Abstract
In discovery of HDAC inhibitors (HDACIs) with improved anticancer potency, structural modification was performed on the previous derived indole-3-butyric acid derivative. Among all the synthesised compounds, molecule I13 exhibited high HDAC inhibitory and antiproliferative potencies in the in vitro investigations. The IC50 values of I13 against HDAC1, HDAC3, and HDAC6 were 13.9, 12.1, and 7.71 nM, respectively. In the cancer cell based screening, molecule I13 showed increased antiproliferative activities in the inhibition of U937, U266, HepG2, A2780, and PNAC-1 cells compared with SAHA. In the HepG2 xenograft model, 50 mg/kg/d of I13 could inhibit tumour growth in athymic mice compared with 100 mg/kg/d of SAHA. Induction of apoptosis was revealed to play an important role in the anticancer potency of molecule I13. Collectively, a HDACI (I13) with high anticancer activity was discovered which can be utilised as a lead compound for further HDACI design.
Keywords: Histone deacetylase, inhibitor, anticancer, indole
Introduction
Histone deacetylases (HDACs) are a family of enzymes involved in the deacetylation of histones and non-histone proteins including transcription factors, Hsp90 and tubulin1–3. To date, a total of 18 different isoforms of HDACs classified into two families and four classes have been identified in human4. The two families are the zinc dependent class I, II, and IV, and the nicotinamide-adenine-dinucleotide (NAD) dependent class III. Class I HDACs contain HDAC1, 2, 3, and 8, and class II HDACs are subdivided into IIa (HDAC4, 5, 7, and 9) and IIb (HDAC6 and 10). Class III HDACs, as a separate family, are a group of NAD dependent silent information regulator 2-related proteins (known as sirtuins, sirt1-7). Class IV HDAC, HDAC11, is structurally different from either class I or class II HDACs.
The acetylation level of histone and non-histone proteins is an opposing activity regulated by HDACs and histone acetyltransferases (HATs)5–7. The epigenetic acetylation plays a key role in the regulation of fundamental cellular functions including protein phosphorylation, signal transduction, cell cycle, proliferation, apoptosis, cardiac development, and so on8–10. The abnormal expression of HDACs is associated with the pathogenesis of a variety of diseases such as diabetes mellitus11, neurodegenerative diseases12,13, inflammatory disorders14, HIV15–17, cardiac diseases18,19, and especially tumor20. Pharmacological inhibition of HDACs and development of HDAC inhibitors (HDACIs) have been widely investigated in the treatment of cancer and other diseases21.
HDAC inhibitors have been extensively studied in the treatment of tumour and other epigenetic disorders20. According to the chemical structure, HDACIs are divided into groups, including hydroxamic acids, aminobenzamides, cyclic peptides, carboxylic acids, and hybrid molecules4. Suberoylanilide hydroxamic acid (SAHA)22 and FK22823 have been approved by US Food and Drug Administration (FDA) for the treatment of refractory cutaneous T-cell lymphoma (CTCL). PDX10124 and LBH58925 have been approved for the treatment of peripheral T-cell lymphoma (PTCL) and multiple myeloma, respectively. Chidamide26 is a benzamide HDACI approved by Chinese Food and Drug Administration (CFDA) for the treatment of relapsed or refractory PTCL.
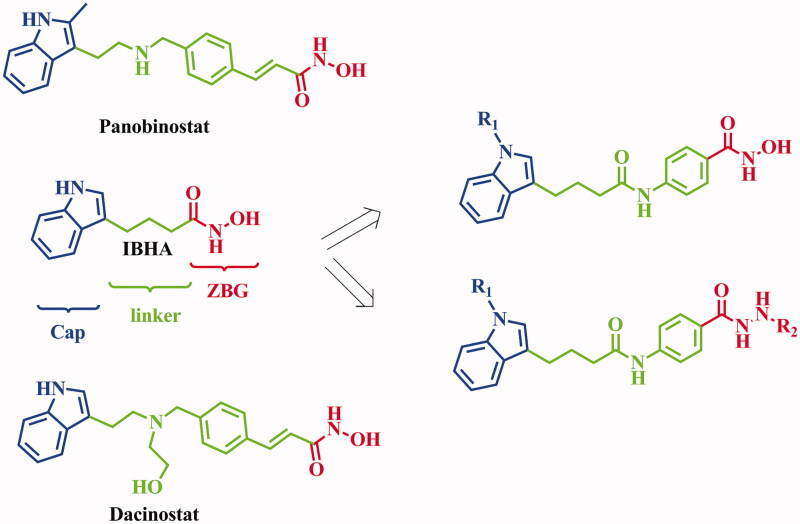
Auxins are a group of plant hormones that play a cardinal role in the plant growth and behavioural processes27. Biological effects of naturally occurring auxins in plants include indole-3-acetic acid (IAA), 4-chloroindole-3-acetic acid (4-Cl-IAA), phenylacetic acid (PAA), indole-3-butyric acid (IBA), and indole-3-propionic acid (IPA), have exhibited inhibitory activities against human tumour cell lines28. However, development of auxins as anticancer agents has been rarely reported. In our previous study, a potent IBHA with antitumor activity was derived by substitution of carboxyl group of IBA with hydroxamic acid29. Compared with SAHA, IBHA exhibited reduced activities in the HDAC enzymatic inhibition and antiproliferative assays. Inspired by structures of high potent HDACIs Panobinostat and Dacinostat which have the 3-alkyl indole pharmacophores in the cap region, structural modification was performed to IBHA. In the present study, to enhance the binding affinity of IBA derivatives with HDACs, and improve the antitumor activity of IBA derivatives, phenyl group was introduced to the linker of IBHA. Substituted groups were introduced to the nitrogen in the indole ring, and hydroxamic acid was utilised as zinc binding group (ZBG). Hydrazide has been reported as ZBG with good HDAC inhibitory activity30. Therefore, to investigate the performance of hydrazide in the present structure, hydroxamic acid groups of several active molecules have been replaced by hydrazide groups (Figure 1). The anticancer activities of the derived molecules were evaluated in the enzymatic assay, in vitro cancer cell based screening, apoptosis, and in vivo anticancer test.
Figure 1.
Design of indole-3-butyric acid derivatives from the structure of IBHA. R1 group is substituted aromatic rings, R2 group is alkanes.
Materials and methods
All commercially available starting materials, reagents and solvents were used without further purification. All reactions were monitored by TLC with 0.25 mm silica gel plates (60GF-254). UV light and ferric chloride were used to visualise the spots. 1H NMR and 13C NMR spectra were recorded on a Bruker DRX spectrometer at 500 MHz, using TMS as an internal standard. High-resolution mass spectra were performed in Weifang Medical University.
Methyl 4-aminobenzoate hydrochloric acid has been synthesised and described in our previous work.
Preparation of I2a and its analogues: Derivatives I3a–I13a were prepared as described for I2a (see below).
4-(1-Benzyl-1H-indol-3-yl)-butyric acid (I2a). To a solution of NaH in DMF, were stirred under Ar2 and 0 °C. After 30 min, the benzyl bromide was slowly added. The reaction solution was stirred at room temperature for 2 h. Then, the solvent was evaporated under vacuum. The residue was acidified with saturated citric acid, and then extracted with EtOAc (3 × 20 ml). The organic layer was combined, washed with brine (3 × 20 ml) dried over MgSO4, and evaporated under vacuum. The desired compound I2a was derived by crystallisation in EtOAc as powder. HRMS m/z: 292.1338 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.03 (s, 1H), 7.53 (d, J = 7.6 Hz, 1H), 7.38 (d, J = 8.2 Hz, 1H), 7.31–7.27 (m, 3H), 7.23 (d, J = 7.2 Hz, 1H), 7.21–7.17 (m, 2H), 7.09–7.05 (m, 1H), 7.00–6.97 (m, 1H), 5.35 (s, 2H), 2.70 (t, J = 7.6 Hz, 2H), 2.27 (t, J = 7.2 Hz, 2H), 1.91–1.83 (m, 2H).
4-(1-Ethyl-1H-indol-3-yl)-butyric acid (I3a). HRMS m/z: 230.11719 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.04 (s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.16 (s, 1H), 7.12–7.09 (m, 1H), 7.00–6.97 (m, 1H), 4.14 (q, J = 7.2 Hz, 2H), 2.68 (t, J = 7.6 Hz, 2H), 2.26 (t, J = 7.2 Hz, 2H), 1.89–1.82 (m, 2H), 1.33 (t, J = 7.2 Hz, 3H).
4-(1-(4-Trifluoromethyl-benzyl)-1H-indol-3-yl)-butyric acid (I4a). HRMS m/z: 360.12186 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.06 (s, 1H), 7.67 (d, J = 8.2 Hz, 2H), 7.55 (d, J = 7.8 Hz, 1H), 7.38–7.31 (m, 4H), 7.11–7.07 (m, 2H), 7.03–6.99 (m, 1H), 5.48 (s, 2H), 2.72 (t, J = 7.6 Hz, 2H), 2.29 (t, J = 7.2 Hz, 2H), 1.92–1.85 (m, 2H).
4-(1-(2-Trifluoromethyl-benzyl)-1H-indol-3-yl)-butyric acid (I5a). HRMS m/z: 360.12039 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.04 (s, 1H), 7.79 (d, J = 7.8 Hz, 1H), 7.60 (d, J = 7.6 Hz, 1H), 7.47 (m, 2H), 7.26 (s, 1H), 7.17 (d, J = 8.0 Hz, 1H), 7.10–7.02 (m, 2H), 6.50 (d, J = 6.9 Hz, 1H), 5.58 (s, 2H), 2.75 (t, J = 7.6 Hz, 2H), 2.30 (t, J = 7.4 Hz, 2H), 1.94–1.86 (m, 2H).
4-(1-(3,4-Difluoro-benzyl)-1H-indol-3-yl)-butyric acid (I6a). HRMS m/z: 328.11359 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.10 (s, 1H), 7.53 (d, J = 7.8 Hz, 1H), 7.41 (d, J = 8.2 Hz, 1H), 7.35 (dd, J = 19.2, 8.4 Hz, 1H), 7.32–7.26 (m, 2H), 7.11–7.08 (m, 1H), 7.02–6.99 (m, 2H), 5.35 (s, 2H), 2.70 (t, J = 7.6 Hz, 2H), 2.27 (t, J = 7.2 Hz, 2H), 1.91–1.83 (m, 2H).
4-(1-(3-Fluoro-benzyl)-1H-indol-3-yl)-butyric acid (I7a). HRMS m/z: 310.12384 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.05 (s, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.33 (dd, J = 14.4, 8.0 Hz, 1H), 7.30 (s, 1H), 7.11–6.97 (m, 5H), 5.38 (s, 2H), 2.71 (t, J = 7.5 Hz, 2H), 2.28 (t, J = 7.4 Hz, 2H), 1.92–1.84 (m, 2H).
4-(1-(3-Bromo-benzyl)-1H-indol-3-yl)-butyric acid (I8a). HRMS m/z: 370.04352 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.07 (s, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.44–7.39 (m, 3H), 7.30 (s, 1H), 7.27–7.24 (m, 1H), 7.15 (d, J = 7.8 Hz, 1H), 7.11–7.07 (m, 1H), 7.03–6.99 (m, 1H), 5.37 (s, 2H), 2.71 (t, J = 7.5 Hz, 2H), 2.28 (t, J = 7.4 Hz, 2H), 1.91–1.84 (m, 2H).
4-(1-(3-Chloro-benzyl)-1H-indol-3-yl)-butyric acid (I9a). HRMS m/z: 326.09418 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.08 (s, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.34–7.28 (m, 3H), 7.23 (s, 1H), 7.13–7.07 (m, 2H), 7.03–6.99 (m, 2H), 5.38 (s, 2H), 2.71 (t, J = 7.5 Hz, 2H), 2.28 (t, J = 7.4 Hz, 2H), 1.91–1.84 (m, 2H).
4-(1-(2,6-Difluoro-benzyl)-1H-indol-3-yl)-butyric acid (I10a). HRMS m/z: 328.11423 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.02 (s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.46–7.39 (m, 2H), 7.17–7.11 (m, 4H), 7.02–6.99 (m, 1H), 7.11–7.07 (m, 1H), 5.37 (s, 2H), 2.66 (t, J = 7.6 Hz, 2H), 2.25 (t, J = 7.4 Hz, 2H), 1.86–1.79 (m, 2H).
4-(1-(3,5-Bis-trifluoromethyl-benzyl)-1H-indol-3-yl)-butyric acid (I11a). HRMS m/z: 428.10791 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.03 (s, 1H), 8.00 (s, 1H), 7.85 (s, 2H), 7.56 (d, J = 7.8 Hz, 1H), 7.48 (d, J = 8.2 Hz, 1H), 7.40 (s, 2H), 7.13–7.10 (m, 1H), 7.05–7.01 (m, 1H), 5.59 (s, 2H), 2.73 (t, J = 7.4 Hz, 2H), 2.26 (t, J = 7.4 Hz, 2H), 1.91–1.84 (m, 2H).
4-(1-Butyl-1H-indol-3-yl)-butyric acid (I12a). HRMS m/z: 258.14868 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.06 (s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.13–7.09 (m, 1H), 7.00–6.97 (m, 1H), 4.01 (q, J = 7.0 Hz, 2H), 2.69 (t, J = 7.4 Hz, 2H), 2.26 (t, J = 7.4 Hz, 2H), 1.90–1.82 (m, 2H), 1.74–1.66 (m, 2H), 1.28–1.18 (m, 2H), 0.87(t, J = 7.4 Hz, 3H).
4-(1-methyl-1H-indol-3-yl)-butyric acid (I13a). HRMS m/z: 216.10153 [M–H]–. 1H NMR (400 MHz, DMSO) δ 12.03 (s, 1H), 7.51 (d, J = 7.9 Hz, 1H), 7.36 (d, J = 8.2 Hz, 1H), 7.14–7.09 (m, 2H), 7.02–6.98 (m, 1H), 3.72 (s, 3H), 2.68 (t, J = 7.5 Hz, 2H), 2.26 (t, J = 7.2 Hz, 2H), 1.89–1.81 (m, 2H).
Preparation of I1b and its analogues: derivatives I2b–I13b were prepared as described for I1b (see below).
Methyl 4-(4-(1H-indol-3-yl)butanamido)benzoate (I1b). To a solution of IBA (a) (1.0 g, 5 mmol) in DCM (50 ml), Et3N (0.55 g, 5.5 mmol) and TBTU (1.8 g, 5.5 mmol) were added in turn. After 20 min, methyl 4-aminobenzoate hydrochloride (0.94 g, 5 mmol) and Et3N (0.50 g, 5 mmol) were added. The reaction solution was stirred at room temperature for 8 h. Then, the solvent was evaporated with the residue being taken up in EtOAc (50 ml). The EtOAc solution was washed with saturated citric acid (3 × 20 ml), NaHCO3 (3 × 20 ml), and brine (3 × 20 ml), dried over MgSO4, and evaporated under vacuum. The desired compound I1b (1.2 g, 72% yield) was derived by crystallisation in EtOAc as white powder. HRMS m/z: 335.13815 [M + H]–. 1H NMR (400 MHz, DMSO) δ 10.78 (s, 1H), 10.23 (s, 1H), 7.90 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 8.7 Hz, 2H), 7.52 (d, J = 7.8 Hz, 1H), 7.33 (d, J = 8.1 Hz, 1H), 7.13 (s, 1H), 7.07–7.04 (m, 1H), 6.98–6.94 (m, 1H), 2.74 (t, J = 7.4 Hz, 2H), 2.41 (t, J = 7.4 Hz, 2H), 2.01–1.94 (m, 2H).
Methyl 4-(4-(1-benzyl-1H-indol-3-yl)butanamido)benzoate (I2b). HRMS m/z: 427.20010 [M + H]+. 1H NMR (400 MHz, DMSO) δ 10.24 (s, 1H), 7.90 (d, J = 8.6 Hz, 2H), 7.74 (d, J = 8.7 Hz, 2H), 7.55 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 7.8 Hz, 1H), 7.30–7.27 (m, 1H), 7.23 (d, J = 7.1 Hz, 1H), 7.18 (d, J = 7.1 Hz, 2H), 7.10–7.06 (m, 1H), 7.01–6.98 (m, 1H), 5.35 (s, 2H), 3.81 (s, 3H), 2.75 (t, J = 7.4 Hz, 2H), 2.43 (t, J = 7.4 Hz, 2H), 2.02–1.95 (m, 2H).
Methyl 4-(4-(1-ethyl-1H-indol-3-yl)butanamido)benzoate (I3b). HRMS m/z: 365.18481 [M + H]+. 1H NMR (400 MHz, DMSO) δ 10.23 (s, 1H), 7.90 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 7.9 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.18 (s, 1H), 7.13–7.09 (m, 1H), 7.00–6.97 (m, 1H), 4.14 (q, J = 7.2 Hz, 2H), 3.81 (s, 3H), 2.73 (t, J = 7.4 Hz, 2H), 2.42 (t, J = 7.4 Hz, 2H), 2.01–1.93 (m, 2H), 1.33 (t, J = 7.2 Hz, 3H).
Methyl 4-(4-(1-(4-trifluoromethyl-benzyl)-1H-indol-3-yl)butanamido)benzoate (I4b). HRMS m/z: 495.18729 [M + H]+. δ 10.24 (s, 1H), 7.90 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 8.7 Hz, 2H), 7.66 (d, J = 7.9 Hz, 2H), 7.57 (d, J = 8.2 Hz, 1H), 7.38–7.32 (m, 4H), 7.11–7.07 (m, 1H), 7.03–6.99 (m, 1H), 5.48 (s, 2H), 3.81 (s, 3H), 2.76 (t, J = 7.4 Hz, 2H), 2.44 (t, J = 7.4 Hz, 2H), 2.03–1.96 (m, 2H).
Methyl 4-(4-(1-(2-trifluoromethyl-benzyl)-1H-indol-3-yl)butanamido)benzoate (I5b). HRMS m/z: 495.18607 [M + H]+. δ 10.24 (s, 1H), 7.90 (d, J = 8.7 Hz, 2H), 7.79 (d, J = 6.9 Hz, 1H), 7.73 (d, J = 8.7 Hz, 2H), 7.62 (d, J = 7.7 Hz, 1H), 7.51–7.44 (m, 2H), 7.28 (s, 1H), 7.17 (d, J = 7.9 Hz, 1H), 7.11–7.02 (m, 2H), 6.51 (d, J = 7.1 Hz, 1H), 5.57 (s, 2H), 3.81 (s, 3H), 2.79 (t, J = 7.2 Hz, 2H), 2.45 (t, J = 7.4 Hz, 2H), 2.04–1.97(m, 2H).
Methyl 4-(4-(1-(3,4-difluoro-benzyl)-1H-indol-3-yl)butanamido)benzoate (I6b). HRMS m/z: 463.18167 [M + H]+. δ 10.24 (s, 1H), 7.90 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 8.6 Hz, 2H), 7.55 (d, J = 7.7 Hz, 1H), 7.41 (d, J = 8.1 Hz, 1H), 7.39–7.23 (m, 3H), 7.12–7.08 (m, 1H), 7.03–6.99 (m, 2H), 5.35 (s, 2H), 3.81 (s, 3H), 2.75 (t, J = 7.6 Hz, 2H), 2.43 (t, J = 7.2 Hz, 2H), 2.02–1.95(m, 2H).
Methyl 4-(4-(1-(3-fluoro-benzyl)-1H-indol-3-yl)butanamido)benzoate (I7b). HRMS m/z: 445.19061 [M + H]+. δ 10.24 (s, 1H), 7.90 (d, J = 8.7 Hz, 2H), 7.74 (d, J = 8.7 Hz, 2H), 7.56 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.36–7.31 (m, 2H), 7.11–6.99 (m, 5H), 5.38 (s, 2H), 3.81 (s, 3H), 2.75 (t, J = 7.5 Hz, 2H), 2.43 (t, J = 7.2 Hz, 2H), 2.02–1.95(m, 2H).
Methyl 4-(4-(1-(3-bromo-benzyl)-1H-indol-3-yl)butanamido)benzoate (I8b). HRMS m/z: 505.11108 [M + H]+. δ 10.24 (s, 1H), 7.89 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 8.7 Hz, 2H), 7.56 (d, J = 7.8 Hz, 1H), 7.44–7.41 (m, 3H), 7.33 (s, 1H), 7.28–7.24 (m, 1H), 7.15 (t, J = 7.8 Hz, 1H), 7.12–7.08 (m, 1H), 7.03–6.99 (m, 1H), 5.37 (s, 2H), 3.81 (s, 3H), 2.75 (t, J = 7.5 Hz, 2H), 2.43 (t, J = 7.4 Hz, 2H), 2.02–1.97(m, 2H).
Methyl 4-(4-(1-(3-chloro-benzyl)-1H-indol-3-yl)butanamido)benzoate (I9b). HRMS m/z: 461.16150 [M + H]+. δ 10.25 (s, 1H), 7.90 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 8.7 Hz, 2H), 7.56 (d, J = 7.7 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.34–7.28 (m, 3H), 7.25 (s, 1H), 7.13–7.08 (m, 2H), 7.03–6.99 (m, 1H), 5.38 (s, 2H), 3.81 (s, 3H), 2.76 (t, J = 7.4 Hz, 2H), 2.43 (t, J = 7.3 Hz, 2H), 2.63–1.95(m, 2H).
Methyl 4-(4-(1-(2,6-difluoro-benzyl)-1H-indol-3-yl)butanamido)benzoate (I10b). HRMS m/z: 463.18112 [M + H]+. δ 10.23 (s, 1H), 7.90 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 7.9 Hz, 1H), 7.47–7.41 (m, 2H), 7.16–7.12 (m, 4H), 7.03–6.99 (m, 1H), 5.39 (s, 2H), 3.81 (s, 3H), 2.71 (t, J = 7.5 Hz, 2H), 2.40 (t, J = 7.4 Hz, 2H), 1.99–1.91(m, 2H).
Methyl 4-(4-(1-(3,5-bis-trifluoromethyl-benzyl)-1H-indol-3-yl)butanamido)benzoate (I11b). HRMS m/z: 563.17468 [M + H]+. δ 10.24 (s, 1H), 8.01 (s, 1H), 7.91–7.87 (m, 4H), 7.73 (d, J = 8.5 Hz, 2H), 7.58 (d, J = 7.8 Hz, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.42 (s, 1H), 7.14–7.10 (m, 1H), 7.05–7.01 (m, 1H), 5.58 (s, 2H), 3.82 (s, 3H), 2.76 (t, J = 7.4 Hz, 2H), 2.43 (t, J = 7.4 Hz, 2H), 2.02–1.95(m, 2H).
Methyl 4-(4-(1-butyl-1H-indol-3-yl)butanamido)benzoate (I12b). HRMS m/z: 393.21597 [M + H]+. δ 10.23 (s, 1H), 7.89 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 8.6 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.16 (s, 1H), 7.12–7.08 (m, 1H), 7.00–6.97 (m, 1H), 4.09 (t, J = 7.0 Hz, 2H), 3.81 (s, 3H), 2.73 (t, J = 7.4 Hz, 2H), 2.41 (t, J = 7.2 Hz, 2H), 2.01–1.93(m, 2H), 1.74–1.66(m, 2H), 1.28–1.91 (m, 2H), 0.87 (t, J = 7.4 Hz, 3H).
Methyl 4-(4-(1-methyl-1H-indol-3-yl)butanamido)benzoate (I13b). HRMS m/z: 351.16901 [M + H]+. δ 10.23 (s, 1H), 7.90 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 7.9 Hz, 1H), 7.37 (d, J = 8.2 Hz, 1H), 7.14–7.12 (m, 2H), 7.02–6.98 (m, 1H), 3.81 (s, 3H), 3.72 (s, 3H), 2.73 (t, J = 7.3 Hz, 2H), 2.41 (t, J = 7.4 Hz, 2H), 2.00–1.93(m, 2H).
Preparation of I1 and its analogues: derivatives I2–I13 were prepared as described for I1 (see below).
4-(4-(1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I1). Compound methyl 4-(4-(1H-indol-3-yl)butanamido)benzoate (I1a) (0.34 g, 1.0 mmol) was dissolved in 14 ml of NH2OK (0.56 g, 24 mmol) methanol solution. After 2 h, the solvent was evaporated under vacuum. The residue was acidified with saturated citric acid, and then extracted with EtOAc (3 × 20 ml). The organic layers were combined, washed with brine (3 × 20 ml) and dried over MgSO4. The desired compound c (0.16 g, 46% yield) was derived by crystallisation in EtOAc as white powder. HRMS (AP-ESI) m/z calcd for C19H20N3O3 [M + H]+ 338.14600, found 338.34195. 1H NMR (400 MHz, DMSO) δ 11.08 (s, 1H), 10.78 (s, 1H), 10.09 (s, 1H), 8.93 (s, 1H), 7.68 (dd, J = 18.4, 8.8 Hz, 4H), 7.53 (d, J = 7.8 Hz, 1H), 7.34 (d, J = 8.0 Hz, 1H), 7.14 (s, 1H), 7.08–7.04 (m, 1H), 6.99–6.95 (m, 1H), 2.74 (t, J = 7.36 Hz, 2H), 2.40 (t, J = 7.4 Hz, 2H), 2.01–1.94 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.14, 164.46, 142.34, 136.79, 128.12, 127.63, 127.38, 122.80, 121.32, 118.77, 118.61, 114.42, 111.81, 36.66, 26.25, 24.73 ppm.
4-(4-(1-benzyl-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I2). HRMS (AP-ESI) m/z calcd for C26H25N3O3 [M + H]+ 428.19295, found 428.19577. 1H NMR (400 MHz, DMSO) δ 11.08 (s, 1H), 10.10 (s, 1H), 8.92 (s, 1H), 7.67 (dd, J = 18.4, 8.8 Hz, 4H), 7.54 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.4 Hz, 1H), 7.30–7.27 (m, 3H), 7.23 (d, J = 7.0 Hz, 1H), 7.18 (d, J = 7.4 Hz, 2H), 7.10–7.06 (m, 1H), 7.01–6.98 (m, 1H), 5.35 (s, 2H), 2.75 (t, J = 7.2 Hz, 2H), 2.41 (t, J = 7.5 Hz, 2H), 2.01–1.96 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.07, 164.44, 142.38, 138.91, 136.58, 128.96, 128.26, 128.12, 127.71, 127.47, 127.39, 126.73, 121.64, 119.20, 118.94, 118.75, 114.51, 110.49, 49.37, 36.68, 26.24, 24.65 ppm.
4-(4-(1-Ethyl-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I3). HRMS (AP-ESI) m/z calcd for C21H23N3O3 [M + H]+ 366.17730, found 366.18027. 1H NMR (400 MHz, DMSO) δ 11.08 (s, 1H), 10.09 (s, 1H), 8.92 (s, 1H), 7.67 (dd, J = 19.0, 8.7 Hz, 4H), 7.53 (d, J = 7.9 Hz, 1H), 7.40 (d, J = 8.3 Hz, 1H), 7.18 (s, 1H), 7.13–7.09 (m, 1H), 7.01–6.97 (m, 1H), 4.14 (q, J = 7.2 Hz, 2H), 2.73 (t, J = 7.4 Hz, 2H), 2.40 (t, J = 7.4 Hz, 2H), 1.98–1.93 (m, 2H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.05, 164.40, 142.35, 136.16, 128.12, 128.07, 127.38, 125.59, 121.38, 119.14, 118.72, 118.66, 113.96, 110.00, 40.45, 36.67, 26.24, 24.66, 15.93 ppm.
4-(4-(1-(4-Trifluoromethyl-benzyl)-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I4). HRMS (AP-ESI) m/z calcd for C27H24F3N3O3 [M + H]+ 496.18033, found 496.18274. 1H NMR (400 MHz, DMSO) δ 11.09 (s, 1H), 10.11 (s, 1H), 8.93 (s, 1H), 7.71–7.64 (m, 6H), 7.57 (d, J = 7.8 Hz, 1H), 7.38–7.33 (m, 4H), 7.11–7.07 (m, 1H), 7.03–7.00 (m, 1H), 5.48 (s, 2H), 2.76 (t, J = 7.2 Hz, 2H), 2.42 (t, J = 7.2 Hz, 2H), 2.01–1.98 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.03, 164.40, 143.83, 142.34, 136.55, 128.48, 128.30, 128.12, 128.00, 127.39, 126.76, 125.91, 121.85, 119.31, 119.16, 118.73, 114.88, 110.41, 48.81, 36.64, 26.14, 24.62 ppm.
4-(4-(1-(2-Trifluoromethyl-benzyl)-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I5). HRMS (AP-ESI) m/z calcd for C27H24F3N3O3 [M + H]+ 496.18033, found 496.18295. 1H NMR (400 MHz, DMSO) δ 11.08 (s, 1H), 10.11 (s, 1H), 8.93 (s, 1H), 7.80 (d, J = 6.9 Hz, 1H), 7.71–7.61 (m, 5H), 7.52–7.45 (m, 2H), 7.29 (s, 1H), 7.18 (d, J = 8.0 Hz, 1H), 7.12–7.03 (m, 2H), 6.52 (d, J = 7.0 Hz, 1H), 5.58 (s, 2H), 2.79 (t, J = 7.6 Hz, 2H), 2.43 (t, J = 7.2 Hz, 2H), 2.04–1.97 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.04, 164.41, 142.35, 137.40, 136.65, 133.48, 128.35, 128.26, 128.13, 127.80, 127.39, 127.12, 126.36, 123.58, 122.12, 119.46, 119.36, 118.74, 115.12, 110.04, 45.95, 36.63, 26.16, 24.61 ppm.
4-(4-(1-(3,4-Difluoro-benzyl)-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I6). HRMS (AP-ESI) m/z calcd for C26H23F2N3O3 [M + H]+ 464.17410, found 464.17673. 1H NMR (400 MHz, DMSO) δ 11.09 (s, 1H), 10.10 (s, 1H), 8.93 (s, 1H), 7.67 (dd, J = 19.2, 8.7 Hz, 4H), 7.55 (d, J = 7.8 Hz, 1H), 7.42 (d, J = 8.2 Hz, 1H), 7.39–7.27 (m, 3H), 7.12–7.08 (m, 1H), 7.03–6.99 (m, 2H), 5.35 (s, 2H), 2.75 (t, J = 7.4 Hz, 2H), 2.41 (t, J = 7.2 Hz, 2H), 2.02–1.95 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.08, 164.46, 150.24, 148.58, 142.33, 136.69, 136.44, 128.31, 128.12, 127.40, 126.57, 121.85, 119.28, 119.16, 118.78, 117.97, 116.53, 114.89, 110.42, 48.27, 36.67, 26.19, 24.61 ppm.
4-(4-(1-(3-Fluoro-benzyl)-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I7). HRMS (AP-ESI) m/z calcd for C26H24FN3O3 [M + H]+ 446.18353, found 446.18613. 1H NMR (400 MHz, DMSO) δ 11.08 (s, 1H), 10.11 (s, 1H), 8.93 (s, 1H), 7.70–7.64 (m, 4H), 7.55 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.36–7.31 (m, 2H), 7.11–6.99 (m, 5H), 5.38 (s, 2H), 2.75 (t, J = 7.5 Hz, 2H), 2.41 (t, J = 7.2 Hz, 2H), 2.02–1.97 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.05, 163.87, 161.45, 143.25, 141.94, 136.53, 130.97, 128.27, 128.19, 127.39, 126.72, 123.45, 119.26, 119.10, 118.74, 114.42, 114.35, 110.45, 48.77, 36.67, 26.20, 24.62 ppm.
4-(4-(1-(3-Bromo-benzyl)-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I8). HRMS (AP-ESI) m/z calcd for C26H24BrN3O3 [M + H]+ 506.39106, found 506.10611. 1H NMR (400 MHz, DMSO) δ 11.09 (s, 1H), 10.11 (s, 1H), 8.93 (s, 1H), 7.67 (dd, J = 18.4, 8.8 Hz, 4H), 7.56 (d, J = 7.8 Hz, 1H), 7.44–7.41 (m, 3H), 7.33 (s, 1H), 7.28–7.24 (m, 1H), 7.16 (d, J = 7.6 Hz, 1H), 7.12–7.08 (m, 1H), 7.03–6.99 (m, 1H), 5.37 (s, 2H), 2.75 (t, J = 7.5 Hz, 2H), 2.42 (t, J = 7.2 Hz, 2H), 2.02–1.95 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.04, 164.44, 142.35, 141.78, 136.52, 131.20, 130.61, 130.15, 128.28, 128.13, 127.41, 126.68, 126.50, 122.24, 121.83, 119.28, 119.13, 118.76, 114.83, 110.43, 48.62, 36.68, 26.21, 24.63 ppm.
4-(4-(1-(3-Chloro-benzyl)-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I9). HRMS (AP-ESI) m/z calcd for C26H24ClN3O3 [M + H]+ 463.14767, found 462.15634. 1H NMR (400 MHz, DMSO) δ 11.08 (s, 1H), 10.11 (s, 1H), 8.92 (s, 1H), 7.70–7.64 (m, 4H), 7.56 (d, J = 7.8 Hz, 1H), 7.41 (d, J = 8.2 Hz, 1H), 7.35–7.29 (m, 3H), 7.25 (s, 1H), 7.13–7.08 (m, 2H), 7.03–6.99 (m, 1H), 5.38 (s, 2H), 2.75 (t, J = 7.2 Hz, 2H), 2.41 (t, J = 7.3 Hz, 2H), 2.02–1.94 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.04, 164.41, 142.35, 141.55, 136.51, 133.59, 130.93, 128.27, 128.12, 127.71, 127.39, 127.26, 126.70, 126.12, 121.82, 119.28, 119.12, 118.73, 114.81, 110.44, 48.66, 36.67, 26.20, 24.62 ppm.
4-(4-(1-(2,6-Difluoro-benzyl)-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I10). HRMS (AP-ESI) m/z calcd for C26H23FN3O3 [M + H]+464.17410, found 464.17679. 1H NMR (400 MHz, DMSO) δ 11.09 (s, 1H), 10.11 (s, 1H), 8.93 (s, 1H), 7.67 (dd, J = 19.6, 8.4 Hz, 4H), 7.53 (d, J = 7.8 Hz, 1H), 7.47–7.39 (m, 2H), 7.16–7.12 (m, 4H), 7.03–6.99 (m, 1H), 5.39 (s, 2H), 2.71 (t, J = 7.4 Hz, 2H), 2.39 (t, J = 7.4 Hz, 2H), 1.98–1.91 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.06, 164.47, 162.57, 160.10, 142.34, 136.35, 131.30, 128.12, 128.03, 127.38, 126.21, 121.91, 119.26, 119.16, 118.78, 114.91, 113.68, 112.52, 112.27, 109.79, 37.28, 36.62, 26.17, 24.52 ppm.
4-(4-(1-(3,5-Bis-trifluoromethyl-benzyl)-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I11). HRMS (AP-ESI) m/z calcd for C28H23F6N3O3 [M + H]+ 564.16772, found 564.17004. 1H NMR (400 MHz, DMSO) δ 11.08 (s, 1H), 10.10 (s, 1H), 8.93 (s, 1H), 8.01 (s, 1H), 7.88(s, 1H), 7.67 (dd, J = 19.4, 8.6 Hz, 4H), 7.58 (d, J = 7.8 Hz, 1H), 7.48 (d, J = 8.2 Hz, 1H), 7.42 (s, 1H), 7.14–7.10 (m, 1H), 7.05–7.01 (m, 1H), 5.58 (s, 2H), 2.76 (t, J = 7.4 Hz, 2H), 2.41 (t, J = 7.2 Hz, 2H), 1.99–1.96 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.02, 164.41, 142.54, 142.34, 136.50, 131.32, 130.99, 130.67, 128.32, 128.12, 127.39, 126.56, 125.03, 122.32, 122.06, 119.39, 118.73, 115.38, 110.32, 48.21, 36.62, 26.25, 24.58 ppm.
4-(4-(1-Butyl-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I12). HRMS (AP-ESI) m/z calcd for C23H27N3O3 [M + H]+ 394.20860, found 394.21143. 1H NMR (400 MHz, DMSO) δ 11.08 (s, 1H), 10.09 (s, 1H), 8.92 (s, 1H), 7.67 (dd, J = 19.6, 8.4 Hz, 4H), 7.53 (d, J = 7.9 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.16 (s, 1H), 7.12–7.08 (m, 1H), 7.00–6.97 (m, 1H), 4.10 (t, J = 6.9 Hz, 2H), 2.72 (t, J = 7.2 Hz, 2H), 2.39 (t, J = 7.4 Hz, 2H), 2.03–1.93 (m, 2H), 1.74–1.67 (m, 2H), 1.28–1.19 (m, 2H), 0.87 (t, J = 7.4 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.06, 164.40, 142.35, 136.49, 128.11, 127.98, 127.38, 126.25, 121.37, 119.12, 118.72, 118.61, 113.81, 110.09, 45.40, 36.66, 32.46, 26.25, 24.64, 20.03, 14.06 ppm.
4-(4-(1-Methyl-1H-indol-3-yl)butanamido)-N-hydroxybenzamide (I13). HRMS (AP-ESI) m/z calcd for C20H21N3O3 [M + H]+ 352.16165, found 352.16403. 1H NMR (400 MHz, DMSO) δ 11.09 (s, 1H), 10.11 (s, 1H), 8.93 (s, 1H), 7.68 (dd, J = 18.4, 8.8 Hz, 4H), 7.54 (d, J = 7.8 Hz, 1H), 7.37 (d, J = 8.2 Hz, 1H), 7.15–7.12 (m, 2H), 7.03–6.99 (m, 1H), 3.73 (s, 3H), 2.73 (t, J = 7.4 Hz, 2H), 2.40 (t, J = 7.3 Hz, 2H), 2.01–1.93 (m, 2H); 13C NMR (400 MHz, DMSO): δ = 172.04, 164.41, 142.34, 137.15, 128.11, 127.94, 127.37, 127.31, 121.46, 119.02, 118.72, 118.70, 113.75, 110.00, 36.61, 32.67, 26.26, 24.56 ppm.
Preparation of I1c and its analogues: Derivatives I2c, I3c, I7c, I10c, I12c, and I13c were prepared as described for I1c (see below).
4-(4-(1H-indol-3-yl)-butyrylamino)-N-benzoylhydrazine (I1c). Compound methyl 4-(4-(1H-indol-3-yl)butanamido)benzoate (I1b) and hydrazine hydrate were mixed in the methanol. Then, the mixture was heated up to 80 °C and held at 80 °C with stirring for 24 h. After cooling to room temperature, the desired compound I1c was derived by crystallisation as white powder. HRMS m/z: 337.16461 [M + H]+. 1H NMR (400 MHz, DMSO) δ 10.79 (s, 1H), 10.10 (s, 1H), 9.64 (s, 1H), 7.78 (d, J = 8.7 Hz, 2H), 7.66 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.34 (d, J = 8.0 Hz, 1H), 7.14 (d, J = 2.1 Hz, 1H), 7.08–7.04 (m, 2H), 6.99–6.95 (m, 1H), 4.44 (s, 3H), 2.75 (t, J = 7.4 Hz, 2H), 2.40 (t, J = 7.4 Hz, 2H), 2.02–1.95 (m, 2H).
4-(4-(1-Benzyl-1H-indol-3-yl)butanamido)-N-benzoylhydrazine (I2c). HRMS m/z: 427.20109 [M + H]+. 1H NMR (400 MHz, DMSO) δ 10.12 (s, 1H), 9.64 (s, 1H), 7.77 (d, J = 8.6 Hz, 2H), 7.65 (d, J = 8.6 Hz, 2H), 7.55 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.31–7.18 (m, 6H), 7.10–7.06 (m, 1H), 7.02–6.98 (m, 1H), 5.35 (s, 2H), 4.44 (s, 2H), 2.75 (t, J = 7.4 Hz, 2H), 2.42 (t, J = 7.4 Hz, 2H), 2.02–1.95 (m, 2H).
4-(4-(1-Ethyl-1H-indol-3-yl)butanamido)-N-benzoylhydrazine (I3c). HRMS m/z: 365.19595 [M + H]+. 1H NMR (400 MHz, DMSO) δ 10.09 (s, 1H), 9.62 (s, 1H), 7.76 (d, J = 8.5 Hz, 2H), 7.64 (d, J = 8.5 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.18 (s, 1H), 7.13–7.09 (m, 1H), 7.01–6.97 (m, 1H), 4.43 (s, 2H), 4.14 (dd, J = 14.3, 7.1 Hz, 2H), 2.73 (t, J = 7.3 Hz, 2H), 2.40 (t, J = 7.3 Hz, 2H), 2.00–1.93 (m, 2H), 1.33 (t, J = 7.2 Hz, 3H).
4-(4-(1-(3-Fluoro-benzyl)-1H-indol-3-yl)butanamido)-N-benzoylhydrazine (I7c). HRMS m/z: 445.20258 [M + H]+. 1H NMR (400 MHz, DMSO) δ 10.10 (s, 1H), 9.62 (s, 1H), 7.76 (d, J = 8.5 Hz, 2H), 7.65 (d, J = 8.6 Hz, 2H), 7.56 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.36–7.31 (m, 2H), 7.11–7.00 (m, 5H), 5.38 (s, 2H), 4.42 (s, 2H), 2.75 (t, J = 7.2 Hz, 2H), 2.41 (t, J = 7.2 Hz, 2H), 2.00–1.97 (m, 2H).
4-(4-(1-(2,6-Difluoro-benzyl)-1H-indol-3-yl)butanamido)-N-benzoylhydrazine (I10c). HRMS m/z: 436.19232 [M + H]+. 1H NMR (400 MHz, DMSO) δ 10.09 (s, 1H), 9.63 (s, 1H), 7.75 (d, J = 8.6 Hz, 2H), 7.64 (d, J = 8.6 Hz, 2H), 7.53 (d, J = 7.9 Hz, 1H), 7.47–7.40 (m, 2H), 7.16–7.12 (m, 4H), 7.03–7.00 (m, 1H), 5.39 (s, 2H), 4.43 (s, 2H), 2.71 (t, J = 7.4 Hz, 2H), 2.39 (t, J = 7.3 Hz, 2H), 1.98–1.90 (m, 2H).
4-(4-(1-Butyl-1H-indol-3-yl)butanamido)-N-benzoylhydrazine (I12c). HRMS m/z: 393.22568 [M + H]+. 1H NMR (400 MHz, DMSO) δ 10.10 (s, 1H), 9.63 (s, 1H), 7.76 (d, J = 8.5 Hz, 2H), 7.64 (d, J = 8.5 Hz, 2H), 7.53 (d, J = 7.6 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.16 (s, 1H), 7.13–7.09 (m, 1H), 7.01–6.97 (m, 1H), 4.10 (t, J = 6.8 Hz, 2H), 2.73 (t, J = 7.2 Hz, 2H), 2.40 (t, J = 7.4 Hz, 2H`), 2.00–1.93 (m, 2H), 1.74–1.67 (m, 2H), 1.29–1.19 (m, 2H), 0.87 (d, J = 7.4 Hz, 2H).
4-(4-(1-methyl-1H-indol-3-yl)butanamido)-N-benzoylhydrazine (I13c). HRMS m/z: 351.18027 [M + H]+. 1H NMR (400 MHz, DMSO) δ 10.08 (s, 1H), 9.63 (s, 1H), 7.76 (d, J = 8.7 Hz, 2H), 7.64 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.37 (d, J = 8.2 Hz, 1H), 7.15–7.11 (m, 2H), 7.02–6.98 (m, 1H), 4.49 (s, 2H), 3.73 (s, 3H), 2.73 (t, J = 7.4 Hz, 2H), 2.39 (t, J = 7.4 Hz, 2H`), 2.00–1.92 (m, 2H).
Preparation of I1e1 and its analogues: derivatives I1e2–I1e4, I2e2, I2e3, I3e2, I3e3, I7e2, I10e2, I12e2, I12e3, I13e2, and I13e3 were prepared as described for I1e1 (see below).
N-[4-(N′-Ethyl-hydrazinocarbonyl)-phenyl]-4-(1H-indol-3-yl)-butyramide (I1e1). HRMS (AP-ESI) m/z calcd for C21H24N4O2 [M + H]+ 365.19328, found 365.19626. 1H NMR (400 MHz, DMSO) δ 10.79 (s, 1H), 10.12 (s, 1H), 9.89 (d, J = 6.3 Hz, 1H), 7.77 (d, J = 8.2 Hz, 2H), 7.66 (d, J = 8.2 Hz, 2H), 7.52 (d, J = 7.7 Hz, 1H), 7.33 (d, J = 8.0 Hz, 1H), 7.13 (s, 1H), 7.08–7.04 (m, 1H), 6.98–6.95 (m, 1H), 5.02 (d, J = 6.0 Hz, 1H), 2.81–2.72 (m, 4H), 2.40 (t, J = 7.0 Hz, 2H), 1.99–1.95 (m, 2H), 1.03 (t, J = 7.0 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.14, 165.38, 142.48, 136.78, 128.29, 127.77, 127.62, 122.80, 121.29, 118.78, 118.63, 118.58, 114.40, 111.81, 46.02, 36.67, 26.25, 24.76, 13.59 ppm.
4-(1H-Indol-3-yl)-N-[4-(N′-propyl-hydrazinocarbonyl)-phenyl]-butyramide (I1e2). HRMS (AP-ESI) m/z calcd for C22H26N4O2 [M + H]+ 379.20893, found 379.21191. 1H NMR (400 MHz, DMSO) δ 10.78 (s, 1H), 10.09 (s, 1H), 9.88 (d, J = 6.5 Hz, 1H), 7.76 (d, J = 8.6 Hz, 2H), 7.65 (d, J = 8.6 Hz, 2H), 7.52 (d, J = 7.7 Hz, 1H), 7.33 (d, J = 8.0 Hz, 1H), 7.13 (s, 1H), 7.07–7.04 (m, 1H), 6.98–6.94 (m, 1H), 5.06–5.01 (m, 1H), 2.75–2.72 (m, 4H), 2.39 (t, J = 7.2 Hz, 2H), 2.01–1.94 (m, 2H), 1.50–1.41 (m, 2H), 0.90 (t, J = 7.4 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.11, 165.37, 142.44, 136.79, 128.31, 127.83, 127.64, 122.81, 121.31, 118.79, 118.65, 118.60, 114.41, 111.81, 53.64, 36.67, 26.23, 24.75, 21.34, 12.15 ppm.
N-[4-(N′-Butyl-hydrazinocarbonyl)-phenyl]-4-(1H-indol-3-yl)-butyramide (I1e3). HRMS (AP-ESI) m/z calcd for C23H28N4O2 [M + H]+ 393.22458, found 393.22726. 1H NMR (400 MHz, DMSO) δ 10.78 (s, 1H), 10.10 (s, 1H), 9.88 (d, J = 6.6 Hz, 1H), 7.76 (d, J = 8.6 Hz, 2H), 7.65 (d, J = 8.6 Hz, 2H), 7.52 (d, J = 7.8 Hz, 1H), 7.33 (d, J = 8.0 Hz, 1H), 7.13 (s, 1H), 7.07–7.04 (m, 1H), 6.98–6.94 (m, 1H), 5.03–4.99 (m, 1H), 2.78–2.72 (m, 4H), 2.39 (t, J = 7.3 Hz, 2H), 2.01–1.93 (m, 2H), 1.46–1.30 (m, 4H), 0.89 (t, J = 7.2 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.10, 165.35, 142.43, 136.78, 128.30, 127.81, 127.63, 122.81, 121.31, 118.79, 118.63, 118.59, 114.40, 111.81, 51.44, 36.66, 30.27, 26.23, 24.75, 20.32, 14.41 ppm.
4-(1H-Indol-3-yl)-N-[4-(N′-isobutyl-hydrazinocarbonyl)-phenyl]-butyramide (I1e4). HRMS (AP-ESI) m/z calcd for C23H28N4O2 [M + H]+ 393.22458, found 393.22717. 1H NMR (400 MHz, DMSO) δ 10.78 (s, 1H), 10.10 (s, 1H), 9.88 (d, J = 6.3 Hz, 1H), 7.76 (d, J = 8.6 Hz, 2H), 7.65 (d, J = 8.6 Hz, 2H), 7.52 (d, J = 7.8 Hz, 1H), 7.33 (d, J = 8.1 Hz, 1H), 7.13 (s, 1H), 7.07–7.04 (m, 1H), 6.98–6.94 (m, 1H), 5.06–5.01 (m, 1H), 2.73 (t, J = 7.4 Hz, 2H), 2.59 (t, J = 6.2 Hz, 2H), 2.39 (t, J = 7.4 Hz, 2H), 2.01–1.93 (m, 2H), 1.79–1.69 (m, 1H), 0.92 (d, J = 6.6 Hz, 6H); 13C NMR (400 MHz, DMSO): δ = 172.10, 165.34, 142.43, 136.78, 128.31, 127.82, 127.63, 122.81, 121.31, 118.79, 118.63, 118.59, 114.40, 111.81, 59.75, 36.67, 26.99, 26.23, 24.75, 21.13, 20.17 ppm.
4-(1-Benzyl-1H-indol-3-yl)-N-[4-(N′-propyl-hydrazinocarbonyl)-phenyl]-butyramide (I2e2). HRMS (AP-ESI) m/z calcd for C29H32N4O2 [M + H]+ 469.25588, found 469.25735. 1H NMR (400 MHz, DMSO) δ 10.10 (s, 1H), 9.88 (s, 1H), 7.77 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 7.55 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.31–7.27 (m, 3H), 7.23 (d, J = 7.2 Hz, 1H), 7.20–7.18 (m, 2H), 7.10–7.06 (m, 1H), 7.01–6.98 (m, 1H), 5.35 (s, 2H), 5.04 (s, 1H), 2.77–2.73 (m, 4H), 2.41 (t, J = 7.4 Hz, 2H), 2.02–1.95 (m, 2H), 1.50–1.41 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.05, 165.35, 142.42, 138.91, 136.59, 128.96, 128.30, 128.26, 127.85, 127.70, 127.46, 126.72, 121.64, 119.19, 118.94, 118.66, 114.51, 110.49, 53.64, 49.37, 36.68, 26.21, 24.65, 21.34, 12.15 ppm.
4-(1-Benzyl-1H-indol-3-yl)-N-[4-(N′-butyl-hydrazinocarbonyl)-phenyl]-butyramide (I2e3). HRMS (AP-ESI) m/z calcd for C30H34N4O2 [M + H]+ 483.27153, found 483.27390. 1H NMR (400 MHz, DMSO) δ 10.10 (s, 1H), 9.88 (d, J = 6.5 Hz, 1H), 7.76 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 7.55 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.31–7.27 (m, 3H), 7.23 (d, J = 7.2 Hz, 1H), 7.20–7.18 (m, 2H), 7.10–7.06 (m, 1H), 7.01–6.98 (m, 1H), 5.35 (s, 2H), 5.00 (dd, J = 12.4, 6.0 Hz, 1H), 2.78–2.73 (m, 4H), 2.41 (t, J = 7.4 Hz, 2H), 2.02–1.94 (m, 2H), 1.50–1.30 (m, 4H), 0.89 (t, J = 7.4 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.04, 165.34, 142.41, 138.92, 136.58, 128.96, 128.29, 128.26, 127.84, 127.71, 127.46, 126.72, 121.64, 119.19, 118.94, 118.65, 114.50, 110.49, 51.44, 49.37, 36.68, 30.27, 26.21, 24.64, 20.31, 14.40 ppm.
4-(1-Ethyl-1H-indol-3-yl)-N-[4-(N′-propyl-hydrazinocarbonyl)-phenyl]-butyramide (I3e2). HRMS (AP-ESI) m/z calcd for C24H30N4O2 [M + H]+ 407.24023, found 407.24222. 1H NMR (400 MHz, DMSO) δ 10.09 (s, 1H), 9.88 (d, J = 5.2 Hz, 1H), 7.76 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.18 (s, 1H), 7.13–7.09 (m, 1H), 7.01–6.97 (m, 1H), 5.04 (d, J = 5.3 Hz, 1H), 2.73 (t, J = 7.4 Hz, 4H), 2.40 (t, J = 7.2 Hz, 2H), 2.01–1.95 (m, 2H), 1.50–1.41 (m, 2H), 1.33 (t, J = 7.2 Hz, 3H), 0.91 (t, J = 7.6 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.06, 165.34, 142.41, 136.16, 128.30, 128.07, 127.82, 125.58, 121.38, 119.14, 118.66, 118.62, 113.96, 109.99, 53.62, 40.45, 36.67, 26.23, 24.67, 21.33, 15.93, 12.15 ppm.
N-[4-(N′-Butyl-hydrazinocarbonyl)-phenyl]-4-(1-ethyl-1H-indol-3-yl)-butyramide (I3e3). HRMS (AP-ESI) m/z calcd for C25H32N4O2 [M + H]+ 421.25588, found 421.25818. 1H NMR (400 MHz, DMSO) δ 10.09 (s, 1H), 9.88 (d, J = 6.6 Hz, 1H), 7.76 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.18 (s, 1H), 7.13–7.09 (m, 1H), 7.01–6.97 (m, 1H), 5.01 (dd, J = 12.4, 6.0 Hz, 1H), 4.14 (q, J = 7.2 Hz, 2H), 2.79–2.71 (m, 4H), 2.40 (t, J = 7.4 Hz, 2H), 2.01–1.93 (m, 2H), 1.47–1.35 (m, 4H), 1.33 (t, J = 7.2 Hz, 3H), 0.89 (t, J = 7.2 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.06, 165.33, 142.42, 136.16, 128.30, 128.07, 127.81, 125.58, 121.38, 119.14, 118.65, 118.63, 113.96, 109.99, 51.43, 40.45, 36.67, 30.27, 26.23, 24.67, 20.31, 15.93, 14.41 ppm.
4-[1-(3-Fluoro-benzyl)-1H-indol-3-yl]-N-[4-(N′-propyl-hydrazinocarbonyl)-phenyl]-butyramide (I7e2). HRMS (AP-ESI) m/z calcd for C29H31FN4O2 [M + H]+ 487.24646, found 487.24854. 1H NMR (400 MHz, DMSO) δ 10.11 (s, 1H), 9.88 (d, J = 6.6 Hz, 1H), 7.76 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 7.56 (d, J = 7.7 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.34–7.31 (m, 2H), 7.11–6.99 (m, 5H), 5.38 (s, 2H), 5.04 (dd, J = 12.4, 6.0 Hz, 1H), 2.77–2.70 (m, 4H), 2.42 (t, J = 7.4 Hz, 2H), 2.02–1.95 (m, 2H), 1.50–1.41 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.05, 165.36, 161.45, 142.39, 141.87, 136.54, 131.05, 130.96, 128.29, 127.85, 126.71, 123.47, 121.78, 119.26, 119.09, 118.66, 114.77, 114.41, 114.09, 110.44, 53.64, 48.79, 36.68, 26.20, 24.63, 21.33, 12.14 ppm.
4-[1-(2,6-Difluoro-benzyl)-1H-indol-3-yl]-N-[4-(N′-propyl-hydrazinocarbonyl)-phenyl]-butyramide (I10e2). HRMS (AP-ESI) m/z calcd for C29H30F2N4O2 [M + H]+ 505.23704, found 505.23914. 1H NMR (400 MHz, DMSO) δ 10.08 (s, 1H), 9.88 (d, J = 5.4 Hz, 1H), 7.76 (d, J = 8.8 Hz, 2H), 7.64 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.43–7.39 (m, 2H), 7.16–7.12 (m, 4H), 7.03–6.99 (m, 1H), 5.39 (s, 2H), 5.04 (d, J = 5.3 Hz, 1H), 2.75–2.69 (m, 4H), 2.39 (t, J = 7.2 Hz, 2H), 1.98–1.90 (m, 2H), 1.50–1.41 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.00, 165.34, 160.03, 142.39, 136.36, 131.30, 128.29, 128.04, 127.85, 126.22, 121.90, 119.26, 119.15, 118.65, 114.90, 113.69, 112.53, 112.28, 109.79, 53.63, 37.29, 36.63, 26.14, 24.53, 21.33, 12.14 ppm.
4-(1-Butyl-1H-indol-3-yl)-N-[4-(N′-propyl-hydrazinocarbonyl)-phenyl]-butyramide (I12e2). HRMS (AP-ESI) m/z calcd for C26H34N4O2 [M + H]+ 435.27153, found 435.27414. 1H NMR (400 MHz, DMSO) δ 10.09 (s, 1H), 9.88 (d, J = 5.3 Hz, 1H), 7.76 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.16 (s, 1H), 7.12–7.08 (m, 1H), 7.00–6.97 (m, 1H), 5.04 (d, J = 5.2 Hz, 1H), 4.10 (t, J = 7.0 Hz, 2H), 2.75–2.71 (m, 4H), 2.40 (t, J = 7.4 Hz, 2H), 2.00–1.93 (m, 2H), 1.74–1.67 (m, 2H), 1.50–1.41 (m, 2H), 1.29–1.19 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.11, 165.36, 142.40, 136.50, 128.29, 127.99, 127.38, 126.24, 121.38, 119.11, 118.67, 118.61, 113.82, 110.08, 53.63, 45.40, 36.67, 32.45, 26.24, 24.63, 21.31, 20.03, 14.04, 12.13 ppm.
N-[4-(N′-Butyl-hydrazinocarbonyl)-phenyl]-4-(1-butyl-1H-indol-3-yl)-butyramide (I12e3). HRMS (AP-ESI) m/z calcd for C27H36N4O2 [M + H]+ 449.28718, found 449.28943. 1H NMR (400 MHz, DMSO) δ 10.09 (s, 1H), 9.88 (d, J = 6.6 Hz, 1H), 7.76 (d, J = 8.7 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H), 7.16 (s, 1H), 7.12–7.09 (m, 1H), 7.00–6.97 (m, 1H), 5.01 (dd, J = 12.4, 6.0 Hz, 1H), 4.10 (t, J = 6.8 Hz, 2H), 2.78–2.71 (m, 4H), 2.39 (t, J = 7.4 Hz, 2H), 2.00–1.93 (m, 2H), 1.74–1.67 (m, 2H), 1.47–1.19 (m, 6H), 0.90–0.86 (m, 6H); 13C NMR (400 MHz, DMSO): δ = 172.11, 165.36, 142.41, 136.50, 128.28, 127.99, 127.82, 126.23, 121.37, 119.11, 118.68, 118.61, 113.83, 110.07, 51.44, 45.40, 36.67, 32.45, 30.25, 26.25, 24.63, 20.03, 20.01, 14.38, 14.04 ppm.
4-(1-Methyl-1H-indol-3-yl)-N-[4-(N′-propyl-hydrazinocarbonyl)-phenyl]-butyramide (I13e2). HRMS (AP-ESI) m/z calcd for C23H28N4O2 [M + H]+ 393.22458, found 393.22690. 1H NMR (400 MHz, DMSO) δ 10.08 (s, 1H), 9.87 (d, J = 6.3 Hz, 1H), 7.76 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.37 (d, J = 8.2 Hz, 1H), 7.15–7.11 (m, 1H), 7.02–6.98 (m, 1H), 5.03 (dd, J = 11.6, 5.6 Hz, 1H), 3.73 (s, 3H), 2.75–2.71 (m, 4H), 2.39 (t, J = 7.4 Hz, 2H), 2.00–1.93 (m, 2H), 1.50–1.41 (m, 2H), 0.90 (t, J = 7.4 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.05, 165.35, 142.42, 137.16, 128.30, 127.95, 127.83, 127.29, 121.45, 119.02, 118.69, 118.63, 113.77, 109.98, 53.63, 36.62, 32.66, 26.25, 24.57, 21.34, 12.15 ppm.
N-[4-(N′-Butyl-hydrazinocarbonyl)-phenyl]-4-(1-methyl-1H-indol-3-yl)-butyramide (I13e3). HRMS (AP-ESI) m/z calcd for C23H28N4O2 [M + H]+ 407.24023, found 407.24243. 1H NMR (400 MHz, DMSO) δ 10.09 (s, 1H), 9.88 (d, J = 6.4 Hz, 1H), 7.76 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 7.53 (d, J = 7.8 Hz, 1H), 7.37 (d, J = 8.2 Hz, 1H), 7.15–7.11 (m, 1H), 7.02–6.98 (m, 1H), 5.01 (q, J = 6.0 Hz, 1H), 3.73 (s, 3H), 2.79–2.71 (m, 4H), 2.39 (t, J = 7.4 Hz, 2H), 2.00–1.93 (m, 2H), 1.52–1.30 (m, 4H), 0.89 (t, J = 7.2 Hz, 3H); 13C NMR (400 MHz, DMSO): δ = 172.05, 165.34, 142.42, 137.16, 128.30, 127.95, 127.81, 127.30, 121.45, 119.02, 118.69, 118.63, 113.76, 109.98, 51.44, 36.62, 32.66, 30.27, 26.25, 24.57, 20.32, 14.41 ppm.
In vitro HDACs inhibitory assay
All of the HDAC enzymes were purchased from BPS Bioscience (San Diego, CA). Briefly, 20 μl of HeLa nucleus extract or recombinant HDAC enzyme solution was mixed with various concentrations of tested compound (20 μl). The mixture was incubated at 30 °C for 1 h (for the time-dependent assay, the mixture was incubated for 15, 30, 60, and 90 min, respectively), then 10 μl of fluorogenic substrate (Boc-Lys (acetyl)-AMC (3 mM) for HDAC1, 2, 3, and 6, Boc-Lys (trifluoroacetyl)-AMC (3 mM) for HDAC 4, 7, 8, and 9) was added. After incubation at 30 °C for 2 h, the catalytic reaction was stopped by addition of 10 μl of developer containing trypsin and trichostatin A (TSA). After 30 min, fluorescence intensity was measured using a microplate reader at excitation and emission wavelengths of 360 and 460 nm, respectively. The inhibition ratios were calculated from the fluorescence intensity readings of tested wells relative to those of control wells, and the IC50 curves and values were determined by GraphPad Prism 6.0 software (La Jolla, CA).
In vitro antiproliferative activity
Tumour cell inhibition was determined using the MTT assay using SAHA as the control. U266 and U937 cells were cultured in RPMI-1640 medium supplemented with 10% FBS. The stock solutions of tested compounds were diluted with culture medium. Briefly, the cells were seeded into each well of 96-well plate, which were incubated at 37 °C, 5% CO2 until confluency 90–95%. Then, the cells were treated with compound sample at various concentrations for 48 h. Afterward, 20 MTT working solution (5 mg/ml) was added to each well and incubated for another 4 h. After incubation, the medium formed from MTT was extracted by adding DMSO (200 µl). The optical density (OD) at 490 nm and 630 nm were measured by Universal Microplate Spectrophotometer. The percentage of cell growth inhibition was calculated according to the formula: % inhibition = [1 – (Sample group OD490 – Sample group OD630)/(Control group OD490 – Control group OD630)] × 100%. The IC50 values were calculated with Origin 7.5 software (Originlab Corporation, Northampton, MA), and standard deviations of the IC50 values were obtained through at least 3 independent experiments.
In vivo antitumor activity
For in vivo antitumor efficacy study, 1.8 × 107 HepG2 cells were inoculated subcutaneously in the right shoulder of male athymic nude mice (5–6 weeks old, Slac Laboratory Animal, Shanghai, China). Then days after injection, tumours were palpable and mice were randomised into treatment and control groups (seven mice per group). The treatment groups were administrated with 100 mg/kg/d of SAHA and 50 mg/kg/d of I13 intragastrically, the control group was administrated with equal volume of PBS solution. During treatment, the body weight of mice was monitored regularly. After 16 days of administration, the mice were executed and the tumour weight was measured by an electronic balance.
Annexin V/PI detection
HepG2 cells in logarithmic growth phase were seeded in six-well plates (4 × 105 cells/well) and incubated with different doses of FNA and SAHA (0.2 and 0.8 µM) for 24 h. After the incubation, cells were washed with PBS, collected, resuspended with binding buffer from the Annexin V-FITC kit (Thermo Fisher Co., Waltham, MA), and then added with 5 µl annexin V-FITC and mixed gently. After 10 min of incubation, 1 µl PI was added to each sample and mixed gently. After incubation at room temperature for another 20 min in the dark, cells were subjected to flow cytometer (CytoFLEX, Beckman Coulter, Brea, CA).
Statistics
Statistics were performed with SPSS 17.0 (SPSS Inc., Chicago, IL). Analysis of variance (ANOVA) was used to determine differences among groups. When ANOVA detected significant results, least significant difference (LSD) tests were utilised to compare between groups. Results were considered statistically significant when p < 0.05.
Results and discussion
Chemistry
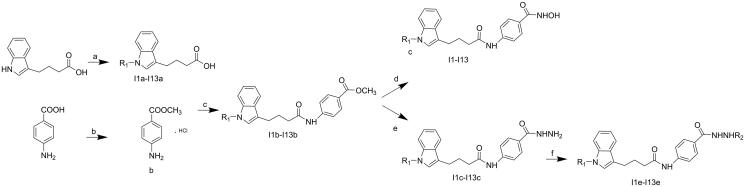
Based on lead structure IBHA, a series of indol-3-ylbutyric acid derivatives were synthesised according to the procedures described in Scheme 1. The starting material IBA was coupled with a series of bromobenzene. The other material, 4-aminobenzoic acid, was protected by a methyl ester. The intermediates I1b–I13b were derived by conjugation of b and I1a–I13a. Target molecules I1–I17 were synthesised by treatment of corresponding intermediates (I1b–I13b) with NH2OK in methanol. The hydrazide compounds were generated by hydrazinolysis, condensation, and reduction reactions.
Scheme 1.
Synthesis of target compounds. Reagents and conditions: (a) substituted bromobenzene, NaH, DMF, Ar2, 0 °C-rt, 3 h; (b) (CO)2Cl, MeOH, 0 °C, overnight; (c) TBTU, Et3N, DCM, 0 °C-rt, overnight; (d) NH2OK, MeOH, 2 h; (e) NH2NH2.H2O, MeOH, reflux, 12–48 h; (f) aliphatic aldehydes, NaBH4, rt, 4 h.
Biological studies
HDAC inhibitory activity assay
To investigate the enzyme inhibitory potency of the synthesised molecules, an enzymatic assay was performed using HeLa nucleus extract containing a mixture of HDAC isoforms. In the screening, percentage inhibition rate (PIR) at the concentration of 1 µM was used as a measure of compound potency. The overall result revealed that molecules with hydroxamic acids as ZBGs have better inhibitory activities (Table 1) than those with hydrazide ZBGs (Table 2). Compound I13 exhibited the highest inhibitory potency with PIR of 82.19. Small sized alkyl substituents in the R1 position showed optimal HDAC inhibitory activity, such as I3 (R1 of ethyl group, PIR of 78.94) and I13 (R1 of methyl group, 82.19). While molecule I12 with bigger sized n-butyl group exhibited reduced activity with PIR of 62.70. Among the aromatic substituted R1 groups, the 1,6-2F and 4-CF3 substitution in the phenyl ring showed good inhibitory activities with PIR values of 79.10 (I10) and 68.50 (I4), respectively. In the hydrazide series, I1e2 and I2e2 with n-propyl substituted R2 group exhibited good performance with PIR of 61.34 and 57.16, respectively, compared with SAHA (PIR of 59.91). The lysate-based enzymatic test method may affect the performance of hydrazide containing molecules. The recombinant HDAC enzyme based assay will be used for further more accurate illustration of derived HDACIs.
Table 1.
Enzyme inhibitory and antiproliferative activities of the derived hydroxamic acid containing compounds. 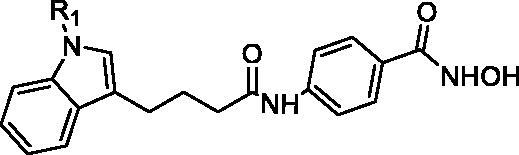
| Compounds | R1 | HDACsa | U937b | U266b | HepG2b |
|---|---|---|---|---|---|
| I1 | –H | 53.81 ± 1.60 | 1.02 ± 0.13 | 0.84 ± 0.09 | 8.98 ± 0.16 |
| I2 |
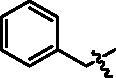
|
78.94 ± 3.41 | 0.95 ± 0.04 | 0.48 ± 0.09 | 3.63 ± 0.26 |
| I3 | –C2H5 | 78.94 ± 2.34 | 0.94 ± 0.04 | 0.24 ± 0.03 | 1.92 ± 0.05 |
| I4 |
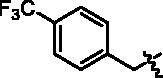
|
68.50 ± 1.22 | 0.97 ± 0.04 | 0.96 ± 0.08 | 4.45 ± 0.25 |
| I5 |
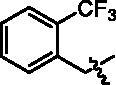
|
19.30 ± 1.60 | 4.42 ± 0.18 | 0.88 ± 0.07 | 6.17 ± 0.21 |
| I6 |
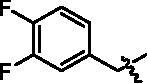
|
16.14 ± 1.07 | 1.25 ± 0.21 | 0.82 ± 0.06 | 7.72 ± 0.34 |
| I7 |
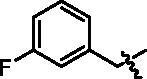
|
46.17 ± 2.94 | 0.95 ± 0.01 | 0.84 ± 0.06 | 4.87 ± 0.12 |
| I8 |
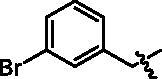
|
15.87 ± 3.27 | 1.94 ± 0.17 | 0.71 ± 0.06 | 6.45 ± 0.28 |
| I9 |
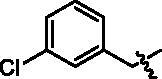
|
56.68 ± 2.42 | 1.00 ± 0.15 | 0.65 ± 0.04 | 5.67 ± 0.06 |
| I10 |
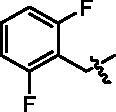
|
79.10 ± 4.65 | 0.63 ± 0.07 | 0.16 ± 0.07 | 3.39 ± 0.25 |
| I11 |
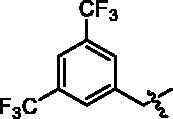
|
52.24 ± 1.10 | 4.58 ± 0.23 | 0.74 ± 0.05 | 4.76 ± 0.61 |
| I12 | –(CH2)3CH3 | 62.70 ± 1.63 | 1.01 ± 0.03 | 0.12 ± 0.02 | 5.76 ± 0.65 |
| I13 | –CH3 | 82.19 ± 1.60 | 0.93 ± 0.08 | 0.16 ± 0.04 | 1.85 ± 0.31 |
| SAHA | 59.91 ± 3.73 | 1.40 ± 0.02 | 0.88 ± 0.08 | 8.68 ± 0.29 |
Percentage inhibition rate (%, at the concentration of 1 µM), HeLa nucleus extract was used for the test. Each value is the mean of at least three experiments.
IC50, µM. Each value is the mean of at least three experiments.
Table 2.
Enzyme inhibitory and antiproliferative activities of the derived hydrazide compounds. 
| Compounds | R1 | R2 | HDACsa | U266b |
|---|---|---|---|---|
| I1e1 | –H | –C2H5 | 50.60 ± 2.02 | 15.97 ± 0.14 |
| I1e2 | –H | –(CH2)2CH3 | 61.34 ± 3.56 | 0.64 ± 0.02 |
| I1e3 | –H | –(CH2)3CH3 | 30.47 ± 1.17 | 1.06 ± 0.05 |
| I1e4 | –H | –CH2CH (CH3)2 | 7.16 ± 1.50 | 15.98 ± 0.67 |
| I2e2 |
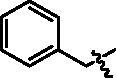
|
–(CH2)2CH3 | 57.46 ± 2.06 | 1.11 ± 0.08 |
| I2e3 |
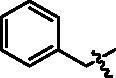
|
–(CH2)3CH3 | 51.41 ± 2.78 | 1.71 ± 0.07 |
| I3e2 | –C2H5 | –(CH2)2CH3 | 36.51 ± 2.93 | 0.48 ± 0.10 |
| I3e3 | –C2H5 | –(CH2)3CH3 | 31.94 ± 1.51 | 1.45 ± 0.10 |
| I7e2 |
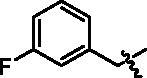
|
–(CH2)2CH3 | 30.45 ± 3.38 | 1.32 ± 0.08 |
| I10e2 |
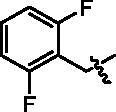
|
–(CH2)2CH3 | 46.85 ± 2.06 | 0.74 ± 0.05 |
| I12e2 | –(CH2)3CH3 | –(CH2)2CH3 | 39.73 ± 1.84 | 1.28 ± 0.12 |
| I12e3 | –(CH2)3CH3 | –(CH2)3CH3 | 42.76 ± 1.56 | 1.44 ± 0.31 |
| I13e2 | –CH3 | –(CH2)2CH3 | 29.68 ± 2.06 | 0.71 ± 0.11 |
| I13e3 | –CH3 | –(CH2)3CH3 | 32.15 ± 1.03 | 1.36 ± 0.06 |
| SAHA | 59.91 ± 3.73 | 0.88 ± 0.08 |
Percentage inhibition rate (%, at the concentration of 1 µM), HeLa nucleus extract was used for the test. Each value is the mean of at least three experiments.
IC50, µM. Each value is the mean of at least three experiments.
To evaluate the isoform selectivity of the most active molecule I13, HDAC1, 2, 3, 4, 6, 7, 8, and 9 were selected for the screening (Table 3). The result revealed that I13 could effectively inhibit the activity of HDAC1, HDAC3, and HDAC6 with IC50 values of 13.9, 12.1, and 7.71 nM, respectively. Compared with SAHA, and the class I HDAC inhibition selective MS275, I13 did not show inhibitory selectivity of a specific HDAC isoform or a particular class. Considering the high potency of I13 against HDAC6, selective HDAC6 inhibitor might be derived by further structural modification of I13.
Table 3.
Enzyme inhibitory activity of I13 compared with MS275 and SAHA (IC50, nMa).
| HDACIs | HDAC1 | HDAC2 | HDAC3 | HDAC4 | HDAC6 | HDAC7 | HDAC8 | HDAC9 |
|---|---|---|---|---|---|---|---|---|
| I13 | 13.9 | 49.9 | 12.1 | >5000 | 7.71 | 326.3 | 284.3 | >5000 |
| MS275 | 53.9 | 108.2 | 77.2 | >5000 | >5000 | >5000 | >5000 | >5000 |
| SAHA | 50.7 | 90.4 | 164.1 | >5000 | 169.5 | >5000 | 4008 | >5000 |
Each value is the mean of at least three experiments.
Antiproliferative activity
To evaluate the antiproliferative activity of hydroxamic acid containing molecules, MTT assays were performed using U937, U266, and HepG2 cell lines. Several synthesised compounds revealed improved inhibitory potency than the positive control SAHA (Table 1). Among them, molecule I3 and I13 which have good performance in the enzyme inhibitory assay, also showed high in vitro antiproliferative activity in the cancer cell based test. The IC50 values of compound I3 and I13 against U937, U266, and HepG2 cells were 0.94 and 0.93 μM, 0.24 and 0.16 μM, 1.92 and 1.85 μM, respectively, compared with SAHA (1.40, 0.88, and 8.68 μM). Molecule I13 exhibited 5.5-fold and 4.7-fold more active than SAHA in the inhibition of U266 and HepG2 cells. Compound I12 also showed good antiproliferative potency in the cell based screening, especially in the inhibition of U266 cells (with IC50 value of 0.12 μM).
The hydrazide molecules showed reduced activity in the enzyme inhibitory assay. Therefore, only the most sensitive cell line U266 was used for the test of hydrazide derivatives. As shown in Table 2, the hydrazide compounds did not exhibit improved antiproliferative activity than the corresponding molecules with hydroxamic acid ZBG. Among the hydrazide series, molecules with -propyl substituted R2 showed good inhibitory potency. The IC50 values of compound I1e2, I3e2, I10e2, and I13e2 were 0.64, 0.48, 0.74, and 0.71 μM, respectively, compared with SAHA (0.88 μM).
A series of tumour cell lines including the lung cancer A549, Calu-3, SPC-A-1, H322, H1299, breast cancer MDA-MB-231, MCF-7, MDA-MB-468, colon carcinoma LoVo, Colo205, ovarian cancer A2780, SKOV3, gastric cancer MKN45, pancreatic cancer PNAC-1, leukemic K562, and multiple myeloma OPM2 cells were cultured for the antiproliferative assay of active compound I13. In the present study, molecule I13 could inhibit the growth of both solid and haematologic tumour cell lines (Table 4). Against most of the tested cell lines, I13 exhibited improved inhibitory activity compared with SAHA. In the antiproliferative test against A549, H1299, MCF-7, LoVo, Colo205, and MKN45 cells, I13 exhibited less potency than SAHA. Remarkably, in inhibition the growth of A2780 and PNAC-1 cells, molecule I13 was revealed to be 5.9-fold (IC50 values of 4.6 μM), and 5.6-fold (IC50 values of 1.17 μM) more active than SAHA with IC50 values of 27.3 μM and 6.57 μM, respectively. The antiproliferative activity of molecule I13 was considered to be restricted by the poor enzyme inhibitory selectivity. Enhancement of selectivity could be a useful strategy to improve the anticancer activity of this group of HDACIs.
Table 4.
Antiproliferative activity of I13 compared with SAHA (IC50, μMa).
| Cell line | Tumour type | I13 | SAHA |
|---|---|---|---|
| A549 | Lung cancer | 3.15 | 1.73 |
| Calu-3 | Lung cancer | 1.26 | 3.18 |
| SPC-A-1 | Lung cancer | 0.81 | 1.11 |
| H322 | Lung cancer | 14.90 | 73.97 |
| H1299 | Lung cancer | 7.67 | 4.57 |
| MDA-MB-231 | Breast carcinoma | 0.78 | 1.11 |
| MCF-7 | Breast carcinoma | 6.34 | 2.78 |
| MDA-MB-468 | Breast carcinoma | 1.34 | 1.44 |
| LoVo | Colon carcinoma | 2.19 | 1.07 |
| Colo205 | Colon carcinoma | 2.42 | 2.01 |
| A2780 | Ovarian cancer | 4.60 | 27.3 |
| SKOV3 | Ovarian cancer | 1.44 | 1.73 |
| MKN45 | Gastric cancer | 18.19 | 16.06 |
| PNAC-1 | Pancreatic cancer | 1.17 | 6.57 |
| K562 | Leukemia | 2.87 | 4.32 |
| OPM2 | Multiple myeloma | 0.23 | 0.56 |
Each value is the mean of at least three experiments.
In vivo antitumor activity assay
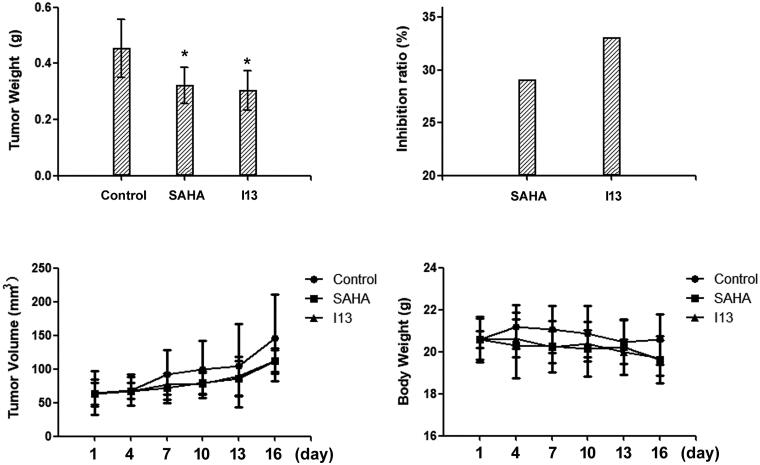
To further investigate anticancer potency of the derived molecules, molecule I13 exhibited both good water solubility and high antiproliferative potency was selected for the in vivo anti-proliferation assay. Considering the tumourigenicity and sensitivity to the synthesised molecules, HepG2 cell line was selected for the inoculation of xenograft model. To preliminarily investigate the in vivo anti-proliferation ability of molecule I13, only one concentration (50 mg/kg/d) was utilised and SAHA (100 mg/kg/d) was used as a positive control. After 16 days of intragastric administration, the mice were executed, and the tumour weight was measured. The mean weight of tumours in the negative control group was 0.453 g (SD 0.10), and the values of SAHA groups and I13 group were 0.322 g (SD 0.06) and 0.303 g (SD 0.07), respectively (Figure 2(a)). The inhibitory ratio of molecule I13 was 33.1% compared with SAHA with a value of 29.0% (Figure 2(b)). The result showed that I13 exhibited anti-tumour activity at dose of 50 mg/kg/d compared with a higher dose of SAHA (100 mg/kg/d). The tumour volume was measured every two days, and the result has correlation with the tumour weight (Figures 2(c) and 3). No evidence of mice body weight (measured every two days) loss indicated that molecule I13 is safe (Figure 2(d)). Moreover, no obvious toxic signs in liver and spleen were detected.
Figure 2.
(a) Tumour weight plot of negative control group, positive control group (SAHA), and I13 group; (b) inhibitory ratio plot of SAHA and I13; (c) tumour volume plot of these experimental groups (negative control, SAHA, and I13); (d) mice body weight plot of these experimental groups (negative control, SAHA, and I13). *Statistically different from control (n = 7).
Figure 3.
Illustration of dissected tumour tissues.
Cell apoptosis assay
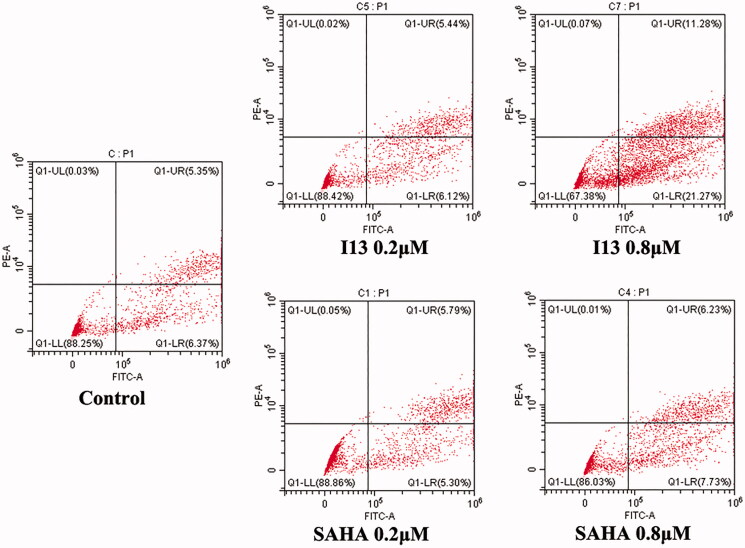
Apoptosis which is a form of programmed cell death maintains the healthy survival/death balance in human cells. Increasing apoptosis rate could be utilised as a strategy for the treatment of cancer. To further evaluate the effect of I13 on apoptosis, flow cytometry analysis was performed by staining HepG2 cells with annexin V-FITC/PI. The results revealed that both I13 and SAHA treatment induced HepG2 cell apoptosis in a dose dependent manner (Figure 4). After treatment with difference doses of I13, the ratio of apoptotic cells was 11.56% and 33.55% at dose of 0.2 and 0.8 µM, respectively, compared with SAHA (11.09% and 13.96% at concentration of 0.2 and 0.8 µM, respectively). It is indicated that induction of cell apoptosis makes contributions to the antitumor effect of I13.
Figure 4.
Pro-apoptotic effect of molecule I13.
Conclusions
Structural modification was performed on the previous IBA based HDACI. Compounds with hydroxamic acid and hydrazide groups as ZBGs were synthesised and evaluated in the enzyme inhibitory assay and in vitro antiproliferative screening. Among the derived compounds, molecule I13 exhibited high HDAC inhibitory activity against HDAC1 (IC50 value of 13.9 nM), HDAC3 (IC50 value of 12.1 nM), and especially HDAC6 (IC50 value of 7.71 nM). The in vitro antiproliferative results revealed that molecule I13 can effectively inhibit the growth of both haematologic and solid tumour cell lines. Compared with SAHA, molecule I13 showed high potency in the growth inhibition of U937, U266, HepG2, A2780, and PNAC-1 cells. In the further in vivo assay, 50 mg/kg/d of I13 can inhibit growth of xenograft tumour in athymic mice compared with 100 mg/kg/d of SAHA. Induction of apoptosis was revealed to play a role in the anticancer activity of molecule I13.The results suggested that HDACIs with high anticancer potency could be derived by further structural modification of the lead compound I13.
Funding Statement
This work was supported by Science and Technology Support Plan for Youth Innovation in Universities of Shandong Province [Grant No. 2019KJM001], Natural Foundation of Shandong Province [Youth Found, Grant No. ZR2019QH005], and National Natural Science Foundation of China [Youth Found, Grant No. 81803343].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Bernstein BE, Tong JK, Schreiber SL.. Genomewide studies of histone deacetylase function in yeast. Proc Natl Acad Sci U S A 2000;97:13708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Ruijter AJM, Van Gennip AH, Caron HN, et al. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 2003;370:737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foglietti C, Filocamo G, Cundari E, et al. Dissecting the biological functions of drosophila histone deacetylases by RNA interference and transcriptional profiling. J Biol Chem 2006;281:17968–76. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Han YT, Jiang QX, et al. Trend of histone deacetylase inhibitors in cancer therapy: isoform selectivity or multitargeted strategy. Med Res Rev 2015;35:63–84. [DOI] [PubMed] [Google Scholar]
- 5.Gronroos E, Hellman U, Heldin CH, Ericsson J.. Control of Smad7 stability by competition between acetylation and ubiquitination. Mol Cell 2002;10:483–93. [DOI] [PubMed] [Google Scholar]
- 6.Giandomenico V, Simonsson M, Gronroos E, Ericsson J.. Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors. Mol Cell Biol 2003;23:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin YH, Jeon EJ, Li QL, et al. Transforming growth factor-beta stimulates p300-dependent RUNX3 acetylation, which inhibits ubiquitination-mediated degradation. J Biol Chem 2004;279:29409–17. [DOI] [PubMed] [Google Scholar]
- 8.Bode AM, Dong ZG.. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 2004;4:793–805. [DOI] [PubMed] [Google Scholar]
- 9.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 2004;5:392–401. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs JJ, Murphy PJM, Gaillard S, et al. HDAC6 regulates hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 2005;18:601–7. [DOI] [PubMed] [Google Scholar]
- 11.Patel MM, Patel BM.. Repurposing of sodium valproate in colon cancer associated with diabetes mellitus: role of HDAC inhibition. Eur J Pharm Sci 2018;121:188–99. [DOI] [PubMed] [Google Scholar]
- 12.Soragni E, Xu C, Cooper A, et al. Evaluation of histone deacetylase inhibitors as therapeutics for neurodegenerative diseases. Methods Mol Biol 2011;793:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mai A, Rotili D, Valente S, Kazantsev AG.. Histone deacetylase inhibitors and neurodegenerative disorders: holding the promise. Curr Pharm Des 2009;15:3940–57. [DOI] [PubMed] [Google Scholar]
- 14.Cantley MD, Haynes DR.. Epigenetic regulation of inflammation: progressing from broad acting histone deacetylase (HDAC) inhibitors to targeting specific HDACs. Inflammopharmacology 2013;21:301–7. [DOI] [PubMed] [Google Scholar]
- 15.Saiyed ZM, Gandhi N, Agudelo M, et al. HIV-1 Tat upregulates expression of histone deacetylase-2 (HDAC2) in human neurons: implication for HIV-associated neurocognitive disorder (HAND). Neurochem Int 2011;58:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS 2011;6:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ylisastigui L, Archin NM, Lehrman G, et al. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS 2004;18:1101–8. [DOI] [PubMed] [Google Scholar]
- 18.Gallo P, Latronico MV, Gallo P, et al. Inhibition of class I histone deacetylase with an apicidin derivative prevents cardiac hypertrophy and failure. Cardiovasc Res 2008;80:416–24. [DOI] [PubMed] [Google Scholar]
- 19.Kook H, Lepore JJ, Gitler AD, et al. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein hop. J Clin Invest 2003;112:863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marks PA, Rifkind RA, Richon VM, et al. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 2001;1:194–202. [DOI] [PubMed] [Google Scholar]
- 21.Bian J, Zhang LH, Han YT, et al. Histone deacetylase inhibitors: potent anti-leukemic agents. Curr Med Chem 2015;22:2065–74. [DOI] [PubMed] [Google Scholar]
- 22.Richon VM, Emiliani S, Verdin E, et al. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci USA 1998;95:3003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda H, Nakajima H, Hori Y, et al. Fr901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J Antibiot (Tokyo) 1994;47:301–10. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Xue XW, Zhang YH.. Simple and efficient synthesis of belinostat. Synth Commun 2010;40:2520–4. [Google Scholar]
- 25.Neri P, Bahlis NJ, Lonial S. Panobinostat for the treatment of multiple myeloma. Expert Opin Investig Drugs 2012;21:733–47. [DOI] [PubMed] [Google Scholar]
- 26.Dong M, Ning Z, Newman MJ, et al. Phase I study of chidamide (CS055/HBI-8000), a novel histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. J Clin Oncol 2009;27:3529. [Google Scholar]
- 27.Enders TA, Strader LC.. Auxin activity: past, present, and future. Am J Bot 2015;102:180–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kline BE, Rusch HP.. Tumor inhibitor studies. II. The effect of plant hormones on tumor growth. Cancer Res 1943;3:702–5. [Google Scholar]
- 29.Bian J, Luan Y, Wang C, Zhang L.. Discovery of n-hydroxy-4-(1h-indol-3-yl)butanamide as a histone deacetylase inhibitor. Drug Discov Ther 2016;10:163–6. [DOI] [PubMed] [Google Scholar]
- 30.McClure J, Zhang C, Inks E, et al. Development of allosteric hydrazide-containing class I histone deacetylase inhibitors for use in acute myeloid leukemia. J Med Chem 2016;59:9942–59. [DOI] [PMC free article] [PubMed] [Google Scholar]