Abstract
Aim
To evaluate the efficacy, safety and tolerability of a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) in patients with hypothalamic obesity (HO).
Materials and Methods
A two‐arm, randomized, multicentre, double‐blind, placebo‐controlled trial was conducted in 10‐ to 25‐year‐olds with hypothalamic injury following intracranial tumour and HO. Participants were randomized to once‐weekly subcutaneous injections of a GLP‐1 RA exenatide 2 mg (ExQW) or placebo for 36 weeks. The primary efficacy endpoint was 36‐week % change in body mass index (BMI). Secondary outcomes included change in body composition (by dual energy x‐ray absorptiometry).
Results
Forty‐two participants were randomized to ExQW (n = 23) or placebo (n = 19). Participants were 5 ± 2 years (mean ± SD) postdiagnosis and development of HO (BMI 37.3 ± 7.1 kg/m2). In intention‐to‐treat analysis, the effect of 36‐week ExQW vs. placebo on % Δ BMI was not significant (estimated treatment difference −1.7 ± 1.8%, 95% CI −4.1 to 0.6%, P = .40); however, total body fat mass was reduced (estimated treatment difference −3.1 ± 1.4 kg, 95% CI −5.7 to −0.4 kg, P = .02). There was a significant reduction in waist circumference (estimated effect of treatment −3.5 [95% CI −5.5 to −1.6] cm, P = .004). All patients treated with placebo increased % of adipose tissue, while 50% treated with ExQW had reductions (P < .001). Mean HbA1c, glucose tolerance and serum lipids did not change significantly with therapy. ExQW was well tolerated. The most frequent adverse events were transient gastrointestinal disturbances (ExQW vs. placebo: nausea 6/23 vs. 3/18, vomiting 4/23 vs. 4/18 and diarrhoea 7/23 vs. 3/18).
Conclusions
GLP‐1 RAs are a promising and safe treatment to improve or stabilize HO in children and young adults.
Keywords: antiobesity drug, exenatide, randomized trial
1. INTRODUCTION
Mediobasal hypothalamic tumours are frequently associated with excessive weight gain and hypothalamic obesity (HO). Craniopharyngiomas (CP) 1 are the most common cause; ~50% of patients with CP develop HO and are at an increased risk of developing type 2 diabetes and cardiovascular disease following tumour therapy. 2 HO can also occur following other suprasellar tumours, radiation, trauma or surgical insult to the hypothalamus. Recognized risk factors for HO include intracranial tumours or lesions affecting medial and posterior hypothalamic nuclei that impact satiety‐signalling pathways. 3 , 4 , 5 , 6 , 7 Structural damage in these nuclei often leads to hyperphagia, rapid weight gain, central insulin and leptin resistance, low energy expenditure and increased energy storage in adipose tissue. 8 , 9
Early and effective management of obesity is vital for this population 1 , 6 , 10 ; however, treatment for HO remains more challenging than treatment for common obesity. 3 , 11 , 12 , 13 , 14 , 15
Multiple therapies have been studied for HO 16 ; however, to date, none have proven to be both safe and effective. Stimulant medications with anorectic properties were not beneficial in decreasing body mass index (BMI). 16 Medications targeting hyperinsulinism (octreotide, metformin) both as monotherapy and in conjunction with diazoxide or fenofibrate, have also failed to improve HO. 16 A novel methionine‐aminopeptidase‐2 inhibitor was shown to be beneficial in phase 2 trials; however, development of this medication was abruptly halted because of safety concerns. 16
Glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) are known to cause weight loss in adults 17 , 18 , 19 , 20 and adolescents 21 , 22 through mechanisms independent of intact hypothalamic structures. Data from a rodent model supported potential benefit from GLP‐1 RA therapy for HO. 23 A prior open‐label trial and two open‐label case reports of GLP‐1 RAs in adolescents and adults with HO found promising reductions in body weight. 20 , 24 , 25 The current multicentre study was organized to test the effect of the GLP‐1 RA exenatide once‐weekly extended‐release (ExQW) on clinical outcomes and metabolic function using a 36‐week double‐blind, placebo‐controlled randomized trial followed by an 18‐week open‐label extension. The hypothesis was drugs causing weight loss via hindbrain signalling, 26 such as GLP‐1 RAs, would reduce body weight in people with severe obesity caused by HO, providing a desperately needed non‐surgical option for treatment of HO. The primary objective was to explore the ability of ExQW to reduce the BMI in patients suffering from HO as well as establish the safety and tolerability of ExQW.
2. MATERIALS AND METHODS
2.1. Trial design and participants
The Energy Balance and Weight Loss in Craniopharyngioma‐related or Other Hypothalamic Tumours in Hypothalamic Obesity (ECHO) trial was an investigator‐initiated multisite, 36‐week, double‐blind, placebo‐controlled randomized trial to evaluate the efficacy and safety of ExQW for BMI reduction in adolescents and young adults with HO.
Enrolled participants were randomized 1:1 to ExQW or placebo. Total study duration was 56 weeks, including a 2‐week placebo run‐in, 36 weeks of randomized treatment with ExQW or placebo and an 18‐week open‐label extension, during which all participants received ExQW (Figure S1 ).
The study was approved by the Institutional Review Board at each site and conducted in accordance with the Declaration of Helsinki and human use guidelines for Good Clinical Practice. This study was registered at ClinicalTrials.gov (ECHO trial, identifier NCT02664441). A data and safety monitoring board (DSMB) followed the progress and safety data at regular intervals. As this was an off‐label application of ExQW (Bydureon), its use was approved under the US Food and Drug Administration (FDA) Investigational New Drug protocol 122 971. At the time of funding submission, no GLP‐1 agents were FDA‐approved in the paediatric population; Bydureon (2 mg) weekly was selected for its safety profile and effects on weight loss.
Patients aged 10‐25 years of age with a diagnosis of HO following treatment for CP were recruited from paediatric endocrinology outpatient clinics at the three research sites (Seattle Children's Hospital, Seattle, WA, USA; Children's Minnesota, St. Paul, MN, USA; and Vanderbilt University Medical Center, Nashville, TN, USA) and HO internet support groups. HO was defined as rapid weight gain related to tumour onset or treatment, as assessed by a paediatric endocrinologist with expertise in HO. To address low enrolment, three patients with HO following treatment for other suprasellar tumours were subsequently included. Prior to commencing study activities, age‐appropriate written informed consent/assent was obtained.
Study eligibility included: age‐ and sex‐adjusted BMI ≥95 percentile or BMI ≥32 kg/m2 if aged 18 years or older; evidence of hypothalamic injury by MRI confirmed by one central neuroradiologist (Seattle); 6 months or longer postsurgical or radiation treatment; weight stable or increasing over 3 months; and stable hormone replacement for at least 3 months. Exclusion criteria included: a family history of multiple endocrine neoplasia type 2 or familial medullary thyroid carcinoma; metabolic disorders, insulin‐treated diabetes, poorly controlled type 2 diabetes (HbA1c ≥ 10%); other chronic serious medical conditions; renal impairment (glomerular filtration rate <60 mL/min/1.73m2); history of gastroparesis; pancreatitis or gallstones (unless status postcholecystectomy); initiation of weight‐loss medications within 3 months; previous blood donation greater than 10% of estimated blood volume within 3 months of study; current warfarin use; untreated thyroid or adrenal disorder; history of or planned bariatric surgery; pregnancy, lactation or an expectation to conceive during the study period. Sexually active female participants were required to use birth control, unless gonadotropin deficiency was documented.
2.2. Procedures
ExQW (Bydureon) 2 mg or placebo was administered subcutaneously once‐weekly in the abdomen, thigh or upper arm. Identical‐appearing drug and placebo kits were supplied by Astra Zeneca under a Clinical Trial Agreement. There was a 2‐week placebo run‐in to test protocol adherence followed by assignment to a treatment arm using a permuted‐block randomization (1:1) with varying block sizes stratified by study site, age (10‐14, 15‐25 years) and sex. Randomization was administered by the research pharmacist. All co‐investigators and study staff were blinded to treatment assignment until study completion. The primary study statistician (KBW) was partially unblinded to facilitate DSMB reporting.
2.3. Outcomes
Participants were seen for study visits at baseline, weeks 6, 18 and 36 (randomized trial), and at 54 weeks (end of open‐label drug treatment). The end of the randomized trial (36 weeks) was the primary study endpoint (see Figure S1 for procedures and timeline of interventions).
2.3.1. Efficacy
The primary outcome was 36‐week % change in BMI. At all study visits, body weight was assessed in light clothing using a calibrated scale. Height was assessed using a stadiometer. BMI was calculated as kg/m2 and BMI % changes were calculated. Secondary outcomes included 36‐week change in BMI, BMI Z‐score, waist circumference, waist‐to‐hip ratio and waist‐to‐height ratio. Waist and hip circumference were measured according to guidelines (http://www.cdc.gov/nchs/nhanes.htm). Additional measures of adiposity including total fat and lean body mass were obtained via dual energy x‐ray absorptiometry (DXA) at 0 and 36 weeks, adjusted for height, age and sex as covariates (GE Lunar Prodigy Advance; GE Lunar iDXA; and GE Hologic Discovery and Horizon). There were too few non‐white patients to adjust for race as a covariate.
The following metabolic outcomes were assessed after a 10‐12‐hour overnight fast: glucose, insulin, lipid panel, high sensitive C‐reactive protein and HbA1c. Samples were tested at a central laboratory (Northwest Lipid Research Laboratories, Seattle, WA, USA). Glucose tolerance was determined at 0, 18 and 36 weeks by a 2‐hour oral glucose tolerance test (75 g) with sample collection at 0, 30, 60, 90 and 120 minutes for measurement of glucose and insulin.
2.3.2. Safety assessments
Electrocardiogram (ECG) was assessed at weeks 0, 18, 36 and 54. Resting heart rate and blood pressure were measured manually according to the guidelines of the National High Blood Pressure Education Program. 27 Laboratory measures—including blood cell count with differential, serum sodium, potassium, amylase, fasting glucose, transaminases (alanine transaminase or ALT, aspartate transaminase or AST), creatinine, calcitonin and thyroid hormones—were routinely monitored and reported to the DSMB. In participants without known hypogonadotropic hypogonadism, gonadotropins were also measured. All females had urine pregnancy testing prior to treatment.
2.3.3. Pubertal development
Pubertal staging was performed by a paediatric endocrinologist. Bone age x‐rays were performed in patients who had not achieved epiphyseal closure.
2.4. Statistical analysis
Assuming attrition of 20%, target enrolment for this study was 48 participants (38 evaluable), randomized (1:1) to treatment with GLP‐1 RAs or placebo. We estimated that this sample size would provide 85% power to detect relative treatment effect sizes of 1 or greater (in absolute value) for % change in BMI over 3 months with type I error rate (α) of 0.05. This effect size was consistent with relative effect sizes observed in preceding pilot studies 20 for % change in BMI, absolute change in BMI, change in glucose AUC (per 100) and change in insulin (mU/L).
Primary and secondary efficacy outcomes were analysed in the intention‐to‐treat (ITT) population, which included all subjects who were successfully randomized and received at least one dose of assigned treatment, using a last observation carried forward (LOCF) approach for measures with mid‐treatment assessments. Outcomes assessed only pretreatment and post‐treatment were analysed without imputation. Sensitivity analyses were conducted using both the ITT population with a LOCF approach and the population of participants who completed the 36‐week randomized trial. The safety population included all subjects who received at least one dose of assigned therapy during the randomized trial.
2.4.1. Efficacy
For the primary efficacy analysis, treatment, time and treatment‐by‐time interaction were entered into a linear mixed effects model (LMM), using restricted maximum likelihood estimation, predicting longitudinal % change in BMI with the participant included as a random intercept. Time points were baseline and after 6, 18 and 36 weeks of treatment. Randomization was stratified by age group, sex and site; these were included as fixed covariates. Model‐based custom contrasts were defined to estimate mean 36‐week % change in BMI for ExQW and placebo; and to determine the significance of ExQW treatment 36‐week % change in BMI compared with placebo (difference of differences). All continuous secondary efficacy endpoints were analysed using similar LMM to those previously described for the primary outcome. Sensitivity analyses utilized analysis of covariance (ANCOVA) models.
2.4.2. Safety
Adverse events (AEs) were summarized, by type and treatment group, as the frequency and % of subjects with at least one AE. AE rates associated with ExQW treatment were compared with placebo using Fisher's exact test. Safety laboratory tests were analysed using ANCOVA modelling with LOCF imputation to estimate the mean 36‐week change from baseline.
Analyses were performed in STATA (StataCorp LLC, College Station, TX, USA) and SAS (SAS Institute, Cary, NC, USA). Two‐sided alternatives were assumed, with α = 0.05.
3. RESULTS
3.1. Patient disposition
A total of 43 patients were screened and 42 patients were enrolled (Figure 1). All 23 patients randomized to ExQW received at least one dose of study medication. Of 19 patients randomized to placebo, 18 received at least one dose and one was withdrawn prior to receiving drug because of the emergence of health issues unrelated to the study. Overall, 35/42 patients (83%) completed the 36‐week randomized trial; and 31/42 (74%) completed the additional open label extension (Figure S2). Reasons for withdrawal included emergence of new medical concerns unrelated to study (n = 2), patient request (n = 2), difficulty with injectable medication (n = 1) and hypersensitivity to the study medication (n = 1). In addition, one patient was withdrawn because of an increase in seizure activity, and one patient died in a traffic accident that was deemed to be unrelated to treatment (both ExQW group). Adherence to treatment was high for both groups (excluding parent report: ExQW 89% ± 14%, placebo 85% ± 20%; including parent report: ExQW 96% ± 8%, placebo 93% ± 14%).
FIGURE 1.
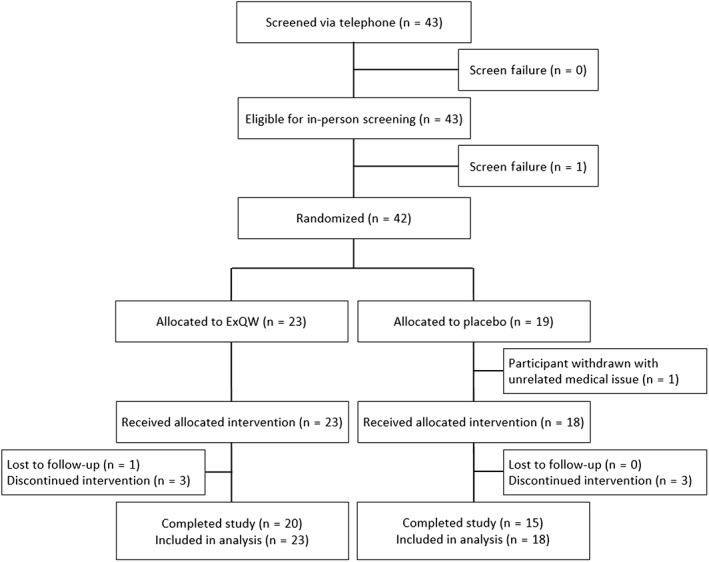
Consort diagram
Treatment arm demographic and clinical characteristics were similar at baseline. Hormonal supplementation was common and as expected given hypothalamic damage (Table 1). Only one patient had type 2 diabetes (an HbA1c of 7% at screening), which was well controlled with metformin. The average HbA1c at time of screening was 5.4% ± 0.5% with no difference in group means (P = .96).
TABLE 1.
Baseline characteristics by treatment
| Demographics | ExQW (N = 23) | Placebo (n = 18) | P‐value |
|---|---|---|---|
| Age, y | 16.9 (4.3) | 16.9 (4.8) | .99 |
| Sex, no. (%) | >.99 | ||
| Male | 9 (39%) | 7 (39%) | |
| Female | 14 (61%) | 11 (61%) | |
| Race, no. (%) | .55 | ||
| Asian | 1 (6%) | ||
| Black or African American | 1 (6%) | ||
| White or Caucasian | 21 (91%) | 15 (82%) | |
| More than 1 race | 2 (9%) | 1 (6%) | |
| Ethnicity, no. (%) | .50 | ||
| Hispanic or Latino | 2 (9%) | ||
| Not Hispanic or Latino | 21 (91%) | 18 (100%) | |
| Type of tumour, no. (%) | .08 | ||
| Craniopharyngioma | 23 (100%) | 15 (82%) | |
| Mixed germ cell tumour | 1 (6%) | ||
| Suprasellar ganglioglioma | 1 (6%) | ||
| Suprasellar germinoma | 1 (6%) | ||
| Time from HO diagnosis – Yr. | 6.7 (4.7) a | 8.0 (4.2) | .34 |
| Anthropometrics and body composition | |||
| Height, cm | 160.9 (15.2) | 161.3 (13.3) | .93 |
| Weight, kg | 95.1 (27.9) | 103.7 (30.0) | .35 |
| Waist circumference, cm | 112.8 (17.4) | 119.7 (16.2) | .20 |
| Waist/hip ratio | 0.98 (0.06) | 0.99 (0.07) | .85 |
| Waist/height ratio | 0.70 (0.08) | 0.74 (0.08) | .10 |
| Body mass index, kg/m2 | 35.8 (6.6) | 39.2 (7.3) | .13 |
| BMI z‐score [<20 years of age] | 2.21 (0.30) | 2.39 (0.29) | .12 |
| BMI >120% of the 95th percentile (age <20 y) Or in adults with BMI >35 kg/m2, no. (%) | 15 (65%) | 14 (78%) | .38 |
| DXA | |||
| % adipose tissue | 47.4 (4.9) a | 49.0 (4.5) | .27 |
| % lean tissue | 50.4 (4.9) a | 48.8 (4.5) | .31 |
| Heart rate, bpm | 75.8 (13.7) | 86.5 (13.2) | .02 |
| Systolic BP, mmHg | 106.0 (13.0) | 106.1 (9.1) | .98 |
| Diastolic BP, mmHg | 69.4 (7.3) | 68.8 (5.6) | .79 |
| Concomitant medications | |||
| Growth hormone, no. (%) | 13 (57%) | 11 (61%) | >.99 |
| Thyroid hormone, no. (%) | 19 (83%) | 15 (83%) | >.99 |
| Hydrocortisone, no. (%) | 19 (83%) | 15 (83%) | >.99 |
| DDAVP/desmopressin, no. (%) | 19 (83%) | 15 (83%) | >.99 |
| Testosterone/oestrogen, no. (%) | 15 (65%) | 15 (83%) | .29 |
| Dextroamph, no. (%) | 5 (22%) | 2 (11%) | .43 |
| Methylphenidate, no. (%) | 3 (13%) | 1 (6%) | .62 |
Abbreviations: BMI, body mass index; BP, blood pressure; bpm, beats per minute; DXA, dual energy x‐ray absorptiometry; ExQW, exenatide once weekly; HO, hypothalamic obesity.For BMI z‐score: ExQW, n = 16; Placebo, n = 14. Data are expressed as means (SD) or count (% of group total) as appropriate. Statistical analyses were performed using Fisher's exact test for categorical or counted variables and Student's t‐test for continuous variables.
Data unavailable for one subject.
3.2. BMI and weight‐related endpoints
ITT analysis indicated significant 36‐week % change in BMI for both the ExQW (estimated effect of treatment 1.7% ± 0.8%, 95% CI 0.2% to 3.3%, P = .03) and placebo (estimated effect of treatment 3.5% ± 0.9%, 95% CI 1.7% to 5.2%, P < .001) treated groups; the difference between groups for BMI change was not significant (estimated treatment difference −1.7% ± 1.2%, 95% CI −4.1% to 0.6%, P = .40). Similar results were observed for change in BMI (estimated effect of treatment: ExQW: 0.6 ± 0.3 kg/m2, 95% CI 0.1 to 1.1 kg/m2, P = .03; placebo: 1.4 ± 0.3 kg/m2, 95% CI 0.8 to 2.0 kg/m2; P < .001), where the difference between groups was not significant (estimated treatment difference −0.8 ± 0.4 kg/m2, 95% CI −1.6 to 0.1 kg/m2, P = .23). In the placebo group, BMI changes in the three subjects with other suprasellar tumours were similar to changes observed in subjects with CP.
Overall, in the ExQW group, two subjects had reductions of BMI by more than 5% and in four subjects BMI was reduced by 2.5% or more at 36 weeks. In the placebo group, no subjects had reductions of BMI by more than 5% and in two subjects BMI was reduced by 2.5% or more at 36 weeks (Figure 2; Figure S2).
FIGURE 2.
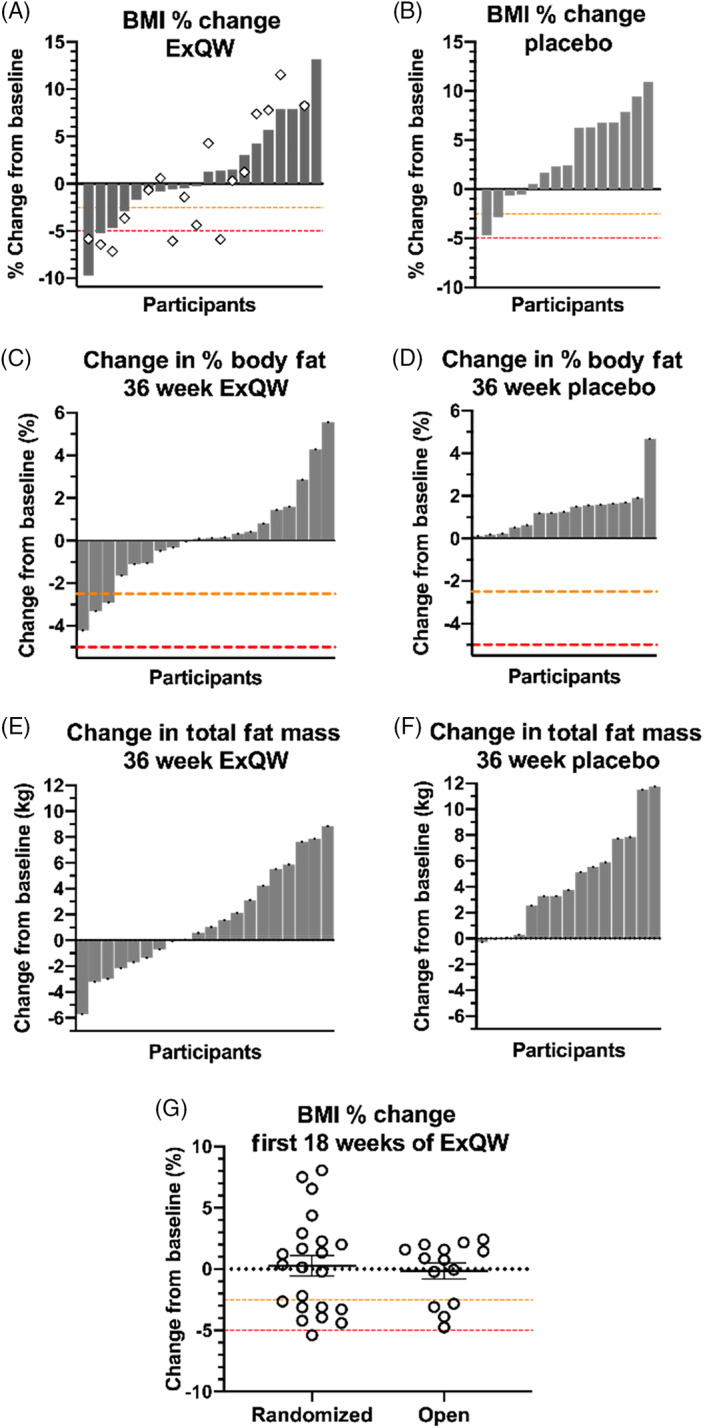
Changes of weight‐related endpoints. Waterfall plots for A, exenatide once‐weekly (ExQW) and B, placebo treated patients outlining the % change in BMI from baseline to the end of the randomized treatment arm (36 weeks; grey bars) and the end of the open‐label arm (54 weeks; ◇, ExQW group only). Change in C and D, % body fat, and E and F, total body fat, during the randomized treatment. G, change in BMI over the first 18 weeks of ExQW during randomized versus open‐label treatment phases
In ITT analysis, the estimated treatment difference between ExQW and placebo for total body fat was −3.1 kg (95% CI −5.7 to −0.4 kg, P = .02). All patients treated with placebo exhibited an increase in % adipose tissue (minimum change 0.13%, estimated effect of treatment 1.3% ± 1.5%, 95% CI 0.3% to 2.3%; P = .008), while 50% of patients treated with ExQW saw reductions in their % adipose tissue (Fisher Exact P < .001). Similar effects were observed in waist circumference where ExQW‐treated patients exhibited no mean change (estimated effect of treatment 0.1 ± 0.7 cm, 95% CI −1.2 to 1.4 cm) and placebo‐treated patients exhibited significant increases (estimated effect of treatment 3.6 ± 0.7 cm, 95% CI 2.2 to 5.1 cm; P = .004) between the groups. While ExQW treatment yielded no change in waist‐to‐height ratio (estimated effect of treatment −0.006 ± 0.004, 95% CI −0.015 to 0.002), placebo‐treated patients had significant increases (estimated effect of treatment 0.013 ± 0.005, 95% CI 0.003 to 0.022), yielding a significant treatment difference (estimated treatment difference −0.018 ± 0.006, 95% CI −0.031 to −0.006, P = .02). No change in waist‐to‐hip ratio was observed for either treatment group (Table 2).
TABLE 2.
Estimated 36‐week change of outcomes by treatment
| ExQW (n = 23) | Placebo (n = 18) | Estimated Tx difference, ExQW versus placebo (95% CI) | P‐value | |
|---|---|---|---|---|
| Body mass index (kg/m2) | 0.6 ± 0.3 | 1.4 ± 0.3 | −0.8 (−1.6 to 0.1) | .23 |
| % change in BMI | 1.7 ± 0.8 | 3.5 ± 0.9 | −1.7 (−4.1 to 0.6) | .40 |
| ≥0% change in BMI | 50% | 26.7% | ||
| ≥2.5% change in BMI | 18.2% | 13.3% | ||
| ≥5% change in BMI | 9.1% | |||
| BMI z‐score a | −0.04 ± 0.02 | 0.03 ± 0.03 | −0.07 (−0.14 to 0.002) | .13 |
| Waist circumference (cm) | 0.1 ± 0.7 | 3.6 ± 0.7 | −3.5 (−5.5 to −1.6) | .004 |
| Waist/hip ratio | −0.01 ± 0.01 | −0.01 ± 0.01 | −0.01 (−0.03 to 0.02) | .37 |
| Waist/height ratio | −0.01 ± 0.004 | 0.01 ± 0.005 | −0.02 (−0.03 to −0.01) | .003 |
| DXA b | ||||
| Total adipose tissue (kg) | 1.5 ± 0.9 | 4.6 ± 1.0 | −3.1 (−5.7 to −0.4) | .02 |
| % adipose tissue | 0.1 ± 0.4 | 1.3 ± 0.5 | −1.2 (−2.5 to 0.0) | .06 |
| Total lean tissue (kg) | 1.7 ± 0.7 | 1.3 ± 0.9 | 0.4 (−1.8 to 2.6) | .73 |
| % lean tissue | −0.1 ± 0.4 | −1.3 ± 0.5 | 1.2 (−0.1 to 2.5) | .06 |
| Bone mineral | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 (−0.1 to 0.1) | .75 |
| Heart rate (bpm) | 5.7 ± 3.0 | −4.8 ± 3.4 | 10.5 (1.5 to 19.4) | .03 |
| Blood pressure (mmHg) | ||||
| Systolic | −1.6 ± 2.3 | 7.8 ± 2.6 | −9.4 (−16.3 to −2.5) | .052 |
| Diastolic | −2.7 ± 1.9 | 3.2 ± 2.1 | −6.0 (−11.6 to −0.4) | .15 |
| Respiratory rate (bpm) | 0.1 ± 0.7 | −0.3 ± 0.8 | 0.4 (−1.7 to 2.6) | .20 |
| Cholesterol (mg/dL) c | ||||
| Total | 1.00 (0.96 to 1.05) | 1.03 (0.97 to 1.08) | 0.98 (0.91 to 1.05) | .81 |
| HDL | 1.01 (0.94 to 1.08) | 1.01 (0.93 to 1.09) | 1.00 (0.91 to 1.11) | .82 |
| LDL | 1.01 (0.90 to 1.14) | 1.01 (0.88 to 1.15) | 1.00 (0.84 to 1.20) | .88 |
| VLDL | 0.98 (0.91 to 1.06) | 1.01 (0.93 to 1.10) | 0.97 (0.86 to 1.08) | .84 |
| Triglycerides | 1.00 (0.89 to 1.13) | 1.01 (0.88 to 1.15) | 1.00 (0.83 to 1.19) | .92 |
| Adiponectin (ng/mL) c | 1.03 (0.93 to 1.13) | 0.94 (0.85 to 1.05) | 1.09 (0.94 to 1.25) | .35 |
| Leptin (ng/mL) c | 0.98 (0.88 to 1.08) | 1.03 (0.92 to 1.16) | 0.95 (0.81 to 1.10) | .69 |
Abbreviations: BMI, body mass index; bpm, beats per minute; DXA, dual energy x‐ray absorptiometry; ExQW, exenatide once‐weekly; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LOCF, last‐observation‐carried‐forward; VLDL, very low‐density lipoprotein.Data are estimated effects of treatment, expressed as means ± standard error, with corresponding estimated treatment differences and 95% confidence intervals, unless otherwise stated, using available data from the full‐analysis set with LOCF imputation. Statistical analyses were performed using linear mixed‐effects modelling with randomization stratification variables (site, sex and age group) as covariates. The full dataset included all subjects who were randomized and received at least one drug treatment administration.
Data are limited to individuals aged less than 20 years of age at start of study drug treatment.
Measurements were only assessed pretreatment and post‐treatment and represent only collected data; no imputations were used.
Data were log‐transformed prior to analysis. Results were back‐transformed through exponentiation and presented as geometric mean ratios with 95% confidence intervals.
3.3. Cardiovascular and metabolic endpoints
Analysis indicated a significant effect of treatment for heart rate (estimated treatment difference 10.5 ± 4.6 bpm, 95% CI 1.5 to 19.4 bpm, P = .03) with ExQW treatment resulting in an increase in heart rate in trend (5.7 ± 3.0 bpm, 95% CI −0.2 to 11.6 bpm, P = .06). There was no statistically significant overall effect of treatment for either systolic or diastolic blood pressure. However, placebo‐treated patients experienced a significant increase in systolic blood pressure (estimated effect of treatment 7.8 ± 2.6, 95% CI 2.7 to 13.0, P = .003). Changes observed in the subgroup of patients who were treated per protocol (>85% compliance) were similar compared with results in subgroups with LOCF imputation or 36‐week completers (Table S2).
There were no significant changes in HbA1c (estimated effect of treatment: ExQW −0.05% ± 0.09%, placebo 0.06% ± 0.10%, P = .89) or metabolic measures in either group (Tables S1 and S2). There was a significant overall effect of treatment on the inflammatory marker serum C‐reactive protein (Tables S1 and S2) because of a mean increase among placebo‐treated patients.
3.4. Sensitivity analysis
Sensitivity analysis outcomes were comparable for BMI, anthropometric and metabolic‐related outcomes relative to the primary analysis. The primary analysis outcomes were confirmed by the completed treatment sensitivity analysis.
Several differences were found between the primary and sensitivity analysis results for cardiovascular outcomes. While the estimated effects of treatment for both systolic and diastolic blood pressure were comparable between the primary and both of the sensitivity analyses, the differences between the groups post‐treatment were considered significant for both measures in each of the sensitivity analyses (Table S2). Adherence to study medication was not associated with the rate of BMI or body fat reduction.
3.5. Safety and tolerability
Overall, 36/41 patients (88%) reported at least one AE. Patients assigned to ExQW reported similar rates of AE (21/23, 91%) compared with placebo (15/18, 83%) (P = .64). During the randomized arm, gastrointestinal tract‐related events were most common, including abdominal pain (ExQW 39%, placebo 11%), nausea (ExQW 26%, placebo 17%), vomiting (ExQW 17%, placebo 22%) and diarrhoea (ExQW 30%, placebo 17%). Injection site reactions were common (ExQW 30%, placebo 22%). One patient on ExQW was withdrawn from the study because of an increasingly severe localized cutaneous sensitivity reaction. Additional AEs reported included constipation, dizziness and headache (Table S3).
Two subjects (both receiving ExQW) had increases of circulating lipase levels, which were not accompanied by clinical evidence for pancreatitis and normalized spontaneously without interruption of study intervention. There were no treatment‐related increases of calcitonin (Table 3) and no occurrence of thyroid AEs.
TABLE 3.
Estimated 36‐week change by treatment of laboratory safety measures
| ExQW | Placebo | ||||
|---|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | P‐value | |
| Lipase (IU/L) | 23.7 | (−19.1 to 66.5) | −7.9 | (−20.1 to 4.3) | .15 * |
| Amylase (IU/L) | 7.5 | (−2.5 to 17.5) | −2.1 | (−4.6 to 0.4) | .03 * |
| Fasting glucose (mg/dL) | −2.5 | (−10.3 to 5.2) | 2.0 | (−11.9 to 15.9) | .99 * |
| White blood cell (K/cmm) | −0.3 | (−1.1 to 0.6) | −0.9 | (−1.9 to 0.2) | .94 * |
| Haematocrit (%) | −0.4 | (−1.4 to 0.6) | 0.6 | (−0.5 to 1.7) | .14 * |
| Sodium (mEq/L) | 0.2 | (−1.3 to 1.8) | 0.7 | (−0.3 to 1.7) | .81 |
| Potassium (mEq/L) | 0.1 | (−0.1 to 0.2) | −0.1 | (−0.2 to 0.1) | .32 |
| Aspartate aminotransferase (IU/L) | 6.2 | (0.7 to 11.8) | −2.4 | (−9.3 to 4.6) | .17 * |
| Alanine aminotransferase (IU/L) | 13.2 | (1.7 to 24.8) | −0.7 | (−9.6 to 8.2) | .09 * |
| Calcitonin (pg/mL) | −0.1 | (−0.6 to 0.5) | 0.0 | (−0.1 to 0.0) | .76 * |
| Carcinoembryonic antigen (ng/mL) | 0.1 | (0.0 to 0.2) | −0.1 | (−0.1 to 0.0) | .003 * |
Abbreviation: ExQW, exenatide once‐weekly.
Data are from the full intent to treat analysis set with last observation carried forward imputation. Corresponding means (95% CI) are from analyses using non‐transformed data to provide insight into magnitude of change.
P‐values are from non‐normally distributed data that were transformed for analyses.
Six serious AEs (SAEs) requiring hospitalization were reported among four patients in the ExQW group during the study: one patient with increased seizure activity (age 23 years; at weeks 19 and 25 of treatment); one patient with altered mental status, hypernatremia and suspicion of seizure activity (age 12 years; weeks 38 and 43 of treatment); one patient with migraine headache (age 21 years; week 46 of treatment); and one patient with cellulitis of the right arm requiring IV antibiotic therapy (age 11 years; week 36 of treatment, determined as definitely unrelated to study drug). Two SAEs were reported in one patient from the placebo group; both were for hypernatremic dehydration (age 22 years; weeks 15 and 29 of treatment) requiring hospitalization. One patient (age 23) from the placebo group developed cholecystitis 13 weeks into the open‐label extension and was admitted for cholecystectomy (Table S3). Overall, there was no difference in the rates of patients with SAEs during the randomized arm of the study (ExQW 9%, placebo 6%; P > .99).
Bone age increased as expected for age and similarly in both treatment arms (ExQW: mean bone age increased 0.9 years, 95% CI 0 to 1.8; placebo: mean 1.3 years, 95% CI 0.2 to 2.4). Odds of abnormal 36‐week ECG results compared with baseline were not significantly greater for either treatment.
4. DISCUSSION
Severe obesity occurs in 50% of CP survivors despite optimal endocrine management of hypopituitarism, and is associated with profound morbidity, mortality and reduced functional capacity and quality of life. 28 HO is one of the most refractory types of childhood obesity and novel treatment options are necessary. 16 , 28 , 29 GLP‐1 RAs are well established for the treatment of type 2 diabetes in patients older than 10 years, and are associated with significant reductions in body weight and fat mass in adults and children; they are potent stimulators of glucose‐dependent insulin secretion and modulators of satiety and energy intake. 19 , 30
GLP‐1 RA treatment is a novel approach for HO intervention because the mechanism of action is not entirely dependent on intact hypothalamic structures. GLP‐1 binds to receptors in the vagus nerve as well as to appetite‐related sites in the hindbrain (nucleus of the solitary tract, area postrema) and the hypothalamus (arcuate and dorsomedial nuclei). 31 GLP‐1 functions as a satiety hormone, promoting reduced food intake and more rapid meal termination, 32 as well as modulating activity in appetite‐ and reward‐related brain areas. 33 , 34
Our randomized clinical trial shows the effects of GLP‐1 RA therapy to reduce or stabilize BMI and body fat compared with steady increases in the placebo group. The change in body fat between the two groups appears clinically relevant and confirms that some GLP‐1 RA actions are independent of intact hypothalamic pathways. These results further support the role of the hindbrain in feeding regulation. It is not clear why the change in body fat was greater than the change in BMI. Interestingly, similar findings have been seen in pilot studies of weight loss interventions for Prader‐Willi syndrome. 35 , 36 Because BMI is a measure of total weight adjusted for height, it includes both fat and fat‐free body mass. While the change in lean mass was not statistically significant, the decrease seen in the placebo group may have been enough to affect BMI and offset the change seen in BMI between the ExQW and placebo groups. In short, BMI alone may not adequately assess the change in fat mass. 36
The majority of AEs were expected and similar to those reported for GLP‐1 RA medications. Additional potential safety concerns with GLP‐1 RAs include early pubertal changes because of activation of central pathways. 37 We saw neither pubertal advancement nor changes in bone maturation in our study group. This study supports the safety of GLP‐1 RAs in peripubertal children.
Waist circumference showed a statistically significant difference in changes between the treatment groups. Waist circumference has repeatedly been shown to be a reliable predictor for risk of cardiovascular disease, and is associated with low HDL, and high triglycerides and fasting insulin. 38 Reduction of waist circumference is a clinically meaningful change related to ExQW therapy. Lowering the risk for development of cardiovascular disease is important for adolescents with obesity.
This study has several strengths. A double‐blind, randomized, placebo‐controlled study design was used and we successfully enrolled a large population of paediatric patients. Subjects had high adherence to study medication and ExQW was well tolerated. The analysis included an ITT approach and evaluation of data in completers. Body fat was assessed by DXA scan and metabolic variables including glucose tolerance testing were obtained.
There are potential study limitations. First, we did not completely reach our enrolment goal. However, the dropout rate was low, 16.7% in the ExQW group and 21% in the placebo group. There was a wide range of obesity severity at baseline. Furthermore, we used a comparatively low, fixed dose in this group of heterogenous patients with extreme obesity. Dose escalation with either this medication, or another GLP‐1 RA, could achieve greater efficacy. Indeed, in a recent publication in adolescents with severe obesity, escalation to a high dose of liraglutide (3 mg per day) over a 56‐week intervention showed a 5% BMI reduction in 43% versus 19% in the placebo‐treated group. 22 There may be a difference in hypothalamic injury that impacts the response to therapy. Previous studies indicate that the degree of injury impacts the degree of HO. 5 , 6 , 7 , 8 Further studies in this population may benefit from additional stratification based on the degree of hypothalamic damage.
In summary, this is the first randomized controlled clinical trial testing a GLP‐1 RA in adolescent and young adult subjects with HO because of hypothalamic injury. The shown GLP‐1 RA effects in patients with hypothalamic dysfunction not only open new avenues for intervention in a difficult‐to‐treat form of obesity but also contribute to a better understanding of GLP‐1 physiology. While the overall results are encouraging, as we observed relative reductions in body fat mass, the heterogeneity of response to ExQW therapy suggests that some patients are more probable to respond. More research is needed to understand the factors that have an impact on or may predict treatment success as well as strategies for identifying the optimal drug dose.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
CLR, MJA and AHS performed the study as investigators. FAP evaluated all neuroimaging scans as well as DXA results. JAY gave advice in planning and conducting the trial. KBW and CE performed statistical analyses, created figures and tables, and summarized the statistical approach and results in the paper. CLR wrote the first draft of the paper, which was further developed and edited by MJA, AHS, KBW and FAP. JAY reviewed and edited the final draft. AHS and MJA contributed equally to this study.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14224.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGEMENTS
We thank the patients and their families for participating in this study. We are also grateful to our study coordinators who organized all study visits and collected data. Finally, we thank our team for statistical analysis and the data monitoring team for oversight of potential safety concerns of the study. Astra Zeneca supported our study by providing the active drug and matching placebo but played no role in the design, interpretation of results, or decision to publish. This study was supported by R01DK104936 (CLR, MJA and AHS). AHS was supported by K23DK101689. CLR was also supported by unrelated studies of treatments of obesity R01DK098466 and R41 DK120236. CLR, AHS and JAY report receiving grant funds for unrelated studies of treatments for rare genetic forms of obesity from Rhythm Pharmaceuticals, Inc.; MJA, AHS and JAY report receiving grant funds for unrelated studies from Soleno Therapeutics, Inc.; JAY reports receiving grant support from the Intramural Research Program of NICHD, NIH for studies of obesity.
Roth CL, Perez FA, Whitlock KB, et al. A phase 3 randomized clinical trial using a once‐weekly glucagon‐like peptide‐1 receptor agonist in adolescents and young adults with hypothalamic obesity. Diabetes Obes Metab. 2021;23:363–373. 10.1111/dom.14224
Funding information National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: R01DK104936
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Muller HL. Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow‐up. Horm Res. 2008;69(4):193‐202. [DOI] [PubMed] [Google Scholar]
- 2. Simoneau‐Roy J, O'Gorman C, Pencharz P, Adeli K, Daneman D, Hamilton J. Insulin sensitivity and secretion in children and adolescents with hypothalamic obesity following treatment for craniopharyngioma. Clin Endocrinol. 2010;72(3):364‐370. [DOI] [PubMed] [Google Scholar]
- 3. Bereket A, Kiess W, Lustig RH, et al. Hypothalamic obesity in children. Obes Rev. 2012;13(9):780‐798. [DOI] [PubMed] [Google Scholar]
- 4. Elowe‐Gruau E, Beltrand J, Brauner R, et al. Childhood craniopharyngioma: hypothalamus‐sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab. 2013;98(6):2376‐2382. [DOI] [PubMed] [Google Scholar]
- 5. Muller HL, Gebhardt U, Teske C, et al. Post‐operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3‐year follow‐up. Eur J Endocrinol. 2011;165(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 6. Muller HL, Merchant TE, Puget S, Martinez‐Barbera JP. New outlook on the diagnosis, treatment and follow‐up of childhood‐onset craniopharyngioma. Nat Rev Endocrinol. 2017;13(5):299‐312. [DOI] [PubMed] [Google Scholar]
- 7. Roth CL, Eslamy H, Werny D, Elfers C, et al. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity. 2015;23(6):1226‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lustig RH. Hypothalamic obesity after craniopharyngioma: mechanisms, diagnosis, and treatment. Front Endocrinol. 2011;2:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roth CL. Hypothalamic obesity in patients with craniopharyngioma: profound changes of several weight regulatory circuits. Front Endocrinol. 2011;2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875‐880. [DOI] [PubMed] [Google Scholar]
- 11. Greenway FL, Bray GA. Treatment of hypothalamic obesity with caffeine and ephedrine. Endocr Pract. 2008;14(6):697‐703. [DOI] [PubMed] [Google Scholar]
- 12. Hamilton JK, Conwell LS, Syme C, Ahmet A, Jeffery A, Daneman D. Hypothalamic obesity following Craniopharyngioma surgery: results of a pilot trial of combined Diazoxide and metformin therapy. Int J Pediatr Endocrinol. 2011;2011:417949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lustig RH, Hinds PS, Ringwald‐Smith K, et al. Octreotide therapy of pediatric hypothalamic obesity: a double‐blind, placebo‐controlled trial. J Clin Endocrinol Metab. 2003;88(6):2586‐2592. [DOI] [PubMed] [Google Scholar]
- 14. Danielsson P, Janson A, Norgren S, Marcus C. Impact sibutramine therapy in children with hypothalamic obesity or obesity with aggravating syndromes. J Clin Endocrinol Metab. 2007;92(11):4101‐4106. [DOI] [PubMed] [Google Scholar]
- 15. Ismail D, O'Connell MA, Zacharin MR. Dexamphetamine use for management of obesity and hypersomnolence following hypothalamic injury. J Pediatr Endocrinol Metab. 2006;19(2):129‐134. [DOI] [PubMed] [Google Scholar]
- 16. Abuzzahab MJ, Roth CL, Shoemaker AH. Hypothalamic obesity: prologue and promise. Horm Res Paediatr. 2019;91(2):128‐136. [DOI] [PubMed] [Google Scholar]
- 17. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of Liraglutide in weight management. N Engl J Med. 2015;373(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 18. Rosenstock J, Klaff LJ, Schwartz S, et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre‐diabetes. Diabetes Care. 2010;33(6):1173‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double‐blind, placebo and active controlled, dose‐ranging, phase 2 trial. Lancet. 2018;392(10148):637‐649. [DOI] [PubMed] [Google Scholar]
- 20. Lomenick JP, Buchowski MS, Shoemaker AH. A 52‐week pilot study of the effects of exenatide on body weight in patients with hypothalamic obesity. Obesity. 2016;24(6):1222‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weghuber D, Forslund A, Ahlstrom H, et al. A 6‐month randomized, double‐blind, placebo‐controlled trial of weekly exenatide in adolescents with obesity. Pediatr Obes. 2020;15(7):e12624. [DOI] [PubMed] [Google Scholar]
- 22. Kelly AS, Auerbach P, Barrientos‐Perez M, et al. A randomized, controlled trial of Liraglutide for adolescents with obesity. N Engl J Med. 2020;382(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 23. Elfers CT, Simmons JH, Roth CL. Glucagon‐like peptide‐1 agonist exendin‐4 leads to reduction of weight and caloric intake in a rat model of hypothalamic obesity. Horm Res Paediatr. 2012;78(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 24. Simmons JH, Shoemaker AH, Roth CL. Treatment with glucagon‐like peptide‐1 agonist exendin‐4 in a patient with hypothalamic obesity secondary to intracranial tumor. Horm Res Paediatr. 2012;78(1):54‐58. [DOI] [PubMed] [Google Scholar]
- 25. Zoicas F, Droste M, Mayr B, Buchfelder M, Schofl C. GLP‐1 analogues as a new treatment option for hypothalamic obesity in adults: report of nine cases. Eur J Endocrinol. 2013;168(5):699‐706. [DOI] [PubMed] [Google Scholar]
- 26. Morton GJ, Blevins JE, Williams DL, et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115(3):703‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flynn JT, Mitsnefes M, Pierce C, et al. Blood pressure in children with chronic kidney disease: a report from the chronic kidney disease in children study. Hypertension. 2008;52(4):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muller HL. Childhood craniopharyngioma–current concepts in diagnosis, therapy and follow‐up. Nat Rev Endocrinol. 2010;6(11):609‐618. [DOI] [PubMed] [Google Scholar]
- 29. Roth CL. Hypothalamic obesity in Craniopharyngioma patients: disturbed energy homeostasis related to extent of hypothalamic damage and its implication for obesity intervention. J Clin Med. 2015;4(9):1774‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 31. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP‐1 receptor agonist liraglutide‐dependent weight loss. J Clin Invest. 2014;124(10):4473‐4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma X, Bruning J, Ashcroft FM. Glucagon‐like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci. 2007;27(27):7125‐7129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Farr OM, Sofopoulos M, Tsoukas MA, et al. GLP‐1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP‐1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo‐controlled trial. Diabetologia. 2016;59(5):954‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Bloemendaal L, RG IJ, Ten Kulve JS, et al. GLP‐1 receptor activation modulates appetite‐ and reward‐related brain areas in humans. Diabetes. 2014;63(12):4186‐4196. [DOI] [PubMed] [Google Scholar]
- 35. Kimonis V, Surampalli A, Wencel M, Gold JA, Cowen NM. A randomized pilot efficacy and safety trial of diazoxide choline controlled‐release in patients with Prader‐Willi syndrome. PLoS One. 2019;14(9):e0221615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wells JC, Coward WA, Cole TJ, Davies PS. The contribution of fat and fat‐free tissue to body mass index in contemporary children and the reference child. Int J Obes Relat Metab Disord. 2002;26(10):1323‐1328. [DOI] [PubMed] [Google Scholar]
- 37. Farkas I, Vastagh C, Farkas E, et al. Glucagon‐like peptide‐1 excites firing and increases GABAergic miniature postsynaptic currents (mPSCs) in gonadotropin‐releasing hormone (GnRH) neurons of the male mice via activation of nitric oxide (NO) and suppression of Endocannabinoid signaling pathways. Front Cell Neurosci. 2016;10:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist‐to‐height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Relat Metab Disord. 2000;24(11):1453‐1458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


