Leishmania, the causative agent of leishmaniasis, is an intracellular pathogen that thrives in the insect gut and mammalian macrophages to complete its life cycle. Apart from temperature difference (26 to 37°C), it encounters several harsh conditions, including oxidative stress, inflammatory reactions, and low pH. Heat shock proteins (HSPs) play essential roles in cell survival by strategically reprogramming cellular processes and signaling pathways. HSPs assist cells in multiple functions, including differentiation, adaptation, virulence, and persistence in the host cell.
KEYWORDS: heat shock proteins, leishmania, parasitic infection, therapeutics, chaperone inhibitors, kala azar
ABSTRACT
Leishmania, the causative agent of leishmaniasis, is an intracellular pathogen that thrives in the insect gut and mammalian macrophages to complete its life cycle. Apart from temperature difference (26 to 37°C), it encounters several harsh conditions, including oxidative stress, inflammatory reactions, and low pH. Heat shock proteins (HSPs) play essential roles in cell survival by strategically reprogramming cellular processes and signaling pathways. HSPs assist cells in multiple functions, including differentiation, adaptation, virulence, and persistence in the host cell. Due to cyclical epidemiological patterns, limited chemotherapeutic options, drug resistance, and the absence of a vaccine, control of leishmaniasis remains a far-fetched dream. The essential roles of HSPs in parasitic differentiation and virulence and increased expression in drug-resistant strains highlight their importance in combating the disease. In this review, we highlighted the diverse physiological importance of HSPs present in Leishmania, emphasizing their significance in disease pathogenesis. Subsequently, we assessed the potential of HSPs as a chemotherapeutic target and underlined the challenges associated with it. Furthermore, we have summarized a few ongoing drug discovery initiatives that need to be explored further to develop clinically successful chemotherapeutic agents in the future.
INTRODUCTION
Leishmaniasis is a neglected tropical disease primarily caused by more than 20 species of an obligate, intracellular pathogen Leishmania (1). The clinical manifestations of leishmaniasis vary from nonfatal cutaneous leishmaniasis to life-threatening visceral leishmaniasis. According to well-curated epidemiological data, up to 0.4 million visceral leishmaniasis cases and 1.2 million cases of cutaneous leishmaniasis occur every year (2). Despite decades of research, treatment of the disease is still a challenge before the clinicians. Limited therapeutic options, the severe side effects of currently used anti-leishmanial drugs (3, 4), the absence of vaccines (5), drug resistance (6), suboptimal diagnostics (7), cyclical epidemiological patterns (1), and knowledge gaps in the disease pathogenesis continue to obstruct global progress in restricting the disease (8).
The pathogen, Leishmania, is transmitted from the midgut of female phlebotomine sandflies to macrophages of mammalian hosts through a sandfly bite to complete its digenetic life cycle. The transmission of parasites from sandfly (vector) to mammals (host) exposes them to a variety of environmental changes (9, 10). The cell copes with it by undergoing several morphological changes to adapt to diverse environments (11). There are two primary morphological forms in which the parasite exists: one in the sandfly midgut as promastigotes and the other in mammalian macrophages as amastigotes (12, 13). Promastigote form is spindle-shaped, noninfective flagellated, and motile, whereas amastigote appears in the round, nonflagellated, and immobile form (14). Also, in sandfly midgut, promastigote matures from noninfectious procyclic form to highly infectious metacyclic ones (15). During blood meal or sandfly bite, these metacyclic promastigotes enter the mammalian body through the host dermis and are engulfed by the macrophages (10). Soon after their entry, the virulent parasites start differentiating into nonmotile amastigote form, which multiplies and persists in the macrophages’ phagolysosomal compartment, ultimately causing the disease (10, 12).
Several studies suggest that the induction of heat shock proteins (HSPs) in several Leishmania species is essential for their differentiation during distinct life cycle stages and adaptation to high temperatures (16). Expression of HSPs is essential for the cell survival and proliferation of leishmanial cells in both the stages of life. It imparts thermotolerance, increased virulence, and better adaptability to the adverse host environment (17–19). These proteins enable the parasite to perceive sudden changes in the environment and respond at least partially by transcriptional regulation of HSPs (16, 20). Additional mechanisms of gene regulation in the heat shock responses also exist in the parasite, including regulation of gene expression at the level of mRNA processing, mRNA stability, translation rate, posttranslation modification, and protein metabolism (21). Leishmania has a highly flexible genome with genes organized into long polycistronic gene clusters (22, 23). Environmental stresses can impact the expression level of different genes in diverse ways. It has been frequently observed that the onset of stress does not necessarily influence the mRNA transcription; instead, it increases the mRNA half-life resulting in upregulated protein synthesis (24). Therefore, instead of the transcriptional upregulation, cells choose to increase the protein content by enhancing the mRNA’s stability in the cell.
Transfer of Leishmania from sandfly midgut at ∼26°C to mammalian macrophages at ∼37°C induces a heat shock response in the cells characterized by the upregulation of a variety of HSPs to rescue cells from environmental stresses like high temperature (25). In Leishmania, HSPs like HSP70, HSP83, and HSP100 are expressed constitutively during both stages of the life cycle. However, as soon as the parasite enters host macrophages and starts differentiating into amastigotes, the transcriptional levels of these HSPs elevate (24, 26, 27). This transcriptional control in Leishmania is often modulated by aneuploidy, gene copy number variations, or gene dosage to adapt to the diverse environment during their digenetic life cycle (28–30). Nutrient availability and favorable environmental conditions allow aneuploidy evident at in vitro stage of the parasite, whereas lower levels of copy number variations are observed in harsh or stress conditions (30).
Owing to the essential role of HSPs in almost all phases of cell growth, these proteins have gained considerable attention as an excellent target for chemotherapeutic interventions of parasitic diseases, including leishmaniasis (31). Multiple studies have underlined the importance of heat shock response for organismal survival by investigating the lethal complications caused due to their suppression or pharmacological inhibition (32–35). A recent trend of repurposing HSP inhibitor drugs previously approved to treat other diseases has been observed (36). Nevertheless, more profound insights into the mechanisms of heat shock machinery linked with immune evasion, host-pathogen interaction, and disease progression in the context of leishmaniasis is required to accelerate these drug discovery initiatives.
This review presents an overview of significant roles of various heat shock proteins in cell differentiation, infectivity, encountering drug resistance, and the overall survival of the Leishmania. The article examines the merits and challenges of heat shock machinery of Leishmania as a potential druggable target, along with summarizing ongoing drug discovery initiatives exploring the potential of heat shock protein inhibition in leishmaniasis. In addition, the challenges that one can encounter in targeting these proteins are also discussed.
HSPs AND LEISHMANIA: A NOTORIOUS CONNECTION
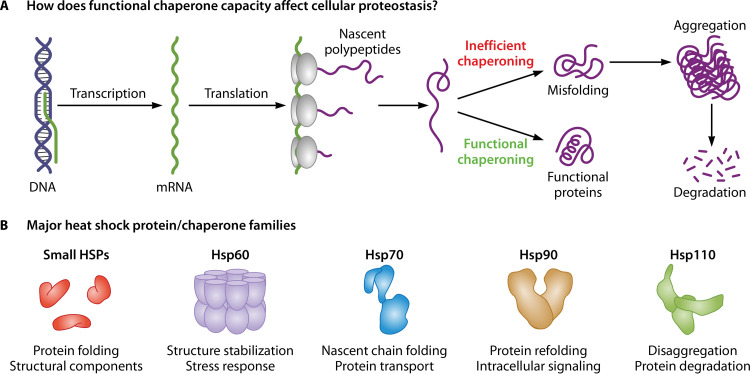
Heat shock proteins are a family of proteins secreted ubiquitously in most cell types to provide the correct three-dimensional shape to all the newly synthesized polypeptides and prevent misfolding and aggregation of all the proteins (37, 38). As shown in Fig. 1, HSPs, in eukaryotes, are broadly classified into different subgroups: HSP110, HSP90, HSP70, HSP60, small HSP (sHSP), etc., based on molecular weights, functions, structural arrangements, and subcellular localization (39). Under physiological conditions, HSPs interact with client proteins with the help of auxiliary proteins, such as Aha1 (activator of HSP90 ATPase 1) and P23, to regulate their maturation, activation, and functioning (40, 41). In Leishmania, HSP100, HSP83, and HSP70 have been extensively studied and have been of immense importance in both the cell cycle stages. These HSPs and the auxiliary proteins (also referred to as cochaperones) play an essential role in the parasite survival and differentiation at the mammalian body temperature (42).
FIG 1.
Overview of heat shock proteins. In a eukaryotic cell, the role of HSPs starts soon as the newly synthesized polypeptides exit through the ribosomal exit site into the cytosol. Several specific chaperones start acting on the linear chains and fold them into a specific three-dimensional shape needed for most cellular proteins to localize and perform their duties. Under several stress-like conditions, proteins may deviate from their folding paths and may end up in semifolded or misfolded species, which may generate toxicity inside the cells. To eliminate such proteins, chaperones may further attempt to refold the misfolded proteins or disaggregate, if they start polymerizing under disease or environmental distress conditions. If all attempts fail, the chaperones may also direct specific proteins toward degradation pathways in association with cellular proteolytic systems, including the ubiquitin-proteasome system and autophagy (152, 153). (B) The lower subsection shows a representative structure and significant functions attributed to five prominent chaperone families (154).
Survival under diverse physiological conditions requires parasites to modulate their metabolic pathways and maintain the proteome’s functional capabilities, which is widely achieved by molecular chaperones (43). The correlation between the HSPs of various parasitic organisms and their virulence potential is also widely acknowledged (20, 44, 45). A basic representation of the diverse roles of HSPs at different cell cycle stages is shown in Fig. 2. Emerging pieces of evidence highlight the role of posttranslational modifications, such as phosphorylation in the regulation of HSPs at different stages of the life of the parasite (46). It has been determined that when the promastigotes infect mammalian cells, an increase in the level of proteins, including the HSPs, occurs at both mRNA and protein levels. Subsequently, the increased protein synthesis rate is lowered by increasing the phosphorylation of eIF2α in amastigotes and attenuating the translation machinery at the mRNA level (21). A summary of all the characterized HSPs in Leishmania and their diverse physiological roles in various aspects of parasite survival is provided in Table 1.
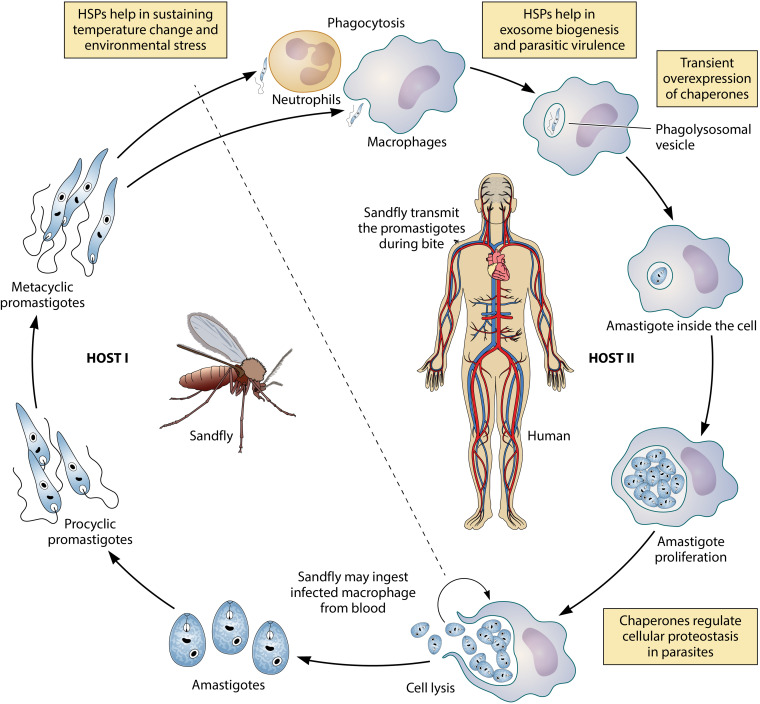
FIG 2.
Diagram of the digenetic life cycle of Leishmania sp. Transient overexpression of HSPs is observed during the transformation of promastigote-to-amastigote differentiation and helps in the survival and proliferation of amastigotes inside host macrophages. Macrophage infection is facilitated by the release of virulence factors by promastigotes well packaged into exosomes. HSPs are crucial for exosomal packaging as well. In addition, HSPs are essential in maintaining the proteostasis and regulation of signaling pathways in the parasite.
TABLE 1.
Role of heat shock proteins in Leishmaniaa
| HSP | Location(s) | Function(s) | Physiological role(s) | Reference(s) |
|---|---|---|---|---|
| Aha1 | Cytoplasm | Mediates HSP90 functions | Intracellular survival; cell uptake by macrophages | 97 |
| Calreticulin | ER | Protein folding | Intracellular survival; pathogenicity | 87 |
| CPN10 | Mitochondria | Protein folding | Parasite internalization; intracellular survival | 111 |
| CPN60 | Mitochondria | Protein folding; solubilization of aggregates | Differentiation | 110 |
| Grp94 | ER | Phosphoglycan synthesis | Virulence | 155 |
| Grp78 | ER | Unfolded protein response; translocation of secretory proteins | Virulence | 79 |
| HSP90 | Cytoplasm | Protein maturation and activation | Survival; virulence | 114 |
| HSP83 | Cytoplasm | Protein maturation cell signaling | Cell cycle; differentiation | 20, 66 |
| HSP83 | Cytoplasm | Protein maturation cell signaling | Resistance | 124 |
| HSP83 | Cytoplasm | Protein maturation cell signaling | Virulence | 156 |
| HSP83 | Cytoplasm | Protein maturation cell signaling | Immunogenicity | 157 |
| HSP70 | Cytoplasm | Protein folding; translocation | Resistance | 119, 127 |
| HSP70 | Cytoplasm | Protein folding; translocation | Oxidative stress tolerance | 79 |
| HSP70 | Cytoplasm | Protein folding; translocation | Immunogenicity | 157 |
| HSP100 | Cytoplasm | Unfolding; proteolysis; solubilization of aggregates | Infection | 49, 55 |
| HSP100 | Cytoplasm | Unfolding; proteolysis; solubilization of aggregates | Stabilizing the amastigote stage | 49, 55 |
| HSP23 | Cytoplasm | Thermoprotection; membrane homeostasis | Virulence; survival | 17 |
| HSP20 | - | - | Immunogenicity | 158 |
| HSP78 | - | - | Infection persistence | 35 |
| p23 | Cytoplasm | Mediates HSP90 functions | Protection against HSP90-mediated growth arrest | 100, 102, 105 |
| STI1 | Nucleus; cytoplasm | Mediates HSP90 functions | Proliferation | 18 |
| SGT | Cytoplasm | Client protein recognition; assist large chaperones | Intracellular survival | 19 |
Abbreviations used in table: Aha1, activator of HSP90 ATPase 1; ER, endoplasmic reticulum; CPN, chaperonins; Grp94, glucose-regulated protein 94; HSP, heat shock proteins (large); SGT, small glutamine-rich tetratricopeptide repeat; STI1, stress-inducible protein 1.
ROLES OF HSPs IN CELL GROWTH, DIFFERENTIATION, AND VIRULENCE OF LEISHMANIA
Leishmania’s infection is mediated by several virulence factors that undergo folding, processing, and trafficking before being secreted from the cells. Many chaperones (HSP100, HSP90, and calreticulin) play crucial roles in the maturation process (47–51). Correct intracellular processing of virulence proteins followed by their secretion is critical for the survival of the parasites in the hosts; therefore, it can be well utilized as a therapeutic target (52). Similarly, inhibition of protein kinases, which mediate stage-specific phosphorylation of HSPs to regulate their expression, could also be a promising strategy for chemotherapeutic intervention (46). To provide an in-depth understanding of the roles of HSPs in different facets of the parasite life, we summarize essential aspects of a few prominent families of HSPs.
HSP100 Family.
HSP100 is an essential class of molecular chaperones expressed in eukaryotic organisms, including Leishmania. These proteins are well recognized for their roles in stress tolerance. Unlike other HSPs, such as HSP70 and HSP83, which have multiple gene copies, HSP100 is encoded by a single copy of clpB genes in Leishmania (53). Interestingly, induction of HSP100 in Leishmania occurs only in the presence of thermal stress, not by stresses caused due to acidic conditions or other chemical stimuli, such as hypoxia, oxidative or nitrosative stress, etc. (49, 54). HSP100 does not affect the cell viability of the parasite at the promastigote stage. It plays an essential role in proliferation and survival inside macrophages and prevents amastigote-to-promastigote differentiation, thereby strictly maintaining the parasite’s amastigote form (55). This is partially achieved by controlling the expression of the amastigote-specific A2 protein family (56, 57). Although HSP100 is essential for maintaining the amastigote stage, the protein’s absence does not entirely block its differentiation. In contrast to Saccharomyces cerevisiae, compensation for the loss of HSP100 by overexpression of HSP70 has not been reported in Leishmania (53, 58). However, loss of HSP100 in Leishmania is compensated partially by the overexpression of a nonessential virulence factor P46 (59, 60). Interestingly, P46 restores the parasite virulence caused due to absence of HSP100 as a form of phenotypic compensation.
In Leishmania, HSP100 is vital for the parasite virulence, although the precise mechanism is not well known. Silverman et al. demonstrated that HSP100 mediates the packaging of specialized proteins (including virulence factors) in the exosomes (61). These exosomes deliver virulence proteins to the host macrophages (62). Exposure of host cells with exosomes causes immunomodulation in the host cells by inhibiting the production of proinflammatory cytokines, like gamma interferon (IFN-γ), interleukin-8 (IL-8), tumor necrosis factor alpha (TNF-α), and activation of IL-10, an anti-inflammatory cytokine. It is a widely accepted notion that macrophages use the T-cell-mediated immune response to counter intracellular infections wherein greater levels of T-helper 1 (Th1) cytokines, such as IFN-γ and IL-12, and suppressed levels of a regulatory cytokine, e.g., IL-10, are strategically used by the host cells to eliminate the parasite burden (63, 64). Exosome-mediated inhibition of IFN-γ and TNF-α and the enhanced levels of IL-10 in null mutants of HSP100 promote Th2 polarization of macrophages, favoring disease pathogenesis (61).
HSP90 Family.
HSP90 is one of the highly abundant classes of intracellular HSPs in eukaryotes. These 90-kDa proteins have a central role in processing several client proteins that are hubs of signaling networks in the cell and appear to participate in almost all kinds of cellular functions (40). In other words, HSP90 acts as a connecting link between regulatory circuits of the cell and environmental stresses (65). HSP90 accomplishes its functions by forming large protein complexes with different sets of cochaperones (47). In Leishmania, the HSP90 homolog is also referred to as HSP83 (66, 67). There are up to 18 gene copies of HSP83 in Leishmania, comprising 2.8 to 3% of the total protein content in the promastigote stage (18, 24, 68). The protein content rises further with an increase in temperature (24, 69). The parasite-stage differentiation accompanies changes in the expression of HSPs at the transcriptional level (27).
HSP90 interacts with various cyclin-dependent proteins and plays a significant role in cell cycle progression (70). The inhibition of HSP90 implies growth arrest at the G2 phase (20). It is essential to maintain both promastigote and amastigote stages, even under normal stress-free conditions (71). These proteins are constitutively expressed in general and are upregulated on the arrival of stress conditions (24). Inhibition of HSP90 could be compensated by enhanced expression of HSP100, which induces the synthesis of amastigote-specific proteins and directs differentiation of the parasite toward the amastigote stage (20). Glucose-regulated protein 94 (Grp94), the endoplasmic reticulum (ER) variant, influences parasitic virulence by affecting the synthesis of lipophosphoglycan (LPG), a known virulence protein (11, 26, 72, 73). Inactivation of GRP94 results in reduced LPG synthesis, culminating in decreased virulence of the parasite (26).
HSP70 Family.
HSP70 is a family of highly conserved 70-kDa proteins that play an essential role in protein folding and trafficking of client proteins (74). It carries out its chaperoning function in an ATP-dependent manner with the help of a nucleotide exchange factor and HSP40, a J protein (DnaJ) (75). HSP70 loci in Leishmania are well conserved and contain two genes (HSP70I and HSP70II), except in L. braziliensis, which lacks HSP70-II (67). Several isoforms of HSP70 are known to exist in Leishmania; for instance, the ER-resident isoform Grp78, the mitochondrial isoform Grp75, and three unique HSP70 variants (HSP70.4, HSP70.b, and HSP70.c), which are absent in human beings (76, 77). All of these isoforms are constitutively expressed (24). HSP70 is upregulated in promastigotes upon infection into the host macrophages, indicating an essential role of the protein in host-pathogen interaction and parasite differentiation (78).
Overexpression of HSP70 helps the promastigotes to neutralize the highly oxidative environment of host cells and renders tolerance to the pathogen against temperature changes and environmental stresses, including free radicals (79). The ER variant of HSP70 (Grp78) has also been found to have an indirect role in parasitic virulence (80). The single-copy gene of HSP70 from L. braziliensis is a probable candidate for serodiagnosis since it presents itself as an antigen, well recognized in the sera of patients (81). Parasite HSP70 is also being used in species determination, exploiting its polymorphic sequence through PCR amplification, followed by restriction fragment length polymorphisms (RFLP) analysis, a sensitive and rapid tool that can be directly applied on the clinical samples (82–84). Further examination of the chaperone complexes in Leishmania revealed that several proteins belonging to RNA metabolism (27%) and the translation process (7%), e.g., elongation factor-1α (EF-1α), EF-2, eukaryotic initiation factors-4A (eIF4A), initiation factor-4A (IF-4A), etc., interact with HSP70, which in turn affects a plethora of pathways inside the cell (85).
Calreticulin.
Calreticulin, a conserved calcium-binding protein with an inherent RNA-binding activity, is predominantly found in the ERs of eukaryotic cells, except erythrocytes (86). This multifunctional protein is involved in multiple cellular processes, such as chaperoning, signaling, enhancement of phagocytosis, gene expression regulation, etc. (86, 87). The chaperoning function of this protein is necessary to ensure the correct folding of glycoproteins in the cell (87). Calreticulin also helps the parasite in responding to the changing environment during promastigote-to-amastigote differentiation (48). Calreticulin is known to undergo several posttranslational modifications (PTMs), such as glycosylation and phosphorylation, that may have implications in the organism’s heat shock response (88). However, the characterization of calreticulin in Leishmania demonstrated that the protein mostly gets glycosylated for its activity, a feature distinct from human calreticulin, where phosphorylation is mainly involved (48).
There are several secretory proteins in Leishmania that act as virulence factors and are essential for host immune response evasion and pathogenesis (51, 62, 89). Calreticulin mediates the proper folding of secretory proteins, including acid phosphatases, a virulence factor essential for cell survival and adaptation to the host environment (62, 89–91). It has been anticipated that calreticulin modulates macrophage defense mechanisms in favor of the parasite, and its overexpression leads to reduced parasite survival in the host cells (91). The exact mechanism remains elusive; however, any change and/or impairment in the calreticulin-mediated functions may adversely impact parasite virulence and survival in macrophages, thus proving lethal for the parasite (87, 91).
Cochaperones.
Apart from the chaperones (both large and small), cells contain a cohort of cochaperone proteins that participate in the formation of large chaperone complexes, regulate ATPase activity of HSPs, and mediate processing of client proteins (92). Leishmanial genome also encodes many cochaperones that help the organism cope with environmental stress and allow cell proliferation inside the macrophages (77, 93). Major cochaperones known to be found in Leishmania are activator of HSP90 ATPase 1 (Aha1), p23, stress-inducible protein 1 (STI1), and small glutamine-rich tetratricopeptide repeat protein (SGT) (94–97). Aha1 is an ∼38-kDa protein known for stimulating the ATPase activity of HSP90 (97). This protein has been characterized in Leishmania and found to interact with leishmanial HSP90 stimulating its ATPase activity by ∼10-fold (98). Aha1 is a nonessential protein that does not affect cell viability, but changes in its expression level negatively impact the parasite infectivity (99).
Another cochaperone SGT binds both HSP70 and HSP90 to form large and stable chaperoning complexes (96). The peculiar orthologues of SGT characterized in Leishmania are constitutively expressed in both the life cycle stages and are known to have an essential role in the growth and survival of promastigotes (19). STI1 is a conserved protein commonly induced under stress conditions (94). Interaction of STI1 and HSP90 in Leishmania is crucial for cell proliferation, multiplication, and heat shock response in both promastigote and amastigote stages (18). Studies regarding its subcellular localization suggest that it continuously shuttles back and forth across the nucleus (70). The protein p23 is the smallest cochaperone to participate in the chaperoning pathway of HSP90 (95). It binds with the HSP90 ATPase domain and mediates the release of client proteins (100). The protein p23 is expressed constitutively and facilitates the maturation of client proteins (65). It primarily arrests HSP90 ATPase activity, thus preventing aggregation of denatured proteins (101, 102). In Leishmania, two isoforms have been identified with a 30% similarity with the human orthologues (103). Although p23 itself is not essential for the survival or virulence of the parasite, it plays a vital role in protecting parasites from HSP90 inhibitor-mediated cell cycle arrest by binding to the ATP-bound form of HSP90 dimer and preventing the ATP hydrolysis (104, 105).
sHSPs are small (∼12 to 43 kDa), ubiquitous, and conserved ATP-dependent molecular chaperones (106). They form complexes with misfolded/unfolded proteins and prevent their aggregation, thus relieving cells from proteotoxic stress (107). In the context of leishmanial viability in both the stages of the life cycle, two sHSPs, namely, HSP23 and HSP40, are of utmost importance. HSP23 in Leishmania is essential for the survival of parasite inside macrophages. It confers tolerance against heat and chemical stress and is an important virulence factor required in the infection process (17). Under stress conditions, cells exhibit rapid expression of HSP23, and any loss of expression or function could be lethal due to the enhanced susceptibility of the amastigotes to the extracellular stress at 37°C (17).
Although the Leishmania genome contains multiple genes of HSP40, the protein is not well characterized (77, 93). Proteomic investigations have revealed that HSP40 gets phosphorylated during differentiation from promastigotes to amastigotes (77, 108). Furthermore, the upregulation of HSP40 has been observed in artemisinin-resistant cells (109). The presence of both phosphosites and the methyltransferase domain in the protein highlights that the protein undergoes a range of posttranslational modifications. More studies are required to unravel the regulatory role and functional relevance of HSP40 in the life cycle of the parasite.
Another important class of cochaperones is Cpn60, a 60-kDa GroEL homologs in Leishmania. It has two Cpn60 gene family members (Cpn60.1 and Cpn60.2), considered HSP60 orthologues in trypanosomatids. During heat stress, Cpn60.2 of Leishmania has been found to upregulate by 2.5-fold (110). Similarly, CPN10, a GroES homolog in Leishmania, is required for the survival of intracellular form and negatively controls the internalization of the parasite by the human macrophages (111). This observation is supported by the increased expression of the parasite in the amastigote stage (112).
PHOSPHORYLATION OF HSPs: A THERAPEUTIC CONTEXT
Recent shreds of evidence suggest that differentiation of parasite from promastigote to amastigote is regulated by stage-specific differential phosphorylation of a few chaperone proteins residing inside the parasitic cells, e.g., HSP70, STI1, and HSP90 (46). Signals that can activate the differentiation process in the parasite primarily include high temperature and reduced pH, leading to the phosphorylation of many cellular proteins, including protein kinases (108). In Leishmania, the chaperones and cochaperones have also been subjected to increased phosphorylation during differentiation to amastigote stages (113–115). Recently, HSP90 and HSP70 have been discovered as essential substrates of mitogen-activated protein kinase 1 of L. donovani in addition to other proteins of the HSP90-foldosome complex, namely, STI and SGT. It influences the expression of crucial HSPs by regulating the stability and activity of HSP90-foldosome proteins employing phosphorylation (116).
Leishmania also possesses phosphatases, such as PP5, which influences parasite pathogenicity (117). Even though PP5 is nonessential, it impacts metacyclogenesis (differentiation of noninfectious promastigotes to the infectious metacyclic form) and adaptation upon stress conditions most probably through dephosphorylating HSP83 since loss of PP5 in the parasite attenuates its virulence potential. Phosphorylation of HSP90 is required for fine-tuning the chaperone cycle of various client proteins (115). Any alteration in these posttranslational modifications may hamper HSP90 function, thereby affecting an organism’s adaptability to the diverse environments at distinct life cycle stages (47, 118). It is presumed that HSPs coordinate with specific protein kinases to cope with changing environments during differentiation and phosphorylation of cochaperones, such as STI1, that are essential for parasitic viability, especially during the pathogenic stage (18, 46). Based on these observations, it could be assumed that HSPs and their respective protein kinases may provide promising drug targets against these parasitic diseases; however, more studies needed to elucidate their mechanistic role (114).
ROLE OF HSPs IN THE DEVELOPMENT OF DRUG RESISTANCE IN LEISHMANIA
Previous studies have presented several evidences that suggests a strong correlation between the expression of HSPs and drug resistance in Leishmania. An elevated level of HSPs has often been observed in different drug-resistant strains (119–121). Overexpression of HSPs, such as HSP70 in the case of L. tarentolae and both HSP70 and HSP83 in the case of clinical isolates of L. donovani (122), confers increased resistance to pentavalent antimony (the first-line drug for treating leishmaniasis) (121), antifolate methotrexate resistance (123), and nelfinavir resistance (120). Clinical isolates from drug-resistant leishmaniasis patients have shown HSP90 accumulation (124). Similarly, amphotericin B resistance is characterized by high expression levels of HSP90, HSP60, and HSP70 (125).
Similarly, a comparison of proteomes of antimony-resistant and -susceptible strains of three Leishmania species (L. braziliensis, L. infantum, and L. chagasi lines) has demonstrated the accumulation of HSPs in the antimony-resistant cells (126). Surprisingly, miltefosine resistance is correlated with the downregulation of HSP70 (HSPA9B) (127). Since several evidences highlight the upregulation of HSPs in case of drug resistance, it is presumed that inhibition of HSPs may reverse the resistance to their respective drugs. Although the underlying mechanism is not well understood, the fundamental role of HSPs in the physiology of Leishmania cannot be undermined. The upregulation of HSPs in response to drug resistance indicates that HSP accumulation somehow assists the cell in tolerating drug-induced shock in a better manner (119). Diverse opinions regarding the underlying mechanism exist throughout the literature. Some of them advocate that HSP70 and HSP83 may inactivate the apoptotic pathway that is usually activated by drugs like antimony in the parasite (119). Since the induction of apoptosis is a common death mechanism affected by most antileishmanial drugs (128, 129), the induction of HSPs may lead to either suppressing apoptosis or interfering with the activities of proapoptotic proteins (124). In-depth examination of the overexpression of these stress proteins under drug resistance conditions may help in the development of future antileishmanial chemotherapeutic strategies.
EXPLORING HSP INHIBITORS AS POTENT ANTILEISHMANIAL AGENTS: CURRENT STATUS
The heat shock response is a highly coordinated event wherein multiple chaperones participate together in the form of complexes to accomplish their specified functions (40, 47, 128). Inhibiting chaperone machinery not only perturbs the proteostasis inside the cells but also impairs several activities essential for pathogenesis and virulence. In Leishmania, inhibition of HSPs may hinder with the transitioning between different stages of the life cycle (105, 129). In addition, human HSP90 differs from the parasite homolog in the domain region from amino acids 50 to 75 in terms of hydrophobicity, allowing the design of specific inhibitors (130). The critical role of HSPs in cell growth, differentiation, drug resistance, and phenotypic variation from their human orthologues have made them a popular drug target (131, 132). Figure 3 depicts a few possible consequences of HSP inhibition on the survival of both the stages of the parasite as well as disease progression. Therefore, targeting the parasitic organisms’ chaperone system to combat the diseases they cause holds promising future perspectives.
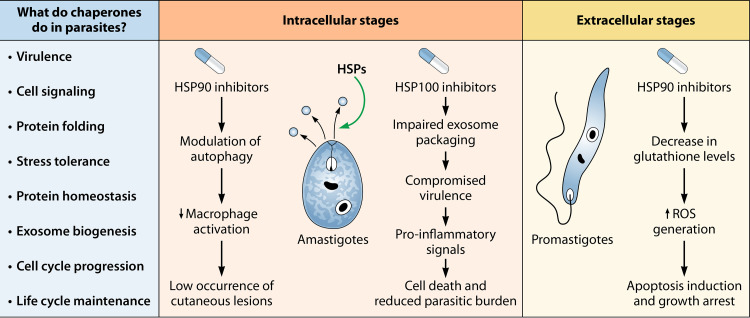
FIG 3.
Summary of possible consequences of HSP inhibition in both intracellular and extracellular stages of the parasite. Inhibition of parasite HSP90 in promastigotes results in the induction of apoptosis and cell growth arrest, whereas in amastigotes HSP90 inhibition causes autophagic alterations and downregulation of proinflammatory cytokine molecules, such as TNF-α and IL-6, which in turn prevents the activation of macrophages (34, 145). Subsequently, the hypoactive macrophage suppresses the formation of cutaneous lesions, thereby limiting the progression of cutaneous leishmaniasis. HSP inhibition may also adversely impact the packaging of exosomes essential for pathogenicity and result in a dramatic reduction in the parasite burden (61).
HSPs are already an attractive target in diseases like cancer and neurodegeneration, where the stress response pathways have achieved encouraging milestones; however, its relevance for parasitic infections has been realized lately and is still under investigation (133–135). A number of ongoing studies have paved the way toward designing and examining the therapeutic potential of HSP inhibitors as an antileishmanial agent (32, 43). A list of inhibitors tested against HSPs in Leishmania is given in Table 2. However, we still fall short when it comes to efficacy and translation of these inhibitors to clinical levels, especially in leishmaniasis. Geldanamycin (GA), radicicol, 17-aminoallyl-geldanamycin (17-AAG), and reblastatin are prominent HSP inhibitors that have already been tested in treating diseases such as cancer (136). Repurposing them in the treatment of neglected tropical diseases has been widely considered these days. GA, an ansamycin-benzoquinone antibiotic, is one of the most recognized HSP90 inhibitors. It acts by competing with ATP for binding to the ATP pocket of HSP90. It causes cell cycle arrest and apoptosis induction in the cells (129).
TABLE 2.
HSP inhibitors of Leishmaniaa
| Inhibitor | Target HSP | Organism | IC50 | Reference(s) |
|---|---|---|---|---|
| Ap5A | HSP78 | L. donovani | 15 μM | 35 |
| CKI-7 | CK | L. donovani | >100 μM | 159 |
| D4476 | CK | L. donovani | 30 μM | 159 |
| EGCG | HSP70 | L. braziliensis | 278.8 μM | 160 |
| Geldanamycin | HSP83 | L. amazonensis | 0.2 μM | 33 |
| IC261 | CK | L. donovani | 78 μM | 159 |
| Liposomal 17-AAG | HSP83 | L. amazonensis | 0.006 nM | 148 |
| HSP83 | L. braziliensis | 65 nM | 145 | |
| Novobiocin | HSP83 | L. donovani | 242 μg/ml | 142, 161 |
| PBCANP-polymyxin B | HSP70 | L. amazonensis | 4.6 μM | 162 |
| Polymyxin B | HSP70 | L. infantum | 169 nM | 162 |
| HSP70 | L. amazonensis | 59.2 μM | 162 | |
| 17-AAG | HSP83 | L. braziliensis | 65 nM | 34 |
| HSP83 | L. amazonensis | 65 nM | 34 | |
| HSP83 | L. major | 79 nM | 34 | |
| 17-DMAG | HSP83 | L. amazonensis | 0.0861 μM | 33 |
Abbreviations used in table: Ap5A, P1, P5-di(adenosine-5′)-penta-phosphate ammonium; CK, casein kinase; EGCG, epigallocatechin-3-gallate; PBCANP, poly(n-butylcyanoacrylate) nanoparticles; 17-AAG, 17-allylamino-17-demethoxygeldanamycin.
Treatment of L. donovani cells with high GA concentrations arrests cell growth in the G2 phase and prevents stage differentiation by causing apoptosis (137). It has also been known to inhibit HSP90 phosphorylation (115). Since HSP90 phosphorylation is highly significant in terms of regulation of the heat shock response in the parasite, its inhibition by GA imparts a detrimental effect on the growth and differentiation of the parasite. Radicicol, a macrocyclic fungal antibiotic, is another classical HSP90 inhibitor that binds to ATP-binding domain of the protein with greater affinity than geldanamycin and interferes with the binding of p23 with HSP90 and the formation of chaperone complex (138). The compound is unstable and ineffective in vivo (139), but its derivatives, such as oxime derivatives (e.g., KF58333) and resorcinol-containing derivatives (e.g., NVP-AUY922 and STA-1474), have been effective in cancer cell lines both in vitro and in vivo (59, 140, 141). Radicicol has also been shown to possess strong antitrypanosomal activity at nanomolar concentrations both in vitro and in vivo (142). The effects of GA and radicicol exposure on Leishmania resulted in the induction of HSP100 and amastigote-specific proteins (the A2 family), along with arresting the cell cycle in the G2 phase (20).
17-AAG is another well-known HSP90 inhibitor that has already been successfully tested in cancer (143, 144). The molecule is also found to be active against Leishmania both in vitro and in vivo (34, 145). Even at a low dosage, the inhibitor reduces parasite load in the macrophages by severely compromising the production of proinflammatory cytokines and oxidative moieties (34). The antileishmanial effects of 17-AAG are comparable to the most accepted second-line leishmaniasis drug, amphotericin B (145). 17-AAG induces cell cycle arrest in promastigotes at the G0/G1 stage. A combination of 17-AAG with edelfosine (a phospholipid) has been proposed to kill the parasite via an apoptosis-like cell death pathway (146, 147). Despite its efficacy, 17-AAG failed to progress through clinical trials because of hepatotoxicity and low solubility (134). Therefore, to address these pharmacokinetic challenges, a liposomal formulation encapsulating hydroxypropyl-β-cyclodextrin complexed with 17-AAG has been prepared that presents a higher therapeutic index with improved solubility (148).
Recently, an in vivo study has shown that P1,P5-di(adenosine-5′)-penta-phosphate ammonium salt (Ap5A), an inhibitor of HSP78 in L. donovani, could be an excellent antileishmanial drug candidate at least at the preclinical level (35). However, the use of HSP inhibitors to treat intracellular parasitic infections is appealing only if it is exclusively directed against parasitic protein targets without affecting the human counterparts. This can be achieved by exploiting the differences in the active site of the orthologues. In this regard, a recent docking analysis has shown that HSP90 inhibitors, such as GA and its derivatives, such as 17-AAG and 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17-DMAG), have a greater affinity to HSP83/90 of L. amazonensis than to human HSP90 (33).
Alkynyl derivatives of reblastatin, prepared by a chemobiosynthetic approach, have also been found to exhibit remarkable efficacy against HSP90 in L. braziliensis, with moderate differences in binding to their human counterparts (130). Another preliminary investigation has identified and screened several small molecules capable of inhibiting ATPase activity of HSP90 from L. braziliensis through a coupled molecular modeling and in vitro assay approach (32). The encouraging outcomes of this study could lay a firm foundation for future drug development initiatives. However, a few challenges make targeting this protein family complicated, such as the ability to functionally compensate for the inhibition of one heat shock protein by modulating the expression level of another (20, 149) and safeguarding parasitic cells from inhibitor-mediated growth arrest through altering expression levels of cochaperones such as p23 and Aha1 (105).
Moreover, responding to environmental stresses by copy number, variations, or aneuploidy for antagonizing the external stress in favor of the parasite poses difficulties in therapeutic intervention (22). The flexibility of the genome and changes in the copy numbers are not always correlated with changes at the transcript level but are regulated through additional mechanisms that remain to be deciphered (29, 150). A more in-depth insight into the structural and functional aspects of chaperones in Leishmania and a firm understanding of the prevalent compensatory mechanisms in the cell are required to overcome the existing challenges and to develop HSPs as useful pharmaceutical targets.
CONCLUSIONS
Kinetoplastids encode the whole range of HSPs responsible for a wide range of functions, from protein folding to preventing deposition of protein aggregates and maintaining protein homeostasis under both normal and stress conditions in the cell (77). Decades of understanding of these proteins and their mechanism of actions have revealed the significance of heat shock response machinery in the digenetic life cycle of Leishmania. Some components are essential, while others are required for parasitic virulence, thereby presenting an excellent drug target (45, 55, 71). Inhibition of such versatile cellular machinery is promising, and targeted inhibition of parasite HSPs could provide novel solutions and cure this neglected tropical disease, leishmaniasis. However, there is still a long road ahead before we can reliably use HSP inhibition for clinical purposes.
In order to target HSPs, the existing compensatory mechanisms should be studied in detail for significant inhibition. For instance, GA and radicicol, the inhibitors of Hsp90, may have opposing effects on the expression of a cochaperone, Aha1, that protects the parasite under stressed conditions by regulating the activities of HSP90 in cooperation with p23 (99). In a different study, the overexpression of p23 confers the protective effect by interfering with the inhibitor’s binding with the HSP90 protein (105). A similar mechanism may occur in the case of other cochaperones. Such observations need to be substantiated with future studies for better therapeutic intervention. A proteomic study of L. braziliensis has identified several hypothetical proteins interacting with HSP70 (85). Their functions and possible impact on physiology and pathogenicity of Leishmania are yet to be studied. Moreover, HSPs are also linked with antileishmanial drug resistance and tolerance (124, 127). Combination therapies should be encouraged to target multiple HSPs or foldosome complexes at the same time to counter HSP compensation mechanisms and drug resistance.
The importance of PTMs such as phosphorylation in chaperoning function should also be studied more to understand the regulatory checkpoints for effective chemotherapeutic interventions (114, 151). A number of inhibitors effective in other protozoan parasites remain to be tested in Leishmania. The development of compounds for selective inhibition of HSPs in both parasite stages is ideal for antileishmanial therapies. However, the biggest problem in targeting HSPs is posed by the structural similarity of parasite HSPs to human orthologues. Future studies should focus on evaluating selectivity and binding studies of currently tested inhibitors on both human and parasite proteins. Studying conformational changes caused by the binding of inhibitors to the domains, such as the N-terminal domains in HSP90, might help to achieve the selectivity of inhibitors (130). Another knowledge gap is evident regarding HSP inhibition downstream events or, in other words, underlying mechanisms of cell death due to HSP inhibition are not clear.
Based on studies of the toxicity of HSP inhibition in humans, it can be anticipated that, most probably, dysregulation of signaling pathways due to HSP inhibition is responsible. Nonetheless, more studies are required to substantiate the anticipated notions (142). Phosphorylation enzyme (e.g., casein kinase and phosphatases such as PP5)-mediated parasitic intervention requires an in-depth understanding of the mechanism by which enzyme-HSP interactions influence client proteins’ fate, thus determining parasite pathogenicity. Finally, the majority of HSP inhibitors have only been tested at in vitro levels. Translation of these in vitro-tested inhibitors to clinical levels is a big challenge, especially in the case of neglected tropical diseases. Furthermore, the therapeutic index of the drug is a crucial parameter that seeks attention as a low therapeutic index, leading to adverse drug reactions that may compromise drug usage despite remarkable efficacy. Recently, liposomal approaches have been applied to 17-AAG that increased its therapeutic index along with improving pharmacokinetic parameters (148). These efforts should be encouraged further.
ACKNOWLEDGMENTS
We thank the National Institute of Pharmaceutical Education and Research, Hajipur, and the Central University of Rajasthan for providing the required facilities during the preparation of the manuscript.
P.P. received a fellowship from the Ministry of Chemicals and Fertilizers, Government of India.
We have no conflicts of interest to declare.
Biographies
Pragya Prasanna obtained her B.Sc. degree and M.Sc. degree in Biotechnology from Devi Ahilya Vishvavidyalaya Indore and the University of Lucknow India. Later, she earned her Ph.D. at the Department of Biotechnology, National Institute of Pharmaceutical Education and Research (NIPER), Hajipur, India. Her research mainly focuses on nanoparticle-based drug discovery for the treatment of visceral leishmaniasis. She is currently investigating the potential and mechanism of action of functionalized gold nanoparticles on both sensitive and amphotericin B-resistant strains of L. donovani.
Arun Upadhyay worked on the structural biology of proteins while an Assistant Professor at the Central University of Rajasthan, Ajmer, India. He is currently working on proteostasis regulation in different pathological conditions at the Feinberg School of Medicine, Northwestern University, Chicago, IL. His doctoral research is aimed at the modulation of cellular protein quality control pathways under different stress conditions. He has worked on various proteinopathies and associated disease pathways and looked at different ways to suppress the aggregation of proteins under pathological conditions. His primary research areas include understanding the evolution of protein sequences and characterization of highly evolved protein structures of therapeutic importance. He is currently investigating these topics using the novel, state-of-the-art proteomics approaches.
REFERENCES
- 1.Burza S, Croft SL, Boelaert M. 2018. Leishmaniasis. Lancet 392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, de Boer M, WHO Leishmaniasis Control Team. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundar S, Jaya J. 2010. Liposomal amphotericin B and leishmaniasis: dose and response. J Glob Infect Dis 2:159–166. doi: 10.4103/0974-777X.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabra R, Branch RA. 1990. Amphotericin B nephrotoxicity. Drug Saf 5:94–108. doi: 10.2165/00002018-199005020-00003. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava S, Shankar P, Mishra J, Singh S. 2016. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit Vectors 9:277. doi: 10.1186/s13071-016-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, López-Vélez R, García-Hernández R, Pountain AW, Mwenechanya R, Papadopoulou B. 2017. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 11:e0006052. doi: 10.1371/journal.pntd.0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundar S, Rai M. 2002. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol 9:951–958. doi: 10.1128/cdli.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muniaraj M. 2014. The lost hope of elimination of Kala-azar (visceral leishmaniasis) by 2010 and cyclic occurrence of its outbreak in India, blame falls on vector control practices or coinfection with human immunodeficiency virus or therapeutic modalities? Trop Parasitol 4:10–19. doi: 10.4103/2229-5070.129143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zilberstein D, Shapira M. 1994. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol 48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]
- 10.De Almeida MC, Vilhena V, Barral A, Barral-Netto M. 2003. Leishmanial infection: analysis of its first steps, a review. Mem Inst Oswaldo Cruz 98:861–870. doi: 10.1590/s0074-02762003000700001. [DOI] [PubMed] [Google Scholar]
- 11.Handman E. 1999. Cell biology of Leishmania. Adv Parasitol 44:1–39. doi: 10.1016/s0065-308x(08)60229-8. [DOI] [PubMed] [Google Scholar]
- 12.Liévin-Le Moal V, Loiseau PM. 2016. Leishmania hijacking of the macrophage intracellular compartments. FEBS J 283:598–607. doi: 10.1111/febs.13601. [DOI] [PubMed] [Google Scholar]
- 13.Zakai HA, Chance ML, Bates PA. 1998. In vitro stimulation of metacyclogenesis in Leishmania braziliensis, L. donovani, L. major, and L. mexicana. Parasitology 116:305–309. doi: 10.1017/S0031182097002382. [DOI] [PubMed] [Google Scholar]
- 14.Sunter J, Gull K. 2017. Shape, form, function, and Leishmania pathogenicity: from textbook descriptions to biological understanding. Open Biol 7:170165. doi: 10.1098/rsob.170165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bates PA. 2007. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int J Parasitol 37:1097–1106. doi: 10.1016/j.ijpara.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence F, Robert-Gero M. 1985. Induction of heat shock and stress proteins in promastigotes of three Leishmania species. Proc Natl Acad Sci U S A 82:4414–4417. doi: 10.1073/pnas.82.13.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hombach A, Ommen G, MacDonald A, Clos J. 2014. A small heat shock protein is essential for thermotolerance and intracellular survival of Leishmania donovani. J Cell Sci 127:4762–4773. doi: 10.1242/jcs.157297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hombach A, Ommen G, Chrobak M, Clos J. 2013. The Hsp90-Sti1 interaction is critical for Leishmania donovani proliferation in both life cycle stages. Cell Microbiol 15:585–600. doi: 10.1111/cmi.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ommen G, Chrobak M, Clos J. 2010. The co-chaperone SGT of Leishmania donovani is essential for the parasite’s viability. Cell Stress Chaperones 15:443–455. doi: 10.1007/s12192-009-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiesgigl M, Clos J. 2001. Heat shock protein 90 homeostasis controls stage differentiation in Leishmania donovani. Mol Biol Cell 12:3307–3316. doi: 10.1091/mbc.12.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahav T, Sivam D, Volpin H, Ronen M, Tsigankov P, Green A, Holland N, Kuzyk M, Borchers C, Zilberstein D, Myler PJ. 2011. Multiple levels of gene regulation mediate differentiation of the intracellular pathogen Leishmania. FASEB J 25:515–525. doi: 10.1096/fj.10-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadopoulou B, Ouellette M, Laffitte MCN, Leprohon P. 2016. Plasticity of the Leishmania genome leading to gene copy number variations and drug resistance. F1000Res 5:2350. doi: 10.12688/f1000research.9218.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worthey EA, Martinez-Calvillo S, Schnaufer A, Aggarwal G, Cawthra J, Fazelinia G, Fong C, Fu G, Hassebrock M, Hixson G, Ivens AC, Kiser P, Marsolini F, Rickel E, Rickell E, Salavati R, Sisk E, Sunkin SM, Stuart KD, Myler PJ. 2003. Leishmania major chromosome 3 contains two long convergent polycistronic gene clusters separated by a tRNA gene. Nucleic Acids Res 31:4201–4210. doi: 10.1093/nar/gkg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandau S, Dresel A, Clos J. 1995. High constitutive levels of heat shock proteins in human-pathogenic parasites of the genus Leishmania. Biochem J 310:225–232. doi: 10.1042/bj3100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Der Ploeg LHT, Giannini SH, Cantor CR. 1985. Heat shock genes: regulatory role for differentiation in parasitic protozoa. Science 228:1443–1446. doi: 10.1126/science.4012301. [DOI] [PubMed] [Google Scholar]
- 26.Larreta R, Soto M, Alonso C, Requena JM. 2000. Leishmania infantum: gene cloning of the GRP94 homologue, its expression as recombinant protein, and analysis of antigenicity. Exp Parasitol 96:108–115. doi: 10.1006/expr.2000.4553. [DOI] [PubMed] [Google Scholar]
- 27.Rastrojo A, Corvo L, Lombraña R, Solana JC, Aguado B, Requena JM. 2019. Analysis by RNA-seq of transcriptomic changes elicited by heat shock in Leishmania major. Sci Rep 9:6919. doi: 10.1038/s41598-019-43354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ubeda JM, Légaré D, Raymond F, Ouameur AA, Boisvert S, Rigault P, Corbeil J, Tremblay MJ, Olivier M, Papadopoulou B, Ouellette M. 2008. Modulation of gene expression in drug-resistant Leishmania is associated with gene amplification, gene deletion and chromosome aneuploidy. Genome Biol 9:R115. doi: 10.1186/gb-2008-9-7-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumetz F, Imamura H, Sanders M, Seblova V, Myskova J, Pescher P, Vanaerschot M, Meehan CJ, Cuypers B, De Muylder G, Späth GF, Bussotti G, Vermeesch JR, Berriman M, Cotton JA, Volf P, Dujardin JC, Domagalska MA. 2017. Modulation of aneuploidy in Leishmania donovani during adaptation to different in vitro and in vivo environments and its impact on gene expression. mBio 8:e00599-17. doi: 10.1128/mBio.00599-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leprohon P, Légaré D, Raymond F, Madore É, Hardiman G, Corbeil J, Ouellette M. 2009. Gene expression modulation is associated with gene amplification, supernumerary chromosomes and chromosome loss in antimony-resistant Leishmania infantum. Nucleic Acids Res 37:1387–1399. doi: 10.1093/nar/gkn1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pallavi R, Roy N, Nageshan RK, Talukdar P, Pavithra SR, Reddy R, Venketesh S, Kumar R, Gupta AK, Singh RK, Yadav SC, Tatu U. 2010. Heat shock protein 90 as a drug target against protozoan infections: biochemical characterization of HSP90 from Plasmodium falciparum and Trypanosoma evansi and evaluation of its inhibitor as a candidate drug. J Biol Chem 285:37964–37975. doi: 10.1074/jbc.M110.155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batista FAH, Ramos SL, Tassone G, Leitão A, Montanari CA, Botta M, Mori M, Borges JC. 2020. Discovery of small molecule inhibitors of Leishmania braziliensis Hsp90 chaperone. J Enzyme Inhib Med Chem 35:639–649. doi: 10.1080/14756366.2020.1726342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palma LC, Ferreira LFGR, Petersen AL, de OA, Dias BRS, de Menezes JPB, de Moreira DR, Hernandes MZ, Veras PST. 2019. A docking-based structural analysis of geldanamycin-derived inhibitor binding to human or Leishmania Hsp90. Sci Rep 9:14756. doi: 10.1038/s41598-019-51239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen Al de OA, Guedes CES, Versoza CL, Lima JGB, de Freitas LAR, Borges VM, Veras PST. 2012. 17-AAG kills intracellular Leishmania amazonensis while reducing inflammatory responses in infected macrophages. PLoS One 7:e49496. doi: 10.1371/journal.pone.0049496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das S, Banerjee A, Kamran M, Ejazi SA, Asad M, Ali N, Chakrabarti S. 2020. A chemical inhibitor of heat shock protein 78 (HSP78) from Leishmania donovani represents a potential antileishmanial drug candidate. J Biol Chem 295:9934–9947. doi: 10.1074/jbc.RA120.014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Murillo-Solano C, Kirkpatrick MG, Antoshchenko T, Park H, Pizarro JC. 2018. Repurposing drugs to target the malaria parasite unfolding protein response. Sci Rep 8:10333. doi: 10.1038/s41598-018-28608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU. 2010. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb Perspect Biol 2:a004390. doi: 10.1101/cshperspect.a004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartl FU. 1996. Molecular chaperones in cellular protein folding. Nature 381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 39.Jee H. 2016. Size dependent classification of heat shock proteins: a mini-review. J Exerc Rehabil 12:255–259. doi: 10.12965/jer.1632642.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wayne N, Mishra P, Bolon DN. 2011. Hsp90 and client protein maturation. Methods Mol Biol 787:33–44. doi: 10.1007/978-1-61779-295-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer P, Prodromou C, Hu B, Vaughan C, Roe SM, Panaretou B, Piper PW, Pearl LH. 2003. Structural and functional analysis of the middle segment of Hsp90: implications for ATP hydrolysis and client protein and cochaperone interactions. Mol Cell 11:647–658. doi: 10.1016/s1097-2765(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 42.Larreta R, Soto M, Quijada L, Folgueira C, Abanades DR, Alonso C, Requena JM. 2004. The expression of HSP83 genes in Leishmania infantum is affected by temperature and by stage-differentiation and is regulated at the levels of mRNA stability and translation. BMC Mol Biol 5:3. doi: 10.1186/1471-2199-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zininga T, Shonhai A. 2019. Small molecule inhibitors targeting the heat shock protein system of human obligate protozoan parasites. Int J Mol Sci 20:5930. doi: 10.3390/ijms20235930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller CM, Smith NC, Johnson AM. 1999. Cytokines, nitric oxide, heat shock proteins, and virulence in Toxoplasma. Parasitol Today 15:418–422. doi: 10.1016/S0169-4758(99)01515-X. [DOI] [PubMed] [Google Scholar]
- 45.Neckers L, Tatu U. 2008. Molecular chaperones in pathogen virulence: emerging new targets for therapy. Cell Host Microbe 4:519–527. doi: 10.1016/j.chom.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales MA, Watanabe R, Dacher M, Chafey P, Osorio Y Fortéa J, Scott DA, Beverley SM, Ommen G, Clos J, Hem S, Lenormand P, Rousselle JC, Namane A, Späth GF. 2010. Phosphoproteome dynamics reveal heat-shock protein complexes specific to the Leishmania donovani infectious stage. Proc Natl Acad Sci U S A 107:8381–8386. doi: 10.1073/pnas.0914768107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson JL, Brown C. 2009. Plasticity of the Hsp90 chaperone machine in divergent eukaryotic organisms. Cell Stress Chaperones 14:83–94. doi: 10.1007/s12192-008-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joshi M, Pogue GP, Duncan RC, Lee NS, Singh NK, Atreya CD, Dwyer DM, Nakhasi HL. 1996. Isolation and characterization of Leishmania donovani calreticulin gene and its conservation of the RNA binding activity. Mol Biochem Parasitol 81:53–64. doi: 10.1016/0166-6851(96)02676-x. [DOI] [PubMed] [Google Scholar]
- 49.Krobitsch S, Brandau S, Hoyer C, Schmetz C, Hübel A, Clos J. 1998. Leishmania donovani heat shock protein 100: characterization and function in amastigote-stage differentiation. J Biol Chem 273:6488–6494. doi: 10.1074/jbc.273.11.6488. [DOI] [PubMed] [Google Scholar]
- 50.Bangs JD, Brouch EM, Ransom DM, Roggy JL. 1996. A soluble secretory reporter system in Trypanosoma brucei: studies on endoplasmic reticulum targeting. J Biol Chem 271:18387–18393. doi: 10.1074/jbc.271.31.18387. [DOI] [PubMed] [Google Scholar]
- 51.Silverman JM, Chan SK, Robinson DP, Dwyer DM, Nandan D, Foster LJ, Reiner NE. 2008. Proteomic analysis of the secretome of Leishmania donovani. Genome Biol 9:R35. doi: 10.1186/gb-2008-9-2-r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McConville MJ, Mullin KA, Ilgoutz SC, Teasdale RD. 2002. Secretory pathway of trypanosomatid parasites. Microbiol Mol Biol Rev 66:122–154. doi: 10.1128/mmbr.66.1.122-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hübel A, Krobitsch S, Hörauf A, Clos J. 1997. Leishmania major Hsp100 is required chiefly in the mammalian stage of the parasite. Mol Cell Biol 17:5987–5995. doi: 10.1128/mcb.17.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clos J, Brandau S, Hoyer C. 1998. Chemical stress does not induce heat shock protein synthesis in leishmania donovani. Protist 149:167–172. doi: 10.1016/S1434-4610(98)70021-5. [DOI] [PubMed] [Google Scholar]
- 55.Krobitsch S, Clos J. 1999. A novel role for 100-kDa heat shock proteins in the parasite Leishmania donovani. Cell Stress Chaper 4:191. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang WW, Matlashewski G. 1997. Loss of virulence in Leishmania donovani deficient in an amastigote-specific protein, A2. Proc Natl Acad Sci U S A 94:8807–8811. doi: 10.1073/pnas.94.16.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCall LI, Matlashewski G. 2010. Localization and induction of the A2 virulence factor in Leishmania: evidence that A2 is a stress response protein. Mol Microbiol 77:518–530. doi: 10.1111/j.1365-2958.2010.07229.x. [DOI] [PubMed] [Google Scholar]
- 58.Vogel JL, Parsell DA, Lindquist S. 1995. Heat-shock proteins Hsp104 and Hsp70 reactivate mRNA splicing after heat inactivation. Curr Biol 5:306–317. doi: 10.1016/s0960-9822(95)00061-3. [DOI] [PubMed] [Google Scholar]
- 59.McCleese JK, Bear MD, Fossey SL, Mihalek RM, Foley KP, Ying W, Barsoum J, London CA. 2009. The novel HSP90 inhibitor STA-1474 exhibits biologic activity against osteosarcoma cell lines. Int J Cancer 125:2792–2801. doi: 10.1002/ijc.24660. [DOI] [PubMed] [Google Scholar]
- 60.Reiling L, Chrobak M, Schmetz C, Clos J. 2010. Overexpression of a single Leishmania major gene enhances parasite infectivity in vivo and in vitro. Mol Microbiol 76:1175–1190. doi: 10.1111/j.1365-2958.2010.07130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, Reiner NE. 2010. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol 185:5011–5022. doi: 10.4049/jimmunol.1000541. [DOI] [PubMed] [Google Scholar]
- 62.Silverman JM, Clos J, de’Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE. 2010. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci 123:842–852. doi: 10.1242/jcs.056465. [DOI] [PubMed] [Google Scholar]
- 63.Kaye P, Scott P. 2011. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol 9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 64.Gordon S. 2003. Alternative activation of macrophages. Nat Rev Immunol 3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 65.Taipale M, Jarosz DF, Lindquist S. 2010. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 66.Wiesgigl M, Clos J. 2001. The heat shock protein 90 of Leishmania donovani. Med Microbiol Immunol 190:27–31. doi: 10.1007/s004300100074. [DOI] [PubMed] [Google Scholar]
- 67.Folgueira C, Cañavate C, Chicharro C, Requena JM. 2007. Genomic organization and expression of the HSP70 locus in New and Old World Leishmania species. Parasitology 134:369–377. doi: 10.1017/S0031182006001570. [DOI] [PubMed] [Google Scholar]
- 68.Graefe SEB, Wiesgigl M, Gaworski I, Macdonald A, Clos J. 2002. Inhibition of HSP90 in Trypanosoma cruzi induces a stress response but no stage differentiation. Eukaryot Cell 1:936–943. doi: 10.1128/ec.1.6.936-943.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Argaman M, Aly R, Shapira M. 1994. Expression of heat shock protein 83 in Leishmania is regulated posttranscriptionally. Mol Biochem Parasitol 64:95–110. doi: 10.1016/0166-6851(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 70.Longshaw VM, Chapple JP, Balda MS, Cheetham ME, Blatch GL. 2004. Nuclear translocation of the Hsp70/Hsp90 organizing protein mSTI1 is regulated by cell cycle kinases. J Cell Sci 117:701–710. doi: 10.1242/jcs.00905. [DOI] [PubMed] [Google Scholar]
- 71.Hombach A, Clos J. 2014. No stress: Hsp90 and signal transduction in Leishmania. Parasitology 141:1156–1166. doi: 10.1017/S0031182013002151. [DOI] [PubMed] [Google Scholar]
- 72.Turco SJ, Descoteaux A. 1992. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol 46:65–92. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 73.McConville MJ, Ferguson MA. 1993. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J 294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernández-Fernández MR, Valpuesta JM. 2018. Hsp70 chaperone: a master player in protein homeostasis. F1000Res 7:1497 2018. doi: 10.12688/f1000research.15528.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schroder H, Langer T, Hartl FU, Bukau B. 1993. DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J 12:4137–4144. doi: 10.1002/j.1460-2075.1993.tb06097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daugaard M, Rohde M, Jäättelä M. 2007. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett 581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 77.Requena JM, Montalvo AM, Fraga J. 2015. Molecular chaperones of Leishmania: central players in many stress-related and -unrelated physiological processes. Biomed Res Int 2015:301326. doi: 10.1155/2015/301326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sousa R, Andrade VM, Bair T, Ettinger NA, Guimarães L, Andrade L, Guimarães LH, Machado PRL, Carvalho EM, Wilson ME, Schriefer A. 2018. Early suppression of macrophage gene expression by Leishmania braziliensis. Front Microbiol 9:2464. doi: 10.3389/fmicb.2018.02464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller MA, McGowan SE, Gantt KR, Champion M, Novick SL, Andersen KA, Bacchi CJ, Yarlett N, Britigan BE, Wilson ME. 2000. Inducible resistance to oxidant stress in the protozoan Leishmania chagasi. J Biol Chem 275:33883–33889. doi: 10.1074/jbc.M003671200. [DOI] [PubMed] [Google Scholar]
- 80.Jensen ATR, Curtis J, Montgomery J, Handman E, Theander TG. 2001. Molecular and immunological characterization of the glucose regulated protein 78 of Leishmania donovani. Biochim Biophys Acta Protein Struct Mol Enzymol 1549:73–87. doi: 10.1016/S0167-4838(01)00240-0. [DOI] [PubMed] [Google Scholar]
- 81.Zurita AI, Rodríguez J, Piñero JE, Pacheco R, Carmelo E, del Castllo A, Valladares B. 2003. Cloning and characterization of the Leishmania (Viannia) braziliensis HSP70 gene: diagnostic use of the C-terminal fragment rlb70(513–663). J Parasitol 89:372–378. doi: 10.1645/0022-3395(2003)089[0372:CACOTL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 82.Garcia L, Kindt A, Bermudez H, Llanos-Cuentas A, De Doncker S, Arevalo J, Tintaya KWQ, Dujardin JC. 2004. Culture-independent species typing of neotropical leishmania for clinical validation of a PCR-based assay targeting heat shock protein 70 genes. J Clin Microbiol 42:2294–2297. doi: 10.1128/jcm.42.5.2294-2297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van der Auwera G, Maes I, De Doncker S, Ravel C, Cnops L, Van Esbroeck M, Van Gompel A, Clerinx J, Dujardin J. 2013. Heat-shock protein 70 gene sequencing for Leishmania species typing in European tropical infectious disease clinics. Euro Surveill 18:20543. doi: 10.2807/1560-7917.es2013.18.30.20543. [DOI] [PubMed] [Google Scholar]
- 84.Montalvo AM, Fraga J, Tirado D, Blandón G, Alba A, Van der Auwera G, Vélez ID, Muskus C. 2017. Detection and identification of Leishmania spp.: application of two hsp70-based PCR-RFLP protocols to clinical samples from the New World. Parasitol Res 116:1843–1848. doi: 10.1007/s00436-017-5454-6. [DOI] [PubMed] [Google Scholar]
- 85.Ramírez CA, Dea-Ayuela MA, Gutiérrez-Blázquez MD, Bolas-Fernández F, Requena JM, Puerta CJ. 2013. Identification of proteins interacting with HSP70 mRNAs in Leishmania braziliensis. J Proteomics 94:124–137. doi: 10.1016/j.jprot.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 86.Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. 1999. Calreticulin: one protein, one gene, many functions. Biochem J 344 :281–292. doi: 10.1042/bj3440281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferreira V, Molina MC, Valck C, Rojas Á, Aguilar L, Ramírez G, Schwaeble W, Ferreira A. 2004. Role of calreticulin from parasites in its interaction with vertebrate hosts. Mol Immunol 40:1279–1291. doi: 10.1016/j.molimm.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 88.Jethmalani SM, Henle KJ. 1998. Calreticulin associates with stress proteins: implications for chaperone function during heat stress. J Cell Biochem 69:30–43. doi:. [DOI] [PubMed] [Google Scholar]
- 89.Fernandes ACS, Soares DC, Saraiva EM, Meyer-Fernandes JR, Souto-Padrón T. 2013. Different secreted phosphatase activities in Leishmania amazonensis. FEMS Microbiol Lett 340:117–128. doi: 10.1111/1574-6968.12080. [DOI] [PubMed] [Google Scholar]
- 90.Braakman I, Hebert DN. 2013. Protein folding in the endoplasmic reticulum. Cold Spring Harb Perspect Biol 5:a013201. doi: 10.1101/cshperspect.a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Debrabant A, Lee N, Pogue GP, Dwyer DM, Nakhasi HL. 2002. Expression of calreticulin P-domain results in impairment of secretory pathway in Leishmania donovani and reduced parasite survival in macrophages. Int J Parasitol 32:1423–1434. doi: 10.1016/s0020-7519(02)00134-0. [DOI] [PubMed] [Google Scholar]
- 92.Li J, Soroka J, Buchner J. 2012. The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones. Biochim Biophys Acta 1823:624–635. doi: 10.1016/j.bbamcr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 93.Folgueira C, Requena JM. 2007. A postgenomic view of the heat shock proteins in kinetoplastids. FEMS Microbiol Rev 31:359–377. doi: 10.1111/j.1574-6976.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- 94.Webb JR, Campos-Neto A, Skeiky YAW, Reed SG. 1997. Molecular characterization of the heat-inducible LmSTI1 protein of Leishmania major. Mol Biochem Parasitol 89:179–193. doi: 10.1016/S0166-6851(97)00115-1. [DOI] [PubMed] [Google Scholar]
- 95.Felts SJ, Toft DO. 2003. p23, a simple protein with complex activities. Cell Stress Chaper 8:108–113. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu FH, Wu SJ, Hu SM, Hsiao CD, Wang C. 1999. Specific interaction of the 70-kDa heat shock cognate protein with the tetratricopeptide repeats. J Biol Chem 274:34425–34432. doi: 10.1074/jbc.274.48.34425. [DOI] [PubMed] [Google Scholar]
- 97.Panaretou B, Siligardi G, Meyer P, Maloney A, Sullivan JK, Singh S, Millson SH, Clarke PA, Naaby-Hansen S, Stein R, Cramer R, Mollapour M, Workman P, Piper PW, Pearl LH, Prodromou C. 2002. Activation of the ATPase activity of Hsp90 by the stress-regulated cochaperone Aha1. Mol Cell 10:1307–1318. doi: 10.1016/S1097-2765(02)00785-2. [DOI] [PubMed] [Google Scholar]
- 98.Seraphim TV, Alves MM, Silva IM, Gomes FER, Silva KP, Murta SMF, Barbosa LRS, Borges JC. 2013. Low-resolution structural studies indicate that the activator of Hsp90 ATPase 1 (Aha1) of Leishmania braziliensis has an elongated shape which allows its interaction with both N- and M-domains of Hsp90. PLoS One 8:e66822. doi: 10.1371/journal.pone.0066822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bartsch K, Hombach-Barrigah A, Clos J. 2017. Hsp90 inhibitors radicicol and geldanamycin have opposing effects on Leishmania Aha1-dependent proliferation. Cell Stress Chaperones 22:729–742. doi: 10.1007/s12192-017-0800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young JC, Hartl FU. 2000. Polypeptide release by Hsp90 involves ATP hydrolysis and is enhanced by the co-chaperone p23. EMBO J 19:5930–5940. doi: 10.1093/emboj/19.21.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Freeman BC, Toft DO, Morimoto RI. 1996. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science 274:1718–1720. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- 102.McLaughlin SH, Sobott F, Yao ZP, Zhang W, Nielsen PR, Grossmann JG, Laue ED, Robinson CV, Jackson SE. 2006. The co-chaperone p23 arrests the Hsp90 ATPase cycle to trap client proteins. J Mol Biol 356:746–758. doi: 10.1016/j.jmb.2005.11.085. [DOI] [PubMed] [Google Scholar]
- 103.Batista FAH, Almeida GS, Seraphim TV, Silva KP, Murta SMF, Barbosa LRS, Borges JC. 2015. Identification of two p23 co-chaperone isoforms in Leishmania braziliensis exhibiting similar structures and Hsp90 interaction properties despite divergent stabilities. FEBS J 282:388–406. doi: 10.1111/febs.13141. [DOI] [PubMed] [Google Scholar]
- 104.Grenert JP, Johnson BD, Toft DO. 1999. The importance of ATP binding and hydrolysis by Hsp90 in formation and function of protein heterocomplexes. J Biol Chem 274:17525–17533. doi: 10.1074/jbc.274.25.17525. [DOI] [PubMed] [Google Scholar]
- 105.Hombach A, Ommen G, Sattler V, Clos J. 2015. Leishmania donovani P23 protects parasites against HSP90 inhibitor-mediated growth arrest. Cell Stress Chaperones 20:673–685. doi: 10.1007/s12192-015-0595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haslbeck M, Weinkauf S, Buchner J. 2019. Small heat shock proteins: simplicity meets complexity. J Biol Chem 294:2121–2132. doi: 10.1074/jbc.REV118.002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ungelenk S, Moayed F, Ho CT, Grousl T, Scharf A, Mashaghi A, Tans S, Mayer MP, Mogk A, Bukau B. 2016. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat Commun 7:13673–13614. doi: 10.1038/ncomms13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsigankov P, Gherardini PF, Helmer-Citterich M, Späth GF, Myler PJ, Zilberstein D. 2014. Regulation dynamics of leishmania differentiation: deconvoluting signals and identifying phosphorylation trends. Mol Cell Proteomics 13:1787–1799. doi: 10.1074/mcp.M114.037705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Verma A, Ghosh S, Salotra P, Singh R. 2019. Artemisinin-resistant Leishmania parasite modulates host cell defense mechanism and exhibits altered expression of unfolded protein response genes. Parasitol Res 118:2705–2713. doi: 10.1007/s00436-019-06404-9. [DOI] [PubMed] [Google Scholar]
- 110.Schlüter A, Wiesgigl M, Hoyer C, Fleischer S, Klaholz L, Schmetz C, Clos J. 2000. Expression and subcellular localization of cpn60 protein family members in Leishmania donovani. Biochim Biophys Acta Gene Struct Expr 1491:65–74. doi: 10.1016/S0167-4781(00)00028-2. [DOI] [PubMed] [Google Scholar]
- 111.Colineau L, Clos J, Moon KM, Foster LJ, Reiner NE. 2017. Leishmania donovani chaperonin 10 regulates parasite internalization and intracellular survival in human macrophages. Med Microbiol Immunol 206:235–257. doi: 10.1007/s00430-017-0500-7. [DOI] [PubMed] [Google Scholar]
- 112.Zamora-Veyl FB, Kroemer M, Zander D, Clos J. 2005. Stage-specific expression of the mitochondrial co-chaperonin of Leishmania donovani, CPN10. Kinetoplastid Biol Dis 4:3. doi: 10.1186/1475-9292-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muller P, Ruckova E, Halada P, Coates PJ, Hrstka R, Lane DP, Vojtesek B. 2013. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene 32:3101–3110. doi: 10.1038/onc.2012.314. [DOI] [PubMed] [Google Scholar]
- 114.Hombach-Barrigah A, Bartsch K, Smirlis D, Rosenqvist H, MacDonald A, Dingli F, Loew D, Späth GF, Rachidi N, Wiese M, Clos J. 2019. Leishmania donovani 90-kDa heat shock protein: impact of phosphosites on parasite fitness. Sci Rep 9:1–16. doi: 10.1038/s41598-019-41640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao YG, Gilmore R, Leone G, Coffey MC, Weber B, Lee PWK. 2001. Hsp90 phosphorylation is linked to its chaperoning function: assembly of the reovirus cell attachment protein. J Biol Chem 276:32822–32827. doi: 10.1074/jbc.M105562200. [DOI] [PubMed] [Google Scholar]
- 116.Kaur P, Garg M, Hombach-Barrigah A, Clos J, Goyal N. 2017. MAPK1 of Leishmania donovani interacts and phosphorylates HSP70 and HSP90 subunits of foldosome complex. Sci Rep 7:10202. doi: 10.1038/s41598-017-09725-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Norris-Mullins B, Krivda JS, Smith KL, Ferrell MJ, Morales MA. 2018. Leishmania phosphatase PP5 is a regulator of HSP83 phosphorylation and essential for parasite pathogenicity. Parasitol Res 117:2971–2985. doi: 10.1007/s00436-018-5994-4. [DOI] [PubMed] [Google Scholar]
- 118.Zuehlke AD, Reidy M, Lin C, Lapointe P, Alsomairy S, Lee DJ, Rivera-Marquez GM, Beebe K, Prince T, Lee S, Trepel JB, Xu W, Johnson J, Masison D, Neckers L. 2017. An Hsp90 co-chaperone protein in yeast is functionally replaced by site-specific posttranslational modification in humans. Nat Commun 8:15328. doi: 10.1038/ncomms15328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brochu C, Haimeur A, Ouellette M. 2004. The heat shock protein HSP70 and heat shock cognate protein HSC70 contribute to antimony tolerance in the protozoan parasite Leishmania. Cell Stress Chaperones 9:294–303. doi: 10.1379/csc-15r1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trudel N, Garg R, Messier N, Sundar S, Ouellette M, Tremblay MJ. 2008. Intracellular survival of Leishmania species that cause visceral leishmaniasis is significantly reduced by HIV-1 protease inhibitors. J Infect Dis 198:1292–1299. doi: 10.1086/592280. [DOI] [PubMed] [Google Scholar]
- 121.Biyani N, Singh AK, Mandal S, Chawla B, Madhubala R. 2011. Differential expression of proteins in antimony-susceptible and -resistant isolates of Leishmania donovani. Mol Biochem Parasitol 179:91–99. doi: 10.1016/j.molbiopara.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 122.Kumar A, Sisodia B, Misra P, Sundar S, Shasany AK, Dube A. 2010. Proteome mapping of overexpressed membrane-enriched and cytosolic proteins in sodium antimony gluconate (SAG) resistant clinical isolate of Leishmania donovani. Br J Clin Pharmacol 70:609–617. doi: 10.1111/j.1365-2125.2010.03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drummelsmith J, Girard I, Trudel N, Ouellette M. 2004. Differential protein expression analysis of Leishmania major reveals novel roles for methionine adenosyltransferase and S-adenosylmethionine in methotrexate resistance. J Biol Chem 279:33273–33280. doi: 10.1074/jbc.M405183200. [DOI] [PubMed] [Google Scholar]
- 124.Vergnes B, Gourbal B, Girard I, Sundar S, Drummelsmith J, Ouellette M. 2007. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol Cell Proteomics 6:88–101. doi: 10.1074/mcp.M600319-MCP200. [DOI] [PubMed] [Google Scholar]
- 125.Brotherton MC, Bourassa S, Légaré D, Poirier GG, Droit A, Ouellette M. 2014. Quantitative proteomic analysis of amphotericin B resistance in Leishmania infantum. Int J Parasitol Drugs Drug Resist 4:126–132. doi: 10.1016/j.ijpddr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matrangolo FSV, Liarte DB, Andrade LC, De Melo MF, Andrade JM, Ferreira RF, Santiago AS, Pirovani CP, Silva-Pereira RA, Murta SMF. 2013. Comparative proteomic analysis of antimony-resistant and-susceptible Leishmania braziliensis and Leishmania infantum chagasi lines. Mol Biochem Parasitol 190:63–75. doi: 10.1016/j.molbiopara.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 127.Vacchina P, Norris-Mullins B, Carlson ES, Morales MA. 2016. A mitochondrial HSP70 (HSPA9B) is linked to miltefosine resistance and stress response in Leishmania donovani. Parasites Vectors 9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kampinga HH, Craig EA. 2010. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim HR, Lee CH, Choi YH, Kang HS, Kim HD. 1999. Geldanamycin induces cell cycle arrest in K562 erythroleukemic cells. IUBMB Life 48:425–428. doi: 10.1080/713803539. [DOI] [PubMed] [Google Scholar]
- 130.Mohammadi-Ostad-Kalayeh S, Stahl F, Scheper T, Kock K, Herrmann C, Heleno Batista FA, Borges JC, Sasse F, Eichner S, Ongouta J, Zeilinger C, Kirschning A. 2018. Heat shock proteins revisited: using a mutasynthetically generated reblastatin library to compare the inhibition of human and Leishmania Hsp90s. Chembiochem 19:562–574. doi: 10.1002/cbic.201700616. [DOI] [PubMed] [Google Scholar]
- 131.Sõti C, Nagy E, Giricz Z, Vígh L, Csermely P, Ferdinandy P. 2005. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol 146:769–780. doi: 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Evans CG, Chang L, Gestwicki JE. 2010. Heat shock protein 70 (Hsp70) as an emerging drug target. J Med Chem 53:4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chatterjee S, Burns TF. 2017. Targeting heat shock proteins in cancer: a promising therapeutic approach. Int J Mol Sci 18:1978. doi: 10.3390/ijms18091978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jhaveri K, Taldone T, Modi S, Chiosis G. 2012. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta 1823:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Badhwar R, Upadhyay A. 2020. Modulation of cellular protein quality control pathways using small natural molecules, p 609–641. In Bioactive natural products in drug discovery. Springer, New York, NY. [Google Scholar]
- 136.Biamonte MA, Van De Water R, Arndt JW, Scannevin RH, Perret D, Lee WC. 2010. Heat shock protein 90: inhibitors in clinical trials. J Med Chem 53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 137.Li Q, Zhou Y, Yao C, Ma X, Wang L, Xu W, Wang Z, Qiao Z. 2009. Apoptosis caused by Hsp90 inhibitor geldanamycin in Leishmania donovani during promastigote-to-amastigote transformation stage. Parasitol Res 105:1539–1548. doi: 10.1007/s00436-009-1582-y. [DOI] [PubMed] [Google Scholar]
- 138.Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM. 1998. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaper 3:100–108. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Neckers L, Workman P. 2012. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Soga S, Sharma SV, Shiotsu Y, Shimizu M, Tahara H, Yamaguchi K, Ikuina Y, Murakata C, Tamaoki T, Kurebayashi J, Schulte TW, Neckers LM, Akinaga S. 2001. Stereospecific antitumor activity of radicicol oxime derivatives. Cancer Chemother Pharmacol 48:435–445. doi: 10.1007/s002800100373. [DOI] [PubMed] [Google Scholar]
- 141.Soga S, Shiotsu Y, Akinaga S, Sharma S. 2003. Development of radicicol analogues. Curr Cancer Drug Targets 3:359–369. doi: 10.2174/1568009033481859. [DOI] [PubMed] [Google Scholar]
- 142.Meyer KJ, Shapiro TA. 2013. Potent antitrypanosomal activities of heat shock protein 90 inhibitors in vitro and in vivo. J Infect Dis 208:489–499. doi: 10.1093/infdis/jit179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pacey S, Gore M, Chao D, Banerji U, Larkin J, Sarker S, Owen K, Asad Y, Raynaud F, Walton M, Judson I, Workman P, Eisen T. 2012. A phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Invest New Drugs 30:341–349. doi: 10.1007/s10637-010-9493-4. [DOI] [PubMed] [Google Scholar]
- 144.Goetz MP, Toft D, Reid J, Ames M, Stensgard B, Safgren S, Adjei AA, Sloan J, Atherton P, Vasile V, Salazaar S, Adjei A, Croghan G, Erlichman C. 2005. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol 23:1078–1087. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 145.Santos DM, Petersen ALOA, Celes FS, Borges VM, Veras PST, de Oliveira CI. 2014. Chemotherapeutic potential of 17-AAG against cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis. PLoS Negl Trop Dis 8:e3275. doi: 10.1371/journal.pntd.0003275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Varela-M RE, Mollinedo-Gajate C, Muro A, Mollinedo F. 2014. The HSP90 inhibitor 17-AAG potentiates the antileishmanial activity of the ether lipid edelfosine. Acta Trop 131:32–36. doi: 10.1016/j.actatropica.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 147.Varela-M RE, Villa-Pulgarin JA, Yepes E, Müller I, Modolell M, Muñoz DL, Robledo SM, Muskus CE, López-Abán J, Muro A, Vélez ID, Mollinedo F. 2012. In vitro and in vivo efficacy of ether lipid edelfosine against Leishmania spp. and SbV-resistant parasites. PLoS Negl Trop Dis 6:e1612. doi: 10.1371/journal.pntd.0001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.de Almeida Petersen AL, Campos TA, dos Santos Dantas DA, de Souza Rebouças J, da Silva JC, de Menezes JPB, Formiga FR, de Melo JV, Machado G, Veras PST. 2018. Encapsulation of the HSP-90 chaperone inhibitor 17-AAG in stable liposome allow increasing the therapeutic index as assessed, in vitro, on Leishmania amazonensis amastigotes hosted in mouse CBA macrophages. Front Cell Infect Microbiol 8:303. doi: 10.3389/fcimb.2018.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Griffin TM, Valdez TV, Mestril R. 2004. Radicicol activates heat shock protein expression and cardioprotection in neonatal rat cardiomyocytes. Am J Physiol Heart Circ Physiol 287:H1081–H1088. doi: 10.1152/ajpheart.00921.2003. [DOI] [PubMed] [Google Scholar]
- 150.Iantorno SA, Durrant C, Khan A, Sanders MJ, Beverley SM, Warren WC, Berriman M, Sacks DL, Cotton JA, Grigg ME. 2017. Gene expression in Leishmania is regulated predominantly by gene dosage. mBio 8:e01393-17. doi: 10.1128/mBio.01393-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Späth GF, Drini S, Rachidi N. 2015. A touch of Zen: posttranslational regulation of the Leishmania stress response. Cell Microbiol 17:632–638. doi: 10.1111/cmi.12440. [DOI] [PubMed] [Google Scholar]
- 152.Hartl FU, Bracher A, Hayer-Hartl M. 2011. Molecular chaperones in protein folding and proteostasis. Nature 475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 153.Amanullah A, Upadhyay A, Joshi V, Mishra R, Jana NR, Mishra A. 2017. Progressing neurobiological strategies against proteostasis failure: challenges in neurodegeneration. Prog Neurobiol 159:1–38. doi: 10.1016/j.pneurobio.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 154.Kumar CMS, Mande SC, Mahajan G. 2015. Multiple chaperonins in bacteria: novel functions and non-canonical behaviors. Cell Stress Chaperones 20:555–574. doi: 10.1007/s12192-015-0598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Descoteaux A, Avila HA, Zhang K, Turco SJ, Beverley SM. 2002. Leishmania LPG3 encodes a GRP94 homolog required for phosphoglycan synthesis implicated in parasite virulence but not viability. EMBO J 21:4458–4469. doi: 10.1093/emboj/cdf447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Salotra P, Ralhan R, Bhatnagar R. 1994. Differential expression of stress proteins in virulent and attenuated promastigotes of Leishmania donovani. Biochem Mol Biol Int 33:691–697. [PubMed] [Google Scholar]
- 157.Rico AI, Angel SO, Alonso C, Requena JM. 1999. Immunostimulatory properties of the Leishmania infantum heat shock proteins HSP70 and HSP83. Mol Immunol 36:1131–1139. doi: 10.1016/s0161-5890(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 158.Montalvo-Álvarez AM, Folgueira C, Carrión J, Monzote-Fidalgo L, Cañavate C, Requena JM. 2008. The Leishmania HSP20 is antigenic during natural infections, but, as DNA vaccine, it does not protect BALB/c mice against experimental L. amazonensis infection. J Biomed Biotechnol 2008:695432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rachidi N, Taly JF, Durieu E, Leclercq O, Aulner N, Prina E, Pescher P, Notredame C, Meijer L, Späth GF. 2014. Pharmacological assessment defines Leishmania donovani casein kinase 1 as a drug target and reveals important functions in parasite viability and intracellular infection. Antimicrob Agents Chemother 58:1501–1515. doi: 10.1128/AAC.02022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Inacio JDF, Gervazoni L, Canto-Cavalheiro MM, Almeida-Amaral EE. 2014. The effect of (–)-epigallocatechin 3-O-gallate in vitro and in vivo in Leishmania braziliensis: involvement of reactive oxygen species as a mechanism of action. PLoS Negl Trop Dis 8:e3093. doi: 10.1371/journal.pntd.0003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Singh G, Jayanarayan KG, Dey CS. 2005. Novobiocin induces apoptosis-like cell death in topoisomerase II overexpressing arsenite-resistant Leishmania donovani. Mol Biochem Parasitol 141:57–69. doi: 10.1016/j.molbiopara.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 162.Souza Ribeiro Costa J, Medeiros M, Yamashiro-Kanashiro EH, Rocha MC, Cotrim PC, Stephano MA, Lancellotti M, Tavares GD, Oliveira-Nascimento L. 2019. Biodegradable nanocarriers coated with polymyxin B: evaluation of leishmanicidal and antibacterial potential. PLoS Negl Trop Dis 13:e0007388. doi: 10.1371/journal.pntd.0007388. [DOI] [PMC free article] [PubMed] [Google Scholar]