Abstract
Atypical teratoid/rhabdoid (AT/RT) tumors are the most common malignant brain tumor of infancy and have a poor prognosis. We have previously identified very high expression of LIN28A and/or LIN28B in AT/RT tumors and showed that AT/RT have corresponding increased expression of the mitogen-activated protein (MAP) kinase pathway. Binimetinib is a novel inhibitor of mitogen-activated protein kinase (MAP2K1 or MEK), and is currently in pediatric phase II clinical trials for low-grade glioma. We hypothesized that binimetinib would inhibit growth of AT/RT cells by suppressing the MAP kinase pathway. Binimetinib inhibited AT/RT growth at nanomolar concentrations. Binimetinib decreased cell proliferation and induced apoptosis in AT/RT cells and significantly reduced AT/RT tumor growth in flank xenografts. Our data suggest that MAP kinase pathway inhibition could offer a potential avenue for treating these highly aggressive tumors.
Keywords: INI1, Malignant rhabdoid tumor, MEK162, Pediatric brain tumor, RAS
INTRODUCTION
Atypical teratoid/rhabdoid tumors (AT/RT) are a group of rare pediatric embryonal brain tumors with limited therapeutic options. Even with multimodal therapy the 2-year event-free survival for children with AT/RT is only ∼50% (1), supporting the urgency to search for more effective therapeutic strategies. The hallmark genetic alteration of AT/RTs is mutation in the epigenetic regulator SMARCB1/INI1. Recent epigenetic profiling suggests 3 subtypes with different profiles and sensitivities to therapy (2, 3). Termed as AT/RT-TYR, AT/RT-SHH, and AT/RT-MYC by Johann et al and as groups 1, 2A, and 2B by Torchia et al, these groups differ in methylation pattern, location, age, and genetic pathways. Each subgroup of AT/RT has distinctive RNA expression profiles, suggesting that there may be subgroup-specific targeted therapies.
However, we have previously identified increased phospho-ERK in >85% of AT/RT primary tumors, suggesting that activation of the mitogen-activated protein (MAP) kinase pathway may be a common output of the various upstream molecular drivers (4). While there are several drugs available to target the MAP kinase pathway, and some are used in the context of clinical trials in BRAF-driven pediatric brain tumors (5), to the best of our knowledge none have been investigated in clinical trials for AT/RT.
The MEK inhibitor binimetinib (MEK162) is a potent inhibitor of MEK1/2 and has been FDA approved for the treatment of NRAS mutant melanoma in combination with the BRAF inhibitor encorafenib (6). A multicenter phase II trial of binimetinib is underway in progressive low-grade glioma in children after demonstrating acceptable safety profile in phase I studies (NCT02285439). We therefore hypothesized that binimetinib would target the MEK pathway and inhibit the growth of AT/RT.
MATERIALS AND METHODS
Cell Culture
AT/RT cell lines BT12, BT37, CHLA-06-ATRT, CHLA-266, CHLA-05-ATRT, and CHLA-02-ATRT have been described before (7, 8). BT12 and CHLA-266 were obtained through the Children’s Oncology Group cell repository (9). BT37 was derived from a human xenograft originating at St. Jude Children’s Research Hospital as described previously (10). CHLA-06-ATRT, CHLA-05-ATRT and CHLA-02-ATRT were obtained from Children’s Hospital of Los Angeles (4, 8, 10). All cell lines were grown in complete growth media as described previously supplemented with 1% Penicillin/Streptomycin. Cell lines were routinely tested for mycoplasma contamination.
Drug Treatments
Binimetinib was purchased through AdooQ Biosciences (Irvine, CA) and dissolved in dimethyl sulfoxide (DMSO) for in vitro experiments and 1% carboxymethylcellulose with 0.5% Tween80 for in vivo experiments. Trametinib was purchased from Selleckchem and dissolved in DMSO for our experiments. Negative control cell lines were treated with maximum concentration of DMSO (0.1%, v/v).
Cell Growth and Proliferation Assays
To assess proliferation effects, bromodeoxyuridine incorporation assays were performed by incubating cells with 100 µM 5-bromo-2′-deoxyuridine ([BrdU]; Sigma-Aldrich, St. Louis, MO) for 6 hours as described previously (4). Anti-BrdU antibody was used per the manufacturer’s direction (#5292; Cell Signaling Technology, Danvers, MA) at 1:1000 dilution. To assess relative growth, cells were plated in 96-well plates in triplicate at densities between 2000 and 8000 cells per well, and absorbance was measured at 0, 3, and 6 days using the colorimetric CellTiter 96 MTS assay (Promega, Madison, WI).
Cell Death Assays
Immunofluorescence assays were performed as previously described (4), using cleaved caspase-3 primary antibody at a dilution of 1:400 (#9661; Cell Signaling Technology).
Western Blotting
Western blots were performed as previously described. Specific antibodies were used as per the manufacturer’s instructions: Cleaved poly(ADP-ribose) polymerase (cPARP) (9541), LIN28A (3978), MAPK/ERK (9102), pMAPK/p-ERK (T202/Y204; 4370), phosphorylated Retinoblastoma (phospho-RB) ser-780(9307) (all from Cell Signaling Technologies), and ACTIN (47778) (Santa Cruz Biotechnology, Dallas, TX). Densitometry was performed using ImageJ v1.440 software as previously described (11, 12).
Xenograft Tumors
For animal care and anesthesia, “Principles of Laboratory Animal Care” (NIH publication No. 86-223, revised 1985) was followed, using a protocol approved by the Johns Hopkins Animal Care and Use Committee, in compliance with United States Animal Welfare Act regulations and Public Health Service Policy. Intracranial xenografts were produced in anesthetized animals as previously described (13). Intracranial injection guide holes were produced by twirling an 18-gauge beveled needle and 2.5 × 104 viable cells (CHLA-06-AT/RT) were injected in 3 µL of growth medium into the right striatum through a needle connected to a Hamilton syringe. Cells were injected using the following coordinates: anteroposterior = 3 mm; mediolateral = 2 mm; dorsoventral = 3 mm. For intracranial xenografts the day after injection, mice were treated either with vehicle or 30 mg/kg binimetinib orally twice daily 4 days a week (Monday, Tuesday, Thursday, Friday). Animals were killed upon signs of distress, such as neurologic deficits, poor grooming, and cachexia suggestive of an intracranial mass lesion. Immediately after death, areas of tumor infiltration were excised and snap frozen in liquid nitrogen and subsequently processed for Western blotting. Flank xenografts were produced by injecting 2 × 106 cells (CHLA-06) or 3 × 106 (CHLA-266) cells per flank in 200 µL of growth medium diluted 50:50 in Matrigel (Corning, NY). For flank injections, tumors were allowed to grow to ∼0.5 cm in diameter (typically ∼1 week after injections) and then treated with either 10 mg/kg binimetinib dissolved in 1% carboxymethylcellulose or vehicle alone orally twice daily 5 days a week.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism or Excel (Microsoft) software. All tests were 2 sided unless indicated otherwise, p values <0.05 were considered significant. Results are shown from 3 biological repeats, each having 3 or more technical repeats, unless indicated otherwise.
RESULTS
Binimetinib Decreases p-ERK Expression in AT/RT Cells and Decreases Cell Growth
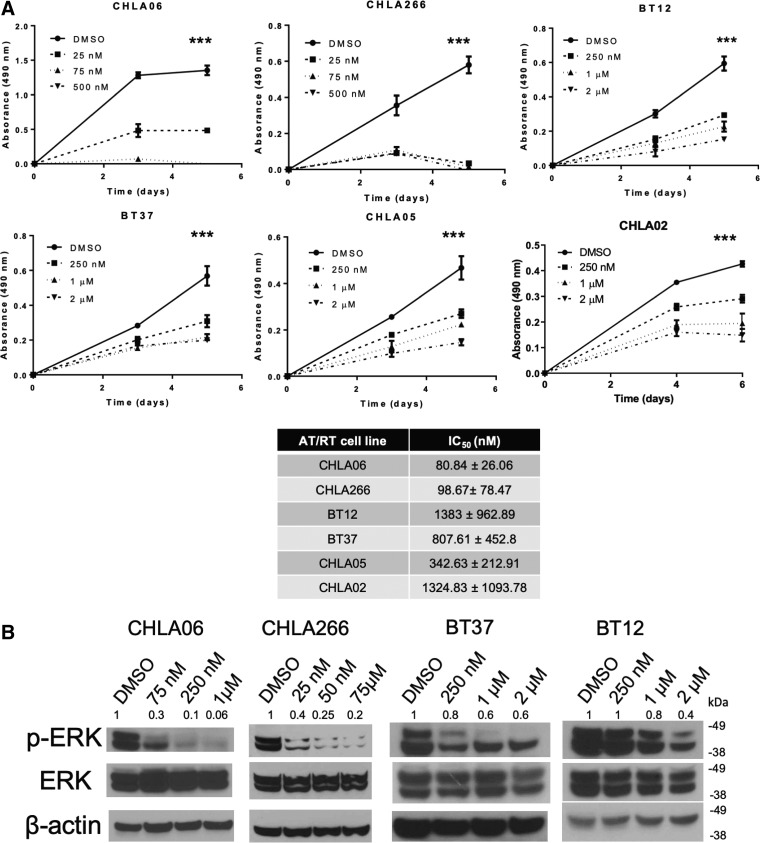
Our lab has previously demonstrated that almost all primary AT/RTs have increased expression of p-ERK and that the MEK inhibitor selumetinib inhibits AT/RT proliferation and increases apoptosis in vitro (4). We hypothesized that binimetinib (MEK162), an FDA-approved, newer MEK inhibitor with potentially better blood-brain barrier penetration would be efficacious in AT/RT. We first treated the AT/RT cell lines CHLA-06, CHLA-266, BT37, BT12, CHLA-05, and CHLA-02 with varying amounts of binimetinib (range 25 nM–2 µM) or with DMSO (vehicle) and found that increasing concentrations of binimetinib led to decreasing growth for each of these cell lines (Fig. 1A). In most cell lines, we saw significant decrease in cell viability even at low nanomolar concentrations. To further validate this pathway in AT/RT, we also tested the FDA-approved MEK inhibitor trametinib, and found similar sensitivities in CHLA-06, BT12, and BT37 (Supplementary Data Fig. S1). Based on the binimetinib IC50 values, we decided to further investigate the cell lines CHLA-06, CHLA-266, BT12, and BT37. Torchia et al recently identified 3 different subgroups of AT/RT based on genome-wide methylation profiling and extended the classification to AT/RT cell lines (14). CHLA-06 and BT12 are group 2, and CHLA-266 has features of both groups 1 and 2. BT37 has not yet been subtyped, but lacks MYC expression, suggesting it is not group 2B/MYC (7). As shown in Figure 1B, 6 hours of treatment with binimetinib leads to inhibition of phospho-ERK in all AT/RT cell lines.
FIGURE 1.
Binimetinib decreases AT/RT cell viability and p-ERK expression at nanomolar concentrations. (A) MTS assays measuring cell viability demonstrates significantly reduced viability with increasing concentration of binimetinib (**p < 0.05 by t-test; ***p < 0.005 by t-test comparing the absorbance from day 5 highest dose vs. DMSO; representative assay from at least 3 different biological replicates is shown). The IC50 values for each of the cell lines are also listed. (B) Dose dependent inhibition of p-ERK expression in the AT/RT cell lines CHLA-06, CHLA-266, BT37, and BT12 after 6 hours of treatment with binimetinib at each concentration or with DMSO. Normalized relative pERK expression as assessed by densitometry is also shown above each blot.
Binimetinib Decreases Proliferation of AT/RT Cells
Since the MAP kinase pathway is intricately connected with regulation of cell cycle proteins, we hypothesized that MEK inhibition could lead to a decrease in cell proliferation. Exposure to binimetinib led to smaller and fewer neurospheres (Fig. 2A), as expected with growth inhibition. When we investigated the underlying mechanism for reduced growth, we saw a significant downregulation of BrdU incorporation (CHLA-06 and BT37) 72 hours after treatment with binimetinib (Fig. 2B), suggesting reduced proliferation and cell cycle arrest. Similarly, we found a decrease in expression of phosphorylated Rb protein, specifically at S780, known to be involved in cell cycle regulation (15), supporting the notion that cell cycle is inhibited by binimetinib (Fig. 2C).
FIGURE 2.
Binimetinib decreases proliferation of AT/RT cells. (A) Photomicrographs of CHLA-06 and BT37 cells demonstrating smaller and fewer number of neurospheres with increasing binimetinib dose. (B) Immunofluorescence assay demonstrating decreased incorporation of BrdU with increasing concentration of binimetenib in CHLA-06 and BT37. On the left are representative images from one of 3 biological replicates. Cells stained with DAPI are in blue, while cells tagged with BrdU antibody are red. Calculated fraction of BrdU-positive cells in this particular biological replicate is shown on the right (*p < 0.05 by Student t-test, n = minimum of 3 technical replicates). (C) Western blot demonstrating decreasing S780 phospho-Rb expression with increasing concentrations of binimetinib compared with vehicle treated AT/RT cells. Relative phospho-RB expression levels as assessed by densitometry are shown above each image.
Treatment With Binimetinib Increases Apoptosis
We next hypothesized that the downregulation of the MAP kinase pathway and subsequent cell cycle arrest would lead to an increase in cells undergoing apoptosis. To test this hypothesis, we checked cell lysates collected after 6 hours of binimetinib treatment for the induction of cleaved PARP and performed cleaved caspase 3 immunofluorescence assays in 2 different AT/RT cell lines (CHLA-06 and BT37) 72 hours following treatment with binimetinib. Increasing doses of binimetinib led to higher number of cells undergoing apoptosis as evidenced by increasing levels of cleaved PARP (Fig. 3A) and higher percentage of cleaved caspase 3-positive cells (Fig. 3B).
FIGURE 3.
Binimetinib treatment induces apoptosis in AT/RT. (A) Western blots showing induction of cleaved PARP expression after 6 hours of binimetinib treatment at different concentrations compared with DMSO. Representative images from at least 3 different biological replicates are shown. Relative expression level of cPARP for each concentration as measured by densitometry is shown above each blot. (B) Immunofluorescence assay demonstrating increased incorporation of cleaved caspase 3 with increasing concentration of binimetenib in CHLA-06 and BT37. The upper panel shows representative images from one of 3 biological replicates. Cells stained with DAPI are in blue, while cells tagged with anticleaved caspase 3 antibody are red. The lower panel denotes the calculated fraction of cleaved caspase 3-positive cells in this particular biological replicate (*p < 0.05 and **p < 0.005 by Student t-test, n = minimum of 3 technical replicates).
Binimetinib Treatment Decreases Tumor Growth In Vivo in Heterotopic Models But Not in Orthotopic Models
To test whether the decreased cell proliferation and increased apoptosis caused by binimetinib would lead to decreased tumorigenesis in vivo, we first injected nude mice with AT/RT cells in the flanks. When these flank tumors reached ∼0.5 cm in diameter we treated the mice orally with binimetinib 10 mg/kg twice a day or with vehicle control only. Mice were killed after ∼2 weeks of treatment when tumor sizes were prohibitive for continued treatment. There was a statistically significant (p < 0.005) decrease in the CHLA-06 flank tumor sizes when mice were treated with binimetinib compared with the control group. In the CHLA-266 injected mice, there was a trend of reduced flank tumor size with binimetinib treatment compared with the vehicle control. However, the trend did not reach statistical significance (p = 0.066) (Fig. 4). For both cell lines, tumor weight at the end of treatment was statistically significantly lower in the binimetinib treated group compared with the control animals. We next tested binimetinib at 10 mg/kg × 5 days a week and 30 mg/kg twice daily × 4 days per week in mice bearing orthotopic intracranial CHLA-06 xenografts. Although we saw significant reductions in CHLA-06 flank tumor growth in mice with binimetinib treatment, we did not detect any difference in survival in intracranially xenografted animals in 2 separate experiments using cohorts of 5 mice each (data not shown).
FIGURE 4.
Binimetinib treatment reduces tumor growth in vivo. Nude mice were injected in the flanks with AT/RT cell lines CHLA-266 (A) or CHLA-06 (B) and then treated with 10 mg/kg binimetinib or 1% carboxymethylcellulose (vehicle control) twice a day for 14 days before tumors were harvested. Tumor volume is plotted with standard error. Binimetinib causes significant decrease in tumor growth (multiple comparison t-test p = 0.066 for CHLA-266; p < 0.005 for CHLA-06 using the Holm-Sidak method) as indicated by normalized volumes plotted in these graphs. (C) Representative images of flanks from mice injected with CHLA-06 cells and treated with either vehicle (top row) or binimetinib (bottom row) resected after 15 days of drug treatment. (D) Flank tumor mass (in grams) at the time of death (mean ± SEM). *p < 0.05.
DISCUSSION
On the most recently concluded COG trial using multimodal therapy (surgery, radiation, and 6 agent chemotherapy with 3 consolidations cycles with PBSC rescue) for children with AT/RT the 2-year event-free survival was 42% (16). Another multi-institutional trial from 2004 to 2006 using protocols developed at Dana Farber achieved an event-free survival of 53% in a smaller number of patients (1). In those patients who were cured, toxicity from therapy often leads to lifelong consequences. There is thus an urgent need to develop more targeted therapeutic approaches. Part of the challenge in targeting AT/RT has been finding that molecularly these tumors tend to be relatively silent except for mutations in SMARCB1/INI1. However, recent work from different groups has demonstrated that epigenetically AT/RTs can be subtyped by different enhancer landscapes (3, 14). The discovery by our group that majority of AT/RTs display activation of the MAP kinase pathway also opens the possibility of targeting these tumors with inhibitors of this pathway (4).
Here, we demonstrate that the MEK inhibitor binimetinib acts as a potent and effective inhibitor of AT/RT cells at nanomolar doses by specifically targeting the MAP kinase pathway. While there are several MEK inhibitors in clinical trials, we chose to use binimetinib since it is already in phase II clinical trial in pediatric patients and has had acceptable safety data from phase I studies. Our study demonstrates that binimetinib is able to significantly reduce tumor burden in mice bearing flank xenografts suggesting that MAP kinase pathway inhibitors may be useful in targeting this tumor in vivo. Our results build on our prior study demonstrating in vitro activity of selumetinib against AT/RT (4). We also here show activity of trametinib and binimetinib, suggesting a broad class effect against AT/RT cells derived from different subgroups.
While in several of our cell lines we demonstrate drug sensitivity at nanomolar levels, some of the cell lines were more resistant to therapy and required higher doses to achieve sufficient reduction in growth. Although we did not specifically investigate the factors influencing sensitivity of the different cell lines in this study, this finding may reflect the inherent heterogeneity in AT/RTs in activation of the MAP kinase pathway as demonstrated in our previous work (4).
One of the challenges of using targeted therapy in brain tumors is the limited blood-brain barrier penetration of many agents. Binimetinib is being tested against pediatric low-grade glioma tumors, which are avidly gadolinium contrast enhancing, suggesting a leaky blood-tumor barrier. AT/RT tumors generally do not show gadolinium enhancement to the degree of low-grade gliomas, suggesting that the blood brain and blood tumor barrier may limit binimetinib’s activity in AT/RT tumors in the brain. The preclinical data for most MEK inhibitors tend to suggest low levels of CNS drug penetration. In the adult phase 3 COLUMBUS trial evaluating efficacy of combination of binimetinib with the BRAFV600E inhibitor encorafenib compared with single agent vemurafenib, combination therapy led to better progression-free survival except for the small subset of patients with brain metastasis at baseline (6, 17), suggesting that this MEK inhibitor is not able to achieve sufficient drug levels in the CNS. We also did not observe any activity of binimetinib as a single agent against CHLA-06 orthotopic xenografts. However, a newer MEK inhibitor E6201 demonstrated improved BBB penetrance in preclinical model (18) and is currently being investigated in phase I clinical trial in adult patients with CNS metastasis from melanoma (NCT03332589) with early clinical data being encouraging (19). A recent phase 1b/2 study combining the CDK4/6 inhibitor LEE011 with binimetinib in NRAS mutant melanoma patients has shown promising results in patients with brain metastasis (20). The CDK4/6 inhibitor abemaciclib is being investigated in a phase I study in pediatric patients with DIPG and other recurrent brain tumors, and has potentially improved brain distribution by saturating the efflux channels at the BBB (NCT02644460). Intriguingly, Meel et al recently demonstrated that the BBB may be disrupted in certain models of AT/RT xenografts and that combination of the MEK inhibitor trametinib with a MELK inhibitor OTSSP167 lengthened survival of orthotopic tumor-bearing mice (21). Of note, trametinib as a single agent did not improve survival of mice bearing orthotopic AT/RT xenografts.
Our study demonstrates that binimetinib can successfully suppress tumor growth in vivo at tolerable doses (the 10 mg/kg dose used in our flank studies are equivalent to the MTD established from adult clinical trials and the phase II recommended dose of 32 mg/m2/dose in pediatric LGG trials). Although binimetinib did not demonstrate activity against orthotopic xenografts, we have extended our prior findings of activity of MEK inhibitors in AT/RT, suggesting that additional agents targeting the MAP kinase pathway that have improved blood brain barrier penetration may demonstrate efficacy in AT/RT.
Supplementary Material
This study was supported by Alex’s Lemonade Stand Foundation (E.H.R.); Giant Food Pediatric Cancer Research Fund; National Cancer Institute Core Grant to the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (P30CA006973); and NCI T32 training grant 5T32CA060441 (S.S.).
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Chi SN, Zimmerman MA, Yao X, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol 2009;27:385–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torchia J, Picard D, Lafay-Cousin L, et al. Molecular subgroups of atypical teratoid rhabdoid tumours in children: An integrated genomic and clinicopathological analysis. Lancet Oncol 2015;16:569–82 [DOI] [PubMed] [Google Scholar]
- 3. Johann PD, Erkek S, Zapatka M, et al. Atypical teratoid/rhabdoid tumors are comprised of three epigenetic subgroups with distinct enhancer landscapes. Cancer Cell 2016;29:379–93 [DOI] [PubMed] [Google Scholar]
- 4. Weingart MF, Roth JJ, Hutt-Cabezas M, et al. Disrupting LIN28 in atypical teratoid rhabdoid tumors reveals the importance of the mitogen activated protein kinase pathway as a therapeutic target. (Research Support, Non-U.S. Gov’t). Oncotarget 2015;6:3165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: A multicentre, phase 2 trial. Lancet Oncol 2019;20:1011–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2018;19:1315–27 [DOI] [PubMed] [Google Scholar]
- 7. Wang SZ, Poore B, Alt J, et al. Unbiased metabolic profiling predicts sensitivity of high MYC-expressing atypical teratoid/rhabdoid tumors to glutamine inhibition with 6-diazo-5-oxo-l-norleucine. Clin Cancer Res 2019;25:5925–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Erdreich-Epstein A, Robison N, Ren X, et al. PID1 (NYGGF4), a new growth-inhibitory gene in embryonal brain tumors and gliomas. Clin Cancer Res 2014;20:827–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arcaro A, Doepfner KT, Boller D, et al. Novel role for insulin as an autocrine growth factor for malignant brain tumour cells. Biochem J 2007;406:57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaur H, Hutt-Cabezas M, Weingart MF, et al. The chromatin-modifying protein HMGA2 promotes atypical teratoid/rhabdoid cell tumorigenicity. J Neuropathol Exp Neurol 2015;74:177–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abramoff MD, Magaljaes PJ, Ram SJ. Image processing with ImageJ. Biophoton Int 2004;11:36–42 [Google Scholar]
- 13. Rubens JA, Wang SZ, Price A, et al. The TORC1/2 inhibitor TAK228 sensitizes atypical teratoid rhabdoid tumors to cisplatin-induced cytotoxicity. Neuro Oncol 2017;19:1361–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torchia J, Golbourn B, Feng S, et al. Integrated (epi)-genomic analyses identify subgroup-specific therapeutic targets in CNS rhabdoid tumors. Cancer Cell 2016;30:891–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rubin SM. Deciphering the retinoblastoma protein phosphorylation code. Trends Biochem Sci 2013;38:12–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reddy A, Strother D, Judkins A, et al. Efficacy of High-Dose Chemotherapy and Three-Dimensional Conformal Radiation for Atypical Teratoid/Rhabdoid Tumor: A Report From the Children's Oncology Group Trial ACNS0333. J Clin Oncol 2020;38:1175–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018;19:603–15 [DOI] [PubMed] [Google Scholar]
- 18. Gampa G, Kim M, Cook-Rostie N, et al. Brain distribution of a novel MEK inhibitor E6201: Implications in the treatment of melanoma brain metastases. Drug Metab Dispos 2018;46:658–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Babiker HM, Byron SA, Hendricks WPD, et al. E6201, an intravenous MEK1 inhibitor, achieves an exceptional response in BRAF V600E-mutated metastatic malignant melanoma with brain metastases. Invest New Drugs 2019;37:636–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sosman JA, Kittaneh M, Lolkema M, et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity. J Clin Oncol 2014;32:9009 [Google Scholar]
- 21. Meel MH, Guillén Navarro M, de Gooijer MC, et al. MEK/MELK inhibition and blood-brain barrier deficiencies in atypical teratoid/rhabdoid tumors. Neuro Oncol 2020;22:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.