Abstract
We examined the association between established risk factors for breast cancer and microcalcification clusters and their asymmetry. A cohort study of 53 273 Swedish women aged 30 to 80 years, with comprehensive information on breast cancer risk factors and mammograms, was conducted. Total number of microcalcification clusters and the average mammographic density area were measured using a Computer Aided Detection system and the STRATUS method, respectively. A polygenic risk score for breast cancer, including 313 single nucleotide polymorphisms, was calculated for those women genotyped (N = 7387). Odds ratios (ORs) and 95% confidence intervals (CIs), with adjustment for potential confounders, were estimated. Age was strongly associated with microcalcification clusters. Both high mammographic density (>40 cm2), and high polygenic risk score (80‐100 percentile) were associated with microcalcification clusters, OR = 2.08 (95% CI = 1.93‐2.25) and OR = 1.22 (95% CI = 1.06‐1.48), respectively. Among reproductive risk factors, life‐time breastfeeding duration >1 year was associated with microcalcification clusters OR = 1.22 (95% CI = 1.03‐1.46). The association was confined to postmenopausal women. Among lifestyle risk factors, women with a body mass index ≥30 kg/m2 had the lowest risk of microcalcification clusters OR = 0.79 (95% CI = 0.73‐0.85) and the association was stronger among premenopausal women. Our results suggest that age, mammographic density, genetic predictors of breast cancer, having more than two children, longer duration of breast‐feeding are significantly associated with increased risk of microcalcification clusters. However, most lifestyle risk factors for breast cancer seem to protect against presence of microcalcification clusters. More research is needed to study biological mechanisms behind microcalcifications formation.
Keywords: cohort study, mammographic feature, mammographic microcalcifications
Short abstract
What's new?
Mammographic microcalcifications are one of the earliest mammographic signs of breast cancer. However, little is known on the predictors of mammographic microcalcifications and the mechanisms behind their formation. This large population‐based cohort study explored the association between established breast cancer risk factors and the risk of mammographic microcalcifications among pre‐ and postmenopausal women using an FDA‐approved method for detection of suspicious microcalcifications. The findings suggest that most established risk factors for breast cancer, with exception of age, mammographic density, familial history, polygenic risk score of breast cancer, having more children, and longer duration of breast‐feeding are protective against microcalcification clusters.
Abbreviations
- BI‐RADS
breast imaging‐reporting and data system
- BMI
body mass index
- CAD
computer aided detection
- CI
confidence interval
- FDA
Food and Drug Administration
- KARMA
Karolinska Mammography Project for Risk Prediction of Breast Cancer
- OR
odds ratio
1. INTRODUCTION
Microcalcifications are small calcium deposits with a diameter of less than 1 mm, found in the breast tissue. Microcalcifications play a crucial role in breast cancer screening. Presence of microcalcifications is associated with both ductal carcinoma in situ 1 and invasive breast cancers. 2 Approximately 50% of non‐palpable breast cancers are detected through identification of malignant microcalcifications on a mammogram. 3 Breast microcalcifications are normally described through their morphology, size and distribution. 4 Number of microcalcifications tend to increase with age, and they range from common benign to rare malignant alterations, there could be several mechanisms behind the formation of microcalcifications. One such mechanism is epithelial‐mesenchymal transition. 5 Epithelial‐mesenchymal transition is induced by a number of stimuli, including proinflammatory cytokines, hypoxia, changes in extracellular matrix and mechanical properties. During epithelial‐mesenchymal transition, epithelial cells gain several properties of mesenchymal cells such as migratory and invasive properties. 5 It has been hypothesized that epithelial cells that acquire mesenchymal characteristics become capable of producing breast microcalcifications. 5 In contrast, the formation of benign microcalcifications are considered to be explained by cell necrosis and debris. 6
The few studies that have attempted to investigate predictors of microcalcifications 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 have important limitations, such as including only breast cancer cases, 9 , 11 , 12 using case‐control or case‐report study designs rather than prospective cohort design, 7 , 9 , 11 , 12 using a qualitative and crude measure of microcalcifications (Breast Imaging‐Reporting and Data System [BI‐RADS score]), 9 , 11 , 12 or inability to exclude breast arterial calcifications. 7 , 8 , 10 , 13 , 14 Breast arterial calcifications are potential surrogate marker of atherosclerotic cardiovascular disease but are not associated with breast cancer. 15
While predictors of mammographic density have been studied extensively over the years, little is known about the predictors of microcalcifications despite being a clinically established mammographic sign of breast cancer for decades. 16 Mammographic density is associated with an increased risk of breast cancer 17 and is the radiographic appearance of epithelial and fibrous tissue that appears white on a mammogram. In contrast, the dark part of a mammogram represents the fatty tissue. 17 After age and body mass index (BMI) is taken into consideration, women with very dense breasts have four to six times greater risk of breast cancer compared to women with low density. 18 , 19 High age, postmenopausal status, parity, early pregnancy and high BMI are associated with lower mammographic density. 20 , 21 , 22 In contrast, high intake of alcohol and use of menopausal hormone therapy are associated with higher mammographic density. 23
To our knowledge, our study is the first large population‐based cohort study that addresses all previous described limitations. We included 53 273 women from the unique prospective Karolinska Mammography Project for Risk Prediction of Breast Cancer cohort (KARMA), 24 and used a novel Computer Aided Detection software (iCAD) to detect microcalcification clusters 25 , 26 and the STRATUS method to measure mammographic density. 27 We investigated predictors of microcalcification clusters and their asymmetry, that is, the clinically recognized relationship between an uneven distribution of microcalcifications between the breasts and the risk of breast cancer. 28 Additionally, we presented the results separated by menopausal status.
2. MATERIALS AND METHODS
2.1. Study population
KARMA is a Swedish population‐based prospective screening cohort of 70 874 women attending one of four Swedish mammography units as part of the national mammography screening program during January 2011 to March 2013. 24 The final analyses included 53 273 women aged 30 to 80 years, and reasons for exclusions are given in Figure 1. All participants signed an informed consent form and Stockholm ethical review board approved the study.
FIGURE 1.
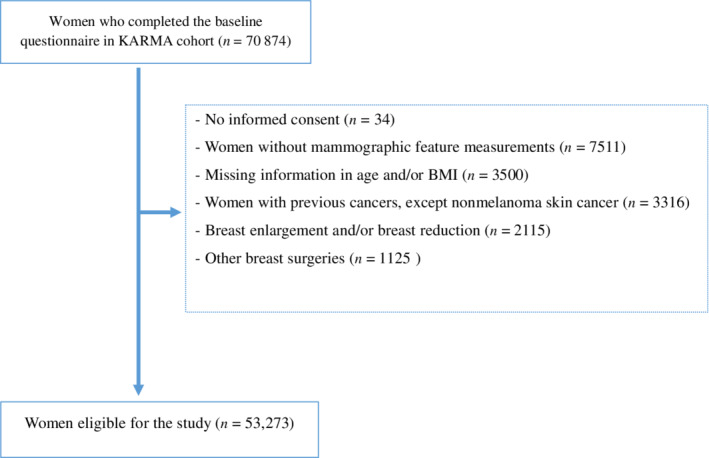
Flow chart describing the exclusion criteria for 70 874 women in KARMA cohort [Color figure can be viewed at wileyonlinelibrary.com]
2.2. Measurement of mammographic features
Raw mammograms from the mediolateral oblique and cranio‐caudal views of left and right breasts were collected. The CAD system used for identification of microcalcifications (iCAD; M‐Vu iCAD, Nashua, NH) 29 is a Food and Drug Administration (FDA) approved class 3 device (PMA number P010038) with an accuracy of 92%. 25 The algorithm of the system identifies suspicious microcalcification clusters that corresponds to microcalcifications with malignant morphology as defined by the BI‐RADS 3‐5 scores 25 , 26 (Supplementary Materials and Methods). The total number of microcalcification clusters of the breasts was calculated and the asymmetry was defined as the absolute difference between the numbers of microcalcification clusters between the breasts. Figure 2 illustrates how microcalcification clusters are marked on cranio‐caudal views using the iCAD software. We used microcalcification clusters rather than single microcalcifications since clusters are more likely a sign of cancer. 30 , 31 From here onward, suspicious microcalcification clusters are referred to as microcalcification clusters.
FIGURE 2.
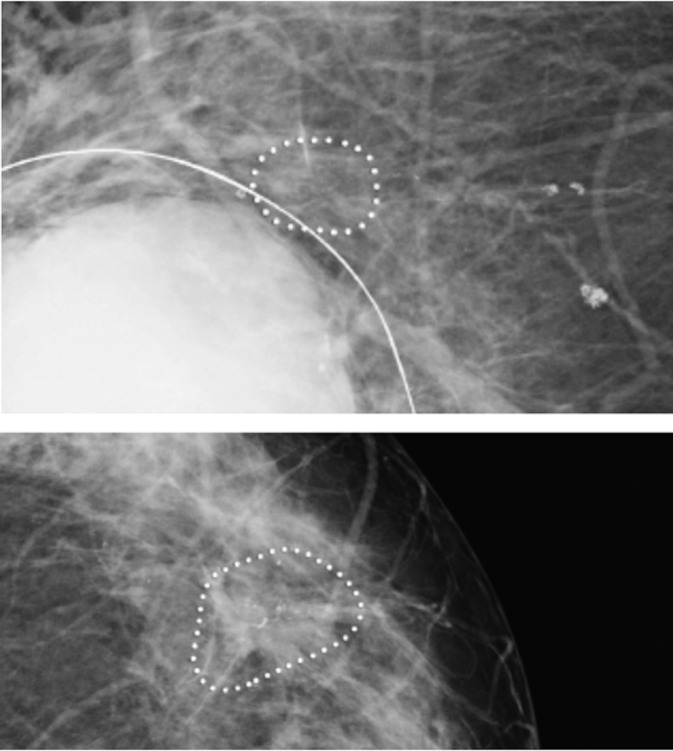
Illustration of suspicious microcalcification clusters using iCAD software on cranio‐caudal views of a 74 years old woman with a lump in the right breast. iCAD software identified microcalcification clusters with suspicious morphology (iCAD Inc. Mammography: benefits of computer aided detection. Clinical case study; 2016. Accessed August 13, 2020. https://www.icadmed.com/assets/dmm223_mammography_benefit_of_computer‐aided_detection_reva_01.pdf)
Mammographic density was measured as the average dense areas (cm2) of left and right breasts using the STRATUS method. 27 STRATUS is a fully automated tool developed to analyze digital and analogue images using an algorithm that measures density on all types of images regardless of vendor. STRATUS measures the mammographic dense area and the breast area and calculates the percent density from these measures. 27
2.3. Covariates
Participants of the KARMA cohort completed a detailed web‐based questionnaire within 3 months of conducting the baseline mammogram. Established risk factors were categorized as: age at baseline (<50, 50‐60, >60 years), baseline mammographic density divided in to quartiles (<9.0, 9.0‐19.9, 20.0‐40.0, >40 cm2), BMI (20.0‐24.9, 25.0‐29.9, ≥30), smoking status (never, former, current), alcohol consumption (none, 0.1‐10, >10 g/d), physical activity (<40, 40‐44.9, 45.0‐49.9, ≥50 metabolic equivalent of task hours per day), age at first birth (<20, 20‐25, >25 years), number of birth (0, 1‐2, >2), breast‐feeding among parous women (0, 1‐5, 6‐12, >12 months), time since last birth (<10, ≥10 years), age at menarche (<13, ≥13), oral contraceptive use (no, yes), menopausal hormone therapy use (never, former, current) and first‐degree family history of breast cancer (no, yes).
Genotyping of a random subset of healthy KARMA participants was performed using either a custom Illumina iSelect genotyping array chip, which included 200 K single nucleotide polymorphisms (SNPs), or the Oncoarry chip, which included 500 K SNPs. A weighted polygenic risk score for breast cancer was calculated for each genotyped woman using the recently published 313 SNPs that reached genome‐wide significance. 32 The polygenic risk score of women was divided into quintiles (0%‐20%, 20%‐40%, 40%‐60%, 60%‐80%, 80%‐100%).
Women reporting no natural menstruation over the past 12 months before study entry or no mensuration due to oophorectomy were considered postmenopausal. Women with missing information on menstruation status or having no menstruation due to gynecological surgeries other than oophorectomy were considered premenopausal if they were age 50 years or younger and postmenopausal if older than 50 years.
2.4. Statistical analyses
T test and chi‐square tests were used to compare characteristics of pre‐ and postmenopausal women and tests were performed at the two‐sided .05 significance level. Logistic regression analyses were used to estimate odds ratios (OR), to quantify the association between breast cancer risk factors and the risk of having microcalcification clusters and their asymmetric distributions between the breasts. Microcalcification clusters asymmetry was coded as 0 (for women with no microcalcification clusters in any breast and/or women with symmetrical microcalcification cluster distribution between the breasts) and 1 (for women with an asymmetric distribution of microcalcification clusters between the breasts). We additionally performed a sensitivity analysis where we excluded 705 women with symmetrical microcalcification clusters. All models were adjusted for age, BMI and menopausal status at baseline. For regression models including alcohol as a covariate, smoking was adjusted for. All statistical tests were two‐sided and P‐value of less than .05 were considered statistically significant.
Finally, we compared how established risk factors for breast cancer were associated with microcalcification clusters, mammographic density and risk of breast cancer risk using previous findings from the KARMA cohort 20 , 33 , 34 and a most up‐to‐date and comprehensive breast cancer polygenic risk score. 32
3. RESULTS
3.1. Baseline characteristics
The mean age of women included in our study was 54.1 years and more than half of the women in the study were postmenopausal (53.9%; Table 1). The majority of women had no microcalcification clusters (82.7%) and postmenopausal women had a greater mean number of microcalcification clusters than premenopausal women. The mean baseline mammographic dense area was higher in premenopausal (37.1 cm2) women compared to postmenopausal women (20.9 cm2) (Table 1). A polygenic risk score was calculated for the random subset of KARMA participants that were genotyped. Of the genotyped women 52.5% were postmenopausal and 47.4% were premenopausal. To account for the risk of bias due to the slightly skewed age distribution, we adjusted all analyses for age and menopausal status at baseline.
TABLE 1.
Characteristics of 53 273 women included in the final analyses, stratified by menopausal status
| Characteristics | Total | Premenopausal women | Postmenopausal women | P‐value* |
|---|---|---|---|---|
| No. of women (%) | 53 273 | 24 537 (46.0) | 28 736 (53.9) | |
| Mean age at baseline (SD) | 54.1 (9.7) | 45.4 (4.2) | 61.5 (6.6) | <.001 |
| Microcalcification clusters (%) | ||||
| 0 | 44 088 (82.7) | 21 595 (88.0) | 22 493 (78.2) | |
| ≥1 | 9167 (17.2) | 2934 (11.9) | 6233 (21.6) | |
| <.001 | ||||
| Missing | 18 (0.03) | |||
| Microcalcification clusters asymmetry (%) | ||||
| 0 | 44 793 (84.0) | 1866 (7.6) | 22 493 (91.6) | |
| ≥1 | 8462 (15.8) | 580 (2.3) | 6233 (21.6) | |
| 0.02 | ||||
| Missing | 18 (0.03) | |||
| Mean mammographic dense area (cm2) at baseline (SD) | 28.3 (23.8) | 37.1 (25.2) | 20.9 (19.6) | <.001 |
| Mammographic dense area (cm2) at baseline (%) | ||||
| <9.0 | 12 443 (23.3) | 2932 (11.9) | 9511 (33.0) | |
| 9.0‐19.9 | 10 943 (20.5) | 3603 (14.6) | 7340 (25.5) | |
| 20.0‐40.0 | 15 748 (29.5) | 8261 (33.6) | 7487 (26.0) | |
| >40 | 13 607 (25.5) | 9526 (38.8) | 4081 (14.2) | |
| <.001 | ||||
| Missing | 532 (0.9) | |||
| Mean BMI (kg/m2) (SD) | 25.1 (4.1) | 25.3 (4.0) | 24.9 (4.2) | <.001 |
| BMI (kg/m2) (%) | ||||
| 20‐24.9 | 27 123 (50.9) | 13 123 (53.4) | 14 000 (48.7) | |
| 25‐29.9 | 16 763 (31.4) | 6964 (28.3) | 9799 (34.1) | |
| ≥30 | 6460 (12.1) | 2935 (11.9) | 3525 (12.2) | |
| <.001 | ||||
| Smoking status (%) | ||||
| Never | 25 386 (47.6) | 13 558 (55.2) | 11 828 (41.6) | |
| Former | 20 912 (39.2) | 7977 (32.5) | 12 935 (45.0) | |
| Current | 6236 (11.7) | 2714 (11.0) | 3522 (12.2) | |
| <.001 | ||||
| Missing | 739 (1.3) | |||
| Mean alcohol consumption (gram/day) (SD) | 7.1 (8.5) | 7.9 (8.0) | 9.5 (9.2) | <.001 |
| Alcohol consumption (gram/day) (%) | ||||
| 0 | 9742 (18.2) | 4573 (18.6) | 5169 (17.9) | |
| 0.1‐10 | 32 426 (60.8) | 15 606 (63.0) | 16 820 (58.5) | |
| >10 | 9865 (18.5) | 3905 (15.9) | 5960 (20.7) | |
| <.001 | ||||
| Missing | 1240 (2.3) | |||
| Mean physical activity, (MET‐h per day) (SD) | 42.4 (6.2) | 43.1 (6.6) | 41.8 (5.8) | <.001 |
| Physical activity (MET‐h per day) (%) | ||||
| <40 | 18 492 (34.7) | 7811 (31.8) | 10 681 (37.1) | |
| 40.0‐44.9 | 18 326 (34.4) | 8113 (33.0) | 10 213 (35.5) | |
| 45.0‐49.9 | 9320 (17.4) | 4910 (20.0) | 4410 (15.3) | |
| ≥50.0 | 5114 (9.5) | 2944 (12.0) | 2170 (7.5) | |
| <.001 | ||||
| Missing | 2021 (3.7) | |||
| Mean age at first birth (SD) | 27.7 (5.2) | 28.7 (5.1) | 25.9 (5.0) | <.001 |
| Age at first birth (%) | ||||
| <20.0 | 2450 (4.5) | 497 (2.0) | 1953 (6.7) | |
| 20.0‐25.0 | 15 856 (29.7) | 5456 (22.2) | 10 400 (36.1) | |
| >25.0 | 27 505 (51.6) | 15 214 (62.0) | 12 291 (42.7) | |
| <.001 | ||||
| Missing | 7462 (14.0) | |||
| Mean number of births (SD) | 1.9 (1.0) | 2.1 (0.7) | 2.2 (0.8) | <.001 |
| Number of births (%) | ||||
| 0 | 6644 (12.4) | 3354 (13.6) | 4162 (14.4) | |
| 1‐2 | 32 824 (61.6) | 12 189 (49.6) | 13 119 (45.6) | |
| >2 | 13 012 (24.4) | 4629 (18.8) | 5815 (20.2) | |
| <.001 | ||||
| Missing | 793 (1.4) | |||
| Mean breast‐feeding duration (months) (SD) | 18.8 (10.0) | 20.6 (9.7) | 18.1 (9.6) | <.001 |
| Duration of breast‐feeding (months) (%) | ||||
| 0 | 911 (1.7) | 222 (0.9) | 689 (2.3) | |
| 1‐5 | 1214 (2.2) | 327 (1.3) | 887 (3.0) | |
| 6‐12 | 6558 (12.3) | 2168 (8.8) | 4390 (15.2) | |
| >12 | 33 830 (63.5) | 16 212 (66.0) | 17 618 (61.3) | |
| <.001 | ||||
| Missing | 9478 (17.7) | |||
| Mean time since last birth (years) (SD) | 22.5 (12.1) | 12.7 (6.6) | 30.9 (8.9) | <.001 |
| Time since last birth (years) (%) | ||||
| <10 | 7926 (14.8) | 7695 (31.3) | 231 (0.8) | |
| ≥10 | 38 444 (72.1) | 13 656 (55.6) | 24 788 (86.2) | |
| <.001 | ||||
| Missing | 6110 (11.4) | |||
| Mean age at menarche (SD) | 13.1 (1.4) | 12.9 (1.4) | 13.2 (1.4) | <.001 |
| Age at menarche (%) | ||||
| <13 | 17 782 (33.3) | 9109 (37.1) | 8673 (30.1) | |
| ≥13 | 33 876 (63.5) | 14 776 (60.2) | 19 100 (66.4) | |
| <.001 | ||||
| Missing | 1615 (3.0) | |||
| Oral contraceptives use (%) | ||||
| Never | 7512 (14.1) | 2142 (8.7) | 5370 (18.6) | |
| Ever | 44 441 (83.4) | 22 114 (90.1) | 22 327 (77.6) | |
| <.001 | ||||
| Missing | 1320 (2.4) | |||
| MTH use (%) | ||||
| Never user | 39 960 (75.0) | 22 410 (91.3) | 17 550 (61.0) | |
| Former user | 7373 (13.8) | 779 (3.1) | 6594 (22.9) | |
| Current user | 1879 (3.5) | 355 (1.4) | 1524 (5.3) | |
| <.001 | ||||
| Missing | 4061 (7.6) | |||
| Family history of breast cancer (%) | ||||
| No | 44 422 (83.3) | 20 844 (84.9) | 23 578 (82.0) | |
| Yes | 7211 (13.5) | 2989 (12.1) | 4222 (14.6) | |
| <.001 | ||||
| Missing | 1640 (3.0) | |||
| No of women with a PRS (%) | 7387 | 3505 (47.4) | 3882 (52.5) | |
Note: The number of women should be added to the number of missing.
Abbreviations: BMI, body mass index; MET, the metabolic equivalent of task; MHT, menopausal hormone therapy; PRS, polygenic risk score.
P value for t test of means or χ2 test of proportions between premenopausal and postmenopausal women; tests were performed at the two‐sided .05 significance level.
3.2. Age and lifestyle predictors of microcalcification clusters
Women above 60 years of age at examination had two times higher risk of having microcalcification clusters (OR = 2.51; 95% confidence interval [CI] = 2.28‐2.77) compared to younger women (<50 years). Overweight (BMI, 25.0‐29.9 kg/m2) and obese (≥30 kg/m2) women had approximately 20% lower risk compared to women with a normal BMI, OR = 0.84 (95% CI = 0.80‐0.88) and OR = 0.79 (95% CI = 0.73‐0.85), respectively (Table 2). The influence of BMI was more pronounced in premenopausal compared to postmenopausal women (Supplementary Table 1).
TABLE 2.
Predictors of microcalcification clusters risk and their asymmetry in the 53 273 women included in the final analyses
| Predictors | OR (95% CI) a | P‐value* | P‐value* | OR (95% CI) a | P‐value* | P‐value* |
|---|---|---|---|---|---|---|
| All women | All women | |||||
| Clustered microcalcifications | Asymmetry | |||||
| Age baseline (years) b | ||||||
| <50 | 1.00 | Ref. | 1.00 | Ref. | ||
| 50‐60 | 1.47 (1.36‐1.60) | <.001 | 1.46 (1.34‐1.58) | <.001 | ||
| >60 | 2.51 (2.28‐2.77) | <.001 | 2.36 (2.14‐2.61) | <.001 | ||
| Continuous | <.001 | <.001 | ||||
| BMI (kg/m2) c | ||||||
| 20.0‐24.9 | 1.00 | Ref. | 1.00 | Ref. | ||
| 25.0‐29.9 | 0.84 (0.80‐0.88) | <.001 | 0.86 (0.81‐0.91) | <.001 | ||
| ≥30.0 | 0.79 (0.73‐0.85) | <.001 | 0.83 (0.76‐0.89) | <.001 | ||
| Continuous | <.001 | <.001 | ||||
| Smoking status | ||||||
| Never | 1.00 | Ref. | 1.00 | Ref. | ||
| Former | 0.87 (0.83‐0.92) | <.001 | 0.88 (0.84‐0.93) | <.001 | ||
| Current | 0.89 (0.82‐0.96) | .003 | 0.89 (0.82‐0.96) | .005 | ||
| Alcohol consumption (gram/day) d | ||||||
| 0 | 1.00 | Ref. | 1.00 | Ref. | ||
| 0.1‐10 | 0.87 (0.82‐0.92) | <.001 | 0.88 (0.82‐0.94) | <.001 | ||
| >10 | 0.90 (0.84‐0.97) | .01 | 0.91 (0.84‐0.99) | .02 | ||
| Continuous | .18 | .28 | ||||
| Physical activity (MET‐h per day) | ||||||
| <40 | 1.00 | Ref. | 1.00 | Ref. | ||
| 40‐44.9 | 0.97 (0.92‐1.02) | .34 | 0.97 (0.92‐1.03) | .36 | ||
| 45.0‐49.9 | 0.92 (0.86‐1.04) | .25 | 0.92 (0.86‐1.05) | .24 | ||
| ≥50.0 | 0.94 (0.86‐1.03) | .22 | 0.93 (0.85‐1.02) | .16 | ||
| Continuous | .12 | .15 | ||||
| Age at first birth (year) | ||||||
| <20 | 1.00 | Ref. | 1.00 | Ref. | ||
| 20‐25 | 0.82 (0.74‐0.91) | <.001 | 0.85 (0.77‐0.95) | .004 | ||
| >25 | 0.72 (0.65‐0.79) | <.001 | 0.75 (0.68‐0.84) | <.001 | ||
| Continuous | <.001 | <.001 | ||||
| Number of children | ||||||
| 0 | 1.00 | Ref. | 1.00 | Ref. | ||
| 1‐2 | 0.97 (0.90‐1.04) | .42 | 0.97 (0.90‐1.05) | .50 | ||
| >2 | 1.11 (1.02‐1.20) | .009 | 1.10 (1.01‐1.20) | .01 | ||
| Continuous | <.001 | <.001 | ||||
| Breast feeding duration (month) | ||||||
| 0 | 1.00 | Ref. | 1.00 | Ref. | ||
| 1‐5 | 1.14 (0.91‐1.43) | .24 | 1.16 (0.92‐1.47) | .19 | ||
| 6‐12 | 1.07 (0.89‐1.29) | .45 | 1.06 (0.88‐1.29) | .50 | ||
| >12 | 1.22 (1.03‐1.46) | .02 | 1.21 (1.01‐1.46) | .03 | ||
| Continuous | <.001 | <.001 | ||||
| Time since last birth (year) | ||||||
| <10 | 1.00 | Ref. | 1.00 | Ref. | ||
| ≥10 | 1.06 (0.97‐1.16) | .08 | 1.08 (0.98‐1.19) | .09 | ||
| Continuous | .07 | .08 | ||||
| Age at menarche (year) | ||||||
| <13 | 1.00 | Ref. | 1.00 | Ref. | ||
| ≥13 | 0.92 (0.88‐0.97) | .002 | 0.93 (0.88‐0.98) | .007 | ||
| Continuous | <.001 | <.001 | ||||
| Oral contraceptive use | ||||||
| Never | 1.00 | Ref. | 1.00 | Ref. | ||
| Ever | 0.84 (0.79‐0.89) | <.001 | 0.85 (0.79‐0.90) | <.001 | ||
| MHT status | ||||||
| Never user | 1.00 | Ref. | 1.00 | Ref. | ||
| Former user | 0.91 (0.85‐0.97) | .007 | 0.90 (0.84‐0.97) | .006 | ||
| Current user | 0.94 (0.83‐1.06) | .33 | 0.97 (0.85‐1.09) | .62 | ||
| Baseline mammographic area (cm2) | ||||||
| <9.0 | 1.00 | Ref. | 1.00 | Ref. | ||
| 9.0‐19.9 | 1.23 (1.14‐1.32) | <.001 | 1.19 (1.11‐1.28) | <.001 | ||
| 20.0‐40.0 | 1.61 (1.50‐1.72) | <.001 | 1.56 (1.45‐1.68) | <.001 | ||
| >40 | 2.08 (1.93‐2.25) | <.001 | 2.00 (1.84‐2.15) | <.001 | ||
| Continuous | <.001 | <.001 | ||||
| Family history of breast cancer | ||||||
| No | 1.00 | Ref. | 1.00 | Ref. | ||
| Yes | 1.13 (1.06‐1.22) | <.001 | 1.13 (1.06‐1.21) | <.001 | ||
| Overall PRS percentile | ||||||
| 0%‐20% | 0.93 (0.76‐1.14) | .51 | 0.93 (0.75‐1.14) | .50 | ||
| 20%‐40% | 1.05 (0.86‐1.29) | .57 | 1.09 (0.89‐1.34) | .38 | ||
| 40%‐60% | 1.00 | Ref. | 1.00 | Ref. | ||
| 60%‐80% | 1.06 (0.86‐1.29) | .56 | 1.09 (0.88‐1.33) | .40 | ||
| 80%‐100% | 1.22 (1.06‐1.48) | .04 | 1.27 (1.04‐1.56) | .01 | ||
| Continuous | .001 | <.001 | ||||
Abbreviations: BMI, body mass index; CI, confidence interval; MET, the metabolic equivalent of task; MHT, menopausal hormone therapy; PRS, polygenic risk score; Ref., reference.
Adjusted Models: age, BMI and menopausal status at baseline.
Not adjusted for age at baseline.
Not adjusted for BMI at baseline.
Adjusted for age, BMI, menopausal status and smoking.
P‐value is performed at the two‐sided .05 significance level.
Former and current smokers had approximately 10% lower risk of microcalcification clusters than never smokers (Table 2), an association confined to postmenopausal women (Supplementary Table 1). Moderate alcohol consumption (0.1‐10 g/day) reduced the risk of microcalcification clusters (OR = 0.87; 95% CI = 0.82‐0.92; Table 2). There was no evidence of an association between physical activity and microcalcification clusters (Table 2).
When assessing the associations between age and lifestyle factors with asymmetry of microcalcification clusters, similar results as for the association between age and lifestyle factors with microcalcification clusters were observed (Table 2).
3.3. Reproductive and exogenous hormone predictors of microcalcification clusters
Age at first birth >25 years was significantly associated with lower risk (OR = 0.72; 95% CI = 0.65‐0.79) of microcalcification clusters (Table 2), but only among postmenopausal women (OR = 0.68; 95% CI = 0.61‐0.76; Supplementary Table 1). Women with >2 children or more than 1 year of breastfeeding had significantly increased risks of microcalcification clusters compared to nulliparous women and women who never breast‐fed (Table 2). The results were only seen among postmenopausal women (Supplementary Table 1). Higher age at menarche (≥13 years) was associated with 8% lower risk of having microcalcification clusters (OR = 0.92; 95% CI = 0.88‐0.97) (Table 2), and the association was only seen among postmenopausal women (Supplementary Table 1). There was no evidence of an association between time since last birth and microcalcification clusters (Table 2). Both oral contraceptives and menopausal hormone therapy significantly decreased the risk of having microcalcification clusters compared to never users (Table 2).
Similar results as for microcalcification clusters were found when assessing the association of reproductive and exogenous hormonal factors with asymmetry of microcalcification clusters (Table 2).
3.4. Mammographic density and genetic predictors of microcalcification clusters
Women with dense area >40 cm2 had two times higher risk of microcalcification clusters (OR = 2.08; 95% CI = 1.93‐2.25) compared to women with dense area < 9.0 cm2 (Table 2), an association not influenced by menopausal status (Supplementary Table 1).
Women with a family history of breast cancer had a significantly increased risk of microcalcification clusters (OR = 1.13; 95% CI = 1.06‐1.22) (Table 2). The result was confined to postmenopausal women (OR = 1.16; 95% CI = 1.07‐1.25) (Supplementary Table 1). Women in the highest quintile (80th‐100th percentile) of the polygenic risk score, compared to those in the middle quintile (40th‐60th percentile), had a significantly 22% higher risk of microcalcification clusters (Table 2). When stratifying the effect of the polygenic risk score by menopausal status, stronger association was observed in premenopausal women with 34% increased risk of microcalcification clusters, however the results did not reach the statistical significance (Supplementary Table 1).
Mammographic density and genetic factors influenced the asymmetry of microcalcification clusters in a similar manor risk as the risk of microcalcification clusters (Table 2).
When excluding the 705 women with symmetrical distribution of microcalcification clusters, no substantial differences between point estimates were seen compared to the results as when we treated women with symmetrical microcalcification same as the comparison group.
Table 3 shows a summary of associations between established breast cancer risk factors, including polygenic risk score, microcalcification clusters, mammographic density and breast cancer based on our previous findings using the KARMA cohort 20 , 34 , 35 and a most up‐to‐date and comprehensive study on breast cancer polygenic risk score. 32 A detailed description of each study is given in Supplementary Table 2. Increasing age, high mammographic density, family history of breast cancer and high polygenic risk score were the only factors that both increased the risk of microcalcification clusters and risk of breast cancer (Table 3). Nearly all other established risk factors for breast cancer that are known to increase the risk of breast cancer, decreased the risk of microcalcification clusters. Several factors (alcohol, high age at first birth, few children, use of oral contraceptive and menopausal hormonal therapy) were associated with fewer microcalcification clusters but higher mammographic density and higher risk of breast cancer. In contrast, some factors (postmenopausal obesity, tobacco use and short period of breast‐feeding) were associated with lower risk of microcalcifications clusters, lower mammographic density but increased breast cancer risk (Table 3). Lastly, increasing age is associated with more microcalcifications and risk of breast cancer but a decrease in mammographic density.
TABLE 3.
Summary of direction of associations between established breast cancer risk factors with microcalcification cluster risk, mammographic density and breast cancer risk
| Established risk factors for breast cancer | Suspicious microcalcification clusters | Mammographic density | Breast cancer risk |
|---|---|---|---|
| High age | Higher | Lower | Higher |
| High MD | Higher | — | Higher |
| High PRS | Higher | Higher | Higher |
| Family history of breast cancer | Higher | Higher | Higher |
| More children | Higher | Lower | Lower |
| Longer period of breast feeding | Higher | Higher | Lower |
| High BMI a | Lower | Lower | Higher |
| Current smoking | Lower | Lower | Higher |
| Alcohol consumption | Lower | Higher | Higher |
| Physical activity | Lower | Lower | Lower |
| Late menarche | Lower | Higher | Higher |
| High age at first birth | Lower | Higher | Higher |
| Oral contraceptive use b | Lower | Lower | Lower |
| MHT use | Lower | Higher | Higher |
Notes: The summary direction of associations are based on the previous studies using KARMA cohort 20 , 34 , 35 and a most up‐to‐date and comprehensive breast cancer polygenic risk score. 32 A detailed description of this table with point estimates is presented in Supplementary Table 2.
Abbreviations: BMI, body mass index; MD, mammographic density; MHT, menopausal hormone therapy; PRS, polygenic risk score.
Increased risk for breast cancer only seen among postmenopausal women.
We found an opposite direction of association between oral contraceptive use with the risk of breast cancer compared to previous evidence, 36 however, the result was not statistically significant.
4. DISCUSSION
Using a large prospective cohort, we found high age, high baseline mammographic density, family history of breast cancer, high polygenic risk score of breast cancer, having more than two children and breast feeding more than 1 year, to be associated with an increased risk of microcalcification clusters. In contrast, other established breast cancer risk factors such as high BMI, smoking, alcohol consumption, high age at first birth, oral contraceptive and menopausal hormone therapy use were all significantly associated with lower risk of microcalcification clusters. The association between most lifestyle, reproductive and genetic risk factors for breast cancer and microcalcifications clusters were confined to postmenopausal women.
Our finding of an association between microcalcification clusters and high age agrees with previous studies. 7 , 8 , 10 , 13 , 14 However, only one study focused on the presence of mammographic microcalcifications, 7 while the other studies included breast arterial calcifications. 8 , 10 , 13 , 14
The higher prevalence of microcalcification clusters at older age supports the epithelial mesenchymal transition hypothesis since it has been shown that the transition increases with age. 37 The reason for the age dependency could partly be explained by the inhibitory effect estrogen has on epithelial mesenchymal transition. 38 , 39 Epithelial‐mesenchymal transition is a complex biological process in which epithelial cells acquire invasive characteristics of mesenchymal cells and it is suggested as plausible explanation to the formation of malignant microcalcifications. 5
The finding of an association between higher mammographic density and microcalcification clusters are in line with previous studies. 7 , 40 , 41 The biological mechanism behind the association is largely unknown but an increase in matrix proteoglycans 41 and changes of collagen genesis in the extracellular matrix 42 have been suggested as explanations. A simpler but not contradicting hypothesis is that a higher epithelial component and increased matrix rigidity induces epithelial‐mesenchymal transition. 43
Both a family history of breast cancer and high polygenic risk score were associated with microcalcification clusters. These results are in agreement with our previous study which found 23% heritability in microcalcifications. 34 Given the strong inheritance of breast cancer and the link between microcalcifications and breast cancer, this is not a surprising result, but the findings merit further investigation, for example, conducting a genome‐wide association of microcalcification clusters.
Several factors, all indirectly (high BMI, smoking, alcohol use) or directly (use of exogenous hormones) linked to an increase in estrogen exposure are associated with fewer microcalcification clusters (Table 3). It could be described as if estrogens have a “protective” effect against the formation of microcalcifications. At the same time, these factors are (apart from high BMI and smoking) associated with higher mammographic density and increased risk of breast cancer. Previously exogenous estrogens have been shown to be negatively associated with microcalcifications, 44 , 45 so has tobacco. 7 , 10 , 13 , 46 However, the majority of these studies investigated the breast arterial calcifications.
Other factors that decrease the prevalence of microcalcification clusters are higher age at first birth, few children and shorter period of breast feeding. These associations have previously been described in other studies on arterial breast calcifications. 7 , 8 , 10 , 13 , 14 Three other case‐report studies showed post‐lactational increase of suspicious microcalcifications. 9 , 11 , 12 Pregnancy associated histological changes in the mammary tissue are induced by steroid hormones and growth factors. 12 When this influence diminishes, epithelial cells undergo a massive programmed cell death and tissue remodeling, so called post‐lactational involution. 47 It is hypothesized that post‐lactational involution increases the number of microcalcification clusters.
To our knowledge, this is the first large population‐based study examining the predictors of microcalcification clusters with malignant potential among healthy women using a fully automated software. The CAD system used in our study has been developed to identify potential malignant calcifications, previously found to be associated with breast cancer, 48 and not arterial calcifications. 29 Nevertheless, the study had a number of limitations. Information on the breast cancer risk factors was based on a self‐reported questionnaire and is therefore prone to information bias. However, a differential misclassification is unlikely since women were not aware of the presence of microcalcifications in their breasts. Even though we used an FDA approved software for identifying suspicious microcalcification, it is possible that some of microcalcifications were breast arterial calcifications. However, given the quite substantial risk of breast cancer seen previously in women with microcalcification clusters, we believe that the majority of identified calcifications were not breast arterial calcifications. 48 Strengths of our study are the prospective population‐based design, the large sample size, detailed information of the established breast cancer risk factors and access to mammograms for measurement of mammographic density using the fully automated STRATUS tool.
To conclude, our results indicate that most established risk factors for breast cancer, with exception of age, mammographic density, familial history, polygenic risk score of breast cancer, having more children and longer duration of breast‐feeding seems to protect against microcalcification clusters. However, the mechanism by which microcalcification clusters are formed in the breast tissues is unclear. Microcalcifications ranges from benign harmless alterations to signs of malignancy and more research are needed to understand the mechanism behind the latter entity.
CONFLICT OF INTEREST
Per Hall, Kamila Czene and Mikael Eriksson are collaborating with iCAD to develop a fully automated risk tool that takes mammographic density and microcalcifications in to consideration when assessing the risk of breast cancer.
ETHICS STATEMENT
The study was approved by the ethical review board in Stockholm (2010/958‐31/1). Informed consent was obtained from all individual participants included in the study. All experiments comply with the current Swedish laws.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
The authors thank all the participants in the KARMA study, and personnel for their devoted work during data collection. They also would like to acknowledge José Tapia for helping in data management. This work was supported by “Märit and Hans Rausing's Inititive Against Breast Cancer.” The funding agency had no role in the study design, data collection, analyses and data interoperation, in writing the manuscript or in the decision to submit the manuscript for publication.
Azam S, Eriksson M, Sjölander A, et al. Predictors of mammographic microcalcifications. Int. J. Cancer. 2021;148:1132–1143. 10.1002/ijc.33302
Funding information “Märit and Hans Rausing's Inititive Against Breast Cancer.”
REFERENCES
- 1. Henrot P, Leroux A, Barlier C, Genin P. Breast microcalcifications: the lesions in anatomical pathology. Diagn Interv Imaging. 2014;95:141‐152. [DOI] [PubMed] [Google Scholar]
- 2. Stomper PC, Geradts J, Edge SB, Levine EG. Mammographic predictors of the presence and size of invasive carcinomas associated with malignant microcalcification lesions without a mass. AJR Am J Roentgenol. 2003;181:1679‐1684. [DOI] [PubMed] [Google Scholar]
- 3. Cox RF, Hernandez‐Santana A, Ramdass S, McMahon G, Harmey JH, Morgan MP. Microcalcifications in breast cancer: novel insights into the molecular mechanism and functional consequence of mammary mineralisation. Br J Cancer. 2012;106:525‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernández P, Estrada T, Pizarro A, Tapia M. Breast calcifications: description and classification according to BI‐RADS 5th edition. Chil J Radiol. 2016;22:80‐91. [Google Scholar]
- 5. Scimeca M, Giannini E, Antonacci C, Pistolese CA, Spagnoli LG, Bonanno E. Microcalcifications in breast cancer: an active phenomenon mediated by epithelial cells with mesenchymal characteristics. BMC Cancer. 2014;14:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tot T, Gere M, Hofmeyer S, Bauer A, Pellas U. The clinical value of detecting microcalcifications on a mammogram. Semin Cancer Biol. 2019;14:S1044‐579X(19)30356‐6. [DOI] [PubMed] [Google Scholar]
- 7. Ali MCK, Hall P, Humphreys K. Association of microcalcification clusters with short‐term invasive breast cancer risk and breast cancer risk factors. Sci Rep. 2019;9:14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bielak LF, Whaley DH, Sheedy PF 2nd, Peyser PA. Breast arterial calcification is associated with reproductive factors in asymptomatic postmenopausal women. J Women's Health. 2010;19:1721‐1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giron GL, Boolbol SK, Gross J, Cohen JM, Feldman S. Postlactational microcalcifications. Breast J. 2004;10:247‐252. [DOI] [PubMed] [Google Scholar]
- 10. Maas AH, van der Schouw YT, Beijerinck D, Deurenberg JJ, Mali WP, van der Graaf Y. Arterial calcifications seen on mammograms: cardiovascular risk factors, pregnancy, and lactation. Radiology. 2006;240:33‐38. [DOI] [PubMed] [Google Scholar]
- 11. Mercado CL, Koenigsberg TC, Hamele‐Bena D, Smith SJ. Calcifications associated with lactational changes of the breast: mammographic findings with histologic correlation. AJR Am J Roentgenol. 2002;179:685‐689. [DOI] [PubMed] [Google Scholar]
- 12. Stucker DT, Ikeda DM, Hartman AR, et al. New bilateral microcalcifications at mammography in a postlactational woman: case report. Radiology. 2000;217:247‐250. [DOI] [PubMed] [Google Scholar]
- 13. Trimboli RM, Codari M, Guazzi M, Sardanelli F. Screening mammography beyond breast cancer: breast arterial calcifications as a sex‐specific biomarker of cardiovascular risk. Eur J Radiol. 2019;119:108636. [DOI] [PubMed] [Google Scholar]
- 14. Zafar AN, Khan S, Zafar SN. Factors associated with breast arterial calcification on mammography. J Coll Physicians Surg Pak. 2013;23:178‐181. [PubMed] [Google Scholar]
- 15. Bui QM, Daniels LB. A review of the role of breast arterial calcification for cardiovascular risk stratification in women. Circulation. 2019;139:1094‐1101. [DOI] [PubMed] [Google Scholar]
- 16. Ferranti C, Coopmans de Yoldi G, Biganzoli E, et al. Relationships between age, mammographic features and pathological tumour characteristics in non‐palpable breast cancer. Br J Radiol. 2000;73:698‐705. [DOI] [PubMed] [Google Scholar]
- 17. Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133‐1144. [PubMed] [Google Scholar]
- 18. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227‐236. [DOI] [PubMed] [Google Scholar]
- 19. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta‐analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159‐1169. [DOI] [PubMed] [Google Scholar]
- 20. Azam S, Sjolander A, Eriksson M, Gabrielson M, Czene K, Hall P. Determinants of mammographic density change. JNCI Cancer Spectr. 2019;3:pkz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boyd N, Martin L, Stone J, Little L, Minkin S, Yaffe M. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol Biomarkers Prev. 2002;11:1048‐1053. [PubMed] [Google Scholar]
- 22. Boyd NF, Martin LJ, Sun L, et al. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2086‐2092. [DOI] [PubMed] [Google Scholar]
- 23. Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control. 2000;11:653‐662. [DOI] [PubMed] [Google Scholar]
- 24. Gabrielson M, Eriksson M, Hammarstrom M, et al. Cohort profile: the Karolinska mammography project for risk prediction of breast cancer (KARMA). Int J Epidemiol. 2017;46:1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Food and Drug Administration . Summary of safety and effectiveness data: Mammoreader. iCAD; 2007.
- 26. Jeffrey C, WehnesJames P, MonacoDavid S, HardingJames H, PikeAnbinh T, HoLawrence MH. Microcalcification detection classification in radiographic images. United States Patent and Trademark; 2014.
- 27. Eriksson M, Li J, Leifland K, Czene K, Hall P. A comprehensive tool for measuring mammographic density changes over time. Breast Cancer Res Treat. 2018;169:371‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eriksson M, Czene K, Pawitan Y, Leifland K, Darabi H, Hall P. A clinical model for identifying the short‐term risk of breast cancer. Breast Cancer Res. 2017;19:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. iCAD . Breast health solutions: advanced cancer detection built on artificial intelligence. September 27, 2019.
- 30. Shao Y‐Z, Liu L‐Z, Bie M‐J, et al. Characterizing the clustered microcalcifications on mammograms to predict the pathological classification and grading: a mathematical modeling approach. J Digit Imaging. 2011;24:764‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sickles EA. Breast calcifications: mammographic evaluation. Radiology. 1986;160:289‐293. [DOI] [PubMed] [Google Scholar]
- 32. Mavaddat N, Michailidou K, Dennis J, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019;104:21‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Azam S, Eriksson M, Sjölander A, et al. Mammographic density change and risk of breast cancer. J Natl Cancer Inst. 2020;112:391‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holowko N, Eriksson M, Kuja‐Halkola R, et al. Heritability of mammographic breast density, density change, microcalcifications, and masses. Cancer Res. 2020;80:1590‐1600. [DOI] [PubMed] [Google Scholar]
- 35. Azam S, Eriksson M, Sjolander A, et al. Mammographic density change and risk of breast cancer. J Natl Cancer Inst. 2019;112:391‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228‐2239. [DOI] [PubMed] [Google Scholar]
- 37. Santos F, Moreira C, Nóbrega‐Pereira S, Bernardes de Jesus B. New insights into the role of epithelial−mesenchymal transition during aging. Int J Mol Sci. 2019;20:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang X, Belguise K, Kersual N, et al. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat Cell Biol. 2007;9:470‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ye Y, Xiao Y, Wang W, et al. ERalpha signaling through slug regulates E‐cadherin and EMT. Oncogene. 2010;29:1451‐1462. [DOI] [PubMed] [Google Scholar]
- 40. Naseem M, Murray J, Hilton JF, et al. Mammographic microcalcifications and breast cancer tumorigenesis: a radiologic‐pathologic analysis. BMC Cancer. 2015;15:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skandalis SS, Labropoulou VT, Ravazoula P, et al. Versican but not decorin accumulation is related to malignancy in mammographically detected high density and malignant‐appearing microcalcifications in non‐palpable breast carcinomas. BMC Cancer. 2011;11:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Azari F, Vali H, Guerquin‐Kern JL, et al. Intracellular precipitation of hydroxyapatite mineral and implications for pathologic calcification. J Struct Biol. 2008;162:468‐479. [DOI] [PubMed] [Google Scholar]
- 43. Rice AJ, Cortes E, Lachowski D, et al. Matrix stiffness induces epithelial‐mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis. 2017;6:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lochner DM, Brubaker KL. Incidence of malignancy in hormone therapy users with indeterminate calcifications on mammogram. Am J Obstet Gynecol. 2006;194:82‐85. [DOI] [PubMed] [Google Scholar]
- 45. Schnatz PF, Rotter MA, Hadley S, Currier AA, O'Sullivan DM. Hormonal therapy is associated with a lower prevalence of breast arterial calcification on mammography. Maturitas. 2007;57:154‐160. [DOI] [PubMed] [Google Scholar]
- 46. Hendriks EJ, de Jong PA, van der Graaf Y, Mali WP, van der Schouw YT, Beulens JW. Breast arterial calcifications: a systematic review and meta‐analysis of their determinants and their association with cardiovascular events. Atherosclerosis. 2015;239:11‐20. [DOI] [PubMed] [Google Scholar]
- 47. Bambhroliya A, Van Wyhe RD, Kumar S, et al. Gene set analysis of post‐lactational mammary gland involution gene signatures in inflammatory and triple‐negative breast cancer. PLoS One. 2018;13:e0192689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Azam SEM, Sjölander A, Hellgren R, Gabrielson M, Czene K, Hall P. Mammographic microcalcifications and risk of breast cancer; 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


