Abstract
Introduction
We use multilevel modeling to parse out the effects of time-varying smoking abstinence and baseline depression (history and severity) on depression severity over 1 year.
Aims and Methods
Participants were 1000 smokers recruited worldwide for an online randomized controlled tobacco cessation trial. We examined whether changes in depression severity over time were associated with self-reported 7-day point prevalence smoking status assessed at 1-, 3-, 6-, and 12-month follow-up (FU) using baseline major depressive episode (MDE) history and baseline depression severity as time-invariant covariates. We present depression severity means and smoking abstinence at each FU.
Results
Regardless of concurrent abstinence status, baseline MDE history was significantly related to depression severity over time: those reporting a past MDE had worse depressive symptoms over time compared with those reporting no MDE history. Baseline depression severity interacted significantly with time-varying abstinence status: for every 1-unit increase in baseline scores on the Center for Epidemiological Studies—Depression Scale (CES-D), individuals who were smoking at FU reported CES-D scores that were 0.17 points higher than those who were abstinent. In this context, nicotine dependence, gender, age, or marital status did not affect depression severity.
Conclusions
In the context of cessation, having an MDE history plays a significant role in the trajectory of depression severity over the course of 1 year, regardless of abstinence status. Abstinence is related to lower depressive symptoms at each FU, and this effect was stronger at higher levels of baseline depression severity.
Implications
This study indicates that depressive symptoms are not exacerbated among individuals who are quitting smoking at 1-, 3-, 6-, and 12-month FUs. Depression severity is worse with a baseline history of MDE. Further, those with high baseline depression severity who continue smoking have worse depressive symptoms throughout a 1-year period compared with their abstinent counterparts.
Introduction
Globally, tobacco use is the main cause of preventable death and disproportionately affects individuals with mental disorders.1 Guidelines recommend the integration of tobacco cessation into routine mental health care settings.2 Prior to this integration however, it is critical to understand the effect of abstinence on depression symptoms, while also parsing out the roles of baseline depression history and baseline depression severity. If abstinence exacerbates depression, clinicians need to be aware of and prepared to address this in a clinical context. If abstinence improves depression, this might increase the engagement of both clinicians and patients in tobacco cessation in these settings. If either of these relationships are affected by baseline depression history and baseline depression severity, clinicians should tailor cessation integration accordingly.
Many studies have explored the relationship between abstinence and depressive symptoms categorically—whether or not tobacco cessation resulted in the later development of a major depressive episode (MDE)—while also accounting for the special risk among those with a prior MDE history. Generally, those who successfully quit smoking but also had an MDE history were at risk for developing another MDE after cessation compared with those who did not have an MDE history.3–7 For example, at 3-month follow-up (FU) in a cessation study, the incidence of a new MDE was 2%, 17%, and 30% among abstinent individuals with no history of depression, single major depression, or recurrent major depression, respectively.4 At 3- and 6-month FU, heavy smokers with a history of depression who abstained from smoking in the final 2 weeks of treatment were more likely to develop a recurrent episode of depression compared with those who continued to smoke.6 At 12-month FU, the incidence of MDE was 14.1% for the entire sample after smoking cessation treatment and the development of a later MDE was significantly different based on baseline history of MDE—23.7% 12-month MDE incidence among those who had a history of depression compared with 9.7% among those with no history of depression.7 At 1-, 3-, 6-, and 12-month FU, in a previous analysis that included a subsample of all recruited participants from our study who did not meet criteria for a current MDE at baseline, a past MDE—but not abstinence—was associated with development of a later MDE.8 In another study, smokers with an MDE history who returned to smoking had a higher risk of later developing an MDE and abstinence did not increase risk of MDE incidence but rather was associated with nonsignificant lower risk.9
One potential limitation to these traditional approaches is that they do not capture variation of depressive symptoms at different intervals, which may be more important and more informative clinically than a categorical diagnosis. Also, the majority of prior studies did not capture the time-varying dimension of abstinence itself. Quitting smoking, after all, typically includes periods of smoking and abstinence over time and is rarely a linear process or one-time event.10 These limitations have made it difficult to disentangle whether MDE history, abstinence, or both, contribute to later depressive symptoms.
There is now a growing body of studies which have begun to capture the time-varying association between tobacco cessation and depression severity using advanced statistical models, such as multilevel modeling (MLM). This approach can help with understanding the nuanced relationship between MDE history, time-varying depressive symptoms, and time-varying abstinence using longitudinal data. One of the studies9 mentioned earlier explored the relationship between depression and abstinence in the course of smoking cessation that incorporated cognitive-behavioral treatment for depression and found that abstinence was related to reduced depression severity a year later. In another longitudinal study, composed of heavy drinking smokers, abstinence was associated with a reduction in depressive symptom severity, an MDE history was not related to abstinence patterns, and an MDE history did appear related to later depression symptom severity.11 In a longitudinal study of perinatal women, abstinence was associated with a reduction in depression symptom severity; however, this study did not look at those seeking smoking cessation treatment.12 A recent study exploring a combined treatment for smoking cessation in heavy drinkers found that abstinence from smoking was associated with significantly lower depressive severity over a 26-week period.13
In the current study, using data from a previous Internet-based worldwide smoking cessation trial, we use MLM to examine the relationship between time-varying abstinence and depression symptoms measured at 1-, 3-, 6-, and 12-month FU, accounting for a history of MDE assessed at baseline and baseline depression severity. Based on a systematic review of previous studies on the relationship between smoking and mental health,14 we also control for differences in nicotine dependence, gender, ethnicity, and educational status. Therefore, our questions are as follows: (1) in the context of a cessation trial, what are the effects of time-varying smoking abstinence and time-invariant baseline depression (history and severity) on depression severity over time? (2) In this context, do nicotine dependence, gender, ethnicity, and education moderate changes in depression severity?
Materials and Methods
Data were collected as part of an international, web-based randomized control trial for tobacco cessation in which 500 Spanish-speaking and 500 English-speaking adult Internet users (smoking at least 5 cigarettes/day and intending to quit in the next month) were recruited online from 68 countries. Details of this study are available elsewhere.15,16 Briefly though, participants were recruited using a Google AdWords campaigns targeted at English- and Spanish-speaking smokers from any country. Smokers came to the site via search engines after entering relevant key words, links from other Web sites, media stories, and word of mouth. The website was described as a “Free online University of California Stop Smoking Study” which entailed an 8-week program for tobacco smokers who were ready to quit. Participants were informed that they would be contacted at 1-, 3-, 6-, and 12-month FU after their study entry to answer a brief questionnaire.
Study Procedures
Interested individuals logged onto a website and responded to an 11-item eligibility questionnaire, which included being 18 years of age, smoking five or more cigarettes daily, using e-mail at least once weekly, and planning to quit within the next 3 months. Those eligible were presented with an online institutional review board-approved consent form. Consenting participants provided baseline demographics, smoking characteristics, lifetime and current MDE symptoms, and depressive symptom severity using established measures. Those not eligible or not consenting could access a smoking cessation guide online.
To screen out those merely browsing and unlikely to return, potential participants who completed the baseline questionnaire were asked to log daily cigarette use on an online cigarette counter on three separate days within the following week. E-mail reminders were sent daily until the third entry or the seventh day. After their third entry, participants were asked to set their initial quit date within the next 30 days. FUs were keyed to the initial quit date, although users could change it later. Those who logged cigarettes smoked on 3 days within a week and set a quit date were randomized to one of four conditions and taken to an individualized home page. Participants could access their designated interventions throughout the 12-month FU period. Self-reported 7-day abstinence was defined as a “‘no’” response to the question, ”Have you smoked 1 or more cigarettes in the last 7 days?”
Each condition added new elements: Condition 1 was the static National Cancer Institute evidence-based Guide to Stop Smoking17 which covers reasons to quit, cessation strategies, relapse prevention and management, information about pharmacological aids, and how to help a smoker quit. They were also provided an online journal to record experiences while quitting. Condition 2 consisted of Condition 1 materials plus e-mail reminders to return to the site. Condition 3 consisted of Condition 2 materials plus cognitive-behavioral mood management strategies, which were an extended version of an intervention tested previously.18 Condition 4 consisted of Condition 3 materials plus a “virtual group” (an asynchronous bulletin board for mutual support and suggestions).
Participants
Baseline measures were collected from participants about standard smoking characteristics, sociodemographic characteristics (eg, age, gender, marital and status, ethnicity, race, and educational attainment), and depressive symptoms (eg, MDE history and symptom severity). Participants reported smoking status and completed the depressive symptoms measures at baseline and at FUs at 1, 3, 6, and 12 months. Demographic, smoking, and clinical characteristics of our sample are shown in Table 1 and additional details can be found in a prior publication.15 Each of the four conditions had roughly a quarter of the total sample, with 24.7% individuals in Condition 1 and 25.1% in Conditions 2–4. Slightly more than half were men (55.5%). At baseline, 69.7% of individuals reported no MDE history, 17.3% a past MDE, and 12.9% a current MDE.
Table 1.
Baseline Characteristics of 1000 Cigarette Smokers from 68 Countries, by History of Major Depressive Episode (MDE), Randomized to Tomando Control Study (2005–2007)
| Variable | Total N | N (%) | No MDEa | Past MDEa | Current MDEa |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| 1000 | 697 (69.7%) | 173 (17.3%) | 129 (12.9%) | ||
| Gender | |||||
| % Women | 996 | 443 (44.6) | 295 (42.6) | 94 (54.7) | 54 (41.9) |
| % Men | 553 (55.4) | 402 (57.4) | 79 (45.3) | 75 (58.1) | |
| Language | |||||
| % Spanish speakers | 998 | 499 (50.0) | 343 (49.3) | 73 (42.2) | 83 (64.3) |
| % English speakers | 499 (50.0) | 354 (50.7) | 100 (57.8) | 46 (35.7) | |
| Marital status | |||||
| % Partneredb | 997 | 539 (54.1) | 403 (57.9) | 84 (48.8) | 52 (40.3) |
| % Non-partnered | |||||
| 458 (45.9) | 294 (42.1) | 89 (51.2) | 77 (59.7) | ||
| Ethnicity | |||||
| % Hispanic or Latino/a | 973 | 515 (52.9) | 356 (52.8) | 74 (43.3) | 85 (66.4) |
| % Not Hispanic/Latino/a | 458 (47.1) | 341 (47.2) | 99 (56.7) | 44 (33.6) | |
| Variable | Total N | Overall | No MDEa | Past MDEa | Current MDEa |
| Mean (SD)d | Mean (SD)d | Mean (SD)d | Mean (SD)d | ||
| Age (years) | 999 | 37.9 (11.3) | 38.4 (22.7) | 35.8 (10.7) | 35.3 (11.1) |
| Education (years) | 993 | 13.1 (2.1) | 13.1 (2.2) | 13.0 (1.86) | 13.1 (1.82) |
| Smoking history | |||||
| Age (years), first cigarette | 999 | 15.6 (3.30) | 15.7 (3.1) | 15.2 (2.8) | 15.8 (4.7) |
| Age (years), regular smoker | 993 | 18.3 (4.0) | 18.4 (3.9) | 17.3 (3.7) | 18.7 (4.8) |
| Years smoked | 1000 | 21.4 (11.7) | 21.9 (11.9) | 20.6 (10.9) | 19.4 (11.7) |
| Cigarettes per day | 1000 | 19.8 (10.1) | 19.5 (10.4) | 20.4 (9.5) | 19.9 (9.4) |
| FTNDe | 997 | 5.2 (2.5) | 5.0 (2.5) | 5.6 (2.4) | 5.7 (2.5) |
| Depression | |||||
| CES-Df (baseline) | 996 | 16.0 (11.6) | 12.2 (8.4) | 17.6 (9.6) | 34.2 (10.9) |
aMDE = major depressive episode.
bPartnered = married or living with partner.
cMestizo = person of mixed Spanish and indigenous ancestry.
dSD = standard deviation.
eFTND = Fagerström Test for Nicotine Dependence.
fCES-D = Center for Epidemiological Studies—Depression Scale.
Measurements
Depression Severity
Center for Epidemiologic Studies—Depression Scale (CES-D).19 Depressive symptoms were determined by the CES-D, a continuous measure of self-reported depressive symptoms in the general population.20 Scores range from 0 to 60 with higher scores indicating more depression and scores of 16 or higher representing clinically significant depression. Previous studies have demonstrated the validity and reliability of this measure administered over the Internet.21 The CES-D was administered at baseline, and at 1-, 3-, 6-, and 12-month FU periods and, as noted in Table 2, CES-D completion rates were 72.5%, 65.1%, 55.8%, and 68.3%, respectively.
Table 2.
Depression Severity Scores by Self-reported 7-Day Smoking Abstinence and Follow-up Period, Tomando Control Study, 2005–2007
| Total N | No MDEa history | Past MDE | Current MDE | ||||
|---|---|---|---|---|---|---|---|
| Smoking | Abstinent | Smoking | Abstinent | Smoking | Abstinent | ||
| % | % | % | % | % | % | ||
| Baseline | 1000 | 100 | 0 | 100 | 0 | 100 | 0 |
| 1 month | 725 (72.5) | 76.1 | 23.9 | 79.2 | 20.8 | 75.0 | 25.0 |
| 3 months | 651 (65.1) | 75.1 | 24.9 | 72.8 | 27.2 | 72.3 | 27.7 |
| 6 months | 558 (55.8) | 73.7 | 26.3 | 74.8 | 25.3 | 72.7 | 27.3 |
| 12 months | 683 (68.3) | 69.2 | 30.8 | 70.3 | 29.8 | 71.9 | 28.1 |
| CES-D | Mean (SDb) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Baseline | 1000 | 12.20 (8.45) | — | 17.60 (9.57) | — | 34.21 (10.88) | — |
| 1 month | 725 (72.5) | 13.17 (10.48) | 11.88 (9.87) | 20.04 (12.28) | 14.54 (11.48) | 26.12 (13.63) | 19.57 (12.43) |
| 3 months | 651 (65.1) | 12.04 (9.81) | 10.50 (10.35) | 17.63 (12.71) | 13.70 (8.90) | 26.76 (12.01) | 18.26 (12.98) |
| 6 months | 558 (55.8) | 11.45 (9.92) | 8.97 (7.12) | 18.89 (12.97) | 12.29 (12.09) | 24.98 (14.25) | 18.80 (10.50) |
| 12 months | 683 (68.3) | 11.45 (10.92) | 9.31 (9.91) | 18.51 (13.92) | 10.74 (11.01) | 25.00 (14.73) | 15.20 (13.21) |
aMDE = major depressive episode; CES-D = Center for Epidemiological Studies—Depression Scale.
bSD = standard deviation.
MDE History
The MDE Screener22 is an 18-item measure designed to screen for the presence and absence of current and past MDEs. It assesses the presence of nine symptoms of depression according to the Diagnostic and Statistical Manual, Fourth Edition23 over a period of 2 weeks or more and assesses whether significant functional impairment is met within the same time span. All participants reported whether they had ever experienced any of the nine MDE symptoms during a 2-week period (lifetime MDE) and then whether any of the symptoms ever experienced for 2 weeks were currently present (current MDE). Those screening positive for a lifetime MDE but not for a current MDE were designated as having a past MDE. This resulted in three non-overlapping categories: past MDE, current MDE, and no MDE history. The screener has good agreement with established measures,24,25 and with clinician-administered diagnostic interviews.26 The screener was completed at baseline and all FU periods; for our purposes, we were interested only in this assessment at baseline. As noted above, this categorical variable was assumed to not be as good a dependent variable as a continuous measure (CES-D) to capture fluctuations in depression severity over time.
Nicotine Dependence and Smoking History
The Fagerström Test for Nicotine Dependence (FTND)27 is a standardized ordinal measure of nicotine dependence related to cigarette smoking and assesses quantity of cigarette consumption, compulsion to use, and dependence. At baseline, smokers were asked to complete the FTND, as well as indicate their smoking history and length of time of smoking. These data are presented in Table 1.
Smoking Status
Abstinence was assessed as self-reported 7-day point prevalence, which has been established by expert consensus as an appropriate outcome measure for smoking cessation28,29 and generally corresponds well with bioverification measures.30 The SRNT Subcommittee on Biochemical Verification also found that biochemical verification is not required and may not be desirable in large-scale studies where the optimal data collection methods are through the Internet.31
Data Analysis Plan
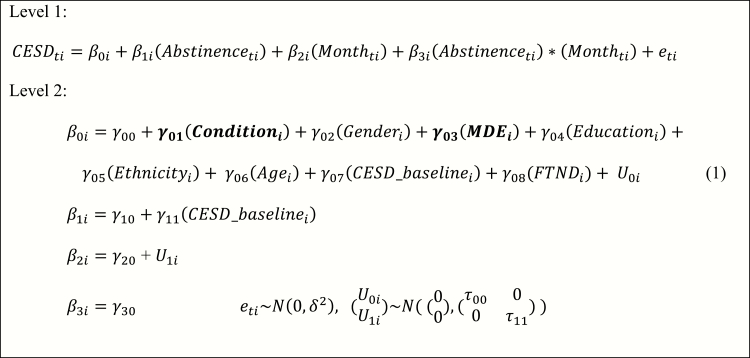
We applied MLM estimated using SAS PROC GLIMMIX to explore whether abstinence at any FU period was associated with concurrent CES-D scores. Our model accounted for those who at baseline identified as having a past, current, or no history of an MDE and demographic variables such as gender, age, ethnicity, FTND, and education as covariates. Table 2 provides CES-D means and smoking abstinence rates at 1-, 3-, 6-, and 12-month FU. We examined the overall pattern of and individual differences in CES-D scores over the FU periods. Restricted maximum likelihood was used for model estimation; −2 log-likelihood, Akaike’s Information Criterion (AIC) and Bayesian Information Criterion (BIC) were reported as model fit indices; and degrees of freedom were estimated using the Satterthwaite method. We selected an alpha level of .05 and assumed data were missing at random (data were missing for various reasons despite our best efforts to gather complete data using extensive FU procedures)32; under this assumption, MLM uses maximum likelihood estimation method to handle missing data, yielding consistent and asymptotically efficient estimations.33 A polynomial model was fit to estimate the effect of the intervention on the CES-D scores across the FU periods. Time was centered at the first FU period and the intercept represented the CES-D score at 1 month. The first of the five observations was at baseline (or month 0); as we examined change, we were interested in the four subsequent FU periods and change was expected as a linear (rather than quadratic or higher) form of time. To select appropriate polynomial trends, we visually inspected spaghetti diagrams,34 which revealed possible variation in change rate across smokers. Thus, we fit two unconditional models (ie, with and without a random slope) with only time as the fixed effect. The model with a random slope fit better than the model without a random slope, χ2(1) = 3.88, p = .05. For this reason, both the random intercept and random slope were included in the random portion of the model. We applied a top-down approach to model building (see Figure 1 for full details). Cohen’s f235 and the significance of the fixed effects were criteria of model selection. The resultant final model is shown in Table 3.
Figure 1.
Final model equations. In the Level 1 model, Center for Epidemiological Studies—Depression (CES-D) scores are a function of within-person abstinence status (smoking vs. abstinent), within-person follow-up month, and their interaction with the effect of β3i. In the Level 2 model, the individual intercept is a function of the number of between-person predictors including condition (1, 2, 3, 4), gender (female vs. male), Major Depressive Episode (MDE) history (none, past, current), education in years, ethnicity (Hispanic/Latino vs. Not Hispanic/Latino), age, baseline CES-D, and Fagerström Test for Nicotine Dependence. We also include the interaction between abstinence and baseline CES-D. Bolded variables are vectors. Please see Supplementary Materials for more details for the model and the model building process.
Table 3.
Model Results of Predicting Depression Symptoms on the CES-D
| Estimate | SEa | p | |
|---|---|---|---|
| Fixed effects | |||
| Intercept (γ00) | 13.73 | 1.25 | — |
| Condition 4b (γ01, reference = guide + e-mail + mood management + virtual group) | |||
| 1 = Guide (γ011) | −1.04 | 1.01 | .30 |
| 2 = Guide + e-mail (γ012) | −1.56 | 1.01 | .13 |
| 3 = Guide + e-mail + mood management (γ013) | −1.38 | 1.01 | .17 |
| Gender (γ02 reference = male) | −0.54 | 0.72 | .45 |
| MDEc history (γ03, reference = no MDE history) | |||
| Past MDE (γ031) | 2.61 | 0.98 | .01 |
| Current MDE (γ032) | 1.65 | 1.37 | .23 |
| Educationd (γ04) | −0.20 | 0.17 | .24 |
| Ethnicity (γ05 reference = not Hispanic or Latina/o) | 1.50 | 0.73 | .04 |
| Age (γ06) | 0.04 | 0.03 | .19 |
| Baseline CES-De (γ07) | 0.37 | 0.06 | <.01 |
| FTNDf (γ08) | 0.09 | 0.15 | .55 |
| Abstinence status (γ10 reference = abstinent) | 0.76 | 1.02 | .46 |
| Baseline CES-D × abstinence status (γ11) | 0.17 | 0.06 | .003 |
| Follow-up month (γ20) | −0.29 | 0.12 | .01 |
| Follow-up month × abstinence status (γ30) | 0.28 | 0.14 | .05 |
| Random effect | |||
| Random intercept (τ00) | 34.23 | 4.72 | <.01 |
| Random slope (τ11) | 0.22 | 0.08 | <.01 |
| Residual (δ2) | 54.07 | 3.33 | |
| Model fit | |||
| −2 log-likelihood | 8769.11 | ||
| AICg | 8775.11 | ||
| BICh | 8788.46 |
aSE = standard error.
bSee text for more details about each of the four conditions.
cMDE = major depressive episode.
dEducation in years.
eCES-D = Center for Epidemiological Studies—Depression Scale.
fFTND = Fagerström Test for Nicotine Dependence.
gAIC = Akaike’s Information Criterion.
hBIC = Bayesian Information Criterion.
Results
Intraclass Correlations and Baseline Longitudinal Model
MLM parses out variance due to between-person (ie, cross-sectional) versus within-person (ie, changes in an individual over time) differences. An empty model (ie, random intercept-only) was fit to determine the intraclass correlation of the outcome variable. Between-person variance was 81.7 and within-person variance was 58.5. The intraclass correlation demonstrated that 58.2% of the variance is due to between-person dependency and 41.8% to within-person dependency, indicating that depression severity varies at the within-person level and thus, the suitability of MLM for these data. As shown in Table 3, the random intercept was significant (p < .01), indicating significant variation in intercept across individuals. The overall intercept indicated that the initial CES-D score is 13.73 for those who at baseline have no MDE history and are abstinent at 1-month FU. Further, 95% of the individual intercepts fell within the interval of 13.73 ± 11.47.
Condition and Time on Depression Severity
Prior to our analyses, we tested the effect of condition on depression severity. As the primary focus of the intervention was on tobacco cessation, we sought to clarify that groups did not vary significantly in their depression severity scores based on condition. The omnibus test for condition was not significant F(3,258)=1, p = .40.
Baseline Depression History and Concurrent Abstinence Status on Depression Severity
To address our first hypothesis, the interaction between the covariate baseline MDE history and abstinence status on depression severity at each FU period was not significant in Model 2, p = .78. That is, the effect of concurrent abstinence status on depression severity at any FU period was not significantly different among those who at baseline reported no MDE history, a past MDE, and a current MDE. See Table 2 for abstinence status and depression severity scores at each FU period by baseline MDE history. Note that depression scores were lower for those who were abstinent at each assessment period for all three depression history groups. The main effect of baseline MDE history, however, was significant in the final model: compared with those who at baseline indicated no MDE history, those who at baseline reported a past MDE had a CES-D score that was 2.61 points higher than those reporting no MDE history at baseline, p = .01. Compared with those who at baseline indicated no MDE history, those who at baseline reported a current MDE had a higher depression severity score at each FU point, though this was not significant (p = .23).
Baseline Depression Severity and Concurrent Abstinence Status on Depression Severity
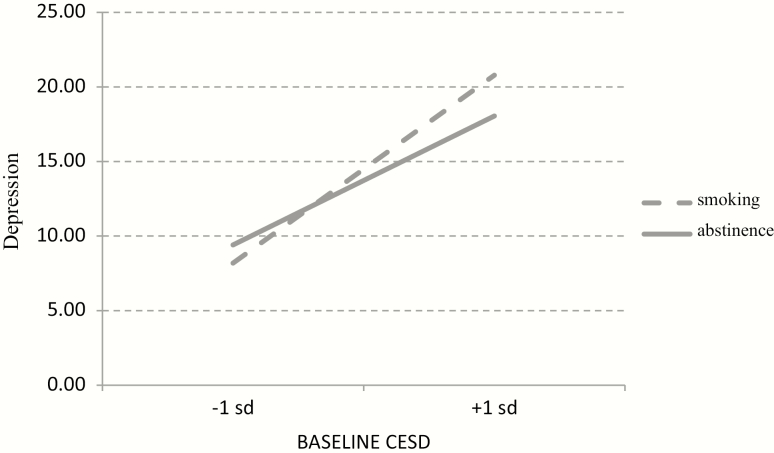
The interaction between baseline CES-D scores and abstinence status was significant, p < .0005. For every 1-unit increase in baseline CES-D scores, individuals who were smoking reported 0.17 higher concurrent CES-D scores at FU periods compared with those who were abstinent, as shown in Figure 2. That is, smokers with higher CES-D scores at baseline had higher CES-D scores at each FU if they were smoking at that FU than if they were abstaining. Those who were smoking scored 0.76 points higher on concurrent CES-D than those who were abstinent at 1-month FU, but this was not significant, p = .46. Over time, however, this increased by 0.28 points per month, p = .05, revealing that the interaction between abstinence status and FU period was significant. For example, at 12-month FU, a smoker scored 3.36 (.28*12) points higher on their concurrent CES-D than those who were abstinent. To ensure baseline MDE history or baseline CES-D scores did not contribute to this, we explored whether this effect was explained by 3-way interactions (ie, baseline MDE history × time × abstinence or baseline CES-D × time × abstinence status); neither of these was significant.
Figure 2.
Interaction between baseline CES-D score and abstinence status on depression severity. CES-D = Center for Epidemiological Studies—Depression.
Demographic and Smoking History Factors
Within this context and as seen in Table 3, FTND was not significant in the final model. Gender and education were not significant predictors of depression severity (p = .45 and p = .24, respectively). Ethnicity was significant, however: individuals who identified as Hispanic or Latino/a had a 1.50 higher CES-D score over time compared with those who identified as not Hispanic or Latina/o, p = .04.
Discussion
This study contributes to our understanding of the effects of time-varying abstinence and baseline current or past depression and severity of depressive symptoms on later depression severity in four main ways. First, the effect of time-varying abstinence on concurrent depression severity did not differ between those with and without a baseline MDE history. Second, compared with those who indicated no MDE history at baseline, those who reported a past MDE at baseline had higher depression severity scores at each FU. An MDE history appears related to worsened depression severity over time. These results are consistent with another report which found that a past MDE, and not abstinence, was associated with development of a later MDE.8 Our results indicate that this holds true for continuous measures of depression symptom severity.
Third, among those with higher depressive symptoms at the outset, smoking rather than abstinence appears related to exacerbated depression symptoms. This is consistent with a study from smoking heavy drinkers who used a similar analytical approach: those reporting higher baseline depression scores had higher depressive symptoms at FU if they were concurrently smoking compared with their abstinent counterparts.11 Another study among heavy drinkers similarly found smoking abstinence to be associated with reduced depressive symptoms.13
Finally, in our study, gender, education, and smoking history were not related to exacerbations in depression symptom severity. The negative findings of gender and education are consistent with a recent systematic review14 which found inconsistent findings on these factors. Ethnicity however, was related to depressive symptom severity: those identifying as Latina/o or Hispanic reported worse depressive symptoms over time. Past research has noted differences in the unique mental health needs of this population in the United States versus elsewhere.36,37 Our work adds to this larger conversation in the context of tobacco cessation and mental health.
Our results are also consistent with a systematic review38 on broader mental health systems which concluded that across 26 studies, abstinence is associated with improved mental health symptoms compared with those who continue to smoke. Furthermore, using MLM, we were able to capture the within-person variability in depression severity and smoking cessation that go beyond traditional methods of between-person change. This method has several advantages, which have been described by others11 and our work adds to a growing body of literature using these methods to capture complexity in the tobacco cessation process. Our study also adds encouraging results that among those who endorse high baseline depression severity, incorporating tobacco cessation can improve depression symptoms over time.
This study also adds to a slowly growing body of literature highlighting the importance of prioritizing tobacco cessation for those with mental health problems, ideally integrating such services into routine mental health care through adjunctive in-person or technological-based services. Major professional and public health organizations like the World Health Organization have published recommendations exhorting mental health providers to address tobacco cessation with every patient.39 Despite this, many mental health clinicians continue to believe this may be harmful to patients, a low priority, or not achievable by patients with severe mental disorders.40 This also goes against our own findings: depression improved with abstinence across all MDE history groups across FU periods (Table 2). Utilizing technology may help with access to these interventions and may provide an unprecedented opportunity to disseminate resources.41,42 Special attention to tobacco cessation for clinical populations with high rates of smoking (eg, individuals with severe mental illness) will also likely prove valuable. Future studies should explore the integration of technology-based tobacco cessation into routine mental health care.
Limitations
It is worth noting that in our sample, baseline CES-D scores for all three depression history groups were higher than in the general population, which have been reported to have a mean of 8.7 (standard deviation [SD] = 8.4).43,44 This suggests that either smokers or at least smokers seeking smoking cessation tools online may have higher depression levels than nonsmokers, which has been shown in prior studies.45 We did not explore the specificity of time-varying abstinence trajectories (eg, smoking to abstinent, abstinent to smoking, continuous smoking, and continuous abstinence) or latent class trajectories on depressive symptoms, which have been elegantly modeled by others.9,11 It is possible that specific characteristics of abstinence trajectories (eg, long stretches of smoking followed by abstinence or long stretches of abstinence followed by smoking) may have had an impact on depression severity symptoms that were not captured by our analyses. Although medication and other methods used to quit were assessed in our sample,15 this was not a focus of the current analysis. It is possible that our results may have been affected by nonstudy methods used to quit. We also did not have biochemical verification of abstinence status and relied on self-report for smoking status. This method has been recommended for large-scale studies without face-to-face contact.31 As noted above, our finding that identifying as Hispanic/Latino/a in a worldwide sample was associated with high depressive symptoms over time regardless of smoking status appears different from prior studies based solely in the United States.36 Future studies should further explore the replicability of this finding.
Our results highlight the differences in measuring depression diagnosis categorically (presence/absence of MDE history) versus as a continuous symptom variable (CES-D) that captures severity. It is possible such granularity is helpful for parsing out how depression and abstinence status interact in a more fine-grained way; however, it is also possible that these fluctuations in CES-D scores may represent statistically but not clinically significant change.46 Continuous measures may help us understand in finer detail the reductions and exacerbation of symptoms; whereas the diagnostic variables help us understand the clinical significance of these impacts. Our study was unable to clearly delineate this. Future studies should explore the clinical significance of mood changes over time.
Although our data were comprehensive in depression severity, we did not have information on whether participants were receiving concurrent psychological or pharmacological depression treatment at each time period. Nevertheless, our results are similar to another worldwide online randomized controlled trial, which explored as an outcome variable the prevalence of depression prescriptions and found that compared with current smokers, those who were abstinent reported a lower prevalence of depression prescriptions.47
This study addresses the concern that abstinence from smoking may negatively affect mood. Our data support earlier studies showing that successful abstinence at a specific timepoint is associated with lower depressive symptoms at the same timepoint. These findings should encourage the integration of tobacco cessation resources into routine mental health care settings and should be an encouragement to clinicians and smokers alike. Quitting smoking is associated with improved mood.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Supplementary data are available at Nicotine & Tobacco Research online.
Funding
This research was supported by grant 13RT-0050 from the Tobacco-Related Disease Research Program (Muñoz PI), which helped establish the University of California, San Francisco/San Francisco General Hospital Internet World Health Research Center (www.health.ucsf.edu), and by an infrastructure grant from the University of California Committee on Latino Research to the UCSF/SFGH Latino Mental Health Research Program, directed by Muñoz. Dr. Pérez-Stable’s time was funded by grant no. TW05935 from the Tobacco Research Network Program, Fogarty International 1033 Nicotine & Tobacco Research, Volume 11, Number 9 (September 2009) Center, National Cancer Institute, National Institute on Drug Abuse, National Institutes of Health, and by a grant from the National Cancer Institute, Redes en Acción, U01-CA86117..
Declaration of Interests
None declared.
Acknowledgments
The authors thank the Center for Health and Community (Nancy Adler, Director) for providing office space and additional resources to target Latino smokers, and Alinne Z. Barrera, Kevin Delucchi, Carlos Penilla, Leslie Lenert, and especially Leandro D. Torres, for having made major contributions to the implementation of the original study. Special thanks go to Google, Inc for awarding us an AdWords grant, which provided us with the ability to recruit smokers worldwide using Google sponsored links. Finally, we want to thank the following research assistants who made the follow-up phone calls 24-h a day to reach smokers around the world: Yasmin Lozano, Erika Torres, Alejandra Calderon, Jasmine Alvarez, Elma Lorenzo, Yunuen Mora, María José Herrera, Jessica Z. Borja, Luis Flores, Sam Ruiz, Wendy Zepeda-Diaz, Karina Alcalá, Celia P. Casco, Ritika Dhami, and Shelley K. Dhillon. This work was performed at the University of California, San Francisco, Department of Psychiatry at San Francisco General Hospital. The findings and conclusions in this article are those of the authors and do not necessarily represent the views or the official position(s) of the National Institutes of Health.
References
- 1. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. R Coll Phys R Coll Psychiatr. Smoking and Mental Health. London: R Coll. Phys; 2013. [Google Scholar]
- 3. Glassman AH, Stetner F, Walsh BT, et al. Heavy smokers, smoking cessation, and clonidine. Results of a double-blind, randomized trial. JAMA. 1988;259(19):2863–2866. [PubMed] [Google Scholar]
- 4. Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. Am J Psychiatry. 1997;154(2):263–265. [DOI] [PubMed] [Google Scholar]
- 5. Glassman AH, Helzer JE, Covey LS, et al. Smoking, smoking cessation, and major depression. JAMA. 1990;264(12):1546–1549. [PubMed] [Google Scholar]
- 6. Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet. 2001;357(9272):1929–1932. [DOI] [PubMed] [Google Scholar]
- 7. Tsoh JY, Humfleet GL, Muñoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000;157(3):368–374. [DOI] [PubMed] [Google Scholar]
- 8. Torres LD, Barrera AZ, Delucchi K, Penilla C, Pérez-Stable EJ, Muñoz RF. Quitting smoking does not increase the risk of major depressive episodes among users of Internet smoking cessation interventions. Psychol Med. 2010;40(3):441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kahler CW, Brown RA, Ramsey SE, et al. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. J Abnorm Psychol. 2002;111(4):670–675. [DOI] [PubMed] [Google Scholar]
- 10. Asma S, Gonghuan Y, Samet J, et al. Tobacco. In Detels R, Beaglehole R, Lansang MA, Guilliford M, eds. Oxford Textbook of Public Health. 4th ed.Oxford, UK: Oxford University Press; 2009;1277–1302. [Google Scholar]
- 11. Kahler CW, Spillane NS, Busch AM, Leventhal AM. Time-varying smoking abstinence predicts lower depressive symptoms following smoking cessation treatment. Nicotine Tob Res. 2011;13(2):146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munafò MR, Heron J, Araya R. Smoking patterns during pregnancy and postnatal period and depressive symptoms. Nicotine Tob Res. 2008;10(11):1609–1620. [DOI] [PubMed] [Google Scholar]
- 13. Lechner WV, Sidhu NK, Cioe PA, Kahler CW. Effects of time-varying changes in tobacco and alcohol use on depressive symptoms following pharmaco-behavioral treatment for smoking and heavy drinking. Drug Alcohol Depend. 2019;194:173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fluharty M, Taylor AE, Grabski M, Munafò MR. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob Res. 2017;19(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muñoz RF, Barrera AZ, Delucchi K, Penilla C, Torres LD, Pérez-Stable EJ. International Spanish/English Internet smoking cessation trial yields 20% abstinence rates at 1 year. Nicotine Tob Res. 2009;11(9): 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muñoz RF, Lenert LL, Delucchi K, et al. Toward evidence-based Internet interventions: a Spanish/English Web site for international smoking cessation trials. Nicotine Tob Res. 2006;8(1):77–87. [DOI] [PubMed] [Google Scholar]
- 17. Pérez-Stable EJ, Sabogal F, Marín G, Marín BV, Otero-Sabogal R. Evaluation of “Guia para Dejar de Fumar,” a self-help guide in Spanish to quit smoking. Public Health Rep. 1991;106(5):564–570. [PMC free article] [PubMed] [Google Scholar]
- 18. Muñoz RF, Marín BV, Posner SF, Pérez-Stable EJ. Mood management mail intervention increases abstinence rates for Spanish-speaking Latino smokers. Am J Community Psychol. 1997;25(3):325–343. [DOI] [PubMed] [Google Scholar]
- 19. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 20. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. [DOI] [PubMed] [Google Scholar]
- 21. Cuijpers P, Bolujit P, van Straten A. Screening of adolescents through the Internet: sensitivity and specificity of two screening questionnaires. Eur Child Adolesc Psychiatry. 2008;17(1):32–38. [DOI] [PubMed] [Google Scholar]
- 22. Muñoz RF. (1998). The Major Depression Episode (MDE) Screener.https://i4health.paloaltou.edu/resources.html.
- 23. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: Author; 1994. [Google Scholar]
- 24. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- 25. Muñoz RF, McQuaid JR, Gonzalez GM, Dimas J, Rosales VA. Depression screening in a women’s clinic: using automated Spanish- and English-language voice recognition. J Cons Clin Psychol. 1 999;67(4): 502–510. [DOI] [PubMed] [Google Scholar]
- 26. Vázquez FL, Muñoz RF, Blanco V, López M. Validation of Muñoz’s mood screener in a nonclinical Spanish population. Eur J Psychol Assess. 2008;24(1):57–64. [Google Scholar]
- 27. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 28. Cheung KL, de Ruijter D, Hiligsmann M, et al. Exploring consensus on how to measure smoking cessation. A Delphi study. BMC Public Health. 2017;17(1):890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 30. McFall M, Saxon AJ, Malte CA, et al. ; CSP 519 Study Team Integrating tobacco cessation into mental health care for posttraumatic stress disorder: a randomized controlled trial. JAMA. 2010;304(22): 2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res. 2019:ntz132, doi: 10.1093/ntr/ntz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Little RJ, Rubin D.. Statistical Analysis with Missing Data. 2nd ed Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- 33. Goldstein H. Multilevel Statistical Models. 4th ed. New York: John Wiley; 2011. [Google Scholar]
- 34. Kwok OM, Underhill AT, Berry JW, Luo W, Elliott TR, Yoon M. Analyzing longitudinal data with multilevel models: an example with individuals living with lower extremity intra-articular fractures. Rehabil Psychol. 2008;53(3):370–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A practical guide to calculating Cohen’s f(2), a measure of local effect size, from PROC MIXED. Front Psychol. 2012;3:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castro Y, Costello TJ, Correa-Fernández V, et al. Differential effects of depression on smoking cessation in a diverse sample of smokers in treatment. Am J Prev Med. 2011;41(1):84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alegría M, Canino G, Shrout PE, et al. Prevalence of mental illness in immigrant and non-immigrant U.S. Latino groups. Am J Psychiatry. 2008;165(3):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ. 2014;348:g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prochaska JJ. Smoking and mental illness—breaking the link. N Engl J Med. 2011;365(3):196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muñoz RF, Bunge EL, Chen K, et al. Massive Open Online Interventions: a novel model for delivering behavioral-health services worldwide. Clin Psychol Sci. 2016;4(2):194–205. [Google Scholar]
- 42. Muñoz RF, Chavira DA, Himle JA, et al. Digital apothecaries: a vision for making health care interventions accessible worldwide. Mhealth. 2018;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. National Center for Health Statistics. Basic data on depressive symptomatology. United States Vital and Health Statistics. Series 11, No. 216.DHEW Publication No. (PHS) 80-1666, US GPO; 1980. [Google Scholar]
- 44. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. [DOI] [PubMed] [Google Scholar]
- 45. Pérez-Stable EJ, Marín G, Marín BV, Katz MH. Depressive symptoms and cigarette smoking among Latinos in San Francisco. Am J Public Health. 1990;80(12):1500–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Busch AM, Wagener TL, Gregor KL, Ring KT, Borrelli B. Utilizing reliable and clinically significant change criteria to assess for the development of depression during smoking cessation treatment: the importance of tracking idiographic change. Addict Behav. 2011;36(12):1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shahab L, Andrew S, West R. Changes in prevalence of depression and anxiety following smoking cessation: results from an international cohort study (ATTEMPT). Psychol Med. 2014;44(1):127–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.