Abstract
Background: We aimed at analysing the association between dietary fibre intake during childhood and cardiovascular health markers. Methods: We used observational longitudinal analysis and recorded diet using 3-day diaries at the ages of 3, 4, 5, 6, and 8 years in children from the EU Childhood Obesity Project Trial. At the age of 8, waist circumference, systolic and diastolic blood pressure (SBP and DBP) and biochemical analyses (lipoproteins, triglycerides and homeostasis model for insulin resistance (HOMA-IR)) were evaluated. Those parameters were combined into a cardiometabolic risk score through the sum of their internal z-scores. Results: Four-hundred children (51.8% girls) attended to the 8-year visit with a 3-day diary. Adjusted linear regression models showed that children who repeatedly stayed in the lowest tertile of fibre intake during childhood had higher HOMA-IR (p = 0.004), higher cardiometabolic risk score (p = 0.02) and a nonsignificant trend toward a higher SBP at 8 years. The higher the dietary intake of soluble fibre (from fruits and vegetables) at 8 years, the lower the HOMA-IR and the cardiometabolic risk score (p = 0.002; p = 0.004). SBP was directly associated with fibre from potatoes and inversely with fibre from nuts and pulses. Conclusion: A diet rich in dietary fibre from fruits, vegetables, pulses and nuts from early childhood was associated to a healthier cardiovascular profile, regardless of children’s weight.
Keywords: dietary fibre, children, cardiovascular risk, soluble fibre, insoluble fibre, resistant starch, fibre food sources
1. Introduction
An optimal diet during childhood must be adequate to support normal growth and development. At the same time, the diet must be aimed at reducing the risk of diet-related chronic diseases during adulthood. Starting proper dietary habits from early childhood could help to maintain them throughout life [1]. Dietary fibre intake is frequently related to the digestive and cardiovascular health of the adult and child population [2,3]. Physiological benefits of fibre on health are produced by several of its properties, such as viscosity, solubility and fermentability. The main effects derived from the viscosity of soluble fibre are responsible for reducing lipid absorption, slowing carbohydrate absorption, producing satiety and providing part of its anticarcinogenic potential. Insoluble fibres produce an increase in faecal mass that accelerates intestinal transit, which is useful in the treatment and prevention of chronic constipation and colon cancer [4,5]. More recent studies have highlighted the fermentability of the fibre, in contact with the colonic microflora, as the most important property, since a multitude of local and systemic effects derive from it [6]. The short-chain fatty acids, which result from the fermentation process [7], are responsible for several functions related to the cardiovascular and digestive tract health, such as an anti-inflammatory action, protection against colonic carcinogenesis, a direct effect on the synthesis of cholesterol and glucose, a reduction of peripheral insulin resistance and a prebiotic effect [8,9,10,11,12,13,14,15].
In adults, a low dietary fibre intake has been related to risk indicators of cardiovascular disease such as hypercholesterolemia, diabetes mellitus, high blood pressure and obesity, as well as gastrointestinal diseases including constipation, irritable bowel syndrome, ulcerative colitis, diverticular disease and colorectal cancer [16,17,18,19]. Currently, it is proposed that a dietary fibre intake between 25–35 g a day in adults may contribute to reducing the prevalence of some of these noncommunicable diseases [20,21,22].
Studies in children analysing the association between dietary fibre intake and cardiovascular health are scarce and have been performed mainly in adolescence [23,24]. Therefore, studies are needed to evaluate the relationship of dietary fibre intake with health indicators in young children. We aimed at analysing the relation of the dietary fibre intake in children from five European countries at the ages of 3 to 8 years to cardiovascular health markers such as obesity, blood lipids, blood pressure and glucose metabolism.
2. Materials and Methods
2.1. Study Design and Population
This was an observational longitudinal analysis assessing the association between dietary fibre intakes during childhood, from 3 to 8 years, on cardiovascular health at 8 years. This study is a secondary analysis of data collected in the EU-CHOP Childhood Obesity Project trial (NCT00338689), carried out in Germany, Belgium, Italy, Poland and Spain. The EU-CHOP was a double-blind, randomized dietary intervention trial that recruited children from birth (October 2002 to July 2004) and followed their dietary intake until the age of 8 years. Recruited infants were healthy, full-term and born from uncomplicated pregnancies with a normal weight for gestational age. Formula-fed infants were randomly assigned to either a lower or a higher protein content formula, with the aim to assess the effect on later obesity risk. A group of breastfed infants was recruited and followed up as the observational gold standard group. Details of the clinical trial have been previously published [25,26]. The data used for this study were collected prospectively during the post-intervention follow-up visits.
2.2. Dietary Intake Assessment
Dietary intake was recorded using 3-day diet diaries at the ages of 3, 4, 5, 6, and 8 years. The amounts of recorded foods were coded by trained research nutritionists following standardized procedures [27,28] and were introduced in a dedicated program for the conversion to nutrients. The program contained the German BLS II.2 food composition database [29], and the nutrient content of local foods from the different countries was added using national food composition databases. The average intakes of energy (kcal/day), fibre (g/day, g/1000 kcal) and macronutrients (g/day, g/1000 kcal and as % of total energy) of the 3 days were calculated. Furthermore, the amount of fibre (g, g/1000 kcal) was quantified according to its food source as that from cereals and derivatives (as an approximation to insoluble fibre), fruits and vegetables (as an approximation to soluble fibre), potatoes and tubers (as an approximation to resistant starch) and that from legumes and nuts that contain different types of soluble and insoluble fibres and resistant starch [30].
2.3. Health Variables
At the age of 8, we measured anthropometry. The main health outcome measures were blood pressure and blood sample parameters related to cardiovascular risk.
2.3.1. Anthropometry
At 8 years of age, the measurements of weight (kg), height (cm), calculation of the body mass index (BMI) (kg/m2) and waist circumference (cm) were analysed. Weight was measured in underwear on a SECA 702/703 digital scale (10 g precision). Height was measured with a SECA 242 digital statiometer (1 mm precision). Standardized procedures for measuring the child’s height included having the feet slightly apart, with the back of the head, shoulder blades, buttocks and heels touching the vertical board whenever possible. The mother or caregiver was asked to hold the child’s knees and ankles to help keep the legs straight while the researcher held the child’s head in the Frankfort plane and read the measurement. Weight, height and BMI z-scores were calculated using World Health Organization (WHO) references [31].
2.3.2. Blood Pressure
Systolic and diastolic blood pressures (mmHg) were measured at 8 years of age using a Dinamap ProCare 100/200 digital blood pressure monitor following a standardized procedure. Blood pressure was measured after at least 15 min from arrival at the centre and after at least 5 min of rest. The measurement was taken in duplicate and on the left arm supported by a slightly elevated horizontal support (that is, close to the level of the heart). Both measurements were taken separated by at least 5 min, and the mean between them was used for statistical analysis. Systolic and diastolic blood pressure are presented as raw measurements (mmHg). Furthermore, they were standardized as percentiles for height and sex using the references from the American Academy of Pediatrics, 2017 [32].
2.3.3. Blood Sample Parameters
At 8 years of age, a fasting venous blood sample was drawn. Total cholesterol, low-density lipoprotein cholesterol (LDL cholesterol), high-density lipoprotein cholesterol (HDL cholesterol), triglycerides, insulin and glucose were analysed. Total cholesterol (mg/dL), LDL cholesterol (mg/dL), HDL cholesterol (mg/dL), triglycerides (mg/dL), and glucose (mg/dL) were assessed in the clinical chemistry laboratories of study centres with routine methods used for clinical diagnosis [33]. Total and HDL cholesterol, triglycerides and glucose were analysed by indirect or enzymatic potentiometric methods. LDL cholesterol values were calculated by the Friedewald equation [34]. Insulin (AIU/mL) was quantified at the Department of Biochemistry, Radioimmunology and Experimental Medicine of the Institute for Children’s Memorial Health using the immunoradiometric assay (DiaSource, Nivelles, Bélgica) [35]. The evaluation of the homeostasis model for insulin resistance (HOMA-IR) was calculated as an approximation for insulin resistance [36,37].
2.3.4. Cardiometabolic Risk Assessment
The cardiometabolic risk factor was calculated as a continuous variable equal to the sum of the internal z-scores for both sexes separately of the waist circumference, HDL cholesterol (multiplied by -1 to equate the sense of its beneficial effect to the other parameters), LDL cholesterol, triglycerides, HOMA-IR, DBP percentile and SBP percentile, as described in other studies [2,38,39]. A higher score was indicative of a less favourable cardiometabolic profile. This score could be summed to a maximum of around ±14 points.
2.4. Ethics
The study complied with the ethical requirements of the Declaration of Helsinki [40]. The study was approved by the ethics committees of all research centres and parents or legal guardians obtained the study information and signed the informed consent to participate.
2.5. Statistical Analyses
Continuous normally distributed variables are shown as mean and standard deviation, and continuous not normally distributed variables are presented as median and interquartile range. The distribution of the sample was assessed by means of graphical representation. Categorical variables are described as n and percentage of the total. Differences between sexes in continuous variables were assessed either through Student’s t-test or Mann-Whitney U-test according to the distribution of the variables. A cross-sectional analysis of fibre intake (g/1000 kcal) and its relationship with health variables at 8 years was performed using linear regression analysis, in which fibre intake (total or by subtypes) was adjusted by country of origin (Germany, Belgium, Italy, Poland and Spain), sex, average energy intake (kcal/day), maternal education (high, medium or low) and BMI (when this was not the dependent variable of the model). The feeding type (intervention low vs. high protein content during the first year) and breastfeeding (at least 3 months) was considered for adjustment in linear regression models but discarded after checking that there was no effect on the cardiovascular health related markers at that age.
The relationship between repeatedly staying in a low fibre intake tertile throughout childhood (at least 3 timepoints out of 5 between the 3 and 8 years of age) and health variables at 8 years was analysed using one-way ANOVA models adjusted for country of origin, maternal education, sex and BMI. For this analysis, participants who had reported fibre intakes in the lowest tertile on at least 3 occasions were considered.
Statistical significance was accepted at the level of p < 0.05.
All statistical analyses have been conducted with IBM SPSS Statistics for Windows, Version 26.0 Released 2019 (IBM Corp. Armonk, NY: IBM Corp).
3. Results
A total of 587 children attended the visit at age 8 years (277 boys, 310 girls). Blood pressure was obtained in 556 children (53.2% were girls). Among those, 400 participants (51.8% girls) completed the 3-day dietary diary. From these, 361 (49.9% girls) fasted blood samples were obtained.
3.1. Dietary Intake
Table 1 describes the daily intakes of energy, macronutrients and fibre by age. Stratification and comparison by sex is shown in Appendix A, Table A1.
Table 1.
Energy, macronutrient and fibre intakes by age group.
| Energy (kcal/Day) Mean (±SD) |
Proteins (g/Day) Mean (±SD) |
Lipids (g/Day) Mean (±SD) |
Carbohydrates (g/Day) Mean (±SD) |
Total Fibre (g/Day) Mean (±SD) |
Total Fibre (g/1000 kcal) Mean (±SD) |
|
|---|---|---|---|---|---|---|
| 3 years, n = 534 |
1221 (243) | 47.0 (12.2) | 47.1 (12.8) | 152.6 (37.2) | 8.3 (3.3) | 6.9 (2.7) |
| 4 years, n = 504 |
1317 (249) | 50.0 (13.1) | 52.0 (14.4) | 163.2 (34.5) | 9.2 (3.4) | 7.1 (2.4) |
| 5 years, n = 447 |
1394 (268) | 52.1 (13.6) | 54.7 (14.4) | 174.5 (41.1) | 9.9 (3.5) | 7.2 (2.2) |
| 6 years, n = 469 |
1479 (253) | 55.2 (12.7) | 58.1 (14.3) | 184.8 (39.5) | 10.8 (3.6) | 7.3 (2.4) |
| 8 years, n = 400 |
1598 (304) | 61.1 (15.4) | 65.4 (17.8) | 192.4 (42.1) | 12.1 (4.0) | 7.7 (2.3) |
Table 2 describes the total fibre intake and that adjusted for energy according to the food source and in all age groups. The sources of consumed dietary fibre were divided into four food groups based on the corresponding fibre type. Stratification and comparison by sex is shown in Appendix A, Table A2.
Table 2.
Fibre intake by age according to food source.
| Insoluble | Soluble | Resistant Starch | Fibre from Pulses and Nuts | |||||
|---|---|---|---|---|---|---|---|---|
| g/Day | g/1000 kcal | g/Day | g/1000 kcal | g/Day | g/1000 kcal | g/Day | g/1000 kcal | |
| 3 years, n = 531 |
3.32 (2.32, 4.41) | 2.73 (1.99, 3.62) | 3.27 (1.99, 4.98) | 2.72 (1.71, 4.08) | 0.36 (0.10, 0.66) | 0.30 (0.09, 0.54) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| 4 years, n = 503 |
3.68 (2.63, 4.94) | 2.86 (2.15, 3.69) | 3.80 (2.41, 5.24) | 2.87 (1.79, 3.89) | 0.39 (0.07, 0.73) | 0.30 (0.05, 0.53) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) |
| 5 years, n = 445 |
4.15 (3.21, 5.38) | 3.06 (2.41, 3.73) | 3.76 (2.43, 5.47) | 2.65 (1.77, 3.84) | 0.39 (0.07, 0.73) | 0.27 (0.05, 0.52) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) |
| 6 years, n = 468 |
4.78 (3.64, 5.85) | 3.17 (2.57, 3.94) | 3.85 (2.46, 5.93) | 2.65 (1.73, 3.85) | 0.41 (0.10, 0.87) | 0.30 (0.07, 0.59) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) |
| 8 years, n = 399 |
5.68 (4.53, 7.23) | 3.60 (2.94, 4.49) | 4.03 (2.61, 5.80) | 2.58 (1.72, 3.65) | 0.55 (0.18, 1.02) | 0.35 (0.11, 0.63) | 0.00 (0.00, 0.02) | 0.00 (0.00, 0.01) |
Data presented as median (IQR). Insoluble fibre includes fibre from cereals, pasta, rice, cookies, cakes and convenience foods. Soluble fibre includes that from fruits and vegetables. Resistant starch includes fibre from potatoes and potato products. Pulses and nuts are groups separately, containing soluble, insoluble and resistant starch.
3.2. Association between Dietary Fibre Intake and Health in Children—Cross-Sectional Analyses
The cardiovascular health-related variables (anthropometric values, the biochemical parameters and blood pressure) at 8 years are described in Table 3. Linear regression analyses shows that children who ate a higher amount of fibre at 8 years of age had a higher BMI, but the model explains that only the 4.8% of total BMI at 8 years (Appendix B, Table A3). No further associations were found in cross-sectional analyses between fibre intake and health outcomes.
Table 3.
Cardiovascular health-related parameters at age 8 years.
| All | Boys | Girls | |
|---|---|---|---|
| Anthropometry | Mean (±SD) | Mean (±SD) | Mean (±SD) |
| Weight (kg) | 28.6 (6.1) | 28.7 (6.6) | 28.6 (5.7) |
| Height (cm) | 129.6 (5.7) | 130.2 (5.8) | 129.0 (5.6) * |
| BMI (kg/m2) | 16.9 (2.6) | 16.8 (2.8) | 17.0 (2.4) |
| Abdominal circumference (cm) | 59.4 (7.3) | 59.5 (7.5) | 59.3 (7.1) |
| Weight for age (z score) | 0.59 (1.19) | 0.59 (1.31) | 0.60 (1.07) |
| Height for age (z score) | 0.43 (0.99) | 0.47 (1.03) | 0.39 (0.96) |
| BMI for age (z score) | 0.46 (1.21) | 0.40 (1.37) | 0.52 (1.04) |
| Biochemical Parameters | Mean (±SD) | Mean (±SD) | Mean (±SD) |
| Glucose (mg/dL) | 84 (8) | 84 (7) | 83 (8) * |
| Total cholesterol (mg/dL) | 167 (27) | 164 (27) | 169 (27) |
| HDL cholesterol (mg/dL) | 60 (15) | 61 (16) | 59 (14) |
| LDL cholesterol (mg/dL) | 94 (25) | 91 (24) | 97 (25) * |
| Triglycerides (mg/dL) | 60 (26) | 55 (22) | 64 (29) ǂ |
| Insulin (µIU/mL) | 8.75 (3.15) | 8.43 (2.96) | 9.09 (3.32) * |
| HOMA-IR § | 1.82 (0.70) | 1.78 (0.66) | 1.88 (0.74) |
| Blood Pressure | Mean (±SD) | Mean (±SD) | Mean (±SD) |
| Systolic blood pressure (mmHg) | 100 (10) | 100 (9) | 100 (10) |
| Diastolic blood pressure (mmHg) | 57 (7) | 56 (7) | 58 (7) * |
| Systolic blood pressure (percentile) | 57.8 (27.5) | 55.6 (27.9) | 59.7 (28.2) |
| Diastolic blood pressure (percentile) | 44.5 (21.8) | 41.5 (21.4) | 47.2 (21.9) * |
| Cardiovascular Risk | Mean (±SD) | ||
| Cardiometabolic score | −0.20 (3.85) | −0.03 (3.87) | −0.39 (3.86) |
* p < 0.05, ǂ p < 0.01, p-value for Student’s t-test for comparison boys vs. girls. Skewed variables tested in logarithmic form; § Not normally distributed variable, median 1.70 (IQR: 1.31, 2.19); boys median 1.66 (IQR: 1.32, 2.10); girls median 1.78 (IQR: 1.31, 2.25).
3.3. Fibre Intake According to Dietary Source and Association to Health—Cross-Sectional Analyses
When relating dietary fibre intake according to its source, a higher intake of soluble fibre (fibre from fruit and vegetables) at 8 years was related to lower HOMA-IR and cardiometabolic risk score (Table 4). Systolic blood pressure was inversely associated with the intake of fibre from nuts and legumes and was directly associated with the consumption of resistant starch. Triglycerides levels were not associated to dietary fibre intakes from different food sources.
Table 4.
Intake of dietary fibre from different sources and cardiovascular health parameters at age 8 y.
| HOMA-IR | Systolic Blood Pressure | Triglycerides | Cardiometabolic Risk Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI (Min, Max) |
p-Value | B | 95% CI (Min, Max) |
p-Value | B | 95% CI (Min, Max) |
p-Value | B | 95% CI (Min, Max) |
p-Value | ||
| Unadjusted analyses | Insoluble fibre | 0.005 | (−0.008, 0.017) | 0.445 | 1.351 | (0.13, 2.56) | 0.030 | 0.006 | (−0.006, 0.017) | 0.346 | 0.148 | (−0.130, 0.426) | 0.296 |
| Resistant starch | 0.028 | (−0.009, 0.065) | 0.137 | 9.698 | (4.67, 14.72) | <0.001 | 0.015 | (−0.020, 0.050) | 0.397 | 1.323 | (0.495, 2,151) | 0.002 | |
| Soluble fibre | −0.010 | (−0.018, −0.001) | 0.028 | −0.358 | (−1.41, 0.69) | 0.505 | −0.001 | (−0.009, 0.006) | 0.709 | −0.068 | (−0.261, 0.124) | 0.486 | |
| Fibre from pulses and nuts | 0.015 | (−0.004, 0.034) | 0.131 | −1.917 | (−4.49, 0.65) | 0.144 | −0.008 | (−0.026, 0.010) | 0.381 | −0.301 | (−0.734, 0.131) | 0.171 | |
| R2 = 0.016 | R2 = 0.040 | NS | R2 = 0.37 | ||||||||||
| Adjusted analyses | Insoluble fibre | 0.000048 | (−0.010, 0.010) | 0.982 | −0.48 | (−1.70, 0.74) | 0.440 | 0.006 | (−0.005, 0.016) | 0.281 | 0.059 | (−0.14, 0.26) | 0.566 |
| Resistant starch | −0.014 | (−0.046, 0.017) | 0.370 | 4.60 | (0.06, 9.19) | <0.0496 | −0.023 | (−0.057, 0.011) | 0.104 | 0.087 | (−0.56, 0.74) | 0.793 | |
| Soluble fibre | −0.008 | (−0.016,−0.001) | 0.025 | −0.19 | (−1.16, 0.77) | 0.694 | −0.005 | (−0.014, 0.001) | 0.182 | −0.159 | (−0.30, −0.00) | 0.037 | |
| Fibre from pulses and nuts | 0.012 | (−0.004,0.028) | 0.148 | −2.24 | (−4.53, 0.05) | 0.055 | 0.012 | (−0.007, 0.027) | 0.183 | −0.045 | (−0.37, 0.20) | 0.784 | |
| R2 = 0.346 | R2 = 0.252 | R2 = 0.207 | R2 = 0.489 | ||||||||||
Cross-sectional analyses on the intakes of different fibre types adjusted by energy (g/1000 kcal) at 8 years old on health outcome parameters at the same age. All fibre types entered together in a linear regression model, unadjusted and adjusted by country, sex, average energy intake at 8 years old, maternal education and BMI. NS: Overall goodness of fit of the model was not significant.
3.4. Association between Dietary Fibre Intake and Health in Children—Longitudinal Analyses
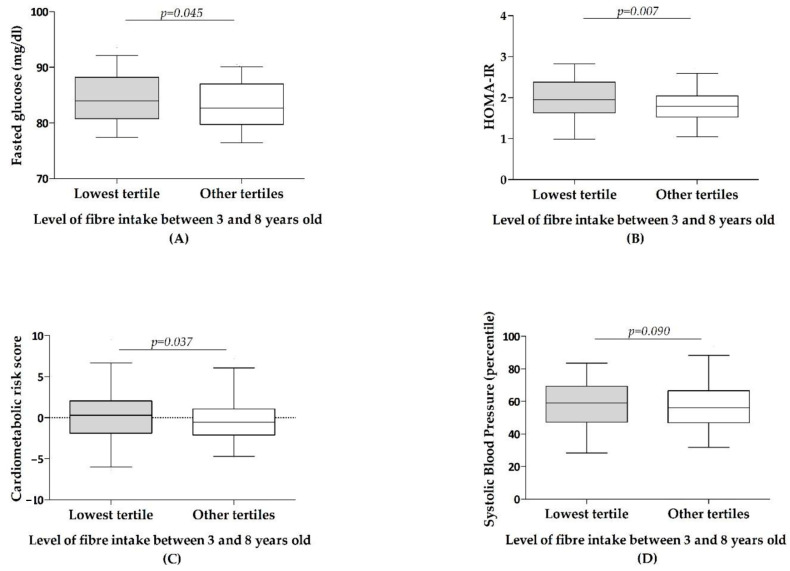
Of the total of 400 children who attended the 8-year visit with dietary diary, 89 had repeatedly remained in the lowest tertile of dietary fibre intake between 3 and 8 years (at least three times out of five in that period). Children staying repeatedly in the lowest tertile of fibre intake throughout childhood, at 8 years, exhibited statistically significant higher glucose levels, higher HOMA-IR, higher cardiometabolic risk score and a trend for a higher systolic blood pressure, as shown by ANOVA adjusted models (Figure 1). Models carried out on triglycerides, HDL and LDL cholesterol and diastolic blood pressure did not reveal any significant association.
Figure 1.
Cardiovascular health parameters according to the level of fibre intake throughout childhood. Children classified as being repeatedly in the lowest tertile of fibre intake between 3 and 8 years old (at least three times out of five in this period) or in other tertiles and/or not repeatedly in the lowest tertile. Results from one-way ANOVA models adjusted by country, sex, body mass index and maternal education for glucose (A), HOMA-IR (B), Cardiometabolic risk (C) and systolic blood pressure (D).
4. Discussion
This is the first multicentre European study investigating the association of dietary fibre intake and its sources with cardiovascular health in young children.
Fibre dietary intakes at 8 years old did not show any significant association to cardiovascular health at the same age. However, maintaining low fibre intakes during young childhood was associated with a worse glucose tolerance, systolic blood pressure and overall cardiometabolic risk at the age of 8. Not all fibre types were associated with these benefits. The consumption of fibre from fruits and vegetables was associated with a lower insulin resistance index and cardiometabolic risk, and the consumption of fibre from legumes and nuts was associated with a trend to lower SBP. These associations were not explained by differences in energy intake, as fibre intake was adjusted for total energy intake. Insoluble fibre intake was not associated to health outcome parameters and resistant starch was associated with a trend to a higher SBP.
Different prospective studies and clinical trials in adults have shown that following a diet rich in fibre is related to a lower cardiometabolic risk by improving the lipid profile, reducing blood pressure and insulin resistance [18,41,42,43]. Although the metabolic syndrome is well defined in adults, there is no universal and uniform definition for children and adolescents [44,45] and frequency in this age group is low. This was the reason why we used a continuous score as a cardiometabolic risk score, using common variables of the metabolic syndrome for the analyses of our study [38].
In children, studies that link dietary fibre intake and cardiometabolic health are scarce and mostly in adolescents or pre-adolescents [46,47,48,49,50]. We found only two published studies that analysed the relationship between dietary fibre intake and cardiometabolic risk in young children, which reported associations to lipid profiles but not to glucose metabolism [2,23].
In adults, effects of fibre intake on weight control and lower obesity risk have been attributed to different mechanisms, including the satiating power of fibres [10,51]. In children, concern was raised that a high fibre intake could decrease energy intake and the absorption of critical nutrients, thereby inducing potential harm. Data from the several publications have shown that high fibre intakes do not cause growth problems or lack of nutrients in healthy children [49,52]. In our study, the children who consumed more fibre, regardless of its source, had a higher BMI (without reaching pathological variations). This association was independent of the total energy intake, and the results obtained in our analyses associating dietary fibre consumption and cardiovascular health were independent of the BMI. Therefore, increasing the intake of dietary fibre can improve cardiovascular health risk markers in the short and long term, even in young children independently of weight.
Dietary fibre intake improves glucose homeostasis by causing a decrease in blood glucose, a reduction in hyperinsulinism and an improvement in insulin resistance [17,43]. This effect has been classically attributed to soluble fibre (which, through the formation of viscous gels in the intestine, reduces glucose absorption and decreases insulin secretion), but some studies have found significant effects from insoluble fibre as well [10,51,53]. In children, the relationship between fibre intake and glycaemic control had not been well demonstrated [24]. Our results are novel, as we have observed a similar trend to that from adults at an early age. Thus, a sustained low fibre intake from childhood could be associated to a poorer glucose tolerance. Furthermore, a diet with a higher amount of soluble fibre at the age of 8 from fruits and vegetables could reduce insulin resistance index as several studies in adults have previously shown [10,17,54].
Similar to studies previously published that have focused on children, adolescents and adults [23,49,50], we found a trend for a lower LDL cholesterol at 8 years of age with higher fibre intakes. The improved lipid profile may occur through several mechanisms, such as the reduction of cholesterol absorption, the blocking of its hepatic synthesis (through propionate derived from fibre fermentation) and the reduction of circulating cholesterol (due to increased cholesterol utilisation for the synthesis of bile acids, stimulated by intestinal bile acid sequestration by fibre) [55].
Higher fibre intakes have been related to lower systolic and diastolic BP in adults, as well as an improvement in BP levels in hypertensive patients [56,57,58]. In children, studies linking dietary fibre intake and BP are very scarce. Our study corroborates the association found in adults for the first time in children. Children with low fibre intakes maintained over time exhibited higher figures of systolic blood pressure. One explanation that could partly explain this association is that fibre-rich vegetables could be poorer in sodium and rich in potassium (depending on its preparation). In any case, a mechanism that could explain this association between fibre and blood pressure could be the renal sodium absorption, increased activity of the sympathetic nervous system, and sensitivity induced by free fatty acids to adrenergic stimuli and the antagonized vasorelaxation of nitric oxide that produces hyperinsulinism [59]. In line with previous studies, this protective association of fibre with blood pressure was mainly related with soluble fibre and that derived from pulses and nuts [58,60,61,62,63,64,65]. Nuts also contain high levels of unsaturated fatty acids and bioactive compounds that could contribute to the improvement of blood pressure and endothelial function [66,67]. On the other hand, pulses have a high content of dietary fibre, vegetable protein and potassium, which confer reducing effects on blood pressure [68]. The intake of resistant starch was associated with higher SBP. Resistant starch mainly comes from potatoes, and potatoes consumption, particularly as fries or chips, could be frequently accompanied by a high salt intake.
The amount of salt could not be quantified, which is considered a limitation in this study. Other dietary factors that could be related to cardiovascular risk, such as the proportion of sugars and fats, were not included in these analyses. However, all of the analyses were adjusted by overall energy intake. Another possible limitation is that the analyses did not account for physical activity, which might be related to the health outcomes as well. Other possible limitations of the study are the decreasing number of participants with increasing age, especially those who provided all dietary data. A strength of the study is that this is the only longitudinal European multicentre study in prepubertal children in which fibre and its food sources are analysed in relation with different cardiovascular health items and cardiometabolic risk.
5. Conclusions
In summary, children who consistently consumed fewer amounts of fibre had indications of poorer glucose tolerance and overall higher cardiometabolic risk score at 8 years. These better glucose tolerance levels and overall higher cardiometabolic risk scores were mainly associated with a higher intake of fibre from fruits and vegetables. These results support the importance of acquiring a healthy diet from childhood that includes dietary fibre as an important component. A diet rich in dietary fibre from fruits, vegetables, legumes and nuts should be promoted from an early age, regardless of the weight and health status of children, due to its apparent short- and long-term benefits.
Acknowledgments
CHOP study group: J. Beyer, M. Fritsch, G. Haile, U. Handel, I. Hannibal, B. Koletzko, S. Kreichauf, I. Pawellek, S. Schiess, S. Verwied-Jorky, R. von Kries, M. Weber (Children’s University Hospital, University of Munich Medical Center, Munich, Germany); R. Closa-Monasterolo, J. Escribano, N. Ferré, V. Luque, M. Gispert-Llauradó, C. Rubio-Torrents, M. Zaragoza-Jordana (Pediatrics, Nutrition and Development Research Unit, Universitat Rovira i Virgili, IISPV, Reus, Spain); A. Dobrzańska, D. Gruszfeld, R. Janas, A. Wierzbicka, P. Socha, A. Stolarczyk, J. Socha (Children’s Memorial Health Institute, Warsaw, Poland); C. Carlier, E. Dain, P. Goyens, J.N. Van Hees, J. Hoyos, J.P. Langhendries, F. Martin, P. Poncelet, A. Xhonneux (ULB, Bruxelles, Belgium, and CHC St. Vincent, Liège-Rocourt, Belgium); E. Perrin (Danone Research Centre for Specialised Nutrition, Schiphol, The Netherlands), and C. Agostoni, M. Giovannini, A. Re Dionigi, E. Riva, S. Scaglioni, F. Vecchi, E. Verducci (University of Milan).
Appendix A. Description of The Study Sample and Comparison by Sex
Table A1.
Energy, macronutrient and fibre intake by age group and sex.
| Energy (kcal/Day) Mean (±SD) |
Proteins (g/Day) Mean (±SD) |
Lipids (g/Day) Mean (±SD) |
Carbohydrates (g/Day) Mean (±SD) |
Total Fibre (g/Day) Mean (±SD) |
Total Fibre (g/1000 kcal) Mean (±SD) |
|
|---|---|---|---|---|---|---|
| Boys | ||||||
| 3 years, n = 253 | 1264 (1086, 1437) | 48.82 (39.62, 56.45) | 48.02 (39.36, 57.40) | 154.19 (129.74, 183.76) | 8.36 (6.04, 11.26) | 6.83 (5.19, 8.41) |
| 4 years, n = 253 | 1340 (1183, 1513) | 49.28 (41.15, 58.24) | 51.86 (43.20, 63.20) | 165.34 (143.97, 190.78) | 9.23 (7.08, 11.11) | 6.71 (5.33, 8.20) |
| 5 years, n = 217 | 1436 (1253, 1622) | 52.58 (45.62, 61.71) | 54.63 (44.61, 65.60) | 180.19 (150.28, 208.32) | 9.58 (7.48, 11.46) | 6.72 (5.36, 7.97) |
| 6 years, n = 227 | 1476 (1322, 1680) | 55.31 (47.28, 63.72) | 56.71 (48.88, 66.77) | 186.89 (162.88, 212.34) | 10.50 (8.56, 12.99) | 6.92 (5.83, 8.48) |
| 8 years, n = 193 | 1690 (1464, 1850) | 62.50 (52.87, 73.80) | 68.81 (56.48, 79.93) | 201.43 (172.33, 229.58) | 11.95 (9.88, 14.51) | 7.33 (6.07, 8.58) |
| Girls | ||||||
| 3 years, n = 281 | 1172 (1028, 1341) Φ | 44.71 (37.64, 52.32) ‡ | 45.89 (37.19, 53.65) ‡ | 144.72 (125.29, 168.39) ‡ | 7.67 (6.02, 9.98) | 6.66 (5.22, 8.13) |
| 4 years, n = 251 | 1262 (1138, 1405) ‡ | 47.54 (40.94, 56.31) | 50.00 (41.66, 57.80) * | 156.66 (137.69, 178.85) ‡ | 8.82 (7.01, 10.92) | 6.89 (5.44, 8.45) |
| 5 years, n = 230 | 1378 (1202, 1536) ‡ | 49.73 (41.11, 58.74) * | 53.60 (43.96, 61.65) | 170.42 (141.46, 194.76) ‡ | 9.48 (7.38, 12.01) | 7.13 (5.96, 8.48) |
| 6 years, n = 242 | 1465 (1270, 1615) | 54.36 (45.64, 61.35) | 56.39 (47.22, 66.33) | 179.88 (153.72, 203.71) * | 10.50 (8.12, 12.76) | 7.38 (5.75, 8.42) |
| 8 years, n = 207 | 1532 (1360, 1689) Φ | 56.36 (48.54, 64.86) Φ | 59.23 (51.10, 72.61) Φ | 182.05 (156.95, 210.66) Φ | 11.04 (9.17, 13.66) * | 7.19 (6.18, 9.16) |
* p < 0.05, ǂ p < 0.01, Φ p < 0.001, p-value for Student’s t-test for comparison boys vs. girls.
Table A2.
Fibre intake by age according to food source in boys and girls.
| Insoluble | Soluble | Resistant Starch | Fibre from Pulses and Nuts | |||||
|---|---|---|---|---|---|---|---|---|
| g/Day | g/1000 kcal | g/Day | g/1000 kcal | g/Day | g/1000 kcal | g/Day | g/1000 kcal | |
| Boys | ||||||||
| 3 years, n = 253 | 3.55 (2.31, 4.55) | 2.77 (1.91, 3.69) | 3.38 (1.96, 5.10) | 2.70 (1.64, 4.16) | 0.39 (0.11, 0.75) | 0.32 (0.09, 0.56) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| 4 years, n = 253 | 3.67 (2.63, 4.89) | 2.79 (2.10, 3.64) | 3.79 (2.41, 5.42) | 2.78 (1.76, 3.85) | 0.43 (0.14, 0.78) | 0.32 (0.11, 0.57) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) |
| 5 years, n = 217 | 4.16 (3.30, 5.37) | 2.99 (2.39, 3.56) | 3.88 (2.39, 5.55) | 2.66 (1.62, 3.78) | 0.41 (0.12, 0.78) | 0.29 (0.08, 0.53) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) |
| 6 years, n = 227 | 4.89 (3.85, 6.04) | 3.18 (2.65, 3.96) | 3.66 (2.41, 6.01) | 2.59 (1.69, 3.80) | 0.47 (0.14, 0.95) | 0.32 (0.09, 0.66) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| 8 years, n = 193 | 5.94 (4.67, 7.49) | 3.55 (2.85, 4.31) | 4.19 (2.83, 5.98) | 2.57 (1.76, 3.58) | 0.59 (0.17, 1.09) | 0.35 (0.10, 0.63) | 0.00 (0.00, 0.08) | 0.00 (0.00, 0.03) |
| Girls | ||||||||
| 3 years, n = 281 | 3.17 (2.34, 4.31) | 2.70 (2.08, 3.58) | 0.35 (0.09, 0.61) | 0.29 (0.08, 0.51) | 3.19 (2.02, 4.75) | 2.76 (1.78, 3.94) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| 4 years, n = 251 | 3.69 (2.63, 5.04) | 2.90 (2.26, 3.73) | 3.81 (2.39, 5.14) | 2.97 (1.82, 3.91) | 0.37 (0.00, 0.64) * | 0.28 (0.00, 0.48) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| 5 years, n = 230 | 4.14 (3.12, 5.39) | 3.12 (2.43, 3.91) | 3.63 (2.43, 5.34) | 2.69 (1.88, 3.93) | 0.35 (0.00, 0.72) | 0.25 (0.00, 0.51) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) |
| 6 years, n = 242 | 4.63 (3.38, 5.73) | 3.16 (2.45, 3.94) | 3.95 (2.47, 5.90) | 2.74 (1.77, 3.86) | 0.39 (0.08, 0.78) * | 0.29 (0.05, 0.53) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) |
| 8 years, n = 207 | 5.54 (4.45, 7.06) | 3.60 (2.95, 4.57) | 2.58 (1.68, 3.80) | 3.93 (2.38, 5.45) | 0.52 (0.18, 0.95) | 0.34 (0.12, 0.67) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) |
Data are presented as median and IQR. * p < 0.05, p-value for Student’s t-test for comparison boys vs. girls.
Appendix B
Table A3.
Linear regression analyses on the association between dietary fibre intakes at age 8 years on cardiovascular health outcomes.
| B | 95% CI (Min, Max) | p-Value | R2 | ||
|---|---|---|---|---|---|
| Unadjusted analyses | BMI z score | 0.076 | (0.000, 0.151) | 0.049 | 0.011 |
| Glucose (mg/dL) | −0.004 | (−0.006, −0.001) | 0.002 | 0.032 | |
| LDL cholesterol (mg/dL) | −1.434 | (−2.928, 0.060) | 0.060 | 0.010 | |
| HDL cholesterol (mg/dL) | −0.004 | (−0.011, 0.003) | 0.260 | 0.001 | |
| Triglycerides (mg/dL) | −0.004 | (−0.014, 0.005) | 0.392 | −0.001 | |
| HOMA-IR | −0.009 | (−0.019, 0.001) | 0.092 | 0.007 | |
| Systolic Blood Pressure (mmHg) | −2.126 | (−3.770, −0.482) | 0.011 | 0.020 | |
| Cardiometabolic risk score | −0.195 | (−0.432, 0.042) | 0.107 | 0.006 | |
| Adjusted analyses | BMI z score | 0.078 | (0.004, 0.152) | 0.039 | 0.048 |
| Glucose (mg/dL) | −0.002 | (−0.004, 0.000) | 0.064 | 0.257 | |
| LDL cholesterol (mg/dL) | −1.417 | (−2.922, 0.087) | 0.065 | 0.026 | |
| HDL cholesterol (mg/dL) | −0.006 | (−0.012, 0.001) | 0.075 | 0.180 | |
| Triglycerides (mg/dL) | −0.001 | (−0.010, 0.008) | 0.857 | 0.191 | |
| HOMA-IR | −0.006 | (−0.015, 0.003) | 0.167 | 0.347 | |
| Systolic Blood Pressure (mmHg) | −1.996 | (−3.495, −0.496) | 0.009 | 0.255 | |
| Cardiometabolic risk score | −0,149 | (−0.327, 0.029) | 0.101 | 0.489 | |
Linear regression models unadjusted and adjusted by country, sex, average energy intake at 8 years old, maternal education and BMI (if was not the dependent variable). Each line depicts a separate linear regression model.
Author Contributions
Conceptualization, V.L. and J.E.; methodology, V.L., J.E. and V.G.; formal analysis, S.L. and V.L.; investigation, V.L., V.G., R.C.-M., N.F., B.K., D.G., E.V., A.X. and J.E.; resources, funding acquisition and supervision R.C.-M., B.K. and J.E.; data curation, V.G.; writing—original draft preparation, S.L. and V.L; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
The Childhood Obesity Project was funded by the 5th Framework Program from the European Union [grants number QLRT-2001-00389 and QLK1-CT-2002-30582]. The follow up of the participants was funded by the 6th Framework Program (with contract number FOOD-CT-2005-007036) and also by the 7th Framework Program (FP7-KBBE-2007-1, ref. No. 212652; and FP7-289346-EarlyNutrition and its Brain Mobility Program). This manuscript does not necessarily reflect the views of the Commission and in no way anticipates the future policy in this area. The work of VG and BK has been supported by the European Commission, H2020 Programmes Lifecycle-733206 and CoreMD, the Erasmus Plus Programmes Early Nutrition eAcademy Southeast Asia-573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP and Capacity Building to Improve Early Nutrition and Health in South Africa-598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP, and the European Joint Programming Initiative Projects NutriPROGRAM and EndObesity supported by the German Ministry of Education and Research, Berlin. BK is the Else Kröner-Seniorprofessor of Paediatrics co-funded by the Else Kröner-Fresenius-Foundation, Bad Homburg, Germany, and the LMU University Hospitals Munich, Germany.
Data Availability Statement
Data available on request due to ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luque V., Escribano J., Closa-Monasterolo R., Zaragoza-Jordana M., Ferré N., Grote V., Koletzko B., Totzauer M., Verduci E., ReDionigi A., et al. Unhealthy Dietary Patterns Established in Infancy Track to Mid-Childhood: The EU Childhood Obesity Project. J. Nutr. 2018;148:752–759. doi: 10.1093/jn/nxy025. [DOI] [PubMed] [Google Scholar]
- 2.Van Gijssel R.M.A., Braun K.V.E., Kiefte-de Jong J.C., Jaddoe V.W.V., Franco O.H., Voortman T. Associations between dietary fiber intake in infancy and cardiometabolic health at school age: The generation R study. Nutrients. 2016;8:531. doi: 10.3390/nu8090531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephen A.M., Champ M.M.J., Cloran S.J., Fleith M., Van Lieshout L., Mejborn H., Burley V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017;30:149–190. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- 4.Aggett P.J., Agostoni C., Axelsson I., Edwards C.A., Goulet O., Hernell O., Koletzko B., Lafeber H.N., Micheli J.L., Michaelsen K.F., et al. Nondigestible carbohydrates in the diets of infants and young children: A commentary by the ESPGHAN committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2003;36:329–337. doi: 10.1097/00005176-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y. AGA technical review: Impact of dietary fiber on colon cancer occurrence. Gastroenterology. 2000;118:1235–1257. doi: 10.1016/S0016-5085(00)70377-5. [DOI] [PubMed] [Google Scholar]
- 6.Escudero Álvarez E., González Sánchez P. La fibra dietética. Nutr. Hosp. 2006;21:61–72. [PubMed] [Google Scholar]
- 7.Peris G.P., Lesmes B., Cuerda C.M., Alvarez C. Metabolismo colónico de la fibra. Nutr. Hosp. 2002;17:11–16. [PubMed] [Google Scholar]
- 8.Trautwein E.A., Kunath-Rau A., Erbersdobler H.F. Increased fecal bile acid excretion and changes in the circulating bile acid pool are involved in the hypocholesterolemic and gallstone-preventive actions of psyllium in hamsters. J. Nutr. 1999;129:896–902. doi: 10.1093/jn/129.4.896. [DOI] [PubMed] [Google Scholar]
- 9.Gibson G.R. Fibre and effects on probiotics (the prebiotic concept) Clin. Nutr. Suppl. 2004;1:25–31. doi: 10.1016/j.clnu.2004.09.005. [DOI] [Google Scholar]
- 10.Galisteo M., Duarte J., Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 2008;19:71–84. doi: 10.1016/j.jnutbio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Velázquez O.C., Zhou D., Seto R.W., Jabbar A., Choi J., Lederer H.M., Rombeau J.L. In vivo crypt surface hyperproliferation is decreased by butyrate and increased by deoxycholate in normal rat colon: Associated in vivo effects on c-fos and c-jun expression. J. Parenter. Enter. Nutr. 1996;20:243–250. doi: 10.1177/0148607196020004243. [DOI] [PubMed] [Google Scholar]
- 12.Inan M.S., Rasoulpour R.J., Yin L., Hubbard A.K., Rosenberg D.W., Giardina C. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118:724–734. doi: 10.1016/S0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- 13.Eckel R.H., Grundy S.M., Zimmet P.Z. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 14.Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson H.L., Campbell B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015;42:158–179. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andoh A., Tsujikawa T., Fujiyama Y. Role of Dietary Fiber and Short-Chain Fatty Acids in the Colon. Curr. Pharm. Des. 2005;9:347–358. doi: 10.2174/1381612033391973. [DOI] [PubMed] [Google Scholar]
- 17.Chandalia M., Garg A., Lutjohann D., Von Bergmann K., Grundy S.M., Brinkley L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2000;342:1392–1398. doi: 10.1056/NEJM200005113421903. [DOI] [PubMed] [Google Scholar]
- 18.Davy B.M., Melby C.L. The effect of fiber-rich carbohydrates on features of Syndrome X. J. Am. Diet. Assoc. 2003;103:86–96. doi: 10.1053/jada.2003.50005. [DOI] [PubMed] [Google Scholar]
- 19.Murphy N., Norat T., Ferrari P., Jenab M., Bueno-de-Mesquita B., Skeie G., Dahm C.C., Overvad K., Olsen A., Tjønneland A., et al. Dietary fibre intake and risks of cancers of the colon and rectum in the European prospective investigation into cancer and nutrition (EPIC) PLoS ONE. 2012;7:e39361. doi: 10.1371/journal.pone.0039361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slavin J.L. Position of the American Dietetic Association: Health implications of dietary fiber. J. Am. Diet. Assoc. 2008;108:1716–1731. doi: 10.1016/j.jada.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 21.European Food Safety Authority Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010;8:1462. [Google Scholar]
- 22.Amine E.K., Baba N.H., Belhadj M., Deurenberg-Yap M., Djazayery A., Forrestre T., Galuska D.A., Herman S., James W.P.T., M’Buyamba Kabangu J.R., et al. Diet, nutrition and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003;916:1–149. [PubMed] [Google Scholar]
- 23.Fulgoni V.L., Brauchla M., Fleige L., Chu Y.F. Association of whole-grain and dietary fiber intake with cardiometabolic risk in children and adolescents. Nutr. Health. 2020;26:243–251. doi: 10.1177/0260106020928664. [DOI] [PubMed] [Google Scholar]
- 24.White J., Jago R., Thompson J.L. Dietary risk factors for the development of insulin resistance in adolescent girls: A 3-year prospective study. Public Health Nutr. 2012;17:361–368. doi: 10.1017/S1368980012004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber M., Grote V., Closa-monasterolo R., Escribano J., Langhendries J., Dain E., Giovannini M., Verduci E., Gruszfeld D., Socha P., et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014;99:1041–1051. doi: 10.3945/ajcn.113.064071. [DOI] [PubMed] [Google Scholar]
- 26.Koletzko B., Kries V., Closa R., Escribano J., Scaglioni S., Giovannini M., Beyer J., Demmelmair H., Gruszfeld D., Dobrzanska A., et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009;89:1836–1845. doi: 10.3945/ajcn.2008.27091. [DOI] [PubMed] [Google Scholar]
- 27.Verwied-Jorky S., Schiess S., Luque V., Grote V., Scaglioni S., Vecchi F., Martin F., Stolarczyk A., Koletzko B. Methodology for longitudinal assessment of nutrient intake and dietary habits in early childhood in a transnational multicenter study. J. Pediatr. Gastroenterol. Nutr. 2011;52:96–102. doi: 10.1097/MPG.0b013e3181f28d33. [DOI] [PubMed] [Google Scholar]
- 28.Luque V., Escribano J., Mendez-Riera G., Schiess S., Koletzko B., Verduci E., Stolarczyk A., Martin F., Closa-Monasterolo R. Methodological Approaches for Dietary Intake Assessment in Formula-fed Infants. J. Pediatr. Gastroenterol. Nutr. 2013;56:320–327. doi: 10.1097/MPG.0b013e3182779a60. [DOI] [PubMed] [Google Scholar]
- 29.Dehne L.I., Klemm C., Henseler G., Hermann-Kunz E. The German Food Code and Nutrient Data Base (BLS II.2) Eur. J. Epidemiol. 1999;15:355–358. doi: 10.1023/A:1007534427681. [DOI] [PubMed] [Google Scholar]
- 30.Food and Nutrition Board. Institute of Medicine of the National Academies . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) The National Academies Press; Washington, DC, USA: 2005. Dietary, functional and total fiber; pp. 339–421. [Google Scholar]
- 31.De Onis M., Onyango A.W., Borghi E., Siyam A., Nishida C., Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flynn J.T., Falkner B.E. New clinical practice guideline for the management of high blood pressure in children and adolescents. Hypertension. 2017;70:683–686. doi: 10.1161/HYPERTENSIONAHA.117.10050. [DOI] [PubMed] [Google Scholar]
- 33.Miller W.G., Myers G.L., Sakurabayashi I., Bachmann L.M., Caudill P., Dziekonski A., Edwards S., Kimberly M.M., Korzum W.J., Leary E.T., et al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin. Chem. 2015;56:977–986. doi: 10.1373/clinchem.2009.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 35.Besch W., Woltanski W., Keilacker H., Díaz-Alonso J.M., Schulz B., Amendt P., Kohnert K.D., Ziegler M. Measurement of Insulin in Human Sera Using a New RIA Kit. Insulin determination in the absence of insulin antibodies–Conventional assay and micro modification. Exp. Clin. Endocrinol. 1987;90:271–277. doi: 10.1055/s-0029-1210700. [DOI] [PubMed] [Google Scholar]
- 36.Matthews D.R., Hosker J.R., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C., Infirmary R. Homeostasis model assessment: Insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 37.Mclaughlin T., Abbasi F., Cheal K., Chu J., Lamendola C., Reaven G. Use of Metabolic Markers To Identify Overweight individuals who are insulin resistant. Ann. Intern. Med. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 38.Eisenmann J.C. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc. Diabetol. 2008;7:1–6. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voortman T., van den Hooven E.H., Tielemans M.J., Hofman A., Kiefte-de Jong J.C., Jaddoe V.W.V., Franco O.H. Protein intake in early childhood and cardiometabolic health at school age: The Generation R Study. Eur. J. Nutr. 2016;55:2117–2127. doi: 10.1007/s00394-015-1026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Medical Association . WMA Declaration of Helsinki—Ethica Principles for Medical Research Involving Human Subjects. World Medical Association; Ferney-Voltaire, France: 2013. [Google Scholar]
- 41.Reynolds A., Mann J., Cummings J., Winter N., Mete E., Te Morenga L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet. 2019;393:434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 42.Pereira M.A., O’Reilly E., Augustsson K., Brown M.M. Dietary fiber and risk of coronary heart disease: A pooled analysis of cohort studies. Arch. Intern. Med. 2004;164:370–376. doi: 10.1001/archinte.164.4.370. [DOI] [PubMed] [Google Scholar]
- 43.Threapleton D.E., Greenwood D.C., Evans C., Cleghorn C.L., Nykjaer C., Woodhead C., Burley V.J. Dietary fibre intake and diabetes risk: A systematic review and meta-analysis of prospective studies. Proc. Nutr. Soc. 2013;72:2020. doi: 10.1017/S0029665113002784. [DOI] [Google Scholar]
- 44.Battista M., Murray R.D., Daniels S.R. Use of the metabolic syndrome in pediatrics: A blessing and a curse. Semin. Pediatr. Surg. 2009;18:136–143. doi: 10.1053/j.sempedsurg.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Ford E.S., Li C. Defining the Metabolic Syndrome in Children and Adolescents: Will the Real Definition Please Stand Up? J. Pediatr. 2008;152:160–164. doi: 10.1016/j.jpeds.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 46.Kynde I., Johnsen N.F., Wedderkopp N., Bygbjerg I.C., Helge J.W., Heitmann B.L. Intake of total dietary sugar and fibre is associated with insulin resistance among Danish 8-10- and 14-16-year-old girls but not boys. European Youth Heart Studies i and II. Public Health Nutr. 2010;13:1669–1674. doi: 10.1017/S1368980010000285. [DOI] [PubMed] [Google Scholar]
- 47.Gopinath B., Flood V.M., Rochtchina E., Baur L.A., Smith W., Mitchell P. Diet and Blood Pressure Influence of High Glycemic Index and Glycemic Load Diets on Blood Pressure During Adolescence. Hypertension. 2012;59:1272–1277. doi: 10.1161/HYPERTENSIONAHA.112.190991. [DOI] [PubMed] [Google Scholar]
- 48.Veldhuis L., Koppes L.L.J., Driessen M.T., Samoocha D., Twisk J.W.R. Effects of dietary fibre intake during adolescence on the components of the metabolic syndrome at the age of 36 years: The Amsterdam Growth and Health Longitudinal Study. J. Hum. Nutr. Diet. 2010;23:601–608. doi: 10.1111/j.1365-277X.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 49.Ruottinen S., Lagström H.K., Niinikoski H., Rönnemaa T., Saarinen M., Pahkala K.A., Hakanen M., Viikari J.S.A., Simell O. Dietary fiber does not displace energy but is associated with decreased serum cholesterol concentrations in healthy children. Am. J. Clin. Nutr. 2010;91:651–661. doi: 10.3945/ajcn.2009.28461. [DOI] [PubMed] [Google Scholar]
- 50.Schiess S., Grote V., Scaglioni S., Luque V., Martin F., Stolarczyk A., Vecchi F., Koletzko B. Introduction of complementary feeding in 5 European countries. J. Pediatr. Gastroenterol. Nutr. 2010;50:92–98. doi: 10.1097/MPG.0b013e31819f1ddc. [DOI] [PubMed] [Google Scholar]
- 51.Satija A., Hu F.B. Cardiovascular Benefits of Dietary Fiber. Curr. Atheroscler. Rep. 2012;14:505–514. doi: 10.1007/s11883-012-0275-7. [DOI] [PubMed] [Google Scholar]
- 52.Edwards C.A., Parrett A.M. Dietary fibre in infancy and childhood. Proc. Nutr. Soc. 2003;62:17–23. doi: 10.1079/PNS2002231. [DOI] [PubMed] [Google Scholar]
- 53.Yao B., Fang H., Xu W., Yan Y., Xu H., Liu Y., Mo M., Zhang H., Zhao Y. Dietary fiber intake and risk of type 2 diabetes: A dose-response analysis of prospective studies. Eur. J. Epidemiol. 2014;29:79–88. doi: 10.1007/s10654-013-9876-x. [DOI] [PubMed] [Google Scholar]
- 54.Almaraz R.S., Fuentes M.M., Milla S.P., Plaza B.L. Indicaciones de diferentes tipos de fibra en distintas patologías. Nutr. Hosp. 2015;31:2372–2383. doi: 10.3305/nh.2015.31.6.9023. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez M.L. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr. Opin. Lipidol. 2001;12:35–40. doi: 10.1097/00041433-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Hollenberg N.K. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet: Editor’s comments. Curr. Hypertens. Rep. 2001;3:373. [Google Scholar]
- 57.Ndanuko R.N., Tapsell L.C., Charlton K.E., Neale E.P., Batterham M.J. Dietary patterns and blood pressure in adults: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2016;7:76–89. doi: 10.3945/an.115.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiavaroli L., Viguiliouk E., Nishi S.K., Mejia S.B., Rahelić D., Kahleová H., Salas-Salvadó J., Kendall C.W.C., Sievenpiper J.L. DASH dietary pattern and cardiometabolic outcomes: An umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. doi: 10.3390/nu11020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Després J.P., Lamarche B., Mauriège P., Cantin B., Dagenais G.R., Moorjani S., Lupien P.J. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 60.Burke V., Hodgson J.M., Beilin L.J., Giangiulioi N., Rogers P., Puddey I.B. Dietary Protein and Soluble Fiber Reduce Ambulatory Blood Pressure in Treated Hypertensives. Hypertension. 2001;38:821–826. doi: 10.1161/hy1001.092614. [DOI] [PubMed] [Google Scholar]
- 61.Evans C.E.L., Greenwood D.C., Threapleton D.E., Cleghorn C.L., Nykjaer C., Woodhead C.E., Gale C.P., Burley V.J. Effects of dietary fibre type on blood pressure: A systematic review and meta-analysis of randomized controlled trials of healthy individuals. J. Hypertens. 2015;33:897–911. doi: 10.1097/HJH.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 62.Mejia S.B., Kendall C.W.C., Viguiliouk E., Augustin L.S., Ha V., Cozma A.I., Mirrahimi A., Maroleanu A., Chiavaroli L., Leiter L.A., et al. Effect of tree nuts on metabolic syndrome criteria: A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2013-004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Del Gobbo L.C., Falk M.C., Feldman R., Lewis K., Mozaffarian D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015;102:1347–1356. doi: 10.3945/ajcn.115.110965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jayalath V.H., De Souza R.J., Sievenpiper J.L., Ha V., Chiavaroli L., Mirrahimi A., Di Buono M., Bernstein A.M., Leiter L.A., Kris-Etherton P.M., et al. Effect of dietary pulses on blood pressure: A systematic review and meta-analysis of controlled feeding trials. Am. J. Hypertens. 2014;27:56–64. doi: 10.1093/ajh/hpt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim Y., Keogh J., Clifton P.M. Nuts and cardio-metabolic disease: A review of meta-analyses. Nutrients. 2018;10:1935. doi: 10.3390/nu10121935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Souza R.G.M., Gomes A.C., Naves M.M.V., Mota J.F. Nuts and legume seeds for cardiovascular risk reduction: Scientific evidence and mechanisms of action. Nutr. Rev. 2015;73:335–347. doi: 10.1093/nutrit/nuu008. [DOI] [PubMed] [Google Scholar]
- 67.Kim Y., Keogh J.B., Clifton P.M. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: Multiple potential mechanisms of actions. Nutrients. 2017;9:1271. doi: 10.3390/nu9111271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee Y.P., Puddey I.B., Hodgson J.M. Protein, fibre and blood pressure: Potential benefit of legumes. Clin. Exp. Pharmacol. Physiol. 2008;35:473–476. doi: 10.1111/j.1440-1681.2008.04899.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to ethical restrictions.