Abstract
Introduction
Toxic tobacco smoke residue, also known as thirdhand smoke (THS), can persist in indoor environments long after tobacco has been smoked. This study examined the effects of different cleaning methods on nicotine in dust and on surfaces.
Aims and Methods
Participants had strict indoor home smoking bans and were randomly assigned to: dry/damp cleaning followed by wet cleaning 1 month later (N = 10), wet cleaning followed by dry/damp cleaning (N = 10) 1 month later, and dry/damp and wet cleaning applied the same day (N = 28). Nicotine on surfaces and in dust served as markers of THS and were measured before, immediately after, and 3 months after the cleaning, using liquid chromatography with triple quadrupole mass spectrometry (LC–MS/MS).
Results
Over a 4-month period prior to cleaning, surface nicotine levels remained unchanged (GeoMean change: −11% to +8%; repeated measures r = .94; p < .001). Used separately, dry/damp and wet cleaning methods showed limited benefits. When applied in combination, however, we observed significantly reduced nicotine on surfaces and in dust. Compared with baseline, GeoMean surface nicotine was 43% lower immediately after (z = −3.73, p < .001) and 53% lower 3 months later (z = −3.96, p < .001). GeoMean dust nicotine loading declined by 60% immediately after (z = −3.55, p < .001) and then increased 3 months later to precleaning levels (z = −1.18, p = .237).
Conclusions
Cleaning interventions reduced but did not permanently remove nicotine in dust and on surfaces. Cleaning efforts for THS need to address persistent pollutant reservoirs and replenishment of reservoirs from new tobacco smoke intrusion. THS contamination in low-income homes may contribute to health disparities, particularly in children.
Implications
Administered sequentially or simultaneously, the tested cleaning protocols reduced nicotine on surfaces by ~50% immediately after and 3 months after the cleaning. Nicotine dust loading was reduced by ~60% immediately after cleaning, but it then rebounded to precleaning levels 3 months later. Cleaning protocols were unable to completely remove THS, and pollutants in dust were replenished from remaining pollutant reservoirs or new secondhand smoke intrusion. To achieve better outcomes, cleaning protocols should be systematically repeated to remove newly accumulated pollutants. New secondhand smoke intrusions need to be prevented, and remaining THS reservoirs should be identified, cleaned, or removed to prevent pollutants from these reservoirs to accumulate in dust and on surfaces.
Introduction
Toxic tobacco smoke residue can persist for extended periods of time after tobacco has been smoked in homes, used cars, rental cars, hotel rooms, casinos, and in educational and medical settings where tobacco smoke pollutants have been brought in from the outside.1–12 Also known as thirdhand smoke (THS), this residue accumulates in dust and on surfaces and can become embedded in materials where it creates long-term reservoirs of pollutants.13,14 From these reservoirs, volatile compounds can be reemitted and particulate matter can become resuspended, allowing THS pollutants to cause exposure and human health risks through inhalation, dermal transfer, and ingestion long after smoking has occurred.15,16 Young children are especially at risk due to hand-to-mouth behavior, small body size, increased inhalation rate, immature organ and immune systems, and activity near surfaces where pollutants accumulate.17
Secondhand smoke (SHS) is a dynamic mixture of more than 7000 particulate and gas-phase compounds, and this mixture is the precursor of THS.18 The deposition, adsorption, and accumulation of SHS and its transition to THS have been studied in controlled chambers and in real-world field studies, and these processes are a function of smoking behavior (eg, frequency, duration, and type of tobacco products), the properties and interactions of the chemical constituents (eg, volatility, pH, and reactivity), and the environment in which they are emitted (eg, volume, surface material, humidity, and air exchange rate).15,19–24 The dynamic interaction of chemicals with surfaces in indoor environments is particularly significant for semi-volatile organic compounds (SVOCs) and their secondary pollutants, many of which are known biologically active toxicants and federally regulated as hazardous air pollutants and California state-regulated toxic air contaminants.15,24–30
While the pervasiveness and persistence of THS pollutants have been demonstrated across a wide variety of indoor environments, very little is known about how to reduce or remove THS pollution in contaminated environments. Existing research suggests that routine cleaning efforts in private homes and cars,2,31 and in hotels are unable to remove THS pollutants.3 This limitation may be in part because many household cleaners are alkaline (eg, soap and ammonia) causing some compounds to be volatilized (eg, free-base nicotine) and reemitted from their reservoirs and subsequently adsorb to other surfaces.32,33 Moreover, although cleaning may remove some THS from surfaces and dust, SVOCs embedded in materials (eg, carpets, upholstery, and walls) may simply reemit into the air, partition into the particulate phase, deposit back on surfaces, and accumulate in dust.34,35
One strategic consideration for cleaning up THS pollution is to target two of its major reservoirs: dust accumulation and adsorption to surfaces. For THS compounds that have accumulated in settled house dust, removal through vacuuming, washing, and wiping should significantly reduce dust loading and, therefore, human exposure. For SVOCs in THS (eg, nicotine) that have adsorbed to material surfaces (eg, fibers of carpets, drapes, desks, and cabinets), the sequential use of alkaline (ie, ammonia), and acidic (eg, white vinegar) solutions may be more effective than either an alkaline or acidic cleaning solution alone. While the alkaline solution volatilizes nicotine bound on surfaces, the acidic solution turns the free nicotine compound into a stable salt that can then be more easily dissolved and removed.32
This study was designed to examine whether common household cleaning methods have a short-term and long-term impact on THS levels in THS polluted apartments as measured by nicotine levels in dust and on surfaces. Nicotine was used as a marker for THS as it is specific to tobacco smoke, validated and sensitive methods for detection on surfaces and in dust were available and levels are highly correlated with other toxic compounds in THS.13,14,36,37 Since smoking prevalence is higher among lower-income populations and SHS is higher in multiunit housing, this study focused on THS polluted apartments in low-income housing. We were specifically interested in examining the separate and combined impacts of dust removal using vacuuming and wiping surfaces (ie, dry/damp methods) and deep carpet cleaning and washing of materials and surfaces (ie, wet methods).38–40
Methods
Participants
After approval from the San Diego State University Institutional Review Board, participants were recruited into the study from prescreened multiunit housing homes (N = 220) found to have elevated levels of THS pollution operationalized as nicotine surface concentrations ≥3.1 µg/m2. This cut-off is the lower-bound of the 95% confidence interval (CI) of the geometric mean (GeoM) from a previous study of nonsmokers moving into homes previously occupied by smokers (GeoM = 10.0 μg/m2, 95% CI [3.1; 28.6]).2 This cut-off is just above the upper bound of the 95% CI of the GeoM in nonsmokers’ homes who had lived there ≥6 months and allowed no smoking or electronic cigarette use by residents or visitors (GeoM = 1.5 μg/m2, 95% CI [0.4; 3.0]).2 Procedures for recruiting participants into the screening phase of the study were previously described.41 Of the 220 prescreened homes, 79 had nicotine levels above the 3.1 µg/m2 cut-off. Of those, N = 74 had strict indoor home smoking bans and planned to live in their homes for the next 6 months, and N = 38 agreed to participate in the baseline, posttest, follow-up measures, and the cleaning intervention. None of the participants dropped out after enrolling in the study. Participants signed informed consent and received incentives of up to $120.
Research Design and THS Cleaning Intervention
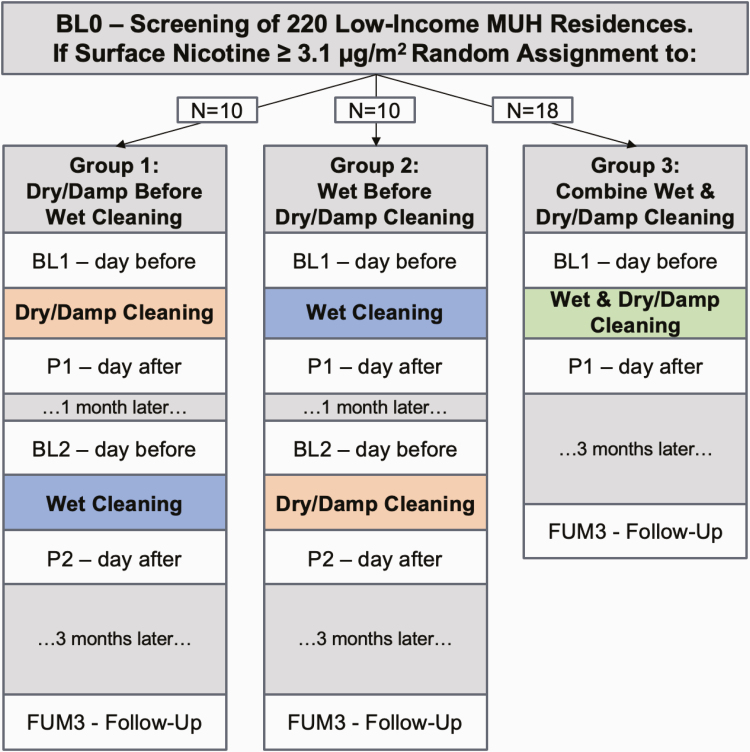
Participants were randomly assigned to one of three THS cleaning protocols. In group 1, N = 10 homes received the “dry/damp cleaning,” followed by the “wet cleaning” one month later. In group 2, N = 10 received the “wet cleaning” first, followed by the “dry/damp cleaning” 1 month later. In group 3, N = 18 homes received all cleaning procedures on the same day. Figure 1 shows an overview of the research design and timing of the screening, baseline, posttest, and follow-up measurements.
Figure 1.
Overview of research design and measurements. MUH: Multiunit Housing; BL0: baseline 0; BL1: baseline 1; P1: posttest 1; BL2: baseline 2; P2; posttest2; FUM3: follow-up month 3
The cleaning was conducted by two different cleaning and restoration companies (D, W) who were given alternating assignments whenever possible. Company D cleaned eight homes in group 1, five in group 2, and nine in group 3. The corresponding assignments for company W were 2, 5, and 9, respectively. Before the cleaning, the crew supervisor and a research assistant met with the study participant at their home to review the planned procedures. The companies were provided with a written protocol and checklist, and an “assurance of work completed” was signed by the study participant, the cleaning crew supervisor, and the company owner after the cleaning was completed. The median cleaning cost per home was $1234 (interquartile range: [$984; $1752]).
Dry-Damp Cleaning
All horizontal and vertical hard surfaces in the home were wiped with alkaline Simple Green All-Purpose Cleaner (1 part Simple Green to 10 parts water), followed by acidic distilled white vinegar (1 part vinegar to 3 parts water). The vinegar solution was left for 1–2 min dwell time on nonporous surfaces, and scrub brushes were used as needed. The cleaned surfaces included floors, walls, counters, ceilings, blinds, windows, and exterior sides of drawers, cabinets, and closets. Interiors of drawers, cabinets, and closets were only cleaned if participants emptied them, and <10% were cleaned. Floors, closets, upholstered furniture, and mattresses were vacuumed with high-efficiency particulate air (HEPA) filters to remove dust.
Wet Cleaning
Carpets, area rugs, and upholstered furniture and mattresses were steam cleaned using standard procedures, including an enzymatic preconditioner and hot water with a pH neutralizer. The study protocol stated that fabric curtains should also be steam cleaned, or taken off site for dry cleaning. Of the 10 homes with curtains, six were steam-cleaned on site, two were dry-cleaned off site, one was vacuumed, and one was deemed too fragile and was not cleaned.
Measures
After the initial screening (BL0) to determine eligibility, there were five additional visits in groups 1 and 2 to collect study measures: 1 day prior to and 1 day after the first part of the cleaning intervention (BL1, P1), 1 day prior to and 1 day after the second part of the cleaning intervention (BL2, P2), and at follow-up 3 months after the final cleaning (FUM3). In group 3, there were three additional visits after the BL0 screening: 1 day prior to and 1 day after the combined cleaning intervention (BL1, P1) and at follow-up 3 months later (FUM3). The average interval between BL0 and Bl1 was 120 days. Home visits took about 60–90 min and were conducted from September, 2016 to April, 2018.
Interviews
Pairs of research assistants visited homes to collect samples and conduct in-person interviews about: tobacco and electronic cigarettes use overall and inside the home during the past 12 months; reported exposure to tobacco and electronic cigarettes away from home and at home during the 7 days prior to each home visit; and knowledge of building smoking restrictions inside apartments, on balconies and porches, and in indoor and outdoor common areas.
Surface Nicotine
Nicotine on surfaces is a specific marker of THS, representing SVOCs in THS on household surfaces. Two wipe samples and a field blank were collected at each home visit for analysis of nicotine using isotope-dilution liquid chromatography/triple quadrupole mass spectrometry (LC-MS/MS). Samples were collected in the room where residents had smelled the strongest tobacco odor, or if no odor was noted, in the living room. The wipe sample preparation and nicotine LC-MS/MS methods were published elsewhere, and additional details are provided in Supplementary Material.4 A wood vertical surface (eg, door panel) and a wood horizontal surface (eg, underneath a table, shelf, or desk) were sampled. In one home there was no wood horizontal surface, so two wood vertical surfaces were sampled. The nicotine levels of the two surface wipes were averaged for statistical analyses. Field blanks were collected in all homes; a random sample of 20% were analyzed. The individual field blank nicotine level (or the average field blank for the batch if the individual field blank was not analyzed) was subtracted from the corresponding sample level to determine the blank-corrected surface nicotine level based on which all data analyses were conducted. Field blank values ranged from <LOQ of 0.30 ng/wipe to 1.31 ng/wipe (GeoM = 0.37 ng/wipe). The median nicotine level of the field blank was 1.2% of the median nicotine wipe sample (interquartile range, IQR: [0.1%; 6.3%]).
Dust loading
Dust samples were collected from a 1-m2 area (or from a larger area if needed to collect ∼1 cm of dust in a collection bottle) at each home visit using a high-volume small-surface sampler cyclone vacuum (CS3, Model HVS4, Venice, FL). The median area vacuumed was 6.7 m2 (IQR:1.0–13.2). Dust loading describes the overall dustiness of a home in grams of sieved dust collected per square meter vacuumed (g/m2).
Dust Nicotine
Nicotine in dust is a specific marker of THS, representing SVOCs in THS that have accumulated in settled house dust, and additional details are provided in the Supplementary Material. The dust sample preparation method and nicotine LC-MS/MS method were published elsewhere.5 Levels of nicotine are reported in micrograms per gram of dust collected (μg/g; ie, concentration) and in micrograms per square meter vacuumed (μg/m2; loading).
Statistical Analyses
Logarithmic transformations were applied to all surface and dust nicotine measures to control for positively skewed distributions and heterogeneous residual variances. We compared group means at baseline using linear regression models to determine whether randomization achieved equivalent groups with respect to participant and apartment characteristics, the time interval between measurements, and nicotine levels prior to the cleaning interventions. Changes in nicotine levels over time were examined separately within each group with mixed linear models, in which home was the random factor and time was the repeated-measures fixed factor. We estimated model parameters using restricted maximum likelihood estimation and allowing for unstructured residual variances. Specific comparisons between timepoints were tested using contrasts following model estimation. All statistical analyses were conducted using Stata version 16, and the Type I error rate was set at 5% (two-sided).42
Results
Characteristics of Participants and Their Homes
The median age of participants was 71.5 years, 74% (N = 28) were women; 39% (N = 15) were Latino/Hispanic, 34% (N = 13) White/Caucasian, 13% (N = 5) Black/African American, 11% (N = 4) Asian American, and 3% (N = 1) bi/multiracial. The median apartment size was 447 sqft (IQR: 370–671), and 76% of apartments were fully or partially carpeted. The apartment types were studio (45%, N = 17), followed by one-bedroom (26%, N = 10) and two-bedroom apartments (18%, N = 7). At the start of the cleaning intervention, participants had lived a median 5.0 years (IQR: 2.7–9.6) in their apartment. Supplementary Table 1 shows participants’: smoking status, home smoking policies, building smoking policies, and reported smoke intrusion from neighbors.
Stability of Surface Nicotine Levels Before Cleaning
Table 1 shows that mean surface nicotine concentrations remained stable during the 4-month period between the BL0 and BL1 prior to the intervention (all p > .50) changing by ≤11% in each of the three groups. Similar to the stability in mean levels, Pearson correlations between the BL0 and BL1 measures show strong linear associations between surface nicotine levels over a 4-month period for the three groups combined (r = .94, p < .001) as well as individually (group 1: r = .87, group 2: r = .94, group 3: r = .95; all p < .001). Supplementary Figure 1 shows the scatterplot of the two measures with a linear fit line. Findings about the linear associations among nicotine levels measured over the course of the cleaning interventions are reported in Supplementary Table 4.
Table 1.
Surface wipe nicotine loading before and after the cleaning interventions
| Percent change | |||||
|---|---|---|---|---|---|
| GeoMean, µg/m2 | 95% Confidence Interval | vs. BL0 | vs. BL1 | vs. BL2 | |
| Group 1 (N = 10) | |||||
| Baseline 0-screening | 7.7a,b,c | [4.5; 12.9] | Ref | ||
| Baseline 1 | 7.2d,e,f | [3.0; 15.7] | −7% | Ref | |
| Dry/damp cleaning | |||||
| Posttest 1 | 5.5g | [2.5; 11.1] | −28% | −23% | |
| Baseline 2 | 4.4a,d,g | [2.1; 8.7] | −42% | −38% | Ref |
| Wet cleaning | |||||
| Posttest 2 | 4.0b,e | [1.8; 7.8] | −10% | ||
| Follow-up month 3 | 4.5c,f | [2.0; 9.0] | 1% | ||
| Group 2 (N = 10) | |||||
| Baseline 0−—-Screening | 57.1a,b | [18.2; 175.3] | Ref | ||
| Baseline 1 | 61.6 | [19.3; 191.9] | 8% | Ref | |
| Wet cleaning | |||||
| Posttest 1 | 59.2c | [11.1; 298.8] | 4% | −4% | |
| Baseline 2 | 48.9c | [9.2; 242.0] | −14% | −21% | Ref |
| Dry/damp cleaning | |||||
| Posttest 2 | 31.0a | [7.6; 118.4] | −37% | ||
| Follow-up month 3 | 25.7b | [7.6 82.5] | −47% | ||
| Group 3 (N = 18) | |||||
| Baseline 0-screening | 21.9a,b | [9.5; 49.0] | Ref | ||
| Baseline 1 | 19.5 | [5.9; 59.8] | −11% | Ref | |
| Combination dry/damp and wet cleaning | |||||
| Posttest 1 | 12.7a | [5.4; 28.4] | −42% | −35% | |
| Follow-up month 3 | 8.1b | [3.6; 17.1] | −63% | −58% | |
Ref: reference group for the calculation of the percent change in GeoMean.a,b,c,d,e,f,g Within each of the cleaning groups, pairs of letters indicate significant differences between GeoMeans (p <.05).
Equivalence of Groups at Baseline
Baseline nicotine levels and the mean number of days between BL0 and BL1 are presented in Supplementary Table 2. Statistical comparisons of group means showed no significant differences (all p > .50) with respect to the time interval between BL0 and BL1, dust loading, dust nicotine concentration, and dust nicotine loading. In contrast, GeoM levels of surface nicotine at BL0 and BL1 differed significantly between cleaning intervention groups (p = .006 and .015) with the lowest levels in group 1 (BL1: 7.15 µg/m2, 95% CI: [2.98; 15.70]), the highest levels in group 2 (BL1: 61.57 µg/m2, 95% CI: [19.30; 191.92]), and intermediate levels in group 3 (BL1: 19.51 µg/m2, 95% CI: [5.92; 59.82]).
Dry/Damp Cleaning
To examine the immediate effects of dry/damp cleaning, we compared pre–post changes in group 1 (BL1 vs. P1) and group 2 (BL2 vs. P2). To examine the longer-term effects, we compared in group 1 the BL1 to the BL2 measures taken ~30 days after the cleaning. Additionally, we compared in group 2 the BL2 to FUM3, the 3-month follow-up measures.
Surface Nicotine Loading
Surface nicotine levels before and after dry/damp cleaning are reported in Table 1. In group 1, dry/damp cleaning was associated with a decrease in GeoM surface nicotine concentration of 23% (BL1 vs. P1; p = .15) and 38% (BL1 vs. BL2; z = −2.65, p = .008) immediately after and 30 days after the cleaning, respectively. In group 2, dry/damp cleaning was associated with a 37% decrease (BL2 vs. P2; p = .26) comparing levels immediately before to immediately after the cleaning. Three months after the cleaning, GeoM surface nicotine levels in group 2 remained at 47% lower levels (BL2 vs. FUM3; p = .20) than before the cleaning.
Dust Nicotine
Dust nicotine loadings before and after dry/damp cleaning are reported in Table 2. In group 1, dry/damp cleaning was associated with a decrease in dust nicotine loading of 34% (BL1 vs. P1; p = .123) and 37% (BL1 vs. BL2; z = −1.89; p = .059) immediately after and 30 days after the cleaning, respectively. In group 2, dry/damp cleaning was associated with a 2% increase comparing levels (BL2 vs. P2; p > .25) immediately before to immediately after the cleaning. Three months after the cleaning, GeoM dust nicotine loading in group 2 remained at 5% higher (BL2 vs. FUM3; p > .25) levels than before the cleaning. None of the observed changes in nicotine concentration (see Supplementary Table 3) were statistically significant (all p > .25).
Table 2.
Dust nicotine loading before and after the cleaning interventions
| Change | ||||
|---|---|---|---|---|
| GeoMean µg/m2 | 95% Confidence Interval | vs. BL1 | vs. BL2 | |
| Group 1 (N = 10) | ||||
| Baseline 1 | 1.23a,b,c | [0.31; 2.82] | Ref | |
| Dry/damp cleaning | ||||
| Posttest 1 | 0.82 | [0.30; 1.53] | −34% | |
| Baseline 2 | 0.78a,d | [0.19; 1.64] | −37% | Ref |
| Wet cleaning | ||||
| Posttest 2 | 0.33b,d | [0.07; 0.65] | −58% | |
| Follow-up month 3 | 0.47c | [0.23; 0.76] | −39% | |
| Group 2 (N = 10) | ||||
| Baseline 1 | 1.05 | [0.05; 3.00] | Ref | |
| Wet cleaning | ||||
| Posttest 1 | 0.58 | [0.05; 1.62] | −45% | |
| Baseline 2 | 0.89 | [0.16; 2.06] | −15% | Ref |
| Dry/damp cleaning | ||||
| Posttest 2 | 0.91 | [0.23; 1.96] | 2% | |
| Follow−up month 3 | 0.93 | [0.34; 1.78] | 5% | |
| Group 3 (N = 18) | ||||
| Baseline 1 | 1.33a | [0.46; 2.72] | Ref | |
| Combination dry/damp and wet cleaning | ||||
| Posttest 1 | 0.42b | [0.04; 0.95] | −68% | |
| Follow-up month 3 | 1.19a,b | [0.51; 2.17] | −11% | |
Ref: reference group for the calculation of the percent change in GeoMean.
a,b,c,d Within each of the cleaning groups, pairs of letters indicate significant differences between GeoMeans (p < .05).
Wet Cleaning
To examine the immediate effects of wet cleaning, we compared pre–post changes in group 1 (BL2 vs. P2) and group 2 (BL1 vs. P1). To examine the longer-term effects, we compared in group 1 the BL2 to the FUM3 measures, the 3-month follow-up. Additionally, we compared in group 2 the BL1 to BL2 taken ~30 days after the cleaning.
Surface Nicotine Loading
Surface nicotine levels before and after wet cleaning are presented in Table 1. In group 1, wet cleaning was associated with a 10% decrease comparing levels immediately before to immediately after the cleaning (BL2 vs. P2). Three months after the cleaning, surface nicotine concentrations in group 2 increased 1% compared with before the cleaning (BL2 vs. FUM3). In group 2, wet cleaning was associated with a decrease in GeoM surface nicotine levels of 4% (BL1 vs. P1) and 21% (BL1 vs. BL2). None of the observed changes in nicotine concentration were statistically significant (all p >.15).
Dust Nicotine
Changes in dust nicotine loadings before and after wet cleaning are shown in Table 2. In group 1, wet cleaning was associated with a 58% decrease (BL2 vs. P2; p = .007), comparing levels immediately before to immediately after the cleaning. Three months after the cleaning, dust nicotine loading in group 1 remained at 39% lower levels than before the cleaning (BL2 vs. FUM3; p = .30). In group 2, wet cleaning was associated with a decrease in GeoM dust nicotine loading of 45% (BL1 vs. P1; p = .22) and 15% (BL1 vs. BL2; p = .73) immediately after and 30 days after the cleaning, respectively.
Dust nicotine concentrations before and after wet cleaning (see Supplementary Table 3) in group 1 was associated with a 32% reduction (p = .019) comparing levels immediately before to immediately after the cleaning. Three months after the cleaning, dust nicotine concentrations in group 1 were 13% lower (p = .643) than levels than before the cleaning. In group 2, wet cleaning was associated with a 4% decrease (p = .78) and 11% increase (p = .23) immediately after and 30 days after the cleaning, respectively.
Combination Dry/Damp and Wet Cleaning
Surface Nicotine Loading
Table 1 shows statistically significant improvements in GeoM surface nicotine levels after combined dry/damp/wet cleaning. In group 3, the combination cleaning was associated with a significant decrease in GeoM surface nicotine loading of 35% (BL1 vs. P1; z = −3.36, p = .001) immediately after the cleaning. At the 3-month follow-up, surface nicotine concentration remained significantly lower than at baseline with a 58% reduction (BL1 vs. FUM3; z = −4.14, p = <.001).
Dust Nicotine
Table 2 shows statistically significant improvements in GeoM dust nicotine loading immediately after combined dry/damp/wet cleaning. In group 3, we observed a significant decrease in dust nicotine loading of 68% immediately after the cleaning (BL1 vs. P1; z = −3.36, p = .001). At the 3-month follow-up, nicotine loading was 11% lower compared with baseline levels (BL1 vs. FUM3; p = .76).
Supplementary Table 3 shows that in group 3, the combination dry/damp/wet cleaning was associated with a significant decrease in dust nicotine concentration of 31% (BL1 vs. P1; z = −2.38, p = .017) immediately after the cleaning. At the 3-month follow-up, nicotine concentration had returned to baseline levels (0% change; p = .99).
Overall Change in THS Associated with the Cleaning Intervention
Because small sample sizes of the individual intervention groups limited the statistical power and because the homes of all three groups experienced the same cleaning regimens—though in different orders and in combination—we examined whether the overall cleaning efforts were associated with changes in surface and dust nicotine levels. Therefore, we pooled the data from the three cleaning groups and compared BL1 with P2 and FUM3 measures.
Table 3 shows the GeoM levels before and after the completion of all cleaning efforts for the three cleaning groups combined. Compared to BL1, surface nicotine levels after the cleaning interventions at P2 showed a significant reduction of 43% (BL1 vs. P2; z = −3.73, p < .001) and a 53% reduction 3 months later (BL1 vs. FUM3; z = −3.96, p < .001). Dust nicotine loading were significantly lower by 60% at the end of the cleaning efforts (BL1 vs. P2; z = −3.55, p < .001), but the loading rebounded 3 months later, when the improvement was only 26% lower than at baseline (BL1 vs. FUM3; z = −1.18, p = .237). Compared to dust nicotine concentration at BL1, the cleaning efforts were not associated with any significant changes at P2 (p = .369) and FUM3 (p = .565).
Table 3.
Surface nicotine loading, dust nicotine loading, and dust nicotine concentration before and after cleaning for the three cleaning groups combined (N = 38)
| Percent change | ||||
|---|---|---|---|---|
| GeoMean µg/m2 | 95% Confidence Interval | vs. BL0 | vs. BL1 | |
| Surface nicotine concentrations (µg/m 2) | ||||
| Baseline 0—screening | 21.58a,b | [12.90; 35.69] | Ref | |
| Baseline 1 | 20.47c,d | [10.56; 38.86] | −5% | Ref |
| Sequential or combined dry/damp and wet cleaning | ||||
| Posttest 2 | 11.59a,c | [6.58; 19.92] | −46% | −43% |
| Follow-up month 3 | 9.58b,d | [5.64; 15.87] | −56% | −53% |
| Dust nicotine loading (µg/m 2) | ||||
| Baseline 1 | 1.23a | [0.62 2.06] | Ref | |
| Sequential or combined dry/damp and wet cleaning | ||||
| Posttest 2 | 0.50a | [0.22 0.83] | −60% | |
| Follow-up month 3 | 0.91 | [0.55 1.35] | −26% | |
| Dust nicotine concentration (µg/g) | ||||
| Baseline 1 | 1.47 | [1.07 1.95] | Ref | |
| Sequential or combined dry/damp and wet cleaning | ||||
| Posttest 2 | 1.30 | [0.87 1.83] | −12% | |
| Follow-up month 3 | 1.64 | [1.18 2.19] | +11% | |
Reference group for the calculation of the percent change in GeoMeans.
a,b,c,d For each of the nicotine measures, pairs of letters indicate significant differences in GeoMeans (p < .05).
Discussion
This is the first study to examine the effects of cleaning interventions for homes polluted with THS residue. Used separately, dry/damp and wet cleaning methods showed only limited benefits with respect to surface and dust nicotine. The combination of the two methods, however, significantly reduced nicotine on surfaces and in dust, but the cleaning methods did not eliminate the THS. Compared to baseline, the cleaning efforts lowered mean surface nicotine levels by ~50% immediately after and 3 months after the cleaning. Mean dust nicotine loadings declined by 60% immediately after cleaning and then increased 3 months later to levels 26% lower than at baseline. There was no change, however, in dust nicotine concentration. The observed reductions are noteworthy because mean surface nicotine levels remained virtually unchanged over a 4-month period prior to cleaning (BL0 vs. BL1), and the correlation between the repeated measures indicated very high stability over time (r = .94).
The lack of change in dust nicotine concentration and the rebound in dust nicotine loading underscores an important limitation of the cleaning methods. As dust accumulates again after the cleaning, nicotine levels in settled house dust appears to be replenished. Given the chemical properties of THS constituents, one likely explanation is that nicotine is being replenished from THS reservoirs that were not removed or not sufficiently depleted during the cleaning. Given the many suitable materials available in a home that can potentially store THS constituents, we suspect that THS reservoirs may be widely distributed in a home and may include carpets, furniture, upholstery, mattresses, blankets, walls, clothes, books, etc. Achieving larger and longer-term reductions in THS levels may require repeated and frequent cleaning to remove dust that re-accumulated and surface deposits that have been replenished. This may also require the identification and removal of important THS reservoirs in a home that cannot be effectively cleaned. Future research is needed to better understand which materials and objects in a home are the primary THS reservoirs that should be targeted for remediation, removal, or replacement.
The observed reduction in nicotine dust loading immediately after cleaning is similar to those reported in studies of cleaning interventions for lead, polycyclic aromatic hydrocarbons (PAHs), and house dust mite allergens.43–46 For instance, Yu et al. examined the effects of HEPA filter vacuuming and dry steam cleaning in homes with wall-to-wall carpet and observed a 59–69% reduction in polycyclic aromatic hydrocarbon dust loading. They found that house dust mite allergens were removed at an even greater efficiency (81–86%), likely due to a larger particle size (>10 µm) that can be more efficiently removed with well-maintained HEPA filter vacuum cleaners.47 As HEPA filters only remove particles, they have only short-term impact on removal of SVOCs in THS (eg, nicotine) that exist in equilibrium of gas and condensed phases and can repartition into the particle phase.11,34,35 Our study indicates that initial gains in THS reduction are lost or reduced 3 months after the initial cleaning. Although likely occurring through different mechanisms, this finding was also made in long-term follow-up studies for lead and dust mites following cleaning.44,48 In combination, these studies point to the critical role of persistent and replenished pollutant reservoirs in limiting the long-term benefits of cleaning interventions.
Two limitations of this study should be noted. While nicotine in dust and on surfaces is validated markers of SVOCs in THS residue, future studies should also include markers of THS particulate- and gas-phase constituents (eg, nicotelline, naphthalene) and de novo THS constituents created through secondary reactions (eg, NNA).14,30 In addition to the plausible hypothesis that repartitioning of nicotine from THS reservoirs occurs into dust and onto surfaces, the rebound in nicotine levels observed after the cleaning intervention could also be the result of new SHS intrusion. The hypothesis that this rebound occurs due to SHS intrusion was not supported by further analysis, however, as we found no association between nicotine levels and SHS intrusion reported by residents (see Online Supplement). Moreover, the observed stability of surface and dust nicotine levels in repeated measures also provides evidence that SHS intrusion does not play a dominant role in the rebound of nicotine on surfaces and in dust. Future studies should include specific markers of SHS (eg, 3-ethynylpyridine) to better rule out SHS intrusion as an alternative explanation.
When tobacco is regularly smoked in indoor environments over long periods of time, its multiple chemical constituents accumulate and leave behind a lasting and toxic legacy in the built indoor environment (eg, doors, walls, and ceilings) as well as in the movable possessions of its residents (eg, furniture, clothes, blankets, pillows, toys, and books). Young children who are actively interacting with their environments are especially at risk of chronic exposure to THS pollutants via oral, dermal, and respiratory routes in a home environment. As previously mentioned, THS contamination of low-income housing is more likely than high-income housing due to higher smoking rates, higher density occupation, smaller homes, poorer quality housing, and frequent moves.49,50 Therefore, exposure to THS in homes may contribute to tobacco-related health disparities associated with housing. Interventions are clearly needed to keep THS pollutant levels as low as possible to protect children and other vulnerable occupants. Due to well-documented exposures through house dust, we recommend frequent vacuuming and dust removal as one step that can be taken by current residents.51 The present study has shown that conventional cleaning methods had limited success in removing nicotine and presumably other toxic THS component and can be costly. As current occupants and new tenants may not be aware of the toxic legacy left behind in their homes by previous residents, the question arises who should bear the responsibility and the cost of identification and remediation. Similar to other hazardous postconsumer waste products (eg, paints, old motor oil, electronic waste, and unused pharmaceuticals), THS is a form of product waste for which manufacturers, suppliers, and retailers should assume responsibility to prevent and mitigate harmful environmental impacts.52 The growing body of research on the pervasiveness, persistence, and toxicity of THS supports legislative initiatives at the national, state, and local levels to broadly ban tobacco use in all indoor settings and to require the beneficiaries of tobacco sales to share in the costs to identify and remediate the toxic legacy of tobacco use.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
The work that provided the basis for this publication was supported by funding under an award (CAHHU0028-15) with the U.S. Department of Housing and Urban Development, with support from the California Tobacco Related Disease Research Program (28PT-0078), and through the National Institute of Environmental Health Sciences at the National Institutes of Health (R01ES027815). The substance and findings of the work are dedicated to the public. The author and publisher are solely responsible for the accuracy of the statements and interpretations contained in this publication. Such interpretations do not necessarily reflect the views of the Government.
Declaration of interests
None declared.
Acknowledgments
The authors express their gratitude to United Women of East Africa including Sahra Abdi and Bethlehem Degu for their collaboration, to Miriam Adam, Korryn Brackin, Brittney Carroll, Elizabeth Davies, Mayra Duran, Nicolette Dux, Irma Hernandez, Lupita Lombardo, Olga Vergara Lozano, Laura Markman, Maria Martinez, Viridiana Mendoza, Kafisa Mohamed, Dr. Ana M. Navarro, Taylor Shrum, and Faiza Warsame for assisting with participant recruitment and data collection, and to Christine Batikian, Linda Chu, Dana Datuin, Laura Markman, and Mansi Vyas for samples preparation. The authors also express their appreciation to Dan MacAiri (D-Mac Restoration) and Nathan Raymond (West Coast Restoration and Cleaning) for their contributions to the cleaning protocols.
References
- 1. Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matt GE, Quintana PJ, Zakarian JM, et al. When smokers move out and non-smokers move in: residential thirdhand smoke pollution and exposure. Tob Control. 2011;20(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matt GE, Quintana PJ, Fortmann AL, et al. Thirdhand smoke and exposure in California hotels: non-smoking rooms fail to protect non-smoking hotel guests from tobacco smoke exposure. Tob Control. 2014;23(3):264–272. [DOI] [PubMed] [Google Scholar]
- 4. Matt GE, Quintana PJE, Hoh E, et al. A Casino goes smoke free: a longitudinal study of secondhand and thirdhand smoke pollution and exposure. Tob Control. 2018;27(6):643–649. [DOI] [PubMed] [Google Scholar]
- 5. Matt GE, Quintana PJE, Zakarian JM, et al. When smokers quit: exposure to nicotine and carcinogens persists from thirdhand smoke pollution. Tob Control. 2016;26(5):548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matt GE, Fortmann AL, Quintana PJ, et al. Towards smoke-free rental cars: an evaluation of voluntary smoking restrictions in California. Tob Control. 2013;22(3):201–207. [DOI] [PubMed] [Google Scholar]
- 7. Matt GE, Quintana PJ, Hovell MF, et al. Residual tobacco smoke pollution in used cars for sale: air, dust, and surfaces. Nicotine Tob Res. 2008;10(9):1467–1475. [DOI] [PubMed] [Google Scholar]
- 8. Santos E Silva SI, Bowdler P, Giltrow D, Riddell S, Honeychurch KC. A simple and rapid method for the determination of nicotine in third-hand smoke by liquid chromatography and its application for the assessment of contaminated outdoor communal areas. Drug Test Anal. 2016;8(7):676–681. [DOI] [PubMed] [Google Scholar]
- 9. Hood NE, Ferketich AK, Klein EG, Pirie P, Wewers ME. Associations between self-reported in-home smoking behaviours and surface nicotine concentrations in multiunit subsidised housing. Tob Control. 2014;23(1):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang S, Qiao S, Chen M, Xia Y, Hang B, Cheng S. [A investigation of thirdhand smoke pollution in 3 types of places of Nanjing, 2014]. Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49(1):31–35. [PubMed] [Google Scholar]
- 11. DeCarlo PF, Avery AM, Waring MS. Thirdhand smoke uptake to aerosol particles in the indoor environment. Sci Adv. 2018;4(5):eaap8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Northrup TF, Khan AM, Jacob P 3rd, et al. Thirdhand smoke contamination in hospital settings: assessing exposure risk for vulnerable paediatric patients. Tob Control. 2016;25(6):619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matt GE, Quintana PJ, Destaillats H, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect. 2011;119(9):1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacob P 3rd, Benowitz NL, Destaillats H, et al. Thirdhand smoke: new evidence, challenges, and future directions. Chem Res Toxicol. 2017;30(1):270–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sleiman M, Logue JM, Luo W, Pankow JF, Gundel LA, Destaillats H. Inhalable constituents of thirdhand tobacco smoke: chemical characterization and health impact considerations. Environ Sci Technol. 2014;48(22):13093–13101. [DOI] [PubMed] [Google Scholar]
- 16. Hang B, Sarker AH, Havel C, et al. Thirdhand smoke causes DNA damage in human cells. Mutagenesis. 2013;28(4):381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts JW, Dickey P. Exposure of children to pollutants in house dust and indoor air. Rev Environ Contam Toxicol. 1995;143:59–78. [DOI] [PubMed] [Google Scholar]
- 18. Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke. Boca Raton (FL): CRC Press, Taylor & Francis Group; 2009. [Google Scholar]
- 19. Daisey JM, Mahanama KR, Hodgson AT. Toxic volatile organic compounds in simulated environmental tobacco smoke: Emission factors for exposure assessment. J Expo Anal Environ Epidemiol. 1998;8(3):313–334. [PubMed] [Google Scholar]
- 20. Klepeis NE, Apte MG, Gundel LA, Sextro RG, Nazaroff WW. Determining size-specific emission factors for environmental tobacco smoke particles. Aerosol Sci Technol. 2003;37:780–790. [Google Scholar]
- 21. Singer BC, Hodgson AT, Guevarra KS, Hawley EL, Nazaroff WW. Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Environ Sci Technol. 2002;36(5):846–853. [DOI] [PubMed] [Google Scholar]
- 22. Singer BC, Hodgson AT, Nazaroff WW. Gas-phase organics in environmental tobacco smoke. 2. Exposure-relevant emission factors and indirect exposures from habitual smoking. Atmos Environ. 2003;37(40):5551–5561. [Google Scholar]
- 23. Sleiman M, Destaillats H, Smith JD, et al. Secondary organic aerosol formation from ozone-initiated reactions with nicotine and secondhand tobacco smoke. Atmos Environ. 2010;44(34): 4191–4198. [Google Scholar]
- 24. Sleiman M, Gundel LA, Pankow JF, Jacob P 3rd, Singer BC, Destaillats H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc Natl Acad Sci USA. 2010;107(15):6576–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Loy MD, Lee VC, Gundel LA, Daisey JM, Sextro RG, Nazaroff WW. Dynamic behavior of semivolatile organic compounds in indoor air. 1. Nicotine in a stainless steel chamber. Environ Sci Technol. 1997;31(9):2554–2561. [Google Scholar]
- 26. Nazaroff WW. Indoor particle dynamics. Indoor Air. 2004;14(Suppl. 7):175–183. [DOI] [PubMed] [Google Scholar]
- 27. Bahl V, Shim HJ, Jacob P 3rd, Dias K, Schick SF, Talbot P. Thirdhand smoke: Chemical dynamics, cytotoxicity, and genotoxicity in outdoor and indoor environments. Toxicol In Vitro. 2016;32:220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Destaillats H, Lunden MM, Singer BC, et al. Indoor secondary pollutants from household product emissions in the presence of ozone: A bench-scale chamber study. Environ Sci Technol. 2006;40(14):4421–4428. [DOI] [PubMed] [Google Scholar]
- 29. Destaillats H, Singer BC, Lee SK, Gundel LA. Effect of ozone on nicotine desorption from model surfaces: Evidence for heterogeneous chemistry. Environ Sci Technol. 2006;40(6):1799–1805. [DOI] [PubMed] [Google Scholar]
- 30. Sheu R, Stönner C, Ditto JC, Klüpfel T, Williams J, Gentner DR. Human transport of thirdhand tobacco smoke: A prominent source of hazardous air pollutants into indoor nonsmoking environments. Sci Adv. 2020;6(10):eaay4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fortmann AL, Romero RA, Sklar M, et al. Residual tobacco smoke in used cars: Futile efforts and persistent pollutants. Nicotine Tob Res. 2010;12(10):1029–1036. [DOI] [PubMed] [Google Scholar]
- 32. Pankow JF, Mader BT, Isabelle LM, Luo W, Pavlick A, Liang C. The conversion of nicotine in tobacco smoke to its volatile and available free-base form through the action of gaseous ammonia. Environ Sci Technol. 1999;33(8):1320–1320. [Google Scholar]
- 33. Summerfield JH. An acid-base chemistry example: conversion of nicotine. J Chem Educ. 1999;76(10):1397–1398. [Google Scholar]
- 34. Weschler CJ, Nazaroff WW. Semivolatile organic compounds in indoor environments. Atmos Environ. 2008;42(40):9018–9040. [Google Scholar]
- 35. Weschler CJ, Nazaroff WW. SVOC partitioning between the gas phase and settled dust indoors. Atmos Environ. 2010;44:3609–3620. [Google Scholar]
- 36. Schick SF, Farraro KF, Perrino C, et al. Thirdhand cigarette smoke in an experimental chamber: evidence of surface deposition of nicotine, nitrosamines and polycyclic aromatic hydrocarbons and de novo formation of NNK. Tob Control. 2014;23(2):152–159. [DOI] [PubMed] [Google Scholar]
- 37. Whitehead TP, Metayer C, Petreas M, Does M, Buffler PA, Rappaport SM. Polycyclic aromatic hydrocarbons in residential dust: sources of variability. Environ Health Perspect. 2013;121(5):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson KM, Klein JD, Blumkin AK, Gottlieb M, Winickoff JP. Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics. 2011;127(1):85–92. [DOI] [PubMed] [Google Scholar]
- 39. Snyder K, Vick JH, King BA. Smoke-free multiunit housing: a review of the scientific literature. Tob Control. 2016;25(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kraev TA, Adamkiewicz G, Hammond SK, Spengler JD. Indoor concentrations of nicotine in low-income, multi-unit housing: associations with smoking behaviours and housing characteristics. Tob Control. 2009;18(6):438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matt GE, Quintana PJE, Hoh E, et al. Persistent tobacco smoke residue in multiunit housing: legacy of permissive indoor smoking policies and challenges in the implementation of smoking bans. Prev Med Rep. 2020;18:101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. StataCorp. Stata statistical software: Release 16. College Station, TX: Stata Corporation; 2019. [Google Scholar]
- 43. Yu CH, Yiin LM, Tina Fan ZH, Rhoads GG. Evaluation of HEPA vacuum cleaning and dry steam cleaning in reducing levels of polycyclic aromatic hydrocarbons and house dust mite allergens in carpets. J Environ Monit. 2009;11(1):205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yiin LM, Rhoads GG, Rich DQ, et al. Comparison of techniques to reduce residential lead dust on carpet and upholstery: the New Jersey assessment of cleaning techniques trial. Environ Health Perspect. 2002;110(12):1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rich DQ, Rhoads GG, Yiin LM, et al. Comparison of home lead dust reduction techniques on hard surfaces: the New Jersey assessment of cleaning techniques trial. Environ Health Perspect. 2002;110(9):889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yiin LM, Yu CH, Ashley P, Rhoads G. Cleaning efficacy of high-efficiency particulate air-filtered vacuuming and “dry steam” cleaning on carpet. J Occup Environ Hyg. 2008;5(2):94–99. [DOI] [PubMed] [Google Scholar]
- 47. Platts-Mills TA, Heymann PW, Longbottom JL, Wilkins SR. Airborne allergens associated with asthma: Particle sizes carrying dust mite and rat allergens measured with a cascade impactor. J Allergy Clin Immunol. 1986;77(6):850–857. [DOI] [PubMed] [Google Scholar]
- 48. Vojta PJ, Randels SP, Stout J, et al. Effects of physical interventions on house dust mite allergen levels in carpet, bed, and upholstery dust in low-income, urban homes. Environ Health Perspect. 2001;109(8):815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jacobs DE, Wilson J, Dixon SL, Smith J, Evens A. The relationship of housing and population health: a 30-year retrospective analysis. Environ Health Perspect. 2009;117(4):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hernández D, Swope CB. Housing as a platform for health and equity: evidence and future directions. Am J Public Health. 2019;109(10):1363–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. U.S. Environmental Protection Agency. Exposure factors handbook 2011 edition (final report). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-09/052F; 2011. [Google Scholar]
- 52. Nash J, Bosso C. Extended producer responsibility in the United States. J Ind Ecol. 2013;17(2):175–185. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.