A treat‐to‐target approach is gaining ground as an effective and efficient strategy for a range of rheumatic diseases (1, 2, 3, 4). It is assumed that a treatment continuously aimed at a single target—abrogation of inflammation, leading to remission—will have “domino effects” on all other treatment goals as well (1). Since the first recommendations were published there have been new insights, and there is a need to revisit the discussion. in this commentary we will reflect on treat‐to‐target in rheumatic diseases from the patient perspective, based on our experiences as patient representatives in research on rheumatoid arthritis (RA), psoriatic arthritis (PsA), and juvenile idiopathic arthritis (JIA).
Overarching principles
All treat‐to‐target recommendations start by formulating a set of overarching principles, including the ultimate goals. For treat‐to‐target in RA, the primary goal is to maximize long‐term health‐related quality of life through control of symptoms (e.g., pain, inflammation, stiffness, and fatigue), prevention of structural damage, normalization of function, and improved/restored ability to participate in social and work‐related activities (2). For JIA, the ultimate treatment goals have been described as follows: “to control signs and symptoms; to prevent structural damage; to avoid comorbid conditions and drug toxicities; and to optimise function, growth and development, quality of life, and social participation” (3). From a patient perspective, the acknowledgment of all goals, including those related to pain, fatigue, activities of daily living, and social participation, is highly valued (5, 6).
In the next overarching principle, abrogation of inflammation is assumed to be essential to reach these goals (2, 3, 4). In the final overarching principle, it is assumed that treatment to target by regularly assessing disease activity and adapting therapy accordingly is important to achieve these goals. The treat‐to‐target recommendations are derived from this last overarching principle.
Reaching all goals
In these treat‐to‐target recommendations abrogation of inflammation, leading to remission, is implicitly assumed to be necessary and sufficient for reaching all treatment goals. This assumption is justified for some of the outcomes directly associated with inflammation, such as number of swollen joints, C‐reactive protein level, and erythrocyte sedimentation rate (7). However, for several of the main symptoms of JIA and RA (pain, fatigue, functional limitations, morning stiffness, and comorbidities), there is compelling evidence that in a substantial proportion of patients, a treat‐to‐target strategy is not enough (6, 8, 9).
Carpenter et al conducted a large‐scale longitudinal meta‐analysis of 46 cohorts of patients with early RA, with sufficient data from 18,046 patients (8). They concluded that “the introduction of more aggressive, treat‐to‐target based therapies coincided with improvements in disease activity and physical function over the last few decades during the first 60‐months of the disease. However, these large‐scale improvements in disease activity did not translate into equally large improvements in patient‐reported outcomes, namely pain, functional disability and mental well‐being.” Furthermore, in a Cochrane review it was concluded that treatment of RA with biologic agents has only a small‐to‐moderate effect on fatigue (9). As a result, some patients whose RA is consdered to be in remission still experience fatigue. Walter et al, for example, reported that at 12 months, despite a strict treat‐to‐target strategy and decreased disease activity, nearly half of their studied patients with early RA (43%) still epxerienced fatigue (10). Finally, there is some indirect evidence of the effects of treat‐to‐target on some of the activities of daily living and social participation goals in RA patients (7, 11). Findings of studies on treat‐to‐target in JIA (6) have been consistent with the findings of these studies in RA. Shiff et al, for example, found that a majority of children with JIA continued to report frequent pain and its debilitating consequences, in spite of effective disease control with biologic therapies (12).
We do believe that a treat‐to‐target strategy is a promising approach. At this point, however, despite the progress that has been made by introducing principles of tight control (6, 7, 8), we find it premature to speak of “dramatic effectiveness” of treat‐to‐target in RA (1), or to state that transferring treat‐to‐target recommendations into clinical practice “will significantly improve the outcomes in JIA” (3). The holy grail has not yet been found.
A bowling analogy may be helpful to clarify this issue. A bowling ball will never directly knock down all 10 pins at once. Therefore, bowlers aim at the so‐called “head pin” in the front, which is knocked down directly by ball impact and then starts a process of “pin action” by which pins interact and knock each other down. The ultimate goal of bowling is a strike: all 10 pins knocked down on the first roll (Figure 1). The success of a bowler is not measured by the impact on the head pin, or the adjacent pins in the middle, but on all pins. Similarly, to measure the success of a treat‐to‐target strategy, a disease activity score will not suffice. A proper “pin count” must be conducted.
Figure 1.
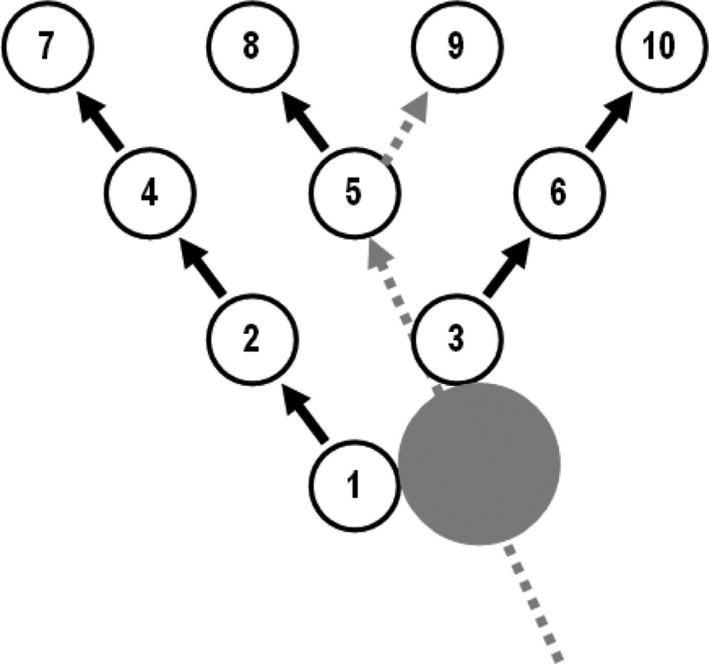
The angle of a bowling ball impacting the head pin and the subsequent pin action leading to a perfect strike.
Unfortunately, in the recommendations for treat‐to‐target in JIA or RA, no such pin count is included (2, 3). Decisions regarding disease management are based almost solely on disease activity scores, and on use of pharmaceutical treatments to affect these scores. The recommendations do not take into account other patient‐relevant outcomes, e.g., pain, fatigue, morning stiffness, and daily functioning, some of which may require other interventions, e.g., exercise or physical therapy, specialized surgery, or psychosocial support, rather than a change in the pharmaceutical treatment (5, 6, 8).
Shared decision‐making
The above illustrates the mismatch between the recommended treatment target (remission) and the emphasis on personal goal setting as the result of shared decision‐making, another important stated overarching principle of treat‐to‐target (2, 3). How can treatment decisions genuinely be shared, when the most relevant outcomes are not discussed?
In our experience, conversations between rheumatologists and patients on treatment success often resemble the confusion of tongues in Babel. For most patients, treatment success is about the whole spectrum of goals in the aforementioned overarching principles. For most rheumatologists, treatment success is a synonym for achieving remission, or—more precisely—what Ferreira et al have coined “biological remission” (5). When lay patients and their caregivers discuss treatment outcomes with the doctor, they often assume that the term remission includes the entire impact of their disease: not only physical signs and symptoms, but also the social and psychological impact. It has been suggested that patients should be educated about the “true” meaning of remission. From a patient perspective, it is instead time for a more widely encompassing definition of remission, including inflammation as well as disease impact, to cover all treatment goals in the overarching principles of treat‐to‐target (5).
Numerous composite indices have been developed to measure disease impact in rheumatic diseases. These measures can be very helpful, as long as they allow assessment of each component separately (in bowling terms, a pin count). This is specifically the case for the Rheumatoid Arthritis Impact of Disease and the Psoriatic Arthritis Impact of Disease, 2 patient‐reported outcomes that were also developed for clinical practice with the explicit purpose of the individual domains being visible to both patient and physician at all times (13). This visibility to the patient and the provider promotes personal goal setting and monitoring in the context of routine clinical care.
Some people may argue that the patient perspective in all its diversity is captured by the patient global assessment. This single‐item question is part of almost all composite indices that are recommended in treat‐to‐target strategies to measure disease impact. However, the patient global assessment has many flaws, as shown in recent studies (5). Furthermore, it provides no insight into which specific goals have been reached.
Future research
We agree with the treat‐to‐target task forces that there is an urgent need for more research to elucidate the causal relationships between the currently designated target and the other goals (2, 3, 4). Trajectory analyses are clearly needed in order to understand the complex domino effects between the various outcomes (12). Using the bowling analogy, an approach aimed at 2 targets may be more effective to start the pin action (5) (see Figure 1). Ultimately, well‐conducted strategic trials will be needed to demonstrate the presumed superiority of the treat‐to‐target strategies with regard to all relevant goals. Unfortunately, thus far in most treat‐to‐target‐trials a measure of disease activity or “biological remission” has been the main, or even the single, end point (5, 6, 7, 8, 9, 10, 11, 12, 14). From the patient perspective, this is clearly insufficient to judge success.
We believe the bowling metaphor helps to clarify the discussions on treat‐to‐target and remission. It demonstrates the importance of focusing on the entire spectrum of patients’ quality of life. However, some limitations are worth noting. The outcomes in bowling are binary: pins can either stand or fall. Most outcomes in rheumatic diseases are continuous variables, although they are often dichotomized using cutoffs. Not all patients with rheumatic disease have the same symptoms: the “pins” for each rheumatic disease, disease stage, and even for each individual patient, may differ. In PsA, for instance, skin and nail disease are essential outcome measures (4, 15). Patients may even add their own individual treatment goals, with reference to their daily life (6). Finally, while in bowling every pin counts for 1, an individual patient will have personal preferences for reaching some of the goals over others. In general, an open discussion of the goals of therapy should be the start of every treatment strategy.
AUTHOR CONTRIBUTIONS
Drs. Schoemaker and Wit drafted the article, revised it critically for important intellectual content, and approved the final version to be published.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Smolen JS. Treat‐to‐target as an approach in inflammatory arthritis [review]. Curr Opin Rheumatol 2016;28:297–302. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravelli A, Consolaro A, Horneff G, Laxer RM, Lovell DJ, Wulffraat NM, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis 2018;77:819–28. [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Schöls M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheumc Dis 2018;77:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferreira RJ, Duarte C, Ndosi M, de Wit M, Gossec L, da Silva JA. Suppressing inflammation in rheumatoid arthritis: does patient global assessment blur the target? A practice‐based call for a paradigm change. Arthritis Care Res (Hoboken) 2018;70:369–78. [DOI] [PubMed] [Google Scholar]
- 6. Schoemaker CG, Swart JF, Wulffraat NM. Treating juvenile idiopathic arthritis to target: what is the optimal target definition to reach all goals? Pediatr Rheumatol Online 2020;18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stoffer MA, Schoels MM, Smolen JS, Aletaha D, Breedveld FC, Burmester G, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 2016;75:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carpenter L, Barnett R, Mahendran P, Nikiphorou E, Gwinnutt J, Verstappen S, et al. Secular changes in functional disability, pain, fatigue and mental well‐being in early rheumatoid arthritis: a longitudinal meta‐analysis. Semin Arthritis Rheum 2020;50:209–19. [DOI] [PubMed] [Google Scholar]
- 9. Almeida C, Choy EH, Hewlett S, Kirwan JR, Cramp F, Chadler T, et al. Biologic interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev 2016:Cd008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walter MJ, Kuijper TM, Hazes JM, Weel AE, Luime JJ. Fatigue in early, intensively treated and tight‐controlled rheumatoid arthritis patients is frequent and persistent: a prospective study. Rheumatol Int 2018;38:1643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wechalekar MD, Quinn S, Lester S, Metcalf RG, Shanahan E, Walker JG, et al. A treat‐to‐target strategy preserves work capacity in a rheumatoid arthritis inception cohort treated with combination conventional DMARD therapy. J Clin Rheumatol 2017;233:131–7. [DOI] [PubMed] [Google Scholar]
- 12. Shiff NJ, Tupper S, Oen K, Guzman J, Lim H, Lee CH, et al. Trajectories of pain severity in juvenile idiopathic arthritis: results from the Research in Arthritis in Canadian Children Emphasizing Outcomes cohort. Pain 2018;159:57–66. [DOI] [PubMed] [Google Scholar]
- 13. Mistry J, Sharif M, Prideaux A, Smith C, Sumbwanyambe M, Sibley M, et al. Use of rheumatoid arthritis impact of disease (RAID) in routine care; identification of DAS28 remission and unmet patient reported outcomes. Rheumatol Adv Pract 2020;4:rkaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muller PH, Brinkman DM, Schonenberg‐Meinema D, van den Bosch WB, Koopman‐Keemink Y, Brederije IC, et al. Treat to target (drug‐free) inactive disease in DMARD‐naive juvenile idiopathic arthritis: 24‐month clinical outcomes of a three‐armed randomised trial. Ann Rheum Dis 2019;78:51–9. [DOI] [PubMed] [Google Scholar]
- 15. Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


