Abstract
Introduction
The LipiDiDiet trial investigates the effects of the specific multinutrient combination Fortasyn Connect on cognition and related measures in prodromal Alzheimer's disease (AD). Based on previous results we hypothesized that benefits increase with long‐term intervention.
Methods
In this randomized, double‐blind, placebo‐controlled trial, 311 people with prodromal AD were recruited using the International Working Group‐1 criteria and assigned to active product (125 mL once‐a‐day drink) or an isocaloric, same tasting, placebo control drink. Main outcome was change in cognition (Neuropsychological Test Battery [NTB] 5‐item composite). Analyses were by modified intention‐to‐treat, excluding (ie, censoring) data collected after the start of open‐label active product and/or AD medication.
Results
Of the 382 assessed for eligibility, 311 were randomized, of those 162 participants completed the 36‐month study, including 81 with 36‐month data eligible for efficacy analysis. Over 36 months, significant reductions in decline were observed for the NTB 5‐item composite (−60%; between‐group difference 0.212 [95% confidence interval: 0.044 to 0.380]; P = 0.014), Clinical Dementia Rating‐Sum of Boxes (−45%; P = 0.014), memory (−76%; P = 0.008), and brain atrophy measures; small to medium Cohen's d effect size (0.25–0.31) similar to established clinically relevant AD treatment.
Discussion
This multinutrient intervention slowed decline on clinical and other measures related to cognition, function, brain atrophy, and disease progression. These results indicate that intervention benefits increased with long‐term use.
Keywords: Alzheimer's disease, atrophy, cognition, dietary intervention, function, hippocampus, mild cognitive impairment, nutrition, omega 3, prodromal, randomized controlled clinical trial, therapy
1. BACKGROUND
Experts consensus opinions on dementia prevention suggested that progression from mild cognitive impairment (MCI) to dementia could be reduced by attention to modifiable risk factors related to lifestyle. 1 , 2 Dietary and nutritional interventions as part of broader lifestyle changes may contribute to improved cognitive performance among individuals at risk of progression to dementia. 3 , 4 Fortasyn Connect (Souvenaid) contains docosahexaenoic acid and eicosapentaenoic acid; uridine monophosphate; choline; vitamins B12, B6, C, E, and folic acid; phospholipids; and selenium. 5 Preclinical research has shown that this specific combination of nutrients (Fortasyn Connect) reduces Alzheimer's disease (AD)‐linked brain pathologies in a neuroprotective manner, 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 and previous clinical studies showed benefits on memory and functional connectivity in patients with mild, but not moderate, AD. 14 , 15 , 16
Recently, a further expert consensus opinion stated that Fortasyn Connect is not recommended for patients with moderate or advanced AD dementia, but should be considered as an option for patients with mild AD dementia or MCI due to AD pathology (prodromal AD), based on the available clinical trials results including LipiDiDiet. 17 The European LipiDiDiet research consortium conducts preclinical research and clinical trials to better understand the impact of nutrition at different stages of AD. Here we studied the prodromal stage of AD as defined using the International Working Group‐1 criteria. 18 These individuals are approaching the onset of overt dementia; they are characterized by typical mild cognitive and functional impairments and biomarker‐validated AD pathology. The LipiDiDiet trial, a randomized, double‐blind, controlled trial, was designed to investigate the effects of Fortasyn Connect versus control on cognition and related measures in this population over a maximum period of 72 months. 19 Analysis of the first 24‐month intervention period showed favorable effects on the secondary endpoints Clinical Dementia Rating‐Sum of Boxes (CDR‐SB) and hippocampal atrophy, and the post‐hoc endpoint AD Composite Score (ADCOMS), but not on the primary endpoint (Neuropsychological Test Battery [NTB] 5‐item composite) in the modified intention‐to‐treat (mITT) population. 19 , 20 Because we observed a much lower than expected cognitive decline in the control group, we hypothesized that the primary endpoint was inadequately powered and that longer intervention duration might lead to more pronounced benefits.
Here we report previously specified primary and secondary outcomes over 36 months of intervention with Fortasyn Connect versus control in participants with prodromal AD.
2. METHODS
Detailed methods were published previously. 19 Additional information can be found in the supporting information.
2.1. Study design and participants
The LipiDiDiet trial is a randomized, controlled, double‐blind, parallel‐group, multicenter trial done primarily in memory clinics at 11 study sites in Finland, Germany, the Netherlands, and Sweden. After the 24‐month intervention, 19 participants could continue in the trial for a maximum total of 72 months of randomized, controlled, double‐blind, parallel‐group intervention. Here we report analyses over a total of 36 months of intervention after the initial randomization. The study protocol and consent forms were approved by the local ethical committees of all participating sites. The study was done in accordance with the Declaration of Helsinki and International Conference of Harmonization Good Clinical Practice guidelines.
We enrolled participants aged 55 to 85 years who had recently undergone routine assessments and fulfilled criteria for prodromal AD. 18 Participants diagnosed with dementia during the study could remain in the study and could start AD treatment according to their clinician's judgement. The protocol was amended to allow participants who progressed to dementia to switch to the active product after it became generally available. 19 Because this trial was designed to investigate the effects of the intervention on drug‐naïve individuals with prodromal AD, data collected after participants started open‐label medication (defined as use of active study product and/or AD medication after dementia diagnosis) were excluded from the efficacy analysis (ie, censored), as predefined in the protocol.
All participants provided written informed consent before study participation. The only criteria for participants, including those progressing to dementia, to remain in the trial beyond 24 months were continued participation of the study site and for the participant to sign the informed consent form annually.
The initial group assignment and double blinding were maintained throughout the entire maximum permitted blinded intervention period of 72 months. Eligible participants were randomly assigned (1:1) to receive either the active or control product once daily.
We enrolled eligible participants at a combined screening and baseline visit or during a separate baseline visit. Efficacy evaluations were done at baseline, 6, and 12 months, and subsequently every 12 months. Participants in the active group received the medical food Souvenaid, a 125 mL once‐a‐day drink containing the specific multinutrient combination (Fortasyn Connect, Table S1 in supporting information). Participants in the control group received a 125 mL once‐a‐day control drink. The control drink is isocaloric, similar in appearance and flavor to the active study product, but without Fortasyn Connect (Table S1). 21
2.2. Outcomes
The endpoints specified for the 24‐month analysis, including the primary outcome, 19 were also used for this analysis of outcomes over 36 months. In short: the primary outcome was an NTB 5‐item composite Z score. Secondary measures included composite Z scores for NTB memory domain, NTB executive function domain, and NTB total based on 16 items; CDR‐SB; hippocampal, ventricular, and whole brain atrophy based on magnetic resonance imaging (MRI; 3D T1‐weighted anatomical scans); and incidence of dementia (diagnosis according to criteria defined in the Diagnostic and Statistical Manual‐IV, the National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer's Disease and Related Disorders Association [ADRDA] Criteria for AD). NTB composite scores were calculated as Z scores standardized to the baseline mean and standard deviation (SD), with higher scores suggesting better performance. Composite Z scores were obtained by averaging the individual NTB items’ Z scores and weighting according to the number of NTB items available. The minimum number of NTB items required was set to four of five for the NTB 5‐item composite, three of three for NTB memory domain, three of four for NTB executive function domain, and 12 of 16 for NTB total.
RESEARCH IN CONTEXT
Systematic review: We searched ClinicalTrials.gov, PubMed, and other sources to find new randomized controlled trials (RCT) of “Souvenaid” or “Fortasyn” published since the initial LipiDiDiet 2017 trial report. No new trials were identified.
Interpretation: This is the first completed RCT in prodromal Alzheimer's disease (AD) with a nutritional intervention for 36 months. With positive results on the highly relevant Clinical Dementia Rating‐Sum of Boxes, supported by other measures of cognition, function, and brain atrophy, including some that appeared only after long‐term intervention, the study shows that this intervention has the potential to alter disease trajectories. Such 3‐year sustainable benefit has not been reported before in prodromal AD. The results further suggest that treatment duration and initiating early in the disease continuum might be factors contributing to the achievable benefits.
Future directions: Future research may assess incremental benefits by integrating with multidomain interventions such as World Wide FINGERS and by combination with pharmaceutical therapies.
Participants were frequently monitored for adverse events, use of concomitant medications, consumption of nutritional supplements, study product compliance, vital signs (heart rate, systolic blood pressure, and diastolic blood pressure), and clinical safety laboratory tests.
2.3. Statistical analyses
Statistical analyses were performed as described previously. 19 In brief, we calculated that a sample size of 300 randomly assigned participants would be sufficient to provide 90% power to detect a 40% difference in NTB score change between groups over 24 months (based on a t test and 5% significance level). 19 Additional power calculations for this follow‐up period were not performed.
Analyses were performed on the mITT population including all participants randomized, but excluding (ie, censoring) data collected after the start of open‐label medication (defined as use of active study product and/or AD medication after dementia diagnosis). Note that although participants who started open‐label medication remained in the study for at least one additional visit, some have discontinued the study at a later stage. Safety analyses were performed for all randomized participants who consumed at least one dose of study product.
As for the 24‐month analysis, we analyzed all outcomes of a continuous type using a predefined linear mixed model for longitudinal data with change from baseline as the response variable and linear time (days since baseline), baseline score, baseline Mini‐Mental State Examination (MMSE), treatment, and time × treatment as fixed effects. Analyses were performed in parallel and validated by the Pentara Corporation (Millcreek, UT, USA). Additionally, we did a planned sensitivity analysis using a joint model, as described previously, 19 , 22 to correct for potential bias from data missing not at random. The joint model comprised a mixed model for the longitudinal outcome and a Cox proportional hazards model for time to dropout, with dropout being defined as missing data due to study discontinuation, the censoring of data collected after the start of open‐label medication, or violation of the predefined visit window (supporting information). By jointly modelling the repeated measurements and the dropout in a single statistical model, joint models can still obtain unbiased estimates if the missingness is not at random and are suitable for early AD analyses. 22 , 23 , 24 The underlying idea is that, in these models, a joint distribution of the dropout times and the repeated measurements is modelled via a set of random effects. These random effects are “shared” between the mixed model and the Cox proportional hazards model, which is assumed to account for the associations between these two outcomes. 23 , 25 , 26 Additional analyses were performed by including apolipoprotein E (APOEε4; carrier vs non‐carrier), baseline cerebrospinal fluid (CSF) amyloid beta (Aβ)‐42, baseline CSF (Aβ‐42/Aβ‐40) × 10, baseline CSF total‐tau, and baseline CSF phospho‐tau as additional covariates to the main mixed model (Table S4 in supporting information). Because CSF data were available for a subset of participants (n = 107), we first repeated the main mixed model analysis in this CSF subgroup (“Reference CSF subgroup”) and used this subgroup as reference for comparison to the “CSF‐covariates subgroup” analyses. For all analyses on MRI ventricular volume data, 36‐month data from one study site were excluded due to technical reasons (supporting information). As a supportive analysis, to investigate whether censoring of data collected after the start of open‐label medication (defined as use of active study product and/or AD medication after dementia diagnosis) would potentially have an impact on the interpretation of the results, we repeated the main mixed model analysis by including the censored data points and adding the time on open‐label medication as an additional covariate to the model.
Effect sizes were shown using Cohen's d standardized effect size calculated based on the mean treatment difference for the change from baseline over 36 months estimated in the mixed model and pooled SD with sample size value based on first follow‐up visit in the mixed model.
P values of <0.05 were deemed statistically significant in comparisons of efficacy and safety data. No corrections for multiple testing were performed. Statistical analyses were performed using SAS software version 9.4. The study is registered with the Dutch Trial Register (NTR1705).
3. RESULTS
During the initial study enrolment period (April 20, 2009 to July 3, 2013), 382 individuals were screened and 311 were randomly assigned to either the active group (n = 153) or the control group (n = 158; Figure 1). Among participants who provided informed consent to participate in each study phase, calculated rates of study discontinuation (including lost to follow‐up) were ≈10% per year in both groups; that is, rates were 33/153 (22%) for the first 24 months and 8/93 (9%) for months 24 to 36 in the active group, and 33/158 (21%) and 10/87 (11%), respectively, in the control group. From the 245 randomized participants who completed the first 24 months, 180 (73%) continued and 162 (85 from the active group and 77 from the control group) completed the 36‐month intervention. A detailed overview is presented in Figure S1 in supporting information. Reasons for study discontinuation during the 36‐month study period were: not opting in after the first 24 months by not providing informed consent (n = 64), site discontinuation after the first 24 months (n = 1), adverse events (n = 18), withdrawal of informed consent (n = 22), protocol deviations (n = 3), other reasons (n = 37; Table S2 in supporting information), or lost to follow‐up (n = 4; Figure S1).
FIGURE 1.
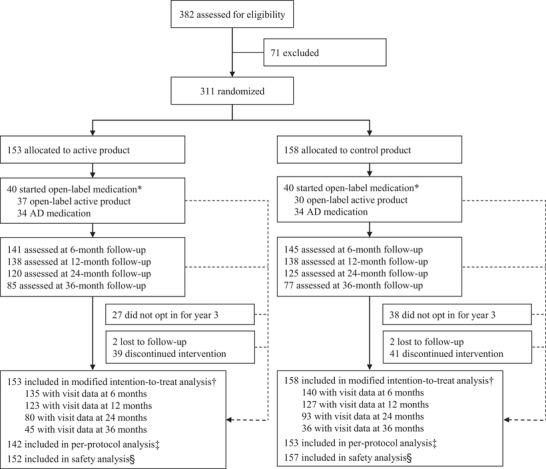
Trial profile *Open‐label medication was defined as the use of active study product and/or Alzheimer's disease medication after dementia diagnosis. Data collected after the start of open‐label medication were excluded (ie, censored) from the efficacy analyses. Note that although participants who started open‐label medication remained in the study for at least one additional visit, some have discontinued the study at a later stage. †All randomly assigned participants, excluding visit data after the start of open‐label medication and visit data in violation of the predefined visit window. Numbers with visit data available for the efficacy analysis (modified intention‐to‐treat) are based on the data available for the Neuropsychological Test Battery 5‐item composite. ‡Respective visits of participants were excluded in case of major protocol deviations; number based on participants with at least one follow‐up visit in the per‐protocol dataset. §All randomly assigned participants, excluding participants that discontinued at baseline and did not receive allocated intervention. Abbrevation: AD, Alzheimer's disease
Over 36 months, a total of 80 participants started open‐label medication (active study product and/or AD medication after dementia diagnosis); 40 in the active group and 40 in the control group (Figure 1). After the censoring of data collected after the start of open‐label medication and exclusion of visit data in violation of the predefined visit window, the mITT analysis included 36‐month visit data from 81 (45 active and 36 control) out of 162 participants completing the 36‐month intervention period.
Table 1 shows baseline characteristics for all randomized participants, and those with 36‐month visit data in the mITT analysis. In the all‐randomized population, active and control groups were well balanced at baseline, except for a slight but significantly lower MMSE for the active group as reported previously (Table 1 and Figure S2A in supporting information). As expected, participants with 36‐month data in the mITT analysis had slightly better baseline scores than the all‐randomized population. There were no statistically significant baseline differences between the active and control groups among the participants with 36‐month data in the mITT analysis (Table 1).
TABLE 1.
Baseline characteristics of all randomized participants and participants with 36‐month data eligible for efficacy analyses
| All randomized participants | Participants with 36‐month data eligible for efficacy analyses | |||
|---|---|---|---|---|
| Control (n = 158) | Active (n = 153) | Control (n = 36) | Active (n = 45) | |
| Age (years) | ||||
| Mean (SD) | 70.7 (6.2) | 71.3 (7.0) | 69.9 (6.6) | 71.9 (7.2) |
| Median (min ‐ max) | 71 (52 to 84) | 72 (50 to 86) | 71 (54 to 84) | 72 (56 to 85) |
| Sex, no. (%) | ||||
| Men | 73 (46%) | 81 (53%) | 19 (53%) | 25 (56%) |
| Women | 85 (54%) | 72 (47%) | 17 (47%) | 20 (44%) |
| Ethnic origin, no. (%) | ||||
| White | 157 (99%) | 152 (99%) | 36 (100%) | 44 (98%) |
| Black | 0 (0%) | 1 (1%) | 0 (0%) | 1 (2%) |
| Other | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Education (years) | 10.7 (3.6) | 10.6 (3.9) | 11.4 (3.8) | 10.5 (3.8) |
| Mini‐Mental State Examination | 26.9 (1.9) | 26.4 (2.1)a | 27.7 (1.7) | 27.2 (2.0) |
| APOE ɛ4 genotypeb, n/N (%) | ||||
| Carrier | 90/143 (63%) | 83/138 (60%) | 22/33 (67%) | 21/42 (50%) |
| Non‐carrier | 53/143 (37%) | 55/138 (40%) | 11/33 (33%) | 21/42 (50%) |
| Cognitive measures (composite Z score) | ||||
| NTB 5‐item | 0.00 (0.68) [156] | −0.00 (0.70) [152] | 0.20 (0.67) [36] | 0.40 (0.50) [45] |
| NTB memory domain | 0.03 (0.82) [156] | −0.02 (0.87) [151] | 0.19 (0.79) [36] | 0.47 (0.61) [44] |
| NTB executive function | −0.01 (0.71) [155] | 0.01 (0.71) [150] | 0.17 (0.68) [35] | 0.27 (0.64) [44] |
| NTB total | −0.02 (0.56) [156] | 0.02 (0.57) [151] | 0.19 (0.52) [36] | 0.33 (0.48) [44] |
| CDR‐SB | 1.75 (1.14) [143] | 1.87 (1.17) [140] | 1.05 (0.78) [32] | 1.18 (0.88) [42] |
| MRI brain volumes (cm3)c | ||||
| Total hippocampal volume | 5.70 (1.25) [115] | 5.62 (1.10) [102] | 5.93 (1.36) [29] | 6.06 (1.10) [37] |
| Whole brain volume | 1377.30 (84.08) [101] | 1370.56 (81.64) [89] | 1438.59 (93.92) [23] | 1398.13 (83.55) [32] |
| Ventricular volume | 53.95 (25.31) [123] | 58.35 (26.66) [114] | 47.27 (22.92) [28] | 50.62 (24.05) [34] |
| CSFc | ||||
| Aβ‐42 (pg/mL) | 401.1 (196.1) | 426.9 (292.7) | 482.8 (232.0) | 626.2 (365.0) |
| (Aβ‐42/Aβ‐40) × 10 | 0.62 (0.25) | 0.65 (0.29) | 0.69 (0.27) | 0.86 (0.32) |
| Total‐tau (pg/mL) | 634.8 (287.7) | 591.9 (260.9) | 603.6 (230.6) | 467.8 (172.5) |
| Phospho‐tau (pg/mL) | 80.3 (30.6) | 74.2 (25.8) | 79.7 (26.9) | 63.6 (21.1) |
Data are mean (SD) or mean (SD) [N] unless stated otherwise.
Slight but statistically significant lower Mini‐Mental State Examination score in the active versus control group (P = 0.038, t test). There were no other statistically significant baseline differences between the active and control groups, both within the all‐randomized participants and the participants with 36‐month data eligible for efficacy analysis.
Data not available for all randomized participants. Percentages are calculated based on number of participants with available data.
Central analysis CSF data available for n = 107 (all randomized participants; control n = 61, active n = 46) and n = 32 (participants with 36‐month data in the mITT analyses; control n = 15, active n = 17); central analyses MRI data available for n = 279 and n = 75, respectively).
Abbreviations: Aβ, amyloid beta; APOE, apolipoprotein E; CDR‐SB, Clinical Dementia Rating‐Sum of Boxes; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; NTB, Neuropsychological Test Battery; SD, standard deviation.
Results for all main outcomes are reported in Table 2 and Figure 2 (mixed model), presented as estimated means rather than observed means to reduce potential bias from the increased level of missing data during the study. Higher scores indicate better performance for all endpoints except for CDR‐SB and ventricular volume.
TABLE 2.
Summary of clinical and magnetic resonance imaging outcomes during the 36‐month intervention
| Mixed model a | Joint model b | |||||
|---|---|---|---|---|---|---|
| Difference Estimate (95% CI) | P value | Effect size Cohen's d c | Difference Estimate (95% CI) | P value | Effect size Cohen's d c | |
| NTB 5‐item composite (Z score) | ||||||
| Modified intention‐to‐treat | 0.212 (0.044 to 0.380) | .014 | 0.26 | 0.219 (0.126 to 0.312) | <.001 | 0.54 |
| Per‐protocol | 0.269 (0.081 to 0.457) | .005 | 0.31 | 0.268 (0.153 to 0.382) | <.001 | 0.56 |
| NTB memory (Z score) | ||||||
| Modified intention‐to‐treat | 0.274 (0.071 to 0.477) | .008 | 0.25 | 0.234 (0.024 to 0.444) | .029 | 0.26 |
| Per‐protocol | 0.352 (0.125 to 0.579) | .003 | 0.32 | 0.316 (0.090 to 0.543) | .006 | 0.34 |
| NTB executive function (Z score) | ||||||
| Modified intention‐to‐treat | 0.006 (−0.169 to 0.182) | .943 | 0.01 | −0.015 (−0.189 to 0.158) | .862 | −0.02 |
| Per‐protocol | 0.076 (−0.107 to 0.258) | .415 | 0.08 | 0.038 (−0.145 to 0.220) | .685 | 0.05 |
| NTB total (Z score) | ||||||
| Modified intention‐to‐treat | 0.086 (−0.046 to 0.218) | .202 | 0.15 | 0.076 (−0.059 to 0.210) | .272 | 0.12 |
| Per‐protocol | 0.141 (−0.001 to 0.283) | .051 | 0.23 | 0.118 (−0.024 to 0.260) | .103 | 0.19 |
| CDR‐SB (score)d | ||||||
| Modified intention‐to‐treat | −0.90 (−1.62 to −0.19) | .014 | 0.31 | −0.83 (−1.47 to −0.19) | .011 | 0.33 |
| Per‐protocol | −1.15 (−1.90 to −0.41) | .003 | 0.41 | −0.95 (−1.59 to −0.30) | .004 | 0.40 |
| MRI hippocampal volume (cm3) | ||||||
| Modified intention‐to‐treat | 0.20 (0.07 to 0.33) | .002 | 0.27 | 0.20 (0.07 to 0.32) | .002 | 0.43 |
| Per‐protocol | 0.21 (0.07 to 0.35) | .004 | 0.25 | 0.21 (0.08 to 0.35) | .003 | 0.44 |
| MRI whole brain volume (cm3) | ||||||
| Modified intention‐to‐treat | 8.70 (1.31 to 16.09) | .021 | 0.26 | 10.82 (2.23 to 19.43) | .014 | 0.36 |
| Per‐protocol | 8.69 (0.59 to 16.79) | .036 | 0.35 | 11.89 (3.33 to 20.46) | .007 | 0.42 |
| MRI ventricular volume (cm3)d, e | ||||||
| Modified intention‐to‐treat | −2.49 (−4.88 to −0.09) | .042 | 0.26 | −2.28 (−4.47 to −0.09) | .028 | 0.30 |
| Per‐protocol | −2.49 (−5.05 to 0.06) | .056 | 0.25 | −2.38 (−4.80 to 0.05) | .026 | 0.30 |
P values are for effect of intervention over 36 months (significant results are indicated in bold typeface).
Mixed model: the difference (active minus control) is based on the estimated mean for change from baseline over 36 months.
Joint model: for comparability with the mixed models, the difference and effect size are estimated using only the effect(s) for time and the interaction effect(s) of intervention by time, thereby ignoring the main intervention effect (ie, the difference between active minus control already present at baseline).
Results are presented so that a positive effect size indicates improved performance in the active versus control group and vice versa and in bold typeface in case of statistical significance in the corresponding statistical model.
Higher scores indicate worse performance; for all other endpoints, higher scores indicate better performance.
For MRI ventricular volume a quadratic trajectory function was used; P values reflect a combined effect for the linear and quadratic term.
Abbreviations: CDR‐SB, Clinical Dementia Rating‐Sum of Boxes; CI, confidence interval; MRI, magnetic resonance imaging; NTB, Neuropsychological Test Battery.
FIGURE 2.
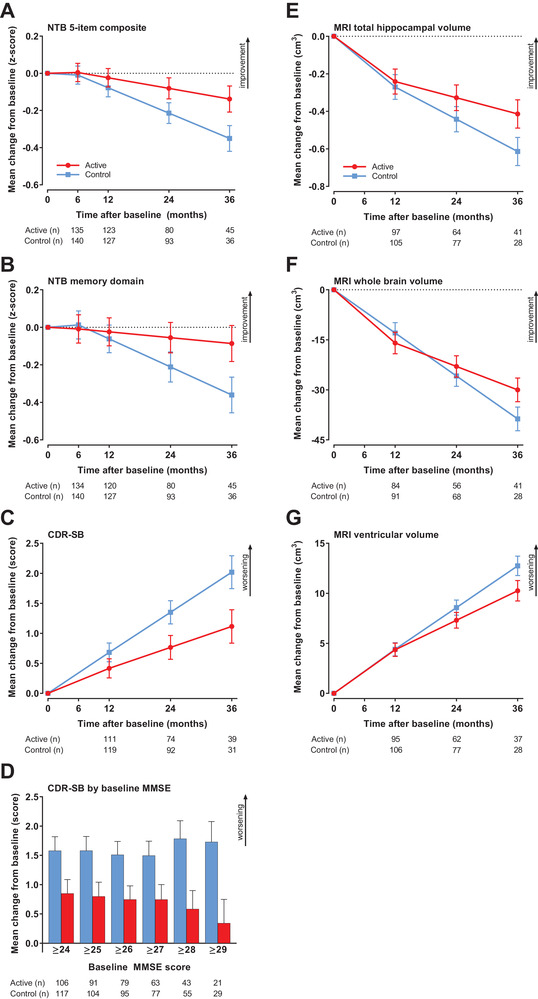
Changes in main endpoints during the 36‐month intervention (mITT). A, NTB 5‐item. B, NTB memory domain. C, CDR‐SB. D, CDR‐SB in subgroups defined by baseline MMSE. E, MRI total hippocampal volume. F, MRI whole brain volume. G, MRI ventricular volume. Data are mean change from baseline as estimated by the mixed model; error bars are standard error. For (A–C) and (E–G), n is the number of participants with change from baseline data in the mixed model; for (D), n is the number of participants with at least one post‐baseline value in the mixed model. Abbreviations: CDR‐SB, Clinical Dementia Rating‐Sum of Boxes; mITT, modified intention‐to‐treat; MMSE, Mini‐Mental State Examination; MRI, magnetic resonance imaging; NTB, Neuropsychological Test Battery
The mean change from baseline to month 36 for the NTB 5‐item composite was −0.138 (standard error [SE] 0.070) in the active group and −0.350 (SE 0.070) in the control group (Figure 2A), with a statistically significant difference between groups favoring active intervention (0.212, 95% confidence interval [CI]: 0.044 to 0.380; P = 0.014; 60% reduction in decline). We found statistically significant between‐group differences for changes from baseline to month 36 in favor of the active intervention for the NTB memory domain (mean treatment difference of 0.274, 95% CI: 0.071 to 0.477; P = 0.008; 76% reduction in decline; Figure 2B). Statistically significant differences between groups in favor of the active group were observed for the CDR‐SB (−0.90, 95% CI: −1.62 to −0.19; P = 0.014; 45% less worsening; Figure 2C). Similar to the 24‐month analysis, 19 the current exploratory analysis of CDR‐SB performance across the spectrum of baseline MMSE (≥24 to ≥29) again suggested that the intervention effect on CDR‐SB was larger for those with higher baseline MMSE scores (Figure 2D, Table S2). No statistically significant between‐group differences were observed for the NTB executive function domain and the NTB total (Table 2).
All brain volume changes were in favor of the active intervention (hippocampal volume: P = 0.002, whole brain volume: P = 0.021, ventricular volume: P = 0.042; Table 2, Figures 2E‐G). The rates of deterioration for hippocampal, whole brain, and ventricular volumes, respectively, were 33%, 22%, and 20% less in the active group than in the control group.
Taken together, between‐group differences observed over 24 months 19 were increased over 36 months and differences in cognition‐related scores between groups were more pronounced in the per‐protocol (PP) analyses than in the mITT analyses (Table 2). The Cohen's d standardized effect sizes increased and reached d = 0.25 to 0.31 for those outcomes with significant separation between groups in the mITT population. Sensitivity joint model analyses confirmed the results (Table 2). Similar results were also obtained with the supportive analyses including censored observations (Figure S3 in supporting information).
The additional analyses including APOE (ε4 carrier vs non‐carrier), baseline CSF Aβ‐42, baseline CSF (Aβ‐42/Aβ‐40) × 10, baseline CSF total‐tau, and baseline CSF phospho‐tau as potential confounders suggested that these covariates were not confounders for the treatment effect in our main mixed model analyses (Table S4).
During the trial, no overall difference was observed between active and control groups in the number of participants diagnosed with dementia over 36 months (66 [43.1%] and 70 [44.3%], respectively) or in the time to dementia using Kaplan‐Meier analysis (Figure S2B). According to main study visit periods, incidence of dementia in the active versus control groups was, respectively,10/15 (67%) versus 5/15 (33%) for the 0 to 6 months period, 13/25 (52%) versus 12/25 (48%) for 6 to 12 months, 39/81 (48%) versus 42/81 (52%) for 12 to 24 months, and 4/15 (27%) versus 11/15 (73%) for the 24 to 36 months period (Figure S2C).
Based on the safety monitoring, there was no suggestion of tolerability or health concerns related to the use of the active product (Fortasyn Connect) taken for 36 months (Table 3). The incidences of adverse events and serious adverse events over the entire 36‐month period in participants on double‐blind treatment were similar between groups (P = 1.000 and P = 0.696), and similar to the results over the 24‐month period as reported previously. 19 None of the serious adverse events was assessed by the investigators as related to the study product. One participant in the control group died during the 24–36‐month study period (intracranial hemorrhage). The rate of study discontinuation due to adverse events was similar for active and control groups (10/153 [7%] and 8/158 [5%] respectively, Table S1).
TABLE 3.
Summary of adverse events in all participants who were randomly assigned and on double‐blind treatment
| Control (n = 157) | Active (n = 152) | |
|---|---|---|
| All events | ||
| At least one adverse event | 139 (88.5%) | 134 (88.2%) |
| At least one serious adverse event | 38 (24.2%) | 40 (26.3%) |
| Most common serious adverse eventsa | ||
| Syncope | 0 (0.0%) | 3 (2.0%) |
| Breast cancer | 1 (0.6%) | 2 (1.3%) |
| Fall | 1 (0.6%) | 2 (1.3%) |
| Vertigo | 1 (0.6%) | 2 (1.3%) |
| Cerebral infarction | 0 (0.0%) | 2 (1.3%) |
| Circulatory collapse | 0 (0.0%) | 2 (1.3%) |
| Femur fracture | 1 (0.6%) | 2 (1.3%) |
| Hip fracture | 0 (0.0%) | 2 (1.3%) |
| Hospitalization | 0 (0.0%) | 2 (1.3%) |
| Osteoarthritis | 4 (2.5%) | 1 (0.7%) |
| (Major) depression | 3 (1.9%) | 1 (0.7%) |
| Acute myocardial infarction | 2 (1.3%) | 1 (0.7%) |
| Cardiac operation | 2 (1.3%) | 0 (0.0%) |
| Cholelithiasis | 2 (1.3%) | 0 (0.0%) |
| Gastrointestinal hemorrhage | 2 (1.3%) | 0 (0.0%) |
| Intervertebral disc protrusion | 2 (1.3%) | 0 (0.0%) |
| Myocardial infarction | 2 (1.3%) | 0 (0.0%) |
| Wrist fracture | 2 (1.3%) | 0 (0.0%) |
| Most common adverse eventsb | ||
| Fall | 10 (6.4%) | 11 (7.2%) |
| Back pain | 7 (4.5%) | 10 (6.6%) |
| Vertigo | 13 (8.3%) | 9 (5.9%) |
| Headache | 12 (7.6%) | 9 (5.9%) |
| Bronchitis | 5 (3.2%) | 8 (5.3%) |
| Cystitis | 12 (7.6%) | 8 (5.3%) |
| Gastroenteritis | 1 (0.6%) | 8 (5.3%) |
| Nasopharyngitis | 18 (11.5%) | 8 (5.3%) |
| Pain in extremity | 8 (5.1%) | 8 (5.3%) |
| Diarrhea | 17 (10.8%) | 7 (4.6%) |
| Respiratory tract infection | 10 (6.4%) | 7 (4.6%) |
| Urinary tract infection | 9 (5.7%) | 7 (4.6%) |
| Arthralgia | 12 (7.6%) | 6 (3.9%) |
| Depression | 8 (5.1%) | 4 (2.6%) |
| Cough | 11 (7.0%) | 3 (2.0%) |
| Osteoarthritis | 8 (5.1%) | 3 (2.0%) |
| Influenza | 8 (5.1%) | 2 (1.3%) |
Data are n (%). Adverse events are presented by Medical Dictionary for Regulatory Activities preferred term.
Only those reported by at least two participants in either group are shown.
Only those reported by at least 5% of participants in either group are shown.
Overall mean compliance over 36 months was 91.4% (SD 10.6) in the active group and 90.8% (SD 13.1) in the control group (mITT).
4. DISCUSSION
The LipiDiDiet clinical trial with Fortasyn Connect in prodromal AD participants showed a significant benefit over a treatment period of 3 years as measured by the majority of the previously defined primary and secondary endpoints, including NTB 5‐item composite score, NTB memory domain, CDR‐SB, and brain atrophy. In addition, a good safety profile was confirmed. Previously, we found a significant reduction in cognitive‐functional decline over 24 months as measured by CDR‐SB and attenuated hippocampal atrophy. However, the cognitive deterioration as measured by the primary endpoint (NTB 5‐item composite) in the control group was markedly less than we had expected over 24 months, rendering the primary endpoint inadequately powered. 19 Now, the cognitive decline over the 36‐month intervention period in the control group was well within the range of the originally anticipated 24‐month decline, and we observed a statistically significant difference on the NTB 5‐item composite favoring the active group. Similarly, over 36 months, the NTB memory domain showed continued decline in the control group, while in the active group we observed a statistically significant reduction in decline. No significant between‐group differences were observed for the other NTB scores. The CDR‐SB reflects real‐life performance and is now widely used as a sensitive and meaningful primary clinical outcome assessment in prodromal/early AD trials. 27 Data from the Alzheimer's Disease Neuroimaging Initiative (ADNI) suggest that changes in CDR‐SB points per year can indicate disease status and trajectory, 28 , 29 , 30 with a worsening of 0.5 and 1.4 points per year reported for MCI individuals with AD biomarkers and patients with mild AD, respectively. For early AD, a reduction by 0.5 or 1.0 in CDR‐SB was proposed to capture both efficacy and clinical relevance. 31 Here, we observed over 3 years an annual progression equivalent to 0.67 points in the control group and 0.37 points in the active group, equivalent to 45% less worsening with treatment. A 3‐year sustainable benefit in CDR‐SB has not been observed before in early AD. Moreover, the benefit in CDR‐SB occurred in conjunction with a benefit in several NTB items, supporting the positive impact of the intervention on cognition.
In addition to the benefits observed on cognition, benefits were also observed on measures of brain volume. In the present study, control group decline rates for hippocampal atrophy appeared well matched with those of MCI/mild AD reported previously. ADNI and Jack et al. reported annualized hippocampal atrophy rates of −3.2% to −3.69% for MCI progressing to AD dementia, and −3.5% to −4.0% for AD, respectively. 32 , 33 Our data showed diminished hippocampal atrophy in the active compared to the control group, with annualized changes equivalent to −2.5% and −3.6%, respectively, providing relevant evidence of potential effect on disease pathology. The between‐group difference in whole‐brain atrophy was statistically significant with longer intervention duration, indicating that the effect on atrophic process was not limited to one brain area. The reduction in ventricular enlargement further corroborated these observations. The finding that long‐term intake of this specific multinutrient combination partially protects brain structures and reduces cognitive and functional decline in prodromal AD indicates that these nutrients play a pivotal role in reducing the neurodegenerative process in AD, suggesting the presence of a nutritional need. The beneficial effect on hippocampal atrophy, with its critical link to memory, might be a basis for the memory benefit reported by several trials with the active intervention in prodromal to mild AD. 14 , 15 , 19
The results on CDR‐SB and brain structures show that while the trajectories in the control group remained on a typical MCI to AD slope, 28 , 30 , 32 , 33 the active group performed significantly better on these endpoints. We found a significant separation between groups for the majority of cognitive and brain atrophy measures, including outcome measures without significant group separation over the 24‐month duration, suggesting that sustained trajectory separation is a pivotal finding of the 3‐year results. Effect size, expressed as Cohen's d value, for established clinically relevant AD treatment is modest (up to or slightly exceeding a value of 0.30) 34 and a similar effect size (0.25–0.31) was observed for all outcome measures with significant between‐group differences in this trial. Our results thus support the current expert opinion on Fortasyn Connect 17 and extend it by highlighting that the potential benefit might be further increased by early and long‐term intervention.
Despite the clear cognitive, functional, and structural benefits observed, neither the cumulative incidence of dementia nor the mean time to dementia diagnosis were different between groups over 36 months. The LipiDiDiet trial was primarily designed for the analysis of changes on continuous scales (the NTB), not for changes on discrete outcomes such as incidence of dementia. We observed a few more dementia cases during the first 6 months in the active group, possibly due to more advanced disease stage at baseline as indicated by a lower mean MMSE (Figure S2C). 19 If longer intervention duration before dementia onset is more effective, a stronger effect would be expected in participants at earlier stages of prodromal AD. The CDR‐SB analysis across the spectrum of baseline MMSE would support such an interpretation. Corroborating this, we observed fewer dementia cases in the active group (n = 4) than in the control group (n = 11) during the third year of intervention. However, the very few cases occurring in the third year limits our ability to draw any conclusion on the dementia incidence parameter.
Compliance and safety results previously observed with the active intervention 14 , 15 , 16 , 19 were confirmed in the current analyses over 36 months, indicating that high tolerability and good safety profile were maintained during longer term use of the active product. Together with the clinical benefits observed, these safety results indicate that the prodromal AD population is amenable to long‐term treatment with this intervention.
This study was designed to investigate the effects of the nutritional intervention in participants with prodromal AD; therefore, data collected from participants who received open‐label medication (defined as use of active study product and/or AD medication after dementia diagnosis) were censored from the efficacy analysis. In addition to the previously reported limitations related to population heterogeneity and demographic restrictions, 19 long‐term follow‐up is expected to add further limitations because the longer the intervention, the more participants will leave the trial and consequentially the population studied will change over time. Indeed, the current analysis of the 36‐month study period was limited by the increasing level of missing data, resulting from either study discontinuation or censoring of data collected after the start of open‐label medication. Also, participants with 36‐month data included in the efficacy analysis tended to have slightly better baseline scores (Table 1) than all randomized participants. This is not surprising because individuals with more advanced prodromal AD at baseline would be expected to develop dementia and start open‐label medication earlier. Moreover, fewer participants with long‐term follow‐up will unavoidably change the relative distribution of key AD biomarkers. For example, in this study, a different numerical percentage of APOEε4 carriers and concentrations of amyloid and tau in CSF were observed. These differences were not statistically significant between the active and control groups, both within the all‐randomized participants and the participants with 36‐month data eligible for efficacy analyses. Despite absence of significance, in a long‐term trial like this one, it would still be conceivable that qualitatively differential dropout potentially played a role in the observed treatment effects. To address these issues, we performed predefined sensitivity analyses using a joint model combining longitudinal and survival data, supportive mixed model analyses including the censored observations, as well as additional mixed model analyses to investigate potential confounders effects. All these analyses confirmed the main results (Table 2 and Table S4), indicating that the intervention effects cannot be explained by bias due to missing data, or by imbalance of APOEε4, CSF amyloid, or CSF tau covariates. In addition, the results from the supportive mixed model analyses including the censored observations indicated that results found for the intervention factor in the other models cannot be explained by the impact of having censored data (Figure S3). This supportive analysis has additional limitations and methodological concerns. It is outside of the original study design, incurs noise due to open‐label data points included, uses a covariate that is collected post‐randomization, and while the treatment effects of the AD medication are arguably small on atrophy outcomes, they are expected to be extensive on the cognitive outcomes. These combined limitations to the supportive mixed model result in a likely dilution (underestimation) of intervention effects. Despite the observation of similar results, comparison with our predefined main and sensitivity models should be done with caution. We therefore restricted interpretation of the supportive model only to address the question of whether the intervention effects found in other models might have been driven by the increased missingness of data due to censoring. The results of the supportive analysis indicate that this is not the case and therefore validated the main conclusions.
The LipiDiDiet trial has several strengths that allow us to assess the potential value of this specific multinutrient intervention from a clinical perspective. First, the active product was evaluated for an extended duration in a well‐characterized prodromal AD population (Table S3 in supporting information). Second, it used a broad range of established and validated outcome measures to show clinically meaningful effects, particularly on CDR‐SB. Furthermore, the specific multinutrient product was shown to be safe and well tolerated. Finally, these benefits were achieved using a novel intervention strategy, potentially broadening the arsenal for the management of this condition.
Future research should determine whether benefits could be further increased by intervening in yet earlier disease stages, for a longer period, as part of multimodal interventions, such as The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), 35 or in combination with pharmaceutical therapies. Indeed, Fortasyn Connect has been used safely in conjunction with AD medication 36 and is currently part of the multimodal World Wide FINGERS intervention study MIND‐AD. 37
In conclusion, the present study provides evidence for potentially altered disease trajectories supporting the positive effects of long‐term multinutrient intervention in prodromal AD. Over 3 years, significant benefits were observed on cognition, function, and brain atrophy, with clinically relevant effect sizes demonstrated. Prolonged intervention resulted in a broader range of endpoints showing statistically significant differences than reported before. Such sustainable benefits lasting for 3 or more years have not been reported before for an intervention in prodromal AD. The totality of our results highlights that the benefits might be increased with early and long‐term intervention.
CONFLICTS OF INTEREST
The LipiDiDiet consortium received funding by Danone Nutricia Research for the intervention period from 25 to 96 months and the consortium distributed the funding to their members to conduct the trial and analysis. SBH received payment for statistical analysis from the LipiDiDiet Consortium and Danone Nutricia Research during the conduct of the study. Additional declaration of interest: HS reports personal fees from ACI and MERCK (advisor), outside the submitted work, and KB has served as a consultant or at advisory boards for Abcam, Axon, Biogen, Lilly, MagQu, Novartis, and Roche Diagnostics, all unrelated to the submitted work. AS, PJV, MK, and TH declare no competing interests.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The research leading to these results was mainly funded by the European Commission under the seventh framework programme of the European Union (grant agreement no. 211696); Danone Nutricia Research funded part of the 36‐months study; additional funding for this study was provided by the EU Joint Programme– Neurodegenerative Disease Research (JPND) MIND‐AD grant to MK, HS, TH; EU Joint Programme– Neurodegenerative Disease Research (JPND) EURO‐FINGERS grant to MK, TH; Kuopio University Hospital, Finland (EVO/VTR grant; HS); Alzheimerfonden Sweden; Swedish Research Council; Stockholm City Council (ALF grant); Center for Innovative Medicine (CIMED) at Karolinska Institute, Sweden; Stiftelsen Stockholms sjukhem, Sweden; European Research Council (grant 804371); and Academy of Finland grants (317465, 287490). The European Commission had no role in study design, data collection, analysis and interpretation, writing of the report, or the decision to submit for publication. Danone Nutricia Research had no role in study design, data collection, and the decision to submit for publication, and as part of the LipiDiDiet consortium was involved in 36‐month data analysis and writing of the current report. All decisions taken on design, analysis, interpretation, and decision to submit for publication were made by the LipiDiDiet Trial Steering Committee, excluding Danone Nutricia Research. HS, AS, PJV, SBH, KB, MK, and TH had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication. We sincerely thank all participants enrolled in the study and their families. We thank Nico Rozendaal, Jose de Bont, Anja Kerksiek, and all investigators and on‐site study staff for their efforts in the conduct of the field work and all preclinical researchers for their design contributions. A full list of collaborators is provided in the supporting information. Editorial support and language correction were provided by Tim Kelly (Medi‐Kelsey Limited), funded by Danone Nutricia Research.
Soininen H, Solomon A, Visser PJ, et al. 36‐month LipiDiDiet multinutrient clinical trial in prodromal Alzheimer's disease. Alzheimer's Dement. 2021;17:29–40. 10.1002/alz.12172
REFERENCES
- 1. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673‐2734. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization; 2019. Licence: CC BY‐NC‐SA 3.0 IGO, ISBN 978‐92‐4‐155054‐3. [PubMed] [Google Scholar]
- 3. Anastasiou CA, Yannakoulia M, Kontogianni MD, et al. Mediterranean lifestyle in relation to cognitive health: results from the HELIAD study. Nutrients. 2018;10:1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lehtisalo J, Ngandu T, Valve P, et al. Nutrient intake and dietary changes during a 2‐year multi‐domain lifestyle intervention among older adults: secondary analysis of the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) randomised controlled trial. Br J Nutr. 2017;118:291‐302. [DOI] [PubMed] [Google Scholar]
- 5. van Wijk N, Broersen LM, de Wilde MC, et al. Targeting synaptic dysfunction in Alzheimer's disease by administering a specific nutrient combination. J Alzheimers Dis. 2014;38:459‐479. [DOI] [PubMed] [Google Scholar]
- 6. de Wilde MC, Hogyes E, Kiliaan AJ, Farkas T, Luiten PG, Farkas E. Dietary fatty acids alter blood pressure, behavior and brain membrane composition of hypertensive rats. Brain Res. 2003;988:9‐19. [DOI] [PubMed] [Google Scholar]
- 7. De Bruin NM, Kiliaan AJ, De Wilde MC, Broersen LM. Combined uridine and choline administration improves cognitive deficits in spontaneously hypertensive rats. Neurobiol Learn Mem. 2003;80:63‐79. [DOI] [PubMed] [Google Scholar]
- 8. de Wilde MC, Penke B, van der Beek EM, Kuipers AA, Kamphuis PJ, Broersen LM. Neuroprotective effects of a specific multi‐nutrient intervention against Abeta42‐induced toxicity in rats. J Alzheimers Dis. 2011;27:327‐339. [DOI] [PubMed] [Google Scholar]
- 9. Broersen LM, Kuipers AA, Balvers M, et al. A specific multi‐nutrient diet reduces Alzheimer‐like pathology in young adult AbetaPPswe/PS1dE9 mice. J Alzheimers Dis. 2013;33:177‐190. [DOI] [PubMed] [Google Scholar]
- 10. Jansen D, Zerbi V, Arnoldussen IA, et al. Effects of specific multi‐nutrient enriched diets on cerebral metabolism, cognition and neuropathology in AbetaPPswe‐PS1dE9 mice. PLoS One. 2013;8:e75393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zerbi V, Jansen D, Wiesmann M, et al. Multinutrient diets improve cerebral perfusion and neuroprotection in a murine model of Alzheimer's disease. Neurobiol Aging. 2014;35:600‐613. [DOI] [PubMed] [Google Scholar]
- 12. Koivisto H, Grimm MO, Rothhaar TL, et al. Special lipid‐based diets alleviate cognitive deficits in the APPswe/PS1dE9 transgenic mouse model of Alzheimer's disease independent of brain amyloid deposition. J Nutr Biochem. 2014;25:157‐169. [DOI] [PubMed] [Google Scholar]
- 13. Grimm MOW, Michaelson DM, Hartmann T. Omega‐3 fatty acids, lipids, and apoE lipidation in Alzheimer's disease: a rationale for multi‐nutrient dementia prevention. J Lipid Res. 2017;58:2083‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scheltens P, Kamphuis PJ, Verhey FR, et al. Efficacy of a medical food in mild Alzheimer's disease: a randomized, controlled trial. Alzheimers Dement. 2010;6:1‐10.e1. [DOI] [PubMed] [Google Scholar]
- 15. Scheltens P, Twisk JW, Blesa R, et al. Efficacy of Souvenaid in mild Alzheimer's disease: results from a randomized, controlled trial. J Alzheimers Dis. 2012;31:225‐236. [DOI] [PubMed] [Google Scholar]
- 16. Shah RC, Kamphuis PJ, Leurgans S, et al. The S‐Connect study: results from a randomized, controlled trial of Souvenaid in mild‐to‐moderate Alzheimer's disease. Alzheimers Res Ther. 2013;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cummings J, Passmore P, McGuinness B, et al. Souvenaid in the management of mild cognitive impairment: an expert consensus opinion. Alzheimers Res Ther. 2019;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS‐ADRDA criteria. Lancet Neurol. 2007;6:734‐746. [DOI] [PubMed] [Google Scholar]
- 19. Soininen H, Solomon A, Visser PJ, et al. 24‐month intervention with a specific multinutrient in people with prodromal Alzheimer's disease (LipiDiDiet): a randomised, double‐blind, controlled trial. Lancet Neurol. 2017;16:965‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hendrix SB, Soininen H, van Hees AMJ, et al. Alzheimer's disease composite score: a post‐hoc analysis using data from the LipiDiDiet trial in prodromal Alzheimer's disease. J Prev Alzheimers Dis. 2019;6:232‐236. [DOI] [PubMed] [Google Scholar]
- 21. Rijpma A, Meulenbroek O, van Hees AM, et al. Effects of Souvenaid on plasma micronutrient levels and fatty acid profiles in mild and mild‐to‐moderate Alzheimer's disease. Alzheimers Res Ther. 2015;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Oudenhoven FM, Swinkels SHN, Hartmann T, Soininen H, van Hees AMJ, Rizopoulos D. Using joint models to disentangle intervention effect types and baseline confounding: an application within an intervention study in prodromal Alzheimer's disease with Fortasyn Connect. BMC Med Res Methodol. 2019;19:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rizopoulos D. JM: an R package for the joint modelling of longitudinal and time‐to‐event data. J Statistical Software. 2010;35:1‐33. [Google Scholar]
- 24. Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6‐year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster‐randomised controlled trial. Lancet. 2016;388:797‐805. [DOI] [PubMed] [Google Scholar]
- 25. Vonesh EF, Greene T, Schluchter MD. Shared parameter models for the joint analysis of longitudinal data and event times. Stat Med. 2006;25:143‐163. [DOI] [PubMed] [Google Scholar]
- 26. Rizopoulos D. Joint Models for Longitudinal and Time‐to‐Event Data: With Applications in R. CRC press; 2012. [Google Scholar]
- 27. Vellas B, Bateman R, Blennow K, et al. Endpoints for pre‐dementia AD trials: a report from the EU/US/CTAD Task Force. J Prev Alzheimers Dis. 2015;2:128‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Delor I, Charoin JE, Gieschke R, Retout S, Jacqmin P. Modeling Alzheimer's disease progression using disease onset time and disease trajectory concepts applied to CDR‐SOB scores from ADNI. CPT Pharmacometrics Syst Pharmacol. 2013;2:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ito K, Hutmacher MM. Predicting the time to clinically worsening in mild cognitive impairment patients and its utility in clinical trial design by modeling a longitudinal clinical dementia rating sum of boxes from the ADNI database. J Alzheimers Dis. 2014;40:967‐979. [DOI] [PubMed] [Google Scholar]
- 30. Samtani MN, Raghavan N, Novak G, Nandy P, Narayan VA. Disease progression model for clinical dementia rating‐sum of boxes in mild cognitive impairment and Alzheimer's subjects from the Alzheimer's Disease Neuroimaging Initiative. Neuropsychiatr Dis Treat. 2014;10:929‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aisen PS, Andrieu S, Sampaio C, et al. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011;76:280‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chincarini A, Sensi F, Rei L, et al. Integrating longitudinal information in hippocampal volume measurements for the early detection of Alzheimer's disease. Neuroimage. 2016;125:834‐847. [DOI] [PubMed] [Google Scholar]
- 33. Jack CR, Jr. , Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rockwood K. Size of the treatment effect on cognition of cholinesterase inhibition in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:677‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. FINGER: The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) (FINLAND). Available at: http://wwfingers.com/finger/. Accessed December 13, 2019.
- 36. Olde Rikkert MG, Verhey FR, Blesa R, et al. Tolerability and safety of Souvenaid in patients with mild Alzheimer's disease: results of multi‐center, 24‐week, open‐label extension study. J Alzheimers Dis. 2015;44:471‐480. [DOI] [PubMed] [Google Scholar]
- 37. MIND‐AD: MIND‐AD: Multimodal preventive trials for Alzheimer's Disease: towards multinational strategies (MIND‐AD) trial. (SWEDEN, FINLAND, FRANCE, GERMANY). Available at: http://wwfingers.com/mind-ad/. Accessed December 13, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.


