Summary
The plant hormone auxin is a key factor for regulation of plant development, and this function was probably reinforced during the evolution of early land plants. We have extended the available toolbox to allow detailed studies of how auxin biosynthesis and responses are regulated in moss reproductive organs, their stem cells and gametes to better elucidate the function of auxin in the morphogenesis of early land plants.
We measured auxin metabolites and identified IPyA (indole‐3‐pyruvic acid) as the main biosynthesis pathway in Physcomitrium (Physcomitrella) patens and established knock‐out, overexpressor and reporter lines for biosynthesis genes which were analyzed alongside previously reported auxin‐sensing and transport reporters.
Vegetative and reproductive apical stem cells synthesize auxin. Sustained stem cell activity depends on an inability to sense the auxin produced while progeny of the stem cells respond to the auxin, aiding in the control of cell division, expansion and differentiation. Gamete precursors are dependent on a certain degree of auxin sensing, while the final differentiation is a low auxin‐sensing process.
Tha data presented indicate that low auxin activity may represent a conserved hallmark of land plant gametes, and that local auxin biosynthesis in apical stem cells may be part of an ancestral mechanism to control focal growth.
Keywords: auxin, moss, Physcomitrium(Physcomitrella)patens, R2D2, reproductive development, stem cell, TAR, YUC
Introduction
The plant hormone auxin is a key factor in the regulation of plant development, and this function was probably reinforced during early land plant evolution. Functional studies in the bryophyte models Physcomitrium (Physcomitrella) patens and Marchantia polymorpha, early diverging land plants separated from the flowering plants roughly 450 million years ago, lend support to this hypothesis (Rensing et al., 2008; Finet & Jaillais, 2012; Bowman et al., 2017; Thelander et al., 2018, 2019; Kato et al., 2018; Morris et al., 2018; Puttick et al., 2018). Homologs to genes encoding the nuclear auxin sensing and response machinery in the flowering plant model Arabidopsis thaliana are present in the model bryophyte genomes (Rensing et al., 2008; Flores‐Sandoval et al., 2015; Kato et al., 2015). Genetic characterization suggests strongly that the bryophyte TIR1/AFB‐AUX/IAA co‐receptors and the three classes of ARF transcription factors regulating auxin responses are functionally conserved (Ashton et al., 1979; Paponov et al., 2009; Prigge et al., 2010; Causier et al., 2012a,b; Lavy et al., 2016, 2012; Sugano et al., 2014; Eklund et al., 2015; Kato et al., 2015, 2017, 2018, 2020). In addition, PIN‐mediated directional auxin transport is important for developmental regulation, not only in flowering plants, but also in bryophytes, as demonstrated for P. patens (Bennett et al., 2014; Viaene et al., 2014).
Studies using exogenous auxin or bryophyte lines affected in auxin sensing, signaling or transport suggest that auxin was important for cell fate transitions early in land plant evolution. In P. patens, the transition from chloronema to caulonema, two filamentous tissue types, is promoted by auxin (Johri & Desai, 1973; Ashton et al., 1979; Cove & Ashton, 1984; Prigge et al., 2010; Hayashi et al., 2012; Lavy et al., 2012, 2016; Viaene et al., 2014; Plavskin et al., 2016). Likewise, the initiation of root hair‐like rhizoids is promoted by exogenous auxin and sensed through the TIR1/AFB‐AUX/IAA co‐receptors, in both bryophyte models (Ashton et al., 1979; Sakakibara et al., 2003; Prigge et al., 2010; Kato et al., 2015; Lavy et al., 2016). Auxin also affects branching in the bryophytes. Changes in auxin signaling by inhibition of auxin biosynthesis or overexpression (OE) of auxin responses in the M. polymorpha thallus caused a decreased or increased bifurcation rate, respectively (Flores‐Sandoval et al., 2015). In addition, reduced auxin transport in P. patens induces branching of the gametophore shoots and the sporophyte (Fujita et al., 2008; Bennett et al., 2014; Coudert et al., 2015).
During the emergence of land plants, many important characters were acquired, including three‐dimensional growth from apical meristems, which in at least flowering plants are dependent on regulated auxin sensing (Du & Scheres, 2018; Wang & Jiao, 2018). Three‐dimensional tissue growth and organ initiation occurs through divisions of a single meristematic apical stem cell in bryophytes (Harrison et al., 2009; Kofuji & Hasebe, 2014; Shimamura, 2016). The formation and maintenance of stem cells in P. patens gametophore shoots, and in M. polymorpha gemma are both affected by changes in auxin sensing (Bennett et al., 2014; Lavy et al., 2016; Kato et al., 2017). However, so far, a limited toolbox has hampered detailed studies of how auxin affects the identity and function of bryophyte apical stem cells. One additional approach is to change the levels of auxin biosynthesis. In flowering plants, the indole‐3‐pyruvate (IPyA) auxin biosynthesis pathway (Ljung, 2013; Kasahara, 2016; Casanova‐Sáez & Voss, 2019) dominates. This is initiated by the conversion of tryptophan to IPyA by TAA1‐related enzymes (TARs), while YUCCA‐related enzymes (YUCs) convert IPyA to the active auxin IAA (Stepanova et al., 2008; Tao et al., 2008; Mashiguchi et al., 2011; Won et al., 2011). The bryophyte genomes encode homologous sequences, and OE of an M. polymorpha YUC homolog results in elevated IAA production, and morphological changes similar to those induced by exogenous IAA treatment were found in both MpTAR and MpYUC OE lines (Eklund et al., 2015).
Thus, to widen our understanding of auxin biosynthesis in early land plant evolution and to establish tools to study spatiotemporal changes in auxin sensing and responses, we investigated the IPyA pathway in P. patens. Representative PpYUC and PpTAR homologs were selected for OE and knock‐out (KO) studies. This revealed that the IPyA pathway is the major P. patens auxin biosynthesis route controlled by largely redundant gene copies, and that the first step is rate limiting. As the genetic redundancy was less prominent in the reproductive organs, the KO lines allowed us to use the initiation and development of gametangia to study the role of auxin in stem cells, as well as in organ and gamete differentiation. Apart from following the effect of reduced auxin production on reproductive development, we also compared the sites of auxin biosynthesis and sensing. We used PpTAR and PpYUC transcriptional reporters as well as the PpR2D2 reporter for AuxRE‐ and ARF‐independent detection of auxin sensing activity (Thelander et al., 2019). These studies revealed that vegetative as well as reproductive gametophore stem cells produce auxin, but that this auxin is not sensed by the stem cells. Instead, auxin sensing increases in daughter cells, aiding in the control of cell division, expansion and thus in the final differentiation/morphogenesis. Early egg and sperm precursor cells are dependent on a certain degree of auxin sensing, while the final differentiation of the gametes appears to be a low auxin sensing process.
Materials and Methods
Plant material, growth conditions, moss transformation and crosses
The Physcomitrium (Physcomitrella) patens ecotype Reute was used as WT and background to all transgenic lines produced (Hiss et al., 2017). The following lines were published previously: PpR2D2‐2 and PpR2D2‐3 (Thelander et al., 2019), PpPINBpro::GFP‐1, PpPINCpro::GFP‐1, PpPINDpro::GFP‐1 and PpPINApro::PpPINA‐GFP‐2 (Viaene et al., 2014, Gransden ecotype). For cultivation, spores were germinated, or protonemal tissue was subcultivated, on MM plates containing BCD medium (Thelander et al, 2007) supplemented with 5 mM ammonium tartrate (Sigma Aldrich A2956) and 0.8% agar (Sigma Aldrich A1296). The protonemal tissue was shaped into 2 mm balls, inoculated on solid BCD medium in 25 mm deep Petri dishes (VWR International PHOE305; Radnor, PA, USA) and grown at 25°C under constant white light from F25T8/TL741 fluorescent tubes (Philips, Somerset, NJ, USA) at 35 µmol m−2 s−1 in a Percival Scientific CU‐41L4 growth chamber (Perry, IA, USA) for 5–6 wk. To induce reproductive organ formation the plates were then transferred to a Sanyo MLR‐350 light chamber with 8 h of light (30 µmol m−2 s−1) per day at 15°C. Protoplast transformation and crosses were carried out as previously described (Schaefer et al., 1991; Thelander et al., 2019). Stable transformants and higher order mutants were selected in the presence of 50 µg ml−1 hygromycin (Duchefa H0192; Haarlem, the Netherlands), G418 (11811023; Thermo Fisher Scientific, Waltham, MA, USA) or zeocin (R250‐01; Thermo Fisher Scientific).
IAA metabolite measurements
Protonemal tissue, blended and re‐grown twice, was grown for 9 d on cellophane (A.A. Packaging Limited, Preston, UK) overlayed MM plates in continuous light, and then harvested, weighed and frozen in liquid nitrogen. At least five independent biological replicates were harvested for the PpTARA OE, PpTARF OE, PpYUCA OE, PpYUCC OE, Pptara‐1, Pptarb‐1 and Pptaratarb‐1 lines and 20 biological samples for WT. The extraction, purification and the LC‐MS analysis of endogenous IAA, its precursors and metabolites were carried out according to Novák et al. (2012). Briefly, c. 25 mg of frozen material per sample was homogenized using a bead mill (27 Hz, 10 min, 4°C; MixerMill, Retsch GmbH, Haan, Germany) and extracted in 1 ml of 50 mM sodium phosphate buffer containing 1% sodium diethyldithiocarbamate and a mixture of 13C6‐ or deuterium‐labeled internal standards. After centrifugation (23 000 g, 15 min, 4°C), the supernatant was divided into two aliquots: the first aliquot was derivatized using cysteamine (0.25 M; pH 8; 1 h; room temperature; Sigma‐Aldrich), and the second aliquot was immediately further processed as follows. The pH of the sample was adjusted to 2.5 by 1 M HCl and applied on preconditioned solid‐phase extraction columns (30 mg 1 ml, Oasis HLB; Waters Inc., Milford, MA, USA). After sample application, the column was rinsed with 2 ml 5% methanol. Compounds of interest were then eluted with 2 ml 80% methanol. The derivatized fraction was purified alike. MS analysis and quantification were performed by using an LC‐MS/MS system comprising a 1290 Infinity Binary LC System coupled to a 6490 Triple Quad LC/MS System with Jet Stream and Dual Ion Funnel technologies (Agilent Technologies, Santa Clara, CA, USA).
Gene inventory and phylogenetic reconstruction
The full complement of TAR and YUC genes in the genome sequences of Arabidopsis (TAIR 10) and P. patens (v.3.3) were identified by Blast searches against the Phytozome database (v.12.1.5) and protein sequences deduced from primary gene models were retrieved (AtTAA1: AT1G70560.1; AtTAR1: AT1G23320.1; AtTAR2: AT4G24670.1; AtTAR3: AT1G34040.1; AtTAR4: AT1G34060.1; AtYUC1: AT4G32540.1; AtYUC2: AT4G13260.1; AtYUC3: AT1G04610.1; AtYUC4: AT5G11320.1; AtYUC5: AT5G43890.1; AtYUC6: AT5G25620.2; AtYUC7: AT2G33230.1; AtYUC8: AT4G28720.1; AtYUC9: AT1G04180.1; AtYUC10: AT1G48910.1; AtYUC11: AT1G21430.1; PpTARA: Pp3c21_15370V3.1; PpTARB: Pp3c18_15140V3.1; PpTARC: Pp3c17_6500V3.1; PpTARD: Pp3c26_12520V3.1; PpTARE: Pp3c25_6670V3.1; PpTARF: Pp3c5_24670V3.1; PpYUCA: Pp3c3_18590V3.1; PpYUCB: Pp3c11_11790V3.1; PpYUCC: Pp3c1_11500V3.1; PpYUCD: Pp3c2_27740V3.1; PpYUCE: Pp3c13_21970V3.1; PpYUCF: Pp3c3_20490V3.1). Phylogenetic reconstructions were produced with the Megax software (v.10.1.5; Kumar et al., 2018): protein alignments produced with the muscle algorithm (default settings) were used to construct trees with the maximum likelihood method (default settings) and 500 replications of bootstrapping.
Construct building and transgenic lines
The generation of new transgenic lines is described in Supporting Information Methods S1–S4, and their molecular verification is shown in Figs S1–S6. For lists of constructs and primers, see Tables S1 and S2, respectively.
RT‐qPCR
Reverse transcriptase quantitative PCR (RT‐qPCR) determination of PpTAR transcript abundance is described in Methods S5.
Mutant phenotyping
Colonies and vegetative shoots were documented using a Leica M205 FA stereo microscope and Las af software (Leica Microsystems, Wetzlar, Germany). Colony diameters were measured in imagej (Schneider et al., 2012). To analyze reproductive organ development, entire shoot apices were harvested at indicated time points and all leaves were detached under a stereo microscope (Leica MZ16; Leica Biosystems, Heidelberg, Germany) leaving the reproductive organs exposed at the apex. The shoot apices were mounted on objective glasses in 30% glycerol and reproductive organs were analyzed using a Leica DMI4000B microscope with differential interference contrast (DIC; Nomarski) optics, a Leica DFC360FX camera, and the Las af (Leica Microsystems) software. Adobe Photoshop CC was used to adjust intensity and contrast, remove background, mark borders and cells, and to visualize entire late‐stage archegonia by merging two to three images taken at 63× or 100× magnification. All presented experiments were performed in at least two independent biological data sets. Microsoft Excel was used to create bar charts, calculate means and SD, and to perform a Student’s t‐test where indicated in the Results section.
Confocal microscopy
For fluorescence reporter signal analysis, shoot apices were harvested as for phenotypic analysis, mounted in water and immediately detected with an inverted Zeiss 780 confocal microscope at 20× (Plan‐Apochromat, NA 0.8) or 63× (C‐Apochromat, water immersion, NA 1.20) magnification. Excitation/detection parameters were 488 nm/491–598 nm for green fluorescent protein (GFP) and 633/647–721 nm for Chl auto‐fluorescence. Both PpTARA::GFP‐GUS lines analyzed had the same signal strength in all analyzed developmental stages and organs, and selected pictures of both are shown in the figures. PpPINB, PpPINC and PpPIND GFP reporter signals were detected with an inverted Zeiss 800 confocal microscope at 40× and 63× magnification. Excitation/detection parameters were 488 nm/410–617 nm for GFP and 655/656–721 nm for Chl auto‐fluorescence. All images are snapshots of a single focal plane with selected channels overlaid using the Zen black software.
PpR2D2 output was detected and quantified as previously described with the addition that late‐stage archegonia were analyzed with the 20× objective while all other organs were analyzed with the 63× objective (Thelander et al., 2019). PpR2D2 output (images as well as signal quantifications) presented within a figure is always directly comparable, but this is not necessarily the case between figures due to differences in microscopy settings and/or image processing.
Results
The IPyA pathway is dominant in P. patens and manipulation of TAR and YUC expression drastically affects auxin levels
To investigate if the IPyA pathway is important for auxin biosynthesis in P. patens, we measured IAA metabolite levels in chloronema‐enriched protonemal tissue. Of the four IAA precursors representing different potential routes from tryptophan (TRP) to IAA, the level of IPyA is high, and tryptamine (TRA) low, while indole‐3‐acetonitrile (IAN) and indole‐3‐acetamide (IAM) are nondetectable, establishing IPyA as the dominant IAA precursor in P. patens (Table 1). Also, the amounts of the oxidative catabolites oxIAA and oxIAA‐glucose are high, of amino acid conjugates extremely low, and of glucose conjugates significant, showing that auxin conjugation occurs in P. patens. Overall, the metabolite profile is similar to that of flowering plants (Novák et al, 2012).
Table 1.
Auxin metabolite profile in Physcomitrium (Physcomitrella) patens chloronema‐enriched protonema.
| Abbreviation | Compound | Concentration (pmol g−1 FW; mean ± SD) |
|---|---|---|
| TRP | Tryptophan | 21 785 ± 5712 |
| TRA (TAM) | Tryptamine | 11.77 ± 3.56 |
| IAN | Indole‐3‐acetonitrile | UDL |
| IAM | Indole‐3‐acetamide | UDL |
| IPyA | Indole‐3‐pyruvic acid | 82.99 ± 17.22 |
| IAA | Indole‐3‐acetic acid | 8.50 ± 1.75 |
| oxIAA | 2‐Oxoindole‐3‐acetic acid | 27.17 ± 5.16 |
| oxIAA‐Glc | oxIAA‐glucose | 46.69 ± 12.41 |
| IAA‐Glc | IAA‐glucose | 7.84 ± 2.08 |
| IAA‐Asp | IAA‐aspartate | 0.28 ± 0.09 |
| IAA‐Gly | IAA‐glycin | 0.58 ± 0.24 |
| IAA‐Glu | IAA‐glutamate | 0.62 ± 0.15 |
| IAA‐Val | IAA‐valine | UDL |
| IAA‐Leu | IAA‐leucine | 0.08 ± 0.03 |
| IAA‐Phe | IAA‐phenylalanine | UDL |
| IAA‐Trp | IAA‐tryptophane | UDL |
FW, fresh weight; UDL, under detection limit.
In accordance with previous inventories and comprehensive phylogenetic reconstructions, we identified multiple tryptophan aminotransferase (TAR) and YUCCA (YUC) genes in P. patens (Yue et al., 2014; Eklund et al., 2015; Poulet & Kriechbaumer, 2017; Romani, 2017). The genome possesses six TAR homologs on separate chromosomes which fall in two distinct phylogenetic groups (Fig. 1a). PpTARA–D cluster with the Arabidopsis genes AtTAA1, AtTAR1 and AtTAR2 demonstrated to play a role in auxin biosynthesis (TAA clade in Fig. 1a; Stepanova et al., 2008; Tao et al., 2008). PpTARE–F cluster with AtTAR3 and AtTAR4 (AtTAR3/4 clade in Fig. 1a) which have been proposed to encode alliinases rather than tryptophan aminotransferases, but a functional characterization is pending (Turnaev et al., 2015). The intron positions within the coding region of all Arabidopsis and P. patens TAR homologs is conserved, suggesting a common descent for all members (Fig. S7).
Fig. 1.
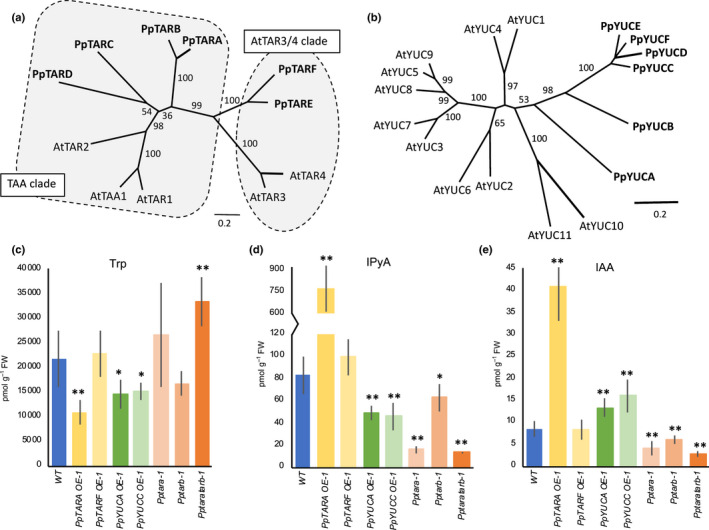
TAR and YUC multigene families promote auxin biosynthesis in Physcomitrium (Physcomitrella) patens. (a, b) Unrooted phylogenetic trees produced by the maximum likelihood method to infer evolutionary relationship of (a) TAR and (b) YUC proteins encoded by Arabidopsis (At) and P. patens (Pp) genes. Branch lengths indicate genetic distance and numbers on branches are bootstrap values (only values above 50% are shown). The TAR proteins fall in two distinct clusters referred to as the TAA and the AtTAR3/4 clades, respectively. (c–e) Level of (c) tryptophan (Trp), (d) indole‐3‐pyruvic acid (IPyA) and (e) IAA in protonemal tissue of P. patens wild type (WT) and stated over‐expressor (OE) and knock‐out mutant lines. Error bars represent the SD of the mean of at least five biological replicates. Asterisks indicate statistically significant differences compared to WT: *, P < 0.05; **, P < 0.01 (Student’s t‐test). See also Supporting Information Table S3.
Of the six YUC homologs, PpYUCA and PpYUCB share an identical exon–intron organization with the majority of the 11 Arabidopsis YUC genes while the four highly similar genes PpYUCC–F lack introns (Figs 1b, S8). With the exception of PpYUCA and PpYUCF, which both reside on chromosome 3, the genes are found on separate chromosomes. Phylogenetic analysis indicates that all PpYUC genes are more similar to each other than to any Arabidopsis gene and, even if the support is low, that their closest Arabidopsis relatives may be AtYUC10 and AtYUC11 as previously reported (Fig. 1b; Poulet & Kriechbaumer, 2017).
Next, we generated OE lines of PpTARA, PpTARF, PpYUCA and PpYUCC to represent the main branches in the TAR and YUC trees (Fig. 1a,b). Two lines of each construct exhibiting high transgene expression in protonema were selected for IAA metabolite profiling (Figs 1c–e, S1; Table S3). As expected, IPyA levels were strongly elevated upon PpTARA OE and significantly reduced by PpYUCA and PpYUCC OE, showing that PpTAR enzymes catalyze the production of IPyA while PpYUC activity consumes it (Fig. 1d). Accordingly, the IAA level is higher in both PpYUC OEs compared to WT, and even further so in PpTARA OE, indicating that PpTAR, but not PpYUC enzymes, represent the major rate‐limiting step in P. patens IAA biosynthesis (Fig. 1e). PpTARF OE does not influence the level of any measured auxin metabolite (Fig. 1c–e; Table S3), suggesting that PpTARF and PpTARE are not involved in IAA biosynthesis.
We also created KO mutants for the four AtTAA1‐like PpTAR genes and all six PpYUC genes. None of the Ppyuc single mutants showed a significant protonemal phenotype, indicating a high degree of PpYUC redundancy, while at least one PpTAR single mutant, Pptara, was significantly affected in colony diameter (Fig. S9). In line with this, both published trascriptome data (ecotype Gransden) and our qPCR analysis (ecotype Reute) show that PpTARA is the most highly expressed PpTAR in chloronema, followed by PpTARB and PpTARC, while PpTARD expression is negligible (Ortiz‐Ramirez et al., 2016, Fig. S10). Based on this, we created Pptaratarb double KO mutants and measured the levels of IAA and IAA metabolites in Pptara, Pptarb and Pptaratarb. Consistent with a role in IPyA‐dependent IAA biosynthesis, the level of both IPyA and IAA were reduced slightly in the WT‐resembling Pptarb, strongly in Pptara and even further in Pptaratarb compared to WT (Fig. 1d,e).
Auxin is synthesized but not sensed in shoot apical stem cells and its immediate cleavage products
Shoot growth in P. patens is sustained by a single apical stem cell which cleaves off daughter cells (Fig. 2a) contributing to stem growth, lateral leaves and hairs (Harrison et al., 2009; Kofuji et al., 2018). qPCR suggests that at least PpTARA and PpTARC are expressed in detached vegetative shoot apices (Fig. 2b). We thus generated and analyzed PpTAR transcriptional reporters as a proxy for where and when auxin biosynthesis takes place in this region. PpTARA expression is relatively strong in the apical stem cells of vegetative adult shoots and somewhat weaker in its most immediate cleavage products (Fig. 2d), while no consistent PpTARC expression could be observed in the stem cell region (data not shown). As the PpTARB and PpTARD reporters failed to detect any expression in the stem cell region or in reproductive organs, they are not further discussed (data not shown). Mapping of auxin sensing in the stem cell region is challenging because promoter‐based reporters (GmGH3, DR5, DR5revV2) are not informative and the ratiometric PpR2D2 reporter is expressed at low levels in these cells (Thelander et al., 2019). Still, the PpR2D2 reporter clearly shows that all cells in the stem cell region display extremely low auxin sensing (Fig. 2e). As Pptaratarb and Pptaratarc double mutants produce functional shoots (data not shown), although the Pptaratarc stem is dwarfed (Fig. S11), the shoot apical stem cell appears unaffected by the reduced auxin biosynthesis. This indicates that the shoot apical stem cell and its immediate cleavage products represent a hotspot for auxin biosynthesis but neither sense the auxin nor depend on it for their function.
Fig. 2.
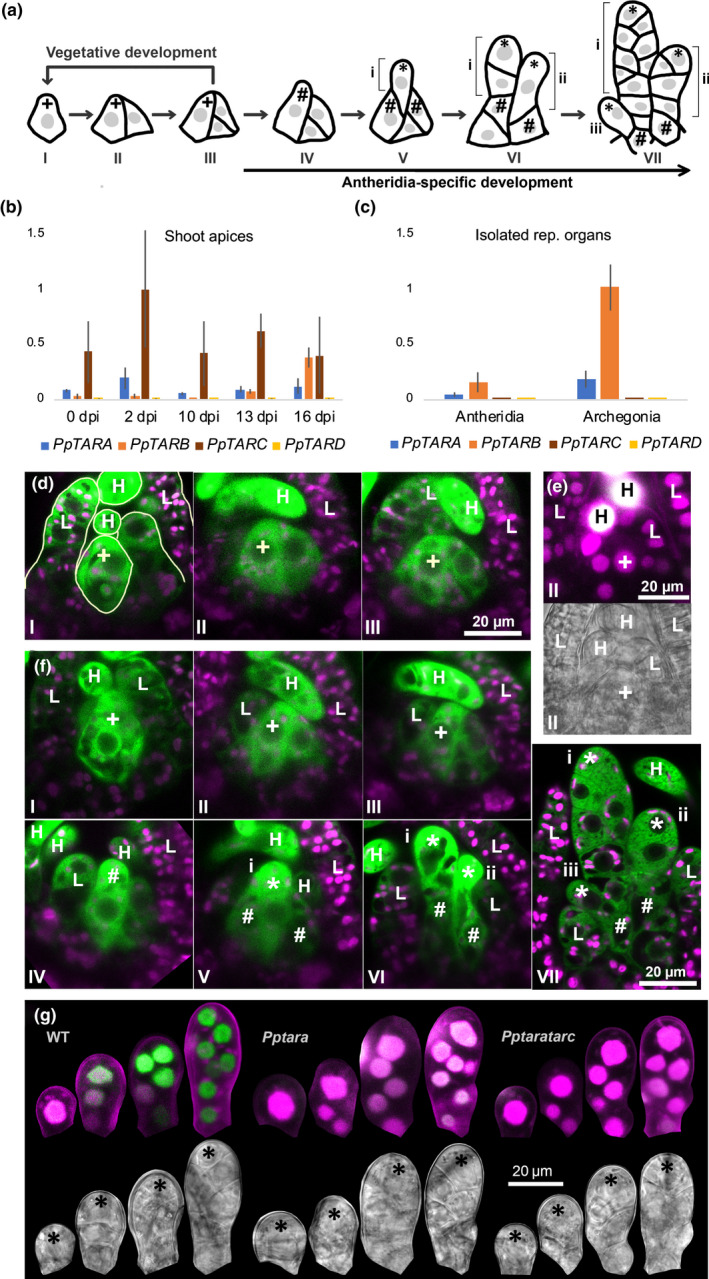
PpTAR expression and auxin sensing during transition from vegetative to early male reproductive development in Physcomitrium (Physcomitrella) patens. (a) Schematic drawing of cell divisions in the shoot apical stem cell region during the transition from vegetative to early male reproductive development. (b, c) Relative PpTAR transcript abundance assayed by qPCR in (b) detached shoot apices harvested at different time points after transfer to inductive conditions (10 dpi apices possess young antheridia; 13 dpi apices possess mid‐stage antheridia and young archegonia; 16 dpi apices possess mature antheridia and mid‐stage archegonia) and (c) in isolated antheridia and archegonia bundles. Each data point represents an average of three independent biological replicates and error bars indicate SD. (d) PpTARA::GFPGUS‐1/2 reporter output in the stem cell region of vegetative shoot apices before the transfer to inductive conditions. (e) Auxin sensing visualized by PpR2D2‐3 output in the stem cell region of a WT vegetative shoot apex before the transfer to inductive conditions. (f) PpTARA::GFPGUS‐1/2 reporter output in shoot apices in different stages of early male reproductive development. (g) Auxin sensing visualized by PpR2D2‐3 output in one‐ to eight‐celled antheridia initials in WT, Pptara (PpR2D2‐3 Pptara‐1) and Pptaratarc (PpR2D2‐3 Pptaratarc‐1) backgrounds. For related signal quantification data, see Fig. S12. In (d, f), merged images of confocal channels detecting green fluorescent protein (green) and chloroplast autofluorescence (magenta) are shown. In (e, g), a maximum‐intensity projection of a Z‐stack with confocal channels detecting mDII‐nVENUS (green) and DII‐nTdTOMATO (magenta) merged (upper) and a differential interference contrast image from a selected Z‐plane (lower) are shown for each item. Microscopy settings and image processing are identical between the different items. A high green : magenta signal ratio indicates high auxin sensing (for details, see Thelander et al., 2019). Key to symbols: +, vegetative shoot apical stem cell; #, antheridium initial stem cell; *, antheridium apical stem cell; I–VII, consecutive developmental stages; i–iii, consecutive antheridia initials; L, leaf initial; H, hair.
Auxin biosynthesis appears dispensable for the primary initiation of reproductive development
The P. patens shoot apex transits from a vegetative to a monoecious reproductive program in response to low temperature and short day‐length (Hohe et al., 2002; Landberg et al., 2013; Hiss et al., 2017; Kofuji et al., 2018). It first triggers the initiation of male antheridia by imposing changes in the behavior of the shoot apical stem cell and its most recent daughter cell, both of which take on new identities as antheridium initial stem cells (Fig. 2a; Kofuji et al., 2018). Unlike other described P. patens stem cell types, antheridium initial stem cells cleave off daughter cells distally rather than proximally, which immediately acquire identities as antheridium apical stem cells driving antheridia development (Fig. 2a; Kofuji et al., 2018). The two antheridium initial stem cells cleave off multiple antheridium apical stem cells in an alternating manner so that the most recent one is positioned outside its predecessors to eventually give rise to bundles with the oldest organ in the middle (Landberg et al., 2013; Kofuji et al., 2018).
Sensing of the environmental conditions inducing the transition to reproductive development appears to be independent of auxin biosynthesis. The first transitional sign, the emergence of a protrusion from the apical stem cell which marks the switch from proximal to distal cell division, occurs with similar timing in WT and PpTAR mutants (data not shown). We were also unable to score significant changes of PpTAR transcript levels, or in the output from the PpTARA and PpR2D2 reporters, in the stem cell region of shoots before or during the transit process (Fig. 2b,d,f; data not shown).
A gradual increase in PpTAR‐dependent auxin sensing is needed for early antheridia development
The newly established antheridium apical stem cell cleaves off daughter cells proximally to form a stage‐2 antheridium consisting of about eight cells arranged in two cell files (Fig. 2a VII; stages here and below according to Landberg et al., 2013; Kofuji et al., 2009). As PpTARA‐C transcripts could be detected in developing reproductive organs by qPCR (Fig. 2b,c), we used our transgenic tools to estimate their role in organ pattern formation. PpTARA is expressed uniformly in stage‐2 organs at levels higher than in the antheridium initial stem cell from which it originates (Fig. 2f). PpR2D2 auxin sensing also increases uniformly in one‐ to three‐celled organs, while further increase is restricted to the apical part in four‐ to eight‐celled organs resulting in a gradient (Figs 2g, S12). This gradual increase in auxin sensing is dependent on auxin biosynthesis as PpR2D2 readout is significantly reduced in Pptara and Pptaratarc young antheridia (Figs 2g, S12).
The lack of increase in auxin sensing in young Pptaratarc antheridia delays the transition from stage 1 to stage 2 by 2–3 d, but the organ then goes through subsequent developmental stages at normal pace (Fig. 3a). This may be the result of a delay in the formation of the antheridium apical stem cell from the antheridium initial stem cell, and/or a delay in the first cell division executed by the newly formed antheridium apical stem cell. The delay in the stage 1 to 2 transition is seen mainly in Pptaratarc, indicating that both PpTARA and PpTARC contribute to auxin biosynthesis at this stage (Fig. 3a). In line with this, auxin sensing is even lower in Pptaratarc than in Pptara early during stage 2 development (Figs 2g, S12). This indicates that the establishment and/or activity of the antheridium apical stem cell requires a minimal PpTAR‐dependent auxin activity.
Fig. 3.
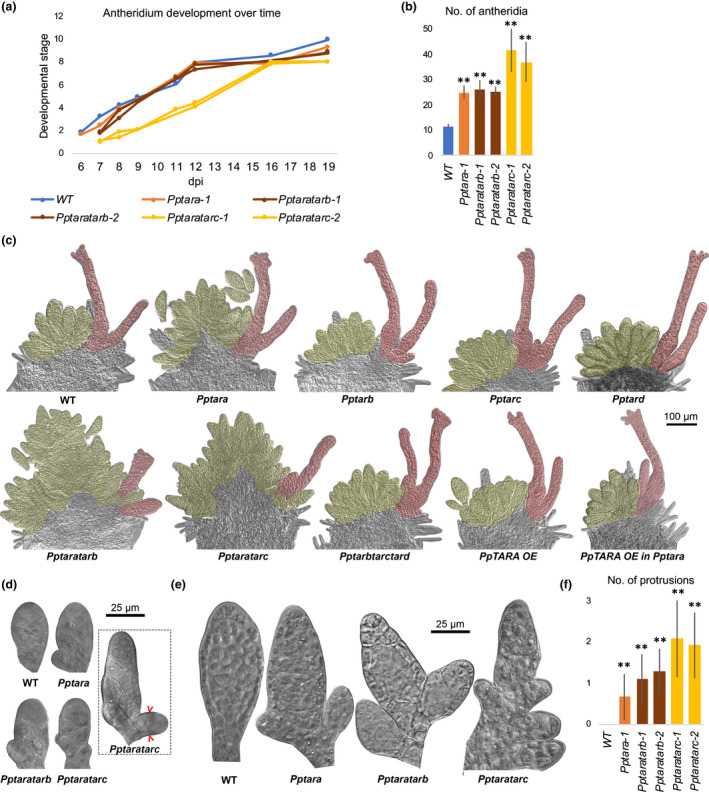
Pptar mutant phenotype relating to the initiation and early development of Physcomitrium (Physcomitrella) patens antheridia. (a) Graph showing the average developmental stage (Landberg et al., 2013) of the most advanced antheridium in shoot apices of selected Pptar mutant lines vs WT at different time points after the transfer to inductive conditions (dpi). The two Pptaratarc lines differ significantly (P < 0.01) from the WT at both 8 and 12 dpi as determined in a Dunnett’s test performed using the R package multicomp (Dunnett, 1955; Hothorn et al., 2008). (b) Bar graph showing the average number of antheridia per bundle when the most advanced antheridium has reached stage 8 in selected Pptar mutant lines vs WT. (c) Mature reproductive shoot apices from Pptar mutants and the WT demonstrating the hyperformation of antheridia and stunted archegonia in Pptara, Pptaratarb and Pptaratarc. Also note that PpTARA OE restores the Pptara phenotype while no phenotype is caused in WT (see also Supporting Information Fig. S13). Antheridia and archegonia have been false shaded in yellow and red, respectively, for clarity. (d) Representative stage 2 antheridia from the WT and selected Pptar mutants. Note outgrowths from the base of mutant organs which eventually will result in ectopic antheridia. The dashed square shows a somewhat later Pptaratarc organ demonstrating that the first division undertaken by ectopic outgrowths is periclinal (marked by red brackets). (e) Representative late‐stage antheridia from the WT and selected Pptar mutants. Note ectopic antheridia outgrowth from the base of mutant organs. (f) Bar graph showing the average frequency of ectopic outgrowths from the basal parts of late‐stage antheridia from WT and selected Pptar mutants. In (b, f), error bars indicate SD and double asterisks indicate a statistically significant difference from the WT (Student’s t‐test: **, P < 0.01).
In addition, the number of antheridia per bundle is significantly increased in Pptara and Pptaratarb, and even further so in Pptaratarc (Fig. 3b,c). This and all other phenotypes discussed below require that PpTARA has been deleted and they can all be at least partially restored by PpTARA OE, while the Pptarb, Pptarc, Pptard and Pptarbtarctard mutants produce functional reproductive organs indistinguishable from WT (Figs 3c, S13). PpTARA thus appears particularly important for reproductive development while PpTARB and PpTARC contribute, at least in the absence of PpTARA. Despite several attempts, we have not been able to generate Pptaratarbtarc triple KO lines, suggesting that these three genes together provide an essential function (data not shown). Antheridia hyperformation is due to ectopic outgrowth of extra organs from basal cells of pre‐existing stage‐2 antheridia, which form a protrusion and undergo a periclinal division (Fig. 3d). The ectopic cell formed acquires the identity of an antheridium apical stem cell which cleaves off cells proximally in two adjacent cell files to form an early ectopic antheridium which develops more or less normally and show the expected PpR2D2 auxin sensing at their tip and jacket cells (Fig. 4c). The ectopic antheridia can either be initiated by basal cells forming the stalk, making them appear as stand‐alone organs, or by basal cells on the body part, resulting in antheridia that appear branched, a phenomenon that is never seen in WT but is frequent in Pptara and even more so in Pptaratarb and Pptaratarc (Fig. 3e,f). The basal part of the antheridium shows significantly lower auxin sensing in Pptara, and even more so in Pptaratarc, compared to WT (Figs 2g, S12). This indicates that PpTAR‐dependent auxin sensing in basal cells of stage‐2 antheridia suppress them from undergoing periclinal/distal divisions to produce antheridium apical stem cells, a task normally carried out only by antheridium initial stem cells.
Fig. 4.
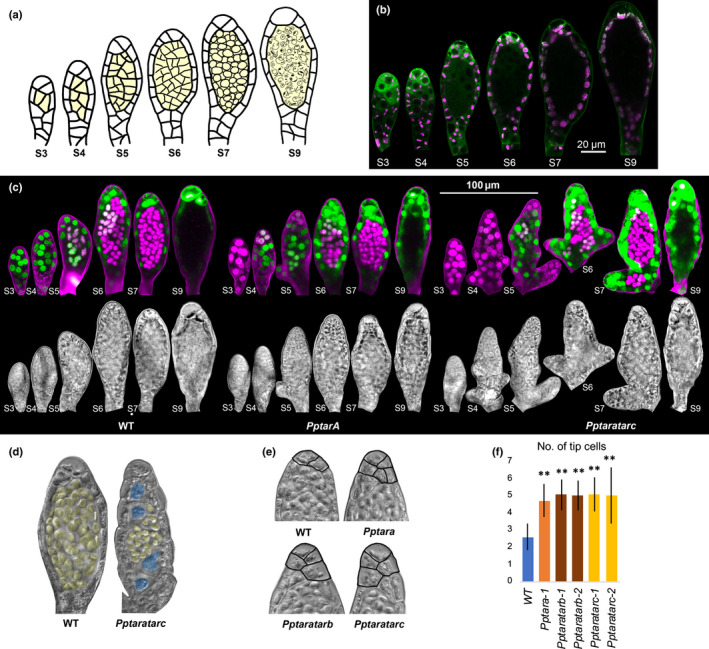
PpTAR expression, auxin sensing and Pptar mutant phenotype in stage 3–9 Physcomitrium (Physcomitrella) patens antheridia. (a) Schematic drawing of stage 3–9 antheridia development. Inner spermatogenous cells are shaded in yellow. (b) PpTARA::GFPGUS‐1/2 reporter output in stage 3–9 antheridia. For each stage, a merged image of confocal channels detecting green fluorescent protein (green) and chloroplast autofluorescence (magenta) is shown. (c) Auxin sensing visualized by PpR2D2‐3 output in stage 3–9 antheridia from WT, Pptara (PpR2D2‐3 Pptara‐1) and Pptaratarc (PpR2D2‐3 Pptaratarc‐1) backgrounds. For each organ a maximum‐intensity projection of a Z‐stack with confocal channels detecting mDII‐nVENUS (green) and DII‐nTdTOMATO (magenta) merged (upper) and a differential interference contrast image from a selected Z‐plane (lower) are shown. Microscopy settings and image processing are identical between the different organs. A high green : magenta signal ratio indicates high auxin sensing (for details, see Thelander et al., 2019). For related signal quantification data, see Supporting Information Fig. S14. (d) Stage 7 antheridia from WT and the Pptaratarc mutant demonstrating that a subset of mutant inner cells fail to proliferate (shaded in blue). Spermatogeneous inner cells have been shaded in yellow. See also Fig. S15. (e) Upper half of stage 7 antheridia from WT and selected Pptar mutants demonstrating ectopic tip cells in the latter. Borders of cells classified as tip cells have been marked for clarity. (f) Bar graph showing the average number of tip cells per stage 8 antheridium in selected Pptar mutants vs WT. Error bars indicate standard deviation and double asterisks indicate a statistically significant difference from the WT (Student’s t‐test: **, P < 0.01).
PpTAR‐dependent auxin sensing controls cell division activity in the antheridium apex and spermatogenesis is associated with minimal auxin sensing
At the start of stage 3, subapical antheridia cells undergo periclinal divisions to set off a few inner cells (Fig. 4a). Simultaneously, the uniform PpTARA expression from stage 2 changes to a gradient with an apical maximum at stages 3 and 4, while the auxin sensing gradient is partly evened out as sensing in the basal parts increases (Figs 4b,c, S14). The new inner cells initially share PpTARA expression and auxin sensing levels with the subapical jacket cell from which they were derived (Figs 4b,c, S14). The inner cells further divide to produce a number of considerably smaller spermatogenous cell initials, each of which go through spermatogenesis during stages 7–9 to form a biflagellated coiled sperm (Fig. 4a; Landberg et al., 2013). While inner cells form normally in all Pptar mutants up until stage 4, a subset of these cells fails to divide in Pptaratarc and never enter the spermatogenesis program (Figs 4d, S15). Auxin sensing is severely reduced in the inner stage 3–4 cells, suggesting that further proliferation of stage 4 inner cells, and thus the production of normal sperm counts, requires a minimal level of auxin sensing which is not always met in Pptaratarc but is apparently satisfied in the Pptara single mutant (Figs 4c, S14).
From stage 5, both PpTARA expression and auxin sensing in inner cells starts to decrease gradually to reach extremely low levels at around the onset of spermatogenesis in stage 7 (Fig. 4b,c). Spermatogenous cells from all Pptar mutants, including the Pptaratarc cells that have entered cell division, are able to complete the spermatogenesis program. Sperm from the two double mutants were able to fertilize egg cells of the male sterile ecotype Gransden, resulting in the production of kanamycin‐resistant heterozygous sporophytes, although at very low frequency. These data suggest that final sperm differentiation is a low‐auxin sensing process.
In parallel with the development of the inner cells, cells in the unicellular jacket layer undergo anticlinal divisions to keep up with organ growth (Fig. 4a). In WT, PpTARA expression in the outer cell layer becomes largely restricted to the extreme tip cells at around stage 6 before it is completely lost before organ opening (Fig. 4b). Auxin sensing remains high and relatively uniform in the tip cells and in other outer cells until organ opening (Figs 4c, S14). As expected, auxin sensing is dramatically reduced in the tip cells of stage 6 Pptara and Pptaratarc antheridia and this correlates with the formation of extra cells at the antheridia tips (Fig. 4e,f). This indicates that in comparison to WT the mutant antheridium apical stem cell remains active for longer and cleaves off more daughter cells after the first inner cell has formed. PpTAR‐dependent auxin sensing above a certain level may therefore be needed to cease cell division activity of the antheridium apical stem cell.
PpTAR expression marks the de novo establishment of a female archegonia bundle at an independent lateral position
Although the first morphological sign of female archegonia emergence only become evident around 10 d post‐induction (dpi) at a position separated from the developing antheridia bundle by a leaf (Landberg et al., 2013), PpTARC expression is activated in a single cell initiating a de novo female stem cell region as early as around 7 dpi (Fig. 5a,b). This cell, situated where the abaxial face of the last leaf formed by the vegetative shoot apex connects to the shoot stem, then undergoes two radial anticlinal divisions to form a broad three‐celled ridge able to produce both archegonia and leaves (Fig. 5a,b). The fact that occasional lateral vegetative shoot branches also emerge from leaf axils suggests that cells in this position may be predisposed for stem cell respecification (Coudert et al., 2015). While a first leaf initial appears to emerge from one of the flanking cells in the triplet, the first archegonium is produced by a distal division of the middle cell in the triplet, which show PpTAR activity (Fig. 5a,b). In the actual organ initial PpTARA expression has become more dominant compared to PpTARC (Figs 5b, 6b). Despite the PpTAR expression the stem cells show extremely low auxin sensing (Fig. 5c) and all Pptar mutants produce archegonia initials at the same positions and timing as WT (data not shown). This suggests that auxin is dispensable for the process, unless additional biosynthesis genes, or pathways, are involved. Their de novo formation from leaf axils may suggest that archegonia bundles represent lateral shoots which instantly enter a female reproductive program.
Fig. 5.
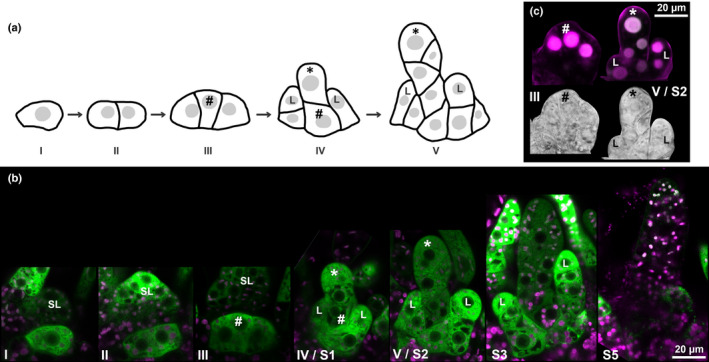
PpTAR expression and auxin sensing during archegonia initiation in Physcomitrium (Physcomitrella) patens. (a) Schematic drawing of cell divisions during the de novo establishment of a stem cell region producing archegonia. (b) PpTARC::GFPGUS‐1 reporter output during early archegonia initiation. For each stage, a merged image of confocal channels detecting green fluorescent protein (green) and chloroplast autofluorescence (magenta) is shown. (c) Auxin sensing during early archegonia initiation as visualized by PpR2D2‐3 output. Both a differential interference contrast image from a selected Z‐plane (lower) and a maximum‐intensity projection of a Z‐stack with confocal channels detecting mDII‐nVENUS (green) and DII‐nTdTOMATO (magenta) merged (upper) are shown. A high green : magenta signal ratio indicates high auxin sensing (for details, see Thelander et al., 2019). Key to symbols: #, probable archegonium initial stem cell; *, archegonium apical stem cell; I–V, consecutive developmental stages; L, leaf initial; SL, leaf separating antheridia and archegonia bundle; S1/2/3/5, archegonia stages according to Landberg et al. (2013).
Fig. 6.
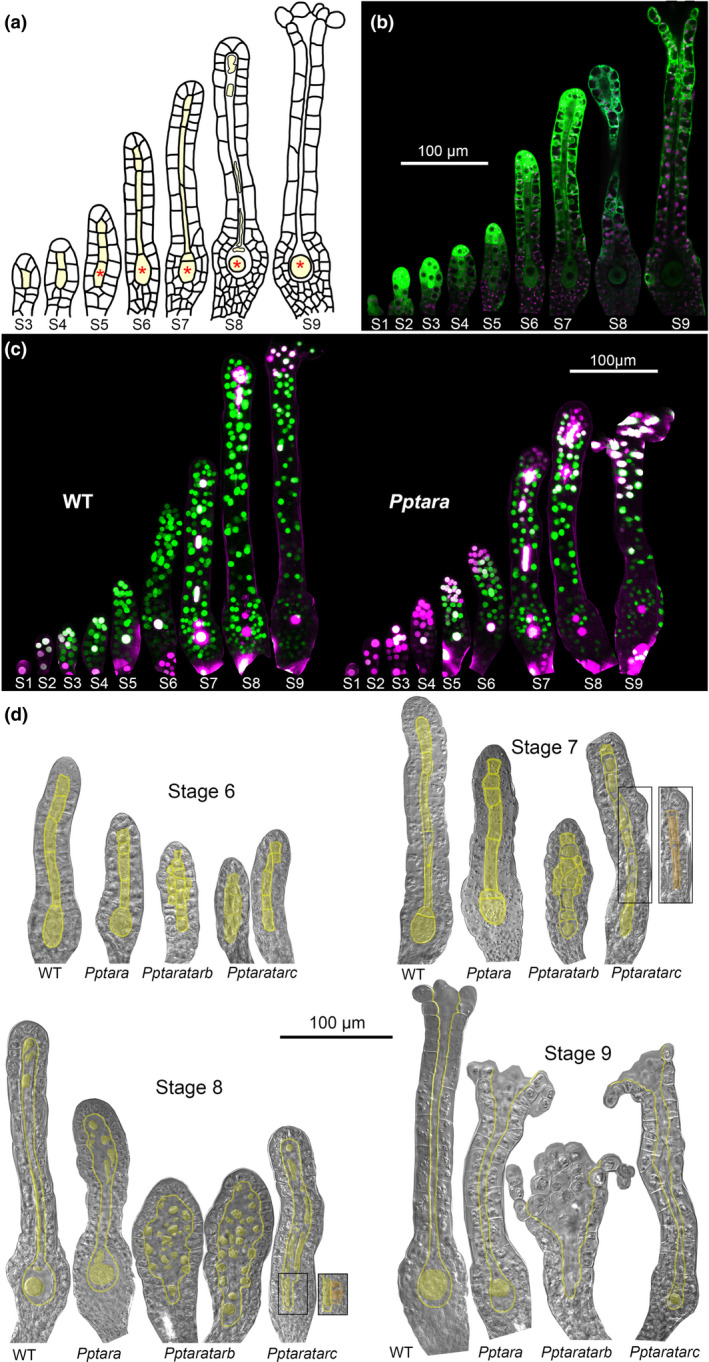
PpTARA expression, auxin sensing and Pptar mutant phenotype in Physcomitrium (Physcomitrella) patens archegonia. (a) Schematic drawing of stage 3–9 archegonia development. Inner cells are shaded in yellow. The pre‐egg/egg is marked with an asterisk. (b) PpTARA::GFPGUS‐1/2 reporter output during WT archegonia development. For each stage, a merged image of confocal channels detecting green fluorescent protein (green) and chloroplast autofluorescence (magenta) is shown. (c) Auxin sensing visualized by PpR2D2‐2 output in different stages of archegonia from WT and Pptara (PpR2D2‐2 Pptara‐1) backgrounds. For each organ, a maximum‐intensity projection of a Z‐stack with confocal channels detecting mDII‐nVENUS (green) and DII‐nTdTOMATO (magenta) merged is shown. Microscopy settings and image processing are identical between the different organs. A high green : magenta signal ratio indicates high auxin sensing (for details, see Thelander et al., 2019). For related signal quantification data, see Supporting Information Figs S16 and S17. (d) Differential interference contrast images of representative stage 6–9 archegonia from WT, Pptara‐1/2, Pptaratarb‐1/2 and Pptaratarc‐1/2 to demonstrate mutant phenotypes. Inner cells and their borders have been shaded in yellow for clarity. Separate boxes show a different focal plane of the boxed area in the neighboring organ. See also Fig. S18.
Auxin sensing dependent on PpTAR‐mediated synthesis controls cell division activity to ensure proper patterning and radial expansion of archegonia
A mature archegonium consists of an ovoid body with a cavity harboring the egg and a slender neck with a central canal connecting the cavity to the outside to allow sperm entrance (Landberg et al., 2013; Fig. 6a). Stage 2 archegonia development is essentially identical to that of antheridia and is driven by the cell division activity of a two‐faced apical stem cell (Fig. 5a). At this stage PpTARA, and to some extent PpTARC, is expressed throughout the WT organ and auxin sensing is successively increased towards the organ apex in a PpTAR‐dependent manner (Figs 5b, 6b,c, S16), largely mirroring the situation in stage 2 antheridia.
Once inner cells form during stages 3–7 they are organized in a uniseriate file typically consisting of six cells where the basal‐most cell is destined to become the egg (Fig. 6a). In WT, inner cells appear to originate from two sequential inward periclinal divisions of the third cell from the organ tip. The first division takes place at stage 3 to produce an inner cell which undergoes transverse anticlinal divisions to give rise to the egg, the upper basal cell and the two longer basal canal cells (Fig. 6a). The second division takes place in stage 5 and produces a short upper canal cell which may duplicate through a transverse anticlinal division (Fig. 6a). The outer cells in the upper part of the Pptaratarb archegonia neck frequently undergo ectopic inwards periclinal divisions from stage 5, suggesting that auxin normally restricts neck‐cell proliferation. Some ectopic cells retain a neck cell identity while others take on a canal cell identify resulting in two or more canal cells positioned alongside each other, often misaligned and with a seemingly stochastic variation in length (Fig. 6d). Ectopic canal cells are also seen in Pptaratarc archegonia but here localized mainly to the basal part of the neck or even adjacent to the egg cell cavity (Fig. 6d). None of these defects could be detected in any single mutant.
At stages 7 and 8, cell division has completely ceased in WT while it is still evident in mutant archegonia tips. In Pptara, a subapical cell now typically undergoes a third periclinal division producing an ectopic apical canal cell while other outer tip cells undergo ectopic radial anticlinal divisions contributing to abnormal organ tip expansion (Fig. 6d). Because PpTARA is expressed in the tips of stage 7 and 8 archegonia where it generates high auxin sensing, we conclude that PpTAR‐mediated auxin sensing is important to terminate tip‐cell division at these stages (Fig. 6b,c; Fig. S17). These defects are enhanced in the two double mutants. At stage 8, before organ opening at the tip, all inner cells except the egg degrade to clear the central neck canal in WT. Degradation of the abnormal Pptaratarb canal cell population typically results in a markedly wide apical cavity instead of the evenly sized canal seen in the WT (Fig. 6d). Most mutant organ tips open in stage 9 even if openings are typically wider and lined by more cells than in the WT (Fig. 6d).
Auxin sensing dependent on PpTAR‐mediated synthesis promotes elongation of archegonia neck cells
The length of the archegonium neck is significantly reduced in Pptara, Pptaratarb and Pptaratarc (Figs 6d, S18a). As the number of outer neck cells along their apical–basal axis is unaffected, the phenotype is due to cell elongation defects (Figs 6d, S18b). This fits well with the weak neck cell signals from the PpTARA reporter and the fact that auxin sensing, largely dependent on PpTARA, peaks in basal neck cells at stages 6–7 when cell elongation takes place (Figs 6b,c, S17). The defect becomes enhanced in Pptaratarb but not in Pptaratarc, suggesting that only PpTARA and PpTARB contribute to neck cell elongation (Figs 6c,d, S18).
Egg maturation is characterized by low auxin activity
At stage 5, the pre‐egg starts to enlarge before it undergoes an asymmetric transverse anticlinal division to produce a large basal egg and small upper basal cell (Fig. 6a). At stage 8, when the upper basal cell and the canal cells above it degrade, the egg loses its cell wall and physical connections to cells surrounding it (Fig. 6a). Auxin sensing in the pre‐egg is relatively high and PpTARA‐dependent at stage 4/5, but becomes reduced to low levels even before the asymmetric division giving rise to the egg cell (Figs 6c, S17). This level is not further decreased in the Pptara mutant (Figs 6c, S17). Auxin sensing remains extremely low in the egg cell until maturity, suggesting that, in line with male gametes, the female gamete maturation and function is linked to low auxin activity (Figs 6c, S17).
The Pptara mutant produces normal looking egg cells and the female fertility rate is still considerable (Fig. 6d; Table 2), which fits with the observation that the low auxin sensing in the WT pre‐egg and egg is not further reduced when PpTARA is deleted (Figs 6c, S17). By contrast, the double mutants both suffer severe egg cell defects and show no (Pptaratarb) or very low (Pptaratarc) female fertility and the few sporophytes formed are malformed or blocked in development (Fig. 6d; Table 2). An obvious pre‐egg (e.g. rounded and enlarged) possible to discriminate from canal cells at stages 5–6 is missing from the majority of double mutant archegonia, which frequently results in empty cavities or cavities containing an abnormal and severely condensed egg cell at stage 9 (Fig. 6d). Although we cannot exclude effects on the differentiation or function of egg cells as such, it appears likely that the frequent lack of egg cells, but not necessarily the egg abnormalities, are indirect consequences of the disturbed pattering caused by ectopic cell division activity already discussed.
Table 2.
Outcome of genetic crosses involving various Physcomitrium (Physcomitrella) patens Pptar mutants and WT.
| Female parent egg donor | Male parent sperm donor | Shoots analyzed | Frequency of shoots initiating sporophyte development (%) | Frequency of immature or malformed sporophytes among those initiated (%) |
|---|---|---|---|---|
| WT | WT | 194 | 99 | 8 |
| Pptara‐1 | Pptara‐1 | 128 | 40 | 14 |
| Pptara‐1 | WT | 98 | 53 | 2 |
| Pptaratarb‐1 | Pptaratarb‐1 | 133 | 0 | – |
| Pptaratarb‐1 | WT | 165 | 0 | – |
| Pptaratarb‐2 | Pptaratarb‐2 | 513 | 0 | – |
| Pptaratarb‐2 | WT | 399 | 0 | – |
| Pptaratarc‐1 | Pptaratarc‐1 | 329 | 5 | 100 |
| Pptaratarc‐1 | WT | 425 | 15 | 98 |
| Pptaratarc‐2 | Pptaratarc‐2 | 322 | 5 | 100 |
| Pptaratarc‐2 | WT | 138 | 13 | 100 |
Discussion
We show that PpTAR and PpYUC homologs encode functional auxin biosynthesis enzymes and constitute a major route for auxin biosynthesis in moss. Together with similar findings from flowering plants and a liverworth, two other deep branches of land plants, this confirms that the IPyA pathway dates back at least to the common ancestors of all land plants (Ljung, 2013; Eklund et al., 2015; Romani, 2017). The pathway is of major importance for developmental regulation in moss, and restriction of PpTAR activity results in decreased auxin sensing linked to severe developmental abnormalities. Preliminary observations of selected PpYUC reporters and mutants fit well with the more complete PpTAR data presented in this study (Fig. S19). While this study focused on reproductive development, the IPyA pathway probably plays important roles throughout the life cycle. Our failure to produce a triple mutant in which the three major PpTAR genes (A–C) are deleted probably reflects an essential function.
The acquirement of nuclear auxin sensing may have provided ancestral plants with a means for intercellular coordination of cell proliferation and differentiation to facilitate focal growth (Flores‐Sandoval et al., 2018). Our data suggest an underlying mechanism for how this works. Proliferation of a moss apical stem cell requires low auxin sensing but at the same time this cell synthesizes auxin to control different aspects of differentiation of its progeny.
The formation of gametangia and leaves at the moss shoot apex is initially driven by two‐faced apical stem cells cleaving off cells backwards in two parallel files. In these cells, auxin sensing is initially extremely low, while continuous PpTAR‐dependent auxin synthesis contributes to a successive build‐up of sensing as organs grow larger (this study; Thelander et al., 2019; Fig. S20). Once sensing has reached a certain threshold, it terminates apical stem cell division, indicating that a successive build‐up of sensing by local auxin biosynthesis could represent a simple general mechanism to control the window during which two‐faced apical stem cells remain active. After inactivation of the two‐faced stem cells, organ development is driven by a mix of divisions, expansions and fate changes affecting the apical stem cell derivatives (Figs 4a, 6a; Harrison et al., 2009). PpTAR‐dependent auxin sensing also regulates these processes by restricting periclinal and radial anticlinal divisions while promoting apical–basal cell elongation.
Unlike gametangia and leaf apical stem cells, the three‐faced apical stem cell of the shoot retains PpTAR expression combined with extremely low auxin sensing indeterminately, probably blocking stem cell arrest. As PpPIN auxin exporters have been hypothesized to remove auxin from the meristem (Bennett et al., 2014), although PpPIN reporters fail to detect their precise localization (data not shown; Viaene et al., 2014), the inability of the stem cell to sense auxin could be caused by auxin drainage in combination with active repression of auxin responses. In M. polymorpha, a repressive type B ARF is active in the apical region of the thallus, which also produce auxin via the IPyA pathway to control, for example, the dormancy of gemmae produced by its progeny (Eklund et al., 2015; Kato et al., 2020). Local IPyA‐mediated auxin biosynthesis may thus represent an ancestral general mechanism for apical stem cells to control where in relation to their own position critical differentiation processes affecting their progeny occur. Where auxin produced by the moss shoot apex is sensed is unclear. However, Pptaratarc shoots, lacking the two PpTAR genes significantly expressed in vegetative shoot apices (Fig. 2b), are dwarfed and the frequency of lateral branching is increased, revealing that auxin promotes cell expansion and suppresses respecification of epidermal cells into competing lateral shoot apical stem cells (Fig. S11; data not shown; Fujita et al., 2008; Coudert et al., 2015). Some support for both functions is found in reproductive organs as PpTAR‐dependent auxin sensing is important to suppress ectopic formation of antheridium apical stem cells and to promote apical–basal cell expansion in the archegonia neck.
Auxin sensing drops dramatically during gamete differentiation, explaining why both eggs and sperm are characterized by strikingly low auxin activity. This coincides with declining PpTAR expression and activation of auxin transport. Spermatogenous cells express PpPIND, a short endoplasmic reticulum‐localized transporter, limiting the pool of available auxin in the nucleus (Fig. S21; Viaene et al., 2014). The pre‐egg and egg instead express the long auxin exporters PpPINA, B and C from stage 6, suggesting that they are actively drained of auxin (Fig. S22; Landberg et al., 2013; Viaene et al., 2014). As PpTAR expression remains active in the egg to stage 7, well after PpPINA‐C activation and the loss of auxin sensing (stage 5), auxin export may prepare the egg and/or surrounding tissues for fertilization, for example by activating degradation of the egg cell wall (Figs 6b,c, S17). This hypothesis is based on the observation that PpPINA expression in antheridia and archegonia tips coincides with organ opening facilitated by cell wall degradation (Landberg et al., 2013).
If the strikingly low auxin activity of the moss gametes is a prerequisite for their development, and/or whether it is critical for successful fertilization or even downstream embryo development is not clear. The Arabidopsis egg and central cell are characterized by low auxin activity, which is broken upon double fertilization to drive development of the embryo and endosperm, but also to trigger growth and development of the surrounding mother tissues (Figueiredo et al., 2016). The malformed/aborted sporophytes produced by Pptaratarc both after selfing and fertilization by WT sperm show that auxin synthesis is important also for moss embryo/sporophyte development, and may be related to problems with the interaction between embryo and the surrounding mother tissues.
Author contributions
JŠ and KLjung conducted and analyzed the auxin metabolite measurements and revised the manuscript. KLandberg and MT conducted all other experiments and analyzed the data. KLandberg, ES and MT designed the experiments, interpreted the results and wrote the manuscript.
Supporting information
Fig. S1 PpTARA, PpTARF, PpYUCA and PpYUCC overexpressor lines in WT background: construct design, PCR verification and expression levels.
Fig. S2 PpTARA overexpressor lines in Pptara‐1 mutant background: construct design, PCR verification and expression levels.
Fig. S3 PpTARA, PpTARB, PpTARC, PpTARD and PpYUCF transcriptional reporter lines in WT background: construct design and PCR verification.
Fig. S4 PpTARA‐D and PpYUCA‐F single knockout lines in WT background: principal construct design and PCR verification.
Fig. S5 PCR verification of PpTAR double and triple knockout lines.
Fig. S6 Confirmation of knockout lines carrying the PpR2D2 reporter.
Fig. S7 Intron positions in coding regions are conserved in Arabidopsis and P. patens TAR genes of both the TAA clade and the AtTAR3/4 clade.
Fig. S8 The number of introns in YUC gene coding regions differ in both Arabidopsis and P. patens but the positions of introns that do exist are conserved.
Fig. S9 The diameter of Pptara protonemal colonies is reduced.
Fig. S10 Relative expression of PpTAR genes in chloronema.
Fig. S11 Pptaratarc shoots are dwarfed but otherwise develop normally.
Fig. S12 Quantitative PpR2D2 output as a measure of auxin sensing during stage 2 antheridia development.
Fig. S13 Complementation of Pptara reproductive organ phenotype by PpTARA overexpression.
Fig. S14 Quantitative PpR2D2 output as a measure of auxin sensing during stage 3–9 antheridia development.
Fig. S15 A subset of inner cells do not proliferate in the Pptaratarc double mutant.
Fig. S16 Quantitative PpR2D2 output as a measure of auxin sensing during stage 2 archegonia development.
Fig. S17 Quantitative PpR2D2 output as a measure of auxin sensing during stage 3–9 archegonia development.
Fig. S18 Archegonia neck lengths are reduced in Pptar mutants due to a cell elongation defect.
Fig. S19 PpYUC expression patterns and knockout phenotype resemble those of PpTAR in reproductive organs.
Fig. S20 Auxin sensing in the apical stem cell of young vegetative leaves is successively increased in a PpTAR‐dependent manner.
Fig. S21 PpPIND is expressed in spermatogenous cells of antheridia.
Fig. S22 Long PpPINs are expressed in the pre‐egg/egg from around stage 5 of archegonia development.
Methods S1 Generation of overexpression constructs and lines.
Methods S2 Generation of transcriptional reporter constructs and lines.
Methods S3 Generation of knockout constructs and lines.
Methods S4 Generation of knockout lines carrying the PpR2D2 reporter.
Methods S5 RT‐qPCR to determine PpTAR transcript abundance.
Table S1 Constructs produced and used in the study.
Table S2 Primers used in study.
Table S3 Level of auxin metabolites in wildtype P. patens and in stated overexpressor and mutant lines.
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
This work was supported by grants from The Knut and Alice Wallenberg Foundation (no. 2012.0087) and the Swedish Research Council (nos. 621‐2014‐4941, 2018‐04068). KLjung and JŠ acknowledge the Knut and Alice Wallenberg Foundation (KAW), the Swedish Governmental Agency for Innovation Systems (Vinnova), the Swedish Research Council (VR) and the Swedish Metabolomics Centre (https://www.swedishmetabolomicscentre.se/) for access to instrumentation. We thank Ulf Lagercrantz for help with statistics, Victoria Sanchez‐Vera for producing the vectors pDONR4r‐3r‐G418 and pDONR4r‐3r‐Zeo, Eric Pedersen for producing the vector pEP54, and Michael Prigge and Mark Estelle for providing the vector pUK‐Pp108 + Hsp‐GW + npt.
References
- Ashton NW, Grimsley NH, Cove DJ. 1979. Analysis of gametophytic development in the moss, Physcomitrella patens, using auxin and cytokinin resistant mutants. Planta 144: 427–435. [DOI] [PubMed] [Google Scholar]
- Bennett T, Liu M, Aoyama T, Bierfreund NM, Braun M, Coudert Y, Dennis RJ, O'Connor D, Wang XY, White CD et al 2014. Plasma membrane‐targeted PIN proteins drive shoot development in a moss. Current Biology 24: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y, Berger F et al 2017. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–304. [DOI] [PubMed] [Google Scholar]
- Casanova‐Sáez R, Voss U. 2019. Auxin metabolism controls developmental decisions in land plants. Trends in Plant Science 24: 741–754. [DOI] [PubMed] [Google Scholar]
- Causier B, Ashworth M, Davies B. 2012. The TOPLESS interactome: a framework for gene expression in Arabidopsis. Plant Physiology 158: 423–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causier B, Lloyd J, Stevens L, Davies B. 2012. TOPLESS co‐repressor interactions and their evolutionary conservation in plants. Plant Signaling & Behavior 7: 327–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert Y, Palubicki W, Ljung K, Novak O, Leyser O, Harrison J. 2015. Three ancient hormonal cues co‐ordinate shoot branching in a moss. eLife 4: e06808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove DJ, Ashton NW. 1984. The hormonal regulation of gamatophytic development in bryophytes In: Dyer AF, Duckett JG, eds. The experimental biology of bryophytes. London, UK: Academic Press, 177–201. [Google Scholar]
- Du Y, Scheres B. 2018. Lateral root formation and the multiple roles of auxin. Journal of Experimental Botany 69: 155–167. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. 1955. A multiple comparison procedure for comparing several treatments with a control. Journal of the American Statistical Association 50: 1096–1121. [Google Scholar]
- Eklund DM, Ishizaki K, Flores‐Sandoval E, Kikuchi S, Takebayashi Y, Tsukamoto S, Hirakawa Y, Nonomura M, Kato H, Kouno M et al 2015. Auxin produced by the indole‐3‐pyruvic acid pathway regulates development and gemmae dormancy in the liverwort Marchantia polymorpha . The Plant Cell 27: 1650–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo DD, Batista RA, Roszak PJ, Hennig L, Köhler C. 2016. Auxin production in the endosperm drives seed coat development in Arabidopsis. eLife 5: e20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finet C, Jaillais Y. 2012. Auxology: when auxin meets plant evo‐devo. Developmental Biology 369: 19–31. [DOI] [PubMed] [Google Scholar]
- Flores‐Sandoval E, Eklund DM, Bowman JL. 2015. A simple auxin transcriptional response system regulates multiple morphogenetic processes in the liverwort Marchantia polymorpha . PLoS Genetics 11: e1005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Sandoval E, Eklund DM, Hong SF, Alvarez JP, Fisher TJ, Lampugnani ER, Golz JF, Vázquez‐Lobo A, Dierschke T, Lin SS et al 2018. Class C ARFs evolved before the origin of land plants and antagonize differentiation and developmental transitions in Marchantia polymorpha . New Phytologist 218: 1612–1630. [DOI] [PubMed] [Google Scholar]
- Fujita T, Sakaguchi H, Hiwatashi Y, Wagstaff SJ, Ito M, Deguchi H, Sato T, Hasebe M. 2008. Convergent evolution of shoots in land plants: lack of auxin polar transport in moss shoots. Evolution & Development 10: 176–186. [DOI] [PubMed] [Google Scholar]
- Harrison J, Roeder A, Meyerowitz E, Langdale J. 2009. Local cues and asymmetric cell divisions underpin body plan transitions in the moss Physcomitrella patens . Current Biology 19: 461–471. [DOI] [PubMed] [Google Scholar]
- Hayashi K‐I, Neve J, Hirose M, Kuboki A, Shimad Y, Kepinski S, Nozaki H. 2012. Rational design of an auxin antagonist of the SCFTIR1 auxin receptor complex. ACS Chemical Biology 7: 590–598. [DOI] [PubMed] [Google Scholar]
- Hiss M, Meyberg R, Westermann J, Haas FB, Schneider L, Schallenberg‐Rudinger M, Ullrich KK, Rensing SA. 2017. Sexual reproduction, sporophyte development and molecular variation in the model moss Physcomitrella patens: introducing the ecotype Reute. The Plant Journal 90: 606–620. [DOI] [PubMed] [Google Scholar]
- Hohe A, Rensing SA, Mildner M, Lang D, Reski R. 2002. Day length and temperature strongly influence sexual reproduction and expression of a novel MADS‐box gene in the moss Physcomitrella patens . Plant Biology 4: 595–602. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Johri MM, Desai S. 1973. Auxin regulation of caulonema formation in moss protonema. Nature New Biology 245: 223–224. [DOI] [PubMed] [Google Scholar]
- Kasahara H. 2016. Current aspects of auxin biosynthesis in plants. Bioscience, Biotechnology, and Biochemistry 80: 34–42. [DOI] [PubMed] [Google Scholar]
- Kato H, Ishizaki K, Kouno M, Shirakawa M, Bowman JL, Nishihama R, Kohchi T. 2015. Auxin‐mediated transcriptional system with a minimal set of components is critical for morphogenesis through the life cycle in Marchantia polymorpha . PLoS Genetics 11: e1005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Kouno M, Takeda M, Suzuki H, Ishizaki K, Nishihama R, Kohchi T. 2017. The roles of the sole activator‐type auxin response factor in pattern formation of Marchantia polymorpha . Plant and Cell Physiology 58: 1642–1651. [DOI] [PubMed] [Google Scholar]
- Kato H, Mutte SK, Suzuki H, Crespo I, Das S, Radoeva T, Fontana M, Yoshitake Y, Hainiwa E, van den Berg W et al 2020. Design principles of a minimal auxin response system. Nature Plants 6: 473–482. [DOI] [PubMed] [Google Scholar]
- Kato H, Nishihama R, Weijers D, Kohchi T. 2018. Evolution of nuclear auxin signaling: lessons from genetic studies with basal land plants. Journal of Experimental Botany 69: 291–301. [DOI] [PubMed] [Google Scholar]
- Kofuji R, Hasebe M. 2014. Eight types of stem cells in the life cycle of the moss Physcomitrella patens . Current Opinion in Plant Biology 17: 13–21. [DOI] [PubMed] [Google Scholar]
- Kofuji R, Yagita Y, Murata T, Hasebe M. 2018. Antheridial development in the moss Physcomitrella patens: implications for understanding stem cells in mosses. Philosophical Transactions of the Royal Society B: Biological Sciences 373: 20160494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji R, Yoshimura T, Inoue H, Sakakibara K, Hiwatashi Y, Kurata T, Aoyama T, Ueda K, Hasebe M. 2009. Gametangia development in the moss Physcomitrella patens . Annual Plant Reviews 36: 167–181. [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35: 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landberg K, Pederson ERA, Viaene T, Bozorg B, Friml J, Jönsson H, Thelander M, Sundberg E. 2013. The moss Physcomitrella patens reproductive organ development is highly organized, affected by the two SHI/STY genes and by the level of active auxin in the SHI/STY expression domain. Plant Physiology 162: 1406–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Prigge M, Tao S, Shain S, Kuo A, Kirchsteiger K, Estelle M. 2016. Constitutive auxin response in Physcomitrella reveals complex interactions between Aux/IAA and ARF proteins. eLife 5: e13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Prigge M, Tigyi K, Estelle M. 2012. The cyclophilin DIAGEOTROPICA has a conserved role in auxin signaling. Development 139: 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K. 2013. Auxin metabolism and homeostasis during plant development. Development 140: 943–950. [DOI] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H et al 2011. The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences, USA 108: 18512–18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JL, Puttick MN, Clark JW, Edwards D, Kenrick P, Pressel S, Wellman CH, Yang Z, Schneider H, Donoghue PCJ. 2018. The timescale of early land plant evolution. Proceedings of the National Academy of Sciences, USA 306: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák O, Hényková E, Sairanen I, Kowalczyk M, Pospíšil T, Ljung K. 2012. Tissue‐specific profiling of the Arabidopsis thaliana auxin metabolome. The Plant Journal 72: 523–536. [DOI] [PubMed] [Google Scholar]
- Ortiz‐Ramirez C, Hernandez‐Coronado M, Thamm A, Catarino B, Wang M, Dolan L, Feijo JA, Becker JD. 2016. A transcriptome atlas of Physcomitrella patens provides insights into the evolution and development of land plants. Molecular Plant 9: 205–220. [DOI] [PubMed] [Google Scholar]
- Paponov IA, Teale W, Lang D, Paponov M, Reski R, Rensing SA, Palme K. 2009. The evolution of nuclear auxin signaling. BMC Evolutionary Biology 9: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavskin Y, Nagashima A, Perroud P‐F, Hasebe M, Quatrano RS, Atwal GS, Timmermans MCP. 2016. Ancient trans‐acting siRNAs confer robustness and sensitivity onto the auxin response. Developmental Cell 36: 276–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet A, Kriechbaumer V. 2017. Bioinformatics analysis of phylogeny and transcription of TAA/YUC auxin biosynthetic genes. International Journal of Molecular Sciences 18: 1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Lavy M, Ashton NW, Estelle M. 2010. Physcomitrella patens auxin‐resistant mutants affect conserved elements of an auxin‐signaling pathway. Current Biology 20: 1907–1912. [DOI] [PubMed] [Google Scholar]
- Puttick MN, Morris JL, Williams TA, Cox CJ, Edwards D, Kenrick P, Pressel S, Wellman CH, Schneider H, Pisani D et al 2018. The interrelationships of land plants and the nature of the ancestral embryophyte. Current Biology 28: 733–745. [DOI] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y et al 2008. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319: 64–69. [DOI] [PubMed] [Google Scholar]
- Romani F. 2017. Origin of TAA genes in charophytes: new insights into the controversy over the origin of auxin biosynthesis. Frontiers in Plant Science 8: 1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara K, Nishiyama T, Sumikawa N, Kofuji R, Murata T, Hasebe M. 2003. Involvement of auxin and a homeodomain‐leucine zipper I gene in rhizoid development of the moss Physcomitrella patens . Development 130: 4835–4846. [DOI] [PubMed] [Google Scholar]
- Schaefer D, Zrÿd J‐P, Knight CD, Cove DJ. 1991. Stable transformation of the moss Physcomitrella patens . Molecular and General Genetics 226: 418–424. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura M. 2016. Marchantia polymorpha: taxonomy, phylogeny and morphology of a model system. Plant and Cell Physiology 57: 230–256. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Robertson‐Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jürgens G, Alonso JM. 2008. TAA1‐mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191. [DOI] [PubMed] [Google Scholar]
- Sugano SS, Shirakawa M, Takagi J, Matsuda Y, Shimada T, Hara‐Nishimura I, Kohchi T. 2014. CRISPR/Cas9‐mediated targeted mutagenesis in the liverwort Marchantia polymorpha L. Plant Cell Physiology 55: 475–481. [DOI] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K et al 2008. Rapid synthesis of auxin via a new tryptophan‐dependent pathway is required for shade avoidance in plants. Cell 133: 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander M, Landberg K, Sundberg E. 2018. Auxin‐mediated developmental control in the moss Physcomitrella patens . Journal of Experimental Botany 69: 277–290. [DOI] [PubMed] [Google Scholar]
- Thelander M, Landberg K, Sundberg E. 2019. Minimal auxin sensing levels in vegetative moss stem cells revealed by a ratiometric reporter. New Phytologist 224: 775–788. [DOI] [PubMed] [Google Scholar]
- Thelander M, Nilsson A, Olsson T, Johansson M, Girod PA, Schaefer DG, Zryd JP, Ronne H. 2007. The moss genes PpSKI1 and PpSKI2 encode nuclear SnRK1 interacting proteins with homologues in vascular plants. Plant Molecular Biology 64: 559–573. [DOI] [PubMed] [Google Scholar]
- Turnaev II, Gunbin KV, Afonnikov DA. 2015. Plant auxin biosynthesis did not originate in charophytes. Trends in Plant Science 20: 463–465. [DOI] [PubMed] [Google Scholar]
- Viaene T, Landberg K, Thelander M, Medvecka E, Pederson E, Feraru E, Cooper ED, Karimi M, Delwiche CF, Ljung K et al 2014. Directional auxin transport mechanisms in early diverging land plants. Current Biology 24: 2786–2791. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiao Y. 2018. Auxin and above‐ground meristems. Journal of Experimental Botany 69: 147–154. [DOI] [PubMed] [Google Scholar]
- Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y. 2011. Conversion of tryptophan to indole‐3‐acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proceedings of the National Academy of Sciences, USA 108: 18518–18523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Hu X, Huang J. 2014. Origin of plant auxin biosynthesis. Trends in Plant Science 19: 764–770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 PpTARA, PpTARF, PpYUCA and PpYUCC overexpressor lines in WT background: construct design, PCR verification and expression levels.
Fig. S2 PpTARA overexpressor lines in Pptara‐1 mutant background: construct design, PCR verification and expression levels.
Fig. S3 PpTARA, PpTARB, PpTARC, PpTARD and PpYUCF transcriptional reporter lines in WT background: construct design and PCR verification.
Fig. S4 PpTARA‐D and PpYUCA‐F single knockout lines in WT background: principal construct design and PCR verification.
Fig. S5 PCR verification of PpTAR double and triple knockout lines.
Fig. S6 Confirmation of knockout lines carrying the PpR2D2 reporter.
Fig. S7 Intron positions in coding regions are conserved in Arabidopsis and P. patens TAR genes of both the TAA clade and the AtTAR3/4 clade.
Fig. S8 The number of introns in YUC gene coding regions differ in both Arabidopsis and P. patens but the positions of introns that do exist are conserved.
Fig. S9 The diameter of Pptara protonemal colonies is reduced.
Fig. S10 Relative expression of PpTAR genes in chloronema.
Fig. S11 Pptaratarc shoots are dwarfed but otherwise develop normally.
Fig. S12 Quantitative PpR2D2 output as a measure of auxin sensing during stage 2 antheridia development.
Fig. S13 Complementation of Pptara reproductive organ phenotype by PpTARA overexpression.
Fig. S14 Quantitative PpR2D2 output as a measure of auxin sensing during stage 3–9 antheridia development.
Fig. S15 A subset of inner cells do not proliferate in the Pptaratarc double mutant.
Fig. S16 Quantitative PpR2D2 output as a measure of auxin sensing during stage 2 archegonia development.
Fig. S17 Quantitative PpR2D2 output as a measure of auxin sensing during stage 3–9 archegonia development.
Fig. S18 Archegonia neck lengths are reduced in Pptar mutants due to a cell elongation defect.
Fig. S19 PpYUC expression patterns and knockout phenotype resemble those of PpTAR in reproductive organs.
Fig. S20 Auxin sensing in the apical stem cell of young vegetative leaves is successively increased in a PpTAR‐dependent manner.
Fig. S21 PpPIND is expressed in spermatogenous cells of antheridia.
Fig. S22 Long PpPINs are expressed in the pre‐egg/egg from around stage 5 of archegonia development.
Methods S1 Generation of overexpression constructs and lines.
Methods S2 Generation of transcriptional reporter constructs and lines.
Methods S3 Generation of knockout constructs and lines.
Methods S4 Generation of knockout lines carrying the PpR2D2 reporter.
Methods S5 RT‐qPCR to determine PpTAR transcript abundance.
Table S1 Constructs produced and used in the study.
Table S2 Primers used in study.
Table S3 Level of auxin metabolites in wildtype P. patens and in stated overexpressor and mutant lines.
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.


