Neuropilin-1 (Nrp-1) contributes to maintaining the stability of CD4+ CD25+ regulatory T cells (Tregs). We investigated the impact of Nrp-1 on the stability of CD4+ CD25+ Tregs, and the underlying signaling pathways, in a model of sepsis. Splenic CD4+ CD25+ Tregs were either treated with anti-Nrp-1, transfected to silence Nrp-1 and inhibitor of NF-κB kinase subunit beta (IKKβ), or administered ammonium pyrrolidine dithiocarbamate (PDTC), followed by recombinant semaphorin 3A (rSema3A), in a simulation of sepsis.
KEYWORDS: sepsis, regulatory T cells, neuropilin-1, NF-κB, immunosuppression, immunoregulation
ABSTRACT
Neuropilin-1 (Nrp-1) contributes to maintaining the stability of CD4+ CD25+ regulatory T cells (Tregs). We investigated the impact of Nrp-1 on the stability of CD4+ CD25+ Tregs, and the underlying signaling pathways, in a model of sepsis. Splenic CD4+ CD25+ Tregs were either treated with anti-Nrp-1, transfected to silence Nrp-1 and inhibitor of NF-κB kinase subunit beta (IKKβ), or administered ammonium pyrrolidine dithiocarbamate (PDTC), followed by recombinant semaphorin 3A (rSema3A), in a simulation of sepsis. After the creation of a sepsis model in mice, anti-Nrp-1 was administered. The expression of the gene encoding forkhead box protein P-3 foxp3-Treg-specific demethylated region (foxp3-TSDR), the apoptosis rate, the expression of Foxp-3, cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), and transforming growth factor β1 (TGF-β1), interleukin 10 (IL-10) and TGF-β1 secretion, and the NF-κB signaling activity of CD4+ CD25+ Tregs were determined. Sepsis simulation with or without rSema3A increased the stability of CD4+ CD25+ Tregs, including an increase in the expression of Foxp-3, CTLA-4, and TGF-β1, decreases in apoptosis and the methylation of foxp3-TSDR, increases in the secretion of TGF-β1 and IL-10, and an increase in the immunosuppressive effect on CD4+ T lymphocytes. Silencing of Nrp-1 or anti-Nrp-1 treatment abrogated lipopolysaccharide (LPS) stimulation with or without an rSema3A-mediated effect. Sepsis simulation increased the DNA-binding activity of NF-κB, as well as the ratios of phosphorylated IKKβ (p-IKKβ) to IKKβ and p-P65 to P65 in vitro and vivo. Silencing of IKKβ expression or PDTC treatment suppressed the stability of CD4+ CD25+ Tregs in LPS-induced sepsis. Weakening Nrp-1 reduced the stability of CD4+ CD25+ Tregs by regulating the NF-κB signaling pathway; thus, Nrp-1 could be a new target for immunoregulation in sepsis.
INTRODUCTION
Sepsis can be defined as a syndrome characterized by life-threatening organ dysfunction caused by the dysregulated host response to an infection (1). As understanding of sepsis pathophysiology has improved, recent guidelines for the diagnosis and treatment of sepsis have emphasized the evaluation of sepsis-induced multiple-organ dysfunction syndromes (MODS) (1).
Sepsis is a medical emergency associated with high morbidity, high mortality, and prolonged after-effects. Sepsis affects one-fifth of patients admitted to intensive care units in mainland China, with a 90-day mortality rate of 35.5% (2). In 2015, 1,937,299 deaths occurred at 605 disease surveillance points in mainland China, and the standardized sepsis-related mortality incidence was 66.7 deaths per 100,000 population (3).
The antagonism between the host and pathogenic microorganisms is a complex pathophysiological reaction, which involves localizing and controlling bacterial invasion while initiating the repair of injured tissue (4). Compelling experimental and clinical evidence has indicated that immunosuppression is the cause of such aggravation, which complicates MODS and can even lead to the death of sepsis patients (5). However, the main pathological mechanism of sepsis-induced immunosuppression is incompletely understood, and therefore, systematic, standardized clinical treatment for sepsis-induced immunosuppression is lacking.
Regulatory T cells (Tregs) are subsets of CD4+ T lymphocytes with negative immunomodulatory function. High expression of the interleukin 2 (IL-2) receptor (CD25) and the transcription factor forkhead box protein P-3 (Foxp-3) are essential for the development and function of Tregs (6). They maintain peripheral immune tolerance to prevent excessive autoimmunity and control immune responses to prevent exaggerated responses to infections and harmless antigens (6, 7). Due to the extensive regulatory role of Tregs in the immune system, Tregs have considerable potential for treating various diseases.
Patients who survive sepsis have chronic immunosuppression and are susceptible to secondary infection (8). In agreement with these lines of evidence, we and other research teams have reported that patients and animals with septic shock or MODS have significantly increased frequencies of circulating Treg populations and strengthened stability of Tregs that correlates with immunosuppression (9, 10). However, in sepsis, the specific pathophysiological mechanisms by which Tregs maintain their stability and participate in sepsis-induced immunosuppression are not known. Sepsis is associated with complications of a deregulated inflammatory response against endotoxin/lipopolysaccharide (LPS)-mediated severe infection. In particular, LPS is released as a consequence of infection with Gram-negative bacteria and maximizes the activation of Toll-like receptor 4 (TLR4) signaling in sepsis. Our previous studies have demonstrated that TLR4 is overexpressed on the surfaces of Tregs during sepsis, suggesting its involvement in affecting the immune microenvironment and immunopathology processes by impacting certain signaling pathways or cytokine networks (9).
Neuropilin-1 (Nrp-1) is regarded primarily as a receptor for semaphorins (Sema), such as Sema3A, and vascular endothelial growth factor (VEGF) family members, expressed by neuronal cells and endothelial cells. Nrp-1 plays an essential part in the establishment of the nervous system and endothelial network during embryogenesis (11, 12). In the immune system, Nrp-1 is expressed mainly by CD4− CD8− double-negative cells, CD4+ CD8+ double-positive cells, and CD4+ CD25+ Tregs but is barely detected in single-positive CD4+ and CD8+ thymocytes and is especially strongly expressed in CD4+ CD25+ Tregs, which deserve our attention (13). Nrp-1 not only contributes to the development of Tregs in the thymus gland but is also a key receptor for peripheral Tregs, enabling them to maintain their stability and have a negative immunomodulatory function (14, 15). By use of a cecal ligation and perforation (CLP) model of sepsis in mice, tuftsin-derived T-peptide (a recombinant ligand for Nrp-1) has been shown to weaken the stability and negative immunoregulation of CD4+ CD25+ Tregs, as well as to improve the survival of mice suffering from sepsis (16). Thus, further investigation of the impact of Nrp-1 on the stability and negative immunoregulation of CD4+ CD25+ Tregs could provide a new target for the study of immune regulation in sepsis (17).
Here, we explored the impact of Nrp-1 on the stability and negative immunoregulation of CD4+ CD25+ Tregs, and the underlying signaling pathways, in LPS-induced sepsis and a CLP model of sepsis in mice. We demonstrated that silencing of Nrp-1 expression or anti-Nrp-1 induction reduced the stability of CD4+ CD25+ Tregs in sepsis and that Nrp-1 contributed to this function by regulating the NF-κB signaling pathway.
RESULTS
Anti-Nrp-1 weakened the stability and activity of the NF-κB signaling pathway of CD4+ CD25+ Tregs in LPS-induced sepsis.
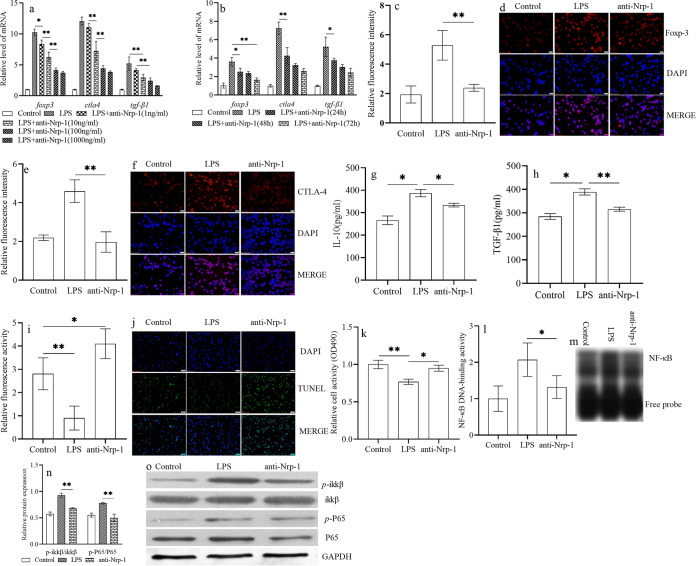
In vitro, after selection, CD4+ CD25+ Tregs were treated with recombinant anti-Nrp-1 in the presence of LPS. Relative to that of the control group, the stability of CD4+ CD25+ Tregs was enhanced significantly following LPS induction. This phenomenon included increased expression of Foxp-3 (Fig. 1a to d), cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) (Fig. 1a, b, e, and f), and transforming growth factor β1 (TGF-β1) (Fig. 1a and b) at the protein and gene levels (P < 0.01), increased secretion levels of TGF-β1 (Fig. 1h) (P < 0.05) and IL-10 (Fig. 1g) (P < 0.05), and decreased apoptosis (Fig. 1i and j) (P < 0.01), as well as (under a coculture condition) an enhanced immunosuppressive effect on CD4+ CD25− T cells (Fig. 1k) (P < 0.01). Upon treatment with anti-Nrp-1 (10, 100, or 1,000 ng/ml) and 24 h of culture (especially at 100 ng/ml), expression of the foxp3, ctla4, and tgf-β1 genes was decreased significantly (Fig. 1a) (P < 0.01), as it was upon culture with anti-Nrp-1 at various time points (24, 48, and 72 h) (Fig. 1b). Also, at the dose of 100 ng/ml, especially at the time point of 24 h, expression of the foxp3, ctla4, and tgf-β1 genes was decreased (Fig. 1b) (P < 0.01). Hence, we chose 100 ng/ml and 24 h of culture as the most appropriate dose and time point, respectively, for subsequent studies. The stability of CD4+ CD25+ Tregs was also reduced significantly relative to that in the LPS induction group after treatment with anti-Nrp-1 at 100 ng/ml and 24 h of culture (Fig. 1c to k) (P, <0.05 or <0.01). Electrophoretic mobility shift assay (EMSA) results (Fig. 1l and m) showed that anti-Nrp-1 inhibited the DNA-binding activity of NF-κB significantly relative to that upon LPS administration alone (P < 0.05). Western blotting (Fig. 1n and o) showed that the ratio of phosphorylated inhibitor of NF-κB kinase subunit beta (p-IKKβ) to IKKβ and the ratio of p-P65 to P65 were enhanced significantly in splenic CD4+ CD25+ Tregs cultured in LPS for 24 h over those in the control group (P < 0.01). However, administration of recombinant anti-Nrp-1 decreased these phosphorylation ratios relative to those obtained with LPS treatment alone (P, <0.05 or <0.01).
FIG 1.
Anti-Nrp-1 weakened the stability of CD4+ CD25+ Tregs through the NF-κB signaling pathway in sepsis. Treatment with recombinant anti-Nrp-1 reduced the mRNA expression of foxp3, ctla4, and tgf-β1 in CD4+ CD25+ Tregs in a dose (a)- and time (b)-dependent manner. The stability of CD4+ CD25+ Tregs was weakened after treatment with anti-Nrp-1 at the 100-ng/ml concentration and at 24 h of culture; this treatment also decreased the expression of Foxp-3 (c, d) and CTLA-4 (e, f) at the protein level, the secretion levels of IL-10 (g) and TGF-β1 (h), and the antiapoptotic level of CD4+ CD25+ Tregs (i, j). The proliferation of CD4+ CD25– T cells was increased when they were cocultured with CD4+ CD25+ Tregs pretreated with anti-Nrp-1 (k). Anti-Nrp-1 significantly inhibited the DNA-binding activity of NF-κB (l, m) and decreased the p-IKKβ/IKKβ and p-P65/P65 ratios (n, o). Administration of recombinant anti-Nrp-1 decreased the ratio of p-IKKβ to IKKβ and the ratio of p-P65 to P65 in splenic CD4+ CD25+ Tregs. Data are presented as means ± SD (n, 4 per group). Asterisks indicate significance (*, P < 0.05; **, P < 0.01) by one-way ANOVA.
Silencing of Nrp-1 expression suppressed the stability of CD4+ CD25+ Tregs during LPS-induced sepsis.
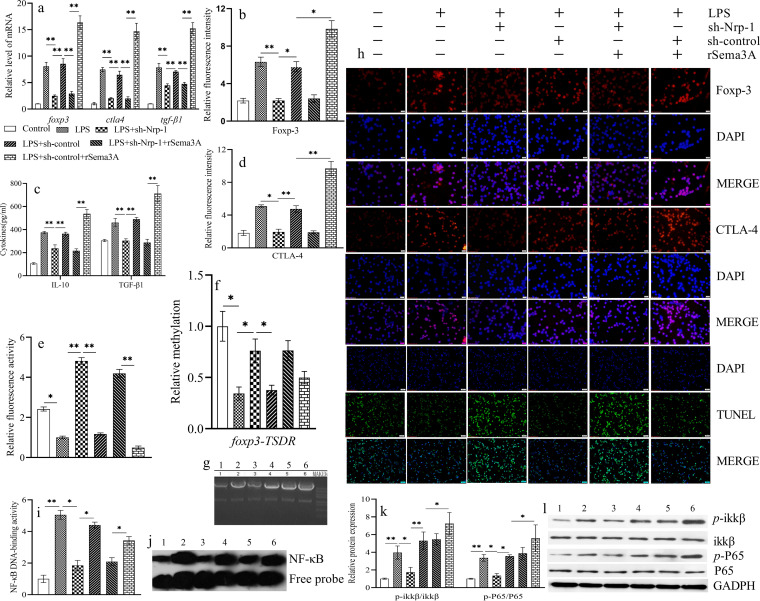
CD4+ CD25+ Tregs were isolated from normal splenic tissue and cultured under LPS-induced (100 ng/ml) sepsis conditions for 24 h. Recombinant Sema3A (rSema3A) treatment significantly upregulated the gene and protein expression levels of Foxp-3 (Fig. 2a, b, and h), CTLA-4 (Fig. 2a, d, and h), and TGF-β1 (Fig. 2a and c) in splenic CD4+ CD25+ Tregs over those in the control group and the LPS induction group (P, <0.05 or <0.01). Knockdown of Nrp-1 expression in splenic CD4+ CD25+ Tregs, by transfection with short hairpin Nrp-1 (sh-Nrp-1) using lentiviral infection, resulted in a significant decrease in the stability of CD4+ CD25+ Tregs, including significantly suppressed expression of Foxp-3 (Fig. 2a, b, and h), CTLA-4 (Fig. 2a, d, and h), and TGF-β1 (Fig. 2a and c) at the protein and gene levels, and an increased apoptosis level (Fig. 2e and h) relative to that with LPS induction alone (P < 0.01) or transfection with the sh-control group (P, <0.05 or <0.01). Furthermore, silencing of Nrp-1 expression significantly suppressed the rSema3A-induced increase in the stability of CD4+ CD25+ Tregs (P < 0.01). Methylation of foxp3-TSDR (encoding forkhead box P-3 [Foxp-3]–Treg-specific demethylated region [TSDR]) (Fig. 2f and g) in CD4+ CD25+ Tregs was decreased by LPS stimulation from that in the control group (P < 0.05). Silencing of Nrp-1 expression upregulated the methylation of foxp3-TSDR (P < 0.05) over that with LPS treatment alone. EMSA and Western blotting (Fig. 2i to l) showed that the silencing of Nrp-1 expression inhibited the DNA-binding activity of NF-κB and reduced p-IKKβ/IKKβ and p-P65/P65 ratios from those obtained with LPS treatment alone (P < 0.05) or transfection with the sh-control group (P < 0.05). Silencing of Nrp-1 expression suppressed the rSema3A-induced increase in the DNA-binding activity of NF-κB, as well as the p-IKKβ/IKKβ and p-P65/P65 ratios (P < 0.05). However, relative to LPS treatment alone, rSema3A did not further enhance the DNA-binding activity of NF-κB (P > 0.05); it only increased the phosphorylation of IKKβ and P65 (P < 0.05).
FIG 2.
Transfection with sh-Nrp-1 using lentiviral infection reduced the mRNA expression of foxp3, ctla4, and tgf-β1 in CD4+ CD25+ Tregs upon LPS stimulation with or without rSema3A (a). Shown are statistical figures and representative immunofluorescence images of Foxp-3 (b, h) and CTLA-4 (d, h) expression at the protein levels, the apoptotic level of CD4+ CD25+ Tregs (e, h), the secretion levels of IL-10 and TGF-β1 (c), the methylation of foxp3-TSDR (f, g), statistical figures and representative EMSA images of the DNA-binding activity of NF-κB (i, j), and statistical figures and representative Western blot images of p-IKKβ/IKKβ and p-P65/P65 ratios (k, l). Data are presented as means ± SD (n, 4 per group). Asterisks indicate significance (*, P < 0.05; **, P < 0.01) by one-way ANOVA. (g, h, j, l) 1: control; 2: LPS; 3: LPS + sh-Nrp-1; 4: LPS + sh-control; 5: LPS + sh-Nrp-1 + rSema3A; 6: LPS + sh-control + rSema3A.
Silencing of IKKβ expression inhibited the rSema3A-mediated increase in the stability of CD4+ CD25+ Tregs in LPS-induced sepsis.
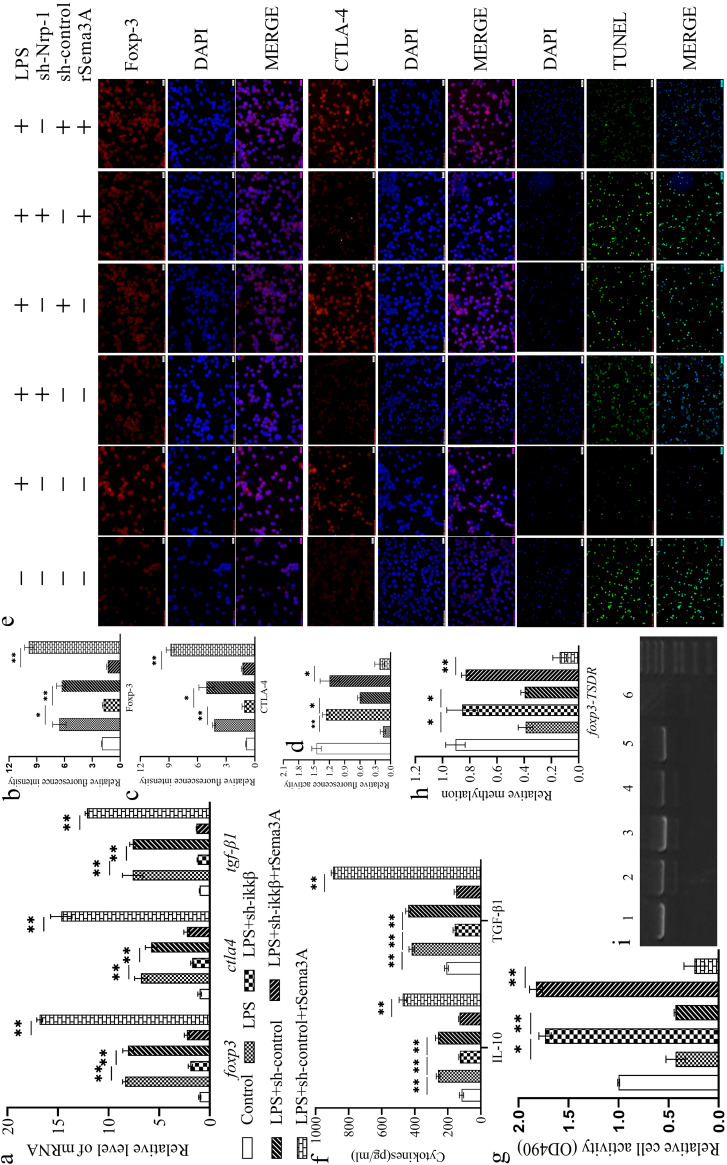
The stability of CD4+ CD25+ Tregs was further enhanced significantly following rSema3A treatment (Fig. 3a to g) over that in the control group and the LPS induction-alone group (P, <0.05 or <0.01). Knockdown of IKKβ expression in splenic CD4+ CD25+ Tregs (by transfection with sh-IKKβ using lentiviral infection) resulted in a significant decrease in the stability of CD4+ CD25+ Tregs from that upon LPS induction alone (P, <0.05 or <0.01) or LPS induction and transfection with the sh-control group (P, <0.05 or <0.01). Furthermore, silencing of IKKβ expression significantly suppressed the rSema3A-induced increase in the stability of CD4+ CD25+ Tregs (P, <0.05 or <0.01). Silencing of IKKβ expression upregulated the methylation of foxp3-TSDR (Fig. 3h and i) over that upon LPS treatment with or without rSema3A (P, <0.05 or <0.01).
FIG 3.
Transfection with sh-IKKβ using lentiviral infection reduced the mRNA expression of foxp3, ctla4, and tgf-β1 in CD4+ CD25+ Tregs stimulated with LPS with or without rSema3A (a). Shown are statistical figures and representative immunofluorescence images of Foxp-3 (b, e) and CTLA-4 (c, e) expression at the protein level, the apoptotic level of CD4+ CD25+ Tregs (d, e), the secretion levels of IL-10 and TGF-β1 (f), the proliferation of CD4+ CD25– T cells when cocultured with CD4+ CD25+ Tregs (g), and the methylation of foxp3-TSDR (h, i). (e, i) 1: control; 2: LPS; 3: LPS + IKKβ; 4: LPS + sh-control; 5: LPS + sh-IKKβ + rSema3A; 6: LPS + sh-control + rSema3A. Data are presented as means ± SD (n, 4 per group). Asterisks indicate significance (*, P < 0.05; **, P < 0.01) by one-way ANOVA.
PDTC suppressed the stability of CD4+ CD25+ Tregs in LPS-induced sepsis.
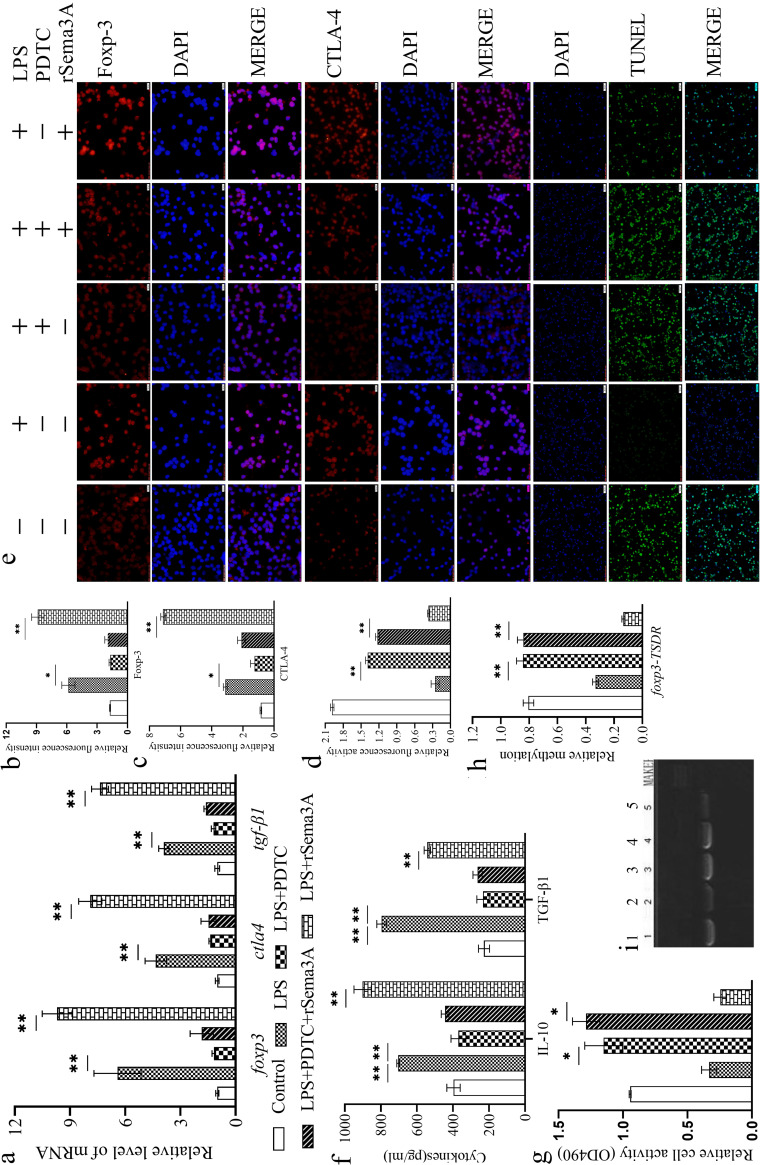
Ammonium pyrrolidine dithiocarbamate (PDTC) is a membrane-permeant moiety that inhibits NF-κB activation in various cell types (18). The stability of CD4+ CD25+ Tregs was enhanced significantly in LPS-induced sepsis with or without rSema3A treatment (Fig. 4a to g), but especially following rSema3A treatment, over that in the control group (P < 0.01). PDTC treatment suppressed the effect of LPS with or without an rSema3A-mediated increase in the stability of CD4+ CD25+ Tregs relative to that obtained upon LPS treatment with or without rSema3A (P, <0.05 or <0.01). PDTC treatment upregulated the methylation of foxp3-TSDR (Fig. 4h and i) over that obtained upon LPS treatment with or without rSema3A (P, <0.05 or <0.01).
FIG 4.
Treatment with PDTC reduced the mRNA expression of foxp3, ctla4, and tgf-β1 in CD4+ CD25+ Tregs stimulated with LPS with or without rSema3A (a). Shown are statistical figures and representative immunofluorescence images of Foxp-3 (b, e) and CTLA-4 (c, e) expression at the protein level, the apoptotic level of CD4+ CD25+ Tregs (d, e), the secretion levels of IL-10 and TGF-β1 (f), the proliferation of CD4+ CD25– T cells when cocultured with CD4+ CD25+ Tregs (g), and the methylation of foxp3-TSDR (h, i). (e, i) 1: control; 2: LPS; 3: LPS + PDTC; 4: LPS + PDTC + rSema3A; 5: LPS + rSema3A. Data are presented as means ± SD (n, 4 per group). Asterisks indicate significance (*, P < 0.05; **, P < 0.01) by one-way ANOVA.
Anti-Nrp-1 alleviated sepsis-induced immunosuppression and renal injury by suppressing the stability of CD4+ CD25+ Tregs in CLP-induced sepsis.
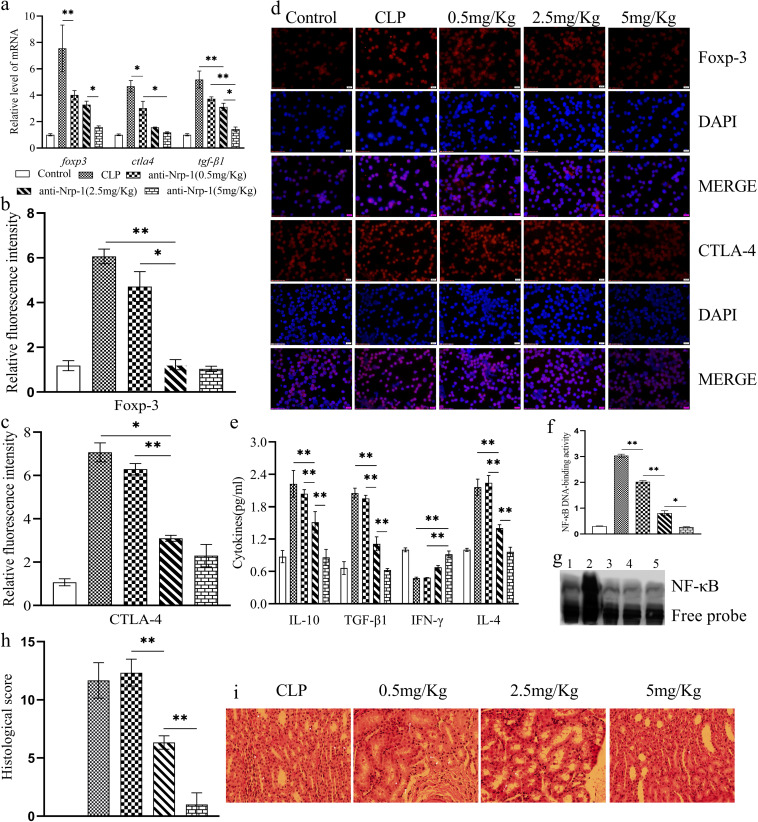
CD4+ CD25+ Tregs were isolated from spleens at 24 h after CLP. Relative to that for the control group, the stability of CD4+ CD25+ Tregs was enhanced significantly in CLP-induced sepsis. This phenomenon was shown by increased expression of Foxp-3 (Fig. 5a, b, and d), CTLA-4 (Fig. 5a, c, and d), and TGF-β1 (Fig. 5a and e) at the protein and gene levels (P < 0.01), increased secretion of IL-10 and TGF-β1 (Fig. 5e) (P < 0.01), and upregulated methylation of foxp3-TSDR (Fig. 5f and g) (P < 0.01). Anti-Nrp-1 treatment suppressed the stability of CD4+ CD25+ Tregs relative to that seen in the CLP-induced sepsis alone group (P, <0.05 or <0.01). We assessed sepsis-induced immunosuppression by measuring the levels of IL-10, TGF-β1, gamma interferon (IFN-γ), and IL-4 in serum (Fig. 5e). Sepsis significantly promoted the secretion of anti-inflammatory cytokines (IL-10, TGF-β1, and IL-4) and inhibited the secretion of a proinflammatory cytokine (IFN-γ) relative to that in the control group (P < 0.01), and anti-Nrp-1 alleviated sepsis-induced immunosuppression (P < 0.01). Acute kidney injury (AKI) is a common injury in sepsis. Histology (Fig. 5h and i) revealed that CLP increased the infiltration of inflammatory cells, tissue edema, and necrosis of renal tubular epithelial cells. Intravenous (i.v.) injection of different concentrations of anti-Nrp-1 could gradually improve tissue performance in sepsis-induced AKI. In particular, administration of anti-Nrp-1 at 5 mg/kg of body weight led to only mild infiltration of inflammatory cells in renal tissue.
FIG 5.
Treatment with anti-Nrp-1 reduced the mRNA expression of foxp3, ctla4, and tgf-β1 in CD4+ CD25+ Tregs in a sepsis model in mice (a). Shown are statistical figures and representative immunofluorescence images of Foxp-3 (b, d) and CTLA-4 (c, d) expression at the protein level, the levels of secretion of IL-10, TGF-β1, IFN-γ, and IL-4 in serum (e), statistical figures and representative EMSA images of the DNA-binding activity of NF-κB (f, g), and the effects of anti-Nrp-1 (administered at different concentrations) on histopathological changes (magnification, ×200) in the renal tissues of mice with CLP-induced sepsis (h, i). (d, g) 1: control; 2: LPS; 3: anti-Nrp-1(0.5 mg/Kg); 4: anti-Nrp-1(2.5 mg/Kg); 5: anti-Nrp-1(5 mg/Kg). Data are presented as means ± SD (n, 4 per group). Asterisks indicate significance (*, P < 0.05; **, P < 0.01) by one-way ANOVA.
DISCUSSION
Tregs are a negative immunomodulatory type of CD4+ T lymphocyte. They must maintain their own stability, adaptability, and immunomodulatory function throughout their lives to suppress excessive inflammation and fatal autoimmunity (6–8). The characteristic transcription factor Foxp-3 is an important determinant for Treg stability (7). Therefore, the stability of Foxp-3 expression is also a key factor in determining the dynamic equilibrium and homeostasis of the immune system. We and other scholars have demonstrated that increased expression of Foxp-3 in Tregs is correlated positively with the mortality of mice suffering from sepsis (9, 16). In the present study, we also showed that sepsis meaningfully increased Foxp-3 expression in CD4+ CD25+ Tregs at both the protein and gene levels. By use of CLP and subsequent Pseudomonas aeruginosa lung infection in a mouse septic model, the stability of Nrp-1+ Foxp-3+ Tregs was increased meaningfully during the early and late phases of murine sepsis (17). Nrp-1 expression in anergic conventional CD4+ T cells was associated with their ability to differentiate into Foxp-3+ Tregs that suppress disease in the immune system (18, 19). We demonstrated that silencing of Nrp-1 expression or anti-Nrp-1 induction reduced the Foxp-3 expression of CD4+ CD25+ Tregs in sepsis. Sema3A is a specific ligand of Nrp-1 and has a key role in neuraxon development through the Sema3A–Nrp-1 signaling pathway (20). In LPS-induced AKI, Sema3A was found in tubular epithelial cells and showed higher expression after LPS treatment (21). Anti-Sema3A antibodies have been shown to improve survival in LPS-induced sepsis in mice (22). Toll-like receptor (TLR) engagement can induce Sema3A expression, thereby completing an “autocrine loop” in LPS-induced sepsis. Sema3A is expressed by activated T cells and downmodulates T-cell activation in vitro (23). By use of a mouse model of collagen-induced arthritis, Sema3A has been shown to increase the ability of CD4+ NP-1+ Tregs to suppress the growth of CD4+ T cells (24). We demonstrated that rSema3A treatment further significantly upregulated the stability of CD4+ CD25+ Tregs, especially Foxp-3 expression, at the protein and gene levels, over that seen with LPS-induced sepsis alone. Silencing of Nrp-1 expression or anti-Nrp-1 induction had helped to weaken the effect of LPS with or without an rSema3A-mediated effect. Treg stability is also reflected in stable expression of surface markers (e.g., CTLA-4) and cytokines (e.g., IL-10, TGF-β1) that have a negative immunomodulatory role, the ability to resist apoptosis, and a stable immunosuppressive effect on CD4+ T lymphocytes and other immune cells (6, 25, 26). Knockdown of Nrp-1 expression in splenic CD4+ CD25+ Tregs (by transfection with sh-Nrp-1 using lentiviral infection) or anti-Nrp-1 induction resulted in significant decreases in the expression of CTLA-4, IL-10, and TGF-β1 at the protein and gene levels, an increase in apoptosis, and a decrease in the immunosuppressive effect on CD4+ T lymphocytes.
The stability of Tregs, including the stability of Foxp-3 expression and the negative immunosuppression function of Tregs, is dependent on the methylation status of foxp3-TSDR (27). Tregs are more stable with an increase in demethylation in this region. This region specifically is demethylated completely in stable Tregs but is heavily methylated in all other types of blood cells (27, 28). The high demethylation of foxp3-TSDR ensures the stability of Foxp-3 expression and the negative immunosuppression function of Tregs (27). Using a new methylation-sensitive real-time reverse transcription-quantitative PCR (RT-qPCR) method and deep amplicon sequencing, Tatura and colleagues demonstrated that natural Tregs and most induced Tregs were stable and exhibited demethylated foxp3-TSDR and that both Treg populations were functionally suppressive in healthy mice and mice suffering from sepsis (17). In the present study, we also found that LPS-induced sepsis alone or with rSema3A meaningfully decreased the methylation status of foxp3-TSDR in CD4+ CD25+ Tregs and increased the demethylation of foxp3-TSDR. We demonstrated that knockdown of Nrp-1 expression or anti-Nrp-1 induction resulted in a significant decrease in the demethylation of foxp3-TSDR and that these treatments could abolish the effects of LPS with or without rSema3A treatment.
Studies have demonstrated that the NF-κB signaling pathway and its transcription factors, in particular the canonical signaling subunits IKKβ, c-Rel, and P65 (RelA), are important in Treg biology, including Treg differentiation in the thymus gland, and that they maintain their own peripheral stability and have a stable negative immunomodulatory function (29–31). Although NF-κB has been reported to bind to the regulatory sequence of foxp3 and to have a complex interaction with foxp3, few studies have shown that NF-κB contributes to the stability of Tregs in sepsis. Using TLR4−/− mice and CLP-induced sepsis, we found that sepsis-induced increases in the quantity and activity of Tregs were attenuated under urinary tract infection (UTI) treatment, but not in TLR4−/− mice, and that this effect could be attributed to the TLR4/NF-κB signaling pathway (9). P65 is critical for the acquisition of the effector state of Tregs independently of the surrounding inflammatory environment. Unexpectedly, P65-deficient Tregs also displayed reduced stability, and cells that lost Foxp-3 produced inflammatory cytokines (30). Mice with a knockout of either Ubc13, an E2 ubiquitin ligase activating IKKβ, or IKKβ itself in Tregs developed a spontaneous autoimmune syndrome, associated with the conversion of Tregs into effector-like T cells and a reduction in Treg survival (31). We also showed that sepsis meaningfully increased the DNA-binding activity of NF-κB, as well as the p-IKKβ/IKKβ and p-P65/P65 ratios in vitro and in vivo. Silencing of IKKβ expression or PDTC treatment suppressed the stability of CD4+ CD25+ Tregs in LPS-induced sepsis. Several Nrp-1 ligands, including tuftsin and MY1340, can regulate downstream NF-κB signaling pathway by binding Nrp-1 to regulate tumor growth (32). We demonstrated that silencing of Nrp-1 expression or anti-Nrp-1 induction reduced the DNA-binding activity of NF-κB, as well as the p-IKKβ/IKKβ and p-P65/P65 ratios, in sepsis and that they could simultaneously reverse induction by LPS and block the effect of rSema3A.
Conclusions.
Silencing of Nrp-1 expression or anti-Nrp-1 treatment could alleviate sepsis-induced immunosuppression and AKI. This effect was related to a reduction in the stability of CD4+ CD25+ Tregs via regulation of the NF-κB signaling pathway. We propose a promising new target for treating the stability of CD4+ CD25+ Tregs, which may reverse (at least in part) sepsis-induced immunosuppression.
MATERIALS AND METHODS
Animals and ethical statement.
Inbred male C57BL/6J mice (6 to 8 weeks old; weight, 20 ± 2 g; catalog no. SCXK-Jing-2014-0004; Laboratory Animal Center of the Chinese Academy of Medical Sciences, Beijing, China) were used. All procedures were undertaken in accordance with the Guide for the Care and Use of Laboratory Animals (33). The study protocol was approved by the Scientific Investigation Board of Tianjin Medical University General Hospital (TMUaMEC2014002) in Tianjin, China.
Isolation of splenic CD4+ CD25+ Tregs.
Spleens were harvested and prepared as single-cell suspensions. Then they were subjected to Ficoll-Paque density gradient centrifugation (Nanjing KeyGen Biotech, Nanjing, China). CD4+ CD25+ Tregs and CD4+ CD25− T cells were isolated from mononuclear cells using mouse CD4+ CD25+ Treg isolation kits (containing a 1-ml cocktail of biotin-conjugated monoclonal antibodies against mouse CD8a, CD11b, CD45R, CD49b, and Ter-119, 2 ml of anti-biotin microbeads, 1 ml of a phycoeryanate [PE]-conjugated mouse CD25 antibody, and 1 ml of anti-PE microbeads) and a MiniMACS separator (both from Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Isolated cells were cultured in RPMI 1640 medium (Nanjing KeyGen Biotech, Nanjing, China) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA).
Sepsis model.
After the induction of anesthesia, a 0.5-cm incision was made on the abdomen of each mouse, and the cecum was exposed. The area between the distal pole of the cecum and the ileocecal junction was ligated. A single puncture was made through the cecum. The diameter of the puncture needle was 0.6 mm, and it was used to induce CLP. The abdominal incision was closed using simple running sutures. Mice were given 0.9% sterile saline solution (40 ml/kg of body weight, subcutaneously [s.c.]) after CLP.
Construction and transfection of shRNA targeting Nrp-1 and IKKβ.
The sequences of the shRNA targeting Nrp-1 (5′-AACCAGACACAGCTTCTTCCCAGTATATTCAAGAGATATACTGGGAAGAAGCTGTGATCTGTTTTTTC-3′) and IKKβ (5′-GATCCAAGACTTGAATGGAACGGTGACTCGAGTCACCGTTCCATTCAAGTCTTTTTTTG-3′) were designed by Sigma-Aldrich. A control shRNA duplex (Mission; Sigma-Aldrich) was used as the negative control. Recombinant retroviruses were packaged using 293T cells by following the protocol of the BD Retro-X universal packaging system (Clontech, Toyobo, Osaka, Japan). After 48 h, the supernatant was collected and diluted serially. The supernatant containing the optimal concentration of viral particles was used for knockdown experiments. Transfection was carried out using the ViraDuctin lentivirus transduction kit (Cell Biolabs, San Diego, CA, USA).
CD4+ CD25+ Tregs were transduced with a sh-Nrp-1 or sh-IKKβ vector according to manufacturer protocols. The transfection efficiencies of sh-Nrp-1 and sh-IKKβ in CD4+ CD25+ Tregs were evaluated by RT-qPCR using a SYBR green PCR mixture. The sequences of the primers (forward and reverse, respectively) used were as follows: 5′-ACACCCACTCCTCCACCTTT-3′ and 5′-TTACTCCTTGGAGGCCATGT-3′ for the mouse glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH), 5′-AAAGGAAACTCACCCTAACTGT-3′ and 5′-TGGAATCTCGTGAAGCGAG-3′ for mouse Nrp-1, and 5′-CGGGATCCGACCACCAGCCTGCCTACC-3′ and 5′-CGGAATTCGTGGAACCCAGTATGAGTG-3′ for mouse ikkβ.
RT-qPCR amplification consisted of 1 min of denaturation at 95°C, followed by 40 cycles of 15 s at 95°C and 40 s at 60°C, and was done in a Sequence Detection System (Agilent Technologies, Santa Clara, CA, USA).
Experimental design.
In vitro, LPS (100 ng/ml; Escherichia coli 0111:B4; Sigma-Aldrich) induction was used for sepsis simulation. Subsequently, splenic CD4+ CD25+ Tregs were seeded onto 96-well cell culture plates (2 × 105 cells/well) and were treated first with anti-CD3e (5 μg/ml; BD Pharmingen, San Diego, CA, USA) and anti-CD28 (2 μg/ml; BD Pharmingen) antibodies for polyclonal activation of T cells and then with a recombinant Nrp-1 polyclonal antibody (10, 100, and 1,000 ng/ml; R&D Systems, Minneapolis, MN, USA) for 24, 48, or 72 h. CD4+ CD25+ Tregs were transfected with sh-Nrp-1or sh-IKKβ to silence Nrp-1 or IKKβ or were administered PDTC (Beyotime, Shanghai, China) at 25 μg/ml to inhibit NF-κB, followed by rSema3A (300 ng/ml; BioVision, Heidelberg, Germany). In vivo, after creation of the sepsis model, different concentrations of anti-Nrp-1 were administered (intravenously [i.v.]) immediately. Then the TSDR, apoptosis rate, Foxp-3/CTLA-4/TGF-β1 expression, secretory capacity (IL-10, TGF-β1), and activity of the NF-κB signaling pathway of CD4+ CD25+ Tregs were determined.
RT-qCR.
Total RNA was purified from 1 × 106 cells/group using the NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. mRNA expression of foxp3/ctla4/tgf-β1 was measured by RT-qPCR using a SYBR green PCR mixture. The sequences of the primers (forward and reverse, respectively) used were as follows: 5′-CAGCTGCCTACAGTGCCCCTAG-3′ and 5′-CATTTGCCAGCAGTGGGTAG-3′ for mouse foxp3, 5′-CGCAGATTTATGTCATTGATCC-3′ and 5′-TTTTCACATAGACCCCTGTTGT-3′ for mouse ctla4, and 5′-AACAATTCCTGGCGTTACCTT-3′ and 5′-GAATCGAAAGCCCTGTATTCC-3′ for mouse tgf-β1.
PCR amplification consisted of 1 min of denaturation at 95°C, followed by 40 cycles of 15 s at 95°C and 40 s at 60°C, and was undertaken in a Sequence Detection System (Agilent Technologies).
Immunofluorescence analyses.
The expression of Foxp-3 and CTLA-4 in CD4+ CD25+ Tregs was examined by immunofluorescence microscopy (Olympus, Tokyo, Japan). CD4+ CD25+ Tregs were seeded on glass coverslips, washed with phosphate-buffered saline (PBS; Nanjing KeyGen Biotech, Nanjing, China), fixed in 4% paraformaldehyde (Nanjing KeyGen Biotech) for 30 min, and then incubated with rabbit anti-mouse Foxp-3/CTLA-4 antibodies (Abcam, Cambridge, MA, USA) for 20 min at 4°C. The cells were subsequently washed and incubated with fluorescein isothiocyanate (FITC)/allophycocyanin (APC)-conjugated goat anti-rabbit IgG (Jackson, Southern Biotechnology Associates, and Molecular Probes) for 30 min at 4°C. For the determination of intranuclear Foxp-3 levels, CD4+ CD25+ Tregs were suspended in 1 ml of a fixation/permeabilization solution (eBioscience, San Diego, CA, USA) for 2 h at 4°C in the dark. Immunofluorescence was assessed by immunofluorescence microscopy (Olympus). Data were collected and processed with ImageJ (Bethesda, MD, USA).
ELISA.
Supernatants or serum samples were collected for the measurement of IL-10, TGF-β1, IFN-γ, and IL-4 levels using enzyme-linked immunosorbent assay (ELISA) kits (ExCell Bio, Shanghai, China) strictly according to manufacturer protocols. Pi (100 μl) was added to terminate the colorimetric reaction. Absorbance was read in a microplate reader (Spectra MR; Dynex Technologies, Chantilly, VA, USA) at 450 nm. Concentration curves using standards for IL-10 and TGF-β1 were plotted from 0 pg/ml to 1,000 pg/ml.
TUNEL assay.
We used a one-step terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) apoptosis assay kit (detection of FITC-dUTP fluorescence; Solarbio, Beijing, China) according to the manufacturer’s instructions. CD4+ CD25+ Tregs were fixed in 4% paraformaldehyde for 25 min. After a wash with PBS, 100 ml of proteinase K (20 pg/ml) was added dropwise to the samples, followed by incubation at room temperature for 5 min. After a wash with PBS, 100 ml of equilibration buffer was added to the cells, which were then incubated for 30 min at room temperature. Finally, 50 ml of TUNEL detection liquid was added dropwise to each sample, followed by incubation for 50 min at 37°C. Immunofluorescence was analyzed by immunofluorescence microscopy (Olympus). The emission wavelength was 525 nm (green fluorescence). Data were collected and processed using ImageJ software.
MTT assay.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Ameresco, New York, NY, USA) was used to evaluate the proliferative ability of CD4+ CD25– T cells. CD4+ CD25+ Tregs, in each group, were cocultured with conventional CD4+ CD25– T cells for 24 h at a 1:1 ratio. The medium was removed and replaced with fresh medium. The assays were initiated by adding 10 ml of the MTT reagent (10 mg/ml) to each well and incubating the cells for 4 h. Finally, the medium was removed, and 100 ml of dimethyl sulfoxide (DMSO; Ameresco) was added to each well. The absorbance was read in a microplate reader (Spectra MR; Dynex, Richfield, MN, USA) at a wavelength of 490 nm.
EMSA.
CD4+ CD25+ Tregs were harvested, nuclear extraction was performed, and extracts were used for the electrophoretic mobility shift assay (EMSA) (Thermo Scientific, Rockford, IL, USA). A biotin-3′-end-labeled DNA oligonucleotide probe (forward, 5′-AGTTGAGGGGACTTTCCCAGGC-biotin-3′; reverse, 5′-GCCTGGGAAAGTCCCCTCAACT-biotin-3′) corresponding to the sequence of the NF-κB binding site was incubated with the nuclear extracts. An anti-NF-κB p50 antibody was used to detect NF-κB activity by DNA binding. To determine whether the shifted bands were specific for NF-κB, a mutated probe and a biotin-free probe were used to perform EMSA. After incubation, both target and free DNA samples were resolved on 5% polyacrylamide gels.
Western blot assay for p-IKKβ/IKKβ and p-P65/P65 ratios.
CD4+ CD25+ Tregs were lysed in 1× NuPAGE LDS lysis buffer (Life Technologies, Carlsbad, CA, USA) with or without phosphatase inhibitors (Life Technologies) and were then incubated for 10 min at 95°C. The bicinchoninic acid (BCA) assay (Life Technologies) was used to measure protein concentrations. Equal amounts of protein extracted from CD4+ CD25+ Tregs were first separated by 10-to-15% SDS–PAGE (Boster, Wuhan, China) and then transferred to nitrocellulose membranes. Membranes were blocked with 3% bovine serum albumin (BSA; Boster) for 2 h at room temperature and then incubated overnight at 4°C with primary antibodies (1:1,000 dilution) against p-IKKβ, IKKβ, p-P65, and P65 (Abcam, Shanghai, China). The membranes were then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (Abcam) secondary antibodies at room temperature for 1.5 h. Data were collected and processed using the LabWorks imaging and analysis system (UVP, CA, USA).
Methylation-specific qPCR.
The methylation of foxp3-TSDR was determined by methylation-specific qPCR as described by Tatura and colleagues (17).
Histology.
Renal tissues were collected and fixed in 4% formaldehyde for 48 h and were then transferred in 70% ethanol. Paraffin-embedded sections were cut, stained with hematoxylin and eosin (H&E), and then blindly analyzed by light microscopy (magnification, ×200; Olympus, Tokyo, Japan).
Statistical analysis.
Data are presented as means ± standard deviations (SD). Data were analyzed by one-way analysis of variance (ANOVA) (n, 4 per group) using SPSS Statistics, version 24 (IBM, Armonk, NY, USA). The Student-Newman-Keuls test was used to evaluate the significance of differences between two groups. A P value of <0.05 or <0.01 was considered significant.
Data availability.
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
ACKNOWLEDGMENTS
We thank Shu-zhang Cui of the Emergency Department of Tianjin Medical University General Hospital for help with the experimental design.
Y.-L.G., C.-X.W., and Y.-F.C. planned the study, wrote the protocol, collected the data, undertook statistical analyses, and contributed to writing the manuscript. Z.-Y.W. and W.-J.L. did the technical work. Z.-Y.W., Y.-C.L., and S.-T.S. helped with data collection and study design and coordinated the study. Y.-F.C. participated in the study design and helped to critically revise the manuscript. All authors have approved the final version of the manuscript.
This work was supported by the National Natural Science Foundation of China (grant 81701931, 81871593) and the National Natural Science Foundation of Tianjin (grant 18JCQNJC10500).
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
REFERENCES
- 1.Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, Pilcher DV, Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). 2017. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA 317:290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 2.Xie J, Wang H, Kang Y, Zhou L, Liu Z, Qin B, Ma X, Cao X, Chen D, Lu W, Yao C, Yu K, Yao X, Shang H, Qiu H, Yang Y, Chinese Epidemiological Study of Sepsis (CHESS) Study Investigators. 2020. The epidemiology of sepsis in Chinese ICUs: a national cross-sectional survey. Crit Care Med 48:e209–e218. doi: 10.1097/CCM.0000000000004155. [DOI] [PubMed] [Google Scholar]
- 3.Weng L, Zeng XY, Yin P, Wang LJ, Wang CY, Jiang W, Zhou MG, Du B, China Critical Care Clinical Trials Group (CCCCTG). 2018. Sepsis-related mortality in China: a descriptive analysis. Intensive Care Med 44:1071–1080. doi: 10.1007/s00134-018-5203-z. [DOI] [PubMed] [Google Scholar]
- 4.Taeb AM, Hooper MH, Marik PE. 2017. Sepsis: current definition, pathophysiology, diagnosis, and management. Nutr Clin Pract 32:296–308. doi: 10.1177/0884533617695243. [DOI] [PubMed] [Google Scholar]
- 5.Stolk RF, Kox M, Pickkers P. 2020. Noradrenaline drives immunosuppression in sepsis: clinical consequences. Intensive Care Med 46:1246–1248. doi: 10.1007/s00134-020-06025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hori S. 2011. Stability of regulatory T-cell lineage. Adv Immunol 112:1–24. doi: 10.1016/B978-0-12-387827-4.00001-2. [DOI] [PubMed] [Google Scholar]
- 7.Ramsdell F, Rudensky AY. 2020. Foxp3: a genetic foundation for regulatory T cell differentiation and function. Nat Immunol 21:708–709. doi: 10.1038/s41590-020-0694-5. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento DC, Melo PH, Piñeros AR, Ferreira RG, Colón DF, Donate PB, Castanheira FV, Gozzi A, Czaikoski PG, Niedbala W, Borges MC, Zamboni DS, Liew FY, Cunha FQ, Alves-Filho JC. 2017. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun 8:14919. doi: 10.1038/ncomms14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao C, Yin C, Chai Y, Jin H, Wang L, Shou S. 2018. Ulinastatin mediates suppression of regulatory T cells through TLR4/NF-κB signaling pathway in murine sepsis. Int Immunopharmacol 64:411–423. doi: 10.1016/j.intimp.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Yoon SJ, Kim SJ, Lee SM. 2017. Overexpression of HO-1 contributes to sepsis-induced immunosuppression by modulating the Th1/Th2 balance and regulatory T-cell function. J Infect Dis 215:1608–1618. doi: 10.1093/infdis/jix142. [DOI] [PubMed] [Google Scholar]
- 11.Gu Z, Ueno M, Klinefelter K, Mamidi M, Yagi T, Yoshida Y. 2019. Skilled movements in mice require inhibition of corticospinal axon collateral formation in the spinal cord by semaphorin signaling. J Neurosci 39:8885–8899. doi: 10.1523/JNEUROSCI.2832-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukushima Y, Nishiyama K, Kataoka H, Fruttiger M, Fukuhara S, Nishida K, Mochizuki N, Kurihara H, Nishikawa SI, Uemura A. 2020. RhoJ integrates attractive and repulsive cues in directional migration of endothelial cells. EMBO J 39:e102930. doi: 10.15252/embj.2019102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbel C, Lemarchandel V, Thomas-Vaslin V, Pelus AS, Agboton C, Roméo PH. 2007. Neuropilin 1 and CD25 co-regulation during early murine thymic differentiation. Dev Comp Immunol 31:1082–1094. doi: 10.1016/j.dci.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA. 2013. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, Shuai Y, Normolle DP, Kirkwood JM, Ferris RL, Delgoffe GM, Bruno TC, Workman CJ, Vignali D. 2017. Interferon-γ drives Treg fragility to promote anti-tumor immunity. Cell 169:1130–1141.e11. doi: 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao YL, Chai YF, Dong N, Han S, Zhu XM, Zhang QH, Yao YM. 2015. Tuftsin-derived T-peptide prevents cellular immunosuppression and improves survival rate in septic mice. Sci Rep 5:16725. doi: 10.1038/srep16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatura R, Zeschnigk M, Hansen W, Steinmann J, Vidigal PG, Hutzler M, Pastille E, Westendorf AM, Buer J, Kehrmann J. 2015. Relevance of Foxp3+ regulatory T cells for early and late phases of murine sepsis. Immunology 146:144–156. doi: 10.1111/imm.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson ST, Commins S, Moynagh PN, Coogan AN. 2015. Lipopolysaccharide-induced sepsis induces long-lasting affective changes in the mouse. Brain Behav Immun 43:98–109. doi: 10.1016/j.bbi.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Kalekar LA, Schmiel SE, Nandiwada SL, Lam WY, Barsness LO, Zhang N, Stritesky GL, Malhotra D, Pauken KE, Linehan JL, O’Sullivan MG, Fife BT, Hogquist KA, Jenkins MK, Mueller DL. 2016. CD4+ T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat Immunol 17:304–314. doi: 10.1038/ni.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita A, Yamashita N, Sasaki Y, Uchida Y, Nakajima O, Nakamura F, Yagi T, Taniguchi M, Usui H, Katoh-Semba R, Takei K, Goshima Y. 2006. Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling. J Neurosci 26:2971–2980. doi: 10.1523/JNEUROSCI.5453-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian X, Gan H, Zeng Y, Zhao H, Tang R, Xia Y. 2018. Inhibition of semaphorin-3a suppresses lipopolysaccharide-induced acute kidney injury. J Mol Med (Berl) 96:713–724. doi: 10.1007/s00109-018-1653-6. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita N, Jitsuki-Takahashi A, Ogawara M, Ohkubo W, Araki T, Hotta C, Tamura T, Hashimoto S, Yabuki T, Tsuji T, Sasakura Y, Okumura H, Takaiwa A, Koyama C, Murakami K, Goshima Y. 2015. Anti-semaphorin 3A neutralization monoclonal antibody prevents sepsis development in lipopolysaccharide-treated mice. Int Immunol 27:459–466. doi: 10.1093/intimm/dxv014. [DOI] [PubMed] [Google Scholar]
- 23.Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, Shirvan A, Barzilai A, Hermine O. 2006. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol 36:1782–1793. doi: 10.1002/eji.200535601. [DOI] [PubMed] [Google Scholar]
- 24.Catalano A. 2010. The neuroimmune semaphorin-3A reduces inflammation and progression of experimental autoimmune arthritis. J Immunol 185:6373–6383. doi: 10.4049/jimmunol.0903527. [DOI] [PubMed] [Google Scholar]
- 25.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. 2010. Stability of the regulatory T cell lineage in vivo. Science 329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knosp CA, Schiering C, Spence S, Carroll HP, Nel HJ, Osbourn M, Jackson R, Lyubomska O, Malissen B, Ingram R, Fitzgerald DC, Powrie F, Fallon PG, Johnston JA, Kissenpfennig A. 2013. Regulation of Foxp3+ inducible regulatory T cell stability by SOCS2. J Immunol 190:3235–3245. doi: 10.4049/jimmunol.1201396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karl M, Sommer C, Gabriel CH, Hecklau K, Venzke M, Hennig AF, Radbruch A, Selbach M, Baumgrass R. 2019. Recruitment of histone methyltransferase Ehmt1 to Foxp3 TSDR counteracts differentiation of induced regulatory T cells. J Mol Biol 431:3606–3625. doi: 10.1016/j.jmb.2019.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MR, Enose-Akahata Y, Massoud R, Ngouth N, Tanaka Y, Oh U, Jacobson S. 2014. Epigenetic modification of the FoxP3 TSDR in HAM/TSP decreases the functional suppression of Tregs. J Neuroimmune Pharmacol 9:522–532. doi: 10.1007/s11481-014-9547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinberg-Bleyer Y, Caron R, Seeley JJ, De Silva NS, Schindler CW, Hayden MS, Klein U, Ghosh S. 2018. The alternative NF-κB pathway in regulatory T cell homeostasis and suppressive function. J Immunol 200:2362–2371. doi: 10.4049/jimmunol.1800042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronin E, Lubrano di Ricco M, Vallion R, Divoux J, Kwon HK, Grégoire S, Collares D, Rouers A, Baud V, Benoist C, Salomon BL. 2019. The NF-κB RelA transcription factor is critical for regulatory T cell activation and stability. Front Immunol 10:2487. doi: 10.3389/fimmu.2019.02487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heuser C, Gotot J, Piotrowski EC, Philipp MS, Courrèges C, Otte MS, Guo L, Schmid-Burgk JL, Hornung V, Heine A, Knolle PA, Garbi N, Serfling E, Evaristo C, Thaiss F, Kurts C. 2017. Prolonged IKKβ inhibition improves ongoing CTL antitumor responses by incapacitating regulatory T cells. Cell Rep 21:578–586. doi: 10.1016/j.celrep.2017.09.082. [DOI] [PubMed] [Google Scholar]
- 32.Mo Z, Yu F, Han S, Yang S, Wu L, Li P, Jiao S. 2018. New peptide MY1340 revert the inhibition effect of VEGF on dendritic cells differentiation and maturation via blocking VEGF–NRP-1 axis and inhibit tumor growth in vivo. Int Immunopharmacol 60:132–140. doi: 10.1016/j.intimp.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 33.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.