Abstract
Aims
In patients without obstructive coronary artery disease (CAD), we examined the prognostic value of risk factors and atherosclerotic extent.
Methods and results
Patients from the long-term CONFIRM registry without prior CAD and without obstructive (≥50%) stenosis were included. Within the groups of normal coronary computed tomography angiography (CCTA) (N = 1849) and non-obstructive CAD (N = 1698), the prognostic value of traditional clinical risk factors and atherosclerotic extent (segment involvement score, SIS) was assessed with Cox models. Major adverse cardiac events (MACE) were defined as all-cause mortality, non-fatal myocardial infarction, or late revascularization. In total, 3547 patients were included (age 57.9 ± 12.1 years, 57.8% male), experiencing 460 MACE during 5.4 years of follow-up. Age, body mass index, hypertension, and diabetes were the clinical variables associated with increased MACE risk, but the magnitude of risk was higher for CCTA defined atherosclerotic extent; adjusted hazard ratio (HR) for SIS >5 was 3.4 (95% confidence interval [CI] 2.3–4.9) while HR for diabetes and hypertension were 1.7 (95% CI 1.3–2.2) and 1.4 (95% CI 1.1–1.7), respectively. Exclusion of revascularization as endpoint did not modify the results. In normal CCTA, presence of ≥1 traditional risk factors did not worsen prognosis (log-rank P = 0.248), while it did in non-obstructive CAD (log-rank P = 0.025). Adjusted for SIS, hypertension and diabetes predicted MACE risk in non-obstructive CAD, while diabetes did not increase risk in absence of CAD (P-interaction = 0.004).
Conclusion
Among patients without obstructive CAD, the extent of CAD provides more prognostic information for MACE than traditional cardiovascular risk factors. An interaction was observed between risk factors and CAD burden, suggesting synergistic effects of both.
Keywords: coronary computed tomography angiography, risk stratification, atherosclerosis, imaging, preventive cardiology
Introduction
Coronary computed tomography angiography (CCTA) is increasingly used to diagnose coronary artery disease (CAD) in patients with low to intermediate cardiovascular risk profile. When obstructive CAD (≥ 50% stenosis) is identified, further non-invasive testing can be used to assess the haemodynamic significance of the stenosis, eventually followed by invasive coronary angiography and percutaneous coronary intervention as recommended in the recent CAD-RADS (Reporting And Data System) consensus document.1 If CCTA does not show obstructive CAD (i.e. no CAD or non-obstructive CAD), optimal medical care is uncertain. The majority of patients who undergo CCTA for suspected CAD belong to this subgroup. As shown in a large registry, approximately two-thirds of the patients do not have obstructive CAD.2 These patients generally have multiple cardiovascular risk factors and are at risk for cardiovascular events. Recently, a large prospective trial evaluating patients with suspected CAD using CCTA showed that the majority of cardiovascular events occurred among patients with non-obstructive CAD.3
Optimal medical treatment strategy of patients without obstructive CAD is unclear. Primary cardiovascular risk prevention guidelines indicate that treatment intensity should be based on clinical risk profile. On the other hand, multiple studies showed that CCTA findings (especially the number of vessels with obstructive CAD) have strong prognostic value.4–7 Also, patients can have multiple cardiovascular risk factors combined with a normal CCTA or absence of risk factors combined with extensive CAD. Accurate estimation of risk for future cardiovascular events is important, since the higher the risk the more intense the medical therapy should be.8 The aim of the current study was to assess which factors (clinical or CCTA findings) are strongest correlated with cardiovascular events in patients without obstructive CAD and should, therefore, determine the intensity of medial therapy.
Methods
Patients
Patients were derived from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry, an open-label, prospective, international, multicenter observational cohort, collecting data from consecutive adults ≥18 years who underwent ≥64-detector row CCTA for suspected CAD; the methodological details of this registry have been described previously.2 The current analysis includes patients from the long-term follow-up CONFIRM cohort, which comprises patients who underwent CCTA at 17 centres in nine countries between 2002 and 2009, with prospective follow-up over 5 years. Of 6620 patients without known CAD [history of myocardial infarction (MI), coronary artery bypass grafting, or coronary revascularization] and obstructive CAD, 2849 patients without information for all clinical endpoints and 224 patients with incomplete coronary stenosis data were excluded, leaving 3547 patients in the current analysis. Institutional review board approval was obtained at each site and patients provided informed consent.
Clinical data
Standardized demographical and clinical patient information were prospectively collected at each study site. Definitions of risk factors for CAD have been reported in earlier reports from the CONFIRM registry.9,10 Diabetes was defined as a fasting glucose of ≥126 mg/dL or the use of insulin and/or oral hypoglycaemic agents. Hypertension was defined as a documented history of high blood pressure or treatment with anti-hypertensive medication. Hypercholesterolaemia was defined as untreated high serum cholesterol or treatment with lipid-lowering medication. Smoking was defined as having smoked in the last 90 days or current smoking. Family history of CAD was defined as a first-degree family member diagnosed with CAD <65 years for women or <55 years for men. Chest pain symptoms were categorized as non-anginal, atypical, or typical chest pain.
CCTA acquisition and interpretation
CCTA acquisition and imaging protocols at each site were in adherence with the Society of Cardiovascular Computed Tomography guidelines.11 Level III-trained experts interpreted the computed tomography images using a 16-segment coronary artery tree model. In each coronary artery segment, the presence of plaque was reported with corresponding stenosis severity.9,10 The stenosis severity of coronary artery plaque was categorized as normal (0% stenosis), non-obstructive (1–49% stenosis), or obstructive CAD (≥50% stenosis) by visual assessment. Based on these data, the segment involvement score (SIS) was calculated as the total number of coronary artery segments exhibiting plaque, irrespective of the degree of stenosis (ranging from 0 to 16).4 Since patients with obstructive CAD were excluded from the current study, the SIS represents the number of non-obstructive coronary plaques per patient.
In addition, the Leiden CCTA score, a comprehensive evaluation of CCTA incorporating plaque presence, extent, severity, and composition, was calculated for each patient. Score creation and calculation have been previously described.12
Outcomes
Primary combined endpoint consisted of major adverse cardiac events (MACE) defined as all-cause mortality, non-fatal MI, and late revascularization (>90 days after CCTA). Late revascularization was included as endpoint since this can be the result of CAD progression causing progressive/new-onset angina or unstable angina among non-obstructive CAD. A follow-up methodology has been previously described in detail.2 The Social Security Index was reviewed for assessment of mortality within the USA or determined through mail or telephone contact with the patients, family, or physician or review of medical records. Other events were collected through a combination of direct interviewing of patients using scripted interview and examination of the patient’s medical files by trained physicians or nurses. Non-fatal MI and late revascularization were further ascertained by reviewing the medical charts.2
Statistical analysis
Continuous variables were presented as mean ± standard deviation; categorical variables as counts with percentages. For the comparison of continuous variables, the Student’s t-test was used; categorical variables were compared with the χ2 test. Cox-proportional hazard analyses were performed to assess the prognostic value of clinical and CCTA variables. SIS categories were defined as 0, 1, 2–3, 4–5, and >5. The highest risk group was defined as SIS >5 based on previous studies demonstrating strong prognostic value of this category.4,13 Hazard ratios (HRs) with their 95% confidence intervals (CIs) were derived. Univariable associates with a P-value <0.10 were entered into the multivariable analysis to determine their independent association with outcome. Furthermore, specific interactions between clinical risk factors and CAD burden were explored. Event-free survival was estimated using the Kaplan–Meier method and compared with the log-rank test. The C-statistic was calculated to assess the incremental discriminatory ability of SIS using MedCalc Statistical Software (version 18, Ostend, Belgium) and compared according to DeLong et al.14 Other analyses were performed using SPSS (version 24, Armonk, NY, USA). A two-sided P-value <0.05 was considered statistically significant.
Results
Patients
A total of 3547 patients were included, with a mean age of 57.9 ± 12.1 years, and 57.8% were male. In total, 460 first events (219 death, 161 non-fatal MI, and 80 late revascularization) occurred during a median follow-up duration of 5.4 years (25–75% interquartile range 5.1–6.0 years). Patients with non-obstructive CAD (N = 1698) were significantly older than patients without CAD (N = 1849; 61.5 vs. 54.6 years, P < 0.001), had more often hypertension and diabetes and displayed higher cardiovascular medication use, as shown in Table 1. Of note, the patients without follow-up information were on average 5 years younger, more frequently female, and had similar presence of diabetes and hypertension as the current study population.
Table 1.
Patient characteristics
| Total (N = 3547) | Normal CCTA (N = 1849) | Non-obstructive CAD (N = 1698) | P-valuea | |
|---|---|---|---|---|
| Age (years) | 57.9 ± 12.1 | 54.6 ± 12.4 | 61.5 ± 10.6 | <0.001 |
| Male gender (%) | 2051 (57.8) | 969 (52.4) | 1082 (63.8) | <0.001 |
| BMI (kg/m2) | 27.2 ± 5.0 | 27.0 ± 4.9 | 27.4 ± 5.1 | 0.026 |
| Chest pain symptoms | 0.004 | |||
| No chest pain (%) | 1344 (43.4) | 678 (41.7) | 666 (45.2) | |
| Non-anginal (%) | 385 (12.4) | 183 (11.3) | 202 (13.7) | |
| Atypical (%) | 1066 (34.4) | 586 (36.1) | 480 (32.6) | |
| Typical (%) | 301 (9.7) | 177 (10.9) | 124 (8.4) | |
| Dyspnoea without chest pain | 155 (12.7) | 75 (11.8) | 80 (13.6) | 0.363 |
| Cardiovascular risk factors | ||||
| Diabetes (%) | 456 (12.9) | 227 (12.3) | 229 (13.5) | 0.293 |
| Hypertension (%) | 1758 (49.7) | 803 (43.9) | 949 (56.1) | <0.001 |
| Hypercholesterolaemia (%) | 1731 (49.0) | 769 (41.7) | 962 (56.8) | <0.001 |
| Family history for CAD (%) | 1029 (29.3) | 554 (30.4) | 475 (28.1) | 0.137 |
| Current smoker (%) | 663 (18.8) | 325 (17.8) | 338 (20.0) | 0.093 |
| Medication use | ||||
| Aspirin (%) | 642 (23.4) | 287 (19.8) | 355 (27.5) | <0.001 |
| Beta blocker (%) | 709 (25.9) | 324 (22.3) | 385 (29.8) | <0.001 |
| ACE-I (%) | 526 (19.2) | 208 (14.3) | 318 (24.7) | <0.001 |
| Statin (%) | 742 (26.9) | 321 (22.0 | 421 (32.3) | <0.001 |
| CCTA findings | ||||
| Segment involvement score | 2.68 ± 2.07 | |||
| 1 | 633 (37.3) | |||
| 2–3 (%) | 649 (38.2) | |||
| 4–5 (%) | 237 (14.0) | |||
| >5 (%) | 179 (10.5) | |||
| Diseased segments | ||||
| LM (%) | 384 (25.0) | |||
| Proximal LAD (%) | 1125 (69.9) | |||
| Proximal LCX (%) | 420 (27.2) | |||
| Proximal RCA (%) | 517 (32.9) | |||
| Stenosis in any proximal segment | 1443 (85.0) |
Comparison between patients with normal CCTA and non-obstructive CAD. CAD, coronary artery disease; CCTA, coronary computed tomography angiography; LAD, left anterior descending artery; LCX, left circumflex; LM, left main; RCA, right coronary artery.
Prognostic value of clinical risk profile vs. coronary atherosclerosis
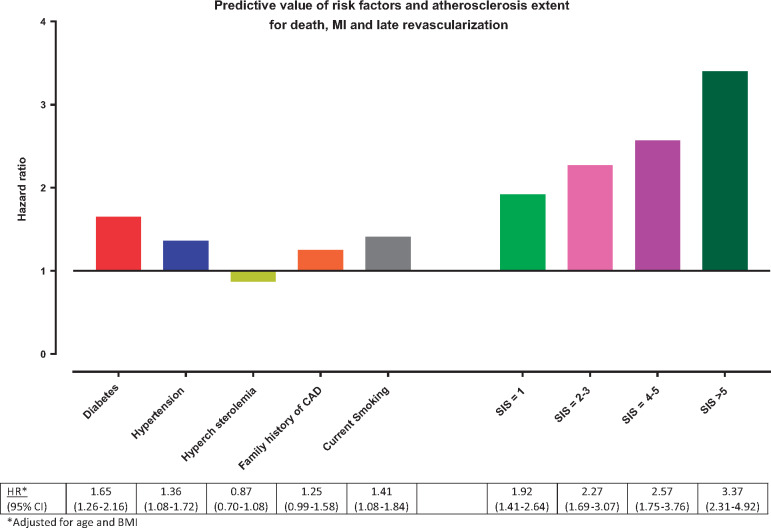
Of the clinical variables, age (HR 1.04, 95% CI 1.03–1.05; P < 0.001), body mass index (BMI) (HR 1.03, 95% CI 1.01–1.05; P = 0.002), diabetes (HR 1.90, 95% CI 1.51–2.38; P < 0.001), and hypertension (HR 1.60 95% CI 1.33–1.93; P < 0.001), were significantly associated with MACE (Table 2). A gradual increase in risk was observed for increasing CAD burden: SIS 1 (HR 1.77, 95% CI 1.35–2.30; P < 0.001) and SIS >5 (HR 3.70, 95% CI 2.65–5.01; P < 0.001). In multivariable analysis of clinical variables and plaque burden, diabetes (HR 1.63, 95% CI 1.23–2.14; P < 0.001) and hypertension (HR 1.27 95% CI 1.01–1.60; P = 0.043) remained predictive, but higher magnitudes of risk for MACE were observed for the SIS subgroups (SIS = 1, HR 1.88, 95% CI 1.37–2.58; P < 0.001 and SIS > 5 HR 3.25, 95% CI 2.22–4.75; P < 0.001), Figure 1. Furthermore, compared with absence of plaque, the adjusted HR for plaque in either left main or proximal left anterior descending artery was numerically higher than for plaque in any coronary segment: HR 2.53 (95% CI 2.06–3.11) and HR 2.17 (95% CI 1.64–2.85).
Table 2.
Clinical profile and CCTA findings associated with major cardiovascular events
| Univariable HR (95% CI) | P-value | Multivariable HR (95% CI)a | P-value | |
|---|---|---|---|---|
| Age (years) | 1.04 (1.03–1.05) | <0.001 | 1.03 (1.01–1.04) | <0.001 |
| Male gender | 0.94 (0.78–1.13) | 0.938 | ||
| BMI (kg/m2) | 1.03 (1.01–1.05) | 0.003 | 1.01 (0.98–1.03) | 0.506 |
| Chest pain symptoms | ||||
| No chest pain | Reference | |||
| Non-anginal | 1.03 (0.74–1.44) | 0.864 | ||
| Atypical | 0.84 (0.65–1.08) | 0.170 | ||
| Typical | 1.21 (0.85–1.72) | 0.284 | ||
| Cardiovascular risk factors | ||||
| Diabetes | 1.90 (1.51–2.38) | <0.001 | 1.59 (1.16–2.18) | 0.004 |
| Hypertension | 1.60 (1.33–1.93) | <0.001 | 1.33 (1.02–1.73) | 0.038 |
| Hypercholesterolaemia | 0.97 (0.81–1.17) | 0.769 | ||
| Family history of CAD | 1.01 (0.82–1.23) | 0.945 | ||
| Current smoker | 1.12 (0.89–1.40) | 0.338 | ||
| CCTA findings | ||||
| Segment involvement score | 1.18 (1.14–1.22) | <0.001 | ||
| 0 | Reference | |||
| 1 | 1.77 (1.35–2.30) | <0.001 | 1.89 (1.32–2.71) | 0.001 |
| 2–3 | 2.53 (1.99–3.21) | <0.001 | 2.48 (1.76–3.47) | <0.001 |
| 4–5 | 3.09 (2.26–4.22) | <0.001 | 2.54 (1.64–3.95) | <0.001 |
| >5 | 3.68 (2.66–5.09) | <0.001 | 3.08 (1.98–4.81) | <0.001 |
| Diseased segments | ||||
| Left main | 1.80 (1.41–2.29) | <0.001 | ||
| Proximal LAD | 2.06 (1.71–2.49) | <0.001 | ||
| Proximal RCA | 1.98 (1.60–2.50) | <0.001 | ||
| Proximal LCX | 2.40 (1.92–2.98) | <0.001 |
Adjusted for statin and/or aspirin use and early revascularization. CAD, coronary artery disease; CCTA, coronary computed tomography angiography; LAD, left anterior descending artery; LCX, left circumflex; LM, left main; RCA, right coronary artery.
Figure 1.
Age and body mass index adjusted hazard ratios are provided for cardiovascular risk factors and the segment involvement score subgroups showing that the number of coronary segments with plaque provide the strongest prognostic information. BMI, body mass index; MI, myocardial infarction; SIS, segment involvement score.
Restricting to asymptomatic individuals showed highest risk for SIS >5 and only hypertension but not diabetes was associated with MACE (Table A1). When excluding late revascularization as an endpoint, the results remained essentially unchanged: HR for diabetes 1.37 (95% CI 0.99–1.88; P = 0.055), 1.36 (95% CI 1.05–1.77 P = 0.020) for hypertension, 1.54 (95% CI 1.08–2.20; P = 0.017) for SIS = 1 and 2.56 (95% CI 1.66–3.93 P < 0.001) for SIS >5 in multivariable analysis. The addition of SIS to a clinical model of age, sex, BMI, diabetes, hypertension, hypercholesterolaemia, smoking, and positive familial history for CAD increased the C-statistic significantly (0.70 vs. 0.67, P = 0.001).
When atherosclerotic burden was defined according to the Leiden CCTA score, a similar stepwise increase in risk was observed with increasing score. Patients with a Leiden CCTA score >12 demonstrated an adjusted HR of 2.64 (95% CI 1.79–3.90), which was comparable to those with SIS >5 (Table A2).
MACE risk according to absence or presence of CAD
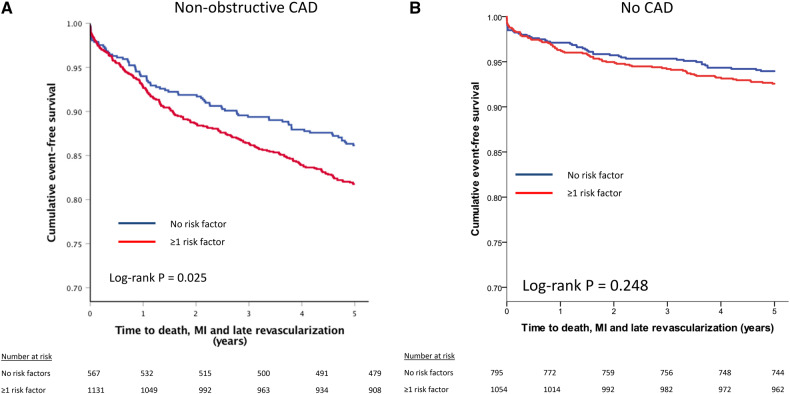
Patients without CAD had a 5-year incidence of MACE of ∼5%; and the absence vs. presence of one or more cardiovascular risk factors did not significantly change this event rate (P = 0.248, Figure 2). Among patients with non-obstructive CAD, 5-year MACE incidence was significantly higher if patients had ≥1 traditional risk factor (P = 0.025, Figure 2). In non-obstructive CAD, cox-regression analysis showed that hypertension and diabetes were among the risk factors significantly associated with increased MACE risk; with a specific higher magnitude of risk for diabetes in the presence of non-obstructive CAD vs. absence of CAD (P-interaction = 0.004, Table 3). Results for CAD defined by the Leiden score are provided in Table A3.
Figure 2.
(A) Five-year cumulative MACE-free Kaplan–Meier survival curves among patients without coronary artery disease showing no difference for absence vs. presence of risk factors. (B) Among patients with non-obstructive CAD, MACE-free survival is worse in the presence of cardiovascular risk factors. MACE, major adverse cardiac events.
Table 3.
Interactions between clinical variables and presence or absence of CAD
| No CAD |
Non-obstructive CAD |
P-interaction | |||
|---|---|---|---|---|---|
| Univariable HR (95% CI) | P-value | Univariable HR (95% CI) | P-value | ||
| Age (years) | 1.02 (1.01–1.04) | 0.003 | 1.03 (1.02–1.05) | <0.001 | 0.182 |
| Male gender | 0.88 (0.64–1.21) | 0.425 | 0.82 (0.65–1.03) | 0.085 | 0.715 |
| BMI (kg/m2) | 1.04 (1.00–1.08) | 0.061 | 1.02 (1.00–1.04) | 0.052 | 0.477 |
| Chest pain symptoms | 0.110 | ||||
| No chest pain | Reference | Reference | |||
| Non-anginal | 1.39 (0.78–2.47) | 0.270 | 0.87 (0.58–1.31) | 0.511 | |
| Atypical | 0.77 (0.48–1.25) | 0.289 | 0.93 (0.69–1.26) | 0.636 | |
| Typical | 0.80 (0.39–1.65) | 0.551 | 1.67 (1.12–2.49) | 0.012 | |
| Cardiovascular risk factors | |||||
| Diabetes | 1.07 (0.66–1.73) | 0.783 | 2.35 (1.82–3.04) | <0.001 | 0.004 |
| Hypertension | 1.61 (1.16–2.22) | 0.004 | 1.38 (1.10–1.75) | 0.006 | 0.486 |
| Hypercholesterolaemia | 0.83 (0.60–1.16) | 0.277 | 0.87 (0.69–1.08) | 0.203 | 0.807 |
| Family history of CAD | 0.92 (0.64–1.32) | 0.655 | 1.10 (0.86–1.40) | 0.467 | 0.447 |
| Current smoker | 1.02 (0.67–1.54) | 0.939 | 1.12 (0.85–1.46) | 0.428 | 0.763 |
CAD, coronary artery disease; CCTA, coronary computed tomography angiography; LAD, left anterior descending artery; LCX, left circumflex; LM, left main; RCA, right coronary artery.
Discussion
The main findings are that patients without obstructive CAD, the extent of CAD on CCTA provides more prognostic information compared with traditional cardiovascular risk factors. Specifically, in absence of CAD, the presence or absence of risk factors did not influence MACE-free survival, but in patients with non-obstructive CAD, hypertension and diabetes provided additional prognostic value.
CCTA for patients with suspected CAD
CCTA is increasingly being used in symptomatic and asymptomatic patients with suspected CAD. From a diagnostic point of view, the identification of obstructive CAD is the target since obstructive lesions may cause myocardial ischaemia and angina which may be alleviated with coronary revascularization. Also, several studies have demonstrated that these patients are at highest cardiovascular event risk and, therefore, require high-intensity medical therapy for prognostic reasons.4,5,15 However, the majority of patients who undergo CCTA do not show obstructive CAD. An earlier report of the CONFIRM registry showed that 26.5% of the 27 125 patients in total had obstructive CAD. Non-obstructive CAD is generally not related to myocardial ischaemia and patients may not need further ischaemia testing or extensive follow-up.1 From a prognostic point of view, it is known that patients with non-obstructive CAD have a more benign prognosis than obstructive CAD, but a worse prognosis than patients without CAD.5 Recently, Hoffmann et al.3 confirmed that patients with non-obstructive CAD are at risk, since the majority of events cardiovascular events occur among these patients. In addition, the recent ICONIC (Incident COroNary Syndromes Identified by Computed Tomography) demonstrated that ∼75% of the lesions that became future acute coronary syndrome culprit lesions were <50% in stenosis at baseline CCTA.16
Risk stratification of patients with no vs. non-obstructive CAD
Absence of CAD on CCTA is associated with excellent long-term outcomes.5–7,17,18 However, patients undergoing CCTA usually have one or more cardiovascular risk factors and the prognostic implications combined with the CAD burden are uncertain. CCTA is a very sensitive technique to detect early atherosclerosis. Compared with histopathology of 322 coronary plaques from 25 human heart specimens, Leschka et al.19 demonstrated that CCTA identified 100% of more advanced plaques (Stary IV–VIII) and only minimal atherosclerotic plaques (Grades I and II) could not reliable be identified. If risk factors are present, but CAD on CCTA absent, it could be hypothesized that these patients represent a subgroup less susceptible to the pro-atherosclerotic and thrombogenic effects of risk factors on the coronary arteries. Indeed, using data from the multi-ethnic study of atherosclerosis, Budoff et al.20 demonstrated that asymptomatic individuals without coronary calcium consistently experienced 10-year MACE rates below the recommended threshold for statin recommendation, irrespective of sex, ethnicity, and age. We demonstrated that among patients without CAD, risk factors did not substantially increase 5-year MACE rates. Reducing medication usage in these patients is likely to improve patient well-being and can reduce medication side effects.
Non-obstructive CAD was associated with more events and having ≥1 risk factor did provide additional prognostic information beyond number of diseased segments. More specifically, independent prognostic value of hypertension and diabetes was observed while adjusting for SIS. Diabetes did not associate with MACE in patients without CAD but only among patients with non-obstructive CAD. This underscores the prognostic importance of CAD burden by CCTA, which resembles a summary of life-long exposure to measurable and unmeasurable risk factors for vascular atherosclerosis. A normal CCTA identifies a subgroup of patients with a more benign phenotype of cardiovascular risk factors. On the other hand, non-obstructive CAD is a marker of more adverse risk factor profile with secondary disease manifestation. Importantly, more prognostic importance of risk factors in non-obstructive CAD is observed despite more use of statin, aspirin, beta blocker, and angiotensin-converting enzyme inhibitor. Hypertension, by an increase in pulse pressure, and diabetes, by chronic glucose disbalance and systemic inflammation, result in endothelial dysfunction which facilitates lipids to enter the blood vessel, initiating the atherosclerotic disease process. Atherosclerosis manifestation as can be observed with CCTA ‘upgrades’ the severity of diabetes or hypertension. Clinically, this means that in the presence of CAD, a synergistic effect exists between risk factors and CAD burden and implies increasing treatment intensity according to number of risk factors and CAD burden.
The translation of improved risk stratification into more appropriate medical care and subsequently improved outcomes has been observed in the SCOT-HEART (Scottish Computed Tomography of the HEART Trial) trial.21 In total, 4146 patients with stable chest pain were randomized to standard care alone or standard care plus CCTA, and rates of coronary heart disease death or myocardial infarction were 41% lower in the CCTA arm. This effect has been explained by the increased use of antiplatelet and statin therapy in patients with non-obstructive and obstructive disease, and potentially by revascularization of high-risk CAD.22 The PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial randomized 10 003 symptomatic patients to CCTA or functional testing. Although the primary endpoint (death, myocardial infarction, hospitalization of unstable angina, or major procedural complications) did not differ between arms, an endpoint of death and myocardial infarction was significantly lower in the CCTA arm at 1 year.23
Limitations
The observational design of the study is a limitation since changes in lifestyle, medical therapy, and revascularization after CCTA may have influenced the results. As such, the observations of this study are based on patients who received treatment for their adverse cardiovascular risk profile according to local guideline recommendations. Not all patients had available follow-up information, but the clinical profile of the missing cohort generally revealed a lower risk for future events. In contemporary clinical practice, guidelines focus on treatment of risk factors not necessarily the quantity of plaque on CCTA. This may have served to enhance the prognostic importance of plaque and diminish that of the cardiovascular risk factors. Also, no independent committee adjudicated the events, which may have limited the accuracy of events. Finally, all-cause death instead of cardiac death was included as an endpoint. More advanced methods for quantifying both calcific and non-calcific plaque burden are being developed which may further improve the prognostic information provided with CCTA.
Conclusion
Among patients without obstructive CAD, the extent of CAD provides more prognostic information for MACE than traditional cardiovascular risk factors. In absence of CAD, the presence of one or more risk factors did not increase risk for MACE. In non-obstructive CAD, the number of diseased segments was predictive, and diabetes and hypertension were further independently associated with MACE, suggesting synergistic effects of plaque burden and risk factors.
Funding
The research reported in this publication was funded, in part, by the National Institute of Health (Bethesda, MD, USA) under award number R01 HL115150. This research was also supported, in part, by the Dalio Institute of Cardiovascular Imaging (New York, NY, USA) and the Michael Wolk Foundation (New York, NY, USA).
Conflict of interest: J.K.M. receives funding from the Dalio Foundation, National Institutes of Health, and GE Healthcare; has serves on the scientific advisory board of Arineta and GE Healthcare; and has an equity interest in Cleerly. B.J.W.C. holds the Saul and Edna Goldfarb Chair in Cardiac Imaging Research; has receives research support from CV Diagnostix and Ausculsciences, educational support from TeraRecon Inc.; and has equity interest in General Electric. K.C. is a non-compensated medical advisory board member of Heartflow Inc. All other authors have no conflict of interest to declare.
Appendix
Table A1.
Prognostic value of risk factors and CCTA findings restricted to asymptomatic individuals
| Univariable HR (95% CI) | P-value | Multivariable HR (95% CI) | P-value | |
|---|---|---|---|---|
| Age (years) | 1.04 (1.02–1.05) | <0.001 | 1.03 (1.01–1.05) | 0.004 |
| Male gender | 0.84 (0.59–1.20) | 0.332 | ||
| BMI (kg/m2) | 1.05 (1.02–1.07) | <0.001 | 1.04 (1.01–1.06) | 0.003 |
| Cardiovascular risk factors | ||||
| Diabetes | 1.52 (0.93–2.48) | 0.092 | 1.08 (0.62–1.90) | 0.778 |
| Hypertension | 2.20 (1.53–3.16) | <0.001 | 1.63 (1.09–2.43) | 0.018 |
| Hypercholesterolaemia | 0.74 (0.52–1.06) | 0.100 | ||
| Family history of CAD | 0.84 (0.55–1.28 | 0.838 | ||
| Current smoker | 1.22 (0.79–1.87) | 0.377 | ||
| CCTA findings | ||||
| Segment involvement score | 1.17 (1.10–1.24) | <0.001 | ||
| 0 | Reference | |||
| 1 | 2.14 (1.27–3.59) | 0.004 | 1.18 (0.84–2.61) | 0.177 |
| 2–3 | 3.02 (1.91–4.76) | <0.001 | 2.13 (1.30–3.49) | 0.003 |
| 4–5 | 2.26 (1.17–4.35) | 0.015 | 1.44 (0.71–2.90) | 0.309 |
| >5 | 4.29 (2.40–7.67) | <0.001 | 2.22 (1.17–4.21) | 0.015 |
CAD, coronary artery disease; CCTA, coronary computed tomography angiography; LAD, left anterior descending artery; LCX, left circumflex; LM, left main; RCA, right coronary artery.
Table A2.
Clinical profile and CCTA Leiden score associated with major cardiovascular events
| Endpoint death, MI, and late revascularization |
Endpoint death and MI |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariable HR (95% CI) | P-value | Multivariable HR (95% CI) | P-value | Univariable HR (95% CI) | P-value | Multivariable HR (95% CI) | P-value | |
| Age (years) | 1.03 (1.02–1.04) | <0.001 | 1.02 (1.01–1.03) | 0.002 | 1.04 (1.03–1.05) | <0.001 | 1.03 (1.01–1.04) | <0.001 |
| Male gender | 0.91 (0.74–1.12) | 0.376 | 0.90 (0.71–1.14) | 0.372 | ||||
| BMI (kg/m2) | 1.03 (1.01–1.05) | 0.006 | 1.02 (1.00–1.04) | 0.072 | 1.03 (1.01–1.05) | 0.013 | 1.02 (1.00–1.05) | 0.052 |
| Chest pain symptoms | 0.033 | 0.074 | 0.007 | 0.012 | ||||
| No chest pain | Reference | Reference | Reference | Reference | ||||
| Non-anginal | 1.05 (0.75–1.49) | 0.769 | 1.03 (0.72–1.47) | 0.868 | 1.03 (0.71–1.50) | 0.868 | 0.98 (0.67–1.45) | 0.935 |
| Atypical | 0.67 (0.50–0.89) | 0.006 | 0.68 (0.50–0.93) | 0.014 | 0.58 (0.42–0.81) | 0.001 | 0.58 (0.41–0.82) | 0.002 |
| Typical | 0.90 (0.59–1.38) | 0.618 | 0.92 (0.58–1.45) | 0.710 | 0.75 (0.46–1.24) | 0.264 | 0.69 (0.40–1.20) | 0.190 |
| Cardiovascular risk factors | ||||||||
| Diabetes | 1.56 (1.18–2.05) | 0.002 | 1.45 (1.04–2.02) | 0.029 | 1.47 (1.07–2.01) | 0.016 | 1.34 (0.92–1.95) | 0.131 |
| Hypertension | 1.61 (1.30–2.00) | <0.001 | 1.49 (1.13–1.95) | 0.005 | 1.69 (1.33–2.14) | <0.001 | 1.60 (1.18–2.18) | 0.003 |
| Hypercholesterolaemia | 1.00 (0.81–1.23) | 0.973 | 0.85 (0.67–1.07) | 0.172 | ||||
| Family history of CAD | 1.00 (0.80–1.26) | 0.985 | 0.97 (0.75–1.25) | 0.808 | ||||
| Current smoker | 1.16 (0.90–1.50) | 0.245 | 1.17 (0.89–1.56) | 0.265 | ||||
| CCTA Leiden score | <0.001 | <0.001 | <0.001 | 0.009 | ||||
| 0 | Reference | Reference | Reference | Reference | ||||
| 0–6 | 1.68 (1.28–2.20) | <0.001 | 1.55 (1.11–2.16) | 0.010 | 1.47 (1.09–1.98) | 0.012 | 1.30 (0.90–1.88) | 0.161 |
| 6–12 | 2.00 (1.50–2.68) | <0.001 | 1.92 (1.35–2.73) | <0.001 | 1.90 (1.39–2.60) | <0.001 | 1.64 (1.11–2.41) | 0.012 |
| >12 | 2.98 (2.17–4.10) | <0.001 | 2.64 (1.79-3.90) | <0.001 | 2.42 (1.68–3.49) | <0.001 | 1.99 (1.28–3.09) | 0.002 |
Results are given from patients with he CCTA Leiden score available (3186). CAD, coronary artery disease; CCTA, coronary computed tomography angiography; LAD, left anterior descending artery; LCX, left circumflex; LM, left main; RCA, right coronary artery; MI, myocardial infarction.
Table A3.
Interactions between clinical variables and presence or absence of CAD
| Leiden score = 0 (N = 1849) | Leiden score 0–6 (N = 656) | Leiden score >6 (N = 681) | P-interaction | ||||
|---|---|---|---|---|---|---|---|
| P-value | P-value | P-value | |||||
| Age (years) | 1.02 (1.01–1.04) | 0.003 | 1.02 (1.00–1.04) | 0.067 | 1.04 (1.02–1.06) | <0.001 | 0.361 |
| Male gender | 0.88 (0.64–1.21) | 0.425 | 0.79 (0.52–1.22) | 0.293 | 0.78 (0.54–1.13) | 0.184 | 0.876 |
| BMI (kg/m2) | 1.04 (1.00–1.08) | 0.061 | 1.00 (0.95–1.05) | 0.903 | 1.03 (1.00–1.05) | 0.027 | 0.451 |
| Chest pain symptoms | 0.272 | 0.088 | 0.102 | 0.174 | |||
| No chest pain | Reference | Reference | |||||
| Non-anginal | 1.39 (0.78–2.47) | 0.270 | 0.48 (0.32–0.97) | 0.042 | 1.49 (0.87–2.56) | 0.147 | |
| Atypical | 0.77 (0.48–1.25) | 0.289 | 0.58 (0.33–1.00) | 0.050 | 0.80 (0.49–1.30) | 0.370 | |
| Typical | 0.80 (0.39–1.65) | 0.551 | 0.86 (0.34–2.16) | 0.744 | 1.64 (0.85–3.14) | 0.140 | |
| Cardiovascular risk factors | |||||||
| Diabetes | 1.07 (0.66–1.73) | 0.783 | 2.24 (1.30–3.86) | 0.004 | 1.89 (1.22–2.94) | 0.005 | 0.104 |
| Hypertension | 1.61 (1.16–2.22) | 0.004 | 1.52 (0.97–2.39) | 0.068 | 1.34 (0.92–1.94) | 0.130 | 0.766 |
| Hypercholesterolaemia | 0.83 (0.60–1.16) | 0.277 | 1.00 (0.65–1.54) | 0.995 | 0.84 (0.58–1.21) | 0.335 | 0.753 |
| Family history of CAD | 0.92 (0.64–1.32) | 0.655 | 0.88 (0.54–1.43) | 0.603 | 1.23 (0.84–1.80) | 0.277 | 0.481 |
| Current smoker | 1.02 (0.67–1.54) | 0.939 | 0.86 (0.49–1.50) | 0.595 | 1.46 (0.98–2.19) | 0.064 | 0.282 |
CAD, coronary artery disease; CCTA, coronary computed tomography angiography; LAD, left anterior descending artery; LCX, left circumflex; LM, left main; RCA, right coronary artery.
References
- 1. Cury RC, Abbara S, Achenbach S, Agatston A, Berman DS, Budoff MJ et al. Coronary Artery Disease–Reporting and Data System (CAD-RADS): an Expert Consensus Document of SCCT, ACR and NASCI: endorsed by the ACC. JACC Cardiovasc Imaging 2016;9:1099–113. [DOI] [PubMed] [Google Scholar]
- 2. Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah MH, Berman DS et al. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: an InteRnational Multicenter) registry. J Cardiovasc Comput Tomogr 2011;5:84–92. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135:2320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–70. [DOI] [PubMed] [Google Scholar]
- 5. Schulman-Marcus J, Hartaigh BO, Gransar H, Lin F, Valenti V, Cho I et al. Sex-specific associations between coronary artery plaque extent and risk of major adverse cardiovascular events: the CONFIRM long-term registry. JACC Cardiovasc Imaging 2016;9:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deseive S, Shaw LJ, Min JK, Achenbach S, Andreini D, Al-Mallah MH et al. Improved 5-year prediction of all-cause mortality by coronary CT angiography applying the CONFIRM score. Eur Heart J Cardiovasc Imaging 2017;18:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanke P, Naoum C, Ahmadi A, Cheruvu C, Soon J, Arepalli C et al. Long-term prognostic utility of coronary CT angiography in stable patients with diabetes mellitus. JACC Cardiovasc Imaging 2016;9:1280–8. [DOI] [PubMed] [Google Scholar]
- 8. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Min JK, Berman DS, Dunning A, Achenbach S, Al-Mallah M, Budoff MJ et al. All-cause mortality benefit of coronary revascularization vs. medical therapy in patients without known coronary artery disease undergoing coronary computed tomographic angiography: results from CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: an InteRnational Multicenter Registry). Eur Heart J 2012;33:3088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58:849–60. [DOI] [PubMed] [Google Scholar]
- 11. Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of Cardiovascular Computed Tomography Guidelines Committee: endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016;10:435–49. [DOI] [PubMed] [Google Scholar]
- 12. van Rosendael AR, Shaw LJ, Xie JX, Dimitriu-Leen AC, Smit JM, Scholte AJ et al. Superior risk stratification with coronary computed tomography angiography using a comprehensive atherosclerotic risk score. JACC Cardiovasc Imaging 2019;12:1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mushtaq S, De Araujo Goncalves P, Garcia-Garcia HM, Pontone G, Bartorelli AL, Bertella E et al. Long-term prognostic effect of coronary atherosclerotic burden: validation of the computed tomography-Leaman score. Circ Cardiovasc Imaging 2015;8:e002332.. [DOI] [PubMed] [Google Scholar]
- 14. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 15. Cho I, Chang HJ, Oh B, Shin S, Sung JM, Lin FY et al. Incremental prognostic utility of coronary CT angiography for asymptomatic patients based upon extent and severity of coronary artery calcium: results from the COronary CT Angiography EvaluatioN For Clinical Outcomes InteRnational Multicenter (CONFIRM) study. Eur Heart J 2015;36:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang HJ, Lin FY, Lee SE, Andreini D, Bax J, Cademartiri F et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol 2018;71:2511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andreini D, Pontone G, Mushtaq S, Gransar H, Conte E, Bartorelli AL et al. Long-term prognostic impact of CT-Leaman score in patients with non-obstructive CAD: results from the COronary CT Angiography EvaluatioN For Clinical Outcomes InteRnational Multicenter (CONFIRM) study. Int J Cardiol 2017;231:18–25. [DOI] [PubMed] [Google Scholar]
- 18. Cheruvu C, Precious B, Naoum C, Blanke P, Ahmadi A, Soon J et al. Long term prognostic utility of coronary CT angiography in patients with no modifiable coronary artery disease risk factors: results from the 5 year follow-up of the CONFIRM International Multicenter Registry. J Cardiovasc Comput Tomogr 2016;10:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leschka S, Seitun S, Dettmer M, Baumuller S, Stolzmann P, Goetti R et al. Ex vivo evaluation of coronary atherosclerotic plaques: characterization with dual-source CT in comparison with histopathology. J Cardiovasc Comput Tomogr 2010;4:301–8. [DOI] [PubMed] [Google Scholar]
- 20. Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J 2018;39:2401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Investigators S-H, Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924–33. [DOI] [PubMed] [Google Scholar]
- 22. Adamson PD, Williams MC, Dweck MR, Mills NL, Boon NA, Daghem M et al. Guiding therapy by coronary CT angiography improves outcomes in patients with stable chest pain. J Am Coll Cardiol 2019;74:2058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]