Summary
Cytosolic Ca2+ levels are maintained at low nanomolar concentrations, and disruption of Ca2+ homeostasis is associated with cell/tissue damage. Thus, methods have been developed to accurately assess cellular Ca2+ levels, each with intrinsic advantages and disadvantages. Here, we present in detail a ratiometric fluorometric method for cytosolic Ca2+ measurement in cultured melanoma cells using Fura 2-AM cell loading and fluorescence microscopy imaging.
For complete details on the use and execution of this protocol, please refer to Esteves et al. (2020).
Subject areas: Cancer, Cell biology, Cell culture, Cell-based assays, Microscopy
Graphical Abstract
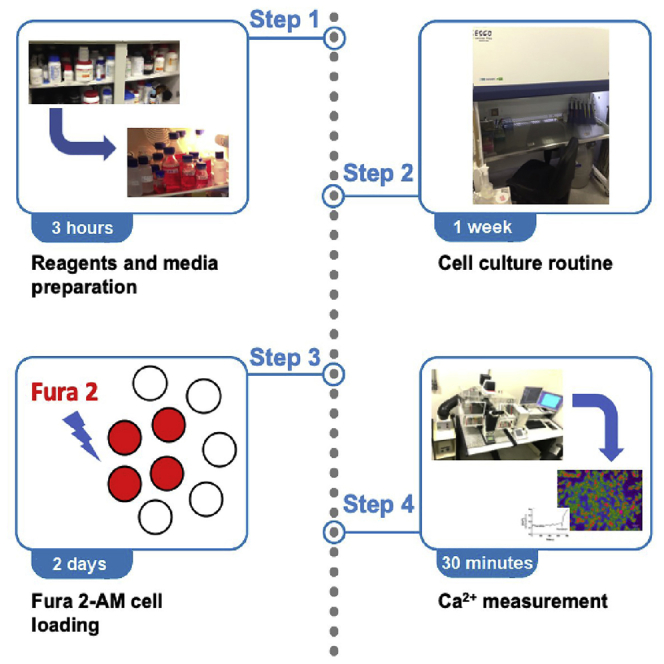
Highlights
-
•
Protocol for real-time intracellular Ca2+ measurement in cultured melanoma cells
-
•
Fura 2 ratiometric Ca2+ measurement minimizes possible artifacts
-
•
Protocol allows evaluation of the immediate effect of chemical addition
Cytosolic Ca2+ levels are maintained at low nanomolar concentrations, and disruption of Ca2+ homeostasis is associated with cell/tissue damage. Thus, methods have been developed to accurately assess cellular Ca2+ levels, each with intrinsic advantages and disadvantages. Here, we present in detail a ratiometric fluorometric method for cytosolic Ca2+ measurement in cultured melanoma cells using Fura 2-AM cell loading and fluorescence microscopy imaging.
Before you begin
Cell culture media and other essential reagents
Timing: 3 h
-
1.Heat inactivation of the fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA)
-
a.Turn on the circulating water bath (15 L capacity, PolyScience AD15H200-A12E, Niles, IL, USA) and set it to 56°C.
-
b.Place the 0.5 L bottle of FBS inside the heating bath.
-
c.Set a timer for 30 min.
-
d.After that, remove it and dry externally the bottle with paper towel.
-
e.Under aseptic conditions (laminar flow cabinet), prepare aliquots in 50 mL conical propylene tubes (Corning, USA) and refreeze at −20°C.
-
a.
-
2.Preparing balanced saline solution free of calcium and magnesium (CMF-BSS).
-
a.After calculations, weigh the reagents (obtained from Sigma-Aldrich Co., St. Louis, MO, USA) on a precision scale (Shimadzu, Kyoto, Japan) to obtain 1.0 L of a solution at the following concentrations: 136.89 mM NaCl, 5.36 mM KCl, 0.70 mM, Na2SO4, 1.12 mM, Na2HPO4, 1.09 mM, KH2PO4, 6.1 mM, glucose, 0.00705 mM, phenol red.
-
b.Add the reagents to a 1 L beaker with 500 mL of ultrapure type I water (Purelab Option-Q, ELGA) and keep it covered and under gentle agitation on a magnetic stirrer (IKA, Staufen, Germany) for 30 min.
-
c.Adjust the pH value with HCl to 7.4 using a pH meter while stirring (Quimis Aparelhos Científicos Ltda, Diadema, SP, Brazil).
-
d.Transfer the solution to a 1 L volumetric flask and complete the volume with ultrapure type I water.
-
e.Filter the solution in a Sterifil Aseptic System (Sigma-Aldrich Co., St. Louis, MO, USA) using a 0.2 μM pore size Whatman membrane filters mixed cellulose ester (Sigma-Aldrich Co., St. Louis, MO, USA).
-
f.Transfer the filtered solution to a previously autoclaved glass bottle and store at 4°C until use.
-
a.
-
3.Preparing the trypsin solution
-
a.Inside the laminar flow cabinet (Esco Technologies Inc., Horsham, PA, USA)10 mL of 10× trypsin solution (Thermo Fisher Scientific, Waltham, MA, USA) and 90 mL of sterile CMF-BSS in a beaker.
-
b.Transfer the solution to a previously autoclaved glass bottle and store at 4°C until use.
-
a.
-
4.Preparing Dulbecco’s modified Eagle’s medium (DMEM) high glucose medium for cell culture
-
a.After calculations, weigh the reagents (obtained from Sigma-Aldrich, St. Louis, MO, USA) on a precision scale (Shimadzu, Kyoto, Japan) to obtain 1.0 L of a solution at the following concentrations: 8.39 mM HEPES and 26.18 mM NaHCO₃.
-
b.Place the above salts into a 1 L beaker and add 10 mL penicillin (100 U/mL)-streptomycin (100 μg/mL) solution (Thermo Fisher Scientific, Waltham, MA, USA), 1 unit of DMEM high glucose cell culture medium (catalog number D5648, Sigma-Aldrich, St. Louis, MO, USA) powder (for 1 L) plus 500 mL of ultrapure type I water, and keep covered and under gentle agitation for 30 min.
-
c.If necessary, adjust the pH to 7.2 with HCl.
-
d.Transfer the solution to a 1 L volumetric flask and complete the volume with ultrapure type I water.
-
e.Inside the laminar flow cabinet, filter the solution through a Sterifil Aseptic System (Sigma-Aldrich Co., St. Louis, MO, USA) using a 0.2 μM pore size Whatman membrane filters mixed cellulose ester (Sigma-Aldrich, St. Louis, MO, USA).
-
a.
-
5.Supplementing the cell culture medium with FBS
-
a.Inside the laminar flow cabinet add 50 mL of heat-inactivated FBS to 450 mL of DMEM medium
-
b.Transfer the filtered solution to a previously autoclaved glass bottle and store at 4°C until use.
-
a.
-
6.Preparing Hanks’ balance salts solution (HBSS)
-
a.Using a precision scale (Shimadzu, Kyoto, Japan), weigh the reagents (Sigma-Aldrich, St. Louis, MO, USA) to prepare 1 L solution at the following concentrations:1.26 mM CaCl2, 5.33 mM KCl, 0.44 mM KH2PO4, 0.50 mM, MgCl2·6H2O, 0.41 mM, MgSO4·7H2O, 138 mM NaCl, 4.0 mM NaHCO3, 0.30 mM Na2HPO4, and 5.60 mM glucose, as described by Hanks and Wallace (1949).
-
b.Add the reagents into a 1 L beaker containing 500 mL of ultrapure type I water and keep it covered and under gentle agitation for 30 min.
-
c.If necessary, adjust the pH to 7.4.
-
d.Transfer the solution to a 1 L volumetric flask and complete the volume with ultrapure type I water.
-
e.Inside the laminar flow cabinet, filter the solution through a Millipore filter using a 0.22 μM pore size MF-Millipore Membrane Filter (Sigma-Aldrich Co., St. Louis, MO, USA).
-
f.Transfer the filtered solution to a previously autoclaved glass bottle and store at 4°C until use.
-
a.
-
7.Preparing fluorescent dye
-
a.Prepare the stock solution of 1.0 mM Fura 2-AM (Thermo Fisher Scientific, Waltham, MA, USA) plus 0.02% poloxamer 407 (Sigma-Aldrich, St. Louis, MO, USA) in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) and store at −20°C protected from light.
-
a.
Cell culture
Timing: 1 week
-
8.Cell thawing
-
a.Pre-warm DMEM high glucose medium in water bath at 37°C (PolyScience, Niles, IL, USA).
-
b.Remove a cryovial containing the cells from liquid nitrogen and place it immediately in the pre-warmed bath at 37°C, rotating the vial gently until all cells thaw (less than 60 s). Each vial was frozen with 1 × 107 cells/mL (SK-MEL-19) in FBS (Thermo Fisher Scientific, Waltham, MA, USA) containing 5% (v/v) DMSO (Sigma-Aldrich, St. Louis, MO, USA).
-
c.Inside the vertical laminar flow cabinet, transfer the vial contents to a 15-mL centrifuge tube containing 5 mL of pre-warmed DMEM high glucose supplemented medium.
-
d.Then, centrifuge the tube at 200 × g for 5 min in a Sorvall ST 16R centrifuge (Thermo Fisher Scientific, Waltham, MA, USA).
-
e.After pellet suspension, transfer the cells to a 75 cm2 cell flask with adhesion treatment (Nest Scientific, Rahway, NJ, USA) containing 10 mL of DMEM high glucose supplemented medium and homogenize it gently.
-
f.Rest the cell flask inside the incubator at 37°C in an atmosphere of 5% CO2 (Panasonic MCO-19AIC, Tokyo, Japan) for 24 h for cell adhesion, which can be checked in an DM IL LED inverted optical microscope (Leica Microsystems, Germany).
-
a.
-
9.Cell subculture
-
a.Warm the culture medium and the trypsin solution bottles by placing them in a water bath at 37°C (PolyScience, Niles, IL, USA). The CMF-BSS solution can be used at 4°C (cold) or 25°C.
-
b.After, position the bottles and all the supplements required for subculture (pipette, pipette tips, and autoclaved centrifuge tubes) inside the laminar flow cabinet.
-
c.Take the cell flask out of the incubator and place it on an inverted optical microscope to check the adherence and polygonal morphology, confluence, and possible microbiological contaminants. For the experiments, cells were grown to reach 70%–80% of confluence.
-
d.Then, inside the laminar flow cabinet carefully remove the culture medium from the cell flask and discard it.
-
e.Gently wash the internal wall of the flask with 5 mL of CMF-BSS.
-
f.Remove the CMF-BSS and add 3 mL of trypsin (for the 75 cm2 flask) and place it in the cell incubator at 37°C for 5 min.
-
g.After, by using optical microscopy, check whether cells detached from the flask.
-
h.Add 6 mL of supplemented DMEM high glucose (twice volume of trypsin) to inactivate the trypsin.
-
i.Transfer the cell suspension to a centrifuge tube and centrifuge it at 200 × g for 5 min.
-
j.Check for pellet formation and discard the supernatant.
-
k.Resuspend the pellet using 1 mL of supplemented culture medium.
-
l.Pipette 10 μL of cell suspension into a 0.5 mL microtube and add 90 μL of 0.4% trypan blue solution (Sigma-Aldrich Co., St. Louis, MO, USA).
-
m.Count the cells in a Neubauer chamber (Neubauer Improved, New Optik, Brazil) and according to the result calculate the number of cells needed to reach a concentration of 1 × 105 cells/mL in a new culture flask containing 15 mL of supplemented medium.
-
n.Return the flask to the cell incubator.
-
o.Subculture the cells every 72 h.
-
p.After two subculture procedures, cells are ready to the experiments.
-
a.
CRITICAL: It is extremely important to disinfect the laminar flow cabinet and all items prior to entering the cabinet with 70% ethanol. Also, the use of appropriate individual protection materials (masks, caps, gloves, and lab coat) is recommended. Remember that cell thawing is a stressful process, so it must be performed quickly (less than 1 min) using a pre-warmed bath (37°C) and always using a pre-warmed cell growth medium. For a better recovery, freeze the cells at a high density, e.g., 1 × 107 cells/mL for SK-MEL-19 in each cryovial. SK-MEL cell lines tend to lose their adherence and consequently their morphology from polygonal to rounded cells after approximately one month of subculture (more than 8 passages), so it is recommended to execute the experiments up to this period. Furthermore, it is extremely important to validate the strains and test them for mycoplasma before adding them to your laboratory catalog. The human melanoma cell line SK-MEL-19 (BRAFV600E mutated) were provided by Prof. Silvya Stuchi Maria-Engler (FCF-USP). The authentication of cell lines was performed by STR profiling and tested mycoplasma free using Hoechst 33342 (Thermo Fisher Scientific, Waltham, MA, USA) staining. Always keep the stock solutions of fluorescent dyes and chemicals protected from light. For this purpose, you can pack the microtube or packaging in aluminum foil.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Fetal bovine serum | Thermo Fisher Scientific | #Cat1265029 |
| Chemicals, peptides, and recombinant proteins | ||
| NaCl | Sigma-Aldrich | #CatS9888; CAS 7647-14-5 |
| KCl | Sigma-Aldrich | #CatP3911; CAS 7447-40-7 |
| Na2SO4 | Sigma-Aldrich | #Cat239313; CAS 7757-82-6 |
| Na2HPO4 | Sigma-Aldrich | #Cats9763; CAS 7558-79-4 |
| KH2PO4 | Sigma-Aldrich | #CatP0662; CAS 7778-77-0 |
| Glucose | Sigma-Aldrich | #CatG7021; CAS 492-62-6 |
| Phenol red | Sigma-Aldrich | #Catp3532; CAS 143-74-8 |
| Trypsin | Thermo Fisher Scientific | #Cat15400054; CAS 9002-07-7 |
| HEPES | Sigma-Aldrich | #CatH4034; CAS 7365-45-9 |
| NaHCO₃ | Sigma-Aldrich | #CatS5761; CAS 144-55-8 |
| DMEM high glucose | Sigma-Aldrich | #CatD5648 |
| Penicillin-streptomycin | Thermo Fisher Scientific | #Cat15140122 |
| CaCl2 | Sigma-Aldrich | #CatC4901; CAS 10043-52-4 |
| MgCl2·6H2O | Sigma-Aldrich | #CatM9272; CAS 7791-18-6 |
| MgSO4·7H2O | Sigma-Aldrich | #Cat230391; CAS 10034-99-8 |
| Sucrose | Sigma-Aldrich | #CatS5016; CAS 57-50-1 |
| Trypan blue | Sigma-Aldrich | #CatT8154; CAS 72-57-1 |
| Fura 2-AM | Thermo Fisher Scientific | #CatF1201; CAS 108964-32-5 |
| Thapsigargin | Sigma-Aldrich | #CatT9033; CAS 67526-95-8 |
| Ionomycin | Sigma-Aldrich | #CatI0634; CAS 56092-82-1 |
| DMSO | Sigma-Aldrich | #Cat472301; CAS 67-68-5 |
| Poloxamer 407 | Sigma-Aldrich | #CatP2443; CAS 9003-11-6 |
| Hoechst 33342 | Thermo Fisher Scientific | #Cat62249; CAS 23491-52-3 |
| Experimental models: cell lines | ||
| Human: SK-MEL-19 cells | Esteves et al., 2020 | https://doi.org/10.1016/j.ceca.2020.102241 |
| Software and algorithms | ||
| GraphPad Prism 6.0 | Esteves et al., 2020 | https://www.graphpad.com/scientific-software/prism/ |
| Materials and equipment | ||
| Circulating water bath | PolyScience | #CatAD15H200-A12 |
| Precision scale | Shimadzu | #CatAX200 |
| Magnetic stirrer | IKA | #CatZ403644 |
| pH meter | Quimis Aparelhos Científicos | #CatQ400A5 |
| Purelab Option-Q | ELGA | #CatLC197 |
| Vertical laminar flow cabinet | Esco Technologies | #CatSVE-4A3 |
| Sterifil aseptic system | Sigma-Aldrich | #Cat171 |
| Whatman membrane filters | Sigma-Aldrich | #Cat10401770 |
| Water bath | PolyScience | #CatWDO5A12E |
| Treated cell culture flask | Nest Scientific | #Cat7080003 |
| MCO-19AIC cell incubator | Panasonic | #CatMCO-19AIC |
| Sorvall ST16R centrifuge | Thermo Fisher Scientific | #CatST16R |
| DM IL LED inverted optical microscope | Leica Microsystems | #CatDMIL-LED |
| Neubauer chamber | New Optik | #Cat1110000 |
| Glass bottom dishes | MatTek | #CatP35G-1.5-14-CGRD |
| Leica AF6000 fluorescence image system | Leica Microsystems | #CatDMI6000B |
| CELLview cell culture dish | Greiner Bio-One | #Cat627860 |
Step-by-step method details
Preparing solutions
| CMF-BSS (for 1 L) | Final concentration (mM) | Amount (g) |
|---|---|---|
| NaCl | 136.89 | 7.99 |
| KCl | 5.36 | 0.40 |
| Na2SO4 | 0.70 | 0.10 |
| Na2HPO4 | 1.12 | 0.16 |
| KH2PO4 | 1.09 | 0.15 |
| Glucose | 6.01 | 1.08 |
| Phenol red | 0.00705 | 0.0025 |
| Total | n/a | n/a |
| DMEM high glucose | Final concentration (mM) | Amount (g) |
|---|---|---|
| DMEM high glucose | n/a | 13.4 |
| HEPES | 8.39 | 2.0 |
| NaHCO₃ | 26.18 | 2.2 |
| Total | n/a | n/a |
| HBBS (for 1 L) | Final concentration (mM) | Amount (mg) |
|---|---|---|
| CaCl2 | 1.26 | 140 |
| MgCl2·6H2O | 0.49 | 100 |
| MgSO4·7H2O | 0.40 | 100 |
| KCl | 5.33 | 400 |
| KH2PO4 | 0.44 | 60 |
| NaHCO3 | 4.16 | 350 |
| NaCl | 137.93 | 8,000 |
| Na2HPO4 | 0.33 | 48 |
| Glucose | 5.55 | 1,000 |
| Total | n/a | n/a |
Alternatives: All reagents are ordinary and can be obtained from different suppliers than specified here, observing the higher purity grade available (>98%). An alternative to substitute CMF-BSS buffer is to use 1× PBS (phosphate saline buffer) composed by 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4.
| PBS buffer (10×) (for 1 L) | Final concentration (M) | Amount (g) |
|---|---|---|
| NaCl | 1.36 | 80 |
| KCl | 0.026 | 2 |
| Na2HPO4 | 0.10 | 14.40 |
| KH2PO4 | 0.017 | 2.40 |
| Total | n/a | n/a |
Cell labeling
Timing: 2 days
At this step, melanoma cells will be subcultured in glass dishes for Fura-2 AM labeling and Ca2+ measurement by fluorescence microscopy. After routine cell subculture including cell counting described above (item 9), cells were plated in special glass dishes for microscopy (CELLview cell culture dish, 35/10 mm, 0.17 mm thin cover glass bottom, 1 compartment, TC, sterile, Greiner Bio-One, Germany).
-
1.Preparation of glass bottom dishes
-
a.Considering that each glass dish has a diameter of 35 mm (radius r = 17.5 mm, area 96.21 cm2), then each dish should contain 6 × 106 cells.
-
b.Plate the cells in the dishes with 3.0 mL of supplemented DMEM high glucose medium.
-
c.Return the dishes to the cell incubator at 37°C in an atmosphere of 5% CO2 and allow them to adhere for 24 h.
-
a.
-
2.Cell labeling
-
a.After cell adhesion, transfer the dishes from the CO2 incubator to the laminar flow cabinet.
-
b.Before starting, check cell morphology (adherent, polygonal), confluence (70%–80%), and possible microbiological contaminants by optical microscopy.
-
c.Wash the cells twice with 1.0 mL HBBS and after add 1.0 mL HBBS.
-
d.For dye loading, pipette 2.0 μL of the stock solution of Fura 2-AM (item 7a) to reach 2.0 μM of final concentration and incubate in the dark for 30 min at 25°C (see Troubleshooting, Problem 1).
-
e.After this, wash the cells twice again to remove unloaded dye with 1.0 mL HBSS and add 1.0 mL HBBS.
-
f.Go to the microscopy room with the glass dishes protected from light by aluminum foil.
-
a.
CRITICAL: Usually, fluorescent dyes are photosensitive and can be degraded and have their ability to emit fluorescence lost when exposed to light. Thus, always keep the microtubes containing the dyes and the glass bottom dishes with labeled cells protected with aluminum foil. Also, considering it is a “live” experiment, it is recommended to stain one dish at a time and keep the others in the CO2 incubator to be stained gradually as needed.
Ratiometric fluorescent Ca2+ measurement
Timing: 30 min per assay/dish
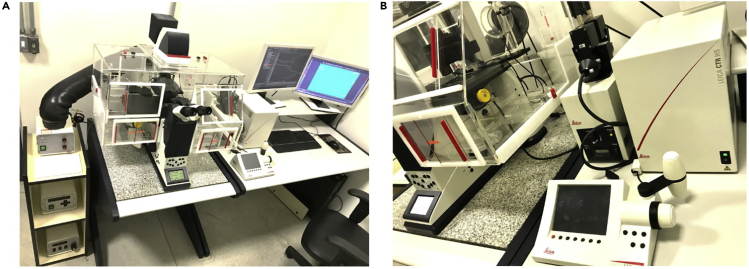
Ratiometric dyes are fluorochromes that shift their fluorescence emission or excitation spectra due to the analyte binding. The main advantages of using ratiometric methods are the artifact corrections during the measurement due to variations of the lamp intensity, focus alterations, and dye bleaching. However, the first difficulty to doing these types of experiments lies in the availability of the apparatus able to excite or to capture the emission in two wavelengths at the same time. Specifically regarding Ca2+ measurements, Fura 2-AM is a cell permeant dye that presents dual excitation, i.e., it needs to be excited at 340 and 380 nm simultaneously and the fluorescence emission acquired at 502–538 nm range. The conjugation of the dye to acetoxymethyl ester (AM) increases its cell permeant properties and improves the cell loading (see Troubleshooting, Problem 2). Thus, once cells are plated and stained as described, a Leica AF6000 Fluorescence Image System (Leica Microsystems, Germany), which was set up to conduct the ratiometric Ca2+ measurements in live cells, was used. As presented in the Figure 1, this system comprises a microscope Leica DMI6000B coupled to an ultrafast high sensitive 1.4 MP monochromatic digital DCF 365 FX camera, with a pixel resolution of 6.45 × 6.45 μm, automatically controlled by the Leica Application Suite (LAS v3.0) software. Although this camera reaches a maximum rate of 21 frames per second, for the experiment presented here it was set to capture one frame at each 5 s. An acrylic environmental chamber was used to maintain the temperature at 37°C during the experiment through the heat unit Tempcontrol 37-2 digital (Carl Zeiss, Germany). Additionally, the internal filter cubes covering the entire spectra from blue to red emission, this apparatus is equipped with an external filter wheel (Figure 2A) to allow the excitation at 340 and 387 nm. The light source used was a Hg lamp HXP R120W/45C VIS (Osram, Brazil) and the images were acquired using an HCX PL Fluotar L 20×/0.40 dry objective (Leica Microsystems, Germany). All functions are controlled by the LAS software, whose commands for set up are very intuitive as observed in the Figure 2B.
-
3.Ratiometric Ca2+ imaging using Fura 2-AM in SK-MEL-19 melanoma cells
-
a.Turn on the microscopy system and set the temperature at 37°C on the heat unit.
-
b.Set the system by selecting the one channel (channel 1) for excitation at 340 nm with red as pseudo color (Figure 2C) and another channel (channel 2) for excitation at 387 nm with green as pseudo color (Figure 2D). Also, the emission filter cube for Fura 2 must be selected with a dichroic mirror at 409 nm and emission band pass of 502–538 nm (Figure 2E).
- c.
-
d.The ratiometric calculation should be channel 1 (340 nm)/channel 2 (387 nm) (Figure 3C).
-
e.The fluorescence background can be corrected in this window and also the relative ratio 340/387 nm can be turned into absolute Ca2+ concentrations by using the Grynkiewicz equation (Grynkiewicz et al., 1985), which is a pre-existing feature in the software, but minimum and maximal fluorescence must be achieved. However, an alternative to quantification of relative ratios under different stimuli, the area under the curve (AUC) can be calculated using this tool in the GraphPad Prism 6.0 software (San Diego, CA, USA).
-
f.Position the glass bottom dish containing the labeled cells and find the focus in the bright field.
-
g.Adjust the fluorescence emission by controlling the exposure and gain using the saturation indicator.
-
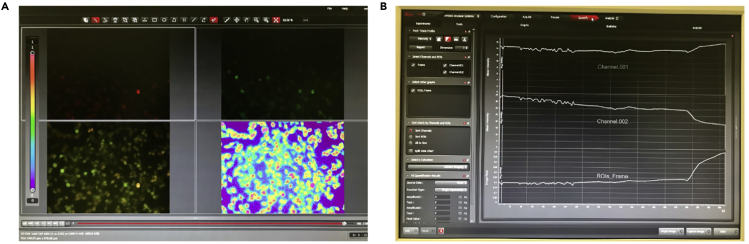
h.Set the time course and read interval. We commonly use a reading of 20 min every each 5 s for melanoma cells under thapsigargin challenge (Esteves et al., 2020). However, the time course of a given experiment depends on the stimuli used (drugs, inhibitors, activators, protein silencing, comparison among different cell lines, etc.) and it must be established during the experimental standardization. An example of an experimental set showing the individual fluorescence in each channel (panel A) and the fluorescence emission intensity graphs (panel B) is presented in Figure 4.
-
i.The drugs/challenges are pipetted directly into the dishes during the measurements and the changes are recorded at real time.
-
a.
CRITICAL: The fluorescence excitation of red pseudo color (channel 1, 340 nm) must be adjusted by changing the exposure and gain to the lowest possible value, since the Ca2+ binding will result in fluorescence increase. The fluorescence excitation of green pseudo color (channel 2, 387 nm) must be adjusted by changing the exposure and gain to the highest possible value without saturation, since the Ca2+ binding will result in fluorescence decrease. For the addition of drugs or modulators during the recording, the stock solution must be diluted in HBSS medium to a given concentration that results in a drop right on top of the excitation light (minimum 25 μL)
Figure 1.
Leica AF6000 fluorescence image system
(A and B) (A) Frontal view showing the complete system and (B) lateral view emphasizing the controller, lamp power, joystick, and external filter wheel.
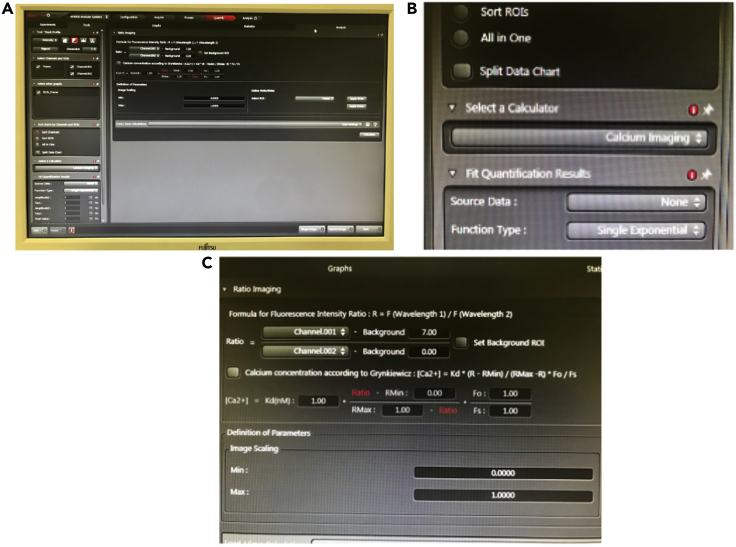
Figure 2.
Adjustments for Ca2+ measurement in Leica Application Suite (LAS) software
(A–E) (A) External filter wheel for specific excitation of Fura 2 at 340/387 nm, (B) overview of “Acquisition” window showing in the left the selection of X, Y, and T modes, camera control, objectives and in the right the selection of channels and cube filters, (C) channel 1 selection at 340 nm and red pseudo color, (D) channel 2 selection at 387 nm and green pseudo color at 387 nm, and (E) Cube filter for Fura 2 emission presenting a dichroic mirror at 409 nm and a band pass at 502–538 nm.
Figure 3.
Calcium imaging application and calculations in Leica Application Suite (LAS) software
(A–C) (A) General overview of the “Quantify” window, (B) selection of the “Calcium Imaging” tool in details, and (C) the equation for channel 1/channel 2 ratio with background subtraction.
Figure 4.
Software demonstration of cytosolic Ca2+ measurement using ratiometric Fura 2 dye
(A) Representative images of the experiment: channel 1 (340 nm) is presented in the upper left panel, channel 2 (387 nm) is in the upper right panel, the overlay channel 1/channel 2 is in the bottom left panel, and the ratio is in the bottom right panel.
(B) Representative traces (Quantify → Graph tabs) referring to the experiment shown in (A).
Expected outcomes
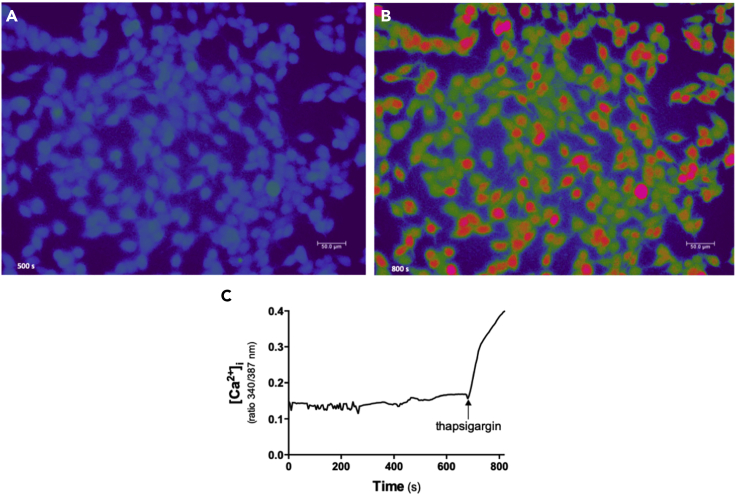
The successful load of Fura 2-AM into the cells allows acquiring relative stable and reproducible fluorescence emission of the dye and also adding chemicals, drugs, and inhibitors without stopping reading. As observed in the Figure 5, the addition of thapsigargin, a sarco/endoplasmic reticulum Ca2+-ATPase inhibitor, immediately increased the cytosolic Ca2+ levels due to the inhibition of endoplasmic reticulum calcium reuptake.
Figure 5.
Cytosolic Ca2+ measurement using ratiometric Fura 2 dye
(A) Representative image of basal level of cytosolic Ca2+ (340/387 nm ratio) in SK-MEL-19 melanoma cells at 500 s (just before adding thapsigargin).
(B) Representative image of thapsigargin-stimulated cytosolic Ca2+ increase (340/387 nm ratio) in SK-MEL-19 melanoma cells at 800 s.
(C) Experimental curve showing the effect of thapsigargin added at 660 s on fluorescence ratio (340/387 nm), representing the intracellular Ca2+ level.
Limitations
A limitation of using this method is the availability of equipment capable of promoting the excitation of the fluorophore Fura 2-AM at the two wavelengths (340/380 nm) simultaneously. Another possible limitation of this protocol is about using non-adherent cells, since the focus may vary during the time of the live experiment, although it is a ratiometric measurement, principally if you want to present representative images. However, it is not impossible to apply this protocol to non-adherent cells, e.g., leukemia cells, but it is recommended to pipette the cells into the glass dishes, load, and then centrifuged it to promote a temporary adhesion with a constant focus during the measurement.
Troubleshooting
Problem 1
Appearance of bright fluorescent spots outside the cells. This problem hinders the proper adjustment of the equipment and the visualization of the cells. This often occurs due to the precipitation of fluorophore crystals due to the evaporation of solvent or spontaneous dye aggregation, especially when it was prepared many months ago.
Potential solution
To avoid the interference of these crystals the dye solution might be filtered. Alternatively, a quick spin of the microtube by centrifugation can be employed, avoiding pipette touching the bottom of the tube.
Problem 2
Low efficiency or unspecific loading of Fura 2-AM.
Potential solution
To solve the low efficiency of Fura 2-AM loading, the stock solution of the dye must be prepared with poloxamer 407, as indicated here in item 7a. The concentration of poloxamer 407 must be titrated to find the best condition for each cell line. Also, to avoid the unspecific entry of the dye into the organelles, such as mitochondria, endoplasmic reticulum, and nucleus, we recommend doing the incubation of the cells with Fura 2-AM at room temperature (25°C) and not inside the CO2 incubator at 37°C. Also, probenecid has been employed as a nonspecific anion transport inhibitor to decrease the possible dye leakage, although its effects on ion channels must be considered (Di Virgilio et al., 1990).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, [Tiago Rodrigues] (tiago.rodrigues@ufabc.edu.br).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate/analyze datasets/code.
Acknowledgments
This work was supported by the Brazilian funding agencies Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2018/25747-5), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 312020/2019-8), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Financial Code 001).
Author contributions
L.S.F. and T.R. designed the study and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- Di Virgilio F., Steinberg T.H., Silverstein S.C. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 1990;11:57–62. doi: 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- Esteves G.N.N., Ferraz L.S., Alvarez M.M.P., Costa C.A.D., Lopes R.M., Tersariol I.L.D.S., Rodrigues T. BRAF and NRAS mutated melanoma: different Ca2+ responses, Na+/Ca2+ exchanger expression, and sensitivity to inhibitors. Cell Calcium. 2020;90:102241. doi: 10.1016/j.ceca.2020.102241. [DOI] [PubMed] [Google Scholar]
- Hanks J.H., Wallace R.E. Relation of oxygen and temperature in the preservation of tissues by refrigeration. Proc. Soc. Exp. Biol. Med. 1949;71:196–200. doi: 10.3181/00379727-71-17131. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets/code.