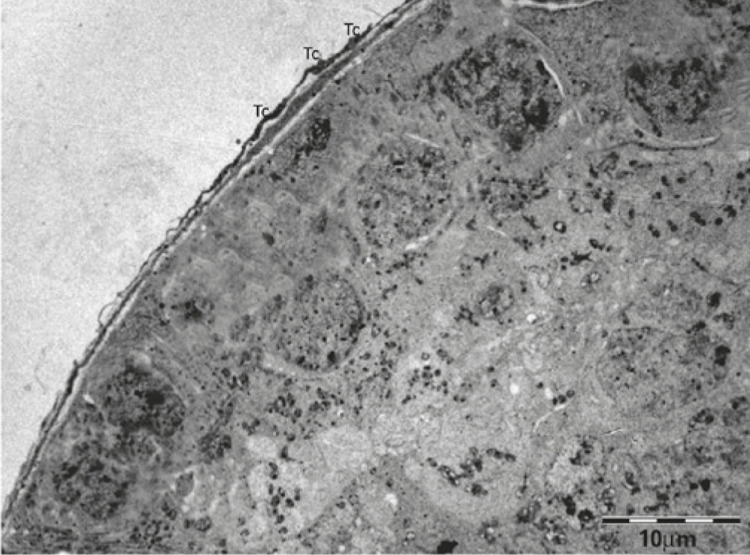
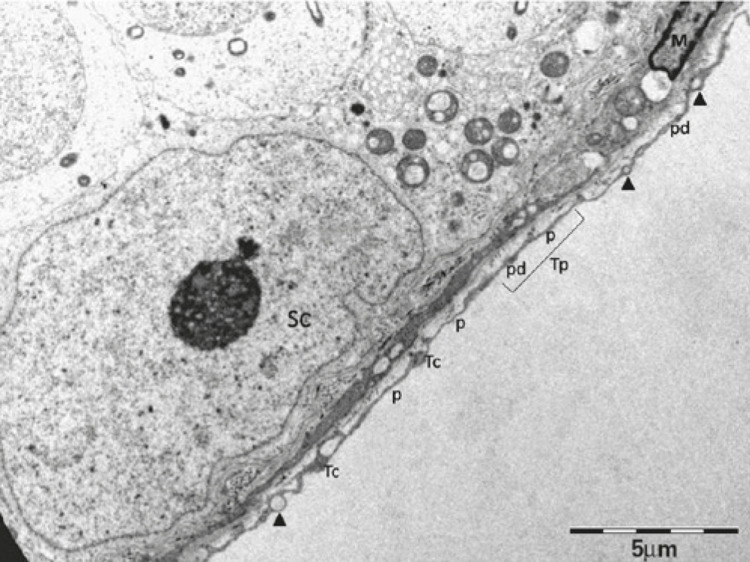
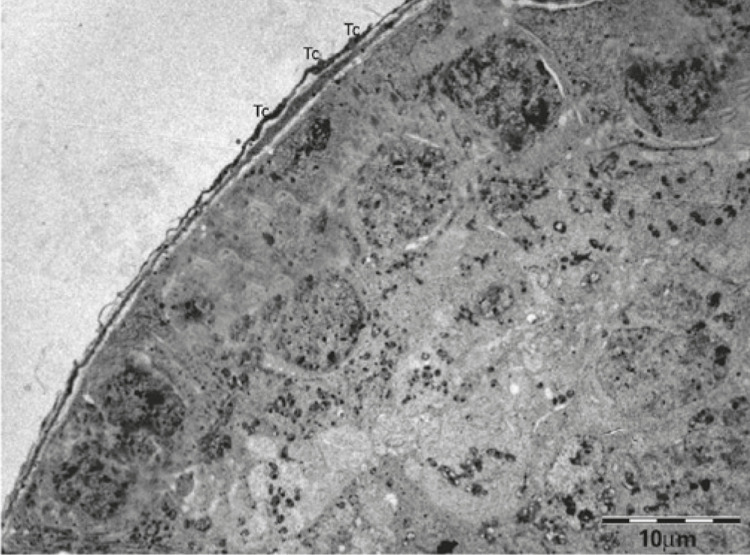
Telocyte (Tc) is a new type of classic interstitial cells, described by Popescu et al., ( 1 ) in the human pancreas. Telocytes are found in several organs, including the human testis. ( 2 ) They are long, thin cells ( Figure 1 ), with small cell bodies, a nucleus containing heterochromatin and presence of mitochondria in the periphery, in addition to a scarcely evident nucleolus. ( 3 - 5 ) They differ from other interstitial cells by their long moniliform cytoplasmic extensions, called telopodes (Tp) ( Figure 2 ), and are usually identified through their ultrastructure by transmission electron microscopy (TEM). ( 1 - 5 ) The long cytoplasmic projections, Tp, comprise thin segments (podomeres) and dilated regions (podoms), containing secretory vesicles and mitochondria ( 4 , 5 ) ( Figure 2 ). Telocytes are located in the peritubular region of the testis and contact myoid cells through cell junctions, while also contacting blood vessels and androgen-producing interstitial cells (Leydig cells) through Tp. ( 2 , 3 , 5 , 6 ) Therefore, it is believed that Tc establish homo- and heterocellular junctions, vesicle release and paracrine and/or autocrine signaling. They also interact and communicate with Leydig myoid cells and blood vessels through Tp, being responsible for the transport of substances between the interstitium (Figure 3A) and the seminiferous tubule, such as testosterone, which is essential for spermatogenesis. ( 2 - 5 )
Figure 1. Telocytes surrounding seminiferous tubule in testis of dystrophic mouse.
Tc: telocyte.
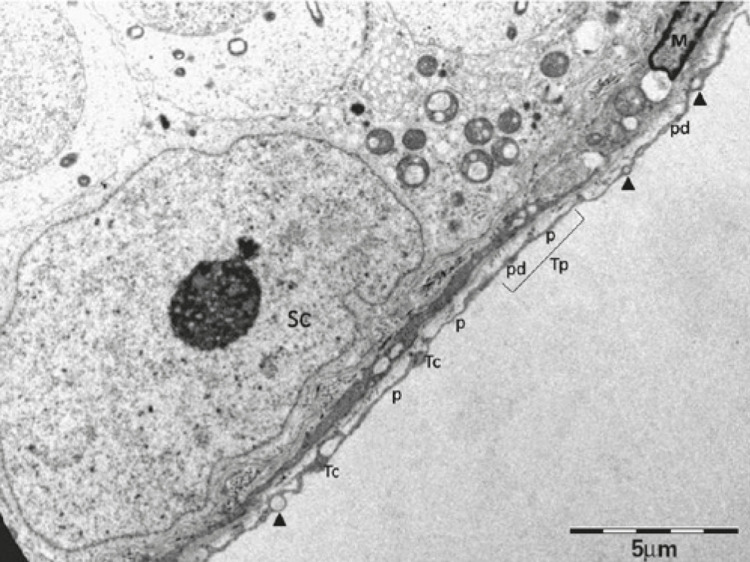
Figure 2. Telocytes in peritubular cells of testis of dystrophic mouse.
Arrow head: vesicles. Tc: telocyte; p: podomer; pd: podom; Tp: telopodes; SC: Sertoli cell; M: single peritubular myoid cell.
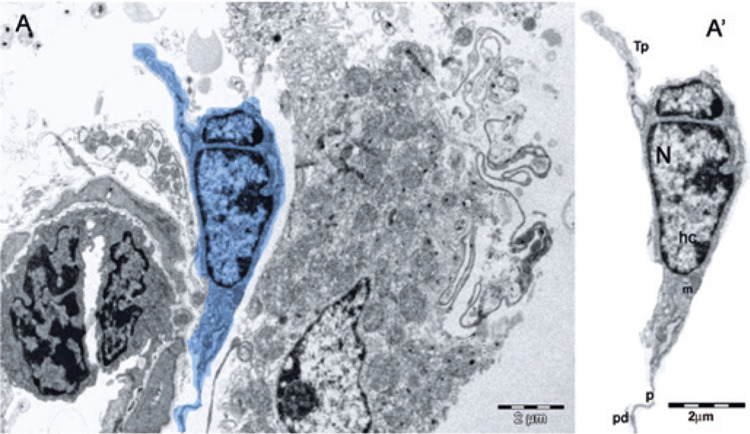
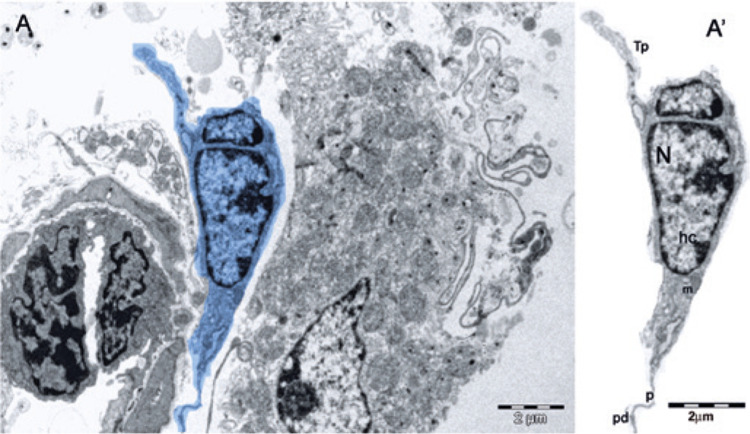
Figure 3. Telocytes in intertubular spaces of testis of dystrophic mouse. (A) digitally colored transmission electron microscope of telocyte (blue); (A’) section of telocyte with oval nucleus and peripheral heterochromatin.
Tp: telopodes; N: nucleus; hc: peripheral heterochromatin; m: mitochondria; p: podomer; pd: podom.
Dystrophic intertubular spaces contain Tc measuring, in average, approximately 8µm. ( 7 ) On the scale of 2µm, it is possible to identify mitochondria, an irregular and oval nucleus with peripheral heterochromatin, telopodes, podom and podomer (Figure 3A’). ( 8 ) In this context, we could demonstrate Tc, for the first time, in 12 testis of mdx mice with Duchenne muscular dystrophy through TEM analyses. Duchenne muscular dystrophy is a degenerative, progressive and disabling genetic disorder linked to the X chromosome, which does not cause infertility. ( 9 ) Thus, evidence of Tc in the testis of dystrophic mice implies in further understanding of spermatogenesis, since these cells help transporting testosterone to the seminiferous tubule.
REFERENCES
- 1.1. Popescu LM, Hinescu ME, Ionescu N, Ciontea SM, Cretoiu D, Ardelean C. Interstitial cells of Cajal in pancreas. J Cell Mol Med. 2005;9(1):169-90. [DOI] [PMC free article] [PubMed]; Popescu LM, Hinescu ME, Ionescu N, Ciontea SM, Cretoiu D, Ardelean C. Interstitial cells of Cajal in pancreas. J Cell Mol Med. 2005;9(1):169–190. doi: 10.1111/j.1582-4934.2005.tb00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2. Marini M, Rosa I, Guasti D, Gacci M, Sgambati E, Ibba-Manneschi L, et al. Reappraising the microscopic anatomy of human testis: identification of telocyte networks in the peritubular and intertubular stromal space. Sci Rep. 2018;8(1):14780. [DOI] [PMC free article] [PubMed]; Marini M, Rosa I, Guasti D, Gacci M, Sgambati E, Ibba-Manneschi L, et al. Reappraising the microscopic anatomy of human testis: identification of telocyte networks in the peritubular and intertubular stromal space. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-33126-2. 14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.3. Pawlicki P, Hejmej A, Milon A, Lustofin K, Płachno BJ, Tworzydlo W, et al. Telocytes in the mouse testicular interstitium: implications of G-protein-coupled estrogen receptor (GPER) and estrogen-related receptor (ERR) in the regulation of mouse testicular interstitial cells. Protoplasma. 2019; 256(2):393-408. [DOI] [PMC free article] [PubMed]; Pawlicki P, Hejmej A, Milon A, Lustofin K, Płachno BJ, Tworzydlo W, et al. Telocytes in the mouse testicular interstitium: implications of G-protein-coupled estrogen receptor (GPER) and estrogen-related receptor (ERR) in the regulation of mouse testicular interstitial cells. Protoplasma. 2019;256(2):393–408. doi: 10.1007/s00709-018-1305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.4. Cretoiu SM, Popescu LM. Telocytes revisited. Biomol Concepts. 2014; 5(5):353-69. Review. [DOI] [PubMed]; Cretoiu SM, Popescu LM. Telocytes revisited. Biomol Concepts. 2014;5(5):353–369. doi: 10.1515/bmc-2014-0029. Review. [DOI] [PubMed] [Google Scholar]
- 5.5. Liu Y, Liang Y, Wang S, Tarique I, Vistro WA, Zhang H, et al. Identification and characterization of telocytes in rat testis. Aging (Albany NY). 2019;11(15):5757-68. [DOI] [PMC free article] [PubMed]; Liu Y, Liang Y, Wang S, Tarique I, Vistro WA, Zhang H, et al. Identification and characterization of telocytes in rat testis. Aging (Albany NY) 2019;11(15):5757–5768. doi: 10.18632/aging.102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.6. Holstein AF, Schulze W, Davidoff M. Understanding spermatogenesis is a prerequisite for treatment. Reprod Biol Endocrinol. 2003;1:107. Review. [DOI] [PMC free article] [PubMed]; Holstein AF, Schulze W, Davidoff M. Understanding spermatogenesis is a prerequisite for treatment. Reprod Biol Endocrinol. 2003;1(107) doi: 10.1186/1477-7827-1-107. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.7. Dawidowicz J, Szotek S, Matysiak N, Mielańczyk Ł, Maksymowicz K. Electron microscopy of human fascia lata: focus on telocytes. J Cell Mol Med. 2015;19(10):2500-6. [DOI] [PMC free article] [PubMed]; Dawidowicz J, Szotek S, Matysiak N, Mielańczyk Ł, Maksymowicz K. Electron microscopy of human fascia lata: focus on telocytes. J Cell Mol Med. 2015;19(10):2500–2506. doi: 10.1111/jcmm.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.8. Kondo A, Kaestner KH. Emerging diverse roles of telocytes. Development. 2019;146(14):dev175018. Review. [DOI] [PMC free article] [PubMed]; Kondo A, Kaestner KH. Emerging diverse roles of telocytes. Development. 2019;146(14) doi: 10.1242/dev.175018. dev175018. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.9. Braz J, Gomes VA, Siman VA, Matta SL, Clebis N, Oliveira M, et al. Spermatogenesis in Mdx mouse model of Duchenne muscular distrophy. Anal Quant Cytopathol Histpathol. 2018;40:125-31.; Braz J, Gomes VA, Siman VA, Matta SL, Clebis N, Oliveira M, et al. Spermatogenesis in Mdx mouse model of Duchenne muscular distrophy. Anal Quant Cytopathol Histpathol. 2018;40:125–131. [Google Scholar]