Abstract
ATP-binding cassette (ABC) transporters are a class of proteins found in living organisms that mediate transmembrane transport by hydrolyzing ATP. They play a vital role in the physiological processes of growth and development in plants. The most numerous sub-type transporter in the ABC transporter family is the ABCG group and which have the most complex function in a plant’s response to abiotic stresses. Our study focused on the effect of ABCG transporters in the adaptation of the pigeon pea to adverse environments (such as drought, salt, temperature, etc.). We conducted a functional analysis of ABCG transporters in the pigeon pea and their role in response to abiotic stresses. A total of 51 ABCG genes (CcABCGs) were identified, and phylogenetic analysis was conducted. We also identified the physicochemical properties of the encoded proteins, predicted their subcellular localization, and identified of the conserved domains. Expression analysis showed that ABCG genes have different expression profiles with tissues and abiotic stresses. Our results showed that CcABCG28 was up-regulated at low temperatures, and CcABCG7 was up-regulated with drought and aluminum stress. The initial results revealed that ABCG transporters are more effective in the abiotic stress resistance of pigeon peas, which improves our understanding of their application in abiotic stress resistance.
Keywords: Pigeon pea (Cajanus Cajan), ABCG transporters, Abiotic stresses, Gene expression
Introduction
ATP-binding cassette (ABC) transporters are a part of the largest and oldest known protein families and are widely found in eukaryotic and prokaryotic organisms (Martinoia et al., 2002; Mosser et al., 1993). ABC transporters play a crucial role in the growth and development of plants by detoxifying exogenous toxins in response to abiotic stress, and transporting metabolites including intercellular peptides, sugars, lipids, alkaloids, inorganic ions and other metabolic substances (Mendez & Salas, 2001; Morris & Zhang, 2006; Mourez, Hofnung & Dassa, 1997). AtABCB1 (also known as AtMDR1) was first identified in Arabidopsis in 1992 (Dudler & Hertig, 1992). ABC transporters are divided into eight subfamilies in the plant genome (ABCA-ABCG and ABCI) according to the Human Genome Organization (HUGO). There are 129 ABC transporters identified in Arabidopsis genomes (Arabidopsis thaliana), 128 identified in rice genomes (Oryza sativa), and 261 identified in soybean (Glycine max) genomes (Mishra et al., 2019; Schulz & Kolukisaoglu, 2006; Sanchez-Fernandez et al., 2001).
The ATP-binding cassette subfamily G is the largest subfamily of the ABC transporter family. The ABCG protein is widely distributed in plants and plays an important role in many fundamental physiological processes (Kretzschmar et al., 2011). Highly conservative amino acids, including 1–2 nucleotide-binding domains (NBDs), a highly hydrophobic transmembrane domain, and 1-2 trans-membrane domains (TMDs) are typical structural features of ABCG transporters (Verrier et al., 2008). The ABCG subfamily was divided into white-brown complexes (WBCs) and pleiotropic drug resistance (PDRs) complexes. WBCs are semi-molecular ABCG transporters that contain one NBD domain and one TMD domain. PDRs belong to the full-molecule ABCG transporter and include two NBD domains and two TMD domains (Jasinski et al., 2009). The NBD domain consists of three highly conserved regions of approximately 200 amino acids and include the Walker A box [GX4GK (ST)], Walker B box [(RK) X3GX3L (hydrophobic)3], and Walker C and are approximately 120 amino acids in length (Walker et al., 1982). The TMD domain contains 4-6 α-helixes, which are the channel for the transport of substrate molecules for transmembrane transport (Hyde et al., 1990; Schneider & Hunke, 1998). It is generally believed that the mechanism of ABCG transporters occurs on the substrate recognition site on the TMD domain and works to recognize and bind transport substrates located near the cell membrane. The NBD domain on the cell membrane hydrolyzes ATP to provide energy for substrate transport and causes conformational changes in the membrane structure (Davidson & Maloney, 2007). SpTUR2, the first full-molecule ABCG transporter gene in plants, was identified in the perennial aquatic plant Spirodella polyrhiza in 2002 (Van den Brule et al., 2002). More than 40 ABCG transporters have been identified in Arabidopsis, rice, and soybean genomes, to date. ABCG transporters are involved with the plant’s many physiological activities. For example, OsABCG31 in rice may decrease evaporation from plant leaves and may be related to the drought stress response of rice (Chen et al., 2011). Arabidopsis AtPDR8 is involved with Na+ excretion, increasing the plant’s tolerance to salt and drought stress (Kim et al., 2007). AtPDR36 also responds to the toxic heavy metal effects in Arabidopsis thaliana and participates in the stomatal self-regulation in leaves (Kim et al., 2010).
Cajanus cajan (L.) Millsp, also known as pigeon pea, is a diploid plant (2n = 22) with a genome size of approximately 858 Mbp. It grows in tropical and subtropical regions and has a stable regulatory system to adapt to conditions that include high temperature, high salinity, and drought (Singh et al., 2013; Varshney et al., 2011; Wu et al., 2011; Yadu et al., 2018). Cooler environmental temperatures have led to the northern migration of the pigeon pea. The pigeon pea grows easily in acidic soil (pH 5-7) and is resistant to aluminum, which has bought it much attention. The numerous secondary metabolites of the pigeon pea play an important role in the adaptation of the plant to its adverse environment (Shepherd & Bhardwaj, 1986). It also possesses medicinal properties and is used widely in the chemical industry (Ogoda, Akubue & Okide, 2002; Pandey & Pandey, 1991).
Our research focuses on the trans-membrane transport of secondary metabolites, antibiotics, and heavy metal ions by ABCG transport protein hydrolysis of ATP (Yazaki, 2006; Badri et al., 2008a; Badri et al., 2008b; Le Hir et al., 2013; Fourcroy et al., 2014). The inhibition of ABCG transporters decreases plant flavonoid content (Morris & Zhang, 2006; Imai et al., 2004). Plants that transport some hormones (such as ABA) can improve their survivability in drought and other adverse conditions (Kuromori et al., 2010; Kang et al., 2010). ABCG transporters could positively affect a plant’s response to adversity by transporting specific substances. We identified family genes, and conducted phylogenetic and expression analyses to reveal the important role of ABCG transporters in resisting environmental stress in the pigeon pea. A total of 51 ABCG transporters were identified and further analyzed. We found cis-acting elements related to the stress response in several genes identified above, suggesting that the ABCG transporter had a related regulatory effect in the pigeon pea. We analyzed the expression of the ABCG gene in different organs and different stress treatments and observed the tissue specificity of the ABCG gene and stress expression response to explore the expression profile of the ABCG gene in the pigeon pea. Our results provide scientific support for exploring the mechanism of ABCG transporters in relation to the resistance of several abiotic stressors of the pigeon pea.
Materials and Methods
Identification of ABCG transporters in the pigeon pea genome
One hundred-twenty nine Arabidopsis ABC protein sequences were downloaded from the Phytozome v12.1 database (https://phytozome.jgi.doe.gov/pz/portal.html). All of the Arabidopsis ABCs were used to identify the ABC transporters in the Cajanus cajan (L.) Millsp (taxid: 3821) database using BLASTP search in the National Center for Biotechnology Information (NCBI) with an initial cut-off e-value of 1.0 e−10 and max target sequence of 500. The Hidden Markov Model (HMM) profiles of ABC transporters (such as PF00005, PF00664, and PF10614) were downloaded from the Pfam database (http://pfam.xfam.org/search#tabview=tab1) and the HMMER search server was used against the pigeon pea proteome with an E-value setting of 1.0 e−5 (https://www.ebi.ac.uk/Tools/hmmer/) (Potter et al., 2018). The resulting protein sequences were further identified by a conserved domain of ABC based on a conserved domain search (CD-search) on the NCBI website with a threshold e-value of 1.0 e−5. We identified members of the ABC transporters using the above approach.
Multiple-sequence alignments were performed on the ABC transporters identified above and several ABC proteins of Arabidopsis thaliana, rice, and soybean, using clustalW with default settings. The ABC transporters’ phylogenetic tree was constructed using the neighbor-joining (NJ) method with 1000 replications of bootstrap and p-distance of a model in MEGA6.0 (https://www.megasoftware.net/) (Tamura et al., 2013). The phylogenetic tree was visualized using the iTOL website (http://itol.embl.de/help.cgi) (Sugiyama et al., 2011). Finally, ABCG transporters were identified through the phylogenetic analysis of the ABC family of the pigeon pea.
Phylogenetic tree construction and chromosome localization
The ABCG genes’ chromosomal information was obtained through the NCBI website (https://www.ncbi.nlm.nih.gov/) and were systematically named based on the number and relative position of the ABCG genes in the pigeon pea chromosome. The phylogenetic tree of the pigeon pea ABCG transporters was constructed in MEGA6.0, as described above.
The chromosomal location of the CcABCGs was mapped using the MapChart software (https://www.wur.nl/en/show/Mapchart.htm) based on the chromosomal location information of the ABCG transporters (Voorrips, 2002).
Protein property prediction, subcellular localization
The exon information of CcABCGs was collected on the NCBI website. The number of amino acid sequences, relative molecular weight, and the isoelectric point (pI) of CcABCGs was predicted in the ExPASy ProtParam server database (http://expasy.org/). We predicted the subcellular localization of ABCG proteins using the Cell-PLoc 2.0 database (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/) (Chou & Shen, 2008).
To verify our predictions of subcellular localization, two-week-old pigeon pea seedlings were used as a cDNA template to amplify the full-length coding region of the CcABCG7 gene. The primers were: F: 5′-ATGGTGATGATATGGGAAAATGTTAC- 3′ and R: 5′-TTATATTGGAAGGTTTGGGGACA- 3′. The recovered PCR production was ligated to the T vector pMD19-T (TaKaRa, Japan). Subsequently, it was cloned into the eGFP-pROK II vector by double digestion using Kpn I/Xb I to construct the CcABCG7-eGFP-pROK II expression vector, and was transformed into the agrobacterium strain GV3101 (Shanghai Weidi Biotechnology, China). One-month-old tobacco seedlings were injected into the agrobacterium liquid on the back of tobacco leaves with a disposable syringe and placed in the dark for 3 days for observation. Approximately 0.5 cm2 of the material was taken around the injection site, observed, and photographed under a Leica SP8 laser confocal microscope (Leica, Germany).
Analysis of motifs and conserved domain
To examine the characteristics and properties of the pigeon pea ABCG subfamily protein domain, the conserved motif of CcABCGs was analyzed on the MEME software http://meme-suite.org/tools/meme with following parameters: the number of motifs was set 10 and the optimum motif width was set between 20 and 200, the other parameters were set to their default (Bailey & Elkan, 1994). Then InterPro program http://www.ebi.ac.uk/interpro/scan.html was used to annotate all 10 motifs.
The conserved domain of CcABCGs was performed using the HMMER servers (https://www.ebi.ac.uk/Tools/hmmer/) and visualized with TBtools software (Chen, 2018). We selected representative WBC and PDR transporter amino acid sequences as target sequences and chose the template with higher homology and better coverage using the Swiss-model server set to automatic (https://swissmodel.expasy.org/interactive). The model results were evaluated using the SAVES server (https://servicesn.mbi.ucla.edu/SAVES/), and the homology model of the CcABCG proteins was visualized by the PyMol software.
Gene structure and cis-elements analysis of CcABCGs
The intron/exon structures of the ABCG genes were analyzed using the gene annotation file (GFF), C.cajan_V1.0, and visualized using TBtools software (Chen, 2018), based on the evolution analysis of CcABCGs. The 2,000 bp upstream region of all identified CcABCGs was extracted from the pigeon pea genome to identify the stress-related or other functional cis-acting regulatory elements of the promoter sequences using TBtools software. All promoter sequences were analyzed using PlantCARE software (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Rombauts et al., 1999).
GO annotation and function prediction
GO (Gene Ontology) integrates and unifies the description and standards of gene products in a database and provides the most comprehensive description of gene functions and gene products. The ABCG protein sequences in the pigeon pea were used for blast alignment in the Swiss-prot database (https://www.uniprot.org/blast/) and the alignment results were annotated and classified by the pigeon pea ABCG gene (Ashburner et al., 2000; Consortium, 2019; Ehlert et al., 2006; Yang et al., 2016).
Plant materials and treatments
Pigeon pea seeds (ICPL87119) were sown in a soil mixture of nutrient soil, vermiculite, and perlite (1:1:1) in the greenhouse at Beijing Forestry University in China with natural light for 10-14 h/day, a temperature of 18−28 °C and a relative humidity of 45–70%. After three months, the roots, stems, leaves, and flowers from pigeon pea trees were selected and stored at −80 °C to extract RNA and further analyze their tissue-specific expression.
The pigeon pea seeds were surface-sterilized with 75% ethanol for 30 s, then soaked in sodium hypochlorite solution for 6 min, and finally washed 5 times with sterile water for 30 s durations. These sterilized seeds were grown in Murashige and Skoog (MS) medium at pH=5.8 in growth chambers at 24 ± 2 °C with a photoperiod of 16 h light/8 h dark, 400 µM m−2 s−1 light intensity. To analyze the expression of the CcABCG genes in various abiotic stresses, the 14-day old pigeon pea seedlings were assigned to different treatments. The treatments were as follows: heat and cold stress, in which the seedlings were cultured at 4 °C and 42 °C in incubators, respectively; salt and metal stress, in which the seedlings were grown in solid MS medium with 200 nmol/L NaCl and 100 µmo/L AlCl3, respectively; drought stress, in which the pigeon pea seedlings were treated with 250 nmol/L mannitol in solid MS medium. The roots and leaves of these seedlings were sampled at 0 h, 6 h, and 12 h after various treatments. Three biological replicates of each sample were immediately frozen in liquid nitrogen and stored at −80 °C until expression analysis of variously abiotic stresses was conducted.
RNA isolation and quantitative real-time PCR analysis
We selected 10 genes containing elements that responded to adversity for tissue specificity analysis and abiotic stress analysis based on the above analysis of cis-elements in the promoter region. Total RNA was isolated using the CTAB method and first-strand cDNA was synthesized from 1 µg of total RNA using a PrimeScript RT kit (Takara) according to the manufacturer’s instructions. The quality and concentration of cDNA were assessed using a Nano Photometer N50 (Implen GmbH, Munich, Germany). We performed expression analysis of the ABCG transporter under different tissues and abiotic stresses using qRT-PCR. Real-time RT-PCR analysis was performed using CFX connect (Bio-Rad, California, USA) with the SYBR Green PCR Master Mix (TaKaRa, Tokyo, Japan) with CcActin as a reference gene. The gene primers selected were synthesized on the Sangon Biotech website (http://www.sangon.com/newPrimerDesign) and are shown in Table S1. All analyses were performed with three biological replicates and qRT-PCR data was analyzed using IBM SPSS 22 software (IBM Corporation, USA).
Results
Identification of ABCG transporter genes in the pigeon pea genome
One hundred-twenty nine Arabidopsis ABC proteins were downloaded and used as queries in the pigeon pea genome database using BLASTP server to identify all members of the pigeon pea ABCG transporters. A total of 222 ABC proteins were identified in the pigeon pea after removing redundancies. The HMMER search was performed using the HMM of ABC transporters (such as PF00005, PF00664, and PF10614) to screen for ABC transporters identified by BLASTP. One hundred fifty-five ABC proteins were eventually confirmed to have NBD/TMD domains (Table S2). To explore the phylogenetic evolutionary relationship between the pigeon pea and other species including Arabidopsis, rice, and soybean, a Neighbor-Joining (NJ) tree was constructed (Fig. S1) based on ABC proteins. A phylogenetic relationship indicated that the pigeon pea ABC gene family was classified into 8 different groups (ABCA-ABCI), and the ABCG subfamily, which contained 51 members, was the largest group of ABC transporters in the pigeon pea.
Phylogenetic tree construction and chromosome localization
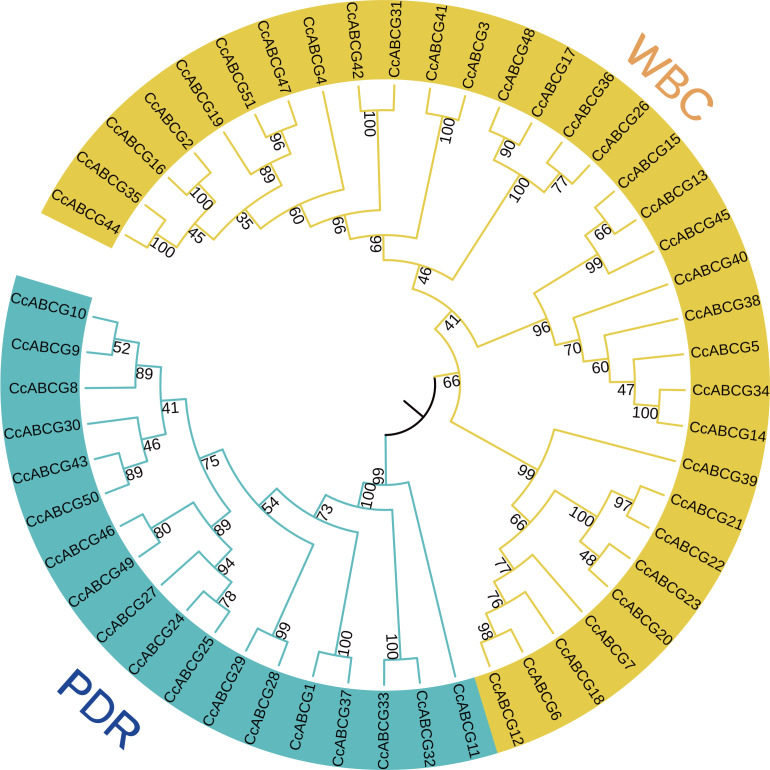
All of the ABCG transporters identified above were named CcABCG1-CcABCG51 based on their chromosomal location. A Neighbor-Joining (NJ) tree was constructed to analyze the phylogenetic relationship of ABCG transporters (Fig. 1). Phylogenetic analysis indicated that the ABCG transporters were further divided into WBC and PDR, in which WBC contained 33 ABCG transporters and PDR contained 18 transporters.
Figure 1. Phylogenetic analysis of the ABCG transporters among pigeon pea.
Using the MEGA6.0 program, the NJ (Neighbor-Joining, NJ) tree was constructed using the amino acid sequence of the pigeon pea ABCG transporters. The numbers beside the branches represent bootstrap values based on 1,000 replications. The outer side of the phylogenetic tree is a branch labeled with two subgroups of the pigeon pea ABCG transporters, and shown in different colors. WBC, white-brown complex; PDR, pleiotropic drug resistance.
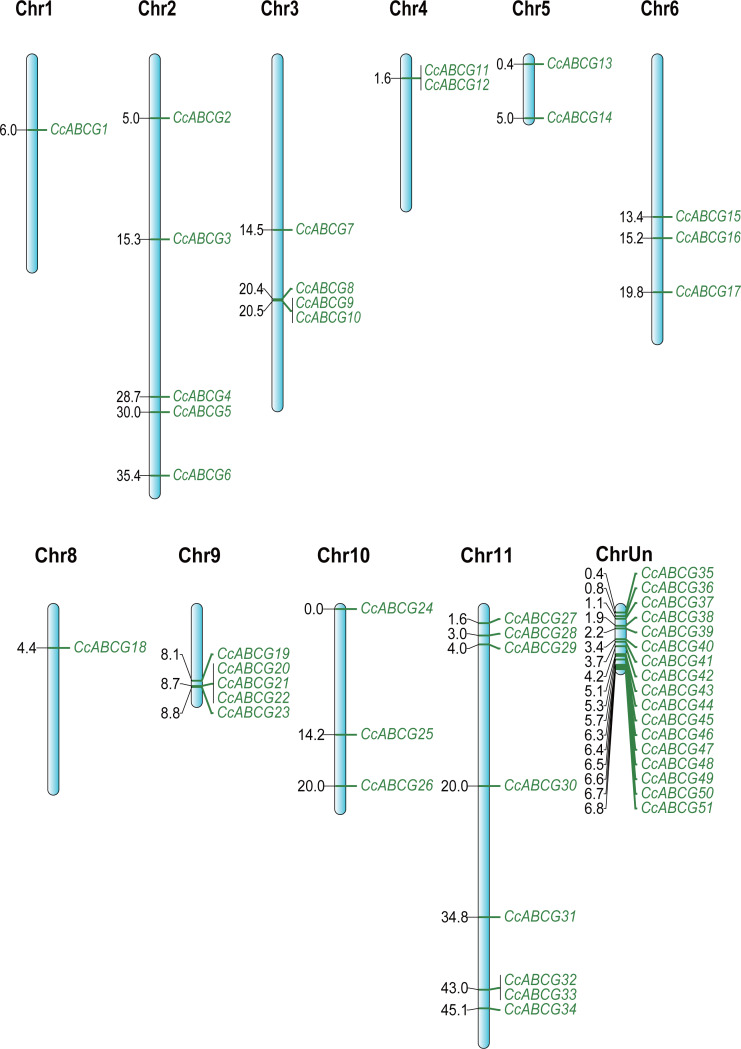
The chromosome localization of ABCG transporters was determined in the pigeon pea genome and all identified CcABCGs were found to be diversely distributed on pigeon pea chromosomes, except for chromosome 7. Chromosomes 1 and 8 both contained one ABCG transporter and all chromosomes of the pigeon pea had the least ABCG transporters. Chromosomes 2, 9, and 11 included 5, 5, and 8 transporters, respectively. Seventeen genes were distributed on unplaced scaffolds and were mapped into a single chromosome, ChrUn (Fig. 2). Two pairs of genes (CcABCG8/9, CcABCG32/33) were closely linked on the chromosome and belonged to the paralogous genes. The ABCG gene locations were annotated using the NCBI website and the information in shown in Table 1.
Figure 2. The chromosomal location of the pigeon pea ABCG genes.
The pigeon pea ABCG genes were named CcABCG1-CcABCG51 based on chromosomal location information. All of ABCG genes were location in all pigeon pea chromosomal except of chromosomal 7. ChrUn contained 17 members, which were mapped based on the length of those unplaced scaffold sequences with 17 genes, respectively.
Table 1. Prediction of physicochemical properties and subcellular localization of ABCG transporters.
| Gene | Accession | Location | Position | Length (aa) | Mw | pI | Chr | Exon | Sub-loc |
|---|---|---|---|---|---|---|---|---|---|
| CcABCG1 | KYP76113 | LOC109804364 | 6,006,382–6,022,606 | 1,421 | 159155.33 | 7.76 | 1 | 23 | Cell membrane |
| CcABCG2 | KYP72481 | LOC109789679 | 5,026,814–5,030,449 | 645 | 72010.85 | 9.08 | 2 | 5 | Cell membrane |
| CcABCG3 | KYP73490 | LOC109810734 | 15,320,209–15,323,794 | 684 | 77318.74 | 8.11 | 2 | 10 | Cell membrane |
| CcABCG4 | KYP74721 | LOC109795335 | 28,723,419–28,730,237 | 668 | 73512.6 | 9.47 | 2 | 3 | Cell membrane |
| CcABCG5 | KYP74838 | LOC109806747 | 29,968,224–29,970,344 | 619 | 69012.83 | 9.4 | 2 | 1 | Cell membrane |
| CcABCG6 | KYP75362 | LOC109815813 | 35,437,420–35,445,042 | 726 | 79570.36 | 8.27 | 2 | 12 | Cell membrane |
| CcABCG7 | KYP70503 | LOC109797228 | 14,492,247–14,496,242 | 660 | 73883.51 | 8.81 | 3 | 9 | Cell membrane |
| CcABCG8 | KYP71088 | LOC109796083 | 20,447,829–20,456,456 | 1,448 | 164560.21 | 8.28 | 3 | 20 | Cell membrane |
| CcABCG9 | KYP71089 | LOC109796084 | 20,469,958–20,479,590 | 1,444 | 163478.51 | 8.27 | 3 | 20 | Cell membrane |
| CcABCG10 | KYP71090 | LOC109796805 | 20,488,744–20,497,576 | 1,454 | 164868.63 | 7.95 | 3 | 20 | Cell membrane |
| CcABCG11 | KYP68041 | LOC109798752 | 1,617,897–1,630,220 | 718 | 80600.64 | 9.1 | 4 | 9 | Cell membrane |
| CcABCG12 | KYP68044 | LOC109798893 | 1,641,751–1,647,610 | 686 | 76872.23 | 8.88 | 4 | 10 | Cell membrane |
| CcABCG13 | KYP67437 | LOC109799601 | 355,237–358,794 | 814 | 91841.52 | 8.95 | 5 | 6 | Cell membrane |
| CcABCG14 | KYP67863 | LOC109799748 | 5,000,555–5,003,051 | 643 | 72113.02 | 7.88 | 5 | 1 | Cell membrane |
| CcABCG15 | KYP66379 | LOC109800436 | 13,422,611–13,426,469 | 721 | 81114.25 | 8.97 | 6 | 3 | Cell membrane |
| CcABCG16 | KYP66524 | LOC109801031 | 15,158,990–15,162,297 | 663 | 74678.78 | 8.6 | 6 | 5 | Cell membrane |
| CcABCG17 | KYP66999 | LOC109800523 | 19,843,419–19,853,680 | 1,092 | 121456.99 | 8.87 | 6 | 14 | Cell membrane |
| CcABCG18 | KYP61725 | LOC109804023 | 4,405,565–4,412,603 | 704 | 78466.57 | 8.83 | 8 | 10 | Cell membrane |
| CcABCG19 | KYP61105 | LOC109804765 | 8,122,601–8,126,214 | 632 | 70593.09 | 9.02 | 9 | 4 | Cell membrane |
| CcABCG20 | KYP61155 | LOC109805263 | 8,663,643–8,668,819 | 658 | 73428.07 | 7.99 | 9 | 8 | Cell membrane |
| CcABCG21 | KYP61157 | LOC109805167 | 8,722,014–8,728,825 | 679 | 75362.12 | 8.36 | 9 | 8 | Cell membrane |
| CcABCG22 | KYP61159 | LOC109805166 | 8,746,304–8,752,313 | 681 | 75328.34 | 8.65 | 9 | 8 | Cell membrane |
| CcABCG23 | KYP61160 | LOC109804802 | 8,756,197–8,761,747 | 648 | 71982.39 | 8.81 | 9 | 9 | Cell membrane |
| CcABCG24 | KYP58337 | LOC109805773 | 11,158–17,707 | 1,427 | 161183.4 | 7.36 | 10 | 21 | Cell membrane |
| CcABCG25 | KYP59575 | LOC109805875 | 14,201,296–14,246,400 | 1,431 | 161968.54 | 7.97 | 10 | 21 | Cell membrane |
| CcABCG26 | KYP60108 | LOC109805696 | 19,992,101–20,040,472 | 1,104 | 123273.83 | 8.89 | 10 | 16 | Cell membrane. Chloroplast. |
| CcABCG27 | KYP53996 | LOC109808825 | 1,626,589–1,635,183 | 1,429 | 162016.24 | 7.32 | 11 | 22 | Cell membrane |
| CcABCG28 | KYP54148 | LOC109808608 | 3,046,631–3,056,753 | 1,482 | 167729.07 | 8.19 | 11 | 23 | Cell membrane. Chloroplast. |
| CcABCG29 | KYP54262 | LOC109807460 | 4,014,294–4,034,321 | 1,500 | 170370.83 | 6.75 | 11 | 26 | Cell membrane. Chloroplast. |
| CcABCG30 | KYP55666 | LOC109807699 | 19,989,558–20,004,511 | 1,418 | 161820.65 | 8.9 | 11 | 26 | Cell membrane |
| CcABCG31 | KYP56982 | LOC109809098 | 34,818,776–34,826,902 | 741 | 81702.72 | 9.05 | 11 | 12 | Cell membrane |
| CcABCG32 | KYP57733 | LOC109807641 | 43,015,398–43,024,752 | 1,449 | 164070.17 | 7.67 | 11 | 24 | Cell membrane |
| CcABCG33 | KYP57734 | LOC109809552 | 43,027,240–43,038,856 | 1,445 | 163218.52 | 8.03 | 11 | 24 | Cell membrane |
| CcABCG34 | KYP57934 | LOC109807560 | 45,075,476–45,077,364 | 609 | 68159.95 | 8.65 | 11 | 1 | Cell membrane |
| CcABCG35 | KYP53034 | LOC109810595 | 391,122–395,936 | 681 | 75466.37 | 8.91 | Un | 5 | Cell membrane |
| CcABCG36 | KYP53068 | LOC109810569 | 840,758–848,796 | 1,115 | 123587.99 | 9.16 | Un | 14 | Cell membrane |
| CcABCG37 | KYP52287 | LOC109811196 | 2,121–13,637 | 1,229 | 138171.93 | 8.81 | Un | 20 | Cell membrane |
| CcABCG38 | KYP52347 | LOC109811227 | 785,413–787,392 | 651 | 73181.09 | 8.45 | Un | 1 | Cell membrane |
| CcABCG39 | KYP51780 | LOC109811628 | 147,015–153,318 | 723 | 80384.61 | 8.89 | Un | 12 | Cell membrane |
| CcABCG40 | KYP51380 | LOC109811918 | 541,176–543,014 | 612 | 68365.89 | 9.39 | Un | 1 | Cell membrane |
| CcABCG41 | KYP49769 | LOC109813109 | 127,353–133,820 | 683 | 77708.58 | 9.05 | Un | 10 | Cell membrane |
| CcABCG42 | KYP49445 | LOC109813363 | 17,796–28,760 | 744 | 82372.18 | 8.91 | Un | 12 | Cell membrane |
| CcABCG43 | KYP48955 | LOC109813737 | 400,317–424,748 | 1,358 | 154173.34 | 8.61 | Un | 21 | Cell membrane |
| CcABCG44 | KYP48157 | LOC109814303 | 92,212–96,948 | 678 | 75455.58 | 9.06 | Un | 5 | Cell membrane |
| CcABCG45 | KYP47060 | LOC109815112 | 59,170–61,921 | 755 | 83546.16 | 9.22 | Un | 1 | Cell membrane |
| CcABCG46 | KYP41536 | LOC109818995 | 187,197–197,961 | 1,445 | 163678.86 | 8.75 | Un | 24 | Cell membrane |
| CcABCG47 | KYP39219 | LOC109788852 | 72,316–76,120 | 624 | 69345.94 | 8.87 | Un | 4 | Cell membrane |
| CcABCG48 | KYP37548 | LOC109790064 | 6,674–12,941 | 1,087 | 120433.13 | 8.96 | Un | 14 | Cell membrane |
| CcABCG49 | KYP36564 | LOC109790672 | 14,766–22,348 | 1,457 | 164896.42 | 8.25 | Un | 24 | Cell membrane |
| CcABCG50 | KYP35274 | LOC109791517 | 44,302–58,642 | 1,473 | 167555.55 | 7.74 | Un | 20 | Cell membrane |
| CcABCG51 | KYP32835 | LOC109793033 | 5,717–9,684 | 628 | 70573.41 | 8.74 | Un | 4 | Cell membrane |
Protein property prediction, subcellular localization of CcABCGs
Fifty-one CcABCGs were identified and their protein properties were predicted (Table 1), including their amino acid lengths, relative molecular weights, and isoelectric points (pIs). Results indicated that the lengths of all ABCG proteins ranged from 609 aa (CcABCG34) to 1500 aa (CcABCG29). Similarly, their relative molecular mass ranged from 68159.95 Kd (CcABCG34) to 170370.83 Kd (CcABCG29) and the theoretical isoelectric points ranged from 6.75 (CcABCG29) to 9.47(CcABCG4). The ABCG genes’ exon numbers changed from 1 to 26.
We performed the subcellular localization of CcABCGs to determine the active sites of the CcABCGs. The prediction of the subcellular localization indicated that all CcABCGs were localized in the cell membrane. Three ABCG transporters (CcABCG26, 28, 29) were found to be localized in the chloroplast and not just on the cell membranes (Table 1). To confirm our prediction, we verified the subcellular localization of the ABCG transporter by transiently expressing CcABCG7-eGFP in tobacco leaves. The results showed that the green fluorescent signal of CcABCG7-eGFP-pROK II was mainly detected in the cell membrane (Fig. S2).
Analysis of motifs and conserved domain of CcABCGs
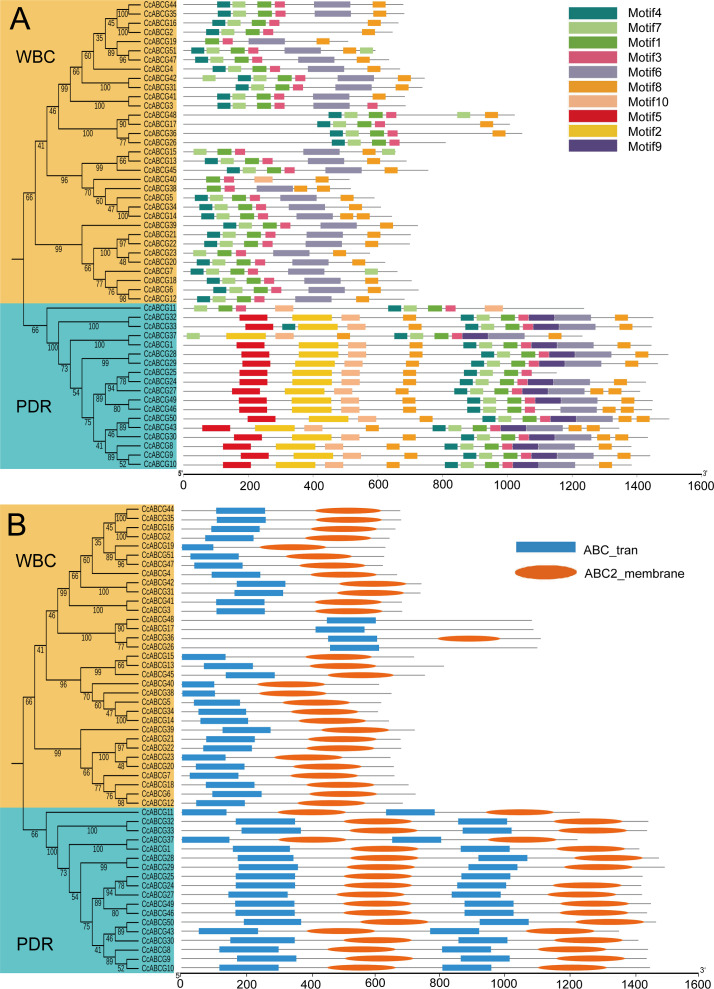
To identify the structure and function of the ABCGs in the pigeon pea, motif analysis was performed based on the phylogenetic analysis (Fig. 3A). A total of 10 conserved motifs were predicted and the width of those motifs ranged from 32 to 123 amino acids. The number of motifs in each amino acid varied from 2 to 10. Results showed that the number of motifs in the WBC was significantly less than the number of motifs detected in the PDR. All 10 motifs were annotated using the InterPro program to identify the motif structure. Annotation analysis demonstrated that Motifs 3, 7, 9, and 10 did not annotate at all; Motif 6 and Motif 8 annotated in the ABC_2_trans structure (IPR013525); Motif 1,2,4,5 annotated in the P_loop_NTPase structure (IPR027417); Motifs 1, 4, and 5 were annotated in the ABC_transporter_like structure (IPR003439). Our results indicated that the motifs of the same annotation were relatively conservative in structure, such as Motifs 1, 4, and 5 (Table S3).
Figure 3. Motifs and conserved domain of CcABCGs.
(A) The 10 conserved motif of CcABCGs on the MEME software. Motif annotation is shown as color legends. Annotates of motif were listed on Table S3. (B) Conserved domain of CcABCGs. Blue indicates the NBD (nucleotide binding domain), and orange indicates the another conserved domain TMD (trans-membrane domains).
We examined the conserved domain of CcABCGs to explore the ABCG transporter’s domain function using HMMER servers (Fig. 3B). The HMME model, “ABC2_membrane” indicated the TMD domain, and “ABC_tran” indicated the NBD domain. The results showed that the ABCG transporters were different from other members of the ABC transporter family with an inverted “TMD-NBD” domain arrangement pattern. All ABCG transporters contained the NBD domain, but several ABCG transporters did not contain the TMD domain. The WBC subgroup contained one NBD domain with a length that increased from 97 to 153 and one or no TMD domains with a length of approximately 200, while the PDR subgroup contained two NBD domain with an amino acid length of 138 to 200 and a domain approximately 200 TMD long.
CcABCG35 was selected for homology modeling to explore the molecular functions of CcABCGs. The best template of CcABCG35 was 6hij.1A (Seq Identity:33.05%, GMQE:0.58, Coverage:0.85) using the Swiss-model program. According to the PROCHECK evaluation results, Ramachandran Plot results in the model evaluation revealed that in each ABCG model >90% of the amino acid residues were distributed in the allowed region, indicating that the quality of the CcABCG protein model obtained by homology modeling was reliable (Fig. S3A). A model of CcABCG35 revealed that Walker A and Walker B were located in the NBD domain, while the TMD domain contained six alpha helices (Fig. S3B).
Gene structure and cis-elements analysis of CcABCGs
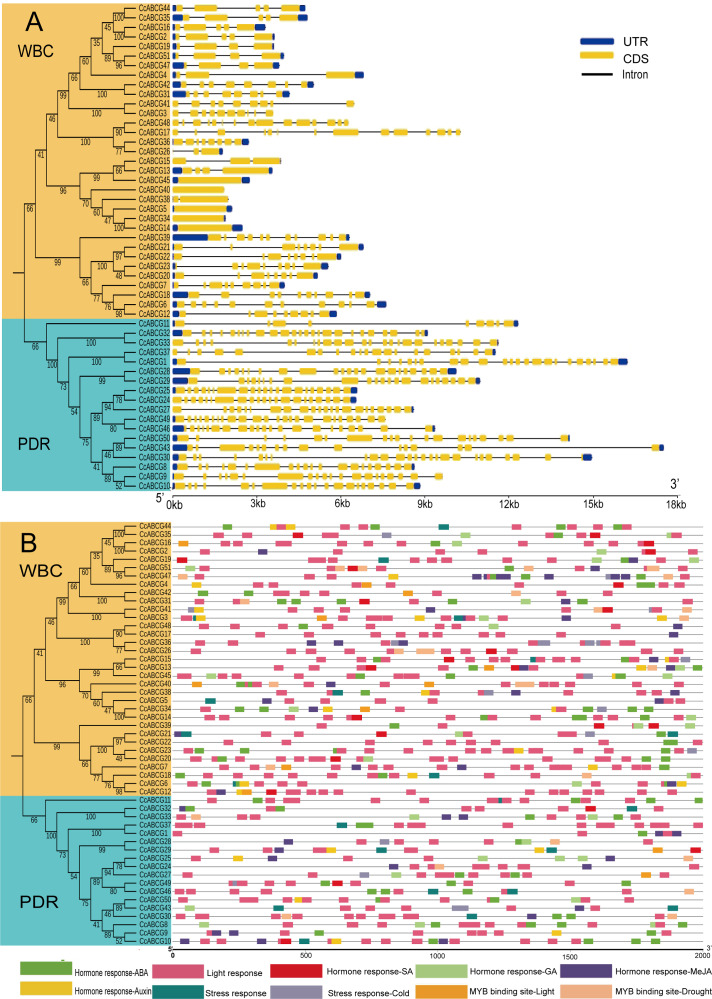
Gene structure was analyzed based on the phylogenetic relationship of the ABCG gene family to better understand their structural evolution (Fig. 4A). The highly conserved exon sequence is an essential sequence for the ABCG transporter to perform gene functions, and the differences in introns may be based on dissimilar regulatory mechanisms for the existence of genes. The results indicated that all ABCG transporters in the pigeon pea contained different numbers of exons and introns. The number of exons in CcPDRs was significantly higher than that in CcWBCs. The number of exons of CcPDRs ranged from 5 to 25, while the number of exons of CcWBCs varied from 1 to 14 and there were large differences in the number of introns. There were approximately 20 introns in CcPDRs and about 5 in CcWBCs.
Figure 4. Gene structure and cis-elements analysis of ABCG genes.
(A) Intron-exon structures of CcABCG genes. Yellow rectangles: coding sequences (CDSs); thin lines: introns; blue rectangles: untranslated regions (UTRs). (B) Putative regulatory cis-elements in the ABCG gene promoters. The relative positions of elements are labeled with capital letters in the figure.
A 2,000 bp region upstream of the promoter was selected for analysis of the cis-acting element of the pigeon pea ABCG gene family in order to explore the expression elements of the promoter region (Fig. 4B). Three cis-acting elements were screened, and focused on hormone-responsive elements, light-responsive elements, and stress-responsive elements. The number of light-responsive elements in the promoter region of the pigeon pea ABCG transporter was found to be relatively large. Hormonal response elements had a large number of copies in the promoter region of the ABCG transporter, including auxin response elements (AuxRR-core, TGA-element, etc.) and gibberellin response elements. There were fewer stress response elements than hormone response elements and some genes did not contain stress response elements (CcABCG14, CcABCG10, etc.). Drought-related cis-acting elements were identified simultaneously in CcABCG7 and CcABCG24. Low-temperature stress-related cis-acting elements (LTR) were identified in CcABCG28 and their elements were highly conserved with short sequences composed of CCGAAA.
Go annotation and function prediction
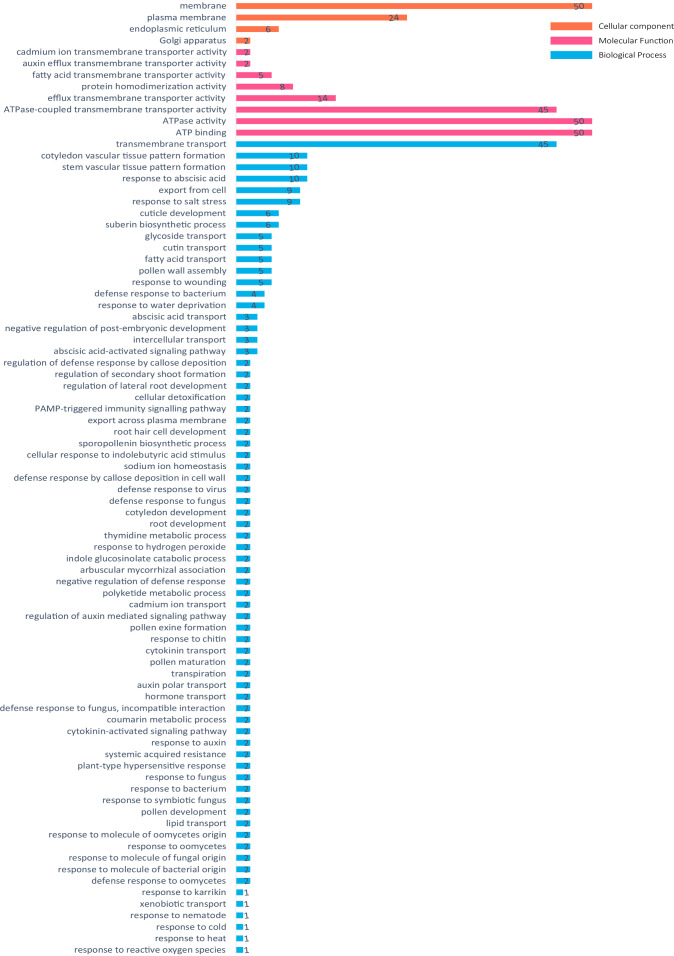
The ABCG gene in pigeon pea was annotated and divided into three categories: molecular function, biological processes, and cell components. The annotation results showed that all 50 genes were annotated into three major categories, but CcABCG48 did not show results from the blast alignment method and UniProtKB ID and all other usable data are provided in Data S5. The transmembrane transport (GO:0055085) had the highest prominence and 45 genes were annotated in the transmembrane transport. CcABCG28 and CcABCG29 were blasted in AB36G_ARATH (UniProtKB ID), and annotated in 42 GO annotations, which included “response to salt stress” (GO:0009651) and “root development” (GO:0048364). Fifty ABCG genes were annotated to the broad category of molecular functions related to ATPase activity and ATP binding. The major category of cellular components found most of the ABCG genes to be annotated on the membrane (Fig. 5).
Figure 5. Go annotation of ABCG transporters in pigeon pea.
GO annotations of ABCG transporters in pigeon pea were predicted using Swiss-prot database serve. Red means molecular function, yellow means cell component and blue means biological process. Displaying only results for FDR P < 0.05.
Expression analysis of the pigeon pea ABCG gene family in different organs
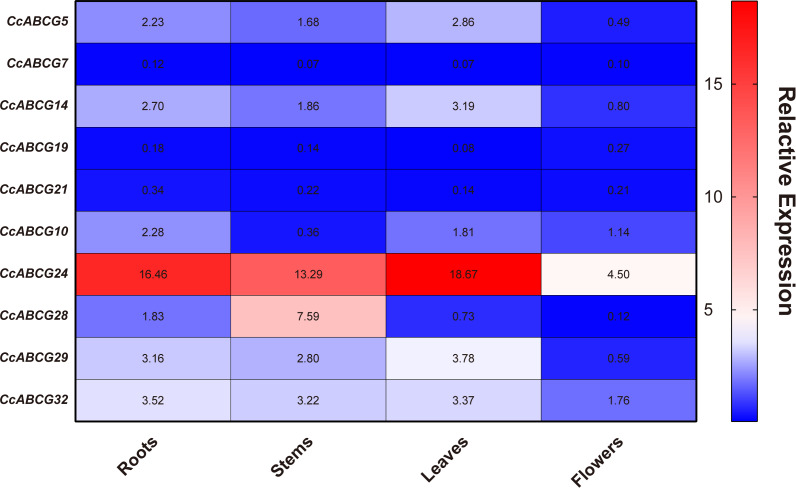
To explore the expression pattern of the ABCG gene in different organs during the growth and development of pigeon peas, the organs (roots, stems, leaves, and flowers) of one-year-old pigeon peas were collected to extract RNA for qRT-PCR analysis for the expression of the ABCG genes. Tissue-specific expression analysis found that the expression of ABCG transporters in different tissues was not significantly different. However, there was an obvious difference in the expression level of the ABCG transporter gene in flowers, which was significantly lower than that in the roots, stems, and leaves. The expression of CcABCG24 was significantly higher than that of any other gene (Fig. 6).
Figure 6. Expression analysis of pigeon pea ABCG gene in different organs.
The expression levels of 10 genes in different tissues are shown in the heat map. Three biological replicates per sample. Values represent means ± SEM.
Expression analysis of the pigeon pea ABCG transporters under different abiotic stresses
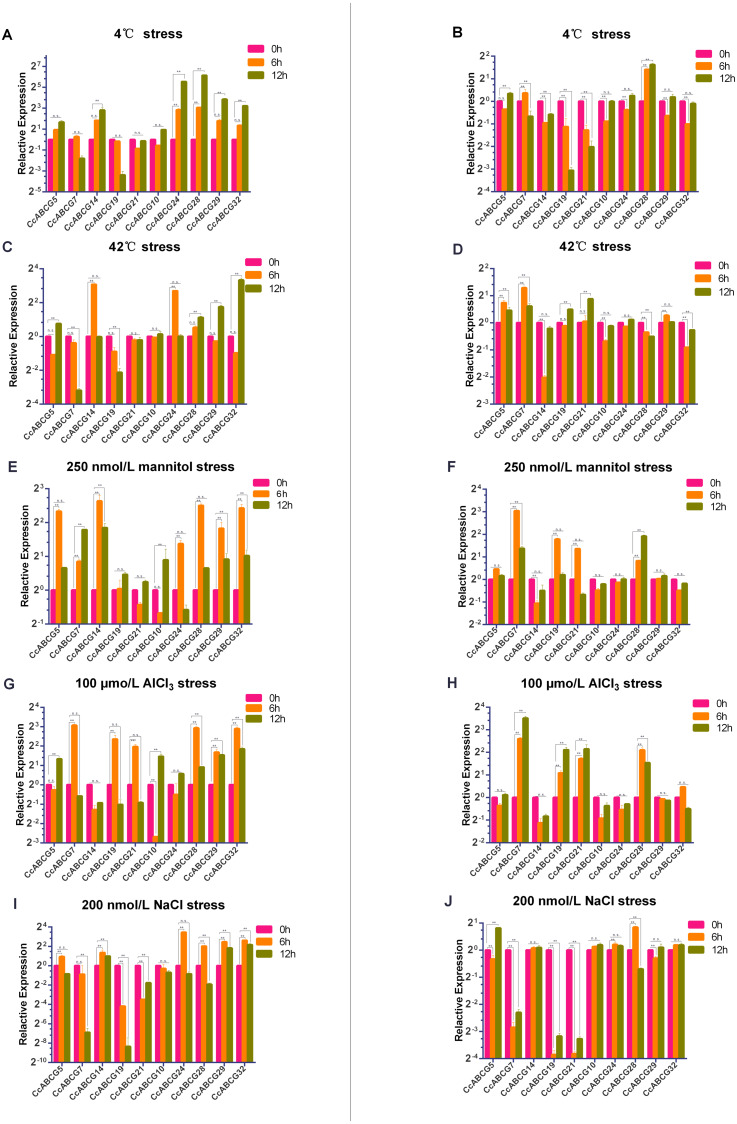
To explore the expression level of the pigeon pea ABCG gene family under different abiotic stresses, two-week-old pigeon pea seedlings were selected and transplanted into the culture environments at 4 °C, 42 °C, 200 nmol/L NaCl, 100 µmol/L AlCl3 and 250 nmol/L mannitol in solid MS medium, respectively. CcABCG5,7,14,19,21 belonged to the WBC subgroup, and CcABCG10,24,28,29,32 belonged to the PDR subfamily. We found that the expression difference of 10 genes in the roots was significantly higher than in the leaves (Fig. 7).
Figure 7. Relative expression analysis of the pigeon pea ABCG genes under different abiotic stresses.
Relative expression level of the ABCG genes in pigeon pea under different abiotic stresses of 4 °C (A, B), 42 °C (C, D), 250 nmol/L mannitol (E, F), 100 µmo/L AlCl3 (G, H), 200 nmol/L NaCl (I, J). Expression analysis was performed using a relative quantitative method 2−ΔΔCq, and CcActin as internal reference gene. Relative expression level of roots: A, C, E, G, I; relative expression level of leaves: B, D, F, H, J. Three biological replicates of each sample. Values represent means ± SEM. Asterisks indicate significant difference as determined by Dunnett’s multiple comparisons test (**, P < 0.01). n.s., no significant difference.
The two major subfamilies of ABCG transporters had significant differences in expression in the roots (Figs. 7A, 7C, 7E, 7G and 7I) under 4 °C stress and NaCl stress. The PDR family genes were significantly up-regulated under both stressors. CcABCG24 was up-regulated 40-fold after 12 h at 4 °C and CcABCG28 increased 65 times. Under the initial NaCl treatment, CcABCG24 increased 10 times. However, it is worth noting that with an increase of the treatment time, the expression of CcABCG24 decreased to below the initial level at 12 h. We found that the relative expression changes of the WBC subgroups under drought and aluminum stress treatments were opposite, as shown in Figs. 7E and 7G. The expression level of CcABCG5 increased under drought stress at 6 h but did not change significantly under aluminum stress. CcABCG19 also exhibited the same pattern of opposite expression in the treatments of mannitol stress and AlCl3 stress. Under high-temperature stress, we observed that the expression of CcABCG7 was down-regulated but the expression of CcABCG7 in leaves was up-regulated.
There was a relatively mild difference in the expression levels in leaves (Figs. 7B, 7D, 7F, 7H and 7J). CcABCG28 experienced up-regulation under 4 °C treatment. However, CcABCG7 was significantly up-regulated after 6 h of drought stress. In the same situation, CcABCG7 was up-regulated 11.3 times after 12 h of aluminum stress treatment. However, under sodium stress, CcABCG7, 19, 21 were down-regulated. The ABCG transporter was found to have different expressions in response to different environmental stresses.
Discussion
ABC transporters are found in animals and plants and have a large number of complex biological functions. A total of 155 ABC transporters were identified in the pigeon pea genome, which were divided into 8 subgroups of ABCA-ABCI (Fig. S1). Among them, the ABCE and ABCF subfamily had no transport function because their proteins were localized to the endoplasmic reticulum without a transporter region. The ABCB subfamily may be involved in the transport of auxins, secondary metabolites, and heavy metal salts (Kang et al., 2011; Verrier et al., 2008). The ABCC subfamily may be involved in plant chlorophyll transport and cell detoxification among other functions (Hashimoto & Yamada, 2003). The ABCG transporters contained 51 members and was the largest group of ABC transporters in the pigeon pea, which was larger than reported for Arabidopsis (44) and rice (50), but smaller than the 116 members of ABCG reported in soybean (Mishra et al., 2019; Jasinski et al., 2003).
As shown in Fig. 1, the ABCG subfamily could be further divided into a full-molecular transporter PDRs and a semi-molecular transporter WBCs, in which PDRs contained 18 ABCG transporters and WBCs included 33 ABCG transporters. The conserved domain of the ABCG transporter is composed of NBD and TMD, and the ABCG transporter is different from the conserved domain arrangement of other ABC transporter subfamilies. The conserved domain composition of the ABCG transporter is a trans-TMD-NBD structure (Fig. 3) (Van den Brule & Smart, 2002). The arrangement of ABC domains and their transmembrane domains are highly conserved, while the number of transmembrane helixes and their arrangements is not necessarily conserved, and determines their functional differences (Andolfo et al., 2015; Goodman, Casati & Walbot, 2004; Locher, 2004). ABCG transporters are involved in many physiological activities of plants, including the transport of small molecular compounds, secondary metabolites, and are active in disease resistance, hormonal regulation, and adaptation to changes in the external environment (Alejandro et al., 2012; Bird et al., 2007; Lee et al., 2010). Arabidopsis cutin and wax secretion require the participation of AtWBC11 (Panikashvili et al., 2007a). Rice OsPDR9 could be induced by methyl jasmonate, and AtPDR8 could be induced by salicylic acid (Kim et al., 2007; Kuromori et al., 2010; Moons, 2008). External stressors on plants cause the signal receptors on plant cells to first sense the external stress signal and produce a second messenger transmitted inside the cell, such as Ca2+, reactive oxygen and ABA. The stress response also mediates proteins, causing phosphorylation to activate downstream transcription factors and stress-related target genes, thereby resisting the destruction of plant cells (Mittler, Finka & Goloubinoff, 2012; Farooq et al., 2009). Many ABCG genes annotated hormone transport and regulation-related functions (Fig. 4B and 5). AtABCG22 was induced by drought stress, possibly by affecting the stomata and increasing transpiration (Kuromori, Sugimoto & Shinozaki, 2011). In our study, CcABCG28 (PDR) may be involved in the response to abiotic stresses including colder temperatures and salinity, while CcABCG7 (WBC) in the pigeon pea tended to respond to drought and aluminum stress, as shown in Fig. 7.
The analysis of the location of the ABCG family in the pigeon pea chromosome found that most of the ABCG gene was located on chromosome 11, as shown in Fig. 2. The major part of most plant genomes consisted of different repeating DNA elements. These sequence elements are essential for the large-scale organization and evolution of the plant genome (Kubis, Schmidt & Heslop-Harrison, 1998). Our results showed that there were two pairs of paralogous genes (CcABCG8/9, CcABCG32/33) closely linked to chromosome 3 and chromosome 11, and the tandem replication led to the expansion of these two genes. Introns are important components in the genome of eukaryotes. The typical 5′-GT...AG- 3′ of intron is an important marker of gene splicing and an important feature of introns in eukaryotic mRNA sequences (Rose et al., 2016; Mukherjee et al., 2018). We performed exon/intron analysis of the ABCG transporter to determine the stress regulation of the splicing process in Fig. 4A. Our results indicated that PDR contained more intron structures than WBC. It is thought that PDR had more variable splices and functions and may respond more frequently to stress. Furthermore, introns may also affect gene expression, and more introns may have a stronger regulatory effect (Rose et al., 2016; Mukherjee et al., 2018). There is no evidence supporting the theory that more exons make them prone to more regulation. PDR/WBC have different regulatory effects, which depends on deeper molecular mechanisms. The cis-acting elements are involved in the binding of transcription factors (TF) and regulate the expression of the gene (Toledo et al., 2011). Our study found that there were many low temperature-related elements in the promoter region of the pigeon pea, which might be induced by chilling in the pigeon pea (Fig. 4B). The promoter region of the pigeon pea ABCG transporter had a large number of hormone-regulated expression elements (abscisic acid responsiveness, MeJA responsiveness), which also demonstrated the important role of ABCG transporters in the regulation of plant hormones (Kuromori et al., 2010; Wu, Lewis & Spalding, 2007). Kuromori, Sugimoto & Shinozaki (2011) found that AtABCG25 is mainly expressed in vascular tissues and can transport ABA out of cells.
ABC transporters are essential for plant development and play a role in the processes of gametogenesis, seed development, seed germination, organ formation, and secondary growth (Do, Martinoia & Lee, 2018). We performed expression analysis of the pigeon pea in different organs and under different abiotic stresses (Figs. 6 and 7). The expression levels of all genes in flowers were not overtly different than in other tissues, while CcABCG24 was more highly expressed in other tissues. CcABCG24 was expressed in the stems of pigeon peas in significantly higher amounts than in other tissues, showing that ABCG transporters are found in active regions such as trans-membrane proteins. AtABCG25 is mainly expressed in vascular tissues, can transport ABA to outside of cells (Kuromori, Sugimoto & Shinozaki, 2011), and is closely related to the formation of plant vascular bundles. We showed that the expression of most genes in the roots was significantly higher than that in the leaves by analyzing the expression levels of the pigeon seedlings under different stresses. Interestingly, the expression of CcABCG28 is up-regulated, regardless of any tissue (root or leaf), especially in the leaves. The up-regulation of this expression is significantly higher than any other gene under low-temperature stress. At present, the regulation mechanism of the CcABCG28 homologous gene in other species is still unclear, but it was shown to play an important role in the growth and development of the pigeon pea. We also found that CcABCG24 was highly expressed under NaCl treatment, and its homologous gene AtPDR12 was of great significance for Pb(II) resistance in Arabidopsis (Lee et al., 2005). CcABCG24 played an important role in response to salt stress in this experiment. In response to drought and salt stress in plants, the cuticle lipid coding ABCG transporter gene was significantly up-regulated, indicating that the ABCG transporter has an important role in adapting plants to drought and saline-alkali environments (Luo et al., 2007; Panikashvili et al., 2007a). The expression level of CcABCG7 was up-regulated in pigeon pea roots under drought and aluminum stress, which proved its important role in stress tolerance.
Conclusions
A total of 51 ABCG transporters were identified and divided into two subgroups: WBC and PDR. We analyzed the protein structure and gene structure cis-elements on the pigeon pea ABCG transporters. The highly conserved NBD domain determines the important function of the ABCG transporter. CcABCG28 was significantly up-regulated under low temperature stress, while CcABCG7 responded to drought stress. In conclusion, our results reveal the role of ABCG transporters in abiotic stress resistance and broaden the research direction in abiotic stress resistance of pigeon peas.
Supplemental Information
A Neighbor-Joining (NJ) tree of ABC transporters was constructed with MEGA6.0 program. The numbers beside the branches represent bootstrap values based on 1000 replications. The red circle indicates ABC transporters in soybean; the green square denotes ABC transporters in rice; the blue triangle indicates ABC transporters in Arabidopsis. All ABC transporters are divided into 8 subgroups (ABCA-ABCG, ABCI) that shown in different color at the inside of the circle.
The CcABCG7 :eGFP plasmid was transformed with the into the leaves of tobacco. Fluorescence was visualized by a confocal laser scanning microscope. Bar = 50µm.
(A) In Ramachandran Plot, the A (red), B (yellow) and L (light yellow) areas represent the most favored, additional allowed and (generously allowed) areas respectively; the other (white) areas are It is a disallowed area. (B) The red and shown regions respectively represent the two motifs of the ABCG transporter NBD domain; where red represents Walker A[GPSGSGKT] and blue represents Walker B [KRVSIGQEML].
Acknowledgments
The authors gratefully acknowledge the support of The College of Forestry, Beijing Forestry University. and Beijing Advanced Innovation Center for Tree Breeding by Molecular Design.
Funding Statement
This work was supported support by the Beijing Forestry University New Teachers Scientific Research Startup Fund Project (BLX201807), the National Natural Science Foundation of China (31930076), Outstanding Young Talent Fund in Beijing Forestry University (2019JQ03009), the National Natural Science Foundation of China (31901281), National Key R&D Program of China (2018YFD1000602, 2019YFD1000605-1), the China Postdoctoral Science Foundation (2019M660505). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Dong Meng, Email: mengdongjlf@163.com.
Yujie Fu, Email: yujie_fu@163.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Lili Niu analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, software, and approved the final draft.
Hanghang Li conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Zhihua Song performed the experiments, prepared figures and/or tables, and approved the final draft.
Biying Dong conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Hongyan Cao conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Tengyue Liu performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Tingting Du, Wanlong Yang and Rohul Amin analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Litao Wang analyzed the data, prepared figures and/or tables, and approved the final draft.
Qing Yang analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Dong Meng and Yujie Fu conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Raw measurements are available in the Supplemental Files.
References
- Alejandro et al. (2012).Alejandro S, Lee Y, Tohge T, Sudre D, Osorio S, Park J, Bovet L, Lee Y, Geldner N, Fernie AR, Martinoia E. AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Current Biology. 2012;22:1207–1212. doi: 10.1016/j.cub.2012.04.064. [DOI] [PubMed] [Google Scholar]
- Andolfo et al. (2015).Andolfo G, Ruocco M, Di Donato A, Frusciante L, Lorito M, Scala F, Ercolano MR. Genetic variability and evolutionary diversification of membrane ABC transporters in plants. BMC Plant Biology. 2015;15:51. doi: 10.1186/s12870-014-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner et al. (2000).Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. Nature Genetics. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri et al. (2008a).Badri DV, Loyola-Vargas VM, Broeckling CD, De-la Peña C, Jasinski M, Santelia D, Martinoia E, Sumner LW, Banta LM, Stermitz F, Vivanco JM. Altered profile of secondary metabolites in the root exudates of Arabidopsis ATP-binding cassette transporter mutants. Plant Physiology. 2008a;146:762–771. doi: 10.1104/pp.107.109587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri et al. (2008b).Badri DV, Loyola-Vargas VM, Du J, Stermitz FR, Broeckling CD, Iglesias-Andreu L, Vivanco JM. Transcriptome analysis of Arabidopsis roots treated with signaling compounds: a focus on signal transduction, metabolic regulation and secretion. New Phytologist. 2008b;179:209–223. doi: 10.1111/j.1469-8137.2008.02458.x. [DOI] [PubMed] [Google Scholar]
- Bailey & Elkan (1994).Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings. International Conference on Intelligent Systems for Molecular Biology. 1994;2:28–36. [PubMed] [Google Scholar]
- Bird et al. (2007).Bird D, Beisson F, Brigham A, Shin J, Greer S, Jetter R, Kunst L, Wu X, Yephremov A, Samuels L. Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant Journal. 2007;52:485–498. doi: 10.1111/j.1365-313X.2007.03252.x. [DOI] [PubMed] [Google Scholar]
- Chen (2018).Chen C. TBtools, a Toolkit for Biologists integrating various biological data handling tools with a user-friendly interface. 2018. [DOI]
- Chen et al. (2011).Chen G, Komatsuda T, Ma JF, Nawrath C, Pourkheirandish M, Tagiri A, Hu YG, Sameri M, Li X, Zhao X, Liu Y, Li C, Ma X, Wang A, Nair S, Wang N, Miyao A, Sakuma S, Yamaji N, Zheng X, Nevo E. An ATP-binding cassette subfamily G full transporter is essential for the retention of leaf water in both wild barley and rice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12354–12359. doi: 10.1073/pnas.1108444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou & Shen (2008).Chou K, Shen H. Cell-PLoc: a package of Web servers for predicting subcellular localization of proteins in various organisms. Nature Protocols. 2008;3:153–162. doi: 10.1038/nprot.2007.494. [DOI] [PubMed] [Google Scholar]
- Consortium (2019).Consortium TGO. The gene ontology resource: 20 years and still going strong. Nucleic Acids Research. 2019;47:D330–D338. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson & Maloney (2007).Davidson AL, Maloney PC. ABC transporters: how small machines do a big job. Trends in Microbiology. 2007;15:448–455. doi: 10.1016/j.tim.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Do, Martinoia & Lee (2018).Do THT, Martinoia E, Lee Y. Functions of ABC transporters in plant growth and development. Plant Biology. 2018;41:32–38. doi: 10.1016/j.pbi.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Dudler & Hertig (1992).Dudler R, Hertig C. Structure of an mdr-like gene from Arabidopsis thaliana. Evolutionary implications. Journal of Biological Chemistry. 1992;267:5882–5888. [PubMed] [Google Scholar]
- Ehlert et al. (2006).Ehlert A, Weltmeier F, Wang X, Mayer CS, Smeekens S, Vicente-Carbajosa J, Dröge-Laser W. Two-hybrid protein-protein interaction analysis in Arabidopsis protoplasts: establishment of a heterodimerization map of group C and group S bZIP transcription factors. The Plant Journal. 2006;46:890–900. doi: 10.1111/j.1365-313X.2006.02731.x. [DOI] [PubMed] [Google Scholar]
- Farooq et al. (2009).Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- Fourcroy et al. (2014).Fourcroy P, Siso-Terraza P, Sudre D, Saviron M, Reyt G, Gaymard F, Abadia A, Abadia J, Alvarez-Fernandez A, Briat JF. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytologist. 2014;201:155–167. doi: 10.1111/nph.12471. [DOI] [PubMed] [Google Scholar]
- Goodman, Casati & Walbot (2004).Goodman CD, Casati P, Walbot V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. The Plant Cell. 2004;16:1812–1826. doi: 10.1105/tpc.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto & Yamada (2003).Hashimoto T, Yamada Y. New genes in alkaloid metabolism and transport. Current Opinion in Biotechnology. 2003;14:163–168. doi: 10.1016/S0958-1669(03)00027-2. [DOI] [PubMed] [Google Scholar]
- Hyde et al. (1990).Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, Gallagher MP, Gill DR, Hubbard RE, Higgins CF. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Imai et al. (2004).Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Research. 2004;64:4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- Jasinski et al. (2009).Jasinski M, Banasiak J, Radom M, Kalitkiewicz A, Figlerowicz M. Full-size ABC transporters from the ABCG subfamily in medicago truncatula. Molecular Plant-Microbe Interactions. 2009;22:921–931. doi: 10.1094/MPMI-22-8-0921. [DOI] [PubMed] [Google Scholar]
- Jasinski et al. (2003).Jasinski M, Ducos E, Martinoia E, Boutry M. The ATP-binding cassette transporters: structure, function, and gene family comparison between rice and Arabidopsis. Plant Physiology. 2003;131:1169–1177. doi: 10.1104/pp.102.014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang et al. (2010).Kang J, Hwang J, Lee M, Kim Y, Assmann SM, Martinoia E, Lee Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2355–2360. doi: 10.1073/pnas.0909222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang et al. (2011).Kang J, Park J, Choi H, Burla B, Kretzschmar T, Lee Y, Martinoia E. Plant ABC transporters. Arabidopsis Book. 2011;9:1–25. doi: 10.1199/tab.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2007).Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant Journal. 2007;50:207–218. doi: 10.1111/j.1365-313X.2007.03044.x. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2010).Kim DY, Jin JY, Alejandro S, Martinoia E, Lee Y. Overexpression of AtABCG36 improves drought and salt stress resistance in Arabidopsis. Physiologia Plantarum. 2010;139:170–180. doi: 10.1111/j.1399-3054.2010.01353.x. [DOI] [PubMed] [Google Scholar]
- Kretzschmar et al. (2011).Kretzschmar T, Burla B, Lee Y, Martinoia E, Nagy R. Functions of ABC transporters in plants. Essays in Biochemistry. 2011;50:145–160. doi: 10.1042/bse0500145. [DOI] [PubMed] [Google Scholar]
- Kubis, Schmidt & Heslop-Harrison (1998).Kubis S, Schmidt T, Heslop-Harrison JSP. Repetitive DNA elements as a major component of plant genomes. Annals of Botany. 1998;82:45–55. doi: 10.1006/anbo.1998.0779. [DOI] [Google Scholar]
- Kuromori et al. (2010).Kuromori T, Miyaji T, Yabuuchi H, Shimizu H, Sugimoto E, Kamiya A, Moriyama Y, Shinozaki K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2361–2366. doi: 10.1073/pnas.0912516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromori, Sugimoto & Shinozaki (2011).Kuromori T, Sugimoto E, Shinozaki K. Arabidopsis mutants of AtABCG22, an ABC transporter gene, increase water transpiration and drought susceptibility. Plant Journal. 2011;67:885–894. doi: 10.1111/j.1365-313X.2011.04641.x. [DOI] [PubMed] [Google Scholar]
- Le Hir et al. (2013).Le Hir R, Sorin C, Chakraborti D, Moritz T, Schaller H, Tellier F, Robert S, Morin H, Bako L, Bellini C. ABCG9, ABCG11 and ABCG14 ABC transporters are required for vascular development in Arabidopsis. The Plant Journal. 2013;76:811–824. doi: 10.1111/tpj.12334. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2010).Lee I, Ambaru B, Thakkar P, Marcotte EM, Rhee SY. Rational association of genes with traits using a genome-scale gene network for Arabidopsis thaliana. Nature Biotechnology. 2010;28:149–156. doi: 10.1038/nbt.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2005).Lee M, Lee K, Lee J, Noh EW, Lee Y. AtPDR12 contributes to lead resistance in Arabidopsis. Plant Physiology. 2005;138:827–836. doi: 10.1104/pp.104.058107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher (2004).Locher KP. Structure and mechanism of ABC transporters. Current Opinion in Structural Biology. 2004;14(4):426–431. doi: 10.1016/j.sbi.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2007).Luo B, Xue X, Hu W, Wang L, Chen X. An ABC transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion. Plant and Cell Physiology. 2007;48:1790–1802. doi: 10.1093/pcp/pcm152. [DOI] [PubMed] [Google Scholar]
- Martinoia et al. (2002).Martinoia E, Klein M, Geisler M, Bovet L, Forestier C, Kolukisaoglu U, Muller-Rober B, Schulz B. Multifunctionality of plant ABC transporters–more than just detoxifiers. Planta. 2002;214:345–355. doi: 10.1007/s004250100661. [DOI] [PubMed] [Google Scholar]
- Mendez & Salas (2001).Mendez C, Salas JA. The role of ABC transporters in antibiotic-producing organisms: drug secretion and resistance mechanisms. Research in Microbiology. 2001;152:341–350. doi: 10.1016/S0923-2508(01)01205-0. [DOI] [PubMed] [Google Scholar]
- Mishra et al. (2019).Mishra AK, Choi J, Rabbee MF, Baek KH. In Silico genome-wide analysis of the ATP-binding cassette transporter gene family in Soybean (Glycine max L.) and their expression profiling. Biomed Research International. 2019;2019:1–14. doi: 10.1155/2019/8150523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler, Finka & Goloubinoff (2012).Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends in Biochemical Sciences. 2012;37:118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Moons (2008).Moons A. Transcriptional profiling of the PDR gene family in rice roots in response to plant growth regulators, redox perturbations and weak organic acid stresses. Planta. 2008;229:53–71. doi: 10.1007/s00425-008-0810-5. [DOI] [PubMed] [Google Scholar]
- Morris & Zhang (2006).Morris ME, Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sciences. 2006;78:2116–2130. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Mosser et al. (1993).Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, Poustka AM, Mandel JL, Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- Mourez, Hofnung & Dassa (1997).Mourez M, Hofnung M, Dassa E. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO Journal. 1997;16:3066–3077. doi: 10.1093/emboj/16.11.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee et al. (2018).Mukherjee D, Saha D, Acharya D, Mukherjee A, Chakraborty S, Ghosh TC. The role of introns in the conservation of the metabolic genes of Arabidopsis thaliana. Genomics. 2018;110:310–317. doi: 10.1016/j.ygeno.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Ogoda, Akubue & Okide (2002).Ogoda OJ, Akubue PI, Okide GB. The kinetics of reversal of pre-sickled erythrocytes by the aqueous extract of Cajanus cajan seeds. Phytotherapy Research. 2002;16:748–750. doi: 10.1002/ptr.1026. [DOI] [PubMed] [Google Scholar]
- Pandey & Pandey (1991).Pandey PC, Pandey V. Urease purification from the seeds of Cajanus cajan and its application in a biosensor construction. Applied Biochemistry and Biotechnology. 1991;31:247–251. doi: 10.1007/BF02921751. [DOI] [PubMed] [Google Scholar]
- Panikashvili et al. (2007a).Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, Höfer R, Schreiber L, Chory J, Aharoni A. The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiology. 2007;145:1345–1360. doi: 10.1104/pp.107.105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter et al. (2018).Potter SC, Luciani A, Eddy SR, Park Y, Lopez R, Finn RD. HMMER web server: 2018 update. Nucleic Acids Research. 2018;46:W200–W204. doi: 10.1093/nar/gky448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombauts et al. (1999).Rombauts S, Dehais P, Van Montagu M, Rouze P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Research. 1999;27:295–296. doi: 10.1093/nar/27.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose et al. (2016).Rose AB, Carter A, Korf I, Kojima N. Intron sequences that stimulate gene expression in Arabidopsis. Plant Molecular Biology. 2016;92:337–346. doi: 10.1007/s11103-016-0516-1. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez et al. (2001).Sanchez-Fernandez R, Davies TG, Coleman JO, Rea PA. The Arabidopsis thaliana ABC protein superfamily, a complete inventory. Journal of Biological Chemistry. 2001;276:30231–30244. doi: 10.1074/jbc.M103104200. [DOI] [PubMed] [Google Scholar]
- Schneider & Hunke (1998).Schneider E, Hunke S. ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiology Reviews. 1998;22:1–20. doi: 10.1111/j.1574-6976.1998.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Schulz & Kolukisaoglu (2006).Schulz B, Kolukisaoglu HU. Genomics of plant ABC transporters: the alphabet of photosynthetic life forms or just holes in membranes? FEBS Letters. 2006;580:1010–1016. doi: 10.1016/j.febslet.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Shepherd & Bhardwaj (1986).Shepherd H, Bhardwaj RK. Moisture-dependent physical properties of Pigeon Pea. Journal of Agricultural Engineering Research. 1986;35(4):227–234. doi: 10.1016/S0021-8634(86)80060-9. [DOI] [Google Scholar]
- Singh et al. (2013).Singh AK, Rai VP, Chand R, Singh RP, Singh MN. Genetic diversity studies and identification of SSR markers associated with Fusarium wilt (Fusariumudum) resistance in cultivated pigeonpea (Cajanus cajan) Journal of Genetics. 2013;92:273–280. doi: 10.1007/s12041-013-0266-7. [DOI] [PubMed] [Google Scholar]
- Sugiyama et al. (2011).Sugiyama M, Inui A, Shin-I T, Komatsu H, Mukaide M, Masaki N, Mizokami M. Easy-to-use phylogenetic analysis system for hepatitis B virus infection. Hepatology Research. 2011;41(10):936–945. doi: 10.1111/j.1872-034X.2011.00859.x. [DOI] [PubMed] [Google Scholar]
- Tamura et al. (2013).Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo et al. (2011).Toledo MA, Schneider DR, Azzoni AR, Favaro MT, Pelloso AC, Santos CA, Saraiva AM, Souza AP. Characterization of an oxidative stress response regulator, homologous to Escherichia coli OxyR, from the phytopathogen Xylella fastidiosa. Protein Expression and Purification. 2011;75:204–210. doi: 10.1016/j.pep.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Van den Brule et al. (2002).Van den Brule S, Muller A, Fleming AJ, Smart CC. The ABC transporter SpTUR2 confers resistance to the antifungal diterpene sclareol. Plant Journal. 2002;30:649–662. doi: 10.1046/j.1365-313X.2002.01321.x. [DOI] [PubMed] [Google Scholar]
- Van den Brule & Smart (2002).Van den Brule S, Smart CC. The plant PDR family of ABC transporters. Planta. 2002;216:95–106. doi: 10.1007/s00425-002-0889-z. [DOI] [PubMed] [Google Scholar]
- Varshney et al. (2011).Varshney RK, Chen W, Li Y, Bharti AK, Saxena RK, Schlueter JA, Donoghue MT, Azam S, Fan G, Whaley AM, Farmer AD, Sheridan J, Iwata A, Tuteja R, Penmetsa RV, Wu W, Upadhyaya HD, Yang SP, Shah T, Saxena KB, Michael T, McCombie WR, Yang B, Zhang G, Yang H, Wang J, Spillane C, Cook DR, May GD, Xu X, Jackson SA. Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource-poor farmers. Nature Biotechnology. 2011;30:83–89. doi: 10.1038/nbt.2022. [DOI] [PubMed] [Google Scholar]
- Verrier et al. (2008).Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu U, Lee Y, Martinoia E, Murphy A, Rea PA, Samuels L, Schulz B, Spalding EJ, Yazaki K, Theodoulou FL. Plant ABC proteins–a unified nomenclature and updated inventory. Trends in Plant Science. 2008;13:151–159. doi: 10.1016/j.tplants.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Voorrips (2002).Voorrips RE. MapChart: software for the graphical presentation of linkage maps and QTLs. Journal of Heredity. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- Walker et al. (1982).Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha-and beta-subunits of ATP synthase, myosin, kinases and ther ATP-requiring enzymes Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO Journal. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2011).Wu N, Kong Y, Fu Y, Zu Y, Yang Z, Yang M, Peng X, Efferth T. In vitro antioxidant properties, DNA damage protective activity, and xanthine oxidase inhibitory effect of cajaninstilbene acid, a stilbene compound derived from pigeon pea [Cajanus cajan (L.) Millsp.] leaves. Journal of Agricultural and Food Chemistry. 2011;59:437–443. doi: 10.1021/jf103970b. [DOI] [PubMed] [Google Scholar]
- Wu, Lewis & Spalding (2007).Wu G, Lewis DR, Spalding EP. Mutations in Arabidopsis multidrug resistance-like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. The Plant Cell. 2007;19:1826–1837. doi: 10.1105/tpc.106.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadu et al. (2018).Yadu B, Chandrakar V, Korram J, Satnami ML, Kumar M, Keshavkant S. Silver nanoparticle modulates gene expressions, glyoxalase system and oxidative stress markers in fluoride stressed Cajanus cajan L. Journal of Hazardous Materials. 2018;353:44–52. doi: 10.1016/j.jhazmat.2018.03.061. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2016).Yang X, Coulombe-Huntington J, Kang S, Sheynkman GM, Hao T, Richardson A, Sun S, Yang F, Shen YA, Murray RR, Spirohn K, Begg BE, Duran-Frigola M, MacWilliams A, Pevzner SJ, Zhong Q, Trigg SA, Tam S, Ghamsari L, Sahni N, Yi S, Rodriguez MD, Balcha D, Tan G, Costanzo M, Andrews B, Boone C, Zhou XJ, Salehi-Ashtiani K, Charloteaux B, Chen AA, Calderwood MA, Aloy P, Roth FP, Hill DE, Iakoucheva LM, Xia Y, Vidal M. Widespread expansion of protein interaction capabilities by alternative splicing. Cell. 2016;164:805–817. doi: 10.1016/j.cell.2016.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki (2006).Yazaki K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Letters. 2006;580:1183–1191. doi: 10.1016/j.febslet.2005.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Neighbor-Joining (NJ) tree of ABC transporters was constructed with MEGA6.0 program. The numbers beside the branches represent bootstrap values based on 1000 replications. The red circle indicates ABC transporters in soybean; the green square denotes ABC transporters in rice; the blue triangle indicates ABC transporters in Arabidopsis. All ABC transporters are divided into 8 subgroups (ABCA-ABCG, ABCI) that shown in different color at the inside of the circle.
The CcABCG7 :eGFP plasmid was transformed with the into the leaves of tobacco. Fluorescence was visualized by a confocal laser scanning microscope. Bar = 50µm.
(A) In Ramachandran Plot, the A (red), B (yellow) and L (light yellow) areas represent the most favored, additional allowed and (generously allowed) areas respectively; the other (white) areas are It is a disallowed area. (B) The red and shown regions respectively represent the two motifs of the ABCG transporter NBD domain; where red represents Walker A[GPSGSGKT] and blue represents Walker B [KRVSIGQEML].
Data Availability Statement
The following information was supplied regarding data availability:
Raw measurements are available in the Supplemental Files.