Abstract
Objective
In the present study, we describe the features and functional properties of a new powder cosmetic ingredient, an amorphous mesoporous magnesium carbonate (MMC, also named Upsalite®) with regard to physical characteristics as well as functional attributes.
Methods
Physical and functional characterization of MMC, as compared to other common powder cosmetic ingredients (silica, mica, kaolin, talc and starch), was assessed using nitrogen gas adsorption, powder X‐ray diffraction, particle size distribution by laser diffraction, scanning electron microscopy (SEM), and oil and moisture uptake tests. The powder ingredients were also applied on human skin and analysed for short‐ and long‐term mattifying effect, and a new method was developed to measure flashback effect. MMC was tested for skin irritation using an in vitro cell model as well as in vivo, through the Human Repeated Insult Patch Test on 50 human volunteers.
Results
Mesoporous magnesium carbonate has a high surface area and pore volume. It has an excellent absorption capacity and can take up both oil and water simultaneously. It provides instant and long‐lasting mattifying effect when applied on human skin without drying or irritating skin and exhibits no measured flashback effect.
Conclusion
Mesoporous magnesium carbonate has good sensory and visual characteristics as well as excellent absorbing and mattifying properties, suggesting that it has great potential to replace other powder ingredients currently used as fillers and absorbers in powder cosmetics.
Color, feel, application and special visual effects along with long wear are the main properties behind all types of powders. MMC has good sensory and visual characteristics as well as excellent absorbing and mattifying properties, suggesting that it has great potential to replace or compliment other powder ingredients currently used as fillers and absorbers in powder cosmetics.
Résumé
Objectif
Dans cette étude, nous décrivons les particularités et les propriétés fonctionnelles d'un nouvel ingrédient pour les poudres cosmétiques, le carbonate de magnésium mésoporeux amorphe (MMC, également appelé Upsalite®), en ce qui concerne ses caractéristiques physiques ainsi que ses attributs fonctionnels.
Méthodes
La caractérisation physique et fonctionnelle du MMC, par rapport aux autres ingrédients courants dans les poudres cosmétiques (silice, mica, kaolin, talc, amidon), a été effectuée en employant l’adsorption d’azote gazeux, la diffraction des rayons X sur poudre, la distribution granulométrique par diffraction laser, la microscopie électronique à balayage (MEB) et des tests d'absorption d'huile et d'humidité. Les ingrédients pour la poudre ont aussi été appliqués sur la peau humaine et analysés quant à l’effet matifiant à court et à long terme, et une méthode nouvelle a été développée pour mesurer la réflexion en photographie au flash, l’effet « flashback ». Le MMC a été testé pour l'irritation cutanée par l'utilisation d'un modèle cellulaire in vitro ainsi qu’in vivo, par le test Human Repeated Insult Patch sur 50 volontaires humains.
Résultats
Le carbonate de magnésium mésoporeux a une surface et un volume de pores élevés. Il a une excellente capacité d'absorption et peut absorber l'huile et l'eau simultanément. Il fournit un effet matifiant instantané et durable lorsqu'on l'applique sur la peau humaine, sans assécher ou irriter la peau, et n'a présenté aucun effet flashback dans nos mesures.
Conclusion
Le carbonate de magnésium mésoporeux a de bonnes caractéristiques sensorielles et visuelles ainsi que d’excellentes propriétés absorbantes et matifiantes, ce qui suggère un grand potentiel pour remplacer d’autres ingrédients qui sont actuellement utilisés comme substances de remplissage et matériaux absorbants dans les poudres cosmétiques.
Introduction
Powder ingredients are widely used in cosmetics as main constituents in loose (flow) and compact (pressed) powder make‐up [1, 2], where they may provide adhesiveness, smoothness, absorbency, coverage, or different sensory or visual attributes to skin and hair [3]. Powder ingredients are also commonly used in liquid formulations in which they can improve the look, feel, cohesion, viscosity or texture of the formulations. The properties of powder make‐up ingredients are analysed and compared in different ways but colour, texture, covering power, perceived skin feel, absorption properties and/or special visual effects along with ease of application and long wear are commonly assessed and reported [3, 4]. In recent years, consumers have also started to look for powders with low flashback effects since some light‐reflecting powder ingredients, mainly silica, can show up as white casts in photographs taken with a strong flash.
The primary consideration in the development of a cosmetic powder is the selection of raw materials. However, the basic formula for loose and pressed powders are often based on the same standard ingredient composition, including fillers, colours, preservatives and binding agents, and there have been few new additions to the list in the last century. Various forms of talc, silica/silicates, magnesium carbonate, mica, modified starches, and kaolin clays are still the most common fillers and absorbing materials. The composition of ingredients, along with their particle size and physical properties (e.g. adhesive or absorbing character), has an impact on the technical quality of the final powder formulation [5].
An amorphous and mesoporous form of magnesium carbonate (MMC, also named Upsalite) was first described in 2013 [6, 7]. MMC has a porous structure with a narrow pore size distribution, centred between 3 and 6 nm. It has an extraordinarily high surface area (up to 700 m2/g) as compared to other alkaline earth metal carbonates and can easily be milled to a fine powder suitable for topical application while maintaining its properties as a porous mineral. Moreover, its capacity to absorb both water‐ and oil‐soluble substances makes it a highly interesting material for cosmetic applications.
In the present study, we characterized finely milled MMC (Cosmetic Grade Upsalite, hereafter just named MMC) as a new powder cosmetic ingredient, and assessed it in parallel with other common powders, with regard to the above‐mentioned characteristics of powder make‐up; including oil and moisture absorbing properties, mattifying effect on skin, and flashback effect. MMC was also dermatologically tested on human subjects to assess skin irritation and sensitization.
We found that MMC provides semi‐translucent coverage and soft skin feel. It has excellent moisture and oil absorbing properties and can absorb moisture even after oil absorption and vice versa. It provides efficient immediate and long‐lasting mattifying effect on skin and exhibits no flashback effect.
Materials and methods
Materials
Mesoporous magnesium carbonate (Cosmetic grade Upsalite C101, Disruptive Materials, Uppsala, Sweden), Silica (fumed silica, TEX‐SILICA‐01, Making Cosmetics, Issaquah, WA, USA), Mica Sericite (GMS‐4C, KOBO Products Inc, South Plainfield, NJ, USA), Kaolin Clay (TKB Trading LLC, Oakland, CA, USA), Talc Ph.Eur (VWR Chemicals, Radnor, PE, USA), Corn Starch (Organic Makers, Malmö, Sweden), Olive oil (Organic Makers, Malmö, Sweden).
Methods
Nitrogen gas adsorption
Pore size, pore volume and BET (Brunauer–Emmett–Teller) specific surface area were determined using nitrogen gas adsorption in a liquid nitrogen bath at 77 K. Measurements were made with a TriStar II Plus, 3030 (Micromeritics, Norcross, GA, USA). Prior to analysis, samples were degassed overnight at 105°C under vacuum using a VacPrep061 (Micromeritics). Pore size distributions were obtained using non‐local density functional theory applied to the adsorption branch of nitrogen sorption isotherms using a Carbon Pore Slit model (ʎ = 0.2). The surface area was determined by applying the BET equation on at least five points in the relative pressure interval 0.05–0.3 [8]. The total pore volume was determined at a relative pressure of 0.97 at the adsorption branch of the isotherm.
Powder X‐ray diffraction (XRD)
Powder X‐ray diffraction (XRD) patterns were obtained on a Bruker D8 Advance diffractometer (Bruker, AXS GmbH, Germany) with Ni‐filtered Cu‐Kα radiation (λ = 1.54 Å), generating XRD patterns through elastic X‐ray scattering. Diffraction angles of 10–70° (2Ɵ) were analysed in steps of 0.02° with 0.2 s per step while rotating the sample. Prior to the analysis, the samples were dispersed with ethanol, mounted onto zero‐background silicon sample holders and dried under a lamp.
Particle size distribution
The particle size distributions were measured using laser diffraction with a Mastersizer 3000 (Malvern Panalytical Ltd, United Kingdom), utilizing an Aero S accessory. The measurement time was set to 10–30 s and the air pressure to 1.5 barg. During the measurement, the feed rate was constantly adjusted so that the obstruction limit was kept between 0.5 and 5%. All measurements were averaged and run at least five times. The light scattering data, converted to particle size distribution, were analysed using Mie‐scattering model, non‐spherical particle type and MgCO3 refractive index (1.717) and adsorption index (0.01).
Oil uptake test
The oil uptake method was adapted from the standard test method ASTM D281‐95 for oil absorption of pigments by spatula rub‐out. Oil uptake was measured by adding approximately 1 g of powder to a plastic weighing ship and then gradually adding olive oil by means of a pipette to the powder. The oil was thoroughly incorporated into the powder with a spatula. Addition of oil was continued until the paste was still hard with a matte finish. At this point, the oil was added drop by drop to the powder and the paste was triturated with the spatula. Addition of oil was stopped when a firm, smooth and glossy paste was obtained. The mass of added oil, when the paste still appeared non‐glossy, was noted. The oil uptake (g g−1) was calculated as the total mass of absorbed oil divided by the weight of the dry powder before oil uptake. The oil uptake test for each material was repeated at least five times.
A weighing ship with the dimension of 36 × 36 × 8 mm was filled with MMC or Silica powder (approximately 8 ml) and compacted with a spatula to make an even surface. 1 ml olive oil was carefully dropped on top of the powder and the time it took for the oil droplet to be fully absorbed into the powder bed was measured.
Moisture uptake
About 1 g powder was portioned into aluminium weighing dishes and dried at a temperature of 105°C overnight. The mass of each sample was noted immediately after removal from the oven. The samples were placed in an environmental climate chamber (Jeio Tech, Daejeon, Republic of Korea) at 20°C and 76% relative humidity (RH) for 24 h to perform a moisture uptake test. The mass of each sample was noted after removal from the climate chamber. The moisture uptake (g g−1) was calculated as the total mass of absorbed water divided by the weight of the dry powder before moisture uptake. The test was performed in triplicates.
Oil and water selectivity
One gram powder was portioned into aluminium weighing dishes and dried at a temperature of 105°C overnight. The mass of each sample was noted immediately after removal from the oven. To determine the moisture uptake in samples containing different amounts of oil, an amount of between 0.1 and 0.5 g olive oil per g powder (g g−1) was added to the dishes with dried powder. The powder and oil mixtures were stirred with a spatula until homogenous mixtures were achieved. The samples were placed in a climate chamber at 20°C and 76% RH for 24 h to perform a moisture uptake test as described above. A reference sample without oil addition was also placed in the climate chamber. The mass of each sample was noted after removal from the climate chamber. The moisture uptake (g g−1) was calculated as the ratio of the mass of absorbed water to the weight of the powder before addition of oil.
To determine the oil uptake in samples containing different amounts of water, an amount of between 0.05 and 0.3 g deionized water per g powder (g g−1) was added to the dishes with the dried powder. The powder and water were stirred with a spatula until homogenous. An oil uptake test, as described above, was performed. The oil uptake (g g−1) was calculated as the mass of absorbed oil divided by the mass of the dry powder before addition of water. The test was performed in triplicates.
Scanning electron microscopy
A high‐resolution Field Emission Gun Scanning Electron Microscope Zeiss Merlin (Zeiss, Germany) was utilized for the high‐resolution images of five different materials: MMC, silica, kaolin, talc and corn starch. All images were obtained using the in‐lens detector at 2 kV acceleration voltage and a probe current of 80 pA. SEM images of the materials were taken before and after oil uptake as long as they remained loose or slightly agglomerated powders. The same amounts of olive oil were added to the materials, and the oil was homogeneously blended into the materials with a spatula. The amounts were 0, 0.25, 0.50, 0.75, 1.00 and 1.25 g g−1 or until the material became a paste. The starch, talc and kaolin formed a paste at 0.50 g oil g−1 material and were therefore analysed in SEM at 0.25 g oil g−1 material. Silica formed a paste at 1.00 g g−1 and MMC formed a paste at 1.25 g g−1. Thus, silica and MMC were analysed in SEM up to 0.75 g g−1. A small amount of powder was applied onto Al stubs with carbon tape by using a spatula. To decrease charging effect, a thin Cu‐foil was applied at the edge of each Al‐stub. Excess powder was blown off using air. A Polaron SC7640 sputter was used to coat the sample with gold. A coating sequence lasting for 40 s under 20 mA was used.
Skin mattifying effect with Glossymeter
The in vivo mattifying effects of MMC, silica, mica, talc and corn starch were determined using a GL 200 W Skin‐Glossymeter (Courage + Khazaka electronic GmbH, Köln, Germany). In the Skin‐Glossymeter, parallel white light is emitted from the light emitting diodes at a 60° angle to the skin surface and the direct reflected light is measured along with the diffuse scattering in separate channels. The values assessed in these channels were used to calculate a Gloss value which was converted into a Gloss value with diffuse scattering correction (Gloss DSC) to eliminate differences in skin colour, texture and brightness. The mattifying effect was calculated as
where Gloss DSCr is the gloss DSC value measured on untreated skin and Gloss DSCm is the gloss DSC value measured on an area on the skin where the material of interest had been applied.
Fourteen human volunteers with normal to oily skin were used as test subjects. The forehead was cleaned and dried before application, and a surgical tape was applied in the middle of the forehead to separate the right and left side. The powder material was applied on the left side of the forehead using a clean brush. The right side was left blank and as internal reference. The surgical tape was removed after application. Glossymeter measurements were performed on 9 different spots on each side of the forehead with the first measurement at 15–25 min after application and then every 2 h up to 8 h after application. The Skin‐Glossymeter probe was wiped off with a tissue paper between measurements. One sample was analysed per subject and day. Each material was tested on between 10 and 14 test subjects, and the mean gloss values (average from 9 measurements per application area) were calculated at each time point. The mean reduction in gloss values as compared to internal references was calculated.
Flashback effect using photoimaging
The flashback effect is when the powder reflects a camera flash and shows up as white cast in a photograph that cannot be seen in real life. The white cast comes from the specular backscatter reflection from the particles on skin. The flashback effect of some cosmetic powders on human skin was determined using photoimaging with a Digital single‐lens reflex camera (Canon, Ōta, Japan). A study with 8 human volunteers was conducted to measure the flashback effect of MMC, silica, mica and talc as follows. The inside of the forearm was cleaned and dried before application, and two rectangles measuring 5 × 8 cm were marked with surgical tape. Samples were applied in one rectangle using a clean brush and the other rectangle was left blank as an internal reference. Photographs, with and without flash, were taken about 15 cm straight above the application area.
The average colour of each region in the photographs was processed using a Blur‐average‐tool in the imaging editing software (Adobe Photoshop CC, San Jose, CA, USA) resulting in one RGB colour code per analysed area. The Y'UV and Y'IQ models were used to convert RGB colour codes to greyscale with possible values from 0 to 255, where 0 defines black and 255 defines white. The flashback effect was calculated as:
where the quotient relates obtained greyscale values with flash for treated and untreated skin to those without flash. Here, Gm* is the greyscale value for the treated skin (where material was applied) and photograph taken with flash, Gr* is the greyscale value for the untreated skin (reference) and photograph taken with flash, Gm is the greyscale value for the treated skin and photograph taken without flash, and Gr is the greyscale value for the untreated skin and photograph taken without flash. The quotient corresponds to the colour change with the applied material, and the value 1 corresponds to normal skin without any applied material.
In vitro EpiDermTM skin irritation test (EPI‐200‐SIT)
An in vitro skin irritation test was performed at the accredited analysis laboratory QACS Ltd (Athens, Greece), according to OECD 439 [9]. In short, MMC was added to a reconstructed human epidermis (RhE) model (MatTek In Vitro Life Science Laboratories, Slovak Republic) followed by a cell viability test as measured by a 4‐h colorimetric MTT assay, according to the manufacturer’s instruction. The RhE cell model consists of normal human‐derived epidermal keratinocytes, which have been cultured to form a multilayered highly differentiated model of the human epidermis, consisting of organized basal, spinous and granular layers, and a multilayer stratum corneum containing intercellular lamellar lipid layers arranged in patterns analogous to these in vivo. Cell viability was measured in triplicates by dehydrogenase conversion of MTT (tetrazolium salt (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide) into a blue formazan salt, which was quantitatively measured photometrically at 500–600 nanometres using a multi‐well spectrophotometer after extraction of tissues. The darker the solution, the greater the number of viable, metabolically active cells. The reduction of the average viability of 3 tissues exposed to the powder in comparison to average viability of 3 negative controls (treated with DPBS without Ca2+ and Mg2+) was used to predict the skin irritation potential. Tissues treated with 5% sodium dodecyl sulphate (SDS) were used as positive controls. According to the European Union (EU) and Globally Harmonized System (GHS) classification (R38/Category 2 or no label), an irritant is predicted if the mean relative tissue viability of three individual tissues exposed to the test substance is reduced below 50% of the mean viability of the negative controls. A relative viability > 50% is classified as not irritant.
Human dermatological tests
Human Repeated Insult Patch Test (HRIPT) was performed by QACS Ltd. The methodology the laboratory use is an adaptation of the Modified Draize human sensitization test [10]. According to the protocol, 0.02 ml MMC powder was applied using patches to the lower or upper back on 50 human volunteer test subjects (with normal or sensitive skin) under occlusive conditions for a 48‐h time‐period. The occluded patches are composed of a small plastic cavity of 0.64 cm2 with a filter tissue at the bottom. The amount of test material applied to each patch is enough to fill the chamber and saturate the pad without overflowing when applied to the skin. A patch without any product, applied in the same conditions as the product to be tested, was used as negative control. The application site was evaluated after 0, 9, 48, 72 and 96 h. The applications were repeated nine times on the same site (induction site) over a period of 3 consecutive weeks, to assess for possible allergy (induction phase). After a 2‐week rest period with no product application, the product was re‐applied, again under patch, to the induction site to reveal a possible induced allergy (challenge phase). A skin examination of the application site was performed by the same dermatologist. Signs of erythema, oedema or dryness (scaling) or any allergic reaction, if any, were recorded.
Statistics
When applicable, a two‐sided t‐test using Excel software was performed to statistically evaluate the null hypothesis between the means at a significance level of 0.95 (*p < 0.05 as compared to control).
Results
Characteristics
Mesoporous magnesium carbonate for cosmetics is a milled white, porous powder with irregular shaped particles as shown in Figure 1a,b. The material is lightweight with a typical bulk density between 0.24 and 0.28 g ml−1. The XRD pattern (Figure 2) of the material was consistent with previously reported X‐ray diffractograms of mesoporous magnesium carbonate (MMC) indicating an amorphous nature with traces of crystalline magnesium oxide and/or magnesium hydroxide [6, 11].
Figure 1.
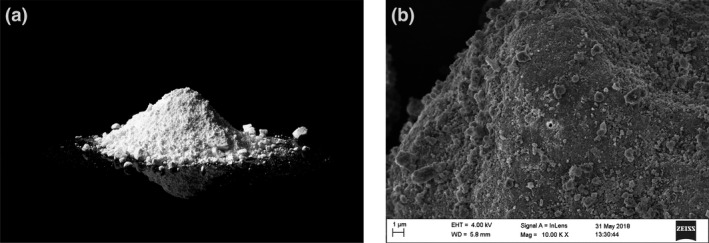
a‐b. Visual assessment of MMC powder: a) MMC powder b) SEM image showing the close‐up structure of an MMC particle.
Figure 2.
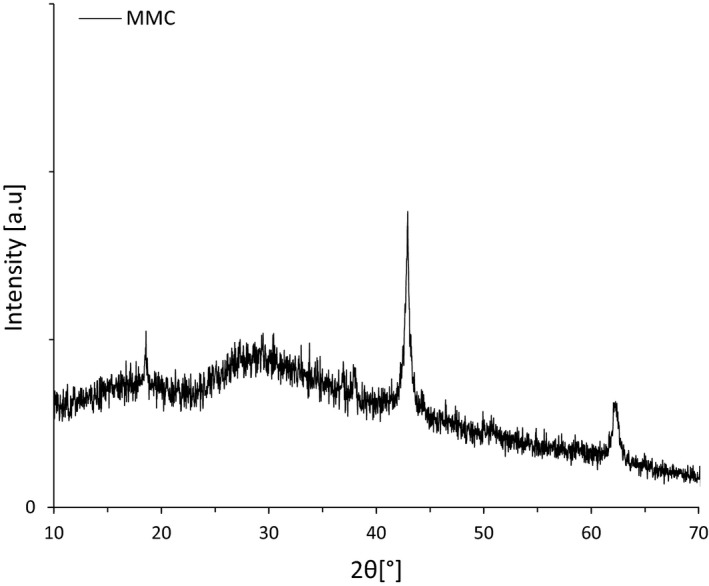
Representative XRD image showing the amorphous nature of MMC. The crystalline peaks correspond to traces of MgO and Mg(OH).
The peak pore size (pore size mode) of MMC was approximately 5 nm, as measured by nitrogen adsorption, whereas the total pore volume and the BET specific surface area were 0.5 cm3 g‐1 and 300 m2 g‐1, respectively (Figure 3a and Table 1). Silica also has high surface area and pore volume, whereas other common powder ingredients exhibit very small surface area and are non‐porous (Table 1). As shown in Table 1 and Figure 3a, silica has a larger peak pore size and a broader pore size distribution as compared to MMC.
Figure 3.
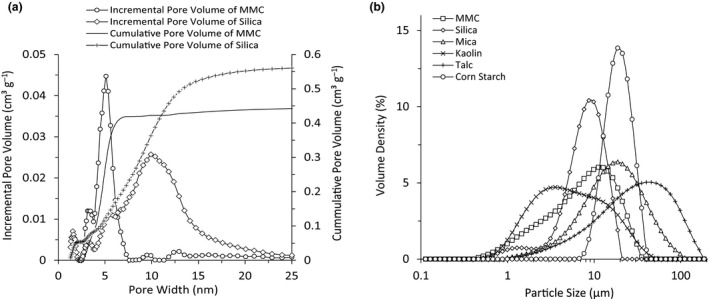
(a) Pore size distribution of MMC and silica presented as incremental and cumulative pore volume. (b) Particle size distribution of MMC, silica, mica, kaolin, talc and corn starch.
Table 1.
Specific surface area and pore volume of cosmetic powders as determined using nitrogen adsorption.
| Ingredient | BET surface area (m2 g‐1) | Pore volume (cm3 g‐1) | Pore size mode (nm) |
|---|---|---|---|
| MMC | 305 ± 20 | 0.49 ± 0.01 | 4.9 ± 0.2 |
| Silica | 291 | 0.58 | 9.9 |
| Mica | 5.7 | Non‐porous | Non‐porous |
| Kaolin | 25 | Non‐porous | Non‐porous |
| Talc | 5.5 | Non‐porous | Non‐porous |
| Corn starch | 2.8 | Non‐porous | Non‐porous |
Data for MMC are mean ± SD from six different batches.
Figure 3b presents the particle size distribution of MMC, silica, mica, kaolin, talc and corn starch. The Dv50 particle size of MMC was found to be 5.1 ± 0.0 µm, and the particle size mode was 7.0 ± 0.5 µm.
Oil and moisture absorption
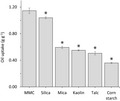
Mesoporous magnesium carbonate can take up both oil and water without any additional modification or surface treatment. The oil uptake capacities of MMC, silica, mica, kaolin, talc and corn starch were assessed using the oil uptake test. MMC absorbed more than 110% of its weight, which is slightly more than silica, whereas the other tested powders were less potent absorbers (Figure 4). Photographic images of the powders mixed with increasing amounts of oil revealed large differences in agglomeration and dryness. Kaolin, talc and corns starch all agglomerated already at 0.25 g g−1 oil and became a paste after addition of 0.50 g g−1 oil, whereas silica did not agglomerate until 0.75 g g−1 oil and formed a paste by addition of 1.00 g g−1 oil. MMC remained as a dry loose powder up to and beyond addition of 0.75 g g−1 oil and started to agglomerate at 1.00 g g−1 oil. It did not form a paste until 1.25 g g−1 oil was added (Figure 5).
Figure 4.
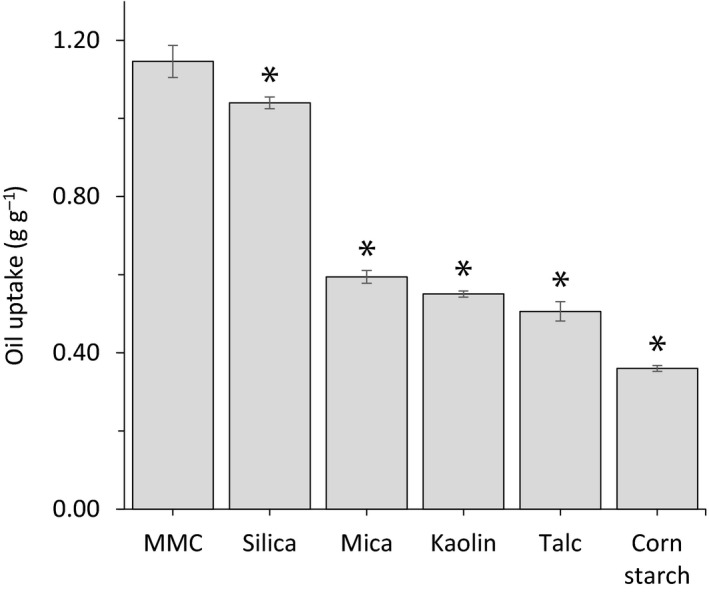
Oil absorption of MMC, silica, mica, kaolin, talc and corn starch as determined using a standard oil uptake test. Values are mean ± SD from at least five replicates. *Indicates p < 0.05 as compared to MMC.
Figure 5.

Photographic images of cosmetic powders mixed with increasing amounts of oil up to 1.0 g oil g−1 material or up to the amount when the powder forms a paste. Kaolin, talc and corn starch agglomerates at 0.25 g g−1 and becomes a paste at 0.5 g g−1, whereas silica and MMC can take up more oil. At 1.0 g g−1 silica has become a paste, whereas MMC is still an agglomerated powder.
The materials were also studied with SEM before and after oil uptake. White or shiny parts that appear in images of material mixed with oil stem from charge build‐up due to the low conductivity of the oil and are therefore a clear sign of oil on the surface of the powder particles. The oil is also visible as a smoothing of the particle surfaces. The SEM images confirmed the visual assessment of the pure and oil‐mixed powders. As shown in Figure 6, MMC remained a completely dry powder consisting of free powder particles up to a load of 0.50 g g−1 oil. At 0.75 g g−1 oil, partial agglomeration occurred but the powder remains non‐oily with no observed shiny or white patches in SEM, suggesting that the oil is encapsulated in the porous structure. In contrast, the non‐porous materials kaolin, talc and corn starch, which all agglomerated at 25 wt% oil, no longer showed well‐defined separate particles, as is the case for the pure materials, and the materials exhibited a clearly oily appearance in SEM images (Figure 6). In agreement with the oil uptake test, silica was capable of higher absorption of oil than the non‐porous materials, remaining a dry powder up to 50 wt% oil but the formation of an oily powder at 75 wt% oil was well visible in SEM showing shiny patches on particles.
Figure 6.
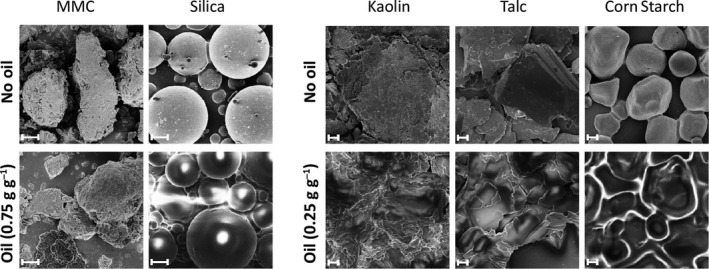
SEM images of pure materials (top) and materials mixed with oil (bottom). SEM images of kaolin, talc and corn starch mixed with 0.25 g g−1 oil are shown on the bottom right, whereas MMC and porous silica mixed with 0.75 g oil g−1 material is shown on the bottom left. The white or shiny parts in the images stem from charge build‐up due to the low conductivity of the oil and are therefore significant of an oily layer formed on top of the powder particles and oil. Note, the dry separated particles of MMC after oil uptake as compared to the shiny, oily appearance of other materials after mix with same or lower amounts of oil. The scale bars represent 1 µm.
Mesoporous magnesium carbonate is an efficient moisture absorber due to the high affinity to water and large surface area. To assess whether MMC can take up oil even if it has already taken up water, an oil uptake test was performed using MMC, pre‐mixed with distilled water. As can be observed in Figure 7a, approximately 90% of its original oil absorption capacity was maintained (from 1.2 g g−1 to 1.1 g g−1) when pre‐loaded with 30 wt% water. In addition, approximately 80% of the moisture uptake capacity was maintained in material pre‐loaded with 0.5 g g−1 oil (Figure 7b). Silica could take up almost as much oil as MMC before and after addition of 0.3 g g−1 distilled water (Figure 7a), but the absorption appeared less efficient as the oil formed a droplet on top of the powder and had to be mixed into the powder to be absorbed in contrast to MMC that readily absorbed the oil droplets. A separate test where an 1 ml drop of oil was placed on a slightly compacted and smooth dry powder surface (approximately 8 ml powder) showed that it took less than 5 min for the oil to be absorbed into MMC, whereas it took almost an hour for silica to be absorbed into the powder bed (results not shown).
Figure 7.
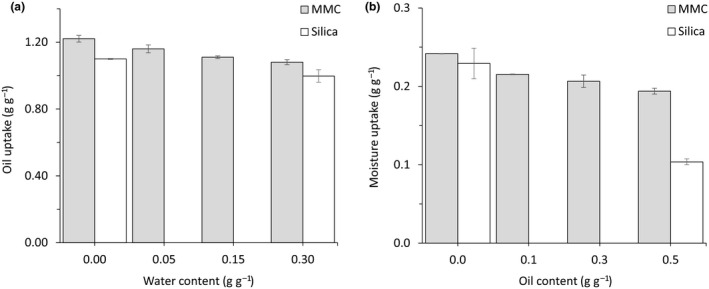
Oil and water selectivity. (a) The capacity of MMC and silica to take up oil before and after addition of water. Oil uptake was measured using a standard oil uptake test and presented as gram oil absorbed per gram dry material (g g−1). (b) The capacity of MMC and silica to adsorb moisture before and after addition of oil. Moisture uptake capacity was measured by calculating the material weight increase after 24 h in a climate chamber at 20°C and 76% RH and presented as gram water absorbed per gram dry material (g g−1). Data are mean ± SD from at least three replicates.
Moisture (humidity) adsorption capacity of silica pre‐loaded with 50 wt% oil was reduced by more than 50% (Figure 7b).
Long‐lasting shine control of skin
A mattified appearance of skin can be achieved by applying a material that efficiently absorbs sebum. Measurement of the gloss value is an important parameter to measure the mattifying effect of a product or powder make‐up ingredient.
The gloss values of naked skin and skin applied with different powder materials were measured and data showed that MMC provides an instant mattifying effect (percent reduction in gloss value as compared to skin with no powder), outperforming all other materials tested except kaolin, which also exhibited a strong instant mattifying effect (Figure 8a). In addition, MMC exhibited a long‐lasting mattifying effect of at least 8 h (Figure 8b). Kaolin, however, quickly lost a lot of its mattifying effect and was the least mattifying ingredient after 2 h (Figure 8b).
Figure 8.
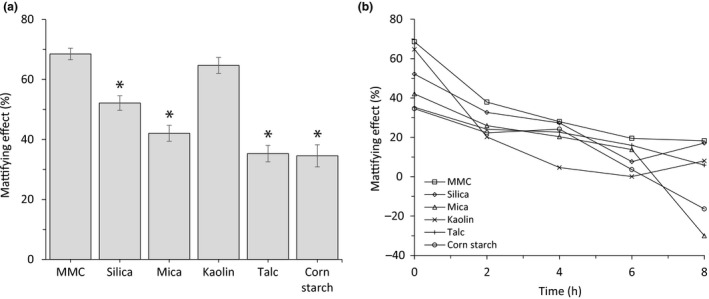
(a) Instant mattifying effect on skin and peak particle size of different cosmetic powders (as measured with a Glossymeter). Data are mean ± SEM from at least 10 independent experiments (human volunteers). *Indicates p < 0.05 as compared to MMC. (b) Mattifying effect of different powders measured with Glossymeter on skin at time 0 and every 2 h over 8 h. Mattifying effect is calculated as per cent reduction of gloss value as compared to internal reference (untreated skin).
Flashback effect
The flashback effect is defined as when reflected light from the camera flash is depicted on the produced photograph. To quantify the flashback effect, a new method was developed as no published standardized method was found. The method may generate both positive and negative values, but only the positive values were considered when quantifying the flashback effect since it is not possible to exhibit negative flashback. A larger positive value is correlated with stronger flashback, and near zero or negative values imply that no flashback arises. The method was used to measure flashback of naked skin as compared to skin applied with different powder materials showed that neither MMC, mica nor talc induced any flashback. However, as confirmed in Figure 9, spherical silica exhibits a clearly measurable flashback effect.
Figure 9.
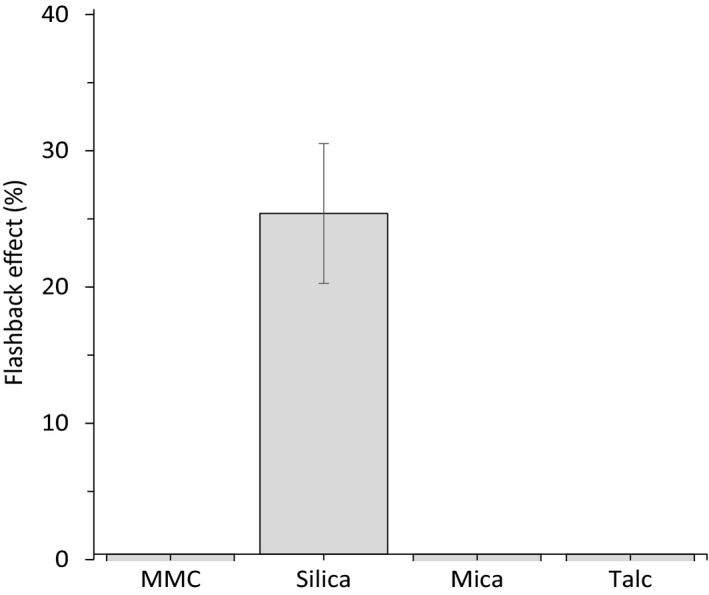
Flashback effect of MMC, silica, mica and talc on skin (image data analysis of photos taken with and without a flash). Silica is the only material with a positive value and therefore the only one to exhibit a flashback effect. Data are mean ± SD from 8 independent experiments (human volunteers).
Dermatological tests
To assess skin irritation, pure MMC materials were sent to an external accredited test laboratory for in vitro and in vivo tests. Firstly, MMC was applied topically to a reconstructed human epidermis model followed by a cell viability testing using the colorimetric MTT assay. As seen in Figure 10, the relative viability of reconstructed human epidermal model exposed to MMC was 85.2 ± 3.5%, which is well above the acceptance criteria of >50%. MMC was therefore classified as 'not irritant/not required classification' in this test. Secondly, skin irritation was assessed in vivo through a Human Repeated Insult Patch Test (HRIPT) on 50 tests subjects with normal to sensitive skin for three consecutive weeks followed by a two‐week rest period. After a resting phase, a challenge was performed through a single exposure on naive skin and potential skin reactions observed. Throughout the study, MMC neither induced any erythema, dryness, oedema nor any other signs of irritation. None of the volunteers presented any allergic reaction. Thus, the external test laboratory concluded that ‘according to the experimental conditions of the study the material can be considered as non sensitizing, or hypoallergenic’.
Figure 10.
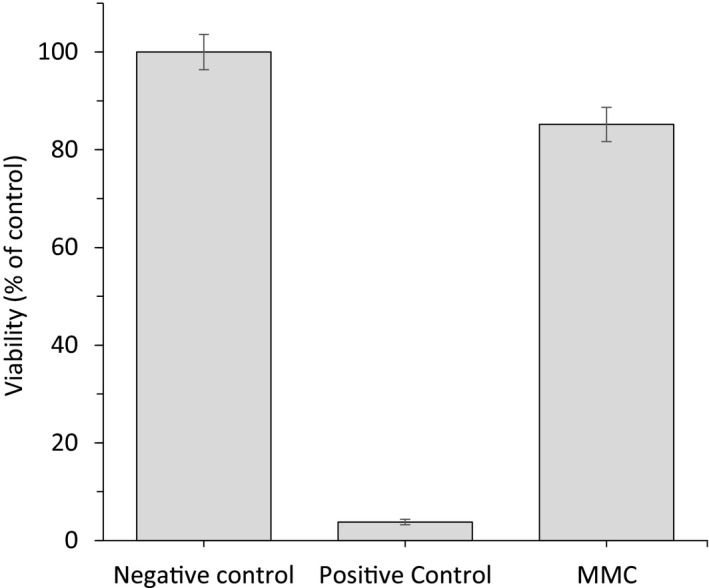
Relative viability of dermal skin model cells exposed to MMC powder as compared to negative control (DPBS). Tissues treated with 5% SDS were used as positive control. Data are mean ± SD from three replicates.
Discussion
The spectrum of basic ingredients available for use when developing cosmetic powders is rather narrow; thus, the functional attributes and quality of each ingredient are important since it will play a major role in the final formulation. Kaolin, silica and starch take up moisture and titanium oxide (not included in this study) provides coverage. Mica provides lustre and enhances skin feel, whereas spherical fillers, such as silica or nylon spheres, enhance skin feel due to the ball‐bearing‐like action of the spheres [4]. Cosmetic silica also has excellent oil absorbing properties. Talc has for long been the basic ingredient of almost all modern face powder formulations [1] due to its smoothness, spreadability (slip) and low to medium covering power. However, since talc and asbestos are often naturally formed alongside each other, geologically, talc deposits may be contaminated with asbestos or asbestiform minerals while being mined. In recent years, this has led to much concern over exposure to contaminated talcum powder products, which have been linked to cases of mesothelioma, lung cancer and ovarian cancer [12, 13]. Talc is therefore starting to be replaced with other materials or synthetic polymers [2], although the use of micro plastics is also controversial. Moreover, in response to the reported concerns with flashback, some brands are also seeking to launch products without silica.
As shown in the present study, MMC (marketed under the brand name Upsalite®) is a welcome addition to the list of cosmetic powder ingredients. It is synthesized from naturally derived minerals and does not cause any skin irritations or sensitization and it does not dry out the skin. MMC has low coverage and a velvety, smooth skin feel and adheres well to the skin (results not shown), but the most prominent feature of MMC is that it is an excellent oil absorber and that the oil is absorbed inside the porous structure leaving the surface of the particles dry and separated. This can be observed both with the naked eye, as presented in Figure 5 showing the dry appearance of MMC when mixed with up to 75 wt% oil but is even more apparent when the particles are studied in close‐up using SEM imaging. In contrast to kaolin, talc and corn starch for which the oil is clearly visible both on the surface and in between particles when mixed with 25 wt% oil, MMC exhibits a similarly dry appearance up to 75 wt% oil as it does without any oil. Silica, which also exhibit good absorbance, remains dry up to 50 wt% oil but looks shiny in SEM images at 75 wt% oil. MMC particles are small and irregular in shape, which may contribute to skin adhesion. Furthermore, they are highly porous, enabling a maximum absorption capacity. These intrinsic properties of MMC provide a dual effect of both hiding the oil inside its structure while also creating the rough surface needed for a matte appearance and excellent setting properties. The inclusion of oil could also explain why MMC not only provides an instant mattifying effect when applied on skin but also has a long‐lasting effect with reduced glossiness after 8 h of use as compared to skin without powder.
The skin contains sebaceous glands that produce sebum to lubricate skin as well as apocrine glands that produce sweat. Sweat production is increased in warm and humid environments and in response to exercise or hot flashes. People that have very oily skin produce an excess amount of sebum. What is seen as unwanted shine on skin is made up of a mixture of sebum, sweat, dead skin cells, and impurities from the surroundings. Thus, an ingredient that exhibit both humidity, sweat and oil absorbing properties and that remains dry after absorption will be better at reducing shine than ingredients that mainly absorb oil or that become wet and cakey after absorbing moisture.
It has previously been shown that MMC has a high water‐absorption capacity [6]. This is expected since MMC is a porous and disordered (x‐ray amorphous) magnesium carbonate with a very high surface area and magnesium is an efficient electron donor, known for its high reactivity with moisture and oxygen [14]. The interaction between MMC surface and water has previously been investigated by dielectric spectroscopy [15]. The study suggests that absorbed water not only reacts with the MMC surface but also penetrates the surface and enter the bulk of the material where it induces crystallization of MgCO3. In the present study, we not only confirm that MMC can take up moisture, but also show that it can absorb water and oil simultaneously. In fact, MMC maintains about 80% of its moisture absorption capacity when loaded with 50 wt% oil and almost 90% of its oil absorption capacity when loaded with 30 wt% water. A possible explanation for this phenomenon is that water and oil are taken up through different mechanisms. When water molecules are adsorbed from gas phase, their mobility is high, and they may readily interact with both the MMC surface and the bulk structure, thereby creating a dual absorption effect. Oil is instead absorbed into the pores through capillary forces. Thus, water and oil are not competing for the same compartmental space.
The cosmetic grade fumed silica, used in this study, is amorphous and porous. Thus, it was interesting to study the capacity of silica to absorb both moisture and oil and compare the result with MMC. Silica exhibits a high oil absorption capacity, but oil absorption takes longer time as compared to MMC (approximately ten times longer). Like MMC, oil absorption capacity of silica was not dramatically affected when pre‐loaded with water, whereas uptake of moisture was reduced by more than half in silica pre‐loaded with oil. These results can be explained by the chemical and physical composition of fumed silica. Synthetic micro‐amorphous silica consists of an inorganic network of tetrahedral SiO4 units bonded via hydrophobic siloxane groups and bearing hydrophilic surface silanol (Si‐OH) groups [16]. Silanol groups present on the surface attract water through hydrogen bonds and the relative amount of silanol is directly related to the water adsorption capacity [16]. Fumed silica (also called pyrogenic silica, made through a thermal process from synthetic amorphous silica) consists of non‐porous, monomers of amorphous silica fused into branched, chainlike, three‐dimensional secondary particles called aggregates, which then agglomerate into spherical tertiary particles [17]. The porous structure of fumed silica is made up of gaps between primary non‐porous particles in aggregates and agglomerates [17]. When silica is loaded with water, the water molecules will only bind the hydrophilic surface silanol groups leaving the pores largely unfilled. Thus, oil absorption through capillary forces and binding to surface siloxane groups is not significantly affected. However, when silica is pre‐loaded with oil, water molecules will have limited binding capacity as the oil binding to siloxane groups may form a hydrophobic layer of the surface that is difficult for the water to penetrate, whereby making the silanol groups less accessible. Moreover, silica is not capable of binding water in the bulk of the material due to the hydrophobicity of the siloxane groups. This contrasts with MMC that can bind water inside the bulk structure of the magnesium carbonate as well as on the surface, as described above.
Talc, mica and kaolin all consist of different complex forms of silicates [18]. They can take up both water and oil but not to the same extent as MMC and silica. Since they are all non‐porous, humidity, perspiration or sebum will only adhere to the outer surface of the particles that will become sticky. This can lead to caking and smearing of make‐up. Talc is hydrophilic and strongly adsorb a monolayer of water molecules but exhibit hydrophobic behaviours as water droplets beads up on the surface and is not absorbed easily without mixing (results not shown). The reason is that the cohesion, that is attraction between water molecules, is stronger than the adhesion between the talc surface and water [19].
Corn starch is a polymeric carbohydrate and can absorb both oil and water and is often used to replace talc. A well‐known defect of starch, however, is that it becomes a sticky paste when moistened with water. Moreover, since it is a food source, it can, in the absence of preservatives, support fungal or bacterial growth when exposed to moisture or sweat, which in turn can cause breakouts and inflammation, in addition to the caking or smearing of make‐up.
In addition to the chemical composition, specific surface area and porosity, colour, size and shape of elementary particles are important affecting setting properties, coverage and, ability to form different degree of transparent or opaque films, colour or gloss. The choice between the different materials when formulating colour cosmetics is made according to certain criteria such as more or less covering/whitening, or more or less mattifying. As can be seen in the SEM images in Figure 6 as well as in the particle size distribution shown in Figure 3b, the shape and size of the different materials tested in this study differ from each other. Silica is smooth and spherical, whereas talc and kaolin have a rougher surface and are more irregular in shape, which may help to increase setting properties. Talc, starch and mica exhibit larger grain size than MMC and silica and therefore become less transparent on skin (results not shown). Kaolin exhibits a small particle size distribution, but it is more difficult to distribute on skin due to its clay‐like structure, which will contribute to a more covering layer. Mica is characterized by its laminated structure, giving shape to shiny flakes of varying size. Due to the shimmer effect, mica is often used to provide gloss or shine but also provides a smooth skin feel due to high slip [18]. MMC exhibit a broad particle size range with rounded, slightly irregular particle shapes and a rough surface structure, providing a semi‐transparent, smooth and matt appearance. It also has good setting properties.
Spherical particles backscatter light to some degree as specular reflection which then reaches the camera lens. Irregular shaped particles, such as MMC, talc and kaolin, mostly reflect light as diffused scattering and therefore, less reflected light reaches the camera lens. The fumed silica often used in cosmetics has spherically shaped particles and is well known to exhibit strong flashback effect. With the surge in finishing powders specifically formulated to provide a blurring effect, such as ‘high definition’ (HD) powders, consumers have become more conscious of ingredients that can cause unwanted flashback effect. Although it is easy to find images showing the effect of flashback from high reflecting ingredients, there is no standardized, quantitative method that measures flashback effect. Therefore, we developed a new method in house, based on image analysis. Our results confirmed that silica induces strong flashback, whereas MMC, talc and mica do not generate any measurable flashback effect. The irregular, dry appearance of the MMC particles, as can be observed in the SEM images, minimizes the risk of inducing any camera flashback effect.
Conclusion
Colour, feel, application and special visual effects along with long wear are the main properties behind all types of powders. MMC has good sensory and visual characteristics (not shown) as well as excellent absorbing and mattifying properties, suggesting that it has great potential to replace or compliment other powder ingredients currently used as fillers and absorbers in powder cosmetics, including the controversial ingredient talc. Moreover, MMC does not dry out skin or induce any skin irritation or sensitization when tested on human subjects.
In conclusion, the present study demonstrates that milled MMC is highly suitable for use in cosmetic powder products. It is lightweight and easily spreadable and provides a soft and semi‐translucent layer when applied on skin. It provides an efficient, instant and long‐lasting mattifying effect when applied on skin without drying out skin or causing any irritation or sensitization and it exhibits no flashback effect. A natural next step would be to evaluate the properties of MMC as a mattifying powder ingredient in liquid cosmetic formulations.
Declaration of interest
This research was performed at and founded by Disruptive Materials AB, Uppsala, Sweden.
Acknowledgements
We thank Snehal Hadawale and Maryia Borys for technical assistance, Caroline Tisénius for preparation of images, and Dr. Shalini Singh, Åsa Frank and Jenny Collby for critically reviewing the manuscript and contributing to the discussion. We also would like to give our gratitude to the volunteers for their participation during the sessions. We thank Division of Nanotechnology and Functional Materials, Department of Materials Science and Engineering, Uppsala University, for the use of analytic equipment and SEM.
References
- 1. Singh SK. ed. Face Powders In: Handbook on Cosmetics (Processes, Formulae with Testing Methods). Asia Pacific Business Press Inc, Delhi: 2010. p. 335–355. [Google Scholar]
- 2. O'Lenick AJ, Siltech LLC, Morante N.Comparatively Speaking: Pressed vs. Loose Powder. Cosmetics and toiletries, August 8, (2012). https://www.cosmeticsandtoiletries.com/formulating/category/color/165338696.html.
- 3. Moussour M, Lavarde M, Pensé‐Lheritier AM, Bouton F. Sensory analysis of cosmetic powders: personal care ingredients and emulsions. Int J Cosmetic Sci. 2017;39:83–89. [DOI] [PubMed] [Google Scholar]
- 4. Schlossman ML. Decorative Products In Barel AO, Paye M, Maibach HI, editors. Handbook of Cosmetic Science and Technology, New York: Marcel Dekker Inc; 2001. p. 645‐701. [Google Scholar]
- 5. Farber L. Face Powders In Balsam MS, editor. Cosmetics‐Science and Technology, New York: Wiley‐Interscience; 1972. p. 335‐353. [Google Scholar]
- 6. Forsgren J, Frykstrand S, Grandfield K, Mihranyan A, Strømme MA. Template‐Free, Ultra‐Adsorbing, High Surface Area. PLoS One. 2013;8:e68486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frykstrand S, Forsgren J, Mihranyan A, Strømme M. On the pore forming mechanism of Upsalite, a micro‐and mesoporous magnesium carbonate. Micropor Mesopor Mat. 2014;190:99–104. [Google Scholar]
- 8. Brunauer S, Emmett P, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60:309–319. [Google Scholar]
- 9. OECD 439 ‐ OECD GUIDELINE FOR THE TESTING OF CHEMICALS, In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method ‐ adopted in 2013.
- 10. Marzulli FN, Maibach HI. Contact allergy: Predictive testing in man. Contact Dermatitis. 1976;2:1–17. [DOI] [PubMed] [Google Scholar]
- 11. Frykstand S, Forsgren J, Mihranyan A, Stromme M. On the pore forming mechanism of Upsalite, a micro‐and mesoporous magnesium carbonate. Micropor Mesopor Mat. 2014;190:99–104. [Google Scholar]
- 12. Moline J, Bevilacqua K, Alexandri M, Gordon R. Mesothelioma associated with the use of cosmetic talc. J Occupational Environ Med. 2020;62:11–17. [DOI] [PubMed] [Google Scholar]
- 13. Paoletti L, Caiazza S, Donelli G, Pocchiari F. Evaluation by electron microscopy techniques of asbestos contamination in industrial, cosmetic, and pharmaceutical talcs. Regulat Toxicol Pharmacol 1984;4:222–235. [DOI] [PubMed] [Google Scholar]
- 14. Goebel MT, Marvel CS. The oxidation of grignard reagents. J Am Chem Soc. 1933;55(4):1693–1696. [Google Scholar]
- 15. Pochard I, Frykstrand S, Ahlström O, Forsgren J, Strømme M. Water and ion transport in ultra‐adsorbing porous magnesium carbonate studied by dielectric spectroscopy. J Appl Phys. 2014;115:044306. [Google Scholar]
- 16. Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf A Physicochem Eng Aspects. 2000;173:1–38. [Google Scholar]
- 17. Gunko VM, Mironyuk IF, Zarko VI et al Morphology and surface properties of fumed silicas. J Colloid Interface Sci. 2005;289:427–445. [DOI] [PubMed] [Google Scholar]
- 18. Le Joliff JC. Silicon and beauty 2: Silica and Silicates in formulation. CosmeticOBS. June 17, 2015. https://cosmeticobs.com/en/articles/ingredients‐50/silicium‐et‐beaute‐2nbsp‐silices‐et‐silicates‐en‐formulation‐2876
- 19. Rotenberg B, Patel AJ, Chandler D. Molecular explanation for why talc surfaces can be both hydrophilic and hydrophobic. J Am Chem Soc. 2011;133(55):20521–20527. [DOI] [PMC free article] [PubMed] [Google Scholar]


