Abstract
Background
The Society for Cardiac Angiography and Interventions (SCAI) Shock Classification has been retrospectively validated by several groups. We sought to prospectively study outcomes of consecutive patients with reference to initial SCAI Shock Stage and therapeutic strategy as well as 24 hr SCAI Shock Stage reassessment.
Methods
Kaplan Meier method was used to describe survival and Cox Proportional hazards modeling used to assess predictors of survival.
Results
Over an 18‐month period, 166 patients were referred for evaluation. Demographics, hemodynamics, and most laboratory findings were similar between SCAI stages, which were assigned by the team. Initial SCAI Stage was a strong predictor of survival. Thirty‐day survival was 100, 65.4, 44.2, and 60% for patients with initial SCAI shock stage B, C, D, and E respectively (p = .0004). Age and initial SCAI Shock Stage were shown to be the strongest predictors of survival by Cox proportional hazards. Mode of mechanical circulatory support (MCS) or lack of such was not a predictor of outcome. Shock stage at 24 hr was also examined. Thirty‐day survival was 100, 96.7, 66.9, 21.6, and 6.2% for patients with 3–4 SCAI stage improvement, 2 stage improvement, 1 stage improvement, no change in SCAI stage and worsening of SCAI stage respectively (p < .0001).
Conclusions
Initial SCAI Shock stage predicts the survival of unselected patients with a variety of MCS interventions and medical therapy alone. The 24‐hr reassessment of shock stage further refines the prognosis.
Keywords: cardiogenic shock, classification, mechanical circulatory support, mortality
1. What is known
The Society for Cardiac Angiography and Intervention (SCAI) Shock classification is simple to apply and can be assessed with a variety of clinical inputs.
This classification has been retrospectively validated in large populations with varying definitions (post hoc).
SCAI stage D indicating deterioration is a poor prognostic sign.
2. What the study adds
First prospective validation of the SCAI Shock staging system.
SCAI Stage at time of shock team consult predicts mortality.
Demographics, hemodynamics, left ventricular ejection fraction, and laboratory values do not clearly correlate with SCAI stage as assigned by the multidisciplinary team
Prognostic value was maintained whether the patient was treated with circulatory support or medical management alone.
24‐hr reassessment of change in SCAI stage further refines mortality prediction.
1. INTRODUCTION
Cardiogenic shock (CS) is defined as a deficit in cardiac output, which results in a state of hypoperfusion, often associated with severe refractory hypotension. CS in the setting of acute myocardial infarction represents approximately half of cases, with others due to exacerbation of systolic heart failure or other causes. The only trial to show a positive effect of an intervention was the, should we emergently revascularize occluded coronaries for cardiogenic shock (SHOCK) trial which was published more than 20 years ago. 1 With the increasing availability of percutaneous mechanical circulatory support (MCS) devices, there has been increasing interest in CS, though no device has shown superior outcomes in a randomized trial. 2 , 3 , 4 , 5 , 6
The American Heart Association recently released a Scientific Statement on CS, which suggested that shock care could be effectively rendered in specialty centers, and that algorithmic approaches to care may have value. 7 Recent work from two centers suggests that improvements in outcomes could be achieved using the current devices with multidisciplinary collaboration and a formal “shock team” approach. 8 , 9 One issue, however, which had not been addressed was the lack of a way to classify the various grades of CS in a standardized fashion.
To address these concerns, the Society for Cardiac Angiography and Intervention (SCAI) assembled a multidisciplinary group and the SCAI Clinical Expert Consensus on the Classification of CS was published in 2019. 10 This document described a 5‐stage schema dividing shock into at risk, beginning shock, classic CS, deteriorating, and extremis stage of CS (stages A‐E) as shown in Figure 1. The intention of the authors was that the schema would be validated, retrospectively, and then prospectively and hopefully serve as a foundation for future trials, which would assign a SCAI shock stage at the time of enrollment. Separating groups of CS patients prospectively might allow insights into utilization of medical and device therapies, and perhaps show which patient groups respond particularly well and which may not be helped by any intervention studied.
FIGURE 1.
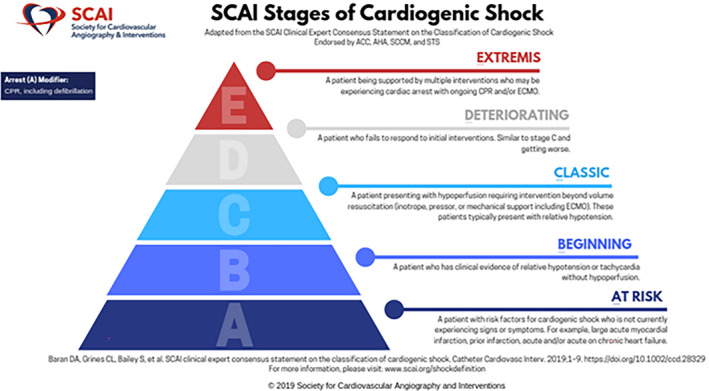
SCAI Shock Stage Classification Pyramid. The SCAI Shock stages are illustrated in this figure which is from the SCAI Shock Center website. It illustrates the stages from A to E, with a pyramid structure reflecting that the number of patients is highest in stage A (at risk) and lowest at the extremes (extremis patients). Used with permission from SCAI (Copyright of SCAI)—Email confirming permission available on request. SCAI, Society for Cardiac Angiography and Interventions [Color figure can be viewed at wileyonlinelibrary.com]
The authors have been prospectively recording the initial and 24‐hr SCAI Shock Stage for all patients referred to their shock team since 2019. The outcomes of patients, stratified by initial SCAI Shock Stage are described along with the initial therapeutic approach chosen.
2. METHODS
Sentara Heart Hospital has a multidisciplinary CS team with representation from interventional cardiology, advanced heart failure, critical care, cardiothoracic surgery, and cardiac critical care nursing. The team receives calls via a central number on a 24 hr/365‐day basis and all team members have access to the shock guideline algorithm that has been developed (Figure 2, Panel A). The patient's SCAI Shock Stage is assessed by consensus and recorded in the initial consult note in the electronic medical record. The Shock coordinator along with the team see each patient daily and a standardized note is placed including SCAI shock stage at 24 hr and thereafter. Data is maintained in a secure quality improvement REDCAP database. Use of this deidentified dataset was authorized via a waiver of informed consent by the Eastern Virginia Medical School Institutional Review Board.
FIGURE 2.
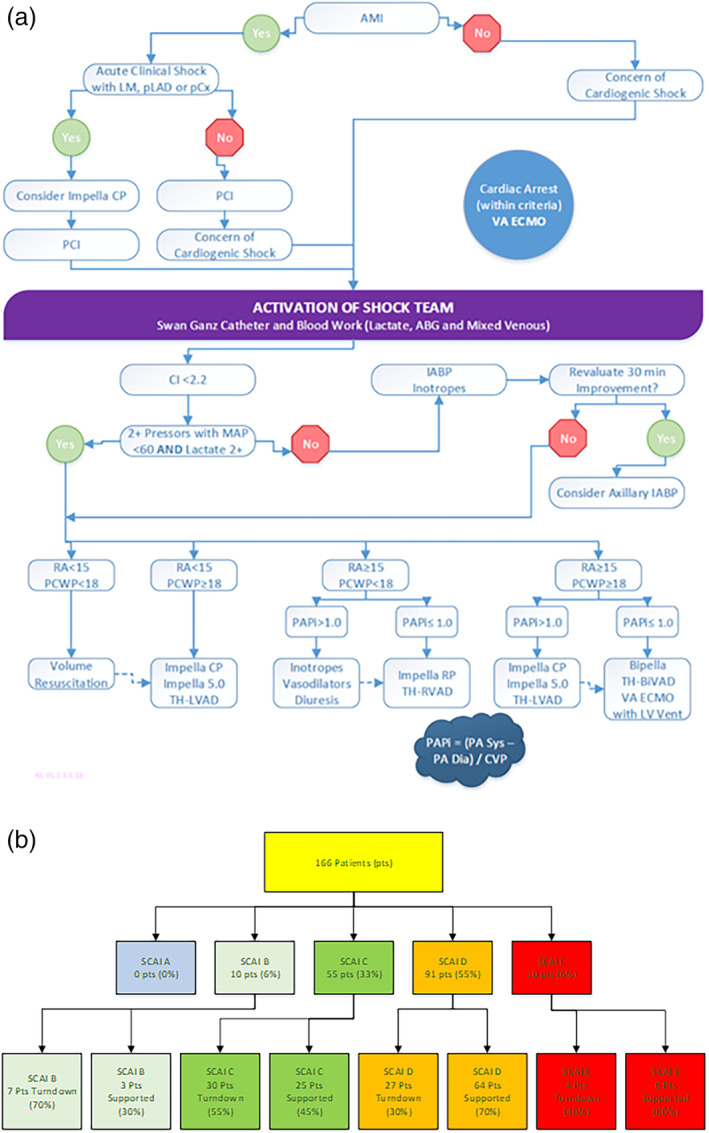
Panel A: Sentara Heart Hospital Shock Team Algorithm. The shock team developed a hybrid algorithm as a guide to operations. This is not intended to be utilized in a rigid fashion but rather to emphasize the importance of hemodynamics and matching support strategy to the particular physiologic disturbances, which are noted. Panel B: Flow diagram of patients seen by the Shock Team. The team evaluated 166 patients and the initial consult indicated the SCAI shock stage. As expected, the team did not receive referrals for stage A (“at risk”) patients who were managed by the general cardiology team. The figure illustrates the percentage of patients who went on to receive mechanical circulatory support and which were followed by a noninvasive route. IABP, intra‐aortic balloon pump; SCAI, Society for Cardiac Angiography and Interventions [Color figure can be viewed at wileyonlinelibrary.com]
Statistics were analyzed with JMP 14.1 (SAS, Cary, NC). The p < .05 was considered significant. Demographics of shock team referrals are reported with descriptive statistics including mean or median and SD or inter‐quartile range as appropriate. Demographics of the groups were compared by Student's t test. Survival from the time of initial referral to last known medical contact was analyzed by Kaplan Meier method, and curves compared with Mantel‐Cox logrank statistics. Cox proportional hazards modeling was employed to assess prognostic value of various factors.
3. RESULTS
From January 4, 2019 to June 3, 2020, the Sentara Shock Team was activated for 166 patient emergencies. All consecutive patients are reported herein without exclusions. Most of the patients had CS but there were some patients where the primary issue was refractory lung failure necessitating veno–veno (VV) extra‐corporeal membrane oxygenation (ECMO). Figure 2, Panel B illustrates the flow of patients through the shock team evaluation including the initial SCAI Stage as assessed. The initial SCAI Shock stages were as follows, no patients in stage A, 10 patients in stage B, 55 patients in stage C, 91 patients in stage D, and 10 patients in stage E (total n = 166). After initial assessment, the Shock Team decided to implement MCS, or use medical therapy alone. Importantly, medical therapy patients were followed by the shock team and managed aggressively with pressors or inotropes as indicated and intensive care, often directed by pulmonary artery catheterization data. All patients were followed throughout hospitalization and with particular attention to a 24‐hr reassessment in each case. Medical therapy was chosen rather than MCS in 70% of stage B patients (7/10), 54.6% of stage C (30/55), 29.7% of stage D (27/91), and 40% of stage E (4/10) with the difference between groups noted to be highly statistically significant (p = .006).
Detailed data about the patient population is presented in Table 1. The average patient age was 58.1 ± 16.2 years old. Most patients were male (60.2%), and ethnicity was split between white (45.2%) and African American (42.2%). Smoking was highly prevalent (either former or current) and only 40% of patients never smoked. Diabetes was present in 38.6% of patients along with hypertension in 75.9%. A history of heart failure was noted in 60% of patients, and 15.8% had a prior sternotomy. STEMI was present in 29.9% of patients and 65.1% of patients were intubated, along with 33.7% who had a cardiac arrest prior to Shock Team activation.
TABLE 1.
Description of patients grouped by initial SCAI Shock stage
| Factor | SCAI B | SCAI C | SCAI D | SCAI E | p‐value for comparison | # pts with data |
|---|---|---|---|---|---|---|
| Number of patients | 10 | 55 | 91 | 10 | – | 166 |
| Turndown for mechanical circulatory support (%) | 70 | 54.5 | 29.7 | 40 | .0006 | 166 |
| Days from admit to shock consult (median, 25th and 75th quartile) | 2, 0–8.25 | 1, 0–6 | 1, 0–3 | 1.5, 0–7.25 | .63 | 166 |
| Age (mean ± SD) | 44.2 ± 15.9 | 57 ± 15.5 | 60 ± 16 | 61.7 ± 17.4 | .02 | 166 |
| Male (%) | 60 | 63.6 | 58.2 | 60 | .94 | 166 |
| Race: White (%) | 20 | 45.5 | 49.5 | 30 | .4 | 75 |
| Race: Black (%) | 70 | 43.6 | 37.4 | 50 | .4 | 70 |
| Height (cm) | 169.7 ± 10.2 | 174.5 ± 9.1 | 171.3 ± 12.5 | 169.9 ± 13.4 | .31 | 166 |
| Weight (kg) | 83.9 ± 35.1 | 90.4 ± 25.4 | 87.5 ± 22.5 | 88.6 ± 19.8 | .8 | 166 |
| Current smoker (%) | 11.1 | 24.5 | 20.5 | 30 | .04 | 155 |
| Former smoker (%) | 44.4 | 39.6 | 32.5 | 70 | .04 | 155 |
| Diabetic (%) | 30 | 43.6 | 31.9 | 80 | .02 | 166 |
| Lung disease (COPD or asthma) (%) | 50 | 45.5 | 40.7 | 80 | .11 | 166 |
| HTN (%) | 80 | 72.7 | 76.9 | 80 | .9 | 166 |
| Heart failure history (%) | 80 | 63.6 | 54.6 | 60 | .36 | 163 |
| Prior sternotomy (%) | 0 | 20 | 13.3 | 30 | .12 | 165 |
| STEMI (%) | 0 | 20 | 37.8 | 40 | .007 | 164 |
| Intubated (%) | 30 | 52.7 | 74.7 | 80 | .003 | 166 |
| Cardiac arrest (%) | 10 | 27.3 | 36.3 | 70 | .02 | 166 |
| Left Main coronary 50% stenosis or higher (%) | 50 | 37 | 64.7 | 75 | .1 | 86 |
| Obstructive CAD (>70%) (%) | 25 | 55.6 | 73.5 | 50 | .13 | 84 |
| ECHO LVEF % | 31.3 ± 23.4 | 31.4 ± 22.4 | 30.8 ± 20.5 | 42.3 ± 21.2 | .45 | 165 |
| ECHO LVEDD (cm) | 5.4 ± 1.3 | 5.5 ± 1.6 | 4.8 ± 1.1 | 4.4 ± 0.8 | .02 | 153 |
| ECHO notes severe RV dysfunction (%) | 11.1 | 14.6 | 10 | 10 | .56 | 164 |
| ECHO notes significant valve disease (%) | 22.2 | 36.5 | 34.5 | 33.3 | .86 | 157 |
| Hemodynamics | ||||||
| Systolic blood pressure (median, 25th and 75th quartile) | 106.5 (101–147) | 109 (95–117) | 101 (91–123) | 107 (83.8–138) | .42 | 166 |
| Diastolic blood pressure (median, 25th and 75th quartile) | 69.5 (60.8–91) | 67 (57–75) | 65 (56–77) | 66.5 (49.8–81.5) | .63 | 166 |
| Mean arterial blood pressure (median, 25th and 75th quartile) | 81 (73.3–114.3) | 78 (73–90) | 77 (70–90) | 79 (58.8–105.3) | .58 | 166 |
| Heart rate (mean ± SD) | 104.7 ± 25.7 | 95.4 ± 20.9 | 99.8 ± 26.1 | 105.5 ± 24.8 | .48 | 165 |
| RA pressure | 11 ± 5.3 | 17.6 ± 8.6 | 17.7 ± 7 | 13 ± 0 | .5 | 51 |
| PA systolic | 49.3 ± 12.5 | 47.2 ± 16.5 | 44.4 ± 15.4 | 55 ± 0 | .83 | 56 |
| PA diastolic | 28 ± 5.6 | 25.9 ± 7.4 | 23.9 ± 8.1 | 30 ± 0 | .6 | 56 |
| Pulmonary capillary wedge pressure | 23.3 ± 6.5 | 26.4 ± 8.2 | 24.1 ± 7.3 | 26 ± 0 | .75 | 49 |
| Pulmonary artery saturation of oxygen | 63.7 ± 11.6 | 47.1 ± 19.2 | 49.9 ± 19.2 | 66 ± 0 | .46 | 28 |
| Cardiac output (thermodilution) | 6.51 ± 3.9 | 5 ± 1.9 | 5 ± 2.3 | 4.8 ± 0 | .9 | 14 |
| Cardiac index (thermodilution) | 3.18 ± 1.6 | 2.2 ± 0.8 | 2.3 ± 1.1 | 1.98 ± 0 | .65 | 14 |
| Cardiac output (Fick method) | 4.7 ± 2.4 | 4.1 ± 1.7 | 4.3 ± 2 | – | .31 | 44 |
| Cardiac index (Fick method) | 2.3 ± 0.9 | 2.2 ± 1 | 2.1 ± 0.9 | – | .77 | 44 |
| CPO (using Fick CO) | 0.77 ± 0.41 | 0.76 ± 0.36 | 0.79 ± 0.38 | – | .97 | 43 |
| Labs | ||||||
| White blood cell count | 10.2 ± 4.2 | 12.1 ± 6.1 | 14.6 ± 8.9 | 12.3 ± 5.9 | .28 | 165 |
| Hemoglobin | 10.6 ± 2.5 | 12.2 ± 2.8 | 10.9 ± 2.7 | 10 ± 3.2 | .02 | 166 |
| Sodium | 138.9 ± 6.2 | 137.1 ± 4.8 | 138.9 ± 6.6 | 139.1 ± 6.8 | .37 | 166 |
| Serum creatinine | 2.8 ± 4.1 | 1.7 ± 1.6 | 1.7 ± 1.6 | 2.1 ± 1.7 | .36 | 166 |
| INR | 1.2 ± 0.2 | 1.5 ± 0.8 | 1.7 ± 0.7 | 2 ± 1.4 | .16 | 78 |
| GFR (mean ± SD) | 43.3 ± 19.1 | 47.7 ± 15.5 | 46.0 ± 15.9 | 43.5 ± 18.3 | .78 | 165 |
| Troponin (median, 25th and 75th quartile) | 0.3 (0.23–0.58) | 0.08 (0.02–0.91) | 0.2 (0.06–2.8) | 3.9 (0.01–7.84) | .53 | 72 |
| Lactate (median, 25th and 75th quartile) | 2.5 (1.2–13.5) | 2.2 (1.35–5.95) | 5.55 (2.4–8.4) | 2.4 (1.35–6.53) | .03 | 81 |
| AST (median, 25th and 75th quartile) | 24 (8–109) | 45 (20–377) | 228 (36–619) | 100 (33–673) | .07 | 87 |
| Procalcitonin (median, 25th and 75th quartile) | 0.3 (0.3–0.3) | 0.65 (0.2–3.1) | 0.5 (0.3–3.2) | 6.9 (0.2–13.6) | .93 | 41 |
| Lactate 24 hr (median, 25th and 75th quartile) | 1.4 (0.52–2.6) | 1.9 (1.3–3.3) | 4.03 (1.4–9.45 | 1.35 (1.1–2.1) | .02 | 74 |
| Procalcitonin 24 hr (median, 25th and 75th quartile) | – | 1 (0.2–2.4) | 9.6 (1.7–69.7) | 4.5 (4.5–4.5) | .01 | 30 |
| Infusions | ||||||
| Norepinephrine drip (initial) (percentage) | 10 | 40 | 61.5 | 30 | .001 | 166 |
| Epinephrine drip (initial) (percentage) | 10 | 27.3 | 44 | 40 | .05 | 166 |
| Neosynephrine drip (initial) (percentage) | 0 | 5.5 | 14.3 | 20 | .11 | 166 |
| Dopamine drip (initial) (percentage) | 0 | 3.6 | 16.5 | 19.1 | .003 | 166 |
| Dobutamine drip (initial) (percentage) | 30 | 14.6 | 22 | 10 | .46 | 166 |
| Milrinone drip (initial) (percentage) | 50 | 30.9 | 23.1 | 20 | .28 | 166 |
| Vasopressin drip (initial) (percentage) | 10 | 25.5 | 34.1 | 10 | .13 | 166 |
| # concurrent vasoactive agents‐initial (median, 25th and 75th quartile) | 1 (0–1.25) | 1 (1–2) | 2 (1–3) | 2 (1–2.3) | .01 | 166 |
| # concurrent inotropic agents‐initial (median, 25th and 75th quartile) | 1 (0–1.25) | 1 (0–1) | 1 (0–1) | 1 (0–1) | .47 | 166 |
| Norepinephrine drip (24 hr) (percentage) | 0 | 49.1 | 65.6 | 50 | .0001 | 165 |
| Epinephrine drip (24 hr) (percentage) | 10 | 36.4 | 57.8 | 30 | .002 | 165 |
| Neosynephrine drip (24 hr) (percentage) | 0 | 12.7 | 16.7 | 10 | .29 | 165 |
| Dopamine drip (24 hr) (percentage) | 0 | 12.7 | 22.2 | 12.9 | .03 | 165 |
| Dobutamine drip (24 hr) (percentage) | 10 | 16.4 | 31.1 | 0 | .01 | 165 |
| Milrinone drip (24 hr) (percentage) | 50 | 43.6 | 28.9 | 10 | .06 | 165 |
| Vasopressin drip (24 hr) (percentage) | 10 | 38.2 | 54.4 | 40 | .02 | 165 |
| # concurrent vasoactive agents‐24 hr (median, 25th and 75th quartile) | 1 (0–1.25) | 2 (1–3) | 3 (2–4) | 2 (1–3) | .0003 | 166 |
| # concurrent inotropic agents‐24 hr (median, 25th and 75th quartile) | 0.5 (0–1.25) | 1 (0–1) | 1 (1–2) | 0 (0–1) | .02 | 166 |
Note: All statistically significant values (p <.05) are in bold.
Abbreviation: SCAI, Society for Cardiac Angiography and Interventions.
In Table 1, patients are compared based on initial SCAI Shock Stage. Younger patients had a lower SCAI stage in general. The ages were 44.2 ± 15.9, 57 ± 15.5, 60 ± 16, and 61.7 ± 17.4 (years ± SD), respectively, for SCAI B, C, D, and E (p = .02). The gender, race, as well as body size did not vary across SCAI stages. ST‐elevation myocardial infarction was significantly more common as SCAI stage increased as was intubation and cardiac arrest. Obstructive coronary disease and left ventricular ejection fraction were not different across groups. The left ventricular end diastolic dimension was lower across SCAI stages however, with smaller left ventricles associated with more severe shock stage (p = .02). However, arterial blood pressure and invasively measured right heart hemodynamics as well as most laboratory values were not significantly different. Lactate was higher in stage D patients, but less than half of patients had this available at initial consult.
The Shock Team made a multidisciplinary consensus treatment plan following the initial consult. For stage B, two patients received an intra‐aortic balloon pump (IABP) and one was placed on VV ECMO. For stage C, one was prioritized for heart transplant but did not have MCS, 20/55 (36.4%) received IABP placement, with one Impella CP (Abiomed, Danvers, MA), one surgical RVAD and two cases of VV ECMO. For stage D, one patient received a durable ventricular assist device, 30/91 (32%) received IABP placement, 10 received Impella CP alone, one underwent Impella CP along with VA ECMO, one percutaneous RVAD, 15 veno‐arterial (VA) ECMO, and six VV ECMO placements. For stage E, one was emergently transplanted without mechanical support, one underwent IABP placement, and four received VA ECMO. The length of time on first chosen support strategy was not statistically different across SCAI stages: SCAI B, 7 ± 2 days, SCAI C, 4.9 ± 4.1, SCAI D, 5.1 ± 4.8 days, and SCAI E, 2.7 ± 0.6 days (p = .69, one way ANOVA).
Use of pressors is noted in Table 1 as well. As SCAI stage increases, the number of vasopressors rises significantly (p = .01) but the use of inotropes (typically one agent) is similar. This relationship is similar at 24 hr with notable reduction in the use of inotropes and vasopressors in patients with a lower SCAI shock stage. Doses are not available in the database.
3.1. Survival
Of 166 patients, 52.4% were discharged alive, 42.8% died in the intensive care unit, and 4.8% died prior to discharge following intensive care unit care. There was a significant difference in outcome by initial SCAI Shock Stage. None of the SCAI B patients died in the hospital, 36.4% of the SCAI C patients died, 58.2% of the SCAI D patients died, and 60% of the SCAI E patients died, (p < .0001). Thirty‐day survival was 100, 65.4, 44.2, and 60% for patients with initial SCAI shock stage B, C, D, and E respectively (p = .0004). Figure 3, Panel A shows the survival curves to one‐year post admission to the hospital.
FIGURE 3.
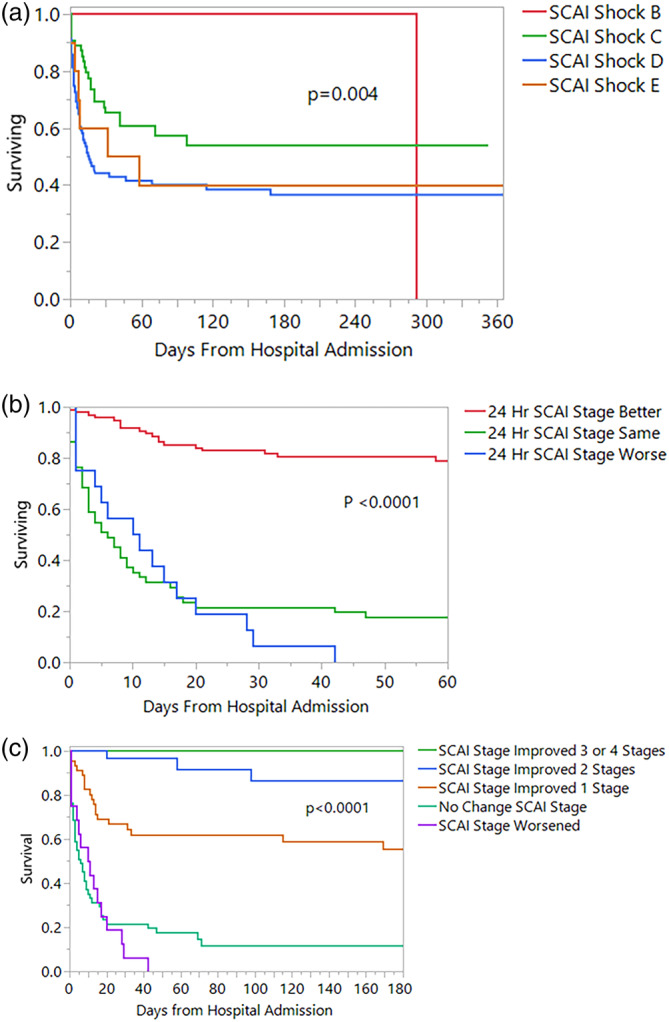
Panel A: Survival by SCAI Shock Stage at initial shock team consult. This Kaplan–Meier plot shows a significant difference in the survival of patients according to initial SCAI Shock stage. There were no early deaths with SCAI stage B patients, with improved survival for SCAI C patients compared to the SCAI D and E groups. This illustrates the potent effect of deterioration or extremis states, which carry a very high mortality. Panel B: This Kaplan–Meier plot demonstrates survival of patients according to the change in SCAI Shock stage as assessed prospectively at 24 hr following initial shock team evaluation. Change is described as SCAI Shock stage better, worse or unchanged. Panel C: This Kaplan–Meier plot demonstrates survival of patients according to the change in SCAI Shock stage as assessed prospectively at 24 hr following initial shock team evaluation. Change is quantitated as the improvement or worsening of SCAI stages. SCAI, Society for Cardiac Angiography and Interventions [Color figure can be viewed at wileyonlinelibrary.com]
We performed Cox proportional hazards modeling of survival. Terms included were patient age, gender, occurrence of cardiac arrest, initial SCAI shock stage, and whether the patient received medical therapy alone or had MCS as the initial strategy. The model was highly significant (p < .0001) with two predictors. The predictors of survival were age (p = .00001) and initial SCAI shock stage (p = .01). The other variables were not significant. Estimates of effect and 95% confidence intervals are in Table 2.
TABLE 2.
Cox proportional hazards modeling of predictors of survival at Shock team activation
| Term | Estimate | SE | Lower 95% | Upper 95% |
|---|---|---|---|---|
| Age | 0.03734 | 0.009119 | 0.02007891 | 0.05583397 |
| Sex (F) | 0.08527 | 0.119301 | −0.15157575 | 0.31749807 |
| Cardiac arres (no) | 0.117319 | 0.123278 | −0.11989669 | 0.36505345 |
| SCAI shock stage first device (B) | −1.228665 | 0.769528 | −3.39490321 | −0.05125674 |
| SCAI shock stage first device (C) | −2.91E‐05 | 0.322929 | −0.57265015 | 0.79316774 |
| SCAI shock stage first device (D) | 0.687328 | 0.302107 | 0.17306336 | 1.45793751 |
| Turndown or not (0) | 0.334332 | 0.206025 | −0.08445871 | 0.72695719 |
| IABP or not (0) | 0.34581 | 0.199021 | −0.06112173 | 0.72390176 |
| Impella CP or not (0) | 0.266371 | 0.250749 | −0.20793441 | 0.78636182 |
| VA ECMO or not (0) | 0.298617 | 0.214314 | −0.12347332 | 0.7234117 |
Abbreviation: SCAI, Society for Cardiac Angiography and Interventions.
4. TRANSITIONS OF SCAI STAGE
The SCAI shock stage at 24 hr was compared to that at initial assessment. The group was divided into patients who have a better (lower) SCAI stage, unchanged and worse (higher) SCAI stage at 24 hr. For patients initially assessed as SCAI Stage B, 80% improved and 20% remained at the same stage. Among stage C patients, 58.2% improved, 16.4% without change, and 25.4% worsened. For the stage D patients, 53.3% improved, 44.4% did not change, and 2.2% worsened. There were only 10 stage E patients, and all improved in SCAI stage at 24 hr. Analysis of 24‐hr change in SCAI shock stage by initial treatment strategy (divided into IABP, Impella, ECMO, other device or medical therapy) showed no significant differences (R = 0.01, p = .91, Chi‐square).
Survival by change in SCAI stage at 24 hr is shown in Figure 3, Panel B. There was a highly significant difference in survival based on improvement, worsening, or no change of SCAI stage at 24 hr, with the differences persisting beyond 60 days following hospital admission. The 30‐day survival was 83, 21.6, and 6.2% for patients whose 24‐hr SCAI Shock stage improved, was unchanged and worsened respectively (p < .0001). In Panel C, change in SCAI shock stage is quantitated (improvement or worsening by number of stages). Thirty‐day survival was 100%, 96.7, 66.9, 21.6, and 6.2% for patients with 3–4 SCAI stage improvement, 2 stage improvement, 1 stage improvement, no change in SCAI stage and worsening of SCAI stage respectively (p < .0001). The survival curves were relatively flat after 50 days, with few late mortalities.
5. DISCUSSION
This is the first prospective study to validate the SCAI Shock Classification system. The SCAI Shock stage is assigned by deriving a gestalt of the patient's status using physical exam, biochemical and hemodynamic information. The lack of rigid definitions is both a strength and a weakness, particularly when trying to apply the SCAI classification retrospectively to a population. 10 However, this flexibility allows its use across the care spectrum including prehospital emergency medical system workers, emergency department, and intensive care unit settings.
The initial SCAI Shock stage was highly predictive of survival. The SCAI stage was not correlated with common variables in Table 1. Patients across SCAI Shock Stages were similar in terms of routine as well as invasive hemodynamics, left ventricular function, presence of STEMI and lab parameters with the exception of lactate, which had a modest difference. This validates the utility of this clinical staging system as opposed to others, which require certain laboratories or specific data elements to provide a score. 11 , 12 , 13
As shown by Cox proportional hazards modeling, only patient age was a stronger predictor of survival than SCAI shock stage. Interestingly, choice of MCS device did not correlate with outcome, nor did blood tests such as lactate or electrolytes or invasive hemodynamics. Reassessment of SCAI stage at 24 hr provided a robust predictor of survival in this cohort. Patients with improved SCAI shock stage at 24 hr experienced markedly better survival than those with unchanged or deteriorating shock stage. It is notable that unchanged shock stage at 24 hr was associated with a high mortality as was worsening shock. This highlights the importance of serial assessment and repeated attempts to improve the hemodynamic and perfusion state of the shock patient in the first 24 hr.
Unlike other series, the outcomes of all shock patients are detailed in this report, even when MCS was not offered. The SHOCK trial registry collected data on patients who were not in the randomized trial, but few have replicated this approach. 14 , 15 In the current investigation, we report that patients managed medically (no MCS) did reasonably well with no worse survival than those who were supported mechanically. A key element is that the Shock Team (usually advanced heart failure and pulmonary critical care) continued to follow these patients and offer guidance. This may have contributed to positive outcomes in the absence of device‐based interventions.
The SCAI Shock classification has now been retrospectively applied by several groups with similar conclusions. Jentzer and colleagues examined a single center intensive care unit population of more than 10,000 patients with comprehensive data and showed that the SCAI Shock stage predicted ICU mortality as well as hospital mortality in escalating fashion. 16 Further analysis from the group showed that post‐discharge mortality was predicted by admission SCAI stage as well. 17 Schrage and colleagues have also validated the SCAI stage to predict 30‐day mortality in more than 1,000 consecutive patients presenting with CS or large acute myocardial infarction. 18 Pareek and colleagues applied the classification to 393 out‐of‐hospital cardiac arrest cases as well, showing similar predictive value of the SCAI Shock Stage. 19
6. LIMITATIONS
This is a prospective series of a relatively small number of patients in a single center, which engenders clear limitations. However, it is a snapshot of a contemporary, high‐functioning CS team, with a defined algorithm, and prospective classification of patients by SCAI Stage. We do not have long‐term follow‐up, which is a limitation. In addition, not all patients had invasive pulmonary artery catheter data measured, and serial measurements of laboratories such as lactic acid were not required or universal.
7. CONCLUSIONS
Assessment of the SCAI Shock stage predicts survival among patients referred to a shock team. Further assessment of shock stage at 24 hr has significant interval prognostic value in this prospective study. The SCAI Shock stage predicted mortality regardless of mode of intervention, including medical therapy or MCS. Utilizing the SCAI Shock stage allows clinicians to rapidly prognosticate as they assess critically ill individuals and help guide the difficult choices, which are necessary in the intensive care unit and catheterization laboratory.
CONFLICT OF INTEREST
Dr Baran has received funds (modest) for consulting with the following firms: Abiomed, Abbott, Livanova, Getinge, MC3, Procyrion. He has been on the speaker's bureau for Pfizer and Novartis in the past. The other authors have no relationships to disclose.
ACKNOWLEDGMENTS
There was no funding for this study. We want to acknowledge and thank the dedicated intensive care and cardiac catheterization laboratory staff who spend many hours taking care of these vulnerable patients. We also acknowledge and thank the many members of the shock team who work tirelessly in the care of our patients and without whom there would be nothing to report.
Baran DA, Long A, Badiye AP, Stelling K. Prospective validation of the SCAI shock classification: Single center analysis. Catheter Cardiovasc Interv. 2020;96:1339–1347. 10.1002/ccd.29319
EDITORIAL COMMENT: Expert Article Analysis for: Towards a standardized classification of cardiogenic shock: Will the new SCAI staging system translate into better clinical practice and research?
DATA AVAILABILITY STATEMENT
If requested, deidentified data will be made available.
REFERENCES
- 1. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic SHOCK. SHOCK investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625‐634. [DOI] [PubMed] [Google Scholar]
- 2. Burkhoff D, O'Neill W, Brunckhorst C, Letts D, Lasorda D, Cohen HA. Feasibility study of the use of the tandemheart percutaneous ventricular assist device for treatment of cardiogenic shock. Catheter Cardiovasc Interv: Off J Soc Cardiac Angiography Interv. 2006;68:211‐217. [DOI] [PubMed] [Google Scholar]
- 3. Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra‐aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69:278‐287. [DOI] [PubMed] [Google Scholar]
- 4. Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short‐term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta‐analysis of randomized trials. Eur Heart J. 2017;38:3523‐3531. [DOI] [PubMed] [Google Scholar]
- 5. Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra‐aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276‐1283. [DOI] [PubMed] [Google Scholar]
- 6. Thiele H, Zeymer U, Neumann FJ, et al. Intra‐aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP‐SHOCK II): final 12 month results of a randomised, open‐label trial. Lancet. 2013;382:1638‐1645. [DOI] [PubMed] [Google Scholar]
- 7. van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232‐e268. [DOI] [PubMed] [Google Scholar]
- 8. Taleb I, Koliopoulou AG, Tandar A, et al. Shock team approach in refractory cardiogenic shock requiring short‐term mechanical circulatory support: a proof of concept. Circulation. 2019;140:98‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized team‐based care for cardiogenic shock. J Am Coll Cardiol. 2019;73:1659‐1669. [DOI] [PubMed] [Google Scholar]
- 10. Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv: Off J Soc Cardiac Angiography Interv. 2019;94:29‐37. [DOI] [PubMed] [Google Scholar]
- 11. Auffret V, Cottin Y, Leurent G, et al. Predicting the development of in‐hospital cardiogenic shock in patients with ST‐segment elevation myocardial infarction treated by primary percutaneous coronary intervention: the ORBI risk score. Eur Heart J. 2018;39:2090‐2102. [DOI] [PubMed] [Google Scholar]
- 12. Harjola VP, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short‐term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17:501‐509. [DOI] [PubMed] [Google Scholar]
- 13. Poss J, Koster J, Fuernau G, et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69:1913‐1920. [DOI] [PubMed] [Google Scholar]
- 14. Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction‐etiologies, management and outcome: a report from the SHOCK trial registry. Should we emergently revascularize occluded coronaries for cardiogenic shock? J Am Coll Cardiol. 2000;36:1063‐1070. [DOI] [PubMed] [Google Scholar]
- 15. Menon V, White H, LeJemtel T, Webb JG, Sleeper LA, Hochman JS. The clinical profile of patients with suspected cardiogenic shock due to predominant left ventricular failure: a report from the SHOCK trial registry. Should we emergently revascularize occluded coronaries in cardiogenic shock? J Am Coll Cardiol. 2000;36:1071‐1076. [DOI] [PubMed] [Google Scholar]
- 16. Jentzer JC, van Diepen S, Barsness GW, et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 17. Jentzer JC, Baran DA, van Diepen S, et al. Admission Society for Cardiovascular Angiography and Intervention shock stage stratifies post‐discharge mortality risk in cardiac intensive care unit patients. Am Heart J. 2020;219:37‐46. [DOI] [PubMed] [Google Scholar]
- 18. Schrage B, Dabboura S, Yan I, et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv: Off J Soc Cardiac Angiography Interv. 2020;96(3):E213‐E219. 10.1002/ccd.28707. [DOI] [PubMed] [Google Scholar]
- 19. Pareek N, Dworakowski R, Webb I, et al. SCAI cardiogenic shock classification after out of hospital cardiac arrest and association with outcome Catheter Cardiovasc Interv: Off J Soc Cardiac Angiography Interv. 2020. 10.1002/ccd.28984. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
If requested, deidentified data will be made available.


