Abstract
Objective
Recent studies have shown a pathophysiologic link between headache and multiple sclerosis (MS), but the prevalence of primary headaches among patients with MS differs substantially across studies. This meta‐analysis aimed to comprehensively gather available evidence to estimate the prevalence of primary headaches among patients with MS.
Method
We systematically searched the electronic databases including PubMed, Embase, and Scopus for cohort, case–control, cross‐sectional studies that measured the prevalence of headache among patients with MS. Two reviewers independently screened titles and abstracts to identify the eligible studies and the full texts of the included studies were reviewed. Newcastle‐Ottawa Scale (NOS) was used to assess the risk of bias of the included literatures. We then conducted a meta‐analysis using Stata Software 15.0 to calculate the pooled prevalence of headaches among patients with MS and assess the source of heterogeneity.
Results
We identified 16 eligible studies covering a total of 3,560 patients with MS. The pooled estimated prevalence of primary headaches among patients with MS was 56%. The statistical heterogeneity was moderate with I 2 of 82.1% (p < .001). Both a visual inspection of the funnel plot and Egger’ regression tests revealed no significant publication bias (p = .44). The pooled estimated prevalence of migraine (55%) was higher in comparison with that of tension‐type headache (20%). The prevalence of migraine subtype was 16% and 10% for migraine without aura and migraine with aura, respectively. The pooled prevalence of primary headache in case–control group (57%) was approximately in line with the cross‐sectional group (56%).
Conclusion
The overall prevalence of primary headaches among patients with MS was considerably high. Clinical screening of headache among patients with MS will be helpful to formulate an individualized treatment plans and alleviate the physical and mental impact of the disease.
Keywords: headache, meta‐analysis, multiple sclerosis, prevalence, systematic review
This meta‐analysis aimed to comprehensively gather available evidence to estimate the prevalence of primary headaches among patients with MS. The overall prevalence of primary headaches among patients with MS was considerably high. Clinical screening of headache among patients with MS will be helpful to formulate a individualized treatment plans and alleviate the physical and metal impact of the disease.
1. INTRODUCTION
Multiple sclerosis (MS) is a chronic and progressive demyelinating disease of the central nervous system (CNS), which is characterized by spatially and temporally multiple lesions of unknown etiology (Hauser & Cree, 2020). Epidemiological studies have shown an increasing prevalence of MS in overall in Europe, Latin America, Middle East, and the Mediterranean Basin (Benito‐León & Bermejo‐Pareja, 2010; Cristiano et al., 2016). On the other hand, recent studies have shown that comorbid diseases such as stroke, epilepsy, and headache can adversely affect the quality of life, treatment outcome, and life expectancies in MS patients (Moisset et al., 2013).
Headache is a common neurological comorbidity in MS. The prevalence of primary headaches among patients with MS has been reported to be high, ranging from 35.5% to 70%, with migraine and tension‐type headache being the most frequent types (Busillo et al., 2014; Foley et al., 2013; Moisset et al., 2013). An autopsy of MS patient demonstrated a lymphatic follicular structure proliferation leading to an inflammatory response of the brain meninges (Koshihara et al., 2014). T‐cell and B‐cell activation in meningitis has been presumed to contribute to the high prevalence of headache, especially migraine headache, in patients with MS (Levy, 2009; Moreno et al., 2018). On the other hand, both headache and MS are associated with the functional or structural alterations of brainstem and cortex (Bourgeais‐Rambur, Beynac, & Villanueva, 2019; Tortorella et al., 2006), suggesting a shared anatomical basis for the comorbid relationship between headache and MS.
The prevalence of both migraine and MS is highest in white race, followed by black race, and finally yellow race (Applebee, 2012). However, epidemiologic evidence from current studies showed low consistence in primary headache prevalence among patients with MS. Therefore, we systematically reviewed and meta‐analyzed the available studies to investigate the overall prevalence of primary headaches among patients with MS and provide guidance for screening and diagnosing the primary headaches comorbid with MS in clinical practice.
2. METHODS
Our meta‐analysis was conducted on the basis of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009).
2.1. Search strategy
We conducted a systematic review and meta‐analysis of research articles to assess the prevalence of primary headaches among patients with MS. Two investigators (Long Wang and Juan Zhang) independently searched published articles indexed in PubMed, Embase, and Scopus database from inception to 31 December 2019. Text words and Medical Subject Headings (MESH) terms were used for primary headache and MS “(headache OR migraine OR tension‐type headache OR cluster headache OR trigeminal‐autonomic cephalalgias OR primary headache) AND (multiple sclerosis OR MS OR demyelinating disease).”
2.2. Eligibility criteria
We included observational studies which fulfill the following inclusion criteria: (a) study design being cohort, case–control, or cross‐sectional study; (b) diagnosis of primary headaches such as migraine (with or without aura), tension‐type headache, cluster headache being based on the criteria of the International Classification of Headache Disorders (ICHD)‐2 or‐3 (Headache Classification Committee of the International Headache Society, 2004; Olesen, 2018); (c) diagnosis of MS being based on the McDonald or Poser's criteria (Polman et al., 2005; Poser et al., 1983), and patient information obtained through questionnaires or clinical interviews; 4) the prevalence of primary headaches among patients with MS being determined from studies published in the English language. We excluded cases series, letters without original data, duplicate studies, reviews, or studies that did not provide data on the odds or risk of headache in subjects with MS.
2.3. Data extraction and quality assessment
One reviewer extracted the data using a standardized data collection form and the information was checked by another reviewer. The following information was extracted from the included studies: surname of the first author, year of publication, country of study, study design, sample size, age of patients, duration of MS, diagnostic criteria, prevalence of headache. The Newcastle‐Ottawa Scale (NOS) was used to assess the quality of the studies (Stang, 2010). We evaluated the following three areas: selection, comparability of studies on the basis of the design, and methodological quality. The maximum score is 9 points. We defined scores of 0–3, 4–6, and 7–9 as low, moderate, and high quality of the included studies, respectively.
2.4. Statistical analysis
Data analysis was performed using Stata 15.0 software (StataCorp, College Station, Texas, USA). I 2 was calculated to quantify the heterogeneity among included studies, A value of I 2 between 0% and 25% represents absence of heterogeneity; 25% ≤I 2 < 50%, low heterogeneity; 50% ≤I 2 < 75%, moderate heterogeneity; I 2 ≥ 75%, substantial heterogeneity (Higgins, Thompson, Deeks, & Altman, 2003). The random‐effect model for meta‐analysis was used to pool the overall prevalence of primary headaches among patients with MS. Funnel plot and Egger's tests were used to evaluate publication bias. We performed subgroups analysis by headache classifying and study designing to check the robustness of the overall results.
3. RESULTS
3.1. Characteristics of included studies
As illustrated in Figure 1, we retrieved a total of articles from our database search. After abstract screening and manual searching of references, we search out 62 articles meeting the criteria for full‐text reviewing. Among the 62 articles, 46 were excluded and 16 research articles were included in the final meta‐analysis (Beckmann & Türe, 2019; Busillo et al., 2014; D'Amico et al., 2004; Doi et al., 2009; Gebhardt, Kropp, Hoffmann, & Zettl, 2018; Gustavsen et al., 2016; Katsiari, Vikelis, Paraskevopoulou, Sfikakis, & Mitsikostas, 2011; Kister et al., 2010; Möhrke, Kropp, & Zettl, 2013; Nicoletti et al., 2008; Özer, Ergün, & İnan, 2018; Putzki et al., 2009; Sorgun, Yucesan, & Yasemin, 2013; Srivastava, Wang, Ugurlu, & Amezcua, 2016; Vacca et al., 2007; Villani et al., 2008).
FIGURE 1.
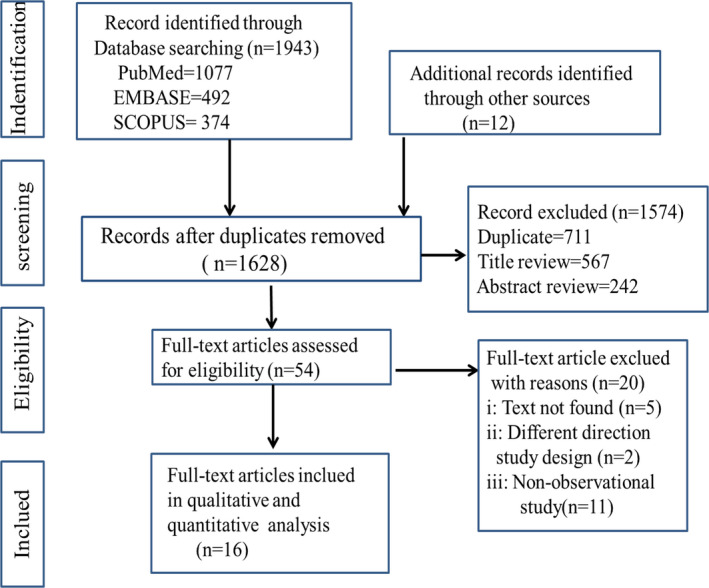
Flowchart of study identification for meta‐analysis
The characteristics of the included studies are shown in Table 1. Of the 16 articles, reporting a total of seven case–control studies, nine were cross‐sectional studies. Most studies were carried out in Europe and North America. For classification of primary headache among patients with MS, migraine and tension‐type headache were evaluated in all sixteen studies, migraine with or without aura in five studies.
TABLE 1.
Information about the included studies
| First author, year | Country | Study design | Sample size | Mean age of MS (years) | Duration of MS (years) | MS diagnosis criteria | Headache diagnosiscriteria | Prevalence of any primary headaches (%) | Prevalence (%) | NOS score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MH | MOA | MA | TTH | ||||||||||
| Beckmann, 2019 | Turkey | Cross‐section | 754 | 36.0 ± NA | NA | McDonald | ICHD‐2 | 40.5 | 26.8 | 18.1 | 8.7 | 13.7 | 8 |
| Özer, 2018 | Turkey | Cross‐section | 100 | 33.9 ± 9.0 | 5.7 ± 4.1 | McDonald | ICHD‐2 | 62.0 | 46.0 | 16.0 | 7 | ||
| Gebhardt, 2018 | Germany | Cross‐section | 50 | 30.0 ± NA | NA | McDonald | MSQ | 61.3 | 47.0 | 14.3 | 8 | ||
| Gustavsen, 2016 | Norway | Case‐control | 510 | 50.7 ± 12.5 | 18.4 ± 11.6 | McDonald | ICHD‐2 | 52.4 | 18.2 | 12.7 | 8 | ||
| Srivastava, 2016 | USA | Cross‐section | 233 | 44.4 ± 12.4 | 11.9 ± 10.4 | McDonald | ICHD‐2 | 49.0 | 36.0 | 5.0 | 8 | ||
| Busillo, 2014 | Italy | Cross‐section | 167 | 45.0 ± 12.0 | 12.5 ± 11.0 | McDonald | ICHD‐2 | 56.3 | 39.5 | 22.8 | 16.7 | 16.8 | 6 |
| Möhrke, 2013 | Germany | Cross‐section | 180 | 43.9 ± 13.1 | 12.3 ± 8.8 | McDonald | ICHD‐2 | 54.4 | 16.7 | 8.9 | 7.8 | 12.8 | 7 |
| Sorgun, 2013 | Turkey | Case‐control | 139 | 37.0 ± 10.2 | 6.7 ± NA | NA | ICHD‐2 | 66.9 | 30.9 | 36.0 | 7 | ||
| Katsiari, 2011 | Greece | Case‐control | 48 | 37.8 ± 11.9 | 7.5 ± 4.8 | McDonald | ICHD‐2 | 50.0 | 22.9 | 27.1 | 9 | ||
| Kister, 2010 | USA | Case‐control | 204 | 45.0 ± 12.0 | 12.5 ± 11.0 | McDonald | ICHD‐2 | 64.2 | 46.1 | 18.1 | 9 | ||
| Putzki, 2009 | Switzerland | Case‐control | 491 | 45.3 ± NA | 11.5 ± NA | McDonald | ICHD‐2 | 56.2 | 24.6 | 37.2 | 9 | ||
| Doi, 2009 | Japan | Cross‐section | 127 | 33.4 + 13.3 | 11.8 + 9.2 | Poser | ICHD‐2 | 50.4 | 20.4 | 16.5 | 3.9 | 30.0 | 6 |
| Nicoletti, 2008 | Italy | Case‐control | 101 | 33.6 ± 10.8 | 10.1 ± 3.0 | Poser | ICHD‐2 | 57.4 | 19.8 | 27.7 | 9 | ||
| Villani, 2008 | Italy | Cross‐section | 102 | 38.7 ± 9.5 | 8.8 ± 7.6 | McDonald | ICHD‐2 | 53.9 | 44.1 | 8.8 | 6 | ||
| Vacca, 2007 | Italy | Case‐control | 238 | 40.0 ± NA | NA | McDonald | ICHD‐2 | 51.3 | 35.7 | 7.1 | 7 | ||
| D'Amico, 2004 | Italy | Cross‐section | 116 | 40.6 ± 11.6 | 13.4 ± 9.4 | McDonald | ICHD‐2 | 57.7 | 25.0 | 16.4 | 8.6 | 31.9 | 6 |
Abbreviations: ICHD‐II, The International Classification of Headache Disorders 2nd edition; MA, Migraine with aura; MH, Migraine Headache, MOA, Migraine without aura; MSQ, Migraine Screening Questionnaire; TTH, Tension‐type headache.
3.2. Quality of included studies
NOS was used to assess the quality of the studies. Of 16 included studies, 13 studies are of high quality (NOS score ≥ 7), 3 studies moderate quality (NOS score between 5 and 6), and 0 study low quality (NOS score < 5) (Table. 1).
3.3. The prevalence of primary headaches among patients with MS
The overall prevalence of primary headaches among patients with MS was 55% (95%CI: 0.51–0.59). Nevertheless, obviously heterogeneity among included studies was showed in our meta‐analysis (I 2 = 82.1%; p < .001, Figure 2).
FIGURE 2.
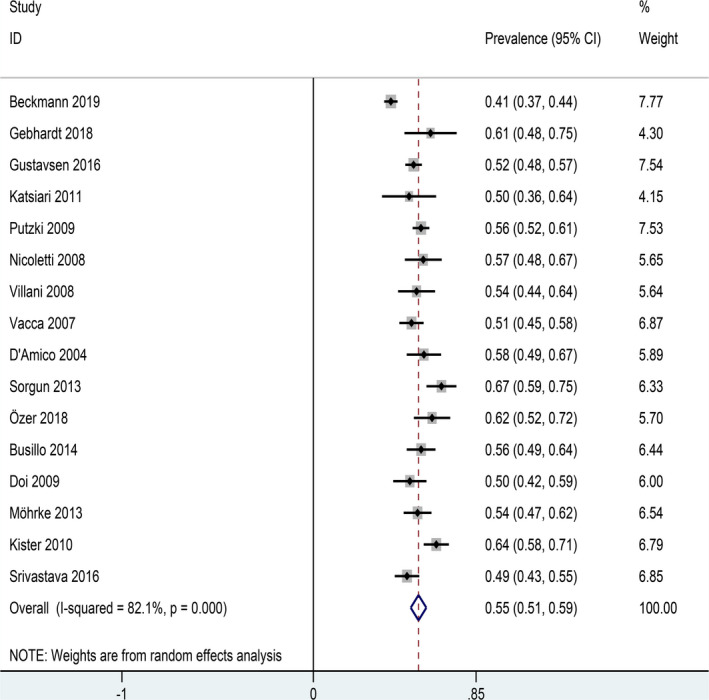
Forest plot for prevalence of primary headaches in patients with MS
3.4. Subgroup analysis
We performed a subgroup analysis using of headache types as a moderator. Results showed pooled prevalence of migraine headache (MH) and tension‐type headache (TTH) was 30% and 22%, respectively (Table 2). Five studies reported MA or MOA prevalence in patients with MS and showed prevalence of headaches was 16% and 10%, respectively. (Figure 3).
TABLE 2.
Subgroup and sensitivity analysis for the prevalence of headaches in patients with MS
| Subgroups | Included studies | Prevalence (%) | 95% CI | Heterogeneity (I 2, Q and p‐value) | ||
|---|---|---|---|---|---|---|
| I 2 (%) | Q‐value | p‐value | ||||
| Types of headache | 30 | |||||
| Migraine | 16 | 30 | 0.25–0.34 | 88.2 | 127.46 | 0.007 |
| Migraine without aura | 5 | 16 | 0.11–0.21 | 81.1 | 21.21 | 0.002 |
| Migraine with aura | 5 | 10 | 0.06–0.14 | 81.0 | 21.06 | 0.002 |
| Tension‐type headache | 16 | 22 | 0.19–0.25 | 94.2 | 259.79 | 0.010 |
| Study design | ||||||
| Case–control | 7 | 57 | 0.53–0.62 | 68.5 | 19.04 | 0.004 |
| Cross‐sectional | 9 | 53 | 0.47–0.59 | 81.6 | 43.51 | 0.000 |
| Regional distribution | ||||||
| Europe | 12 | 55 | 0.53–0.58 | 31.6 | 16.08 | <0.001 |
| Asia | 4 | 55 | 0.41–0.69 | 93.8 | 48.23 | 0.019 |
FIGURE 3.
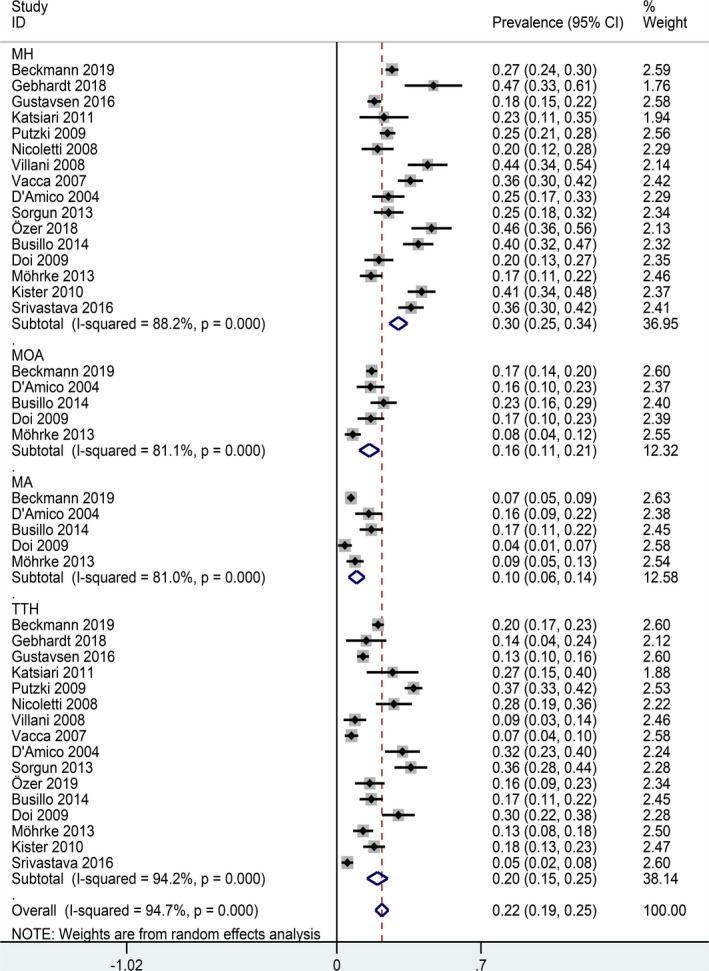
Forest plot for subgroup analysis (types of headache)
We further conducted a subgroup analysis using the design method as a moderator. The prevalence of primary headaches was slightly higher in the case–control study (57%) than cross‐sectional study (53%) (Figure 4).
FIGURE 4.
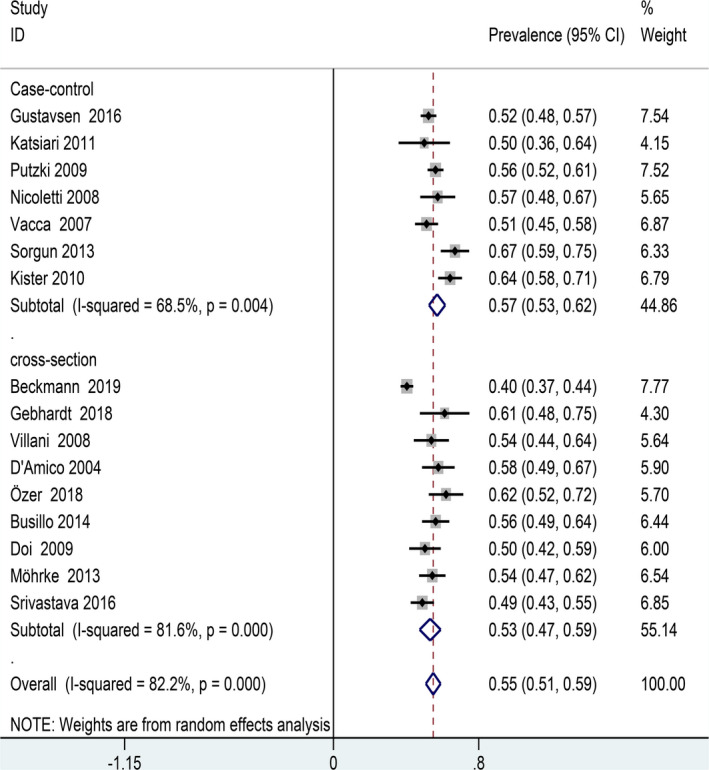
Forest plot for subgroup analysis (study design)
We also conducted a subgroup analysis using the geographical distribution of participants as a moderator. The overall prevalence of primary headaches among patients with MS was 55%, which did not differ from patients in Asian and European countries. The heterogeneity of the included studies was mainly due to the prevalence of primary headaches in Asia (I 2 = 31.6%; p = .019) rather than in Europe (I 2 = 93.8%; p < .001) (Figure 5).
FIGURE 5.
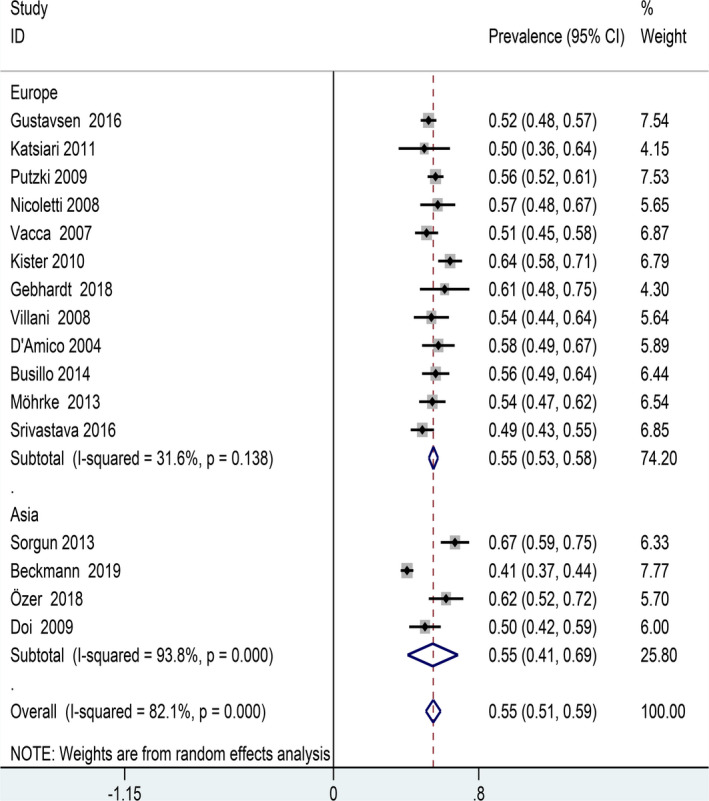
Forest plot for subgroup analysis (regional distribution)
3.5. Publication bias
A visual inspection of the funnel plot revealed no significant publication bias, and a consistent conclusion was indicated by Egger’ regression tests (B = 1.41, SE = 1.79, p = .44, Figure 6).
FIGURE 6.
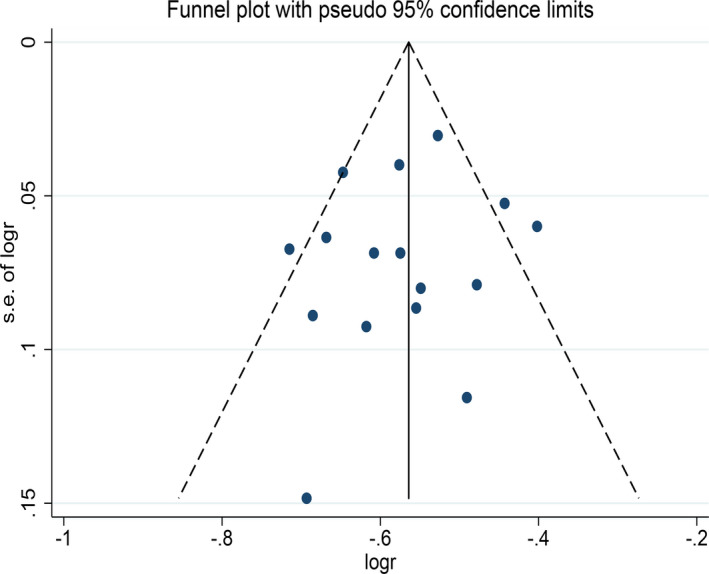
Funnel plot for prevalence of primary headache in patients with MS
4. DISCUSSION
In recent years, the comorbid condition of MS has attracted much attention as emerging evidence indicates that comorbidity is related to the delay of diagnosis, quality of life, course of disease, and progression of disability (Fromont et al., 2013; Marrie et al., 2010, 2015). Headache is a common comorbid condition in MS. However, previous studies vary widely in the prevalence of headaches among patients with MS.
We report, for the first time, the prevalence of primary headaches in patients with MS after conducting systematic review and meta‐analysis of globally available studies. Our study showed that the pooled prevalence of primary headaches in patients with MS (57%) was higher than that of WHO report in 2016 in which the overall primary headache prevalence among MS patients was 50% (World Health Organization, 2016). A reasonable explanation for the discrepancy might be due to the differences of study population and designed methods, as MS is 2 to 3 times more common in women than in men and migraine more common in women than in men in the classification of primary headaches (Artero‐Morales, González‐Rodríguez, & Ferrer‐Montiel, 2018; WenJuan, WeiWei, & Xia, 2017). In our included studies, the gender composition of 72% female patients and 28% male patients gives rise to a female/male ratio of 2.6:1. Therefore, this may increase the prevalence of headaches in patients with MS.
Epidemiological evidence suggested that migraine is most closely related to MS, followed by tension‐type headache (La Mantia & Prone, 2015; Moisset et al., 2013). Nevertheless, tension‐type headache was ever reported to be a most common comorbidity in patients with MS, but there was less evidence to support it (Doi et al., 2009). In our review, the overall prevalence of migraine and tension‐type headache among patients with MS was 27% and 10%, respectively. This indicates that it is common that migraine often co‐occurred with MS. Some studies have shown that migraine commonly presented as an initial symptom in patients with MS, indicating that migraine may be a risk factor for MS (Urits et al., 2019). The underlying mechanism is not clear. It seems that the location of plaques among patients with MS was closely associated with an increased occurrence of migraine (Gee, Chang, Dublin, & Vijayan, 2005). Most common localizations of MS lesions are brainstem and cortex associated with migraine pathophysiology. Previous studies demonstrated that 40%–50% MS patients with headache symptom have either cortical or brainstem lesions (Beckmann & Türe, 2019; Mazhari, 2016). MRI study in patients with MS revealed that demyelination within the periaqueductal gray matter (PAG) are several times more likely to have migraine comparing to those with normal PAG (Gee et al., 2005). Further, inflammation‐mediated cortical demyelination can accelerate cortical spreading depression (CSD) which is also the pathological basis of migraine (Husain, Pardo, & Rabadi, 2018; Merkler et al., 2009; Möhrke et al., 2013). These may be suggested that comorbidity of MS and migraine have a common anatomical basis.
Some case descriptions also indicate an association between MS and another common primary headache, cluster headache (Donat, 2012; Mijajlović, Aleksić, & Covičković Šternić, 2014; Pelikan, McCombe, Kotylak, & Becker, 2016). In our included studies, only D'Amico et al. reported that the prevalence of cluster headache among MS is 0.8% (D'Amico et al., 2004). Studies showed that typical symptoms of cluster headache presents while demyelinating lesions are distributed in the entry area of the trigeminal nerve root in the pons (Gentile, Ferrero, Vaula, Rainero, & Pinessi, 2007).
For the subgroup analysis, the pooled prevalence of primary headache in case–control group (57%) was in line with that in cross‐sectional group (56%). This result ruled out bias due to the inclusion of different study design types on the pooled prevalence of headaches among patients with MS. However, the heterogeneity was presented in these subgroups as bias may occur due to the headache data collection through questionnaire or interview in some studies and bias may occur due to the selection of MS patients and controls. Furthermore, the overall prevalence of primary headaches among patients with MS was 55%, which did not differ between patients from Asia and European countries. These may suggest that the risk of primary headaches among patients with MS is similarly common regardless of geography and economic development. In addition, we found high heterogeneity in the prevalence of headaches patients with MS in Asia counties (I 2 = 93.8) compared to that in European countries (I 2 = 31.6). Further analysis revealed that few literatures included were from Asia, and three of them were from Turkey. The heterogeneity may be in part due to geographical differences.
The strength of our review and meta‐analysis is the high methodological quality of most of the included literatures. In addition, sensitivity analysis verified the stabilization of our study results. However, some limitations need to be noted. Firstly, majority of the included studies were from European countries, which may decrease the accuracy of estimation. Secondly, we did not investigate the ethnic data due to absence of adequate information. Thirdly, nonstandardized data collection tools were used in some studies and this may affect the pooled prevalence of primary headaches.
5. CONCLUSION
We found that the overall prevalence of primary headaches in patients with MS was higher comparing to that in general population. Therefore, we suggest that clinical screening of headache among patients with MS will be helpful to formulate an individualized treatment plan alleviating physical and mental impact of the disease on patients.
CONFLICT OF INTEREST
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
AUTHORS’ CONTRIBUTIONS
LW, ZD, YW: conceptualization. LW and JZ: data curation. LW: formal analysis and funding acquisition. LW, JZ, and MZ: investigation, methodology, resources, and validation. RZ and YW: project administration. LW, ZD, MZ: software. LW, JZ: supervision, writing—review, and editing. LW, JZ, and ZD: writing—original draft.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.1830.
ACKNOWLEDGEMENTS
This work was supported by Natural Science grants to Yu Wang (Grant number: 81671290) from the National Natural Science Foundation of China.
Wang L, Zhang J, Deng Z‐R, Zu M‐D, Wang Y. The epidemiology of primary headaches in patients with multiple sclerosis. Brain Behav.2021;11:e01830 10.1002/brb3.1830
Long Wang and Juan Zhang are contributed equally to this article.
DATA AVAILABILITY STATEMENT
All data generated or analyzed data in study are included in this article.
REFERENCES
- Applebee, A. (2012). The clinical overlap of multiple sclerosis and headache. Headache, 52, 111–116. [DOI] [PubMed] [Google Scholar]
- Artero‐Morales, M. , González‐Rodríguez, S. , & Ferrer‐Montiel, A. (2018). TRP channels as potential as targets for sex‐related differences in migraine pain. Frontiers in Molecular Biosciences, 5, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, Y. , & Türe, S. (2019). Headache characteristics in multiple sclerosis. Multiple Sclerosis and Related Disorders, 27, 112–116. [DOI] [PubMed] [Google Scholar]
- Benito‐León, J. , & Bermejo‐Pareja, F. (2010). Is the epidemiology of multiple sclerosis changing? Revue Neurologique, 51, 385–386. [PubMed] [Google Scholar]
- Bourgeais‐Rambur, L. , Beynac, L. , & Villanueva, L. (2019). Brain network dysfunctions as substrates of primary headaches. Biologie Aujourd'hui, 213, 43–49. [DOI] [PubMed] [Google Scholar]
- Busillo, V. , Pizza, V. , Cassano, D. , Busillo, A. , Somma, M. R. , & Capasso, A. (2014). Headache and multiple sclerosis. Pharmacologyonline, 1, 68–72. [Google Scholar]
- Cristiano, E. , Patrucco, L. , Miguez, J. , Giunta, D. , Peroni, J. , & Rojas, J. I. (2016). Increasing incidence of multiple sclerosis among women in Buenos Aires: A 22 year health maintenance organization based study. Neurological Sciences, 37, 1621–1626. [DOI] [PubMed] [Google Scholar]
- D'Amico, D. , La Mantia, L. , Rigamonti, A. , Usai, S. , Mascoli, N. , Milanese, C. , … Besta, C. (2004). Prevalence of primary headaches in people with multiple sclerosis. Cephalalgia, 24, 980–984. [DOI] [PubMed] [Google Scholar]
- Doi, H. , Matsushita, T. , Isobe, N. , Ishizu, T. , Ohyagi, Y. , & Kira, J. (2009). Frequency of chronic headaches in Japanese patients with multiple sclerosis: With special reference to opticospinal and common forms of multiple sclerosis. Headache, 49, 1513‐1520. [DOI] [PubMed] [Google Scholar]
- Donat, J. (2012). A patient with cluster headache—Due to a brainstem lesion. Headache, 52, 1035–1036. [DOI] [PubMed] [Google Scholar]
- Foley, P. L. , Vesterinen, H. M. , Laird, B. J. , Sena, E. S. , Colvin, L. A. , Chandran, S. , … Fallon, M. T. (2013). Prevalence and natural history of pain in adults with multiple sclerosis: Systematic review and meta‐analysis. Pain, 154, 632–642. [DOI] [PubMed] [Google Scholar]
- Fromont, A. , Binquet, C. , Rollot, F. , Despalins, R. , Weill, A. , Clerc, L. , … Moreau, T. (2013). Comorbidities at multiple sclerosis diagnosis. Journal of Neurology, 260, 2629–2637. [DOI] [PubMed] [Google Scholar]
- Gebhardt, M. , Kropp, P. , Hoffmann, F. , & Zettl, U. K. (2018). Headache at the time of first symptom manifestation of multiple sclerosis: A prospective, longitudinal study. European Neurology, 80, 115–120. [DOI] [PubMed] [Google Scholar]
- Gee, J. R. , Chang, J. , Dublin, A. B. , & Vijayan, N. (2005). The association of brainstem lesions with migraine‐like headache: An imaging study of multiple sclerosis. Headache, 45, 670–677. [DOI] [PubMed] [Google Scholar]
- Gentile, S. , Ferrero, M. , Vaula, G. , Rainero, I. , & Pinessi, L. (2007). Cluster headache attacks and multiple sclerosis. Journal of Headache and Pain, 8, 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsen, M. W. , Celius, E. G. , Winsvold, B. S. , Moen, S. M. , Nygaard, G. O. , & Berg‐Hansen, P. (2016). Migraine and frequent tension‐type headache are not associated with multiple sclerosis in a Norwegian case‐control study. Multiple Sclerosis Journal – Experimental, Translational and Clinical, 2, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, S. L. , & Cree, B. A. C. (2020). Treatment of multiple sclerosis: A review. American Journal of Medicine, 133(12):1380–1390. 10.1016/j.amjmed.2020.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society . (2004). Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia, 24, 1–160. [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain, F. , Pardo, G. , & Rabadi, M. (2018). Headache and its management in patients with multiple sclerosis. Current Treatment Options in Neurology, 20, 10. [DOI] [PubMed] [Google Scholar]
- Katsiari, C. G. , Vikelis, M. , Paraskevopoulou, E. S. , Sfikakis, P. P. , & Mitsikostas, D. D. (2011). Headache in systemic lupus erythematosus vs multiple sclerosis: A prospective comparative study. Headache, 51, 1398–1407. [DOI] [PubMed] [Google Scholar]
- Kister, I. , Caminero, A. B. , Monteith, T. S. , Soliman, A. , Bacon, T. E. , Bacon, J. H. , … Lipton, R. B. (2010). Migraine is comorbid with multiple sclerosis and associated with a more symptomatic MS course. The Journal of Headache and Pain, 11, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshihara, H. , Oguchi, K. , Takei, Y. I. , Kitazawa, K. , Higuchi, K. , & Ohara, S. (2014). Meningeal inflammation and demyelination in a patient clinically diagnosed with acute disseminated encephalomyelitis. Journal of the Neurological Sciences, 346(1–2), 323–327. [DOI] [PubMed] [Google Scholar]
- La Mantia, L. , & Prone, V. (2015). Headache in multiple sclerosis and autoimmune disorders. Neurological Sciences, 36, 75–78. [DOI] [PubMed] [Google Scholar]
- Levy, D. (2009). Migraine pain, meningeal inflammation, and mast cells. Current Pain and Headache Reports, 13, 237–240. [DOI] [PubMed] [Google Scholar]
- Marrie, R. A. , Cohen, J. , Stuve, O. , Trojano, M. , Sørensen, P. S. , Reingold, S. , … Reider, N. (2015). A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: Overview. Multiple Sclerosis Journal, 21, 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie, R. A. , Rudick, R. , Horwitz, R. , Cutter, G. , Tyry, T. , Campagnolo, D. , & Vollmer, T. (2010). Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology, 74, 1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhari, A. (2016). Multiple sclerosis‐related pain syndromes: An imaging update. Current Pain and Headache Reports, 20, 63. [DOI] [PubMed] [Google Scholar]
- Merkler, D. , Klinker, F. , Jürgens, T. , Glaser, R. , Paulus, W. , Brinkmann, B. G. , … Liebetanz, D. (2009). Propagation of spreading depression inversely correlates with cortical myelin content. Annals of Neurology, 66, 355–365. [DOI] [PubMed] [Google Scholar]
- Mijajlović, M. D. , Aleksić, V. M. , & Covičković Šternić, N. M. (2014). Cluster headache as a first manifestation of multiple sclerosis: Case report and literature review. Neuropsychiatric Disease and Treatment, 10, 2269–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. ; Group P . (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ, 339, b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhrke, J. , Kropp, P. , & Zettl, U. K. (2013). Headaches in multiple sclerosis patients might imply an inflammatorial process. PLoS One, 8, e69570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisset, X. , Ouchchane, L. , Guy, N. , Bayle, D. J. , Dallel, R. , & Clavelou, P. (2013). Migraine headaches and pain with neuropathic characteristics: Comorbid conditions in patients with multiple sclerosis. Pain, 154, 2691–2699. [DOI] [PubMed] [Google Scholar]
- Moreno, M. A. , Or‐Geva, N. , Aftab, B. T. , Khanna, R. , Croze, E. , Steinman, L. , & Han, M. H. (2018). Molecular signature of Epstein‐Barr virus infection in MS brain lesions. Neurology‐Neuroimmunology Neuroinflammation, 5(4), e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti, A. , Patti, F. , Fermo, S. L. , Liberto, A. , Castiglione, A. , Laisa, P. , … Contrafatto, D. (2008). Headache and multiple sclerosis: A population‐based case‐control study in Catania, Sicily. Cephalalgia, 28, 1163–1169. [DOI] [PubMed] [Google Scholar]
- Olesen, J. (2018). Headache Classification Committee of the International Headache Society (IHS) the international classification of headache disorders, abstracts. Cephalalgia, 38, 1–211. [DOI] [PubMed] [Google Scholar]
- Özer, G. , Ergün, U. , & İnan, L. E. (2018). Headache in multiple sclerosis from a different perspective. Journal of Clinical Investigation, 9, 9–13. [Google Scholar]
- Pelikan, J. B. , McCombe, J. A. , Kotylak, T. , & Becker, W. J. (2016). Cluster headache as the index event in MS: A case report. Headache, 56, 392–396. [DOI] [PubMed] [Google Scholar]
- Polman, C. H. , Reingold, S. C. , Edan, G. , Filippi, M. , Hartung, H. P. , Kappos, L. , … Sandberg‐Wollheim, M. (2005). Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘‘McDonald Criteria’’. Annals of Neurology, 58, 840–846. [DOI] [PubMed] [Google Scholar]
- Poser, C. M. , Paty, D. W. , Scheinberg, L. , McDonald, W. I. , Davis, F. A. , Ebers, G. C. , … Tourtellotte, W. W. (1983). New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Annals of Neurology, 13, 227–231. [DOI] [PubMed] [Google Scholar]
- Putzki, N. , Pfriem, A. , Limmroth, V. , Yaldizli, Ö. , Tettenborn, B. , Diener, H. C. , & Katsarava, Z. (2009). Prevalence of migraine, tension‐type headache and trigeminal neuralgia in multiple sclerosis. European Journal of Neurology, 16, 262–267. [DOI] [PubMed] [Google Scholar]
- Sorgun, M. H. , Yucesan, C. , & Yasemin, G. (2013). Headache in multiple sclerosis. Turkish Journal of Medical Sciences, 43, 1042–1049. [Google Scholar]
- Srivastava, S. , Wang, S. L. , Ugurlu, C. , & Amezcua, L. (2016). Headaches in multiple sclerosis: Cross‐sectional study of a multiethnic population. Clinical Neurology and Neurosurgery, 143, 71–75. [DOI] [PubMed] [Google Scholar]
- Stang, A. (2010). Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. European Journal of Epidemiology, 25, 603–605. [DOI] [PubMed] [Google Scholar]
- Tortorella, P. , Rocca, M. A. , Colombo, B. , Annovazzi, P. , Comi, G. , & Filippi, M. (2006). Assessment of MRI abnormalities of the brainstem from patients with migraine and multiple sclerosis. Journal of the Neurological Sciences, 244, 137–141. [DOI] [PubMed] [Google Scholar]
- Urits, I. , Adamian, L. , Fiocchi, J. , Hoyt, D. , Ernst, C. , Kaye, A. D. , & Viswanath, O. (2019). Advances in the understanding and management of chronic pain in multiple sclerosis: A comprehensive review. Current Pain and Headache Reports, 23, 59. [DOI] [PubMed] [Google Scholar]
- Vacca, G. , Marano, E. , Morra, V. B. , Lanzillo, R. , De Vito, M. , Parente, E. , & Orefice, G. (2007). Multiple sclerosis and headache co‐morbidity. A case‐control Study. Neurological Sciences, 28, 133–135. [DOI] [PubMed] [Google Scholar]
- Villani, V. , Prosperini, L. , Ciuffoli, A. , Pizzolato, R. , Salvetti, M. , Pozzilli, C. , & Sette, G. (2008). Primary headache and multiple sclerosis: Preliminary results of a prospective study. Neurological Sciences, 29, 146–148. [DOI] [PubMed] [Google Scholar]
- WenJuan, H. , WeiWei, C. , & Xia, Z. (2017). Multiple sclerosis: Pathology, diagnosis and treatments. Experimental and Therapeutic Medicine, 13, 3163–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2016). Headache disorders. https://www.who.int/news‐room/fact‐sheets/detail/headache‐disorders [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed data in study are included in this article.


