Abstract
Background
Smoldering cancer-related inflammation attenuates chemotherapy efficacy and contributes to unsatisfactory outcome for patients of colorectal cancer (CRC). Various inflammation-based biomarkers were reported to predict the survival of the disease, however, it remains unclear which is the best inflammation-based biomarker. The aim of present study was to compare the prognostic role of those biomarkers and to establish superior survival score for post-recurrence survival in radically operative patients with stage II–III CRC.
Patients and Methods
Preoperative peripheral neutrophil, lymphocyte, monocyte, platelet, serum albumin (Alb), pre-Alb, and plasma fibrinogen (Fib) were detected in the discovery and validation cohort which included a total of 1533 stage II–III surgical CRC patients. We calculated and compared fourteen inflammation-based biomarkers for predicting recurrence-free survival (RFS) of the patients with stage II–III CRC.
Results
In this study, the platelet to lymphocyte ratio (PLR), lymphocyte to monocyte (LMR), systemic inflammation response index (SIRI), prognostic nutritional index (PNI), systemic immune-inflammation index (SII), modified systemic inflammation score (mSIS), fibrinogen and neutrophil to lymphocyte ratio score (F-NLR), ratio of Alb to Fib (AFR), and ratio of Fib to pre-Alb (FPR) were all related to the RFS of the patients in both discovery and validation cohorts, however, only the LMR, SIRI, PNI, mSIS, F-NLR, AFR and FPR remained independent predictors for RFS in multivariate analysis. Both the C-index of the FPR (0.629 for 36 months) and the areas under the time-dependent receiver operating characteristic (ROC) curves (0.625 for 12 months, 0.641 for both 24 and 0.637 months) showed that it was superior to the other inflammation-based prognostic scores for predicting the RFS of stage II–III surgical CRC patients. Moreover, elevated FPR was significantly associated with unsatisfactory RFS regardless of TNM stage and primary tumor location. Stage II low FPR patients showed the best RFS regardless of chemotherapy. The better RFS was observed in chemotherapy-treated stage II high FPR patients than those without the treatment, and the outcomes of patients with treatment of XELOX, capecitabine and XELOX were superior to the other regimens to treat patients in stage III low- and high-FPR populations, respectively. Additionally, the carcinoembryonic antigen (CEA)-FPR combined score one (adjusted HR=2.764, 95% CI=2.129–3.589) and two (adjusted HR=3.543, 95% CI=2.317–5.420) were extremely associated with RFS of these patients, and the predicted AUC of the combined score for 12, 24 and 36 months were 0.657, 0.657 and 0.653 in stage II–III patients, which were superior to the single CEA and FPR, respectively.
Conclusion
In conclusion, FPR is superior to the other inflammatory biomarkers as a useful recurrence indicator in stage II–III surgical CRC patients in terms of prognostic ability; it helps to choose the effective chemotherapy regimen and to increase the predicted efficacy of CEA and the combined CEA and FPR score could effectively predict recurrence of the patients.
Keywords: colorectal cancer, fibrinogen to pre-albumin ratio, inflammation-based prognostic biomarker, prognosis
Introduction
Colorectal cancer (CRC) is one of the lethal malignancies worldwide, with China accounting for approximately 0.376 and 0.191 million of cases and deaths, respectively.1 Currently, radical resection is one of the mainstay curative treatments for the disease. Unfortunately, approximately 36% of stage II–III patients show recurrence or distal metastasis in the following five years.2 For this, the recurrence after surgical operation remains a major cause of treatment failure and cancer-related death. It has been well known that chronic inflammation is emerging as a hallmark of CRC,3 and cancer-elicited inflammation is significantly correct with poor differentiation, microvascular invasion, and micro-metastasis of the disease.3–6 Thus, cancer-elicited inflammation may play an important role in predicting clinical efficacy and prognosis in CRC patients.
CRC is a kind of inflammation-derived cancer, and accumulating evidence emphasizes that the host inflammatory response is associated with cancer progression and patient survival.7 Smoldering cancer-related inflammation attenuates chemotherapy efficacy and contributes to the proliferation and survival of CRC cells, angiogenesis, metastasis, and unsatisfactory outcome for patients of CRC.6,8 Furthermore, apart from clinical parameters such as cancer features and common biomarkers, inflammation-related markers are important predictors of prognosis after surgical resection.
Recently, there have been many inflammation-based prognostic biomarkers which constructed on systemic inflammatory responses, such as the Glasgow Prognostic Score (GPS) and modified Glasgow Prognostic Score (mGPS), Prognostic Index (PI), Prognostic Nutritional Index (PNI), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), and systemic immune-inflammation index (SII). These biomarkers have shown prognostic value in several types of cancer, including lung cancer,9,10 biliary tract cancer,11 renal cell carcinoma,12 esophageal cancer,13 and CRC.14 Our previous study illustrated that preoperative fibrinogen (Fib) to pre-albumin (pre-Alb) ratio (FPR) was tightly associated with chemotherapy response and the outcome of advanced CRC.8 However, the predicted ability comparison between these inflammation-based biomarkers for predicting the postoperative recurrence within stage II–III CRC has not been elucidated. There is no study to report which is the best inflammatory biomarker to evaluate the recurrence or distal metastasis of these surgical patients.
In this prospective study, we constructed 14 commonly reported inflammatory scores and compared the clinical utility of them for predicting postoperative recurrence or distal metastasis in 1533 stage II–III CRC patients in two independent cohorts. We also explored the prognostic role of the CEA-FPR combined score in predicting postoperative recurrence in these patients.
Patients and Methods
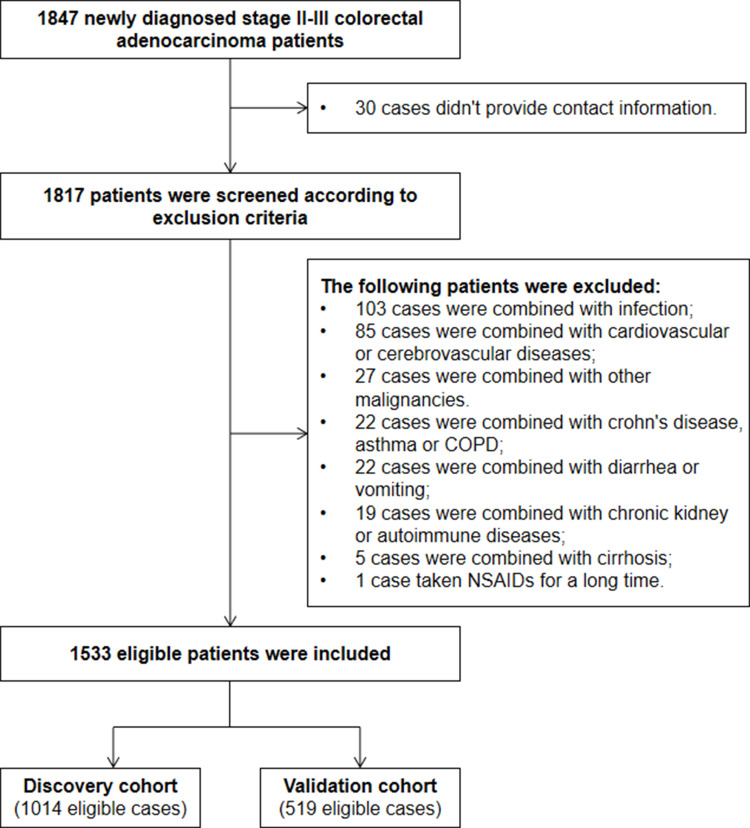
A total of 1847 consecutive CRC patients, who underwent a radical operation at the Second Affiliated Hospital of Nanchang University and Nanjing First Hospital between January 2013 and December 2016, were enrolled in this study. We identified the eligible patients according to the following inclusion and exclusion criteria. The inclusion criteria were as follows: a) stage II–III CRC confirmed by clinical laboratory, imaging, and pathology detection; b) no preoperative treatment for the patient; c) radical operation with tumor-negative resection margins; d) no history of other malignancies; and e) no recent pathogen infection, autoimmune or chronic kidney disease, hematopathy, hepatopathy or cardiovascular and cerebrovascular disease. The exclusion criteria were as follows: a) non-firstly diagnosed CRC in the hospitals; b) hereditary polyposis, ulcerative colitis, emergency surgery and neoadjuvant chemoradiotherapy ahead of the clinical confirmation; c) lost to follow-up within 3 months after surgery; d) diarrhea or undertaking drugs such as antibacterial agent, non-steroidal anti-inflammatory, anti-platelet or anticoagulant or intravenous albumin supplement in recent three months. The eligible patients selected from the Second Affiliated Hospital of Nanchang University consisted of the discovery cohort, and the patients within validation cohort were from Nanjing First Hospital. The study design and flowchart are described in Figure 1. This study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the ethics committees of the two hospitals. All participants were informed and signed a consent form before participating in the study.
Figure 1.
Enrollment flow chart of eligible patients in the present study.
Clinical characteristics and cancer pathological variants were collected after the surgical operation. Preoperative 5mL peripheral blood samples with routine laboratory measurements were collected from 7:00 to 9:00 am a week before the resection. Preoperative whole white cell, neutrophil, lymphocyte, monocyte, and platelet counting were detected using Sysmex HST-302 machine (Sysmex, Tokyo, Japan). Bromcresol green dye method, immunoturbidimetric assay, Clauss assay, and chemiluminescence immunoassay were selected to detect Alb, pre-Alb, Fib and carcinoembryonic antigen (CEA) and carbohydrate antigen 199 (CA199) by OLYMPUS AU5400 (Beckman Coulter, Tokyo, Japan), SYSMEX CA-7000 machine (Sysmex, Tokyo, Japan), and SIEMENS ADVIA Centaur XP machine (Siemens, Erlangen, Germany), respectively. The inter- and intra-batch coefficients of these detections were less than 10%. The NLR, derived-NLR (dNLR), PLR, LMR, nutrition prognostic score (NPS), systemic inflammation response index (SIRI), nutrition prognostic ratio (NPR), PNI, systemic immune-inflammation index (SII), systemic inflammation score (SIS), modified SIS (mSIS), Fib-NLR ratio (F-NLR), Alb to Fib ratio (AFR) and Fib to pre-Alb ratio (FPR) were constructed and calculated as described in Table 1. We constructed a combined score of CEA and FPR, and the score zero, one and two patients were defined as the cases with both low CEA and FPR, a single high CEA or FPR, as well as both high CEA and FPR, respectively.
Table 1.
Definition of the Included Inflammation-Based Biomarkers
| Inflammation-Based Biomarkers | Score |
|---|---|
| NLR | |
| Neutrophil/lymphocyte≤3.12 | 0 |
| Neutrophil/lymphocyte>3.12 | 1 |
| dNLR | |
| (WBC-neutrophil)/lymphocyte≤2.30 | 0 |
| (WBC-neutrophil)/lymphocyte>2.30 | 1 |
| PLR | |
| Platelet/lymphocyte≤176 | 0 |
| Platelet/lymphocyte>176 | 1 |
| LMR | |
| Lymphocyte/monocyte≤4 | 0 |
| Lymphocyte/monocyte>4 | 1 |
| NPS | |
| Neutrophil≤7.5×109/L, platelets≤400×109/L | 0 |
| Either one of them is larger than their cut-offs | 1 |
| Neutrophil>7.5×109/L, platelets>400×109/L | 2 |
| SIRI | |
| NLR×monocyte<1.95 | 0 |
| NLR×monocyte≥1.95 | 1 |
| NPR | |
| NLR/pAlb<295 | 0 |
| NLR/pAlb≥295 | 1 |
| PNI | |
| Alb(g/L) + 5 × lymphocyte (109/L)<45 | 0 |
| Alb(g/L) + 5 × lymphocyte (109/L)≥45 | 1 |
| SII | |
| platelet × NLR<665 | 0 |
| platelet × NLR≥665 | 1 |
| SIS | |
| Alb≥40g/L | 0 |
| Alb<40g/L and LMR≥4 | 1 |
| Alb<40g/L and LMR<4 | 2 |
| mSIS | |
| Alb≥40g/L and LMR≥4 | 0 |
| Alb<40g/L and LMR≥4 or Alb≥40g/L and LMR<4 | 1 |
| Alb<40g/L and LMR<4 | 2 |
| F-NLR score | |
| Fib>3.48 g/L and NLR>2.30 | 0 |
| Fib≤3.48 g/L and NLR>2.30 or Fib>3.48 g/L and NLR≤2.30 | 1 |
| Fib≤3.48 g/L and NLR≤2.30 | 2 |
| AFR | |
| Alb/Fib≤9.2 | 0 |
| Alb/Fib>9.2 | 1 |
| FPR | |
| Fib/pAlb×1000≤18.3 | 0 |
| Fib/pAlb×1000>18.3 | 1 |
Abbreviations: NLR, neutrophil to lymphocyte ratio; dNLR, derived NLR; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; NPS, neutrophil-platelet score; SIRI, systemic inflammation response index; NPR, ratio of NLR to pAlb; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; SIS, systemic inflammation score; mSIS, modified systemic inflammation score; AFR, ratio of Alb to Fib; FPR, ratio of Fib to Alb.
Three years follow-up was carried out after radical resection, with a frequency of three months in the first two years, and six months subsequently until recurrence or metastasis in the third year or the deadline (December 30, 2019). Recurrence-free survival is the main endpoint of the study, and it is defined as the time from surgical operation to the date of recurrence or distal metastasis. Follow-up investigations included a physical examination, common tumor biomarkers (CEA and CA199), and imaging (abdominal computed tomography scan or magnetic resonance imaging) detections or colonoscopy. A contrast-enhanced chest abdominal computed tomography scan and bone scan were used for the detection of lung or bone metastasis, respectively. Recurrence or distal metastasis of the disease was diagnosed according to one of the following criteria: a) colonoscopy examination; b) typical appearances in imaging scan; c) significantly elevated CEA, CA199 after resection.
The optimal cut-off points of each inflammatory score were selected as described previously,9,14–18 or calculated by X-tile software according to RFS, which were summarized in Table 1. The cut-off values of common tumor biomarker, CEA and CA199, were also calculated by the software. Binary variables were summarized as number and frequency, Chi-square test, and Fisher’s exact test were used to analyze the significant difference in the comparisons. Continuous variables were described as the median and quartile, and they were compared by Kolmogorov–Smirnov and Mann–Whitney U-test. Kaplan–Meier curve with a Log rank test was selected to examine RFS difference between the comparisons. Crude and adjusted hazard ratio (HR) and 95% confidence interval (CI) were calculated by univariable and multivariable Cox regression. Time-dependent receiver operating characteristic (ROC) curves and Harrell’s concordance index (C-index) were used to discriminate and to compare the predicted efficacy of these inflammation-based scores. All the statistics were analyzed using SPSS. 22.0 (IBM Corp, Armonk, NY, USA), R 3.5.1 (Institute for Statistics and Mathematics, Vienna, Austria) with packages of “rms”, “survival”, “survivalROC”, and “tdROC”, and GraphPad Prism 8.2.1 (GraphPad Software Inc., San Diego, USA). All analyses were two-sided, and p<0.05 was recognized as significant.
Results
Eligible Patients
A total of 1533 patients suffering stage II–III CRC after surgical resection were included in this study, and the discovery and validation cohort consisted of 1014 and 519 patients, respectively (Figure 1). The study power was 0.918, which was calculated according to the overall sample size, anticipated HR=2, α=0.05, overall probability of event=0.3, and it was significantly higher than the anticipated power (0.8), indicating that the sample size was enough in our study. The detail characteristics, clinical treatment, 14 inflammation-based biomarkers (including the NLR, dNLR, PLR, LMR, NPS, SIRI, NPR, PNI, SII, SIS, mSIS, F-NLR, AFR, and FPR) and recurrence data are described in Table 2. Shown in Table 2, 622 (61.34%) and 348 (67.05%) male patients were included in the two cohorts and approximately half the patients were older than 60 years. The proportions of stage II CRC were 527 (51.97%) and 269 (51.83%) in the discovery and validation cohorts, respectively. All the patients received radical operation, 80.51% and 9.86% of them within the discovery cohort underwent chemotherapy (capecitabine, XELOX, and FOLFOX regimens) and radiotherapy after resection, and the proportions of the patients undergoing the two treatments were 80.54% and 9.25%, respectively, in the validation cohort. After 3-years follow-up, a total of 713 (70.32%) and 345 (66.47%) patients survived without recurrence or distal metastasis, while 301 (29.68%) and 174 (33.53%) patients were confirmed as recurrence in the two cohorts.
Table 2.
Baseline Clinicopathological Characteristics of the Enrolled Patients in Discovery and Validation Cohort
| Parameters | Discovery Cohort (n=1014) n(%) | Validation Cohort (n=519) n(%) | P-value |
|---|---|---|---|
| Gender (male) | 622(61.34%) | 348(67.05%) | 0.028 |
| Age (>60 year) | 460(45.36%) | 328(63.20%) | <0.001 |
| Smoking (yes) | 194(19.13%) | 101(19.46%) | 0.877 |
| Drinking (yes) | 137(13.51%) | 54(10.40%) | 0.081 |
| Diabetes (yes) | 83(8.19%) | 46(8.86%) | 0.651 |
| Hypertension (yes) | 173(17.06%) | 69(13.29%) | 0.056 |
| Proximal colon | 238(23.47%) | 133(25.63%) | |
| Distal colon | 277(27.32%) | 117(22.54%) | 0.070 |
| Rectum | 499(49.21%) | 269(51.83%) | 0.785 |
| TNM stage (II) | 527(51.97%) | 269(51.83%) | 0.958 |
| T stage (T1-2) | 44(4.34%) | 25(4.82%) | 0.669 |
| LN status (N1-2) | 487(48.03%) | 250(48.17%) | 0.958 |
| Differentiation (G1-2) | 868(85.60%) | 454(87.48%) | 0.313 |
| Cancer bulk (>5cm) | 415(40.93%) | 239(46.05%) | 0.055 |
| Radical operation (yes) | 1014(100.00%) | 519(100%) | |
| Chemotherapy (yes) | 814(80.51%) | 418(80.54%) | 0.902 |
| Radiotherapy (yes) | 100(9.86%) | 48(9.25%) | 0.700 |
| CEA (>18.6 ng/mL) | 104(10.26%) | 67(12.91%) | 0.118 |
| CA199 (>55.6 U/mL) | 126(12.43%) | 76(14.64%) | 0.233 |
| NLR (>3.12) | 309(30.47%) | 151(29.09%) | 0.577 |
| dNLR (>2.30) | 335(33.04%) | 130(25.05%) | 0.001 |
| PLR (>176) | 287(28.30%) | 132(25.43%) | 0.002 |
| LMR (<4) | 467(46.06%) | 220(42.39%) | 0.172 |
| NPS (score≥1) | 73(7.20%) | 69(13.29%) | <0.001 |
| SIRI (≥1.95) | 147(14.50%) | 103(19.85%) | 0.007 |
| NPR (>295) | 816(80.47%) | 391(75.34%) | 0.020 |
| PNI (≥45) | 762(75.15%) | 363(69.94%) | 0.029 |
| SII (≥665) | 371(36.59%) | 174(33.53%) | 0.236 |
| SIS (score≥1) | 432(42.60%) | 284(54.72%) | <0.001 |
| mSIS (score≥1) | 680(67.06%) | 373(71.87%) | 0.055 |
| F-NLR (score≥1) | 812(80.08%) | 407(78.42%) | 0.446 |
| AFR (≤9.2) | 121(11.93%) | 89(17.15%) | 0.005 |
| FPR (>18.3) | 329(32.44%) | 203(39.11%) | 0.009 |
| Recurrence rate | 301(29.68%) | 174(33.53%) | 0.124 |
Abbreviations: LN, lymph node; NLR, neutrophil to lymphocyte ratio; dNLR, derived NLR; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; NPS, neutrophil-platelet score; SIRI, systemic inflammation response index; NPR, ratio of NLR to pAlb; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; SIS, systemic inflammation score; mSIS, modified systemic inflammation score; F-NLR, fibrinogen-NLR score; AFR, ratio of Alb to Fib; FPR, ratio of Fib to Alb.
Recurrence-Free Survival and Inflammatory Biomarkers
In discovery cohort, T stage, lymph node (LN) status, cell differentiation, radiotherapy, CEA, CA199, PLR, LMR, SIRI, NPR, PNI, SII, mSIS, F-NLR score, AFR, and FPR were significantly associated with RFS in Kaplan–Meier curve and univariable Cox regression (Table 3). Adjusted by clinical characteristics, tumor biomarkers, pathological characteristics (T and LN stage, differentiation and cancer size) and postoperative therapeutics (chemotherapy and radiotherapy), high CEA (p<0.001, HR=1.782, 95% CI=1.293–2.456), CA199 (p<0.001, HR=1.988, 95% CI= 1.474–2.681), SIRI (p=0.005, HR=1.583, 95% CI=1.146–2.185), mSIS (p=0.007, HR=1.491, 95% CI=1.116–1.992), NPR (p=0.010, HR=1.456, 95% CI=1.096–1.934), and FPR (p<0.001, HR=2.768, 95% CI= 2.141–3.579) were significantly associated with poor RFS of stage II–III CRC patients (Table 3). However, high LMR (p=0.044, HR=0.772, 95% CI=0.600–0.993), PNI (p=0.003, HR=0.660, 95% CI= 0.501–0.869), F-NLR (p<0.001, HR=0.530, 95% CI= 0.399–0.703) and AFR (p<0.001, HR=0.539, 95% CI=0.391–0.743) were still significantly associated with good RFS of stage II–III CRC patients (Table 3).
Table 3.
The Relationship Between Clinicopathological Factors and Survival in Patients with Stage II–III Colorectal Cancer in Discovery and Validation Cohort
| Parameters | Discovery Cohort | Validation Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| p-value | HR(95% CI) | p-value | HR(95% CI) | p-value | HR(95% CI) | p-value | HR(95% CI) | |
| Gender (male) | 0.878 | 0.982(0.779–1.237) | 0.851 | 0.975(0.747–1.272) | 0.888 | 0.978(0.712–1.342) | 0.832 | 0.926(0.456–1.881) |
| Age (>60 year) | 0.769 | 0.967(0.770–1.213) | 0.330 | 1.143(0.874–1.494) | 0.362 | 1.153(0.849–1.565) | 0.601 | 1.199(0.608–2.364) |
| Smoking (yes) | 0.590 | 0.923(0.689–1.236) | 0.958 | 0.990(0.667–1.467) | 0.980 | 0.990(0.427–2.292) | 0.988 | 0.992(0.438–2.471) |
| Drinking (yes) | 0.625 | 0.919(0.654–1.291) | 0.807 | 1.048(0.719–1.527) | 0.874 | 0.934(0.403–2.164) | 0.990 | 0.947(0.456–2.269) |
| Diabetes (yes) | 0.615 | 0.897(0.586–1.372) | 0.311 | 0.782(0.487–1.258) | 0.384 | 1.567(0.570–4.309) | 0.782 | 1.350(0.162–11.232) |
| Hypertension (yes) | 0.383 | 0.871(0.638–1.189) | 0.230 | 0.808(0.570–1.144) | 0.742 | 0.883(0.421–1.851) | 0.435 | 0.546(0.120–2.492) |
| T stage (T1-2) | 0.038 | 0.452(0.214–0.956) | 0.004 | 0.297(0.131–0.674) | 0.174 | 0.540(0.222–1.313) | 0.176 | 0.548(0.235–1.356) |
| LN status (N1-2) | <0.001 | 3.171(2.471–4.070) | 0.751 | 0.721(0.095–5.452) | 0.029 | 1.395(1.034–1.881) | 0.025 | 1.340(1.09–2.012) |
| Differentiation (G1-2) | <0.001 | 0.478(0.343–0.666) | 0.022 | 0.661(0.463–0.943) | 0.765 | 0.906(0.475–1.729) | 0.784 | 0.910(0.468–1.822) |
| Cancer bulk (>5cm) | 0.803 | 0.971(0.771–1.223) | 0.716 | 1.050(0.808–1.365) | 0.399 | 1.168(0.815–1.674) | 0.422 | 1.254(0.820–1.755) |
| Location (right) | 0.141 | 1.214(0.938–1.570) | 0.072 | 1.318(0.975–1.780) | 0.005 | 1.580(1.152–2.167) | 0.023 | 2.700(1.145–6.367) |
| Chemotherapy (yes) | 0.227 | 0.843(0.638–1.113) | 0.001 | 0.582(0.422–0.803) | 0.171 | 0.782(0.550–1.112) | 0.115 | 0.705(0.390–2.098) |
| Radiotherapy (yes) | 0.020 | 0.673(0.483–0.939) | 0.018 | 0.632(0.432–0.925) | 0.047 | 0.305(0.095–0.984) | 0.001 | 0.098(0.025–0.376) |
| CEA (>18.60ng/mL) | <0.001 | 2.111(1.560–2.855) | <0.001 | 1.782(1.293–2.456) | 0.050 | 1.921(1.001–3.687) | 0.038 | 2.464(1.053–5.766) |
| CA199 (>55.60U/mL) | <0.001 | 2.532(1.915–3.349) | <0.001 | 1.988(1.474–2.681) | 0.279 | 1.456(0.737–2.878) | 0.651 | 1.237(0.493–3.103) |
| NLR (>3.12) | 0.066 | 1.525(0.985–1.591) | 0.433 | 1.117(0.847–1.472) | <0.001 | 2.132(1.543–2.944) | <0.001 | 2.035(1.466–2.825) |
| dNLR (>2.30) | 0.631 | 1.062(0.830–1.360) | 0.535 | 1.095(0.822–1.460) | <0.001 | 1.938(1.389–2.704) | <0.001 | 1.874(1.341–2.620) |
| PLR (>176) | 0.007 | 1.377(1.091–1.737) | 0.265 | 1.163(0.891–1.518) | 0.002 | 1.709(1.219–2.394) | 0.011 | 1.590(1.110–2.277) |
| LMR (>4) | 0.002 | 0.697(0.556–0.874) | 0.044 | 0.772(0.600–0.993) | 0.006 | 0.638(0.464–0.877) | 0.019 | 0.680(0.492–0.938) |
| NPS (score≥1) | 0.464 | 1.169(0.770–1.773) | 0.794 | 1.072(0.637–1.805) | 0.298 | 1.248(0.822–1.895) | 0.786 | 1.066(0.673–1.688) |
| SIRI (≥1.95) | <0.001 | 1.770(1.338–2.340) | 0.005 | 1.583(1.146–2.185) | 0.008 | 1.639(1.139–2.359) | 0.016 | 1.565(1.086–2.255) |
| NPR (≥295) | <0.001 | 1.595(1.238–2.057) | 0.010 | 1.456(1.096–1.934) | 0.716 | 1.069(0.744–1.538) | 0.885 | 0.973(0.668–1.416) |
| PNI (≥45) | 0.002 | 0.686(0.537–0.876) | 0.003 | 0.660(0.501–0.869) | <0.001 | 0.402(0.292–0.552) | <0.001 | 0.398(0.284–0.558) |
| SII (≥665) | 0.009 | 1.359(1.080–1.709) | 0.075 | 1.263(0.977–1.632) | 0.012 | 1.516(1.096–2.098) | 0.027 | 1.450(1.044–2.014) |
| SIS (score≥1) | 0.094 | 1.214(0.968–1.522) | 0.366 | 1.130(0.867–1.472) | 0.002 | 1.693(1.219–2.351) | 0.037 | 1.453(1.022–2.065) |
| mSIS (score≥1) | 0.002 | 1.504(1.162–1.946) | 0.007 | 1.491(1.116–1.992) | 0.010 | 1.686(1.134–2.506) | 0.015 | 1.643(1.101–2.452) |
| F-NLR (score≥1) | <0.001 | 0.544(0.424–0.697) | <0.001 | 0.530(0.399–0.703) | 0.014 | 0.638(0.446–0.913) | 0.013 | 0.634(0.443–0.908) |
| AFR (>9.2) | <0.001 | 0.475(0.356–0.632) | <0.001 | 0.539(0.391–0.743) | <0.001 | 0.503(0.357–0.708) | 0.003 | 0.563(0.388–0.819) |
| FPR (>18.3) | <0.001 | 2.855(2.275–3.581) | <0.001 | 2.768(2.141–3.579) | <0.001 | 2.188(1.622–2.953) | <0.001 | 1.846(1.321–2.579) |
Notes: Multivariate cox regression was adjusted by gender, age, tobacco, alcohol, diabetes, hypertension, chemotherapy, radiotherapy, T stage, Node lymph status, differentiation, cancer size.
Abbreviations: LN, lymph node; NLR, neutrophil to lymphocyte ratio; dNLR, derived NLR; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; NPS, neutrophil-platelet score; SIRI, systemic inflammation response index; NPR, ratio of NLR to pAlb; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; SIS, systemic inflammation score; mSIS, modified systemic inflammation score; F-NLR, fibrinogen-NLR score; AFR, ratio of Alb to Fib; FPR, ratio of Fib to Alb; p-value, the value of Cox regression; HR, hazard ratio; CI, confidence interval.
Furthermore, our univariate analysis showed that the following prognostic factors: 1) LN status and primary tumor location as well as radiology; 2) CEA and 12 inflammation-related scores including NLR, dNLR, PLR, LMR, SIRI, PNI, SII, SIS, mSIS, F-NLR, AFR and FPR were obviously related to RFS in validation cohort (Table 3). In addition, multivariable analysis identified that LN status (p=0.025, HR=1.340, 95% CI=1.09–2.012), right location (p=0.023, HR=2.700, 95% CI=1.145–6.367), radiotherapy (p=0.001, HR=0.098, 95% CI=0.025–0.376), CEA (p=0.038, HR=2.464, 95% CI=1.053–5.766), NLR (p<0.001, HR=2.035, 95% CI=1.466–2.825), dNLR (p<0.001, HR=1.874, 95% CI=1.341–2.620), PLR (p=0.011, HR=1.590, 95% CI=1.110–2.277), LMR (p=0.019, HR=0.680, 95% CI=0.492–0.938), SIRI (p=0.016, HR=1.565, 95% CI=1.086–2.255), PNI (p<0.001, HR=0.398, 95% CI=0.284–0.558), SII (p=0.027, HR=1.450, 95% CI=1.044–2.014), SIS (p=0.037, HR=1.453, 95% CI=1.022–2.065), mSIS (p=0.015, HR=1.643, 95% CI=1.101–2.452), F-NLR (p=0.013, HR=0.634, 95% CI=0.443–0.908), AFR (p=0.003, HR=1.775, 95% CI=1.221–2.580) and FPR (p<0.001, HR=1.846, 95% CI=1.321–2.579) were still significantly associated with RFS in the validation cohort (Table 3).
Prognostic Efficacy of FPR
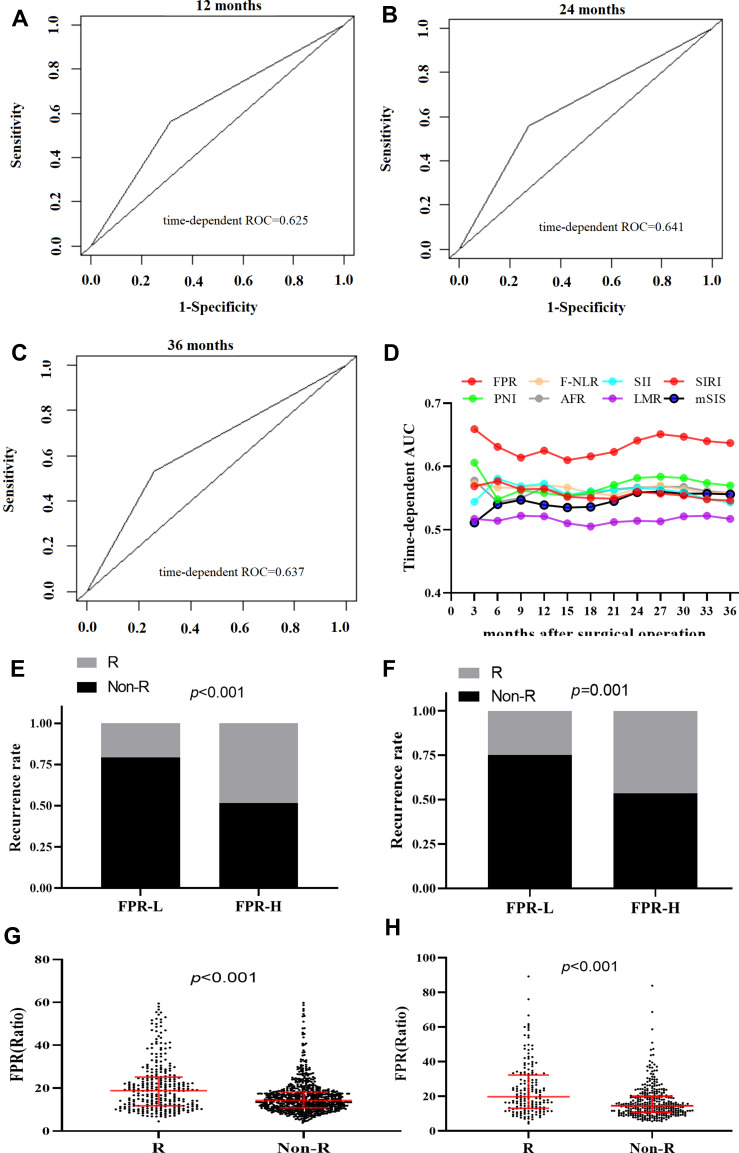
Time-dependent ROC curves at 12, 24, and 36 months of RFS were generated to compare the prognostic performance of the 14 inflammatory biomarkers, and CEA in the overall population. As shown from Figure 2A–D and Table 4, FPR was superior to the other biomarkers in each survival endpoint, and the sequential trends of area under curve (AUC) of FPR (AUC=0.625, 95% CI=0.600–0.656 for 12 months; AUC=0.641, 95% CI=0.628–0.688 for 24 months; AUC=0.637, 95% CI=0.603–0.673 for 36 months) were significantly higher than the other scores, and its sensitivity and specificity were 43.87% and 68.48%, 43.96% and 70.06%, 44.51% and 71.65%, respectively, for predicting 12, 24, and 36 months RFS. The C-index of the three years survival of the FPR for RFS prediction was 0.629 (0.599–0.659), which was obviously higher than that of LMR, SIRI, PNI, SII, mSIS, F-NLR, AFR, and CEA, respectively.
Figure 2.
Preoperative FPR, recurrence, and the area under time-dependent ROC (AUROC) for predicting RFS in the discovery and validation cohorts. (A) AUROC of FPR for 12 months survival in the overall population; (B) AUROC of FPR for 24 months survival in the overall population; (C) AUROC of FPR for 36 months survival in the overall population; (D) AUROC of FPR, F-NLR score, SII, SIRI, PNI, AFR, LMR and mSIS in three years. (E) FPR and the recurrence rate in discovery cohort; (F) FPR and the recurrence rate in validation cohort; (G and H) comparison of circulating FPR between the recurrence and non-recurrence subgroup in discovery (G) and validation (H) cohort.
Table 4.
Comparison of the Performance Discriminative Ability Between the Inflammation-Based Scores in Stage II–III Colorectal Cancer Patients in Overall Population
| Variants | 12-Month Survival | 24-Month Survival | 36-Month Survival | |
|---|---|---|---|---|
| AUROC(95% CI) | AUROC(95% CI) | AUROC(95% CI) | C-index(95% CI) | |
| LMR (<4) | 0.521(0.512–0.604) | 0.514(0.485–0.548) | 0.517(0.488–0.542) | 0.549(0.519–0.579) |
| SIRI (≥1.95) | 0.565(0.538–0.606) | 0.560(0.520–0.580) | 0.546(0.515–0.561) | 0.539(0.515–0.563) |
| PNI (≥45) | 0.558(0.537–0.583) | 0.582(0.530–0.620) | 0.570(0.520–0.651) | 0.538(0.510–0.566) |
| SII (≥665) | 0.573(0.541–0.611) | 0.566(0.528–0.574) | 0.543(0.518–0.563) | 0.520(0.490–0.550) |
| mSIS (score≥1) | 0.539(0.515–0.573) | 0.559(0.540–0.596) | 0.556(0.534–0.572) | 0.546(0.519–0.573) |
| F-NLR (score≥1) | 0.571(0.543–0.603) | 0.565(0.546–0.582) | 0.558(0.541–0.583) | 0.557(0.530–0.584) |
| AFR (≤9.2) | 0.567(0.540–0.588) | 0.567(0.537–0.591) | 0.558(0.540–0.578) | 0.548(0.526–0.570) |
| FPR (>18.3) | 0.625(0.600–0.656) | 0.641(0.628–0.688) | 0.637(0.603–0.673) | 0.629(0.599–0.659) |
| CEA (>18.60ng/mL) | 0.541(0.492–0.594) | 0.543(0.503–0.583) | 0.547(0.513–0.586) | 0.542(0.523–0.561) |
Abbreviations: LMR, lymphocyte to monocyte ratio; SIRI, systemic inflammation response index; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; mSIS, modified systemic inflammation score; F-NLR, fibrinogen-NLR score; AFR, ratio of Alb to Fib; FPR, ratio of Fib to Alb; AUROC, area under time-dependent receiver operating characteristic curve; CI, confidence interval.
FPR and Clinical Characteristics
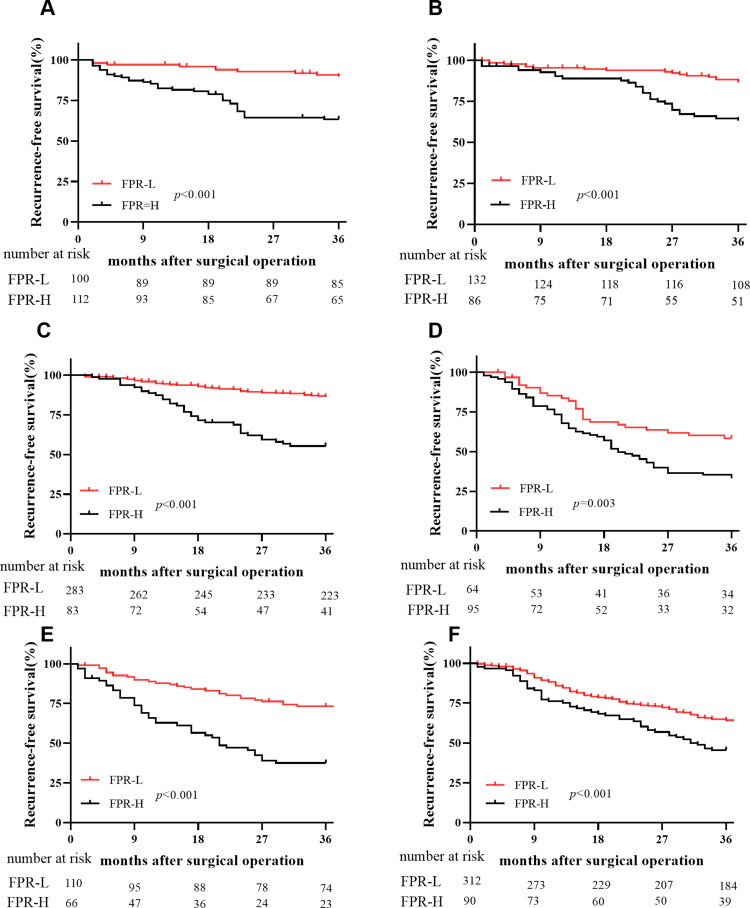
In stage II–III patients, the number of recurrent patients within high-FPR patients was significantly higher than the low-FPR cases in the discovery cohort (p<0.001 for 48.32% vs 20.73%) and validation cohort (p=0.001 for 46.30% vs 25.00%), respectively (Figure 2E and F). Preoperative FPR (all p<0.001) within recurrent patients was significantly higher than those without recurrence in both two cohorts (Figure 2G–H). Moreover, elevated FPR was significantly associated with older individuals (p<0.001), T3-4 stage (p<0.001), poor differentiation (p=0.001), large cancer bulk (p<0.001), high CEA (p<0.001), CA199 (p<0.001), NLR (p<0.001), dNLR(p<0.001), PLR (p<0.001), NPS score (p<0.001), SIRI score (p<0.001), NPR (p<0.001), PNI (p<0.001), SII (p<0.001), SIS (p<0.001), mSIS (p<0.001), F-NLR (p<0.001), and low LMR (p=0.002), AFR (p<0.001), respectively (Table 5). Stratified according to primary tumor location and TNM stage, elevated FPR patients harbored poor RFS comparing to the low-FPR patients in stage II and III proximal, distal colon, and rectal subgroups, respectively (Figure 3).
Table 5.
Preoperative FPR and Clinical Characteristics in Overall Population
| Parameter | FPR(≤18.3)(n=1001) n(%) | FPR(>18.3)(n=532) n(%) | p-value |
|---|---|---|---|
| Gender (male) | 645(64.44%) | 325(61.09%) | 0.196 |
| Age (>60 year) | 460(45.95%) | 328(61.65%) | <0.001 |
| Smoking (yes) | 183(18.28%) | 112(21.05%) | 0.190 |
| Drinking (yes) | 128(12.79%) | 63(11.84%) | 0.594 |
| Diabetes (yes) | 77(7.69%) | 52(9.77%) | 0.162 |
| Hypertension (yes) | 156(15.58%) | 86(16.17%) | 0.766 |
| TNM stage (II) | 515(51.45%) | 281(52.82%) | 0.609 |
| T stage (T1-2) | 60(5.99%) | 9(1.69%) | <0.001 |
| LN status (N1-2) | 486(48.55%) | 251(47.18%) | 0.609 |
| Differentiation (G1-2) | 884(88.31%) | 438(82.33%) | 0.001 |
| Cancer bulk (>5cm) | 336(33.57%) | 318(59.77%) | <0.001 |
| Radical operation (yes) | 1001(100%) | 532(100%) | |
| Chemotherapy (yes) | 830(82.92%) | 402(75.56%) | 0.001 |
| Radiotherapy (yes) | 100(9.99%) | 48(9.02%) | 0.542 |
| CEA (>18.6 ng/mL) | 70(6.99%) | 101(18.98%) | <0.001 |
| CA199 (>55.60U/mL) | 94(9.39%) | 108(20.30%) | <0.001 |
| NLR (>3.12) | 235(23.48%) | 225(42.29%) | <0.001 |
| dNLR (>2.30) | 243(24.28%) | 222(41.73%) | <0.001 |
| PLR (>176) | 233(23.28%) | 186(34.96%) | <0.001 |
| LMR (<4) | 375(37.46%) | 312(58.64%) | <0.001 |
| NPS (score≥1) | 61(6.09%) | 81(15.23%) | <0.001 |
| SIRI (≥1.95) | 110(10.99%) | 140(26.32%) | <0.001 |
| NPR (>295) | 859(85.81%) | 348(65.41%) | <0.001 |
| PNI (≥45) | 848(84.71%) | 277(52.07%) | <0.001 |
| SII (≥665) | 273(27.27%) | 272(51.13%) | <0.001 |
| SIS (score≥1) | 345(34.47%) | 371(69.74%) | <0.001 |
| mSIS (score≥1) | 585(58.44%) | 468(87.97%) | <0.001 |
| F-NLR (score≥1) | 941(94.01%) | 278(52.26%) | <0.001 |
| AFR (≤9.2) | 17(1.70%) | 193(36.28%) | <0.001 |
Abbreviations: LN, lymph node; NLR, neutrophil to lymphocyte ratio; dNLR, derived NLR; PLR, platelet to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; NPS, neutrophil-platelet score; SIRI, systemic inflammation response index; NPR, ratio of NLR to pAlb; PNI, prognostic nutritional index; SII, systemic immune-inflammation index; SIS, systemic inflammation score; mSIS, modified systemic inflammation score; F-NLR, fibrinogen-NLR score; AFR, ratio of Alb to Fib; FPR, ratio of Fib to Alb.
Figure 3.
Kaplan–Meier curve of preoperative FPR and RFS in each subgroup. (A) Stage II surgical proximal colon cancer patients; (B) stage II surgical distal colon cancer patients; (C) stage II surgical rectal cancer patients; (D) stage III surgical proximal colon cancer patients; (E) stage III surgical distal colon cancer patients; (F) stage III surgical rectal cancer patients.
RFS Comparison in the Chemotherapy-Treated Patients
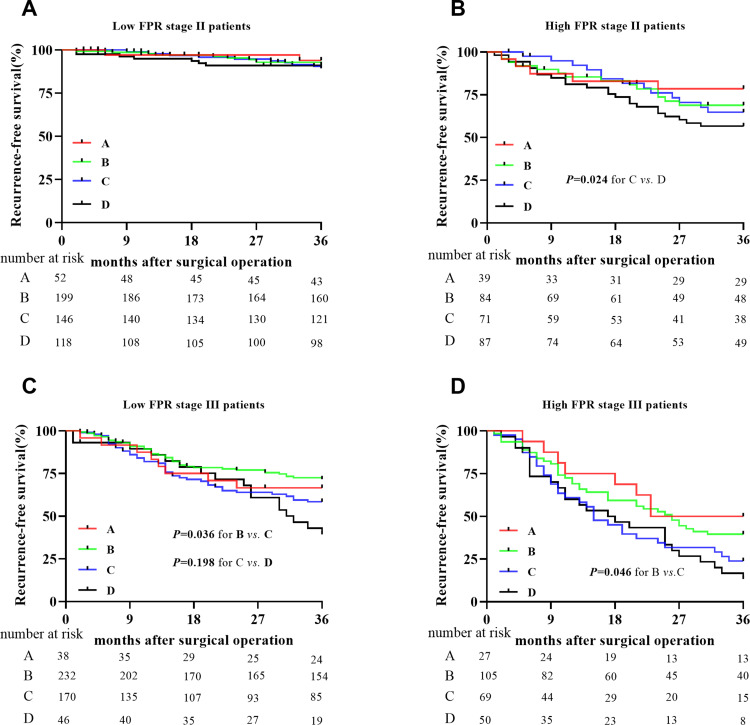
In this study, a total of 591 (74.25%) stage II patients and 641 (86.97%) stage III cases received adjuvant chemotherapy after curable resection. Among them, 156, 620, and 456 cases underwent capecitabine, XELOX (combined capecitabine and oxaliplatin), and FOLFOX (combined leucovorin calcium, fluorouracil, and oxaliplatin) regimens, respectively. Stage II low FPR patients harbored the best RFS in the overall population, and there was no survival difference in these patients with or without chemotherapy (Figure 4A). However, the outcome of chemotherapy-treated stage II high FPR patients was significantly longer than those without the treatment, and no survival difference was observed in the patients with the different chemotherapy regimens (Figure 4B). The XELOX treated patients showed the best RFS in stage III low FPR patients, and the outcome of capecitabine and FOLOFOX treated patients was the same as the non-chemotherapy treated cases (Figure 4C). Although a similar RFS was observed in capecitabine and XELOX treated patients, the outcome of these cases was significantly longer than the FOLFOX treated or non-chemotherapy treated patients (Figure 4D).
Figure 4.
Recurrence-free survival comparisons between the high- and low-FPR patients with or without adjuvant chemotherapy in stage II and stage III population. A=single 5-FU treatment; B=XELOX (combined capecitabine and oxaliplatin) treatment; c=FOLFOX (combined leucovorin calcium, fluorouracil, and oxaliplatin) treatment; D=without treatment of chemotherapy. (A) The low FPR stage II patients; (B) the high FPR stage II patients; (C) the low FPR stage III patients; (D) the high FPR stage III patients.
Combined FPR and CEA Score
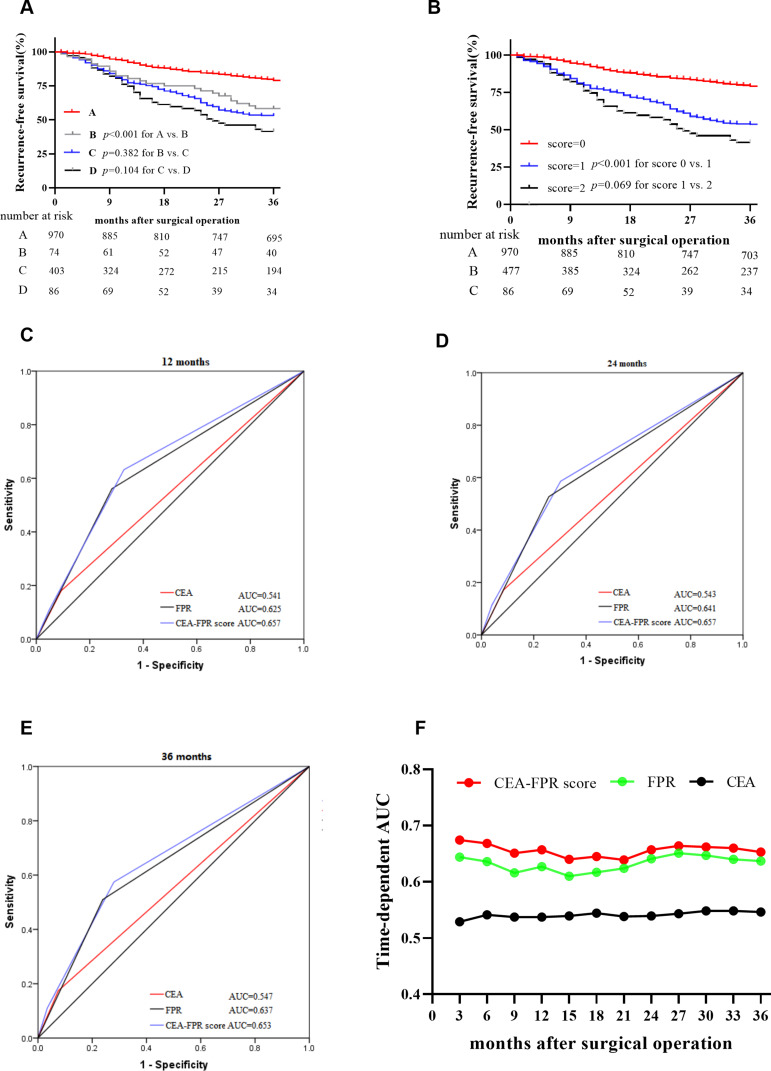
In the overall population, both low CEA and FPR patients and the cases with both high CEA and FPR showed the best and worst RFS, respectively. However, the patients with a single high CEA or FPR showed a similar outcome (plog-rank=0.382) (Figure 5A). Combined with FPR and CEA, the patients were divided into three subgroups according to the combined score, the combined score one (plog-rank<0.001, adjusted HR=2.764, 95% CI=2.129–3.589) and two (plog-rank<0.001, adjusted HR=3.543, 95% CI=2.317–5.420) were extremely associated with RFS of these patients (Figure 5B and Table 6). The predicted AUC of the combined score for 12, 24 and 36 months were 0.657, 0.657 and 0.653 in stage II–III patients, which were superior to the single CEA and FPR, respectively (Figure 5C–F), and the respective sensitivity and specificity of the combined score were 63.23% and 67.24%, 58.61% and 69.74%, 57.46% and 72.00% for 12, 24 and 36 months RFS.
Figure 5.
Survival comparison in subgroup stratified by preoperative CEA, FPR, and CEA-FPR score and their predicted efficacy in the overall population. (A) Kaplan–Meier curve of subgroups stratified by circulating CEA and FPR, A=low CEA and low FPR group; B=high CEA and low FPR group; c=low CEA and high FPR group; D=high CEA and high FPR group; (B) Kaplan–Meier curve of subgroups stratified by CEA-FPR combined score; score 0=both low CEA and low FPR; score 1=single high CEA or FPR; score 2=both high CEA and FPR; (C) AUROC of CEA-FPR combined score for 12 months survival; (D) AUROC of CEA-FPR combined score for 24 months survival; (E) AUROC of CEA-FPR combined score for 36 months survival; (F) AUAUC of CEA, FPR and CEA-FPR combined score in three years.
Table 6.
Cox Analysis of CEA and FPR Combined Score in Stage II–III Surgical CRC Patients
| CEA and FPR | Definition | p-value | Univariate Cox Regression | Multivariate Cox Regression |
|---|---|---|---|---|
| Combined Score | HR(95% CI) | HR(95% CI) | ||
| Score zero | CEA<18.6 and FPR<18.3 | 1 | 1 | |
| Score one | Single CEA≥18.6 or FPR≥18.3 | <0.001 | 2.684(2.153–3.347) | 2.764(2.129–3.589) |
| Score two | CEA≥18.6 and FPR≥18.3 | <0.001 | 3.734(2.624–5.315) | 3.543(2.317–5.420) |
Notes: Multivariate cox regression was adjusted by gender, age, tobacco, alcohol, diabetes, hypertension, chemotherapy, radiotherapy, T stage, Node lymph status, differentiation, cancer size.
Abbreviations: FPR, ratio of Fib to Alb; p-value, the value of Kaplan-Meier curve with Log rank test; HR, hazard ratio; CI, confidence interval.
Discussion
Although a few studies have reported that cancer-related inflammation scores can predict the clinical outcomes of cancer-suffered individual, the best inflammation-based biomarker for RFS of CRC patients after operation remains unclear. In this study, we found that systemic inflammation-based biomarkers, including LMR, SIRI, SII, mSIS, F-NLR, AFR, and FPR could predict the RFS of stage II–III CRC patients in both discovery and validation cohorts. Moreover, FPR was superior to the other biomarkers in prediction, with high-FPR indicating poor RFS. Thus, preoperative FPR could be used as a good prognostic factor for predicting the survival of the patients with stage II–III CRC. Stage II low FPR patients harbored the best RFS regardless of chemotherapy, chemotherapy-treated stage II high FPR patients showed the longer RFS than those without the treatment, and the outcomes of patients with treatment of XELOX, capecitabine and XELOX were superior to the other regimens treated patients in stage III low- and high-FPR populations, respectively. Additionally, FPR could help common biomarkers such as CEA to superiorly predict RFS in those patients. To the best of our knowledge, this is the first study to identify the best inflammation-based prognostic factor for predicting RFS of stage II–III CRC patients.
Lots of inflammatory predicted ratios and scores such as NLR, PLR, LMR, and the cumulative scores neutrophil–lymphocyte score (NLS), platelet–lymphocyte score (PLS), lymphocyte–monocyte score (LMS), neutrophil–platelet score (NPS), modified Glasgow prognostic score (mGPS) have emerged to predict cancer-specific survival and overall survival (OS) of surgery CRC patients and were all proven to be associated with clinical outcome.19–21 In those factors, both the ratios and cumulative scores of the systemic inflammatory response had prognostic value, independent of TNM stage, for stage I–III patients with colon cancer, and the cumulative scores were simpler and more consistent than the ratios for clinical use.20 LMR appeared to be superior to pre-existing biomarkers to predict OS in patients with CRC undergoing curative resection.21 Several studies also reported that C-reactive protein to Alb ratio, PI and PNI, F-NLR score, SII, SIS were survival predictors of surgical CRC patients.19,22–24 However, no study has reported which is the best inflammation-based score to predict recurrence of CRC after curable resection. Thus, we performed this study and found that LMR, SIRI, NPR, PNI, mSIS, F-NLR, AFR and FPR were all independently relevant to RFS in the discovery cohort, while all these scores excluding NPR were verified in the validation cohort. Circulating FPR harbored the best prediction ability compared with the other inflammatory prognostic biomarkers. Furthermore, preoperative elevated FPR was significantly associated with poor RFS regardless of TNM stage and tumor location. Hence, FPR has the potential to be used as an effective recurrence predictor of patients with stage II–III CRC. In addition, the best RFS was observed in stage II low FPR patients with or without chemotherapy, showing that these patients might not receive adjuvant chemotherapy after the operation. RFS of chemotherapy treated stage II high FPR patients was superior to those without the treatment, and the best outcomes were also observed in stage III low and high FPR patients with treatment of XELOX, capecitabine and XELOX, respectively. So, we believe that the stage II high FPR patients and III cases can benefit more from the effective chemotherapy regimen that chooses to rely on FPR.
Cancer is the common condition leading to substantial changes in the plasma concentrations of acute-phase proteins.25 Fib and pre-Alb were the positive and negative acute-phase proteins from the liver. FPR, which is an integrated ratio constructed on circulating Fib and pre-Alb, has been demonstrated to be an effective prognostic predictor for many kinds of malignancies, including surgical gastric cancer, local and advanced CRC as well as hepatocellular carcinoma.7,26,27 In our study, CRC patients with high-FPR had a high recurrence rate, more aggressive features of recurrence, elevated CEA, CA199, NLR, dNLR, PLR, NPS, SIRI, SII, SIS and mSIS score, and low LMR, NPR, PNI, AFR as well as F-NLR score, indicating a high-grade inflammation and hypercoagulable status and poor nutrition response. Fib and pAlb are now understood to play key roles in the acute and chronic phase response triggering by malignancies including CRC.7 Fib appears to be a driver of chronic low-grade inflammation by affecting platelets, leukocyte migration and promoting carcinogenic properties and function as a scaffold for cancer growth, migration, and metastasis.28 Alb and pAlb represent the main energy and nutrition source for tumor growth. Inflammatory cytokines which were produced from CRC microenvironment and Kupfer cells, such as interleukin-1β (IL-1β), IL-6, IL-8, could suppress Alb and pre-Alb synthesized by hepatocytes and regulate complex host inflammatory response to cancer growth and progression of the disease.29–31 The evidence supports our finding that FPR which is based on Fib and pre-Alb, is a useful inflammation-based biomarker for predicting the RFS of CRC patients.
Malnutrition or hypoalbuminemia as consequences of cancer cachexia, and they are commonly clinical symptoms in CRC patients, especially in cases with advanced disease.32,33 High FPR implies hypercoagulability, hypoalbuminemia, emaciation and malnutrition in the CRC patients. Thus, this ratio can illustrate the progression of the disease, and the full understanding of circulating FPR will reveal the association between cancer-associated inflammation, nutrition, and the disease. Moreover, the patients with circulating high FPR harbored significantly higher recurrence rates than the low-FPR patients, and the high FPR is associated with unsatisfactory RFS regardless of TNM stage and primary tumor location. So we believe that combing the biomarker with pathological variables and common tumor biomarkers may contribute to a robust and new model for predicting the prognosis of these patients. Additionally, considerable evidence demonstrated that chemoprevention strategy such as nonsteroidal anti-inflammatory drugs, cyclooxygenase 2 inhibitors and, metformin has been shown their potential for prevention of adenoma recurrence and the cancer onset, particularly CRC.34,35 For this, we believe that high-FPR CRC after surgical resection might benefit from anti-inflammation targeted treatment.
Limitations should be addressed as follows. Although this study was a prospective study, our findings should be validated by a multi-center study with a larger sample size design. On the other hand, no widely accepted cut-off value was found for those inflammation-based factors including FPR, and we selected according to our previous study. Thus, the optimal cut-off of FPR needs to be verified or confirmed if it is necessary for other prospective cohorts.
In summary, prospective FPR is better than the other inflammatory prognostic biomarkers to independently predict recurrence in surgical stage II–III CRC patients in terms of predicting ability. It helps to choose the effective chemotherapy regimen for these surgical patients, especially the stage III patients, and the CEA-FPR combined score can effectively predict the postoperative recurrence of the patients.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (grant number: 81702090), the Natural Science Youth Foundation of Jiangxi Province (grant number: 20171BAB215054) and the Key Technology Research and Development Program of Jiangxi Province (grant number: 20171BBG70049).
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.National Health Commission of the People’s Republic of C.. [Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2020 edition)]. Zhonghua Wai Ke Za Zhi. 2020;58(8):561–585. [DOI] [PubMed] [Google Scholar]
- 2.Kudose Y, Shida D, Ahiko Y, et al. Evaluation of recurrence risk after curative resection for patients with stage i to iii colorectal cancer using the hazard function: retrospective analysis of a single-institution large cohort. Ann Surg. 2020;Publish Ahead of Print. doi: 10.1097/SLA.0000000000004058 [DOI] [PubMed] [Google Scholar]
- 3.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611 [DOI] [PubMed] [Google Scholar]
- 4.Greten FR, Grivennikov SI. Inflammation and Cancer: triggers, Mechanisms, and Consequences. Immunity. 2019;51(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan CA, Garrett WS. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu Rev Microbiol. 2016;70(1):395–411. doi: 10.1146/annurev-micro-102215-095513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. 2017;32:43–53. doi: 10.1016/j.smim.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 7.Li S-Q, You X-H, Sun F, et al. Albumin to fibrinogen ratio and fibrinogen to pre-albumin ratio are economical, simple and promising prognostic factors for solid malignancy. J Thorac Dis. 2019;11(Suppl S15):S2036–S2038. doi: 10.21037/jtd.2019.08.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q-G, Zhang L, Sun F, et al. Elevated FPR confers to radiochemoresistance and predicts clinical efficacy and outcome of metastatic colorectal cancer patients. Aging. 2019;11(6):1716–1732. doi: 10.18632/aging.101864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wuhao H, Shengguang W, Hua Z, Bin Z, Changli W. Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable non-small cell lung cancer. Cancer Biol Med. 2018;15(1):88–96. doi: 10.20892/j.issn.2095-3941.2017.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S, Ahn HJ, Yang M, Kim JA, Kim JK, Park SJ. The prognostic nutritional index and postoperative complications after curative lung cancer resection: A retrospective cohort study. J Thorac Cardiovasc Surg. 2020;160(1):276–285. doi: 10.1016/j.jtcvs.2019.10.105 [DOI] [PubMed] [Google Scholar]
- 11.Salati M, Filippi R, Vivaldi C, et al. The prognostic nutritional index predicts survival and response to first-line chemotherapy in advanced biliary cancer. Liver Int. 2020;40(3):704–711. doi: 10.1111/liv.14314 [DOI] [PubMed] [Google Scholar]
- 12.Hu X, Shao Y-X, Yang Z-Q, Dou W-C, Xiong S-C, Li X. Preoperative systemic immune-inflammation index predicts prognosis of patients with non-metastatic renal cell carcinoma: a propensity score-matched analysis. Cancer Cell Int. 2020;20(1):222. doi: 10.1186/s12935-020-01320-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishibashi Y, Tsujimoto H, Hiraki S, et al. Prognostic Value of Preoperative Systemic Immunoinflammatory Measures in Patients with Esophageal Cancer. Ann Surg Oncol. 2018;25(11):3288–3299. doi: 10.1245/s10434-018-6651-y [DOI] [PubMed] [Google Scholar]
- 14.Abe S, Kawai K, Nozawa H, et al. LMR predicts outcome in patients after preoperative chemoradiotherapy for stage II-III rectal cancer. J Surg Res. 2018;222:122–131. doi: 10.1016/j.jss.2017.09.053 [DOI] [PubMed] [Google Scholar]
- 15.Ying H-Q, Deng Q-W, He B-S, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31(12):305. doi: 10.1007/s12032-014-0305-0 [DOI] [PubMed] [Google Scholar]
- 16.Sun F, Peng H-X, Gao Q-F, et al. Preoperative circulating FPR and CCF score are promising biomarkers for predicting clinical outcome of stage II–III colorectal cancer patients. Cancer Manag Res. 2018;10:2151–2161. doi: 10.2147/CMAR.S167398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watt DG, Proctor MJ, Park JH, Horgan PG, McMillan DC, Grivennikov S. The Neutrophil-Platelet Score (NPS) Predicts Survival in Primary Operable Colorectal Cancer and a Variety of Common Cancers. PLoS One. 2015;10(11):e0142159. doi: 10.1371/journal.pone.0142159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibutani M, Maeda K, Nagahara H, et al. The prognostic significance of the postoperative prognostic nutritional index in patients with colorectal cancer. BMC Cancer. 2015;15:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Climent M, Ryan ÉJ, Stakelum Á, et al. Systemic inflammatory response predicts oncological outcomes in patients undergoing elective surgery for mismatch repair-deficient colorectal cancer. Int J Colorectal Dis. 2019;34(6):1069–1078. doi: 10.1007/s00384-019-03274-6 [DOI] [PubMed] [Google Scholar]
- 20.Dolan RD, McSorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119(1):40–51. doi: 10.1038/s41416-018-0095-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan JCY, Chan DL, Diakos CI, et al. The Lymphocyte-to-Monocyte Ratio is a Superior Predictor of Overall Survival in Comparison to Established Biomarkers of Resectable Colorectal Cancer. Ann Surg. 2017;265(3):539–546. doi: 10.1097/SLA.0000000000001743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, An B, Zhao Q, et al. Combined fibrinogen and neutrophil–lymphocyte ratio as a predictive factor in resectable colorectal adenocarcinoma. Cancer Manag Res. 2018;10:6285–6294. doi: 10.2147/CMAR.S161094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yatabe S, Eto K, Haruki K, et al. Signification of Systemic Immune-Inflammation Index for prediction of prognosis after resecting in patients with colorectal cancer. Int J Colorectal Dis. 2020;35(8):1549–1555. doi: 10.1007/s00384-020-03615-w [DOI] [PubMed] [Google Scholar]
- 24.Suzuki Y, Okabayashi K, Hasegawa H, et al. Comparison of Preoperative Inflammation-based Prognostic Scores in Patients With Colorectal Cancer. Ann Surg. 2018;267(3):527–531. doi: 10.1097/SLA.0000000000002115 [DOI] [PubMed] [Google Scholar]
- 25.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Li S-Q, Liao Z-H, et al. Prognostic value of a novel FPR biomarker in patients with surgical stage II and III gastric cancer. Oncotarget. 2017;8(43):75195–75205. doi: 10.18632/oncotarget.20661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Chen Q-G, Li S-Q, et al. Preoperative fibrinogen to prealbumin ratio as a novel predictor for clinical outcome of hepatocellular carcinoma. Future Oncol. 2019;15(1):13–22. doi: 10.2217/fon-2018-0376 [DOI] [PubMed] [Google Scholar]
- 28.Kwaan HC, Lindholm PF. Fibrin and Fibrinolysis in Cancer. Semin Thromb Hemost. 2019;45(4):413–422. doi: 10.1055/s-0039-1688495 [DOI] [PubMed] [Google Scholar]
- 29.Fanali G, Di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33(3):209–290. doi: 10.1016/j.mam.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 30.Yap SH, Moshage HJ, Hazenberg BP, et al. Tumor necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6 stimulated synthesis of C-reactive protein (CRP) and serum amyloid A (SAA) in primary cultures of human hepatocytes. Biochim Biophys Acta. 1991;1091(3):405–408. doi: 10.1016/0167-4889(91)90207-E [DOI] [PubMed] [Google Scholar]
- 31.Scheede-Bergdahl C, Watt HL, Trutschnigg B, et al. Is IL-6 the best pro-inflammatory biomarker of clinical outcomes of cancer cachexia? Clin Nutr. 2012;31(1):85–88. doi: 10.1016/j.clnu.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 32.Aaldriks AA, van der Geest LGM, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol. 2013;4(3):218–226. doi: 10.1016/j.jgo.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 33.Hu W-H, Eisenstein S, Parry L, Ramamoorthy S. Preoperative malnutrition with mild hypoalbuminemia associated with postoperative mortality and morbidity of colorectal cancer: a propensity score matching study. Nutr J. 2019;18(1):33. doi: 10.1186/s12937-019-0458-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umezawa S, Higurashi T, Komiya Y, et al. Chemoprevention of colorectal cancer: past, present, and future. Cancer Science. 2019;110(10):3018–3026. doi: 10.1111/cas.14149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16(3):173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]