Overview
Introduction
Total knee arthroplasty alleviates pain and restores function in patients with osteoarthritis of the knee, but it is associated with postoperative blood loss resulting in anemia and allogeneic blood transfusion in 10% to 38% of patients.
Step 1: Prepare Solution
Prepare tranexamic acid solution using aseptic technique.
Step 2: Apply Solution
Apply tranexamic acid solution to the open joint and soft-tissue surfaces.
Step 3: Remove Solution and Close
Remove tranexamic acid, keeping the tourniquet inflated until the wound is closed and the dressing is applied.
Results & Preop./Postop. Images
We showed, in a prospective, double-blinded, placebo-controlled randomized trial, that topical application of tranexamic acid directly into the surgical wound prior to closure at the end of a total knee arthroplasty reduces postoperative bleeding by 20% to 25%, or 300 to 400 mL.
What to Watch For
Introduction
Total knee arthroplasty alleviates pain and restores function in patients with osteoarthritis of the knee, but it is associated with postoperative blood loss resulting in anemia and allogeneic blood transfusion in 10% to 38% of patients1,2. Currently available blood conservation modalities to reduce the need for allogeneic blood transfusion do not reduce the amount of intraoperative blood loss or prevent the postoperative reduction in hemoglobin level. Preoperative autologous blood donation is costly; does not eliminate the risks of clerical errors or bacterial contamination; and may be unused, leaving patients anemic3. Intravenous administration of the antifibrinolytic agent tranexamic acid has been shown to reduce postoperative bleeding and the need for transfusion4,5. Tranexamic acid is a synthetic antifibrinolytic drug that prevents the breakdown of fibrin (the primary component of blood clots), thereby stabilizing blood hemostasis and reducing blood loss under conditions that promote fibrinolysis6. Increased fibrinolysis can result in excessive or recurrent bleeding. However, systemic inhibition of fibrinolysis carries the risk of thromboembolic events such as deep-vein thrombosis or pulmonary embolism7,8.
The advantages of topical application of tranexamic acid over intravenous administration are that this method of delivery is both “target-directed” and “safe” to use to reduce postoperative bleeding. Direct application at the site of bleeding attenuates the marked increase in local fibrinolysis associated with surgical trauma and release of the tourniquet9.
The safety of intravenous/systemic use of tranexamic acid to reduce surgical blood loss has been questioned recently10. Minimal systemic absorption of tranexamic acid has occurred when the medication has been applied directly into the surgical wound in total knee arthroplasty11 and when it has been applied locally in other types of surgery12. Topical fibrin sealants reduce blood loss but are costly; also, they are derived from human plasma, so the risk of transmission of infective agents is not completely eliminated13,14. Tranexamic acid is completely synthetic, has no human blood products, and can be used in patients who refuse blood products. Tranexamic acid is a generic and inexpensive medication that is available as a preservative-free liquid, does not need to be reconstituted, and is easy to apply.
The preparation and topical application of tranexamic acid proceeds in three stages:
Prepare tranexamic acid solution.
Apply tranexamic acid.
Remove tranexamic acid.
Step 1: Prepare Solution
Prepare tranexamic acid solution using aseptic technique.
Prepare the 3-g solution by combining three vials of sterile (preservative-free) tranexamic acid with 70 mL of sterile normal saline solution for a total volume of 100 mL. Each 10-mL vial contains 1 g of tranexamic acid (Cyklokapron, 100 mg/mL; Sandoz, Boucherville, Quebec, Canada). If you are using the 1.5-g solution, prepare it by combining 1.5 g of tranexamic acid (15 mL) and 85 mL of sterile normal saline solution for a total volume of 100 mL.
Step 2: Apply Solution
Apply tranexamic acid solution to the open joint and soft-tissue surfaces.
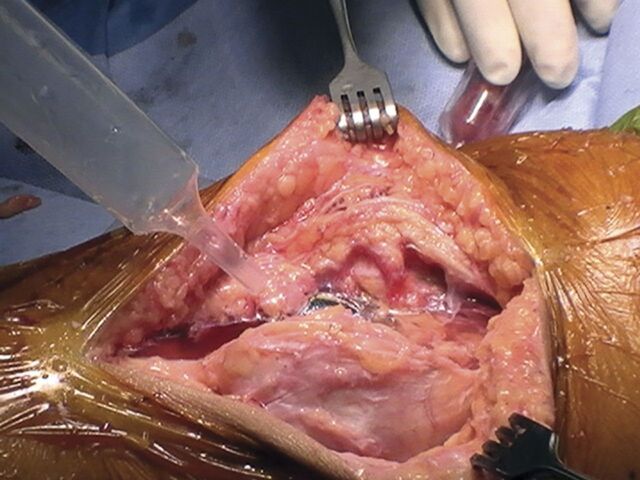
After all components are cemented into place, with the pneumatic tourniquet inflated and the knee in extension, apply the tranexamic acid solution to the open joint and soft-tissue surfaces using a bulb syringe (Fig. 1).
Leave the solution in contact with the tissues for five minutes.
Fig. 1.
Application of tranexamic acid solution to open joint and soft-tissue surfaces with use of a bulb syringe.
Step 3: Remove Solution and Close
Remove tranexamic acid, keeping the tourniquet inflated until the wound is closed and the dressing is applied.
After five minutes, remove the remaining tranexamic acid solution by placing the suction tip on the cemented component without suctioning other parts of the joint and surrounding soft tissues. Some solution may be absorbed into the tissue.
Do not irrigate the wound again.
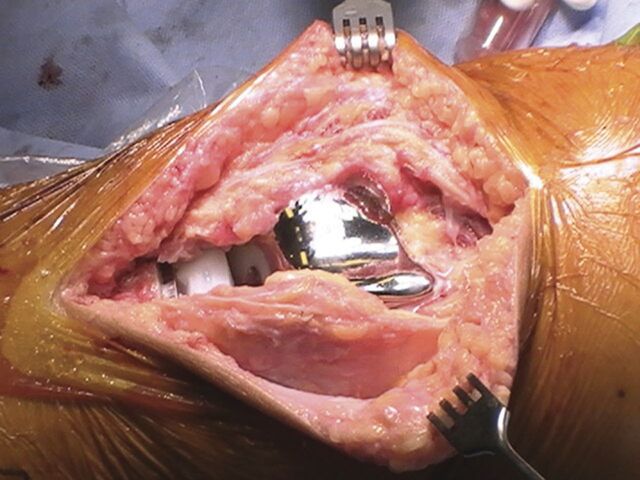
Keep the tourniquet inflated until the wound is closed and the dressing is applied (Fig. 2).
Fig. 2.
With the tourniquet still inflated, wait five minutes and then remove the remaining tranexamic acid solution. Then close the wound without further irrigation.
Results & Preop./Postop. Images
We showed, in a prospective, double-blinded, placebo-controlled randomized trial, that topical application of tranexamic acid directly into the surgical wound prior to closure at the end of a total knee arthroplasty reduces postoperative bleeding by 20% to 25%, or 300 to 400 mL11. This resulted in 16% to 17% higher postoperative hemoglobin levels compared with those in the placebo group. There was minimal systemic absorption, and no difference in the rates of deep-vein thrombosis or pulmonary embolism between patients who received tranexamic acid and those who received the placebo.
What to Watch For
Indications
Primary or revision total knee arthroplasty performed with a pneumatic tourniquet.
Contraindications
Allergy to tranexamic acid.
History of thromboembolic disease (for example, deep-vein thrombosis, pulmonary embolus, or cerebral vascular accident).
Pregnancy and breast-feeding. Tranexamic acid crosses the placenta and is passed into breast milk during lactation.
Disturbance of color vision is a contraindication to use of tranexamic acid, and retinal changes can be caused by long-term use and large doses15.
Renal failure. Topical administration of tranexamic acid is associated with minimal systemic absorption; however, this medication is eliminated by glomerular filtration and can accumulate in patients with renal failure.
Pitfalls & Challenges
The solution should be used within twenty-four hours after preparation.
The volume of study medication used in our study (100 mL) can be too large for the joint space in some patients.
Clinical Comments
In our study, we did not directly investigate the effect of tranexamic acid on the local tissue, prosthetic joint, or healing of the wound. We inferred that the topical application of tranexamic acid did not affect postoperative wound-healing or patient function on the basis of a lack of a significant difference between placebo and tranexamic acid groups with regard to postoperative knee flexion, visual analogue pain scores, length of hospital stay, time to the start of rehabilitation, and improvement in functional scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) six weeks after surgery. A group from the United Kingdom has recently investigated the effect of tranexamic acid on artificial joint materials. The results of their work will help to clarify the effect of tranexamic acid on joint prostheses (Dr. Sattar Alshryda, personal communication). Notably, topical application of tranexamic acid in other types of surgery has not been reported to be associated with adverse effects on wound-healing16-19.
In our study, we did not include patients who had undergone revision total knee arthroplasty as we had a limited number of such patients. However, we believe that patients undergoing revision surgery may receive an even greater benefit from the use of topical tranexamic acid, and we recommend and use the medication in this patient population.
We found that the total volume of medication used in our original study (100 mL) can be too large for the joint space in some patients, and since completing the study we have used a smaller total volume of tranexamic acid solution, usually 80 mL. This volume of tranexamic acid solution still ensures contact of the medication with the tissue surfaces of the knee. The volume of the tranexamic acid solution and the duration for which the medication was left in place in our study were based on studies of topical administration of tranexamic acid in cardiac surgery14. In those randomized trials, the tranexamic acid was diluted with sterile normal saline solution to a volume of 100 mL. The medication or placebo (an equal amount of saline solution) was poured into the pericardial cavity and/or over the mediastinal tissues at the end of the surgery and before the closure of the median sternotomy and was left for two to five minutes. We did not compare different durations for which the medication was left in place. It is possible that a shorter duration of application is also effective for reducing postoperative blood loss.
Supplementary Material
Based on an original article: J Bone Joint Surg Am. 2010;92:2503-13.
Disclosure: None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. None of the authors, or their institution(s), have had any financial relationship, in the thirty-six months prior to submission of this work, with any entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, no author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Bong MR Patel V Chang E Issack PS Hebert R Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19:281-7. [DOI] [PubMed] [Google Scholar]
- 2.Freedman J Luke K Monga N Lincoln S Koen R Escobar M Chiavetta J. A provincial program of blood conservation: The Ontario Transfusion Coordinators (ONTraC). Transfus Apher Sci. 2005;33:343-9. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD Goodnough LT AuBuchon JP Noordsij PG Littenberg B. The cost-effectiveness of preoperative autologous blood donation for total hip and knee replacement. Transfusion. 1993;33:544-51. [DOI] [PubMed] [Google Scholar]
- 4.Zufferey P Merquiol F Laporte S Decousus H Mismetti P Auboyer C Samama CM Molliex S. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105:1034-46. [DOI] [PubMed] [Google Scholar]
- 5.Kagoma YK Crowther MA Douketis J Bhandari M Eikelboom J Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123:687-96. [DOI] [PubMed] [Google Scholar]
- 6.Dunn CJ Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005-32. [DOI] [PubMed] [Google Scholar]
- 7.Ho KM Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31:529-37. [DOI] [PubMed] [Google Scholar]
- 8.Engel JM Hohaus T Ruwoldt R Menges T Jürgensen I Hempelmann G. Regional hemostatic status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesth Analg. 2001;92:775-80. [DOI] [PubMed] [Google Scholar]
- 9.Katsumata S Nagashima M Kato K Tachihara A Wauke K Saito S Jin E Kawanami O Ogawa R Yoshino S. Changes in coagulation-fibrinolysis marker and neutrophil elastase following the use of tourniquet during total knee arthroplasty and the influence of neutrophil elastase on thromboembolism. Acta Anaesthesiol Scand. 2005;49:510-6. [DOI] [PubMed] [Google Scholar]
- 10.Ngaage DL Bland JM. Lessons from aprotinin: is the routine use and inconsistent dosing of tranexamic acid prudent? Meta-analysis of randomised and large matched observational studies. Eur J Cardiothorac Surg. 2010;37:1375-83. [DOI] [PubMed] [Google Scholar]
- 11.Wong J Abrishami A El Beheiry H Mahomed NN Roderick Davey J Gandhi R Syed KA Muhammad Ovais Hasan S De Silva Y Chung F. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92:2503-13. [DOI] [PubMed] [Google Scholar]
- 12.De Bonis M Cavaliere F Alessandrini F Lapenna E Santarelli F Moscato U Schiavello R Possati GF. Topical use of tranexamic acid in coronary artery bypass operations: a double-blind, prospective, randomized, placebo-controlled study. J Thorac Cardiovasc Surg. 2000;119:575-80. [DOI] [PubMed] [Google Scholar]
- 13.Molloy DO Archbold HA Ogonda L McConway J Wilson RK Beverland DE. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. J Bone Joint Surg Br. 2007;89:306-9. [DOI] [PubMed] [Google Scholar]
- 14.Levy O Martinowitz U Oran A Tauber C Horoszowski H. The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am. 1999;81:1580-8. [DOI] [PubMed] [Google Scholar]
- 15.Product monograph: Tranexamic acid injection BP. 2002; Boucherville, Quebec, Canada: Sabex, Inc. Revised 2004. [Google Scholar]
- 16.Abrishami A Chung F Wong J. Topical application of antifibrinolytic drugs for on-pump cardiac surgery: a systematic review and meta-analysis. Can J Anesth. 2009;56:202-12. [DOI] [PubMed] [Google Scholar]
- 17.Jabalameli M Zakeri K. Topical tranexamic acid for bleeding of endoscopic sinus surgery. Can J Anesth. 2005;52Suppl 1:A206. [Google Scholar]
- 18.Krohn CD Sørensen R Lange JE Riise R Bjørnsen S Brosstad F. Tranexamic acid given into the wound reduces postoperative blood loss by half in major orthopaedic surgery. Eur J Surg Suppl. 2003;588:57-61. [PubMed] [Google Scholar]
- 19.Sindet-Pedersen S Ramström G Bernvil S Blombäck M. Hemostatic effect of tranexamic acid mouthwash in anticoagulant-treated patients undergoing oral surgery. N Engl J Med. 1989;320:840-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.