Abstract
Objective:
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. Systemic inflammation and oxidant/antioxidant imbalance has been seen to play a key role in pathogenesis of COPD. The present study investigated the levels of inflammatory and antioxidant/oxidative stress biomarker in COPD patients and healthy subjects.
Materials and Methods:
The present study enrolled seventy COPD patients and seventy healthy controls from Department of Respiratory Medicine at a tertiary care hospital. Vitamin D, C-reactive protein (CRP), superoxide dismutase (SOD), catalase, and malondialdehyde (MDA) levels were measured in both cases and control. GraphPad PRISM version 6.01 was used for analysis of data.
Results:
The levels of Vitamin D, SOD, Catalase, were found to be significantly lower among the COPD patients in comparison to healthy controls while levels of MDA and CRP were significantly higher (P = 0.0001).
Conclusion:
The results showed oxidant/antioxidant imbalance and Vitamin D deficiency in COPD patients. Higher levels of CRP and oxidative stress markers were observed in COPD patients in comparison to healthy controls. A biomarker based study testing the efficacy of novel antioxidant or other agents will be helpful that can modify the course of this disease.
KEYWORDS: Antioxidants, Chronic obstructive pulmonary disease, C-reactive protein, Oxidative stress, Vitamin D
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) a chronic respiratory disease, affecting 210 million people is a leading cause of morbidity and mortality worldwide[1]. WHO predict that by 2020 this disorder will rank as the fifth most prevalent disease and third most common cause of death[2]. COPD is characterized by airway inflammation and progressive airflow limitation and its pathology includes pulmonary inflammation, imbalance of oxidants/antioxidants, protease and antiprotease, both innate and adaptive immunity [3,4,5]. Cigarette smoking is the most important risk factor for COPD. The risk of developing COPD becomes higher as smoking duration increases[6]. It contains >1014 oxidants/free radicals and 4700 reactive chemical and other carcinogens, and it is a risk factor in the development of COPD and lung cancer[7]. Smoking for longer duration causes airway inflammation and elevation in levels of cytokines and C-reactive protein (CRP). CRP is the best studied biomarker, an acute phase reactant secreted by liver in response to infection, inflammation, or tissue damage[8]. Occupational, environmental exposure, and indoor air pollution from biomass cooking are the other risk factors for the development and the progression of COPD.
Cigarette smoking and exacerbations in COPD lead to reduction of antioxidant capacity. It increases the level of oxidants in the lungs, resulting in a decrease of antioxidants. Antioxidants are naturally occurring or synthetic substances that help protect cells from the damaging effects of free radicals[9]. Reactive oxygen species, reactive nitrogen species, and their counterpart antioxidant agents are necessary for physiological signaling as well as host defense, along with persistence of inflammation[10].
Imbalance of reactive oxidant species and antioxidants in the lower respiratory tract leads to oxidative stress, which in turn contributes to various physiological changes including chronic airflow limitation[11,12]. If this airflow limitation is due to oxidative stress arising from increased demand of antioxidants due to disease or due to nutrients deficiency in person with lung disease than, the association between antioxidants and pulmonary function is evident in persons with COPD when compared to healthy persons[13]. Oxidative stress protease - antiprotease imbalance, inflammation, and lung remodeling are the most important pathogenic mechanisms involved in the development of COPD. Lower Vitamin D levels have been related to regulation of each of these processes, that is, higher expression of proteases, modulation of inflammation, and increased oxidative stress[14]. In COPD, the risk of Vitamin D deficiency is higher than expected and is linked with disease severity [15,16,17]. Persistent airway inflammation and airway obstruction are a distinguished characteristics of COPD.
Biomarkers in COPD may be helpful in diagnosis, monitoring the severity of disease and evaluating the effects of drugs. CRP is one of the most important systemic inflammatory biomarker associated with increased risk of hospitalization and death in follow-up study of COPD patients[18]. Due to high prevalence rate of Vitamin D deficiency in these patients and its impact on airways, the present study was designed to evaluate the levels of the serum Vitamin D, CRP, and other antioxidants/oxidative stress biomarkers in COPD patients and healthy controls and study their association with smoking and severity of disease.
MATERIALS AND METHODS
The present study enrolled one hundred and forty individuals, 70 stable COPD patients, and 70 healthy controls. Patients were enrolled from the OPD of Department of Respiratory Medicine of a tertiary care hospital of North India while controls were the healthy attendants of patients or other healthy subjects visiting the department. All patients underwent detailed history, clinical evaluation by a specialized respiratory physician. Chest X-ray was also done. The demographic information was ascertained from self-reported responses to the predesigned questionnaire that includes demographic details, smoking history/pack years, respiratory symptoms, and risk factors for COPD, presence of comorbidities, health status, and limitation of activity. The clinical severity of COPD was determined as per the criteria defined in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines based on the post-bronchodilator forced expiratory volume in 1 s (FEV1) values[19]. For assessing the lung function, pulmonary function test was performed on P. K. Morgan's Medical Pvt. Limited-Pulmolab 435 (Spiro 232) in pulmonary function lab in sitting position and at room temperature in Department of Respiratory medicine. A diagnosis of COPD was established on the basis of largely irreversible airway obstruction, with <12% improvement in FEV1 after inhalation of 200 μg Salbutamol using metered dose inhaler with spacer. Lung functions were again measured 15-20 min after the inhaled bronchodilator for assessment of bronchodilator induced reversibility of bronchoconstriction. Spirometric indices were calculated using the best out of 3 technically satisfactory performances as per recommendations of American Thoracic Society. Patients and controls fulfilling the inclusion and exclusion criteria were recruited only after their informed consent and after approval from the ethics committee. The study was approved by the ethics committee of King George's Medical University, Lucknow (Approval Code No-55 E. C. M. IIB/P11). Informed written consent was obtained from all patients prior to their enrollment in this study.
Inclusion criteria for patients
Subjects with age >35 years and both genders, FEV1/forced vital capacity (FVC) ratio <70% and post bronchodilator FEV1 change <12% and having history of persistent cough, sputum production, dyspnea, and exposure to risk factors for the disease.
Exclusion criteria for patients
Patients having a history of other respiratory illnesses such as acute asthma, pulmonary tuberculosis, bronchiectasis interstitial lung disease, lung cancer, and diabetes. Pregnant women and patients already taking Vitamin D supplements or antioxidants were also excluded. Individuals were classified as current, former, and never smokers on the basis of self-reported smoking history. The subjects were categorized as smokers if they were currently smoking, nonsmokers if they have never smoked during their life time and ex-smokers who had smoking abstinence of 1 year.
The body mass index (BMI) was calculated by dividing the body weight in kilograms by the height in meters square (kg/m2). Dyspnea was assessed by Modified Medical Research Council scale. The presence of Vitamin D deficiency was defined as 25(OH) D levels <10.0 ng/mL, Vitamin D insufficiency as 25(OH) D levels 10 ng/mL-30 ng/mL, and Vitamin D sufficiency as 25(OH) D levels 30 ng/mL-100 ng/mL and toxic >100 ng/mL.
Vitamin-D
Interpretation of result:
<10 ng/mL (Deficiency)
10-30 ng/mL (Insufficiency)
30-100 ng/mL (Sufficiency)
>100 ng/mL (Toxicity).
Determination of biochemical parameters
Sample collection and processing
Venous blood samples were collected from all patients and control subjects in two separate tubes one in plain vial for separation of serum and estimation of Vitamin D, CRP, and other in heparinized vials and centrifuged it at 1000 g for 15 min, and the plasma was removed. Then, erythrocytes were washed with 0.9% NaCl solution three times and kept at −80°C until biochemical determination of other antioxidants.
Estimation of biomarkers
Serum Vitamin D was assessed by The LIAISON® 25 OH Vitamin D TOTAL Assay kit which uses chemiluminescent immunoassay technology for the quantitative determination of 25-hydroxyvitamin D in human serum, by using the LIAISON® analyzer. Serum CRP was also analyzed in serum by commercially available kit as per protocol. Malondialdehyde (MDA) was estimated according to the method of Stocks and Dormandy[20]. Estimation of catalase was done by the method described by Aebi[21]. Estimation of superoxide dismutase (SOD) was done by the method of McCord and Fridovich[22].
Statistical analysis
Graph Pad PRISM version 6.01 (Graph Pad Software Inc, La, Jolla, CA, USA) was used for analysis of data. Values have been represented in mean ± standard deviation (in case of continuous variable) and expressed as number and percentages (in case of categorical variables). Chi-square test has been used to compare proportions whereas analysis of variance was used for comparison of continuous data. P < 0.05 was considered as statistically significant in all analyses.
RESULTS
In the present study, 140 individuals were enrolled, 70 COPD and 70 healthy controls. The demographic and clinical characteristics of individuals are shown in Table 1. Age of patients ranged from 35 to 75 years. There was no significant difference between groups with respect to age. In both the groups, majority of individuals were males. In COPD group, there were 75.8% males and 24.2% females while in control group, there were 71.4% males and 28.5% females.
Table 1.
Baseline demographic and spirometry parameters in chronic obstructive pulmonary disease patients and healthy controls
| Parameters | COPD group (n=70), n (%) | Controls group (n=70), n (%) | P |
|---|---|---|---|
| Age (years) | 55.78±9.8 | 53.5±12.31 | 0.325 |
| Male/female ratio | 53:17 | 50:20 | 0.568 |
| Height (cm) | 159.4±8.37 | 162.86±9.07 | 0.051 |
| Weight (kg) | 53.02±12.59 | 63.02±12.08 | 0.04 |
| BMI (kg/m2) | 20.37±4.21 | 24.34±4.25 | <0.001 |
| Smoker | 31 (44.3) | 25 (35.7) | |
| Ex-smoker | 25 (35.7) | 15 (21.4) | |
| Nonsmoker | 14 (20) | 30 (42.8) | |
| Stage 1 (mild) | 0 (0) | - | |
| Stage 2 (moderate) | 25 (35.7) | - | |
| Stage 3 (severe) | 36 (51.4) | - | |
| Stage 4 (very severe) | 9 (12.8) | - | |
| Post-FVC% | 64.02±17.09 | 88.8±9.0 | <0.001 |
| Post-FEV1/FVC | 56.29±10.3 | 81.4±8.1 | <0.001 |
| Post-FEV1 (%) predicted | 45.14±15.3 | 90.18+8.09 | <0.001 |
Results are expressed as mean±SD or percentages depending on the distribution. P<0.05 considered significant. COPD: Chronic obstructive pulmonary disease, FVC: Forced vital capacity, FEV1: Forced expiratory volume in 1 s, BMI: Body mass index, SD: Standard deviation
The distribution of smokers, nonsmokers and ex-smokers has been given in Table 1. There were nearly 80% patients having smoking history in COPD group there were 31 smokers (44.3%), 14 nonsmokers (20%) and 25 ex-smokers (35.7%) in the COPD group. In control group, there were 25 smokers (35.7%), 30 nonsmokers (42.8%), and 15 ex-smokers (21.4%). Mean weight and BMI were lower in COPD patients as compared to controls, and this difference was statistically significant (P = 0.04, P < 0.001).
Spirometric values such as mean FEV1% predicted, FVC, and FEV1/FVC ratio as mentioned in Table 1 were significantly lower in COPD patients when compared to control group (P < 0.0001). According to GOLD criteria, COPD patients were grouped into three stages based on their severity as there were no patients having mild COPD (stage 1). 25 patients (35.7%) have moderate COPD (stage 2), 36 patients (51.4%) have severe COPD (stage 3) while 9 patients (12.9%) were having very severe COPD (stage 4). Majority of COPD patients were in stage 3 (nearly 50%), i.e., in severe COPD group.
Breathlessness was the main symptom observed in 70 patients (100%) followed by a cough in 62 (94%), chest pain in 5 (7%), wheezing in 8 patients (11.4%), fever in 7 (10%) patients, and pedal edema in 12 patients (17%) [Figure 1].
Figure 1.

Clinical symptoms in chronic obstructive pulmonary disease patients
Mean value of serum Vitamin D levels was significantly lower in the COPD patients as compared to healthy controls (15.5 ± 7.22 vs. 28.3 ± 7.91, P < 0.0001) [Figure 2]. In COPD patients, Vitamin D deficiency was seen in 27% people and insufficiency in 70% COPD patients. In healthy subjects, 55% have sufficient levels while 45% have insufficient levels.
Figure 2.
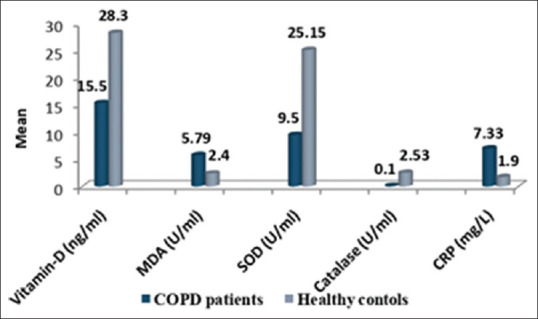
Inflammatory and antioxidant/oxidant parameters in chronic obstructive pulmonary disease patients and healthy controls
Levels of serum Vitamin D between smokers, ex-smoker and nonsmoker COPD patients and control were also compared. The lower Vitamin D level was observed in smokers and ex-smoker as compared to nonsmoker both in COPD and control [Figure 3]. The present study indicates significant association of Vitamin D with smoking. We also found that patients with very low BMI have low Vitamin D levels. With the increasing severity of disease lower levels of Vitamin D were observed. We found that patients with very severe COPD were having Vitamin D deficiency [Figure 4] and significantly lower levels of Vitamin D were observed in stage 3 and 4 patients in comparison to stage 2.
Figure 3.
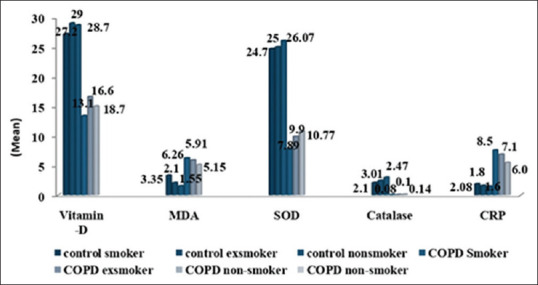
Inflammatory and antioxidants/oxidative stress parameters in chronic obstructive pulmonary disease patients and healthy controls on the basis of smoking status
Figure 4.
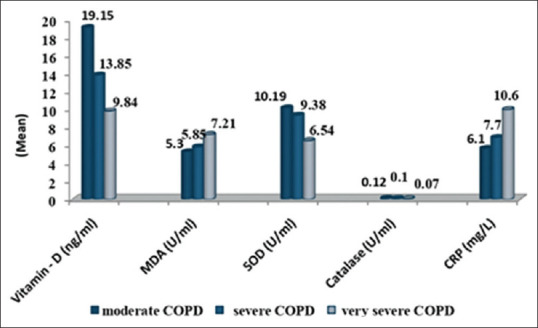
Inflammatory and antioxidants/oxidative stress parameters in chronic obstructive pulmonary disease patients on the basis of severity
Mean serum CRP levels were significantly higher in the COPD group as compared to control group (P < 0.0001) [Figure 2]. Smokers have higher levels of CRP in comparison to nonsmokers [Figure 3]. Elevated CRP levels were observed in patients with increasing severity of disease and were highest in stage 4 COPD patients [Figure 4].
The levels of oxidative stress marker/antioxidants between cases and controls are shown in Figure 2. Mean level of SOD was 25.25 ± 1.4 in cases and 9.44 ± 0.30 in controls while mean level of catalase was 0.10 ± 0.01 in cases and 2.53 ± 0.13 in control. Level of both antioxidant enzymes SOD and catalase were significantly lower in cases as compared to controls (P < 0.0001). MDA level which is a marker of oxidative stress was significantly higher in COPD patients than controls (5.8 ± 0.15 vs. 2.81 ± 0.44), (P < 0.0001).
Figure 3 shows significant difference in the oxidative stress and antioxidant parameters among COPD patients (smoker and nonsmokers and ex-smokers) and healthy control groups (smokers, ex-smokers, and nonsmokers). The antioxidant levels of Catalase, SOD were significantly lower in smoking population in comparison to nonsmoking group (P < 0.001) while significantly higher values of oxidative marker MDA was observed in smoking COPD patients as compared to the nonsmoker COPD individuals (P = 0.009).
The present study shows in Figure 4, antioxidants and oxidative stress parameters in COPD patients grouped on basis of severity into different GOLD stages (moderate, severe, and very severe). Significant differences in the antioxidant parameters among severity of COPD were observed. The statistical analysis revealed that levels of antioxidants SOD and Catalase were significantly (P = 0.0002, P = 0.0006) lower among very severe patients (stage 4) in comparison to the moderate (stage 2) and severe patients (stage 3). MDA was observed to be significantly higher (P = 0.0005) in stage 4 (very severe patients) in comparison to stage 2 (moderate) and stage 3(severe patients) COPD patients.
DISCUSSION
Imbalance of reactive oxidant species and antioxidants in the lower respiratory tract leads to oxidative stress which in turn contributes to various physiological changes, including chronic airflow limitation. Previous studies have shown that Vitamin D can alter the activity of various immune cells, regulate airway smooth muscle, and inhibit inflammatory responses and helps in remodeling of airways[16,17,23]. Holick in his review mentioned that the reasons for low Vitamin D levels might be lack of outdoor activity, lower food intake, reduced synthesis with skin aging, increased catabolism by glucocorticoids, impaired activation because of renal dysfunction, and a lower storage capacity in muscles or fat due to wasting[24].
Oxidative stress protease - antiprotease imbalance, inflammation, and lung remodeling are the most important pathogenic mechanisms involved in the development of COPD although role of Vitamin D in the pathogenesis of COPD is not clear but it is believed that lower Vitamin D levels are related to regulation of each of these processes, that is, higher expression of proteases, modulation of inflammation, and increased oxidative stress[14].
Vitamin D has both immunomodulatory and anti-inflammatory properties[25]. In India, major source of Vitamin D is sun exposure, and food fortification with Vitamin D is not prevalent. The present study found significantly lower levels of Vitamin D in COPD patients in comparison to healthy controls. In people with COPD, the risk of Vitamin D deficiency is higher than expected and is linked with disease severity which is consistent with previous studies[15,16,17,26,27]. We found 27% COPD patients were having Vitamin D deficiency and 70% have an insufficient level which shows that hypovitaminosis D, i.e., both deficiency and insufficiency were found in 97% of COPD patients in our study and more in higher stages of severity (Gold 3 and 4). Førli et al. reported Vitamin D deficiency (20 ng/mL) in >50% of a cohort waiting for lung transplantation[28]. Vitamin D deficiency correlates with the severity of COPD[15,29]. Significant relation between FEV1 and serum 25-hydroxy Vitamin D levels was also seen in previous study[30]. Vitamin D intake improved COPD exacerbations and FEV1 in the patients with severe and very severe COPD[31]. Zhu et al.[32] included 18 studies in meta-analysis and showed that the serum levels of 25(OH) D were lower in COPD patients, and Vitamin D deficiency was associated with COPD severity rather than COPD risk.
Vitamin D is important as an immune system regulator, and many previous studies along with ours have reported low serum 25-OH Vitamin D levels in COPD patients. Intakes of various vitamins have been associated with improvement in symptoms, exacerbations, and airflow limitation in COPD[33]. Therefore, Vitamin D supplementation may be an important therapeutic strategy for COPD which needs more research.
COPD is a multicomponent disease affecting the psychological and physiological conditions as well as social life of patients. It is independently associated with low grade systemic inflammation than that in healthy subjects, and this inflammatory activity increases as severity of disease increases. Our study finds significantly higher levels of CRP in COPD patients as compared to control group (P < 0.0001) which was consistent with many previous studies [34,35,36,37,38]. As the severity of disease become higher, the levels of markers also increased and were highest in very severe patients (stage 4). Similar association was also observed in previous study[39]. Leuzzi et al.[40] in meta-analysis also indicate that baseline higher CRP level is significantly associated with higher late mortality in patients with COPD. The increase in CRP levels with the progression of the disease as seen reflects the severity of the disease and so measuring levels of systemic inflammatory markers like CRP in combination with other biochemical markers at baseline and after anti-inflammatory therapy will be helpful in monitoring disease outcome and also in proper management of disease.
Oxidative stress has also been reported to play an important role in the pathophysiology of COPD[41,42]. The present study found significantly higher levels of oxidative markers (MDA) in COPD patients when compared with control which was similar as seen in study by Cristóvãoa et al.[43] while the levels of antioxidants-Catalase and SOD were significantly lower in patients of COPD which was also observed in previous studies[44,45]. Cigarette smoking has been considered as the major risk factor for developing COPD and prime factor for generation of oxidative free radicals.
Smoking may act as a trigger factor for many people who have COPD and can either cause an exacerbation or flare-up of symptoms. Smoking damages the air sacs, airway, and the lining of the lungs, and due to this, lungs have trouble moving enough air in and out making hard to breathe. However, respiratory infections, inflammation, dust, and air pollution can be attributed to be the factors responsible for oxidative stress in nonsmokers. In our previous study, we found a significant association of smoking status with different stages of COPD[46] and in present study too we found that COPD patients with smoking history (smokers or ex-smokers) had higher levels of oxidative stress markers and reduced levels of antioxidant when compared to nonsmokers which was also reported in previous studies. Higher levels of MDA have also been observed in smokers in comparison to nonsmokers in few studies[47,48]. Nadeem et al.[49] also reported an increase in MDA levels in COPD patients, as compared with healthy nonsmoking controls. Depletion of naturally occurring antioxidants also plays a major role in perpetuation of oxidative stress. An oxidant-antioxidant imbalance which is associated with oxidative stress in COPD patients plays an important role in the progression of disease severity[50].
MDA the main product of lipid peroxidation, one of the key indicators of oxidative stress has been reported to increase in all stages of COPD severity[50,51]. The mean MDA values was found to be significantly increased in all COPD groups according to severity in this study which was also observed in study by Torres-Ramos et al.[52]. They also reported significantly higher levels of MDA in all GOLD Stages of COPD severity, as compared with healthy controls[52]. Increase in MDA levels in COPD patients in comparison to healthy nonsmoking controls has been observed in study by Nadeem et al.
SOD is an intracellular antioxidant enzyme that inhibits superoxide anion and protects aerobic cells against oxidative stress while Catalase, the enzyme responsible for the breakdown of H2O2 is the primary defense against H2O2-mediated toxicity. We found significantly lower levels of antioxidants (Catalase, SOD) in COPD patients in comparison to controls and as the severity increase the level of antioxidants also decreased. Rai and Phadke[53] also reported the same in COPD patients. Nadeem et al. also found an increase in levels of SOD, Catalase in severe COPD patients, as compared with moderate COPD patients[49].
Limitation of study was small size. Estimation of antioxidants and oxidative stress markers before and after dietary intervention and supplementation with antioxidants may have put more light on usefulness of these biomarkers in COPD. Earlier estimation in COPD patients and timely supplementation might help in reducing oxidative stress.
Romieu and Trenga proposed that antioxidant supplementation may be helpful in patients with COPD as a way to reduce oxidative stress and inflammation and improve spirometric values[54]. Higher intake of fruits and vegetables was associated with a lower risk of COPD, lower mortality, and an improvement of spirometric values [55,56,57,58,59]. Multitargeted therapeutic approach with dietary antioxidants along with smoking cessation will be helpful in combating with this disease in the near future.
CONCLUSION
Our study showed that oxidative stress marker (MDA) and inflammatory biomarker (CRP) levels were significantly higher while levels of antioxidants (catalase and SOD) and Vitamin D were lower in our North Indian COPD population in comparison to healthy controls. The results also showed altered oxidant-antioxidant imbalance in COPD patients which increases with the severity of the disease. It was also observed that smokers have elevated levels of CRP and oxidative stress markers in comparison to nonsmokers. A biomarker-based study testing the efficacy of novel antioxidant or other agents will be helpful that can modify the course of this disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are greatly thankful to the Department of Respiratory medicine and Department of Biochemistry for carrying out the study and appreciate patients who participated in the study.
REFERENCES
- 1.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: Current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. COPD: Is there light at the end of the tunnel? Curr Opin Pharmacol. 2004;4:263–72. doi: 10.1016/j.coph.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1304–9. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- 4.Grashoff WF, Sont JK, Sterk PJ, Hiemstra PS, de Boer WI, Stolk J, et al. Chronic obstructive pulmonary disease: Role of bronchiolar mast cells and macrophages. Am J Pathol. 1997;151:1785–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Cristóvão C, Cristóvão L, Nogueira F, Bicho M. Evaluation of the oxidant and antioxidant balance in the pathogenesis of chronic obstructive pulmonary disease. Rev Port Pneumol. 2013;19:70–5. doi: 10.1016/j.rppneu.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: A 25 year follow up study of the general population. Thorax. 2006;61:935–9. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol. 2009;9:375–83. doi: 10.1016/j.coph.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144:266–73. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 10.Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD - Implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis. 2014;9:1207–24. doi: 10.2147/COPD.S51226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacNee W. Oxidants/antioxidants and COPD. Chest. 2000;117:303S–17S. doi: 10.1378/chest.117.5_suppl_1.303s-a. [DOI] [PubMed] [Google Scholar]
- 12.Schünemann HJ, Freudenheim JL, Grant BJ. Epidemiologic evidence linking antioxidant vitamins to pulmonary function and airway obstruction. Epidemiol Rev. 2001;23:248–67. doi: 10.1093/oxfordjournals.epirev.a000805. [DOI] [PubMed] [Google Scholar]
- 13.Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Browne RW, et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur J Clin Nutr. 2006;60:991–9. doi: 10.1038/sj.ejcn.1602410. [DOI] [PubMed] [Google Scholar]
- 14.Ghoneim AH, Al-Azzawi MA, Elmasry SA, Nasr MY, AboZaid Mohamed MM. Association of Vitamin D status in the pathogenesis of chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2015;64:805–12. [Google Scholar]
- 15.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the Vitamin D-binding gene. Thorax. 2010;65:215–20. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee A, Panettieri R., Jr Vitamin D modulates airway smooth muscle function in COPD. Curr Opin Pharmacol. 2012;12:266–74. doi: 10.1016/j.coph.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Janssens W, Mathieu C, Boonen S, Decramer M. Vitamin D deficiency and chronic obstructive pulmonary disease: A vicious circle. Vitam Horm. 2011;86:379–99. doi: 10.1016/B978-0-12-386960-9.00017-4. [DOI] [PubMed] [Google Scholar]
- 18.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–5. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 19.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. [[Last accessed on 2018 May 11]]. Available from: http://goldcopd.org/
- 20.Stocks J, Dormandy TL. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br J Haematol. 1971;20:95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 21.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 22.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 23.Herr C, Greulich T, Koczulla RA, Meyer S, Zakharkina T, Branscheidt M, et al. The role of Vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12:31. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 25.Adams JS, Hewison M. Unexpected actions of Vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gouda E, Zidan M, Gharraf H, Younan DN, Mohamed S. Pattern of Vitamin D in patients with chronic obstructive pulmonary diseases and in patients with bronchial asthma. Egypt J Chest Dis Tuberc. 2016;65:389–96. [Google Scholar]
- 27.Thakuria R, Maitra T, Deka J. Vitamin D deficiency: A factor for exacerbation of COPD: Myth or fact. Int J Contem Med Res. 2016;3:2133–5. [Google Scholar]
- 28.Førli L, Halse J, Haug E, Bjørtuft Ø, Vatn M, Kofstad J, et al. Vitamin D deficiency, bone mineral density and weight in patients with advanced pulmonary disease. J Intern Med. 2004;256:56–62. doi: 10.1111/j.1365-2796.2004.01337.x. [DOI] [PubMed] [Google Scholar]
- 29.Menon B, Mittal A, Nima G, Kaur C, Mittal U, Dogra V. Evaluation of Vitamin D levels in COPD and its correlation with disease severity and frequency of exacerbations. Eur Respir J. 2014;44:3960. [Google Scholar]
- 30.Azargoon AR, Moghadam PK, Shokrollahi S, Ebrahimzadeh F, Pournia Y. Relationship between FEV1 and 25-hydroxy Vitamin D in patients with chronic obstructive pulmonary disease. Trends Med Res. 2011;6:184–90. [Google Scholar]
- 31.Zendedel A, Gholami M, Anbari K, Ghanadi K, Bachari EC, Azargon A. Effects of Vitamin D intake on FEV1 and COPD exacerbation: A randomized clinical trial study. Glob J Health Sci. 2015;7:243–8. doi: 10.5539/gjhs.v7n4p243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu M, Wang T, Wang C, Ji Y. The association between Vitamin D and COPD risk, severity, and exacerbation: An updated systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:2597–607. doi: 10.2147/COPD.S101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsiligianni IG, van der Molen T. A systematic review of the role of Vitamin insufficiencies and supplementation in COPD. Respir Res. 2010;11:171. doi: 10.1186/1465-9921-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto-Plata VM, Müllerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61:23–8. doi: 10.1136/thx.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61:17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Torres JP, Cordoba-Lanus E, López-Aguilar C, Muros de Fuentes M, Montejo de Garcini A, Aguirre-Jaime A, et al. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J. 2006;27:902–7. doi: 10.1183/09031936.06.00109605. [DOI] [PubMed] [Google Scholar]
- 38.Karadag F, Kirdar S, Karul AB, Ceylan E. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med. 2008;19:104–8. doi: 10.1016/j.ejim.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: Data from the third national health and nutrition examination. Am J Med. 2003;114:758–62. doi: 10.1016/s0002-9343(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 40.Leuzzi G, Galeone C, Taverna F, Suatoni P, Morelli D, Pastorino U. C-reactive protein level predicts mortality in COPD: A systematic review and meta-analysis. Eur Respir Rev. 2017;26:pii: 160070. doi: 10.1183/16000617.0070-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soriano JB, Rodríguez-Roisin R. Chronic obstructive pulmonary disease overview: Epidemiology, risk factors, and clinical presentation. Proc Am Thorac Soc. 2011;8:363–7. doi: 10.1513/pats.201102-017RM. [DOI] [PubMed] [Google Scholar]
- 42.MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:258–66. doi: 10.1513/pats.200504-045SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cristóvãoa C, Cristóvão L, Nogueira F, Bicho M. Evaluation of the oxidant and antioxidant balance in the pathogenesis of chronic obstructive pulmonary disease. Rev Port Pneumol. 2013;19:70–5. doi: 10.1016/j.rppneu.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Tavilani H, Nadi E, Karimi J, Goodarzi MT. Oxidative stress in COPD patients, smokers, and non-smokers. Respir Care. 2012;57:2090–4. doi: 10.4187/respcare.01809. [DOI] [PubMed] [Google Scholar]
- 45.Tsukagoshi H, Shimizu Y, Iwamae S, Hisada T, Ishizuka T, Iizuka K, et al. Evidence of oxidative stress in asthma and COPD: Potential inhibitory effect of theophylline. Respir Med. 2000;94:584–8. doi: 10.1053/rmed.2000.0785. [DOI] [PubMed] [Google Scholar]
- 46.Pandey S, Garg R, Kant S, Gaur P, Verma A, Tripathi PM, et al. Association of smoking status with COPD in North Indian population. Int J Life Sci Res. 2018;4:1685–9. [Google Scholar]
- 47.Isik B, Ceylan A, Isik R. Oxidative stress in smokers and non-smokers. Inhal Toxicol. 2007;19:767–9. doi: 10.1080/08958370701401418. [DOI] [PubMed] [Google Scholar]
- 48.Solak ZA, Kabaroǧlu C, Cok G, Parildar Z, Bayindir U, Ozmen D, et al. Effect of different levels of cigarette smoking on lipid peroxidation, glutathione enzymes and paraoxonase 1 activity in healthy people. Clin Exp Med. 2005;5:99–105. doi: 10.1007/s10238-005-0072-5. [DOI] [PubMed] [Google Scholar]
- 49.Nadeem A, Raj HG, Chhabra SK. Increased oxidative stress and altered levels of antioxidants in chronic obstructive pulmonary disease. Inflammation. 2005;29:23–32. doi: 10.1007/s10753-006-8965-3. [DOI] [PubMed] [Google Scholar]
- 50.Ahmad A, Shameem M, Husain Q. Relation of oxidant-antioxidant imbalance with disease progression in patients with asthma. Ann Thorac Med. 2012;7:226–32. doi: 10.4103/1817-1737.102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Vaart H, Postma DS, Timens W, ten Hacken NH. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax. 2004;59:713–21. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres-Ramos YD, García-Guillen ML, Olivares-Corichi IM, Hicks JJ. Correlation of plasma protein carbonyls and C-reactive protein with GOLD stage progression in COPD patients. Open Respir Med J. 2009;3:61–6. doi: 10.2174/1874306400903010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rai RR, Phadke MS. Plasma oxidant-antioxidant status indifferent respiratory disorders. Indian J Clin Biochem. 2006;21:161–4. doi: 10.1007/BF02912934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romieu I, Trenga C. Diet and obstructive lung diseases. Epidemiol Rev. 2001;23:268–87. doi: 10.1093/oxfordjournals.epirev.a000806. [DOI] [PubMed] [Google Scholar]
- 55.Brug J, Schols A, Mesters I. Dietary change, nutrition education and chronic obstructive pulmonary disease. Patient Educ Couns. 2004;52:249–57. doi: 10.1016/S0738-3991(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 56.Watson L, Margetts B, Howarth P, Dorward M, Thompson R, Little P. BThe association between diet and chronic obstructive pulmonary disease in subjects selected from general practice. Eur Respir J. 2002;20:313–8. doi: 10.1183/09031936.02.00256402. [DOI] [PubMed] [Google Scholar]
- 57.Denny SI, Thompson RL, Margetts BM. Dietary factors in the pathogenesis of asthma and chronic obstructive pulmonary disease. Curr Allergy Asthma Rep. 2003;3:130–6. doi: 10.1007/s11882-003-0025-6. [DOI] [PubMed] [Google Scholar]
- 58.Tabak C, Arts IC, Smit HA, Heederik D, Kromhout D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: The MORGEN study. Am J Respir Crit Care Med. 2001;164:61–4. doi: 10.1164/ajrccm.164.1.2010025. [DOI] [PubMed] [Google Scholar]
- 59.Carey IM, Strachan DP, Cook DG. Effects of changes in fresh fruit consumption on ventilatory function in healthy British adults. Am J Respir Crit Care Med. 1998;158:728–33. doi: 10.1164/ajrccm.158.3.9712065. [DOI] [PubMed] [Google Scholar]


