Abstract
Objective
We investigated the effect of arterial stiffness on the severity of enlarged perivascular spaces (EPVSs) and cerebral microbleeds (CMBs) at different brain locations.
Methods
A total of 854 stroke patients underwent both brachial‐ankle pulse wave velocity (baPWV) measurement and brain MRI. The extent of EPVS was separately rated at the levels of the basal ganglia (BG) and centrum semiovale (CS). The CMBs were categorized as strictly lobar CMB and deep CMB. The patients were categorized according to baPWV quartiles, and multivariable logistic regressions were performed to evaluate whether the baPWV increment was independently associated with each cerebral SVD marker at different locations. The odds ratio (OR) with 95% confidence interval (CI) was derived on the reference of the first quartile.
Results
Severe EPVSs at BG and CS were detected in 243 (28.5%) and 353 patients (41.3%), respectively. The increment of baPWV quartiles was associated with both severe BG EPVS burden (Q4: OR = 2.58, CI = 1.45–4.60) and severe CS EPVS burden (Q4: OR = 2.06, CI = 1.24–3.42). Deep CMBs were found in 259 patients (30.3%), and strictly lobar CMBs were found in 170 patients (19.9%). Multivariable logistic regression model revealed deep CMB was independently associated with the baPWV increment (Q4: OR = 2.52, CI = 1.62–3.94). However, strictly lobar CMB had a neutral relationship with baPWV.
Conclusion
Increased arterial stiffness is consistently associated with the presence of deep CMB and severe EPVS burden at the BG and CS, suggesting a common pathophysiologic mechanism.
Keywords: arterial stiffness, enlarged perivascular space, pulse wave velocity, small vessel disease
We investigated the effect of arterial stiffness on the severity of enlarged perivascular spaces (EPVSs) and cerebral microbleeds (CMBs) at different brain locations. A total of 854 stroke patients underwent both brachial‐ankle pulse wave velocity (baPWV) measurement and brain MRI. Increased arterial stiffness is consistently associated with the presence of deep CMB and severe EPVS burden at the BG and CS, suggesting a common pathophysiologic mechanism.

1. INTRODUCTION
Cerebral small vessel disease (SVD) refers to pathological processes that affect the arterioles, venules, and capillaries of the brain. The most common causes are aging, hypertension, and cerebral amyloid angiopathy (Pantoni, 2010). The main imaging features of SVD include lacunar infarction, hemorrhages, lacunes, white matter hyperintensities (WMHs), brain atrophy, cerebral microbleeds (CMBs), and enlarged perivascular spaces (EPVSs) (Wardlaw et al., 2013). Perivascular spaces are cerebrospinal fluid‐filled cavities that surround small penetrating cerebral arterioles. They are assumed to form a network of spaces around cerebral microvessels that act as a conduit for fluid transport, exchange between cerebrospinal fluid and interstitial fluid (Weller et al., 2009). Perivascular spaces are normally microscopic, but EPVSs become visible on T2‐weighted brain magnetic resonance imaging (MRI). EPVSs are one of the novel characteristic MRI features of SVD (Doubal et al., 2010; Pantankar et al., 2005; Potter, Doubal, et al., 2015).
Arterial stiffness exposes cerebral small vessels to abnormally high pulsatile pressure, inducing SVD (Poels et al., 2012). Previous studies have demonstrated a positive correlation between arterial stiffness and the various types of cerebral SVD markers, including CMB and EPVS (Kim et al., 2016; Poels et al., 2012; Riba‐Llena et al., 2018; Xiao et al., 2015; Zhai et al., 2018). The pathophysiology of EPVS and CMB in the deep brain structure and lobar areas is believed to differ (Greenberg et al., 2009; Pollock et al., 1997; Zhu et al., 2010). BG EPVS and deep CMB are typically associated with hypertensive vasculopathy (Greenberg et al., 2009; Zhai et al., 2018), whereas CS EPVS and lobar CMB are related to amyloid angiopathy (Greenberg et al., 2009; Martinez‐Ramirez et al., 2018). We investigated the association between baPWV increment and the location of EPVS and CMB in the deep brain structures and cerebral cortices of Korean stroke patients.
2. METHODS
2.1. Standard protocol approvals, registrations, and patient consents
This study was reviewed and approved by the institutional review boards at Chung‐Ang University Hospital.
2.2. Patient population
Between January 2014 and May 2017, 1,244 consecutive patients with acute ischemic stroke or transient ischemic attack who visited a tertiary university hospital in Seoul, Korea, within seven days of symptom onset were considered for inclusion. Among those, the 854 patients who had undergone both brain MRI and baPWV evaluation during admission were included (412 female patients, mean age = 68.2 ± 12.5 years) (Figure 1). Vascular risk factor profiles and laboratory data were obtained from the prospective stroke registry database. Hypertension was defined as blood pressure ≥ 140/90 mmHg measured on several separate occasions after the patient had been stabilized and started on antihypertensive medications. Diabetes mellitus was defined as receipt of antidiabetic medications before stroke or glycated hemoglobin A1c (HbA1c) exceeding 6.5%. Laboratory data included hematocrit, serum white blood cell count, platelet count, fasting blood sugar and HbA1c, cholesterol, high‐sensitivity C‐reactive protein (hsCRP), and estimated glomerular filtration rate (eGFR). eGFR was calculated by using modification of diet in the renal disease formula: eGFR (ml/min/1.73 m2) = 175 × (serum creatinine)−1.154 × age−0.203 × (0.742 if female).
FIGURE 1.
Flowchart of patient inclusion and exclusion in the study. baPWV, brachial‐ankle pulse wave velocity; MRI, magnetic resonance imaging
2.3. Brachial‐ankle pulse wave velocity (baPWV)
The baPWV was measured with patients in the supine position using a volume‐plethysmography device (VP‐1000; Collin, Komaki, Japan) as reported previously (Tanaka et al., 2009). The device records the phonogram, volume pulse form, and arterial blood pressure at the brachia and ankles bilaterally. The baPWV was automatically calculated as the transmission distance divided by the transmission time. The distance between the right brachium and ankle was estimated on the basis of height. The average of the baPWV values obtained on both sides was used for further analysis.
2.4. Brain MRI protocol and the definition of cerebral small vessel disease markers
Brain MRI was performed with a 3.0‐T MR unit (Avanto, Philips, Eindhoven, The Netherlands), and the definitions were applied according to the standards for reporting vascular changes on neuroimaging (Wardlaw et al., 2013). EPVSs were defined as small, sharply demarcated structures of cerebrospinal fluid intensity on imaging measuring less than 3 mm that followed the pathway of the perforating vessels and ran perpendicular to the brain surface (Wardlaw et al., 2013). At the BG and CS levels, we estimated the slide in the most affected hemisphere. The numbers of EPVS were rated as follows: 0 = no EPVS, 1 = 1 to 10 EPVS, 2 = 11 to 20 EPVS, 3 = 21 to 40 EPVS, and 4 = more than 40 EPVS (Potter, Chappell, et al., 2015). CMBs were visualized as areas of homogenous low signals, either round or oval in shape and less than 10 mm in size on susceptibility‐weighted imaging (Wardlaw et al., 2013). CMBs were categorized as strictly lobar microbleeds or deep microbleeds based on their location. Strictly lobar CMBs were cortical and subcortical regions assessed in the frontal, parietal, temporal, occipital, and insular regions. Deep regions included basal ganglia, thalamus, internal capsule, external capsule, corpus callosum, and periventricular white matter (Gregoire et al., 2009). We categorized patients as either who had microbleeds restricted to a lobar location (strictly lobar microbleeds) or who had microbleeds in a deep location with or without lobar microbleeds (deep microbleeds). Lacunes were defined as round or ovoid, subcortical, fluid‐filled cavities between 3 mm and 15 mm in diameter, which generally had a central CSF‐like intensity on T1‐weighted and T2‐weighted MRI (Wardlaw et al., 2013). WMHs of vascular origin were hyperintense on T2‐weighted sequences (Wardlaw et al., 2013). The total cerebral small vessel disease (SVD) score on an ordinal scale from 0 to 4 was calculated based on a previously described scoring method that incorporates 4 SVD markers (Klarenbeek et al., 2013; Staals et al., 2014). Cerebral small vessel disease markers were rated by the two experienced neurologists (KJM and BJH) who were unaware of the clinical data, and inter‐rater testing showed good inter‐rater reliability with kappa values of 0.785 for EPVS in the basal ganglia, 0.794 for EPVS in the centrum semiovale, 0.805 for WMH, 0.856 for deep CMB, 0.835 for strictly lobar CMB, and 0.834 for lacune.
2.5. Statistical analyses
Statistical analysis was performed using Statistical Package for the Social Sciences version 18.0 (SPSS). First, we examined the relationship between clinical variables and baPWV. The patients were categorized into quartiles according to the baPWV, and demographic and laboratory variables of each quartile group were compared using analysis of variance (ANOVA) testing with the post hoc Bonferroni test or linear‐by‐linear association. Next, to determine whether the effect of arterial stiffness differs according to the anatomic location of the EPVS, we dichotomized the study population according to the EPVS burden at BG and CS into mild (score of 1–2) and severe (score of 3–4) using the previous definitions (Potter, Chappell, et al., 2015). We also dichotomized the study population according to the presence of deep or strictly lobar CMB. Demographic and laboratory information was compared by bivariate analysis with the independent t test or chi‐square test. Multivariable logistic regression analyses were performed by including the variables derived from the bivariate analysis and with biological relevance. With the first quartile as a reference, the odds ratios (OR) of each quartile were derived for cerebral SVD burden with 95% confidence interval (CI).
3. RESULTS
Among the 854 patients, 243 patients (28.5%) had severe BG EPVS and 353 patients (41.3%) had severe CS EPVS; 259 patients (30.3%) had deep CMB, and 170 (19.9%) had strictly lobar CMB. The median value of the total SVD score was 2.0, and the mean baPWV was 20.6 ± 5.6 m/s. The higher baPWV quartile was proportionally associated with old age, female sex, hypertension, diabetes mellitus, previous stroke history, blood pressure, fasting blood sugar, and hsCRP. The proportion of current smokers, estimated glomerular filtration rate, and hematocrit level were inversely related to the baPWV quartile. The extent and severity of each cerebral SVD phenotype tended to increase according to the baPWV quartile, except for strictly lobar CMB (Table 1). The presence of strictly lobar CMB remained relatively stable at approximately 20% throughout each baPWV quartile.
TABLE 1.
Comparison of clinical variables according to the quartiles of mean brachial‐ankle pulse wave velocities
| Q1 | Q2 | Q3 | Q4 | p | |
|---|---|---|---|---|---|
| Patient numbers | 213 | 214 | 213 | 214 | |
| Age, years (SD) | 57.8 (12.6) | 67.4 (10.2) | 70.8 (10.3) | 76.8 (8.1) | <.001 |
| Sex, female, n (%) | 84 (39.4) | 92 (43.0) | 93 (43.7) | 143 (66.8) | <.001 |
| BMI, kg/m2, mean (SD) | 24.3(3.6) | 24.3 (3.3) | 24.2 (3.0) | 24.3 (13.3) | .961 |
| Hypertension, n (%) | 82 (38.4) | 134 (62.6) | 151 (70.9) | 163 (76.2) | <.001 |
| Diabetes mellitus, n (%) | 33 (15.4) | 62 (29.0) | 78 (36.6) | 85 (39.7) | <.001 |
| Smoking, n (%) | 72 (33.8) | 62 (29.0) | 61 (28.6) | 42 (19.6) | <.001 |
| Atrial fibrillation, n (%) | 37 (17.3) | 39 (18.2) | 42 (19.7) | 18 (8.4) | .490 |
| Previous stroke, n (%) | 33 (15.4) | 36 (16.8) | 49 (23.0) | 46 (21.5) | .045 |
| SBP, mm/Hg, mean (SD) | 139 (24) | 149 (26) | 154 (28) | 157 (29) | <.001 |
| DBP, mm/Hg, mean (SD) | 83 (13) | 87 (15) | 86 (16) | 88 (14) | .004 |
| Hematocrit, %, mean (SD) | 41.4 (5.0) | 40.7 (7.0) | 40.4 (5.6) | 39.7 (4.8) | .002 |
| White blood cell, 109/L, mean (SD) | 8.0 (3.1) | 8.1 (3.2) | 8.2 (2.9) | 8.0 (3.3) | .741 |
| Platelet, 109/L, mean (SD) (SD) | 230.0 (53.6) | 236.4 (80.2) | 235.8 (70.3) | 236.8 (85.3) | .405 |
| Fasting blood sugar, mmol/L, mean (SD) | 7.3 (3.4) | 7.5 (2.3) | 8.2 (3.2) | 8.2 (3.6) | <.001 |
| LDL cholesterol, mmol/L, mean (SD) | 2.7 (0.8) | 2.8 (0.8) | 2.7 (0.8) | 2.7 (0.9) | .923 |
| Triacylglycerol, mmol/L, mean (SD) | 3.0 (2.3) | 3.0 (1.8) | 3.3 (2.1) | 3.0 (1.7) | .806 |
| hsCRP, mg/dl, mean (SD) | 4.1(19.4) | 5.2(13.5) | 6.3(18.4) | 8.9(24.3) | .009 |
| eGFR, ml/min/1.73 m2, mean (SD) | 90.2 (23.0) | 82.5 (25.8) | 77.3 (25.8) | 76.5 (27.4) | <.001 |
| Severe WMH, n (%) | 7 (3.3) | 24 (11.2) | 23 (10.8) | 47 (22.0) | <.001 |
| Lacune, n (%) | 100 (46.9) | 138 (64.5) | 157 (73.7) | 183 (85.5) | <.001 |
| Deep CMB, n (%) | 41 (19.2) | 65 (30.4) | 67 (31.5) | 86 (40.2) | <.001 |
| Strictly lobar CMB, n (%) | 35 (16.4) | 43 (20.1) | 44 (20.7) | 48 (22.4) | .129 |
| BG PVS, severe, n (%) | 23 (10.8) | 51 (23.8) | 70 (32.9) | 99 (46.3) | <.001 |
| CS PVS, severe, n (%) | 43 (20.2) | 87 (40.7) | 99 (46.5) | 124 (57.9) | <.001 |
| Total SVD score, median (SD) | 1.2 (1.0) | 1.93 (1.2) | 2.1 (1.0) | 2.6 (0.9) | <.001 |
The cutoff values of mean baPWV for each quartile were 1,663, 1,985, and 2,355 cm/s. p Value was derived from analysis of variance.
Abbreviations: BG, basal ganglia; BMI, body mass index; CMB, cerebral microbleed; CS, centrum semiovale; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; hsCRP, high‐sensitive C‐reactive protein; LDL, low‐density lipoprotein; PVS, perivascular space, SBP, systolic blood pressure; SD, standard deviation; SVD, small vessel disease; WMH, white matter hyperintensities.
When the patients were dichotomized according to the EPVS burden, severe BG EPVS and CS EPVS both were proportionally associated with age, female sex, hypertension, previous stroke history, hematocrit, hsCRP, lower eGFR, and mean baPWV (Table 2). Higher baPWV quartile was associated with both BG EPVS (OR per baPWV quartile, Q2: OR 1.63, 95% CI 0.92–2.88; Q3: 2.11, 1.20–3.71; and Q4: 2.58, 1.45–4.60) and CS EPVS (OR per baPWV quartile, Q2: 1.71, 1.07–2.73; Q3: 1.72, 1.06–2.78; and Q4: 2.06, 1.24–3.42) after adjusting for age, female sex, hypertension, smoking, previous stroke history, hematocrit, platelet, triacylglycerol, hsCRP, and eGFR (Figure 2).
TABLE 2.
Comparison of clinical variables according to the location of EPVS
| Basal ganglia | Centrum semiovale | |||||
|---|---|---|---|---|---|---|
|
None–moderate n = 611 |
Severe n = 243 |
p |
None–moderate n = 501 |
Severe n = 353 |
p | |
| Age, years, mean (SD) | 65.5 (12.7) | 75.0 (8.6) | <.001 | 64.6 (13.1) | 73.3 (9.5) | <.001 |
| Sex, female, n (%) | 270 (44.2) | 142 (58.4) | <.001 | 226 (45.1) | 186 (52.7) | .029 |
| BMI, kg/m2, mean (SD) | 24.5 (8.3) | 23.8 (3.5) | .228 | 24.2 (3.3) | 24.4 (10.5) | .741 |
| Hypertension, n (%) | 351 (57.4) | 179 (73.7) | <.001 | 273 (54.5) | 257 (72.8) | <.001 |
| Diabetes mellitus, n (%) | 184 (30.1) | 74 (30.5) | .923 | 151 (30.1) | 107 (30.3) | .957 |
| Smoking, n (%) | 185 (30.3) | 28 (11.5) | <.001 | 149 (29.7) | 64 (18.1) | <.001 |
| Atrial fibrillation, n (%) | 117 (19.1) | 43 (17.7) | .623 | 98 (19.6) | 62 (17.6) | .461 |
| Previous stroke, n (%) | 94 (15.4) | 70 (28.8) | <.001 | 71 (14.2) | 93 (26.3) | <.001 |
| SBP, mm/Hg, mean (SD) | 149 (27) | 152 (30) | .181 | 149 (28) | 152 (27) | .127 |
| DBP, mm/Hg, mean (SD) | 86 (15) | 86 (15) | .480 | 86 (15) | 86 (14) | .889 |
| Hematocrit, %, mean (SD) | 41.0 (5.4) | 39.4 (6.3) | <.001 | 41.0 (5.4) | 39.9 (6.0) | .005 |
| White blood cell, 109/L, mean (SD) | 8.1 (3.0) | 8.1 (3.5) | .994 | 8.0 (2.7) | 8.2 (3.6) | .242 |
| Platelet, 109/L, mean (SD) | 231.4 (65.3) | 243.5 (89.8) | .029 | 234.6 (64.0) | 235.1 (84.9) | .929 |
| Fasting blood sugar, mmol/L, mean (SD) | 7.8 (3.3) | 7.6 (3.0) | .430 | 7.8 (3.2) | 7.7 (3.2) | .629 |
| LDL cholesterol, mmol/L, mean (SD) | 2.7 (0.8) | 2.7 (0.9) | .546 | 2.8 (0.8) | 2.7 (0.8) | .408 |
| Triacylglycerol, mmol/L, mean (SD) | 3.2 (2.1) | 2.8 (1.7) | .027 | 3.1 (2.0) | 3.0 (2.0) | .538 |
| hsCRP, mg/dl, mean (SD) | 5.1 (16.4) | 8.8 (25.2) | .011 | 4.2 (11.2) | 8.9 (26.8) | <.001 |
| eGFR, ml/min/1.73m2, mean (SD) | 83.4 (25.7) | 77.1 (26.7) | .001 | 84.2 (25.4) | 77.9 (26.6) | <.001 |
| Mean baPWV, m/s, mean (SD) | 2.0 (0.5) | 2.3 (0.6) | <.001 | 1.9 (0.5) | 2.2 (5.7) | <.001 |
Abbreviations: baPWV, brachial‐angle pulse wave velocity; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; hsCRP, high‐sensitive C‐reactive protein; LDL, low‐density lipoprotein, SBP, systolic blood pressure; SD, standard deviation.
FIGURE 2.
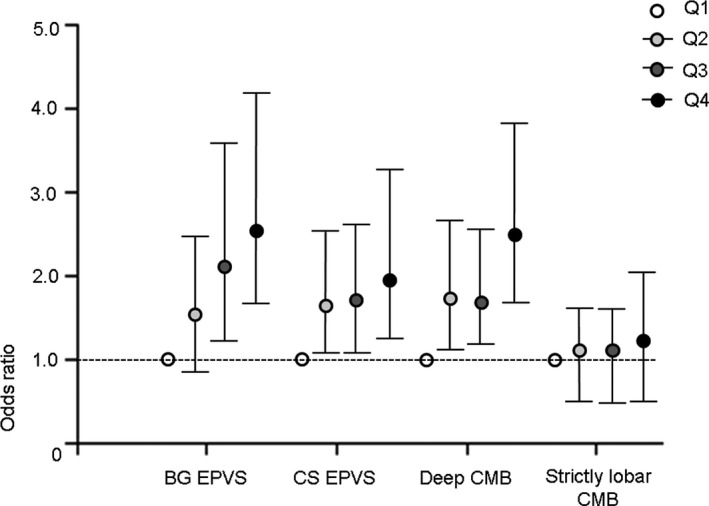
Relationship between arterial stiffness and cerebral small vessel disease markers at different locations. When the patients were grouped according to quartiles of brachial‐ankle pulse wave velocity, enlarged perivascular spaces (EPVS) at the basal ganglia and centrum semiovale and deep CMB showed similar patterns of increase. BG EPVS (OR per baPWV quartile, Q2: 1.63, 95% CI 0.92–2.88; Q3: 2.11, 1.20–3.71; and Q4: 2.58, 1.45–4.60). CS EPVS (OR per baPWV quartile, Q2: 1.71, 1.07–2.73; Q3: 1.72, 1.06–2.78; and Q4: 2.06, 1.24–3.42). Deep CMB (OR per baPWV quartile, Q2: 1.74, 1.10–2.73; Q3: 1.70, 1.08–2.69; and Q4: 2.52, 1.62–3.94). Strictly lobar CMB (OR per baPWV quartile, Q2: 1.13, 0.69–1.85; Q3: 1.14, 0.68–1.93; and Q4: 1.30, 0.73–2.30). The cutoff values of mean brachial‐ankle pulse wave velocity for each quartile were 1,663, 1,985, and 2,355 cm/s. BG, basal ganglia; CMB, cerebral microbleed; CS, centrum semiovale; PVS, perivascular space
Deep CMB was associated with age, hypertension, smoking, previous stroke history, higher blood pressure, lower eGFR, and higher mean baPWV. However, strictly lobar CMB showed an association only with older age (Table 3). Higher baPWV quartile was proportionally associated with deep CMB (OR per baPWV quartile, Q2 1.74, 1.10–2.73; Q3: 1.70, 1.08–2.69; and Q4: 2.52, 1.62–3.94) after adjusting for age, hypertension, smoking, previous stroke history, higher blood pressure, and eGFR (Figure 2).
TABLE 3.
Comparison of clinical variables according to the location of CMB
| Deep CMB | Strictly lobar CMB | |||||
|---|---|---|---|---|---|---|
|
CMB (−) N = 595 |
CMB (+) N = 259 |
p |
CMB (−) N = 684 |
CMB (+) N = 170 |
p | |
| Age, years, mean (SD) | 67.4 (12.7) | 70.2 (11.6) | .002 | 67.8 (12.6) | 70.1 (11.8) | .028 |
| Sex, female, n (%) | 276 (46.4) | 136 (52.5) | .100 | 328 (48.0) | 84 (49.4) | .733 |
| BMI, kg/m2, mean (SD) | 24.1 (3.2) | 24.7 (12.2) | .292 | 24.4 (7.9) | 24.0 (3.4) | .539 |
| Hypertension, n (%) | 355 (59.7) | 175 (67.6) | .029 | 419 (61.3) | 111 (65.3) | .332 |
| Diabetes mellitus, n (%) | 180 (30.3) | 78 (30.1) | .968 | 207 (30.3) | 51 (30.0) | .947 |
| Smoking, n (%) | 161 (27.1) | 52 (20.1) | .030 | 167 (24.4) | 46 (27.1) | .476 |
| Atrial fibrillation, n (%) | 107 (18.0) | 53 (20.5) | .393 | 126 (18.4) | 34 (20.0) | .637 |
| Previous stroke, n (%) | 96 (16.1) | 68 (26.3) | .001 | 127 (18.6) | 37 (21.8) | .344 |
| SBP, mm/Hg, mean (SD) | 148 (26) | 155 (31) | .001 | 150 (28) | 149 (27) | .819 |
| DBP, mm/Hg, mean (SD) | 85 (14) | 88 (16) | .008 | 86 (15) | 86 (16) | .886 |
| Hematocrit, %, mean (SD) | 40.6 (5.2) | 40.5 (6.8) | .747 | 40.7 (5.7) | 39.8 (5.7) | .060 |
| White blood cell, 109/L, mean (SD) | 8.1 (3.0) | 8.1(3.4) | .780 | 8.1 (3.2) | 8.1 (2.8) | .847 |
| Platelet, 109/L, mean (SD) | 233.7 (67.8) | 237.5 (84.6) | .478 | 235.1 (74.2) | 233.9 (69.8) | .849 |
| Fasting blood sugar, mmol/L, mean (SD) | 7.8 (3.2) | 7.7 (3.2) | .596 | 7.8 (3.3) | 7.7 (2.7) | .842 |
| LDL cholesterol, mmol/L, mean (SD) | 2.7 (0.8) | 2.7 (0.9) | .531 | 2.7 (0.8) | 2.7 (0.9) | .551 |
| Triacylglycerol, mmol/L, mean (SD) | 3.1 (2.1) | 3.0 (1.8) | .209 | 3.1 (2.0) | 3.2 (2.2) | .391 |
| hsCRP, mg/dl, mean (SD) | 5.6 (16.1) | 7.3 (25.4) | .236 | 6.2 (20.0) | 5.8 (16.5) | .820 |
| eGFR, ml/min/1.73 m2, mean (SD) | 83.7 (25.1) | 77.0 (27.6) | .001 | 81.2 (26.2) | 83.3 (25.5) | .339 |
| Mean baPWV, m/s, mean (SD) | 2.0 (0.5) | 2.2 (0.6) | <.001 | 2.0 (0.6) | 2.1 (0.5) | .168 |
Abbreviations: baPWV, brachial‐angle pulse wave velocity; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; hsCRP, high‐sensitive C‐reactive protein; LDL, low‐density lipoprotein; SBP, systolic blood pressure; SD, standard deviation.
4. DISCUSSION
This study provides clues about the pathophysiologic mechanisms underlying cerebral SVD phenotypes by illustrating the relationship between arterial stiffness and SVD burden at different brain locations. The burdens of EPVS at the BG and CS levels were consistently related to conventional vascular risk factors and independently associated with increased baPWV. Although the presence of deep CMB was also related to increased baPWV, the strictly lobar CMB did not show any relationship with baPWV, suggesting a different pathophysiologic mechanism beyond hypertensive arteriopathy.
Elastic components of the arterial wall decrease because of aging processes, hypertension, atherosclerosis, and vascular wall calcification, leading to increased pulse wave velocity (Lee & Oh, 2010). Increased arterial stiffness is responsible for a disproportionate increase in systolic arterial pressure and decrease in diastolic pressure, which results in circumferential stretching of the arterial wall, leading to intimal fibrosis, necrosis, remodeling, and atherosclerosis of the vessels (Mitchell et al., 2011). These changes lead to the transmission of higher pulse pressure to distal organs (Laurent et al., 2009). The brain is a distal organ with a large amount of blood flow, and it is particularly susceptible to excessive pulsatile stress because of low cerebral vascular resistance (Mitchell et al., 2011). Increased arterial pulsatility can drive glymphatic influx to the brain parenchyma and blood–brain barrier break down, which causes greater extravasation of fluid into the perivascular space (Kress et al., 2014; Mestre et al., 2017). Multiple studies have consistently shown the association between increased arterial PWV and increased burden of cerebral SVD such as WMH, CMBs, lacunes, and EPVS (Kim et al., 2016; Poels et al., 2012; Riba‐Llena et al., 2018; Zhai et al., 2018).
The pathophysiology of CS EPVS and lobar CMB has been considered to be related to amyloid angiopathy based on analysis of small numbers of postmortem brain specimen (Greenberg et al., 2009; Martinez‐Ramirez et al., 2018). However, more recent studies have reported consistent associations of both BG and CS EPVS with hypertensive arteriopathy, suggesting that overlapping pathophysiology of hypertensive vasculopathy and amyloid angiopathy contribute to CS EPVS (Shams et al., 2017; Yakushiji et al., 2014). Our study results also show that BG EPVS, CS EPVS, and deep CMB are closely related to increased baPWV, sharing arterial stiffness as a main mechanism of injury. However, strictly lobar CMB seemed to be independent of hypertensive vasculopathy, which accords with previous findings.
This study has several limitations. First, some patients who did not undergo brain MRI or baPWV measurement were excluded from the study, which may have caused selection bias. Second, the study was based in a single center and included an ethnically homogeneous population. A causal relationship between baPWV and EPVS could not be ascertained because of the cross‐sectional design. Finally, increased baPWV might be a consequence of decreased physical activity due to cerebral SVD burden.
In conclusion, this study shows that increased baPWV, which is regarded as a marker of arterial stiffness, has an independent association with the presence of EPVS and deep CMB at both the BG and CS, but has a neutral relationship with strictly lobar CMB. This consistent relationship between EPVS at both levels and arterial stiffness suggests a common pathological mechanism related to hypertensive SVD damage.
CONFLICT OF INTEREST
Jae‐Han Bae, MD, reports no disclosures. Jeong‐Min Kim, MD, PhD, is funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF), the Ministry of Education in Republic of Korea (NRF‐2019R1F1A1059455). Kwang‐Yeol Park, MD, PhD, is funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF), the Ministry of Education in Republic of Korea (NRF‐2017R1D1A1B03029909). Su‐Hyun Han, MD, reports no disclosures.
AUTHOR'S CONTRIBUTIONS
Kwang‐Yeol Park and Jeong‐Min Kim participated in the conception and design of the study. Jae‐Han Bae and Su‐Hyun Han collected the data. Kwang‐Yeol Park and Jeong‐Min Kim analyzed the data. Jeong‐Min Kim and Jae‐Han Bae wrote the manuscript.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.1935.
ACKNOWLEDGMENT
The authors thank the patients and clinicians at Chung‐Ang University Hospital, who contributed to this study. This study was supported by the Basic Science Research Program through the National Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF‐2017R1D1A1B03029909 and NRF‐2019R1F1A1059455). The funding has no role in design, collection, analysis, or interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Bae J‐H, Kim J‐M, Park K‐Y, Han S‐H. Association between arterial stiffness and the presence of cerebral small vessel disease markers. Brain Behav.2021;11:e01935 10.1002/brb3.1935
Contributor Information
Jeong‐Min Kim, Email: bellokim1@gmail.com.
Kwang‐Yeol Park, Email: kwangyeol.park@gmail.com.
DATA AVAILABILITY STATEMENT
Anonymized data can be shared at the request of qualified investigators for purposes of replicating procedures and results.
REFERENCES
- Doubal, F. N. , MacLullich, A. M. , Ferguson, K. J. , Dennis, M. S. , & Wardlaw, J. M. (2010). Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke, 41, 450–454. 10.1161/STROKEAHA.109.564914 [DOI] [PubMed] [Google Scholar]
- Greenberg, S. M. , Vernooij, M. W. , Cordonnier, C. , Viswanathan, A. , Al‐Shahi Salman, R. , Warach, S. , Launer, L. J. , Van Buchem, M. A. , & Breteler, M. M. B. (2009). Cerebral microbleeds: A guide to detection and interpretation. The Lancet Neurology, 8, 165–174. 10.1016/S1474-4422(09)70013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire, S. M. , Chaudhary, U. J. , Brown, M. M. , Yousry, T. A. , Kallis, C. , Jager, H. R. , & Werring, D. J. (2009). The microbleed anatomical rating scale (MARS): Reliability of a tool to map brain microbleeds. Neurology, 73, 1759–1766. 10.1212/WNL.0b013e3181c34a7d [DOI] [PubMed] [Google Scholar]
- Kim, Y. B. , Park, K. Y. , Chung, P. W. , Kim, J. M. , Moon, H. S. , & Youn, Y. C. (2016). Brachial‐ankle pulse wave velocity is associated with both acute and chronic cerebral small vessel disease. Atherosclerosis, 245, 54–59. 10.1016/j.atherosclerosis.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Klarenbeek, P. , van Oostenbrugge, R. J. , Rouhl, R. P. , Knottnerus, I. L. , & Staals, J. (2013). Ambulatory blood pressure in patients with lacunar stroke: Association with total MRI burden of cerebral small vessel disease. Stroke, 44, 2995–2999. 10.1161/STROKEAHA.113.002545 [DOI] [PubMed] [Google Scholar]
- Kress, B. T. , Iliff, J. J. , Xia, M. , Wang, M. , Wei, H. S. , Zeppenfeld, D. , Xie, L. , Kang, H. , Xu, Q. , Liew, J. A. , Plog, B. A. , Ding, F. , Deane, R. , & Nedergaard, M. (2014). Impairment of paravascular clearance pathways in the aging brain. Annals of Neurology, 76, 845–861. 10.1002/ana.24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent, S. , Briet, M. , & Boutouyrie, P. (2009). Large and small artery cross‐talk and recent morbidity‐mortality trials in hypertension. Hypertension, 54, 388–392. 10.1161/HYPERTENSIONAHA.109.133116 [DOI] [PubMed] [Google Scholar]
- Lee, H.‐Y. , & Oh, B.‐H. (2010). Aging and arterial stiffness. Circulation Journal, 74, 2257–2262. 10.1253/circj.CJ-10-0910 [DOI] [PubMed] [Google Scholar]
- Martinez‐Ramirez, S. , van Rooden, S. , Charidimou, A. , van Opstal, A. M. Wermer, M. , Gurol, M. E. , Terwindt, G. , van der Grond, J. , Greenberg, S. M. , van Buchem, M. , & Viswanathan, A. (2018). Perivascular Spaces Volume In Sporadic And Hereditary (Dutch‐Type) cerebral amyloid angiopathy. Stroke, 49, 1913–1919. 10.1161/STROKEAHA.118.021137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre, H. , Kostrikov, S. , Mehta, R. I. , & Nedergaard, M. (2017). Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clinical Science (London), 131, 2257–2274. 10.1042/CS20160381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, G. F. , van Buchem, M. A. , Sigurdsson, S. , Gotal, J. D. , Jonsdottir, M. K. , Kjartansson, Ó. , Garcia, M. , Aspelund, T. , Harris, T. B. , Gudnason, V. , & Launer, L. J. (2011). Arterial stiffness, pressure and flow pulsatility and brain structure and function: The age, gene/environment susceptibility‐Reykjavik Study. Brain, 134, 3398–3407. 10.1093/brain/awr253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantankar, T. F. , Mitra, D. , Varma, A. , Snowden, J. , Neary, D. , & Jackson, A. (2005). Dilatation of the Virchow‐Robin Space Is a sensitive indicator of cerebral microvascular disease. American Journal of Neuroradiology, 26, 1512–1520. [PMC free article] [PubMed] [Google Scholar]
- Pantoni, L. (2010). Cerebral small vessel disease from pathogenesis and clinical characteristics to therapeutic challenges. The Lancet Neurology, 9, 689–701. 10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- Poels, M. M. F. , Zaccai, K. , Verwoert, G. C. , Vernooij, M. W. , Hofman, A. , van der Lugt, A. , Witteman, J. C. M. , Breteler, M. M. B. , Mattace‐Raso, F. U. S. , & Ikram, M. A. (2012). Arterial stiffness and cerebral small vessel disease: The Rotterdam Scan Study. Stroke, 43, 2637–2642. 10.1161/STROKEAHA.111.642264 [DOI] [PubMed] [Google Scholar]
- Pollock, H. , Hutchings, M. , Weller, R. O. , & Zhang, E. T. (1997). Perivascular spaces in the basal ganglia of the human brain: Their relationship to lacunes. Journal of Anatomy, 191, 337–346. 10.1046/j.1469-7580.1997.19130337.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, G. M. , Chappell, F. M. , Morris, Z. , & Wardlaw, J. M. (2015). Cerebral perivascular spaces visible on magnetic resonance imaging: Development of a qualitative rating scale and its observer reliability. Cerebrovascular Disease, 39, 224–231. 10.1159/000375153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, G. M. , Doubal, F. N. , Jackson, C. A. , Chappell, F. M. , Sudlow, C. L. , Dennis, M. S. , & Wardlaw, J. M. (2015). Enlarged perivascular spaces and cerebral small vessel disease. International Journal of Stroke, 10, 376–381. 10.1111/ijs.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba‐Llena, I. , Jiménez‐Balado, J. , Castañé, X. , Girona, A. , López‐Rueda, A. , Mundet, X. , Jarca, C. I. , Álvarez‐Sabin, J. , Montaner, J. , & Delgado, P. (2018). Arterial stiffness is associated with basal ganglia enlarged perivascular spaces and cerebral small vessel disease load. Stroke, 49, 1279–1281. 10.1161/STROKEAHA.118.020163 [DOI] [PubMed] [Google Scholar]
- Shams, S. , Martola, J. , Charidimou, A. , Larvie, M. , Granberg, T. , Shams, M. , Kristoffersen‐Wiberg, M. , & Wahlund, L.‐O. (2017). Topography and determinants of magnetic resonance imaging (MRI)‐visible perivascular spaces in a large memory clinic cohort. Journal of the American Heart Association, 6 10.1161/JAHA.117.006279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staals, J. , Makin, S. D. , Doubal, F. N. , Dennis, M. S. , & Wardlaw, J. M. (2014). Stroke subtype, vascular risk factors and total MRI brain small‐vessel disease burden. Neurology, 83, 1228–1234. 10.1212/WNL.0000000000000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, H. , Munakata, M. , Kawano, Y. , Ohishi, M. , Shoji, T. , Sugawara, J. , Tomiyama, H. , Yamashina, A. , Yasuda, H. , Sawayama, T. , & Ozawa, T. (2009). Comparison between carotid‐femoral and brachial‐ankle pulse wave velocity as measures of arterial stiffness. Journal of Hypertension, 27, 2022–2027. 10.1097/HJH.0b013e32832e94e7 [DOI] [PubMed] [Google Scholar]
- Wardlaw, J. M. , Smith, E. E. , Biessels, G. J. , Cordonnier, C. , Fazekas, F. , Frayne, R. , Lindley, R. I. , O'Brien, J. T. , Barkhof, F. , Benavente, O. R. , Black, S. E. , Brayne, C. , Breteler, M. , Chabriat, H. , DeCarli, C. , de Leeuw, F.‐E. , Doubal, F. , Duering, M. , Fox, N. C. , … Dichgans, M. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology, 12, 822–838. 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller, R. O. , Djuanda, E. , Yow, H. Y. , & Carare, R. O. (2009). Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathologica, 117, 1–14. 10.1007/s00401-008-0457-0 [DOI] [PubMed] [Google Scholar]
- Xiao, L. , Lan, W. , Sun, W. , Dai, Q. , Xiong, Y. , Li, L. , Zhou, Y. , Zheng, P. , Fan, W. , Ma, N. , Guo, Z. , Chen, X. , Xie, X. , Xu, L. , Zhu, W. , Xu, G. , & Liu, X. (2015). chronic kidney disease in patients with lacunar stroke: association with enlarged perivascular spaces and total magnetic resonance imaging burden of cerebral small vessel disease. Stroke, 46, 2081–2086. 10.1161/STROKEAHA.114.008155 [DOI] [PubMed] [Google Scholar]
- Yakushiji, Y. , Charidimou, A. , Hara, M. , Noguchi, T. , Nishihara, M. , Eriguchi, M. , Nanri, Y. , Nishiyama, M. , Werring, D. J. , & Hara, H. (2014). Topography and associations of perivascular spaces in healthy adults: The Kashima scan study. Neurology, 2, 2116–2123. 10.1212/WNL.0000000000001054 [DOI] [PubMed] [Google Scholar]
- Zhai, F.‐F. , Ye, Y.‐C. , Chen, S.‐Y. , Ding, F.‐M. , Han, F. , Yang, X.‐L. , Wang, Q. , Zhou, L.‐X. , Ni, J. , Yao, M. , Li, M.‐L. , Jin, Z.‐Y. , Cui, L.‐Y. , Zhang, S.‐Y. , & Zhu, Y.‐C. (2018). Arterial stiffness and cerebral small vessel disease. Frontiers in Neurology, 9, 723 10.3389/fneur.2018.00723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. C. , Tzourio, C. , Soumare, A. , Mazoyer, B. , Dufouil, C. , & Chabriat, H. (2010). Severity of dilated Virchow‐Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: A population‐based study. Stroke, 41, 2483–2490. 10.1161/STROKEAHA.110.591586 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data can be shared at the request of qualified investigators for purposes of replicating procedures and results.


