Abstract
Objective
Increasing magnesium intake might reduce the risk of cardiovascular disease (CVD). Whether potential effects on cortisol contribute to these beneficial effects on cardiovascular health remains unclear. We therefore studied effects of long‐term oral magnesium supplementation on glucocorticoid metabolism, specifically on the excretion of urinary cortisol, cortisone and their metabolites, as well as on the ratios reflecting enzymatic activity of 11β‐hydroxysteroid dehydrogenases (11β‐HSDs) and A‐ring reductases.
Design
A post‐hoc analysis of a randomized trial with allocation to a magnesium supplement (350 mg/day) or a placebo for 24‐week.
Patients
Forty‐nine overweight men and women, aged between 45 and 70 years.
Measurements
Cortisol, cortisone and their metabolites (tetrahydrocortisol [THF], allo‐tetrahydrocortisol [allo‐THF] and tetrahydrocortisone [THE]) were measured in 24‐h urine samples. Enzymatic activities of 11β‐HSD overall and of 11β‐HSD type 2 were estimated as the urinary (THF + allo‐THF [THFs])/THE and cortisol/cortisone ratios, respectively. A‐ring reductase activity was assessed by ratios of THF/allo‐THF, allo‐THF/cortisol, THF/cortisol and THE/cortisone.
Results
After 24‐week, urinary cortisol excretion was decreased in the magnesium group as compared with the placebo group (−32 nmol/24‐h, 95% CI: −59; −5 nmol/24‐h, p = .021). Ratios of THFs/THE and cortisol/cortisone were decreased following magnesium supplementation by 0.09 (95% CI: 0.02; 0.17, p = .018) and 0.10 (95% CI: 0.03; 0.17, p = .005), respectively. No effects were observed on A‐ring reductase activity.
Conclusions
We observed a beneficial effect of magnesium supplementation towards a lower 24‐h urinary cortisol excretion together with an increased activity of 11β‐HSD type 2. Our findings may provide another potential mechanism by which increased magnesium intake lowers CVD risk (ClinicalTrials.gov identifier: NCT02235805).
Keywords: cardiovascular disease, glucocorticoids, magnesium, metabolism, obesity, randomized controlled trial
1. INTRODUCTION
Low dietary magnesium intake has been associated with increased risk of cardiovascular disease (CVD). 1 Specifically, we already observed a clinically relevant improvement in arterial stiffness after long‐term oral magnesium supplementation, 2 which may underlie the beneficial effects of magnesium on CVD events.
Previous studies have also suggested a role for cortisol in CVD risk. 3 , 4 For instance, excess levels of cortisol in patients with Cushing's syndrome have been linked to alterations in the vascular system, including increased arterial stiffness and impaired endothelial function. 5 , 6 Several enzymes, including 11β‐hydroxysteroid dehydrogenases (11β‐HSDs) and A‐ring reductases, regulate intracellular glucocorticoid metabolism and have been suggested to play an important role in the pathogenesis of metabolic syndrome, which is in turn associated with increased risk of CVD. 7 , 8 , 9 11β‐HSDs are primarily responsible for the conversion of inert cortisone to active cortisol and vice versa. In fact, two iso‐enzymes of 11β‐HSD have been identified: type 1 and type 2. The former is mainly expressed in the liver and adipose tissue and converts the inactive cortisone into the active cortisol, 10 while the latter is primarily expressed in the kidney and colon and is responsible for the conversion of cortisol into cortisone. 11 , 12 On the other hand, A‐ring reductases regulate the conversion of cortisol and cortisone to their metabolites tetrahydrocortisol (THF), allo‐tetrahydrocortisol (allo‐THF) and tetrahydrocortisone (THE). The glucocorticoid metabolism is depicted in Figure 1. Enzyme activity of 11β‐HSDs and A‐ring reductases can be quantified by the ratios of 24‐h urinary excretions of cortisol, cortisone and their metabolites, which provides insight into the intracellular production and clearance of cortisol. 13 , 14
FIGURE 1.
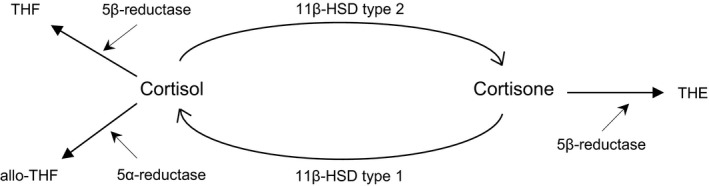
Schematic overview of glucocorticoid metabolism. 11β‐HSD type 1 converts cortisone into cortisol causing increased intracellular cortisol levels, while 11β‐HSD type 2 decreases intracellular cortisol levels by converting cortisol into cortisone. 5α‐ and 5β‐reductase enzymes inactivates cortisol and cortisone to their inactive metabolites THF, allo‐THF and THE, respectively. 11β‐HSD, 11β‐hydroxysteroid dehydrogenase; THF, tetrahydrocortisol; allo; THF, allo‐tetrahydrocortisol; THE, tetrahydrocortisone
Several preclinical studies have shown that magnesium plays a role in glucocorticoid metabolism. 15 , 16 , 17 In fact, dietary magnesium restriction resulted in an altered glucocorticoid metabolism. To date, no human intervention trials have addressed effects of oral magnesium supplementation on glucocorticoid metabolism.
Here, we performed a post‐hoc analysis of a previously performed double‐blind placebo‐controlled intervention trial with the primary aim to study the effect of magnesium supplementation on arterial stiffness, measured by carotid‐to‐femoral pulse wave velocity. In the current study, we investigated effects of long‐term oral magnesium supplementation on glucocorticoid metabolism, specifically on 24‐h urinary excretions of cortisol, cortisone and their metabolites, as well as on enzyme activity of 11β‐HSD and A‐ring reductases.
2. MATERIALS AND METHODS
2.1. Study population
This post‐hoc analysis is part of an intervention trial, in which healthy overweight and slightly obese men and women received either a magnesium citrate supplement or a placebo for 24‐week. Details of the study characteristics have been described before. 2 In brief, eligible participants were men and postmenopausal women, aged between 45 and 70 years old, and with a body mass index (BMI) between 25–35 kg/m2. They had no indications for treatment with cholesterol‐lowering medications; no active cardiovascular disease; no active inflammatory disease; no endocrine disorders, such as Cushing's syndrome, primary aldosteronism or adrenal incidentaloma; and no drug or alcohol abuse. Participants were ineligible for participation if they received proton‐pump inhibitors, anti‐hypertensive medication or drugs known to affect serum lipid or plasma glucose metabolism. All study participants gave written consent before the screening visit. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and was approved by the Medical Ethics Committee of Maastricht University Medical Center. The study was registered at ClinicalTrials.gov as NCT02235805.
2.2. Study design
The intervention trial had a randomized, double‐blind, placebo‐controlled, parallel‐group design. Study participants were allocated to receive either a magnesium citrate supplement or a placebo for 24‐week using a computer‐generated randomization scheme stratified by sex. The total daily dose of magnesium was 350 mg (Magnesium Citrate Complex (Mg 16%). Placebo capsules contained starch (Amylum Solani). Capsules were provided in blister strips. Participants were requested to return all blisters at the next visit, including any unused capsules that were counted as a measure of compliance. Furthermore, participants were requested to maintain their usual diet (including consumption of alcohol), and their physical activity levels during the study period. For the current post‐hoc analysis, we used 24‐h urine samples that were collected at baseline and at the end of the trial.
2.3. Measurements
Participants received strict instructions regarding the collection of 24‐h urine. They discarded the first morning urine samples and collected all urine for the following 24‐h. A separate container was used to collect all urine during the night and the first morning urine sample of the next morning after waking. Participants were requested to empty their bladder completely before going to sleep. On the day before blood sampling, participants were requested not to consume alcohol, nor perform strenuous physical activities. In addition, participants were requested not to consume any foods or drinks (except for water) after 08.00 pm on the day before blood sampling. Measurements were performed at the Metabolic Research Unit Maastricht (MRUM) research facilities. Fasting blood samples were taken from a forearm vein by venipuncture. Blood drawn in vacutainer serum tubes (Becton, Dickinson and Company) was first allowed to clot for at least 30 min at 21°C. Serum separator tubes were then centrifuged at 1300 g for 15 min at 21°C. Serum and urine magnesium were determined photometrically with a Magnesium and Calcium Gen.2 assay (COBAS, Roche Diagnostics GmbH) by the Central Diagnostic Laboratory Maastricht University Medical Center. Cortisol, cortisone, THF, allo‐THF and THE were measured in 24‐h urine samples by isotope dilution liquid chromatography tandem mass spectrometry at the University Medical Center Groningen (UMCG), as previously described. 18 Total production of urinary cortisol, cortisone and their metabolites was calculated as the sum of the following metabolites: (urinary cortisol excretion (nmol/24‐h)/1000) + (urinary cortisone excretion (nmol/24‐h)/1000) + urinary THF excretion (µmol/24‐h) + urinary allo‐THF excretion (µmol/24‐h) + urinary THE excretion (µmol/24‐h). Technicians at the MRUM and at the UMCG were not aware of the treatments of the participants.
2.4. Enzyme activities of 11β‐HSDs and A‐ring reductases
Overall 11β‐HSD enzymatic activity was calculated using the ratio of urinary metabolites of cortisol and cortisone (THF + allo‐THF (THFs))/THE. 13 The ratio of cortisol/cortisone accurately reflects the kidney 11β‐HSD type 2 enzyme activity. 14 A‐ring reductases, including 5α‐ and 5β‐reductase, can be inferred by the ratio of THF/allo‐THF (combined 5α‐ and 5β‐ reductase activity), allo‐THF/cortisol (5α‐reductase activity), THF/cortisol (5β‐reductase activity) and THE/cortisone (5β‐reductase activity). The ratios are summarized in Table 1.
TABLE 1.
Summary of ratios
| Ratio | Index |
|---|---|
| Cortisol/cortisone | 11β‐HSD type 2 activity |
| THFs/THE | 11β‐HSD overall activity |
| THF/allo‐THF | 5α‐ and 5β‐reductase activity |
| allo‐THF/cortisol | 5α‐reductase activity |
| THF/cortisol | 5β‐reductase activity |
| THE/cortisone | 5β‐reductase activity |
Abbreviations: 11β‐HSD, 11β‐hydroxysteroid dehydrogenase; THE, tetrahydrocortisone; THFs, tetrahydrocortisol and allo‐tetrahydrocortisol.
2.5. Statistical analyses
The study was powered on the primary endpoint (carotid‐to‐femoral pulse wave velocity) of the intervention trial. A detailed power calculation can be found in our previous paper. 2 An intention‐to‐treat analysis (using the last observation carried forward approach) was performed. Normality of data was assessed by the Kolmogorov–Smirnov test. Data are presented as means ± SD or percentages. Differences in responses between magnesium and placebo treatments were calculated using a single‐factor ANCOVA, with inclusion of the baseline measurements of the outcome variable as covariate. Sex was considered as a potential confounder and therefore, the analyses were adjusted for sex. Changes were calculated as the difference between the values at the end and the start of the trial. In a sensitivity analysis, we performed a single‐factor ANCOVA with log‐transformed data, because the variables 24‐h urinary cortisol, cortisone and their metabolites as well as the ratios reflecting A‐ring reductase activity followed a skewed distribution. Skewed data are presented as geometric means with 95% confidence intervals. A p < .05 was considered statistically significant. We did not adjust for multiple testing, as the post‐hoc analyses we performed are exploratory and hypothesis generating. Analyses were performed using SPSS version 23.0 for Windows.
3. RESULTS
3.1. Study population
A total of 51 participants completed the initial intervention trial. For the current post‐hoc analysis, data on 24‐h urinary excretions of cortisol, cortisone and their metabolites were available from 49 participants. Baseline characteristics of the 49 participants that were included in the analysis are presented in Table 2. Their mean age was 62 ± 6 years and 22 (44.9%) were female. They had an average BMI of 29 ± 3 kg/m2. Baseline measurements were comparable between the magnesium and placebo group (Table 2). Oral magnesium supplementation led to an increase in 24‐h urinary magnesium excretion, (2.22 mmol/24‐h; 95% CI: 1.38; 3.07 mmol/24‐h, p < .001) and a small, but non‐significant, increase in plasma magnesium (0.01 mmol/L; 95% CI: −0.01; 0.04 mmol/L, p = .170) (Table 3). Overall compliance was excellent, as evident by the increased 24‐h urinary magnesium excretion observed in the magnesium group as well as the capsule counts. Compliance, based on the returned capsules, ranged from 86% to 102% and was on average 99% and 98% for the magnesium group and for the placebo group, respectively. The magnesium supplements were well tolerated. No serious adverse events were observed. Only mild headache and mild gastrointestinal complaints were reported for 7 days by 1 women from the magnesium group (during week 11 of the study).
TABLE 2.
Baseline characteristics of the included participants
| Variable | Magnesium group (n = 25) a | Placebo group (n = 24) a |
|---|---|---|
| Females, % | 48.0 | 41.7 |
| Age, years | 62.6 ± 5.3 | 61.5 ± 6.1 |
| Body mass index, kg/m2 | 29.0 ± 2.5 | 29.8 ± 3.1 |
| Waist circumference, cm | 100 ± 9 | 103 ± 11 |
| Serum triglycerides, mmol/L | 1.33 ± 0.65 | 1.37 ± 0.52 |
| Plasma glucose, mmol/L | 5.52 ± 0.53 | 5.48 ± 0.61 |
| Serum creatinine, µmol/L | 79.8 ± 11.5 | 81.9 ± 13.5 |
| Systolic blood pressure, mm Hg | 134 ± 14 | 134 ± 18 |
| Diastolic blood pressure, mm Hg | 87 ± 8 | 90 ± 11 |
| Heart rate, beats/min | 66 ± 8 | 68 ± 6 |
Values are means ± SDs or percentages.
TABLE 3.
Magnesium and cortisol and cortisone measurements at baseline and after a 24‐week magnesium or placebo treatment
| Magnesium group (n = 25) | Placebo group (n = 24) | Treatment effect | ||||
|---|---|---|---|---|---|---|
| Baseline a | 24 weeks a | Baseline a | 24 weeks a | Adjusted effect estimate b , c | p‐value | |
| Magnesium parameters | ||||||
| Serum magnesium, mmol/L | 0.85 ± 0.05 | 0.87 ± 0.04 | 0.85 ± 0.05 | 0.85 ± 0.05 | 0.01 (−0.01; 0.04) | .170 |
| Urinary magnesium, mmol/24‐h | 4.70 ± 1.16 | 6.46 ± 1.16 | 4.30 ± 1.46 | 4.24 ± 2.10 | 2.22 (1.38; 3.07) | <.001 |
| 24‐h urinary cortisol and cortisone excretions | ||||||
| Cortisol, nmol/24‐h | 204 ± 64 | 188 ± 46 | 226 ± 110 | 220 ± 88 | −32 (−59; −5) | .021 |
| THF, μmol/24‐h | 5.5 ± 1.8 | 4.7 ± 1.3 | 5.2 ± 2.4 | 5.1 ± 2.0 | −0.5 (−1.2; 0.3) | .231 |
| allo‐THF, μmol/24‐h | 3.2 ± 1.9 | 2.9 ± 1.6 | 3.3 ± 1.9 | 3.4 ± 2.1 | −0.5 (−1.1; −0.0) | .065 |
| Cortisone, nmol/24‐h | 272 ± 91 | 271 ± 83 | 285 ± 112 | 272 ± 102 | −1 (−38; 35) | .949 |
| THE, μmol/24‐h | 10.2 ± 4.1 | 9.7 ± 3.9 | 9.9 ± 4.3 | 9.8 ± 4.7 | −0.2 (−1.9; 1.6) | .861 |
| Total production, μmol/24‐h | 19.4 ± 6.4 | 17.7 ± 5.6 | 19.0 ± 7.6 | 18.9 ± 8.2 | −1.2 (−4.0; 1.7) | .420 |
| Index for 11β‐HSDs | ||||||
| Cortisol/cortisone, nmol/nmol | 0.78 ± 0.17 | 0.71 ± 0.15 | 0.80 ± 0.17 | 0.82 ± 0.15 | −0.10 (−0.17; −0.03) | .005 |
| THFs/THE, μmol/μmol | 0.90 ± 0.27 | 0.84 ± 0.22 | 0.94 ± 0.32 | 0.93 ± 0.32 | −0.09 (−0.17; −0.02) | .018 |
| Index for A‐ring reductases | ||||||
| THF/allo‐THF, μmol/μmol | 4.1 ± 9.1 | 3.5 ± 5.9 | 4.2 ± 12.4 | 3.7 ± 9.8 | −0.21 (−0.80; 0.38) | .280 |
| allo‐THF/cortisol, μmol/μmol | 15.8 ± 8.3 | 16.0 ± 8.8 | 16.2 ± 7.1 | 15.0 ± 6.7 | 1.00 (−1.22; 3.21) | .371 |
| THF/cortisol, μmol/μmol | 28.1 ± 8.0 | 25.7 ± 6.5 | 24.8 ± 10.1 | 24.2 ± 7.0 | 1.48 (−0.91; 3.87) | .204 |
| THE/cortisone, μmol/μmol | 39.5 ± 14.3 | 37.0 ± 15.5 | 36.0 ± 12.7 | 37.9 ± 15.9 | −0.8 (−7.0 5.3) | .781 |
| Urine volume, ml | 1377 ± 505 | 1480 ± 517 | 1565 ± 486 | 1269 ± 481 | 212 (−0; 423) | .050 |
Abbreviations: 11β‐HSDs, 11β‐hydroxysteroid dehydrogenases; allo‐THF, allo‐tetrahydrocortisol; THE, tetrahydrocortisone; THF, tetrahydrocortisol.
Values are means ± SDs.
Values are adjusted mean estimates (95% Confidence Intervals) obtained from a one‐way ANCOVA with baseline value of the dependent variable as covariate.
Models were additionally adjusted for sex.
3.2. Twenty‐four‐hour urinary cortisol, cortisone and their metabolites
Effects on 24‐h urinary excretions of cortisol, cortisone and their metabolites are presented in Table 3. After 24‐week of magnesium supplementation, 24‐h urinary cortisol excretion showed a mean decrease of 32 nmol/24‐h in the magnesium group (95% CI: −59; −5 nmol/24‐h, p = .021). Mean 24‐h urinary THF and allo‐THF excretions were not significantly different between the groups after 24‐week (−0.5 μmol/24‐h, 95% CI: −1.2; 0.3 μmol/24‐h, p = .231 and −0.5 μmol/24‐h, 95% CI: −1.1; −0.0 μmol/24‐h, p = .065, respectively). Oral magnesium supplementation did not change 24‐h urinary cortisone and THE excretions after 24‐week (−1 nmol/24‐h, 95% CI: −38; 35 nmol/24‐h, p = .949 and −0.2 μmol/24‐h, 95% CI: −1.9; 1.6 μmol/24‐h, p = .861, respectively). No significant effect on the total secretion was found (−1.2 μmol/24‐h, 95% CI: −4.0; 1.7 μmol/24‐h, p = .420). Additionally, analyses using log‐transformed data were performed, but similar effects on cortisol, cortisone and their metabolites were observed (Table 4).
TABLE 4.
Cortisol and cortisone measurements at baseline and after a 24‐week magnesium or placebo treatment (sensitivity analysis)
| Magnesium group (n = 25) | Placebo group (n = 24) | Treatment effect | ||||
|---|---|---|---|---|---|---|
| Baseline a | 24 weeks a | Baseline a | 24 weeks a | Adjusted effect stimate b , c | p‐value | |
| 24‐h urinary cortisol and cortisone excretion | ||||||
| Log cortisol, nmol/24‐h | 195 (171; 221) | 179 (164; 196) | 204 (172; 241) | 206 (188; 225) | 0.87 (0.77; 0.99) | .032 |
| Log THF, μmol/24‐h | 5.3 (4.7; 6.0) | 4.5 (4.0; 5.0) | 4.7 (3.9; 5.7) | 4.8 (4.3; 5.3) | 0.93 (0.79; 1.10) | .394 |
| Log allo‐THF, μmol/24‐h | 2.4 (1.7; 3.4) | 2.3 (2.1; 2.6) | 2.7 (1.9; 3.6) | 2.5 (2.2; 2.8) | 0.92 (0.78; 1.09) | .351 |
| Log cortisone, nmol/24‐h | 256 (221; 291) | 256 (233; 282) | 261 (217; 309) | 256 (233; 282) | 1.00 (0.87; 1.16) | .985 |
| Log THE, μmol/24‐h | 9.5 (8.1; 10.9) | 8.9 (7.9; 10.0) | 8.8 (7.2; 10.9) | 8.8 (7.8; 9.9) | 1.01 (0.86; 1.19) | .915 |
| Log total production, μmol/24‐h | 18.4 (16.1; 20.9) | 16.6 (15.0; 18.6) | 17.4 (14.7; 20.7) | 17.3 (15.5; 19.2) | 0.97 (0.83; 1.13) | .669 |
| Index for A‐ring reductases | ||||||
| Log THF/allo‐THF, μmol/μmol | 2.1 (1.5; 2.9) | 1.9 (1.7; 2.1) | 1.8 (1.4; 2.6) | 1.9 (1.8; 2.1) | 0.96 (0.85; 1.09) | .572 |
| Log allo‐THF/cortisol, μmol/μmol | 12.5 (8.9; 16.7) | 12.9 (11.7; 14.2) | 12.6 (8.5; 16.6) | 12.2 (11.1; 13.5) | 1.05 (0.91; 1.21) | .471 |
| Log THF/cortisol, μmol/μmol | 25.9 (23.5; 28.4) | 24.7 (22.9; 26.5) | 22.2 (19.7; 25.1) | 23.4 (21.7; 25.2) | 1.05 (0.95; 1.17) | .317 |
| Log THE/cortisone, μmol/μmol | 35.9 (31.1; 41.4) | 34.6 (31.1; 38.4) | 33.2 (28.3; 39.4) | 34.6 (31.0; 38.5) | 1.00 (0.86; 1.16) | .999 |
Abbreviations: allo‐THF, allo‐tetrahydrocortisol; THE, tetrahydrocortisone; THF, tetrahydrocortisol.
Values are geometric means (95% CIs).
Values are geometric mean ratios (95% CIs) obtained from a one‐way ANCOVA with baseline value of the dependent variable as covariate.
Models were additionally adjusted for sex. All variables were log‐transformed before entering the model.
3.3. Enzyme activity of 11β‐HSDs and A‐ring reductases
Effects of magnesium supplementation on enzymatic activities of 11β‐HSDs and A‐ring reductases are shown in Table 3. The cortisol/cortisone ratio, which reflects the kidney 11β‐HSD type 2 activity, was lower after 24‐week in the magnesium group (−0.10 nmol/nmol, 95% CI: −0.17; −0.03 nmol/nmol, p = .005) as compared with the placebo group. Furthermore, the THFs/THE ratio, which reflects the overall measure of 11β‐HSD activity, was decreased by 0.09 μmol/μmol (95% CI: 0.02; 0.17 μmol/μmol, p = .018) following magnesium supplementation. No difference in THF/allo‐THF ratio, as index for 5α‐ and 5β‐reductase activity, was observed between the groups. The ratio allo‐THF/cortisol, as index for 5α ‐reductase activity, and the ratios THF/cortisol and THE/cortisone reflecting 5β‐reductase activity did also not differ. Finally, no effects of magnesium supplementation on enzyme activities of A‐ring reductases were found when we performed the analyses with log‐transformed data (Table 4).
4. DISCUSSION
In this post‐hoc analysis of a randomized, controlled trial with overweight and slightly obese adults, we observed a reduction in 24‐h urinary cortisol excretion after 24‐week of daily supplementation with 350 mg magnesium. Also, changes in THFs/THE and cortisol/cortisone ratios were observed, which reflects an increased kidney 11β‐HSD type 2 activity. These novel findings indicate an increased inactivation of cortisol by the 11β‐HSD type 2 enzyme that may be another potential mechanism by which increased dietary magnesium intake lowers CVD risk.
To the best of our knowledge, this is the first human intervention trial that addressed the effect of oral magnesium supplementation on glucocorticoid metabolism, as assessed by 24‐h urinary measurements of cortisol, cortisone and their metabolites. Effects of magnesium on glucocorticoid metabolism have been previously reported by preclinical studies. A magnesium deficient diet has been shown to affect glucocorticoid metabolism in rats. 15 , 16 The latter study showed that in rats receiving a magnesium deficient diet, corticosterone concentrations were elevated. Also, the expression of the gene encoding the enzyme 11β‐HSD type 1 was increased, whereas the expression of the gene encoding the enzyme 11β‐HSD type 2 was decreased. The mechanism underlying the effect of dietary magnesium restriction on altered 11β‐HSD activity is unknown. Previous studies showed that 11β‐HSDs are regulated by several cytokines and growth factors, including insulin‐like growth factor 1 (IGF‐1), as well as by gonadal steroids. 19 , 20 , 21 Interestingly, a magnesium deficient diet in rats has been associated with reductions in serum IGF‐1. 22 Thus, it might be possible that the potential effects of magnesium restriction on IGF‐1 underlie the effects on altered 11β‐HSD activity. A limited number of clinical studies observed beneficial effects of oral magnesium supplementation on circulating cortisol concentrations. 23 , 24 , 25 , 26 Although evidence regarding the mechanism by which magnesium lowers circulating cortisol levels is somewhat controversial, it might be that the effect is mediated by changes in the hypothalamic‐pituitary‐adrenal (HPA) axis. The HPA axis is an endocrine feedback system in which corticotropin‐releasing hormone (CRH) from the hypothalamus stimulates the anterior pituitary to produce adrenocorticotropic hormone (ACTH), while ACTH stimulates the adrenal cortex to produce cortisol. Indeed, a previous study in mice showed deregulation of the HPA axis induced by a magnesium deficient diet, as evident by an increased transcription of the CRH and increased plasma CRH concentrations. 27 Another study conducted in healthy men found that after magnesium administration, secretion of ACTH was significantly reduced. 28
Glucocorticoids are essential for maintenance of vascular tone. Findings from (pre)clinical studies have shown that 11β‐HSDs are expressed in the vascular wall, presumably in vascular smooth muscle cells. 29 , 30 In fact, a study of Hadoke et al have demonstrated that mice with 11β‐HSD type 2 knock‐out developed endothelial dysfunction. 31 The authors concluded that 11β‐HSD type 2, rather than 11β‐HSD type 1, influences vascular tone in mice. Thus, targeting this enzyme likely reduce cortisol action and may contribute to a lower CVD risk. 10 Indeed, patients with Cushing's syndrome show an increased risk of the metabolic syndrome, 32 which is in turn associated with increased risk of CVD. 9 Alterations in the vascular system may underlie these associations. Baykan et al showed that endothelial function in patients with Cushing's syndrome was impaired. 6 Another study observed increased arterial stiffness in these patients, which was independent of blood pressure elevation. 33
Emerging evidence suggests that increasing dietary magnesium intake could be an important dietary approach to reduce the risk of CVD. 1 Indeed, we previously showed a clinically relevant improvement on arterial stiffness, measured with carotid‐to‐femoral pulse wave velocity, after long‐term magnesium supplementation. 2 In the current study, we found that oral magnesium supplementation significantly increased 11β‐HSD type 2 activity in overweight and slightly obese adults, which may provide a mechanistic link between dietary magnesium intake and cardiovascular health benefits.
It is well known that glucocorticoids contribute to insulin resistance. 34 Interestingly, accumulating evidence suggest a beneficial role of magnesium in insulin sensitivity. 35 Thus, it might be possible that magnesium improves glucocorticoid metabolism, thereby affecting insulin sensitivity, which in turn reduces the risk of CVD. It should be noted that in our previous study, we did not observe an effect of oral magnesium supplementation on insulin sensitivity. 36 The lack of effect could be explained by the fact that the participants had baseline serum magnesium levels within the normal range. In fact, subgroup analyses of a meta‐analysis that summarized the effects of oral magnesium supplementation on fasting glucose, insulin and the HOMA‐IR index revealed that greater reductions of the HOMA‐IR index were found in subjects with hypomagnesaemia at baseline. 37
Major strengths of the present study include the double‐blinded placebo‐controlled design, as well as the long duration of the intervention trial. Another strength includes the use of 24‐h urinary cortisol and cortisone excretion and measurements of their metabolites, which allowed us to study underlying mechanisms. Specifically, the enzyme activity of intracellular 11β‐HSDs and A‐ring reductases can be quantified from 24‐h urine concentrations of cortisol, cortisone and their metabolites. This approach provides important insight into the intracellular regulation of glucocorticoids.
A potential limitation of our study is that we were not able to define effects of oral magnesium supplementation on the 11β‐HSD type 1 activity. The 11β‐HSD type 1 activity can only be inferred from the THFs/THE ratio when the cortisol/cortisone ratios is unchanged. 14 In the current study, the cortisol/cortisone ratio was changed after the treatment period in the magnesium group, which may imply an effect on whole‐body 11β‐HSD activity, rather than an effect on the specific 11β‐HSD type 1 activity. Second, licorice‐derivatives, such as glycyrrhizic acid, inhibit the enzymatic activity of 11β‐HSD type 2. Whether the intake of licorice has influenced our results, is not entirely clear, because no data on licorice intake were available. However, participants were requested not to change their dietary intake pattern during the study. Results from the food frequency questionnaires showed indeed that dietary intake (macro and micro‐nutrient intake) did not change during the study, suggesting that it is unlikely that licorice intake affected our results. 2 Furthermore, we could not differentiate whether the effect on glucocorticoid metabolism was due to supplementation of magnesium or due to potential effects of citrate. However, in our future study, we will address effects of different magnesium supplements (magnesium citrate, magnesium oxide and magnesium sulphate) on cardiovascular risk markers, including arterial stiffness and blood pressure. 38 Finally, as the post‐hoc analyses we performed are exploratory and hypothesis generating, the current findings should be interpreted with caution.
To conclude, we observed a beneficial effect of oral magnesium supplementation towards a lower 24‐h urinary cortisol excretion together with an increased activity of 11β‐HSD type 2. Our findings may indicate improved glucocorticoid metabolism induced by oral magnesium supplementation, suggesting a potential mechanism by which increased dietary magnesium intake lowers CVD risk. Our results provide a basis to further investigate effects of oral magnesium supplementation on CVD risk and underlying mechanisms.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
JCS: Wrote the manuscript and performed the statistical analyses; PJJ and RPM: Designed and/or conducted the original study; IM: Performed biochemical analyses; SJLB: Overall responsibility of the study; All authors: Involved in revising the manuscript critically for important intellectual content.
Schutten JC, Joris PJ, Minović I, et al. Long‐term magnesium supplementation improves glucocorticoid metabolism: A post‐hoc analysis of an intervention trial. Clin Endocrinol (Oxf).2021;94:150–157. 10.1111/cen.14350
Funding informationThe study is supported by funding from the Nedmag Industries Mining and Manufacturing BV and the NIGRAM2+ collaboration project, financed by the PPP Allowance made available by Top Sector Life Sciences & Health to the Dutch Kidney Foundation to stimulate public–private partnerships. The original study was supported by research grant CH001 from Top Institute of Food and Nutrition, a public–private partnership on precompetitive research in food and nutrition. Capsules were provided by Laboratorium Medisan BV (Heerenveen, Netherlands).
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Del Gobbo LC, Imamura F, Wu JHY, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta‐analysis of prospective studies. Am J Clin Nutr. 2013;98(1):160‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joris PJ, Plat J, Bakker SJ, Mensink RP. Long‐term magnesium supplementation improves arterial stiffness in overweight and obese adults: results of a randomized, double‐blind, placebo‐controlled intervention trial. Am J Clin Nutr. 2016;103(5):1260‐1266. [DOI] [PubMed] [Google Scholar]
- 3. Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag. 2005;1(4):291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crawford AA, Soderberg S, Kirschbaum C, et al. Morning plasma cortisol as a cardiovascular risk factor: findings from prospective cohort and Mendelian randomization studies. Eur J Endocrinol. 2019;181(4):429‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battocchio M, Rebellato A, Grillo A, et al. Ambulatory Arterial Stiffness Indexes in Cushing’s Syndrome. Horm Metab Res. 2017;49(03):214‐220. [DOI] [PubMed] [Google Scholar]
- 6. Baykan M, Erem C, Gedikli Ö, et al. Impairment of flow‐mediated vasodilatation of brachial artery in patients with Cushing’s Syndrome. Endocrine. 2007;31(3):300‐304. [DOI] [PubMed] [Google Scholar]
- 7. Hermanowski‐Vosatka A, Balkovec JM, Cheng K, et al. 11β‐HSD1 inhibition ameliorates metabolic syndrome and prevents progression of atherosclerosis in mice. J Exp Med. 2005;202(4):517‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Westerbacka J, Yki‐Järvinen H, Vehkavaara S, et al. Body fat distribution and cortisol metabolism in healthy men: enhanced 5β‐reductase and lower cortisol/cortisone metabolite ratios in men with fatty liver. J Clin Endocrinol Metab. 2003;88(10):4924‐4931. [DOI] [PubMed] [Google Scholar]
- 9. Wilson PWF, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066‐3072. [DOI] [PubMed] [Google Scholar]
- 10. Tomlinson JW, Walker EA, Bujalska IJ, et al. 11β‐hydroxysteroid dehydrogenase type 1: a tissue‐specific regulator of glucocorticoid response. Endocr Rev. 2004;25(5):831‐866. [DOI] [PubMed] [Google Scholar]
- 11. Edwards CR, Stewart PM, Burt D, et al. Localisation of 11 beta‐hydroxysteroid dehydrogenase–tissue specific protector of the mineralocorticoid receptor. Lancet. 1988;2(8618):986‐989. [DOI] [PubMed] [Google Scholar]
- 12. Chapman K, Holmes M, Seckl J. 11β‐hydroxysteroid dehydrogenases: intracellular gate‐keepers of tissue glucocorticoid action. Physiol Rev. 2013;93(3):1139‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kerstens MN, Riemens SC, Sluiter WJ, Pratt JJ, Wolthers BG, Dullaart RP. Lack of relationship between 11beta‐hydroxysteroid dehydrogenase setpoint and insulin sensitivity in the basal state and after 24h of insulin infusion in healthy subjects and type 2 diabetic patients. Clin Endocrinol. 2000;52(4):403‐411. [DOI] [PubMed] [Google Scholar]
- 14. Palermo M, Shackleton CHL, Mantero F, Stewart PM. Urinary free cortisone and the assessment of 11beta‐hydroxysteroid dehydrogenase activity in man. Clin Endocrinol. 1996;45(5):605‐611. [DOI] [PubMed] [Google Scholar]
- 15. Takaya J, Iharada A, Okihana H, Kaneko K. Magnesium deficiency in pregnant rats alters methylation of specific cytosines in the hepatic hydroxysteroid dehydrogenase‐2 promoter of the offspring. Epigenetics. 2011;6(5):573‐578. [DOI] [PubMed] [Google Scholar]
- 16. Thomas AE, Inagadapa PJN, Jeyapal S, Merugu NM, Kalashikam RR, Manchala R. Maternal magnesium restriction elevates glucocorticoid stress and inflammation in the placenta and fetus of WNIN rat dams. Biol Trace Elem Res. 2018;181(2):281‐287. [DOI] [PubMed] [Google Scholar]
- 17. Takaya J, Iharada A, Okihana H, Kaneko K. Down‐regulation of hepatic phosphoenolpyruvate carboxykinase expression in magnesium‐deficient rats. Magnes Res. 2012;25(3):131‐139. [DOI] [PubMed] [Google Scholar]
- 18. Kerkhofs TMA, Kerstens MN, Kema IP, Willems TP, Haak HR. Diagnostic value of urinary steroid profiling in the evaluation of adrenal tumors. Horm Cancer. 2015;6(4):168‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomlinson JW, Moore J, Cooper MS, et al. Regulation of expression of 11β‐hydroxysteroid dehydrogenase type 1 in adipose tissue: tissue‐specific induction by cytokines*. Endocrinology. 2001;142(5):1982‐1989. [DOI] [PubMed] [Google Scholar]
- 20. Garbrecht MR, Klein JM, McCarthy TA, Schmidt TJ, Krozowski ZS, Snyder JM. 11‐β hydroxysteroid dehydrogenase type 2 in human adult and fetal lung and its regulation by sex steroids. Pediatr Res. 2007;62(1):26‐31. [DOI] [PubMed] [Google Scholar]
- 21. Sun K, Yang K, Challis JRG. Regulation of 11β‐hydroxysteroid dehydrogenase type 2 by progesterone, estrogen, and the cyclic adenosine 5′‐monophosphate pathway in cultured human placental and chorionic trophoblasts1. Biol Reprod. 1998;58(6):1379‐1384. [DOI] [PubMed] [Google Scholar]
- 22. Dørup I, Flyvbjerg A, Everts ME, Clausen T. Role of insulin‐like growth factor‐1 and growth hormone in growth inhibition induced by magnesium and zinc deficiencies. Br J Nutr. 1991;66(3):505‐521. [DOI] [PubMed] [Google Scholar]
- 23. Abbasi B, Kimiagar M, Sadeghniiat K, Shirazi MM, Hedayati M, Rashidkhani B. The effect of magnesium supplementation on primary insomnia in elderly: a double‐blind placebo‐controlled clinical trial. J Res Med Sci. 2012;17(12):1161‐1169. [PMC free article] [PubMed] [Google Scholar]
- 24. Golf SW, Happel O, Graef V, Seim KE. Plasma aldosterone, cortisol and electrolyte concentrations in physical exercise after magnesium supplementation. J Clin Chem Clin Biochem. 1984;22(11):717‐721. [DOI] [PubMed] [Google Scholar]
- 25. Zogović D, Pešić V, Dmitrašinović G, et al. Pituitary‐gonadal, pituitary‐adrenocortical hormones and IL‐6 levels following long‐term magnesium supplementation in male students. J Med Biochem. 2014;33(3):291‐298. [Google Scholar]
- 26. Held K, Antonijevic IA, Künzel H, et al. Oral Mg2+ supplementation reverses age‐related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry. 2002;35(4):135‐143. [DOI] [PubMed] [Google Scholar]
- 27. Sartori SB, Whittle N, Hetzenauer A, Singewald N. Magnesium deficiency induces anxiety and HPA axis dysregulation: Modulation by therapeutic drug treatment. Neuropharmacology. 2012;62(1):304‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murck H, Steiger A. Mg 2+ reduces ACTH secretion and enhances spindle power without changing delta power during sleep in men ‐ possible therapeutic implications. Psychopharmacology. 1998;137(3):247‐252. [DOI] [PubMed] [Google Scholar]
- 29. Walker BR, Yau JL, Brett LP, et al. 1l β ‐hydroxysteroid dehydrogenase in vascular smooth muscle and heart: implications for cardiovascular responses to glucocorticoids*. Endocrinology. 1991;129(6):3305‐3312. [DOI] [PubMed] [Google Scholar]
- 30. Smith RE, Maguire JA, Stein‐Oakley AN, et al. Localization of 11 beta‐hydroxysteroid dehydrogenase type II in human epithelial tissues. J Clin Endocrinol Metab. 1996;81(9):3244‐3248. [DOI] [PubMed] [Google Scholar]
- 31. Hadoke PWF, Christy C, Kotelevtsev YV, et al. Endothelial cell dysfunction in mice after transgenic knockout of type 2, but not type 1, 11β‐hydroxysteroid dehydrogenase. Circulation. 2001;104(23):2832‐2837. [DOI] [PubMed] [Google Scholar]
- 32. Ferraù F, Korbonits M. Metabolic comorbidities in Cushing’s syndrome. Eur J Endocrinol. 2015;173(4):M133‐M157. [DOI] [PubMed] [Google Scholar]
- 33. Battocchio M, Rebellato A, Grillo A, et al. Ambulatory arterial stiffness indexes in Cushing’s Syndrome. Horm Metab Res. 2017;49(03):214‐220. [DOI] [PubMed] [Google Scholar]
- 34. Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci. 1999;96(5):513. [DOI] [PubMed] [Google Scholar]
- 35. Gommers LMM, Hoenderop JGJ, Bindels RJM, de Baaij JHF. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes. 2016;65(1):3‐13. [DOI] [PubMed] [Google Scholar]
- 36. Joris PJ, Plat J, Bakker SJL, Mensink RP. Effects of long‐term magnesium supplementation on endothelial function and cardiometabolic risk markers: a randomized controlled trial in overweight/obese adults. Sci Rep. 2017;7(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simental‐Mendia LE, Sahebkar A, Rodriguez‐Moran M, Guerrero‐Romero F. A systematic review and meta‐analysis of randomized controlled trials on the effects of magnesium supplementation on insulin sensitivity and glucose control. Pharmacol Res. 2016;111:272‐282. [DOI] [PubMed] [Google Scholar]
- 38. Schutten JC, Joris PJ, Mensink RP, et al. Effects of magnesium citrate, magnesium oxide and magnesium sulfate supplementation on arterial stiffness in healthy overweight individuals: a study protocol for a randomized controlled trial. Trials. 2019;20(1):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.


