Abstract
Background
Alpha1-microglobulin (A1MG) is a small molecular protein related to oxidation and inflammation. It exists in diverse body fluids, including urine. Results from urine tests are sometimes neglected when predicting in-hospital prognosis. It remains unclear whether urinary A1MG (UA1MG) can predict short-term prognosis of ST-elevated myocardial infarction (STEMI).
Material/Methods
A total of 1854 hospitalized patients with acute STEMI were retrospectively enrolled in our study. Medical records were used to obtain patient demographic and clinical information, UA1MG values (which were used to divide patients into groups of low, medium, or high), and other laboratory parameters. Principal clinical outcomes of interest were all-cause in-hospital deaths, cardiac deaths, and major adverse cardiac events (MACEs).
Results
Among the 1854 enrolled patients, 43 (2.3%) died in the hospital, of which 33 (1.8%) were cardiac deaths. MACEs were noted in 113 patients (6.1%) during hospitalization. The group with the highest UA1MG value showed a significantly higher frequency of in-hospital deaths, cardiac deaths, and MACEs, compared to those of the lowest UA1MG value group (4.4% vs. 1.0%, P<0.001; 3.1% vs. 0.6%, P<0.005; and 8.6% vs. 4.7%, P=0.007, respectively). Multivariate regression analysis revealed that UA1MG levels (odds ratio 1.109, 95% confidence interval (CI) 1.027–1.197, P=0.008) independently predicted all-cause in-hospital mortality. A UA1MG value of 3.23 mg/dL was considered as an optimal cutoff point in STEMI to predict all-cause mortality after receiver operating characteristic curve analysis (area under the curve 0.73, 95% CI 0.65–0.80, P<0.001).
Conclusions
The UA1MG value at hospital admission could be an independent prognostic factor of all-cause in-hospital mortality in patients with STEMI.
MeSH Keywords: alpha-Globins, Hospital Mortality, Myocardial Infarction, Prognosis, Urine Specimen Collection
Background
ST-elevated myocardial infarction (STEMI) is a life-threatening disease resulting from acute occlusion of epicardial coronary arteries and the subsequent cessation of blood flow downstream to the myocardium. Recent decades have witnessed significant improvement of clinical outcomes owing to the implementation of modern therapeutics such as revascularization techniques, antithrombotic therapeutics, and secondary prevention [1]. Nevertheless, existing reports from national registries worldwide indicate the morbidity and in-hospital deaths of patients with STEMI remain substantial [1,2].
Many studies have investigated in-hospital mortality after myocardial infarction and its potential prognosis indicators. Studies demonstrated that increased age, advanced Killip class, abnormal heart rate, arterial hypotension, increased serum creatinine, white blood cell counts, and hemoglobin levels might be meaningful predictors of in-hospital and short-term mortality in patients with STEMI treated with primary percutaneous coronary intervention (PPCI) [3,4]. Laboratory blood tests, especially cardiac enzyme results, enhanced the diagnosis and assessment of patients with STEMI, and some other indexes from blood tests were found to be valuable predictors for in-hospital mortality [5]. However, frequent blood draws and the accompanying increased nursing burden prevent these indexes from becoming better STEM1 monitoring indicators.
Urine tests are relatively easy to perform. However, their prognostic value in the intensive care unit for patients with STEMI has been neglected. Alpha1-microglobulin (A1MG) is a highly conserved glycoprotein exclusively synthesized in the liver and broadly distributed in the serum, monocytes, synovial fluid, cerebrospinal fluid, heart, skin, liver, gut, kidneys, and brain before being excreted from the kidneys [6]. The prognostic role of urinary A1MG (UA1MG) in patients with STEMI remains unknown. Therefore, we evaluated the predictive role of UA1MG in mortality among hospitalized patients with STEMI.
Material and Methods
Study design and population
From January 2013 to December 2019, a total of 2101 patients with STEMI were consecutively admitted to the Department of Cardiology, Beijing Friendship Hospital, Capital Medical University. Detailed clinical information of these patients was retrospectively evaluated from the China Beijing Friendship Hospital database. The study’s inclusion criteria were derived from a previous study or guidelines [7,8] and were as follows: (1) The onset of ischemic symptoms lasted >30 min, and the symptoms included various combinations of chest, upper extremity, mandibular, or epigastric discomfort or an ischemic equivalent, such as dyspnea or fatigue [7,8]. (2) Electrocardiographic alterations included new ST elevation in 2 contiguous leads with a cut point ≥1 mm in all leads other than leads V2–V3, where the following cut points applied: ≥2 mm in men ≥40 years; ≥2.5 mm in men <40 years; and ≥1.5 mm in women regardless of age [7,8]. Also, a new left bundle branch block with ischemic repolarization patterns was included [7,8]. Lastly, (3) cardiac troponin values rose with at least 1 value above the 99th percentile upper reference limit [7,8]. To avoid interactions of comorbidities with the level of A1MG or with the in-hospital prognosis of patients, 155 patients were excluded owing to ongoing infection, active cancer, uncontrolled hepatobiliary diseases, end-stage renal disease, hematological diseases, inflammatory or autoimmune disorders, or history of glucocorticoid therapy. Ninety-two patients with incomplete records of UA1MG on admission were eliminated. A total of 1854 patients were enrolled and classified into 3 subgroups based on tertiles of baseline UA1MG value. Tertile 1 was classified as the low UA1MG group, tertile 2 was classified as the median UA1MG group, and tertile 3 was classified as the high UA1MG group. A flowchart illustrates the study design (Figure 1). This study protocol was approved by Beijing Friendship Hospital’s Ethics Committee.
Figure 1.
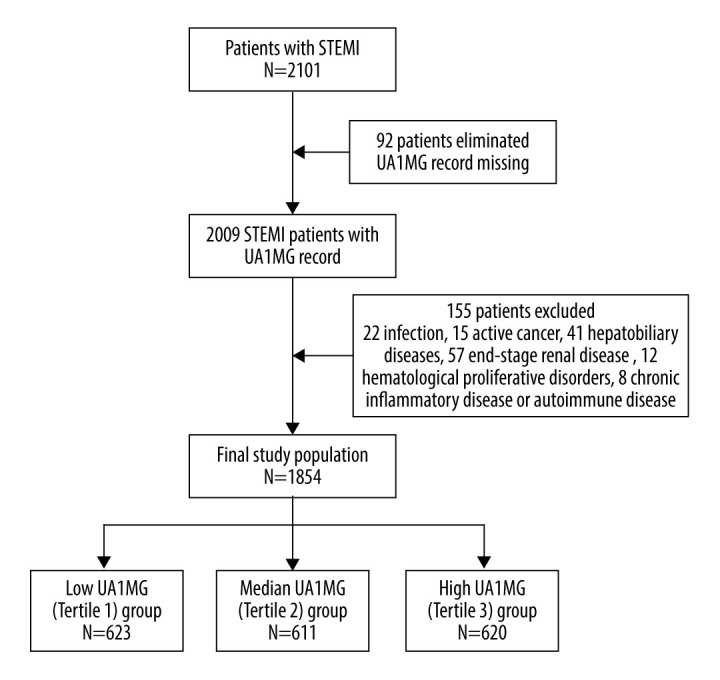
Flow chart illustrating the selection of study population. The final cohort consisted of 1854 patients with ST-elevated myocardial infarction (STEMI). UA1MG – urinary alpha1-microglobulin.
Laboratory analysis and UA1MG test
The UA1MG level was tested using the overnight clean midstream urine, which was sampled in the morning between 6: 00 a.m. and 11: 00 a.m., within 24 h of hospital admission, and sent to the Laboratory Medicine Department within 1 h for testing, as previously reported [9]. The nephelometry assay kit was used to test the UA1MG level with the BN II protein analyzer (Siemens, Germany), with a normal reference range provided by the manufacturer of 0.0 to 1.2 mg/dL. As previously described [7], patients had their peripheral blood draw sampled immediately on admission. Complete blood cell counts were measured using the fluorescent dyeing flowcytometry method with the XN3000 analyzer (Sysmex, Japan). The lipid panel, glycated hemoglobin A1c, and high-sensitivity C-reactive protein (hs-CRP) levels were collected on the morning of the second day after at least 8 h of fasting. The levels of cardiac enzymes involving troponin I (cTnI), creatine kinase myocardial band (CK-MB), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were collected on admission and every morning according to the hospital protocol. Blood biochemistry parameters and cardiac biomarkers were evaluated using test kits with AU5821 biochemistry analyzing equipment (Beckman Coulter Company), according to the manufacturer’s instructions.
Data collection and clinical outcomes
The patients’ medical records were carefully reviewed to extract demographic characteristics and possible cardiovascular risk factors, including known coronary artery disease, dyslipidemia, diabetes mellitus, hypertension, and smoking history [7]. The first recorded blood pressure, heart rate, and the Killip class on admission were considered as baseline. Before discharge, the Simpson method was used to quantitatively evaluate left ventricular contractive function, and the left ventricular ejection fraction was calculated. In-hospital mortality was defined as the frequency of any death occurring in the hospital. Deaths from cardiac causes including myocardial infarction, heart failure, arrhythmia, and unexplained sudden death were defined as cardiac deaths, as previously reported [7]. Protocol-defined MACEs included a composite of cardiovascular mortality, re-infarction, malignant ventricular arrhythmias, repeat target vessel revascularization, and stroke.
Revascularization procedure and medications
In accordance with established guidelines [10,11], dual antiplatelets at loading doses (300 mg aspirin and 300–600 mg clopidogrel) were administrated before PPCI, and the patients who did not receive PPCI were also prescribed dual antiplatelets as defined above once STEMI was diagnosed. Choosing whether patients with STEMI who did not receive PPCI underwent elective PCI, and the appropriate time point of the elective PCI, also depended on the guidelines [10,11] and the decision of the clinical team. PPCI procedures were performed with standard techniques and the appropriate strategy, according to the guidelines. All enrolled patients without contradictions were prescribed antiplatelets, β-blockers, angiotensin-converting enzyme inhibitors, and statins during their hospitalization and after discharge.
Statistical analysis
Categorical variables are presented as percentages and numerical variables were presented as mean±standard deviation or the median (interquartile range). Kolmogorov-Smirnov statistics were adopted to test for the assumption of normality of continuous variables. Variance analysis, the LSD post hoc test, or the Mann-Whitney U test were used to compare among the different tertile subgroups and conduct pairwise comparisons. The predictive variables for higher in-hospital mortality were evaluated by univariate and multivariate binary logistic regression analysis. Multivariate logistic regression analysis was applied for significant variables (when P<0.05 or variables were of clinical importance) to identify the independent prognostic indicators for in-hospital death. The cutoff point of UA1MG for in-hospital mortality was determined with a receiver operating curve. Spearman rank analysis was used to evaluate the correlation between UA1MG and hs-CRP and creatinine. A P value <0.05 was considered statistically significant. We used SPSS software, version 25.0 (SPSS, Chicago, Illinois) for all statistical analyses.
Results
Patients were classified into 3 tertile subgroups according to their baseline UA1MG values. Of the 1854 patients, 623 were in the low UA1MG subgroup (mean age 60.92±11.74 years, 73.4% men), 611 were in the moderate UA1MG subgroup (mean age 62.69±12.88 years, 77.7% men), and 620 were in the high UA1MG subgroup (mean age 66.57±12.77 years, 76.8% men). Detailed comparisons of the demographic characteristics, clinical manifestations, and laboratory parameters of the 3 subgroups are listed in Table 1.
Table 1.
Basic demographic, clinical, and laboratory factors of the tertile subgroups of the admission UA1MG.
| Characteristics | Tertile 1, n=623 <0.77 |
Tertile 2, n=611 0.77–1.89 |
Tertile 3, n=620 >1.89 |
p1 value | p2 value |
|---|---|---|---|---|---|
| Age, years | 60.92±11.74 | 62.69±12.88 | 66.57±12.77 | <0.001 | <0.001 |
| Male gender, n (%) | 457 (73.4) | 475 (77.7) | 476 (76.8) | 0.165 | 0.164 |
| PPCI, n (%) | 368 (62.0) | 362 (63.3) | 306 (58.3) | 0.216 | 0.211 |
| Medical histories | |||||
| CHD, n (%) | 181 (29.2) | 171 (28.1) | 186 (30.2) | 0.728 | 0.700 |
| Hypertension, n (%) | 342 (55.0) | 354 (58.0) | 397 (64.7) | 0.002 | 0.001 |
| DM, n (%) | 140 (22.5) | 170 (27.9) | 223 (36.1) | <0.001 | <0.001 |
| Dyslipidemia, n (%) | 274 (44.1) | 278 (45.6) | 264 (42.9) | 0.631 | 0.672 |
| Smoke, n (%) | 327 (52.7) | 326 (53.4) | 286 (46.4) | 0.259 | 0.044 |
| SBP, mmHg | 124.43±19.90 | 125.16±21.91 | 123.70±22.33 | 0.493 | 0.550 |
| DBP, mmHg | 73.16±12.66 | 72.91±12.66 | 72.45±13.24 | 0.622 | 0.336 |
| HR, bpm | 72.26±12.84 | 75.90±15.36 | 78.40±16.99 | <0.001 | <0.001 |
| Killip ≥III n (%) | 20 (3.2) | 39 (6.4) | 83 (13.7) | <0.001 | <0.001 |
| Laboratory index | |||||
| WBC, ×109/L | 8.79±2.95 | 9.17±3.09 | 9.94±3.30 | <0.001 | <0.001 |
| Neutrophil, ×109/L | 5.92 (4.26–7.76) | 6.32 (4.82–8.32) | 6.99 (5.47–9.44) | <0.001 | <0.001 |
| Hemoglobin, g/L | 140.92±17.69 | 140.37±19.00 | 136.09±20.84 | <0.001 | <0.001 |
| platelets, ×109/L | 230.45±62.67 | 227.02±67.89 | 220.20±76.32 | 0.029 | 0.009 |
| RDW, % | 12.7 (12.0–13.7) | 12.7 (12.0–13.6) | 12.9 (12.1–13.8) | 0.120 | 0.286 |
| PDW, % | 13.58±2.68 | 13.74±2.85 | 14.17±2.79 | <0.001 | <0.001 |
| Glucose, mmol/L | 7.3 (6.2–9.2) | 7.91 (6.47–10.80) | 8.62 (6.95–12.54) | <0.001 | <0.001 |
| Creatinine, μmol/L | 76.8 (66.9–86.3) | 80.4 (70.8–90.4) | 87.1 (74.4–103.0) | <0.001 | <0.001 |
| ALT, U/L | 23.0 (16.0–33.0) | 24.0 (17.0–36.0) | 25.0 (17.0–39.0) | 0.047 | 0.006 |
| Albumin, g/L | 39.81±3.84 | 39.35±4.02 | 38.26±4.79 | <0.001 | <0.001 |
| T-BIL, μmol/L | 11.4 (8.6–15.3) | 12.4 (9.20–17.0) | 12.8 (9.2–18.3) | <0.001 | <0.001 |
| TC, mmol/L | 4.57±0.99 | 4.43±1.04 | 4.39±1.07 | 0.010 | <0.001 |
| LDL-C, mmol/L | 2.69±0.73 | 2.57±0.76 | 2.54±0.79 | 0.002 | 0.001 |
| HDL-C, mmol/L | 1.02 (0.9–1.17) | 1.03 (0.88–1.21) | 1.01 (0.88–1.21) | 0.538 | 0.487 |
| TG, mmol/L | 1.42 (1.06–1.99) | 1.38 (0.99–2.12) | 1.30 (0.99–1.86) | 0.035 | 0.012 |
| hsCRP, mg/dL | 4.2 (1.8–10.9) | 6.7 (2.3–16.0) | 13.6 (4.3–30.5) | <0.001 | <0.001 |
| HbA1c, % | 6.26±1.37 | 6.45±1.47 | 6.74±1.67 | <0.001 | <0.001 |
Categorical variable was presented as percentage and numeric variable was presented as mean±standard deviation or the median (interquartile range). P1 value was obtained from comparing three subgroups. P2 value was obtained from comparing the tertile 3 subgroup with the tertile 1 subgroup. PPCI – primary percutaneous coronary intervention; CHD – coronary heart disease; DM – diabetic mellitus; DBP – diastolic blood pressure; SBP – systolic blood pressure; HR – heart rate; WBC – white blood cells; RDW – red blood cell distribution width; PDW – platelet distribution width; ALT – alanine aminotransferase; TG – triglyceride; T-BIL – total bilirubin; TC – total cholesterol; HDL-C – high-density-lipoprotein cholesterol; LDL-C – low-density-lipoprotein cholesterol; hs-CRP – high sensitive C-reactivity protein; HbA1c – glycated hemoglobin A1c; UA1MG – urinary alpha1-microglobulin.
Overall, there were 43 (2.3%) in-hospital deaths, 33 cardiac deaths (1.8%), and 113 MACEs (6.1%). Patients in the high UA1MG group had elevated peak values of CK-MB, cTnI, and NT-proBNP and reduced left ventricular ejection fraction compared to the low UA1MG group (P<0.05, Table 2). Concordantly, patients had significantly higher in-hospital mortality, more cardiac deaths and MACEs in the high UA1MG group than in the low UA1MG group (4.4% vs. 1.0%, P<0.001; 3.1% vs. 0.6%, P<0.005; 8.6% vs. 4.7%, P=0.007, respectively; Table 2).
Table 2.
In-hospital outcomes, myocardial injuries, and left ventricular function of STEMI patients based on the tertiles of UA1MG.
| Characteristics | Tertile 1, n=623 <0.77 |
Tertile 2, n=611 0.77–1.89 |
Tertile 3, n=620 >1.89 |
p1 value | p2 value |
|---|---|---|---|---|---|
| In-hospital death, % | 6 (1.0) | 10 (1.7) | 27 (4.4) | <0.001 | <0.001 |
| Cardiac death, % | 4 (0.6) | 10 (1.7) | 19 (3.1) | 0.005 | 0.001 |
| MACE, % | 29 (4.7) | 31 (5.1) | 53 (8.6) | 0.007 | 0.005 |
| Cardiac markers | |||||
| Peak NTproBNP, pg/mL | 1164.0 (503.0–2706.0) | 1613.0 (701.0–3907.0) | 3825.0 (1639.5–9982.0) | <0.001 | <0.001 |
| Peak CK-MB, ng/mL | 74.7 (11.9–202.0) | 95.9 (17.7–215.0) | 100.0 (21.1–273.8) | 0.001 | 0.004 |
| Peak cTnI, ng/mL | 10.9 (2.5–28.7) | 15.1 (4.0–37.7) | 23.9 (7.2–50.0) | <0.001 | <0.001 |
| LVEF | 0.59±0.09 | 0.57±0.09 | 0.54±0.10 | <0.001 | <0.001 |
Categorical variable was presented as percentage and numeric variable was presented as mean±standard deviation or the median (interquartile range). P1 value was obtained from comparing three subgroups. P2 value was obtained from comparing the tertile 3 subgroup with the tertile 1 subgroup. MACE – major adverse cardiac events; CK-MB – creatine kinase MB; cTnI – cardiac troponin I; NT-proBNP – N-terminal pro-B-type Natriuretic Peptide; LVEF – left ventricular ejection fraction; UA1MG – urinary alpha1-microglobulin.
Ten statistically significant factors identified by univariate analysis or by clinical significance (age, male sex, PPCI, heart rate, neutrophil count, and hemoglobin, glucose, albumin, total bilirubin, and UA1MG levels at admission) were chosen to be evaluated by multivariate logistic regression analysis. A high UA1MG level was significantly correlated with in-hospital mortality (odds ratio [OR] 1.109, 95% confidence interval [CI] 1.027–1.197, P=0.008, Table 3). Also, age was an independent predictor for all-cause in-hospital mortality (OR 1.052, 95% CI 1.008–1.099, P=0.022, Table 3).
Table 3.
Effects of factors on in-hospital all-cause mortality in univariate and multivariate logistic regression analysis.
| Variables | Unadjusted OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Age | 1.089 | 1.065–1.114 | <0.001 | 1.052 | 1.008–1.099 | 0.022 |
| Male gender | 1.759 | 1.058–2.924 | 0.029 | 1.364 | 0.509–3.655 | 0.537 |
| PPCI | 0.697 | 0.328–1.481 | 0.348 | 0.755 | 0.297–1.919 | 0.554 |
| CHD | 0.853 | 0.508–1.431 | 0.546 | |||
| Hypertension | 0.514 | 0.298–0.888 | 0.017 | |||
| DM | 0.732 | 0.441–1.215 | 0.228 | |||
| Smoke | 1.668 | 1.215–2.291 | 0.002 | |||
| SBP | 0.993 | 0.981–1.004 | 0.201 | |||
| HR | 1.026 | 1.013–1.039 | <0.001 | 0.992 | 0.965–1.02 | 0.569 |
| Killip Class | 4.893 | 3.811–6.283 | <0.001 | |||
| WBC | 1.116 | 1.043–1.194 | 0.001 | |||
| Neutrophil | 1.137 | 1.058–1.221 | <0.001 | 1.06 | 0.909–1.235 | 0.457 |
| Hemoglobin | 0.979 | 0.968–0.99 | <0.001 | 0.991 | 0.963–1.019 | 0.525 |
| Platelets | 1 | 0.996–1.003 | 0.898 | |||
| RDW | 1.022 | 0.991–1.053 | 0.164 | |||
| PDW | 1.006 | 0.922–1.098 | 0.886 | |||
| Glucose | 1.088 | 1.039–1.14 | <0.001 | 1.038 | 0.947–1.138 | 0.427 |
| Creatinine | 1.018 | 1.013–1.023 | <0.001 | |||
| Albumin | 0.898 | 0.853–0.947 | <0.001 | 0.952 | 0.845–1.072 | 0.417 |
| ALT | 1.011 | 1.001–1.021 | 0.039 | |||
| T-BIL | 1.038 | 1.011–1.066 | 0.005 | 1.015 | 0.964–1.069 | 0.575 |
| TC | 0.631 | 0.472–0.844 | 0.002 | |||
| LDL-C | 0.544 | 0.368–0.805 | 0.002 | |||
| TG | 0.676 | 0.461–0.99 | 0.044 | |||
| HsCRP | 1.043 | 1.026–1.06 | <0.001 | |||
| HbA1c | 1.009 | 0.842–1.209 | 0.924 | |||
| UA1MG | 1.155 | 1.094–1.22 | <0.001 | 1.109 | 1.027–1.197 | 0.008 |
OR – odds ratio; CI – confidence interval; PPCI – primary percutaneous coronary intervention; CHD – coronary heart disease; DM – diabetic mellitus; DBP – diastolic blood pressure; SBP – systolic blood pressure; HR – heart rate; WBC – white blood cells; RDW – red blood cell distribution width; PDW – platelet distribution width; ALT – alanine aminotransferase; TG – triglyceride; T-BIL – total bilirubin; TC – total cholesterol; HDL-C – high-density-lipoprotein cholesterol; LDL-C – low-density-lipoprotein cholesterol; hs-CRP – high sensitive C-reactivity protein; HbA1c – glycated hemoglobin A1c; UA1MG – urinary alpha1-microglobulin.
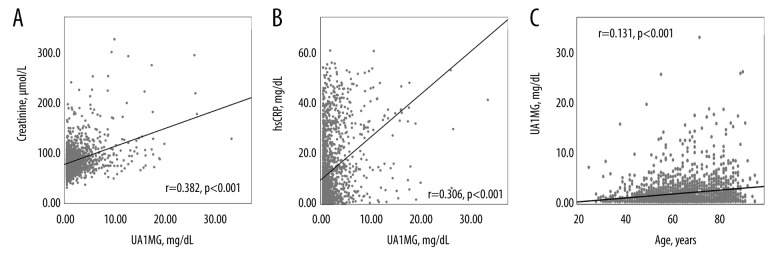
A UA1MG value of 3.23 mg/dL was identified by the receiver operating characteristic curve analysis as a meaningful and effective cutoff point in patients with STEMI to predict all-cause in-hospital mortality (area under the curve=0.73, 95% CI 0.65–0.80, P<0.001; Figure 2). To find possible reasons for the predictive value in UAIMG, we performed Spearman correlation analysis. A significant correlation between UA1MG and hs-CRP (r=0.306, P<0.001) and a significant correlation between UA1MG and creatinine (r=0.382, P<0.001) were identified. Age was also correlated with the UA1MG level (r=0.131, P<0.001; Figure 3).
Figure 2.
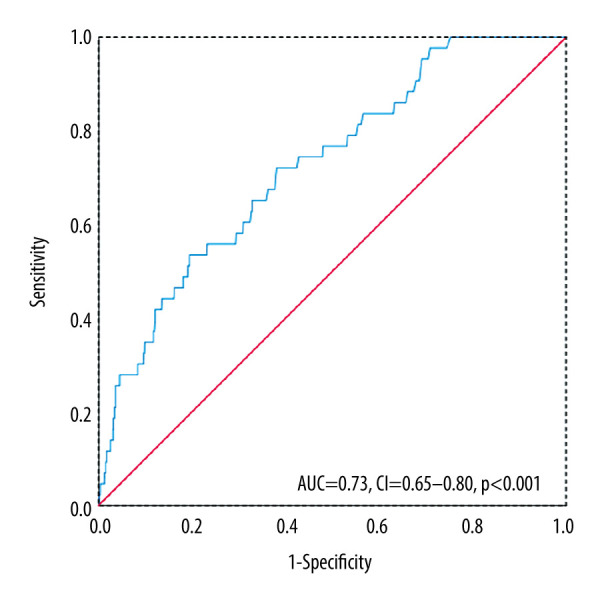
Receiver operating characteristic curve of urinary alpha1-microglobulin (UA1MG) value. UA1MG value was a prognostic indicator for in-hospital mortality in ST-elevated myocardial infarction (STEMI) by receiver operating characteristic curve analysis. AUC – area under the curve; CI – confidence interval.
Figure 3.
(A–C) Spearman correlation analysis of urinary alpha1-microglobulin (UA1MG) and highly sensitive C-reactive protein (hs-CRP), creatinine, and age. UA1MG and hs-CRP, UA1MG, and creatinine, as well as UA1MG and age, were significantly correlated.
Discussion
Few studies have investigated the role of UA1MG in the prognosis of patients with coronary heart disease. Our study demonstrated that the UA1MG value on admission of patients with STEMI independently predicted all-cause in-hospital mortality.
Evidence-based reperfusion therapy and optimized medicine improve the prognosis in patients with STEMI; however, in-hospital mortality remains considerably variable. Individualized patient risk evaluation allows for more accurate clinical care in regard to therapeutic strategy and allocation of medical resources. Blood tests provide timely and direct assessment and give doctors information about individualized risk; however, venipuncture creates additional work for nurses and unwanted pain for patients, which limits its application in continuous monitoring of patient clinical condition. Routine urinary tests do not have the above shortcomings. Furthermore, UA1MG could work as an effective tool for risk assessment when blood laboratory testing is difficult to perform, underscoring that our findings may provide for the convenience of testing certain conditions, such as in underdeveloped areas with an inadequate medical or nursing care workforce.
A1MG, present in various body fluids, is a small molecular weight protein (26 kDa), which functionally has been shown to affect heme-induced intracellular oxidation in vitro and reduce structural damage in hemoglobin- or heme-induced rat kidney injury and ewe preeclampsia models [6,12,13]. A few studies in cardiovascular fields have investigated the role of UA1MG. In a study with a small sample size, Holm et al. reported that the urinary excretion of A1MG in patients with myocardial infarction was significantly elevated and the levels were positively correlated with higher plasma levels of CRP and troponin I [14]. Recently, Garimella et al. demonstrated UA1MG levels are associated with cardiovascular events and mortality among nondiabetic persons with chronic kidney disease [15], indicating that UA1MG might be associated with renal function and inflammation. Our present findings further revealed the high UA1MG group had higher levels of cardiac troponin, CK-MB, and NT-proBNP, in agreement with the above study.
Renal function is a known risk factor of in-hospital clinical endpoints of patients with myocardial infarction. Many acknowledged risk models such as the Global Registry of Acute Coronary Events score [16], a recent parsimonious risk model [2], and a laboratory stratification model [5] include blood creatinine as an important predictor of prognosis. Oliguria, anuria, and renal replacement lead to unexpected alterations of UA1MG; therefore, patients with end-stage renal disease were excluded from our present study. Nevertheless, UA1MG was positively correlated with creatinine level. This might be one explanation for the prognostic value of UA1MG in in-hospital mortality. Moreover, UA1MG freely passes through the glomerular membranes, of which almost 99% is reabsorbed and catabolized by the proximal tubular cells [17]. Alpha1-microglobulinuria can be induced by glomerular dysfunction and reabsorption dysfunction of renal tubules [17,18]. In other words, UA1MG reflects not only glomerular function but also reflects tubular reabsorbing function. It should be noted that we did not focus on other parameters related to reabsorbing function such as beta 2-microglobulin or N-acetyl-beta-D-glucosaminidase in the present study; hence, the mechanism by which tubular function influences UA1MG needs further investigation. Even so, UA1MG might be a more comprehensive factor than blood creatinine levels for prognosis related to renal function.
Unlike the contents in serum such as cardiac enzymes, which change rapidly during the course of STEMI, urinary indexes reflect only general changes during the past several hours because of the storage nature of the bladder. Although the level of UA1MG in myocardial infarction has not been studied sufficiently, it might be influenced by the length of time urine is in the bladder. A previous study indicated that urinary indexes including myoglobin, beta 2-microglobulin, and albumin are significantly elevated in the first 3 days during myocardial infarction, especially during the first day [19]. Hence, based on previous studies [9,19], we chose the first overnight urine as our study object. It was found that the urinary myoglobin excretion pattern varies greatly among patients and is not correlated with peak serum enzyme levels or with peak serum myoglobin levels [19]. In contrast, in the present study, we found a difference in cardiac biomarkers among the 3 UA1MG groups, which was supported by another report [14]. However, owing to the limitation of retrospective analyses, the blood level of A1MG was not tested, and the UA1MG level was tested only once during hospitalization. Future studies should focus on the fluctuation of UA1MG during the whole disease course of myocardial infarction.
A recent study indicated that the value of UA1MG was sex-dependent, and the 95th percentiles were 2.64 mg/dL and 0.86 mg/dL in men and women, respectively [17]. Regardless, there were no differences by sex among the tertile groups in the present study, and sex was not found to be statistically significant in the multivariate logistic regression analysis. Consequently, in the present study, our conclusions may not be affected by sex. Moreover, a statistically significant correlation between age and UA1MG was revealed in our study, which agreed with a previous report [17]. Nevertheless, UA1MG remained a statistically significant predictor after adjustment by age and other risk factors in the multivariate logistic regression analysis.
Inflammation closely correlates with life-threatening cardiovascular events and clinical complications in patients with myocardial infarction. Several inflammation-related indicators are closely associated with short-term and long-term mortality in cardiovascular patients. For instance, neutrophil count, red blood cell distribution width, ratios of neutrophil to lymphocyte or CRP to albumin glowed with fluorescence intensity [20–23]. Recent studies have indicated that UA1MG also participates in the inflammation process. As mentioned above, elevated UA1MG was related to higher plasma levels of CRP in patients with type 2 diabetes mellitus and myocardial infarction [14,18]. Although previous studies tended to describe A1MG as an antioxidant molecule, an increasing number of studies have questioned its protective role. Administration of A1MG fails to decrease non-heme-induced injury and surprisingly worsens renal injury [24]. Also, Zhang et al. found a significant association between UA1MG levels and nonalcoholic fatty liver disease in the Chinese population [25]. Recently, Hakuno et al. were the first to show that A1MG induces cardiac toxicity via cardio-hepatic interactions and to uncover the mechanisms involved [26]. In the mouse myocardial infarction model used in the Hakuno et al. study, A1MG was transiently distributed in the infarct and border zones during the acute phase, reflecting the infiltration of macrophages. A1MG stimulation activated Akt, nuclear factor-κB signaling, and enhanced inflammation and macrophage migration and polarization. Intramyocardial A1MG administration exacerbated macrophage infiltration, inflammation, and matrix metalloproteinase 9 mRNA expression in the infarct and border zones, whereas it disturbed fibrotic repair. The investigators suggested that targeting the A1MG pathway would be a novel pharmacological tool to mitigate adverse myocardial remodeling in myocardial infarction [26].
The percentages of PPCI among the 3 tertile subgroups in the present study were not significantly different, indicating that PPCI therapy did not affect prognosis in the 3 tertile subgroups. PPCI did not reach statistical significance in univariate analysis, which might be owing to the study’s limited population size. However, according to the experiences from clinical practice, it was indeed a strong protective indicator; therefore, we included PPCI in the multivariate logistic regression model. Owing to the correlation between UA1MG and creatinine and hs-CRP, creatinine and CRP were not included in the multivariate logistic regression. UA1MG was still an independent prognostic factor of all-cause mortality in the multivariate logistic regression analysis, which was adjusted with 9 risk factors.
To the best of our knowledge, UA1MG serves as an indicator of renal function and inflammation. Markers of these kinds from routine blood tests and urine tests, including UA1MG, are objective laboratory results and could be conveniently acquired. However, the mortality of patients with STEMI varies greatly. Many factors, such as increased age, Killip class, treatment strategy, time delay to treatment, and diabetes mellitus, could influence disease prognosis. In addition, this study excluded many conditions that might also be related to heart problems, so it is necessary to know that the conclusion is not applied in the STEMI population with the above comorbidities excluded.
Conclusions
In conclusion, our study demonstrated that the UA1MG level at hospital admission independently predicted in-hospital mortality among patients diagnosed with STEMI, probably because UA1MG indicates the presence of renal function and inflammation. This retrospective analysis indicated that patients with STEMI with a high UA1MG value should be carefully monitored for severe cardiovascular events and complications. To better illuminate and examine the clinical implications of our findings, further prospective studies are needed.
Acknowledgements
We thank Shanshan Wu and Guoliang Zhao for conducting the statistical interpretation and data collection in the study.
Footnotes
Conflicts of interests
None.
Source of support: This work was supported by the National Natural Science Foundation (No. 81603425), Seed Plan Program of Beijing Friendship Hospital (YYZZ2017A06), Beijing Talents Fund (No. 2016000021469G221), and Beijing Key Clinical Subject Program
References
- 1.Cui Y, Hao K, Takahashi J, et al. Age-specific trends in the incidence and in-hospital mortality of acute myocardial infarction over 30 years in Japan – Report from the Miyagi AMI Registry Study. Circ J. 2017;81(4):520–28. doi: 10.1253/circj.CJ-16-0799. [DOI] [PubMed] [Google Scholar]
- 2.McNamara RL, Kennedy KF, Cohen DJ, et al. predicting in-hospital mortality in patients with acute myocardial infarction. J Am Coll Cardiol. 2016;68(6):626–35. doi: 10.1016/j.jacc.2016.05.049. [DOI] [PubMed] [Google Scholar]
- 3.Puymirat E, Simon T, Steg PG, et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA. 2012;308(10):998–1006. doi: 10.1001/2012.jama.11348. [DOI] [PubMed] [Google Scholar]
- 4.Tanik VO, Cinar T, Arugaslan E, et al. The predictive value of PRECISE-DAPT score for in-hospital mortality in patients with ST-Elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2019;70(5):440–47. doi: 10.1177/0003319718807057. [DOI] [PubMed] [Google Scholar]
- 5.Yanishi K, Nakamura T, Nakanishi N, et al. A simple risk stratification model for ST-elevation myocardial infarction (STEMI) from the combination of blood examination variables: Acute Myocardial Infarction-Kyoto Multi-Center Risk Study Group. PLoS One. 2016;11(11):e0166391. doi: 10.1371/journal.pone.0166391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Åkerström B, Gram M. A1M, an extravascular tissue cleaning and housekeeping protein. Free Radic Biol Med. 2014;74:274–82. doi: 10.1016/j.freeradbiomed.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Cui H, Ding X, Li W, et al. The neutrophil percentage to albumin ratio as a new predictor of in-hospital mortality in patients with ST-segment elevation myocardial infarction. Med Sci Monit. 2019;25:7845–52. doi: 10.12659/MSM.917987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2018;40(3):237–69. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Wang Y, Li H. Elevated admission microalbuminuria predicts poor myocardial blood flow and 6-month mortality in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. Clin Cardiol. 2012;35(4):219–24. doi: 10.1002/clc.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 11.Chinese Society of Cardiology of Chinese Medical Association. Editorial Board of Chinese Journal of Cardiology. [2019 Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of patients with ST segment elevation myocardial infarction]. Zhonghua Xin Xue Guan Bing Za Zhi. 2019;47(10):766–83. doi: 10.3760/cma.j.issn.0253-3758.2019.10.003. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 12.Sverrisson K, Axelsson J, Rippe A, et al. Extracellular fetal hemoglobin induces increases in glomerular permeability: Inhibition with α1-microglobulin and tempol. Am J Physiol Renal Physiol. 2014;306(4):F442–48. doi: 10.1152/ajprenal.00502.2013. [DOI] [PubMed] [Google Scholar]
- 13.Olsson MG, Allhorn M, Larsson J, et al. Up-regulation of A1M/α1-microglobulin in skin by heme and reactive oxygen species gives protection from oxidative damage. PLoS One. 2011;6(11):e27505. doi: 10.1371/journal.pone.0027505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holm J, Ravn J, Ingemann Hansen S. Urinary excretion of alpha1-microglobulin and albumin in acute myocardial infarction. Correlation with plasma concentrations of troponin I and C-reactive protein. Scand J Urol Nephrol. 2006;40(4):339–44. doi: 10.1080/00365590600750136. [DOI] [PubMed] [Google Scholar]
- 15.Garimella PS, Lee AK, Ambrosius WT, et al. Markers of kidney tubule function and risk of cardiovascular disease events and mortality in the SPRINT trial. Eur Heart J. 2019;40(42):3486–93. doi: 10.1093/eurheartj/ehz392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Araújo Gonçalves P, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: Sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J. 2005;26(9):865–72. doi: 10.1093/eurheartj/ehi187. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Jiang X, Cui XF, Liu R. A study on the biological reference interval of urinary alpha 1-microglobulin in a group of Chinese people. J Clin Lab Anal. 2018;32(3):e22305. doi: 10.1002/jcla.22305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan X, Zhang L, Gu H, et al. The association of serum hsCRP and urinary alpha1-microglobulin in patients with type 2 diabetes mellitus. BioMed Res Int. 2019;2019 doi: 10.1155/2019/6364390. 6364390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson F, Roxin L, Venge P, Wibell L. Evaluation of urinary myoglobin assay test for myocardial infarction. Scand J Clin Lab Invest. 1978;38(8):717–21. doi: 10.1080/00365517809104878. [DOI] [PubMed] [Google Scholar]
- 20.Pan W, Zhao D, Zhang C, et al. Application of neutrophil/lymphocyte ratio in predicting coronary blood flow and mortality in patients with ST-elevation myocardial infarction undergoing percutaneous coronary intervention. J Cardiol. 2015;66(1):9–14. doi: 10.1016/j.jjcc.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Celik T, Balta S, Demir M, et al. Predictive value of admission red cell distribution width-platelet ratio for no-reflow phenomenon in acute ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiol J. 2016;23(1):84–92. doi: 10.5603/CJ.a2015.0070. [DOI] [PubMed] [Google Scholar]
- 22.Tian J, Liu Y, Liu Y, et al. Prognostic association of circulating neutrophil count with no-reflow in patients with ST-segment elevation myocardial infarction following successful primary percutaneous intervention. Dis Markers. 2017;2017 doi: 10.1155/2017/8458492. 8458492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karabag Y, Cagdas M, Rencuzogullari I, et al. Usefulness of the C-reactive protein/albumin ratio for predicting no-reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur J Clin Invest. 2018;48(6):e12928. doi: 10.1111/eci.12928. [DOI] [PubMed] [Google Scholar]
- 24.Zager RA, Johnson AC, Frostad K. An evaluation of the antioxidant protein α1-microglobulin as a renal tubular cytoprotectant. Am J Physiol Renal Physiol. 2016;311(3):F640–51. doi: 10.1152/ajprenal.00264.2016. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Zhang X, Zhao Y, Lv G. Association between urinary alpha1-microglobulin levels and nonalcoholic fatty liver disease: A cross-sectional study. Ann Nutr Metab. 2018;72(1):30–36. doi: 10.1159/000484255. [DOI] [PubMed] [Google Scholar]
- 26.Hakuno D, Kimura M, Ito S, et al. Hepatokine α1-microglobulin signaling exacerbates inflammation and disturbs fibrotic repair in mouse myocardial infarction. Sci Rep. 2018;8(1):16749. doi: 10.1038/s41598-018-35194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]