Significance
Extensive care of offspring by mothers is a fundamental trait of all mammals, including humans, and the loss of a mother can be catastrophic for offspring. Here, we identify previously undocumented ways in which the death of a mother affects her offspring, using long-term, longitudinal data from seven primate species. First, females that experience early maternal loss are often less able, as adults, to produce offspring that survive to maturity. Second, in some species young offspring are more likely to die if their mother is facing impending death (death in the next few years), even while she is still alive. This work has important implications for our understanding of the evolution of the long life spans that primates exhibit.
Keywords: maternal death, maternal effects, intergenerational effects, maternal condition, maternal grief
Abstract
Primate offspring often depend on their mothers well beyond the age of weaning, and offspring that experience maternal death in early life can suffer substantial reductions in fitness across the life span. Here, we leverage data from eight wild primate populations (seven species) to examine two underappreciated pathways linking early maternal death and offspring fitness that are distinct from direct effects of orphaning on offspring survival. First, we show that, for five of the seven species, offspring face reduced survival during the years immediately preceding maternal death, while the mother is still alive. Second, we identify an intergenerational effect of early maternal loss in three species (muriquis, baboons, and blue monkeys), such that early maternal death experienced in one generation leads to reduced offspring survival in the next. Our results have important implications for the evolution of slow life histories in primates, as they suggest that maternal condition and survival are more important for offspring fitness than previously realized.
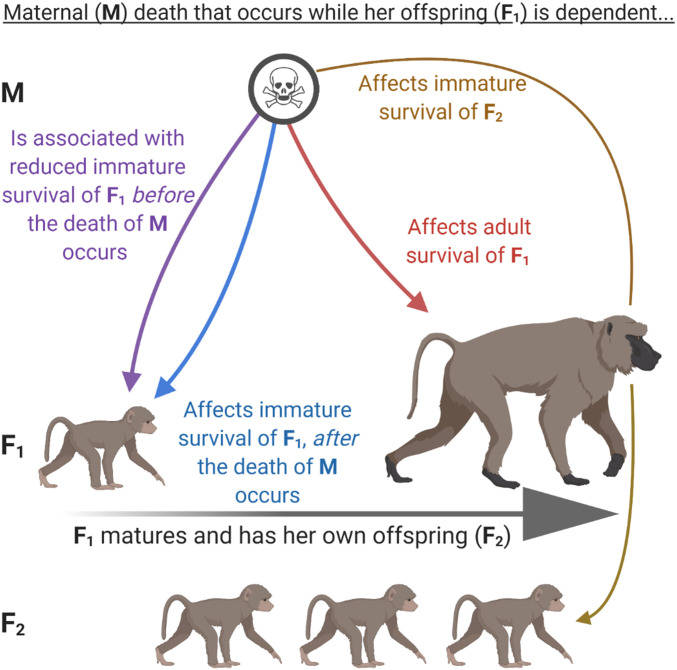
Mammalian life history is marked by a strong dependent relationship between offspring and their mothers (1). The quantity or quality of maternal allocation to offspring, particularly during the gestation and lactation periods, is often related to maternal physical condition, and a range of offspring fitness outcomes are compromised if gestating or lactating mothers are in poor condition (2–8). In addition, infants that experience maternal loss prior to weaning face an enormous, acute risk of death in both nonhuman mammals and in humans (9–13) (Fig. 1, blue arrow).
Fig. 1.
Four ways in which the death of a female primate mother (M) may be linked to her offspring’s fitness (F1), if the death of M occurs while F1 is still dependent on M. First, F1 should display reduced survival during the immature period, following the death of M (especially before weaning but also after), because F1 will lack the critically important social, nutritional, and/or protective resources that M provided (blue arrow). Second, F1 should display reduced survival in the period before M actually dies, because, on average, mothers are in worse condition shortly before their death compared to mothers that survive the same period. We therefore expect M to provide lower-quality maternal care to F1 during the weeks to years immediately preceding M’s death (purple arrow). Third, if F1 survives these first two challenges, she is likely to be in chronically worse condition during adulthood because of reductions in maternal allocation that she received during development (red arrow). F1 should therefore face reduced survival in adulthood, years (or even decades) after the death of M occurred. Fourth, this chronic reduction in F1’s condition may have an intergenerational effect, such that F2 (F1’s offspring) also experience reduced immature survival (gold arrow). The blue and red arrows have been previously tested in several species; the analyses presented here focus on the purple and gold arrows.
In some species, including primates, hyenas, whales, and some ungulates, mothers and offspring continue to associate after weaning, and mothers may provide substantial social and energetic input as well as protection during some or all of the remainder of the predispersal, immature period (hereafter the “immature period”) (12, 14–21). Thus, loss of the mother can continue to heighten the risk of death even in weaned, immature offspring (17, 19, 21) (Fig. 1, blue arrow). However, because offspring are less dependent on mothers during this phase of life, the effects of maternal loss after weaning can be sublethal (9, 16, 22). If an offspring that is weaned (but still partially dependent on its mother) survives its mother’s death, the offspring may experience long-lasting negative effects, including adverse behavioral or social outcomes in adolescence or adulthood [humans (23, 24); nonhumans (19, 20, 25–31)]. In baboons, chimpanzees, and elephants, motherless offspring may experience reduced survival during adolescence and adulthood, well after the maternal loss occurs, presumably because maternal loss results in a chronic reduction in body condition (13, 21, 26, 32) (Fig. 1, red arrow).
These observations (see citations in previous paragraphs) combine to provide a strong framework that describes the dependent relationship between mammalian offspring and their mothers and allows us to make predictions about the expected effects of maternal death on offspring fitness. This framework relies on the following assumptions, which are based on the observations described above: Mammalian offspring are critically dependent on their mothers for nutrition, protection, transport, and learning. In many species, the period of dependence is not restricted to infancy and may extend well past weaning. During this dependent period, poor maternal body condition (defined here as an unmeasured physical state that predicts an animals’ ability to perform functions necessary for reproduction and survival) can lead to reduced maternal allocation to offspring and hence poor offspring body condition. Poor offspring condition, in turn, may have both immediate and later-life consequences for offspring fitness outcomes, including survival. As a result, mothers in poor condition (even those that survive to wean their offspring) are likely to produce offspring in poor condition that experience compromised survival. Maternal death at any time during this dependent period can therefore result in both short-term and chronic reductions in offspring physical condition and survival.
This framework yields four main predictions about how maternal death experienced in early life affects offspring fitness outcomes across the life span. First, immature offspring that lose their mother will face reduced survival throughout the remainder of their immature period (Fig. 1, blue arrow). Although the impact of maternal loss will be especially strong if the mother dies before the offspring is weaned, the immature offspring may continue to face reduced survival if its mother dies any time before the offspring matures. Second, because the loss of the mother in early life results in developmental constraints that persist throughout the offspring’s lifetime, offspring that experienced early maternal loss will continue to experience reduced survival in adulthood, leading to shortened adult life spans (Fig. 1, red arrow). These two predictions are important for offspring fitness outcomes; they have been previously tested in several species (see above summary) and are therefore not the focus of our study.
We focus instead on two additional predictions, which have received little previous attention. First, we expect offspring to face reduced survival if their mothers are going to die in the near future, because, on average, a mother whose death is imminent is more likely to be in poor condition compared to those mothers that survive the same period. Thus, in this prediction, imminent maternal death serves as a proxy for poor maternal condition. We can test this prediction by measuring the association between offspring survival and impending maternal death, while the mother is still alive (Fig. 1, purple arrow). Second, we predict an intergenerational effect of early maternal loss on offspring survival (Fig. 1, gold arrow). That is, we predict that female offspring that experience maternal loss but still survive to adulthood (F1 generation in Fig. 1) will produce offspring with compromised survival (F2 generation). We expect the proximate mechanism leading to this intergenerational effect to be that F1’s compromised condition causes her to be less able to allocate adequate resources to her offspring.
These latter two predictions about survival patterns have been previously confirmed in wild baboons (33), but otherwise we have little knowledge of the generality of these two links between maternal survival and the survival of offspring (F1 generation) and grand offspring (F2 generation) in natural populations of primates or other mammals. Here, we leverage long-term longitudinal data from eight wild populations of seven primate species to assess 1) the extent to which offspring suffer reduced survival when their mothers will soon die (Fig. 1, purple arrow), and 2) the extent to which the effects of early maternal loss carry over from one generation to the next, resulting in reduced immature survival for offspring whose mothers experienced early maternal loss (Fig. 1, gold arrow).
Results
The Primate Life Histories Database (PLHD) is a collection of demographic data from seven primate species across eight long-term studies of wild populations. The PLHD contains data from taxonomically diverse primates, including great apes (eastern chimpanzees [Pan troglodytes schweinfurthii] and mountain gorillas [Gorilla beringei beringei]), Old World monkeys (yellow baboons [Papio cynocephalus] and blue monkeys [Cercopithecus mitis]), New World monkeys (northern muriquis [Brachyteles hypoxanthus] and two populations of white-faced capuchins [Cebus capucinus imitator]), and indriids (Verreaux’s sifakas [Propithecus verreauxi]). Each of the studies and the database have been described elsewhere (34–36). Of critical importance for the present study, data are available on the timing of major life history events, including birth and death dates for individuals from all species. Because we rarely observe maternal or offspring death directly, we are unable to assess cause of death for most individuals in our dataset. Sample sizes ranged from 71 to 1,123 offspring from a given population, depending on the analysis and on the eligibility of offspring for inclusion (Methods and SI Appendix, Table S1).
First Prediction: Offspring Are More Likely to Die if Their Mothers Face Impending Death.
To measure the association between offspring survival and impending maternal death, we built mixed effects Cox proportional hazards models of offspring survival during the first 2 y of life. The predictor of interest is a binary fixed effect indicator of whether the mother died within 4 y after the offspring’s birth; in constructing this model, we do not imply that the future event causes the past event, but instead we view impending maternal death as a proxy for poor maternal condition, the actual cause of infant death. This predictor represents our estimate of impending maternal death. We also included random effects of maternal ID to eliminate concerns about pseudoreplication while also partially controlling for genetic differences that might explain our results. It would be valuable for future studies to use the “animal model” (37) to identify the extent to which genetics influences offspring survival in these populations. We are unable to perform this analysis here because of incomplete pedigree information in our shared database. We also included random effects of site-specific birth year to control for cohort effects in particular years in each study site and, thus, partially control for shared environmental experiences of mothers and offspring (Methods). For offspring whose mothers died before the offspring or on the same day as the offspring, we right-censored the offspring’s survival at the day of maternal death. That is, we included in the model of offspring survival only those periods of the offspring’s first 2 y of life when mothers were alive; the model did not consider offspring survival after maternal death occurred, because we were interested in determining whether offspring faced reduced survival in the period only before impending maternal death.
We built species-specific models using data from each of the seven species. Each species is represented in the database by a single population, except for capuchins, which are studied both at Santa Rosa (SR) and Lomas Barbudal (LB). When modeling capuchin survival, we therefore built both population-specific models and a combined-population model that additionally included a fixed effect of study site ID. Finally, we built a combined species model that considered data from all seven species together and a model that combined data from all species except baboons, which made up about one-third of all records. In addition to maternal ID and site-specific birth year, these combined-species models also included a random effect of study site ID.
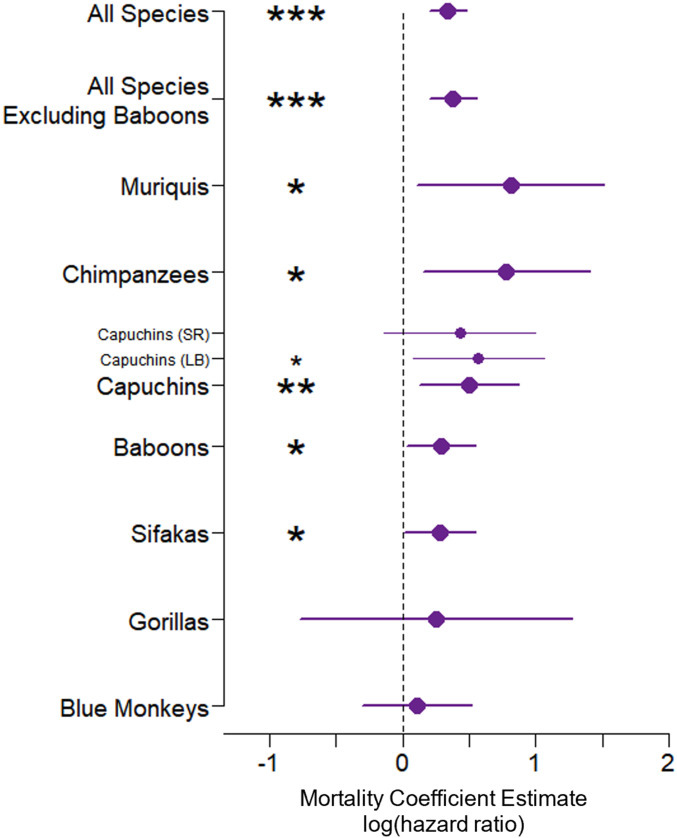
In our species-level models, five species showed statistically significant associations between offspring survival during years 0 to 2 and impending maternal death. Specifically, offspring of muriquis (hazard ratio [HR] = 2.24, z = 2.24, P = 0.03), capuchins (HR = 1.65, z = 2.64, P = 0.008), chimpanzees (HR = 2.18, z = 2.49, P = 0.01), baboons (HR = 1.34, z = 2.17, P = 0.03), and sifakas (HR = 1.33, z = 2.07, P = 0.045) were less likely to survive their first 2 y of life if their mothers were going to die in the near future (note that a HR greater than 1 indicates an increase in mortality and hence a reduction in survival). The other two species (gorillas and blue monkeys) did not show statistically significant associations when considered on their own, but the coefficient estimates were both in the expected direction (Table 1).
Table 1.
Results of mixed effects Cox proportional hazards models that predict offspring survival in years 0 to 2 as a function of impending maternal death
| Species | Coefficient estimate | SE | z value | P value | HR estimate [95% CI] | In expected direction? |
| All species combined* | 0.35 | 0.08 | 4.54 | <0.0001 | 1.42 [1.22, 1.65] | Yes |
| All species, except baboons* | 0.39 | 0.09 | 4.16 | <0.0001 | 1.47 [1.22, 1.76] | Yes |
| Muriquis | 0.81 | 0.36 | 2.24 | 0.03 | 2.24 [1.11, 4.55] | Yes |
| Chimpanzees | 0.78 | 0.32 | 2.49 | 0.01 | 2.18 [1.17, 4.08] | Yes |
| Capuchins (Santa Rosa) | 0.45 | 0.30 | 1.49 | 0.14 | 1.57 [0.87, 2.82] | Yes |
| Capuchins (Lomas Barbudal) | 0.57 | 0.25 | 2.29 | 0.02 | 1.77 [1.08, 2.89] | Yes |
| Capuchins (combined)† | 0.50 | 0.19 | 2.64 | 0.008 | 1.65 [1.14, 2.39] | Yes |
| Baboons | 0.29 | 0.13 | 2.17 | 0.03 | 1.34 [1.04, 1.72] | Yes |
| Sifakas | 0.28 | 0.14 | 2.01 | 0.045 | 1.33 [1.01, 1.76] | Yes |
| Gorillas | 0.29 | 0.53 | 0.55 | 0.59 | 1.34 [0.47, 3.78] | Yes |
| Blue monkeys | 0.08 | 0.22 | 0.38 | 0.70 | 1.08 [0.70, 1.67] | Yes |
All models include site-specific random effects of birth year and random effects of maternal ID. Bold values refer to a statistically significant effect (P < 0.05) or an estimate in the expected direction (“In expected direction?” is “Yes”).
Model also included random effect of study ID.
Model also included fixed effect of study ID.
When we built a single model with data from all species, the association between offspring survival and impending maternal death was strong and highly statistically significant (HR = 1.41, z = 4.59, P < 0.0001). This association remained strong and significant even when we excluded baboons (the largest sample size in the database) from the analysis (HR = 1.46, z = 4.21, P < 0.0001; Fig. 2, Table 1, and SI Appendix, Fig. S1).
Fig. 2.
Impending maternal death (a proxy for maternal condition) predicts offspring survival in five of seven primate species, and in combined-species models. The closed circles show, for each primate population, the magnitude and direction of the mortality coefficient estimates (equivalent to the log of the hazard ratio) for the association between offspring mortality (from birth to 2 y) and impending maternal death, with 95% CIs. The vertical dashed line at a coefficient estimate of zero indicates no effect, with positive values indicating increased mortality and hence reduced survival. Significance levels are indicated as follows: ***P < 0.001; **P < 0.01; *P ≤ 0.05.
Our definition of offspring deaths linked to impending maternal death included offspring that died as neonates but whose mothers did not die until nearly 4 y later. Is it reasonable to expect an association to exist between infant death and maternal death when the events are separated by such long periods? To examine this question, we conducted an additional analysis considering offspring survival during the first 6 mo of life only, and including only offspring whose mothers either 1) survived for the entire 4 y following offspring birth or 2) died 3.5 to 4 y after offspring birth, i.e., quite a long time after offspring birth. In a combined-species model of offspring survival, our single predictor was an indicator of whether the mother would die during the period 3.5 to 4 y after offspring birth. Remarkably, we found a strong and significant association between offspring survival in the first 6 mo of life and maternal death in years 3.5 to 4 after offspring birth (HR [95% CI] = 1.7 [1.1,2.6]; P = 0.02). In other words, young infants are likely to die if their mothers are going to die more than 3 y later (SI Appendix, Fig. S2). While impending maternal death predicts offspring death, offspring born to mothers whose deaths are especially imminent (e.g., whose mothers die within 1 y of the offspring birth) are not more likely to die (SI Appendix, Fig. S3).
A possible explanation for the negative association between immature survival and impending maternal death is that offspring may have died as a result of being born to old mothers, as older mothers may have been more likely to die in the near future. To test this possibility, we added mothers’ chronological ages to the combined-species model, as well as to the species-level models for baboons, chimpanzees, muriquis, capuchins, and sifakas. We used z-scored chronological age for each study site because of differences in life expectancy across studies. In no cases did we identify a significant effect of mothers’ chronological age on offspring survival after controlling for the timing of maternal death relative to offspring birth. The models for chimpanzee, capuchin, baboon, and combined species all continued to indicate a statistically significant effect of impending maternal death on offspring survival after controlling for maternal chronological age (SI Appendix, Table S2), and although the coefficient estimates in muriquis and sifakas were no longer significant with the addition of maternal age, the estimated effect of impending maternal death on offspring survival was little changed by the addition of maternal chronological age (muriquis: HR = 2.05 with and HR = 2.24 without; sifakas: HR = 1.29 with and HR = 1.33 without).
Our results suggest that females are, on average, less able to keep their offspring alive when their own death is imminent. However, this effect occurs regardless of the chronological age of the mother: An offspring that is born to a mother who will soon die is at an elevated risk of death regardless of whether that mother is old or young. This result suggests that maternal senescence does play a critical role in offspring outcomes, but that senescence begins at different ages or proceeds at different rates in different mothers (see also ref. 38, Fig. 3, for related analyses on baboons).
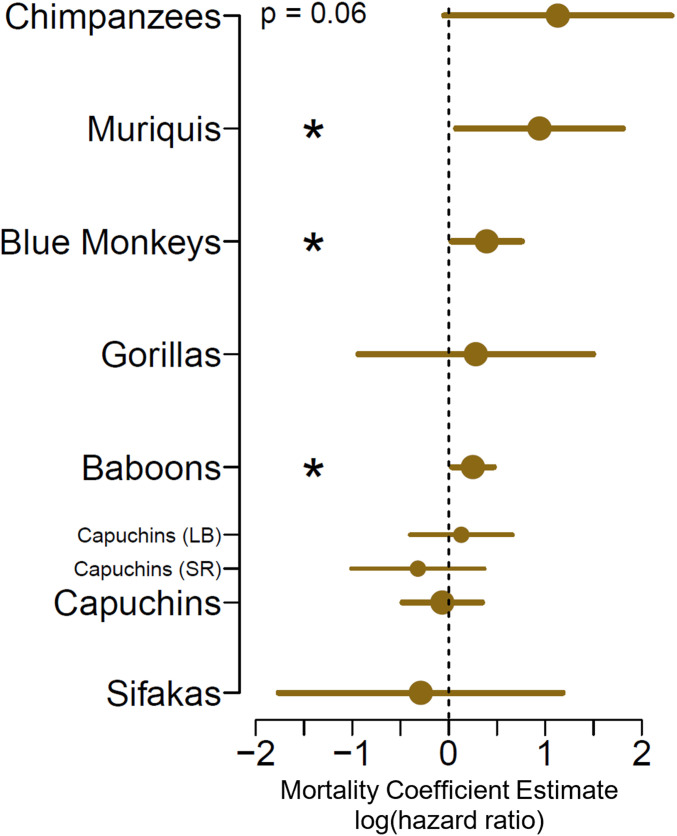
Fig. 3.
Maternal loss experienced by F1 mothers results in increased mortality for F2 offspring. The closed circles show, for each primate population, the magnitude and direction of the mortality coefficient estimates (equivalent to the log of the hazard ratio) for the association between F2 offspring mortality and maternal loss experienced by the F1 generation, 95% CIs. The vertical dashed line at a coefficient estimate of zero indicates no effect, with positive values indicating increased mortality and hence reduced survival. *P ≤ 0.05.
Another possible explanation for the negative association between immature survival and impending maternal death is that this association is driven by covariation in the offspring’s and the mother’s extrinsic mortality risk. This could occur, for instance, if the social groups that they belong to experience higher predation pressure or occupy lower-quality home ranges compared to other social groups. To test this possibility, we performed a post hoc analysis in which we replaced the random effect of study site-specific birth year with a random effect of social group-specific birth year (each study site has within it multiple social groups). This change did not result in any substantial changes to the coefficient estimates of the effect of impending maternal death in any models, and did not affect qualitative results for seven of the eight populations. This change did cause the P value associated with the sifaka model to increase from 0.045 to 0.051, but the magnitude of the coefficient estimate for the sifaka model actually increased slightly (0.29 vs. 0.28; see SI Appendix, Table S3 for full results). Having found no evidence that the association between offspring survival and impending maternal death was explained by the mother’s chronological age or by differences in offspring exposure to extrinsic mortality risk, we report the results in Fig. 2 and Table 1 (which do not account for maternal age or differences in extrinsic mortality risk) as our final results.
Second Prediction: F1 Offspring that Experience Maternal Loss Will Produce F2 Offspring with Compromised Survival.
To test for intergenerational effects of early maternal loss (Fig. 1, gold arrow), we built species-level mixed effects Cox proportional hazards models of offspring survival until the end of a species-specific immature period. The predictor of interest in these models is a binary fixed effect indicator of whether the F1 female had experienced maternal loss when she was young, i.e., whether the mother of F1 (M generation, the grandmother of F2) died before the F1 female (the mother of F2) had reached a species-specific age (SI Appendix, Table S1). As in previous models, we included random effects of maternal ID as well as birth year at each study site. We also built a capuchin model that combined data from both populations, along with a fixed effect of study site ID.
Three species (muriquis, blue monkeys, and baboons) showed statistically significant intergenerational effects of early maternal loss on offspring survival, such that F1 females who experienced early maternal loss produced F2 offspring who were more likely to die during their immature period compared to F2 offspring whose mothers did not experience early maternal loss (muriquis: HR = 2.55, P = 0.03; blue monkeys: HR = 1.48, P = 0.049; baboons: HR = 1.28, P = 0.03). Overall, chimpanzees showed the strongest estimated effect, although this relationship did not meet the threshold for statistical significance (HR = 3.1, P = 0.06), likely because of relatively small sample size (n = 7) of F2 offspring having been born to F1 mothers that experienced early maternal loss. Orphaned F1 females chimpanzees reach sexual maturity at a later age (31), and the small sample of F2 chimpanzees may be the result of this and other orphaning effects on F1 reproduction or fertility, combined with female dispersal in this species. No species showed a strong or statistically significant association in the unexpected direction (Table 2 and see SI Appendix, Fig. S4 for species-level survival curves). The intergenerational effects that we observed are not the result of the loss of direct grandmaternal care nor the result of F2 offspring being more likely to experience maternal (F1) death directly (SI Appendix, Supplemental Analyses).
Table 2.
Model outputs of mixed effects models of offspring survival throughout the predispersal immature period as a function of whether the mother of the offspring experienced early maternal loss
| Species | Coefficient estimate | SE | z value | P value | HR estimate [95% CI] | In expected direction? |
| Chimpanzees | 1.13 | 0.60 | 1.88 | 0.06 | 3.10 [0.96, 10.0] | Yes |
| Muriquis | 0.94 | 0.44 | 2.05 | 0.03 | 2.55 [1.07, 6.05] | Yes |
| Blue monkeys | 0.39 | 0.20 | 1.97 | 0.049 | 1.48 [1.00, 2.19] | Yes |
| Gorillas | 0.28 | 0.62 | 0.45 | 0.65 | 1.33 [0.39, 4.46] | Yes |
| Baboons | 0.25 | 0.12 | 2.14 | 0.03 | 1.28 [1.01, 1.62] | Yes |
| Capuchins (Santa Rosa) | −0.32 | 0.35 | −0.92 | 0.36 | 0.73 [0.37, 1.44] | No |
| Capuchins (Lomas Barbudal) | 0.13 | 0.27 | 0.48 | 0.63 | 1.14 [0.67, 1.92] | Yes |
| Capuchins (combined)* | −0.07 | 0.21 | −0.32 | 0.75 | 0.93 [0.62, 1.41] | No |
| Sifakas | −0.29 | 0.75 | −1.28 | 0.20 | 0.75 [0.17, 3.25] | No |
All models included site-specific random effects of birth year and maternal ID. Bold values refer to a statistically significant effect (P < 0.05) or an estimate in the expected direction (“In expected direction?”). Italics indicate an estimate where 0.05 < P < 0.1.
Model also included fixed effect of study ID.
Discussion
We have shown that, in several primate species, offspring face reduced fitness prospects if they are born to mothers that will die in the near future (i.e., within a few years) or if they are born to mothers that themselves had experienced early maternal loss. Our first analysis shows that, in five species, offspring whose mothers will die in the near future face reduced fitness prospects before maternal death actually occurs, presumably because they experience reductions in maternal allocation (Fig. 2 and Table 1). This negative association between impending maternal death and offspring survival is also strong and significant when data from all seven species are considered together. Furthermore, in three of the species considered, the effects of early maternal loss carry over from one generation to the next (Fig. 3 and Table 2), as offspring are less likely to survive the immature period if their mothers had experienced early maternal loss. We interpret this intergenerational effect to be the result of chronic reductions in the physical condition of female offspring that experience early maternal loss. While drawing causal inferences from correlational analyses is always challenging, these results are fully consistent with our hypothesis that experiencing maternal loss in early life has both chronic and long-term consequences for offspring outcomes.
What Explains the Association between Offspring Death and Impending Maternal Death?
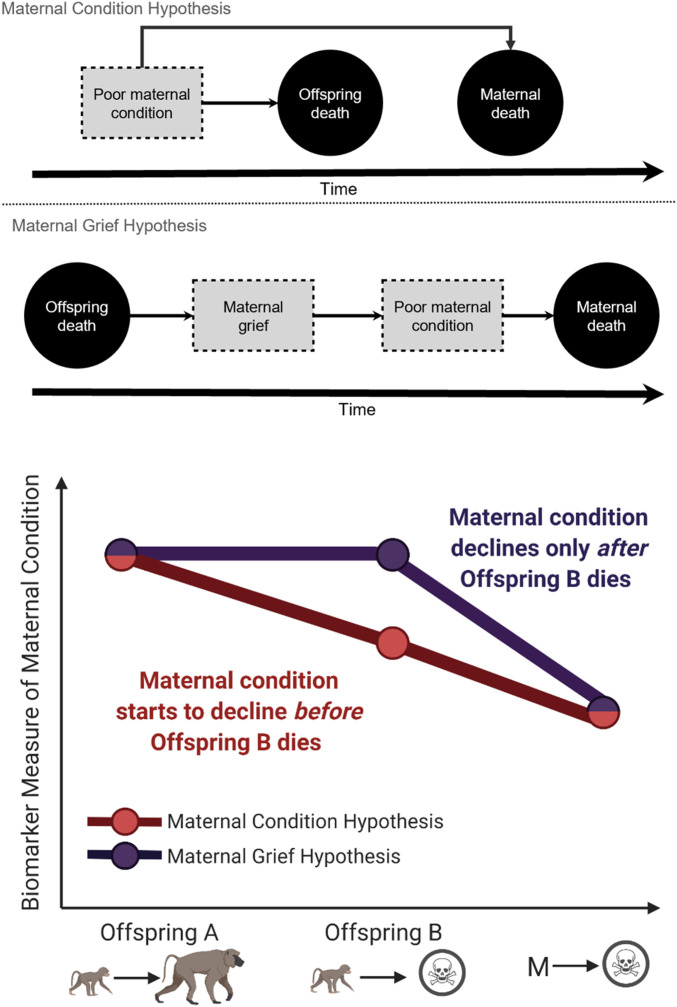
Our results show that offspring death in early life is often followed by the death of the offspring’s mother shortly thereafter. Thus far, our analyses have been motivated by the hypothesis that offspring survival and future maternal death are both affected by an unmeasured variable: the physical condition of the mother in the years shortly after offspring birth. Under this interpretation (hereafter the “maternal condition hypothesis,” Fig. 4), mothers who die are often in poor physical condition for several years before their death, and this reduction in condition has negative effects on their offspring, presumably through reductions in the quantity or quality of maternal care. The maternal condition hypothesis is consistent with our understanding of the mother–offspring relationships in our study systems and is also consistent with findings from humans that young children experience reduced survival if their mother is critically ill (39).
Fig. 4.
(Top) Schematic representations of the maternal condition and maternal grief hypotheses. According to both hypotheses, maternal death is caused by poor maternal condition. However, under the maternal condition hypothesis, poor maternal condition also causes offspring death, whereas under the maternal grief hypothesis poor maternal condition is caused by the grief resulting from offspring death. (Bottom) Alternative predictions of the maternal condition and maternal grief hypotheses. In this example, offspring A survives to adulthood, while offspring B does not, and the mother dies at some point shortly after the death of offspring B. Under the maternal grief hypothesis, we predict that biomarkers of maternal condition decline (compared to their baseline levels during the early life of offspring A), only after the death of offspring B. Under the maternal condition hypothesis, we predict that the same biomarkers should start to decline before the death of offspring B.
An alternative hypothesis to explain the observed association between offspring survival and subsequent maternal death is that offspring death during the earliest stage of its life has a causal effect on future maternal survival. Under this interpretation, mothers face increased psychological stress and reduced physical condition as a result of the loss of the offspring, which in turn results in an increased likelihood of maternal death (hereafter the “maternal grief hypothesis,” Fig. 4). Studies of humans have found that parents who experience the death of a child face reduced health and survival for years afterward (40–45), including an increase in the likelihood of deaths associated with grief [e.g., deaths by suicide (46–48)].
Distinguishing between the Maternal Condition and Maternal Grief Hypotheses.
We therefore seek to distinguish between the maternal condition hypothesis and the maternal grief hypothesis to the extent that we are able. Although causes of death data are not available for all individuals in our dataset, some of the data regarding the causes of death for infant chimpanzees are generally consistent with the maternal condition hypothesis. Of the 13 infant chimpanzees who died within the first 2 y of life and whose mothers died shortly thereafter, 3 were cases in which an offspring was born to a simian immunodeficiency virus-positive mother, who died shortly after her offspring died. In a fourth case, the mother was known to be very ill with a different disease at the time of the offspring’s death. These cases are consistent with similar data from humans regarding survival of infants born to HIV-positive mothers (39), which implicate poor maternal health in offspring death.
The impact of grief on wild animal behavior and survival are much more challenging to identify in natural populations as compared to humans, but the last decade has seen efforts to identify these effects (49–51). Within the populations studied here, an example of the death of a white-faced capuchin mother from the Lomas Barbudal population is consistent with our expectations of what grief might look like in a wild primate. This female (Chaos) had lost three infants in less than 3 y, with male-perpetrated infanticide suspected to be the cause in each case. In the weeks following the final infant death, Chaos wandered the forest on her own, rarely appearing in daily censuses of her social group. She eventually died, less than 5 wk after the death of the third infant. At the same time, Chaos faced additional social challenges, including the deaths of her mother and sister and her immigration into a new social group. So, while we cannot state with certainty that grief resulting from the violent killings of her last three infants contributed to Chaos’ death, her case is fully consistent with the maternal grief hypothesis.
Chaos’ death represents a single case, but it underscores the importance of distinguishing between the maternal condition and maternal grief hypotheses. While Chaos’ death following the killing of her infants is suggestive of maternal grief, other cases of infanticide followed by maternal death could be consistent with the maternal condition hypothesis, if mothers in poor condition are less able to protect their offspring from infanticidal threats. Thus, observations of infanticide followed by maternal death could be consistent with either hypothesis.
We are optimistic that the maternal condition and maternal grief hypotheses can be distinguished through the collection of longitudinal data of various biomarkers before and after the death of offspring. As an example, imagine two offspring that are born to a mother (Fig. 4). The first offspring (offspring A) survives to adulthood, while the second offspring (offspring B) does not, and the mother dies at some point relatively soon after the death of offspring B. Importantly, offspring A (born before offspring B) survives in this example; the mother’s risk of experiencing grief due to the death of an infant occurs only after offspring B dies. If the maternal grief hypothesis is correct, we would expect biomarker measures of maternal condition to remain relatively constant between the birth of offspring A and the death of offspring B, and then decline only after the death of offspring B. Alternatively, if the maternal condition hypothesis is correct, we would expect biomarker measures of maternal condition to be in decline (relative to baseline levels measured during the early life of offspring A) before the death of offspring B, and continue to decline thereafter (Fig. 4).
In sum, we are not yet able to distinguish between the maternal condition and maternal grief hypotheses. Distinguishing between these hypotheses will require the collection of continuous behavioral and physiological sampling of focal mothers across their life spans. For now, we argue that researchers of human populations should not assume that the association between offspring death and future maternal death can be fully explained by maternal grief following offspring death. Instead, we highlight the potential effects of maternal condition on offspring health prior to the offspring’s death, and their potential importance in developing a complete understanding of the mother–child relationship, which has important implications for both child and maternal health. Similarly, researchers of nonhuman animals should not assume that these associations can be entirely explained without considering the possibility that the grief associated with offspring death has a causal, negative effect on future maternal survival.
Variation in the Effects of Early Maternal Loss.
Several patterns emerge from the results depicted in Figs. 2 and 3. First, links between maternal survival and offspring and grandoffspring survival may covary within species. Chimpanzees and muriquis displayed the highest point estimates for both the effects of impending maternal death and the intergenerational effects of maternal loss on offspring survival, and baboons showed significant effects in both analyses. Gorillas were the only species to show neither a significant effect of impending maternal death nor an intergenerational effect of maternal death, which is consistent with a study from this population that found (in contrast to baboons and chimpanzees) no detectable fitness consequences for offspring that lose their mothers after the age of 2 (52).
What might explain this variation in the effects of maternal loss across species? One possibility is that the effects of maternal loss are reduced in species that display higher levels of allomaternal care from kin and nonkin males or females. If the mother of the F1 individual dies when F1 is young, but F1 continues to receive care from other individuals, this allomaternal care might mitigate the long-term effects of early maternal loss. Simultaneously, allomaternal care provided to the F2 generation could further mitigate any negative effects of reductions in F1 condition. White-faced capuchins displayed one of the lowest coefficient estimates for the intergenerational effect, and are also known to display relatively high levels of allomaternal care compared to the other species considered here (53–55). Immature gorillas that suffer early maternal loss display an increase in social connectedness after maternal death, suggesting that their social relationships may act as a buffer, reducing the effects of maternal loss (52). Detailed comparative examination of this hypothesis will require data from many more species than are contained here, and future work should consider the potential role of allomaternal care in mitigating the costs of maternal loss in wild primates. It is also unclear exactly what other social factors (e.g., effects of social rank) mediate the effects described here, and identifying these influences in individual populations would be a valuable avenue of future research.
Implications for the Evolution of Life Histories.
Our results have important implications for understanding the evolution of primate life histories. We have shown that an offspring’s fitness is influenced by the longevity of its mother in ways not previously considered. Life history theory assumes that females face trade-offs between allocating resources to maintenance (which promotes better physical condition and greater longevity) versus to reproduction (56). If we assume that the allocation of resources to maintenance is at least partially under genetic control, then increased maintenance effort may have an indirect genetic effect on offspring survival and reproductive success (57, 58). Because mothers and offspring are closely related, it follows that mutations that increase females’ maintenance allocation (and therefore their longevity) would increase females’ fitness not only by extending their reproductive life span, but also by decreasing the likelihood that their offspring will experience the detrimental effects of declining maternal condition or maternal loss during development. We would therefore expect stronger selection on longevity in species in which maternal survival has downstream effects on offspring survival and reproductive success (12, 59, 60). If mothers face an evolutionary trade-off in which increased maintenance allocation leads to longer life spans but less frequent reproduction (56), we would expect the associations that we present here to select for a slower, longer life history in female primates compared to species where links between maternal survival and offspring fitness are weaker or nonexistent. Rigorously evaluating such a possibility would require a more formal theoretical approach that is beyond the scope of this paper, but primates’ relatively slow life histories are consistent with this hypothesis (56, 61, 62).
We hope that our results encourage other researchers to publish similar demographic analyses that will allow for a broader comparative examination of predictors of whether these effects occur. For example, we predict that all effects of maternal loss will be reduced in species in which allomaternal care is relatively common and important for offspring survival, as other kin and nonkin members of the social group may mitigate the loss of maternal allocation. We also predict that these associations will be strongest in mammal species in which offspring continue to receive support from their mothers beyond the age of weaning, as is the case for most primate species analyzed here. For example, we would expect these associations to be strong in species where mothers and offspring coreside, such as hyenas and elephants, and less strong in species in which mothers are likely to disperse before the offspring matures, such as gorillas (52). Furthermore, we expect these associations (especially the blue and purple arrows in Fig. 1) to be present across a wide range of the mammalian taxonomy. Assessing these predictions will rely on both publication of similar demographic analyses to those presented here, as well as publication of behavioral data regarding the extent of maternal and allomaternal care before and after weaning.
More generally, our results reveal unexpected and additional ways in which offspring fitness depends on maternal condition and have important implications for our understanding of life history evolution. Additional studies by behavioral ecologists will be important to confirm if these results are generalizable to other mammal species. Furthermore, our results would benefit from theoretical studies of parent–offspring interactions (63) that can help characterize the microevolutionary dynamics of intergenerational fitness effects.
Methods
For this study, we analyzed the lives of all offspring included in the PLHD (Results) that met particular inclusion criteria, which we present separately below for the two major analyses that we performed.
Inclusion Criteria for Analysis 1: Association between Offspring Death and Impending Maternal Death.
To be included in this analysis, three pieces of information needed to be known with certainty: 1) if and when the offspring died prior to age 2 y, 2) whether the mother of the offspring died within 4 y of the offspring’s birth, and (3) in cases where the mother died within 4 y of the offspring’s birth, whether she died before or after her offspring. For mothers and offspring that died on the same day, we make the conservative assumption that the mother died before the offspring. Because we are interested only in offspring survival during the period when the mother is alive, we right-censored the lives of any offspring whose mothers died before them, assigning them a censor date equal to the maternal death date. Offspring were also right-censored at age 2 y, if they survived beyond age 2. The age cutoff of 2 y is meant to capture a period of life that corresponds to a period of heightened offspring vulnerability for all species considered here. Although the seven species vary in life history patterns, such that some offspring are weaned before 2 y of age and others are not, offspring of all species are most vulnerable during these first 2 y of life.
Within the database, many individuals have estimated birth and death dates, with some uncertainty around the dates. If uncertainty in the estimates of demographic events created any uncertainty in the inclusion criteria, those records were excluded from analysis. SI Appendix, Table S1 contains the number of offspring included in and excluded from this analysis for each species as well as the number of offspring deaths that contribute to each analysis.
Inclusion Criteria for Analysis 2: Intergenerational Effects of Early Maternal Loss on Immature Survival.
To be included in this analysis, two pieces of information needed to be known with certainty: 1) if and when the offspring (F2 generation) died prior to the end of the species-specific immature period (described in SI Appendix, Table S1) and 2) whether the mother (F1 generation) of the F2 offspring had experienced early maternal loss while she was immature (i.e., whether the grandmother, M generation, of the F2 offspring died while the F1 female was immature). As above, if uncertainty in the dates of any demographic events caused ambiguity in any of these areas, records were excluded from analysis. The numbers of offspring included in and excluded from this second analysis also appear in SI Appendix, Table S1.
To determine whether the F1 female had experienced early maternal loss, it was first necessary to calculate species-specific estimates of the end of the immature period. For two species (baboons and chimpanzees), we used the median age at which females achieve menarche, which can be directly observed via the appearance of sexual swellings (31, 64). For sifakas, capuchins, and blue monkeys, menarche is not directly observable. We instead estimated the end of the immature period as the median age of first birth, minus 1 SD (65–67). For gorillas, we estimated the end of the immature period as the median age of first birth, minus 2 y of “adolescent sterility” (68). Finally, for muriquis, we used the approximate median age of female dispersal the end of the immature period (69). Each of these species-specific estimates of the end of the immature period appear in SI Appendix, Table S1.
Statistical Analyses.
For both analyses, we built mixed effects Cox proportional hazards models using the R 3.5.0 (70) packages survival (71) and coxme (72).
For all models built for analysis 1, the response variable was time elapsed between offspring birth and offspring death or censoring during the first 2 y of life. The only fixed effect was a binary indicator of whether the mother died within 4 y of the offspring’s birth. We included random effects of maternal ID and birth year to control for cohort effects in particular years for each species. For baboons, chimpanzees, and gorillas, we considered the birth year to begin January 1 of each calendar year. Birth years for the other species began after the period that included the fewest births (73) (muriquis: March 1; blue monkeys: November 1; sifakas: July 15; capuchins: October 1). We also built combined species models that contained data from all seven species (or all species except baboons, see Results) along with an additional random effect of study site.
For all models built for analysis 2, the response variable was time elapsed between F2 birth and F2 death or censoring during the species-specific immature period (SI Appendix, Table S1). The only fixed effect was a binary indicator of whether the F1 female who was the mother of the F2 offspring experienced maternal loss/death when she was immature. As in analysis 1, we also included random effects of maternal ID and birth year at each site in the models.
For those species in which we identified a significant intergenerational effect, we also built post hoc models that additionally included a time-varying fixed effect of maternal presence in the offspring’s life. This addition allowed us to explore the extent to which the intergenerational effect of early maternal death on F2 survival is independent of an increased likelihood of F1 absence during F2 early life (i.e., to what extent the gold and red arrows in Fig. 1 are independent). All models were assessed for violations of the proportional hazards assumption and were generally found not to violate the assumption (with a single exception; see SI Appendix for details).
Supplementary Material
Acknowledgments
The Primate Life Histories Working Group was funded by the National Evolutionary Synthesis Center, the National Center for Ecological Analysis and Synthesis, the Princeton Centers for Health and Wellbeing and the Demography of Aging, and the Princeton Environmental Institute. The governments of Brazil, Costa Rica, Kenya, Madagascar, Rwanda, and Tanzania provided permission to conduct the field studies. We thank the many students, colleagues, field assistants, and technicians who have contributed to collecting and managing the long-term data stored in the PLHD. We also thank Fernando Colchero, Robert Seyfarth (editor), and three anonymous reviewers for providing helpful comments that improved the manuscript. Specific acknowledgments for each study, including funding agencies, can be found at https://docs.google.com/document/d/1rjUon48QburrO-xHd_2qxiC9wMQwZf4fZQ9RdbalYtU/edit?usp=sharing. Figs. 1 and 4 were created with BioRender.com.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2015317118/-/DCSupplemental.
Data Availability.
An anonymized CSV with demographic data has been deposited in the Duke Data Repository, and can be accessed at https://doi.org/10.7924/r44f1tk8k (74).
References
- 1.Clutton-Brock T. H., The Evolution of Parental Care (Princeton University Press, Princeton, NJ, ed. 1, 1991). [Google Scholar]
- 2.Cameron R. D., Smith W. T., Fancy S. G., Gerhart K., White R., Calving success of female caribou in relation to body weight. J. Zool. (Lond.) 71, 480–486 (1993). [Google Scholar]
- 3.Keech M. A., et al. , Life-history consequences of maternal condition in Alaskan moose. J. Wildl. Manage. 64, 450–462 (2000). [Google Scholar]
- 4.Bales K., French J. A., Dietz J. M., Explaining variation in maternal care in a cooperatively breeding mammal. Anim. Behav. 63, 453–461 (2002). [Google Scholar]
- 5.Altmann J., Alberts S. C., Growth rates in a wild primate population: Ecological influences and maternal effects. Behav. Ecol. Sociobiol. 57, 490–501 (2005). [Google Scholar]
- 6.Théoret-Gosselin R., Hamel S., Côté S. D., The role of maternal behavior and offspring development in the survival of mountain goat kids. Oecologia 178, 175–186 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Clutton-Brock T. H., Major M., Albon S. D., Guinness F. E., Early development and population dynamics in red deer. I. Density-dependent effects on juvenile survival. J. Anim. Ecol. 56, 53–67 (1987). [Google Scholar]
- 8.Fairbanks L. A., McGuire M. T., Maternal condition and the quality of maternal care in vervet monkeys. Behaviour 132, 733–754 (1995). [Google Scholar]
- 9.Hasegawa T., Hiraiwa M., Social interactions of orphans observed in a free-ranging troop of Japanese monkeys. Folia Primatol. (Basel) 33, 129–158 (1980). [DOI] [PubMed] [Google Scholar]
- 10.Sear R., Mace R., Who keeps children alive? A review of the effects of kin on child survival. Evol. Hum. Behav. 29, 1–18 (2008). [Google Scholar]
- 11.Balshine S., “Patterns of parental care in vertebrates” in The Evolution of Parental Care, Royle N. J., Smiseth P. T., Kolliker M., Eds. (Oxford University Press, Oxford, 2012), pp. 62–80. [Google Scholar]
- 12.van Noordwijk M. A., “From maternal investment to lifetime maternal care” in The Evolution of Primate Societies, Mitani J. C., Call J., Kappeler P. M., Palombit R. A., Silk J. B., Eds. (University of Chicago Press, Chicago, 2012), pp. 321–342. [Google Scholar]
- 13.Lahdenperä M., Mar K. U., Lummaa V., Short-term and delayed effects of mother death on calf mortality in Asian elephants. Behav. Ecol. 27, 166–174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pusey A. E., Mother-offspring relationships in chimpanzees after weaning. Anim. Behav. 31, 363–377 (1983). [Google Scholar]
- 15.Green W. C. H., Griswold J. G., Rothstein A., Post-weaning associations among bison mothers and daughters. Anim. Behav. 38, 847–858 (1989). [Google Scholar]
- 16.Kojola I., Mother’s dominance status and differential investment in reindeer calves. Anim. Behav. 38, 177–185 (1989). [Google Scholar]
- 17.Watts H. E., Tanner J. B., Lundrigan B. L., Holekamp K. E., Post-weaning maternal effects and the evolution of female dominance in the spotted hyena. Proc. R Soc. B 276, 2291–2298 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster E. A., et al. , Adaptive prolonged postreproductive life span in killer whales. Science 337, 1313 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Andres D., et al. , Sex differences in the consequences of maternal loss in a long-lived mammal, the red deer (Cervus elaphus). Behav. Ecol. Sociobiol. 67, 1249–1258 (2013). [Google Scholar]
- 20.Lea A. J., Learn N. H., Theus M. J., Altmann J., Alberts S. C., Complex sources of variance in female dominance rank in a nepotistic society. Anim. Behav. 94, 87–99 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanton M. A., Lonsdorf E. V., Murray C. M., Pusey A. E., Consequences of maternal loss before and after weaning in male and female wild chimpanzees. Behav. Ecol. Sociobiol. 74, 1–11 (2020). [Google Scholar]
- 22.Holand Ø., et al. , Induced orphaning reveals post-weaning maternal care in reindeer. Eur. J. Wildl. Res. 58, 589–596 (2012). [Google Scholar]
- 23.Rosenbaum-Feldbrügge M., Debiasi E., The impact of parental death on the timing of first marriage: Evolutionary versus social explanations (The Netherlands, 1850–1940). Demogr. Res. 40, 799–834 (2019). [Google Scholar]
- 24.Rosenbaum-Feldbrügge M., The impact of parental death in childhood on sons’ and daughters’ status attainment in young adulthood in the Netherlands, 1850–1952. Demography 56, 1827–1854 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Archie E. A., Tung J., Clark M., Altmann J., Alberts S. C., Social affiliation matters: Both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc. R Soc. B 281, 20141261 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tung J., Archie E. A., Altmann J., Alberts S. C., Cumulative early life adversity predicts longevity in wild baboons. Nat. Commun. 7, 11181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson J. A., Dominance rank in juvenile olive baboons, Papio anubis: The influence of gender, size, maternal rank and orphaning. Anim. Behav. 35, 1694–1708 (1987). [Google Scholar]
- 28.Goldenberg S. Z., Wittemyer G., Orphaned female elephant social bonds reflect lack of access to mature adults. Sci. Rep. 7, 14408 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldenberg S. Z., Wittemyer G., Orphaning and natal group dispersal are associated with social costs in female elephants. Anim. Behav. 143, 1–8 (2018). [Google Scholar]
- 30.Botero M., Macdonald S. E., Miller R. S., Anxiety-related behavior of orphan chimpanzees (Pan troglodytes schweinfurthii) at Gombe National Park, Tanzania. Primates 54, 21–26 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Walker K. K., Walker C. S., Goodall J., Pusey A. E., Maturation is prolonged and variable in female chimpanzees. J. Hum. Evol. 114, 131–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura M., Hayaki H., Hosaka K., Itoh N., Zamma K., Brief communication: Orphaned male chimpanzees die young even after weaning. Am. J. Phys. Anthropol. 153, 139–143 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Zipple M. N., Archie E. A., Tung J., Altmann J., Alberts S. C., Intergenerational effects of early adversity on survival in wild baboons. eLife 8, e47433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strier K. B., et al. , The primate life history database: A unique shared ecological data resource. Methods Ecol. Evol. 1, 199–211 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bronikowski A. M., et al. , Aging in the natural world: Comparative data reveal similar mortality patterns across primates. Science 331, 1325–1328 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry S., Godoy I., Lammers W., “The Lomas Barbudal monkey project: Two decades of research on Cebus capucinus” in Long-Term Field Studies of Primates, Kappeler P., Watts D., Eds. (Springer, Berlin, 2012), pp. 141–163. [Google Scholar]
- 37.Wilson A. J., et al. , An ecologist’s guide to the animal model. J. Anim. Ecol. 79, 13–26 (2010). [DOI] [PubMed] [Google Scholar]
- 38.McLean E. M., Archie E. A., Alberts S. C., Lifetime fitness in wild female baboons: Trade-offs and individual heterogeneity in quality. Am. Nat. 194, 745–759 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark S. J., et al. , Young children’s probability of dying before and after their mother’s death: A rural South African population-based surveillance study. PLoS Med. 10, e1001409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Hansen D., Mortensen P. B., Olsen J., Myocardial infarction in parents who lost a child: A nationwide prospective cohort study in Denmark. Circulation 106, 1634–1639 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Espinosa J., Evans W. N., Maternal bereavement: The heightened mortality of mothers after the death of a child. Econ. Hum. Biol. 11, 371–381 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Valdimarsdóttir U. A., et al. , The mother’s risk of premature death after child loss across two centuries. eLife 8, e43476 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schorr L., et al. , Mortality, cancer incidence, and survival in parents after bereavement. Ann. Epidemiol. 26, 115–121 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Rostila M., Saarela J., Kawachi I., Mortality in parents following the death of a child: A nationwide follow-up study from Sweden. J. Epidemiol. Community Health 66, 927–933 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Harper M., O’Connor R. C., O’Carroll R. E., Increased mortality in parents bereaved in the first year of their child’s life. BMJ Support. Palliat. Care 1, 306–309 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Chen C.-C., Kuo C.-C., Wu T.-N., Yang C.-Y., Death of a son is associated with risk of suicide among parous women in Taiwan: A nested case-control study. J. Epidemiol. 22, 532–536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin P., Mortensen P. B., The impact of parental status on the risk of completed suicide. Arch. Gen. Psychiatry 60, 797–802 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Agerbo E., Midlife suicide risk, partner’s psychiatric illness, spouse and child bereavement by suicide or other modes of death: A gender specific study. J. Epidemiol. Community Health 59, 407–412 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson J. R., Responses to death and dying: Primates and other mammals. Primates 61, 1–7 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Lonsdorf E. V., et al. , Why chimpanzees carry dead infants: An empirical assessment of existing hypotheses. R. Soc. Open Sci. 7, 200931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson C. L., et al. , Callitrichid responses to dead and dying infants: The effects of paternal bonding and cause of death. Primates 61, 707–716 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Morrison R. E., Eckardt W., Colchero F., Vecellio V., Stoinski T. S., Social groups buffer maternal loss in mountain gorillas. 10.1101/2020.09.01.276956 (1 September 2020). [DOI] [PMC free article] [PubMed]
- 53.Fedigan L. M., Carnegie S. D., Jack K. M., Predictors of reproductive success in female white-faced capuchins (Cebus capucinus). Am. J. Phys. Anthropol. 137, 82–90 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Sargeant E. J., Wikberg E. C., Kawamura S., Fedigan L. M., Allonursing in white-faced capuchins (Cebus capucinus) provides evidence for cooperative care of infants. Behaviour 152, 1841–1869 (2015). [Google Scholar]
- 55.Perry S., Female‐female social relationships in wild white‐faced capuchin monkeys, Cebus capucinus. Am. J. Primatol. 40, 167–182 (1996). [Google Scholar]
- 56.Charnov E. L., Life History Invariants (Oxford University Press, Oxford, 1993). [Google Scholar]
- 57.Wolf J. B., Brodie Iii E. D., Cheverud J. M., Moore A. J., Wade M. J., Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 13, 64–69 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Stadler P. F., Stephens C. R., Landscapes and effective fitness. Comments Theor. Biol. 8, 389–431 (2003). [Google Scholar]
- 59.Cyrus Chu C. Y., Lee R. D., The co-evolution of intergenerational transfers and longevity: An optimal life history approach. Theor. Popul. Biol. 69, 193–201 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee R., Chu C. Y. C., The evolution of transfers and life histories. Exp. Gerontol. 47, 803–806 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charnov E. L., Berrigan D., Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evol. Anthropol. 1, 191–194 (1993). [Google Scholar]
- 62.Moses M. E., Brown J. H., Allometry of human fertility and energy use. Ecol. Lett. 6, 295–300 (2003). [Google Scholar]
- 63.Hadfield J., “The quantitative genetic theory of parental effects” in The Evolution of Parental Care, Royle N. J., Smiseth P. T., Kolliker M., Eds. (Oxford University Press, Oxford, 2012), pp. 267–284. [Google Scholar]
- 64.Charpentier M. J. E., Tung J., Altmann J., Alberts S. C., Age at maturity in wild baboons: Genetic, environmental and demographic influences. Mol. Ecol. 17, 2026–2040 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Cords M., Chowdhury S., Life history of Cercopithecus mitis stuhlmanni in the Kakamega forest, Kenya. Int. J. Primatol. 31, 433–455 (2010). [Google Scholar]
- 66.Richard A. F., Dewar R. E., Schwartz M., Ratsirarson J., Life in the slow lane? Demography and life histories of male and female sifaka (Propithecus verreauxi verreauxi). J. Zool. (Lond.) 256, 421–436 (2002). [Google Scholar]
- 67.Perry S., The behavior of wild white-faced capuchins. Demography, life history, social relationships, and communication. Adv. Stud. Behav. 44, 135–181 (2012). [Google Scholar]
- 68.Watts D. P., Mountain gorilla reproduction and sexual behavior. Am. J. Primatol. 24, 211–225 (1991). [DOI] [PubMed] [Google Scholar]
- 69.Strier K. B., Mendes S. L., “The northern muriqui (Brachyteles hypoxanthus): Lessons on behavioral plasticity and population dynamics from a critically endangered species” in Long-Term Field Studies of Primates, Kappeler P. M., Watts D. P., Eds. (Springer, Berlin, 2012), pp. 125–140. [Google Scholar]
- 70.R Development Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2018). https://www.R-project.org. Accessed 1 May 2018.
- 71.Therneau T., Lumley T., Package “survival,” Version 3.2-2. R Top. Doc. 128 (2015). https://cran.uib.no/web/packages/survival/survival.pdf. Accessed 10 September 2015.
- 72.Therneau T. M., coxme: Mixed effects cox models, Version 2.3-3. R Package (2012). https://cran.uib.no/web/packages/coxme/coxme.pdf. Accessed 1 June 2018.
- 73.Campos F. A., et al. , Does climate variability influence the demography of wild primates? Evidence from long-term life-history data in seven species. Glob. Change Biol. 23, 4907–4921 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Zipple M., et al. , Data from: Beyond orphaned infants: novel effects of maternal death in wild primates. Duke Research Data Repository. 10.7924/r44f1tk8k. Deposited 26 October 2020. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
An anonymized CSV with demographic data has been deposited in the Duke Data Repository, and can be accessed at https://doi.org/10.7924/r44f1tk8k (74).