Key Points
Question
Does breast cancer–specific mortality among US women with estrogen receptor–positive, axillary node–negative breast cancer differ by race/ethnicity within risk categories defined by the 21-gene Oncotype DX Breast Recurrence Score test, and does the prognostic accuracy of the recurrence score differ by race/ethnicity?
Findings
Using the Surveillance, Epidemiology, and End Results Oncotype database, this retrospective cohort study found that compared with non-Hispanic White women, Black women were more likely to have a high-risk recurrence score and had higher breast cancer–specific mortality within each risk category. The recurrence score also provided less prognostic information for Black women.
Meaning
The recurrence score should be calibrated in populations with greater racial/ethnic diversity.
Abstract
Importance
Given the widespread use of the 21-gene recurrence score for identifying candidates for adjuvant chemotherapy, it is important to examine the performance of the Oncotype DX Breast Recurrence Score test in diverse patient populations to validate this approach for tailoring treatment in women in racial/ethnic minority groups.
Objective
To examine whether breast cancer–specific mortality for women with hormone-dependent breast cancer differs by race/ethnicity across risk categories defined by the Oncotype DX Breast Recurrence Score test and whether the prognostic accuracy of the 21-gene recurrence score differs by race/ethnicity.
Design, Setting, and Participants
This retrospective, population-based cohort study used the Surveillance, Epidemiology, and End Results Oncotype DX 2004-2015 database to obtain breast cancer–specific survival data on US women 18 years and older who were diagnosed with first primary stage I to III, estrogen receptor–positive breast cancer between January 1, 2004, and December 31, 2015, and had tumor testing through the Genomic Health Clinical Laboratory. Data were analyzed from April 20 to September 27, 2020.
Main Outcomes and Measures
The primary outcome was breast cancer–specific mortality among women from different racial/ethnic groups stratified by the 21-gene recurrence score risk categories. Secondary analyses compared the prognostic accuracy of the recurrence score among the different racial/ethnic groups.
Results
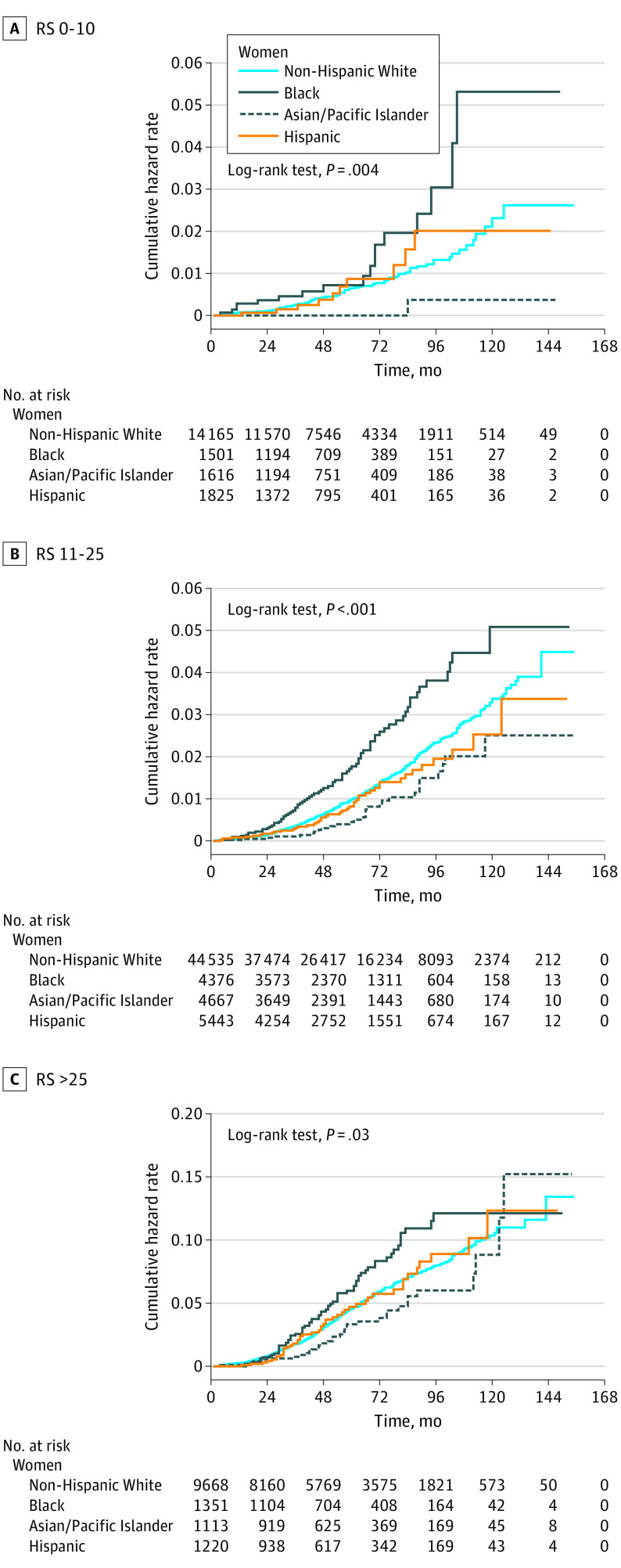
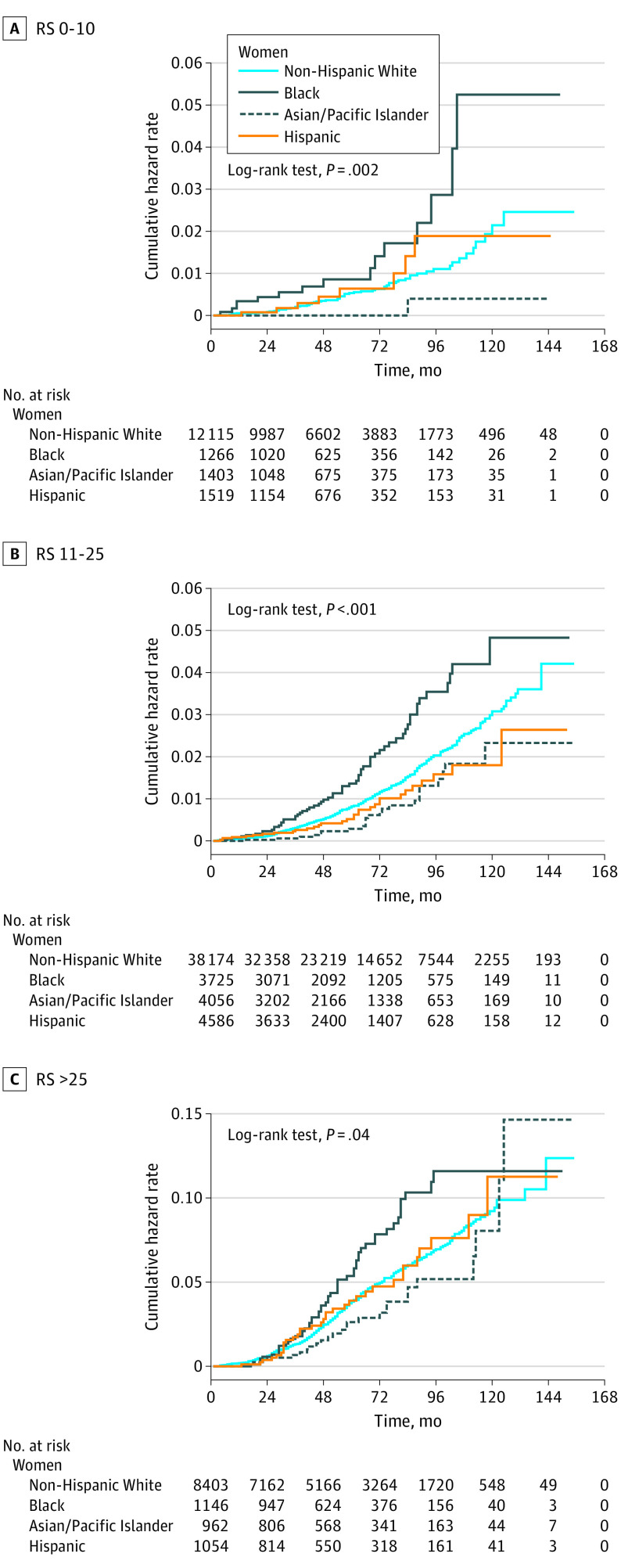
A total of 86 033 patients with breast cancer (mean [SD] age, 57.6 [10.6] years) with Oncotype DX Breast Recurrence Score test information were available for the analysis, including 64 069 non-Hispanic White women (74.4%), 6719 non-Hispanic Black women (7.8%), 7944 Hispanic women (9.2%), 6950 Asian/Pacific Islander women (8.0%), and 351 American Indian/Alaska Native women (0.4%). Black women were significantly more likely than non-Hispanic White women to have a recurrence score greater than 25 (17.7% vs 13.7%; P < .001). Among women with axillary node–negative tumors, competing risk models adjusted for age, tumor characteristics, and treatment found higher breast cancer–specific mortality for Black compared with non-Hispanic White women within each recurrence score risk stratum, with subdistribution hazard ratios of 2.54 (95% CI, 1.44-4.50) for Black women with recurrence scores of 0 to 10, 1.64 (95% CI, 1.23-2.18) for Black women with recurrence scores of 11 to 25, and 1.48 (95% CI, 1.10-1.98) for Black women with scores greater than 25. The prognostic accuracy of the recurrence score was significantly lower for Black women, with a C index of 0.656 (95% CI, 0.592-0.720) compared with 0.700 (95% CI, 0.677-0.722) (P = .002) for non-Hispanic Whites.
Conclusions and Relevance
In this cohort study, Black women in the US were more likely to have a high-risk recurrence score and to die of axillary node–negative breast cancer compared with non-Hispanic White women with comparable recurrence scores. The Oncotype DX Breast Recurrence Score test has lower prognostic accuracy in Black women, suggesting that genomic assays used to identify candidates for adjuvant chemotherapy may require model calibration in populations with greater racial/ethnic diversity.
This cohort study examines whether breast cancer–specific mortality for women with hormone-dependent breast cancer and the prognostic accuracy of a 21-gene recurrence score differ by race/ethnicity.
Introduction
Prognostic gene expression profiles are firmly established as an integral component of treatment planning for women with early-stage, estrogen receptor (ER)–positive, ERBB2 (eg, formerly HER2 or HER2/neu)–negative breast cancer. Endorsement of these genomic biomarkers in treatment guidelines1,2,3 and their widespread adoption in clinical practice4,5 are the result of concerted efforts to establish the clinical validity and utility of these tests with retrospective5,6,7,8,9,10 and prospective11,12,13,14,15 validation studies. The most commonly ordered genomic assay for obtaining prognostic information on the risk of distant recurrence and for predicting the likelihood of benefit from adjuvant chemotherapy in women with ER-positive breast cancer is the 21-gene recurrence score (RS) on the Oncotype DX Breast Recurrence Score test.4 The gene set included in the expression profile and the algorithm for determining the RS were developed using tumors from a subset of participants enrolled in the National Surgical Adjuvant Breast and Bowel Project B-20 trial5,16 and from single-institution case series.17 A key principle of biomarker development is that the cohorts used for discovery and validation should be representative of the populations targeted for clinical application of the biomarker.18 The racial/ethnic distribution of the tumors used to develop the RS was not reported, but overall only 6% of B-20 participants were Black.16 The landmark study6 validating the prognostic accuracy of the RS in a subset of participants from the National Surgical Adjuvant Breast and Bowel Project B-14 trial included 5% Black women. Underrepresentation of women from racial/ethnic minority groups in the development and validation of the RS raises questions about the prognostic accuracy of the Oncotype DX Breast Recurrence Score test in populations other than non-Hispanic White people. This issue is especially relevant in light of persistent racial disparities in survival among Black women with ER-positive breast cancer.19
Given the widespread use of the RS for identifying candidates for adjuvant chemotherapy,4 it is important to examine the performance of the Oncotype DX Breast Recurrence Score test in diverse patient populations to validate this approach for tailoring treatment in women from racial/minority groups. Until recently, no studies have reported the accuracy of the RS separately for different racial/ethnic groups. A recently published study4 using the National Cancer Database found that the prognostic accuracy of the RS for predicting overall survival was lower in Black women compared with non-Hispanic White women. However, we are unaware of any studies that validate the accuracy of the RS for predicting breast cancer–specific survival in racial/ethnic minority groups or that compare breast cancer–specific survival between racial/ethnic groups for each of the risk categories defined by the Oncotype DX Breast Recurrence Score test using nationally representative data. We report an in-depth analysis of breast cancer–specific mortality according to race/ethnicity and the RS in diverse women with ER-positive breast cancer using data from the Surveillance, Epidemiology, and End Results (SEER) Oncotype registry, and we compare the prognostic accuracy of the RS for predicting breast cancer–specific mortality among the different racial/ethnic groups.
Methods
Study Design and Population
We conducted a population-based, retrospective cohort study using the SEER Oncotype DX Database.20 This specialized database includes variables generated from the 21-gene RS assay that are provided by linkage of test orders and results from the Genomic Health Clinical Laboratory with invasive breast cancer cases in the SEER registry diagnosed from January 1, 2004, to December 31, 2015, with follow-up for survival through December 31, 2016. The study was approved by the institutional review board at the University of Illinois at Chicago, which granted a waiver of consent because the study did not involve human participants research. All data were deidentified. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
We included women 18 years and older diagnosed with a first primary breast cancer that was American Joint Committee on Cancer (AJCC) stage I to III and estrogen receptor positive. Information on age at diagnosis, race/ethnicity, year of diagnosis, AJCC stage, tumor size, involvement of axillary lymph nodes, tumor grade, hormone receptor status, surgery, and receipt of radiotherapy and any chemotherapy as part of the first course of therapy were collected from SEER records. Race/ethnicity was categorized per SEER recoding of race and Hispanic ethnicity as non-Hispanic White, non-Hispanic Black, American Indian/Alaska Native, non-Hispanic Asian/Pacific Islander, and Hispanic (any race). Nodal status was classified as negative (N0 or N0[i+]) or positive (N1mi or N1-N3). Vital status and cause of death were determined from longitudinal cancer registry follow-up information. The RS variables included the continuous measure (0- to 100-point scale, with 0 indicating the lowest risk of distant recurrence and 100 indicating the highest risk of distant recurrence) provided by the Genomic Health Clinical Laboratory, month and year of testing, and months from diagnosis to multigene testing. Patients were grouped according to the RS categories established for the Trial Assigning Individualized Options for Treatment (TAILORx) because those cutoffs are the most clinically relevant after publication of that landmark trial.12,13 Women were separated into low-risk (RS, 0-10), intermediate-risk (RS, 11-25), and high-risk (RS, >25) groups. Patients with no race/ethnicity or RS information, longitudinal follow-up for survival, or vital status information were excluded from the study. Information on ERBB2 status was only available for cases diagnosed in years 2010 and later. Women with ERBB2-positive or borderline status were excluded from our analysis. We conducted a sensitivity analysis in patients diagnosed in 2010 to 2015 whose tumors were confirmed as ERBB2 negative. The outcome of interest was death from breast cancer. Women were followed up from the month of breast cancer diagnosis until death or the end of the study period.
Statistical Analysis
Data were analyzed from April 20 to September 27, 2020. We estimated Nelson-Aalen cumulative hazard functions21,22 by race/ethnicity across low-, intermediate-, and high-risk subgroups, with stratification by nodal status, and we tested for equality of hazard functions using stratified log-rank tests.
Relative hazards of breast cancer–specific mortality were estimated using multivariable Fine and Gray competing risks regression models accounting for other-cause death as a competing event.23 Subdistribution hazard ratios (HRs) and robust 95% CIs were estimated comparing Black, American Indian/Alaska Native, Asian/Pacific Islander, and Hispanic women with non-Hispanic White women. Models were stratified by RS risk groups and status of axillary lymph nodes. Multivariable estimates were adjusted for age (continuous), year of diagnosis (2004-2007, 2008-2011, or 2012-2015), tumor size (<2, 2-5, or >5 cm), progesterone receptor status (negative, positive, or unknown), type of surgery (none, breast-conserving, mastectomy, or unknown), and administration of radiation therapy (none/unknown or any) and chemotherapy (none/unknown or any).
Performance of the RS for predicting risk of breast cancer–specific mortality was evaluated in multivariable Fine and Gray models by comparing women with scores greater than 25 with those with scores of 0 to 10 and by each 10-unit increase in the RS. We calculated race/ethnicity-specific C index measures from rank parameter Harrell C with symmetric 95% CIs for the crude HR. Pairwise comparisons of C indexes were performed with a reference group of non-Hispanic White women, and differences in the C index with 95% CIs were estimated using bootstrap samples.24
We determined crude and adjusted associations between race/ethnicity and likelihood of having an RS greater than 25 using modified Poisson regression models with robust SEs.25 Crude and multivariable rate ratios and 95% CIs were estimated with adjustment for age, year of diagnosis, tumor size, nodal status (negative, positive, or unknown), progesterone receptor status, and tumor grade (1, 2, 3, or unknown).
All relevant tests were 2-sided, and associations were considered statistically significant with an α of .05. All analyses were conducted using Stata statistical software, version 16.1 (StataCorp LLC).
Results
A total of 86 033 patients with breast cancer (mean [SD] age, 57.6 [10.6] years) with Oncotype DX Breast Recurrence Score test information were available for the analysis (eFigure 1 in the Supplement), including 64 069 non-Hispanic White women (74.4%), 6719 Black women (7.8%), 7944 Hispanic women (9.2%), 6950 Asian/Pacific Islander women (8.0%), and 351 American Indian/Alaska Native women (0.4%). Overall, 85 118 patients (98.7%) were diagnosed with AJCC stages I to II disease, and 74 002 (85.6%) had axillary lymph node–negative tumors. Adjuvant chemotherapy was administered to 874 women (4.8%) with a low-risk RS, 10 075 (18%) with an intermediate-risk RS, and 8011 (65.8%) with a high-risk RS (eTable 1 in the Supplement). eTable 2 in the Supplement presents data on treatment according to the RS category and race/ethnicity.
Racial/Ethnic Differences in the RS
Demographic and tumor characteristics for each of the risk categories defined by the RS are presented in eTable 1 in the Supplement. Black women were significantly more likely than non-Hispanic White women to have an RS greater than 25 in univariate analysis (17.7% vs 13.7%; P < .001). A high-risk RS was also associated with age younger than 40 years, larger tumor size, higher AJCC stage and tumor grade, and a progesterone receptor–negative tumor (eTable 1 in the Supplement). The relative risk of an RS greater than 25 for Black women compared with non-Hispanic White women was 1.30 (95% CI, 1.23-1.37; P < .001) in unadjusted Poisson regression models and 1.21 (95% CI, 1.15-1.28; P < .001) after adjusting for age, year of diagnosis, tumor size, and nodal status (Table 1). The racial/ethnic difference remained after further adjustment for tumor biologic factors (progesterone receptor status and tumor grade).
Table 1. Association of Race/Ethnicity With Recurrence Score Greater Than 25.
| Race/ethnicity | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| Non-Hispanic | ||||||
| White | 1.00 [Reference] | NA | 1.00 [Reference] | NA | 1.0 [Reference] | NA |
| Black | 1.30 (1.23-1.37) | <.001 | 1.21 (1.15-1.28) | <.001 | 1.11 (1.06-1.17) | <.001 |
| American Indian/Alaska Native | 1.27 (1.01-1.60) | .04 | 1.36 (1.08-1.70) | .009 | 1.24 (1.01-1.53) | .04 |
| Asian/Pacific Islander | 1.07 (1.01-1.14) | .02 | 1.04 (0.98-1.10) | .19 | 0.98 (0.92-1.03) | .40 |
| Hispanic | 1.01 (0.95-1.07) | .82 | 1.00 (0.68-1.13) | .32 | 0.95 (0.90-1.01) | .08 |
Abbreviations: NA, not applicable; RR, rate ratio.
Model 1: unadjusted Poisson regression model with robust SEs.
Model 2: multivariable Poisson regression model with robust SEs adjusted for age, year of diagnosis, tumor size, and nodal status.
Model 3: model 2 plus adjustment for progesterone receptor status and tumor grade.
Breast Cancer–Specific Mortality
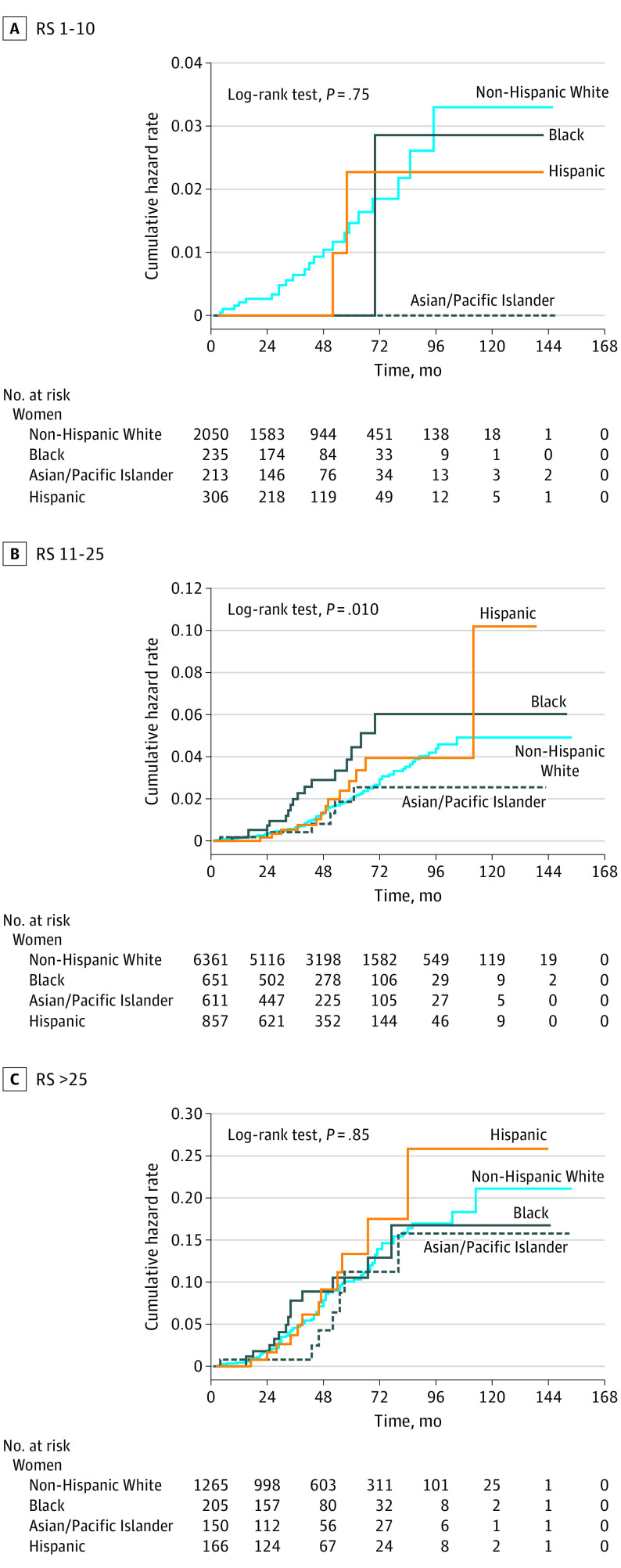
The median follow-up time for the entire cohort was 54 months (interquartile range, 30-83 months). Figure 1 shows cumulative hazard functions for breast cancer–specific mortality for each RS category, stratified by race/ethnicity. The cumulative hazard of mortality was significantly higher for Black women compared with non-Hispanic White women within each RS group. When analyzed according to the status of axillary lymph nodes, the mortality disparity for Black women persisted in all RS risk groups among node-negative patients (Figure 2). Table 2 lists the hazards of breast cancer–specific mortality for node-negative women from racial/minority groups compared with non-Hispanic White women with competing risk regression models adjusted for age, year of diagnosis, tumor size, progesterone receptor status, type of surgery, and administration of radiotherapy and chemotherapy. There was a significantly increased hazard of breast cancer–specific mortality for Black women compared with non-Hispanic White women across all RS risk strata, with subdistribution HRs of 2.54 (95% CI, 1.44-4.50) for RSs of 0 to 10, 1.64 (95% CI, 1.23-2.18) for RSs of 11 to 25, and 1.48 (95% CI, 1.10-1.98) for RSs greater than 25. A sensitivity analysis confined to axillary node–negative patients with tumors that were documented as ERBB2 negative confirmed the findings of the main analysis, with subdistribution HRs that were in the same direction and had similar effect sizes (eTable 3 in the Supplement). No significant racial difference was found in breast cancer–specific mortality among node-positive cases (Figure 3 and eTable 4 in the Supplement).
Figure 1. Cumulative Hazard of Breast Cancer–Specific Mortality by Race/Ethnicity and Recurrence Score (RS).
Figure 2. Cumulative Hazard of Breast Cancer–Specific Mortality by Race/Ethnicity and Recurrence Score (RS) in Axillary Node–Negative Patients.
Table 2. Risk of Breast Cancer–Specific Mortality Among Node-Negative Patients, According to the Recurrence Score and Race/Ethnicity.
| Race/ethnicity | No. of patients | No. of deaths from breast cancer | Subdistribution HR (95% CI) | |
|---|---|---|---|---|
| Age-adjusted model | Multivariable modela | |||
| All node-negative patients | ||||
| Non-Hispanic | ||||
| White | 54 945 | 782 | 1 [Reference] | 1 [Reference] |
| Black | 5697 | 126 | 1.82 (1.51-2.20) | 1.66 (1.37-2.02) |
| American Indian/Alaska Native | 295 | 7 | 1.48 (0.62-3.57) | 1.51 (0.63-3.65) |
| Asian/Pacific Islander | 6033 | 50 | 0.73 (0.55-0.97) | 0.67 (0.50-0.90) |
| Hispanic | 6688 | 73 | 1 (0.78-1.27) | 0.94 (0.73-1.19) |
| Patients with recurrence scores of 0-10 | ||||
| Non-Hispanic | ||||
| White | 11 453 | 65 | 1 [Reference] | 1 [Reference] |
| Black | 1192 | 15 | 2.45 (1.39-4.31) | 2.54 (1.44-4.50) |
| American Indian/Alaska Native | 59 | 1 | 4.27 (0.59-30.91) | 5.15 (0.71-37.44) |
| Asian/Pacific Islander | 1318 | 1 | 0.18 (0.02-1.28) | 0.18 (0.02-1.29) |
| Hispanic | 1434 | 8 | 1.42 (0.68-2.96) | 1.15 (0.50-2.67) |
| Patients with recurrence scores of 11-25 | ||||
| Non-Hispanic | ||||
| White | 35 866 | 402 | 1 [Reference] | 1 [Reference] |
| Black | 3498 | 58 | 1.75 (1.33-2.30) | 1.64 (1.23-2.18) |
| American Indian/Alaska Native | 185 | 2 | 1.26 (0.31-5.07) | 1.3 (0.32-5.20) |
| Asian/Pacific Islander | 3830 | 23 | 0.7 (0.46-1.06) | 0.65 (0.42-0.99) |
| Hispanic | 4315 | 31 | 0.84 (0.58-1.21) | 0.81 (0.56-1.17) |
| Patients with recurrence score >25 | ||||
| Non-Hispanic | ||||
| White | 7606 | 315 | 1 [Reference] | 1 [Reference] |
| Black | 1007 | 53 | 1.53 (1.14-2.05) | 1.48 (1.10-1.98) |
| American Indian/Alaska Native | 51 | 2 | 1.09 (0.27-4.38) | 1.11 (0.28-4.49) |
| Asian/Pacific Islander | 885 | 26 | 0.79 (0.53-1.18) | 0.76 (0.51-1.13) |
| Hispanic | 939 | 34 | 1.08 (0.76-1.54) | 1.06 (0.74-1.51) |
Abbreviation: HR, hazard ratio.
Multivariable Fine-Gray model with robust SEs adjusted for age, year of diagnosis, tumor size, progesterone receptor status, type of surgery, and administration of radiotherapy and chemotherapy.
Figure 3. Cumulative Hazard of Breast Cancer–Specific Mortality by Race/Ethnicity and Recurrence Score (RS) in Axillary Node–Positive Patients.
Prognostic Accuracy of the RS in Women With Node-Negative Disease
The RS provided statistically significant prognostic information on breast cancer–specific mortality in each racial/ethnic group in competing risk models adjusted for age, year of diagnosis, tumor size, progesterone receptor status, type of surgery, and administration of radiotherapy and chemotherapy, with the exception of American Indian/Alaska Native women (eTable 5 in the Supplement). However, significant differences were seen among racial/ethnic groups. The subdistribution HR for women with RSs of 0 to 10 compared with RSs greater than 25 were 5.79 (95% CI, 4.29-7.82) for non-Hispanic White women and 3.48 (95% CI, 1.83-6.63) for Black women. Each 10-unit increase in the RS provided significantly more prognostic information for non-Hispanic White women, with a subdistribution HR of 1.71 (95% CI, 1.60-1.83) and a C index of 0.700 (95% CI, 0.677-0.722) compared with a subdistribution HR of 1.58 (95% CI, 1.35-1.84) and a C index of 0.656 (95% CI, 0.592-0.720) (P = .002) for Black women. The CI for the difference in C indexes does not cross zero (difference, –0.044; 95% CI, –0.085 to –0.002), confirming a significant racial/ethnic difference in the prognostic accuracy of the RS.
Discussion
To our knowledge, this cohort study is the first population-based study using a nationally representative data set to compare breast cancer–specific mortality across racial/ethnic groups according to risk strata defined by the Oncotype DX assay. This study found that Black women are more likely to have a high-risk RS and to die of axillary node–negative breast cancer compared with women of other race/ethnicity with the same RS. These findings also indicate that the validity of the Oncotype DX test for determining the need for adjuvant chemotherapy in Black women with node-negative tumors must be further investigated.
Collin et al26 used data from the Georgia Cancer Registry to examine racial/ethnic differences in the RS in 4418 non-Hispanic white women and 1332 Black women with ER-positive breast cancer and reported findings similar to those of the current study, with HRs for breast cancer–specific mortality for Black women compared with non-Hispanic White women ranging from 1.56 to 2.57 across RS risk categories. However, several key differences exist between that study26 and the current one, in addition to the larger sample size in the present analysis. First, the study by Collin et al26 used population-based data from a single state rather than a nationwide sample. There is evidence that racial/ethnic disparities in breast cancer mortality vary by region27; therefore, the SEER data set used in this report may better reflect the overall racial disparity in this country. Second, the study by Collin et al26 does not report HRs separately for node-negative and node-positive patients. The current study found that the disparity is primarily observed among women with node-negative disease.
These findings partially confirm preliminary findings from the TAILORx trial that were reported in abstract form.28 The TAILORx investigators found that Black women with an intermediate-risk RS had worse overall survival compared with non-Hispanic White women despite comparable treatment. The current study found a hazard of mortality for Black women with an intermediate-risk RS that was nearly identical to that in the TAILORx report (a subdistribution HR of 1.64 compared with an HR of 1.67 in TAILORx). However, important differences exist between this study and the TAILORx study.28 First, the present study reports racial/ethnic differences in breast cancer–specific mortality, whereas the TAILORx study reported overall and disease-free survival. Breast cancer–specific mortality provides a more direct measure of racial/ethnic differences in prognosis and treatment effect because it is not influenced by mortality from comorbid conditions that are disproportionately prevalent in the Black population.29 Second, the current analysis used data from the SEER registry rather than from a clinical trial; therefore, it may be more representative of the breast cancer population in this country. Third, the current study reports a significant racial/ethnic disparity in survival in all 3 RS risk groups, whereas the TAILORx study found a difference only among women with an RS of 11 to 25. This finding may be the result of the 10-fold larger sample size in the current analysis (6719 Black participants compared with 693 in TAILORx), which provided more robust statistical power for subgroup analyses. However, it is difficult to speculate on reasons for the discordant findings because a detailed analysis of racial/ethnic differences in the TAILORx trial has not been published to date.
The current study found that the RS underperforms for Black women with axillary node–negative tumors compared with non-Hispanic White women, with a lower hazard of breast cancer mortality for high- vs low-risk groups and a lower hazard for each 10-unit increase in the RS, with significantly lower C indexes30 for both measures. To our knowledge, only 1 other study4 compared the performance of the RS in diverse racial/ethnic groups. Ibraheem et al4 reported similar findings using data from the National Cancer Database with overall survival as the outcome. The limitations of that outcome for studying cancer disparities are noted above. Taken together, these data suggest that the RS may not be well calibrated for racial/ethnic minority populations. This disparity underscores the need for additional work to formally test the calibration of genomic prognostic assays in diverse populations to determine whether the models require recalibration for racial/ethnic minority patients.
Finally, the finding that Black women are more likely to have a high-risk RS confirms the results of 2 earlier studies31,32 that examined data from local cancer registries. Furthermore, other reports33,34 indicate that Black women with ER-positive tumors have higher scores on the PAM50 risk of recurrence assay. These data, along with emerging molecular investigations,35 suggest that Black women disproportionately develop biologically aggressive hormone-dependent tumors. The data also suggest that racial/ethnic differences in prognosis are only partially reflected in the RS. The underlying mechanisms contributing to worse survival in Black women with ER-positive breast cancer are not known. Multilevel social determinants of health, including the effects of living in poverty, residential segregation, experiences of racism and discrimination, and inequitable access to timely and high-quality cancer screening and treatment, are consequences of racial/ethnic stratification in our society and are root causes of health disparities.36 It is likely that these factors contributed to the findings of the current study. This report highlights the need for more studies to illuminate the intersection of social determinants of health and tumor biology.37
Strengths and Limitations
This study has strengths and limitations. It is, to our knowledge, the largest study of racial/ethnic difference in survival across RS risk strata published to date. Strengths include an adequate sample size of women from racial/ethnic minority groups to allow detailed subgroup analyses, a sample that is broadly representative of patients with breast cancer in the US, breast cancer–specific mortality as the outcome, and the use of competing risk models. However, the study also has limitations. Data in the SEER registry are from patients who were treated nonuniformly, which could introduce bias. However, treatment received, including type of surgery and receipt of adjuvant radiotherapy and chemotherapy, was adjusted for in multivariable models, but factors that were not accounted for could confound the analysis. For example, the SEER registry does not collect data on adherence to adjuvant endocrine therapy. Any racial/ethnic differences in adherence could confound survival analyses. Studies38,39,40,41,42 that examined racial/ethnic differences in early discontinuation of endocrine therapy report conflicting results. The higher discontinuation rate among Black women reported in some claims-based studies can be explained by a higher rate of early recurrence in these women.43 An observational study44 and a clinical trial45 that documented adherence to adjuvant treatment found that nonadherence to endocrine therapy does not explain racial/ethnic differences in survival. Similarly, a modeling study46 concluded that differences in the use of adjuvant therapies do not entirely explain the racial/ethnic disparity in breast cancer mortality. Therefore, it is unlikely that the differential use of adjuvant endocrine therapy explains the results of this study. Finally, although relevant patient and tumor characteristics were adjusted for, it is possible that factors not included in these models influenced recommendations for Oncotype DX testing and introduced selection bias.
Conclusions
This study suggests that Black women in the US with ER-positive, ERBB2-negative, axillary lymph node–negative breast cancer are more likely to have a high-risk RS and to experience breast cancer–specific mortality compared with non-Hispanic White women within the same risk group. In addition, the RS provides less prognostic information for Black women. The findings suggest that Black women disproportionately develop aggressive ER-positive tumors and that the Oncotype DX Breast Recurrence Score test incompletely defines prognosis in these women. Genomic prognostic assays may require recalibration for racial/ethnic minority groups. These factors could contribute to racial/ethnic disparities in breast cancer mortality and require further study.
eFigure 1. CONSORT Diagram of Study Cohort Selection
eTable 1. Characteristics of Study Participants
eTable 2. Treatment of Node-Negative Participants
eTable 3. Sensitivity Analysis: Risk of Breast Cancer–Specific Mortality According to the Recurrence Score and Race/Ethnicity Restricted to Node-Negative Tumors That Are ER-Positive and HER2-Negative
eTable 4. Risk of Breast Cancer-Specific Mortality Among Node-Positive Patients, According to the Recurrence Score and Race/Ethnicity
eTable 5. Performance of the Recurrence Score in Axillary Lymph Node–Negative Patients, by Race/Ethnicity
eReference
References
- 1.Krop I, Ismaila N, Stearns V. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice focused update guideline summary. J Oncol Pract. 2017;13(11):763-766. doi: 10.1200/JOP.2017.024646 [DOI] [PubMed] [Google Scholar]
- 2.Andre F, Ismaila N, Henry NL, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update—integration of results from TAILORx. J Clin Oncol. 2019;37(22):1956-1964. doi: 10.1200/JCO.19.00945 [DOI] [PubMed] [Google Scholar]
- 3.Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(4):452-478. doi: 10.6004/jnccn.2020.0016 [DOI] [PubMed] [Google Scholar]
- 4.Ibraheem A, Olopade OI, Huo D. Propensity score analysis of the prognostic value of genomic assays for breast cancer in diverse populations using the National Cancer Data Base. Cancer. 2020;126(17):4013-4022. doi: 10.1002/cncr.32956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petkov VI, Miller DP, Howlader N, et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer. 2016;2:16017. doi: 10.1038/npjbcancer.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817-2826. doi: 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 7.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726-3734. doi: 10.1200/JCO.2005.04.7985 [DOI] [PubMed] [Google Scholar]
- 8.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829-1834. doi: 10.1200/JCO.2009.24.4798 [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Barlow WE, Shak S, et al. ; Breast Cancer Intergroup of North America . Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55-65. doi: 10.1016/S1470-2045(09)70314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sestak I, Buus R, Cuzick J, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):545-553. doi: 10.1001/jamaoncol.2017.5524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso F, van’t Veer LJ, Bogaerts J, et al. ; MINDACT Investigators . 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717-729. doi: 10.1056/NEJMoa1602253 [DOI] [PubMed] [Google Scholar]
- 12.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005-2014. doi: 10.1056/NEJMoa1510764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111-121. doi: 10.1056/NEJMoa1804710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gluz O, Nitz UA, Christgen M, et al. West German Study Group Phase III PlanB Trial: first prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol. 2016;34(20):2341-2349. doi: 10.1200/JCO.2015.63.5383 [DOI] [PubMed] [Google Scholar]
- 15.Stemmer SM, Steiner M, Rizel S, et al. Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017;3:32. doi: 10.1038/s41523-017-0033-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89(22):1673-1682. doi: 10.1093/jnci/89.22.1673 [DOI] [PubMed] [Google Scholar]
- 17.Cobleigh MA, Tabesh B, Bitterman P, et al. Tumor gene expression and prognosis in breast cancer patients with 10 or more positive lymph nodes. Clin Cancer Res. 2005;11(24 Pt 1):8623-8631. doi: 10.1158/1078-0432.CCR-05-0735 [DOI] [PubMed] [Google Scholar]
- 18.Pepe MS, Feng Z, Janes H, Bossuyt PM, Potter JD. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008;100(20):1432-1438. doi: 10.1093/jnci/djn326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438-451. doi: 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 20.Surveillance, Epidemiology, and End Results (SEER) Program. Oncotype DX Database (2004-2015). National Cancer Institute. April 2019. Accessed February 17, 2020. https://seer.cancer.gov/seerstat/databases/oncotype-dx/index.html
- 21.Nelson W. Theory and applications of hazard plotting for censored failure data. Technometrics. 1972;14(4):945-966. doi: 10.1080/00401706.1972.10488991 [DOI] [Google Scholar]
- 22.Aalen O. Nonparametric inference for a family of counting processes. Ann Stat. 1978;6(4):701-726. doi: 10.1214/aos/1176344247 [DOI] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 24.Kang L, Chen W, Petrick NA, Gallas BD. Comparing two correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Stat Med. 2015;34(4):685-703. doi: 10.1002/sim.6370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 26.Collin LJ, Yan M, Jiang R, et al. Oncotype DX recurrence score implications for disparities in chemotherapy and breast cancer mortality in Georgia. NPJ Breast Cancer. 2019;5:32. doi: 10.1038/s41523-019-0129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitman S, Orsi J, Hurlbert M. The racial disparity in breast cancer mortality in the 25 largest cities in the United States. Cancer Epidemiol. 2012;36(2):e147-e151. doi: 10.1016/j.canep.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 28.Albain KS, Gray RJ, Makower DF JA, et al. Race, ethnicity and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer in the randomized TAILORx trial. J Natl Cancer Inst. Published online September 28, 2020. doi: 10.1093/jnci/djaa148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izano M, Satariano WA, Tammemagi MC, et al. Long-term outcomes among African-American and white women with breast cancer: what is the impact of comorbidity? J Geriatr Oncol. 2014;5(3):266-275. doi: 10.1016/j.jgo.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 30.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128-138. doi: 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holowatyj AN, Cote ML, Ruterbusch JJ, et al. Racial differences in 21-gene recurrence scores among patients with hormone receptor-positive, node-negative breast cancer. J Clin Oncol. 2018;36(7):652-658. doi: 10.1200/JCO.2017.74.5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund MJ, Mosunjac M, Davis KM, et al. 21-Gene recurrence scores: racial differences in testing, scores, treatment, and outcome. Cancer. 2012;118(3):788-796. doi: 10.1002/cncr.26180 [DOI] [PubMed] [Google Scholar]
- 33.Huo D, Hu H, Rhie SK, et al. Comparison of breast cancer molecular features and survival by African and European ancestry in The Cancer Genome Atlas. JAMA Oncol. 2017;3(12):1654-1662. doi: 10.1001/jamaoncol.2017.0595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troester MA, Sun X, Allott EH, et al. Racial differences in PAM50 subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst. 2018;110(2). doi: 10.1093/jnci/djx135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byun JS, Singhal SK, Park S, et al. Racial differences in the association between luminal master regulator gene expression levels and breast cancer survival. Clin Cancer Res. 2020;26(8):1905-1914. doi: 10.1158/1078-0432.CCR-19-0875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608-1615. doi: 10.2105/AJPH.2006.102525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linnenbringer E, Gehlert S, Geronimus AT. Black-white disparities in breast cancer subtype: the intersection of socially patterned stress and genetic expression. AIMS Public Health. 2017;4(5):526-556. doi: 10.3934/publichealth.2017.5.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120-4128. doi: 10.1200/JCO.2009.25.9655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farias AJ, Du XL. Racial differences in adjuvant endocrine therapy use and discontinuation in association with mortality among Medicare breast cancer patients by receptor status. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1266-1275. doi: 10.1158/1055-9965.EPI-17-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am J Public Health. 2015;105(suppl 3):e4-e15. doi: 10.2105/AJPH.2014.302490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farias AJ, Wu WH, Du XL. Racial differences in long-term adjuvant endocrine therapy adherence and mortality among Medicaid-insured breast cancer patients in Texas: findings from TCR-Medicaid linked data. BMC Cancer. 2018;18(1):1214. doi: 10.1186/s12885-018-5121-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602-606. doi: 10.1200/JCO.2003.07.071 [DOI] [PubMed] [Google Scholar]
- 43.Menashe I, Anderson WF, Jatoi I, Rosenberg PS. Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. J Natl Cancer Inst. 2009;101(14):993-1000. doi: 10.1093/jnci/djp176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33(20):2254-2261. doi: 10.1200/JCO.2014.57.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sparano JA, Wang M, Zhao F, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104(5):406-414. doi: 10.1093/jnci/djr543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Ravesteyn NT, Schechter CB, Near AM, et al. Race-specific impact of natural history, mammography screening, and adjuvant treatment on breast cancer mortality rates in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(1):112-122. doi: 10.1158/1055-9965.EPI-10-0944 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. CONSORT Diagram of Study Cohort Selection
eTable 1. Characteristics of Study Participants
eTable 2. Treatment of Node-Negative Participants
eTable 3. Sensitivity Analysis: Risk of Breast Cancer–Specific Mortality According to the Recurrence Score and Race/Ethnicity Restricted to Node-Negative Tumors That Are ER-Positive and HER2-Negative
eTable 4. Risk of Breast Cancer-Specific Mortality Among Node-Positive Patients, According to the Recurrence Score and Race/Ethnicity
eTable 5. Performance of the Recurrence Score in Axillary Lymph Node–Negative Patients, by Race/Ethnicity
eReference