Abstract
Aim
This study aims to evaluate the intracorporeal pressures immediately after the insertion of the catheters for urodynamic testing with a water‐filled urodynamic pressure transducer system to determine the relevance of the International Continence Society (ICS) zeroing principles.
Methods
Here, a retrospective analysis of a random series of urodynamic recordings is performed. The initial pressures, immediately after the insertion of the catheters, have been compared with the pressures after some milliliters of filling and flushing away of the gel, used with insertion, and/or the mucus and debris from the inserted catheters. Differences of initially recorded intravesical and intrarectal pressures from those after flushing and filling are analyzed and associated with the ICS standard practice of zeroing.
Results
Statistically and clinically significant differences between the initial pressures and the pressures after filling and flushing are observed, with nonphysiological initial pressures in 62% of the studies. Some filling (20 ml or more in the bladder) and flushing of the pressure channels resulted in the registration of physiological pressures and synchronous response from both lines on abdominal pressure increases.
Conclusions
The pressure signal quality of a water‐filled urodynamic system immediately after catheter insertion is low with inaccurately displayed pressure values, but it changes to normal after flushing the pressure channels and some filling. Rezeroing of the intracorporeal pressures immediately after catheter insertion for cystometry is the inappropriate correction procedure that misleadingly modifies the false initial pressures, resulting in ongoing unrealistic urodynamic study pressures.
Keywords: clinical practice standard, diagnostic techniques, healthcare quality, lower urinary tract function, lower urinary tract physiology, urodynamic testing
1. INTRODUCTION
Urodynamic testing is the gold standard for objective diagnosis of the lower urinary tract function. 1 , 2 Reliable recordings of intravesical (p ves) and intrarectal (abdominal) pressure (p abd) during cystometry are essential. Both pressures are relevant in themselves, but they are also pertinent to ascertain a reliable subtracted detrusor pressure (p det). Intravesical pressure is measured directly, usually via a transurethral catheter, but the measurement of intra‐abdominal pressure is somewhat indirect through the insertion of a catheter in the rectum or alternatively in the vagina. The rectal activity is commonly visible and affects the subtracted detrusor pressure pattern. Increases of p ves during the filling phase, visible in the subtracted p det, are interpreted as a sign ofphasicdetrusor muscle contraction activity or of an insufficient adaptation to intravesical volume increase, depending on the pattern. Reliable and realistic pressures are relevant for the grading of bladder outflow obstruction and detrusor contraction as well as for leak point pressure assessment.
The International Continence Society (ICS) recommends measuring p ves and p abd with respect to the atmospheric pressure around the patient at the level of the symphysis pubis. This is done by setting the transducer to zero before the start of cystometry with the pressure lines opened to the environment “zeroing.” 3 The urodynamic machines available to date are equipped with a set‐zero button for this purpose. An educational article has accurately described the urodynamic procedure and has stated: [cited] It is important to allow the bladder to fill with a small volume of fluid before completing this initial assessment. The initial assessment was defined in that manuscript as checking for a balanced cough response. 4
Despite the ICS good urodynamic practice (GUP) standardization and recommendations, the system has intrinsic sources of error and inaccuracies. 5 It is, however, also noteworthy that when graphs are published in scientific literature or on the Internet, the urodynamic pressures are often not displayed relative to atmospheric pressure. 6 , 7 Also a poststudy quality assessment of 123 urodynamic graphs submitted for prospective multicenter studies has shown that in 30% of cases, the pressures were, despite the ICS‐GUP standard, zeroed in the body cavities. Furthermore, the pressures have been outside the expected range in 30% of the studies. 8
Zeroing is discussed in the earliest ICS‐GUP document, 3 which explains: [Citations] Many important aspects of quality and plausibility control, such as typical resting value ranges at different patient position, are based on the proper recording of pressures, and will not apply if pressures are not recorded according to ICS standards. Also, it is only meaningful to subtract one pressure from the other, for example (p ves − p abd = p det ), when both are recorded to the same reference level. The standard stated that: …It is often argued that it does not make a difference for the most relevant parameter, p det, if the same error is introduced to p ves and p abd, as they tend to cancel each other out. This is not an acceptable argument. [End of citations.]. However, it has not been clarified why the argument is not acceptable; also, it is not explained in this document how likely it is that the same error is introduced to p ves and p abd. Indeed, p det is a relative pressure (p ves relative to p abd) and the diagnoses of detrusor overactivity or reduced compliance are based more on cystometry patterns than on quantities. The barometric or gauge pressures (pressures relative to atmosphere) are clinically relevant, as mentioned above, for leak point pressures and for the grading of bladder outflow obstruction and detrusor contraction, in addition to the technical quality and plausibility monitoring of each of the pressure channels.
The pressure that rests inside the abdomen on a patient's pelvic floor and on the bladder and the rectum (in cmH2O) while seated is loosely related to the height of the intra‐abdominal mass, which actually corresponds to the distance from the diaphragm to the pelvic floor. As the relative weight of the human body is approximately equal to water, the typical resting value range of p ves and p abd in seated adult persons is expected to be 30–40 cmH2O. Although the abdominal mass is not homogenous and also not exactly liquid water, this theoretical explanation corresponds with clinical observations. 3 , 9 , 10 , 11 In supine patients, the pressures in the pelvis are, thus, expected to be lower, and they are expected to be somewhat higher in the standing position, due to the additional abdominal muscle contraction. 9
This retrospective study analyzing the standard ICS practice answers the following research question: Are both pressure recordings (p ves and p abd) of an external water‐filled pressure transducer system within limits of physiological plausibility and adequately responsive immediately after the insertion of the cystometry catheters?
2. MATERIALS AND METHODS
We took a retrospective, random, sample of 136 (out of the 516 = 26%) adult (normal size) patient cystometries for all indications performed at our department in one calendar year. International Continence Society Good Urodynamic Practice (ICS‐GUP)‐transurethral cystometry in a sitting position with 25–50ml/min room temperature saline and with tubes to external pressure domes and transducers (Ellipse, modular urodynamics system, Andromeda, Medizinische Systeme GmbH; Figure 1) was done in all patients included in this cohort. 12 All patients received an information leaflet about the study that also contained our recommendation to come for the test with an empty rectum, if possible. After catheter‐free uroflowmetry and subsequent complete emptying of the remaining urine with a 14Ch (hydrophilic coating) disposable catheter, we performed cystometry catheterization. Some amount of intraurethral gel (2–10 ml) was used to introduce the 8Ch double‐lumen PVC cystometry (p ves) catheter. An open, 12Ch feeding tube with side holes, also with some gel, was introduced (10–15 cm) in the rectum to measure intrarectal p abd. Both catheters were attached as close as possible to the body orifices with an adhesive plaster strip. Three‐way stopcocks between the horizontally mounted pressure domes and the tubes were used for connection and reference to the atmospheric pressure. To remove all air before insertion of the catheters, the connecting tubes, the domes, and the catheters were filled before the testing from an infusion bottle with a fluid level approximately 80 cm above the pressure domes (see Figure 1). Pressure domes were adjusted to the level of the symphysis pubis of every patient. We start and display the digital recording of the urodynamic test before the filling pump is switched on, thus we include the initial zero to atmosphere in the urodynamic graph, in every patient. We also flushed both pressure lines with water at the beginning of the cystometry in every patient, as previously advised 3 , 4 , 7 and recently again 8 (see Figures 2 and 3).
Figure 1.
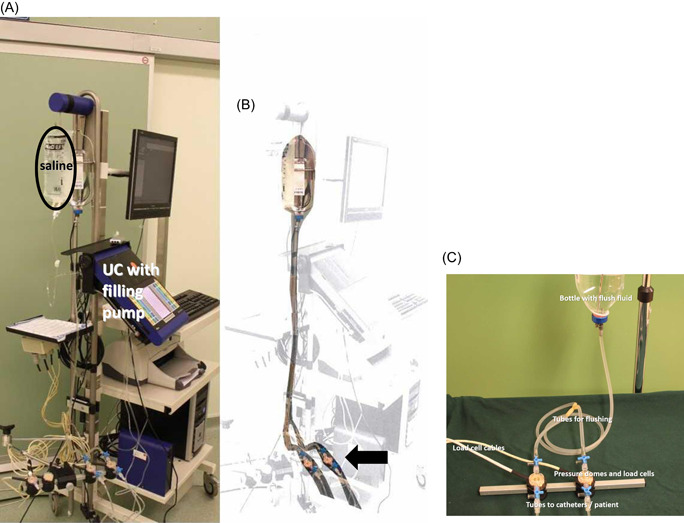
The flushing system: (A) a copy of the photo with the flush system highlighted. (B) A bottle with a liquid level about 80 cm above the pressure domes connected to the tubes split just before the pressure domes (arrow). Visible (original photograph—B): The bag (saline) for filling the bladder, with a separate tube to pump (integrated in the console of the urodynamics system: UC). (C) The flush system in more detail, disconnected from the urodynamics device
Figure 2.
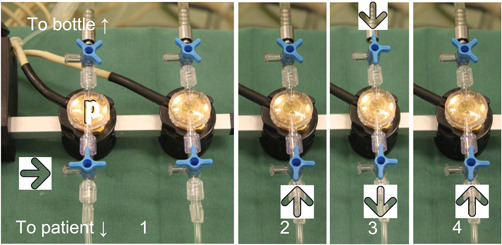
The start‐up procedure (note that filling tube is not shown). Compare with Figure 3 to see the effect of the different steps in the urodynamic graphs.
Step 1: Pressure domes (both shown) are connected to atmospheric pressure (large arrow) and closed to the bottle while the system is zeroed.
Step 2: Pressures (only one shown) are connected to the patient (arrow = pressure from patient to dome) before flushing = Init‐P.
Step 3: Flushing the pressure line, with fluid from the bottle (opened stopcock at the top) via dome to the catheter inside the patient (bottom). Simultaneously, the filling pump is switched on.
Step 4: The pressure dome is only connected to the body cavity pressure again = Flush‐P. Flush‐P, flushed pressure (step 4); Init‐P, initial pressure (step 2)
Figure 3.
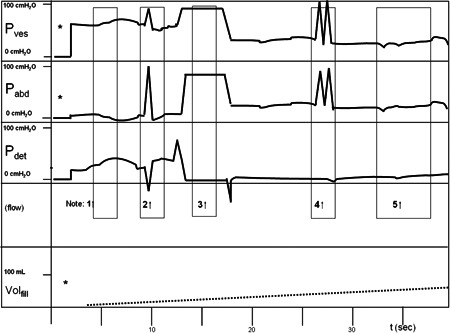
A cartoon example of the observations in the first 40 s of UDI.
*Pressure lines zeroed to atmospheric pressure after catheter insertion. (Step 1; Figure 2)
Notes:
-
1.Immediately after insertion of the catheters and zeroing*, with the patient placed in the sitting position, p ves appears to be outside the physiological range and p abd is relatively low. Consequently, p det is relatively far above zero. This is Init‐P. (Table 1).
-
2.When the patient coughs, the response is unbalanced, probably adequate in p abd but relatively low in p ves. Consequently, p det shows a negative peak (Step 2; Figure 2).
-
3.p ves and p abd are flushed from the infusion bottle approximately 80 cm above the pressure domes (Step 3; Figure 2). The subtracted pressure of both pressures (from the same infusion bottle) is therefore zero. The filling pump is switched on.
-
4.(Step 4; Figure 2). Coughing is balanced now in both pressure channels.
-
5.After flushing, both p ves and p abd are in the physiological range and both responsive (responding to patient movements). This is Flush‐P, which was established in this cartoon case at 20 s and 15 ml of infusion (Volfill). Flush‐P, flushed pressure; Init‐P, initial pressure
The recorded p ves and p abd pressures immediately after ICS‐GUP zeroing to atmosphere, before filling and flushing, were retrospectively determined and compared with the pressures after catheter flushing and filling (see Figures 2 and 3) We flushed both catheters and filled the bladder until both pressures were in the physiological range 8 with a balanced response on coughing. Less than 10 cmH2O peak difference in p det while coughing and synchronous responses to patient breathing, talking, and movements are labeled as a balanced response.
Pressures after insertion but before flushing were reported as initial pressure (Init‐P) and pressures after flushing and filling were reported as flushed pressure (Flush‐P); differences were calculated. The intravesical volume and time span until physiological and similarly responsive pressures were determined. We started our analysis with a sample size of 100 patients and found that the statistical results did not change when we included another 36 patients, confirming that the sample size was adequate.
This retrospective and anonymized patient chart review of standard practice is exempted from formal ethical approval under current law in our country.
3. RESULTS
The mean age of patients was 55 years (range: 19–9; SD = 17 years); 65% were male. No patient had fecal urge or rectal filling, and intravaginal p abd was not used in any patient.
Table 1 shows the initial (before flushing and filling) pressures (Init‐p ves, Init‐p abd, and Init‐p det); after flushing and some filling Flush‐p ves, Flush‐p abd, and Flush‐p det, and also the differences (Init‐P − Flush‐P); all values are expressed in cmH2O. It can be noted that statistically significant differences were recorded in both pressure lines, comparing the values before and after flushing.
Table 1.
Average pressures in cmH2O, recorded immediately after insertion (Init‐P) and after flushing and some filling (Flush‐P)
| N = 136 | Init‐P mean; SD (range) | Flush‐P mean; SD (range) | Difference (Init‐P − Flush‐P) mean; SD (range) | t‐Test |
|---|---|---|---|---|
| p ves | 27.4; 12.7 (−4 to 60) | 37.3; 5.9 (22–51) | −9.9; 12.4 (−13 to 41) | 0.000 |
| p abd | 34.7; 8.7 (0–54) | 36.8; 6.5 (23–55) | 2.2; 6.1 (−15 to 25) | 0.000 |
| p det | −7.2; 13.8 (−41 to 24) | 0.5; 2.8 (−7 to 12) | −7.7; 13.4 (−41 to 18) | 0.000 |
Abbreviations: Flush‐P, flushed pressure; Init‐P, initial pressure.
In 11 patients (8%), we did not flush. To obtain reliable pressures in both channels, one flush was needed in 99 (73%) patients, two flushes in 23 (17%), and three in 3 (2%) of patients. No patient had detrusor activity at the beginning of the study before the intravesical pressure was in the physiological range, and six had rectal contractions.
The mean volume infused and flushed until reliable pressures was 16 ml (range: 1–108 ml) and the corresponding time was, on average, 30 s (range: 1–120 s). The results within the ranges were presented in a left‐skewed distribution (compared with Gaussian distribution), with modal values of ≈10 ml and ≈20 s for volume and time, respectively. The mean cystometric end fill capacity of this cohort was 437 ml (SD = 66 ml), without a correlation between time or volume to physiological pressures and cystometric capacity.
In 67% of cases, the p ves difference before and after flushing was more than plus or minus 5 cmH2O; p abd showed this difference in 29% of cases. In 62% of patients, imbalanced and/or no physiological initial resting p det (difference ≥ 5 cmH2O above or below zero) was observed at the onset of the cystometry (before flushing).
4. DISCUSSION
This analysis shows that the intracorporeal pressures that are recorded with an external water‐filled pressure transducer system, after standard ICS‐GUP zeroing to atmospheric pressure and before the start of filling, have been unrealistic in more than 60% of the patients. The obtained intracorporeal pressure, immediately after catheter insertion, is unphysiological without flushing of debris, mucus, gel, and remaining air bubbles from the catheter. Even with prefilling before insertion, some air bubbles will inevitably be present at the tip of the catheters due to the manipulations before or during the insertion. Only a few drops of water are usually necessary to replace these when the catheter is located inside the patient. The rest of the water that is required to obtain reliable pressures, as observed in this study, is necessary to flush away the gel and the debris from the catheter holes. In addition, however, a small (8Ch) double‐lumen intravesical catheter in the emptied bladder is likely to be kinked, and the pressure channel side hole of such a catheter has a good chance of being blocked, at least in part, against the bladder wall and the pleated inner surface. Kinks are less likely to play a role in the rectum if the catheter is inserted gently, but flushing is also relevant here. Both the kinking and the blocked side holes, thus, may also cause a false registration of the pressure. In the usual set‐up of a cystometry, it is therefore very plausible that some water filling of the bladder is required to allow proper transmission of the pressures and ensure technically adequate recording. We believe flushing is the most important action to improve pressure registration. With this retrospective study, we have demonstrated the effect of our standard practice. We explained in the methods section that we have flushed the pressure channels and at the same time turned on the filling pump after inserting the catheters, immediately after zeroing the system to atmospheric pressure in all patients. We cannot prove or reject from our data that bladder filling has a significant separate effect on pressure stabilization. We show that it is important to have open pressure channels, free from the walls of the body cavities, and to have fluid around the catheters.
The strategy to start a cystometry, after insertion of the catheters, should simply be as follows: Start by zeroing the system to atmosphere pressure, then start filling and flushing (1 flush: 1‐2 sec.), continue filling and waiting, and flushing again, if necessary. Usually, the pressures corrects themselves without using any button on the urodynamics machine. If a zero button or a pressure‐equalizing button is pushed after catheters insertion, before or without flushing (see Figure 3), within the first 10 s, it will result in a p det around zero, but the study will probably continue with unphysiological and unbalanced pressures. In such a case (Figure 3, with blocked intravesical catheter), p abd will be overcorrected and p det will continue to show extremely low pressures, resulting in an underestimation of bladder outlet obstruction or amplitude of detrusor overactivity (when considered relevant). If we had pushed zero in all the patients who are included in this study, before the flushing and filling, we would have had extremely low p det values (adjusted or wrongly corrected from initial resting p det values of more than −10 cmH2O) in 34% (47/136) of patients and extremely high p det values (on the basis of a difference more than 10 cmH2O) in 9 (7%) of all patients.
In an external water‐filled pressure transducer system, some filling of the bladder is usually required to prevent underestimation of intravesical pressure due to the catheter kinking in the empty bladder. In addition, all lumens of the catheters should be flushed when inside patient to remove remaining air bubbles at the tip of the catheters and to remove insertion gel and debris from the catheter holes. Flushing alone is sufficient in a number of such cases, but our method did not allow examination of this. These results, in agreement with ICS‐GUP recommendation, suggest that intracorporeal zeroing should be discouraged, 13 because the pressures in the initial (empty bladder) phase of the cystometry are imperfectly recorded and inherently unreliable.
5. CONCLUSIONS
This study, using a large number of randomly selected urodynamic tests, showed that the initial cystometry pressures were not realistic in the majority of patients. The intravesical pressure was false in 67% of the patients and the intra‐abdominal pressure in almost 30% of the cases. The ICS standard water‐filled urodynamic catheter system is generally unable to record reliable pressures before some amount of water through the catheters has entered the body cavities and before flushing of gel and debris from both catheters after insertion. Zeroing of the intracorporeal pressures or equalizing the pressures immediately after catheter insertion for cystometry after initial zeroing is an erroneous correction procedure, which misleadingly alters the false Init‐Ps, resulting in ongoing unrealistic intravesical and intrarectal abdominal pressures during the urodynamic study. Consequently, extremely low or extremely high detrusor pressures will be obtained, with possible clinical consequences. This provides solid evidence for the validity of the ICS‐GUP recommendation for not zeroing the pressures to intracorporeal pressure directly after catheter insertion but zeroing to atmospheric pressure, followed by filling, flushing, and waiting. Practitioners should adhere to ICS‐GUP regarding the atmospheric pressure as the zero reference for cystometric testing, and the observations, reported in this study, must be included in future GUP teaching, standards, and protocols.
Rosier PFWM. Good urodynamic practice: Pressure signal quality immediately after catheter insertion for cystometry with a water‐filled pressure transducer system and its relevance for the ICS zero procedure. Neurourology and Urodynamics. 2021;40:319–325. 10.1002/nau.24561
REFERENCES
- 1. Rosier PF, Gajewski JB, Sand PK, et al. Executive summary: The International Consultation on Incontinence 2008—Committee on: “Dynamic Testing”; for urinary incontinence and for fecal incontinence. Part 1: Innovations in urodynamic techniques and urodynamic testing for signs and symptoms of urinary incontinence in female patients. Neurourol Urodyn. 2010;29(1):140–145. [DOI] [PubMed] [Google Scholar]
- 2. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub‐committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–178. [DOI] [PubMed] [Google Scholar]
- 3. Schäfer W, Abrams P, Liao L, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure‐flow studies. Neurourol Urodyn. 2002;21(3):261–274. [DOI] [PubMed] [Google Scholar]
- 4. Raz O, Tse V, Chan L. Urodynamic testing: physiological background, setting‐up, calibration and artefacts. BJU Int. 2014;114(Suppl 1):22–28. 10.1111/bju.12633 [DOI] [PubMed] [Google Scholar]
- 5. Gammie A. The accuracy of static pressure measurement with water‐filled urodynamic systems. Neurourol Urodyn. 2018;37(2):626–633. 10.1002/nau.23358 [DOI] [PubMed] [Google Scholar]
- 6. Rosier PFWM, Bosch JLHR. A picture can tell… The quality of urodynamics as shown in scientific papers. Neurourol Urodyn. 2010;29(6):869–870. [Google Scholar]
- 7. Rosier PFWM. Comparison of the technical quality of urodynamic graphs acquired via Google search engine on the internet with graphs acquired via PubMed [ICS abstract 190 Presentation ICI annual Meeting Philadelphia]. Neurourol Urodyn. 2018;37(S5):127 10.1002/nau.23760 [DOI] [Google Scholar]
- 8. Aiello M, Jelski J, Lewis A, et al. Quality control of uroflowmetry and urodynamic data from two large multicenter studies of male lower urinary tract symptoms. Neurourol Urodyn. 2020;39(4):1170–1177. 10.1002/nau.24337 [DOI] [PubMed] [Google Scholar]
- 9. Sriram R, Ojha H, Farrar DJ. An audit of urodynamic standardization in the West Midlands, UK. BJU Int. 2002;90(6):537–539. [DOI] [PubMed] [Google Scholar]
- 10. Sullivan J, Lewis P, Howell S, Williams T, Shepherd AM, Abrams P. Quality control in urodynamics: a review of urodynamic traces from one centre. BJU Int. 2003;91(3):201–207. [DOI] [PubMed] [Google Scholar]
- 11. Gammie A, Drake M, Swithinbank L, Abrams P. Absolute versus relative pressure. Neurourol Urodyn. 2009;28(5):468 10.1002/nau.20716 [DOI] [PubMed] [Google Scholar]
- 12. Rosier PFWM, Schaefer W, Lose G, et al. International Continence Society Good Urodynamic Practices and Terms 2016: urodynamics, uroflowmetry, cystometry, and pressure-flow study. Neurourol Urodyn. 2017;36(5):1243–1260. 10.1002/nau.23124 [DOI] [PubMed] [Google Scholar]
- 13. Sullivan JG, Swithinbank L, Abrams P. Defining achievable standards in urodynamics‐ a prospective study of initial resting pressures. Neurourol Urodyn. 2012;31(4):535–540. [DOI] [PubMed] [Google Scholar]


