Abstract
In diabetic animal models, high plasma/tissue levels of methylglyoxal (MG) are implicated in atherosclerosis. N-acetylcysteine (NAC) is a cysteine prodrug that replenishes intracellular glutathione (GSH) levels, which can increase the elimination of MG in diabetes mellitus (DM). The present study investigated the anti-atherosclerotic role of NAC in DM and aimed to determine whether the mechanism involved GSH-dependent MG elimination in the aorta. Apolipoprotein-E knockdown (ApoE−/−) mice injected with streptozotocin for 5 days exhibited enhanced atherosclerotic plaque size in the aortic root; notably, a high-lipid diet aggravated this alteration. NAC treatment in the drinking water for 12 weeks decreased the size of the atherosclerotic lesion, which was associated with a reduction in MG-dicarbonyl stress and oxidative stress, as indicated by decreased serum malondialdehyde levels, and increased superoxide dismutase-1 and glutathione peroxidase-1 levels in the diabetic aorta. Endothelial damage was also corrected by NAC, as indicated by an increase in the expression levels of phosphorylated (p-)Akt and p-endothelial nitric oxide synthase (eNOS) in the aorta, as well as nitric oxide (NO) in the serum. In addition, MG-treated human umbilical vein endothelial cells (HUVECs) exhibited increased reactive oxygen species and decreased antioxidant enzyme expression levels. NAC treatment corrected the alteration in HUVECs induced by MG, whereas the protective role of NAC was blocked via inhibition of GSH. These findings indicated that the diabetic aorta was more susceptible to atherosclerotic lesions compared with non-diabetic ApoE−/− mice. Furthermore, NAC may offer protection against atherosclerotic development in DM by altering aortic and systemic responses via correcting GSH-dependent MG elimination, leading to decreased oxidative stress and restoration of the p-Akt/p-eNOS pathway in the aorta.
Keywords: N-acetylcysteine, diabetes, glutathione, methylglyoxal, dicarbonyl stress, atherosclerosis
Introduction
Diabetes mellitus (DM) is one of the most important global health threats (1). Accelerated and aggressive atherosclerosis leading to severe cardiovascular events, such as ischemic heart disease and stroke, accounts for an important cause of morbidity and mortality due to cardiovascular disease in patients with DM (2). Notably, metabolic disease is characterized by hyperglycemia, and lowering blood glucose levels by diet, exercise and medication is an important foundation of DM management (3). However, tight glucose control has been reported to limit early microvascular diseases, such as retinopathy and nephropathy, but not macrovascular atherosclerosis, according to the Diabetes Control and Complication Trial study (4–6). In addition, more than half the increased risk of cardiovascular disease in DM cannot be fully explained by well-known risk factors (7), such as hypertension and abnormal cholesterol, thus indicating that other elements underlying DM may contribute to macrovascular atherosclerosis (7,8).
Methylglyoxal (MG) is a highly reactive dicarbonyl compound, which is a key contributor to atherosclerosis. MG is derived from the glucose metabolic process, and is a precursor of most of the functionally important spontaneous protein and DNA modifications (9,10). Circulating and tissue MG are 3–5-fold higher in diabetic models (11). In patients with DM and vascular complications, plasma MG levels are further elevated (12,13). While limited data are available on the alteration of aortic MG levels during DM, abundant literature supports the concept that MG is important in the pathogenesis of atherosclerosis (14,15). A previous report demonstrated that exposure to exogenous MG or inhibition of glyoxalase-1 to increase endogenous MG levels was able to increase vascular endothelial adhesion and augment atherogenesis in apolipoprotein E (ApoE) knockout mice (16). Furthermore, Goto-Kakizaki rats (a type 2 DM model) treated with MG in drinking water exhibited aggravated endothelial dysfunction and oxidative stress (17,18). Overall, these studies suggested a potential benefit for targeting MG in diabetic individuals.
Glutathione (GSH) is a rate-limiting co-factor in the glyoxalase elimination pathway for MG. High glucose levels have been reported to decrease the intracellular levels and rate of uptake of cysteine, which is a rate-limiting factor that maintains GSH levels in human aortic vascular smooth muscle cells (19). Reduced GSH content was further detected in the aortic tissue of diabetic rats with hyperglycemia in vivo (20). N-acetylcysteine (NAC) is an effective agent to replenish GSH in deficient cells. Previously, it was demonstrated that DM imbalanced the MG/GSH ratio in brains of a stroke model, whereas NAC corrected this ratio, and decreased dicarbonyl levels and oxidative stress to reduce cerebral injury in a diabetic ischemia/reperfusion model (21,22). These findings suggested a preventive role of NAC in endothelial dysfunction, which may be associated with the correction of MG/GSH levels and oxidative stress (21,22). Since oxidative stress and endothelial dysfunction have been identified as common upstream events that mediate the atherogenic effects of hyperglycemia (23,24), the present study aimed to investigate the anti-atherosclerotic role of NAC in DM and whether the mechanism involved decreased oxidative stress and endothelial damage via GSH-dependent MG elimination in the aorta.
Materials and methods
Materials
MG (cat. no. M0252), GSH (cat. no. G4251), NAC (cat. no. A7250), streptozotocin (STZ; cat. no. S0130), the GSH synthesis inhibitor buthionine sulfoximine (BSO; cat. no. B2640) and guanidine hydrochloride (cat. no. G7294) were purchased from Sigma-Aldrich (Merck KGaA). Rabbit anti-glutathione peroxidase-1 (GPX-1; cat. no. ab22604), rabbit anti-superoxide dismutase-1 (SOD-1; cat. no. ab51254), rabbit anti-endothelial nitric oxide synthase (eNOS; cat. no. ab5589), anti-phosphorylated (p)-eNOS (cat. no. ab215717) and HRP-conjugated goat-anti-rabbit IgG (cat. no. ab150077) were purchased from Abcam. Rabbit anti-Akt (cat. no. sc-8312) and anti-p-Akt (cat. no. sc-7985 R) were obtained from Santa Cruz Biotechnology, Inc. Mouse anti-actin antibody (cat. no. 612656) was obtained from BD Biosciences. HRP-conjugated sheep-anti-mouse IgG (cat. no. NA931) was purchased from Amersham (Cytiva). Enhanced chemiluminescence (ECL) reagent (cat. no. 1705060) was acquired from Bio-Rad Laboratories, Inc. Nitric oxide (NO; cat. no. A012), which was used for the nitrate reductase method according to the instructions of the NO assay kit, and the malondialdehyde (MDA) assay kit (cat. no. A003) were obtained from Nanjing Jiancheng Bioengineering Institute.
Animal preparation
Male ApoE−/− mice (age, 5 weeks; weight, 20–25 g; Jackson Laboratory, n=40) were fed at the Animal Center of Huazhong University of Science and Technology (Wuhan, China), following the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (25). Rearing temperature was between 22–25°C, relative humidity was 60%, and light-dark cycle was 12 h. Mice were supplied with continuous access to drinking water and a normal diet. After acclimatization for 1 week, the mice were randomly divided into four groups: i) control group (n=10); ii) DM group (n=10); iii) diabetic mice fed a high-lipid diet (HLD) (DM + HLD group, n=10); and iv) diabetic mice fed a HLD and administered NAC (DM + HLD + NAC group, n=10). Experimental diabetic mice were achieved via an intraperitoneal injection of 50 mg/kg STZ for 5 consecutive days, whereas control mice were injected with citrate buffer for 5 consecutive days. At day 7, those mice whose plasma glucose was >300 mg/dl were deemed to be diabetic. In total, 20 diabetic mice were placed on a HLD containing 1.25% cholesterol and 10% coconut oil. A total of 10 mice from the DM + HLD group received 2 mmol/l NAC in their drinking water, whereas the remaining mice in DM + HLD group received normal drinking water. All mice were fed for 12 weeks. After 12 weeks, blood was obtained from the carotid artery under anesthesia with ketamine (125 mg/kg, intraperitoneal) and xylazine (6.25 mg/kg, intraperitoneal). The doses of ketamine and xylazine were selected according to previous studies (26–29), and were appropriately modified to anesthetize mice and obtain blood for blood lipid, NO and MDA assays. Subsequently, the mice were sacrificed by cervical dislocation under deep anesthesia. The aortas were harvested and immersed in liquid nitrogen for high-performance lipid chromatography (HPLC), protein carbonyl contents assay, western blotting and atherosclerotic lesion evaluation.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated from neonatal umbilical cords, as described in our previous study (30). HUVECs were cultured in M199 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Beijing Solarbio Science & Technology Co., Ltd.), 1% insulin-transferrin-sodium selenite (Sigma-Aldrich; Merck KGaA) and 1% penicillin-streptomycin at 37°C in an atmosphere containing 5% CO2. HUVECs at passages 2–4 were divided into four groups: i) control group; ii) MG group, where HUVECs were incubated with 1 mM MG for 24 h; iii) NAC group, where HUVECs were pretreated with 1 mM NAC overnight, and then incubated with 2 mM NAC and 1 mM MG for 24 h; and iv) BSO group, where HUVECs were pretreated with 1 mM NAC and 50 µM BSO overnight, and then incubated with 2 mM NAC, 300 µM BSO and 1 mM MG for 24 h. For the reactive oxygen species (ROS) assay, 5×104 HUVECs were cultured in 96-well plates. For western blot analyses, HUVECs were cultured in 6-well plates at 1×106 cells/well.
Evaluation of atherosclerotic lesions
For the evaluation of aortic lesions, 5-µm cryosections of the thoracic aortic root were stained with oil red O at 37°C for 20 min, and were assessed using the Paigen's method with Image Pro Plus 7.0 software (Media Cybernetics, Inc.) (31). For each animal, five sections were used to assess the mean lesion area under a light microscope with Image-Pro Plus 7.0 software.
Blood lipid measurement
An automatic biochemistry analyzer (Olympus AU2700; Olympus Corporation) was used to measure the levels of total cholesterol (TC), triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C), according to the manufacturer's protocols. The levels of low-density lipoprotein cholesterol (LDL-C) were calculated using the Friedewald formula (32).
HPLC for quantification of GSH and MG in the aorta
Determination of GSH was performed as previously described (22,33). Briefly, trichloroacetic acid (TCA)-soluble acid supernatants of the aorta in PBS were derived using 6 mmol/l iodoacetic acid (pH 7–8) and 1% 2,4-dinitrophenyl fluorobenzene (pH 7.0) to produce S-carboxymethyl and 2,4-dinitrophenyl derivatives, respectively. GSH derivatives were separated using buffer [solvent A, 4:1 methanol:water (v/v); solvent B, 272 g sodium acetate trihydrate, 122 ml water and 378 ml glacial acetic acid were mixed, and 200 ml of the resulting solution was added to 800 ml solvent A] on a 250×4.6-mm Alltech Lichrosorb NH2 10 µm anion-exchange column (LC-20AB; Shimadzu Corporation) and detected at UV-365 nm at room temperature [sample quantity, 50 µl; flow rate, 1 ml/min; solvent A/solvent B, 90/10; running time 30 min; internal standards, GSH and oxidized glutathione (GSSG)]. The aortic GSH contents, expressed as nmol/mg protein, were quantified by comparison to standard derivatives in the same manner.
Determination of MG was performed as previously described (22,34). Briefly, aortic homogenates in PBS were treated with 60% perchloric acid (29:1 v/v), and the acidic supernatants were derivatized with 0.1 mol/l o-phenylenediamine (100:1 v/v) for 24 h. MG derivatives were separated on a 250×4.6-mm Chromegabond Ultra C-18 reversed phase column (Shimadzu Corporation), and detected at UV-315 nm. Aortic MG contents, expressed as nmol/mg protein, were quantified by comparison to MG standard derivatives with o-phenylenediamine.
Assessment of protein carbonyls
Total protein carbonyls were determined in aortic homogenates as previously described (35). Briefly, homogenates were incubated with 10 mmol/l 2,4-dinitrophenylhydrazine in 2 mol/l hydrochloric acid (1:2 v/v) and precipitated with 10% TCA, and then centrifuged at 11,000 × g at room temperature for 3 min. The supernatants were removed and the pellets were washed with 1 ml ethanol-ethyl acetate (1:1) at room temperature (1:1 volume ratio) three times (10 min each). Then, protein pellets were re-dissolved in 600 µl of 6 mol/l guanidine hydrochloride containing 20 mmol/l potassium phosphate (pH 2.3) and incubated for 15 min at 37°C, and supernatants were obtained following centrifugation at 11,000 × g at room temperature for 3 min. Absorbance of the supernatants was measured at 366 nm. Protein carbonyl contents were quantified using an extinction coefficient of 22,000 M−1 cm−1.
ELISA for determination of serum NO and MDA levels
Whole blood was placed for 2 h at room temperature and centrifuged to obtain serum at 1,500 × g for 10 min at 4°C. Serum NO and MDA levels were detected using commercial kits, according to the manufacturer's protocols. The kits were based on colorimetric methods and the samples were evaluated using a microplate reader.
Measurement of intracellular ROS levels
Intracellular ROS levels were measured using 2′,7′-dichlorofluorescein diacetate (DCFH-DA), according to the method described by Zhu et al (36); briefly, 10 µM DCFH-DA was added to the medium. DCFH-DA is converted to dichlorofluorescein (DCF) in proportion to ROS concentration and remains trapped inside the cell. Prior to fluorescence analysis, cells were incubated with DCFH-DA for 30 min at 37°C in the dark and washed three times with 1X PBS. Subsequently, DCF fluorescence was determined using excitation/emission wavelengths of 488/610 nm. ROS levels were digitally quantified using Image Pro Plus 7.0 software (Media Cybernetics, Inc.).
Western blot analysis of p-Akt, p-eNOS, GPX-1 and SOD-1
Aortic tissues or HUVECs were homogenized with RIPA lysis buffer containing 50 mM Tris (pH 8.0), 150 mM sodium chloride, 0.5% sodium deoxycholate, 0.1% SDS and a protease inhibitor cocktail (cat. no. P9599; Sigma-Aldrich; Merck KGaA). Total proteins (30 µg) were separated by SDS-PAGE on 15% gels at 120 V for 140 min for actin, SOD-1 and GPX-1, and on 10% gels at 120 V for 120 min for actin, Akt, p-Akt, eNOS and p-eNOS. Proteins were then transferred onto PVDF membranes at 100 V for 1 h. The membranes were blocked in 5% skimmed milk in TBS with Tween-20 buffer [20 mM Tris, 137 mM NaCl and 0.1% Tween-20 (pH 7.6)] for 1 h at room temperature, and were then incubated with rabbit anti-Akt and p-Akt antibodies (1:500), rabbit anti-eNOS and p-eNOS antibodies (1:500), rabbit anti-GPX-1 antibody (1:2,000), rabbit anti-SOD-1 antibody (1:2,000) and mouse anti-actin antibody (1:5,000) at 4°C overnight with agitation, followed by incubation with the corresponding HRP-conjugated secondary antibody (1:5,000) for 2 h at room temperature. Protein expression was detected using ECL (Bio-Rad Laboratories, Inc.), according to the manufacturer's instructions. Protein expression levels were normalized to actin (in the case of SOD-1 and GPX-1), total Akt (in the case of p-Akt) or total eNOS (in the case of p-eNOS) with Image-Pro Plus 7.0 software.
Statistical analysis
All experiments were repeated four to five times. Data are presented as the mean ± SEM. Significant differences were assessed by one-way ANOVA followed by Tukey's post hoc test (for multiple comparisons) using GraphPad Prism 5 software (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
NAC inhibits atherosclerotic development in STZ-induced diabetic ApoE−/− mice fed a HLD
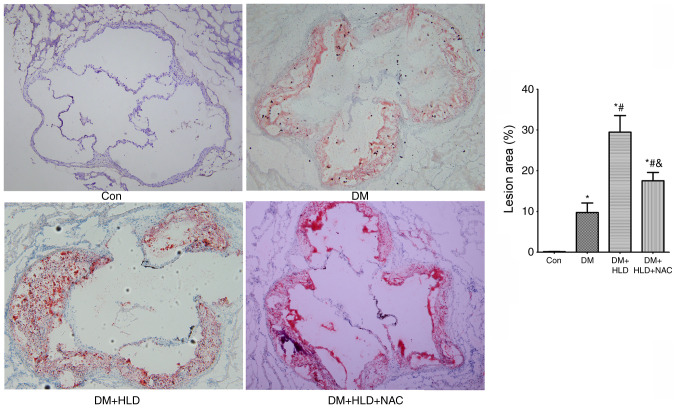
At 12 weeks, the atherosclerotic plaque area of the aortic root in diabetic mice was larger compared with that in the normal diet control group (Fig. 1). The atherosclerotic lesion area induced by the addition of a HLD was 3-fold larger than the area induced by DM alone (Fig. 1). Conversely, NAC treatment for 12 weeks significantly attenuated the atheroma by nearly half in diabetic mice fed a HLD compared with that in the DM + HLD group (Fig. 1).
Figure 1.
Atherosclerotic lesion areas in the aortic root were stained with oil red O (magnification, ×100) in ApoE−/− mice (Con group), STZ-injected ApoE−/− mice (DM group), STZ-injected ApoE−/− mice fed a HLD (DM + HLD group), and STZ-injected ApoE−/− mice fed a HLD and administered NAC-containing water (DM + HLD + NAC group). *P<0.05 vs. Con group; #P<0.05 vs. DM group; &P<0.05 vs. DM + HLD group (n=5/group). ApoE, apolipoprotein E; Con, control; DM, diabetes mellitus; HLD, high-lipid diet; NAC, N-acetylcysteine; STZ, streptozotocin.
NAC does not alter blood lipid levels or lipid profiles
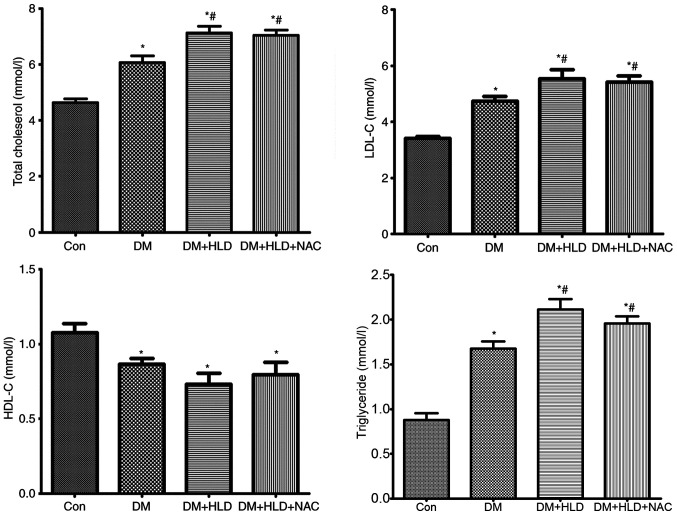
High plasma LDL-C is a well-known risk factor for the development and progression of atherosclerosis (37,38). Low HDL-C is also a contributor to atherosclerotic lesions. To ascertain if NAC affected cholesterol content to inhibit atherosclerosis in DM, total lipids and their composition were analyzed. The levels of TC, LDL-C and TG were elevated, whereas HDL-C was decreased in diabetic mice at 12 weeks compared with the lipid profiles in normal control mice. Mice fed a HLD for 12 weeks exhibited further increased TC, LDL-C and TG levels compared with DM group; however, this diet did not alter the levels of HDL-C in diabetic mice. NAC treatment did not affect total lipid levels or cholesterol composition compared with the observations in diabetic mice fed a HLD (Fig. 2). These results indicated that alteration of lipid or lipid profiles was not associated with the mechanism underlying the anti-atherosclerotic ability of NAC.
Figure 2.
Blood lipid levels (total cholesterol, LDL-C, HDL-C and triglyceride) were assessed using an automatic biochemistry analyzer in ApoE−/− mice (Con group), STZ-injected ApoE−/− mice (DM group), STZ-injected ApoE−/− mice fed a HLD (DM + HLD group), and STZ-injected ApoE−/− mice fed a HLD and administered NAC-containing water (DM + HLD + NAC group). *P<0.05 vs. Con group; #P<0.05 vs. DM group (n=8/group). LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ApoE, apolipoprotein E; Con, control; DM, diabetes mellitus; HLD, high-lipid diet; NAC, N-acetylcysteine; STZ, streptozotocin.
NAC corrects the upregulation of MG and carbonyl proteins in diabetic ApoE−/− mice fed a HLD
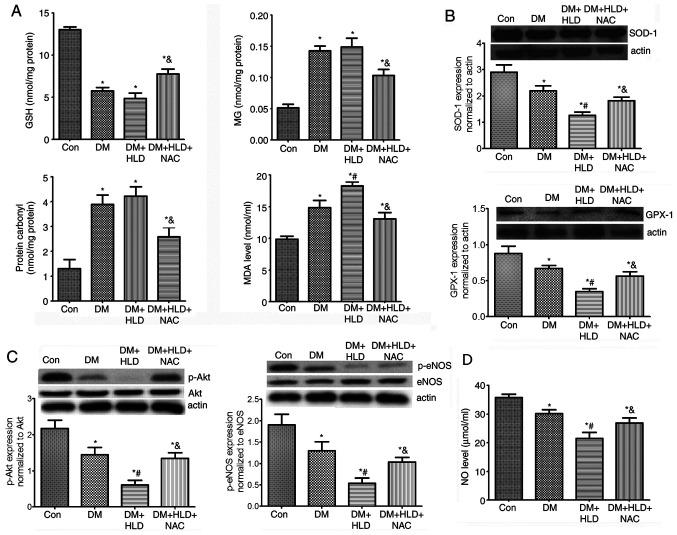
To ascertain whether NAC affected aortic MG and dicarbonyl stress, MG and carbonyl protein levels were detected in thoracic aorta samples. As expected, the MG and carbonyl protein contents in the aorta of diabetic mice were increased ~3-fold compared with those in the control group (Fig. 3A). Mice fed a HLD exhibited a slight elevation in MG and carbonyl protein contents in the aorta compared with those in the DM group; however, there were no significant differences between the DM and DM + HLD groups. After treatment with NAC for 12 weeks, DM + HLD + NAC mice exhibited reduced free MG and protein carbonyl contents in the thoracic aorta compared with those in the DM + HLD group (Fig. 3A). These results suggested that NAC corrected the increased dicarbonyl stress detected in ApoE−/− mice in the DM + HLD group.
Figure 3.
Effects of NAC on oxidative stress, p-Akt/p-eNOS protein expression and serum NO levels in diabetic ApoE−/− mice fed a HLD. (A) Aortic protein expression of GSH, MG, protein carbonyl contents and serum MDA levels (n=5 repeats/group). (B) Aortic protein expression levels of antioxidant enzymes (SOD-1 and GPX-1) (n=4 repeats/group). (C) Aortic protein expression levels of p-Akt and p-eNOS (n=4 repeats/group). (D) Serum levels of NO (n=4 repeats/group) in ApoE−/− mice (Con group), STZ-injected ApoE−/− mice (DM group), STZ-injected ApoE−/− mice fed a HLD (DM + HLD group), and STZ-injected ApoE−/− mice fed a HLD and administered NAC-containing water (DM + HLD + NAC group). *P<0.05 vs. Con group; #P<0.05 vs. DM group; &P<0.05 vs. DM + HLD group. ApoE, apolipoprotein E; Con, control; DM, diabetes mellitus; HLD, high-lipid diet; NAC, N-acetylcysteine; STZ, streptozotocin; GSH, glutathione; MG, methylglyoxal; p-, phosphorylated; eNOS, endothelial nitric oxide synthase; NO, nitric oxide.
NAC scavenges oxidative stress in diabetic ApoE−/− mice fed a HLD
To determine the levels of oxidative stress in DM, serum MDA, and aortic SOD-1 and GPX-1 expression levels were detected in mice. Serum MDA levels in DM mice were 1.5-fold higher than those in the control group. Mice fed a HLD exhibited a further increase in serum MDA levels compared with those detected in the DM group (Fig. 3A). DM markedly decreased aortic SOD-1 and GPX-1 protein expression levels, which were further aggravated by a HLD. NAC treatment decreased serum MDA, but increased aortic SOD-1 and GPX-1 expression levels compared with the findings in ApoE−/− mice in the DM + HLD group (Fig. 3A and B). These findings indicated that oxidative stress was increased in ApoE−/− mice in the DM + HLD group. Conversely, NAC scavenged oxidative stress partly by decreasing serum MDA and promoting the expression levels of antioxidant enzymes in diabetic ApoE−/− mice fed a HLD.
NAC improves the reduction in aortic p-Akt/p-eNOS protein expression, and corrects serum NO levels in diabetic ApoE−/− mice fed a HLD
To further ascertain the endothelial function affected by MG-dicarbonyl and oxidative stress, alterations in p-Akt/eNOS and serum NO levels were detected. The phosphorylation of Akt was significantly decreased in the aorta of mice in the DM group compared with that in the aorta of control mice. The levels of p-Akt in the aorta were further decreased in the DM + HLD group. After treatment with NAC in diabetic ApoE−/− mice fed a HLD, p-Akt expression was significantly enhanced compared with that detected in the DM + HLD group. The changes in p-eNOS expression in aorta samples were similar to those in p-Akt expression. NO levels were significantly decreased in the DM group compared with the control mice, which were further decreased in the DM + HLD group. Whereas, after treatment with NAC, NO levels increased. These results suggested that DM may damage endothelial function and decrease serum NO levels, which were corrected by treatment with NAC for 12 weeks via promoting Akt/eNOS phosphorylation (Fig. 3C and D).
MG induces ROS expression in HUVECs
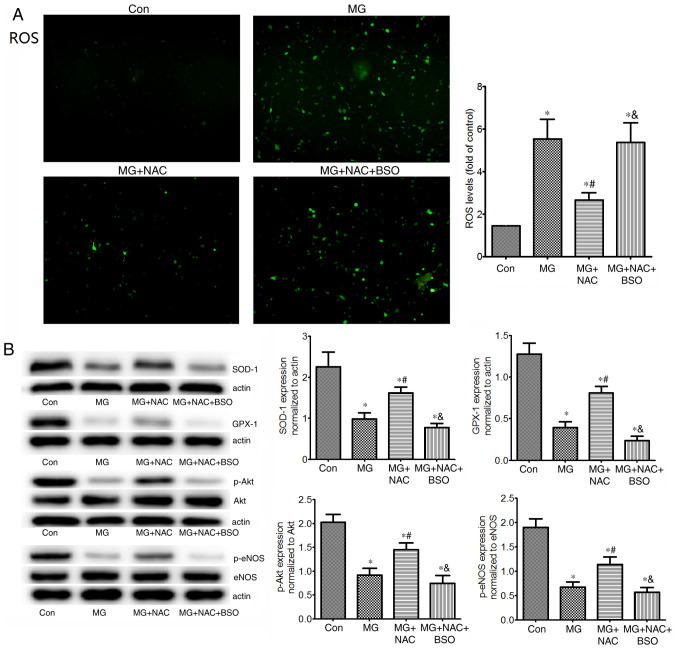
To explore whether MG induced oxidative stress, ROS levels and antioxidant protein expression levels (SOD-1 and GPX-1) were detected in HUVECs cultured with MG. In addition, p-Akt and p-eNOS protein expression levels were detected in HUVECs (Fig. 4). As expected, 1 mM MG significantly increased ROS, and decreased SOD-1 and GPX-1 expression levels in vitro compared with in the control group. MG treatment also markedly reduced p-Akt and p-eNOS protein expression levels in HUVECs. The results in HUVECs were consistent with those in the aortas of mice, and suggested that MG induced oxidative stress by increasing ROS, and decreasing SOD-1 and GPX-1. Furthermore, excessive MG may result in endothelial dysfunction by destructing the p-Akt/p-eNOS pathway.
Figure 4.
Effects of NAC on ROS levels, SOD-1, GPX-1 and p-Akt/p-eNOS protein expression in HUVECs. (A) Intracellular ROS levels (magnification, ×100), and (B) protein expression levels of antioxidant enzymes (SOD-1 and GPX-1), p-Akt and p-eNOS in untreated HUVECs, or in HUVECs exposed to 1 mM MG, with or without NAC, or with NAC + BSO (glutathione inhibitor). Intracellular ROS levels were measured using 2′,7′-dichlorofluorescein diacetate. Protein expression levels were assessed by western blotting (n=4 repeats/group). *P<0.05 vs. control group; #P<0.05 vs. MG group; &P<0.05 vs. MG + NAC group. HUVECs, human umbilical vein endothelial cells; NAC, N-acetylcysteine; MG, methylglyoxal; BSO, buthionine sulfoximine; ROS, reactive oxygen species; SOD-1, superoxide dismutase 1; GPX-1, glutathione peroxidase 1; p-, phosphorylated; eNOS, endothelial nitric oxide synthase.
Inhibition of GSH reverses the effects of NAC on protection of HUVECs against MG-induced oxidative stress. GSH is a rate-limiting enzyme in the elimination of MG. To determine the role of GSH in the antioxidative effects of NAC, aortic GSH levels were determined in mice. As expected, mice in the DM group exhibited reduced levels of GSH in the aorta. In addition, a HLD led to a slight reduction in aortic GSH compared with that in the DM group; however, there was no significant difference between the DM and DM + HLD groups. After treatment with NAC for 12 weeks, diabetic ApoE−/− mice fed a HLD exhibited enhanced aortic GSH levels compared with those in the DM + HLD group (Fig. 3A). BSO is an inhibitor of GSH (39). In vitro, NAC significantly reduced MG-induced ROS elevation compared with that in the MG group. However, administration of BSO increased ROS to the levels of the MG group (Fig. 4A). These results indicated that NAC inhibited MG-induced ROS by increasing GSH synthesis. In addition, in cells treated with NAC, the protein expression levels of SOD-1, GPX-1, p-Akt and p-eNOS were significantly increased compared with those in the MG group (Fig. 4B). Conversely, BSO reversed the effects of NAC on these proteins, suggesting that the protective role of NAC on these proteins may be due to increasing the levels of GSH.
Discussion
The present study demonstrated that mice in the DM group exhibited a larger atherosclerotic area in the aorta compared with that detected in control mice, which was associated with the GSH-dependent ability to eliminate aortic MG. Additionally, the elevation of MG in diabetic aortas was associated with an increase in carbonyl and oxidative stress, as well as a decrease in serum NO levels. NAC provides cysteine for GSH production, and may therefore be effective at decreasing MG and oxidative stress, increasing serum NO and preventing DM-induced atherosclerosis.
In the present study, MG levels in the aortas of ApoE−/− mice injected with STZ were elevated 3-fold compared with those in the control mice. The increase in MG levels in the aortas of experimental diabetic mice was consistent with the elevated plasma MG levels that have previously been described in this model (16). Furthermore, GSH levels were markedly reduced in diabetic mice aortas compared with those in the aortas of control mice, which suggested that MG elimination was decreased in the aorta of diabetic mice. Notably, neither MG nor GSH content in the aorta was affected further by a HLD, suggesting that hyperglycemia rather than hypercholesterolemia may be the major cause of alterations in MG and GSH levels in the aorta. Although there are limited data to support the alteration of MG in the aorta during DM, previous studies have reported that hyperglycemia reduces GSH content in aortic tissue (20,40), which is consistent with the present results.
While anti-diabetic treatment can control the increased production of MG caused by hyperglycemia, it is difficult to correct for the decreased elimination of MG caused by low GSH levels. NAC, a precursor of GSH, can increase MG elimination (22). In the present animal study, NAC treatment attenuated atherosclerosis in mice in the DM + HLD group, indicating that aortic MG elevation may have an important role in atherogenesis. NAC administration may therefore be considered an effective anti-atherosclerotic method to decrease MG levels and MG-dicarbonyl stress in the aorta.
Activation of receptor for advanced glycation end products (RAGE) has been widely implicated in the pro-atherosclerotic effects of MG, whereas RAGE deletion cannot completely prevent vascular inflammation and damage (41). Increased oxidative stress has been identified as a common upstream event that mediates the atherogenic effects of hyperglycemia (42–44). In line with this, diabetic ApoE−/− mice exhibited higher serum MDA, and lower aortic SOD and GPX-1 expression levels compared with those in the control group. In addition, a HLD aggravated oxidative stress. Conversely, NAC-induced reduction of MG corrected the oxidative stress detected in the aorta, suggesting that MG may induce aortic oxidative stress in DM. This hypothesis was further validated by MG-treated HUVECs, which exhibited an increase in ROS production and a decrease in antioxidant levels. All of these effects were significantly attenuated by pretreatment with NAC, but were blocked by inhibition of GSH. These findings indicated that MG acted as a strong stimulator that could induce an imbalance between ROS and antioxidant enzymes, thus upregulating oxidative stress, whereas NAC was dependent on GSH to reverse MG-induced dicarbonyl and oxidative stress. Whereas NAC-restored GSH could act as an antioxidant and/or induce elimination of MG, our previous findings in platelets revealed that the protection offered by NAC in STZ-treated mice was caused by its role as an eliminator of MG instead of as an antioxidant, since GSSG levels were unchanged when GSH and MG were significantly altered in platelets (45). In the present study, the GSSG levels in the aorta were not detected. Whether NAC acts as an eliminator of MG rather than as an antioxidant requires further investigation.
Alterations in Akt/eNOS signaling, which can inhibit ROS-induced endothelial damage and improve NO release, also serve an important role in atherosclerosis (21,22). The present study detected a reduction in aortic Akt phosphorylation, and a subsequent decrease in eNOS phosphorylation and serum NO levels in diabetic mice, suggesting endothelial dysfunction in the DM group. These changes were further aggravated by a HLD. NAC afforded protection to increase serum NO. In line with the present results, a previous study revealed that MG increased oxidative stress and/or AGEs formation alongside a decrease in NO bioavailability, thus inducing or aggravating endothelial dysfunction in normal Wistar rats or in Goto-Kakizaki rats (7,18). Overall, based on the aforementioned results, it may be hypothesized that MG-dicarbonyl stress induces endothelial dysfunction by altering Akt and eNOS phosphorylation, and aggravates endothelial dysfunction by increasing oxidative stress. NAC may enhance the phosphorylation of Akt and increase NO levels to reverse such dysfunction.
In the present study, lipids, particularly LDL-C, were altered in mice in the DM and DM + HLD groups, whereas NAC had no effect on blood lipid profiles. The present results differ from those reported in other studies. For example, NAC has previously been shown to reduce TC, TG, HDL and very-LDL levels without affecting LDL-C levels in rats following a splenectomy (46). NAC also enhanced HDL-C levels in patients with hyperlipidemia (47). The possible reasons for these discrepancies may be different experimental species, disease status and NAC dosage. Lipid peroxidation by ROS has a critical role in atherogenesis, particularly the early stage of atherosclerosis (48). However, the alteration of lipid peroxidation was not measured in the present model and requires further research in the future.
At present, no specific antioxidant treatment has been recommended to prevent atherosclerotic progression (49). The present study confirmed that NAC possessed anti-atherosclerotic potential in experimental DM via the elevation of GSH in the aorta to inhibit dicarbonyl and oxidative stress. This finding may lead to a novel antioxidant strategy in the treatment of atherosclerosis in DM, in addition to the treatment of established risk factors.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- DM
diabetes mellitus
- GPX-1
glutathione peroxidase-1
- GSH
glutathione
- GSSG
oxidized glutathione
- HDL-C
high-density lipoprotein cholesterol
- HUVECs
human umbilical vein endothelial cells
- LDL-C
low-density lipoprotein cholesterol
- MG
methylglyoxal
- MDA
malondialdehyde
- NAC
N-acetylcysteine
- NO
nitric oxide
- p-eNOS
phosphorylated endothelial nitric oxide synthase
- ROS
reactive oxygen species
- SOD-1
superoxide dismutase-1
- STZ
streptozotocin
- TC
total cholesterol
- TG
triglyceride
Funding
The present study was supported by a grant from the Hubei Natural Science Foundation of China (grant no. WJ2017M117).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BW conceived and designed the experiments. SZ and MZ performed the experiments. LL analyzed the data. XF performed the experiments and drafted the paper. XF, SZ and BW confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Tongji Medical School, Huazhong University of Science and Technology (Wuhan, China; approval no. S1297). Written informed consent was obtained from patients prior to the use of umbilical cord tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat Rev Endocrinol. 2016;12:616–622. doi: 10.1038/nrendo.2016.105. [DOI] [PubMed] [Google Scholar]
- 2.Bornfeldt KE. 2013 Russell Ross memorial lecture in vascular biology: Cellular and molecular mechanisms of diabetes mellitus-accelerated atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:705–714. doi: 10.1161/ATVBAHA.113.301928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bairey Merz CN, Alberts MJ, Balady GJ, Ballantyne CM, Berra K, Black HR, Blumenthal RS, Davidson MH, Fazio SB, Ferdinand KC, et al. American College of Cardiology Foundation; American Heart Association; American College of Physicians Task Force on Competence and Training (Writing Committee to Develop a Competence and Training Statement on Prevention of Cardiovascular Disease); American Academy of Neurology; American Association of Cardiovascular and Pulmonary Rehabilitation; American College of Preventive Medicine; American Diabetes Association; American Society of Hypertension; Association of Black Cardiologists; National Lipid Association; Preventive Cardiovascular Nurses Association: ACCF/AHA/ACP 2009 competence and training statement: a curriculum on prevention of cardiovascular disease: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Competence and Training (Writing Committee to Develop a Competence and Training Statement on Prevention of Cardiovascular Disease): developed in collaboration with the American Academy of Neurology; American Association of Cardiovascular and Pulmonary Rehabilitation; American College of Preventive Medicine; American College of Sports Medicine; American Diabetes Association; American Society of Hypertension; Association of Black Cardiologists; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute; National Lipid Association; and Preventive Cardiovascular Nurses Association J Am Coll Cardiol. 2009;54:1336–1363. doi: 10.1016/j.jacc.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 5.Lachin JM, Genuth S, Cleary P, Davis MD, Nathan DM, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;(2):CD009122. doi: 10.1002/14651858.CD009122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt AM, Stern D. Atherosclerosis and diabetes: The RAGE connection. Curr Atheroscler Rep. 2000;2:430–436. doi: 10.1007/s11883-000-0082-4. [DOI] [PubMed] [Google Scholar]
- 8.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, et al. ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabbani N, Xue M, Thornalley PJ. Methylglyoxal-induced dicarbonyl stress in aging and disease: First steps towards glyoxalase 1-based treatments. Clin Sci (Lond) 2016;130:1677–1696. doi: 10.1042/CS20160025. [DOI] [PubMed] [Google Scholar]
- 10.Turk Z. Glycotoxines, carbonyl stress and relevance to diabetes and its complications. Physiol Res. 2010;59:147–156. doi: 10.33549/physiolres.931585. [DOI] [PubMed] [Google Scholar]
- 11.Watson AM, Soro-Paavonen A, Sheehy K, Li J, Calkin AC, Koitka A, Rajan SN, Brasacchio D, Allen TJ, Cooper ME, et al. Delayed intervention with AGE inhibitors attenuates the progression of diabetes-accelerated atherosclerosis in diabetic apolipoprotein E knockout mice. Diabetologia. 2011;54:681–689. doi: 10.1007/s00125-010-2000-9. [DOI] [PubMed] [Google Scholar]
- 12.Lapolla A, Flamini R, Dalla Vedova A, Senesi A, Reitano R, Fedele D, Basso E, Seraglia R, Traldi P. Glyoxal and methylglyoxal levels in diabetic patients: Quantitative determination by a new GC/MS method. Clin Chem Lab Med. 2003;41:1166–1173. doi: 10.1515/CCLM.2003.180. [DOI] [PubMed] [Google Scholar]
- 13.McLellan AC, Thornalley PJ, Benn J, Sonksen PH. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci (Lond) 1994;87:21–29. doi: 10.1042/cs0870021. [DOI] [PubMed] [Google Scholar]
- 14.Rabbani N, Godfrey L, Xue M, Shaheen F, Geoffrion M, Milne R, Thornalley PJ. Glycation of LDL by methylglyoxal increases arterial atherogenicity: A possible contributor to increased risk of cardiovascular disease in diabetes. Diabetes. 2011;60:1973–1980. doi: 10.2337/db11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigro C, Leone A, Raciti GA, Longo M, Mirra P, Formisano P, Beguinot F, Miele C. Methylglyoxal-glyoxalase 1 balance: the root of vascular damage. Int J Mol Sci. 2017;18:188. doi: 10.3390/ijms18010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tikellis C, Pickering RJ, Tsorotes D, Huet O, Cooper ME, Jandeleit-Dahm K, Thomas MC. Dicarbonyl stress in the absence of hyperglycemia increases endothelial inflammation and atherogenesis similar to that observed in diabetes. Diabetes. 2014;63:3915–3925. doi: 10.2337/db13-0932. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Juurlink BH. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension. 2002;39:809–814. doi: 10.1161/hy0302.105207. [DOI] [PubMed] [Google Scholar]
- 18.Sena CM, Matafome P, Crisóstomo J, Rodrigues L, Fernandes R, Pereira P, Seiça RM. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol Res. 2012;65:497–506. doi: 10.1016/j.phrs.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Tachi Y, Okuda Y, Bannai C, Okamura N, Bannai S, Yamashita K. High concentration of glucose causes impairment of the function of the glutathione redox cycle in human vascular smooth muscle cells. FEBS Lett. 1998;421:19–22. doi: 10.1016/S0014-5793(97)01526-3. [DOI] [PubMed] [Google Scholar]
- 20.Tachi Y, Okuda Y, Bannai C, Bannai S, Shinohara M, Shimpuku H, Yamashita K, Ohura K. Hyperglycemia in diabetic rats reduces the glutathione content in the aortic tissue. Life Sci. 2001;69:1039–1047. doi: 10.1016/S0024-3205(01)01183-3. [DOI] [PubMed] [Google Scholar]
- 21.Sung HJ, Kim J, Kim Y, Jang SW, Ko J. N-acetyl cysteine suppresses the foam cell formation that is induced by oxidized low density lipoprotein via regulation of gene expression. Mol Biol Rep. 2012;39:3001–3007. doi: 10.1007/s11033-011-1062-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Aw TY, Stokes KY. The protection conferred against ischemia-reperfusion injury in the diabetic brain by N-acetylcysteine is associated with decreased dicarbonyl stress. Free Radic Biol Med. 2016;96:89–98. doi: 10.1016/j.freeradbiomed.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotur-Stevuljevic J, Memon L, Stefanovic A, Spasic S, Spasojevic-Kalimanovska V, Bogavac-Stanojevic N, Kalimanovska-Ostric D, Jelić-Ivanovic Z, Zunic G. Correlation of oxidative stress parameters and inflammatory markers in coronary artery disease patients. Clin Biochem. 2007;40:181–187. doi: 10.1016/j.clinbiochem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council, corp-author. National Academy Press; Washington, DC: 1996. Guide for the Care and Use of Laboratory Animals; pp. 8–78. [Google Scholar]
- 26.Traslavina RP, King EJ, Loar AS, Riedel ER, Garvey MS, Ricart-Arbona R, Wolf FR, Couto SS. Euthanasia by CO2 inhalation affects potassium levels in mice. J Am Assoc Lab Anim Sci. 2010;49:316–322. [PMC free article] [PubMed] [Google Scholar]
- 27.Overmyer KA, Thonusin C, Qi NR, Burant CF, Evans CR. Impact of anesthesia and euthanasia on metabolomics of mammalian tissues: Studies in a C57BL/6J mouse model. PLoS One. 2015;10:e0117232. doi: 10.1371/journal.pone.0117232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuentes JM, Talamini MA, Fulton WB, Hanly EJ, Aurora AR, De Maio A. General anesthesia delays the inflammatory response and increases survival for mice with endotoxic shock. Clin Vaccine Immunol. 2006;13:281–288. doi: 10.1128/CVI.13.2.281-288.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol. 1999;277:H1967–H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- 30.Guan S, Wang B. Effects of fosinopril and valsartan on expressions of ICAM-1 and NO in human umbilical vein endothelial cells. Chin Med J (Engl) 2003;116:923–927. [PubMed] [Google Scholar]
- 31.Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 32.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 33.Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 34.Dhar A, Desai K, Liu J, Wu L. Methylglyoxal, protein binding and biological samples: Are we getting the true measure? J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1093–1100. doi: 10.1016/j.jchromb.2009.02.055. [DOI] [PubMed] [Google Scholar]
- 35.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- 36.Zhu ZX, Cai WH, Wang T, Ye HB, Zhu YT, Chi LS, Duan YM, Sun CC, Xuan YH, Jin LT. bFGF-regulating MAPKs are involved in high glucose-mediated ROS production and delay of vascular endothelial cell migration. PLoS One. 2015;10:e0144495. doi: 10.1371/journal.pone.0144495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannel WB, Castelli WP, Gordon T, McNamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med. 1971;74:1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- 38.Keys A. Coronary heart disease in seven countries. 1970. Nutrition. 1997;13:250–252; discussion 249, 253. doi: 10.1016/S0899-9007(96)00410-8. [DOI] [PubMed] [Google Scholar]
- 39.Valdovinos-Flores C, Limón-Pacheco JH, León-Rodríguez R, Petrosyan P, Garza-Lombó C, Gonsebatt ME. Systemic L-buthionine-S-R-sulfoximine treatment increases plasma NGF and upregulates L-cys/L-cys2 transporter and γ-glutamylcysteine ligase mRNAs through the NGF/TrkA/Akt/Nrf2 pathway in the striatum. Front Cell Neurosci. 2019;13:325. doi: 10.3389/fncel.2019.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nascimento NR, Costa-e-Forti A, Peter AA, Fonteles MC. Free radical scavengers improve the impaired endothelium-dependent responses in aorta and kidneys of diabetic rabbits. Diabetes Res Clin Pract. 2003;61:145–153. doi: 10.1016/S0168-8227(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 41.Muniyappa R, Srinivas PR. Dicarbonyl stress and atherosclerosis: Is it all RAGE? Diabetes. 2014;63:3587–3589. doi: 10.2337/db14-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: is the Total Antioxidant Capacity the right tool? Redox Rep. 2004;9:145–152. doi: 10.1179/135100004225004814. [DOI] [PubMed] [Google Scholar]
- 43.Peluso I, Morabito G, Urban L, Ioannone F, Serafini M. Oxidative stress in atherosclerosis development: The central role of LDL and oxidative burst. Endocr Metab Immune Disord Drug Targets. 2012;12:351–360. doi: 10.2174/187153012803832602. [DOI] [PubMed] [Google Scholar]
- 44.Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B, Yee Aw T, Stokes KY. N-acetylcysteine attenuates systemic platelet activation and cerebral vessel thrombosis in diabetes. Redox Biol. 2018;14:218–228. doi: 10.1016/j.redox.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sit M, Yilmaz EE, Tosun M, Aktas G. Effects of N-acetyl cysteine on lipid levels and on leukocyte and platelet count in rats after splenectomy. Niger J Clin Pract. 2014;17:343–345. doi: 10.4103/1119-3077.130237. [DOI] [PubMed] [Google Scholar]
- 47.Franceschini G, Werba JP, Safa O, Gikalov I, Sirtori CR. Dose-related increase of HDL-cholesterol levels after N-acetylcysteine in man. Pharmacol Res. 1993;28:213–218. doi: 10.1006/phrs.1993.1124. [DOI] [PubMed] [Google Scholar]
- 48.Violi F, Loffredo L, Carnevale R, Pignatelli P, Pastori D. Atherothrombosis and oxidative stress: mechanisms and management in elderly. Antioxid Redox Signal. 2017;27:1083–1124. doi: 10.1089/ars.2016.6963. [DOI] [PubMed] [Google Scholar]
- 49.Pignatelli P, Menichelli D, Pastori D, Violi F. Oxidative stress and cardiovascular disease: New insights. Kardiol Pol. 2018;76:713–722. doi: 10.5603/KP.a2018.0071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.