The majority of Gram-negative bacteria elicit a potent immune response via recognition of lipid A expressed on the outer bacterial membrane by the host immune receptor Toll-like receptor 4 (TLR4). However, some Gram-negative bacteria evade detection by TLR4 or alter the outcome of TLR4 signaling by modification of lipid A species.
KEYWORDS: bacterial pathogenesis, Porphyromonas gingivalis, TLR4, antigen cross-presentation, dendritic cells, inflammation, lipid A
ABSTRACT
The majority of Gram-negative bacteria elicit a potent immune response via recognition of lipid A expressed on the outer bacterial membrane by the host immune receptor Toll-like receptor 4 (TLR4). However, some Gram-negative bacteria evade detection by TLR4 or alter the outcome of TLR4 signaling by modification of lipid A species. Although the role of lipid A modifications on host innate immunity has been examined in some detail, it is currently unclear how lipid A remodeling influences host adaptive immunity. One prototypic Gram-negative bacterium that modifies its lipid A structure is Porphyromonas gingivalis, an anaerobic pathobiont that colonizes the human periodontium and induces chronic low-grade inflammation that is associated with periodontal disease as well as a number of systemic inflammatory disorders. P. gingivalis produces dephosphorylated and deacylated lipid A structures displaying altered activities at TLR4. Here, we explored the functional role of P. gingivalis lipid A modifications on TLR4-dependent innate and adaptive immune responses in mouse bone marrow-derived dendritic cells (BMDCs). We discovered that lipid A 4′-phosphate removal is required for P. gingivalis to evade BMDC-dependent proinflammatory cytokine responses and markedly limits the bacterium’s capacity to induce beta interferon (IFN-β) production. In addition, lipid A 4′-phosphatase activity prevents canonical bacterium-induced delay in antigen degradation, which leads to inefficient antigen cross-presentation and a failure to cross-prime CD8 T cells specific for a P. gingivalis-associated antigen. We propose that lipid A modifications produced by this bacterium alter host TLR4-dependent adaptive immunity to establish chronic infections associated with a number of systemic inflammatory disorders.
INTRODUCTION
A number of host-adapted microorganisms have evolved mechanisms to evade detection by the host immune system or, in some instances, to manipulate host immune responses for their own benefit (1, 2). In the case of Gram-negative bacteria, this is frequently accomplished by modification of lipid A, the immune-stimulatory component of lipopolysaccharide (LPS). Canonical lipid A from bacteria such as Escherichia coli is a hexa-acylated bisphosphorylated glucosamine disaccharide that acts as a potent Toll-like receptor 4 (TLR4) agonist to drive a robust inflammatory response. In contrast, host-adapted bacteria can modify their lipid A to modulate the extent and consequences of detection by TLR4 (3, 4). Modifications include acyl chain length, number, and positioning, as well as the number and positioning of polar functional groups (5). In addition to effects on TLR4 signaling, altering lipid A structure can render bacteria resistant to innate immune effector molecules, such as cationic antimicrobial peptides (CAMPs) (4). Examples of pathogenic bacteria that modify lipid A include Yersinia pestis (6), Francisella spp. (7), and Helicobacter pylori (8). The altered lipid A molecules produced by these bacteria poorly activate TLR4 signaling (8–10). When lipid A modification enzymes are inactivated, these organisms fail to establish productive infection (8, 10–13). Similar to host-adapted pathogens, commensal bacteria of the mammalian intestine also alter their lipid A structure, and such modifications are critical for long-term colonization (14).
One organism that produces a particularly wide array of lipid A structures is the Gram-negative anaerobic oral pathobiont Porphyromonas gingivalis (15). P. gingivalis colonizes the subgingival oral mucosa and is associated with periodontal disease, a chronic oral inflammatory disorder that leads to the destruction of tooth-supporting bone and tissue. P. gingivalis is also associated with a number of systemic inflammatory disorders, including cardiovascular disease (16–21), rheumatoid arthritis (22–26), nonalcoholic fatty liver disease (27, 28), and cancer (29, 30).
P. gingivalis synthesizes a penta-acylated bisphosphorylated lipid A that, compared to lipid A from E. coli, only modestly activates TLR4. This structure can be modified by the action of three enzymes, a C1-lipid A phosphatase, a C4′-lipid A phosphatase, and a recently identified 3′-O lipid A deacylase, to give rise to a variety of lipid A structures with different activities at TLR4 (15, 31–33). These include two tetra-acylated forms, one of which is nonphosphorylated and inert at TLR4, and one of which is monophosphorylated at C-1 and acts as a weak TLR4 agonist that is able to block the potent TLR4 stimulatory activity of E. coli lipid A (15, 31–33). Due to the ability of the monophosphorylated lipid A to block the activity of more potent lipid A species, this lipid A structure is designated as an antagonist. The structures of these P. gingivalis lipid A moieties, determined by mass spectrometry (31, 33, 34), are depicted in Fig. S1 in the supplemental material. The production of these variant lipid A structures is regulated by environmental cues, including hemin (31, 35) and temperature (36).
To facilitate studies on the impact of these variant lipid A structures on host immune responses, we recently constructed P. gingivalis strains with targeted deletions of either the C1- or C4′-lipid A phosphatase genes (33). The lipid A C1-phosphatase mutant (designated 1773381) expresses primarily the antagonist lipid A moiety (Fig. S1B), along with a smaller amount of the diphosphorylated penta-acylated agonist structure (Fig. S1D) (33). The lipid A C4′-phosphatase mutant (designated 1587381) produces penta-acylated mono- or diphosphorylated TLR4 agonist structures (Fig. S1C and D) (33). This mutant produces penta-acylated structures because generation of tetra-acylated structures by activity of the 3′-O lipid A deacylase requires the removal of the C4′-phosphate (31, 32). Mutant and wild-type strains display similar growth rates in culture, have a similar surface appearance by electron microscopy, express similar levels of the major fimbria protein, and are equally able to induce TLR2-dependent NF-κB activation in HEK cells expressing murine TLR2 and TLR1 along with an NF-κB reporter construct (33). However, only the lipid A C4′-phosphatase mutant strain, 1587381, was effective at inducing TLR4-dependent NF-κB activity in HEK cells expressing murine TLR4, MD2, and the NF-κB reporter construct (33). Nonetheless, compared to E. coli, strain 1587381 is a weak TLR4 agonist. In studies with bone marrow-derived macrophages (BMDMs), we showed that the P. gingivalis wild-type and C1-phosphatase-deficient strains, which express inert or antagonist lipid A structures, elicited reduced levels of proinflammatory mediators from macrophages compared to the C4′-phosphatase-deficient strain (agonist) (33). Expression of TLR4 inert/antagonist lipid A was also associated with a failure to induce inflammasome activation, resulting in impaired interleukin-1β (IL-1β) secretion, reduced induction of macrophage cell death, and increased intracellular bacterial survival (33). The nomenclature and properties of the P. gingivalis strains used in this study are summarized in Table 1. Despite our studies (33), as well as studies with other host-adapted Gram-negative species (8–11, 13, 37), which examined the impact of lipid A modifications on innate immune responses (3, 4), little is known about the impact of lipid A modifications on adaptive immune responses. Evasion or manipulation of adaptive immunity is important for long-term colonization by pathogens such as P. gingivalis that cause chronic, low-grade inflammation. Furthermore, effects of P. gingivalis lipid A remodeling on T cell responses may directly promote disease, as the systemic pathologies associated with P. gingivalis are driven in part by dysregulated T cell responses (38–48).
TABLE 1.
Phenotypes of P. gingivalis wild-type and lipid A phosphatase mutant strains
The impact of lipid A structure on regulating adaptive immunity is likely mediated by interactions with dendritic cells (DCs). DCs are specialized antigen-presenting cells (APCs) that initiate and control immunity and tolerance (49). They are the peripheral sentinels of the immune system, equipped with a large repertoire of pattern recognition receptors, including TLR4, for detection of microbes, along with the unique ability to activate naive antigen-specific T cells. In the current study, we examined the impact of P. gingivalis lipid A modifications on TLR4-dependent DC functions, including production of proinflammatory cytokines and upregulation of maturation markers. We also assessed the impact of variant lipid A structures on cross-presentation of a P. gingivalis-associated antigen. We demonstrate that expression of modified lipid A structures limits the induction of proinflammatory cytokines by BMDCs and promotes avoidance of a P. gingivalis-specific CD8 T cell response.
RESULTS
Impact of variant lipid A structures on induction of inflammatory cytokines and BMDC viability.
An important consequence of TLR4 signaling in dendritic cells is the production of proinflammatory mediators. TLR4 signals from the cell surface through the adaptor MyD88 and, after internalization, from endosomes through the adaptor TRIF (50). Both MyD88 and TRIF are required for maximal induction of most genes induced through TLR4. However, the relative contribution of each adaptor varies, depending on the gene and cell type (51–55).
To determine the effect of variant lipid A structures on the induction of proinflammatory cytokines, we first examined the effect of variant lipid A structures on the induction of IL-6, tumor necrosis factor alpha (TNF-α), and IL-12p70. In dendritic cells, the induction of these proteins through TLR4 is largely MyD88 dependent (52). We treated BMDCs differentiated from C57BL/6 or TLR4−/− mice with each of the three P. gingivalis strains and measured secretion of IL-6 (Fig. 1A), TNF-α (Fig. 1B), and IL-12p70 (Fig. 1C). Induction of all three cytokines was observed in response to all of the P. gingivalis strains, with P. gingivalis 1587381 (agonist) exhibiting the strongest activity (Fig. 1A to C). In all cases, cytokine secretion was reduced, but not eliminated, in TLR4−/− BMDCs, indicating that P. gingivalis-induced secretion of these cytokines is substantially, but not entirely, mediated by TLR4. TLR4-independent cytokine induction is likely downstream of TLR2, which recognizes P. gingivalis major fimbriae (56), as well as an LPS-associated lipoprotein (57). Secretion of proinflammatory cytokines in response to P. gingivalis is impaired in the absence of TLR2 (58). As expected, the induction of all three cytokines by treatment of BMDCs with E. coli LPS was entirely dependent on TLR4 (see Fig. S2 in the supplemental material), while Pam3CSK4 treatment of BMDCs led to low levels of proinflammatory cytokine production that were largely independent of TLR4 (Fig. S2). Consistent with its ability to more strongly induce inflammatory cytokine production, P. gingivalis 1587381 induced more activation of mitogen-activated protein kinases (MAPKs) and NF-κB than either the wild-type P. gingivalis or 1773381 strain (see Fig. S3 in the supplemental material).
FIG 1.
Induction of cytokine secretion by P. gingivalis strains. BMDCs differentiated from bone marrow of C57BL/6 or TLR4−/− mice were untreated (medium) or treated for 18 h with P. gingivalis 381 (red), 1773381 (blue), or 1587381 (green) at an MOI of 10, and supernatants were collected and analyzed by ELISA for (A) IL-6, (B) TNF-α, or (C) IL-12p70. Shown are averages from biological triplicates of one experiment (± standard deviation), representative of two independent experiments. Square brackets indicate statistical comparison of P. gingivalis 1587381-treated versus 381- or 1773381-treated C57BL/6 BMDCs. Straight horizontal lines indicate statistical comparison for each treatment of TLR4−/− BMDCs to the correspondingly treated C57BL/6 BMDCs. Statistical analysis was by two-way ANOVA with a Tukey’s multiple-comparison post hoc test. ****, P < 0.0001.
We next assessed the effect of variant lipid A structures on the induction of IFN-β. Induction of IFN-β by LPS requires TRIF, although MyD88 is required for maximal induction (53). C57BL/6 or TLR4−/− BMDCs were treated with increasing multiplicities of infection (MOI) of each P. gingivalis strain, and IFN-β secretion was measured by enzyme-linked immunosorbent assay (ELISA) (Fig. 2). We observed minimal IFN-β secretion in response to P. gingivalis strains 381 and 1773381, which express inert and/or antagonist lipid A, while P. gingivalis 1587381, which expresses agonistic lipid A, induced TLR4-dependent IFN-β secretion as effectively as E. coli (Fig. 2). These results support a critical role for lipid A 4′-phosphatase activity in conferring the capacity of P. gingivalis to efficiently evade TRIF-dependent TLR4 signaling (59). LPS plays a key role in the induction of pyroptotic cell death by Gram-negative bacteria, either by providing signal 1 for formation of the canonical inflammasome, or signals 1 and 2 for assembly of the noncanonical inflammasome (60, 61). To determine if variant lipid A structures impact the induction of BMDC death, cells were cultured with the P. gingivalis strains, and cell death was monitored over a 16-h time period (see Fig. S4 in the supplemental material). As a positive control, cells were pretreated with E. coli LPS, and cell death was followed after the addition of ATP. While treatment with E. coli LPS and ATP resulted in significant cell death, stimulation with P. gingivalis was less toxic, with little cell death induced at an MOI of 10 or 25 (Fig. S4). There was no major effect of variant lipid A structures on the extent of cell death (Fig. S4).
FIG 2.

Induction of IFN-β secretion and TLR4 internalization by P. gingivalis strains. BMDCs differentiated from bone marrow of C57/BL6 or TLR4−/− mice were left untreated or were treated for 18 h with P. gingivalis 381 (red), 1773381 (blue), or 1587381 (green) or with heat-killed E. coli cells (black) at the indicated MOI. Supernatants were then collected and analyzed for IFN-β by ELISA. Shown are averages from biological duplicates of one experiment ± standard deviation, representative of four independent experiments. Statistical comparisons are between 1587381 and both 381 and 1773381. Analysis was by two-way ANOVA with a Tukey’s multiple-comparison post hoc test. ****, P < 0.00001.
Impact of variant lipid A structures on BMDC maturation.
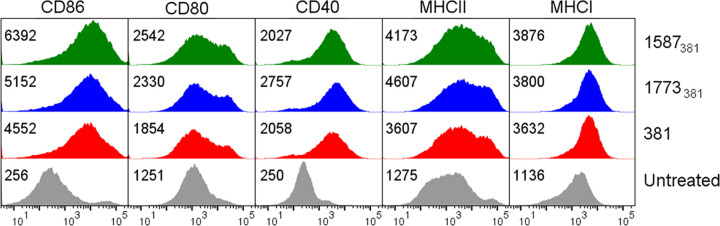
Upon encounter with a microbe, dendritic cells undergo a complex process termed maturation, during which they become competent to efficiently take up, process, and present antigens to T cells and prime an appropriate T cell response. This process includes the upregulation of a number of surface proteins important for DC antigen presentation and T cell priming functions, including major histocompatibility complex class I (MHC-I) and MHC-II and the costimulatory molecules CD40, CD80, and CD86. TLR4 is a key mediator of these events when they occur in response to Gram-negative bacteria such as E. coli, which produce lipid A that potently activates TLR4. To determine how the expression by P. gingivalis of inert and/or antagonistic lipid A affects these processes, we treated BMDCs with each of the P. gingivalis strains and examined the acquisition of maturation markers by flow cytometry. As shown in Fig. 3, all P. gingivalis strains induced comparable surface expression of costimulatory molecules CD40, CD80, and CD86, as well as of MHC-I and MHC-II. Thus, the ability of P. gingivalis to modify its lipid A is not a mechanism by which it avoids induction of BMDC maturation.
FIG 3.
Upregulation of costimulatory molecules is unaffected by expression of P. gingivalis variant lipid A structures. Surface expression of CD86, CD80, CD40, MHC-II, and MHC-I was assessed by flow cytometry in untreated BMDCs (gray) or after treatment with P. gingivalis 381 (red), 1773381 (blue), or 1587381 (green) for 18 h at an MOI of 10. The numbers in each panel are the geometric mean fluorescent intensity. Shown are results from one experiment representative of three independent experiments.
Impact of variant lipid A structures on cross-presentation of P. gingivalis-associated antigens to antigen-specific CD8 T cells.
TLR signaling from phagosomes stimulates cross-presentation of peptides derived from phagocytosed microbes (62). To determine the impact of lipid A modifications on cross-presentation of P. gingivalis-associated antigens, we treated BMDCs obtained from C57BL/6 or TLR4−/− mice with heat-killed P. gingivalis strains coated with ovalbumin (OVA) and assessed their ability to cross-present OVA to T cell receptor (TCR) transgenic, OVA-specific, CD8+ OT-I T cells. Flow cytometry analysis demonstrated that OVA coatings were comparable among strains (see Fig. S5 in the supplemental material). As a measure of cross-presentation efficiency, we determined the percentage of OT-1 T cells that became CD69+ after coculture with BMDCs that had been treated with OVA-coated P. gingivalis strains (Fig. 4). The percentage of CD8 T cells induced to express CD69 has been shown to be proportional to the amount of presented antigenic peptide (63). Since CD69 is induced solely by TCR cross-linking, independent of any other BMDC-derived signals, the percentage of CD69+ cells in our studies reflects the efficiency with which BMDCs were able to take up, process, and cross-present the antigenic OVA peptide. Stimulation of BMDCs with all P. gingivalis strains led to a TLR4- and dose-dependent increase in the efficiency of cross-presentation, with P. gingivalis 1587381 inducing the highest percentage of CD69+ T cells (Fig. 4).The preprocessed SIINFEKL peptide was used as a control for cross-presentation and, as expected, was presented with equal levels of efficiency by BMDCs obtained from C57BL/6 and TLR4−/− mice.
FIG 4.

Effect of lipid A variation on antigen cross-presentation. BMDCs derived from bone marrow of C57BL/6 or TLR4−/− mice were untreated (gray) or treated for 4.5 h with OVA-coated, heat-killed P. gingivalis cells of strain 381 (red), 1773381 (blue), or 1587381 (green) at MOI of 10, 25, or 50 and then cocultured with OT-I T cells. CD69 expression on OT-I T cells was assessed by flow cytometry following an 18-h incubation. As a positive control, unstimulated BMDCs were pulsed with 0.01 ng/ml SIINFEKL peptide before incubation with OT-I cells (black). Data are averages from biological duplicates from one experiment (± standard deviation) representative of three independent experiments. Square brackets indicate statistical comparison of P. gingivalis 1587381-treated versus 381- or 1773381-treated C57BL/6 BMDCs. Straight lines indicate statistical comparison for each treatment of TLR4−/− BMDCs to the correspondingly treated C57BL/6 BMDCs. Statistical analysis was by two-way ANOVA with a Tukey’s multiple-comparison post hoc test. ****, P < 0.0001.
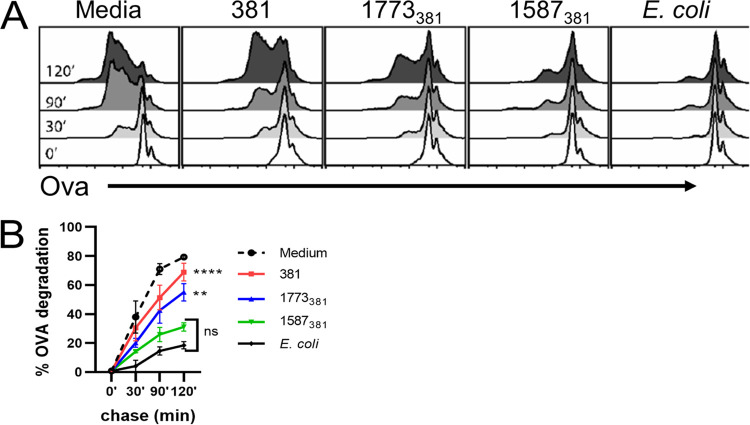
It is possible that the increased ability of P. gingivalis 1587381 to induce cross-presentation results from more efficient uptake of this strain by BMDCs than P. gingivalis strains 381 and 1773381. However, this was not the case, as all three P. gingivalis strains were internalized to the same degree (Fig. 5). An important TLR4-induced event critical for efficient antigen cross-presentation is inhibition of phagosome-lysosome fusion (64). This results in delayed degradation of internalized antigen and allows for loading of the antigen onto MHC-I. To determine if variant lipid A structures affect the rate of antigen degradation, we treated BMDCs with the three P. gingivalis strains or E. coli for 16 to 18 h and then pulsed cultures with beads coated with OVA. Degradation of OVA on internalized beads was then assessed at various times by flow cytometry. As predicted, pretreatment of BMDCs with E. coli inhibited the degradation of OVA on phagocytosed beads (Fig. 6A and B). We also observed that pretreatment with P. gingivalis 1587381 inhibited OVA degradation (Fig. 6A and B). P. gingivalis strains 381 and 1773381 were less effective at inhibiting antigen degradation than P. gingivalis 1587381 (Fig. 6A and B), suggesting that lipid A 4′-phosphatase activity is essential for wild-type P. gingivalis and strain 1773381 to avoid induction of a delay in antigen degradation, resulting in impaired cross-presentation of P. gingivalis-associated antigens by BMDCs.
FIG 5.

Variation of lipid A structure does not affect uptake of P. gingivalis by BMDCs. C57BL/6 BMDCs were treated with FITC-labeled P. gingivalis strains (MOI of 10) for 30, 60, or 90 min, and bacterial uptake was assessed by flow cytometry. Trypan blue was added to the samples before acquisition to quench extracellular fluorescence due to any membrane-bound bacteria. Shown is one experiment representative of two independent experiments.
FIG 6.
Effect of lipid A variation on preservation of internalized antigen. C57BL/6 BMDCs were left untreated (media) or treated with P. gingivalis strains or heat-killed E. coli cells at an MOI of 10 for 16 to 18 h. Bacteria were removed, and cells were pulsed for 10 min with latex beads covalently coupled with OVA. After different periods of time, cells were lysed, and the amount of OVA remaining on beads was assessed by flow cytometry after staining beads for OVA. (A) Histograms showing OVA staining on gated beads for one experiment representative of two independent experiments. (B) Averaged results from two experiments (± standard deviation). The percentage of degradation is 100 − the percentage of undegraded OVA at each time point relative to the percentage undegraded at time zero. Statistical comparisons are for 381 and 1773381 versus 1587381 at 120 min using a two-way ANOVA and Tukey’s multiple-comparison post hoc test. **, P < 0.001; ****, P < 0.0001. There was no statistically significant difference (ns) between 1587381 and E. coli at any time point.
Impact of variant lipid A structures on cross-priming antigen-specific CD8 T cells by P. gingivalis-treated BMDCs.
While the combination of antigen processing and presentation is sufficient to induce TCR cross-linking and CD69 upregulation, it is not sufficient to induce sustained, productive T cell activation, proliferation, and effector function. Productively activated T cells produce IL-2, which is dependent on receipt of costimulatory signals and required for T cell proliferation. We therefore measured IL-2 in supernatants obtained from OT-I–BMDC cocultures in which the BMDCs had been pretreated with heat-killed OVA-coated P. gingivalis strains (Fig. 7A). OT-I T cells incubated with P. gingivalis 381- or 1773381-treated BMDCs produced low levels of IL-2 that did not significantly differ from the levels produced by OT-I T cells incubated with untreated BMDCs (Fig. 7A). In contrast, in cultures incubated with P. gingivalis 1587381-treated BMDCs, OT-I T cells secreted high levels of IL-2 (Fig. 7A). The complete inability of BMDCs treated with P. gingivalis strains 381 and 1773381 to induce OT-I IL-2 production was in contrast with their more modestly impaired ability to induce CD69 on OT-I cells (Fig. 4). This suggests a nonlinear relationship between the degree of cross-presentation and cross-priming. Activation of CD8 T cells by TLR4 ligand-treated BMDCs generally results in the production of gamma interferon (IFN-γ). Thus, as an additional measure of productive T cell activation, we measured IFN-γ in supernatants of the cocultures. As with IL-2, only OT-1 T cells cocultured with P. gingivalis strain 1587381-treated BMDCs produced IFN-γ (Fig. 7B). Both secretion of IL-2 and that of IFN-γ were dependent on TLR4 (Fig. 7A and B). Taken together, our results indicate that, by modifying its lipid A, P. gingivalis avoids suppression of phagosome-lysosome fusion, resulting in both inefficient cross-presentation of P. gingivalis-associated antigens and a significantly limited induction of productive CD8 T cell responses, which promotes bacterial survival.
FIG 7.
Effect of lipid A variation on cross-priming of OT-I T cells. BMDCs obtained from C57/BL6 or TLR4−/− mice were untreated (gray) or treated for 4.5 h with OVA-coated heat-killed P. gingivalis 381 (red), 1773381 (blue), or 1587381 (green) at MOI of 10, 25, or 50 and then cocultured with OT-I T cells. As a positive control, BMDCs were pulsed with SIINFEKL peptide before addition of T cells (black). Supernatants were collected after an 18-h incubation and analyzed by ELISA for IL-2 (A) and IFN-γ (B). Shown are averages of biological duplicates from one experiment (± standard deviation) representative of three independent experiments. Statistical comparisons are between 1587381 and both 381 and 1773381 using a two-way ANOVA and Tukey’s multiple-comparison post hoc test. # signifies not detected because the IFN-γ concentration was below the level of detection. ****, P < 0.0001.
DISCUSSION
We demonstrate here that the ability of P. gingivalis to modify its lipid A structure allows for evasion of TLR4-mediated dendritic cell responses. Most strikingly, P. gingivalis strains that express tetra-acylated lipid A species (wild-type and 1773381 strains) failed to induce BMDCs to cross-prime a P. gingivalis-specific CD8 T cell response. This was based on our finding that BMDCs treated with OVA-coated P. gingivalis wild-type or 1773381 failed to induce IL-2 or IFN-γ production by cocultured OVA-specific CD8+ OT-I T cells. In contrast, BMDCs treated with OVA-coated P. gingivalis 1587381 induced OT-I T cells to secrete significant levels of both IL-2 and IFN-γ, indicative of functional T cell activation. The failure of BMDCs to induce a CD8 T cell response after treatment with the P. gingivalis wild-type or strain 1773381 could have been due to a failure of these strains to induce upregulation of costimulatory molecules. However, all three P. gingivalis strains induced expression of CD80, CD86, CD40, MHC-I, and MHC-II to the same extent. It is possible that impaired cross-priming is due to the induction of a coinhibitory molecule. However, our finding that P. gingivalis wild-type and 1773381 strains were inefficient at inducing cross-presentation of P. gingivalis-associated antigens suggests that impaired induction of cross-presentation underlies the failure of these strains to induce cross-priming of a CD8 T cell response. All P. gingivalis strains were internalized at the same rate and to the same extent by the BMDCs. Thus, differential antigen uptake did not account for differences in cross-presentation efficiency. However, the strains differed in their abilities to induce a delay in the degradation of bacterium-associated antigens. A TLR4-dependent delay in antigen degradation is important for promoting antigen cross-presentation by LPS-treated BMDCs (64). In splenic CD8α+ DCs, delayed degradation of antigen has been shown to be promoted by type I interferon, with a concomitant increase in cross-presentation activity (65). We found that treatment of BMDCs with P. gingivalis 1587381 induced considerably more IFN-β secretion than treatment with either the P. gingivalis wild-type or 1773381 strain. This likely contributes to the greater ability of P. gingivalis 1587381 to promote delayed degradation of antigen and, in turn, antigen cross-presentation and T cell cross-priming. TLR4 signaling also facilitates cross-presentation of bacterial antigens by inducing alterations in vesicle trafficking patterns that are necessary to bring MHC-I molecules to the appropriate compartment for loading with endocytosed bacterial antigens (62). It is possible that expression of P. gingivalis lipid A variant structures may impact vesicle trafficking, and this will be pursued in future studies.
Lipid A modification is not the only strategy evolved by P. gingivalis for manipulation of DC-primed T cell responses. A number of studies have established a role for P. gingivalis major and minor fimbriae in DC responses to P. gingivalis (66–72). Human monocyte-derived DCs (MoDCs) treated with a P. gingivalis wild-type strain secreted inflammatory cytokines, upregulated costimulatory molecules, and induced proliferation and Th1 polarization of cocultured autologous naive CD4+ T cells (72). In contrast, MoDCs treated with a mutant strain that expressed only minor fimbriae induced a Th2 response when cocultured with T cells (72). Other studies have characterized the interactions of P. gingivalis with noncanonical pathogen-differentiated DCs (PDDCs) (70, 73). In contrast to conventionally differentiated MoDCs (using granulocyte-macrophage colony-stimulating factor [GM-CSF] and IL-4), which induce markers of Th1 differentiation in cocultured autologous T cells, PDDCs induce expression of IL-4R and IL-10, suggestive of an immunosuppressive phenotype (73). Taken together, these results indicate that P. gingivalis has evolved multiple strategies to manipulate host T cell responses. However, to our knowledge, our study is the first to examine the impact of P. gingivalis alternative lipid A structures on antigen cross-presentation and priming by dendritic cells.
In addition to antigen presentation functions, DCs respond to pathogen encounter with the production of cytokines and chemokines that help shape the immune response. TLR4 signals from the cell surface through the adaptor MyD88 to activate NF-κB and MAPKs, leading to the rapid upregulation of proinflammatory mediators. Subsequently, TLR4 is endocytosed and signals from endosomes through the adaptor TRIF. This results in a slightly delayed activation of NF-κB and MAPKs, as well as the activation of TBK-1, IRF3, and transcription of IFN-β (50). A major finding in the present study is that P. gingivalis strains expressing predominantly tetra-acylated lipid A and lacking a 4′-phosphate (wild type and 1773381) were weak inducers of IFN-β secretion. Removal of the lipid A 4′-phosphate is required for subsequent lipid A 3′-O-deacylation (31). It is likely that both removal of the lipid A 4′-phosphate and removal of the 3′-O-acyl group are key structural modifications required for the combined evasion of TRIF-dependent and MyD88-dependent TLR4 signaling (31, 32). Similarly, treatment of BMDCs with the P. gingivalis wild-type and 1773381 strains induced reduced TLR4-dependent secretion of the MyD88-dependent cytokines IL-6, IL-12p70, and TNF-α compared to treatment with P. gingivalis strain 1587381. The effect of lipid A structure on IL-6 and IL-12p70 secretion was statistically significant but quite modest (<2-fold reduced induction after treatment with P. gingivalis wild-type and 1773381 strains compared to P. gingivalis strain 1587381). TNF-α secretion, in contrast, was more dramatically affected (4-fold reduced with the wild-type or 1773381 strain compared to 1587381). Variable, cytokine-specific effects of lipid A modifications on secretion of proinflammatory cytokines were also observed in a study with THP1 monocyte cells treated with engineered E. coli lipid A variants that varied with respect to number and position of phosphorylations, number of acyl chains, and presence or absence of acyl chain modifications (37).
Our finding that the P. gingivalis wild-type strain induced production of proinflammatory cytokines by BMDCs (albeit at reduced levels compared to strain 1587381) contrasts with results reported by a different group (Abdi et al. [74]). These investigators found that a P. gingivalis wild-type strain failed to induce the expression of several cytokines, including IL-6, TNF-α, and IL-12p40/p35 in BMDCs, at both the RNA and protein levels (74). The discrepancy between the two results is likely due to the use of different P. gingivalis strains. We used the unencapsulated P. gingivalis strain 381, while Abdi et al. used encapsulated P. gingivalis strain W50. The presence of a capsule has been shown to suppress P. gingivalis-induced production of proinflammatory cytokines by human gingival fibroblasts (75). Consistent with our finding, P. gingivalis strain 381 induced secretion of IL-6, TNF-α, and IL-12p70 by human MoDCs (72).
Recently, a chemically modified monophosphorylated derivative of lipid A from Salmonella enterica serovar Minnesota (MPLA) was approved for use as a TLR4-based vaccine adjuvant because of its ability to induce potent adaptive immune responses without concomitant high-level production of proinflammatory mediators (59, 76). This has fueled enthusiasm for developing other TLR4-based adjuvants (37, 76). Our finding that P. gingivalis 1587381, which expresses a monophosphorylated, penta-acylated lipid A, is able to induce antigen cross-priming, suggests that this lipid A structure may warrant consideration for development as an adjuvant for vaccines against intracellular pathogens.
The ability of P. gingivalis to avoid induction of a CD8 T cell response has potential consequences for its pathogenicity. Although P. gingivalis is a component of the subgingival dental biofilm, we and others have shown that it invades and survives within epithelial and endothelial cells (77–81). We have also demonstrated that P. gingivalis can enter and survive in human MoDCs in vitro and can be detected in vivo within circulating human myeloid DCs (mDCs) and in mDCs that had infiltrated the oral submucosa or atherosclerotic lesions (67, 68). Together these results suggest that intracellular survival of P. gingivalis may be critical to the ability of this pathobiont to evade immunity, resulting in low-grade chronic inflammation. Consistent with this, we previously showed that lipid A modifications allow P. gingivalis to evade killing by macrophages and that strain 1587381, which produces a TLR4-stimulatory lipid A, is impaired in its ability to induce vascular inflammation compared to the other two strains (33). The finding that lipid A modifications promote the evasion of a CD8 T cell response provides an additional mechanism by which P. gingivalis can ensure its own intracellular survival, leading to chronic inflammation, and a variety of systemic inflammatory disorders.
MATERIALS AND METHODS
Animals and cells.
C57BL/6J (WT; Jax no. 000664), Cd14−/− (Jax no. 003726), Tlr4−/− (Jax no. 007227), Tlr2−/− (Jax no. #004650), Trif−/− (Jax no. 005037), Myd88−/− (Jax no. 009088), and OT-I (Jax no. 00383) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed under specific-pathogen-free conditions and used at 6 to 10 weeks of age. All animal procedures were approved by the Tufts University School of Medicine Institutional Animal Care and Use Committee.
To generate monocyte-derived DCs, single-cell suspensions of red blood cell-depleted bone marrow cells were cultured in RPMI 1640 (Corning) containing 10% fetal bovine serum (FBS; HyClone), 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 1× nonessential amino acids, 1× sodium pyruvate, 50 μM β-mercaptoethanol, 10 mM HEPES (all from Life Technologies), and 20 ng/ml recombinant mouse GM-CSF (Peprotech). After 8 days, nonadherent cells were collected and used for experiments. For all experiments, DC purity was greater than 90%, as assessed by flow cytometry. Maturation was induced by 16 to 18 h of treatment with bacteria (MOI of 10), ultrapure E. coli O111:B4 LPS (100 ng/ml; Invivogen), or Pam3CSK4 (1 μg/ml; Invivogen). CD8+ T cells specific for the SIINFEKL peptide presented by the H-2Kb MHC-I molecule were isolated from spleens and lymph nodes of OT-I mice by negative selection (Stemcell Technologies) according to the manufacturer’s instructions.
Bacteria.
Lipid A phosphatase mutants were generated by gene deletion in the parental strain P. gingivalis 381 (ATCC BAA-1703) and were previously characterized (33). Frozen stocks of wild-type P. gingivalis and the isogenic lipid A mutant strains were streaked onto blood agar plates (BD BBL) and grown anaerobically at 37°C for 3 to 5 days. Plate-grown organisms were used to inoculate liquid cultures of brain heart infusion broth (BD BBL) supplemented with yeast extract (0.5%; BD BBL), hemin (10 μg/ml; Sigma), and menadione (1 μg/ml; Sigma). Stationary-phase liquid cultures (18 to 24 h) were harvested by centrifugation (7,000 × g for 8 min), washed three times in phosphate-buffered saline (PBS), and resuspended at an optical density at 660 nm (OD660) of 1.0 (∼1 × 109 CFU/ml) prior to use. Live P. gingivalis cells were used for all experiments, with the exception of cross-presentation assays, where heat-killed bacteria were coated with OVA.
Heat-killed E. coli cells were used as a control for bacterial stimulation. E. coli was grown at 37°C in LB broth under standard conditions. Overnight cultures were harvested by centrifugation, resuspended in PBS at an OD600 of 1.0, and heat killed at 65°C for 15 min. Heat-killed bacteria were washed three times with PBS prior to use.
Phagocytosis of P. gingivalis strains.
Binding and internalization of fluorescently labeled P. gingivalis strains were determined as previously described (82). Briefly, P. gingivalis strains were labeled with 0.01 mg/ml fluorescein isothiocyanate–N-hydroxysuccinimide ester (FITC-NHS) in PBS at room temperature for 30 min on a rotating wheel. Bacteria were washed three times with PBS to remove unreacted dye and then added to DCs (MOI of 10). At the indicated times, phagocytosis was stopped by addition of ice-cold PBS, and unbound bacteria were removed by washing. Cells were then analyzed by flow cytometry to determine bacterial uptake. To distinguish between bound and internalized bacteria, 2% trypan blue (Merck) was added to samples before acquisition to quench extracellular fluorescence.
Phagosomal degradation.
DCs were collected from culture dishes, washed twice in PBS, and resuspended in CO2-independent medium at a density of 2 × 107 cells/ml. Cells were pulsed with latex beads covalently coupled to OVA (bead to cell ratio of 5:1) for 10 min at 37°C. Ice-cold PBS was added to stop internalization, followed by three rounds of fetal calf serum (FCS) flotation to remove beads that were not internalized. DCs were resuspended in complete medium and incubated at 37°C. Aliquots of cell suspension were collected 0, 30, 90, and 120 min after bead pulsing and lysed in buffer containing protease inhibitors (Roche) overnight at 4°C in a 96-well V-bottom plate. Beads were stained with a polyclonal rabbit anti-OVA antibody followed by a goat anti-rabbit secondary antibody conjugated with Alexa 647, and fluorescence was analyzed by flow cytometry. The percentage of degradation was calculated by determining the amount of remaining OVA on beads (mean fluorescent intensity [MFI]) relative to time zero.
Cross-presentation of SIINFEKL from OVA-coated P. gingivalis.
Ninety-six-well round-bottom plates were seeded with DCs (1 × 104) and pulsed with OVA-coated P. gingivalis for 4.5 h. Cells were washed with PBS and then cocultured with OT-I T cells (5 × 104) for 18 h. CD69 expression on T cells was examined by flow cytometry. Secretion of IL-2 and IFN-γ was measured by ELISA.
To prepare OVA-coated P. gingivalis, stationary-phase liquid cultures were harvested by centrifugation, resuspended in PBS at an OD660 of 1.0 and heat killed for 15 min at 65°C. Cells of heat-killed P. gingivalis strains (109/ml) were washed twice with PBS and coated with 2 mg/ml of OVA overnight at 4°C on a rotating wheel. OVA-coated strains were washed three times with PBS prior to use. To ensure even coating, bacteria were stained with a 1/750 dilution of a polyclonal rabbit anti-OVA antibody (Sigma) followed by an Alexa 647-conjugated anti-rabbit IgG (Life Technologies) and analyzed by flow cytometry.
Immunoblotting.
Cells were subjected to the indicated treatments and lysed in radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher) containing a protease/phosphatase inhibitor cocktail (Roche). Cleared lysates were electrophoresed, transferred to polyvinylidene difluoride (PVDF) membranes, and immunoblotted with the primary antibodies using standard conditions. The primary antibodies used were anti-stress-activated protein kinase/Jun N-terminal protein kinase (anti-SAPK/JNK; catalog no. 9252), anti-p-SAPK/JNK (T183/Y185) (catalog no. 9255S), anti-p38 MAPK (catalog no. 9212), anti-p-p38 MAPK (T180/Y182) (catalog no. 9211S), anti-NF-κB p65 (catalog no. 8242), and anti-p-NF-κB p65 (S536) (catalog no. 3033S) from Cell Signaling Technology and anti-β-actin (AC-74) (catalog no. A2228) from Sigma. Membranes were probed with species-specific secondary antibodies conjugated to near-infrared (near-IR) fluorescent dyes (Licor), and protein expression was quantified using an Odyssey CLx imager (Licor).
Flow cytometry.
Cells were washed with PBS containing 0.5% bovine serum albumin (PBS/BSA) and incubated with Fc block (anti-mouse CD16/32; eBioscience clone 93) or 2% rat serum for 15 min at 4°C to block nonspecific binding of antibodies. Incubations with fluorochrome-conjugated antibodies were performed on ice for 30 min in the dark. The stained cells were washed twice and resuspended in PBS/BSA for analysis using a BD LSRI II. The antibodies used were anti-CD40 (eBioscience, clones 1C10 and HM40-3), CD69 (eBioscience, clone H1.2F3), CD80 (eBioscience, clone 16-10A1), CD86 (eBioscience, clone GL-1), MHC-I (eBioscience, clone AF6-88.5.5.3), MHC-II (I-A/I-E) (eBioscience, clone M5/114.15.2), polyclonal rabbit anti-OVA (Sigma, catalog no. C6534), Alexa 488-conjugated goat anti-rabbit IgG (H+L) (Invitrogen), and Alexa Fluor 647-conjugated goat anti-rabbit IgG (H+L) (Invitrogen).
Kinetic cytotoxicity assay.
The Cytation3 automated microscope was used to maintain temperature at 37°C and 5% CO2 for kinetic cytotoxicity assays. Glass bottom plates 0.17 mm thick were seeded with cells, stimulated as indicated, and imaged at 15-min intervals with ×4 magnification to capture 3,000 to 4,000 cells per field of view. Propidium iodide (10 μg/ml; Life Technologies, P3566) incorporation was detected via 535-nm excitation and 617-nm emission.
ELISA.
Levels of IL-1β, IL-2, IL-6, IL-12p-70, IFN-γ, and TNF in cell culture supernatants were measured by ELISA using commercially available kits (BD Biosciences). A sandwich ELISA was used to detect mouse IFN-β (Biolegend).
Statistical analysis.
Statistical analyses were performed using GraphPad Prism. Data were analyzed by two-way analysis of variance (ANOVA) with Tukey’s multiple-comparison post hoc test. A P value of <0.05 was considered significant. Error bars are ± standard deviation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jennifer Snyder-Cappione (BUSM), Paola Massari (TUSM), Alexander Poltorak (TUSM), and Joseph Sarhan (TUSM) for insightful conversations and critical review of the manuscript.
This work was supported in part by NIH grant AI142628 to C.A.G.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ayres JS. 2016. Cooperative microbial tolerance behaviors in host-microbiota mutualism. Cell 165:1323–1331. doi: 10.1016/j.cell.2016.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sansonetti PJ, Di Santo JP. 2007. Debugging how bacteria manipulate the immune response. Immunity 26:149–161. doi: 10.1016/j.immuni.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Simpson BW, Trent MS. 2019. Pushing the envelope: LPS modifications and their consequences. Nat Rev Microbiol 17:403–416. doi: 10.1038/s41579-019-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Needham BD, Trent MS. 2013. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol 11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raetz CR, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebeil R, Ernst RK, Jarrett CO, Adams KN, Miller SI, Hinnebusch BJ. 2006. Characterization of late acyltransferase genes of Yersinia pestis and their role in temperature-dependent lipid A variation. J Bacteriol 188:1381–1388. doi: 10.1128/JB.188.4.1381-1388.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunn JS, Ernst RK. 2007. The structure and function of Francisella lipopolysaccharide. Ann N Y Acad Sci 1105:202–218. doi: 10.1196/annals.1409.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, Trent MS. 2011. Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog 7:e1002454. doi: 10.1371/journal.ppat.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjostedt A, Edebro H, Forsman M, Bystrom M, Pelletier M, Wilson CB, Miller SI, Skerrett SJ, Ernst RK. 2006. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun 74:6730–6738. doi: 10.1128/IAI.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter RJ, Goguen JD, Lien E. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol 7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 11.Kanistanon D, Hajjar AM, Pelletier MR, Gallagher LA, Kalhorn T, Shaffer SA, Goodlett DR, Rohmer L, Brittnacher MJ, Skerrett SJ, Ernst RK. 2008. A Francisella mutant in lipid A carbohydrate modification elicits protective immunity. PLoS Pathog 4:e24. doi: 10.1371/journal.ppat.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanistanon D, Powell DA, Hajjar AM, Pelletier MR, Cohen IE, Way SS, Skerrett SJ, Wang X, Raetz CR, Ernst RK. 2012. Role of Francisella lipid A phosphate modification in virulence and long-term protective immune responses. Infect Immun 80:943–951. doi: 10.1128/IAI.06109-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Ribeiro AA, Guan Z, Abraham SN, Raetz CR. 2007. Attenuated virulence of a Francisella mutant lacking the lipid A 4′-phosphatase. Proc Natl Acad Sci U S A 104:4136–4141. doi: 10.1073/pnas.0611606104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH, Booth CJ, Yu H, Goodman AL. 2015. Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347:170–175. doi: 10.1126/science.1260580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain S, Darveau RP. 2010. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontol 2000 54:53–70. doi: 10.1111/j.1600-0757.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahekar AA, Singh S, Saha S, Molnar J, Arora R. 2007. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J 154:830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Gibson FC III, Hong C, Chou HH, Yumoto H, Chen J, Lien E, Wong J, Genco CA. 2004. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi C, Viereck J, Hua N, Phinikaridou A, Madrigal AG, Gibson FC III, Hamilton JA, Genco CA. 2011. Porphyromonas gingivalis accelerates inflammatory atherosclerosis in the innominate artery of ApoE deficient mice. Atherosclerosis 215:52–59. doi: 10.1016/j.atherosclerosis.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. 2008. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med 23:2079–2086. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain A, Batista EL Jr, Serhan C, Stahl GL, Van Dyke TE. 2003. Role for periodontitis in the progression of lipid deposition in an animal model. Infect Immun 71:6012–6018. doi: 10.1128/IAI.71.10.6012-6018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kebschull M, Demmer RT, Papapanou PN. 2010. “Gum bug, leave my heart alone!”—epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res 89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arvikar SL, Collier DS, Fisher MC, Unizony S, Cohen GL, McHugh G, Kawai T, Strle K, Steere AC. 2013. Clinical correlations with Porphyromonas gingivalis antibody responses in patients with early rheumatoid arthritis. Arthritis Res Ther 15:R109. doi: 10.1186/ar4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Z, Meade J, Mankia K, Emery P, Devine DA. 2017. Periodontal disease and periodontal bacteria as triggers for rheumatoid arthritis. Best Pract Res Clin Rheumatol 31:19–30. doi: 10.1016/j.berh.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Chukkapalli S, Rivera-Kweh M, Gehlot P, Velsko I, Bhattacharyya I, Calise SJ, Satoh M, Chan EK, Holoshitz J, Kesavalu L. 2016. Periodontal bacterial colonization in synovial tissues exacerbates collagen-induced arthritis in B10.RIII mice. Arthritis Res Ther 18:161. doi: 10.1186/s13075-016-1056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, van de Loo FA, Pruijn GJ, Marijnissen RJ, Walgreen B, Helsen MM, van den Bersselaar LA, de Molon RS, Avila Campos MJ, Cunha FQ, Cirelli JA, van den Berg WB. 2014. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J Immunol 192:4103–4111. doi: 10.4049/jimmunol.1301970. [DOI] [PubMed] [Google Scholar]
- 26.Potempa J, Mydel P, Koziel J. 2017. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol 13:606–620. doi: 10.1038/nrrheum.2017.132. [DOI] [PubMed] [Google Scholar]
- 27.Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, Gotoh K, Motooka D, Nakamura S, Iida T, Yamazaki K. 2014. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep 4:4828. doi: 10.1038/srep04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoneda M, Naka S, Nakano K, Wada K, Endo H, Mawatari H, Imajo K, Nomura R, Hokamura K, Ono M, Murata S, Tohnai I, Sumida Y, Shima T, Kuboniwa M, Umemura K, Kamisaki Y, Amano A, Okanoue T, Ooshima T, Nakajima A. 2012. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol 12:16. doi: 10.1186/1471-230X-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaud DS, Lu J, Peacock-Villada AY, Barber JR, Joshu CE, Prizment AE, Beck JD, Offenbacher S, Platz EA. 2018. Periodontal disease assessed using clinical dental measurements and cancer risk in the ARIC study. J Natl Cancer Inst 110:843–854. doi: 10.1093/jnci/djx278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitmore SE, Lamont RJ. 2014. Oral bacteria and cancer. PLoS Pathog 10:e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, To TT, Goodlett DR, Ernst RK, Darveau RP. 2009. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4'-phosphatase activities. Cell Microbiol 11:1587–1599. doi: 10.1111/j.1462-5822.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain S, Chang AM, Singh M, McLean JS, Coats SR, Kramer RW, Darveau RP. 2019. Identification of PGN_1123 as the gene encoding lipid A deacylase, an enzyme required for Toll-like receptor 4 evasion, in Porphyromonas gingivalis. J Bacteriol 201:e00683-18. doi: 10.1128/JB.00683-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slocum C, Coats SR, Hua N, Kramer C, Papadopoulos G, Weinberg EO, Gudino CV, Hamilton JA, Darveau RP, Genco CA. 2014. Distinct lipid A moieties contribute to pathogen-induced site-specific vascular inflammation. PLoS Pathog 10:e1004215. doi: 10.1371/journal.ppat.1004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumada H, Haishima Y, Umemoto T, Tanamoto K. 1995. Structural study on the free lipid A isolated from lipopolysaccharide of Porphyromonas gingivalis. J Bacteriol 177:2098–2106. doi: 10.1128/jb.177.8.2098-2106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Qutub MN, Braham PH, Karimi-Naser LM, Liu X, Genco CA, Darveau RP. 2006. Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect Immun 74:4474–4485. doi: 10.1128/IAI.01924-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curtis MA, Percival RS, Devine D, Darveau RP, Coats SR, Rangarajan M, Tarelli E, Marsh PD. 2011. Temperature-dependent modulation of Porphyromonas gingivalis lipid A structure and interaction with the innate host defenses. Infect Immun 79:1187–1193. doi: 10.1128/IAI.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS. 2013. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc Natl Acad Sci U S A 110:1464–1469. doi: 10.1073/pnas.1218080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. 1999. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun 67:2804–2809. doi: 10.1128/IAI.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker PJ, Evans RT, Roopenian DC. 1994. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol 39:1035–1040. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 40.Baker PJ, Garneau J, Howe L, Roopenian DC. 2001. T-cell contributions to alveolar bone loss in response to oral infection with Porphyromonas gingivalis. Acta Odontol Scand 59:222–225. doi: 10.1080/00016350152509247. [DOI] [PubMed] [Google Scholar]
- 41.Baker PJ, Howe L, Garneau J, Roopenian DC. 2002. T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunol Med Microbiol 34:45–50. doi: 10.1111/j.1574-695X.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 42.Silva-Santos B, Serre K, Norell H. 2015. gammadelta T cells in cancer. Nat Rev Immunol 15:683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 43.Speiser DE, Ho PC, Verdeil G. 2016. Regulatory circuits of T cell function in cancer. Nat Rev Immunol 16:599–611. doi: 10.1038/nri.2016.80. [DOI] [PubMed] [Google Scholar]
- 44.Ivanova EA, Orekhov AN. 2015. T helper lymphocyte subsets and plasticity in autoimmunity and cancer: an overview. Biomed Res Int 2015:327470. doi: 10.1155/2015/327470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lintermans LL, Stegeman CA, Heeringa P, Abdulahad WH. 2014. T cells in vascular inflammatory diseases. Front Immunol 5:504. doi: 10.3389/fimmu.2014.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nevius E, Gomes AC, Pereira JP. 2016. Inflammatory cell migration in rheumatoid arthritis: a comprehensive review. Clin Rev Allergy Immunol 51:59–78. doi: 10.1007/s12016-015-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chackelevicius CM, Gambaro SE, Tiribelli C, Rosso N. 2016. Th17 involvement in nonalcoholic fatty liver disease progression to non-alcoholic steatohepatitis. World J Gastroenterol 22:9096–9103. doi: 10.3748/wjg.v22.i41.9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paquissi FC. 2016. Immune imbalances in non-alcoholic fatty liver disease: from general biomarkers and neutrophils to interleukin-17 axis activation and new therapeutic targets. Front Immunol 7:490. doi: 10.3389/fimmu.2016.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 50.Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 51.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 52.Kolb JP, Casella CR, SenGupta S, Chilton PM, Mitchell TC. 2014. Type I interferon signaling contributes to the bias that Toll-like receptor 4 exhibits for signaling mediated by the adaptor protein TRIF. Sci Signal 7:ra108. doi: 10.1126/scisignal.2005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen H, Tesar BM, Walker WE, Goldstein DR. 2008. Dual signaling of MyD88 and TRIF is critical for maximal TLR4-induced dendritic cell maturation. J Immunol 181:1849–1858. doi: 10.4049/jimmunol.181.3.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weighardt H, Jusek G, Mages J, Lang R, Hoebe K, Beutler B, Holzmann B. 2004. Identification of a TLR4- and TRIF-dependent activation program of dendritic cells. Eur J Immunol 34:558–564. doi: 10.1002/eji.200324714. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 56.Davey M, Liu X, Ukai T, Jain V, Gudino C, Gibson FC III, Golenbock D, Visintin A, Genco CA. 2008. Bacterial fimbriae stimulate proinflammatory activation in the endothelium through distinct TLRs. J Immunol 180:2187–2195. doi: 10.4049/jimmunol.180.4.2187. [DOI] [PubMed] [Google Scholar]
- 57.Jain S, Coats SR, Chang AM, Darveau RP. 2013. A novel class of lipoprotein lipase-sensitive molecules mediates Toll-like receptor 2 activation by Porphyromonas gingivalis. Infect Immun 81:1277–1286. doi: 10.1128/IAI.01036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papadopoulos G, Weinberg EO, Massari P, Gibson FC III, Wetzler LM, Morgan EF, Genco CA. 2013. Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss. J Immunol 190:1148–1157. doi: 10.4049/jimmunol.1202511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 60.Diamond CE, Khameneh HJ, Brough D, Mortellaro A. 2015. Novel perspectives on non-canonical inflammasome activation. Immunotargets Ther 4:131–141. doi: 10.2147/ITT.S57976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Moltke J, Ayres JS, Kofoed EM, Chavarria-Smith J, Vance RE. 2013. Recognition of bacteria by inflammasomes. Annu Rev Immunol 31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 62.Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, Overholtzer M, Roche PA, Tampe R, Brown BD, Amsen D, Whiteheart SW, Blander JM. 2014. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell 158:506–521. doi: 10.1016/j.cell.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balyan R, Gund R, Ebenezer C, Khalsa JK, Verghese DA, Krishnamurthy T, George A, Bal V, Rath S, Chaudhry A. 2017. Modulation of naive CD8 T cell response features by ligand density, affinity, and continued signaling via internalized TCRs. J Immunol 198:1823–1837. doi: 10.4049/jimmunol.1600083. [DOI] [PubMed] [Google Scholar]
- 64.Alloatti A, Kotsias F, Pauwels AM, Carpier JM, Jouve M, Timmerman E, Pace L, Vargas P, Maurin M, Gehrmann U, Joannas L, Vivar OI, Lennon-Dumenil AM, Savina A, Gevaert K, Beyaert R, Hoffmann E, Amigorena S. 2015. Toll-like receptor 4 engagement on dendritic cells restrains phago-lysosome fusion and promotes cross-presentation of antigens. Immunity 43:1087–1100. doi: 10.1016/j.immuni.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Lorenzi S, Mattei F, Sistigu A, Bracci L, Spadaro F, Sanchez M, Spada M, Belardelli F, Gabriele L, Schiavoni G. 2011. Type I IFNs control antigen retention and survival of CD8alpha(+) dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J Immunol 186:5142–5150. doi: 10.4049/jimmunol.1004163. [DOI] [PubMed] [Google Scholar]
- 66.Arjunan P, El-Awady A, Dannebaum RO, Kunde-Ramamoorthy G, Cutler CW. 2016. High-throughput sequencing reveals key genes and immune homeostatic pathways activated in myeloid dendritic cells by Porphyromonas gingivalis 381 and its fimbrial mutants. Mol Oral Microbiol 31:78–93. doi: 10.1111/omi.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carrion J, Scisci E, Miles B, Sabino GJ, Zeituni AE, Gu Y, Bear A, Genco CA, Brown DL, Cutler CW. 2012. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J Immunol 189:3178–3187. doi: 10.4049/jimmunol.1201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Awady AR, Miles B, Scisci E, Kurago ZB, Palani CD, Arce RM, Waller JL, Genco CA, Slocum C, Manning M, Schoenlein PV, Cutler CW. 2015. Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS Pathog 10:e1004647. doi: 10.1371/journal.ppat.1004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jotwani R, Cutler CW. 2004. Fimbriated Porphyromonas gingivalis is more efficient than fimbria-deficient P. gingivalis in entering human dendritic cells in vitro and induces an inflammatory Th1 effector response. Infect Immun 72:1725–1732. doi: 10.1128/iai.72.3.1725-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miles B, Scisci E, Carrion J, Sabino GJ, Genco CA, Cutler CW. 2013. Noncanonical dendritic cell differentiation and survival driven by a bacteremic pathogen. J Leukoc Biol 94:281–289. doi: 10.1189/jlb.0213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miles B, Zakhary I, El-Awady A, Scisci E, Carrion J, O'Neill JC, Rawlings A, Stern JK, Susin C, Cutler CW. 2014. Secondary lymphoid organ homing phenotype of human myeloid dendritic cells disrupted by an intracellular oral pathogen. Infect Immun 82:101–111. doi: 10.1128/IAI.01157-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeituni AE, Jotwani R, Carrion J, Cutler CW. 2009. Targeting of DC-SIGN on human dendritic cells by minor fimbriated Porphyromonas gingivalis strains elicits a distinct effector T cell response. J Immunol 183:5694–5704. doi: 10.4049/jimmunol.0901030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tyagi RK, Miles B, Parmar R, Garg NK, Dalai SK, Baban B, Cutler CW. 2017. Human IDO-competent, long-lived immunoregulatory dendritic cells induced by intracellular pathogen, and their fate in humanized mice. Sci Rep 7:41083. doi: 10.1038/srep41083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdi K, Chen T, Klein BA, Tai AK, Coursen J, Liu X, Skinner J, Periasamy S, Choi Y, Kessler BM, Palmer RJ, Gittis A, Matzinger P, Duncan MJ, Singh NJ. 2017. Mechanisms by which Porphyromonas gingivalis evades innate immunity. PLoS One 12:e0182164. doi: 10.1371/journal.pone.0182164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brunner J, Scheres N, El Idrissi NB, Deng DM, Laine ML, van Winkelhoff AJ, Crielaard W. 2010. The capsule of Porphyromonas gingivalis reduces the immune response of human gingival fibroblasts. BMC Microbiol 10:5. doi: 10.1186/1471-2180-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gregg KA, Harberts E, Gardner FM, Pelletier MR, Cayatte C, Yu L, McCarthy MP, Marshall JD, Ernst RK. 2017. Rationally designed TLR4 ligands for vaccine adjuvant discovery. mBio 8:e00492-17. doi: 10.1128/mBio.00492-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deshpande RG, Khan MB, Genco CA. 1998. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun 66:5337–5343. doi: 10.1128/IAI.66.11.5337-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dorn BR, Burks JN, Seifert KN, Progulske-Fox A. 2000. Invasion of endothelial and epithelial cells by strains of Porphyromonas gingivalis. FEMS Microbiol Lett 187:139–144. doi: 10.1111/j.1574-6968.2000.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 79.Duncan MJ, Nakao S, Skobe Z, Xie H. 1993. Interactions of Porphyromonas gingivalis with epithelial cells. Infect Immun 61:2260–2265. doi: 10.1128/IAI.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olsen I, Progulske-Fox A. 2015. Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J Oral Microbiol 7:28788. doi: 10.3402/jom.v7.28788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sandros J, Papapanou PN, Nannmark U, Dahlen G. 1994. Porphyromonas gingivalis invades human pocket epithelium in vitro. J Periodontal Res 29:62–69. doi: 10.1111/j.1600-0765.1994.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 82.Papadopoulos G, Shaik-Dasthagirisaheb YB, Huang N, Viglianti GA, Henderson AJ, Kantarci A, Gibson FC III. 2017. Immunologic environment influences macrophage response to Porphyromonas gingivalis. Mol Oral Microbiol 32:250–261. doi: 10.1111/omi.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.