Abstract
Background
SII and SIRI are two novel systemic inflammation indexes that were suggested in predicting poor outcomes in cancers. However, no studies have examined their effect on cardiovascular diseases (CVDs) and all-cause mortality. Thus, this study aims to investigate associations between SII, SIRI, and the risks for CVDs and all-cause mortality.
Methods
A total of 85,154 participants from the Kailuan cohort were included and followed up for incidents of CVDs (including MI, stroke) and all-cause death for 10 years. Multiple Cox regression was used to calculate the adjusted hazard ratios (HRs).
Results
During the follow-up period, 4262 stroke events, 1233 MI events, and 7225 all-cause deaths were identified, respectively. Compared with the lowest quantile (Q1) of SII or SIRI, after adjusted for most cardiovascular risk factors, both indexes showed positive associations with the risk for stroke (adjusted HRs in Q4 were 1.264 (95% CI: 1.157,1.382) for SII, 1.194 (95% CI: 1.087,1.313) for SIRI), and all-cause death (adjusted HRs in Q4 were 1.246 (95% CI: 1.165,1.331) for SII, 1.393 (95% CI: 1.296,1.498) for SIRI). Additionally, higher SII and SIRI are also associated with increased risk of hemorrhagic stroke and ischemic stroke. Higher SIRI but not SII exhibited a higher MI risk, the adjusted HR in Q4 was 1.204 (1.013,1.431). The significant association remained after additional adjustment for CRP. Subgroup analysis and sensitivity analysis displayed consistent results except for SIRI with MI, where the association did not arrive at significance in subjects aged ≥60.
Conclusion
Elevated SII and SIRI increased the risk of stroke, two stroke subtypes, and all-cause death. Higher SIRI, but not SII associated with increased MI incidence, and the association of SIRI was only significant in subjects aged <60.
Keywords: systemic inflammation, prospective study, risk factors, cardiovascular events, mortality
Introduction
Cardiovascular diseases (CVDs) are the most common non-communicable diseases globally responsible for an estimated 17.7 million deaths in 2017.1 Much evidence has suggested that chronic low-grade inflammation plays a critical role in the pathogenesis of atherosclerosis and CVDs.2,3 Vascular endothelial injury, oxidative stress, thrombosis may be potentially underlying the mechanisms.4,5 Several prospective studies also demonstrated that WBC and its elements, including neutrophil, monocyte, lymphocyte, as inexpensive and easily accessible system inflammatory biomarkers, were associated with an increased risk of coronary heart diseases,6–8 stroke,9,10 and all-cause mortality.11–13 Additionally, by integrating two different complementary immune pathways, neutrophil to lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were found to have a greater predictive value in the prognosis of CVDs and its-related mortality.14–18 Previous work also reported that higher CRP was an independent predictor for the risk of CVDs.19,20
Recently, two novel inflammatory markers, systemic immune inflammation index (SII) and system inflammation response index (SIRI), composed of platelet and three subtypes of WBC, have been proposed to be associated with poor outcomes of cancers, and display a better predictive ability than NLR and PLR.21–24 However, their associations with CVDs and all-cause death have not been elucidated yet, thus this study aims to determine the associations between SII, SIRI with CVDs, and all-cause mortality.
Methods
Study Population
The participants of this study were recruited from the Kailuan cohort study, which is a large, prospective, population-based study. The details of this cohort were previously reported elsewhere.25 In brief, from June 2006 to October 2007, a total of 101,510 participants (81,110 men and 20,400 women) aged 18–98 years were recruited. All participants were allowed to complete a health examination including biennial questionnaire, physical examination, and biochemical tests. In this present study, participants were excluded if they had at least one of the following: i) with acute inflammation response or too low WBC count, defined as the WBC counts >10*109/L, or CRP >10.00 mg/L, or WBC counts <4*109/L; ii) without information of questionnaire, physical examination, biochemical tests; iii) with baseline CVDs including stroke, myocardial infarction (MI), or cancer; iv) with baseline chronic liver diseases or kidney diseases; v) with inflammation-related diseases including pneumonia, bronchitis, rhinitis, enterogastritis, cholecystitis, allergic dermatitis, phlebophlogosis, pleuritis, parotitis, angiitis, urocystitis, pancreatitis, pharyngitis, or periodontitis. Finally, a total of 85,154 subjects were included in the final analysis (Figure 1).
Figure 1.
Flow chart of the participants.
The study was approved jointly by the Ethics Committee of Kailuan General hospital and School of Medicine, Zhejiang University. All the participants gave written informed consent. The study was conducted according to the provisions of the Declaration of Helsinki.
Baseline Data Collection
The baseline data were obtained from a standardized questionnaire, variables including age, gender, marriage status (married, divorced, others), education level (primary school, above primary school), physical activity (active, inactive), income level (<1000¥/month, >1000¥/month), smoking status (never, former/quit, occasionally, often smoking), drinking status (never, former, current drinker), a family history of MI and stroke, and a history of type 2 diabetes and hypertension were collected by trained doctors and nurses, a history of stroke or MI was determined by self-reported physician diagnosis. Type 2 diabetes was defined as a fasting blood glucose level ≥7.0 mmol/L, taking oral hypoglycemic agents or insulin, or self-reported physician diagnosis. Hypertension was defined as self-reported use of antihypertensive medication, having a history of hypertension, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg. Height, weight, and blood pressure were measured according to standard procedures. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m2). With a mercury sphygmomanometer, blood pressure was measured twice on the left arm after at least 5 min rest, if the difference between the two measurements was more than 5 mmHg, then the third measurement was taken, the average of the measurements was used for the final analysis.
Fasting blood samples were collected in the morning, biochemical parameters, including fasting blood glucose, total triglyceride, total cholesterol, and high-density lipoprotein cholesterol levels were measured with an auto-analyzer (Hitachi 747; Hitachi, Tokyo, Japan) at the central laboratory of Kailuan General Hospital. Fasting blood glucose (FBG) was determined using the hexokinase/glucose-6-phosphate dehydrogenase method. Triglycerides (TGs), total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured using the direct test method (Mind Bioengineering Co. Ltd., Shanghai, China). Routine blood tests including platelet, neutrophil, lymphocyte, and monocyte counts were determined by a full blood count analyzer (Sysmex XT-1800i, Sysmex Corporation). High‐sensitivity C‐reactive protein (CRP) was measured using a high-sensitivity, particle-enhanced immunonephelometric assay (Cias Latex CRP‐H, Kanto Chemical, Tokyo, Japan).
Definition of SII and SIRI
SII is defined as (P x N)/L, where P, N, and L represent the peripheral platelet, neutrophil, and lymphocyte counts, respectively. SIRI is defined as (N x M)/L, where N, M, and L represent, respectively, the counts of peripheral neutrophil, monocyte, and lymphocyte.
Outcomes Ascertainment
The participants were followed up from the baseline examination to December 31, 2017, or the first occurrence of the outcomes including nonfatal and fatal MI, stroke, or all-cause death. The outcomes information was collected from medical records and discharge register from 11 Kailuan hospitals and the Municipal Social Insurance Institution. Death was verified by death certificates from local vital statistics offices. The diagnostic criteria for MI was based on combinations of chest pain symptoms, electrocardiogram changes, and cardiac enzyme levels26. Stroke was diagnosed according to the World Health Organization criteria,27 which requires symptoms of neurological deficits, brain computed tomography, or magnetic resonance imaging. Strokes were further classified into two types in this current study, the ischemic stroke (cerebral infarction) and hemorrhage stroke (including intracerebral hemorrhage and subarachnoid hemorrhage).
All the outcomes were validated by the Data Safety Monitoring Board and the Arbitration Committee for Clinical Outcomes.
Statistical Analysis
The population was, respectively, divided into four groups according to their baseline SII, SIRI levels. Continuous variables were described as mean ± standard deviation, or median (25th percentile, 75th percentile) for skew distribution, and were compared among groups with one‐way ANOVA test or Kruskal–Wallis test. Categorical variables were described as percentage and were compared with the Chi‐Square test.
Cox proportional hazards regression model was used to estimate the associations of three system inflammatory markers with the incidence of stroke, MI, and all-cause death, the reference group was the first quantile. The proportional-hazards assumption was checked with the likelihood ratio test. The hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated in each group. The Cochran–Armitage test was applied to examine the linear trends between levels of system inflammation and the risk for CVDs and death. Three Cox models were applied: Model 1 was unadjusted; Model 2 was adjusted for age, gender, BMI, smoking, drinking, education, marriage, income, physical activity, family history of cardiovascular disease; Model 3 was adjusted the variables in model2 plus CRP. The subgroup analysis by gender or age was conducted. To confirm the robustness of our findings, several sensitivity analyses were performed by i) excluding the events that occurred within the first 2 years of follow-up; ii) excluding current or former smokers and drinkers. The model2 was applied in the subgroup analysis and sensitivity analysis.
The statistical analysis was performed with IBM SPSS 21.0 software. A two-tailed P<0.05 was regarded as statistically significant.
Results
Characteristics of the Study Population
A total of 85,154 participants were included in this study. The mean age of the subjects was 51.3±12.5 years old and 79.66% were male, the mean BMI was 23.97. During the ten-year follow-up, we found 4262 stroke incident patients (including 849 patients of hemorrhagic stroke and 3546 cases with ischemic stroke), 1233 MI events, and 7225 for deaths. The incidence rates were 49.30/10,000 (person-year) for stroke, 15.56/10,000 for MI, 84.66/10,000 all-cause death, respectively. The baseline characteristics of the participants are summarized in Table 1, the medians (25th percentiles, 75th percentiles) of SII and SIRI were 332.32 (137.56,527.08), 0.6 (0.13,1.07). The baseline characteristics by quantiles of SII and SIRI were correspondingly described in Online Tables 1 and 2.
Table 1.
Baseline Characteristics of Subjects (n=85,154)
| Characteristics | Overall |
|---|---|
| Age (years) | 48.6±11.7 |
| Men (%) | 79.15 |
| BMI (kg/m2) | 23.97±3.21 |
| Married (%) | 95.11 |
| Primary education (%) | 7.56 |
| Income>¥1000/Month (%) | 5.89 |
| Smoker (%) | |
| Never | 64.51 |
| Former/quit | 5.25 |
| Occasionally | 3.16 |
| Often | 27.08 |
| Current drinking (%) | 17.91 |
| Antihypertensive drugs (%) | 16.40 |
| Hypoglycemic drugs (%) | 4.53 |
| Lipid-lowering drugs (%) | 1.29 |
| Active physical (%) | 12.31 |
| Family history of CVD (%) | 5.27 |
| TG (mmol/L) | 1.27 (0.90, 1.93) |
| TC (mmol/L) | 4.82±1.15 |
| LDL-C (mmol/L) | 2.34±0.76 |
| HDL-C (mmol/L) | 1.58±0.40 |
| SBP (mmHg) | 127.16±19.73 |
| DBP (mmHg) | 82.08±11.48 |
| FPG (mmol/L) | 5.29±1.36 |
| Platelet counts (10^9/L) | 203.84±62.68 |
| Neutrophil counts (10^10/L) | 3.50±1.22 |
| Lymphocyte counts (10^11 L) | 2.25±0.70 |
| Monocyte counts (10^12/L) | 0.40±0.45 |
| CRP (mg/L) | 0.74 (0.30, 1.87) |
| SII | 333.32 (137.56, 527.08) |
| SIRI | 0.60 (0.13, 1.07) |
Notes: Data was presented as mean ± SD, median (25th, 75th percentiles), or percentage.
Abbreviations: BMI, body mass index; CRP, C-reactive protein; CVDs, cardiovascular diseases; DBP, diastolic blood pressure; FPG, fast plasma glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; SBP, systolic blood pressure; SII, systematic inflammation index, SIRI, systematic inflammation response index.
The Associations of SII and SIRI with the Risk of CVDs and All-Cause Death
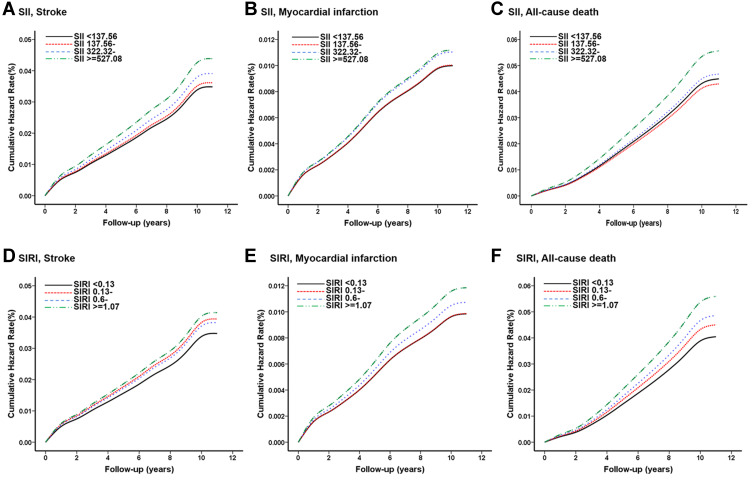
Compared with the reference group, after adjusted for the cardiovascular risk factors, with increasing quartiles of SII, significant dose–response relationships with the risk for stroke, stroke subtypes, and all-causes death were observed, with all p for trend <0.001 (Table 2, Figure 2A, 2C). Significantly higher risk of stroke was found in the Q3 and Q4 groups of SII with respective HRs were 1.124 (1.026, 1.231), 1.264 (1.157, 1.382), marginal significance was found in the Q2 group. For all-cause death, only the Q4 group displayed significant higher risk with HR 1.246 (1.165, 1.331). No significant difference was found in the quantiles of SII with MI risk (Table 2, Figure 2B). In addition, the associations persisted after additionally adjusted for CRP levels (Table 2).
Table 2.
Hazard Ratios of Stroke, Subtypes of Stroke, MI, All-Cause Mortality According to Quantiles of SII
| Q1 (n=20,054) | Q2 (n=20,151) | Q3 (n=20,148) | Q4 (n=20,045) | p for Trend | |
|---|---|---|---|---|---|
| Levels of SII | <137.56 | 137.56- | 333.32- | ≥527.08 | |
| The number of incident stroke events (n) | 963 | 992 | 1048 | 1146 | |
| Incidence density | 46.25 | 47.44 | 50.20 | 55.37 | |
| Model1 | 1 (reference) | 1.025 (0.938, 1.120) | 1.085 (0.994, 1.184) | 1.196 (1.098, 1.303) | <0.001 |
| Model2 | 1 (reference) | 1.038 (0.947, 1.138) | 1.124 (1.026, 1.231) | 1.264 (1.157, 1.382) | <0.001 |
| Model3 | 1 (reference) | 1.038(0.947,1.138) | 1.129(1.03,1.236) | 1.274(1.166,1.393) | <0.001 |
| The number of incident Hemorrhage stroke events (n) | 183 | 201 | 202 | 243 | |
| Incidence density | 8.79 | 9.61 | 9.68 | 11.74 | |
| Model1 | 1 (reference) | 1.093 (0.895, 1.336) | 1.100 (0.900, 1.344) | 1.333 (1.100, 1.615) | 0.004 |
| Model2 | 1 (reference) | 1.136 (0.922, 1.399) | 1.167 (0.947, 1.437) | 1.383 (1.132, 1.690) | 0.002 |
| Model3 | 1 (reference) | 1.146 (0.931, 1.411) | 1.180 (0.958, 1.454) | 1.382 (1.130, 1.691) | 0.002 |
| The number of incident Ischemic stroke events (n) | 808 | 823 | 880 | 937 | |
| Incidence density | 38.81 | 39.36 | 42.15 | 45.28 | |
| Model1 | 1 (reference) | 1.014 (0.920, 1.117) | 1.086 (0.987, 1.195) | 1.166 (1.061, 1.281) | <0.001 |
| Model2 | 1 (reference) | 1.020 (0.922, 1.129) | 1.122 (1.016, 1.240) | 1.239 (1.125, 1.366) | <0.001 |
| Model3 | 1 (reference) | 1.021 (0.923, 1.130) | 1.121 (1.015, 1.239) | 1.254 (1.138, 1.383) | <0.001 |
| The number of incident MI events (n) | 297 | 297 | 317 | 322 | |
| Incidence density | 14.05 | 13.98 | 14.94 | 15.26 | |
| Model1 | 1 (reference) | 0.995 (0.847, 1.169) | 1.063 (0.907, 1.245) | 1.085 (0.927, 1.271) | 0.218 |
| Model2 | 1 (reference) | 1.004 (0.848, 1.188) | 1.105 (0.937, 1.304) | 1.120 (0.950, 1.321) | 0.100 |
| Model3 | 1 (reference) | 1.023 (0.865, 1.209) | 1.133 (0.960, 1.337) | 1.190 (1.009, 1.404) | 0.020 |
| The number of incident All-cause mortality events (n) | 1761 | 1600 | 1687 | 2027 | |
| Incidence density | 85.77 | 77.23 | 81.66 | 99.73 | |
| Model1 | 1 (reference) | 0.900 (0.841, 0.963) | 0.951 (0.890, 1.017) | 1.163 (1.091, 1.240) | <0.001 |
| Model2 | 1 (reference) | 0.955 (0.890, 1.025) | 1.042 (0.972, 1.117) | 1.246 (1.165, 1.331) | <0.001 |
| Model3 | 1 (reference) | 0.952 (0.887, 1.022) | 1.038 (0.968, 1.113) | 1.237 (1.157, 1.323) | <0.001 |
Notes: Data was presented as HR (95% CI). The incidence rate was presented as per 10,000 person years. The multiple Cox analysis was conducted by quantiles of SII. Model 1: unadjusted. Model 2: adjusted for age, gender, BMI, smoking, drinking, education, marriage, income level, physical activity, family history of cardiovascular disease, triglycerides, high-density lipoproteins, type 2 diabetes and hypertension. Model 3: model 2 plus C-reactive protein.
Abbreviations: CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; SII, systemic immune-inflammation index.
Figure 2.
(A–C) The Kaplan–Meier curves for 10-year incident of stroke (A), myocardial infarction (B), and all-cause mortality (C) by quartiles of SII. (D–F) The Kaplan–Meier curves for 10-year incident of stroke (D), myocardial infarction (E), and all-cause mortality (F) by quartiles of SIRI. The cumulative hazard risk was calculated according to the multiple Cox regression adjusted for age, gender, BMI, smoking, drinking, education, marriage, income level, physical activity, family history of cardiovascular disease, TG, HDL-C, a history of hypertension and type 2 diabetes.
Abbreviations: SII, systemic immune-inflammation index; SIRI, systemic inflammation response index.
For SIRI, after controlled for the cardiovascular risk factors, increasing quartile of SIRI showed elevated risks of overall stroke, stroke subtypes, all-cause death, as well as MI in a dose-dependent manner (Table 3, Figure 2D–F). The Q2 and Q4 group of SIRI displayed increased risk for stroke with HRs 1.135 (1.030, 1.251) and 1.194 (1.087, 1.313), only the Q4 group of SIRI had a higher risk of MI with HRs 1.204 (1.013, 1.431) (Table 3). All three groups of SIRI exhibited a higher risk for all-cause death than the reference group, the adjusted HRs were 1.115 (1.033, 1.204), 1.206 (1.118, 1.300), and 1.393 (1.296, 1.498). Also, the associations of SIRI were independent of the CRP levels (Table 3).
Table 3.
Hazard Ratios of Stroke, Subtypes of Stroke, MI, All-Cause Mortality According to Quantiles of SIRI
| Q1 (n=17,833) | Q2 (n=20,896) | Q3 (n=20,870) | Q4 (n=20,382) | p for Trend | |
|---|---|---|---|---|---|
| Levels of SIRI | <0.13 | 0.13- | 0.60- | ≥1.07 | |
| The number of incident stroke events (n) | 756 | 1046 | 1098 | 1235 | |
| Incidence density | 40.78 | 48.26 | 50.75 | 58.74 | |
| Model1 | 1 (reference) | 1.184 (1.078, 1.300) | 1.245 (1.135, 1.366) | 1.441 (1.316, 1.578) | <0.001 |
| Model2 | 1 (reference) | 1.135 (1.030, 1.251) | 1.101 (0.999, 1.212) | 1.194 (1.087, 1.313) | 0.001 |
| Model3 | 1 (reference) | 1.124 (1.012, 1.248) | 1.061 (0.957, 1.176) | 1.152 (1.040, 1.276) | 0.028 |
| The number of incident Hemorrhage stroke events (n) | 137 | 197 | 225 | 265 | |
| Incidence density | 7.39 | 9.09 | 10.40 | 12.60 | |
| Model1 | 1 (reference) | 1.229 (0.989, 1.529) | 1.407 (1.138, 1.740) | 1.703 (1.385, 2.093) | <0.001 |
| Model2 | 1 (reference) | 1.198 (0.956, 1.503) | 1.267 (1.014, 1.583) | 1.498 (1.207, 1.859) | <0.001 |
| Model3 | 1 (reference) | 1.212(0.968,1.517) | 1.274(1.025,1.583) | 1.487(1.201,1.84) | <0.001 |
| The number of incident Ischemic stroke events (n) | 640 | 871 | 914 | 1012 | |
| Incidence density | 34.52 | 40.19 | 42.25 | 48.13 | |
| Model1 | 1 (reference) | 1.165 (1.052, 1.290) | 1.225 (1.107, 1.355) | 1.396 (1.264, 1.541) | <0.001 |
| Model2 | 1 (reference) | 1.109 (0.998, 1.233) | 1.071 (0.964, 1.190) | 1.138 (1.026, 1.262) | 0.042 |
| Model3 | 1 (reference) | 1.145 (1.040, 1.261) | 1.091 (0.993, 1.199) | 1.201 (1.094, 1.318) | 0.001 |
| The number of incident MI events (n) | 234 | 289 | 327 | 379 | |
| Incidence density | 12.46 | 13.11 | 14.87 | 17.68 | |
| Model1 | 1 (reference) | 1.053 (0.886, 1.251) | 1.195 (1.010, 1.413) | 1.420 (1.206, 1.671) | <0.001 |
| Model2 | 1 (reference) | 1.002 (0.835, 1.202) | 1.090 (0.913, 1.301) | 1.204 (1.013, 1.431) | 0.014 |
| Model3 | 1 (reference) | 1.116 (0.930, 1.339) | 1.226 (1.031, 1.458) | 1.366 (1.150, 1.621) | <0.001 |
| The number of incident All-cause mortality events (n) | 1260 | 1655 | 1880 | 2246 | |
| Incidence density | 68.64 | 77.00 | 88.05 | 109.00 | |
| Model1 | 1 (reference) | 1.122 (1.043, 1.208) | 1.284 (1.196, 1.379) | 1.593 (1.486, 1.706) | <0.001 |
| Model2 | 1 (reference) | 1.115 (1.033, 1.204) | 1.206 (1.118, 1.300) | 1.393 (1.296, 1.498) | <0.001 |
| Model3 | 1 (reference) | 1.126 (1.043, 1.215) | 1.187 (1.103, 1.278) | 1.372 (1.277, 1.474) | <0.001 |
Notes: Data was presented as HR (95% CI). The incidence rate was presented as per 10,000 person years. The multiple Cox analysis was conducted by quantiles of SIRI. Model 1: unadjusted. Model 2: adjusted for age, gender, BMI, smoking, drinking, education, marriage, income level, physical activity, family history of cardiovascular disease, triglycerides, high-density lipoproteins, type 2 diabetes and hypertension. Model 3: model 2 plus C-reactive protein.
Abbreviations: CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; SIRI, systematic inflammation response index.
Subgroup Analysis
Subgroup analysis by sex described similar associations except for the marginal associations between SIRI and MI incident in both genders (Online Table 3). Subgroup analysis by age also documented consistent associations of SII; however, for SIRI, its correlation with MI incident became nonsignificant in subjects aged >60 (Online Table 4). Sensitivity analysis showed that after excluding the events occurred at the first two years of the follow-up, the association of SII with the outcomes remained unchanged, but in the analysis of SIRI, its correlation with MI was close to the significance, the p-value for trend was 0.075 (Online Table 5). Among subjects without smoking and drinking, the results were consistent with the overall population (Online Table 6).
Discussions
In this large-scale general population study, we investigated the associations of two novel systemic inflammatory indexes of SII, SIRI with the risk of CVDs and all-cause death. We found both indexes associated with the risk for overall stroke and all-cause death. Moreover, these associations were independent of the CRP levels. Elevated SIRI, but not SII displayed an independent association with the risk for MI, especially in subjects aged <60. Also, after excluding events occurred at the first two years of the follow-up, a marginal association between SIRI and the risk of MI was observed, future studies need to replicate this finding.
SII and SIRI were firstly proposed to be predictors of adverse prognosis in cancer, which can reflect the system inflammatory response of body. Although there was no study examined the associations of SII and SIRI with CVDs, its different components, including neutrophil, monocyte, lymphocyte, platelet have been broadly discussed in stroke, MI, and all-cause death.11,28–30 Previous studies have suggested that neutrophils played a critical role in the inflammation response of atherosclerosis, which could secret amounts of inflammation mediators, chemotactic agents, and oxygen-free radicals to induce endothelial cell injury and subsequent tissue ischemia.4,31–33 The activation of monocytes and their transformation into lipid-laden macrophages are essential processes of the atherosclerotic lesion formation.34 Conversely, lymphocytes have a regulatory function in inflammation and may exert an inhibitory effect on atherosclerosis.35 Therefore, SIRI, calculated as (N*M/L), was biologically plausible to associate with CVDs and all-causes. Some perspective and observational studies also supported these findings, which showed higher monocyte, neutrophils, and low lymphocytes associated with higher CVDs risk.6,8,34,36–39
SII, different from the monocyte of SIRI, was calculated as (N*P/L). Platelets also have a central role in thrombosis, which have been implicated in CVDs prognosis,5,40 its interaction with monocytes, and neutrophils as well reflect the process of vascular endothelial injury.4,30,41 Hence, SII also seemed to correlate with a higher risk of stroke, MI, and all-cause death. However, only increased stroke and all-causes death risk, but not MI, was observed in subjects with higher SII, which may partly attribute to the effect of the platelet. A recent Mendelian randomization study also demonstrated that higher platelet counts associated with increased stroke risk but not MI,42 another population-based study also supported this finding.43 A possible reason was that the thrombus formation caused by platelet aggregation in the two diseases was different, for the diameter of cerebral arteries was generally smaller than coronary arteries.
Another notable finding of this study was that both indexes showed positive associations with the risk of stroke subtypes. Most population-based studies found that higher WBC or higher neutrophils increased the risk of ischemic stroke risk. However, inconclusive associations were found in hemorrhagic stroke.9,10,31,44 In concordant with this study, the CKB study showed that higher systemic inflammation measured by CRP was positively associated with the risk of hemorrhagic stroke,44 while other prospective studies did not find the association between WBC and the incident of hemorrhagic stroke. A possible explanation was that the sample size in previous studies was insufficient to examine the relationships with the risk for hemorrhagic stroke owning to its relatively low incidence. Given both the subgroup analysis and sensitivity analysis did not alter the results, we speculated that compared with the single WBC subtype, the SII and SIRI may better reflect the inflammation response in hemorrhagic stroke, much efforts need to be conducted to explain the results.
Subgroup analysis by age showed that in subjects aged ≥60, the association between SIRI and MI did not arrive at the significance. Consistent findings have been observed in previous studies of CRP.45 The relatively smaller sample size in subjects aged ≥60 may be a likely reason. In subjects aged <60, a positive correlation was observed between SIRI and the risk of MI, which indicated that earlier exposure or the younger exposure to high levels of chronic inflammation had a high risk of long-term outcome. The same trend was also found in metabolic syndrome.
In addition, our results also showed that both indexes had CRP-independent effects on the outcomes, indicating that SII and SIRI may reflect different biological aspects of inflammation response from CRP, and the two novel indexes may provide additional information in the risk assessment of CVDs.
Strengths and Limitations
A major strength of this study is the large prospective population-based design, which included a broad age range and a long follow-up period, as well as a fair number of CVDs cases. Besides, we also analyzed the associations of different stroke subtypes with the indexes. And to the best of our knowledge, this is the first study to report the associations between two system inflammatory indexes and CVDs and all-cause mortality.
There are several limitations in our study. Firstly, the blood cell-based tests were conducted only once, during the follow-up, the concentrations of these blood cell may change; secondly, a single measurement of the blood cell counts could be affected by other factors, such as special medication, which may result in residual confounding, cautions need to be given in interpreting the results; thirdly, in the prospective study, an inherent problem is the change of exposure factors over time, like the smoking status and physical activity, which could influence the observed associations; finally, since the Kailuan cohort was from an occupational population, most of them (almost 80%) was males, which may affect the generalization of the results.
Conclusion
Elevated SII and SIRI increased the risk of stroke, stroke subtypes, and all-cause death. SIRI, but not SII positively associated with the MI incidence, and the association was only significant in subjects aged <60.
Acknowledgments
We also would like to thank all the participants and investigators that took part in this study.
Funding Statement
This work was supported by the grants from National Key Research and Development Program of China (2017YFC0907004) and Hangzhou Science and Technology Project (20171226Y27). The funders have no role in the design of the study, collection, analysis, and interpretation of data.
Abbreviations
SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; CRP, C-reactive protein; MI, myocardial infarction; CVDs, cardiovascular diseases; WBC, white blood cell.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. doi: 10.1016/S0140-6736(19)32008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaptoge S, Di Angelantonio E, Pennells L, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367(14):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan RC, Frishman WH. Systemic inflammation as a cardiovascular disease risk factor and as a potential target for drug therapy. Heart Disease. 2001;3(5):326–332. doi: 10.1097/00132580-200109000-00009 [DOI] [PubMed] [Google Scholar]
- 4.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44(10):1945–1956. doi: 10.1016/j.jacc.2004.07.056 [DOI] [PubMed] [Google Scholar]
- 5.Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: in-depth review and update. Texas Heart Institute j. 2013;40(1):17–29. [PMC free article] [PubMed] [Google Scholar]
- 6.Wheeler JG, Mussolino ME, Gillum RF, Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30,374 individuals. Eur Heart J. 2004;25(15):1287–1292. doi: 10.1016/j.ehj.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 7.Mary C, Alice MA, Bruce MP, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women the cardiovascular health study. Circulation. 2005;112(1):25–31. doi: 10.1161/circulationaha.104.504159. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Lim S, Park KS, Jang HC, Choi SH. Total and differential WBC counts are related with coronary artery atherosclerosis and increase the risk for cardiovascular disease in Koreans. PLoS One. 2017;12(7):e0180332. doi: 10.1371/journal.pone.0180332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zia E, Melander O, Björkbacka H, Hedblad B, Engström G. Total and differential leucocyte counts in relation to incidence of stroke subtypes and mortality: a prospective cohort study. J Intern Med. 2012;272(3):298–304. doi: 10.1111/j.1365-2796.2012.02526.x [DOI] [PubMed] [Google Scholar]
- 10.Wu TH, Chien KL, Lin HJ, et al. Total white blood cell count or neutrophil count predict ischemic stroke events among adult Taiwanese: report from a community-based cohort study. BMC Neurol. 2013;13:7. doi: 10.1186/1471-2377-13-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abete I, Lu Y, Lassale C, Verschuren M, van der Schouw Y, Bueno-de-Mesquita B. White cell counts in relation to mortality in a general population of cohort study in the Netherlands: a mediating effect or not? BMJ Open. 2019;9(10):e030949. doi: 10.1136/bmjopen-2019-030949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Jiang CQ, Xu L, et al. White blood cell count and all-cause and cause-specific mortality in the Guangzhou biobank cohort study. BMC Public Health. 2018;18(1):1232. doi: 10.1186/s12889-018-6073-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinholt PJ, Hvas AM, Frederiksen H, Bathum L, Jørgensen MK, Nybo M. Platelet count is associated with cardiovascular disease, cancer and mortality: A population-based cohort study. Thromb Res. 2016;148:136–142. doi: 10.1016/j.thromres.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 14.Tokgoz S, Kayrak M, Akpinar Z, Seyithanoglu A, Guney F, Yuruten B. Neutrophil lymphocyte ratio as a predictor of stroke. J Stroke Cerebrovasc Dis. 2013;22(7):1169–1174. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 15.Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11(1):55–59. doi: 10.1586/erc.12.159 [DOI] [PubMed] [Google Scholar]
- 16.Kaya H, Ertas F, Islamoglu Y, et al. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thromb Hemost. 2014;20(1):50–54. doi: 10.1177/1076029612452116 [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Zhang G, Jiang X, Zhu H, Lu Z, Xu L. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: a meta-analysis of observational studies. Atherosclerosis. 2014;234(1):206–213. doi: 10.1016/j.atherosclerosis.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–1643. doi: 10.1016/j.jacc.2005.02.054 [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Han W, Gong D, Man C, Fan Y. Hs-CRP in stroke: A meta-analysis. Clin Chim Acta. 2016;453:21–27. doi: 10.1016/j.cca.2015.11.027 [DOI] [PubMed] [Google Scholar]
- 20.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive services task force. Ann Intern Med. 2009;151(7):483–495. doi: 10.7326/0003-4819-151-7-200910060-00009 [DOI] [PubMed] [Google Scholar]
- 21.Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272. doi: 10.3748/wjg.v23.i34.6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu H, Zheng J, Cai J, et al. Systemic immune-inflammation index (sii) is useful to predict survival outcomes in patients after liver transplantation for hepatocellular carcinoma within hangzhou criteria. Cell Physiol Biochem. 2018;47(1):293–301. doi: 10.1159/000489807 [DOI] [PubMed] [Google Scholar]
- 23.Xie QK, Chen P, Hu WM, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med. 2018;16(1):273. doi: 10.1186/s12967-018-1638-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geng Y, Zhu D, Wu C, et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2018;65:503–510. doi: 10.1016/j.intimp.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 25.Wu S, Huang Z, Yang X, et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. 2012;5(4):487–493. doi: 10.1161/CIRCOUTCOMES.111.963694 [DOI] [PubMed] [Google Scholar]
- 26.Lu L, Liu M, Sun R, Zheng Y. Myocardial Infarction: symptoms and Treatments. Cell Biochem Biophys. 2015;72(3):865–867. doi: 10.1007/s12013-015-0553-4 [DOI] [PubMed] [Google Scholar]
- 27.Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the who task force on stroke and other cerebrovascular disorders. Stroke. 1989;20(10):1407–1431. doi: 10.1161/01.STR.20.10.1407 [DOI] [PubMed] [Google Scholar]
- 28.Lassale C, Curtis A, Abete I, et al. Elements of the complete blood count associated with cardiovascular disease incidence: findings from the EPIC-NL cohort study. Sci Rep. 2018;8(1):3290. doi: 10.1038/s41598-018-21661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welsh C, Welsh P, Mark PB, et al. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK biobank. Arterioscler Thromb Vasc Biol. 2018;38(6):1415–1423. doi: 10.1161/ATVBAHA.118.310945 [DOI] [PubMed] [Google Scholar]
- 30.Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am J Epidemiol. 2001;154(8):758–764. doi: 10.1093/aje/154.8.758 [DOI] [PubMed] [Google Scholar]
- 31.Huh JY, Ross GW, Chen R, et al. Total and differential white blood cell counts in late life predict 8-year incident stroke: the Honolulu Heart Program. J Am Geriatr Soc. 2015;63(3):439–446. doi: 10.1111/jgs.13298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: A Caliber cohort study. J Am Coll Cardiol. 2017;69(9):1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62(17):1541–1551. doi: 10.1016/j.jacc.2013.07.043 [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Lee YJ, Park B. Higher monocyte count with normal white blood cell count is positively associated with 10-year cardiovascular disease risk determined by Framingham risk score among community-dwelling Korean individuals. Medicine. 2019;98(17):e15340. doi: 10.1097/MD.0000000000015340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106(4):470–476. doi: 10.1016/j.amjcard.2010.03.062 [DOI] [PubMed] [Google Scholar]
- 36.Sweetnam PM, Thomas HF, Yarnell JW, Baker IA, Elwood PC. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am J Epidemiol. 1997;145(5):416–421. doi: 10.1093/oxfordjournals.aje.a009123 [DOI] [PubMed] [Google Scholar]
- 37.Rana JS, Boekholdt SM, Ridker PM, et al. Differential leucocyte count and the risk of future coronary artery disease in healthy men and women: the EPIC-Norfolk prospective population study. J Intern Med. 2007;262(6):678–689. doi: 10.1111/j.1365-2796.2007.01864.x [DOI] [PubMed] [Google Scholar]
- 38.Waterhouse DF, Cahill RA, Sheehan F, McCreery C. Prediction of calculated future cardiovascular disease by monocyte count in an asymptomatic population. Vasc Health Risk Manag. 2008;4(1):177–187. doi: 10.2147/VHRM.S2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: a CALIBER cohort study. Open Heart. 2016;3(2):e000477. doi: 10.1136/openhrt-2016-000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurtul A, Ornek E. Platelet to lymphocyte ratio in cardiovascular diseases: a systematic review. Angiology. 2019;70(9):802–818. doi: 10.1177/0003319719845186 [DOI] [PubMed] [Google Scholar]
- 41.Chien KL, Su TC, Hsu HC, et al. Constructing the prediction model for the risk of stroke in a Chinese population: report from a cohort study in Taiwan. Stroke. 2010;41(9):1858–1864. doi: 10.1161/STROKEAHA.110.586222 [DOI] [PubMed] [Google Scholar]
- 42.Gill D, Monori G, Georgakis MK, Tzoulaki I, Laffan M. Genetically determined platelet count and risk of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2018;38(12):2862–2869. doi: 10.1161/ATVBAHA.118.311804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klovaite J, Benn M, Yazdanyar S, Nordestgaard BG. High platelet volume and increased risk of myocardial infarction: 39,531 participants from the general population. J Thrombosis Haemostasis. 2011;9(1):49–56. doi: 10.1111/j.1538-7836.2010.04110.x [DOI] [PubMed] [Google Scholar]
- 44.Karim MA, Kartsonaki C, Bennett DA, et al. Systemic inflammation is associated with incident stroke and heart disease in East Asians. Sci Rep. 2020;10(1):5605. doi: 10.1038/s41598-020-62391-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66(2):265–275. doi: 10.1016/j.cardiores.2004.12.026 [DOI] [PubMed] [Google Scholar]