Abstract
Aims
Insulin glargine 300 U/mL (Gla‐300) contains the same units versus glargine 100 U/mL (Gla‐100) in three‐fold lower volume, and higher subcutaneous (SC) doses are required in people with diabetes. To investigate blood glucose (BG) lowering potency, Gla‐300 and Gla‐100 were compared after intravenous (IV, for 4 h) and SC (for 24 h) injection in healthy Beagle dogs.
Materials and methods
The dose of 0.15 U/kg Gla‐300 and Gla‐100 was injected IV in 12 dogs. BG, C‐peptide, glucagon and the active metabolite 21A‐Gly‐human insulin (M1; liquid chromatography‐tandem mass spectrometry method) were measured. Twelve other dogs were studied after SC injection of 0.3 U/kg Gla‐300 and Gla‐100.
Results
After IV injection, Gla‐300 and Gla‐100 were equally potent [BG_AUC0‐4 h ratio 1.01 (95% confidence interval, 0.94; 1.09)]. After SC injection, BG decreased slower and less with Gla‐300. Similar metabolism of Gla‐300 and Gla‐100 to M1 occurred with IV dosing [M1_AUC0‐1 h ratio 0.99 (95% confidence interval, 0.82; 1.22)], but with SC dosing M1_Cmax and AUC0‐24h were 44% and 17% lower; mean residency time and bioavailability were 32% longer and 50% lower, with Gla‐300.
Conclusions
IV Gla‐300 and Gla‐100 have the equivalent of BG‐lowering potency and M1 metabolism. SC Gla‐300 has lower M1 bioavailability with a reduced BG‐lowering effect and need for greater doses versus Gla‐100.
Keywords: glargine M1, glargine M2, glargine metabolism, glargine parent M0, glargine subcutaneous, intravenous glargine insulin
1. INTRODUCTION
The second‐generation long‐acting (basal) insulin analogue glargine 300 U/mL (Gla‐300) has advantages as compared with first‐generation insulin glargine 100 U/mL (Gla‐100) in people with type 1 and type 2 diabetes. 1 , 2 , 3 , 4 , 5 The flatter pharmacokinetics (PK) and pharmacodynamics (PD) of Gla‐300, more evenly distributed over the 24 h at the clinical, individual doses used by people with type 1 diabetes, 2 , 3 explain the reduced risk for hypoglycaemia with Gla‐300, including severe episodes as compared with Gla‐100, 6 for similarly improved glycaemic control. 7 , 8 , 9
In clinical trials, Gla‐300 requires higher doses as compared with Gla‐100 in type 1 and type 2 diabetes. 2 , 4 , 5 , 7 , 8 , 9 , 10 , 11 In experimental euglycaemic clamp studies in type 1 diabetes, the same subcutaneous (SC) dose of Gla‐300 at steady state showed reduced blood glucose (BG)‐lowering effects versus Gla‐100 by 27%, 1 and by 14%‐30% versus degludec, 12 , 13 over the 24 h post‐dosing. Consequently, Gla‐300 is referred to as a basal insulin with lower potency versus comparators. 12
However, the differential BG‐lowering effect observed with Gla‐300 as compared with Gla‐100 after SC dosing, might be explained also by the different mechanism of action. Gla‐300 contains the same glargine molecule soluble at acidic pH as Gla‐100, which becomes insoluble and forms micro‐precipitates at the neutral pH of the SC tissue injection site. In people with type 1 and type 2 diabetes, Gla‐300 and Gla‐100 undergo the same, nearly total biotransformation of glargine parent (M0) primarily to 21A‐Gly‐human insulin (metabolite M1) and, to a lesser extent, to 21A‐Gly‐des‐30B‐Thr‐human insulin (metabolite M2), which both drive the insulin effect of glargine in type 1 diabetes 14 , 15 and in type 2 diabetes. 16 , 17 , 18 However, as compared with 100 U/mL glargine, in 300 U/mL glargine, the same units are contained in one‐third of the volume thereby forming a smaller insulin depot after SC injection with half of the surface area and consequently slower absorption rate. The longer residency time in the SC tissue before absorption of Gla‐300 versus Gla‐100 might favour the activity of local protease enzymes with greater, local degradation of former versus latter glargine formulation. If so, while the slower and more prolonged SC absorption would explain the more physiological PD of Gla‐300, its lower bioavailability would account for the reduced BG‐lowering activity as compared with Gla‐100 and IDeg, and explain the need to increase the dose in people with type 1 diabetes to match the BG‐lowering effect. 2
Interestingly, because glargine remains soluble with intravenous (IV) dosing because of immediate dilution, Gla‐100 fully reproduces the PD of human regular insulin in people with type 1 diabetes 19 and normal subjects, 20 as shown by euglycaemic clamp studies. In theory, if IV administration of Gla‐300 reproduced fully the PD effects of Gla‐100, this would show equivalence of the BG‐lowering effects of the 300 and 100 U formulations of glargine. In turn, this would show that different bioavailability rather than different potency, is the mechanism by which Gla‐300 has lower, in addition to different, PD over the 24 h after SC dosing versus Gla‐100. However, no such data are available at present.
The aim of this study was to explore the equivalence of BG lowering and glargine metabolism of the same dose of Gla‐300 versus Gla‐100 after IV dosing, to compare the results with those observed after SC dosing of the two glargine formulations and to calculate the mean residency time and bioavailability of M1. For this purpose, an experimental model in dogs was used where BG was allowed to decrease.
2. MATERIALS AND METHODS
2.1. Study design
The study was performed in 24 healthy, normoglycaemic, male Beagle dogs (10‐14 kg). The first 12 dogs were studied using the IV route of either Gla‐300 (N = 6) or Gla‐100 (N = 6) administration, whereas the second 12 dogs underwent the SC route of either Gla‐300 (N = 6) or Gla‐100 (N = 6) administration, so that each dog was studied only once (first injection of Gla‐300 and Gla‐100 both IV and SC). The animals were kept under standardized conditions (light cycle from 06:00 to 18:00 h) and on a standard diet (ssnif, Soest, Germany). The animals were fasted for 16 h before the study start and throughout the experiment with free access to tap water. Gla‐100 and Gla‐300 solutions were administered as a single injection at a dose of 0.15 U/kg (for IV injection into the jugular vein) or 0.3 U/kg (for SC injection into a skinfold of the lateral flank to avoid intramuscular penetration) with Hamilton syringes to ensure accuracy of tiny microliter volumes, in the morning (about 07:00 h). After IV or SC insulin injection, BG was allowed to decrease and no glucose was infused. The duration of the IV study was 4 h, and that of the SC study 24 h.
2.2. Methods
Blood samples for glucose analysis were taken at time point 0 and at time points 5, 10, 15, 30, 45, 60, 90, 120, 180 and 240 min (IV, total blood drawn 33 mL) or every hour for 24 h (SC, mean total blood volume 75 mL) after injection. BG concentration was determined enzymatically from 5 μL whole blood haemolysed with 250 μL haemolysate.
The study was approved by the local ethics committee and was conducted in accordance with EU law, including the use and care of laboratory animals, under the condition of using a blood volume not >100 mL per dog. Therefore, each dog could not be studied twice over a short period, but only once.
PK of parent insulin glargine (M0) and its metabolites [M1 (21A‐Gly‐human insulin) and M2 (21A‐Gly‐Des‐30B‐human insulin)] were determined by measuring the concentrations of M0, M1 and M2 in plasma by an exploratory liquid chromatography‐tandem mass spectrometry assay method, as previously described, 14 with previous immunoaffinity enrichment of samples, with a lower limit of quantification (LLOQ) of 0.2 ng/mL. The active moieties of M0, M1 and M2 were quantified by measuring peak area ratios using tandem mass spectrometry detection. Quantification of M0, M1 and M2 in plasma was unaffected by the presence of haemolysed blood (3%) or by the presence of canine insulin. The results were expressed in ng/mL with subsequent conversion in μU/mL. The PK parameters were calculated using a non‐compartmental approach using the linear trapezoidal calculation method. Nominal sampling times were used. Samples less than the LLOQ were taken as equal to 0 for calculations. C‐peptide was determined from 100 μL dog plasma using a commercial canine C‐peptide RIA assay (Millipore CCP‐24HK, Darmstadt, Germany) with an LLOQ of 0.172 ng/L. All values below LLOQ were set to LLOQ = 0.173 ng/L.
Glucagon was measured from 25 μL dog plasma by a commercially available glucagon enzyme‐linked immunosorbent assay assay (Mercodia 10‐1271‐01, Uppsala, Sweden) with an LLOQ of 5.3 ng/L. All values below LLOQ were set to LLOQ = 5.3 ng/L.
2.3. Statistical analysis and calculations
The linear trapezoidal rule was used to calculate the area under the concentration‐time curve (AUC) for BG. The minimum BG concentration (BG‐Cmin) and the time to reach BG‐min, BG‐Tmin, were read directly from the curve. The BG‐AUC0‐24h and (BG‐Cmin) taken to indicate the extent and rate of glucose excursions, respectively, were the parameters of interest (main outcome variables) in the comparison between Gla‐300 and Gla‐100 following IV insulin injection. Secondary outcome variables were glargine's metabolites M0, M1, M2 (both 300 U/mL and 100 U/mL formulations), C‐peptide and glucagon. For these variables AUC, Cmax, Tmax were calculated. Finally, other secondary outcomes were BG‐AUC, BG‐Cmin, Tmin, glargine's metabolites M0, M1 and M2 following SC injection. For these variables AUC, Cmax, Tmax, F (bioavailability) and mean residence time (MRT) were calculated. The bioavailability (F) refers to the fraction of insulin after SC administration that reaches the systemic circulation. It is calculated as AUC 0‐inf._SC/AUC 0‐inf._IV after dose normalization. MRT, useful index of the average time a drug remains in the body, is calculated as AUMC 0‐inf./AUC 0‐inf.. PK parameters were calculated using non‐compartmental analysis. 21
Equivalence testing was conducted using the two one‐sided test procedure on log‐transformed data and re‐transformations by determining whether the upper and lower bounds of the 90% confidence interval (CI90%) for the AUCGla‐300/AUCGla‐100 ratios and Cmin_Gla‐300/Cmin_Gla‐100 ratios were contained within the intervals 0.80‐1.25 and 0.70‐1.43, respectively. 22 Tmin and Tmax metrics and those variables for which a log‐transformation could not be performed because of zero values were analysed non‐parametrically using Wilcoxon's rank‐sum test and Hodges‐Lehmann estimates of the median treatment differences computed with CI90%. Data are expressed as arithmetic means (SD), median (CI95%) and geometric means (CI95%) in text and tables, and as means and SE in figures. Statistical analysis was usually performed using NCSS20 (NCSS, LLC. Kaysville, UT, USA) 23 and PKSolver. 24
3. RESULTS
3.1. Intravenous dosing
The extent of BG changes 1, 2 and 4 h following IV dosing was equivalent between Gla‐300 and Gla‐100, as also shown by individual cases over the full 4‐h period (Figure S1). BG_Cmin was 11% lower with Gla‐300 as compared with Gla‐100. Tmin value was achieved between 15 and 45 min, with a median time of 30 min for both insulins (Figure 1, Table 1, and Figures S1 and S2).
FIGURE 1.
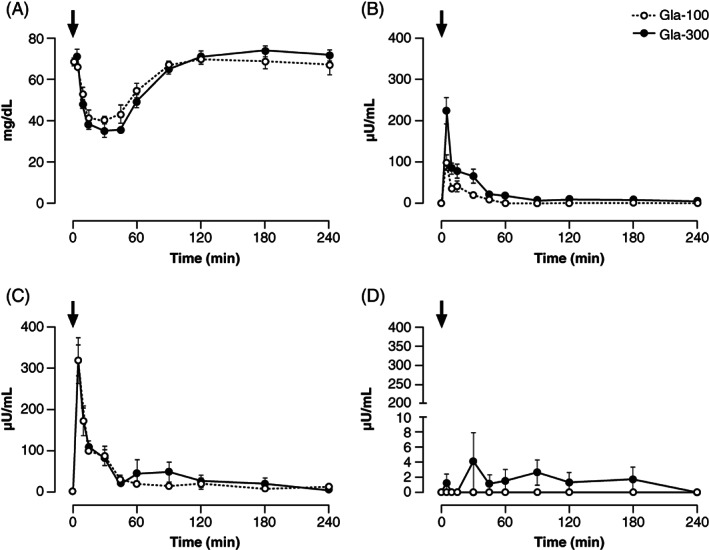
Concentrations of A, blood glucose, B, plasma M0, C, plasma M1 and D, plasma M2, after IV administration of 0.15 U/kg Gla‐300 and Gla‐100. Black arrows indicate the time of dosing. IV, intravenous; M0, metabolite M0; M1, metabolite M1; M2, metabolite M2
TABLE 1.
Pharmacodynamic and pharmacokinetic results following IV and SC of either Gla‐300 or Gla‐100 injections
| Gla‐300 | Gla‐100 | Estimate and 90% CI for mean ratios (Gla‐300/Gla‐100) b | |
|---|---|---|---|
| IV injection | (0.15 U/kg) | (0.15 U/kg) | |
| BG AUC0‐1 h (mg/h/dL) | 38.3 (35.1; 41.9) | 42.3 (36.4; 49.4) | 0.91 (0.8; 1.03) |
| BG AUC0‐2 h (mg/h/dL) | 96 (91.3; 101.5) | 102 (93; 113) | 0.94 (0.87; 1.02) |
| BG AUC0‐4 h (mg/h/dL) | 249 (234; 266) | 247 (226; 270) | 1.01 (0.94; 1.09) |
| BG Cmin (mg/dL) | 31.3 (28.4; 34.5) | 35.2 (30.6; 40.6) | 0.89 (0.79; 1.0) |
| BG Tmin (h) a | 0.50 (0.25; 0.50) | 0.50 (0.25; 0.75) | 0.0 (−15; 0.0) |
| M1 AUC0‐1 h (μU/h/mL) | 87.6 (68; 112) | 87.8 (77; 100) | 0.99 (0.82; 1.22) |
| M1 AUC0‐4 h (μU/h/mL) | 137 (72; 259) | 116 (76; 178) | 1.18 (0.68; 2.03) |
| M1 Cmax (μU/mL) | 316 (240; 416) | 313 (208; 470) | 1.01 (0.71; 1.43) |
| M1 Tmax (min) a | 5 (5; 60) | 5 (5; 30) | 0 (0; 0) |
| M0 AUC0‐1 h (μU/h/mL) | 59.5 (41; 86) | 21.3 (10; 45) | 2.79 (1.55; 5.01) |
| M0 AUC0‐4 h (μU/h/mL) | 83.9 (56; 126) | 21.3 (10; 45) | 3.94 (2.17; 7.16) |
| M0 Cmax (μU·mL−1) | 211.8 (143; 315) | 84.8 (39; 185) | 2.5 (1.35; 4.63) |
| M0 Tmax (min) a | 5 (5; 5) | 6 (3.6; 9.6) | 0 (0; 0) |
| M2 AUC0‐1 h (μU/h/mL) a | 0 (0; 24) | 0 (0; 0) | 0 (0; 7.1) |
| M2 AUC0‐4 h (μU/h/mL) a | 0 (0; 31) | 0 (0; 0) | 0 (0; 4) |
| M2 Cmax (μU/mL−1) a | 0 (0; 24) | 0 (0; 0) | 0 (0; 7.1) |
| M2 Tmax (min) a | 0 (0; 30) | 0 (0; 0) | 0 (0; 5) |
| Glucagon AUC0‐4 h (ng/h/L) | 88.5 (58; 135) | 74.5 (50; 110) | 1.19 (0.79; 1.78) |
| Glucagon Cmax (ng/L) | 72.3 (54; 97) | 47 (25; 89) | 1.55 (0.95; 2.53) |
| Glucagon Tmax (min) a | 31.8 (27; 38) | 31.8 (17.4; 58.4) | 0 (0; 25) |
| C‐Pep AUC0‐4 h (ng/h/L) a | 0.80 (0.62; 1.03) | 0.79 (0.64; 0.98) | 1.01 (0.8; 1.27) |
| C‐Pep Cmax (ng/L) | 0.33 (0.19; 0.55) | 0.39 (0.26; 0.59) | 0.84 (0.52; 1.34) |
| C‐Pep Tmax (h) a | 0 (0; 4) | 0.04 (0; 4) | 0 (−0.5; 0.08) |
| M0/(M0 + M1) c AUC0‐2 h | 0.37 (0.29; 0.49) | 0.15 (0.06; 0.40) | 2.52 (1.23; 5.21) |
| SC injection | (0.30 U/kg) | (0.30 U/kg) | |
| BG AUC0‐24 h (mg/h/dL) | 1599 (1484; 1722) | 1667 (1589; 1748) | 0.96 (0.90; 1.02) |
| BG AUC0‐12 h (mg/h/dL) | 743 (645; 849) | 673 (602; 752) | 1.10 (0.98; 1.25) |
| BG AUC12‐24 h (mg/h/dL) | 851 (773; 937) | 991 (964; 1021) | 0.86 (0.80; 0.92) |
| BG Cmin (mg/dL) | 43.9 (35.1; 55.2) | 36.2 (31.7; 41.5) | 1.21 (1.01; 1.46) |
| BG Tmin (h) a | 8.5(6; 12) | 6.5 (5; 8) | 2 (1; 4) |
| M1 AUC0‐24 h (μU/h/mL) | 78.2 (56.7; 104) | 94.7 (74.5; 120) | 0.83 (0.63; 1.09) |
| M1 AUC0‐12 h (μU/h/mL) | 76.7 (56.7; 104) | 94.7 (74.5; 120) | 0.81 (0.62; 1.05) |
| M1 AUC12‐24 h (μU/h/mL) | 1.6 | 0 | ‐ |
| M1 Cmax (μU/mL) | 9.9 (8.3; 12) | 17.7 (11; 28.7) | 0.56 (0.38; 0.83) |
| M1 Tmax (h) a | 6 (1; 9) | 5 (2; 7) | 1 (−3; 4) |
| F | 0.25 (0.09; 0.68) | 0.50 (0.33; 0.75) | 0.50 (0.25; 0.99) |
| MRT (h) | 12 (9.4; 18.1) | 9.4 (4.6; 18.9) | 1.32 (0.74; 2.34) |
| Glucagon AUC0‐24 h (ng/h/L) | 272 (193; 383) | 221 (153; 320) | 1.23 (0.86; 1.75) |
| Glucagon Cmax (ng/L) | 20.2 (14.3; 28.4) | 18.6 (12.2; 28.5) | 1.08 (0.74; 1.59) |
| Glucagon Tmax (h) a | 5.5 (2; 23) | 4.5 (2; 7) | 1 (−2; 11) |
| C‐Pep AUC0‐24h (ng/h/L) | 0.36 (0.23, 0.56) | 0.34 (0.21; 0.54) | 1.07 (0.65; 1.75) |
| C‐Pep (ng/L) | 0.36 (0.23, 0.56) | 0.34 (0.21; 0.54) | 1.07 (0.65; 1.75) |
| C‐Pep Tmax (h) a | 17.5 (14; 21) | 17.0 (17; 23) | 0.5 (−1; 2) |
Note: Data are geometric mean (95% CI) or
median (95% CI).
Abbreviations: AUC, area under the curve; BG, blood glucose; Cmax, maximum concentration; Cmin, minimum concentration; C‐Pep, C‐peptide; CI, confidence interval; F, bioavailability; IV, intravenous; LLOQ, lower limit of quantification; M0, metabolite M0; M1, metabolite M1; M2, metabolite M2; MRT, mean residence time; SC, subcutaneous; Tmax, time at which Cmax is observed; Tmin, time at which Cmin is observed.
Point estimates of treatment ratios with 90% CIs were calculated using t test based on log‐transformed data and re‐transformations. Bold type: those PK/PD parameters that met equivalence criteria following statistical equivalence testing or that were statistically not different from zero following non‐parametric analysis. Following SC injection of both Gla‐300 and Gla‐100, M0 and M2 were <LLOQ. In one dog in the Gla‐300 SC group M1 was <LLOQ throughout.
Percentage of M0 relative to total plasma insulin (M0 + M1).
M1, M2 and M0 were not present in plasma before either Gla‐300 or Gla‐100 administration. One hour after dosing, M1_AUC0‐1h was equivalent between Gla‐300 and Gla‐100, with a similar peak (Table 1), although the 90% CI of the geometric ratio extended outside the equivalence range. M1_Tmax was reached at a similar median time of 5 min. M1_AUC0‐4h was 18% greater with Gla‐300 as compared with Gla‐100 (Table 1).
M2 was undetectable with Gla‐100, and measurable with Gla‐300 only in two of the six dogs at few time points (Figure 1, Table 1). M0 increased to a peak at 5 and 6 min with Gla‐300 and Gla‐100, respectively, but more so with Gla‐300 as compared with Gla‐100 (212 vs. 85 μU/mL, respectively) (Table 1). M0 was measurable in three dogs until 240 min with Gla‐300, but only up to 45 min with Gla‐100 in three other dogs (Figure 1). M0_AUC0‐4h was nearly four times higher with Gla‐300 versus Gla‐100 (Table 1). The percentage of total serum insulin (M0 + M1) represented by M0 was 37% with Gla‐300 and 15% with Gla‐100 (Table 1).
C‐peptide was similarly suppressed after Gla‐300 and Gla‐100 between 5 and 120 min, then similarly recovered by the end of studies, and was equivalent over the 4 h study (Figure S2, Table 1). Baseline plasma glucagon was 40% (−32% to 191%) higher with Gla‐300 as compared with Gla‐100. Consequently, Glucagon_ AUC0‐4h and Glucagon_Cmax were greater with Gla‐300 as compared with Gla‐100 (19% and 55%, respectively). However, Glucagon_Tmax occurred at the same median time of 31.8 min (Figure S2, Table 1).
3.2. Subcutaneous dosing
BG decreased more slowly with Gla‐300 and Gla‐100 after SC compared with IV dosing, as expected, despite doubling the insulin dose (Figure 2, Table 1, and Figures S1 and S3). However, with Gla‐300 baseline‐to‐nadir BG decrease was slower by 64% (42%‐78%) as compared with Gla‐100. In fact, the rate of BG fall was 3.1 mg/h/dL (1.6‐6.0) and 8.37 mg/h/dL (7.2‐10.6) (P = .012) for Gla‐300 and Gla‐100, respectively. BG_Cmin was 21% greater (44 vs. 36 mg/dL) and was reached 2 h later (8.5 vs. 6.5), with Gla‐300 as compared with Gla‐100, indicating a slower absorption rate with the former as compared with the latter. The post‐nadir recovery from hypoglycaemia was slower with Gla‐300 as compared with Gla‐100 (Figure 2, Table 1).
FIGURE 2.
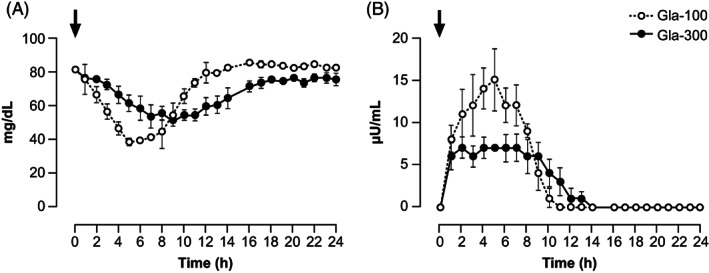
Concentrations of A, blood glucose and B, plasma M1, after SC administration of 0.30 U/kg Gla‐300 and Gla‐100. Data for M0 and M2 are not shown (M0 was detectable only in two dogs for up to 3 h only, M2 was never detectable, see Results). Black arrows indicate the time of dosing. M0, metabolite M0; M1, metabolite M1; M2, metabolite M2; SC, subcutaneous
Overall, in contrast to IV Gla‐300 and Gla‐100 bolus, which resulted in an equivalent glucose lowering potency (i.e., similar AUC, Cmin and Tmin), SC injection of Gla‐300 and Gla‐100 produced a different distribution of the rate and extent of glucose excursions (BG fell less rapidly and to a smaller extent with Gla‐300), based on which equivalence was not proven (Table 1).
M1, M2 and M0 were not present in plasma before the administration of either Gla‐300 or Gla‐100. After dosing, M1 increased with both insulins within 1 h. With Gla‐300, M1 concentrations averaged 6.5 μU/mL (6.1‐6.9) between 1 and 9 h, with a peak of 9.9 μU/mL (11‐28.7) at 7 h and then it decreased slowly until 14 h, after which time it was no longer detectable. In contrast, with Gla‐100, M1 continued to increase to a greater peak of 17.7 μU/mL (11‐28.7) at 5 h, after which it decreased slowly until 12 h, after which it was undetectable (Figure 2). Bioavailability (F) of M1 after SC administration was 25% and 50% with Gla‐300 and Gla‐100, respectively. The relative bioavailability of M1 following SC administration of Gla‐300 was 50% lower as compared with that of Gla‐100. MRT was 32% longer for Gla‐300 than Gla‐100, indicating a longer residence time for Gla‐300 in subcutaneous tissue. The overall M1‐AUC0‐24h with Gla‐300 was 17% lower than that of Gla‐100, and 19% lower in the first 12 h post‐dosing (Table 1 and Figure S1). M0 was undetectable with Gla‐300, whereas with Gla‐100 it was detectable only in two of the six dogs for the initial 3 h post‐dosing (concentrations 10‐27 μU/mL), after which it was no longer detectable (data not shown). M2 was not detectable in any dog with either Gla‐300 or Gla‐100 (data not shown).
Plasma C‐peptide was similarly suppressed for the first 16 h post‐dosing with both Gla‐300 and Gla‐100, after which time it increased to similar values until 24 h, and was equivalent over the 0‐24 h with the two insulins (Table 1 and Figure S3). Baseline plasma glucagon was similar with Gla‐300 (14.9 ± 5.7 ng/mL) and Gla‐100 (10.9 ± 2.9 ng/mL) (P = .231). The response of glucagon to the slow fall of BG with SC dosing of Gla‐300 and Gla‐100 was less pronounced as compared with IV dosing. As baseline plasma glucagon levels were 30% greater (−10 to 90) with Gla‐300 as compared with Gla‐100, Glucagon_AUC0‐24 and Glucagon_Cmax were also greater by 23% and 8%, respectively, with Gla‐300 as compared with Gla‐100 (Table 1 and Figure S3).
4. DISCUSSION
The present study in a dog model where BG was allowed to decrease after insulin dosing, demonstrates that the BG‐lowering effect of Gla‐300 and Gla‐100 given IV is equivalent, and that the PK of M1, the main metabolite of the glargine parent M0, is equivalent with the two glargine formulations. This is a novel observation. The present study also shows that, as compared with IV dosing, with SC dosing the BG‐lowering effect with both insulins is slower and prolonged, as expected, but more so with Gla‐300. With Gla‐300, the BG initially decreased more slowly to a nadir, which remains higher, and recovered more slowly versus Gla‐100. BG_AUC0‐24h of Gla‐300 and Gla‐100 was similar, but Cmin and Tmin were different (Table 1), and therefore, in contrast to IV dosing, equivalence of the BG‐lowering effect of Gla‐300 and Gla‐100 could not be demonstrated with SC dosing. In fact, the BG‐lowering effect was 10% lower in the first 12 h, and 14% greater in the second 12 h after SC dosing with Gla‐300 versus Gla‐100 (Table 1).
The results of BG dynamics in the present SC dosing study are consistent with the PD (glucose infusion rate, GIR) observed with Gla‐300 and Gla‐100 in people with type 1 diabetes. However, in contrast to the present study, in previous studies BG was not allowed to decrease (euglycaemic clamp) after the SC administration of fixed doses, either the first injection 25 or at steady state, 1 as well as at steady state with individual doses. 2 The euglycaemic clamp technique makes it possible to study PK/PD of injected insulin without inducing hypoglycaemia and stimulating hormonal counter‐regulation to hypoglycaemia. 26 This is different from the present study where BG was allowed to fall spontaneously and to elicit counter‐regulatory responses. The approach used in dogs in the present study with hypoglycaemia prolonged for several hours, is not feasible in humans for ethical reasons, and has strengths and limitations as compared with the euglycaemic clamp technique. One strength is the low complexity of the procedure, and the easy understanding of results of injected insulin such as lowering of BG. This is different from the more complex methodology of the euglycaemic clamp where the interpretation is based on GIR in the context of actual BG. 26 The main limitation of the current approach is the fact that BG changes are affected not only by injected insulin, but also by counter‐regulatory responses to hypoglycaemia, primarily hormonal, and by residual endogenous insulin secretion in non‐diabetic dogs such as those of the present study. Of note, with IV dosing, the equivalent BG‐lowering effect and BG dynamics with the two insulins induced a similar suppression of C‐peptide and responses of glucagon (Figure S2, Table 1), and this was probably the case for the other important counter‐regulatory hormones such as catecholamines (not measured in the present study). Thus, in the IV study, it may be assumed that the BG changes represented quite specifically the PD effect of injected insulin. On the other hand, the interpretation of the results with SC dosing might be more complex. Here the BG‐lowering effect of the two insulins was not equivalent, hypoglycaemia occurred earlier and the BG nadir was lower with Gla‐100 versus Gla‐300 (Figure 2), possibly eliciting differential counter‐regulatory responses. Suppression of endogenous insulin secretion appeared to be similar with the two insulins (Figure S3, Table 1), whereas the response of glucagon tended to higher with Gla‐300, although the different baseline makes the comparison difficult. It is probable that the responses of catecholamines, 27 growth hormone 28 and cortisol, 29 not measured in the present study, were greater with the more rapid fall to a lower nadir with Gla‐100 versus Gla‐300. If so, this would contribute to higher post‐nadir BG recovery, in addition to earlier waning of the insulin effect, with Gla‐100 versus Gla‐300. If so, the calculation of BG_AUC12‐24h would be overestimated with Gla‐100 versus Gla‐300, and the overall 0‐24 BG‐lowering effect reduced with Gla‐300 versus Gla‐100.
A second, novel finding in this study is that, in contrast to IV dosing, with SC dosing there is no equivalence of M1 with Gla‐300 and Gla‐100, in line with the differential effects of BG lowering. With Gla‐300, M1 is 17% lower over 24 h post‐injection, 19% lower during first 12 h post‐injection versus Gla‐100 (Figure 2, Table 1). Assuming that the hypoglycaemia with Gla‐300 and Gla‐100 did not affect glargine metabolism, this finding is coherent with the reduced BG‐lowering effect of Gla‐300 versus Gla‐100 SC observed in the present study. These results with the first injection in dogs are also in line with those reported with the first injection in people with type 1 diabetes where INS_AUC0‐24h was 38% lower (Japanese population) and 45% lower (European population), and the GIR_AUC was reduced in the euglycaemic clamp 47% (0‐36 h) to 75% (0‐24 h) with Gla‐300 versus Gla‐100, although serum insulin, not M1 was not measured in that study. 25 With the premise of species differences, the present study confirms the flatter PK of Gla‐300 versus Gla‐100 and its more even distribution over the 24‐h period observed in humans. 1 , 2 It would be interesting to compare Gla‐300 with the intraperitoneal insulin infusion that has shown the benefits of lower glucose variability and risk reduction of hypoglycaemia. 30
The observed difference of PK of M1 with SC dosing in the present study may be the consequence of the slower absorption of the more versus less concentrated glargine. 1 A slower and more prolonged SC absorption of glargine not only flattens and extends the serum insulin concentration beyond the 24 h, but also opens the possibility that the prolonged residency time in SC tissue favours greater local degradation. This concept has recently been supported by the new observation that precipitates of glargine in the SC at pH 7.4, present as particles with diameters surprisingly greater and surface area proportionally lower in relation to increasing concentration of glargine injected (Sanofi, data on file). In addition, the kinetics of glargine studied in an experimental model of precipitation and dissolution in vitro indicate that the process of re‐dissolution takes a longer time with Gla‐300 versus Gla‐100. Finally, measurement of the absorption rate in vivo from the estimate of the depot size reduction in SC tissue with magnetic resonance imaging in rats, indicates a slower process with Gla‐300 versus Gla‐100 (Sanofi, data on file). Taken together, these in vitro and in vivo observations give strength to the hypothesis that the slower SC absorption of Gla‐300 is the driving factor not only for the slower increase of M1 in plasma after SC dosing of Gla‐300, and its longer steady state in serum, but at the same time for its lower bioavailability because of greater local degradation in the SC tissue following longer residency time.
In fact, the present study originally shows that Gla‐300 has a 32% greater MRT and a 50% lower bioavailability as compared with Gla‐100, which contribute to lower M1 concentrations and reduced BG‐lowering effect of Gla‐300 SC.
Notably, in the present study in dogs, the SC absorption of glargine, both 100 and 300 U/mL, as measured by plasma M1, appears faster and lasts for a shorter time as compared with PK in people with type 1 diabetes given similar glargine doses as the first injection. 25 , 31 , 32 It is possible that the difference is explained by species differences in SC tissue structure and composition. In dogs, the SC tissue is particularly rich in vascularization, 33 although head‐to‐head comparisons with SC tissue in humans are not available. A relatively faster SC absorption of long‐acting insulin PZI, which precipitates the SC like glargine, has been reported in dogs. 34
A new, unprecedented observation is the elevated percentage of M0 after IV dosing of glargine (Gla‐300 and Gla‐100) as compared with total plasma insulin (M0 + M1) (Figure 1). With Gla‐300, the M0 fraction was nearly twice that of Gla‐100 (Table 1). In contrast, after SC injection M0 was undetectable with Gla‐300, whereas it was detectable only in two of the six dogs for the initial 3 h post‐dosing with Gla‐100. These findings are in line with those observed in humans following SC of therapeutic doses of glargine in humans, both Gla‐300 and Gla‐100, with M0 representing less than 5%‐10% of circulating insulin. 14 , 15 , 16 , 17 , 18
However, it should be noted that in the present IV studies the insulin dose was large (0.15 U/kg) and resulted into pharmacological serum M1 concentrations (>300 μU/mL, more than 10 times higher than therapeutic concentrations). This possibly saturated the proteolytic enzymes that rapidly convert M0 to M1. Similarly, the elevated M1 concentrations in the IV experiments probably favoured conversion of M1 to M2 to a larger extent than usually observed with SC therapeutic doses in humans. 14 , 15 , 16 , 17 , 18
Limitations of the present study have been mentioned and include investigation in normal, non‐diabetic dogs with endogenous insulin secretion, the model of the first injection rather than steady state and the low number of dogs studied. Because of the limited blood volume allowed to be drawn in each dog, the study was unpaired. The strengths are the IV dosing experiment (the only experiment so far with Gla‐300 IV); measurement of M1, which is not confounded by endogenous insulin secretion; and 24 h observation of hypoglycaemia after SC dosing of the two glargine formulations, mimicking the clinical hypoglycaemia in humans because of basal insulin administration.
In conclusion, the present study in dogs demonstrates equivalent BG‐lowering effects and PK of M1 with Gla‐300 versus Gla‐100 given IV, and, in contrast, greater MRT in SC tissue with lower bioavailability of M1 with Gla‐300 versus Gla‐100 after SC dosing, with reduced BG‐lowering effects of the former versus the latter. These results indicate that the reduced BG‐lowering effects with SC doses of Gla‐300 versus Gla‐100 reported in humans 1 , 2 are explained not by the different potency of the two glargine formulations, but by greater MRT and lower bioavailability with of Gla‐300 versus Gla‐100 SC dosing. In turn, this explains the clinical need in people with type 1 diabetes of 10%‐30% greater doses of Gla‐300 SC to match the glucose‐lowering efficacy versus Gla‐100 2 , 4 , 5 , 7 , 8 , 9 , 10 , 11 and versus other basal insulins such as degludec, which do not precipitate. 35
CONFLICT OF INTEREST
U.W. is an employee and stock holder of Sanofi; N.T is an employee and stock holder of Sanofi; C.G.F. has received honoraria for lecturing and consultations from Sanofi, Lilly and travel grants from Menarini; G.B.B. has received honoraria for lectures, consultation and research grant from Sanofi, Menarini and Lilly.
AUTHOR CONTRIBUTIONS
U.W. designed and conducted the experiments, and performed the statistical analyses. N.T. designed and conducted the experiments. C.G.F. performed the statistical analyses. All authors contributed to writing and reviewing drafts of the manuscript and approved the final draft for submission.
Supporting information
Figure S1. BG_AUC and M1_AUC for individual dogs after administration of Gla‐300 and Gla‐100. A, IV, 0.15 U/kg, and B, SC, 0.3 U/kg
Figure S2. Concentrations of A, plasma glucagon and B, plasma C‐peptide after 0.15 U/kg IV Gla‐300 and Gla‐100 injection. Black arrows indicate the time of dosing
Figure S3. Concentrations of A, Plasma glucagon and B, plasma C‐peptide after 0.30 U/kg SC Gla‐300 and Gla‐100 injection. Black arrows indicate the time of dosing
ACKNOWLEDGMENTS
This study was conducted by Sanofi (Frankfurt, Germany). No funding/financial support was provided for the development of this manuscript.
Werner U, Tennagels N, Fanelli CG, Bolli GB. Equipotency of insulin glargine 300 and 100 U/mL with intravenous dosing but differential bioavailability with subcutaneous dosing in dogs. Diabetes Obes Metab. 2021;23:166–174. 10.1111/dom.14212
Results from preliminary analyses of the data presented in this manuscript have been presented in abstract and poster form at the 9th International Conference on Advanced Technologies & Treatments for Diabetes, 3‐6 February 2016, Milan, Italy.
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request. To gain access, data requestors will need to sign a data access agreement.
REFERENCES
- 1. Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units·mL‐1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units·mL‐1. Diabetes Care. 2015;38:637‐643. [DOI] [PubMed] [Google Scholar]
- 2. Porcellati F, Lucidi P, Candeloro P, et al. Pharmacokinetics, pharmacodynamics, and modulation of hepatic glucose production with insulin glargine U300 and glargine U100 at steady state with individualized clinical doses in type 1 diabetes. Diabetes Care. 2019;42:85‐92. [DOI] [PubMed] [Google Scholar]
- 3. Lucidi P, Porcellati F, Cioli P, et al. Greater suppression of glucagon, lipolysis, and ketogenesis with insulin glargine U300 as compared with glargine U100 in type 1 diabetes mellitus. Diabetes Technol Ther. 2020;22:57‐61. [DOI] [PubMed] [Google Scholar]
- 4. Ritzel R, Roussel R, Bolli GB, et al. Patient‐level meta‐analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terauchi Y, Koyama M, Cheng X, et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 2). Diabetes Obes Metab. 2016;18:366‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danne T, Matsuhisa M, Sussebach C, et al. Lower risk for severe hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL in participants with type 1 diabetes: a meta‐analysis of 6‐month phase 3 clinical trials. Diabetes Obes Metab. 2020;22:1880‐1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsuhisa M, Koyama M, Cheng X, et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 1). Diabetes Obes Metab. 2016;18:375‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergenstal RM, Bailey TS, Rodbard D, et al. Comparison of insulin glargine 300 Units/mL and 100 Units/mL in adults with Type 1 diabetes: continuous glucose monitoring profiles and variability using morning or evening injections. Diabetes Care. 2017;40:554‐560. [DOI] [PubMed] [Google Scholar]
- 9. Pettus J, Gill J, Paranjape S, et al. Efficacy and safety of a morning injection of insulin glargine 300 units/mL versus insulin glargine 100 units/mL in adult patients with type 1 diabetes: a multicentre, randomized controlled trial using continuous glucose monitoring. Diabetes Obes Metab. 2019;21:1906‐1913. [DOI] [PubMed] [Google Scholar]
- 10. Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 Units/mL versus glargine 100 Units/mL in people with type 1 diabetes: a randomized, phase 3a, open‐label clinical trial (EDITION 4). Diabetes Care. 2015;38:2217‐2225. [DOI] [PubMed] [Google Scholar]
- 11. Danne T, Tamborlane WV, Malievsky OA, et al. Efficacy and safety of insulin glargine 300 units/mL (Gla‐300) versus insulin glargine 100 units/mL (Gla‐100) in children and adolescents (6‐17 years) with type 1 diabetes: results of the EDITION JUNIOR randomized controlled trial. Diabetes Care. 2020;43:1512‐1519. 10.2337/dc19-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heise T, Nørskov M, Nosek L, Kaplan K, Famulla S, Haahr HL. Insulin degludec: lower day‐to‐day and within‐day variability in pharmacodynamic response compared with insulin glargine 300 U/mL in type 1 diabetes. Diabetes Obes Metab. 2017;19:1032‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bailey TS, Pettus J, Roussel R, et al. Morning administration of 0.4U/kg/day insulin glargine 300U/mL provides less fluctuating 24‐hour pharmacodynamics and more even pharmacokinetic profiles compared with insulin degludec 100U/mL in type 1 diabetes. Diabetes Metab. 2018;44:15‐21. [DOI] [PubMed] [Google Scholar]
- 14. Bolli GB, Hahn AD, Schmidt R, et al. Plasma exposure to insulin glargine and its metabolites M1 and M2 after subcutaneous injection of therapeutic and supratherapeutic doses of glargine in subjects with type 1 diabetes. Diabetes Care. 2012;35:2626‐2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinstraesser A, Schmidt R, Bergmann K, Dahmen R, Becker RHA. Investigational new insulin glargine 300 U/ml has the same metabolism as insulin glargine 100 U/ml. Diabetes Obes Metab. 2014;16:873‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lucidi P, Porcellati F, Rossetti P, et al. Metabolism of insulin glargine after repeated daily subcutaneous injections in subjects with Type 2 diabetes mellitus. Diabetes Care. 2012;35:2647‐2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lucidi P, Porcellati F, Candeloro P, et al. Glargine metabolism over 24 hours following its subcutaneous injection in subjects with type 2 diabetes mellitus: a dose‐response study. Nutr Metab Cardiovasc Dis. 2014;24:709‐716. [DOI] [PubMed] [Google Scholar]
- 18. Lucidi P, Porcellati F, Yki‐Järvinen H, et al. Low levels of unmodified insulin glargine in plasma of people with type 2 diabetes mellitus requiring high doses of basal insulin. Diabetes Care. 2015;38:e96‐e97. [DOI] [PubMed] [Google Scholar]
- 19. Mudaliar S, Mohideen P, Deutsch R, et al. Intravenous glargine and regular insulin have similar effects on endogenous glucose output and peripheral activation/deactivation kinetic profiles. Diabetes Care. 2002;25:1597‐1602. [DOI] [PubMed] [Google Scholar]
- 20. Scholtz HE, Pretorius SG, Wessels DH, Venter C, Potgieter MA, Becker RH. Equipotency of insulin glargine and regular human insulin on glucose disposal in healthy subjects following intravenous infusion. Acta Diabetol. 2003;40:156‐162. [DOI] [PubMed] [Google Scholar]
- 21. Gabrielsson J, Weiner D. Non‐compartmental analysis. Methods Mol Biol. 2012;929:377‐389. [DOI] [PubMed] [Google Scholar]
- 22. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐investigation‐bioequivalence‐rev1_en.pdf. Accessed April 4, 2020.
- 23. NCSS 2020 . Statistical Software. Kaysville, UT: NCSS, LLC; 2020. ncss.com/software/ncss. Accessed March 11, 2020. [Google Scholar]
- 24. Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add‐in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft excel. Comput Methods Programs Biomed. 2010;99:306‐314. [DOI] [PubMed] [Google Scholar]
- 25. Shiramoto M, Eto T, Irie S, et al. Single‐dose new insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab. 2015;17:254‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Porcellati F, Lucidi P, Bolli GB, Fanelli CG. How to accurately establish pharmacokinetics/dynamics of long‐acting insulins in humans: relevance to biosimilar insulins. Diabetes Care. 2015;38:2237‐2240. [DOI] [PubMed] [Google Scholar]
- 27. De Feo P, Perriello G, Torlone E, et al. Contribution of adrenergic mechanisms to glucose counterregulation in humans. Am J Physiol. 1991;261:E725‐E736. [DOI] [PubMed] [Google Scholar]
- 28. De Feo P, Perriello G, Torlone E, et al. Contribution of cortisol to glucose counterregulation in man. Am J Physiol. 1989;257:E35‐E42. [DOI] [PubMed] [Google Scholar]
- 29. De Feo P, Perriello G, Torlone E, et al. Demonstration of a role for growth hormone in glucose counterregulation. Am J Physiol. 1989;256:E835‐E843. [DOI] [PubMed] [Google Scholar]
- 30. Van Dijk PR, Logtenberg SJJ, Gans ROB, Bilo HJG, Kleefstra N. Intraperitoneal insulin infusion: treatment option for type 1 diabetes resulting in beneficial endocrine effects beyond glycaemia. Clin Endocrinol (Oxf). 2014;81:488‐497. [DOI] [PubMed] [Google Scholar]
- 31. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long‐acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142‐2148. [DOI] [PubMed] [Google Scholar]
- 32. Porcellati F, Rossetti P, Ricci NB, et al. Pharmacokinetics and pharmacodynamics of the long‐acting insulin analog glargine after 1 week of use compared with its first administration in subjects with type 1 diabetes. Diabetes Care. 2007;30:1261‐1263. [DOI] [PubMed] [Google Scholar]
- 33. Budras K‐D. Skin Anatomy of the Dog. In Budras K‐D, McCarthy PH, Fricke W, Richter R, eds. Hannover, Germany: Schueltersche, Verlag Gesellschaft mbH & Co. KG; 2007:4‐5. [Google Scholar]
- 34. Clark N, Thomaseth K, Heist M, Hoenig M. Pharmacokinetics and pharmacodynamics of protamine zinc recombinant human insulin in healthy dogs. J Vet Pharmacol Ther. 2912;35:342‐350. [DOI] [PubMed] [Google Scholar]
- 35. Lucidi P, Porcellati F, Candeloro P, Cioli P, Pascucci C, Bolli GB, Fanelli CG. A PK/PD and metabolic head‐to‐head comparison of clinical doses of insulin glargine U300 and degludec in type 1 diabetes. 79th Scientific Sessions of the American Diabetes Association, San Francisco, 2019, Poster #1088.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. BG_AUC and M1_AUC for individual dogs after administration of Gla‐300 and Gla‐100. A, IV, 0.15 U/kg, and B, SC, 0.3 U/kg
Figure S2. Concentrations of A, plasma glucagon and B, plasma C‐peptide after 0.15 U/kg IV Gla‐300 and Gla‐100 injection. Black arrows indicate the time of dosing
Figure S3. Concentrations of A, Plasma glucagon and B, plasma C‐peptide after 0.30 U/kg SC Gla‐300 and Gla‐100 injection. Black arrows indicate the time of dosing
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request. To gain access, data requestors will need to sign a data access agreement.


