor located in Benin and Togo (Salzmann & Hoelzmann, 2005), is less clear. Differences in species diversity are less marked between these two forest blocks than between West/Central and East Africa (Linder et al., 2012; Droissart et al., 2018). Even though there are high levels of taxonomic endemicity in West Africa (Linder, 2001; Penner et al., 2011), numerous species are common between both regions (Linder et al., 2012; Droissart et al., 2018). In addition, diversity studies in plants or animals still do not agree on where the biogeographic separation lies between West and Central Africa (e.g. Volta and Niger rivers, Dahomey gap or the Cross River region in eastern Nigeria), and this is probably species dependent (Booth, 1958; White, 1979; Nicolas et al., 2010; Penner et al., 2011; Linder et al., 2012; Droissart et al., 2018). This suggests a closer biogeographic link between these regions than between West/Central and East Africa. Numerous phases of savanna expansions are documented for the last 7 Myr (Dupont et al., 2000; Bonnefille, 2010), linking and unlinking west and central forests, potentially allowing recent floristic and faunistic exchanges. Estimated ages of vicariance, based on dated phylogenies between animal species on either side of West and Central Africa, span the Late Miocene and Plio‐Pleistocene (e.g. Nicolas et al., 2006, 2019; Hassanin et al., 2015; Huntley & Voelker, 2016; Gaubert et al., 2018; Jongsma et al., 2018). The late Pliocene–early Pleistocene, between 3 and 2 Ma, appears to concentrate most of these vicariance events across studies. Indeed, this period is marked by a sudden and strong increase in savanna across West Africa (see Section III.6). Finally, more recent forest fragmentation (last 150 Kyr; Dupont et al., 2000) mainly impacted within‐species genetic diversity structuring (e.g. Nicolas et al., 2012; Fuchs & Bowie, 2015; Demenou, Doucet, & Hardy, 2018; Huntley et al., 2019; Leaché et al., 2019).
(b). The golden age of mangroves
The warm Paleocene and Eocene climates were favourable for mangrove vegetation (Morley, 2000). Probably, the most striking geological feature of the Paleocene was the presence of the epicontinental Sahara Sea in northern Africa (Fig. 2A). It was connected to the Tethys Ocean to the north and at its maximum extent reached western Africa in present‐day northern Nigeria (Luger, 2003; Guiraud et al., 2005; Ye et al., 2017a). This marine incursion originated during the middle Cretaceous (ca. 98 Ma) and disappeared during the middle Eocene (Guiraud et al., 2005). The influence of this incursion on African biodiversity has been little studied, possibly because it is just too old to have had lasting effects on present‐day biodiversity (Fig. 3D, E) in contrast to a similar event during the Early Miocene in the Amazon region (the Pebas system; Hoorn et al., 2010). Nevertheless, the presence of marine‐like herring fishes in east and west African lakes has been linked to the existence of this palaeo‐sea (Wilson, Teugels, & Meyer, 2008). It was also suggested to have provided a passage between northern and western Africa for fossil ostracod taxa (Luger, 2003) and marine fishes such as lamniform sharks and rays (Murray, 2000). This extended shoreline of the Tethys sea was inferred to be the origin of the mangrove vegetation (Descombes et al., 2018), which became well established during the Paleocene and Eocene across Africa based on palynological data (Morley, 2000). During the Eocene, mangrove taxa represented up to 20% of plant diversity in certain sites around the Benue River catchment (Utescher & Mosbrugger, 2007). The Paleocene and Eocene correspond to a global increase and diversification of mangroves worldwide and models suggest a strong presence of mangrove taxa along most of the African coast at that time (Descombes et al., 2018).
(3). ‘Descent into the icehouse’: Eocene–Oligocene transition (34.1–33.6 Ma)
Following the MECO, global temperatures decreased gradually, a trend that culminated with abrupt cooling at the EOT (Zachos et al., 2008). During this time, Earth switched from a greenhouse to an ‘icehouse’ climate state (Thomas, 2008), characterized by a permanent ice sheet over Antarctica (Fig. 3A; Zachos et al., 2008; Thomas, 2008; Inglis et al., 2015). The onset of the Antarctic glaciation is attributed to a decrease in pCO2 (Ladant et al., 2014) and/or continental reconfiguration opening the southern seaways (the Drake passage and the Tasman seaway), ultimately modifying ocean heat transport (Lear & Lunt, 2016). How the EOT altered the African climate remains unclear mainly because of uncertainties in pCO2 reconstructions during the Eocene and Oligocene (Steinthorsdottir et al., 2016). While a cooling trend has been recorded by ocean proxies, continental indicators have shown contradictory results (Pound & Salzmann, 2017). Numerical simulations suggest that the intensification of the Atlantic meridional overturning circulation associated with the EOT also caused a northward shift of the Inter Tropical Convergence Zone (ITCZ), increasing precipitation over northern Africa (Elsworth et al., 2017). The inception of Antarctic glaciation is also thought to have produced a ~70‐m sea‐level drop (Miller et al., 2005). Meanwhile, the growth of the Hoggar swell in northern Africa (Fig. 2B, C) led to the establishment of a modern‐like west African drainage geometry (Grimaud et al., 2017). Alluvial deposits in the Niger Basin, as well as along the northern African coast, testify to humid conditions and rivers flowing both towards the Atlantic and Tethys oceans during the early Oligocene (Fig. 2C). The Sahara Sea slowly shrank due to doming, leaving large lakes in huge depressions in western Africa from Mali to Chad (Fig. 2B). In East Africa, the onset of volcanic activity is dated to 45–40 Ma (Roberts et al., 2012; Prave et al., 2016) but reached a peak with the outpouring of important magma ca. 31 Ma leading to formation of the Ethiopian traps (Figs 2C, 3B).
As for the rest of the Paleogene, the Oligocene is poor in fossil sites for animals and plants as well as palaeoclimate proxy records (Murray, 2000; Jacobs et al., 2010; Seiffert, 2010; Gardner & Rage, 2016). The Kwa‐Kwa palaeoflora core near present‐day Douala in Cameroon documents an important turnover of the vegetation at or around the EOT, with numerous taxa disappearing followed by a rapid increase in new, mainly angiosperm taxa (Salard‐Cheboldaeff, 1979). Morley (2000, p. 87), based on a compilation of west African palaeoflora data (Salard‐Cheboldaeff, 1990), documents a decrease in overall plant diversity immediately after the EOT. This decrease in rain forest palaeodiversity appears to be a tropical‐wide phenomenon at the EOT, with similar patterns reported in the Neotropics (Jaramillo, Rueda, & Mora, 2006). Fossil data document considerable extinction in palms, more so than across the KPB (Morley, 2000; Pan et al., 2006), for example with the mangrove palm Nypa disappearing from records across Africa.
Diversification analyses using dated phylogenies also document (mass) extinction around the EOT in several clades, such as climbing palms (Faye et al., 2016b ) and the legume tribe Podalyrieae (Crisp & Cook, 2009). By contrast, other groups did not show signs of mass extinction across the EOT, for example in the mainly African legume tree clade Detarioideae, although extinction rates were inferred to be generally quite high in this clade between 45 and 15 Ma (de la Estrella et al., 2017). The EOT also marked an important evolutionary turn in grasses (Poaceae), which shifted and subsequently diversified from their ancestrally closed habitats into open ones (Bouchenak‐Khelladi et al., 2010b ; Bouchenak‐Khelladi, Muasya, & Linder, 2014a), although it does not correlate with the well‐studied origin of C4 metabolism in grasses (Edwards et al., 2010).
Overall, rain forests are thought to have retracted significantly during the EOT, breaking up the Eocene pan‐African forest that potentially persisted until then (see Section III.2a). In northern Africa, there is fossil evidence for the extinction of tropical taxa and the appearance of savannah‐ and woodland‐associated ones (Boureau et al., 1983). This pan‐African fragmentation had an important impact on the distribution of present‐day diversity, leading to the first vicariance of once‐widespread groups into west/central and east clades and the origin of endemic East African genera (Couvreur et al., 2008; Tolley et al., 2013).
The EOT led to what is known as the ‘Grande Coupure’ for primates, a sudden reduction in their diversity mainly documented in the fossil record of Europe and North America. Interestingly, molecular diversification analyses either failed to find support for a turnover of primate palaeodiversity overall (Springer et al., 2012; Herrera, 2017) or detected moderate support for declining diversification rates at the EOT (Herrera, 2017). In Africa, despite the few fossil sites available, the EOT potentially led to a gradual reduction in primate diversity, linked to a continent‐wide contraction of rain forests, although only a few major lineages went extinct (Seiffert, 2007). It also marked the origin of the oldest present‐day primates, the Galagidae or bush babies, which started to diversify at 33 Mya just after the EOT (Pozzi, Disotell, & Masters, 2014; Pozzi, 2016).
More favourable conditions after the EOT might have led to a renewed expansion of rain forests, reconnecting the west and east forest blocks (Morley, 2000). Indeed, analyses of palaeosurface formed during the Late Oligocene (29–24 Ma) depict a hot climate with seasonal precipitation in West Africa (Beauvais & Chardon, 2013) and increased humidity (Robert & Chamley, 1987). This is consistent with the northward drift of Africa and the position of the equator south of western Africa, above the present‐day Gulf of Guinea. Rain forest‐resembling fossil taxa are documented from Ethiopia and Cameroon (Bonnefille, 2010; Jacobs et al., 2010). Although palm diversity never recovered after the EOT, palm fossils remained an important component of the few documented Oligocene palaeofloras (Salard‐Cheboldaeff, 1979; Pan et al., 2006). Finally, dated molecular phylogenies support the idea that the post‐EOT period marked an important phase of diversification for certain reptile groups such as burrowing snakes (Aparallactinae; Portillo et al., 2018) and chameleons (Tolley et al., 2013) and for major clades in skinks (Scincidae; Medina et al., 2016).
(4). Renewed warm climates: early Miocene to the middle Miocene climatic optimum (~17–14.7 Ma)
The Miocene (ca. 23–5.3 Ma) is considered one of the most pivotal periods for tropical Africa (Plana, 2004), with several climatic, geological and physiographic changes hypothesized to have led to a complex evolution of African biodiversity (White, 1981; Morley, 2000; Senut, Pickford, & Ségalen, 2009; Bonnefille, 2010). How African vegetation responded to these changes is far from clear, since (i) absolute dating of the fossil record is rare for the early and Middle Miocene of Africa, and (ii) numerous factors, either proximal, like mountain uplift and rifting, giant lakes and palaeodrainage upheavals, or remote, like pCO2 variations, closure of tropical seaways (e.g. Hamon et al., 2013; Sepulchre et al., 2014) and orbital cycles, altogether altered the tropical climate of Africa during this period (Linder, 2017).
The global long‐term cooling trend initiated after the EECO is less marked in the early Miocene deep‐sea record (Fig. 3A), and is obscured by the major interruption of the Middle Miocene Climatic Optimum (MCO; ca. 17–14.7 Ma, Fig. 3A). This interval was characterized by global temperatures about 3–8°C higher than the pre‐industrial period of the late Holocene, similar to those of the late Oligocene (You et al., 2009; Holbourn et al., 2015), and an increase in pCO2 when compared to the Oligocene–Miocene transition (Kürschner, Kvaček, & Dilcher, 2008). Given the lack of constraints on palaeobotanic dates and the absence of direct continental palaeoclimate proxies for the Middle Miocene in Africa, inferring how the ca. 2 million‐year‐long warming of the MCO influenced the fate of tropical African biodiversity remains very challenging.
In western Africa, the fossil record documents the presence of rain forests and the reappearance of mangrove vegetation following its EOT demise (Salard‐Cheboldaeff, 1979; Jacobs et al., 2010). In addition, the lack of charred grass cuticles and pollen indicates the absence of widespread open habitats (Morley & Richards, 1993). There is also fossil evidence of early Miocene (ca. 19 Ma) rain forest assemblages from Kivu in the East Democratic Republic of the Congo (Jacobs et al., 2010). In addition, unfavourable conditions for dry‐adapted plants during the Early Miocene led to the first vicariance events inferred within some elements of the Rand Flora (Pokorny et al., 2015; Mairal, Sanmartín, & Pellissier, 2017), an assemblage of unrelated drought‐adapted taxa co‐distributed around the subtropical and drier margins of Africa (Sanmartín et al., 2010).
In East Africa, the picture is even less clear, with several fossil sites documenting the presence of rain forest, a mix of rain forest and grassland patches, woodland or grasslands (Andrews & Van Couvering, 1975; Bobe, 2006; Bonnefille, 2010; Jacobs et al., 2010; Wichura et al., 2015; Linder, 2017). This heterogeneity in the East African early Miocene fossil record could either reflect stronger climate variability, or an early role of changing elevations leading to different palaeoenvironmental, geomorphological, and palaeohydrogeological settings. Indeed, although the overall elevation of the African continent was still lower than present‐day (Fig. 2C), the East African surface underwent large‐scale doming during the Early to Middle Miocene, and changes in basin configuration were initiated in the western branch (Lake Albert) of the East African Dome during the Early Miocene (17 Ma; Simon et al., 2017; Guillocheau et al., 2018).
The extent of the Early to Middle Miocene rain forests in East Africa remains controversial (Bonnefille, 2010; Fer et al., 2017; Linder, 2017), and the question is open as to whether a pan‐African rain forest was once again in place. Climate and vegetation modelling have produced a variety of results, depending on the experimental design (You et al., 2009; Henrot et al., 2010, 2017; Hamon et al., 2012; Goldner, Herold, & Huber, 2014). Henrot et al. (2017) showed an increase in temperature and rainfall in East Africa during the MCO, but no clear signal could be extracted amongst the five models tested regarding a continuous rain forest band across tropical Africa. By contrast, other experiments with low topography suggested numerous combinations of rainfall and temperatures which could have allowed the presence of a pan‐African rain forest (Fer et al., 2017). However, the above‐mentioned models are based on an homogeneous East African Dome ranging from 500 to 800 m asl (Herold et al., 2008) and are likely over‐simplifications, since evidence of high elevations (1400 m asl) shortly after the MCO (13.4 Ma) suggests a very rapid uplift in this region during the Middle Miocene (Wichura et al., 2010).
Several Oligocene to early Miocene fossil sites suggest the presence of rain forest in Eastern Africa (Ethiopia, Kenya, and Uganda). Interestingly, these palaeofloras and faunas were shown to have elements linked to West/Central African forests (Andrews & Van Couvering, 1975; Vincens, Tiercelin, & Buchet, 2006; Jacobs et al., 2010; Wichura et al., 2015; Linder, 2017). The presence of a 17‐Myr‐old whale fossil (Wichura et al., 2015) from the now 600 m high Turkana Basin (northern Kenya, Fig. 1A) attests to an active eastward‐directed drainage basin linking the African interior with the Indian Ocean. This, coupled with fossil pollen evidence for closed‐canopy vegetation and humid (rainfall >1000 mm/year) conditions (Vincens et al., 2006), suggests a possible role of the Turkana Basin as an important corridor for faunal and floral transcontinental connections (Feibel, 1993).
In addition, the Eastern Arc Mountains, an ancient crystalline mountain chain ranging from East Tanzania to south‐east Kenya (Lovett, 1993) could have played a crucial role in connecting west/central and east forests. Indeed, this mountain range has been suggested as climatically stable on a multimillion year scale, probably continuously harbouring forests since the Miocene (Lovett et al., 2005). Dated molecular phylogenies of certain Eastern Arc clades find support for Oligocene–Miocene origins and long‐term persistence in these forests (Tolley et al., 2011; Dimitrov, Nogués‐Bravo, & Scharff, 2012; Loader et al., 2014; Grebennikov, 2017). This stability has been linked to the proximity of the mountain range to the Indian Ocean, providing significant and constant moisture through time (Lovett et al., 2005; Finch, Leng, & Marchant, 2009).
Finally, the presence of rain forest habitat in East Africa is also suggested by the evolutionary history of forest‐restricted lineages that diversified extensively during the Early Miocene, such as chameleons (Matthee, Tilbury, & Townsend, 2004; Tolley et al., 2011). Thus, even though a continuous pan‐African forest might not have persisted throughout the entire Early Miocene (Bonnefille, 2010; Linder, 2017), evidence from vegetation and climate models, fossil sites, and dated molecular phylogenies favours the hypothesis of a rain forest band reconnecting east and west forests blocks after the EOT fragmentation (Andrews & Van Couvering, 1975; Morley, 2000; Couvreur et al., 2008).
(5). The middle Miocene climate transition (15–13 Ma)
Shortly after the MCO, global cooling resumed (Fig. 3A) and the marine isotopic record suggests a phase of important Antarctic ice sheet expansion (Shevenell, Kennett, & Lea, 2008), termed the Middle Miocene Climate Transition (MCT; ca. 15–13 Ma; Fig. 3A). Amongst the hypothesized drivers of this cooling are (i) tropical seaway constrictions, in particular Tethys sea closure around 14 Ma (Zhang et al., 2011; Hamon et al., 2013), (ii) a major pCO2 decrease between 15 and 14 Ma (Kürschner et al., 2008), and (iii) tectonic uplift at a global scale.
Climate modelling shows that the changing topography of East Africa dramatically influenced climate at the continental scale. Sensitivity experiments to elevation change of the EARS showed that the first‐order response to uplift was a precipitation reduction in tropical East Africa (Sepulchre et al., 2006). Altering air mass dynamics also had remote consequences such as the drying of the Congo Basin (Sepulchre, Ramstein, & Schuster, 2009; Prömmel, Cubasch, & Kaspar, 2013; Sommerfeld, Prömmel, & Cubasch, 2016). Another interesting geological development was the uplift of the Central African Atlantic Swell (Fig. 1A), a low mountain range (max. 1200 m asl) stretching from Ngovayang massif (South Cameroon) to the Mayombe massif (South Republic of the Congo), possibly since the Middle Miocene (ca. 16 Ma; Guillocheau et al., 2015).
The reconnection of Africa and Eurasia via the closure of the Tethys seaway (20–14 Ma) (Hamon et al., 2013) ended the 80 million‐year‐long isolation of Africa. This led to major faunal interchanges via the Arabian plate. Turnover of previous African lineages, that had evolved in isolation within Africa (Springer et al., 1997), with northern migrants are evidenced from the fossil record in East Africa already at the start of the reconnection during the Oligocene–Miocene transition and later during the Miocene–Pliocene transition (Leakey et al., 2011). Several dispersal events between Africa and Asia are also recorded (e.g. Lecompte et al., 2008).
Several authors infer that during the Middle Miocene, overall drier conditions led to the expansion of open habitats such as grasslands and woodlands, providing diversification opportunities for numerous dry‐adapted plant and animal taxa (Retallack, Dugas, & Bestland, 1990; Morley & Richards, 1993; Morley, 2000; Davis et al., 2002; Senut et al., 2009; Jacobs et al., 2010). The Middle Miocene corresponds to the first inferred shifts of forest‐adapted species into open and drier habitats followed by subsequent diversification (Davis et al., 2002; Bouchenak‐Khelladi et al., 2010a; Armstrong et al., 2014; Veranso‐Libalah et al., 2018). This period also marks the presence of C4 carbon fixation in grasses, or C4 grasses, in Africa, a dominant component of present‐day African savannas which evolved independently in numerous Poaceae (Bobe, 2006; Ségalen, Lee‐Thorp, & Cerling, 2007; Bouchenak‐Khelladi et al., 2009, 2014b ; Edwards et al., 2010; Uno et al., 2011).
Globally, the Middle Miocene marks the retraction of rain forest towards the equator and the expansion of savannas (Morley, 2007). In Africa, the lowland rain forest which may have connected east and west forest blocks during the Early Miocene (see Section III.4) retracted again, as evidenced by semi‐arid conditions in the Congo Basin in the Middle Miocene (Senut et al., 2009). In East Africa, rain forests greatly reduced with a marked increase in grassland and gallery forests (Retallack et al., 1990; Morley, 2000; Jacobs et al., 2010). This fragmentation was suggested to have spurred diversification in forest‐dwelling animals, such as guenons (tribe Cercopithecini; Guschanski et al., 2013). Numerous independent molecular‐dating studies support vicariance within forest‐restricted clades around the MCT (15–13 Ma) in plants (Davis et al., 2002; Couvreur et al., 2008; Dimitrov et al., 2012; Pokorny et al., 2015; Tosso et al., 2018; Brée et al., 2020), snakes (Menegon et al., 2014; Greenbaum et al., 2015), amphibians (Loader et al., 2007; Bell et al., 2017), birds (Voelker, Outlaw, & Bowie, 2010) and rodents (Bryja et al., 2017). Once again, these studies strongly support the idea of continental‐wide pan‐African forest fragmentation (Couvreur et al., 2008) as a main driver of east/west disjunctions rather than random long‐distance dispersals.
During the Middle Miocene, the continued uplift of the East African Plateau is contemporaneous with the first radiations of the tropical alpine or Afrotemperate/Afromontane (White, 1981; Linder, 2017) frost‐tolerant clades (Galley et al., 2007; Antonelli, 2009; Linder et al., 2013). However, these resulted in lower numbers of species (Cox et al., 2014; Gehrke & Linder, 2014) compared with other tropical Alpine regions like the Andes (Hughes & Eastwood, 2006). The East African Plateau provided an important migration route linking north and south Africa, allowing Cape elements to disperse northwards (Galley et al., 2007), and Eurasian elements to disperse southwards (White, 1981; Gehrke & Linder, 2009; Mairal et al., 2015; Gizaw et al., 2016), favouring longitudinal transcontinental exchanges (Galley et al., 2007). Diversification also occurred in the Cameroon Volcanic Line for certain montane clades such as puddle frogs which find their origins in the mountain range during the Early Miocene (Zimkus & Gvoždík, 2013). Other typical Afromontane clades also started to diverge during this time, such as the conifer montane‐restricted genus Podocarpus (Quiroga et al., 2016). Fossil pollen evidence of Podocarpus is recorded off the Somali coast as early as 11 Ma (Feakins et al., 2013) however clear presence of this genus in continental Africa dates only to 2.7 Ma from West Africa (Morley, 2011).
(6). The end of equable climates: from the late Miocene to the mid‐Pleistocene (11–1.5 Ma)
The last 11 Myr appear critical in the evolution of tropical African biodiversity, as most extant species or genera have originated during this time interval (Fig. 3D, E). In terms of climate, sea‐surface temperature reconstructions depict a global and sustained cooling from 11 Ma to 5.3 Ma, with a steeper decrease in temperatures between ca. 7 and 5.4 Ma, the so‐called Late Miocene Cooling (LMC), that was very likely driven by a decrease in atmospheric pCO2 (Herbert et al., 2016). Between 11 Ma and the end of the LMC (5.4 Ma), high‐latitude temperatures dropped by as much as 13°C to reach near‐modern values, whereas cooling was less marked in the tropics. The resulting increase in the temperature latitudinal gradient is expected to have reinforced and contracted the Hadley cells (atmosphere circulations around the tropics), thereby expanding arid areas in the subtropics (Herbert et al., 2016). Between 6 Ma and 5.4 Ma, multiple glacial‐to‐interglacial fluctuations have been inferred from the isotopic record, with a precession‐like periodicity (Hodell et al., 2001), likely explaining Late Miocene evidence for partial glacial and ephemeral glaciation in Greenland (Larsen et al., 1994). The LMC also partly overlapped with the Messinian Salinity Crisis (MSC, 5.97–5.33 Ma), during which the Mediterranean turned into deep desiccated basins, with partial or full closure of the Gibraltar Strait (Krijgsman et al., 2018). However the consequences of the MSC on the tropical climate of Africa remain hard to quantify (Murphy et al., 2009).
At the scale of the African continent, the Late Miocene cooling is thought to have triggered a progressive aridification, and overall the Late Miocene palaeovegetation records depict a trend to more open habitats and the rise of grasslands. However, stating that the African biota responded linearly to global climate changes would be an oversimplification, as major proximal factors (e.g. topography, Paratethys retreat) likely altered temperature and precipitation patterns, driving various biota responses during the last 11 Myr. Previous reviews of the Neogene continental and marine palaeobotanical records (Jacobs, 2004; Bonnefille, 2010) show strong heterogeneity of the Miocene ecosystems of tropical Africa. Pollen data also suggest that savannah expansion occurred at ca. 10 Ma in East Africa, whereas it would have occurred later in western Africa (8–7 Ma). The northern Chad record shows that between 7.5 and 7 Ma, the vegetation cover of the region was characterized by a “mosaic environment, including closed forest patches, palm groves, and mixed/grassland formations” (Novello et al., 2017, p. 66) whereas a grass‐dominated signal appears only during the Pliocene, after 4.5 Ma. The same region has also provided the earliest firm evidence for a Sahara desert, dated at 7 Ma (Schuster et al., 2006), the onset of which is inferred by climate simulations triggered by the retreat of the Tethys Sea (Zhang et al., 2014). Still, fluctuations among lacustrine, swamp and arid environments in the Chad Basin during the Late Miocene testify to higher‐frequency, maybe orbitally paced, climate variations during the Late Miocene in northern Africa (Vignaud et al., 2002). Extensive tropical rain forests were unlikely in north‐East Africa any time during the last 12 Ma (Feakins et al., 2013) and Ethiopia was more likely covered by seasonal, deciduous woodland dominated by a diversified Fabaceae family before grassland expansion (Bonnefille, 2010; Feakins et al., 2013). The rise to dominance of C4 photosynthesis is complex and decoupled from the earliest evolutionary origins of C4 grasses during the EOT (Bouchenak‐Khelladi et al., 2014b ). The transition to C4 grass‐dominated biomes has been discontinuous and spatially heterogeneous, with at least two phases of C4 grass biomass increase (11–9 Ma and 4.3–1.4 Ma; Ségalen et al., 2007; Feakins et al., 2013). A similar trend is seen in the clade Amaranthaceae/Chenopodiaceae, a group of plants characteristic of arid lands and with the largest diversity of C4 eudicot plants (Kadereit, Ackerly, & Pirie, 2012), where two main peaks are recorded across northern East Africa: Late Miocene 8–6 Ma and Pliocene 5.5–2.5 Ma (Bonnefille, 2010). In addition, C4‐dominated ecosystems rose abruptly in north‐western and East Africa around 10 Ma (Uno et al., 2016). Finally, it has recently been suggested that this transition happened in the absence of any significant aridification signal, rather suggesting a major role for cooling and pCO2 decrease in this process (Polissar et al., 2019).
In animals, the evolutionary shift to C4‐grazing amongst large mammalian herbivores seems to have been immediate for some lineages like the proboscideans (elephants), which started to include C4 plants in their (browsing) diet as early as 9.9 Ma and became grazers at 7 Ma (Uno et al., 2016), and more gradual for others (Ségalen et al., 2007; Uno et al., 2011). In particular, there is a documented rise in herbivorous mammals during the Late Miocene in East Africa (Bobe, 2006) followed by a clear decline in megaherbivores from 7 Ma onwards (Faith, Rowan, & Du, 2019). By contrast, large carnivore species richness declines after 3 Ma possibly linked to the decrease in megaherbivores across East Africa and the expansion of C4‐dominated ecosystems (Faith et al., 2019). In addition, numerous animal clades are suggested to have progressively diversified during the Late Miocene in relation to more‐open ecosystems such as bush crickets (Voje et al., 2009), gazelles (tribe Antilopini; Hassanin et al., 2012), and burrowing snakes (subfamilly Aparallactinae; Portillo et al., 2018).
At lower latitudes, offshore marine pollen data from the Niger delta document a possible forested wet phase between 7.5 and 7.0 Ma (Morley, 2000; Bonnefille, 2010). This is in agreement with vegetation simulations of the Turonian period (11.61–7.25 Ma) where rain forests were likely in West, Central and East Africa (Ethiopia and Somalia; Pound et al., 2011).
In the rift system in Kenya, vegetation patterns are biogeographically complex throughout the last 12 Ma, suggesting that palaeobotanical change from wet forest to savanna was not unidirectional (Jacobs et al., 2010). This is likely due to increased topographic complexity linked to ongoing rifting throughout the region during the Late Miocene and the Pliocene.
The transition from the Miocene to the Pliocene depicts a renewal of warm climate at the global scale. Temperatures peaked during the early Pliocene (ca. 4 Ma) to reach values globally ~4°C greater than the preindustrial, and 1°C warmer than the following mid‐Pliocene warm period (also referred to as the mid‐Piacenzian warm period; see Haywood et al., 2013). Numerical simulations suggest this time interval, besides ephemeral cold events [e.g. the Marine Isotope Stage (MIS) M2, 3.31–3.26 Ma; Tan et al., 2017], was characterized by a slowdown of the Hadley circulation that led to increased precipitation over subtropical regions of Africa (Brierley et al., 2009), and a strengthening of the African summer monsoon (Zhang et al., 2016). The early to mid‐Pliocene interval was termed the ‘Golden Age’ with tropical rain forests re‐expanding and savannas contracting (Morley, 2000). Indeed, several fossil sites from East Africa document the presence of moist‐adapted taxa and forest between 5 and 3 Ma (Morley, 2000; Pickford, Senut, & Mourer‐Chauviré, 2004; Jacobs et al., 2010; Linder, 2017; Joordens et al., 2019). The East African coastal forests were suggested to extend from southern Africa to the Horn of Africa prior to 3 Ma (Joordens et al., 2019). Once again, this favourable climate possibly allowed west/central and east rain forest blocks to reconnect, either as a continuous forest block (Fer et al., 2017) or via moist vegetation corridors linking East and West/Central regions (Joordens et al., 2019). For example, the Turkana gap fossil site in southern Ethiopia dated to 3.4–3.3 Ma documents the presence of evergreen or semi‐deciduous forests (Hernández Fernández & Vrba, 2006; Bonnefille, 2010) with the presence of plant (Antrocaryon, Anacardiaceae) and animal (Potadoma, Pachychilidae) taxa known today only from Central African rain forests (Bonnefille & Letouzey, 1976; Williamson, 1985). Isotopic data on pedogenic carbonates also indicate increased woody plant (tree) cover in the Awash Valley and north Turkana Basin in north East Africa (Cerling et al., 2011). Interestingly, this period might also have led to reversals from open to forested habitats in some Mimosoideae (Fabaceae) clades (Bouchenak‐Khelladi et al., 2010a ).
Following the mid‐Pliocene warm period, the climate gradually cooled during a time interval referred to as the Pliocene–Pleistocene Transition (PPT, 3.6–1.4 Ma; see Fig. 3A). PPT cooling was marked by the intensification of Northern Hemisphere glaciation (iNHG; e.g. Haug et al., 2005). Starting from 2.7 Ma onwards, the Earth system entered full glacial/interglacial cycles with hemispheric glaciations, in contrast to the previous ephemeral ice sheets waxing and waning that characterized the Miocene and the Pliocene. These fluctuations between glacial and interglacial periods had a strong impact on all vegetation types across Africa during the Pleistocene (Trauth et al., 2009). Interestingly, 2.7 Ma also coincides with a marked shift in both western and eastern African pollen records during which a minimum in tree cover density is reached (Bonnefille, 2010) indicating a hypothetical link between the hemispheric‐scale iNHG and vegetation in tropical Africa. Indeed, the iNHG and associated growth of massive ice sheets likely altered atmospheric dynamics through orographic and radiative effects, but did not coincide with any major change in tropical sea surface temperature (SST) patterns (Ravelo et al., 2004). Aridification is inferred from the increased abundance of sub‐desertic pollen taxa and C4 plants (e.g. Amaranthaceae s.l.) at the expense of grasses and arboreal taxa in west and east Africa (Feakins et al., 2013; Liddy, Feakins, & Tierney, 2016), and from the increase of terrestrial dust flux off the east, north and west African coasts (Trauth et al., 2009). The numerous palaeoenvironmental records of East Africa [see Maslin et al. (2014) for a review] also showed a transition from C3 to C4 plants during the Plio‐Pleistocene in East Africa, that was attributed to “a gradual progression towards a more variable climate with intensified arid periods” (Maslin et al., 2014, p. 5). Palaeosol data from the Awash valley and the Omo‐Turkana Basin depict a transition from woodland/bushland to wooded grasslands during the PPT (Cerling et al., 2011), but the trend to aridification and the increase in variability of the tropical African climate are subject to ongoing debates regarding their pace and driving mechanisms (e.g. stepwise or gradual; deMenocal, 2004; Trauth et al., 2009). The difficulty comes from the hard task of deciphering between (i) the long‐term secular trend to more open environments recorded since the Late Miocene and (ii) the orbital‐scale vegetation variations recorded in the marine cores or inferred from the cycles of rift lake fluctuations in East Africa (Trauth et al., 2009; Joordens et al., 2011). Indeed, palaeoenvironmental records potentially include (i) threshold effects linked to the ongoing uplifting and rifting in the EARS and (ii) changes in moisture availability and rainfall seasonality driven by the local solar heating, ultimately paced by precession forcing (Larrasoaña et al., 2003; Trauth et al., 2009). Interestingly, the analysis of biomarkers retrieved from the eastern Mediterranean Basin for two time slices at 3.05 and 1.75 Ma suggests no significant increase in C4‐plant cover in the eastern Sahara between those two intervals, while showing large orbital‐scale variability within each interval (Rose et al., 2016). The latter authors suggest that the Pleistocene expansion of C4 vegetation could have been restricted to the East African domain and was not a pan‐African vegetation transition. This could be explained by the onset of a modern‐like Walker circulation at 1.9–1.6 Ma (Ravelo et al., 2004), that would have changed SST patterns in the tropics and ultimately increased variability and aridity over East Africa, without influencing the eastern Saharan environments.
The major climatic shifts described above have greatly impacted vegetation and herbivore communities. In West Africa, these changes are suggested to have triggered speciation in certain animal clades (e.g. mammals; Nicolas et al., 2019) and also led to vicariant speciation between West and Central species as discussed above (see Section III.2a). Interestingly, these changes appear to have had little impact on mammal diversification in East Africa, with speciation and extinction rates estimated from the fossil record to have been generally continuous during the Plio‐Pleistocene (Bibi & Kiessling, 2015). Nevertheless, about two thirds of the extant African biota for which we complied age estimates (1482 events) originated during the last 5 million‐years (Fig. 3D). How did the secular trends and orbital oscillations combine and influence diversification? On the one hand, the increase in aridification could have led to novel ecological niches which spurred the radiation of dry‐adapted clades in animals (e.g. Mus; Bryja et al., 2014) and plants [e.g. Coccinia (Holstein & Renner, 2011), Guibourtia (Tosso et al., 2018), Melastomateae (Veranso‐Libalah et al., 2018)]. On the other hand, cycles of forest expansion and contraction during the Plio‐Pleistocene could have increased allopatric speciation rates for forest‐adapted lineages such as birds (Voelker et al., 2010), frogs (Portik et al., 2019), insects (Hemp et al., 2015), and plants (Couvreur et al., 2011b ). Overall, oscillating climates during the last 10 Ma, between relatively stable warm and wet conditions with colder and drier ones appears to have spurred the evolution of the tropical African biota in general, and of hominid evolution in particular (deMenocal, 2004; Joordens et al., 2019).
IV. MAJOR SPECIATION MODELS OF TROPICAL AFRICAN BIODIVERSITY
It is within the above‐described geodiversity matrix, with dramatic climatic shifts, continental drifting, rifting and mountain uplifts, that the modern tropical African biota evolved. We now review diversification and molecular‐dating studies providing insights into the different speciation mechanisms possibly involved across tropical Africa (see Appendix S1, Tables S1 and S2). In most cases, the cited studies do not explicitly test these speciation models but their results are generally concordant with them. Based on our review, we also find that most animal or plant genera show mixed vegetation zonation, with species occupying two or more zones (lowland, 0–700 m; premontane, 701–1500 m; montane, 1501–3000 m; alpine, <3000 m; see Appendix S2, Table S2). Note that several speciation mechanisms might act together within clades with mixed zonation.
Speciation is the process during which new species are formed as a result of reproductive isolation. Although there are numerous speciation models that could apply to tropical fauna and flora (e.g. Hill & Hill, 2001), we here consider three major model types, each of which have nuanced, underlying mechanisms that could apply depending on the clade, temporal scale or environment considered (Table 1, Fig. 4): (i) the geographic model primarily driven by allopatric speciation; (ii) the ecological model primarily driven by ecological speciation (Orr & Smith, 1998; Givnish, 2010); and (iii) the genomic model primarily driven by genome duplication. For each model, we discuss below the proposed mechanisms linked to spatial and/or temporal factors relating to tropical Africa.
Table 1.
Main diversification models and mechanisms documented in tropical Africa, with phylogenetic and ecological predictions. Citations refer to studies of African biota.
| Model | Mechanism | General phylogenetic predictions | Specific geographic and/or phylogenetic predictions | Geographic locality | Selected references for Africa |
|---|---|---|---|---|---|
| Geographic | Pleistocene lowland forest refugia | Sister species have similar ecologies and allopatric/parapatric distributions; high phylogenetic niche conservatism; evidence of past fragmentation or separation | Speciation predominant during the Pleistocene; young species in lowland rain forests; evidence of population contraction/expansion | Lowland rain forests of West, Central and East Africa; savanna | Johnston & Anthony (2012); Bell et al. (2017) |
| Fragmentation – refugium (see Fig. 4) | Speciation throughout the Cenozoic | Lowland rain forests of West, Central and East Africa; savannas of West and East Africa | Couvreur et al. (2008); Tolley et al. (2013) | ||
| Riverine barrier | Sister species occur on opposite sides of river; no evidence of population contraction/expansion | Along major river systems of Africa, Congo Basin, East and West African deltas | Voelker et al. (2013) | ||
| Montane refugia | Sister species occur on different mountain blocks (allopatry) and have overlapping altitudinal ranges, speciation is temporally decoupled from mountain orogeny, but congruent with climatic fluctuations | Montane regions, East African Rift, Eastern Arc Mountains, Cameroon Volcanic Line, Guinea rise | Voelker et al. (2010); Tolley et al. (2011) | ||
| Ecological | Ecotone speciation (see Fig. 4) | Sister species have different ecologies and sympatric/parapatric (sometimes allopatric) distributions; moderate to low phylogenetic niche conservatism; evidence of ecological selection | Sister species parapatric; numerous transitions between habitats across clades | Vegetation gradients, Congo Basin, West Africa; mountain regions of Africa | Smith et al. (1997) |
| Montane gradient speciation | Sister species co‐occur on same mountain block and have non‐overlapping elevational distributions; speciation concordant with mountain orogeny | Gradient in montane regions, East African Rift, Eastern Arc mountains, Cameroon Volcanic Line, Guinea rise | Voje et al. (2009); Cox et al. (2014) | ||
| Peripatric | Species with restricted distributions sister to more widely distributed species (strong asymmetrical distributions); sister species have different ecologies; genetic signals of founder events | Potentially everywhere, but more likely in dynamic ecosystems, especially high‐elevation regions | Lawson et al. (2015) | ||
| Vanishing refugia (see Fig. 4) | Sister species have allopatric/parapatric or disjunct distributions; evidence of habitat fragmentation at time of speciation | Highly dynamic ecosystems, East Africa, savannah–forest ecosystems in Central Africa | Barratt et al. (2018) | ||
| Rapid adaptive radiation | Key innovation leading to ecological opportunities; numerous species originating in a short period of time; convergent evolution expected, with similar phenotypes originating in geographic isolation, resulting in independent adaptations to similar ecological conditions | Newly formed ecosystems, lakes, savannas, montane regions | Salzburger (2018) | ||
| Genomic | Polyploidization | Sister species have different ecologies but not necessarily sympatric/parapatric | Evidence of genome duplication prior to speciation | Potentially everywhere | Evans et al. (2015); Donkpegan et al. (2017) |
Fig 4.
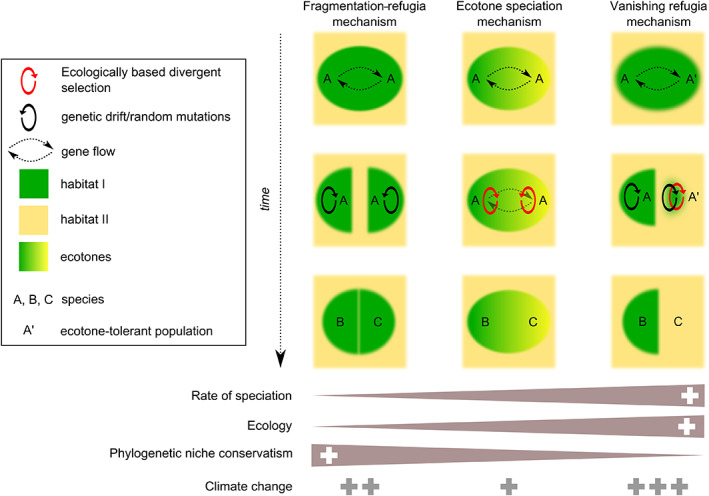
Schematic representations of three selected mechanisms of speciation relevant to tropical Africa. The fragmentation–refugia mechanism is an example of the geographic model, the ecotone speciation mechanism is an example of the ecological model, and the vanishing refugia mechanism has elements of both model types. The figure provides predictions in relation to rate of speciation, and the roles of ecology, phylogenetic niche conservatism and climate change in the speciation processes (see Table 1 for further details). The time axis is not equivalent between mechanisms.
(1). The geographic model
In the geographic model of speciation, widespread species become geographically disconnected to form isolated populations, with vicariance impeding gene flow resulting in allopatric speciation (Coyne & Orr, 2004). This vicariance can be caused by the appearance of environmental barriers such as novel vegetation types or the formation of, for example, rivers, mountains or arid valleys due to climatic or geological changes. Vicariance can also arise due to biotic factors such as competition, predation or diseases fragmenting an initially widespread population into disconnected areas. Although multiple factors could be involved during allopatric speciation (Gavrilets, 2003), we highlight below mechanisms that could drive the speciation process through random genetic drift and mutation (i.e. in the absence of direct selection). Genetic drift can be accentuated in founder events, but will also occur in large populations (as a result of vicariance) and can lead to different allele frequencies given sufficient generations since disruption of gene flow (Gavrilets, 2003). These allopatric species will remain adapted to their ancestral habitat (Table 1). The geological and climatic history of Africa has provided numerous opportunities for allopatric speciation.
One major mechanism by which the geographic model can lead to speciation is via repeated fragmentation and contraction of once‐continuous populations into refugia areas [we use the term refugium/refugia rather than refuge, see Keppel et al. (2011) for a definition], leading to diversification via allopatric speciation (Fig. 4). Several different variants of this mechanism could have led to speciation in tropical Africa, and these are considered in detail below.
(a). Pleistocene lowland forest refugia mechanism
One potential explanation for the large number of species in lowland tropical rain forests is the Pleistocene lowland refugia mechanism (Haffer, 2008). Alternation between humid and dry climatic phases during the Pleistocene (2.58–0.01 Ma) is linked to orbitally paced glacial–interglacial Milankovitch cycles resulting in cyclical variation of insolation. These phases have been hypothesized to fragment continuous lowland forest vegetation into refugia in which populations of forest‐adapted organisms can persist during adverse climatic periods. Long‐term vicariance of these forest patches will promote allopatric speciation between isolated populations. This mechanism was applied to tropical African species based on studies of diversity/endemism patterns and palaeobotanical data (Aubréville, 1975; Diamond & Hamilton, 1980; Mayr & O'Hara, 1986; Hamilton & Taylor, 1992; Sosef, 1994; Maley, 1996; Robbrecht, 1996; Plana, 2004). However, the impact of Pleistocene climatic fluctuations on rain forest fragmentation across tropical Africa is contested (Cowling et al., 2008; Hardy et al., 2013; Levinsky et al., 2013; Lézine et al., 2019).
Under this mechanism, we expect to find phases of allopatric speciation in lowland rain forests during the Pleistocene (<2.58 Ma) across multiple taxa (Table 1). Indeed, several dated phylogenetic studies across a suite of animal groups have provided support for this. In mammals, Old World fruit bats (megabats, Pteropodidae) show a strong Pleistocene signal of speciation (Nesi et al., 2013; Cunha Almeida, Giannini, & Simmons, 2016). In particular, the forest‐restricted tribes Myonycterini (11 species) and Scotonycterini (four species) originated during the last 2.8 Myr, with allopatric speciation linked to rain forest refugia (Nesi et al., 2013; Hassanin et al., 2015). Species of the largely forest‐restricted guenons (tribe Cercopithecini), a diverse clade of African primates (63 species), were inferred to have diversified mainly via allopatric speciation during the Pleistocene, but also during the Late Miocene (Guschanski et al., 2013). Other examples of allopatric speciation linked to isolation in refugia during the Pleistocene have been reported in mammals (Johnston & Anthony, 2012; Missoup et al., 2012; Bohoussou et al., 2015; Gaubert et al., 2018; Nicolas et al., 2019, 2020) and frogs (Bell et al., 2017). In insects, this mechanism was suggested for the East African Coastal forests of Tanzania and Kenya, where the origin of 25 species of East African flightless grasshoppers (Parepistaurus) was dated to the Pleistocene and attributed to allopatric speciation linked to climatic fluctuations (Hemp et al., 2015).
For plants, there is less evidence for this mechanism. Molecular dating of the African genus Begonia indicated that around half of the species sampled originated during the Pleistocene, with the other half originating earlier, during the Pliocene/Miocene (Plana et al., 2004). Because Begonia species are generally restricted to lowland rain forests and are poor dispersers (Sosef, 1994), this supports, at least in part, a role of Pleistocene cycles in generating plant biodiversity. In the Zingiberales lowland rain forest herbaceous genus Aframomum, most speciation events were initially proposed to have taken place during the Pleistocene (Harris et al., 2000), although a revised temporal framework for this genus indicated that only a few species originated during the last 2.5 Myr (Auvrey et al., 2010). A worldwide sampling of the tropical and subtropical montane forest genus Impatiens dated part of its diversification to the Pleistocene, although no specific study was undertaken in Africa (Janssens et al., 2009). Most tree species of the genera Carapa (Meliaceae) and Piptostigma (Annonaceae) originated during the Pleistocene (Koenen et al., 2015; Brée et al., 2020). Within the tribe Coffeeae (Rubiaceae), which are mainly trees, about half of the species were dated to have originated during the Pleistocene (Kainulainen et al., 2017).
While most studies to date have focused on testing forest refugia, Pleistocene refugia for savanna‐restricted clades have been suggested to occur in Sudanian and Zambezian regions, linked to savanna fragmentation (e.g. primates: Dolotovskaya et al., 2017). However, it is unclear whether the savanna biome simply shifted in latitude in response to glacial/interglacial fluctuations, rather than becoming fragmented. Nevertheless, intra‐specific genetic structuring within savanna species (Lorenzen, Heller, & Siegismund, 2012; Odee et al., 2012; Engelbrecht et al., 2020) linked to Pleistocene climatic fluctuations supports historical fragmentation and expansion cycles of this vegetation type.
The Pleistocene was an important period for speciation across tropical Africa for both animal and some herbaceous plant clades (Fig. 3D, E). However, these speciation events generally occurred in clades that were already diversifying (Fig. 3D, E). Thus, it seems unlikely that the Pleistocene lowland forest refugia mechanism was the primary driver of diversity across African rain forests. This mechanism may be more relevant in explaining phylogeographic patterns observed within species rather than diversification at the species level or above (Nicolas et al., 2011; Hardy et al., 2013; Faye et al., 2016a; Portik et al., 2017).
(b). Fragmentation–refugia mechanism
The Pleistocene lowland refugia mechanism discussed above was focussed on the Pleistocene (last 2.58 Myr). The fragmentation–refugia mechanism (Fig. 4) extends this across the Cenozoic. Indeed, dated molecular phylogenies demonstrate that speciation events for some extant plant and animal groups in tropical Africa can be dated at least to the Oligocene, although the majority of species‐level diversification appears to have taken place from the Late Miocene to Pliocene (Fig. 3D, E) (Plana, 2004; Couvreur et al., 2008; Voelker et al., 2010; Tolley et al., 2013; Koenen et al., 2015; Barlow et al., 2019; Portik et al., 2019; Brée et al., 2020). As reviewed above, Africa is characterized by numerous alternating phases of marked climatic change throughout the Cenozoic. Such climate cycles could lead to a similar pattern to that posited for the Pleistocene of fragmentation of vegetation types into refugia followed by re‐expansion. This repeated habitat fragmentation and contraction could promote allopatric speciation through vicariance, particularly for the Oligocene and Miocene epochs as suggested by numerous dated molecular studies (Fjeldså, 1994; Plana, 2004; Couvreur et al., 2008; Voelker et al., 2010; Branch, Bayliss, & Tolley, 2014; Hughes et al., 2018). It has been invoked to explain major faunistic and floristic disjunctions between Guineo‐Congolian and East African rain forest species (see Section III.2a), presumably resulting from climatic shifts from the Oligocene through the Pliocene (Loader et al., 2007; Couvreur et al., 2008). Evidence for this fragmentation mechanism is abundant in rain forest‐restricted animal lineages. For example, speciation has been linked to forest fragmentation during the Oligocene and Miocene for at least three genera of chameleons from tropical Africa (Tolley et al., 2013; Branch et al., 2014; Ceccarelli et al., 2014; Hughes et al., 2018). In birds, recurrent forest fragmentation from the Miocene through the Pliocene has been implicated as the main factor impacting diversification (Fjeldså et al., 2007; Njabo, Bowie, & Sorenson, 2008; Voelker et al., 2010). In African woodpeckers, despite the absence of an absolute time frame, the main process of diversification proposed was repeated cycles of fragmentation followed by allopatric speciation (Fuchs, Pons, & Bowie, 2017). Frog lineages also show a strong pre‐Pleistocene diversification pattern, especially from the Late Miocene into the Pliocene (Evans et al., 2015; Bittencourt‐Silva et al., 2016; Larson et al., 2016; Liedtke et al., 2016; Zimkus et al., 2017; Portik et al., 2019). For example, speciation in clawed frogs started during the Late Miocene, and high diversity in central Africa was linked to persistence of forest refugia that remain today (Evans et al., 2015). Finally, Miocene and Pliocene speciation was suggested to explain diversification of several rodent clades (Demos et al., 2014; Bryja et al., 2017; Nicolas et al., 2020) and within African colobines (Ting, 2008).
In plants, Miocene speciation due to lowland rain forest fragmentation was suggested for Annonaceae trees (Couvreur et al., 2008, 2011b ). Most sister species in this family are allopatric in distribution and show strong ecological similarities (Couvreur et al., 2011b ) supporting pre‐Pleistocene allopatric speciation. Numerous other studies have dated speciation to before the Pleistocene in palms (Faye et al., 2016a; Faye et al., 2016b ), trees (Tosso et al., 2018; Migliore et al., 2019; Monthe et al., 2019; Brée et al., 2020) and herbs (Plana et al., 2004; Auvrey et al., 2010), although to date there have been no attempts to link this to vicariance and allopatric speciation.
Fragmentation as a mechanism for speciation has been suggested for other habitats in tropical Africa that contracted due to climatic shifts. A study of West African lizards showed that ecotone speciation potentially supports a savannah refugia model (Leaché et al., 2014). Vicariance was posited to explain the present‐day distribution of the dry Rand Flora elements (Mairal et al., 2015; Pokorny et al., 2015) via the fragmentation of ancestral populations linked with the formation of the Sahara desert during the Late Miocene (Mairal et al., 2017). Given that tropical Africa has undergone substantial habitat shifts over the Cenozoic, fragmentation of habitats into refugia could apply to a wide range of taxa and circumstances. However, the greatest signal in the existing data appears for allopatric speciation in forest specialists, most likely because of the increasing loss of forest during the Cenozoic (Kissling et al., 2012).
(c). Montane refugia mechanism
Tropical mountains harbour exceptional biodiversity (Barthlott et al., 2005, 2007) and have been described as ‘evolutionary arenas’ (Muellner‐Riehl, 2019). Mountains are topologically complex regions with high levels of geodiversity which has been shown to correlate with high levels of biodiversity (Antonelli et al., 2018b ; Rahbek et al., 2019). In tropical Africa, the East African Rift System, the Eastern Arc Mountains and the Cameroon Volcanic Line are exceptional in terms of their species diversity and endemicity at a global scale (Fjeldså & Lovett, 1997; Barthlott et al., 2005; Burgess et al., 2007; Antonelli et al., 2018b ; Hoorn, Perrigo, & Antonelli, 2018b; Dagallier et al., 2020). The evolutionary processes leading to high biodiversity in (tropical) mountain regions are complex (see Hoorn, Antonelli, & Perrigo, 2018a) but have recently been summarized under the mountain‐geobiodiversity hypothesis (MGH) (Mosbrugger et al., 2018; Muellner‐Riehl, 2019). The MGH posits that (i) steep ecological gradients along elevation zones allow adaptation and ecological speciation of species to new environments or immigration of pre‐adapted taxa; (ii) climatic fluctuations leading to cycles of disconnection and reconnection of populations could drive allopatric speciation via vicariance (‘species pumps’); and (iii) there is a lower risk of local extinction under climate change (compared with lowland species) because a change in temperature can be compensated by an elevation shift, requiring limited horizontal displacement (Fjeldså et al., 2007; Mosbrugger et al., 2018). Thus, tropical mountains may be ‘cradle’ regions where taxa can diversify and/or ‘museum’ regions allowing taxa to persist over evolutionary time. This has been shown to apply to tropical African mountains, mainly in the east, for both animals and plants (Fjeldså & Lovett, 1997; Dagallier et al., 2020).
In the present context, the montane refugia mechanism refers to speciation of montane taxa by vicariance linked to climatic fluctuations (condition b of the MGH; Moritz et al., 2000; Mosbrugger et al., 2018; Rahbek et al., 2019), rather than by ecological speciation and adaptation linked to the evolution of novel habitats appearing during geological events such as mountain orogeny or volcano formation (condition a of the MGH; Mosbrugger et al., 2018; Rahbek et al., 2019). The latter condition is referred to herein as the montane gradient speciation mechanism and is discussed in Section IV.2b. Under the montane refugia mechanism (see Table 1), we expect speciation or diversification of clades to be congruent with periods of significant climatic fluctuations (Voje et al., 2009; Voelker et al., 2010; Muellner‐Riehl et al., 2019).
Numerous studies have provided evidence for this mechanism in tropical African mountains. The exceptional and unique biodiversity of the ancient Eastern Arc Mountains of Tanzania and Kenya (Burgess et al., 2007) was suggested to be driven by long‐term persistence of montane forests together with recurrent connections and disconnections between montane isolates since the Oligocene–Miocene (Lovett, 1993; Lovett et al., 2005; Fjeldså & Bowie, 2008; Voelker et al., 2010; Loader et al., 2014). This mechanism was inferred for several clades such as songbirds (Passeriformes) (Bowie et al., 2004; Fjeldså & Bowie, 2008; Voelker et al., 2010; Fjeldså, Bowie, & Rahbek, 2012), rodents (Mizerovská et al., 2019; Nicolas et al., 2020), forest‐restricted chameleons (Tolley et al., 2011; Ceccarelli et al., 2014), brevicipitid frogs (Loader et al., 2014), various insect groups such as Orthoptera (Voje et al., 2009; Hemp et al., 2010) and weevils (Grebennikov, 2017), and plants (Dimitrov et al., 2012). Evidence suggests that most montane sister species in the Eastern Arc Mountains are allopatric but located on different montane areas, refuting in situ speciation (Hemp et al., 2010; Voelker et al., 2010; Missoup et al., 2012; Ceccarelli et al., 2014; Taylor et al., 2014). This mechanism has also been proposed in other mountain regions of Africa such as the Albertine Rift and Kenyan Highlands (Demos et al., 2014; Hughes et al., 2018), the Cameroon Volcanic Line (Zimkus & Gvoždík, 2013; Taylor et al., 2014; Missoup et al., 2016) and the inselbergs of northern Mozambique (Branch et al., 2014; Bittencourt‐Silva et al., 2016).
(d). Riverine barrier mechanism
Wide river systems can limit the distribution of terrestrial animals or zoochorous, balochorous or non‐water‐dispersed plant species and serve as barriers to gene flow leading to allopatric speciation (Wallace, 1852; Moritz et al., 2000; Plana, 2004; Voelker et al., 2013). Tropical Africa is home to several large rivers systems (Fig. 1A) such as the Niger, Volta and Cross River in West Africa, the Sanaga in Cameroon, the Ogooué in Gabon, the Congo in the Democratic Republic of Congo (the second longest river in Africa after the Nile), and the Zambezi in East Africa amongst others (Goudie, 2005).
In tropical Africa, the role of river systems in speciation remains ambiguous and few studies have explicitly tested this mechanism above the species level. In vertebrates, river systems appear to be important barriers delimiting the distribution of some extant species but not historically [e.g. Colyn, Gautier‐Hion, & Verheyen, 1991; Louette, 1992; Katuala et al., 2008; Nicolas et al. (2011) and references therein; Kennis et al., 2011]. Some studies have shown that barriers provided by rivers such as the Congo or Ogooué could explain some species divergences, for example in Amnirana (Ranidae) frogs (Jongsma et al., 2018), between bonobos (Pan paniscus) and chimpanzees (P. troglodytes) (Gonder et al., 2011), or certain rodent groups (e.g. Praomys; Kennis et al., 2011). The timing of speciation events was not congruent within and among clades (Jongsma et al., 2018), which could be linked with the highly dynamic nature of river basins and substantial changes in their courses during the Cenozoic (Goudie, 2005).
To test whether rivers represent an effective barrier to gene flow in animals, several intra‐specific studies of genetic variation within taxa occurring on both sides of major rivers have been carried out (Anthony et al., 2007; Nicolas et al., 2011; Olayemi et al., 2012; Voelker et al., 2013; Jacquet et al., 2014; Bell et al., 2017; Huntley et al., 2019). The results are mixed. In a study of 10 bird species distributed north and south of the Congo River (near Kisangani), Voelker et al. (2013) found genetic variation across only four understorey species, providing limited support for the riverine barrier mechanism (see also Huntley & Voelker, 2016). Rivers were not found to be important barriers within certain frog species complexes [Hyperolius (Bell et al., 2017); Chiromantis rufescens (Leaché et al., 2019)] or in the common pangolin Manis tricuspis (Gaubert et al., 2018). By contrast, rivers were shown to be intra‐species barriers in several other animal groups including insects (Simard et al., 2009), mammals (Nicolas et al., 2011; Guschanski et al., 2013; Jacquet et al., 2014; Huntley et al., 2019; Mizerovská et al., 2019), reptiles (Leaché & Fujita, 2010) and certain bird clades (Huntley et al., 2018, 2019).
For terrestrial plants in tropical Africa, there is no evidence that rivers play a role in diversification. This is possibly because rivers are poor barriers to seed dispersal (Muloko‐Ntoutoume et al., 2000) as confirmed by recent work on trees (Hardy et al., 2013) and herbs (Ley et al., 2014).
The riverine barrier mechanism has yet to be tested properly above the species level in tropical Africa. Intra‐specific studies of animals suggest that rivers might play a role in limiting gene flow, a possible precursor to speciation, depending on the biological traits of that species (e.g. specialists versus generalists, water tolerant versus water intolerant, dispersal capacity, body size). Moreover, detailed information about African river systems and their history in terms of riverbed position or water level fluctuations remain poorly documented, limiting our understanding of whether rivers played a significant barrier role.
(2). The ecological model
Ecological speciation is defined as a process by which gene flow between populations is suppressed as a result of ecologically based divergent selection (Orr & Smith, 1998; Rundle & Nosil, 2005; Givnish, 2010). In contrast to the geographical model, ecologically dependent traits (e.g. habitat, pollinators, feeding/mating systems) drive speciation. Ecological speciation can occur in allopatry, parapatry or sympatry (Coyne & Orr, 2004). Although research on ecological speciation in the tropics is relatively scarce (Beheregaray et al., 2015), several studies have been carried out in Africa. Several different mechanisms can lead to speciation under this model, either acting alone or in concert.
(a). Ecotone speciation mechanism
This mechanism (Fig. 4) postulates that adaptation via natural selection to different habitats along ecological gradients (i.e. ecotones) drives phenotypic diversification and ultimately speciation (Smith et al., 1997; Schluter, 1998; Moritz et al., 2000). Parapatric populations occurring along an ecotone progressively adapt to different habitats leading to speciation in the presence of gene flow (Fig. 4). Without physical barriers between these populations, speciation can occur through divergent selection on different ecological traits, if selection is stronger than the homogenizing effects of gene flow (Moritz et al., 2000; Smith, Schneider, & Holder, 2001). Different factors can induce or enhance reproductive isolation (e.g. phenological shift in plants, changes in behaviour in animals). In addition, models have shown that selection gradients of intermediate strength along the ecotone promote speciation (Doebeli & Dieckmann, 2003). In tropical Africa, ecotones are commonly found on the periphery of west/central rain forests that gradually give way to drier habitats such savanna/woodland/gallery forests, or elevational gradients in mountainous regions. Increased aridity in Africa since the Miocene has led to novel arid ecosystems (see Section III.6), and this may have been an important driver of speciation in both animals and plants (Matthee & Davis, 2001; Davis et al., 2002; Voje et al., 2009). The latter has been linked to the evolution of the C4 photosynthetic pathway in plants (Bouchenak‐Khelladi et al., 2009).
In birds, high levels of recent lineage diversification were identified in forest/savannah ecotones at the periphery of rain forests in the Congo Basin and West Africa, implying that the ecotone speciation mechanism may be relevant (Fjeldså, 1994; Smith et al., 2001). Based on a global phylogenetic analysis, ecological speciation was suggested as the possible mechanism by which the evolution of more‐open vegetation could have promoted the origin and diversification of the parrot genus Poicephalus from forest‐dependent ancestors (Schweizer, Seehausen, & Hertwig, 2011). Speciation linked to ecological gradients was also suggested for some rodents (Cricetomys, Nesomyidae; Olayemi et al., 2012) and shrews (Nicolas et al., 2019). Two sister species with very different ecologies in the duiker genus Cephalopus, the central African forest‐dwelling C. nigrifrons and the Sahel savanna species C. rufilatus, were found to have diverged during the Pleistocene (Johnston & Anthony, 2012). Another interesting case might be the forest versus savanna elephant species, estimated to have diverged during the Pliocene (Rohland et al., 2010; Brandt et al., 2012). In the latter two cases at least, rapid speciation via ecological selection to contrasting ecologies might have played a fundamental role.
Detailed population‐level studies have provided evidence for the ecotone speciation mechanism in Central and West Africa (Smith et al., 1997). Populations of the little greenbul (Adropadus virens) distributed along an ecological gradient in Cameroon showed positive selection for certain morphological traits (Smith et al., 1997, 2001) and local adaptation to different habitats even in the presence of gene flow (Zhen et al., 2017). In another study, genomic evidence for early adaptive diversification to different habitats along a rain forest–savanna ecotone in Cameroon was suggested for the lizard species Trachylepis affinis (Freedman et al., 2010). Although these studies do not document species‐level diversification per se, they do provide evidence of morphological and/or genetic adaptation to different habitats within a species, a prerequisite for this mechanism to operate (Coyne & Orr, 2004, p. 184).
There are examples in plant clades of frequent transitions between closed/forest and open/savanna habitats. Although explicit tests have not been carried out, phylogenetic analyses suggest that these transitions took place by ecological adaptation of ancestral wet‐forest species to dry woodland or savanna regions throughout the Miocene. Several transitions between an inferred ancestral forested habitat to a dry forest/savannah ecosystem have been found across a wide range of families and genera such as Coccinia (Cucurbitaceae; Holstein & Renner, 2011), Guibourtia (Fabaceae; Tosso et al., 2018), Erythrophleum (Fabaceae; Duminil et al., 2015), Acridocarpus (Malpighiaceae; Davis et al., 2002), African Melastomataceae (Veranso‐Libalah et al., 2018), Entandrophragma (Meliaceae; Monthe et al., 2019) and Manilkara (Sapotaceae; Armstrong et al., 2014).
(b). Montane gradient speciation mechanism
Mountains concentrate high topographic complexity and habitat heterogeneity, potentially leading to ecological speciation (Graham et al., 2018). In contrast to the montane refugia mechanism (Section IV.1c), in this case biodiversity arises from within‐mountain (in situ) diversification as populations adapt to the variety of different micro‐habitats or along latitudinal/elevational gradients (condition a of the MGH; Moritz et al., 2000; Graham et al., 2018; Mosbrugger et al., 2018;Muellner‐Riehl, 2019; Rahbek et al., 2019). Mountain or volcano formation provides a wide range of new niches which could also allow, in the latter case rapid, ecologically driven diversification (Muellner‐Riehl, 2019; Rahbek et al., 2019).
Evidence for this mechanism in African mountain biota remains poor, with most available studies supporting the montane refugia mechanism (see Section IV.1c). One study focusing on the east African montane white‐eyes (Zosteropidae) found evidence of niche divergence between species suggesting ecological speciation (Cox et al., 2014), although no clear mechanism was concluded.
(c). Peripatric speciation mechanism
Under this mechanism, a small peripheral population becomes isolated and diverges from the source population (Losos & Glor, 2003). The main driver behind peripatric speciation is the geographic isolation of small populations, however this might also be accompanied by shifts into novel habitats, which would involve ecological speciation after physical isolation (Coyne & Orr, 2004). These peripheral isolates originate via founder events where either a few individuals disperse to different areas, or the appearance of a geographical barrier isolates (vicariance) a small population from the larger population (Coyne & Orr, 2004). The newly formed species should show signs of severe population contraction (bottleneck) at the time of the divergence. This mechanism has been poorly documented in nature, and appears to be rare (Losos & Glor, 2003). We include it here under the ecological model because the few cases reported in tropical Africa have also involved ecological adaptation of peripheral populations.
An interesting case of peripatric speciation was suggested for the six species of the spiny‐throated reed frog (Hyperolius) complex distributed in the East Arc Mountains (Lawson et al., 2015). Species with restricted distributions were recovered as sister to more widely distributed species. However, two out of three species pairs showed a difference in ecology: the peripheral species had adapted to rain forest, montane grassland or forest mosaics. This implies a role of ecology during speciation, which is not incompatible with peripatric speciation (Losos & Glor, 2003). Peripatric speciation was proposed as the main speciation mechanism in the rat genus Otomys across the Afromontane regions (Taylor et al., 2014).
In plants, there is little evidence for peripatric speciation, although this may be because it has never been explicitly tested. There are, however, numerous examples of widespread species with sister relationships to range‐restricted species. For example, the East African range‐restricted Monodora hastipetala (Annonaceae) was inferred to be sister to the widely distributed M. junodii (Couvreur et al., 2011b ) and although not explicitly discussed this could be due to peripatric speciation.
(d). Vanishing refugia mechanism
The vanishing refugia mechanism (VRM; Vanzolini & Williams, 1981) is an explicit mechanism (Fig. 4) whereby ecotone speciation (Section IV.2a) occurs in concert with peripatric or allopatric speciation (Section IV.2c). Under the VRM, a forest gradually contracts and fragments and is replaced by open habitat vegetation with some or all of the forest patches eventually vanishing. Forest‐adapted species become trapped in the vanishing forest refugia. These peripatric populations will either go extinct, or adapt to their new conditions through ecological speciation through directional selection. Concurrently, some populations might persist long‐term in more stable patches of core forest habitat and remain adapted to that core habitat. Initially, gene flow could occur between the stable core patch and the contracting fragments, but as the fragments become fully isolated, populations undergo allopatric diversification through mutation‐order speciation (Nosil & Flaxman, 2011).
The diminishing patches of forest refugia would be surrounded by ecotonal vegetation and embedded in a matrix of novel vegetation. As the forest patch completely disappears, the ecotone habitat initially increases, but eventually gives way to the new habitat. Trapped populations would first be under directional selection for the ecotone, and later for the novel habitat. The distinguishing feature of the VRM from the ecotone speciation mechanism is that gene flow between the core forest patch and the forest fragments has ceased due to vicariance. This provides a clear mechanism for the diversification that is not implicit in the ecotonal mechanism where gene flow still occurs. The VRM requires one of the isolated refugial populations to persist and adapt to the new habitat after which it can expand into the new habitat. The resulting sister species should be genetically, morphologically, functionally and ecologically divergent and are separated by an ecological barrier that prevents subsequent gene flow (Vanzolini & Williams, 1981; Damasceno et al., 2014).
Whether the VRM has influenced the biota of tropical Africa is not known, as it has not been explicitly tested. Testing would require evidence for the timing of habitat shifts, interpreted with respect to the date of diversification through either phylogenetic studies or population‐level genetics. The latter approach can also be used to examine whether there has been a population expansion in the newly adapted species, and coalescent methods can be used to examine population‐level divergence with absence of gene flow. Validation of the VRM also requires evidence for adaptation to the new habitat, such as differing morphological features that are linked to functional traits that are optimal for the respective habitats (Vanzolini & Williams, 1981; Damasceno et al., 2014). It is, however, possible that some of the examples of speciation discussed above could have been driven by this mechanism, such as Coccinia (Holstein & Renner, 2011), several frog taxa where allopatric sister groups occur in different habitats (Bell et al., 2017), and white‐eyes (Zosterops) from East Africa where closely related species show niche divergence (Cox et al., 2014). In plants, the VRM was suggested as a potential driver of intra‐specific genetic differentiation within the semi‐deciduous forest tree species Erythrophleum suaveolens (Duminil et al., 2015), although this has not led to full speciation.
(e). Adaptive radiation
Adaptive radiation is a special case of species diversification where a single ancestor rapidly gives rise to numerous descendant species that are adapted to novel habitats through ecological opportunity [Schluter, 2000; Linder, 2008; Rundell & Price, 2009; but see Gillespie et al. (2020) for an in depth discussion]. Although it has some similarities to ecotone speciation, adaptive radiation does not rely on ecological gradients but rather on ecological opportunity, that is the presence of non‐exploited resources or habitats. Adaptive radiation requires colonization of, or dispersal to, new habitats or to habitats vacated following extinctions, or the evolution of key innovations allowing rapid exploitation of these new or existing niches (Givnish, 2010; Gillespie et al., 2020).
Besides the classic example of adaptive radiation of cichlid fishes in the East African Great Lakes (Salzburger, Van Bocxlaer, & Cohen, 2014), adaptive radiations have been suggested as a mechanism for diversification in other terrestrial tropical African animal clades. For example, the megabat tribe Epomophorini (Rousettinae) radiated into 12 species during the last 2.5 Myr. These species now occur across a number of different habitats, such as deciduous and montane forests, and savanna woodlands, and are thought to have arisen from an ancestral species inhabiting rain forest (Cunha Almeida et al., 2016). Adaptive radiation has also been reported in the diverse clade of Afrobatrachian frogs (Portik & Blackburn, 2016), especially in the Hyperoliidae family, linked to the origin of sexual dichromatism, suggesting that speciation by sexual selection triggered this radiation (Portik et al., 2019). Although sexual selection is assumed to be decoupled from ecological speciation, and thus considered a non‐ecological process (Coyne & Orr, 2004), others have recognized a correlation between habitat type and sexually selected traits (Kraaijeveld, Kraaijeveld‐Smit, & Maan, 2011), highlighting the role of sexual selection in adaptive radiations.
Compared to plant clades in southern Africa (Linder, 2003), there are relatively few clear cases of plant adaptive radiations for tropical Africa. Adaptive radiations have however, been suggested for the tropical‐alpine flora (e.g. East African Rift; Linder, 2014; Hughes & Atchison, 2015) resulting from novel habitats created during the uplift of East Africa (Linder, 2017). Adaptive radiations were proposed for genera such as Alchemilla (Rosaceae; Gehrke et al., 2008), Lychnis (Caryophyllaceae; Gizaw et al., 2016) and giant senecios (Dendrosenecio, Astercaeae; Knox & Palmer, 1995; but see Kandziora, Kadereit, & Gehrke, 2016), although neither of these clades are particularly speciose (Gehrke & Linder, 2014). A final example is the woody genus Coffea (Rubiaceae) which was suggested to have radiated in lowland and high‐altitude forests of tropical Africa and shows probable convergent evolution in caffeine production (Anthony et al., 2010; Hamon et al., 2017).
(3). The genomic model: polyploidization
Polyploidization, the duplication of entire genomes either via hybridization between different species (allopolyploidy) or within single species (autopolyploidy), is recognized as an important mode of speciation especially in plants (Estep et al., 2014; Vamosi et al., 2018). Polyploids are suggested to have higher genome plasticity (Leitch & Leitch, 2008) allowing adaptation to different environments in both plants (Leitch & Leitch, 2008; te Beest et al., 2012; Diallo et al., 2016; Han et al., 2020) and animals (Schoenfelder & Fox, 2015). Thus, polyploidization can be a first step in ecological speciation and adaptive radiation (Rundle & Nosil, 2005). To date, few studies have provided indisputable links between polyploidization and ecological speciation in tropical Africa.
Polyploidization appears less common in animals than in plants (Van de Peer, Mizrachi, & Marchal, 2017). The African clawed frogs (Xenopus, Silurana, Pipidae) provide an unusual case where allopolyploid species have arisen on multiple occasions (Evans et al., 2004, 2015). Over half of the diversity of these frogs is concentrated in Central Africa, and there are several species with high ploidy levels (octoploids and dodecaploids), which has been suggested to have led to selective advantages (Evans et al., 2004, 2015).
Similarly, very few studies report on the impact of polyploidization on speciation in tropical African plants. In the genus Afzelia (Leguminosae), which contains four rain forest tetraploids and two dry forest diploid species, there appears to be a strong association between polyploidization and specialization to different habitats (Donkpegan et al., 2017). Diploids and polyploids were also documented in the tree genera Guibourtia (Tosso et al., 2018), Adansonia (Pettigrew et al., 2012), and Acacia (Diallo et al., 2016) but were not linked to biome shifts. Finally, the origin of coffee (Coffea arabica, Rubiaceae; allotetraploid) might be the result of recent polyploidization between two wild diploid species: C. eugenioides and C. canephora (Lashermes et al., 1999). More studies are needed to clarify how polyploidization events have affected the evolution of tropical African biodiversity. In particular it would be interesting to test if polyploidization events in plants and animals enabled successful ecological shifts into novel habitats across Africa (e.g. Han et al., 2020) or occur randomly.
V. CONCLUSIONS
Tropical Africa has undergone a long and complex evolution, resulting in a spectacular and unique biodiversity (Fig. 3). Modelling past climate, topography and vegetation coupled with the fossil record and dated molecular phylogenies of plants and animals provides a wealth of data allowing us to consider the evolutionary history and diversification processes behind this biodiversity.
Africa underwent numerous climatic fluctuations at different timescales, linked to tectonic, greenhouse gas, and orbital forcing. One major impact was the fragmentation of African rain forests leading to multiple vicariant speciation events. While the presence of a pan‐African rain forest remains subject to debate, there is little doubt from the fossil record, vegetation simulations and dated molecular phylogenies that West/Central and East African rain forests were connected and disconnected several times during the Cenozoic, even after the uplift of the East African rift valley. Evidence for such connections should not only be sought in present‐day east Africa but also further north given the northward movement of the African continent during the Cenozoic.
Compared to other tropical regions, Africa is characterized by significantly increased aridification since the Late Eocene. This led to a number of extinction events generally invoked to explain the lower species diversity across Africa compared to other tropical regions (Kissling et al., 2012; Couvreur, 2015). These events also provided numerous opportunities for speciation and radiation within the newly evolved drier ecosystems (Davis et al., 2002), with the evolution of C4 plant‐dominated ecosystems contributing significantly to the diversification of the African megafauna.
We discuss three main speciation models (geographic, ecological and genomic) and 10 mechanisms that may apply across tropical Africa (Table 1, Fig. 4). Allopatric speciation via vicariance of fragmenting vegetation (rain forests, savannas or montane biota) is likely to be one of the most important mechanisms, linked to the large‐scale climate changes during the entire Cenozoic. Overall, these mechanisms are generally implied rather than tested within a phylogenetic and biogeographic framework. This is an important first step, but more detailed studies need to be undertaken to clarify their role in generating biodiversity. In addition, numerous studies underline that several different mechanisms may have led to diversity within the same clade (Tolley et al., 2011; Cox et al., 2014; Bell et al., 2017; Barratt et al., 2018). Thus, like in other tropical regions such as Madagascar (Brown et al., 2014), no single model will be sufficient to explain patterns of diversification and diversity across tropical Africa. These models and mechanisms have similarly been suggested to explain biodiversity in other tropical regions such as the Neotropics (Gentry, 1989; Antonelli et al., 2018a ), Madagascar (Vences et al., 2009) and South East Asia (Lohman et al., 2011; Kooyman et al., 2019). It will be interesting to compare the roles of these mechanisms among continents to explain the origin of tropical diversity at global scales.
We still lack fundamental biodiversity information for tropical African taxa, including accurate taxonomy, ecological studies and estimates of distribution, compared to temperate or other tropical regions. Recent efforts to compile and synthesize currently available data (Klopper et al., 2007; Tolley et al., 2016; Sosef et al., 2017; Stévart et al., 2019) have led to the identification of both well‐inventoried regions and important knowledge gaps. Continued efforts to acquire primary data from the field will remain an important challenge across tropical Africa.
Despite numerous improvements in terms of data and modelling during the last decade, constraining models of the geophysical evolution of the African continent throughout the Cenozoic remains challenging. First, additional field data are required to qualify environmental and topography changes at multimillion year scales. New numerical simulations using Earth System Models, forced by surface conditions, will be required to quantify trends in African climate through time. As computing power increases and geological field and model data improve, more realistic climate simulations will be possible. However, linking these simulated climate changes to biotic evolution requires consideration of the spatial scale. Reconciling the coarse spatial resolution of climate models with biotic phenomena calls for downscaling techniques that currently are only applied to future climate projections. Such a framework will allow us to address questions regarding geologic–climatic–biotic evolution in Africa.
Next‐generation sequencing is providing a massive amount of data leading to larger and more robust phylogenies (Ojeda et al., 2019; Brée et al., 2020; Koenen et al., 2020; Streicher et al., 2020), but has yet to be applied widely to the African biota. Increased sequence data, together with better fossil calibrations, will provide a more precise understanding of the evolution of African biodiversity. In addition, sequence data from different genomic regions (e.g. plastid versus nuclear) can lead to different age estimates (e.g. Tosso et al., 2018) which will need to be resolved. Future studies should also perform demographic modelling at intra‐specific levels to examine alternative scenarios of population divergences (Portik et al., 2017). Phylogenomic data together with more refined divergence time estimates and additional testing of demographic scenarios will allow a re‐evaluation of our understanding of the timing and diversification of tropical African biodiversity.
Finally, we need to integrate data from Earth and life sciences better, in order to synthesize patterns between major living clades. A huge amount of data has been gathered in recent decades, but this is often only loosely integrated in biogeographic studies. Better interactions between these fields will take us a step closer to ‘a trans disciplinary’ biogeography (Antonelli et al., 2018a ) in the geodiverse region of tropical Africa.
Supporting information
Appendix S1. List of dated molecular phylogeny studies used to generate Fig. 3D,E.
Fig. S1. Palaeoclimate during the Eocene across Africa.
Table S1. Studies used to estimate the origin of extant species within groups with dated molecular phylogenies.
Appendix S2. Assignment of genera to elevation zones as presented in Table S2.
Table S2. Studies used to estimate crown and stem nodes for genera or clades, and to estimate vegetation zonation following methodology provided in Appendix S2.
VI. ACKNOWLEDGEMENTS
This synthesis stems from several meetings within the RAINBIO project funded by the French Foundation for Research on Biodiversity (FRB) and the Provence‐Alpes‐Côte d'Azur (PACA) region through the Centre for Synthesis and Analysis of Biodiversity data (CESAB) program. J.‐C.S. considers this work a contribution to his VILLUM Investigator project “Biodiversity Dynamics in a Changing World” funded by VILLUM FONDEN (grant 16549). T.L.P.C. was supported by the Agence Nationale de la Recherche ANR (AFRODYN project; ANR‐15‐ CE02‐0002‐01). The map in Fig. 1B was created using NOAA Ferret software (https://ferretop.pmel.noaa.gov/Ferret/), embedded in iPython notebooks (https://ipython.org/notebook.html) using the ferretmagic package (https://github.com/PBrockmann/ipython_ferretmagic) developed at LSCE; maps in Fig. 2 were compiled by F.G. and C.R. within the framework of ANR project TopoAfrica (ANR‐08‐BLAN‐0247). Finally, we are grateful to three anonymous reviewers and Simon Loader for providing important comments that improved the quality of this review, and Alison Cooper for carefully reading the final version of this manuscript.
REFERENCES
Asterisks indicate studies that are used only in the online supporting information.
- Andrews, P. & Van Couvering, J. A. (1975). Palaeoenvironments in the east African Miocene In Appraoches to primate paleobiology (ed. Szalay F.), pp. 62–103. Karger, Basel. [PubMed] [Google Scholar]
- Anthony, N. M. , Johnson‐Bawe, M. , Jeffery, K. , Clifford, S. L. , Abernethy, K. A. , Tutin, C. E. , Lahm, S. A. , White, L. J. T. , Utley, J. F. , Wickings, E. J. & Bruford, M. W. (2007). The role of Pleistocene refugia and rivers in shaping gorilla genetic diversity in Central Africa. Proceedings of the National Academy of Sciences of the United States of America 104, 20432–20436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony, F. , Diniz, L. E. C. , Combes, M.‐C. & Lashermes, P. (2010). Adaptive radiation in Coffea subgenus Coffea L. (Rubiaceae) in Africa and Madagascar. Plant Systematics and Evolution 285, 51–64. [Google Scholar]
- Antonelli, A. (2009). Have giant lobelias evolved several times independently? Life form shifts and historical biogeography of the cosmopolitan and highly diverse subfamily Lobelioideae (Campanulaceae). BMC Biology 7, 82 10.1186/1741-7007-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli, A. , Ariza, M. , Albert, J. , Andermann, T. , Azevedo, J. , Bacon, C. , Faurby, S. , Guedes, T. , Hoorn, C. , Lohmann, L. G. , Matos‐Maraví, P. , Ritter, C. D. , Sanmartín, I. , Silvestro, D. , Tejedor, M. , ter Steege, H. , Tuomisto, H. , Werneck, F. P. , Zizka, A. & Edwards, S. V. (2018a). Conceptual and empirical advances in Neotropical biodiversity research. PeerJ 6, e5644 10.7717/peerj.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli, A. , Kissling, W. D. , Flantua, S. G. A. , Bermúdez, M. A. , Mulch, A. , Muellner‐Riehl, A. N. , Kreft, H. , Linder, H. P. , Badgley, C. , Fjeldså, J. , Fritz, S. A. , Rahbek, C. , Herman, F. , Hooghiemstra, H. & Hoorn, C. (2018b). Geological and climatic influences on mountain biodiversity. Nature Geoscience 11, 718–725. [Google Scholar]
- Armstrong, K. E. , Stone, G. N. , Nicholls, J. A. , Valderrama, E. , Anderberg, A. A. , Smedmark, J. , Gautier, L. , Naciri, Y. , Milne, R. & Richardson, J. E. (2014). Patterns of diversification amongst tropical regions compared: a case study in Sapotaceae. Frontiers in Genetics 5 362 10.3389/fgene.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- As‐Saruri, M. L. , Whybrow, P. J. & Collinson, M. E. (1999). Geology, fruits, seeds, and vertebrates (? Sirenia) from the Kaninah formation (middle Eocene), Republic of Yemen In Fossil Vertebrates of Arabia: With emphasis on the Late Miocene faunas, geology, and palaeoenvironments of the Emirate of Abu Dhabi, United Arab Emirates, pp. 443–453. Yale University Press, New Haven. [Google Scholar]
- Aubréville, A. (1975). Essais sur l'origine et l'histoire des flores tropicales africaines. Application de la théorie des origines polytopiques des angiospermes tropicales. Adansonia 15, 31–56. [Google Scholar]
- Auvrey, G. , Harris, D. J. , Richardson, J. E. , Newman, M. F. & Särkinen, T. E. (2010). Phylogeny and dating of Aframomum (Zingiberaceae) In Diversity, Phylogeny, and Evolution in the Monocotyledons (eds Seberg O., Peterson G., Barfod A. and Davis J. I.), pp. 287–305. Aarhus University Press, Aarhus. [Google Scholar]
- Axelrod, D. I. & Raven, P. H. (1978). Late cretaceous and tertiary vegetation history of Africa In Biogeography and Ecology of Southern Africa (ed. Werger M. J. A.), pp. 77–130. W. Junk bv Publishers, The Hague. [Google Scholar]
- Baker, W. J. & Couvreur, T. L. P. (2013). Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. I. Historical biogeography. Journal of Biogeography 40, 274–285. [Google Scholar]
- Barlow, A. , Wüster, W. , Kelly, C. M. R. , Branch, W. R. , Phelps, T. & Tolley, K. A. (2019). Ancient habitat shifts and organismal diversification are decoupled in the African viper genus Bitis (Serpentes: Viperidae). Journal of Biogeography 46, 1234–1248. [Google Scholar]
- Barnet, J. S. K. , Littler, K. , Kroon, D. , Leng, M. J. , Westerhold, T. , Röhl, U. & Zachos, J. C. (2018). A new high‐resolution chronology for the late Maastrichtian warming event: establishing robust temporal links with the onset of Deccan volcanism. Geology 46, 147–150. [Google Scholar]
- Barratt, C. D. , Bwong, B. A. , Jehle, R. , Liedtke, H. C. , Nagel, P. , Onstein, R. E. , Portik, D. M. , Streicher, J. W. & Loader, S. P. (2018). Vanishing refuge? Testing the forest refuge hypothesis in coastal East Africa using genome‐wide sequence data for seven amphibians. Molecular Ecology 27, 4289–4308. [DOI] [PubMed] [Google Scholar]
- Barthlott, W. , Mutke, J. , Rafiqpoor, D. , Kier, G. & Kreft, H. (2005). Global centers of vascular plant diversity. Nova Acta Leopoldina 92, 61–83. [Google Scholar]
- Barthlott, W. , Hostert, A. , Kier, G. , Küper, W. , Kreft, H. , Mutke, J. , Rafiqpoor, M. D. & Sommer, J. H. (2007). Geographic patterns of vascular plant diversity at continental to global scales. Erdkunde 61, 305–315. [Google Scholar]
- Beauvais, A. & Chardon, D. (2013). Modes, tempo, and spatial variability of Cenozoic cratonic denudation: the west African example. Geochemistry, Geophysics, Geosystems 14, 1590–1608. [Google Scholar]
- te Beest, M. , Le Roux, J. J. , Richardson, D. M. , Brysting, A. K. , Suda, J. , Kubešová, M. & Pyšek, P. (2012). The more the better? The role of polyploidy in facilitating plant invasions. Annals of Botany 109, 19–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beheregaray, L. B. , Cooke, G. M. , Chao, N. L. & Landguth, E. L. (2015). Ecological speciation in the tropics: insights from comparative genetic studies in Amazonia. Frontiers in Genetics 5 477 10.3389/fgene.2014.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, R. C. , Parra, J. L. , Badjedjea, G. , Barej, M. F. , Blackburn, D. C. , Burger, M. , Channing, A. , Dehling, J. M. , Greenbaum, E. , Gvoždík, V. , Kielgast, J. , Kusamba, C. , Lötters, S. , McLaughlin, P. J. , Nagy, Z. T. , Rödel, M. O. , Portik, D. M. , Stuart, B. L. , VanDerWal, J. , Zassi‐Boulou, A. G. & Zamudio, K. R. (2017). Idiosyncratic responses to climate‐driven forest fragmentation and marine incursions in reed frogs from Central Africa and the Gulf of Guinea Islands. Molecular Ecology 26, 5223–5244. [DOI] [PubMed] [Google Scholar]
- Bibi, F. & Kiessling, W. (2015). Continuous evolutionary change in Plio‐Pleistocene mammals of eastern Africa. Proceedings of the National Academy of Sciences of the United States of America 112, 10623–10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt‐Silva, G. B. , Conradie, W. , Siu‐Ting, K. , Tolley, K. A. , Channing, A. , Cunningham, M. , Farooq, H. M. , Menegon, M. & Loader, S. P. (2016). The phylogenetic position and diversity of the enigmatic mongrel frog Nothophryne Poynton, 1963 (Amphibia, Anura). Molecular Phylogenetics and Evolution 99, 89–102. [DOI] [PubMed] [Google Scholar]
- Blach‐Overgaard, A. , Balslev, H. , Dransfield, J. , Normand, S. & Svenning, J.‐C. (2015). Global‐change vulnerability of a key plant resource, the African palms. Scientific Reports 5, 12611 10.1038/srep12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobe, R. (2006). The evolution of arid ecosystems in eastern Africa. Journal of Arid Environments 66, 564–584. [Google Scholar]
- Bohoussou, K. H. , Cornette, R. , Akpatou, B. , Colyn, M. , Peterhans, J. K. , Kennis, J. , Šumbera, R. , Verheyen, E. , N'Goran, E. , Katuala, P. & Nicolas, V. (2015). The phylogeography of the rodent genus Malacomys suggests multiple Afrotropical Pleistocene lowland forest refugia. Journal of Biogeography 42, 2049–2061. [Google Scholar]
- Bonnefille, R. (2010). Cenozoic vegetation, climate changes and hominid evolution in tropical Africa. Global and Planetary Change 72, 390–411. [Google Scholar]
- Bonnefille, R. & Letouzey, R. (1976). Fruits fossiles d'Antrocaryon dans la vallée de L'Omo (Ethiopie). Adansonia 16, 65–82. [Google Scholar]
- Booth, A. H. (1958). The Niger, the Volta and the Dahomey gap as geographic barriers. Evolution 12, 48–62. [Google Scholar]
- Bouchenak‐Khelladi, Y. , Verboom, G. A. , Hodkinson, T. R. , Salamin, N. , Francois, O. , Chonghaile, G. N. & Savolainen, V. (2009). The origins and diversification of C4 grasses and savanna‐adapted ungulates. Global Change Biology 15, 2397–2417. [Google Scholar]
- Bouchenak‐Khelladi, Y. , Maurin, O. , Hurter, J. & van der Bank, M. (2010a). The evolutionary history and biogeography of Mimosoideae (Leguminosae): an emphasis on African acacias. Molecular Phylogenetics and Evolution 57, 495–508. [DOI] [PubMed] [Google Scholar]
- Bouchenak‐Khelladi, Y. , Verboom, G. A. , Savolainen, V. & Hodkinson, T. R. (2010b). Biogeography of the grasses (Poaceae): a phylogenetic approach to reveal evolutionary history in geographical space and geological time. Botanical Journal of the Linnean Society 162, 543–557. [Google Scholar]
- Bouchenak‐Khelladi, Y. , Muasya, A. M. & Linder, H. P. (2014a). A revised evolutionary history of Poales: origins and diversification. Botanical Journal of the Linnean Society 175, 4–16. [Google Scholar]
- Bouchenak‐Khelladi, Y. , Slingsby, J. A. , Verboom, G. A. & Bond, W. J. (2014b). Diversification of C4 grasses (Poaceae) does not coincide with their ecological dominance. American Journal of Botany 101, 300–307. [DOI] [PubMed] [Google Scholar]
- Boureau, E. , Cheboldaeff‐Salard, M. , Koeniguer, J.‐C. & Louvet, P. (1983). Evolution des flores et de la végétation Tertiaires en Afrique, au nord de l'Equateur. Bothalia 14, 355–367. [Google Scholar]
- Bowie, R. C. K. , Fjeldså, J. , Hackett, S. J. & Crowe, T. M. (2004). Systematics and biogeography of double‐collared sunbirds from the eastern Arc Mountains, Tanzania. The Auk 121, 660–681. [Google Scholar]
- Branch, W. R. , Bayliss, J. & Tolley, K. A. (2014). Pygmy chameleons of the Rhampholeon platyceps complex (Squamata: Chamaeleonidae): description of four new species from isolated ‘sky islands’ of northern Mozambique. Zootaxa 3814, 1–36. [DOI] [PubMed] [Google Scholar]
- Brandt, A. L. , Ishida, Y. , Georgiadis, N. J. & Roca, A. L. (2012). Forest elephant mitochondrial genomes reveal that elephantid diversification in Africa tracked climate transitions. Molecular Ecology 21, 1175–1189. [DOI] [PubMed] [Google Scholar]
- Brée, B. , Helmstetter, A. J. , Bethune, K. , Ghogue, J.‐P. , Sonké, B. & Couvreur, T. L. P. (2020). Diversification of African rainforest restricted clades: Piptostigmateae and Annickieae (Annonaceae). Diversity 12, 227 10.3390/d12060227. [DOI] [Google Scholar]
- Brierley, C. M. , Fedorov, A. V. , Liu, Z. , Herbert, T. D. , Lawrence, K. T. & LaRiviere, J. P. (2009). Greatly expanded tropical warm pool and weakened Hadley circulation in the early Pliocene. Science 323, 1714–1718. [DOI] [PubMed] [Google Scholar]
- Brown, J. L. , Cameron, A. , Yoder, A. D. & Vences, M. (2014). A necessarily complex model to explain the biogeography of the amphibians and reptiles of Madagascar. Nature Communications 5, 5046 10.1038/ncomms6046. [DOI] [PubMed] [Google Scholar]
- Bryja, J. , Mikula, O. , Šumbera, R. , Meheretu, Y. , Aghová, T. , Lavrenchenko, L. A. , Mazoch, V. , Oguge, N. , Mbau, J. S. , Welegerima, K. , Amundala, N. , Colyn, M. , Leirs, H. & Verheyen, E. (2014). Pan‐African phylogeny of Mus (subgenus Nannomys) reveals one of the most successful mammal radiations in Africa. BMC Evolutionary Biology 14, 256 10.1186/s12862-014-0256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja, J. , Šumbera, R. , Peterhans, K. , Julian, C. , Aghová, T. , Bryjová, A. , Mikula, O. , Nicolas, V. , Denys, C. & Verheyen, E. (2017). Evolutionary history of the thicket rats (genus Grammomys) mirrors the evolution of African forests since late Miocene. Journal of Biogeography 44, 182–194. [Google Scholar]
- Burgess, N. D. , Butynski, T. M. , Cordeiro, N. J. , Doggart, N. H. , Fjeldså, J. , Howell, K. M. , Kilahama, F. B. , Loader, S. P. , Lovett, J. C. , Mbilinyi, B. , Menegon, M. , Moyer, D. C. , Nashanda, E. , Perkin, A. , Rovero, F. , Stanley, W. T. & Stuart, S. N. (2007). The biological importance of the eastern Arc Mountains of Tanzania and Kenya. Biological Conservation 134, 209–231. [Google Scholar]
- * Cardoso, D. , Harris, D. J. , Wieringa, J. J. , São‐Mateus, W. M. B. , Batalha‐Filho, H. , Torke, B. M. , Prenner, G. & de Queiroz, L. P. (2017). A molecular‐dated phylogeny and biogeography of the monotypic legume genus Haplormosia, a missing African branch of the otherwise American‐Australian Brongniartieae clade. Molecular Phylogenetics and Evolution 107, 431–442. [DOI] [PubMed] [Google Scholar]
- Carmichael, M. J. , Lunt, D. J. , Huber, M. , Heinemann, M. , Kiehl, J. , LeGrande, A. , Loptson, C. A. , Roberts, C. D. , Sagoo, N. , Shields, C. , Valdes, P. J. , Winguth, A. , Winguth, C. & Pancost, R. D. (2016). A model–model and data–model comparison for the early Eocene hydrological cycle. Climate of the Past 12, 455–481. [Google Scholar]
- Carruthers, T. & Scotland, R. W. (2020). Insights from empirical analyses and simulations on using multiple fossil calibrations with relaxed clocks to estimate divergence times. Molecular Biology and Evolution 37, 1508–1529. [DOI] [PubMed] [Google Scholar]
- Cascales‐Miñana, B. & Cleal, C. J. (2014). The plant fossil record reflects just two great extinction events. Terra Nova 26, 195–200. [Google Scholar]
- Ceccarelli, F. S. , Menegon, M. , Tolley, K. A. , Tilbury, C. R. , Gower, D. J. , Laserna, M. H. , Kasahun, R. , Rodriguez‐Prieto, A. , Hagmann, R. & Loader, S. P. (2014). Evolutionary relationships, species delimitation and biogeography of eastern Afromontane horned chameleons (Chamaeleonidae: Trioceros). Molecular Phylogenetics and Evolution 80, 125–136. [DOI] [PubMed] [Google Scholar]
- Cerling, T. E. , Wynn, J. G. , Andanje, S. A. , Bird, M. I. , Korir, D. K. , Levin, N. E. , Mace, W. , Macharia, A. N. , Quade, J. & Remien, C. H. (2011). Woody cover and hominin environments in the past 6 million years. Nature 476, 51–56. [DOI] [PubMed] [Google Scholar]
- Chorowicz, J. (2005). The east African rift system. Journal of African Earth Sciences 43, 379–410. [Google Scholar]
- Coetzee, J. A. (1993). African flora since the terminal Jurassic In Biological Relationships between Africa and South America (ed. Goldblatt P.), pp. 37–61. Yale University Press, New Haven. [Google Scholar]
- Colyn, M. , Gautier‐Hion, A. & Verheyen, W. (1991). A re‐appraisal of palaeoenvironmental history in Central Africa: evidence for a major fluvial refuge in the Zaire Basin. Journal of Biogeography 18, 403–407. [Google Scholar]
- Courtillot, V. & Fluteau, F. (2014). A review of the embedded time scales of flood basalt volcanism with special emphasis on dramatically short magmatic pulses In Volcanism, Impacts, and Mass Extinctions: Causes and Effects (eds Keller G. and Kerr A. C.), pp. 301–317. Geological Society of America, Boulder. [Google Scholar]
- Couvreur, T. L. P. (2015). Odd man out: why are there fewer plant species in African rain forests? Plant Systematics and Evolution 301, 1299–1313. [Google Scholar]
- Couvreur, T. L. P. , Chatrou, L. W. , Sosef, M. S. & Richardson, J. E. (2008). Molecular phylogenetics reveal multiple tertiary vicariance origins of the African rain forest trees. BMC Biology 6, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur, T. L. P. , Forest, F. & Baker, W. J. (2011a). Origin and global diversification patterns of tropical rain forests: inferences from a complete genus‐level phylogeny of palms. BMC Biology 9, 44 10.1186/1741-7007-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur, T. L. P. , Porter‐Morgan, H. , Wieringa, J. J. & Chatrou, L. W. (2011b). Little ecological divergence associated with speciation in two African rain forest tree genera. BMC Evolutionary Biology 11, 296 10.1186/1471-2148-11-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling, S. A. , Cox, P. M. , Jones, C. D. , Maslin, M. A. , Peros, M. & Spall, S. A. (2008). Simulated glacial and interglacial vegetation across Africa: implications for species phylogenies and trans‐African migration of plants and animals. Global Change Biology 14, 827–840. [Google Scholar]
- Cox, S. C. , Prys‐Jones, R. P. , Habel, J. C. , Amakobe, B. A. & Day, J. J. (2014). Niche divergence promotes rapid diversification of east African sky Island white‐eyes (Aves: Zosteropidae). Molecular Ecology 23, 4103–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, J. A. & Orr, H. A. (2004). Speciation. Sinauer, Sunderland. [Google Scholar]
- Crisp, M. D. & Cook, L. G. (2009). Explosive radiation or cryptic mass extinction? Interpreting signatures in molecular phylogenies. Evolution 63, 2257–2265. [DOI] [PubMed] [Google Scholar]
- Cunha Almeida, F. , Giannini, N. P. & Simmons, N. B. (2016). The evolutionary history of the African fruit bats (Chiroptera: Pteropodidae). Acta Chiropterologica 18, 73–90. [Google Scholar]
- Dagallier, L.‐P. M. J. , Janssens, S. B. , Dauby, G. , Blach‐Overgaard, A. , Mackinder, B. A. , Droissart, V. , Svenning, J.‐C. , Sosef, M. S. M. , Stévart, T. , Harris, D. J. , Sonké, B. , Wieringa, J. J. , Hardy, O. J. & Couvreur, T. L. P. (2020). Cradles and museums of generic plant diversity across tropical Africa. New Phytologist 225, 2196–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasceno, R. , Strangas, M. L. , Carnaval, A. C. , Rodrigues, M. T. & Moritz, C. (2014). Revisiting the vanishing refuge model of diversification. Frontiers in Genetics 5, 353 https://www.frontiersin.org/articles/10.3389/fgene.2014.00353/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Damm, S. , Dijkstra, K.‐D. B. & Hadrys, H. (2010). Red drifters and dark residents: the phylogeny and ecology of a Plio‐Pleistocene dragonfly radiation reflects Africa's changing environment (Odonata, Libellulidae, Trithemis). Molecular Phylogenetics and Evolution 54, 870–882. [DOI] [PubMed] [Google Scholar]
- Davis, C. C. , Bell, C. D. , Fritsch, P. W. & Mathews, S. (2002). Phylogeny of Acridocarpus‐Brachylophon (Malpighiaceae): implications for tertiary tropical floras and Afroasian biogeography. Evolution 56, 2395–2405. [DOI] [PubMed] [Google Scholar]
- Dee, D. P. , Uppala, S. M. , Simmons, A. J. , Berrisford, P. , Poli, P. , Kobayashi, S. , Andrae, U. , Balmaseda, M. A. , Balsamo, G. , Bauer, P. , Bechtold, P. , Beljaars, A. C. M. , van de Berg, L. , Bidlot, J. , Bormann, N. , Delsol, C. , Dragani, R. , Fuentes, M. , Geer, A. J. , Haimberger, L. , Healy, S. B. , Hersbach, H. , Hólm, E. V. , Isaksen, L. , Kållberg, P. , Köhler, M. , Matricardi, M. , McNally, A. P. , Monge‐Sanz, B. M. , Morcrette, J. J. , Park, B. K. , Peubey, C. , de Rosnay, P. , Tavolato, C. , Thépaut, J. N. & Vitart, F. (2011). The ERA‐interim reanalysis: configuration and performance of the data assimilation system. Quarterly Journal of the Royal Meteorological Society 137, 553–597. [Google Scholar]
- deMenocal, P. (2004). African climate change and faunal evolution during the Pliocene–Pleistocene. Earth and Planetary Science Letters 220, 3–24. [Google Scholar]
- Demenou, B. B. , Doucet, J.‐L. & Hardy, O. J. (2018). History of the fragmentation of the African rain forest in the Dahomey gap: insight from the demographic history of Terminalia superba . Heredity 120, 547–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos, T. C. , Kerbis Peterhans, J. C. , Agwanda, B. & Hickerson, M. J. (2014). Uncovering cryptic diversity and refugial persistence among small mammal lineages across the eastern Afromontane biodiversity hotspot. Molecular Phylogenetics and Evolution 71, 41–54. [DOI] [PubMed] [Google Scholar]
- Descombes, P. , Gaboriau, T. , Albouy, C. , Heine, C. , Leprieur, F. & Pellissier, L. (2018). Linking species diversification to palaeo‐environmental changes: a process‐based modelling approach. Global Ecology and Biogeography 27, 233–244. [Google Scholar]
- Diallo, A. M. , Nielsen, L. R. , Kjær, E. D. , Petersen, K. K. & Ræbild, A. (2016). Polyploidy can confer superiority to west African Acacia Senegal (L.) Wild trees. Frontiers in Plant Science 7, 821 https://www.frontiersin.org/articles/10.3389/fpls.2016.00821/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, A. W. & Hamilton, A. C. (1980). The distribution of forest passerine birds and quaternary climatic change in tropical Africa. Journal of Zoology 191, 379–402. [Google Scholar]
- Dimitrov, D. , Nogués‐Bravo, D. & Scharff, N. (2012). Why do tropical mountains support exceptionally high biodiversity? The eastern Arc Mountains and the drivers of Saintpaulia diversity. PLoS One 7, e48908 10.1371/journal.pone.0048908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebeli, M. & Dieckmann, U. (2003). Speciation along environmental gradients. Nature 421, 259–264. [DOI] [PubMed] [Google Scholar]
- Dolotovskaya, S. , Torroba Bordallo, J. , Haus, T. , Noll, A. , Hofreiter, M. , Zinner, D. & Roos, C. (2017). Comparing mitogenomic timetrees for two African savannah primate genera (Chlorocebus and Papio). Zoological Journal of the Linnean Society 181, 471–483. [Google Scholar]
- Donkpegan, A. S. L. , Doucet, J.‐L. , Migliore, J. , Duminil, J. , Dainou, K. , Piñeiro, R. , Wieringa, J. J. , Champluvier, D. & Hardy, O. J. (2017). Evolution in African tropical trees displaying ploidy‐habitat association: the genus Afzelia (Leguminosae). Molecular Phylogenetics and Evolution 107, 270–281. [DOI] [PubMed] [Google Scholar]
- Doucouré, C. M. & de Wit, M. J. (2003). Old inherited origin for the present near‐bimodal topography of Africa. Journal of African Earth Sciences 36, 371–388. [Google Scholar]
- Droissart, V. , Dauby, G. , Hardy, O. J. , Deblauwe, V. , Harris, D. J. , Janssens, S. , Mackinder, B. A. , Blach‐Overgaard, A. , Sonké, B. , Sosef, M. S. M. , Stévart, T. , Svenning, J.‐C. , Wieringa, J. J. & Couvreur, T. L. P. (2018). Beyond trees: biogeographical regionalization of tropical Africa. Journal of Biogeography 45, 1153–1167. [Google Scholar]
- * Dubey, S. , Salamin, N. , Ruedi, M. , Barrière, P. , Colyn, M. & Vogel, P. (2008). Biogeographic origin and radiation of the Old World crocidurine shrews (Mammalia: Soricidae) inferred from mitochondrial and nuclear genes. Molecular Phylogenetics and Evolution 48, 953–963. [DOI] [PubMed] [Google Scholar]
- * Duchen, P. & Renner, S. S. (2010). The evolution of Cayaponia (Cucurbitaceae): repeated shifts from bat to bee pollination and long‐distance dispersal to Africa 2–5 million years ago. American Journal of Botany 97, 1129–1141. [DOI] [PubMed] [Google Scholar]
- Duminil, J. , Mona, S. , Mardulyn, P. , Doumenge, C. , Walmacq, F. , Doucet, J.‐L. & Hardy, O. J. (2015). Late Pleistocene molecular dating of past population fragmentation and demographic changes in African rain forest tree species supports the forest refuge hypothesis. Journal of Biogeography 42, 1443–1454. [Google Scholar]
- Dupont, L. M. , Jahns, S. , Marret, F. & Ning, S. (2000). Vegetation change in equatorial West Africa: time‐slices for the last 150 ka. Palaeogeography Palaeoclimatology Palaeoecology 155, 95–122. [Google Scholar]
- Edwards, E. J. , Osborne, C. P. , Strömberg, C. A. E. & Smith, S. A. (2010). The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328, 587–591. [DOI] [PubMed] [Google Scholar]
- Elsworth, G. , Galbraith, E. , Halverson, G. & Yang, S. (2017). Enhanced weathering and CO2 drawdown caused by latest Eocene strengthening of the Atlantic meridional overturning circulation. Nature Geoscience 10, 213–216. [Google Scholar]
- Engelbrecht, H. M. , Branch, W. R. , Greenbaum, E. , Burger, M. , Conradie, W. & Tolley, K. A. (2020). African herald snakes, Crotaphopeltis, show population structure for a widespread generalist but deep genetic divergence for forest specialists. Journal of Zoological Systematics and Evolutionary Research. 10.1111/jzs.12361. [DOI] [Google Scholar]
- Erwin, T. L. (1991). An evolutionary basis for conservation strategies. Science 253, 750–752. [DOI] [PubMed] [Google Scholar]
- Estep, M. C. , McKain, M. R. , Diaz, D. V. , Zhong, J. , Hodge, J. G. , Hodkinson, T. R. , Layton, D. J. , Malcomber, S. T. , Pasquet, R. & Kellogg, E. A. (2014). Allopolyploidy, diversification, and the Miocene grassland expansion. Proceedings of the National Academy of Sciences of the United States of America 111, 15149–15154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Estrella, M. , Forest, F. , Wieringa, J. J. , Fougère‐Danezan, M. & Bruneau, A. (2017). Insights on the evolutionary origin of Detarioideae, a clade of ecologically dominant tropical African trees. New Phytologist 214, 1722–1735. [DOI] [PubMed] [Google Scholar]
- Evans, B. J. , Kelley, D. B. , Tinsley, R. C. , Melnick, D. J. & Cannatella, D. C. (2004). A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Molecular Phylogenetics and Evolution 33, 197–213. [DOI] [PubMed] [Google Scholar]
- Evans, B. J. , Carter, T. F. , Greenbaum, E. , Gvoždík, V. , Kelley, D. B. , McLaughlin, P. J. , Pauwels, O. S. G. , Portik, D. M. , Stanley, E. L. , Tinsley, R. C. , Tobias, M. L. & Blackburn, D. C. (2015). Genetics, morphology, advertisement calls, and historical records distinguish six new polyploid species of African clawed frog (Xenopus, Pipidae) from west and Central Africa. PLoS One 10, e0142823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith, J. T. , Rowan, J. & Du, A. (2019). Early hominins evolved within non‐analog ecosystems. Proceedings of the National Academy of Sciences of the United States of America 116, 21478–21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathy, D. , Wagreich, M. , Gier, S. , Mohamed, R. S. A. , Zaki, R. & El Nady, M. M. (2018). Maastrichtian oil shale deposition on the southern Tethys margin, Egypt: insights into greenhouse climate and paleoceanography. Palaeogeography, Palaeoclimatology, Palaeoecology 505, 18–32. [Google Scholar]
- Faye, A. , Deblauwe, V. , Mariac, C. , Richard, D. , Sonké, B. , Vigouroux, Y. & Couvreur, T. L. P. (2016a). Phylogeography of the genus Podococcus (Palmae/Arecaceae) in central African rain forests: climate stability predicts unique genetic diversity. Molecular Phylogenetics and Evolution 105, 126–138. [DOI] [PubMed] [Google Scholar]
- Faye, A. , Pintaud, J.‐C. , Baker, W. J. , Vigouroux, Y. , Sonke, B. & Couvreur, T. L. P. (2016b). Phylogenetics and diversification history of African rattans (Calamoideae, Ancistrophyllinae). Botanical Journal of the Linnean Society 182, 256–271. [Google Scholar]
- Feakins, S. J. , Levin, N. E. , Liddy, H. M. , Sieracki, A. , Eglinton, T. I. & Bonnefille, R. (2013). Northeast African vegetation change over 12 m.y. Geology 41, 295–298. [Google Scholar]
- Feduccia, A. (2014). Avian extinction at the end of the cretaceous: assessing the magnitude and subsequent explosive radiation. Cretaceous Research 50, 1–15. [Google Scholar]
- Feibel, C. S. (1993). Freshwater stingrays from the Plio‐Pleistocene of the Turkana Basin, Kenya and Ethiopia. Lethaia 26, 359–366. [Google Scholar]
- Feng, Y.‐J. , Blackburn, D. C. , Liang, D. , Hillis, D. M. , Wake, D. B. , Cannatella, D. C. & Zhang, P. (2017). Phylogenomics reveals rapid, simultaneous diversification of three major clades of Gondwanan frogs at the cretaceous–Paleogene boundary. Proceedings of the National Academy of Sciences of the United States of America 114, E5864–E5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fer, I. , Tietjen, B. , Jeltsch, F. & Trauth, M. H. (2017). Modelling vegetation change during late Cenozoic uplift of the east African plateaus. Palaeogeography, Palaeoclimatology, Palaeoecology 467, 120–130. [Google Scholar]
- Finch, J. , Leng, M. J. & Marchant, R. (2009). Late quaternary vegetation dynamics in a biodiversity hotspot, the Uluguru Mountains of Tanzania. Quaternary Research 72, 111–122. [Google Scholar]
- Fjeldså, J. (1994). Geographical patterns for relict and young species of birds in Africa and South America and implications for conservation priorities. Biodiversity and Conservation 3, 207–226. [Google Scholar]
- Fjeldså, J. & Bowie, R. C. K. (2008). New perspectives on the origin and diversification of Africa's forest avifauna. African Journal of Ecology 46, 235–247. [Google Scholar]
- Fjeldså, J. & Lovett, J. C. (1997). Geographical patterns of old and young species in African forest biota: the significance of specific montane areas as evolutionary centers. Biodiversity and Conservation 6, 325–346. [Google Scholar]
- Fjeldså, J. , Johansson, U. S. , Lokugalappatti, L. G. S. & Bowie, R. C. K. (2007). Diversification of African greenbuls in space and time: linking ecological and historical processes. Journal of Ornithology 148, 359–367. [Google Scholar]
- Fjeldså, J. , Bowie, R. C. K. & Rahbek, C. (2012). The role of mountain ranges in the diversification of birds. Annual Review of Ecology, Evolution, and Systematics 43, 249–265. [Google Scholar]
- Flowers, R. M. & Schoene, B. (2010). (U‐Th)/he thermochronometry constraints on unroofing of the eastern Kaapvaal craton and significance for uplift of the southern African plateau. Geology 38, 827–830. [Google Scholar]
- Foster, G. L. , Hull, P. , Lunt, D. J. & Zachos, J. C. (2018). Placing our current ‘hyperthermal’ in the context of rapid climate change in our geological past. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 376, 20170086 10.1098/rsta.2017.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, A. H. , Thomassen, H. A. , Buermann, W. & Smith, T. B. (2010). Genomic signals of diversification along ecological gradients in a tropical lizard. Molecular Ecology 19, 3773–3788. [DOI] [PubMed] [Google Scholar]
- Fuchs, J. & Bowie, R. C. (2015). Concordant genetic structure in two species of woodpecker distributed across the primary west African biogeographic barriers. Molecular Phylogenetics and Evolution 88, 64–74. [DOI] [PubMed] [Google Scholar]
- Fuchs, J. , Pons, J.‐M. & Bowie, R. C. K. (2017). Biogeography and diversification dynamics of the African woodpeckers. Molecular Phylogenetics and Evolution 108, 88–100. [DOI] [PubMed] [Google Scholar]
- Galley, C. , Bytebier, B. , Bellstedt, D. U. & Linder, H. P. (2007). The cape element in the Afrotemperate flora: from cape to Cairo? Proceedings of the Royal Society of London B: Biological Sciences 274, 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * García‐Aloy, S. , Vitales, D. , Roquet, C. , Sanmartín, I. , Vargas, P. , Molero, J. , Kamau, P. , Aldasoro, J. J. & Alarcón, M. (2017). North‐West Africa as a source and refuge area of plant biodiversity: a case study on Campanula kremeri and Campanula occidentalis . Journal of Biogeography 44, 2057–2068. [Google Scholar]
- Gardner, J. D. & Rage, J.‐C. (2016). The fossil record of lissamphibians from Africa, Madagascar, and the Arabian plate. Palaeobiodiversity and Palaeoenvironments 96, 169–220. [Google Scholar]
- * Gaubert, P. & Cordeiro‐Estrela, P. (2006). Phylogenetic systematics and tempo of evolution of the Viverrinae (Mammalia, Carnivora, Viverridae) within feliformians: implications for faunal exchanges between Asia and Africa. Molecular Phylogenetics and Evolution 41, 266–278. [DOI] [PubMed] [Google Scholar]
- Gaubert, P. , Antunes, A. , Meng, H. , Miao, L. , Peigné, S. , Justy, F. , Njiokou, F. , Dufour, S. , Danquah, E. , Alahakoon, J. , Verheyen, E. , Stanley, W. T. , O'Brien, S. J. , Johnson, W. E. & Luo, S.‐J. (2018). The complete phylogeny of pangolins: scaling up resources for the molecular tracing of the most trafficked mammals on earth. Journal of Heredity 109, 347–359. [DOI] [PubMed] [Google Scholar]
- Gavrilets, S. (2003). Perspective: models of speciation: what have we learned in 40 years? Evolution 57, 2197–2215. [DOI] [PubMed] [Google Scholar]
- Gehrke, B. & Linder, H. P. (2009). The scramble for Africa: pan‐temperate elements on the African high mountains. Proceedings of the Royal Society of London B: Biological Sciences 276, 2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke, B. & Linder, H. P. (2014). Species richness, endemism and species composition in the tropical Afroalpine flora. Alpine Botany 124, 165–177. [Google Scholar]
- Gehrke, B. , Bräuchler, C. , Romoleroux, K. , Lundberg, M. , Heubl, G. & Eriksson, T. (2008). Molecular phylogenetics of Alchemilla, Aphanes and Lachemilla (Rosaceae) inferred from plastid and nuclear intron and spacer DNA sequences, with comments on generic classification. Molecular Phylogenetics and Evolution 47, 1030–1044. [DOI] [PubMed] [Google Scholar]
- Gentry, A. (1989). Speciation in tropical forests In Tropical Forests: Botanical Dynamics, Speciation and Diversity (eds Holm‐Nielsen L. B., Nielsen I. C. and Balslev H.), pp. 113–134. Academic Press, London. [Google Scholar]
- Gerland, P. , Raftery, A. E. , Ševčíková, H. , Li, N. , Gu, D. , Spoorenberg, T. , Alkema, L. , Fosdick, B. K. , Chunn, J. , Lalic, N. , Bay, G. , Buettner, T. , Heilig, G. K. & Wilmoth, J. (2014). World population stabilization unlikely this century. Science 346, 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, R. G. , Bennett, G. M. , De Meester, L. , Feder, J. L. , Fleischer, R. C. , Harmon, L. J. , Hendry, A. P. , Knope, M. L. , Mallet, J. , Martin, C. , Parent, C. E. , Patton, A. H. , Pfennig, K. S. , Rubinoff, D. , Schluter, D. , et al. (2020). Comparing adaptive radiations across space, time, and taxa. Journal of Heredity 111, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish, T. J. (2010). Ecology of plant speciation. Taxon 59, 1326–1366. [Google Scholar]
- Gizaw, A. , Brochmann, C. , Nemomissa, S. , Wondimu, T. , Masao, C. A. , Tusiime, F. M. , Abdi, A. A. , Oxelman, B. , Popp, M. & Dimitrov, D. (2016). Colonization and diversification in the African ‘sky islands’: insights from fossil‐calibrated molecular dating of Lychnis (Caryophyllaceae). New Phytologist 211, 719–734. [DOI] [PubMed] [Google Scholar]
- Goldner, A. , Herold, N. & Huber, M. (2014). The challenge of simulating the warmth of the mid‐Miocene climatic Optimun in CESM1. Climate of the Past 10, 523–536. [Google Scholar]
- Gonder, M. K. , Locatelli, S. , Ghobrial, L. , Mitchell, M. W. , Kujawski, J. T. , Lankester, F. J. , Stewart, C.‐B. & Tishkoff, S. A. (2011). Evidence from Cameroon reveals differences in the genetic structure and histories of chimpanzee populations. Proceedings of the National Academy of Sciences of the United States of America 108, 4766–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie, A. S. (2005). The drainage of Africa since the cretaceous. Geomorphology 67, 437–456. [Google Scholar]
- Graham, C. H. , Parra, M. , Mora, A. & Higuera, C. (2018). The interplay between geological history and ecology in mountains In Mountains, Climate and Biodiversity (eds Hoorn C., Perrigo A. and Antonelli A.), pp. 231–243. Wiley‐Blackwell, John Wiley & Sons, Hoboken. [Google Scholar]
- Grebennikov, V. V. (2017). Phylogeography and sister group of Lupangus, a new genus for three new flightless allopatric forest litter weevils endemic to the eastern Arc Mountains, Tanzania (Coleoptera: Curculionidae, Molytinae). Fragmenta Entomologica 49, 37–55. [Google Scholar]
- Greenbaum, E. , Portillo, F. , Jackson, K. & Kusamba, C. (2015). A phylogeny of central African Boaedon (Serpentes: Lamprophiidae), with the description of a new cryptic species from the Albertine rift. African Journal of Herpetology 64, 18–38. [Google Scholar]
- Griffiths, C. J. (1993). The geological evolution of eastern Africa In Biogeography and Ecology of the Rain Forest of Eastern Africa (eds Lovett J. C. and Wasser S. K.), pp. 9–21. Cambridge University Press, Cambridge. [Google Scholar]
- Grimaud, J.‐L. , Rouby, D. , Chardon, D. & Beauvais, A. (2017). Cenozoic sediment budget of West Africa and The Niger delta. Basin Research 30, 169–186. [Google Scholar]
- Guillocheau, F. , Chelalou, R. , Linol, B. , Dauteuil, O. , Robin, C. , Mvondo, F. , Callec, Y. & Colin, J.‐P. (2015). Cenozoic landscape evolution in and around The Congo Basin: constraints from sediments and planation surfaces In Geology and Resource Potential of The Congo Basin (eds de Wit M. J., Guillocheau F. and de Wit M. C. J.), pp. 271–313. Springer, Berlin. [Google Scholar]
- Guillocheau, F. , Simon, B. , Baby, G. , Bessin, P. , Robin, C. & Dauteuil, O. (2018). Planation surfaces as a record of mantle dynamics: the case example of Africa. Gondwana Research 53, 82–98. [Google Scholar]
- Guiraud, R. , Bosworth, W. , Thierry, J. & Delplanque, A. (2005). Phanerozoic geological evolution of northern and Central Africa: an overview. Journal of African Earth Sciences 43, 83–143. [Google Scholar]
- Guschanski, K. , Krause, J. , Sawyer, S. , Valente, L. M. , Bailey, S. , Finstermeier, K. , Sabin, R. , Gilissen, E. , Sonet, G. , Nagy, Z. T. , Lenglet, G. , Mayer, F. & Savolainen, V. (2013). Next‐generation museomics disentangles one of the largest primate radiations. Systematic Biology 62, 539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffer, J. (2008). Hypotheses to explain the origin of species in Amazonia. Brazilian Journal of Biology 68, 917–947. [DOI] [PubMed] [Google Scholar]
- Hamilton, A. C. & Faden, R. B. (1974). The history of the vegetation In East African Vegetation (eds Lind E. M. and Morrison M. E. S.), pp. 188–209. Longman, London. [Google Scholar]
- Hamilton, A. C. & Taylor, D. (1992). History of climate and forests in tropical Africa during the last 8 million years In Tropical Forests and Climate (ed. Myers N.), pp. 65–78. Springer, Dordrecht. [Google Scholar]
- Hamon, N. , Sepulchre, P. , Donnadieu, Y. , Henrot, A.‐J. , François, L. , Jaeger, J.‐J. & Ramstein, G. (2012). Growth of subtropical forests in Miocene Europe: the roles of carbon dioxide and Antarctic ice volume. Geology 40, 567–570. [Google Scholar]
- Hamon, N. , Sepulchre, P. , Lefebvre, V. & Ramstein, G. (2013). The role of eastern Tethys seaway closure in the middle Miocene climatic transition (ca. 14 ma). Climate of the Past 9, 2687–2702. [Google Scholar]
- Hamon, P. , Grover, C. E. , Davis, A. P. , Rakotomalala, J.‐J. , Raharimalala, N. E. , Albert, V. A. , Sreenath, H. L. , Stoffelen, P. , Mitchell, S. E. , Couturon, E. , Hamon, S. , de Kochko, A. , Crouzillat, D. , Rigoreau, M. , Sumirat, U. , Akaffou, S. & Guyot, R. (2017). Genotyping‐by‐sequencing provides the first well‐resolved phylogeny for coffee (Coffea) and insights into the evolution of caffeine content in its species: GBS coffee phylogeny and the evolution of caffeine content. Molecular Phylogenetics and Evolution 109, 351–361. [DOI] [PubMed] [Google Scholar]
- Han, T.‐S. , Zheng, Q.‐J. , Onstein, R. E. , Rojas‐Andrés, B. M. , Hauenschild, F. , Muellner‐Riehl, A. N. & Xing, Y.‐W. (2020). Polyploidy promotes species diversification of Allium through ecological shifts. New Phytologist 225, 571–583. [DOI] [PubMed] [Google Scholar]
- Handley, L. , O'Halloran, A. , Pearson, P. N. , Hawkins, E. , Nicholas, C. J. , Schouten, S. , McMillan, I. K. & Pancost, R. D. (2012). Changes in the hydrological cycle in tropical East Africa during the Paleocene–Eocene thermal maximum. Palaeogeography, Palaeoclimatology, Palaeoecology 329–330, 10–21. [Google Scholar]
- Hansen, J. , Sato, M. , Kharecha, P. , Beerling, D. , Berner, R. , Masson‐Delmotte, V. , Pagani, M. , Raymo, M. , Royer, D. L. & Zachos, J. C. (2008). Target atmospheric CO2: where should humanity aim? The Open Atmospheric Science Journal 2, 217–231. [Google Scholar]
- Hardy, O. J. , Born, C. , Budde, K. , Daïnou, K. , Dauby, G. , Duminil, J. , Ewédjé, E.‐E. B. , Gomez, C. , Heuertz, M. , Koffi, G. K. , Lowe, J. A. , Micheneau, C. , Ndiade‐Bourobou, D. , Piñeiro, R. & Poncet, V. (2013). Comparative phylogeography of African rain forest trees: a review of genetic signatures of vegetation history in the Guineo‐Congolian region. Comptes Rendus Geoscience 345, 284–296. [Google Scholar]
- Harris, D. J. , Poulsen, A. D. , Frimodt‐Møller, C. , Preston, J. & Cronk, Q. C. B. (2000). Rapid radiation in Aframomum (Zingiberaceae): evidence from nuclear ribosomal DNA internal transcribed spacer (ITS) sequences. Edinburgh Journal of Botany 57, 377–395. [Google Scholar]
- Hassanin, A. , Delsuc, F. , Ropiquet, A. , Hammer, C. , Jansen van Vuuren, B. , Matthee, C. , Ruiz‐Garcia, M. , Catzeflis, F. , Areskoug, V. , Nguyen, T. T. & Couloux, A. (2012). Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. Comptes Rendus Biologies 335, 32–50. [DOI] [PubMed] [Google Scholar]
- Hassanin, A. , Khouider, S. , Gembu, G.‐C. , Goodman, S. , Kadjo, B. , Nesi, N. , Pourrut, X. , Nakouné, E. & Bonillo, C. (2015). The comparative phylogeography of fruit bats of the tribe Scotonycterini (Chiroptera, Pteropodidae) reveals cryptic species diversity related to African Pleistocene forest refugia. Comptes Rendus Biologies 338, 197–211. [DOI] [PubMed] [Google Scholar]
- Haug, G. H. , Ganopolski, A. , Sigman, D. M. , Rosell‐Mele, A. , Swann, G. E. A. , Tiedemann, R. , Jaccard, S. L. , Bollmann, J. , Maslin, M. A. , Leng, M. J. & Eglinton, G. (2005). North Pacific seasonality and the glaciation of North America 2.7 million years ago. Nature 433, 821–825. [DOI] [PubMed] [Google Scholar]
- Haywood, A. M. , Hill, D. J. , Dolan, A. M. , Otto‐Bliesner, B. L. , Bragg, F. , Chan, W.‐L. , Chandler, M. A. , Contoux, C. , Dowsett, H. J. , Jost, A. , Kamae, Y. , Lohmann, G. , Lunt, D. J. , Abe‐Ouchi, A. , Pickering, S. J. , Ramstein, G. , Rosenbloom, N. A. , Salzmann, U. , Sohl, L. , Stepanek, C. , Ueda, H. , Yan, Q. & Zhang, Z. (2013). Large‐scale features of Pliocene climate: results from the Pliocene model Intercomparison project. Climate of the Past 9, 191–209. [Google Scholar]
- Hemp, C. , Kehl, S. , Heller, K.‐G. , Wägele, J. W. & Hemp, A. (2010). A new genus of African Karniellina (Orthoptera, Tettigoniidae, Conocephalinae, Conocephalini): integrating morphological, molecular and bioacoustical data. Systematic Entomology 35, 581–595. [Google Scholar]
- Hemp, C. , Kehl, S. , Schultz, O. , Wägele, J. W. & Hemp, A. (2015). Climatic fluctuations and orogenesis as motors for speciation in East Africa: case study on Parepistaurus Karsch, 1896 (Orthoptera). Systematic Entomology 40, 17–34. [Google Scholar]
- Henrot, A.‐J. , François, L. , Favre, E. , Butzin, M. , Ouberdous, M. & Munhoven, G. (2010). Effects of CO2, continental distribution, topography and vegetation changes on the climate at the middle Miocene: a model study. Climate of the Past 6, 675–694. [Google Scholar]
- Henrot, A.‐J. , Utescher, T. , Erdei, B. , Dury, M. , Hamon, N. , Ramstein, G. , Krapp, M. , Herold, N. , Goldner, A. , Favre, E. , Munhoven, G. & François, L. (2017). Middle Miocene climate and vegetation models and their validation with proxy data. Palaeogeography, Palaeoclimatology, Palaeoecology 467, 95–119. [Google Scholar]
- Herbert, T. D. , Lawrence, K. T. , Tzanova, A. , Peterson, L. C. , Caballero‐Gill, R. & Kelly, C. S. (2016). Late Miocene global cooling and the rise of modern ecosystems. Nature Geoscience 9, 843–847. [Google Scholar]
- Hernández Fernández, M. & Vrba, E. S. (2006). Plio‐Pleistocene climatic change in the Turkana Basin (East Africa): evidence from large mammal faunas. Journal of Human Evolution 50, 595–626. [DOI] [PubMed] [Google Scholar]
- Herold, N. , Seton, M. , Müller, R. D. , You, Y. & Huber, M. (2008). Middle Miocene tectonic boundary conditions for use in climate models. Geochemistry, Geophysics, Geosystems 9, 1–10. https://agupubs.onlinelibrary.wiley.com/doi/abs/10.1029/2008GC002046. [Google Scholar]
- Herrera, J. P. (2017). Primate diversification inferred from phylogenies and fossils. Evolution 71, 2845–2857. [DOI] [PubMed] [Google Scholar]
- Hill, J. L. & Hill, R. A. (2001). Why are tropical rain forests so species rich? Classifying, reviewing and evaluating theories. Progress in Physical Geography 25, 326–354. [Google Scholar]
- Hoag, C. & Svenning, J.‐C. (2017). African environmental change from the Pleistocene to the Anthropocene. Annual Review of Environment and Resources 42, 27–54. [Google Scholar]
- Hodell, D. A. , Curtis, J. H. , Sierro, F. J. & Raymo, M. E. (2001). Correlation of late Miocene to early Pliocene sequences between the Mediterranean and North Atlantic. Paleoceanography 16, 164–178. [Google Scholar]
- Holbourn, A. , Kuhnt, W. , Kochhann, K. G. D. , Andersen, N. & Meier, K. J. S. (2015). Global perturbation of the carbon cycle at the onset of the Miocene climatic optimum. Geology 43, 123–126. [Google Scholar]
- Holstein, N. & Renner, S. S. (2011). A dated phylogeny and collection records reveal repeated biome shifts in the African genus Coccinia (Cucurbitaceae). BMC Evolutionary Biology 11, 28 10.1186/1471-2148-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorn, C. , Wesselingh, F. P. , ter Steege, H. , Bermudez, M. A. , Mora, A. , Sevink, J. , Sanmartín, I. , Sanchez‐Meseguer, A. , Anderson, C. L. , Figueiredo, J. P. , Jaramillo, C. , Riff, D. , Negri, F. R. , Hooghiemstra, H. , Lundberg, J. , Stadler, T. , Särkinen, T. & Antonelli, A. (2010). Amazonia through time: Andean uplift, climate change, landscape evolution. Biodiversity Science 330, 927–931. [DOI] [PubMed] [Google Scholar]
- Hoorn C., Antonelli A. & Perrigo A. (eds) (2018a). Mountains, Climate, and Biodiversity. Wiley‐Blackwell, Hoboken. [Google Scholar]
- Hoorn, C. , Perrigo, A. & Antonelli, A. (2018b). Mountains, climate and biodiversity: an introduction In Mountains, Climate and Biodiversity, pp. 1–13. Wiley‐Blackwell, Hoboken. [Google Scholar]
- Huber, M. & Caballero, R. (2011). The early Eocene equable climate problem revisited. Climate of the Past 7, 603–633. [Google Scholar]
- Huber, B. T. , MacLeod, K. G. , Watkins, D. K. & Coffin, M. F. (2018). The rise and fall of the cretaceous hot greenhouse climate. Global and Planetary Change 167, 1–23. [Google Scholar]
- Hughes, C. E. & Atchison, G. W. (2015). The ubiquity of alpine plant radiations: from the Andes to the Hengduan Mountains. New Phytologist 207, 275–282. [DOI] [PubMed] [Google Scholar]
- Hughes, C. E. & Eastwood, R. (2006). Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences of the United States of America 103, 10334–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, D. F. , Tolley, K. A. , Behangana, M. , Lukwago, W. , Menegon, M. , Dehling, J. M. , Stipala, J. , Tilbury, C. R. , Khan, A. M. & Kusamba, C. (2018). Cryptic diversity in Rhampholeon boulengeri (Sauria: Chamaeleonidae), a pygmy chameleon from the Albertine rift biodiversity hotspot. Molecular Phylogenetics and Evolution 122, 125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley, J. W. & Voelker, G. (2016). Cryptic diversity in afro‐tropical lowland forests: the systematics and biogeography of the avian genus Bleda . Molecular Phylogenetics and Evolution 99, 297–308. [DOI] [PubMed] [Google Scholar]
- * Huntley, J. W. & Voelker, G. (2017). A tale of the nearly tail‐less: the effects of Plio‐Pleistocene climate change on the diversification of the African avian genus Sylvietta . Zoologica Scripta 46, 523–535. [Google Scholar]
- Huntley, J. W. , Harvey, J. A. , Pavia, M. , Boano, G. & Voelker, G. (2018). The systematics and biogeography of the bearded Greenbuls (Aves: Criniger) reveals the impact of Plio‐Pleistocene forest fragmentation on afro‐tropical avian diversity. Zoological Journal of the Linnean Society 183, 672–686. [Google Scholar]
- Huntley, J. W. , Keith, K. D. , Castellanos, A. A. , Musher, L. J. & Voelker, G. (2019). Underestimated and cryptic diversification patterns across afro‐tropical lowland forests. Journal of Biogeography 46, 381–391. [Google Scholar]
- Im, E.‐S. & Eltahir, E. A. B. (2018). Simulations of the observed ‘jump’ in the west African monsoon and its underlying dynamics using the MIT regional climate model. International Journal of Climatology 38, 841–852. [Google Scholar]
- Inglis, G. N. , Farnsworth, A. , Lunt, D. , Foster, G. L. , Hollis, C. J. , Pagani, M. , Jardine, P. E. , Pearson, P. N. , Markwick, P. , Galsworthy, A. M. J. , Raynham, L. , Taylor, K. W. R. & Pancost, R. D. (2015). Descent toward the icehouse: Eocene Sea surface cooling inferred from GDGT distributions. Paleoceanography 30, 1000–1020. [Google Scholar]
- Jacobs, B. F. (2004). Palaeobotanical studies from tropical Africa: relevance to the evolution of forest, woodland and savannah biomes. Philosophical Transactions of the Royal Society B: Biological Sciences 359, 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, B. F. & Herendeen, P. S. (2004). Eocene dry climate and woodland vegetation in tropical Africa reconstructed from fossil leaves from northern Tanzania. Palaeogeography, Palaeoclimatology, Palaeoecology 213, 115–123. [Google Scholar]
- Jacobs, B. F. , Pan, A. P. & Scotese, C. R. (2010). A review of the Cenozoic vegetation history of Africa In Cenozoic Mammals of Africa (eds Werdelin L. and Sanders J.), pp. 57–72. University of California Press, Berkeley. [Google Scholar]
- Jacquet, F. , Nicolas, V. , Colyn, M. , Kadjo, B. , Hutterer, R. , Decher, J. , Akpatou, B. , Cruaud, C. & Denys, C. (2014). Forest refugia and riverine barriers promote diversification in the west African pygmy shrew (Crocidura obscurior complex, Soricomorpha). Zoologica Scripta 43, 131–148. [Google Scholar]
- Janssens, S. B. , Knox, E. B. , Huysmans, S. , Smets, E. F. & Merckx, V. (2009). Rapid radiation of Impatiens (Balsaminaceae) during Pliocene and Pleistocene: result of a global climate change. Molecular Phylogenetics and Evolution 52, 806–824. [DOI] [PubMed] [Google Scholar]
- Jaramillo, C. , Rueda, M. J. & Mora, G. (2006). Cenozoic plant diversity in the Neotropics. Science 311, 1893–1896. [DOI] [PubMed] [Google Scholar]
- Jarvis, E. D. , Mirarab, S. , Aberer, A. J. , Li, B. , Houde, P. , Li, C. , Ho, S. Y. W. , Faircloth, B. C. , Nabholz, B. , Howard, J. T. , Suh, A. , Weber, C. C. , da Fonseca, R. R. , Li, J. , Zhang, F. , et al. (2014). Whole‐genome analyses resolve early branches in the tree of life of modern birds. Science 346, 1320–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins, C. N. , Pimm, S. L. & Joppa, L. N. (2013). Global patterns of terrestrial vertebrate diversity and conservation. Proceedings of the National Academy of Sciences of the United States of America 110, E2602–E2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K. R. & Ellis, B. (2002). A tropical rainforest in Colorado 1.4 million years after the cretaceous‐tertiary boundary. Science 296, 2379–2383. [DOI] [PubMed] [Google Scholar]
- Johnson, S. P. , Cutten, H. N. C. , Muhongo, S. & De Waele, B. (2003). Neoarchaean magmatism and metamorphism of the western granulites in the central domain of the Mozambique belt, Tanzania: U–Pb shrimp geochronology and PT estimates. Tectonophysics 375, 125–145. [Google Scholar]
- Johnston, A. R. & Anthony, N. M. (2012). A multi‐locus species phylogeny of African forest duikers in the subfamily Cephalophinae: evidence for a recent radiation in the Pleistocene. BMC Evolutionary Biology 12, 120 10.1186/1471-2148-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma, G. F. M. , Barej, M. F. , Barratt, C. D. , Burger, M. , Conradie, W. , Ernst, R. , Greenbaum, E. , Hirschfeld, M. , Leaché, A. D. , Penner, J. , Portik, D. M. , Zassi‐Boulou, A.‐G. , Rödel, M.‐O. & Blackburn, D. C. (2018). Diversity and biogeography of frogs in the genus Amnirana (Anura: Ranidae) across sub‐Saharan Africa. Molecular Phylogenetics and Evolution 120, 274–285. [DOI] [PubMed] [Google Scholar]
- Joordens, J. C. A. , Vonhof, H. B. , Feibel, C. S. , Lourens, L. J. , Dupont‐Nivet, G. , van der Lubbe, J. H. J. L. , Sier, M. J. , Davies, G. R. & Kroon, D. (2011). An astronomically‐tuned climate framework for hominins in the Turkana Basin. Earth and Planetary Science Letters 307, 1–8. [Google Scholar]
- Joordens, J. C. A. , Feibel, C. S. , Vonhof, H. B. , Schulp, A. S. & Kroon, D. (2019). Relevance of the eastern African coastal forest for early hominin biogeography. Journal of Human Evolution 131, 176–202. [DOI] [PubMed] [Google Scholar]
- Kadereit, G. , Ackerly, D. & Pirie, M. D. (2012). A broader model for C4 photosynthesis evolution in plants inferred from the goosefoot family (Chenopodiaceae s.s.). Proceedings of the Royal Society B: Biological Sciences 279, 3304–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainulainen, K. , Razafimandimbison, S. G. , Wikström, N. & Bremer, B. (2017). Island hopping, long‐distance dispersal and species radiation in the Western Indian Ocean: historical biogeography of the Coffeeae alliance (Rubiaceae). Journal of Biogeography 44, 1966–1979. [Google Scholar]
- Kandziora, M. , Kadereit, J. W. & Gehrke, B. (2016). Frequent colonization and little in situ speciation in Senecio in the tropical alpine‐like islands of eastern Africa. American Journal of Botany 103, 1483–1498. [DOI] [PubMed] [Google Scholar]
- Katuala, P. G. B. , Kennis, J. , Nicolas, V. , Wendelen, W. , Hulselmans, J. , Verheyen, E. , Houtte, N. V. , Dierckx, T. , Dudu, A. M. & Leirs, H. (2008). The presence of Praomys, Lophuromys, and Deomys species (Muridae, Mammalia) in the forest blocks separated by The Congo River and its tributaries (Kisangani region, Democratic Republic of Congo). Mammalia 72, 223–228. [Google Scholar]
- Kennis, J. , Nicolas, V. , Hulselmans, J. , Katuala, P. G. B. , Wendelen, W. , Verheyen, E. , Dudu, A. M. & Leirs, H. (2011). The impact of The Congo River and its tributaries on the rodent genus Praomys: speciation origin or range expansion limit? Zoological Journal of the Linnean Society 163, 983–1002. [Google Scholar]
- Keppel, G. , Van Niel, K. P. , Wardell‐Johnson, G. W. , Yates, C. J. , Byrne, M. , Mucina, L. , Schut, A. G. T. , Hopper, S. D. & Franklin, S. E. (2011). Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecology and Biogeography 21, 393–404. [Google Scholar]
- Kissling, W. D. , Eiserhardt, W. L. , Baker, W. J. , Borchsenius, F. , Couvreur, T. L. P. , Balslev, H. & Svenning, J.‐C. (2012). Cenozoic imprints on the phylogenetic structure of palm species assemblages worldwide. Proceedings of the National Academy of Sciences of the United States of America 109, 7379–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerk, H. M. D. , Crowe, T. M. , Fjeldså, J. & Burgess, N. D. (2002). Biogeographical patterns of endemic terrestrial Afrotropical birds. Diversity and Distributions 8, 147–162. [Google Scholar]
- Klopper, R. R. , Gautier, L. , Chatelain, C. , Smith, G. F. & Spichiger, R. (2007). Floristics of the angiosperm Flora of sub‐Saharan Africa: an analysis of the African plant checklist and database. Taxon 56, 201–208. [Google Scholar]
- Knox, E. B. & Palmer, J. D. (1995). Chloroplast DNA variation and the recent radiation of the giant senecios (Asteraceae) on the tall mountains of eastern Africa. Proceedings of the National Academy of Sciences of the United States of America 92, 10349–10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen, E. J. M. , Clarkson, J. J. , Pennington, T. D. & Chatrou, L. W. (2015). Recently evolved diversity and convergent radiations of rainforest mahoganies (Meliaceae) shed new light on the origins of rainforest hyperdiversity. New Phytologist 207, 327–339. [DOI] [PubMed] [Google Scholar]
- Koenen, E. J. M. , Ojeda, D. I. , Bakker, F. T. , Wieringa, J. J. , Kidner, C. , Hardy, O. J. , Pennington, R. T. , Herendeen, P. S. , Bruneau, A. & Hughes, C. E. (2020). The origin of the legumes is a complex paleopolyploid phylogenomic tangle closely associated with the Cretaceous‐Paleogene (K‐Pg) mass extinction event. Systematic Biology syaa041 10.1093/sysbio/syaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyman, R. M. , Morley, R. J. , Crayn, D. M. , Joyce, E. M. , Rossetto, M. , Slik, J. W. F. , Strijk, J. S. , Su, T. , Yap, J.‐Y. S. & Wilf, P. (2019). Origins and assembly of Malesian rainforests. Annual Review of Ecology, Evolution, and Systematics 50, 119–143. [Google Scholar]
- Kraaijeveld, K. , Kraaijeveld‐Smit, F. J. L. & Maan, M. E. (2011). Sexual selection and speciation: the comparative evidence revisited. Biological Reviews 86, 367–377. [DOI] [PubMed] [Google Scholar]
- Krijgsman, W. , Capella, W. , Simon, D. , Hilgen, F. J. , Kouwenhoven, T. J. , Meijer, P. T. , Sierro, F. J. , Tulbure, M. A. , van den Berg, B. C. J. , van der Schee, M. & Flecker, R. (2018). The Gibraltar corridor: Watergate of the Messinian salinity crisis. Marine Geology 403, 238–246. [Google Scholar]
- Küper, W. , Sommer, J. H. , Lovett, J. C. , Mutke, J. , Linder, H. P. , Beentje, H. J. , Van Rompaey, R. , Chatelain, C. , Sosef, M. & Barthlott, W. (2004). Africa's hotspots of biodiversity redefined. Annals of the Missouri Botanical Garden 91, 525–535. [Google Scholar]
- Kürschner, W. M. , Kvaček, Z. & Dilcher, D. L. (2008). The impact of Miocene atmospheric carbon dioxide fluctuations on climate and the evolution of terrestrial ecosystems. Proceedings of the National Academy of Sciences of the United States of America 105, 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladant, J.‐B. , Donnadieu, Y. , Lefebvre, V. & Dumas, C. (2014). The respective role of atmospheric carbon dioxide and orbital parameters on ice sheet evolution at the Eocene‐Oligocene transition. Paleoceanography 29, 810–823. [Google Scholar]
- Larrasoaña, J. C. , Roberts, A. P. , Rohling, E. J. , Winklhofer, M. & Wehausen, R. (2003). Three million years of monsoon variability over the northern Sahara. Climate Dynamics 21, 689–698. [Google Scholar]
- Larsen, H. C. , Saunders, A. D. , Clift, P. D. , Beget, J. , Wei, W. & Spezzaferri, S. (1994). Seven million years of glaciation in Greenland. Science 264, 952–955. [DOI] [PubMed] [Google Scholar]
- Larson, T. R. , Castro, D. , Behangana, M. & Greenbaum, E. (2016). Evolutionary history of the river frog genus Amietia (Anura: Pyxicephalidae) reveals extensive diversification in central African highlands. Molecular Phylogenetics and Evolution 99, 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashermes, P. , Combes, M.‐C. , Robert, J. , Trouslot, P. , D'Hont, A. , Anthony, F. & Charrier, A. (1999). Molecular characterisation and origin of the Coffea arabica L. genome. Molecular and General Genetics 261, 259–266. [DOI] [PubMed] [Google Scholar]
- Lawson, L. P. , Bates, J. M. , Menegon, M. & Loader, S. P. (2015). Divergence at the edges: peripatric isolation in the montane spiny throated reed frog complex. BMC Evolutionary Biology 15, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaché, A. D. & Fujita, M. K. (2010). Bayesian species delimitation in west African forest geckos (Hemidactylus fasciatus). Proceedings of the Royal Society B: Biological Sciences 277, 3071–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaché, A. D. , Wagner, P. , Linkem, C. W. , Böhme, W. , Papenfuss, T. J. , Chong, R. A. , Lavin, B. R. , Bauer, A. M. , Nielsen, S. V. , Greenbaum, E. , Rödel, M.‐O. , Schmitz, A. , LeBreton, M. , Ineich, I. , Chirio, L. , Ofori‐Boateng, C. , Eniang, E. A. , Baha el Din, S. , Lemmon, A. R. & Burbrink, F. T. (2014). A hybrid phylogenetic–phylogenomic approach for species tree estimation in African Agama lizards with applications to biogeography, character evolution, and diversification. Molecular Phylogenetics and Evolution 79, 215–230. [DOI] [PubMed] [Google Scholar]
- Leaché, A. D. , Portik, D. M. , Rivera, D. , Rödel, M.‐O. , Penner, J. , Gvoždík, V. , Greenbaum, E. , Jongsma, G. F. M. , Ofori‐Boateng, C. , Burger, M. , Eniang, E. A. , Bell, R. C. & Fujita, M. K. (2019). Exploring rain forest diversification using demographic model testing in the African foam‐nest treefrog Chiromantis rufescens . Journal of Biogeography 46, 2706–2721. [Google Scholar]
- Leakey, M. , Grossman, A. , Gutiérrez, M. & Fleagle, J. G. (2011). Faunal change in the Turkana Basin during the late Oligocene and Miocene. Evolutionary Anthropology: Issues, News, and Reviews 20, 238–253. [DOI] [PubMed] [Google Scholar]
- Lear, C. H. & Lunt, D. J. (2016). How Antarctica got its ice. Science 352, 34–35. [DOI] [PubMed] [Google Scholar]
- Lecompte, E. , Aplin, K. , Denys, C. , Catzeflis, F. , Chades, M. & Chevret, P. (2008). Phylogeny and biogeography of African Murinae based on mitochondrial and nuclear gene sequences, with a new tribal classification of the subfamily. BMC Evolutionary Biology 8, 199 10.1186/1471-2148-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch, A. R. & Leitch, I. J. (2008). Genomic plasticity and the diversity of Polyploid plants. Science 320, 481–483. [DOI] [PubMed] [Google Scholar]
- Lévêque, C. , Oberdorff, T. , Paugy, D. , Stiassny, M. L. J. & Tedesco, P. A. (2007). Global diversity of fish (Pisces) in freshwater In Freshwater Animal Diversity Assessment. Developments in Hydrobiology (eds Balian E. V., Lévêque C., Segers H. and Martens K.), pp. 545–567. Springer, Dordrecht. [Google Scholar]
- Levinsky, I. , Araújo, M. B. , Nogués‐Bravo, D. , Haywood, A. M. , Valdes, P. J. & Rahbek, C. (2013). Climate envelope models suggest spatio‐temporal co‐occurrence of refugia of African birds and mammals. Global Ecology and Biogeography 22, 351–363. [Google Scholar]
- Ley, A. C. , Dauby, G. , Köhler, J. , Wypior, C. , Röser, M. & Hardy, O. J. (2014). Comparative phylogeography of eight herbs and lianas (Marantaceae) in central African rainforests. Frontiers in Genetics 5, 403 10.3389/fgene.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lézine, A.‐M. , Izumi, K. , Kageyama, M. & Achoundong, G. (2019). A 90,000‐year record of Afromontane forest responses to climate change. Science 363, 177–181. [DOI] [PubMed] [Google Scholar]
- Liddy, H. M. , Feakins, S. J. & Tierney, J. E. (2016). Cooling and drying in Northeast Africa across the Pliocene. Earth and Planetary Science Letters 449, 430–438. [Google Scholar]
- Liedtke, H. C. , Müller, H. , Rödel, M.‐O. , Menegon, M. , Gonwouo, L. N. , Barej, M. F. , Gvoždík, V. , Schmitz, A. , Channing, A. , Nagel, P. & Loader, S. P. (2016). No ecological opportunity signal on a continental scale? Diversification and life‐history evolution of African true toads (Anura: Bufonidae). Evolution 70, 1717–1733. [DOI] [PubMed] [Google Scholar]
- Linder, H. P. (2001). Plant diversity and endemism in sub‐Saharan tropical Africa. Journal of Biogeography 28, 169–182. [Google Scholar]
- Linder, H. P. (2003). The radiation of the cape flora, southern Africa. Biological Reviews 78, 597–638. [DOI] [PubMed] [Google Scholar]
- Linder, H. P. (2008). Plant species radiations: where, when, why? Philosophical Transactions of the Royal Society of London B: Biological Sciences 363, 3097–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder, H. P. (2014). The evolution of African plant diversity. Frontiers in Ecology and Evolution 2, 38 10.3389/fevo.2014.00038. [DOI] [Google Scholar]
- Linder, H. P. (2017). East African Cenozoic vegetation history. Evolutionary Anthropology: Issues, News, and Reviews 26, 300–312. [DOI] [PubMed] [Google Scholar]
- Linder, H. P. , Lovett, J. , Mutke, J. M. , Barthlott, W. , Jürgens, N. , Rebelo, T. & Küper, W. (2005). A numerical re‐evaluation of the sub‐Saharan phytochoria of mainland Africa. Biologiske Skrifter 55, 229–252. [Google Scholar]
- Linder, H. P. , de Klerk, H. M. , Born, J. , Burgess, N. D. , Fjeldså, J. & Rahbek, C. (2012). The partitioning of Africa: statistically defined biogeographical regions in sub‐Saharan Africa. Journal of Biogeography 39, 1189–1372. [Google Scholar]
- Linder, H. P. , Antonelli, A. , Humphreys, A. M. , Pirie, M. D. & Wüest, R. O. (2013). What determines biogeographical ranges? Historical wanderings and ecological constraints in the danthonioid grasses. Journal of Biogeography 40, 821–834. [Google Scholar]
- * Liu, X.‐Q. , Ickert‐Bond, S. M. , Chen, L.‐Q. & Wen, J. (2013). Molecular phylogeny of Cissus L. of Vitaceae (the grape family) and evolution of its pantropical intercontinental disjunctions. Molecular Phylogenetics and Evolution 66, 43–53. [DOI] [PubMed] [Google Scholar]
- Loader, S. P. , Pisani, D. , Cotton, J. A. , Gower, D. J. , Day, J. J. & Wilkinson, M. (2007). Relative time scales reveal multiple origins of parallel disjunct distributions of African caecilian amphibians. Biology Letters 3, 505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loader, S. P. , Ceccarelli, F. S. , Menegon, M. , Howell, K. M. , Kassahun, R. , Mengistu, A. A. , Saber, S. A. , Gebresenbet, F. , de Sá, R. , Davenport, T. R. B. , Larson, J. G. , Müller, H. , Wilkinson, M. & Gower, D. J. (2014). Persistence and stability of eastern Afromontane forests: evidence from brevicipitid frogs. Journal of Biogeography 41, 1781–1792. [Google Scholar]
- Lohman, D. J. , de Bruyn, M. , Page, T. , von Rintelen, K. , Hall, R. , Ng, P. K. L. , Shih, H.‐T. , Carvalho, G. R. & von Rintelen, T. (2011). Biogeography of the indo‐Australian archipelago. Annual Review of Ecology, Evolution, and Systematics 42, 205–226. [Google Scholar]
- Lorenzen, E. D. , Heller, R. & Siegismund, H. R. (2012). Comparative phylogeography of African savannah ungulates. Molecular Ecology 21, 3656–3670. [DOI] [PubMed] [Google Scholar]
- Losos, J. B. & Glor, R. E. (2003). Phylogenetic comparative methods and the geography of speciation. Trends in Ecology & Evolution 18, 220–227. [Google Scholar]
- Louca, S. & Pennell, M. W. (2020). Extant timetrees are consistent with a myriad of diversification histories. Nature 580, 502–505. [DOI] [PubMed] [Google Scholar]
- Louette, M. (1992). Barriers, contact zones and subspeciation in central equatorial Africa. Bulletin of the British Ornithologists’ Club 122A, 209–216. [Google Scholar]
- Lovett, J. C. (1993). Eastern arc moist forest flora In Biogeography and Ecology of the Rain Forests of Eastern Africa (eds Lovett J. C. and Wasser S. K.), pp. 33–55. Cambridge University Press, Cambridge. [Google Scholar]
- Lovett, J. C. , Marchant, R. , Taplin, J. R. D. & Küper, W. (2005). The oldest rainforests in Africa: stability or resilience for survival and diversity? In Phylogeny and Conservation (eds Purvis A. and Gittleman J. L.), pp. 198–229. Cambridge University Press, Cambridge. [Google Scholar]
- * Lu, L. , Wang, W. , Chen, Z. & Wen, J. (2013). Phylogeny of the non‐monophyletic Cayratia Juss. (Vitaceae) and implications for character evolution and biogeography. Molecular Phylogenetics and Evolution 68, 502–515. [DOI] [PubMed] [Google Scholar]
- Luger, P. (2003). Paleobiogeography of late early cretaceous to early Paleocene marine Ostracoda in Arabia and north to equatorial Africa. Palaeogeography, Palaeoclimatology, Palaeoecology 196, 319–342. [Google Scholar]
- Lunt, D. J. , Dunkley Jones, T. , Heinemann, M. , Huber, M. , LeGrande, A. , Winguth, A. , Loptson, C. , Marotzke, J. , Roberts, C. D. , Tindall, J. , Valdes, P. & Winguth, C. (2012). A model–data comparison for a multi‐model ensemble of early Eocene atmosphere–ocean simulations: EoMIP. Climate of the Past 8, 1717–1736. [Google Scholar]
- Macgregor, D. (2015). History of the development of the east African rift system: a series of interpreted maps through time. Journal of African Earth Sciences 101, 232–252. [Google Scholar]
- Mairal, M. , Pokorny, L. , Aldasoro, J. J. , Alarcón, M. & Sanmartín, I. (2015). Ancient vicariance and climate‐driven extinction explain continental‐wide disjunctions in Africa: the case of the Rand Flora genus Canarina (Campanulaceae). Molecular Ecology 24, 1335–1354. [DOI] [PubMed] [Google Scholar]
- Mairal, M. , Sanmartín, I. & Pellissier, L. (2017). Lineage‐specific climatic niche drives the tempo of vicariance in the Rand Flora. Journal of Biogeography 44, 911–923. [Google Scholar]
- Maley, J. (1996). The African Rain forest main characteristics of changes in vegetation and climate from the upper Cretaceous to the Quaternary In Essays on the ecology of the Guinea ‐ Congo rain forest (eds Alexander I., Swaine M. D. and Watling R.), pp. 31–37. Royal Society of Edinburgh Proceedings, Edinburgh. [Google Scholar]
- Marzoli, A. , Piccirillo, E. M. , Renne, P. R. , Bellieni, G. , Iacumin, M. , Nyobe, J. B. & Tongwa, A. T. (2000). The Cameroon volcanic line revisited: Petrogenesis of continental basaltic magmas from lithospheric and Asthenospheric mantle sources. Journal of Petrology 41, 87–109. [Google Scholar]
- Maslin, M. A. , Brierley, C. M. , Milner, A. M. , Shultz, S. , Trauth, M. H. & Wilson, K. E. (2014). East African climate pulses and early human evolution. Quaternary Science Reviews 101, 1–17. [Google Scholar]
- Matthee, C. A. & Davis, S. K. (2001). Molecular insights into the evolution of the family Bovidae: a nuclear DNA perspective. Molecular Biology and Evolution 18, 1220–1230. [DOI] [PubMed] [Google Scholar]
- Matthee, C. A. , Tilbury, C. R. & Townsend, T. (2004). A phylogenetic review of the African leaf chameleons: genus Rhampholeon (Chamaeleonidae): the role of vicariance and climate change in speciation. Proceedings of the Royal Society of London B: Biological Sciences 271, 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Maurin, O. , Muasya, A. M. , Catalan, P. , Shongwe, E. Z. , Viruel, J. , Wilkin, P. & van der Bank, M. (2016). Diversification into novel habitats in the Africa clade of Dioscorea (Dioscoreaceae): erect habit and elephant's foot tubers. BMC Evolutionary Biology 16, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, G. (2009). Paleogene Fossil Birds. Springer, Berlin. [Google Scholar]
- Mayr, E. & O'Hara, R. J. (1986). The biogeographic evidence supporting the Pleistocene forest refuge hypothesis. Evolution 40, 55–67. [DOI] [PubMed] [Google Scholar]
- McClean, C. J. , Lovett, J. C. , Küper, W. , Hannah, L. , Sommer, J. H. , Barthlott, W. , Termansen, M. , Smith, G. E. , Tokamine, S. & Taplin, J. R. D. (2005). African plant diversity and climate change. Annals of the Missouri Botanical Garden 92, 139–152. [Google Scholar]
- McElwain, J. C. & Punyasena, S. W. (2007). Mass extinction events and the plant fossil record. Trends in Ecology & Evolution 22, 548–557. [DOI] [PubMed] [Google Scholar]
- Medina, M. F. , Bauer, A. M. , Branch, W. R. , Schmitz, A. , Conradie, W. , Nagy, Z. T. , Hibbitts, T. J. , Ernst, R. , Portik, D. M. , Nielsen, S. V. , Colston, T. J. , Kusamba, C. , Behangana, M. , Rödel, M.‐O. & Greenbaum, E. (2016). Molecular phylogeny of Panaspis and Afroablepharus skinks (Squamata: Scincidae) in the savannas of sub‐Saharan Africa. Molecular Phylogenetics and Evolution 100, 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon, M. , Loader, S. P. , Marsden, S. J. , Branch, W. R. , Davenport, T. R. B. & Ursenbacher, S. (2014). The genus Atheris (Serpentes: Viperidae) in East Africa: phylogeny and the role of rifting and climate in shaping the current pattern of species diversity. Molecular Phylogenetics and Evolution 79, 12–22. [DOI] [PubMed] [Google Scholar]
- * Merckx, V. , Chatrou, L. W. , Lemaire, B. , Sainge, M. N. , Huysmans, S. & Smets, E. F. (2008). Diversification of myco‐heterotrophic angiosperms: evidence from Burmanniaceae. BMC Evolutionary Biology 8, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Merckx, V. , Bakker, F. T. , Huysmans, S. & Smets, E. (2009). Bias and conflict in phylogenetic inference of myco‐heterotrophic plants: a case study in Thismiaceae. Cladistics 25, 64–77. [DOI] [PubMed] [Google Scholar]
- Meredith, R. W. , Janečka, J. E. , Gatesy, J. , Ryder, O. A. , Fisher, C. A. , Teeling, E. C. , Goodbla, A. , Eizirik, E. , Simão, T. L. L. , Stadler, T. , Rabosky, D. L. , Honeycutt, R. L. , Flynn, J. J. , Ingram, C. M. , Steiner, C. , et al. (2011). Impacts of the cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334, 521–524. [DOI] [PubMed] [Google Scholar]
- Migliore, J. , Kaymak, E. , Mariac, C. , Couvreur, T. L. P. , Lissambou, B.‐J. , Piñeiro, R. & Hardy, O. J. (2019). Pre‐Pleistocene origin of phylogeographical breaks in African rain forest trees: New insights from Greenwayodendron (Annonaceae) phylogenomics. Journal of Biogeography 46, 212–223. [Google Scholar]
- * Mikula, O. , Šumbera, R. , Aghová, T. , Mbau, J. S. , Katakweba, A. S. , Sabuni, C. A. & Bryja, J. (2016). Evolutionary history and species diversity of African pouched mice (Rodentia: Nesomyidae: Saccostomus). Zoologica Scripta 45, 595–617. [Google Scholar]
- Miller, K. G. , Kominz, M. A. , Browning, J. V. , Wright, J. D. , Mountain, G. S. , Katz, M. E. , Sugarman, P. J. , Cramer, B. S. , Christie‐Blick, N. & Pekar, S. F. (2005). The Phanerozoic record of Global Sea‐level change. Science 310, 1293–1298. [DOI] [PubMed] [Google Scholar]
- Missoup, A. D. , Nicolas, V. , Wendelen, W. , Keming, E. , Bilong, C. F. B. , Couloux, A. , Atanga, E. , Hutterer, R. & Denys, C. (2012). Systematics and diversification of Praomys species (Rodentia: Muridae) endemic to the Cameroon volcanic line (west Central Africa). Zoologica Scripta 41, 327–345. [Google Scholar]
- Missoup, A. D. , Nicolas, V. , Eiseb, S. , Chung, E. K. & Denys, C. (2016). Phylogenetic position of the endemic mount Oku rat, Lamottemys okuensis (Rodentia: Muridae), based on molecular and morphological data. Zoological Journal of the Linnean Society 177, 209–226. [Google Scholar]
- Mittermeier, R. A. , Turner, W. R. , Larsen, F. W. , Brooks, T. M. & Gascon, C. (2011). Global biodiversity conservation: the critical role of hotspots In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas (eds Zachos F. E. and Habel J. C.), pp. 3–22. Springer, Heidelberg. [Google Scholar]
- Mizerovská, D. , Nicolas, V. , Demos, T. C. , Akaibe, D. , Colyn, M. , Denys, C. , Kaleme, P. K. , Katuala, P. , Kennis, J. , Kerbis Peterhans, J. C. , Laudisoit, A. , Missoup, A. D. , Šumbera, R. , Verheyen, E. & Bryja, J. (2019). Genetic variation of the most abundant forest‐dwelling rodents in Central Africa (Praomys jacksoni complex): evidence for Pleistocene refugia in both montane and lowland forests. Journal of Biogeography 46, 1466–1478. [Google Scholar]
- Monthe, F. K. , Migliore, J. , Duminil, J. , Bouka, G. , Demenou, B. B. , Doumenge, C. , Blanc‐Jolivet, C. , Ekué, M. R. M. & Hardy, O. J. (2019). Phylogenetic relationships in two African Cedreloideae tree genera (Meliaceae) reveal multiple rain/dry forest transitions. Perspectives in Plant Ecology, Evolution and Systematics 37, 1–10. [Google Scholar]
- Moreau, R. E. (1966). The Bird Faunas of Africa and its Islands. Academic Press, London. [Google Scholar]
- Moritz, C. , Patton, J. L. , Schneider, C. J. & Smith, T. B. (2000). Diversification of rainforest faunas: an integrated molecular approach. Annual Review of Ecology and Systematics 31, 533–563. [Google Scholar]
- Morley, R. J. (2000). Origin and Evolution of Tropical Rain Forests. John Wiley & Sons, New York. [Google Scholar]
- Morley, R. J. (2007). Cretaceous and tertiary climate change and the past distribution of megathermal rainforests In Tropical Rainforest Responses to Climatic Changes (eds Bush M. B. and Flenley J.), pp. 1–31. Praxis Publishing, Chichester. [Google Scholar]
- Morley, R. J. (2011). Dispersal and paleoecology of tropical podocarps In Ecology of the Podocarpaceae in Tropical Forests (eds Turner B. L. and Cernusak L. A.), pp. 21–41. Smithsonian Institution Scholarly Press, Washington, DC. [Google Scholar]
- Morley, R. J. & Richards, K. (1993). Gramineae cuticle: a key indicator of late Cenozoic climatic change in The Niger Delta. Review of Palaeobotany and Palynology 77, 119–127. [Google Scholar]
- Morlon, H. (2014). Phylogenetic approaches for studying diversification. Ecology Letters 17, 508–525. [DOI] [PubMed] [Google Scholar]
- Mosbrugger, V. , Favre, A. , Muellner‐Riehl, A. N. , Päckert, M. & Mulch, A. (2018). Cenozoic evolution of geo–biodiversity in the Tibeto–Himalayan region In Mountains, Climate, and Biodiversity (eds Hoorn C., Antonelli A. and Perrigo A.), pp. 429–448. Wiley‐Blackwell, Hoboken. [Google Scholar]
- Muellner, A. N. , Savolainen, V. , Samuel, R. & Chase, M. W. (2006). The mahogany family ‘out‐of‐Africa’: divergence time estimation, global biogeographic patterns inferred from plastid rbcL DNA sequences, extant, and fossil distribution of diversity. Molecular Phylogenetics and Evolution 40, 236–250. [DOI] [PubMed] [Google Scholar]
- Muellner‐Riehl, A. N. (2019). Mountains as evolutionary arenas: patterns, emerging approaches, paradigm shifts, and their implications for plant Phylogeographic research in the Tibeto‐Himalayan region. Frontiers in Plant Science 10, 195 10.3389/fpls.2019.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellner‐Riehl, A. N. , Schnitzler, J. , Kissling, W. D. , Mosbrugger, V. , Rijsdijk, K. F. , Seijmonsbergen, A. C. , Versteegh, H. & Favre, A. (2019). Origins of global mountain plant biodiversity: testing the ‘mountain‐geobiodiversity hypothesis’. Journal of Biogeography 46, 2826–2838. [Google Scholar]
- Muhongo, S. & Lenoir, J.‐L. (1994). Pan‐African granulite‐facies metamorphism in the Mozambique Belt of Tanzania: U‐Pb zircon geochronology. Journal of the Geological Society 151, 343–347. [Google Scholar]
- Muhongo, S. , Kröner, A. & Nemchin, A. A. (2001). Single zircon evaporation and SHRIMP ages for granulite‐facies rocks in the Mozambique Belt of Tanzania. The Journal of Geology 109, 171–189. [Google Scholar]
- Muloko‐Ntoutoume, N. , Petit, R. J. , White, L. & Abernethy, K. (2000). Chloroplast DNA variation in a rainforest tree (Aucoumea klaineana, Burseraceae) in Gabon. Molecular Ecology 9, 359–363. [DOI] [PubMed] [Google Scholar]
- Murphy, L. N. , Kirk‐Davidoff, D. B. , Mahowald, N. & Otto‐Bliesner, B. L. (2009). A numerical study of the climate response to lowered Mediterranean Sea level during the Messinian salinity crisis. Palaeogeography, Palaeoclimatology, Palaeoecology 279, 41–59. [Google Scholar]
- Murray, A. M. (2000). The Palaeozoic, Mesozoic and early Cenozoic fishes of Africa. Fish and Fisheries 1, 111–145. [Google Scholar]
- Myers, N. , Mittermeier, R. A. , Mittermeier, C. G. , da Fonseca, G. A. B. & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. [DOI] [PubMed] [Google Scholar]
- Nesi, N. , Kadjo, B. , Pourrut, X. , Leroy, E. , Pongombo Shongo, C. , Cruaud, C. & Hassanin, A. (2013). Molecular systematics and phylogeography of the tribe Myonycterini (Mammalia, Pteropodidae) inferred from mitochondrial and nuclear markers. Molecular Phylogenetics and Evolution 66, 126–137. [DOI] [PubMed] [Google Scholar]
- New, M. , Lister, D. , Hulme, M. & Makin, I. (2002). A high‐resolution data set of surface climate over global land areas. Climate Research 21, 1–25. [Google Scholar]
- Newmark, W. D. (2002). Conserving Biodiversity in East African Forests: A Study of the Eastern Arc Mountains. Springer, New York. [Google Scholar]
- Nichols, D. J. & Johnson, K. R. (2008). Plants and the K‐T Boundary. Cambridge University Press, Cambridge. [Google Scholar]
- Nicolas, V. , Quérouil, S. , Verheyen, E. , Verheyen, W. , Mboumba, J. F. , Dillen, M. & Colyn, M. (2006). Mitochondrial phylogeny of African wood mice, genus Hylomyscus (Rodentia, Muridae): implications for their taxonomy and biogeography. Molecular Phylogenetics and Evolution 38, 779–793. [DOI] [PubMed] [Google Scholar]
- Nicolas, V. , Akpatou, B. , Wendelen, W. , Kerbis Peterhans, J. , Olayemi, A. , Decher, J. , Missoup, A.‐D. , Denys, C. , Barriere, P. , Cruaud, C. & Colyn, M. (2010). Molecular and morphometric variation in two sibling species of the genus Praomys (Rodentia: Muridae): implications for biogeography. Zoological Journal of the Linnean Society 160, 397–419. [Google Scholar]
- Nicolas, V. , Missoup, A. D. , Denys, C. , Peterhans, J. K. , Katuala, P. , Couloux, A. & Colyn, M. (2011). The roles of rivers and Pleistocene refugia in shaping genetic diversity in Praomys misonnei in tropical Africa. Journal of Biogeography 38, 191–207. [Google Scholar]
- Nicolas, V. , Missoup, A.‐D. , Colyn, M. , Cruaud, C. , Denys, C. & Jansen van Vuuren, B. (2012). West‐central African Pleistocene lowland forest evolution revealed by the phylogeography of Misonne's soft‐furred mouse. African Zoology 47, 100–112. [Google Scholar]
- Nicolas, V. , Jacquet, F. , Hutterer, R. , Konečný, A. , Kan Kouassi, S. , Durnez, L. , Lalis, A. , Colyn, M. & Denys, C. (2019). Multilocus phylogeny of the Crocidura poensis species complex (Mammalia, Eulipotyphla): influences of the palaeoclimate on its diversification and evolution. Journal of Biogeography 46, 871–883. [Google Scholar]
- Nicolas, V. , Fabre, P.‐H. , Bryja, J. , Denys, C. , Verheyen, E. , Missoup, A.‐D. , Olayemi, A. , Katuala, P. , Dudu, A. & Colyn, M. (2020). The phylogeny of the African wood mice (Muridae, Hylomyscus) based on complete mitochondrial genomes and five nuclear genes reveals their evolutionary history and undescribed diversity. Molecular Phylogenetics and Evolution 144, 106703 10.1016/j.ympev.2019.106703. [DOI] [PubMed] [Google Scholar]
- * Nie, Z.‐L. , Sun, H. , Manchester, S. R. , Meng, Y. , Luke, Q. & Wen, J. (2012). Evolution of the intercontinental disjunctions in six continents in the Ampelopsis clade of the grape family (Vitaceae). BMC Evolutionary Biology 12, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njabo, K. Y. , Bowie, R. C. K. & Sorenson, M. D. (2008). Phylogeny, biogeography and taxonomy of the African wattle‐eyes (Aves: Passeriformes: Platysteiridae). Molecular Phylogenetics and Evolution 48, 136–149. [DOI] [PubMed] [Google Scholar]
- Nosil, P. & Flaxman, S. M. (2011). Conditions for mutation‐order speciation. Proceedings of the Royal Society B: Biological Sciences 278, 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novello, A. , Barboni, D. , Sylvestre, F. , Lebatard, A.‐E. , Paillès, C. , Bourlès, D. L. , Likius, A. , Mackaye, H. T. , Vignaud, P. & Brunet, M. (2017). Phytoliths indicate significant arboreal cover at Sahelanthropus type locality TM266 in northern Chad and a decrease in later sites. Journal of Human Evolution 106, 66–83. [DOI] [PubMed] [Google Scholar]
- Odee, D. W. , Telford, A. , Wilson, J. , Gaye, A. & Cavers, S. (2012). Plio‐Pleistocene history and phylogeography of Acacia Senegal in dry woodlands and savannahs of sub‐Saharan tropical Africa: evidence of early colonisation and recent range expansion. Heredity 109, 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda, D. I. , Koenen, E. , Cervantes, S. , de la Estrella, M. , Banguera‐Hinestroza, E. , Janssens, S. B. , Migliore, J. , Demenou, B. B. , Bruneau, A. , Forest, F. & Hardy, O. J. (2019). Phylogenomic analyses reveal an exceptionally high number of evolutionary shifts in a florally diverse clade of African legumes. Molecular Phylogenetics and Evolution 137, 156–167. [DOI] [PubMed] [Google Scholar]
- Olayemi, A. , Nicolas, V. , Hulselmans, J. , Missoup, A. D. , Fichet‐Calvet, E. , Amundala, D. , Dudu, A. , Dierckx, T. , Wendelen, W. , Leirs, H. & Verheyen, E. (2012). Taxonomy of the African giant pouched rats (Nesomyidae: Cricetomys): molecular and craniometric evidence support an unexpected high species diversity. Zoological Journal of the Linnean Society 165, 700–719. [Google Scholar]
- O'Leary, M. A. , Bloch, J. I. , Flynn, J. J. , Gaudin, T. J. , Giallombardo, A. , Giannini, N. P. , Goldberg, S. L. , Kraatz, B. P. , Luo, Z.‐X. , Meng, J. , Ni, X. , Novacek, M. J. , Perini, F. A. , Randall, Z. S. , Rougier, G. W. , Sargis, E. J. , Silcox, M. T. , Simmons, N. B. , Spaulding, M. , Velazco, P. M. , Weksler, M. , Wible, J. R. & Cirranello, A. L. (2013). The placental mammal ancestor and the post–K‐Pg radiation of placentals. Science 339, 662–667. [DOI] [PubMed] [Google Scholar]
- Orr, M. R. & Smith, T. B. (1998). Ecology and speciation. Trends in Ecology & Evolution 13, 502–506. [DOI] [PubMed] [Google Scholar]
- Pan, A. D. , Jacobs, B. F. , Dransfield, J. & Baker, W. J. (2006). The fossil history of palms (Arecaceae) in Africa and new records from the late Oligocene (28‐27 Mya) of North‐Western Ethiopia. Botanical Journal of the Linnean Society 151, 69–81. [Google Scholar]
- Penner, J. , Wegmann, M. , Hillers, A. , Schmidt, M. & Rödel, M.‐O. (2011). A hotspot revisited–a biogeographical analysis of west African amphibians. Diversity and Distributions 17, 1077–1088. [Google Scholar]
- Pettigrew, J. D. , Bell, K. L. , Bhagwandin, A. , Grinan, E. , Jillani, N. , Meyer, J. , Wabuyele, E. & Vickers, C. E. (2012). Morphology, ploidy and molecular phylogenetics reveal a new diploid species from Africa in the baobab genus Adansonia (Malvaceae: Bombacoideae). Taxon 61, 1240–1250. [Google Scholar]
- Pickford, M. , Senut, B. & Mourer‐Chauviré, C. (2004). Early Pliocene Tragulidae and peafowls in the Rift Valley, Kenya: evidence for rainforest in East Africa. Comptes Rendus Palevol 3, 179–189. [Google Scholar]
- Plana, V. (2004). Mechanisms and tempo of evolution in the African Guineo‐Congolian rainforest. Philosophical Transactions of the Royal Society B‐Biological Sciences 359, 1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plana, V. , Gascoigne, A. , Forrest, L. L. , Harris, D. & Pennington, R. T. (2004). Pleistocene and pre‐Pleistocene Begonia speciation in Africa. Molecular Phylogenetics and Evolution 31, 449–461. [DOI] [PubMed] [Google Scholar]
- Pokorny, L. , Riina, R. , Mairal, M. , Meseguer, A. S. , Culshaw, V. , Cendoya, J. , Serrano, M. , Carbajal, R. , Ortiz, S. , Heuertz, M. & Sanmartín, I. (2015). Living on the edge: timing of Rand Flora disjunctions congruent with ongoing aridification in Africa. Frontiers in Genetics 6, 154 10.3389/fgene.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polissar, P. J. , Rose, C. , Uno, K. T. , Phelps, S. R. & deMenocal, P. (2019). Synchronous rise of African C4 ecosystems 10 million years ago in the absence of aridification. Nature Geoscience 12, 657–660. [Google Scholar]
- Portik, D. M. & Blackburn, D. C. (2016). The evolution of reproductive diversity in Afrobatrachia: a phylogenetic comparative analysis of an extensive radiation of African frogs. Evolution 70, 2017–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portik, D. M. , Leaché, A. D. , Rivera, D. , Barej, M. F. , Burger, M. , Hirschfeld, M. , Rödel, M.‐O. , Blackburn, D. C. & Fujita, M. K. (2017). Evaluating mechanisms of diversification in a Guineo‐Congolian tropical forest frog using demographic model selection. Molecular Ecology 26, 5245–5263. [DOI] [PubMed] [Google Scholar]
- Portik, D. M. , Bell, R. C. , Blackburn, D. C. , Bauer, A. M. , Barratt, C. D. , Branch, W. R. , Burger, M. , Channing, A. , Colston, T. J. , Conradie, W. , Dehling, J. M. , Drewes, R. C. , Ernst, R. , Greenbaum, E. , Gvoždík, V. , Harvey, J. , Hillers, A. , Hirschfeld, M. , Jongsma, G. F. M. , Kielgast, J. , Kouete, M. T. , Lawson, L. P. , Leaché, A. D. , Loader, S. P. , Lötters, S. , Meijden, A. V. D. , Menegon, M. , Müller, S. , Nagy, Z. T. , Ofori‐Boateng, C. , Ohler, A. , Papenfuss, T. J. , Rößler, D. , Sinsch, U. , Rödel, M. O. , Veith, M. , Vindum, J. , Zassi‐Boulou, A. G. & McGuire, J. A. (2019). Sexual dichromatism drives diversification within a major radiation of African amphibians. Systematic Biology 68, 859–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo, F. , Branch, W. R. , Conradie, W. , Rödel, M.‐O. , Penner, J. , Barej, M. F. , Kusamba, C. , Muninga, W. M. , Aristote, M. M. , Bauer, A. M. , Trape, J.‐F. , Nagy, Z. T. , Carlino, P. , Pauwels, O. S. G. , Menegon, M. , Burger, M. , Mazuch, T. , Jackson, K. , Hughes, D. F. , Behangana, M. , Zassi‐Boulou, A. G. & Greenbaum, E. (2018). Phylogeny and biogeography of the African burrowing snake subfamily Aparallactinae (Squamata: Lamprophiidae). Molecular Phylogenetics and Evolution 127, 288–303. [DOI] [PubMed] [Google Scholar]
- Pound, M. J. & Salzmann, U. (2017). Heterogeneity in global vegetation and terrestrial climate change during the late Eocene to early Oligocene transition. Scientific Reports 7, 43386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound, M. J. , Haywood, A. M. , Salzmann, U. , Riding, J. B. , Lunt, D. J. & Hunter, S. J. (2011). A Tortonian (late Miocene, 11.61–7.25 ma) global vegetation reconstruction. Palaeogeography, Palaeoclimatology, Palaeoecology 300, 29–45. [Google Scholar]
- Pozzi, L. (2016). The role of forest expansion and contraction in species diversification among galagos (Primates: Galagidae). Journal of Biogeography 43, 1930–1941. [Google Scholar]
- Pozzi, L. , Disotell, T. R. & Masters, J. C. (2014). A multilocus phylogeny reveals deep lineages within African galagids (Primates: Galagidae). BMC Evolutionary Biology 14, 72 10.1186/1471-2148-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prave, A. R. , Bates, C. R. , Donaldson, C. H. , Toland, H. , Condon, D. J. , Mark, D. & Raub, T. D. (2016). Geology and geochronology of the Tana Basin, Ethiopia: LIP volcanism, super eruptions and Eocene–Oligocene environmental change. Earth and Planetary Science Letters 443, 1–8. [Google Scholar]
- Prömmel, K. , Cubasch, U. & Kaspar, F. (2013). A regional climate model study of the impact of tectonic and orbital forcing on African precipitation and vegetation. Palaeogeography, Palaeoclimatology, Palaeoecology 369, 154–162. [Google Scholar]
- Quiroga, M. P. , Mathiasen, P. , Iglesias, A. , Mill, R. R. & Premoli, A. C. (2016). Molecular and fossil evidence disentangle the biogeographical history of Podocarpus, a key genus in plant geography. Journal of Biogeography 43, 372–383. [Google Scholar]
- Rahbek, C. , Borregaard, M. K. , Antonelli, A. , Colwell, R. K. , Holt, B. G. , Nogues‐Bravo, D. , Rasmussen, C. M. Ø. , Richardson, K. , Rosing, M. T. , Whittaker, R. J. & Fjeldså, J. (2019). Building mountain biodiversity: geological and evolutionary processes. Science 365, 1114–1119. [DOI] [PubMed] [Google Scholar]
- Ravelo, A. C. , Andreasen, D. H. , Lyle, M. , Lyle, A. O. & Wara, M. W. (2004). Regional climate shifts caused by gradual global cooling in the Pliocene epoch. Nature 429, 263–267. [DOI] [PubMed] [Google Scholar]
- Retallack, G. J. , Dugas, D. P. & Bestland, E. A. (1990). Fossil soils and grasses of a middle Miocene east‐African grassland. Science 247, 1325–1328. [DOI] [PubMed] [Google Scholar]
- Richards, P. W. (1973). Africa, the ‘odd man out’ In Tropical Forest Ecosystems of Africa and South America: A Comparative Review (eds Meggers B. J., Ayensu E. S. and Duckworth W. D.), pp. 21–26. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- Ring, U. , Albrecht, C. & Schrenk, F. (2018). The east African rift system: tectonics, climate and biodiversity In Mountains, Climate and Biodiversity (eds Hoorn M. C., Perrigo A. and Antonelli A.), pp. 391–406. Wiley‐Blackwell, John Wiley & Sons, Hoboken. [Google Scholar]
- Robbrecht, E. (1996). Geography of African Rubiaceae with reference to glacial rain forest refuges In The Biodiversity of African Plants, pp. 564–581. Springer, New York. [Google Scholar]
- Robert, C. & Chamley, H. (1987). Cenozoic evolution of continental humidity and paleoenvironment, deduced from the kaolinite content of oceanic sediments. Palaeogeography, Palaeoclimatology, Palaeoecology 60, 171–187. [Google Scholar]
- Roberts, E. M. , Stevens, N. J. , O'Connor, P. M. , Dirks, P. H. G. M. , Gottfried, M. D. , Clyde, W. C. , Armstrong, R. A. , Kemp, A. I. S. & Hemming, S. (2012). Initiation of the western branch of the east African rift coeval with the eastern branch. Nature Geoscience 5, 289–294. [Google Scholar]
- Rohland, N. , Reich, D. , Mallick, S. , Meyer, M. , Green, R. E. , Georgiadis, N. J. , Roca, A. L. & Hofreiter, M. (2010). Genomic DNA sequences from mastodon and woolly mammoth reveal deep speciation of forest and savanna elephants. PLoS Biology 8, e1000564 10.1371/journal.pbio.1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, C. , Polissar, P. J. , Tierney, J. E. , Filley, T. & deMenocal, P. B. (2016). Changes in northeast African hydrology and vegetation associated with Pliocene–Pleistocene sapropel cycles. Philosophical Transactions of the Royal Society B: Biological Sciences 371, 20150243 10.1098/rstb.2015.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell, R. J. & Price, T. D. (2009). Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends in Ecology & Evolution 24, 394–399. [DOI] [PubMed] [Google Scholar]
- Rundle, H. D. & Nosil, P. (2005). Ecological speciation. Ecology Letters 8, 336–352. [Google Scholar]
- Sagoo, N. , Valdes, P. , Flecker, R. & Gregoire, L. J. (2013). The early Eocene equable climate problem: can perturbations of climate model parameters identify possible solutions? Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 371, 20130123 10.1098/rsta.2013.0123. [DOI] [PubMed] [Google Scholar]
- * Sahoo, R. K. , Lohman, D. J. , Wahlberg, N. , Müller, C. J. , Brattström, O. , Collins, S. C. , Peggie, D. , Aduse‐Poku, K. & Kodandaramaiah, U. (2018). Evolution of Hypolimnas butterflies (Nymphalidae): out‐of‐Africa origin and Wolbachia‐mediated introgression. Molecular Phylogenetics and Evolution 123, 50–58. [DOI] [PubMed] [Google Scholar]
- Salard‐Cheboldaeff, M. (1979). Palynologie maestrichtienne et tertiaire du Cameroun. Etude qualitative et repartition verticale des principales especes. Review of Palaeobotany and Palynology 28, 365–388. [Google Scholar]
- Salard‐Cheboldaeff, M. (1990). Intertropical African palynostratigraphy from cretaceous to late quaternary times. Journal of African Earth Sciences and the Middle East 11, 1IN1–24IN6. [Google Scholar]
- Salzburger, W. (2018). Understanding explosive diversification through cichlid fish genomics. Nature Reviews Genetics 19, 705–717. [DOI] [PubMed] [Google Scholar]
- Salzburger, W. , Van Bocxlaer, B. & Cohen, A. S. (2014). Ecology and evolution of the African Great Lakes and their faunas. Annual Review of Ecology, Evolution, and Systematics 45, 519–545. [Google Scholar]
- Salzmann, U. & Hoelzmann, P. (2005). The Dahomey gap: an abrupt climatically induced rain forest fragmentation in West Africa during the late Holocene. The Holocene 15, 190–199. [Google Scholar]
- Sanmartín, I. & Meseguer, A. S. (2016). Extinction in phylogenetics and biogeography: from timetrees to patterns of biotic assemblage. Frontiers in Genetics 7, 35 10.3389/fgene.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartín, I. , Anderson, C. L. , Alarcon, M. , Ronquist, F. & Aldasoro, J. J. (2010). Bayesian Island biogeography in a continental setting: the Rand Flora case. Biology Letters 6, 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauquet, H. (2013). A practical guide to molecular dating. Comptes Rendus Palevol 12, 355–367. [Google Scholar]
- Sayre, R. G. , Comer, P. , Hak, J. , Josse, C. , Bow, J. , Warner, H. , Larwanou, M. , Kelbessa, E. , Bekele, T. , Kehl, H. , Amena, R. , Andriamasimanana, R. , Ba, T. , Benson, L. , Boucher, T. , et al. (2013). A new map of standardized terrestrial ecosystems of Africa. African Geographical Review, 32(S1), 1–24. [Google Scholar]
- * Schaefer, H. & Renner, S. S. (2010). A three‐genome phylogeny of Momordica (Cucurbitaceae) suggests seven returns from dioecy to monoecy and recent long‐distance dispersal to Asia. Molecular Phylogenetics and Evolution 54, 553–560. [DOI] [PubMed] [Google Scholar]
- Schluter, D. (1998). Ecological causes of speciation In Endless Forms: Species and Speciation, pp. 3–15. Oxford University Press, Oxford. [Google Scholar]
- Schluter, D. (2000). The Ecology of Adaptive Radiation. Oxford University Press, Oxford. [Google Scholar]
- Schoene, B. , Samperton, K. M. , Eddy, M. P. , Keller, G. , Adatte, T. , Bowring, S. A. , Khadri, S. F. R. & Gertsch, B. (2015). U‐Pb geochronology of the Deccan traps and relation to the end‐cretaceous mass extinction. Science 347, 182–184. [DOI] [PubMed] [Google Scholar]
- Schoenfelder, K. P. & Fox, D. T. (2015). The expanding implications of polyploidy. Journal of Cell Biology 209, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte, P. , Alegret, L. , Arenillas, I. , Arz, J. A. , Barton, P. J. , Bown, P. R. , Bralower, T. J. , Christeson, G. L. , Claeys, P. , Cockell, C. S. , Collins, G. S. , Deutsch, A. , Goldin, T. J. , Goto, K. , Grajales‐Nishimura, J. M. , Grieve, R. A. F. , Gulick, S. P. S. , Johnson, K. R. , Kiessling, W. , Koeberl, C. , Kring, D. A. , MacLeod, K. G. , Matsui, T. , Melosh, J. , Montanari, A. , Morgan, J. V. , Neal, C. R. , Nichols, D. J. , Norris, R. D. , Pierazzo, E. , Ravizza, G. , Rebolledo‐Vieyra, M. , Reimold, W. U. , Robin, E. , Salge, T. , Speijer, R. P. , Sweet, A. R. , Urrutia‐Fucugauchi, J. , Vajda, V. , Whalen, M. T. & Willumsen, P. S. (2010). The Chicxulub asteroid impact and mass extinction at the cretaceous‐Paleogene boundary. Science 327, 1214–1218. [DOI] [PubMed] [Google Scholar]
- Schuster, M. , Duringer, P. , Ghienne, J.‐F. , Vignaud, P. , Mackaye, H. T. , Likius, A. & Brunet, M. (2006). The age of the Sahara Desert. Science 311, 821–821. [DOI] [PubMed] [Google Scholar]
- Schweizer, M. , Seehausen, O. & Hertwig, S. T. (2011). Macroevolutionary patterns in the diversification of parrots: effects of climate change, geological events and key innovations. Journal of Biogeography 38, 2176–2194. [Google Scholar]
- Ségalen, L. , Lee‐Thorp, J. A. & Cerling, T. (2007). Timing of C4 grass expansion across sub‐Saharan Africa. Journal of Human Evolution 53, 549–559. [DOI] [PubMed] [Google Scholar]
- Seiffert, E. R. (2007). Evolution and extinction of afro‐Arabian Primates near the Eocene‐Oligocene boundary. Folia Primatologica 78, 314–327. [DOI] [PubMed] [Google Scholar]
- Seiffert, E. R. (2010). Chronology of Paleogene mammal localities In Cenozoic Mammals of Africa (eds Werdelin L. and Sanders J.), pp. 19–26. University of California Press, Berkeley. [Google Scholar]
- Senut, B. , Pickford, M. & Ségalen, L. (2009). Neogene desertification of Africa. Comptes Rendus Geoscience 341, 591–602. [Google Scholar]
- Sepulchre, P. , Ramstein, G. , Fluteau, F. , Schuster, M. , Tiercelin, J. J. & Brunet, M. (2006). Tectonic uplift and eastern Africa aridification. Science 313, 1419–1423. [DOI] [PubMed] [Google Scholar]
- Sepulchre, P. , Ramstein, G. & Schuster, M. (2009). Modelling the impact of tectonics, surface conditions and sea surface temperatures on Saharan and sub‐Saharan climate evolution. Comptes Rendus Geoscience 341, 612–620. [Google Scholar]
- Sepulchre, P. , Arsouze, T. , Donnadieu, Y. , Dutay, J. ‐C. , Jaramillo, C. , le Bras, J. , Martin, E. , Montes, C. & Waite, A. J. (2014). Consequences of shoaling of the central American seaway determined from modeling Nd isotopes. Paleoceanography 29, 176–189. [Google Scholar]
- Shevenell, A. E. , Kennett, J. P. & Lea, D. W. (2008). Middle Miocene ice sheet dynamics, deep‐sea temperatures, and carbon cycling: a Southern Ocean perspective. Geochemistry, Geophysics, Geosystems 9, Q02006 10.1029/2007GC001736. [DOI] [Google Scholar]
- Silvestro, D. , Cascales‐Miñana, B. , Bacon, C. D. & Antonelli, A. (2015). Revisiting the origin and diversification of vascular plants through a comprehensive Bayesian analysis of the fossil record. New Phytologist 207, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro, D. , Warnock, R. C. M. , Gavryushkina, A. & Stadler, T. (2018). Closing the gap between palaeontological and neontological speciation and extinction rate estimates. Nature Communications 9, 5237 10.1038/s41467-018-07622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard, F. , Ayala, D. , Kamdem, G. C. , Pombi, M. , Etouna, J. , Ose, K. , Fotsing, J.‐M. , Fontenille, D. , Besansky, N. J. & Costantini, C. (2009). Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecology 9, 17 10.1186/1472-6785-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, B. , Guillocheau, F. , Robin, C. , Dauteuil, O. , Nalpas, T. , Pickford, M. , Senut, B. , Lays, P. , Bourges, P. & Bez, M. (2017). Deformation and sedimentary evolution of the Lake Albert rift (Uganda, east African rift system). Marine and Petroleum Geology 86, 17–37. [Google Scholar]
- Smith, T. B. , Wayne, R. K. , Girman, D. J. & Bruford, M. W. (1997). A role for ecotones in generating rainforest biodiversity. Science 26, 1855–1857. [Google Scholar]
- Smith, T. B. , Schneider, C. J. & Holder, K. (2001). Refugial isolation versus ecological gradients. Genetica 112–113, 383–398. [DOI] [PubMed] [Google Scholar]
- Sommerfeld, A. , Prömmel, K. & Cubasch, U. (2016). The east African rift system and the impact of orographic changes on regional climate and the resulting aridification. International Journal of Earth Sciences 105, 1779–1794. [Google Scholar]
- Sosef, M. S. M. (1994). Refuge begonias—taxonomy, phylogeny and historical biogeography of Begonia sect. Loasibegonia and sect. Scutobegonia in relation to glacial rain forest refuges in Africa. Studies in Begonia V, Wageningen Agricultural University Papers 94, 1–306. [Google Scholar]
- Sosef, M. S. M. , Dauby, G. , Blach‐Overgaard, A. , van der Burgt, X. , Catarino, L. , Damen, T. , Deblauwe, V. , Dessein, S. , Dransfield, J. , Droissart, V. , Duarte, M. C. , Engledow, H. , Fadeur, G. , Figueira, R. , Gereau, R. E. , Hardy, O. J. , Harris, D. J. , de Heij, J. , Janssens, S. , Klomberg, Y. , Ley, A. C. , Mackinder, B. A. , Meerts, P. , van de Poel, J. L. , Sonké, B. , Stévart, T. , Stoffelen, P. , Svenning, J. C. , Sepulchre, P. , Zaiss, R. , Wieringa, J. J. & Couvreur, T. L. P. (2017). Exploring the floristic diversity of tropical Africa. BMC Biology 15, 15 10.1186/s12915-017-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer, R. A. & Collinson, M. E. (2014). Plants and floral change at the cretaceous–Paleogene boundary: three decades In Volcanism, Impacts, and Mass Extinctions: Causes and Effects (eds Keller G. and Kerr A. C.), pp. 117–132. Geological Society of America, Boulder. [Google Scholar]
- Springer, M. S. , Cleven, G. C. , Madsen, O. , de Jong, W. W. , Waddell, V. G. , Amrine, H. M. & Stanhope, M. J. (1997). Endemic African mammals shake the phylogenetic tree. Nature 388, 61–64. [DOI] [PubMed] [Google Scholar]
- Springer, M. S. , Meredith, R. W. , Janecka, J. E. & Murphy, W. J. (2011). The historical biogeography of Mammalia. Philosophical Transactions of the Royal Society B: Biological Sciences 366, 2478–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, M. S. , Meredith, R. W. , Gatesy, J. , Emerling, C. A. , Park, J. , Rabosky, D. L. , Stadler, T. , Steiner, C. , Ryder, O. A. , Janečka, J. E. , Fisher, C. A. & Murphy, W. J. (2012). Macroevolutionary dynamics and historical biogeography of primate diversification inferred from a species supermatrix. PLoS One 7, e49521 10.1371/journal.pone.0049521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir, M. , Porter, A. S. , Holohan, A. , Kunzmann, L. , Collinson, M. & McElwain, J. C. (2016). Fossil plant stomata indicate decreasing atmospheric CO2 prior to the Eocene‐Oligocene boundary. Climate of the Past 12, 439–454. [Google Scholar]
- Stévart, T. , Dauby, G. , Lowry, P. P. , Blach‐Overgaard, A. , Droissart, V. , Harris, D. J. , Mackinder, B. A. , Schatz, G. E. , Sonké, B. , Sosef, M. S. M. , Svenning, J.‐C. , Wieringa, J. J. & Couvreur, T. L. P. (2019). A third of the tropical African flora is potentially threatened with extinction. Science Advances 5, eaax9444 10.1126/sciadv.aax9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher, J. W. , Loader, S. P. , Varela‐Jaramillo, A. , Montoya, P. & de Sá, R. O. (2020). Analysis of ultraconserved elements supports African origins of narrow‐mouthed frogs. Molecular Phylogenetics and Evolution 146, 106771 10.1016/j.ympev.2020.106771. [DOI] [PubMed] [Google Scholar]
- Tan, N. , Ramstein, G. , Dumas, C. , Contoux, C. , Ladant, J.‐B. , Sepulchre, P. , Zhang, Z. & De Schepper, S. (2017). Exploring the MIS M2 glaciation occurring during a warm and high atmospheric CO2 Pliocene background climate. Earth and Planetary Science Letters 472, 266–276. [Google Scholar]
- Taylor, P. J. , Maree, S. , Cotterill, F. P. D. , Missoup, A. D. , Nicolas, V. & Denys, C. (2014). Molecular and morphological evidence for a Pleistocene radiation of laminate‐toothed rats (Otomys: Rodentia) across a volcanic archipelago in equatorial Africa. Biological Journal of the Linnean Society 113, 320–344. [Google Scholar]
- Thomas, E. (2008). Descent into the icehouse. Geology 36, 191–192. [Google Scholar]
- * Thornhill, A. H. , Ho, S. Y. W. , Külheim, C. & Crisp, M. D. (2015). Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Molecular Phylogenetics and Evolution 93, 29–43. [DOI] [PubMed] [Google Scholar]
- Thuiller, W. , Broennimann, O. , Hughes, G. , Alkemade, J. R. M. , Midgley, G. F. & Corsi, F. (2006). Vulnerability of African mammals to anthropogenic climate change under conservative land transformation assumptions. Global Change Biology 12, 424–440. [Google Scholar]
- Ting, N. (2008). Mitochondrial relationships and divergence dates of the African colobines: evidence of Miocene origins for the living colobus monkeys. Journal of Human Evolution 55, 312–325. [DOI] [PubMed] [Google Scholar]
- Tolley, K. A. , Tilbury, C. R. , Measey, G. J. , Menegon, M. , Branch, W. R. & Matthee, C. A. (2011). Ancient forest fragmentation or recent radiation? Testing refugial speciation models in chameleons within an African biodiversity hotspot. Journal of Biogeography 38, 1748–1760. [Google Scholar]
- Tolley, K. A. , Townsend, T. M. & Vences, M. (2013). Large‐scale phylogeny of chameleons suggests African origins and Eocene diversification. Proceedings of the Royal Society B: Biological Sciences 280, 20130184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolley, K. A. , Alexander, G. J. , Branch, W. R. , Bowles, P. & Maritz, B. (2016). Conservation status and threats for African reptiles. Biological Conservation 204, 63–71. [Google Scholar]
- * Tosh, J. , Dessein, S. , Buerki, S. , Groeninckx, I. , Mouly, A. , Bremer, B. , Smets, E. F. & De Block, P. (2013). Evolutionary history of the afro‐Madagascan Ixora species (Rubiaceae): species diversification and distribution of key morphological traits inferred from dated molecular phylogenetic trees. Annals of Botany 112, 1723–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosso, F. , Hardy, O. J. , Doucet, J.‐L. , Daïnou, K. , Kaymak, E. & Migliore, J. (2018). Evolution in the Amphi‐Atlantic tropical genus Guibourtia (Fabaceae, Detarioideae), combining NGS phylogeny and morphology. Molecular Phylogenetics and Evolution 120, 83–93. [DOI] [PubMed] [Google Scholar]
- Trauth, M. H. , Larrasoana, J. C. & Mudelsee, M. (2009). Trends, rhythms and events in Plio‐Pleistocene African climate. Quaternary Science Reviews 28, 399–411. [Google Scholar]
- Turner, S. K. (2018). Constraints on the onset duration of the Paleocene–Eocene thermal maximum. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 376, 20170082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno, K. T. , Cerling, T. E. , Harris, J. M. , Kunimatsu, Y. , Leakey, M. G. , Nakatsukasa, M. & Nakaya, H. (2011). Late Miocene to Pliocene carbon isotope record of differential diet change among east African herbivores. Proceedings of the National Academy of Sciences of the United States of America 108, 6509–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno, K. T. , Polissar, P. J. , Jackson, K. E. & deMenocal, P. B. (2016). Neogene biomarker record of vegetation change in Eastern Africa. Proceedings of the National Academy of Sciences of the United States of America 113, 6355–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utescher, T. & Mosbrugger, V. (2007). Eocene vegetation patterns reconstructed from plant diversity — a global perspective. Palaeogeography, Palaeoclimatology, Palaeoecology 247, 243–271. [Google Scholar]
- Vajda, V. & Bercovici, A. (2014). The global vegetation pattern across the cretaceous–Paleogene mass extinction interval: a template for other extinction events. Global and Planetary Change 122, 29–49. [Google Scholar]
- Vamosi, J. C. , Magallón, S. , Mayrose, I. , Otto, S. P. & Sauquet, H. (2018). Macroevolutionary patterns of flowering plant speciation and extinction. Annual Review of Plant Biology 69, 685–706. [DOI] [PubMed] [Google Scholar]
- Van Bocxlaer, I. , Roelants, K. , Biju, S. D. , Nagaraju, J. & Bossuyt, F. (2006). Late cretaceous vicariance in Gondwanan amphibians. PLoS One 1, e74 10.1371/journal.pone.0000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Peer, Y. , Mizrachi, E. & Marchal, K. (2017). The evolutionary significance of polyploidy. Nature Reviews Genetics 18, 411–424. [DOI] [PubMed] [Google Scholar]
- Vanzolini, P. E. & Williams, E. (1981). The vanishing refuge: a mechanism for ecogeographic speciation. Papéis Avulsos de Zoologia 34, 251–255. [Google Scholar]
- * van Velzen, R. , Wahlberg, N. , Sosef, M. S. M. & Bakker, F. T. (2013). Effects of changing climate on species diversification in tropical forest butterflies of the genus Cymothoe (Lepidoptera: Nymphalidae). Biological Journal of the Linnean Society 108, 546–564. [Google Scholar]
- Vences, M. , Wollenberg, K. C. , Vieites, D. R. & Lees, D. C. (2009). Madagascar as a model region of species diversification. Trends in Ecology & Evolution 24, 456–465. [DOI] [PubMed] [Google Scholar]
- Veranso‐Libalah, M. C. , Kadereit, G. , Stone, R. D. & Couvreur, T. L. P. (2018). Multiple shifts to open habitats in Melastomateae (Melastomataceae) congruent with the increase of African Neogene climatic aridity. Journal of Biogeography 45, 1420–1431. [Google Scholar]
- Vignaud, P. , Duringer, P. , Mackaye, H. T. , Likius, A. , Blondel, C. , Boisserie, J.‐R. , de Bonis, L. , Eisenmann, V. , Etienne, M.‐E. , Geraads, D. , Guy, F. , Lehmann, T. , Lihoreau, F. , Lopez‐Martinez, N. , Mourer‐Chauviré, C. , Otero, O. , Rage, J. C. , Schuster, M. , Viriot, L. , Zazzo, A. & Brunet, M. (2002). Geology and palaeontology of the upper Miocene Toros‐Menalla hominid locality, Chad. Nature 418, 152–155. [DOI] [PubMed] [Google Scholar]
- Vincens, A. , Tiercelin, J.‐J. & Buchet, G. (2006). New Oligocene–early Miocene microflora from the southwestern Turkana Basin: Palaeoenvironmental implications in the northern Kenya rift. Palaeogeography, Palaeoclimatology, Palaeoecology 239, 470–486. [Google Scholar]
- Viste, E. & Sorteberg, A. (2013). Moisture transport into the Ethiopian highlands. International Journal of Climatology 33, 249–263. [Google Scholar]
- Viste, E. , Korecha, D. & Sorteberg, A. (2013). Recent drought and precipitation tendencies in Ethiopia. Theoretical and Applied Climatology 112, 535–551. [Google Scholar]
- Voelker, G. , Outlaw, R. K. & Bowie, R. C. K. (2010). Pliocene forest dynamics as a primary driver of African bird speciation. Global Ecology and Biogeography 19, 111–121. [Google Scholar]
- Voelker, G. , Marks, B. D. , Kahindo, C. , A'genonga, U. , Bapeamoni, F. , Duffie, L. E. , Huntley, J. W. , Mulotwa, E. , Rosenbaum, S. A. & Light, J. E. (2013). River barriers and cryptic biodiversity in an evolutionary museum. Ecology and Evolution 3, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voje, K. L. , Hemp, C. , Flagstad, Ø. , Sætre, G.‐P. & Stenseth, N. C. (2009). Climatic change as an engine for speciation in flightless Orthoptera species inhabiting African mountains. Molecular Ecology 18, 93–108. [DOI] [PubMed] [Google Scholar]
- Walker, N. D. (1990). Links between south African summer rainfall and temperature variability of the Agulhas and Benguela current systems. Journal of Geophysical Research: Oceans 95, 3297–3319. [Google Scholar]
- Wallace, A. R. (1852). On the monkeys of the Amazon. Proceedings of the Zoological Society of London 20, 107–110. [Google Scholar]
- Washington, R. , James, R. , Pearce, H. , Pokam, W. M. & Moufouma‐Okia, W. (2013). Congo Basin rainfall climatology: can we believe the climate models? Philosophical Transactions of the Royal Society B: Biological Sciences 368, 20120296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * van Welzen, P. C. , Strijk, J. S. , van Konijnenburg‐van Cittert, J. H. A. , Nucete, M. & Merckx, V. S. F. T. (2014). Dated phylogenies of the sister genera Macaranga and Mallotus (Euphorbiaceae): congruence in historical biogeographic patterns? PLoS One 9, e85713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdelin, L. & Sanders, J. (2010). Cenozoic Mammals of Africa. University of California Press, Berkeley. [Google Scholar]
- White, F. (1979). The Guineo‐Congolian region and its relationships to other phytochoria. Bulletin du Jardin botanique National de Belgique 49, 11–55. [Google Scholar]
- White, F. (1981). The history of the Afromontane archipelago and the scientific need for its conservation. African Journal of Ecology 19, 33–54. [Google Scholar]
- White, F. (1983). The Vegetation of Africa, a Descriptive Memoir to Accompany the UNESCO/AETFAT/UNSO Vegetation Map of Africa. UNESCO, Paris. [Google Scholar]
- Wichura, H. , Bousquet, R. , Oberhänsli, R. , Strecker, M. R. & Trauth, M. H. (2010). Evidence for middle Miocene uplift of the east African plateau. Geology 38, 543–546. [Google Scholar]
- Wichura, H. , Jacobs, L. L. , Lin, A. , Polcyn, M. J. , Manthi, F. K. , Winkler, D. A. , Strecker, M. R. & Clemens, M. (2015). A 17‐my‐old whale constrains onset of uplift and climate change in East Africa. Proceedings of the National Academy of Sciences of the United States of America 112, 3910–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, P. G. (1985). Evidence for an early Plio‐Pleistocene rainforest expansion in East Africa. Nature 315, 487–489. [Google Scholar]
- Willis, K. J. & McElwain, J. (2014). The Evolution of Plants, 2nd Edition. Oxford University Press, Oxford. [Google Scholar]
- Wilson, A. B. , Teugels, G. G. & Meyer, A. (2008). Marine incursion: the freshwater herring of Lake Tanganyika are the product of a marine invasion into West Africa. PLoS One 3, e1979 10.1371/journal.pone.0001979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, J. , Chardon, D. , Rouby, D. , Guillocheau, F. , Dall'asta, M. , Ferry, J.‐N. & Broucke, O. (2017a). Paleogeographic and structural evolution of northwestern Africa and its Atlantic margins since the early Mesozoic. Geosphere 13, 1254–1284. [Google Scholar]
- * Ye, Z. , Zhen, Y. , Zhou, Y. & Bu, W. (2017b). Out of Africa: biogeography and diversification of the pantropical pond skater genus Limnogonus Stål, 1868 (Hemiptera: Gerridae). Ecology and Evolution 7, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, Y. , Huber, M. , Müller, R. D. , Poulsen, C. J. & Ribbe, J. (2009). Simulation of the middle Miocene climate optimum. Geophysical Research Letters 36, L04702 10.1029/2008GL036571. [DOI] [Google Scholar]
- Zachos, J. C. , Dickens, G. R. & Zeebe, R. E. (2008). An early Cenozoic perspective on greenhouse warming and carbon‐cycle dynamics. Nature 451, 279–283. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Nisancioglu, K. H. , Flatøy, F. , Bentsen, M. , Bethke, I. & Wang, H. (2011). Tropical seaways played a more important role than high latitude seaways in Cenozoic cooling. Climate of the Past 7, 801–813. [Google Scholar]
- Zhang, Z. , Ramstein, G. , Schuster, M. , Li, C. , Contoux, C. & Yan, Q. (2014). Aridification of the Sahara desert caused by Tethys Sea shrinkage during the late Miocene. Nature 513, 401–404. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Zhang, Z. , Jiang, D. , Yan, Q. , Zhou, X. & Cheng, Z. (2016). Strengthened African summer monsoon in the mid‐Piacenzian. Advances in Atmospheric Sciences 33, 1061–1070. [Google Scholar]
- Zhang, L. , Wang, C. , Wignall, P. B. , Kluge, T. , Wan, X. , Wang, Q. & Gao, Y. (2018). Deccan volcanism caused coupled pCO2 and terrestrial temperature rises, and pre‐impact extinctions in northern China. Geology 46, 271–274. [Google Scholar]
- Zhen, Y. , Harrigan, R. J. , Ruegg, K. C. , Anderson, E. C. , Ng, T. C. , Lao, S. , Lohmueller, K. E. & Smith, T. B. (2017). Genomic divergence across ecological gradients in the central African rainforest songbird (Andropadus virens). Molecular Ecology 26, 4966–4977. [DOI] [PubMed] [Google Scholar]
- Zhou, L. , Su, Y. C. F. , Thomas, D. C. & Saunders, R. M. K. (2012). ‘Out‐of‐Africa’ dispersal of tropical floras during the Miocene climatic optimum: evidence from Uvaria (Annonaceae). Journal of Biogeography 39, 322–335. [Google Scholar]
- Zimkus, B. M. & Gvoždík, V. (2013). Sky Islands of the Cameroon volcanic line: a diversification hot spot for puddle frogs (Phrynobatrachidae: Phrynobatrachus). Zoologica Scripta 42, 591–611. [Google Scholar]
- Zimkus, B. M. , Rödel, M.‐O. & Hillers, A. (2010). Complex patterns of continental speciation: molecular phylogenetics and biogeography of sub‐Saharan puddle frogs (Phrynobatrachus). Molecular Phylogenetics and Evolution 55, 883–900. [DOI] [PubMed] [Google Scholar]
- Zimkus, B. M. , Lawson, L. P. , Barej, M. F. , Barratt, C. D. , Channing, A. , Dash, K. M. , Dehling, J. M. , Du Preez, L. , Gehring, P.‐S. , Greenbaum, E. , Gvoždík, V. , Harvey, J. , Kielgast, J. , Kusamba, C. , Nagy, Z. T. , et al. (2017). Leapfrogging into new territory: how Mascarene ridged frogs diversified across Africa and Madagascar to maintain their ecological niche. Molecular Phylogenetics and Evolution 106, 254–269. [DOI] [PubMed] [Google Scholar]
- * Zizka, A. , Silvestro, D. , Andermann, T. , Azevedo, J. , Duarte Ritter, C. , Edler, D. , Farooq, H. , Herdean, A. , Ariza, M. & Scharn, R. (2019). Coordinate cleaner: standardized cleaning of occurrence records from biological collection databases. Methods in Ecology and Evolution 10, 744–751. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. List of dated molecular phylogeny studies used to generate Fig. 3D,E.
Fig. S1. Palaeoclimate during the Eocene across Africa.
Table S1. Studies used to estimate the origin of extant species within groups with dated molecular phylogenies.
Appendix S2. Assignment of genera to elevation zones as presented in Table S2.
Table S2. Studies used to estimate crown and stem nodes for genera or clades, and to estimate vegetation zonation following methodology provided in Appendix S2.


