Abstract
Background
Few studies have investigated the association of magnesium levels with incident peripheral artery disease (PAD) despite emerging evidence of magnesium contributing to vascular calcification. Moreover, no data are available on whether the magnesium–PAD relationship is independent of or modified by kidney function.
Methods
A cohort of 11 839 participants free of PAD in the Atherosclerosis Risk in Communities Study at Visit 2 (1990–92) was studied. We investigated the association of serum magnesium and other bone–mineral metabolism markers [calcium, phosphorus, intact parathyroid hormone (iPTH) and intact fibroblast growth factor-23] with incident PAD using multivariable Cox proportional hazards regression.
Results
Over a median of 23 years, there were 471 cases of incident PAD. The hazard ratio for incident PAD in Quartile 1 (<1.5 mEq/L) versus Quartile 4 (>1.7 mEq/L) of magnesium was 1.96 (95% confidence interval 1.40–2.74) after adjustment for potential confounders. Lower magnesium levels were associated with greater incidence of PAD, particularly in those with estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 (n = 11 606). In contrast, the association was largely flat in those with eGFR <60 mL/min/1.73 m2 (n = 233) with P-for-interaction 0.03. Among bone–mineral metabolism markers, only higher iPTH showed an interaction with kidney function (P-for-interaction 0.01) and iPTH >65 pg/mL was significantly related to PAD only in those with eGFR <60 mL/min/1.73 m2.
Conclusions
Lower magnesium was independently associated with incident PAD, but this association was significantly weaker in those with reduced kidney function. In contrast, higher iPTH levels were particularly related to PAD risk in this clinical population.
Keywords: bone–mineral metabolism, chronic kidney disease, epidemiology, peripheral artery disease
INTRODUCTION
Peripheral artery disease (PAD) affects >200 million individuals around the world [1]. PAD is especially important in persons with chronic kidney disease (CKD) since it occurs more frequently than myocardial infarction or stroke in this population [2]. There is a growing interest in studying risk factors of PAD since the contributions of risk factors to the development of PAD compared with other atherosclerotic diseases are known to be different (e.g. diabetes is more closely related to PAD than coronary artery disease) [3, 4].
Serum magnesium is a promising potential risk factor of PAD for several reasons. A number of cellular pathways have been elucidated by which magnesium may play a role in the inhibition of vascular calcification [5–7]. Magnesium can bind to phosphate and protect against the formation of calcium–phosphate nanocrystals, which can contribute to vascular calcification, particularly in the setting of reduced kidney function. In vitro and animal studies have demonstrated a strong association between magnesium deficiency and systemic inflammation, a condition known to promote atherosclerosis [8, 9]. In patients with Type 2 diabetes mellitus, hypomagnesemia has been associated with more rapid kidney function decline including progression to end-stage kidney disease [10, 11].
Although several studies have reported the associations of hypomagnesemia with cardiovascular phenotypes such as heart failure [12], atrial fibrillation [13] and sudden cardiac death [14], data on the prospective associations of magnesium with PAD are limited. To our knowledge, a single study reported hypomagnesemia associated with greater incident PAD in the general population [15]. However, despite the tight pathophysiological link of magnesium to kidney function and calcium–phosphate metabolism, the study did not fully account for bone–mineral metabolism markers and explore potential interaction with kidney function in understanding the etiological association of magnesium and PAD. Furthermore, magnesium at replete or high levels can inhibit intact parathyroid hormone (iPTH), whereas several rodent models have demonstrated hypomagnesemia as a driver of intact fibroblast growth factor-23 (FGF-23) upregulation.
Identifying biomarkers of PAD can be particularly important since most individuals with PAD are asymptomatic or have atypical leg symptoms and thus are often diagnosed later in the disease course. In some patients, ischemic ulcers or gangrene can be the first manifestation of PAD in its most severe form, a condition known as critical limb ischemia (CLI). The prognosis of CLI is devastating, with half of the patients either dying or losing their limbs within a year from the time of diagnosis [16].
Therefore, we sought to investigate comprehensively the prospective association of serum magnesium levels with incidence of clinical PAD in a large African American and Caucasian community-based study, the Atherosclerosis Risk in Communities (ARIC) Study. We explored whether the adjustment for several bone–mineral metabolism markers (e.g. calcium, phosphorus, iPTH and FGF-23) attenuated the association of magnesium and PAD risk and whether the magnesium–PAD relationship was modified by kidney function. We also investigated the associations of bone–mineral metabolism markers with incident PAD risk by kidney function. We hypothesized that hypomagnesemia would be independently, inversely associated with incident PAD and CLI, with potential interaction by kidney function. We further hypothesized that bone–mineral metabolism markers would be independently associated with incident PAD in participants with estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2.
MATERIALS AND METHODS
Study design and population
The ARIC Study is an ongoing prospective cohort study of 15 792 individuals aged between 45 and 64 years at Visit 1 (between 1987 and 1989) from four communities in the USA (Forsyth County, NC, USA; Jackson, MS, USA; suburban Minneapolis, MN, USA; and Washington County, MD, USA) [17].
We analyzed data from Visit 2 in this study due to the availability of relevant variables including magnesium and bone–mineral metabolism markers, with a starting population of 14 348 participants. We excluded Visit 2 participants of nonblack or nonwhite race (n = 42), those with prevalent PAD (n = 641) and those with missing other covariates (n = 1826) for a final analytic population of 11 839 (Figure 1). Prevalent PAD was defined as self-reported leg pain with ambulation, self-reported leg artery revascularization or an ankle-brachial index ≤0.9.
FIGURE 1.
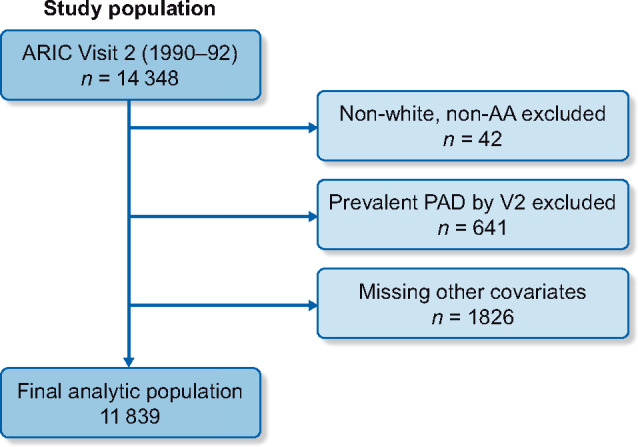
Flowchart of ARIC Study Visit 2 analytic population. Covariates: education, biochemical markers, BMI, smoking status, alcohol status, medical comorbidities. AA, African American; V2, Visit 2.
Magnesium
Serum magnesium was measured by the Gindler and Heth procedure, using metallochromic dye calmagite [1-(1-hydroxy-4-methyl-2-phenylazo)-2-napthol-4sulfonic acid]. When samples were split and measured a week apart, within-participant laboratory coefficient of variation (CV) was 3.6% [12].
Bone–mineral metabolism markers
Serum calcium was measured using o-cresolphthalein complexone with a CV of 1.1%. Serum phosphorus was measured using ammonium molybdate with CV 7.6%. iPTH was measured via sandwich immunoassay method using a Roche Elecsys 2010 Analyzer (Roche Diagnostics), with a reported CV of 9.7% [18]. FGF-23 was measured via a two-site enzyme-linked immunosorbent assay ( FGF-23 ELISA Kit; Kainos Laboratories, Tokyo, Japan) with CV 16.6% from split paired samples and 8.8% from internal laboratory quality control samples at 41.4 pg/mL [18]. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation based on age, gender, race and serum creatinine [19]. Serum creatinine was measured by a modified kinetic Jaffe method and then calibrated to isotope dilution mass spectrometry( IDMS) reference measurement [20].
Covariates
Information on demographic and medical history was obtained from in-person interviews at ARIC Visit 2. Self-reported data included age, race, smoking status and alcohol status (current, former or never). Body mass index (BMI) was calculated as weight (in kilograms) divided by height squared (in meters). Blood pressures were measured in triplicate after 5-min rest, for each participant in a seated position, with the mean of the last two measurements analyzed. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg or the use of antihypertensive medications. Diabetes was defined as a fasting glucose level of ≥126 mg/dL, a nonfasting glucose level ≥200 mg/dL, self-reported physician diagnosis or the use of antidiabetic medications. Other biochemical data measured at Visit 2 included total and high-density lipoprotein (HDL) cholesterols [12, 21]. Prevalent coronary heart disease was defined by self-reported clinical history or evidence of prior myocardial infarction by electrocardiogram at Visit 1 as well as through chart adjudication by a physician panel for myocardial infarction between Visits 1 and 2. Similarly, prevalent stroke was defined by self-report at Visit 1 and additionally by chart adjudication for stroke between Visits 1 and 2.
Outcomes
Incident PAD was defined based on PAD-related hospitalizations identified with the following International Classification of Diseases (ICD) codes at any position based on previous literature [22, 23]: atherosclerosis of native arteries of the extremities, unspecified (440.20), atherosclerosis of native arteries of the extremities with intermittent claudication (440.21), atherosclerosis of native arteries of the extremities with rest pain (440.22), atherosclerosis of native arteries of the extremities with ulceration (440.23), atherosclerosis of native arteries of the extremities with gangrene (440.24), other atherosclerosis of native arteries of the extremities (440.29), atherosclerosis of bypass graft of the extremities (440.3), chronic total occlusion of artery of the extremities (440.4), atherosclerosis of other specified arteries (440.8) and leg artery revascularization (38.18, 39.25, 39.29 and 39.50).
Leveraging a large sample size with long follow-up in ARIC, we also assessed CLI as a severe outcome of PAD. Among PAD cases, those based on 440.22, 440.23 and 440.24 and those with coexisting codes of leg amputation (84.1x), lower extremity ulcer (707.1x) and gangrene (785.4) were considered CLI [23, 24]. Participants free of incident PAD were followed until the date of death, date of last contact or 30 September 2015, whichever came first.
Statistical analysis
We summarized baseline characteristics, including demographic information and medical comorbidities, across quartiles of magnesium level. Given the small differences in serum magnesium level by quartile, the number of participants within each quartile of magnesium was not uniform.
For our survival analysis, we used Cox proportional hazards regression to model the risk of incident PAD and CLI over time in relation to magnesium level, using Quartile 4 (>1.7 mEq/L) as a reference. We confirmed proportional hazards using Schoenfeld residuals. We ran a few models to assess the impact of potential confounders. Model 1 adjusted for baseline demographic data including age, sex, race and education level. Model 2 further accounted for other major atherosclerotic factors, including BMI, smoking status (current, former or never), total and HDL cholesterols, and a history of hypertension, diabetes, coronary heart disease and stroke. Model 3 additionally adjusted for eGFR and bone–mineral metabolism markers (calcium, phosphorus, potassium, iPTH and FGF-23). Given the potential impact of the competing risk of death, we conducted sensitivity analysis using Fine and Gray’s proportional sub-hazards models [25].
Subsequently, we modeled magnesium levels using piece-wise linear spline models with knots at the 25th, 50th and 75th percentiles to evaluate potentially nonlinear relationships across the full spectrum of magnesium levels, evaluating effect modification between magnesium and kidney function (eGFR ≥60 versus <60 mL/min/1.73 m2) given the kidney’s role in magnesium homeostasis. We also analyzed the association of bone–mineral metabolism markers with both incident PAD and CLI by kidney function. We analyzed calcium, phosphorus, iPTH and FGF-23 dichotomously (above and below median) given the limited size of comparison groups by quartile. Given the expected rise in levels of all these markers in the setting of CKD, we did further analysis dichotomizing calcium, phosphorus, iPTH and FGF-23 at the cut points used in previous literature, 10.2 mg/dL, 4.5 mg/dL, 65 pg/mL and 60.6 pg/mL, respectively [26, 27].
Finally, we evaluated whether the addition of magnesium level can improve model performance and risk prediction. Specifically, we assessed the Akaike information criterion, calibration plots (predicted versus observed risk across deciles of predicted risk), Harrell’s C-statistics (a measure of discrimination) and categorical net reclassification improvement (NRI). For NRI, given the lack of established risk categories for PAD, we used risk thresholds in a previous study: 20-year risk of 10% and 20% for PAD and 3.5% and 7.0% for CLI [28]. As the base model, we used predictors in a PAD prediction model previously published: age, sex, race, hypertension, diabetes, smoking, total cholesterol and prevalent coronary heart disease [29].
RESULTS
Baseline characteristics
Mean baseline age was 57.3 (SD 5.7) years. Compared with participants with higher serum magnesium levels, participants in Quartiles 1 and 2 were more likely to be female, be African American, to have a higher BMI and have less formal education (Table 1). They were also more likely to be current smokers and to have more baseline medical comorbidities overall, including hypertension, diabetes, coronary heart disease and prior strokes. There were no evident patterns in the levels of bone–mineral metabolism marker across serum magnesium, although FGF-23 was higher in Quartile 4 compared with the other three quartiles. We further reported characteristics of participants by baseline eGFR (Supplementary data, Table S1).
Table 1.
Baseline characteristics of participants at Visit 2 by magnesium quartile
| Variablea | Totals | Q1 (<1.5 mEq/L) | Q2 (1.5–1.59 mEq/L) | Q3 (1.6–1.7 mEq/L) | Q4 (>1.7 mEq/L) |
|---|---|---|---|---|---|
| N | 11 839 | 1587 | 2294 | 5805 | 2153 |
| Age (years) | 57.3 (5.7) | 57.5 (5.8) | 57.0 (5.9) | 57.3 (5.6) | 57.6 (5.7) |
| Male | 5271 (44.5) | 586 (36.9) | 1019 (44.4) | 2639 (45.5) | 1027 (47.7) |
| African American | 2891 (24.4) | 685 (43.2) | 676 (29.5) | 1172 (20.2) | 358 (16.6) |
| BMI | 28.0 (5.4) | 29.2 (6.2) | 28.0 (5.5) | 27.3 (4.9) | 27.0 (4.6) |
| Education | |||||
| <HS graduate | 2461 (20.8) | 464 (29.2) | 505 (22.0) | 1093 (18.8) | 399 (18.5) |
| HS graduate | 4963 (41.9) | 628 (39.6) | 938 (40.9) | 2481 (42.7) | 916 (42.6) |
| >HS education | 4415 (37.3) | 495 (31.2) | 851 (37.1) | 2231 (38.4) | 838 (38.9) |
| Cigarette use | |||||
| Current | 2542 (21.4) | 382 (24.1) | 502 (21.9) | 1196 (20.6) | 462 (21.5) |
| Former | 4493 (38.0) | 541 (34.1) | 877 (38.2) | 2207 (38.0) | 868 (40.3) |
| Never | 4804 (40.6) | 664 (41.8) | 915 (39.9) | 2402 (41.4) | 823 (38.2) |
| Alcohol use | |||||
| Current | 6763 (57.1) | 762 (48.0) | 1280 (55.8) | 3412 (58.8) | 1309 (60.8) |
| Former | 2428 (20.5) | 384 (24.2) | 504 (22.0) | 1145 (19.7) | 395 (18.4) |
| Never | 2648 (22.4) | 441 (27.8) | 510 (22.2) | 1248 (21.5) | 449 (20.8) |
| Hypertension | 3439 (29.1) | 709 (44.7) | 719 (31.3) | 1503 (25.9) | 508 (23.6) |
| Diabetes | 1338 (11.3) | 438 (27.6) | 329 (14.3) | 448 (7.7) | 123 (5.7) |
| Prevalent coronary heart disease | 651 (5.5) | 100 (6.3) | 139 (6.1) | 301 (5.2) | 111 (5.2) |
| Prevalent stroke | 205 (1.7) | 51 (3.2) | 43 (1.9) | 81 (1.4) | 30 (1.4) |
| Total cholesterol (mmol/L) | 5.42 (1.02) | 5.40 (1.08) | 5.38 (1.02) | 5.41 (0.991) | 5.50 (1.03) |
| HDL cholesterol (mmol/L) | 0.91 (0.28) | 0.891 (0.31) | 0.904 (0.29) | 0.920 (0.28) | 0.926 (0.28) |
| eGFR-Cr (mL/min/1.73 m2) | 96.4 (15.5) | 100.3 (18.5) | 98.0 (15.7) | 95.9 (14.5) | 93.1 (15.4) |
| Potassium (mEq/L) | 4.18 (0.40) | 4.06 (0.42) | 4.15 (0.38) | 4.20 (0.39) | 4.24 (0.40) |
| Calcium (mg/dL) | 9.35 (0.43) | 9.37 (0.47) | 9.32 (0.43) | 9.34 (0.42) | 9.37 (0.42) |
| Phosphorus (mg/dL) | 3.53 (0.49) | 3.56 (0.50) | 3.51 (0.49) | 3.52 (0.48) | 3.53 (0.48) |
| iPTH (pg/mL) | 42.5 (27.5) | 42.5 (18.5) | 42.7 (18.0) | 42.2 (27.5) | 43.3 (39.1) |
| FGF-23 (pg/mL) | 58.1 (909) | 50.5 (109) | 45.2 (16.6) | 58.7 (1088) | 81.6 (1188) |
Categorical variables measured in n (%); continuous variables measured in mean (SD). Q, quartile; HS, high school; Cr, creatinine.
Association of magnesium with PAD
Over a median follow-up of 23.2 years, we identified 471 incident PAD cases and 175 cases of CLI. Table 2 displays the hazard ratio (HR) of incident PAD and CLI by magnesium quartile.
Table 2.
HR of incident PAD and CLI by magnesium quartile, Visit 2
| Outcome | Model | Serum magnesium |
|||
|---|---|---|---|---|---|
| Q1 (<1.5 mEq/L) | Q2 (1.5 mEq/L) | Q3 (1.6–1.7 mEq/L) | Q4 (>1.7 mEq/L) | ||
| N | 1587 | 2294 | 5805 | 2153 | |
| Events | 105 | 107 | 199 | 60 | |
| PAD | Model 1 | 2.57 (1.86–3.55) | 1.77 (1.29–2.44) | 1.26 (0.95–1.68) | Ref. |
| Model 2 | 1.64 (1.18–2.28) | 1.48 (1.07–2.04) | 1.23 (0.92–1.64) | Ref. | |
| Model 3 | 1.96 (1.40–2.74) | 1.67 (1.20–2.31) | 1.35 (1.01–1.80) | Ref. | |
| CLI | Model 1 | 2.50 (1.54–4.06) | 1.23 (0.73–2.06) | 0.89 (0.56–1.41) | Ref. |
| Model 2 | 1.25 (0.76–2.06) | 0.89 (0.53–1.50) | 0.82 (0.52–1.31) | Ref. | |
| Model 3 | 1.59 (0.95–2.66) | 1.04 (0.61–1.78) | 0.95 (0.58–1.53) | Ref. | |
Model 1: adjusted for sex, race and education. Model 2: further adjustment for smoking status, drinking status, hypertension, diabetes mellitus, prevalent coronary heart disease, prevalent stroke, total cholesterol, HDL cholesterol. Model 3: further adjustment for serum electrolytes (potassium, calcium and phosphate), eGFR, iPTH and FGF-23. Q, quartile.
In Model 1, with adjustments for demographic data, those participants in either Quartile 1 or 2 of magnesium level had a significantly higher risk of incident PAD compared with those in Quartile 4. The higher risk of incident PAD persisted in Models 2 and 3. The HR for incident PAD for participants in Quartile 1 compared with Quartile 4 was 1.96 [95% confidence interval (CI) 1.40–2.74] after full adjustment for baseline medical comorbidities and other potential confounders. Compared with PAD, the results for CLI were similar in Model 1 but weaker in Models 2 and 3.
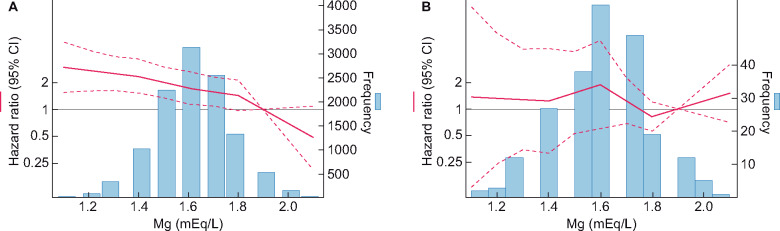
Out of 11 839 total participants, 233 participants had an eGFR <60 mL/min/1.73 m2 at baseline. Similar to the overall study population, lower magnesium levels were significantly associated with higher risk of incident PAD in participants with eGFR ≥60 mL/min/1.73 m2, after full adjustment (Figure 2A). In contrast, the association between magnesium and incident PAD was largely flat in those with eGFR <60 mL/min/1.73 m2 (Figure 2B; P-for-interaction 0.03). For CLI, weaker but similar patterns were observed (Supplementary data, Figure S1, P-for-interaction 0.003). A competing risk analysis for death showed similar results (Supplementary data, Table S2).
FIGURE 2.
Risk of PAD by serum magnesium level, in (A) the 436/11 606 participants with eGFR ≥60 mL/min/1.73 m2 and in (B) the 35/233 participants with eGFR <60 mL/min/1.73 m2. P-for-interaction 0.03.
Association of bone–mineral metabolism markers with PAD
We evaluated whether the association between bone–mineral metabolism markers and incident PAD and CLI was modified by kidney function (Table 3; Supplementary data, Table S3). With bone–mineral metabolism markers categorized into above versus below their median values, notably, there was no interaction by kidney function, and no association of incident PAD with any of bone–mineral metabolism biomarkers regardless of kidney function. However, when we dichotomized the bone–mineral metabolism markers according to their clinical thresholds, we observed a significant interaction by kidney function only for iPTH (P-for-interaction 0.01), with greater risk of incident PAD only in participants with eGFR <60 mL/min/1.73 m2 [HR (95% CI) = 2.24 (0.94–5.36) versus 1.09 (0.76–1.55) in eGFR ≥60 mL/min/1.73 m2].
Table 3.
HR of incident PAD by eGFR, Visit 2
| eGFR ≥60 |
eGFR <60 |
P-for-interaction | |||
|---|---|---|---|---|---|
|
N
|
11 606 |
233 |
|||
| Events |
436 |
35 |
|||
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Higher calciuma | 1.06 (0.86–1.29) | 0.60 | 0.68 (0.31–1.48) | 0.33 | 0.17 |
| Higher phosphorusa | 1.01 (0.83–1.24) | 0.90 | 1.22 (0.53–2.78) | 0.64 | 0.95 |
| Higher iPTHa | 1.07 (0.88–1.30) | 0.52 | 0.51 (0.22–1.22) | 0.13 | 0.93 |
| Higher FGF-23a | 1.09 (0.90–1.32) | 0.39 | 1.40 (0.42–4.73) | 0.58 | 0.84 |
| Higher calciumb | 0.59 (0.33–1.07) | 0.08 | 2.10 (0.44–9.97) | 0.35 | 0.43 |
| Higher phosphorusb | 2.01 (1.36–2.95) | <0.001 | 1.87 (0.64–5.49) | 0.25 | 0.82 |
| Higher iPTHb | 1.09 (0.76–1.55) | 0.64 | 2.24 (0.94–5.36) | 0.07 | 0.01 |
| Higher FGF-23b | 1.43 (1.10–1.86) | 0.01 | 0.94 (0.37–2.39) | 0.90 | 0.60 |
Adjusted for age, sex, race, education, smoking status, drinking status, hypertension, diabetes mellitus, prevalent coronary heart disease, total cholesterol and HDL cholesterol.
Calcium, phosphorus, iPTH and FGF-23 above versus below their median values.
Calcium, phosphorus, iPTH and FGF-23, above versus below clinical cut-points.
Model performance and prediction of PAD with and without magnesium
The base model with established predictors of PAD demonstrated decent calibration visually (Supplementary data, Figure S2), although Hosmer–Lemeshow χ2 test showed statistical significance (P = 0.001) for PAD. The addition of magnesium did not alter the overall calibration. Similarly, the risk discriminations of PAD and CLI were excellent in the base model (c-statistic 0.839 and 0.920, respectively), and the addition of magnesium did not improve it (Supplementary data, Table S4). NRIs for both PAD and CLI were not significant [0.004 (95% CI −0.022, 0.031) and 0.000 (−0.027, 0.028), respectively] either. However, the overall model fit was better when we added magnesium (AIC with versus without magnesium, 8033 versus 8047 for PAD and 2841 versus 2842 for CLI).
DISCUSSION
In this community-based cohort study with more than two decades of follow-up, lower serum magnesium levels were independently associated with a greater risk of incident PAD. Specifically, those individuals in Quartile 1 with serum magnesium level <1.5 mEq/L had approximately two times higher risk of PAD compared with those in Quartile 4, even after accounting for several potential confounders including kidney function and bone–mineral metabolism markers. However, this association was not evident in the relatively small number of participants with eGFR <60 mL/min/1.73 m2, and the addition of magnesium did not necessarily improve risk prediction of PAD beyond a base model with traditional predictors. Among the bone–mineral metabolism markers, only higher iPTH dichotomized by a clinical cut point showed a significant interaction with kidney function, as a result of an elevated PAD risk only in those with eGFR <60 mL/min/1.73 m2.
These results extend the current literature of the magnesium–PAD relationship in several aspects. First, although there have been some cross-sectional studies [30, 31], our study is one of very few showing a prospective inverse association of serum magnesium with incident PAD [15, 32]. While the study by Sun et al. [15] also investigated the association of magnesium with incident PAD, we used a more specific definition of PAD and uniquely investigated CLI as an outcome. Second, we confirmed that the association is independent of kidney function and bone–mineral metabolism markers. Third, we observed that the inverse association is generally consistent for both overall PAD and CLI. Fourth, we uniquely observed that the magnesium–PAD relationship is significantly modified by kidney function, with a largely flat risk gradient in individuals with reduced eGFR. Finally, our results indicate that the predictive value of magnesium for PAD is limited.
There are several possible mechanisms by which lower magnesium levels may play a role in the development of PAD. Magnesium can inhibit formation of calcium–phosphate apatite by forming a more soluble compound as well as competitively binding with the calcium-sensing receptor similar to a calcimimetic. Furthermore, magnesium deficiency has been shown to inhibit endothelial proliferation, in the setting of increases in interleukin-1 and upregulation of both vascular cell adhesion molecule-1 and plasminogen activator inhibitor-1 [33]. Thus, lower magnesium levels may indicate a milieu prone to vascular calcification and systemic inflammation [5, 6, 8]. In multiple animal studies, augmentation of dietary magnesium has been associated with a decrease in vascular calcification both in central arteries such as the aorta as well as cardiac and renal arteries [34–36]. Similarly, low-magnesium diets increased the risk of vascular calcification in animal models [36].
We observed a lack of any inverse association between serum magnesium levels and incident PAD in participants with reduced eGFR. The kidneys serve as the primary regulator of magnesium homeostasis in the body. Roughly 70% of serum magnesium is filtered by the glomerulus, of which the vast majority is reabsorbed and only ∼3–5% excreted in the urine [37, 38]. Thus, reduced GFR can induce hypermagnesemia pathologically [35]. In the setting of reduced kidney function, other factors involved in vascular calcification such as calcium, phosphate, iPTH and FGF-23 may play a more important role than magnesium as drivers of vascular calcification [39]. However, since the number of study participants with reduced eGFR in our study was limited, further investigations into these associations of magnesium with PAD and other cardiovascular outcomes in CKD-enriched cohorts would be warranted.
Although hypomagnesemia was not associated with PAD in persons with reduced kidney function, greater iPTH levels were associated with increased PAD risk in this clinical subgroup. While this may have been a chance finding as a result of multiple statistical testing in our study, there are plausible mechanisms to explain this interaction. As kidney function declines, iPTH is upregulated to maintain homeostasis of vitamin D, calcium and phosphorus [39]. Thus, levels of iPTH rise earlier and more rapidly than abnormalities seen in calcium or phosphorus in CKD [39]. Of note, elevations in iPTH level have been independently associated with higher levels of systemic inflammatory markers including C-reactive protein in the general population, as well as aortic stiffness and vascular calcification in individuals with primary hyperparathyroidism [40–42]. A randomized clinical trial in CKD patients demonstrated that the use of cinacalcet and vitamin D analogs for secondary hyperparathyroidism attenuated vascular and valvular calcification [43].
The results of this study may provide areas of increased focus for health-care providers managing patients at high risk for PAD. If our findings are confirmed in other, larger CKD cohorts, monitoring of magnesium may be less informative in this population. Closer monitoring of bone–mineral metabolism markers such as iPTH in the CKD population, instead, may provide added benefit. Our study generates a hypothesis that magnesium supplementation may provide benefit in reducing future PAD risk, especially in persons with hypomagnesemia and preserved kidney function, which could be evaluated in a clinical trial.
This study has several limitations. The measure of serum magnesium in this study may not accurately reflect ionized magnesium levels, which may be more biologically meaningful [44]. Also, the baseline magnesium level was based on a single blood test, which may be prone to misclassification and typically bias estimates to the null. This may be particularly relevant among those with reduced kidney function since magnesium levels may fluctuate as eGFR declines. The relatively high CV of FGF-23 indicates some misclassification, which might bias its associations with PAD and CLI toward null. Our use of ICD codes from hospital discharges to diagnose clinical PAD or CLI may not capture less severe cases of PAD that did not require hospitalization. Additionally, our study population only included Caucasian and African American participants, so our results may have limited generalizability to other racial/ethnic groups. While we have adjusted for many potential confounding variables, there is still the potential for residual confounding in our results as in any observational study. The small sample size particularly in patients with an eGFR <60 mL/min/1.73 m2 limits our statistical power. Furthermore, study participants were middle-aged (45–64 years) at the start of this study, and thus these results may not be simply generalizable to older adults where PAD and CLI are more common.
In summary, lower levels of magnesium were independently associated with increased PAD risk overall. However, this association was significantly weaker in participants with an eGFR <60 mL/min/1.73 m2. In contrast, higher iPTH levels were particularly related to PAD risk in this clinical population. Our findings suggest the complex interplay among magnesium, bone–mineral metabolism markers and kidney function in their contributions to vascular health.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC Study for their important contributions.
FUNDING
S.M. was supported by National Institutes of Health (NIH)/NHLBI grant T32HL007024. E.S. was supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants K24DK106414 and R01DK089174. K.M. was supported by NIH/NHLBI grant R21HL133694. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data are collected by U012U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH [NHLBI, National Institute of Neurological Disorders and Stroke (NINDS), National Institute on Aging (NIA) and National Institute on Deafness and Other Communication Disorders (NIDCD)], and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI.
AUTHORS’ CONTRIBUTIONS
S.M. and K.M. were involved in the research idea and study design. J.C. and K.M. were involved in data acquisition. S.M., N.D., M.E.G., P.L.L., G.H., A.R.F., E.S., J.C., B.G.J. and K.M. were involved in data analysis/interpretation. S.M., N.D. and K.M. were involved in statistical analysis. J.C., B.G.J. and K.M. were involved in supervision and mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
K.M. received research funding and personal fee from Fukuda Denshi and Kyowa Kirin outside of the work. Other authors declare that they have no conflicts of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
(See related article by de Borst and de Baaij. Low serum magnesium as a risk factor for peripheral artery disease in chronic kidney disease: an open verdict. Nephrol Dial Transplant 2020; 35: 1831--1833)
REFERENCES
- 1. Fowkes FG, Rudan D, Rudan I et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382: 1329–1340 [DOI] [PubMed] [Google Scholar]
- 2.Saran R, Robinson B, Abbott KC et al. US Renal Data System 2016 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2017; 69 (Suppl 1): S1–S688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hazarika S, Annex BH. Biomarkers and genetics in peripheral artery disease. Clin Chem 2017; 63: 236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Virani SS, Callaway CW et al. Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation 2018; 137: e67–e492 [DOI] [PubMed] [Google Scholar]
- 5. LeGeros RZ, Contiguglia SR, Alfrey AC. Pathological calcifications associated with uremia: two types of calcium phosphate deposits. Calcif TissueRes 1973; 13: 173–185 [DOI] [PubMed] [Google Scholar]
- 6. Boskey AL, Posner AS. Effect of magnesium on lipid-induced calcification: an in vitro model for bone mineralization. Calcif Tissue Int 1980; 32: 139–143 [DOI] [PubMed] [Google Scholar]
- 7. Massy ZA, Drueke TB. Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis and survival. Clin Kidney J 2012; 5: i52–i61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mazur A, Maier JA, Rock E et al. Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys 2007; 458: 48–56 [DOI] [PubMed] [Google Scholar]
- 9. Nielsen FH. Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res 2018; 11: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pham PC, Pham PM, Pham PA et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63: 429–436 [DOI] [PubMed] [Google Scholar]
- 11. Sakaguchi Y, Shoji T, Hayashi T et al. Hypomagnesemia in type 2 diabetic nephropathy: a novel predictor of end-stage renal disease. Diabetes Care 2012; 35: 1591–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lutsey PL, Alonso A, Michos ED et al. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2014; 100: 756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khan AM, Lubitz SA, Sullivan LM et al. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation 2013; 127: 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peacock JM, Ohira T, Post W et al. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2010; 160: 464–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun X, Zhuang X, Huo M et al. Serum Magnesium and the Prevalence of Peripheral Artery Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 2018; 282: 196–201 [DOI] [PubMed] [Google Scholar]
- 16. Norgren L, Hiatt WR, Dormandy JA et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 2007; 33 (Suppl 1): S1–S75 [DOI] [PubMed] [Google Scholar]
- 17. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989; 129: 687–702 [PubMed] [Google Scholar]
- 18. Alonso A, Misialek JR, Eckfeldt JH et al. Circulating fibroblast growth factor-23 and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities study. J Am Heart Assoc 2014; 3: e001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levin A, Stevens P, Bilous R et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int 2013; 3: 1–150 [Google Scholar]
- 20. Astor BC, Shafi T, Hoogeveen RC et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis 2012; 59: 653–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The AI. Atherosclerosis Risk in Communities Study: Operations Manual 10. Clinical Chemistry Determinations, 1987 [Google Scholar]
- 22. Hicks CW, Yang C, Ndumele CE et al. Associations of obesity with incident hospitalization related to peripheral artery disease and critical limb ischemia in the ARIC study. J Am Heart Assoc 2018; 7: e008644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsushita K, Kwak L, Yang C et al. High-sensitivity cardiac troponin and natriuretic peptide with risk of lower-extremity peripheral artery disease: the Atherosclerosis Risk in Communities (ARIC) Study. Eur Heart J 2018; 39: 2412–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ding N, Kwak L, Ballew SH et al. Traditional and nontraditional glycemic markers and risk of peripheral artery disease: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 2018; 274: 86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509 [Google Scholar]
- 26. Korada SK, Zhao D, Gottesman RF et al. Parathyroid hormone and subclinical cerebrovascular disease: the atherosclerosis risk in communities brain magnetic resonance imaging study. J Stroke Cerebrovasc Dis 2016; 25: 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fyfe-Johnson AL, Alonso A, Selvin E et al. Serum fibroblast growth factor-23 and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. J Hypertens 2016; 34: 1266–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang C, Kwak L, Ballew SH et al. Retinal microvascular findings and risk of incident peripheral artery disease: An analysis from the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 2020; 294: 62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murabito JM, D’Agostino RB, Silbershatz H et al. Intermittent claudication. A risk profile from the Framingham Heart Study. Circulation 1997; 96: 44–49 [DOI] [PubMed] [Google Scholar]
- 30. Rusu M, Cristea V, Frenţiu T et al. Magnesium and selenium in diabetics with peripheral artery disease of the lower limbs. Clujul Med 2013; 86: 235–239 [PMC free article] [PubMed] [Google Scholar]
- 31. Tzanakis I, Virvidakis K, Tsomi A et al. Intra- and extracellular magnesium levels and atheromatosis in haemodialysis patients. Magnes Res 2004; 17: 102–108 [PubMed] [Google Scholar]
- 32. Meema HE, Oreopoulos DG, Rapoport A. Serum magnesium level and arterial calcification in end-stage renal disease. Kidney Int 1987; 32: 388–394 [DOI] [PubMed] [Google Scholar]
- 33. Maier JA, Malpuech-Brugere C, Zimowska W et al. Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta 2004; 1689: 13–21 [DOI] [PubMed] [Google Scholar]
- 34. Henaut L, Massy ZA. Magnesium as a calcification inhibitor. Adv Chronic Kidney Dis 2018; 25: 281–290 [DOI] [PubMed] [Google Scholar]
- 35. Hamano N, Komaba H, Fukagawa M. Magnesium as a new player in CKD: too little is as bad as too much? Kidney Int 2017; 92: 1034–1036 [DOI] [PubMed] [Google Scholar]
- 36. Diaz-Tocados JM, Peralta-Ramirez A, Rodriguez-Ortiz ME et al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int 2017; 92: 1084–1099 [DOI] [PubMed] [Google Scholar]
- 37. Coburn JW, Popovtzer MM, Massry SG et al. The physicochemical state and renal handling of divalent ions in chronic renal failure. Arch Intern Med 1969; 124: 302–311 [PubMed] [Google Scholar]
- 38. Saris NE, Mervaala E, Karppanen H et al. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta 2000; 294: 1–26 [DOI] [PubMed] [Google Scholar]
- 39. Levin A, Bakris GL, Molitch M et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71: 31–38 [DOI] [PubMed] [Google Scholar]
- 40. Cheng SP, Liu CL, Liu TP et al. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm 2014; 2014: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Macfarlane DP, Yu N, Leese GP. Subclinical and asymptomatic parathyroid disease: implications of emerging data. Lancet Diabetes Endocrinol 2013; 1: 329–340 [DOI] [PubMed] [Google Scholar]
- 42. Goettsch C, Iwata H, Aikawa E. Parathyroid hormone: critical bridge between bone metabolism and cardiovascular disease. Arterioscler Thromb Vasc Biol 2014; 34: 1333–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raggi P, Chertow GM, Torres PU et al. ; on behalf of the ADVANCE Study Group. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2011; 26: 1327–1339 [DOI] [PubMed] [Google Scholar]
- 44. Del Giorno R, Riva H, Donato G et al. Ionized and total serum magnesium in hemodialysis: predictors and variability. A longitudinal cross-sectional study. Clin Exp Nephrol 2018; 22: 620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.