Abstract
BACKGROUND
Precise quantification of the fitness cost of synthetic auxin resistance has been impeded by lack of knowledge about the genetic basis of resistance in weeds. Recent elucidation of a resistance‐endowing IAA16 mutation (G73N) in the key weed species kochia (Bassia scoparia), allows detailed characterization of the contribution of resistance alleles to weed fitness, both in the presence and absence of herbicides. Different G73N genotypes from a segregating resistant parental line (9425) were characterized for cross‐resistance to dicamba, 2,4‐d and fluroxypyr, and changes on stem/leaf morphology and plant architecture. Plant competitiveness and dominance of the fitness effects was quantified through measuring biomass and seed production of three F2 lines in two runs of glasshouse replacement series studies.
RESULTS
G73N confers robust resistance to dicamba but only moderate to weak resistance to 2,4‐d and fluroxypyr. G73N mutant plants displayed significant vegetative growth defects: (i) they were 30–50% shorter, with a more tumbling style plant architecture, and (ii) they had thicker and more ovate (versus lanceolate and linear) leaf blades with lower photosynthesis efficiency, and 40–60% smaller stems with less‐developed vascular bundle systems. F2 mutant plants had impaired plant competitiveness, which can lead to 80‐90% less biomass and seed production in the replacement series study. The pleiotropic effects of G73N were mostly semidominant (0.5) and fluctuated with the environments and traits measured.
CONCLUSION
G73N is associated with significant vegetative growth defects and reduced competitiveness in synthetic auxin‐resistant kochia. Management practices should target resistant kochia's high vulnerability to competition in order to effectively contain the spread of resistance.
Keywords: AUX/IAA16, dicamba resistance, fitness cost, kochia (Bassia scoparia), replacement series study; leaf and stem morphology
An IAA16 mutation (G73N) confers robust resistance to dicamba but moderate to low resistance to 2,4‐d and fluroxypyr in key weed species kochia. The mutation also leads to significant leaf and stem morphological changes and diminished plant size, resulting in plants that are highly vulnerable to competition. Vegetative growth defects of G73N mutant plants suggest that weed management practices which subject resistant plants to substantial competition (e.g. cover crops) will potetially be effective.

© 2020 Society of Chemical Industry.
1. INTRODUCTION
Synthetic auxins are important herbicides that function by simulating the principal natural auxin indole‐3‐acetic acid (IAA) in plants. Plants sprayed with synthetic auxins suffer from an imbalance in auxin homeostasis and impaired auxin interactions with other hormones, leading to deregulated plant growth and eventually plant death. 1 , 2 Resistance evolution for synthetic auxins has been relatively slower than other herbicide modes of action (MOA): 41 resistant weed species have been documented after more than 70 years of use. 3 , 4 Owing to the lack of understanding of the MOA of synthetic auxins, the genetic bases of weed resistance were not known for decades. Multiple mechanisms including impaired herbicide translocation and altered metabolism have been reported to play a role in conferring resistance. 5 The first target‐site based synthetic auxin resistance mechanism was recently reported in the key weed species kochia (Bassia scoparia) to be a dominant point mutation (G73N) at IAA16, one of the transcriptional repression factor auxin/indoleacetic acid (AUX/IAA) proteins. 6 , 7
Kochia is a broadleaf summer annual weed in small grains and broadleaf crops in the US Great Plains, western US and Canada. 8 , 9 Kochia has a strong taproot system which allows it to thrive in droughty, nutrient‐deficient saline soils. 10 Kochia is a prolific seed producer capable of producing up to 30 000 seeds per plant, 11 which can be dispersed efficiently through tumbling across the landscape in strong winds. 12 , 13 Furthermore, kochia has a high level of within‐population genetic diversity, 14 resulting in a higher propensity to evolve herbicide resistance. Kochia has evolved resistance to four herbicide MOAs including synthetic auxins. 3 Kochia biotypes that are resistant to at least one MOA, has spread across 20 US states and Canada, 3 , 15 significantly eroding farmers' options to control them.
As a key factor for predicting resistance evolutionary trajectory, fitness costs of herbicide resistance traits have practical implications for managing resistant weeds. The fitness cost of synthetic auxin resistance has been measured in multiple species including kochia. 16 , 17 , 18 , 19 , 20 However, without knowledge of the genetic bases of resistance, it is hard to unequivocally link the pleiotropic effects with herbicide resistance alleles. 21 Furthermore, the resistance evolutionary trajectory is not only influenced by the presence of the fitness cost, but also by dominance of the costs. Dominance of fitness costs measures the fitness of the heterozygous (RS) individuals relative to the homozygous resistant (RR) and sensitive (SS) plants. 22 , 23 , 24 The fitness of RR plants is critical for initial spread and establishment of resistance genes, because newly arisen herbicide resistance mutations are usually in RS forms. 25 Unfortunately, the dominance of the fitness costs of synthetic auxin resistance has not been quantified in weeds.
In this report, we thoroughly investigated the heritability and manifestation of the fitness costs of a synthetic auxin resistance‐endowing mutation G73N, in both parental and F2 plants grown under different growth conditions, because it is known that fitness penalties associated with adaptive traits might fluctuate with the ecological conditions under which they manifest. 26 , 27 We also quantified the dominance of the fitness costs of G73N to provide insights on mitigating resistance during the early phase of resistance evolution. This very first precise measurement of the fitness costs associating with synthetic auxins, will be valuable to guide the design of integrated weed management practices, to mitigate the spread of resistant weeds and preserve the durability of synthetic auxins in kochia management.
2. MATERIALS AND METHODS
2.1. Plant materials and genotyping method
Seeds from a dicamba‐sensitive kochia wild‐type (WT) line were purchased from Herbiseed (www.herbiseed.com/). A resistant parental line (9425) and three F2 lines were derived from a previously characterized dicamba‐resistant kochia line maintained through a single seed descent (SSD) method. 6 , 7 Generation of the parental and F2 lines is illustrated in Fig. 1. Seeds were first sowed onto germination plug trays containing commercial potting media (Premier Pro‐Mix BX mycorrhizae, Premier Horticultural Services, Quakertown, PA), incorporated with slow release granular fertilizer (Osmocote [14‐14‐14], Scotts Company LLC, Marysville, OH) at the rate of 3600 g m−3 (Fig. S1 in Appendix S1). Seedlings were grown in the growth chamber set as follows: 16 h:8 h, light:dark photoperiod, 26 °C/20 °C day/night temperature; and light intensity 550 μm m−2 s−1. One small cotyledon was sampled from seedlings of 3–4 cm height and genotyped through the allelic specific Taqman® assay as described in LeClere et al. 7 Uniform size of genotyped seedlings was selected and transplanted for downstream studies.
Figure 1.
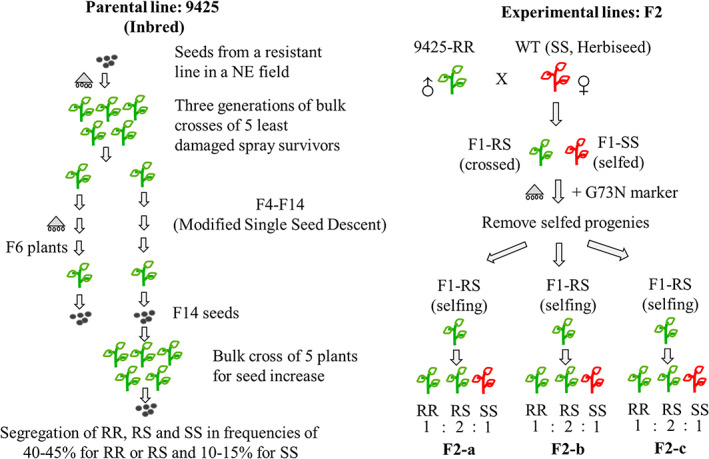
Schematic illustration of the generation of the parental line 9425 and F2 lines. Parental line 9425 was derived from a previously characterized dicamba‐resistant line maintained through a single seed descent (SSD) method. Owing to lack of molecular markers at the time of the bulk cross and morphological similarity between RR and RS plants (Fig. 4), either multiple RS plants were included or self‐pollination of RS plants, could result in a segregating 9425 parental line. To generate F2 lines, WT plants were hand‐pollinated with pollen from 9425‐RR plants, followed by self‐pollinating different F1 crossed progenies confirmed through genotyping and dicamba spray.
2.2. Contribution of G73N to cross‐resistance to broad‐spectrum synthetic auxins
Different G73N genotypes of 9425 (RR, RS, SS) and WT lines were subjected to dose–response studies on three representative auxin herbicides. Plants were grown at a Bayer CropScience glasshouse in St Louis, MO, USA (29/26 ± 3 °C day/night temperatures, 14 h:10 h, light:dark photoperiod, supplemental lighting to maintain ≥1050 μm m−2 s−1 light intensity). Plants at 8–10 cm height were sprayed with 0, 140, 280, 560, 1120 or 2240 g ha−1 of Dicamba (Clarity® BASF Corporation, Florham Park, NJ), or 0, 210, 420, 840, 1680, or 3360 g ha−1 of 2,4‐d (2,4‐D amine® Winfield United, Gibbon, NE), or 0, 39.25, 78.5, 157, 314 or 628 g ha−1 of fluroxypyr (Starane Ultra®, Corteva AgriScience, Wilmington, DE). Herbicides were applied using a research track sprayer equipped with a TTI spray nozzle (TeeJet® spray nozzles, SpraySmarter.com) calibrated to deliver 140 L ha−1 of herbicide solution at 276 kPa, moving at 2.57 km h−1. Plant visual injury was evaluated at 21 days after treatment (DAT) and monitored for another three weeks to see if survivors were able to survive and reproduce.
2.3. Plant architecture, biomass and seed production
Two glasshouse fitness studies were conducted on genotyped 9425 and WT kochia plants (Exp‐1 N = 18 per genotype; Exp‐2, N = 15 per genotype) in May and July 2019. Glasshouse growth conditions are listed in Table S1 in Appendix S1. Genotyped seedlings at 5–7 cm tall were transplanted into 4.5‐in plastic pots (10.2 cm width X 10.2 cm length X 17.8 cm height) filled with the potting mix described above. Plants were watered as needed and fertilized biweekly with Jack's Professional 20–10‐20 general purpose fertilizer (JR Peters Inc., Allentown, PA, USA) at 200 ppm N. Pots were evenly spaced to avoid any interplant shading and re‐randomized weekly.
Plant heights were measured weekly. Plant architecture traits such as changes in gravitropic set point angles (GSAs) of the 6th lateral shoot branch from the soil surface were quantified through images of plants at 10 weeks after planting (WAP) in Fiji. 28 Plants were harvested individually upon natural plant senescence and dried at 40°C for 72 h and weighed for biomass. Harvested plants were threshed and screened through U.S. standard brass sieves (2 mm fb 0.5 mm, Dual Manufacturing Co., Inc., Franklin Park, IL). Seeds were cleaned through a column seed blower (Hoffman Manufacturing, Inc., Corvallis, OR) and weighed. Roots of two‐week‐old seedlings were washed gently under running water and dry weights were measured (N = 6 per genotype).
2.4. Photosynthesis efficiency, vegetative morphology and microscopy
All genotyped 9425 and WT kochia plants from the above glasshouse studies were used to study photosynthesis efficiency, and a subset of the plants were used to study leaf and stem morphology. Five‐mm round leaf punches were taken from the first fully unfolded leaf at 3 WAP, and immediately placed onto 96‐well plates filled with 200 μL leaf disc assay buffer (1 mm MES + 1% sucrose, PH6.5; M2933, 84 097, Sigma‐Aldrich Inc., St. Louis, MO). Plates were dark‐adapted for 20 min and scanned through a chlorophyll fluorescence imager (Technologica CF Imager, Technologica Ltd, Essex, UK), to collect Fv/Fm values, which measure the maximum quantum efficiency of PSII photochemistry/photosynthesis efficiency. 29
Leaf blade width and length of the 1st fully unfolded leaf (N = 32) were measured at 4 WAP. Histological observations were made through hand and microtome transversal sections for stems and leaves, respectively (three to four plants per genotype at 4, 8 and 10 WAP). The stems were sectioned through the internodes at 10–12 cm and 2–4 cm above the soil surface for Exp‐1 and Exp‐2, respectively. Pieces (8 × 9 mm) of leaf blade from three leaves per plant were sampled and fixed in 4% formaldehyde (Electron Microscopy Sciences, Hatfield, PA, cat. no. 15710‐S), infiltrated for 1 h, washed three times in 10% PBS, air‐dried and then embedded in (10 × 10 × 5 mm) vinyl molds filled with OCT compound (Tissue‐Tek; Sakura Finetek USA Inc., Torrance, CA), instantly frozen with liquid N2 and then stored at −80 °C. Transverse 45‐μm sections were made on a microtome and stained for 10 s with toluidine blue (Electron Microscopy Sciences, Hatfield, PA, cat. no. 22050). Observations were made with a standard brightfield microscope (M205 FA, Leica, Wetzlar, Germany) fitted with a digital camera (Leica M205 FA, DFC310 FX) through the Leica application suite X (LAS X) platform. Images from three sections per plant were taken and subjected to leaf thickness (250 μm from the middle vein) and stem diameter measurement in Fiji. 29
2.5. Glasshouse replacement series competition studies on F2 lines
Two glasshouse replacement series competition studies (Exp‐1 N = 480; Exp‐2, N = 320) were conducted on three F2 lines in July and September 2016 (Fig. S2 in Appendix S1). A germination test was carried out on agarose in a growth chamber maintained at 22 °C constant temperature, 16 h:8 h, light:dark. Genotyped seedlings were transplanted into 20‐L plastic pots filled with the potting mix described above. Each pot contained eight plants of two genotypes (RR:SS or RS:SS) either as pure stands or in mixtures at ratios of 8:0 (100%), 6:2 (75%), 4:4 (50%), 2:6 (25%) and 0:8 (0%). All of the pots were randomized weekly and the plants were only watered as needed. Plants were grown in a completely randomized design, with detailed timing and glasshouse conditions listed in Table S1 in Appendix S1. Plant height, biomass and seed production data were collected as described above, except that plants were air‐dried in the glasshouse for six weeks instead of being oven‐dried.
Following replacement series indices derived from biomass and seed production data were calculated using the formulas listed in Table S2 in Appendix S1: Relative yield (RY), competitive ratio (CR), relative crowding coefficient (RCC) and aggressiveness index (AI). These replacement series indices measure the competitiveness and resources niches of different genotypes. 20 , 30 , 31 Dominance of the fitness costs was calculated using the following formula 22 :
| (1) |
where , represent means of plant height, biomass or seed production for F2‐RR, ‐RS and ‐SS plants, respectively. The fitness costs are dominant when h = 1 (RS = RR), semidominant when h = 0.5 (RS = 1/2SS) and recessive when h = 0 (RS=SS).
2.6. Statistical analysis
Dose–response curves were fitted using the four‐parameter log‐logistic model 32 through the DRC package in RStudio (v3.5.1, The R Foundation for Statistical Computing, Vienna, Austria):
| (2) |
where Y is the predicted visual injury (%), x is the herbicide dose, the upper limit d and the lower limit c were fixed, b is the slope, and e is the GR50 (50% growth reduction) for each genotype. A R/S ratio was calculated by dividing the GR50 values of the resistant line by that of the sensitive line. Testing of model fit, parameters estimates and SE were all done through the DRC package.
Violin plots were generated to visualize plant height, biomass and seed production of different genotypes in rstudio. ANOVA was conducted using the linear mixed effect models in SAS (V9.4, SAS Institute, Cary, NC). For parental or F2 line fitness measurements, genotype was the fixed effect. For the replacement series indices (RCC, CR, AI), genotype, competition as well as their interactions were the fixed effects. Detailed models and testing of the assumptions of ANOVA are described in the Appendix S1. All pairwise comparisons between the genotypes were defined within the ANOVA and tested using Student's t‐tests. Overall F‐tests for interactions between competition and genotype also were defined based on the model above. The nonparametric Wilcoxon signed rank test was used for RCC comparison owing to the violation of normality distribution. A two‐sided Student's t‐test was used to determine if CR and AI were different from 1 and 0, respectively; thus, if RY observed values deviate from their expected values at each competition level and if the dominance of resistance costs is different from 0.5 or 1 individually. For the replacement series study, simple linear regression lines were plotted in scatter plots to visualize how plant height, biomass and seed production change in response to increasing resistance frequencies.
3. RESULTS
3.1. The role of G73N in cross‐resistance to broad‐spectrum synthetic auxins
The GR50 of 9425‐RR plants estimated from the dose–response curves were 2187 g, 263 g and 34 g for dicamba, 2,4‐d and fluroxypyr, respectively, which were five‐, three‐ and one‐fold, and 25‐, 11‐ and 11‐fold higher than that of ‐RS and ‐SS plants [Fig. 2(a)–(l)]. However, G73N appeared to confer robust resistance to dicamba (survival at 4×), but only moderate‐to‐weak resistance to 2,4‐d and fluroxypyr [Fig. 2(m)–(o)]. The 9425‐RR plants sprayed with recommended label rates of dicamba (560 g), 2,4‐d (840 g) and fluroxypyr (157 g) were 25%, 70% and 88% injured, respectively. Although most of the RR/RS plants that were sprayed with higher rates (≥2×) of 2,4‐d or fluroxypyr were moderately to highly stunted, the injury levels did appear to be lower than that of 9425‐SS or WT line, and those plants were able to reproduce six weeks after being sprayed (Fig. S3).
Figure 2.
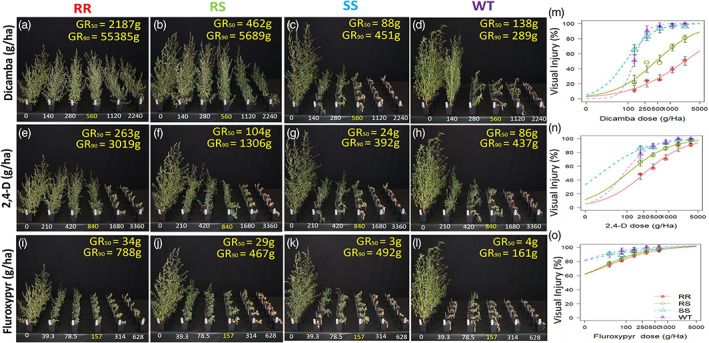
Cross‐resistance of different G73N genotypes to dicamba, 2,4‐d and fluroxypyr. Plants were sprayed with different rates of dicamba (a–d, m), 2,4‐d (e–h, n), fluroxypyr (i‐l, o). Photos were taken 21 days after herbicide treatment (DAT). Dose–response curves were fitted using the four‐parameter log‐logistic model. GR50 and GR90 values were indicated at the top right corner of each photo.
3.2. G73N leads to diminished plant size and altered plant architecture
The fitness effects of G73N also were measured in two glasshouse experiments (Exp‐1, Exp‐2) in different G73N biotypes of parental 9425 and WT lines in the absence of herbicide. No difference was observed on germination rates and seedling mortality as were observed in F2 lines (Fig. S4). Differences in plant height and leaf morphology were obvious and noticeable shortly after emergence [Figs 3(b), S5b and S6a). The 9425‐RR plants were consistently shorter than other biotypes throughout the whole plant growth period (Fig. S6), and were 34%, 47% and 54%, and 29%, 39% and 36% shorter than 9425‐RS, ‐SS and WT plants at 10 WAP in Exp‐1 and Exp‐2, respectively [Table 1; Figs 4 and S7]. We were able to recapitulate dwarf phenotypes in F2 lines by introgression of the G73N mutation into the WT background (Fig. 5), indicating the mutation is associated with the phenotype. Regardless of being significantly shorter, 9425‐RR and ‐RS plants had similar GSAs as the SS plants and reduced apical dominance, resulting in a sphere shape plant architecture (Table 1; Figs 4 and S7). This unique tumbling plant form might allow the resistant plants to bounce and roll more easily in the wind to spread their seeds. The WT plants were similar to 9425‐SS plants in plant height and biomass (Figs 4, 5 and S7), but had much smaller GSAs owing to its more upright lateral branches, showing phenotypic variance among kochia populations of different geographical origins. Differences also were observed in root systems of different biotypes: 9425‐RR plants had fewer lateral roots than ‐RS and ‐SS plants, and an overall reduced root biomass of 50% (Fig. S5c; Table 1).
Figure 3.
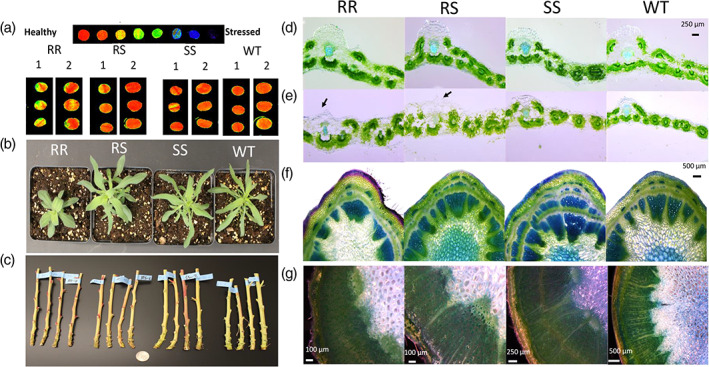
Leaf and stem morphology of different G73N endowing resistant or susceptible genotypes. (a) Example chlorophyll fluorescence images of leaf punches showing different photosynthesis efficiency (1 = Exp‐1, 2 = Exp‐2, 3 leaf punches per genotype); (b) photos of whole plant at three weeks after planting (WAP) from Exp‐1; (c) stems of 9425‐RR, ‐RS, ‐SS and WT plants from Exp‐2 (left to right, four per genotype); (d)–(e) leaf and (f–g) are stem cross‐sections for four‐ and eight‐week‐old plants of different genotypes. In each panel from left to right are 9425‐RR, ‐RS, ‐SS and WT plants, respectively. Arrows in (e) show the distortion of leaf main veins.
Table 1.
Measurement of different plant physiological and morphological traits of different G73N genotypes from the 9425 and WT B. scoparia line
| Fitness measurement | Timing | Genotype | ANOVA † | |||
|---|---|---|---|---|---|---|
| 9425‐RR | 9425‐RS | 9425‐SS | WT | |||
| Fv/Fm | Exp‐1 | 0.630 ± 0.005 (a ‡ ) | 0.645 ± 0.006 (a) | 0.669 ± 0.004 (b) | 0.675 ± 0.008 (b) | <0.0001 |
| Exp‐2 | 0.726 ± 0.005 (a) | 0.739 ± 0.005 (b) | 0.738 ± 0.003 (b) | 0.735 ± 0.004 (ab) | <0.0001 | |
| Leaf thickness (μm) | 4 WAP | 339.6 ± 14.3 (a) | 254.8 ± 5.9 (b) | 291.0 ± 14.8 (bc) | 296.6 ± 16.2 (c) | 0.0007 |
| 8 WAP | 381.1 ± 15.3 (a) | 266.8 ± 17.3 (b) | 217.3 ± 10.4 (c) | 192.1 ± 13.8 (c) | <0.001 | |
| Leaf area (mm2) | 4 WAP | 553.4 ± 21.9 (a) | 530.2 ± 36.5 (a) | 521.0 ± 40.9 (a) | 615.0 ± 52.0 (a) | 0.3437 |
| Leaf length (mm) | 4 WAP | 52.7 ± 1.5 (a) | 66.0 ± 1.6 (b) | 73.4 ± 2.2 (c) | 83.4 ± 1.9 (d) | <0.001 |
| Leaf width (mm) | 4 WAP | 16.6 ± 0.3 (a) | 14.0 ± 0.8 (b) | 12.0 ± 0.4 (c) | 12.0 ± 0.7 (c) | <0.001 |
| Stem diameter (mm) | 4 WAP | 2.8 ± 0.1 (a) | 4.8 ± 0.2 (b) | 5.7 ± 0.2 (c) | 7.1 ± 0.2 (d) | <0.0001 |
| 8 WAP | 4.9 ± 0.1 (a) | 5.7 ± 0.1 (b) | 5.6 ± 0.1 (b) | 6.6 ± 0.3 (c) | <0.0001 | |
| GSA § (°) | Exp‐1 | 64.3 ± 1.8 (a) | 66.3 ± 3.5 (a) | 68.0 ± 2.3 (a) | 43.6 ± 2.5 (b) | <0.0001 |
| Exp‐2 | 65.8 ± 3.0 (a) | 52.9 ± 3.3 (b) | 64.2 ± 2.2 (a) | 36.2 ± 2.3 (c) | <0.0001 | |
| Plant height (cm) | Exp‐1 | 57.7 ± 3.5 (a) | 87.5 ± 5 (b) | 109.7 ± 6.8 (c) | 125.8 ± 3.8 (c) | <0.0001 |
| Exp‐2 | 67.0 ± 2.0 (a) | 94.8 ± 4.3 (b) | 110.2 ± 3.0 (c) | 104.5 ± 4.0 (bc) | <0.0001 | |
| Root weight (g) | 2 WAP | 0.009 ± 0.001 (a) | 0.021 ± 0.003 (b) | 0.024 ± 0.004 (b) | 0.021 ± 0.004 (b) | <0.0001 |
P‐values for ANOVA for the effects of genotype on different fitness traits are listed in the far‐right column.
Different letters represent significant difference among the means at the P = 0.05 level.
Gravitropic set point angles.
Figure 4.
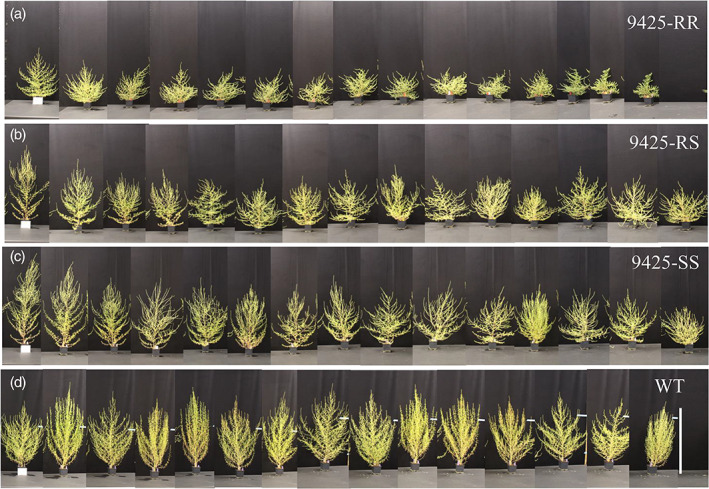
Variations on plant architecture of different G73N genotypes of 9425 and WT kochia lines. Plant biotypes in each panel are (a) homozygous‐resistant (RR), (b) heterozygous‐resistant (RS), (c) homozygous‐sensitive (SS) and (d) WT. Photos were taken 10 weeks after planting. N = 15 per genotype. Scale bar = 100 cm.
Figure 5.
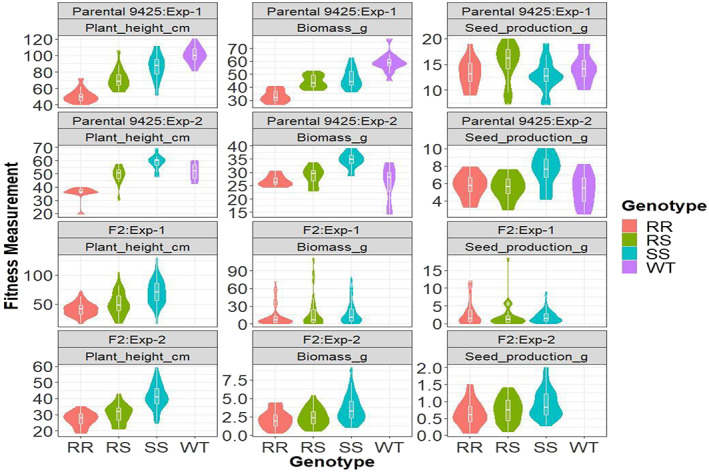
Violin and boxplots of key fitness traits of different G73N genotypes of 9425 and F2 kochia lines. RR, RS and SS stand for homozygous‐resistant (RR), heterozygous‐resistant (RS) and homozygous‐sensitive (SS) plants with or without the G73N mutation. F2 plants were from monoculture pots in the replacement series study (eight plants per pot, total N: Exp‐1, 160; Exp‐2, 128). Parental plants were grown with one plant per pot (total N: Exp‐1, 18; Exp‐2, 15).
3.3. G73N leads to significant leaf and stem morphological changes
In addition to smaller plant statue, significant physiological, morphological and histological changes of leaves and stems also were observed. The leaf blades of 9425‐RR plants were more ovate (20–40% shorter and 20–40% wider), whereas leaves of other biotypes were lanceolate to linear [Table 1; Figs 3(b) and S5(a,b)]. The leaf blades of 9425‐RR plants were 33%, 17% and 14%, and 43%, 75% and 98% thicker than 9425‐RS, ‐SS and WT plants at 4 WAP and 8 WAP, respectively (Table 1). In addition, leaves of 9425‐RR plants also were associated with 2–7% lower photosynthetic efficiency, as indicated by Fv/Fm values [Table 1; Fig. 3(a)]. The Kranz anatomy (thick‐walled mesophyll cells containing large chloroplasts, cluster around bundle‐sheath cells in a ring‐like fashion) of leaves looked largely similar among the genotypes, except that the older leaves (8 WAP) of 9425‐RR and ‐RS plants exhibited obvious disruption on the main veins [Fig. 3(d),(e)].
Furthermore, the stem diameter of 9425‐RR plants was 42%, 51% and 61%, and 12%, 11% and 24% smaller than that of 9425‐RS, ‐SS and WT plants in Exp‐1 and Exp‐2, respectively (Table 1; Fig. S5d–f). Although directionally consistent, the difference in stem diameter between Exp‐1 and Exp‐2 was expected owing to more compact spherical shapes (‘tumbling’) plants observed in Exp‐1 (Figs 4 and S7). Aligned with morphological stem diameter, the cross‐sections indicated that 9425‐RR plants had less developed vascular bundle systems. Both young (4 WAP) and matured stems (8–10 WAP) of 9425‐RR plants had fewer secondary xylem cells and interfascicular fibers, that provide the mechanical support to plant stems, compared to 9425‐SS and WT plants [Figs 3(f),(g) and S5d‐f], resulting in increased lodging propensity [Fig. 4 and S6(c)]. Interestingly, the diminished plant size and vegetative growth defects were translated into lower biomass accumulation but not seed production in both the F2 and parental line (Fig. 5).
3.4. G73N leads to impaired competitiveness in the replacement series study
Replacement series studies on F2 lines indicated that resistant plants were very vulnerable to intraspecies competition [Fig. 6(a); Table 2]. The RY values for the RR biotype were consistently lower than the expected values (0.25, 0.5, 0.75) for both biomass and seed production in both experiments. By contrast, the RY values of the SS biotype were mostly higher than (Exp‐1) or equal to (Exp‐2) the expected values [Fig. 6(a)]. The resulting total RY values (RYT) from the two competing biotypes were equal to or lower than the expected value of 1, indicating that the RR and SS biotypes likely share similar niches and compete fully for the limiting resource(s). Interestingly, RS plants were less competitive than SS biotypes in Exp‐1 [Fig. 6(a)], but were equally competitive with SS plants in Exp‐2, indicating that the manifestation of fitness cost might be environmentally dependent.
Figure 6.
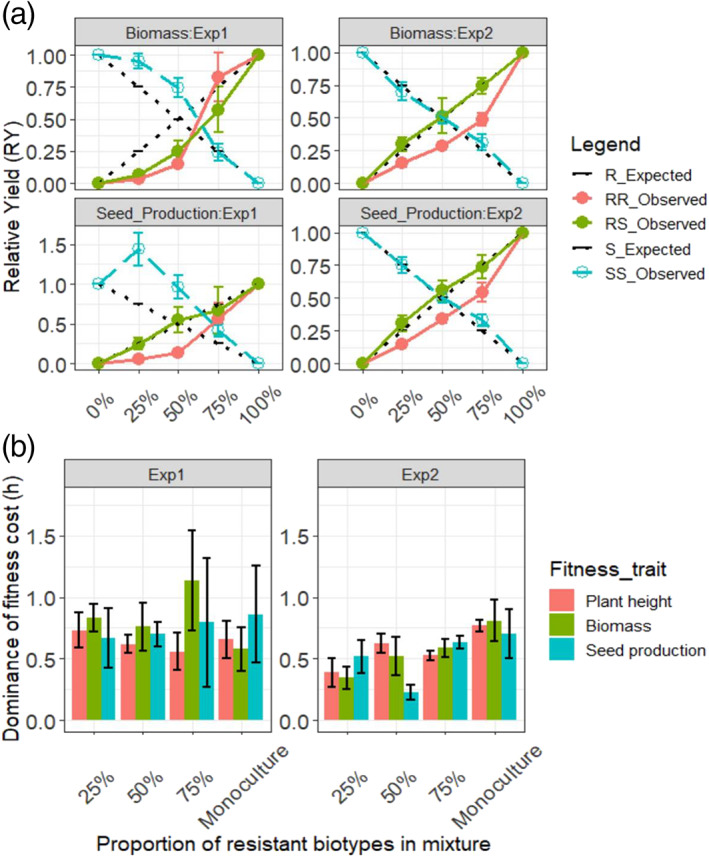
Plant competitiveness and dominance of the resistance costs through the replacement series studies on F2 lines. (a) Relative yield (RY) of different G73N genotypes of F2 line across different competition levels. RY values were calculated from biomass and seed production of two experiments. Dashed lines indicate the expected hypothetical values (H0 = 0, 0.25, 0.5, 0.75, 1) for RY, which indicates the two competing genotypes (RR versus SS or RS versus SS) are equally competitive. (b) Dominance of fitness costs.
Table 2.
Comparison of different replacement indices calculated from biomass and seed production data collected on F2 lines
| Fitness traits | Genotype | Exp‐1 | Exp‐2 | ||||
|---|---|---|---|---|---|---|---|
| Replacement indices (median/mean†) | Replacement indices (median/mean) | ||||||
| RCC | CR | AI | RCC | CR | AI | ||
| Biomass | RR | 0.197 | 3.814 | 0.327 | 0.409 | 1.693 | 0.208 |
| RS | 0.213 | 3.429 | 0.360 | 1.209 | 1.011 | −0.026 | |
| SS | 1.614 | 1.008 | |||||
| Seed production | RR | 0.304 | 5.194 | 0.651 | 0.423 | 1.697 | 0.216 |
| RS | 0.435 | 2.555 | 0.460 | 1.238 | 1.024 | 0.001 | |
| SS | 0.726 | 1.124 | |||||
| Comparison | Fitness cost‐P‐value | Fitness cost‐P‐value | |||||
|---|---|---|---|---|---|---|---|
| RCC | CR > 1 | AI > 0 | RCC | CR > 1 | AI > 0 | ||
| Biomass | RR versus SS | 0.112 | 0.020* | 0.045* | 0.023* | 0.001* | 0.001* |
| RS versus SS | 0.029* ,† | 0.006* | 0.007* | 0.486 | 0.941 | 0.733 | |
| Seed production | RR versus SS | 0.813 | 0.012* | 0.001* | 0.017* | 0.007* | 0.002* |
| RS versus SS | 1.000 | 0.006* | 0.002* | 0.697 | 0.830 | 0.982 | |
Indicate statistical significance at P = 0.05.
Similar results were observed on the secondary replacement series indices, as RCC values of SS plants were consistently higher than RR or RS plants in both experiments, and CR and AI values (Table 2) were significantly greater than 1 and 0, respectively. Interestingly, although competition can lead to 80–90% reduction in biomass in RR plants, they had similar reproductive ability with RS and SS plants when RR become the prominent genotypes (e.g. 75% resistance proportion or in monoculture) (Figs 5 and S8). This suggests that the reduced reproductivity was more likely to have been the result of suppression from plants with bigger sizes other than lower inherent fecundity caused by G73N.
3.5. The fitness costs of G73N is largely semidominant and fluctuate with environments
The quantification of the dominance of the fitness effects of G73N on F2 lines appeared to vary with the fitness traits measured. Plant height and biomass of RS plants were mostly intermediate to those of RR and SS plants, showing a semidominant (h = 0.5) fitness cost [Fig. 6(b)]. Furthermore, the fitness of RS biotypes also varied among the two experiments: In Exp‐1, h was between 0.5 and 1, leaning toward a dominant fitness costs, whereas in Exp‐2, h was generally lower and even leaned toward slightly recessive at higher competition levels when the proportion of RR or RS plants was <50%; however, none of the h values significantly deviated from 0.5. This again suggests that RS plants can have limited or negligible fitness cost, depending on the growth environments.
4. DISCUSSION
Auxin is one of the main regulators of plant development and controls a wide variety of plant responses and stress adaptation. 33 , 34 , 35 Due to the importance of the auxin pathway in plant growth and development, the evolution of synthetic auxin resistance through mutating critical proteins such as auxin receptors was at one point thought to be difficult, and likely associated with a high fitness penalty, if not lethality. 36 The elucidation of a molecular mechanism of synthetic auxin resistance in a natural weed population, provided an unique opportunity for detailed characterization of the resistance gene's contribution to plant fitness, both in the presence and absence of synthetic auxin herbicides.
Dose–response study on cross‐resistance to dicamba, 2,4‐d and fluroxypyr at the genotype level directionally agrees with the molecular and phenotypic data reported previously, 7 except that we observed generally lower R/S ratios especially for fluroxypyr resistance. The difference, we believe, is mainly due to visual injury instead of fresh weight data being used to fit the dose–response models. Highly stunted plants treated with synthetic auxins could still have a substantial amount of fresh weight. Also, kochia is a facultative short‐day plant species whose plant sizes vary with growth conditions (e.g. photoperiod), which could account for some variance on the magnitude of resistance. 37 , 38 Therefore, we believe that visual injury combined with mortality data, as reported in the current study, is a better proxy with which to characterize resistance to synthetic auxin herbicides (Fig. S3). Our results align with the visual injury data collected by LeClere et al. 7 (Appendix S2), as well as the molecular and root length studies. 7 Nevertheless, although G73N might not be the major causative gene for fluroxypyr resistance, it might still present concerns on facilitating the evolution of full resistance to fluroxypyr. A study using sublethal rates of 2,4‐d and recurrently selecting the survivors in Palmer amaranth (Amaranthus palmeri), demonstrated a shift to resistance after three generations, 39 likely resulting from the accumulation of resistance alleles with minor effects. 40 It is likely when the herbicides are applied under suboptimal spraying conditions and/or use of sublethal rates with reduced herbicide effectiveness, resistance allele(s) with minor effects (e.g. G73N to fluroxypyr) might be selected, accumulated and gradually shift a less susceptible population towards resistance.
In the absence of herbicide selection pressure, G73N led to profound vegetative growth defects, altered overall plant architecture and reduced biomass accumulation. This is not unexpected because AUX/IAAs play pivotal roles and are expressed in many tissues and throughout plant growth and development. 6 , 41 It has been well‐documented in the model species Arabidopsis thaliana that AUX/IAA mutants often lead to varied aberrant phenotypes, similar to what we observed in kochia: diminished adult size, decreased shoot apical dominance, abnormal gravitropism (e.g. GSAs of lateral shoots) and fewer lateral roots. 41 , 42 , 43 , 44 Our results also agree with most other fitness studies in synthetic auxin resistance kochia or other weed species which reported up to 90% fitness cost. 16 , 19 , 45 , 46
It is worth mentioning that reduced fitness could be partially due to inbreeding depression because the 9425 parental line was maintained through SSD breeding. 6 , 47 , 48 However, for a prominently outcrossing species such as kochia, multiple generations of self‐pollination also can purge some of the strongly deleterious, nearly recessive mutations endowing inbreeding depression from the genome. 49 , 50 , 51 , 52 , 53 Furthermore, the generation of F2 lines through introgression and shuffling of G73N into an unrelated and highly outcrossing WT line (Fig. 1), might serve as a genetic rescue method that helps bring beneficial variants, minimizing inbreeding depression in the 9425 line. Lastly, we observed highly distinguishable phenotypes (e.g. plant height and architecture, biomass) (Fig. 4) associating with different genotypes of G73N with similar inbreeding levels, and no reduction on fecundity when grown in monoculture 7 (Fig. 5), which are all strong evidences that inbreeding depression was not a major factor that can mask the effects of G73N in our kochia populations.
Through microscopy work and chlorophyll imaging, we identified specific morphological changes in leaf, stem and overall plant architecture that might have contributed to lower vegetative fitness in kochia. First, leaves of G73N mutant plants were associated with reduced photosynthesis efficiency, although this might be transient and limited to seedling stage. Mutant kochia plants also displayed increased leaf thickness and altered leaf shape, which might affect the thermoregulation of leaves under heat stress and the ability of resistant plants to optimize light interception. 54 , 55 , 56 Furthermore, G73N led to less‐developed vascular bundle systems and smaller stem diameter, resulting in spherically shaped plants with higher lodging propensity. Together these changes might compromise the ability of resistant plants to build biomass and produce seeds, especially under competition, as seen in the replacement series study on F2 lines. Interestingly, the spherical and bush shape of RR plants might present some ecological advantages as it might facilitate the spread of resistant alleles across the landscape through tumbling.
Replacement series studies were previously conducted in other kochia populations with unknown resistance mechanisms, in which vegetative traits such as plant height, stem diameter and primary branches were measured. 20 In the current study, we calculated the replacement series indexes (RY, RCC, CR, AI) based on biomass and seed production, which are considered to be better proxies of plant fitness. 57 Our results generally agree with Kumar and Jha 20 in that the resistant plants were much less competitive than the sensitive plants as RY values significantly deviated from their expected values across different growth conditions in the two experiments (Summer versus late Autumn). Furthermore, secondary replacement series indexes such as RCC, CR and AI values also supported higher competitiveness of susceptible plants. The differences in the RCC values were mostly insignificant in Exp‐1. This is likely the consequence of some negative RCC values brought by the heterogenous responses of the plants grown in monoculture and mixture, which suggests that RCC values might not be a very robust index with which to describe the interfering effects in the current study. 31
In this study, we also measured the fitness cost of G73N in both homozygous and heterozygous status and found that deleterious effects of G73N are trait‐dependent and might be magnified or ameliorated by different ecological conditions. 26 It also indicates that employment of weed management to sustain the spread of RS plants might be challenging, and that their effectiveness might vary with environments. Previously, Roux et al. 22 quantified the dominance of the cost of 2,4‐d resistance endowed by three mutations at the same gene in Arabidopsis and reported a wide range of results from recessivity (axr1‐3) to dominance (axr2‐1) and to underdominance (aux1‐7). This indicates that each AUX/IAA mutation might be independent from each other and our current observations on G73N might not predict other naturally occurring mutations for synthetic auxin resistance. It is also possible that G73N might only be a transition step during the resistance evolutionary trajectory, which may be replaced by other resistance mutations or mechanisms with lower fitness cost, as has been found for triazine resistance in common waterhemp (Amaranthus tuberculatus). 58 , 59 , 60
5. CONCLUSIONS
The current study presents the first precise and systemic measurement of the fitness costs of synthetic auxin resistance with known genetic basis, allowing us to unequivocally attribute fitness costs to a specific resistance endowing mutation G73N. We demonstrated that G73N leads to significant vegetative growth defects and impaired competitiveness, which might help explain the relative slower resistance evolution for the herbicide. These results suggest that subjecting G73N mutant kochia plants to substantial competition to restrain light, water and nutrient accessibility (e.g. through the adoption of cover crop or narrower crop row spacing), 61 , 62 will potentially be an effective approach to decrease the frequencies of resistant biotypes. Interestingly, even though resistant plants produced much less biomass and seeds under competition, they appeared to have similar fecundity as sensitive plants in the absence of competition. More study is imminent to determine the impact of G73N on kochia's reproductive growth, especially its unique mating system, to understand the ecological consequences of novel resistance mutations. Nevertheless, it is apparent that there is no single silver bullet to reduce the kochia problem and, thus, that growers must follow integrated weed management approaches that harness evolutionary approaches, as pointed by Neve et al. 63 and Norsworthy et al., 64 to diversify our weed control programs to delay development of synthetic auxin resistance.
AUTHOR CONTRIBUTIONS
CW, SL and RS conceived the project; CW and MP performed the research with assistance from SL and AP; KL and CW conducted statistical analysis; and CW, PW and RS wrote the manuscript with contributions from all other authors.
CONFLICT OF INTEREST
CW, MP, SL, KL and AP are employed by Bayer CropScience.
DATA AVAILABILITY
Data for the current study is available at https://doi.org/10.5061/dryad.2v6wwpzk6.
Supporting information
Appendix S1: Supporting information.
Appendix S2: Supporting information.
ACKNOWLEDGEMENTS
The authors want to thank Adam Davis, Vipan Kumar and Doug Eudy for their valuable discussions on the replacement series study and inbreeding depression; Cindy Trembly, Jeff Haines, Megan Gilley, and Jenny Krebel for plant propagation and herbicide application; Ying Li for helping with leaf cross‐sections; and Chandra Aradhya and Jeff Stein for proof‐reading and editing the manuscript.
The paper was given, in part, at Weed Science Society of America's annual meeting in March 2020.
REFERENCES
- 1. Grossmann K, Auxin herbicides: current status of mechanism and mode of action. Pest Manage Sci 66:113–120 (2010). [DOI] [PubMed] [Google Scholar]
- 2. Grossmann K, Auxin herbicide action. Plant Signal Behav 2:421–423 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heap I, The International survey of herbicide resistant weeds: weeds resistant to synthetic auxins (O/4). Available: http://www.weedscience.org/Summary/MOA.aspx [30 April 2020].
- 4. Kraehmer H, Laber B, Rosinger C and Schulz A, Herbicides as weed control agents: state of the art: I. Weed control research and safener technology: the path to modern agriculture. Plant Physiol 166:1119–1131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Busi R, Goggin DE, Heap I, Horak MJ, Jugulam M, Masters RA et al, Weed resistance to synthetic auxin herbicides. Pest Manage Sci 74:2265–2276 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Preston C, Belles DS, Westra PH, Nissen SJ and Ward SM, Inheritance of resistance to the auxinic herbicide dicamba in Kochia (Bassia scoparia). Weed Sci 57:43–47 (2009). [Google Scholar]
- 7. LeClere S, Wu C, Westra P and Sammons RD, Cross‐resistance to dicamba, 2,4‐D, and fluroxypyr in Bassia scoparia is endowed by a mutation in an AUX/IAA gene. Proc Nat Acad Sci U S A 115:2911–2920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Undersander DJ, Durgan BR, Kaminski AR, Doll JD, Worf GL and Schulte EE, The Alternative field crops manual: kochia. Available: https://hort.purdue.edu/newcrop/afcm/kochia.html [30 April 2020].
- 9. Friesen LF, Beckie HJ, Warwick SI and Van Acker RC, The biology of Canadian weeds. 138. Bassia scoparia (L.) Schrad. Can J Plant Sci 89:141–167 (2009). [Google Scholar]
- 10. Phillips WM and Launchbaugh JL, Preliminary studies of the root system of Bassia scoparia at Hays, Kansas. Weeds 6:19–23 (1958). [Google Scholar]
- 11. Stallings GP, Thill DC, Mallory‐Smith CA and Shafii B, Pollen‐mediated gene flow of sulfonylurea‐resistant kochia (Bassia scoparia). Weed Sci 43:95–102 (1995). [Google Scholar]
- 12. Becker DA, Stem abscission in tumbleweeds of the Chenopodiaceae: kochia. Am J Bot 65:375–383 (1978). [Google Scholar]
- 13. Baker DV, Beck KG, Bienkiewicz BJ and Bjostad LB, Forces necessary to initiate dispersal for three tumbleweeds. Invas Plant Sci Mana 1:59–65 (2008). [Google Scholar]
- 14. Mengistu LW and Messersmith CG, Genetic diversity of kochia. Weed Sci 50:498–503 (2002). [Google Scholar]
- 15. Beckie HJ, Hall LM, Shirriff SW, Martin E and Leeson JY, Triple‐resistant kochia [Kochia scoparia (L.) Schrad.] in Alberta. Can J Plant Sci 99:281–285 (2019). [Google Scholar]
- 16. Hall JC and Romano ML, Morphological and physiological differences between the auxinic herbicide‐susceptible (S) and herbicide‐resistant (R) wild mustard (Sinapis arvensis L.) biotypes. Pestic Biochem Phys 52:149–155 (1995). [Google Scholar]
- 17. Bourdôt GW, Saville DJ and Hurrell GA, Ecological fitness and the decline of resistance to the herbicide MCPA in a population of Ranunculus acris . J Appl Ecol 33:151–160 (1996). [Google Scholar]
- 18. Cranston HJ, Kern AJ, Hackett JL, Miller EK, Maxwell BD and Dyer WE, Dicamba resistance in Kochia. Weed Sci 49:164–170 (2001). [Google Scholar]
- 19. Goss GA and Dyer WE, Physiological characterization of auxinic herbicide‐resistant biotypes of kochia (Bassia scoparia). Weed Sci 51:839–844 (2003). [Google Scholar]
- 20. Kumar V and Jha P, Differences in germination, growth, and fecundity characteristics of dicamba‐fluroxypyr‐resistant and susceptible Bassia scoparia . PLoS One 11:e0161533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coustau C, Chevillon C and ffrench‐Constant R, Resistance to xenobiotics and parasites: can we count the cost? Trends Ecol Evol 15:378–383 (2000). [DOI] [PubMed] [Google Scholar]
- 22. Roux F, Gasquez J and Reboud X, The dominance of the herbicide resistance cost in several Arabidopsis thaliana mutant lines. Genetics 166:449–460 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roux F, Camilleri C, Giancola S, Brunel D and Reboud X, Epistatic interactions among herbicide resistances in Arabidopsis thaliana: the fitness cost of multiresistance. Genetics 171:1277–1288 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roux F and Reboud X, Is the cost of herbicide resistance expressed in the breakdown of the relationships between characters? A case study using synthetic auxin resistant Arabidopsis thaliana mutants. Gen Res Camb 85:101–110 (2005). [DOI] [PubMed] [Google Scholar]
- 25. Sellis D, Callahan BJ, Petrov DA and Messer PW, Heterozygote advantage as a natural consequence of adaptation in diploids. PNAS 108:20666–20671 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gassmann AJ, Onstad DW and Pittendrigh BR, Evolutionary analysis of herbivorous insects in natural and agricultural environments. Pest Manage Sci 65:1174–1181 (2009). [DOI] [PubMed] [Google Scholar]
- 27. Frenkel E, Matzrafi M, Rubin B and Peleg Z, Effects of environmental conditions on the fitness penalty in Brachypodium hybridum . Front Plant Sci 8:94 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al, Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9:676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baker NR, Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113 (2008). [DOI] [PubMed] [Google Scholar]
- 30. Jolliffe PA, The replacement series. J Ecol 88:371–385 (2000). [Google Scholar]
- 31. Weigelt A and Jolliffe P, Indices of plant competition. J Ecol 91:707–720 (2003). [Google Scholar]
- 32. Ritz C, Baty F, Streibig JC and Gerhard D, Dose‐response analysis using R. PLoS One 10:e0146021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saini S, Sharma I, Kaur N and Pati PK, Auxin: a master regulator in plant root development. Plant Cell Rep 32:741–757 (2013). [DOI] [PubMed] [Google Scholar]
- 34. Benjamins R and Scheres B, Auxin: the looping star in plant development. Annu Rev Plant Biol 59:443–465 (2008). [DOI] [PubMed] [Google Scholar]
- 35. Park CM, Auxin homeostasis in plant stress adaptation response. Plant Signal Behav 24:306–307 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Behrens MR, Mutlu N, Chakaborty S, Dumitru R, Wen ZJ, LaVallee BJ et al, Dicamba resistance: enlarging and preserving biotechnology‐based weed management strategies. Science 316:1185–1188 (2007). [DOI] [PubMed] [Google Scholar]
- 37. Ganie ZA, Jugulam M and Jhala AJ, Temperature influences efficacy, absorption, and translocation of 2,4‐D or glyphosate in glyphosate‐resistant and glyphosate‐susceptible common rag‐weed (Ambrosia artemisiifolia) and giant ragweed (Ambrosia trifida). Weed Sci 65:588–602 (2017). [Google Scholar]
- 38. Ou J, Stahlman PW and Jugulam M, Reduced absorption of glyphosate and decreased translocation of dicamba contribute to poor control of kochia (Kochia scoparia) at high temperature. Pest Manage Sci 74:1134–1142 (2018). [DOI] [PubMed] [Google Scholar]
- 39. Ashworth MB, Walsh MJ, Flower KC and Powles SB, Recurrent selection with reduced 2,4‐D amine doses results in the rapid evolution of 2,4‐D herbicide resistance in wild radish (Raphanus raphanistrum L.). Pest Manage Sci 72:2091–2098 (2016). [DOI] [PubMed] [Google Scholar]
- 40. Busi R, Neve P and Powles S, Evolved polygenic herbicide resistance in Lolium rigidum by low‐dose herbicide selection within standing genetic variation. Evol Appl 6:231–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rinaldi MA, Liu J, Enders TA, Bartel B and Strader LC, A gain‐of‐function mutation in IAA16 confers reduced responses to auxin and abscisic acid and impedes plant growth and fertility. Plant Mol Biol 79:359–373 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rogg LE, Lasswell J and Bartel B, A gain‐of‐function mutation in IAA28 suppresses lateral root development. Plant Cell 13:465–480 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park JY, Kim HJ and Kim J, Mutation in domain II of IAA1 confers diverse auxin‐related phenotypes and represses auxin‐activated expression of aux/IAA genes in steroid regulator‐inducible system. Plant J 32:669–683 (2002). [DOI] [PubMed] [Google Scholar]
- 44. Roychoudhry S, Del Bianco M, Kieffer M and Kepinski S, Auxin controls gravitropic setpoint angle in higher plant lateral branches. Curr Biol 23:1497–1504 (2013). [DOI] [PubMed] [Google Scholar]
- 45. Roux F, Giancola S, Durand S and Reboud X, Building of an experimental cline with Arabidopsis thaliana to estimate herbicide fitness cost. Genetics 173:1023–1031 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rahman A, James T and Trolove M, Characteristics and control of dicamba‐resistant common lambsquarters (Chenopodium album). Weed Biol Manage 14:88–98 (2014). [Google Scholar]
- 47. Hedrick PW and Garcia‐Dorado A, Understanding inbreeding depression, purging, and genetic rescue. Trends Ecol Evol 31:940–952 (2016). [DOI] [PubMed] [Google Scholar]
- 48. Roff DA, Inbreeding depression: tests of the overdominance and partial dominance hypotheses. Evolution 56:768–775 (2002). [DOI] [PubMed] [Google Scholar]
- 49. Guttieri MJ, Eberlein CV and Souza EJ, Inbreeding coefficients of field populations of Kochia scoparia using chlorsulfuron resistance as a phenotypic marker. Weed Sci 46:521–525 (1998). [Google Scholar]
- 50. Barrett SCH and Charlesworth D, Effects of a change in the level of inbreeding on the genetic load. Nature 352:522–524 (1990). [DOI] [PubMed] [Google Scholar]
- 51. Johnston MO and Schoen DJ, Correlated evolution of self‐fertilization and inbreeding depression: an experimental study of nine populations of Amsinckia (Boraginaceae). Evolution 50:1478–1491 (1996). [DOI] [PubMed] [Google Scholar]
- 52. Carr DE and Dudash MR, Inbreeding depression in two species of Mimulus (Scrophulariaceae) with contrasting mating systems. Am J Bot 83:586–593 (1996). [Google Scholar]
- 53. Busch JW, Inbreeding depression in self‐incompatible and self‐compatible populations of Leavenworthia alabamica . Heredity 94:159–165 (2005). [DOI] [PubMed] [Google Scholar]
- 54. Leigh A, Sevanto S, Ball MC, Close JD, Ellsworth DS, Knight CA et al, Do thick leaves avoid thermal damage in critically low wind speeds? New Phytol 194:477–487 (2012). [DOI] [PubMed] [Google Scholar]
- 55. Jones CS, Bakker FT, Schlichting CD and Nicotra AB, Leaf shape evolution in the south African genus Pelargonium L' her. (Geraniaceae). Evolution 63:479–497 (2009). [DOI] [PubMed] [Google Scholar]
- 56. Nicotra A, Leigh A, Boyce CK, Jones CD, Niklas KJ, Royer DL et al, The evolution and functional significance of leaf shape in the angiosperms. Funct Plant Biol 38:535–552 (2011). [DOI] [PubMed] [Google Scholar]
- 57. Younginger BS, Sirova D, Cruzan MB and Ballhorn DJ, Is biomass a reliable estimate of plant fitness? Appl Plant Sci 5:1600094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ou J, Stahlman PW and Jugulam M, Reduced absorption of glyphosate and decreased translocation of dicamba contribute to poor control of kochia (Bassia scoparia) at high temperature. Pest Manage Sci 74:1134–1142 (2017). [DOI] [PubMed] [Google Scholar]
- 59. Shyam C, Jhala AJ, Kruger G and Jugulam M, Rapid metabolism increases the level of 2,4‐D resistance at high temperature in common waterhemp (Amaranthus tuberculatus). Sci Rep 9:16695 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu C, Davis AS and Tranel PJ, Limited fitness costs of herbicide‐resistance traits in Amaranthus tuberculatus facilitate resistance evolution. Pest Manage Sci 74:293–301 (2017). [DOI] [PubMed] [Google Scholar]
- 61. Osipitan OA, Dille A, Assefa Y, Radicetti E, Ayeni A and Knezevic SZ, Impact of cover crop management on level of weed suppression: a meta‐analysis. Crop Sci 59:833–842 (2019). [Google Scholar]
- 62. Chauhan BS, Florentine SK, Ferguson JC and Chechetto RG, Implications of narrow crop row spacing in managing weeds in mungbean (Vigna radiata). Crop Prot 95:116–119 (2017). [Google Scholar]
- 63. Neve P, Vila‐Aiub M and Roux F, Evolutionary‐thinking in agricultural weed management. New Phytol 184:783–793 (2009). [DOI] [PubMed] [Google Scholar]
- 64. Norsworthy JK, Ward S, Shaw D, Llewellyn R, Nichols R, Webster T et al, Reducing the risks of herbicide resistance: best management practices and recommendations. Weed Sci 60:31–62 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information.
Appendix S2: Supporting information.
Data Availability Statement
Data for the current study is available at https://doi.org/10.5061/dryad.2v6wwpzk6.


