Abstract
The sanitary status of grapevines has not yet been considered sufficiently in vineyards throughout Bosnia and Herzegovina (BiH). An extensive survey of five major grapevine viruses in the country was carried out in 2019. A total of 630 samples from the two dominant autochthonous cultivars, named Žilavka and Blatina, were tested by DAS-ELISA for the presence of grapevine leafroll-associated viruses (GLRaV-1 and 3), grapevine fleck virus (GFkV), grapevine fanleaf virus (GFLV) and Arabis mosaic virus (ArMV). Eighty-eight % of the samples were positive for at least one virus, and all five viruses were detected, thought with different incidence, i.e. GLRaV-3 (84%), GFLV (43%), GLRaV-1 (14%), GFkV (10%) and ArMV (0.2%). The majority of infected plants (about 75%) were asymptomatic. Specific virus symptoms were observed in the remaining infected plants, together with the reported GLRaV vectors, Planococcus ficus and Parthenolecanium corni, while nematodes of the Xiphinema genus were not found in the GFLV- or ArMV-infected vineyards. The GLRaV-3 CP phylogenetic analyses showed 75–100% nucleotide identity between the BiH and reference isolates, and the BiH isolates clustered into the major group. The dNS/dS ratio indicated a negative selection of the virus population, and the lack of geographical structuring within the population was observed. In addition, putative GLRaV-3 recombinants with breakpoints in the 5’ of the CP gene were detected, while no recombinant strains were identified for the other four viruses. The obtained results indicate a deteriorated sanitary status of the cultivated grapevines, the prevalence and intraspecies genetic diversity of GLRaV-3 throughout the country. The establishment of certified grapevine material and adequate virus vector control is therefore of primary importance to prevent further spread of these viruses. This study presents the results of the first molecular characterisation of grapevine viruses in Bosnia and Herzegovina.
Introduction
Grapevine harvested areas throughout the world cover more than 7 million ha, around half of which is located in Europe, which ranks first in grapevine production [1]. Grapevine is an economically important crop in the Balkan-Mediterranean area, and it ranks third among the cultivated fruit crops in Bosnia and Herzegovina (BiH). Almost 4,500 ha is used for the cultivation of grapevines in the country, with an annual production of around 40,000 tons of grapes in 2019 [1]. The grapevine production in BiH is concentrated in two main vineyard regions, Lištica and Mostar, both located in the Southern part of the country (Herzegovina). Grapevine cultivation in these regions is mainly represented by autochthonous cultivars (cvs.). Two of the most important and commercial cultivars are the white grapevine ’Žilavka’ and the red grapevine ’Blatina’, which have been grown in these regions since the 14th century [2,3]. These two cultivars contribute predominantly to the whole wine production of the country (227,500 hl; Mario Leko, personal communication).
Viruses represent the most dangerous and economically important pathogens of grapevine, and 86 distinct virus species belonging to 17 families and 34 genera have been identified in this crop [4,5]. Viral disease management is often difficult, mainly due to the absence of effective chemical compounds for their control, the rapid evolution of the viruses through mutation and genetic recombination [6–8], their easy adaptation to the local environmental conditions and the breakdown of plant genetic resistance [9]. Therefore, it is very important to know the genetic diversity and dispersion modes of these pathogens, especially in a perspective ecologically sustainable environment [10].
Grapevine leafroll disease (GLD) is the most widespread and economically important grapevine virus disease worldwide, and it is associated with a complex of viruses known as grapevine leafroll-associated viruses (GLRaVs), which belong to the Closteroviridae family (genera Ampelovirus, Closterovirus and Velarivirus) [11]. Grapevine leafroll-associated virus 3 (GLRaV-3) is the most important species within this virus complex and the most predominant virus pathogen of grapevines throughout the world [12–15]. Other economically important and widespread viruses in the Mediterranean region include grapevine leafroll-associated viruses 1 and 2 (GLRaV-1, -2) (other viruses of the GLD complex), grapevine fleck virus (GFkV), grapevine virus A, and grapevine fanleaf virus (GFLV) [16–20].
GFkV belongs to the Tymoviridae family (genus Maculavirus), and it is associated with grapevine fleck disease. Vitis vinifera cultivars infected by GFkV are symptomless, but the virus causes leaf deformation, vein clearing and a vegetative growth reduction in the sensitive indicator Vitis rupestris cv. ’St. George’ [5,19].
GFLV, belonging to the Secoviridae family (genus Nepovirus), is the most widespread and the main causative agent of grapevine fanleaf degeneration disease (GFDD). Another nepovirus associated with GFDD, although to a lesser extent, is Arabis mosaic virus (ArMV) [21]. The main symptoms of the disease are distorted leaves, yellow mosaic stripes, vein clearing and banding, zigzag growth, double nodes, and short/deformed internodes; the disease might also cause the decline and destruction of vinestocks [22,23].
Grapevines may also be infected with viruses without displaying any noticeable symptoms (latent infection) [20,24,25]. Therefore, it is recommended to take samples from both symptomatic and asymptomatic plants to perform virus testing [24]. The correlation of virus infections and symptom expression might be related to various factors, such as environmental conditions, virus strain, or multiple pathogen infections [26].
Grapevine viruses can be transmitted through grafting and by vectors. The current lack of virus-free propagation material of autochthonous cultivars in BiH may enhance the relevance of graft transmission in the spread of grapevine viruses [20]. Many authors have reported mealybugs and soft scale insects (order Hemiptera), as being the vectors of GLRaV-3 and GLRaV-1 [14,27–30]. Dimitrijevic [31] reported the spread of leafroll in vineyards throughout the former Yugoslavia and associated it with the presence of scale insect populations. GFLV can be transmitted by the ectoparasitic nematode Xiphinema index [17,32,33], whose major host is a domesticated grapevine (V. vinifera subsp. vinifera) [34]. ArMV is transmitted by another ectoparasitic soilborne nematode, Xiphinema diversicaudatum [35]. The primary spread of GFkV is known to occur through infected propagation material and no vector has yet been reported for this virus [36].
Upon virus infection, grapevine plants remain infected throughout their life span, and prevention of infection includes the production of certified virus-free and virus-tested plant material. Most vineyards in BiH have been established with Conformitas Agraria Comunitatis (CAC) category of propagation material for autochthonous cultivars. To improve the sanitary status of such grapevines, a decade ago the Federal Agro-Mediterranean Institute of Mostar started a mass selection of the most important cultivars. The selection process involved screening for the presence of five major grapevine viruses, that is GLRaV-1, GLRaV-3, GFkV, GFLV and ArMV, which pursuant to the legislation in the country [37] are to be subjected to mandatory testing for the propagation material. Therefore, the main objective of this study was to monitor for the presence of these five viruses, GLRaV-1, GLRaV-3, GFkV, GFLV and ArMV, in symptomatic and asymptomatic samples derived from BiH commercial vineyards enrolled in local nursery production and used for the propagation of grapevine material. Two other objectives were to perform a molecular characterization of the detected viruses and to investigate the presence of grapevine virus vectors.
Materials and methods
Field survey and sample collection
In order to investigate the sanitary status related to virus infections of the local grapevine cultivars, 278 samples of the cv. Žilavka and 352 samples of the cv. Blatina were collected during late summer in 2019. Field surveys were carried out in 8 commercial vineyards: Višići [collection vineyard, Čapljina (ČA)], Blizanci [Hercegovina vino doo., Čitluk (ČI)], Lopate [Hercegovina vino doo., Kosor, Mostar (MO)], Plantaže-Otok [Erovin doo., Ljubuški (LJB)], Dugolaza-Ražovina (vinarija Nuići doo., Crnopod, LJB), Sovići [private vineyard, Grude (GR)], Buna Stup (private vineyard, MO) and Poprati [private vineyard, Stolac (ST)]. These vineyards are involved in the preparation of local nursery material and in the production of fresh grapes and wine. The field study (number 19_SG) was carried out under permission of the Federal Agromediterranean Institute of Mostar, Mostar, Bosnia and Herzegovina.
A total of 630 samples were collected from 13 to 20-year old Žilavka and Blatina plants grafted onto the Kober 5BB rootstock (Vitis berlandieri x Vitis riparia). A dormant cutting sample of each plant was collected from the basal part of the scion. Each sample was divided into two subsamples (three replicates each) to carry out serological and molecular tests. Both symptomatic and asymptomatic plants were sampled randomly and geo-referenced by means of GPS using the Planthology mobile application [7]. Simple random sampling method based on data of vineyard planting area with 99% confidence interval [38,39] was used to select the sampling points in vineyards, covering 36 vineyards blocks in eight orchards. The field survey also involved a symptomatology assessment and the search for the presence of virus vectors in the vineyards.
Serological test
All the samples were analysed by means of double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) [40] using specific antibodies to GLRaV-1, GLRaV-3, GFLV, GFkV and ArMV (Bioreba AG, Reinach BL 1, Switzerland). Three-hundred mg of cortical scrapings of each sample was mixed and homogenised with 0.5 ml extraction buffer (0.5 M Tris-HCl, 2% PVP-24, 1% PEG 6000, 0.14 M NaCl, 0.05% Tween 20, pH 8.2) and a 1:10 dilution (w/v) of each sample was used for DAS-ELISA, following the manufacturer’s instructions. Lyophilized plant tissue infected by GLRaV-1, GLRaV-3, GFLV, GFkV and ArMV (Bioreba AG), and healthy plant tissue (Bioreba AG) were used as positive and negative controls, respectively. Optical densities (O.D.) were measured at 405 nm, using a Sunrise microplate reader (Tecan, Männedorf, Switzerland) 2 h after the addition of the p-nitro-phenylphosphate substrate. The sample was considered positive if its OD405 value was at least twice the negative control value.
Soil analyses for the presence of nematodes
Soil samples from vineyards in which the nematode transmitted viruses (GFLV and ArMV) were detected by DAS-ELISA were collected and analysed for the presence of X. index and X. diversicaudatum, respectively. Soil samples (ca. 250 g) were investigated for the presence of nematodes using the sieving method, as described by Flegg [41].
RNA extraction
Eighty-one randomly selected samples of Žilavka and Blatina cvs. reacting positively in DAS-ELISA were also tested by means of a multiplex RT-PCR assay. The samples were ground into powder with a mortar and pestle in liquid nitrogen and stored at -20°C for RNA isolation. Lyophilized plant tissue infected by GLRaV-1, GLRaV-3, GFLV, GFkV and ArMV as positive control (Bioreba AG), as well as negative controls (healthy plant tissue; Bioreba AG) were subjected to RNA extraction. Total RNA was extracted using an RNeasy Plant Mini kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions, and eluted in 50 μl RNase-free water. The obtained total RNA was re-suspended in 50 μl of RNase-free water, and the RNA concentration was measured twice with a UV-Vis Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, USA), adjusted to approximately 50 ng/μl and stored at -80°C until use.
Multiplex RT-PCR
One-step multiplex RT-PCR was carried out as described by Gambino and Gribaudo [42]. The assay was performed with a OneStep RT-PCR Kit (Qiagen) in a 25 μl mixture containing 100 ng of total RNA treated with the Ambion™ DNase I (Thermo Fisher Scientific), according to the manufacturer’s instructions, 1× RT-PCR buffer (Qiagen), 0.4 mM dNTPs, 0.5 μl of each primer, and 2 μl of a OneStep RT-PCR enzyme mix (Qiagen). Primers were used at final concentrations of 1.2 μM for GLRaV-3, 0.3 μM for GLRaV-1, 0.2 μM for GFkV, 1.6 μM for GFLV, and 2 μM for ArMV. The cycling conditions were as follows: reverse transcription at 47°C for 50 min, initial denaturation at 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 50°C for 1 min and 72°C for 90 s, and a final extension at 72°C for 5 min. The PCR products were electrophoresed on 1.5% agarose gel, pre-stained with GelRed® Nucleic Acid Gel Stain (1×, Biotium, Hayward, USA), and visualised by means of UV light. In order to ensure the absence of any nonspecific amplification, each RT-PCR run included molecular grade water and a healthy plant sample as negative controls. After the identification of single/mixed infections, RT-PCR assays specific for each of the detected viruses were also carried out on randomly selected samples infected by GLRaV-3 (35), GLRaV-1 (4), GFLV (1), and ArMV (1). In addition, the full-length coat protein (CP) gene of GLRaV-3 and portion of gene sequences of the other three viruses, i.e. partial CP genes for GLRaV-1 and GFLV and the polyprotein gene for ArMV were amplified by RT-PCR, using the conditions and primers listed in S1 Table. Four of the GFkV isolates already had sequences available in the replicase gene [43], which were used for the subsequent phylogenetic analyses.
Sequence analyses
Amplified PCR products of GLRaV-3, GLRaV-1, GFLV and ArMV isolates were purified using a QIAquick PCR purification kit (Qiagen), following the manufacturer’s instructions. The purified PCR products were sequenced in both directions at BMR Genomics (Padua, Italy). The full-length CP sequences of 35 GLRaV-3, partial CP gene sequences of four GLRaV-1 and one GFLV isolate, and partial polyprotein gene sequence of one ArMV isolate were deposited in GenBank under Accession Numbers: MT432352-MT432386, MK526895-MK526898, MW147746, and MW413756, respectively (S1 File). Comparison with sequences available in the GenBank database was performed using the BLAST algorithm (www.ncbi.nlm.nih.gov).
The CLUSTALW program [44] was used to construct a multiple nucleotide (nt) sequence alignment. Phylogenetic relationships were inferred by means of the maximum likelihood estimation method based on the Kimura 2-parameter with 1,000 bootstrap replicates [45,46], and all the analyses were performed using the MEGA X program [47].
The role of natural selection in the evolution of local GLRaV-3 isolates was studied at the molecular level by evaluating the rate of synonymous substitutions per synonymous site (dS) and the rate of nonsynonymous substitutions per nonsynonymous site (dN). These and other genetic diversity parameters were determined by means of DNA Sequence Polymorphism v. 6 software [48]. These values were not calculated for GLRaV-1, GFLV and GFkV due to the limited number of sequenced isolates.
The alignments of the all the sequenced isolates were analyzed, together with the available reference isolates using the RDP4 (v.4.39) software package to detect any potential recombination events [49]. RDP4 parameters were set as default values.
Results
Field survey
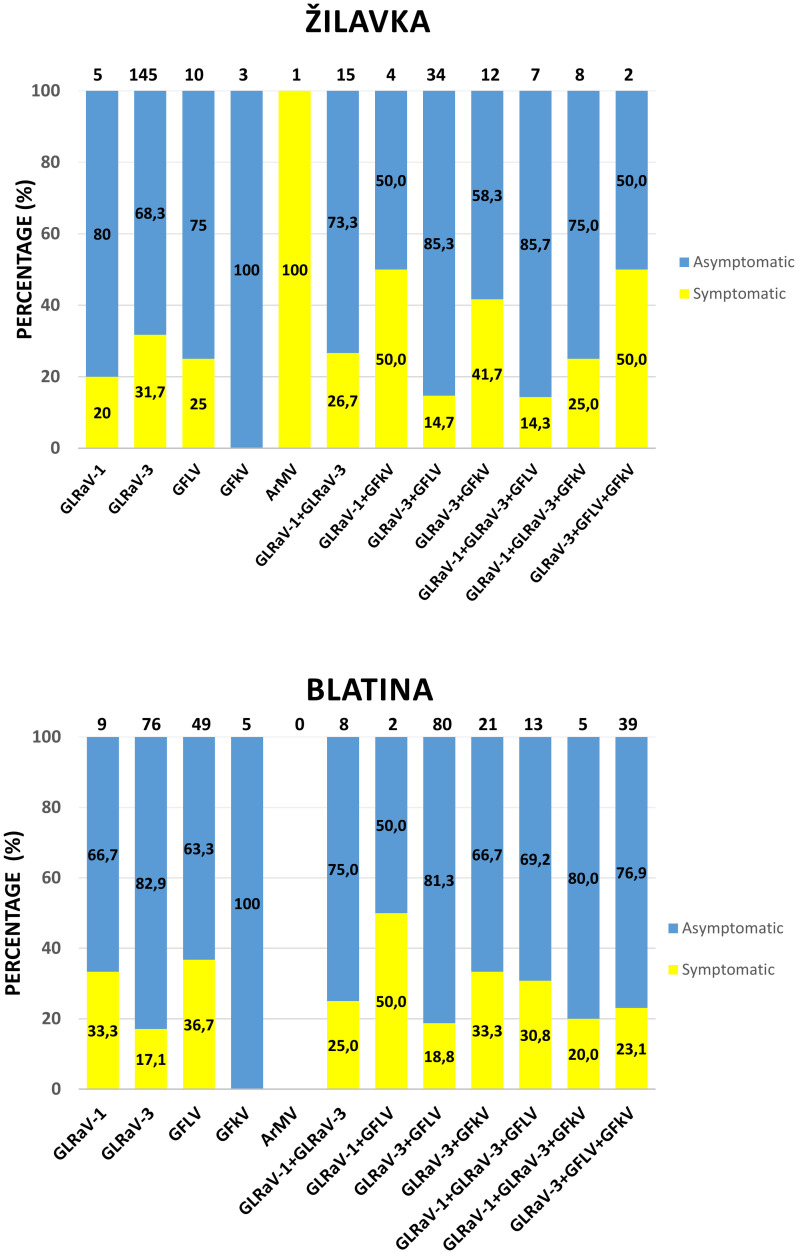
The main symptoms observed during the field survey consisted in downward rolling of leaves followed by reddening (cv. Blatina) or yellowing (cv. Žilavka) of the leaf tissue between the major veins (Fig 1). Leaf-rolling and leaf colour change were observed in all the plants with single (GLRaV-1 or GLRaV-3), double and triple infections (with presence of one or both, GLRaV-1 or GLRaV-3), subsequently detected by DAS-ELISA. Delayed vegetation as well as distorted and asymmetrical leaves were observed in plants with single or mixed infections involving GFLV. Zigzag growth was observed in cv. Blatina, while shortening of the internodes was observed in both cultivars infected by single and mixed GFLV infections. The symptoms detected in the unique plant infected by ArMV consisted in leaf yellowing, while no symptoms were visible in plants with single GFkV infection. Uneven berry ripening was observed in plants infected only by GLRaV-3, whereas delayed ripening was found in the mixed GLRaV-3 + GFLV and GLRaV-1 + GLRaV-3 + GFLV infections. Reduced vigour of the plants and smaller clusters with an increased number of berries were observed in the mixed GFkV + GLRaV-3 and GFkV + GLRaV-3 + GFLV infections (Fig 1). Notably, most (75%) of the surveyed plants were asymptomatic since the vineyards surveyed are used for local nursery production.
Fig 1. Symptomatology observed on infected grapevine plants surveyed in this work.
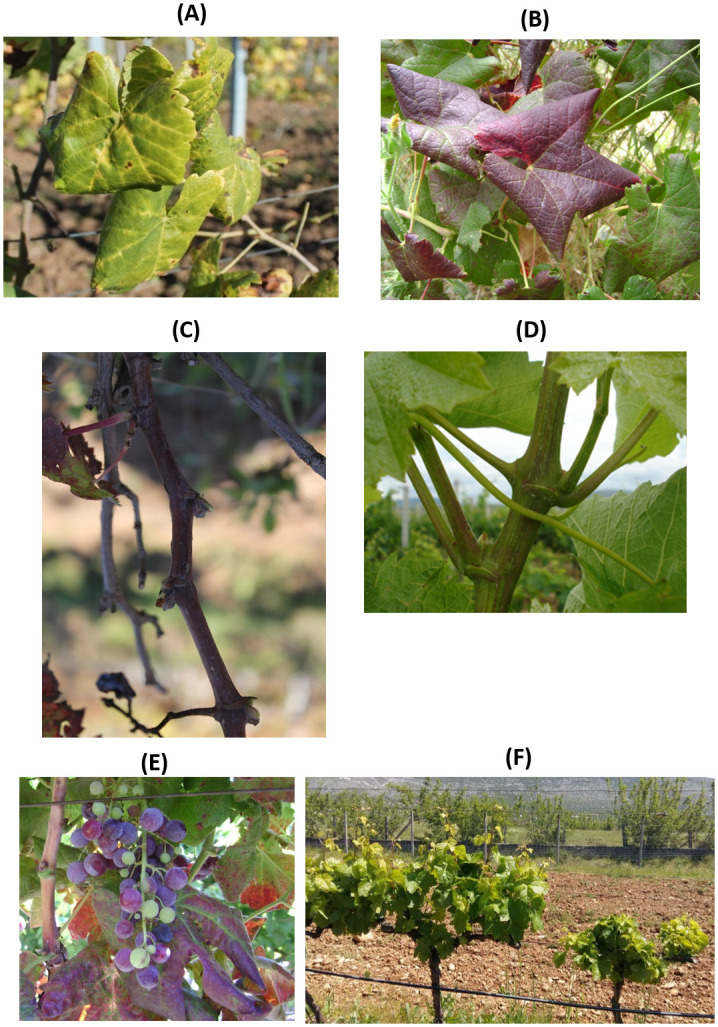
(A) Leafroll and yellowing (cv. Žilavka) associated with grapevine leafroll-associated virus 3 (GLRaV-3). (B) Leafroll and reddening (cv. Blatina) associated with GLRaV-3. (C) Zig-zag growth associated with grapevine fanleaf virus (GFLV). (D) Short internodes associated with GFLV and GLRaV-3. (E) Uneven berry ripening associated with GLRaV-3. (F) Reduced vigour of the plant associated with grapevine fleck virus (GFkV) and GLRaV-3.
The vineyards were also inspected for the presence of mealybugs and soft scale insects, the known vectors of GLRaVs [50,51]. Although the vineyards had been treated with insecticides against these pests, a few mealybugs and soft scale insects were observed. The following species were identified by morphological and morphometric methods [52–54]: Planococcus ficus (Signoret), the Mediterranean vine mealybug (Pseudococcidae family), and the scale insects Parthenolecanium corni (Bouché; the European fruit lecanium), Parthenolecanium persicae (Fabricius; the European peach scale), Pulvinaria vitis (Linnaeus; the cottony grape scale), and Neopulvinaria innumerabilis (Rathvon; the cottony maple scale), all of which have already been reported in other countries as vectors of GRLaV-1 and -3 [28,55–57]. Among the identified species, the most dominant ones were P. ficus and P. corni (Fig 2).
Fig 2. Mealybugs and scale insects observed during the field survey on grapevine leafroll-associated virus (GLRaV)-infected grapevine plants.
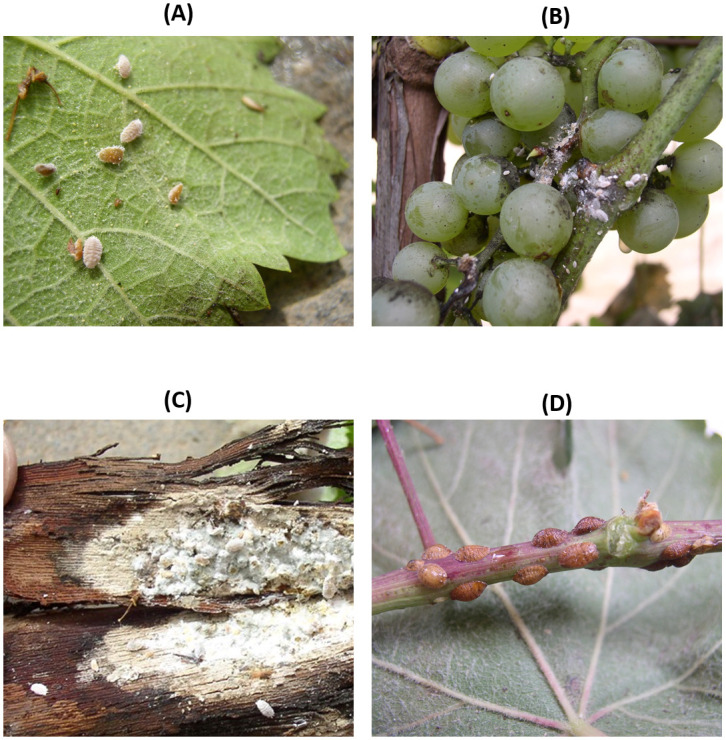
(A-B) Nymphs and adults of Planococcus ficus. (C) Nymphs and adults of Parthenolecanium corni. (D) Adults of P. corni.
Serological test
A total of 278 samples of Žilavka and 352 of Blatina from 8 commercial vineyards were investigated for the presence of five major grapevine viruses. Five hundred and fifty-three (88%) out of the 630 samples, gave positive result for at least one virus (Table 1). Specifically, the detected viruses were: GLRaV-3 (84%), GFLV (43%), GLRaV-1 (14%), GFkV (10%) and ArMV (0.2%) (Table 2). In both cultivars Žilavka and Blatina, GLRaV-3 was the most represented virus, in both single and mixed infections. Mixed infections contributed by 45% (250 samples) of the total infections, with a higher presence in Blatina (55%, 168 samples) than in Žilavka (33%, 82 samples). Double infections of GLRaV-1 + GLRaV-3, GLRaV-3 + GFLV and GLRaV-3 + GFkV, and triple infections of GLRaV-1 + GLRaV-3 + GFLV, GLRaV-1 + GLRaV-3 + GFkV and GLRaV-3 + GFkV + GFLV were identified in both cultivars (S2 and S3 Tables). The most frequent mixed infection was the double infection of GLRaV-3 + GFLV present in 14% of cv. Žilavka samples (S2 Table) and 26% of cv. Blatina (S3 Table). Among the triple infections, the most prevalent were GLRaV-1 + GLRaV-3 + GFkV in cv. Žilavka (3%; S2 Table) and GLRaV-3 + GFLV + GFkV in cv. Blatina (13%; S3 Table).
Table 1. Grapevine virus infection rate detected by double antibody sandwich ELISA (DAS-ELISA) in Žilavka and Blatina cultivars.
| Cultivar | No. of samples | Infection rate (%) | |
|---|---|---|---|
| Tested | Infected | ||
| Žilavka | 278 | 246 | 88.49 |
| Blatina | 352 | 307 | 87.21 |
| Total | 630 | 553 | 87.77 |
Table 2. Relative incidence of grapevine viruses.
| Cultivars | Number of infected samples | GLRaV-3 | GFLV | GLRaV-1 | GFkV | ArMV |
|---|---|---|---|---|---|---|
| Žilavka | 246 | 223 (90.65)* | 53 (21.54) | 39 (15.85) | 29 (11.79) | 1 |
| Blatina | 307 | 242 (78.83) | 183 (59.61) | 37 (12.05) | 25 (22.80) | 0 |
| Total | 553 | 465 | 236 | 76 | 54 | 1 |
| Percentage (%) | 84.09 | 42.67 | 13.74 | 9.77 | 0.18 |
* number in brackets refers to the percentage.
Out of the infected plants, the majority were asymptomatic (72% in cv. Žilavka, and 76% in cv. Blatina). Furthermore, no important differences in virus incidence were observed between samples collected from symptomatic and asymptomatic grapevines (S2 and S3 Tables; Fig 3), i.e. all virus combinations (single and multiple) were detected in both symptomatic and asymptomatic plants, except for samples where only ArMV (symptomatic) or only GFkV (asymptomatic) were present (Fig 3).
Fig 3. Comparative assessment of virus profiles of symptomatic versus asymptomatic infected plants in cvs. Žilavka and Blatina.
Relative incidence of single and mixed viral infections is based on the double antibody sandwich ELISA (DAS-ELISA) results. Numbers on top of the columns indicate the numbers of infected samples.
Soil analyses for the presence of nematodes
The presence of X. index and X. diversicaudatum, the nematode vectors of GFLV and ArMV, respectively [17,32,33,35], was not observed after using the sieving method in the soil samples collected from nepovirus-affected vineyards.
Multiplex RT-PCR and sequence analyses of GLRaV-3 isolates
Based on DAS-ELISA results, 81 positive samples of cvs. Žilavka and Blatina were subjected to a multiplex RT-PCR. This technique confirmed the results of serological assays obtained for both single and mixed infections, without any additionally detected positive infections (S4 Table; S1 Fig).
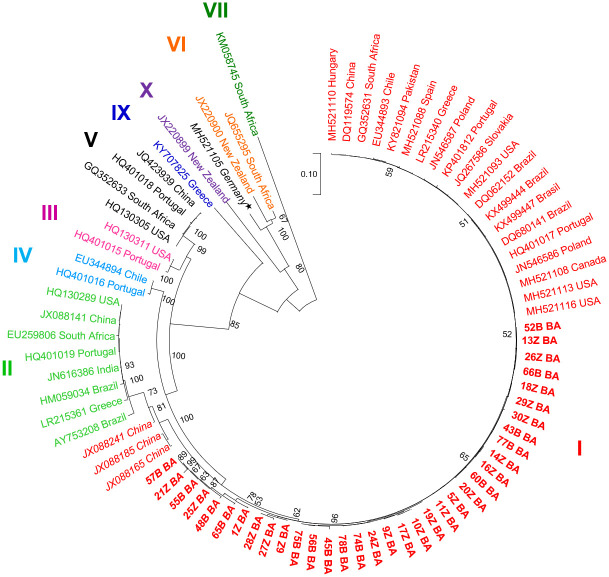
The sequence comparisons and phylogenetic analyses of 35 randomly selected GLRaV-3 samples from the country confirmed that all the sequenced isolates belonged to this most prevalent virus (S1 File). The maximum likelihood phylogenetic tree including 35 GLRaV-3 BiH isolates and 45 reference grapevine isolates from 16 countries showed that they all clustered into 9 distinct phylogenetic groups (groups I-VII, IX and X; Fig 4). The 35 BiH isolates clustered into the major phylogroup (group I) that also included 20 reference isolates. These isolates originated from 14 countries from the Mediterranean Basin (Bosnia and Herzegovina, Spain, Portugal and Greece), Central-Northern Europe (Hungary, Slovakia and Poland), Central-Eastern Asia (China and Pakistan), North America (the USA, Canada), South America (Brazil and Chile) and South Africa. A minor subgroup within the group I contained the BiH isolates, 55B BA, 57B BA, 21Z BA, 25Z BA, 48B BA, 65B BA and 1Z BA (four of which were recombinants), and it was grouped next to the subgroup containing three Chinese divergent isolates (JX088241, JX088165 and JX088185) [58]. The other phylogroups (II-VII, IX and X) included the remaining reference isolates, from 9 countries throughout the world.
Fig 4. Phylogenetic relatedness of grapevine leafroll-associated virus 3 isolates obtained from full-length coat protein sequences, as inferred from a maximum likelihood analysis.
Thirty-five BiH isolates (in bold) and forty-five reference isolates were included in the analysis. The accession number and the origin of each isolate are indicated. The bootstrap consensus tree was inferred from 1000 replicates and the bootstrap values of less than 50% are not presented. Phylogenetic groups are assigned as reported by Maree et al. [59] (groups I-VII) and Diaz-Lara et al. [60] (groups IX and X). Group VIII [59] was not included in phylogenetic analysis because it contained the sequences (JF723301-JF723378) retrieved from the NCBI. The isolate MH521105 (marked with a star) is not assigned to any of phylogenetic group [60], while the divergent isolates from this study and Farooq et al. [58] study (within the group I) are in italics.
The pairwise percent identity of the BiH CP sequences ranged from 96.3–100% (nt) to 97.5–100% (amino acid, aa) (S2 Fig). When the CP sequences included both the BiH and reference isolates, the range was much lower; 75.2–100% (nt) and 87.58–100% (aa) (S3 Fig). The average genetic diversity of BiH GLRaV-3 was 0.010 for the full-length CP gene. The synonymous substitutions (dS) per site (0.3144) were higher than the nonsynonymous substitutions (dNS) (0.0386) and resulted in a dNS/dS ratio of 0.1230 (Table 3). These values indicate the hypothesis of negative or purifying selection. The overall nucleotide diversity (π) value for all the GLRaV-3 isolates was 0.0547, while a value of 0.0096 was found for the isolates from BiH. Tajima’s D, Fu and Li’s D, and Fu and Li’s F tests were negative for CP, thus confirming the occurrence of selective processes or population expansion.
Table 3. Genetic diversity parameters of the full-length coat protein sequences of GLRaV-3 isolates from Bosnia and Herzegovina.
| Isolates | n | d | dS | dNS | dNS/dS | S | π | θ (S) | θ (π) | Tajima’s D | Fu and Li’s D | Fu and Li’s F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 106 | 0.062 | 5.0086 | 0.3448 | 0.0688 | 422 | 0.0547 | 0.1135 | 0.0590 | -1.8660 | -2.3150 | -2. 3870 |
| BiH | 35 | 0.010 | 0.3144 | 0.0386 | 0.1230 | 46 | 0.0096 | 0.0122 | 0.0097 | -0.8584 | -1.0716 | -1.1000 |
| Reference | 71 | 0.057 | 4.6626 | 0.3123 | 0.0669 | 419 | 0.0718 | 0.1224 | 0.0794 | - 1.5803 | - 1.7228 | -1.8135 |
n = number of isolates; d = diversity; dS = synonymous substitutions; dNS = non-synonymous substitutions; S = number of polymorphic sites; π = nucleotide diversity; BiH = Bosnia and Herzegovina.
The full-length BiH GLRaV-3 CP gene sequences were also investigated for the presence of recombination signals using RDP4 software. One putative recombination event involving the isolate 27Z BA was detected with break points at 180 and 239 nt. The isolate 65B BA shared the same recombination event (break points 180–318 nt), while three isolates (1Z BA, 48B BA, 55B BA) also showed traces of the same recombination event (break points 180–330 nt). The isolate 27Z BA had isolates IS2 (Brazil, HM059034) and 45B BA (BiH) as the potential major and minor parents, while the remaining recombinant isolates had isolates 45B BA and IS2 as the putative major and minor parents (Table 4).
Table 4. Recombination event in the coat protein coding region of 35 BiH and 71 reference GLRaV-3 isolates.
Phylogenetic analyses of GLRaV-1, GFLV, ArMV and GFkV
GLRaV-1 phylogenetic analyses, based on the partial CP gene sequences of four BiH isolates and 60 reference isolates from 14 countries, grouped the isolates into eight distinct groups. The BiH isolates clustered into three groups (I, II, and IV), together with reference isolates originating from different continents, while the other five groups included the remaining reference isolates (S4 Fig; S1 File).
GFLV phylogenetic analyses of the partial CP gene divided the studied isolates into two groups. The major group contained the isolates from different European countries, including the BiH 5Z BA isolate (S5 Fig; S1 File). ArMV phylogenetic tree generated from the partial polyprotein gene sequences grouped the BiH ArZ BA isolate within the third minor group together with the German isolates (S6 Fig; S1 File).
GFkV phylogenetic analyses, performed on the partial replicase gene sequences of four BiH isolates and 29 available reference isolates from 7 countries, clustered the isolates into two groups. The BiH isolates were within the major group, inside the subgroup with the Hungarian isolates (S7 Fig; S1 File). No recombinant events were detected in any of the BiH isolates of GLRaV-1, GFLV, ArMV and GFkV.
Discussion
This study was conducted in various vineyards across the main grapevine cultivation areas in Bosnia and Herzegovina in order to investigate the sanitary status of two of the most important autochthonous cultivars, Žilavka and Blatina. The presence of grapevine viruses and a deteriorated sanitary status of vineyards in the country have emerged. All five major grapevine viruses (GLRaV-1, GLRaV-3, GFLV, GFkV and ArMV) have been detected, albeit with different incidences.
The dominant symptoms observed during the field survey were downward rolling with reddening (cv. Blatina) or yellowing (cv. Žilavka) of the leaves between the main green veins. These symptoms indicate the presence of grapevine leafroll-associated viruses (GLRaV-1 and -3), and in particular GLRaV-3, which resulted to be the most prevalent virus in both single and mixed infections. The symptoms here observed fit with those described by Delić et al. [61] on the same cultivars affected by GLD from one vineyard in BiH. The observed delayed growth, leaves with distortions and asymmetry, and shortening of the internodes, all of which are associated with GFDD, were similar to those previously described for GFLV involvement in this disease [62]. No GFkV symptoms were observed in plants that were found infected only by this virus, as expected due to the absence of symptoms of this virus on V. vinifera [19], while the ArMV symptoms detected in one grapevine plant in this study were similar to those described for GFDD. Noteworthy, the majority of the infected plants were asymptomatic and no relevant differences in virus incidence between the symptomatic and asymptomatic grapevines was recorded. This result requires further investigations since the absence of the symptoms may be associated with various factors, such as environmental conditions, virus strain, or mixed virus infections [26,63]. Despite the negative influence of GLD on yield and chemical composition of berries in symptomatic plants compared to asymptomatic plants, Alabi et al. [63] reported important variation of these features between these groups of the plants related to seasonal changes. In our preliminary observations, no evident differences in the overall yield could be observed for both cultivars between asymptomatic and symptomatic plants; it is possible that seasonal impact, but also an evolutionary development of these viruses in the Balkan region, or the relative accumulation of these viruses in the plants are involved in symptom induction. Future investigations will be devoted to quantify the virus accumulation and evaluate their localization in symptomatic and asymptomatic plants, together with a systematic analysis of the chemical composition of the berries and the organoleptic features of wines thereof.
The dominant virus found in our study was GLRaV-3, a result that is in agreement with previous studies in BiH on both cultivars [20,61,64,65]. Similar reports have also been published in neighbouring Balkan countries. Vončina and co-workers [66] reported that GLRaV-3 is the most frequent virus in Croatia, after analysing a panel of native cultivars (including cv. Žilavka) and found a similar level of incidence (79%) to that determined in this study, and to that (70%) reported by Kostadinovska and co-workers [67] for North Macedonia. The prevalence of GLRaV-3 among the major grapevine viruses has also been reported in Montenegro and Serbia, albeit with somewhat lower levels (55% and 31%, respectively) [68,69]. Finally, GLRaV-3 has been reported as the most frequent grapevine virus throughout the Mediterranean basin [16].
The high infection rate by GLRaV-3 found in our study could be explained by using infected plant material and the presence of potential GLD vectors in the vineyards, since GLRaV-3 has been reported to spread rapidly from infected to virus-free plants, reaching around 80% of infection in 8 years (10% of infection per year) [27,70]. In this study, the main hemipteran species observed in vineyards infected with GLRaV-1 and GLRaV-3 were P. ficus and P. corni, known vectors of GLRaV-3 and GLRaV-1 [53,54,71]. Recently, mealybugs and soft scale insects have frequently been reported in Herzegovinian vineyards [72,73]. The prevalence of GLRaV-3 and the presence of mealybugs and scales in infected vineyards require further studies in order to investigate the ability of P. ficus and P. corni to transmit GLRaV-1 and GLRaV-3, as already reported [14,27–30].
The relatively high incidence of GFLV observed in this study is possibly related to the use of infected propagation material, since X. index has not been found in our work, nor was it found in a previous extensive Xiphinema sp. study across BiH in grapevine cultivation areas [74]. Furthermore, some vineyards from our study were established in 2010 and showed a much higher infection rate than older ones planted in 1984 (53% vs. 8%), suggesting that infected propagation material was used in these new vineyards. It also seems that cv. Žilavka is more tolerant to GFLV infection (22% infection rate) than cv. Blatina (60%), since both cultivars were planted in vineyards at the same time, and cv. Blatina showed more infection by this virus in all GFLV affected vineyards. Similar findings were reported in a previous study on Herzegowian vineyards [64].
The finding of only a single plant infected with ArMV out of the 630 tested is not surprising, since its vector, X. diversicaudatum, has not been detected neither in this study nor in previous records pertaining to BiH [74]. The low ArMV incidence could be associated with the absence of the vector and the limited presence of the virus in vegetatively propagated grapevine material. The virus is also rarely present or even absent in neighboring countries [68,75,76], but when the vector is present [74], the incidence of ArMV might increase (> 20%) [66].
When GFkV was present in mixed infections with GLRaV-3 and GFLV in cv. Blatina and with GLRaV-3 in cv. Žilavka, a noticable reduction of the vigour of the plants, and subseqeuntly, decreased yield and quality was observed in infected plants. Such symptomatology was not observed either in the single GFkV infection, or in any other mixed infections. The present result deserves particular attention, since we mainly found GFkV in mixed infections (approx. 90% in both cvs.), and this virus (although symptompless in V. vinifera) influenced negatively the growth, yield and juice quality in V. vinifera cvs. Albana and Trebbiano Romagnolo when combined with other virus diseases such as vein necrosis and vein mosiac [77].
Phylogenetic analyses of the GLRaV-3 CP gene portion, including 35 BiH and forty-five reference GenBank isolates, clustered the isolates into nine already established groups [14,59,60]. The major group I included all the isolates from Bosnia and Herzegovina together with the reference isolates, while the remaining reference isolates were assigned to groups I-VII, IX and X. Group I was exclusive in BiH, as it is predominant in the rest of the world [78,79]. A minor subgroup within the group I contained the BiH isolates (four of which were recombinants), and it was clustered next to the another divergent subgroup with three Chinese isolates (JX088241, JX088165 and JX088185) [58], confirming the high genetic diversity of the virus isolates within this group. The explanation for the prevalence of GLRaV-3 I variants over the other groups is possibly related to a higher fitness of these variants or their more efficient transmission by vectors relative to the other variants [79]. Like the previous studies [25,78,80], we were unable to find any association of the phylogenetic group with the geographic origin or the grapevine cultivar. Although the genetic diversity of the GLRaV-3 BiH isolates of the CP region (0.010) was lower than that found in other similar studies (0.058 in Spain [78]; 0.063 in Portugal [79]), it is noteworthy that we considered only two cultivars, while previous reports were conducted on a higher number of cultivars. Nonetheless, the genetic diversity values obtained in this study, which suggest a negative or purifying selection, indicate that GLRaV-3 isolates have been present in the country since a long period of time, as reported in other countries with long-lasting grapevine tradition of cultivation.
The recombination of RNA viruses, through an exchange of genetic segments, has been associated with the selection of other genomic features that control gene expression, and with the alteration of vector transmission specificity [81]. In our study, we have found recombinant isolates in GLRaV-3, but not for the other studied grapevine viruses. The start and the end recombination break points found in the BiH isolates were between 180 and 330 nt, which are within the recombination junctions (142–531 nt) already reported for all the other recombinants in the CP gene of GLRaV-3 [58,82]. Although the recombinants here detected do not have the same start and end break points with the isolates reported by Turturo et al. [82] and Farooq et al. [58], it is interesting to note that in all three studies the recombination occurred between the isolates of groups I and II (apart from some recombinants between the groups I and III) [58]. Moreover, the recombination occurred at the 5’ portion of the CP gene, which presents the most variable gene part [24]. The occurrence of recombination in the most diverse part of CP could be related to a change in interaction between the viral CP and vector cellular receptors (preferences in the transmission pathways) or to a change in the level of expression of the CP and successive cascade reactions (selection of genomic features which controls a gene expression), both of which require further investigations [79,81].
Considering GLRaV-1, the phylogenetic analyses assigned four of the studied isolates into three groups. This may indicate a lack of geographical distribution, as already reported [25,83–85] and the impact of using infected propagation material in the spread of this virus within the country. Although one GFLV and ArMv isolate from BiH were characterized, the phylogenetic analyses clustered each of them within the phylogenetic groups containing the isolates from different countries, i.e. from various European countries for GFLV and from Germany for ArMV. Since no Xiphinema spp., the vectors of both viruses, have been detected in this and in previous studies in BiH [74], the possible route for their introduction in the country might again be the use of infected propagation material. As far as GFkV is concerned, all the BiH isolates grouped together in the same subgroup with a few virus isolates from Hungary, which could be related to an exchange of infected propagation material, since the vector for this virus is not known [36,86]. Based on these findings, if we exclude in the future the use of infected propagation material, further spread of GLRaV-1 and GLRaV-3 might be facilitated due to the presence of mixed infections and of the mealybugs and scale vectors that could act as potential vectors, while the risk of GFLV, ArMV and GFkV spread would be less envisaged, as no vectors are present in this area.
Overall, this is the first study on the genetic variability of GLRaV-3, the predominant virus in BiH. In spite of the limited number of virus isolates considered, our results show a variability of GLRaV-3, and the local virus isolates clustered together with isolates of different geographic origin. A higher number of clones for each isolate would be useful to investigate the presence of different variants within the same isolate, as already reported in some studies on GLRaV-1, GLRaV-3 and GFLV [22,82,85].
The phytopathological legislation in Bosnia and Herzegovina prescribes testing of grapevine planting material (both scions and rootstocks) for the presence of five viruses (GLRaV-1, GLRaV-3, GFLV, GFkV and ArMV), but their complete absence is not yet mandatory. This has led to a deterioration of the current grapevine sanitary status of autochthonous cultivars in the country, since a high infection rate of GLRaV-3 and GFLV, the difficulty of finding the virus-free mother plants, and the absence of certified planting material probably contribute to further spread the viruses, in particular to new vineyards. In order to prevent this from happening, it is of crucial importance to start producing ‘virus-tested’ and ‘virus-free’ propagation material at the national level, as well as introducing regular monitoring and adequate controls of mealybugs and scale insects and to avoid establishing new vineyards in close proximity to highly infected vineyards. Furthermore, the introduction of next-generation sequencing techniques would be highly recommended for the identification of all the foreign RNA and DNA sequences present in grapevine samples, enabling the detection of new viruses, of agronomically important viruses, and of latent viruses present at low titers. Full virome analyses might also contribute to modify the BiH phytopathological legislation and introduce new candidate target viruses in future surveys of grapevine propagation material [87,88]. All these measures will help to preserve the six century-old autochthonous cultivars used mainly for the production of ‘high-quality’ wines in this country.
In conclusion, our study has revealed a deteriorated sanitary status of the local grapevine industry in Bosnia and Herzegovina. The high genetic diversity among the BiH isolates and the presence of a mixed virus infection highlight the need for both stricter controls on the import of infected grapevine plants, in particular from neighboring countries, and further investigations on the sanitary status of grapevine plants in the presently unmonitored regions of Bosnia and Herzegovina.
Supporting information
Lanes 1, 2, 3, samples infected by GLRaV-3 and GFLV; lane 4, positive control for GLRaV-3 and GFLV; lane 5, sample infected by GFkV; lane 6, sample infected by GLRaV-1 and GFLV; lane 7, positive control for GLRaV-1; lanes 8, 9, 12, samples infected by GLRaV-3, GLRaV-1 and GFLV; lanes 10, 11, samples infected by GLRaV-3 and GLRaV-1; lane 13, positive control for GLRaV-3 and GLRaV-1; lane 14, sample infected by GLRaV-3; lane 15, positive control for GLRaV-1 and GFkV; lanes 16, 18, samples infected by GLRaV-1 and GFkV; lane 17, sample infected by GLRaV-1; lane 19, sample infected by GLRaV-3, GLRaV-1 and GFkV; lanes 20, 22, 23, samples infected by GLRaV-3; lane 24, positive control for GLRaV-3; lane 21, sample infected by ArMV; W, water control; H, healthy grapevine sample; L, 100 bp DNA ladder.
(TIF)
Each colored key represents a percentage to the identity score between two sequences.
(TIF)
Each colored key represents a percentage to the identity score between two sequences.
(TIF)
Four BiH isolates (in bold) and sixty reference isolates were included in the analysis. The accession number and the origin of each isolate are indicated. The bootstrap consensus tree was inferred from 1000 replicates.
(TIF)
One BiH isolate (in bold) and fifty-five reference isolates were included in the analysis. The accession number and the origin of each isolate are indicated. The bootstrap consensus tree was inferred from 1000 replicates.
(TIF)
One BiH isolate (in bold) and seventy-two reference isolates were included in the analysis. The accession number and the origin of each isolate are indicated. The bootstrap consensus tree was inferred from 1000 replicates.
(TIF)
Four BiH isolates (in bold) and twenty-nine reference isolates were included in the analysis. The accession number and the origin of each isolate are indicated. The bootstrap consensus tree was inferred from 1000 replicates.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to remember the co-author Dr. Mladen Gašpar (Federal Agromediterranean Institute of Mostar, Mostar, Bosnia and Herzegovina) passed away on December 27 2020 for complications due SARS-CoV-2. Dr. Mladen Gašpar passed away before the submission of the final version of this manuscript. Prof. Salvatore Davino and Dr. Slavica Matić accept responsibility for the integrity and validity of the data collected and analyzed. The authors thank Prof. I. Ostojić (Faculty of Agriculture and Food Technology, University of Mostar, Mostar, Bosnia and Herzegovina) for providing of some images, and Marguerite Jones for language revision of the manuscript of this paper.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.FAO—Food and agriculture organization of the United Nations; 2019 [cited 2021 January 3]. http://www.fao.org/faostat/en/#home.
- 2.Tomić L, Štajner N, Jovanović Cvetković T, Cvetković M, Javornik B. Collection and genetic characterization of Vitis vinifera ‘ Žilavka’ by microsatellites and AFLP markers. Acta Agric Slov. 2012; 99: 143–150. [Google Scholar]
- 3.Beljo J, Mandić A, Jovanović-Cvetković T, Dodig R, Gašpar M, Ivanković M, et al. Atlas vinogradarstva i vinarstva Bosne i Hercegovine. Mostar: Sveučilište u Mostaru; 2014. [Google Scholar]
- 4.Fuchs M. Grapevine viruses: a multitude of diverse species with simple but overall poorly adopted management solutions in the vineyard. J Plant Pathol. 2020; 102: 643–653. [Google Scholar]
- 5.Martelli GP. Directory of virus and virus-like diseases of the grapevine and their agents. J Plant Pathol. 2014; 96(1S): 1–136. [Google Scholar]
- 6.Matic S, Al Rwahnih M, Myrta A. Diversity of Plum Pox Virus isolates in Bosnia and Herzegovina. Plant Pathol. 2006; 55: 11–17. [Google Scholar]
- 7.Davino S, Panno S, Arrigo M, La Rocca M, Caruso AG, Lo Bosco G. Planthology: an Application System for Plant Diseases Management. Planthology: an application system for plant diseases management. Chem Eng Trans. 2017; 58: 619–624. [Google Scholar]
- 8.Panno S, Caruso AG, Davino S. The nucleotide sequence of a recombinant tomato yellow leaf curl virus strain frequently detected in sicily isolated from tomato plants carrying the Ty-1 resistance gene. Arch Virol. 2018; 163(3): 795–797. 10.1007/s00705-017-3674-9 [DOI] [PubMed] [Google Scholar]
- 9.Elena SF, Fraile A, García-Arenal F. Evolution and emergence of plant viruses. Advances in Virus Res. 2014; 88: 161–191. 10.1016/B978-0-12-800098-4.00003-9 [DOI] [PubMed] [Google Scholar]
- 10.Panno S, Caruso AG, Troiano E, Luigi M, Manglli A, Vatrano T, et al. Emergence of tomato leaf curl New Delhi virus in Italy: estimation of incidence and genetic diversity. Plant Pathol. 2019; 68(3): 601–608. [Google Scholar]
- 11.Naidu R, Rowhani A, Fuchs M, Golino D, Martelli GP. Grapevine Leafroll: A Complex Viral Disease Affecting a High-Value Fruit Crop. Plant Dis. 2014; 98: 1172–1185. 10.1094/PDIS-08-13-0880-FE [DOI] [PubMed] [Google Scholar]
- 12.Martin RR, Eastwell KC, Wagner A, Lamprecht S, Tzanetakis IE. Survey for Viruses of Grapevine in Oregon and Washington. Plant Dis. 2005; 89: 763–766. 10.1094/PD-89-0763 [DOI] [PubMed] [Google Scholar]
- 13.Cabaleiro C, Segura A. Temporal Analysis of Grapevine leafroll associated virus 3 Epidemics. Eur J Plant Pathol. 2006; 114: 441–446. [Google Scholar]
- 14.Maree HJ, Almeida RPP, Bester R, Chooi KM, Cohen D, Dolja VV, et al. Grapevine leafroll-associated virus 3. Front Microbiol. 2013; 4: 82 10.3389/fmicb.2013.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger JT, Maree HJ, Gouveia P, Naidu RA. Grapevine leafroll-associated virus 3 In: Meng B, Martelli GP, Golino DA, Fuchs M, editors. Molecular Biology, Diagnostics and Management. Cham: Springer International Publishing; 2017. pp. 167–195. [Google Scholar]
- 16.Digiaro M, Martelli GP, Savino V. Phloem-limited viruses of the grapevine in the Mediterranean and Near East: a synopsis In: Martelli GP, Digiaro M, editors. Proceedings of the Mediterranean network on grapevine closteroviruses 1992–1997 and the viroses and virus-like diseases of the grapevine a bibliographic report, 1985–1997. Bari: CIHEAM; 1999. pp. 83–92. [Google Scholar]
- 17.Andret-Link P, Laporte C, Valat L, Ritzenthaler C, Demangeat G, Vigne E, et al. Grapevine fanleaf virus: still a major threat to the grapevine industry. J Plant Pathol. 2004; 86:183–195. [Google Scholar]
- 18.Delić D, Jovanović-Cvetković T, Đurić G. Prisustvo i rasprostranjenost Grapevine Leafroll-associated Virus—1 i 3 u Bosni i Hercegovini. Pestic Fitomed Beogr. 2007; 22:45–50. [Google Scholar]
- 19.Martelli GP. An Overview on Grapevine Viruses, Viroids, and the Diseases They Cause In: Meng B, Martelli GP, Golino DA, Fuchs M, editors. Molecular Biology, Diagnostics and Management. Cham: Springer International Publishing; 2017. pp. 31–46. [Google Scholar]
- 20.Karačić A. Pojavnost viroza u autohtonim kultivarima vinove loze u hercegovačkom vinogorju. Zagreb: University of Zagreb; 2015. [Google Scholar]
- 21.Oliver JE, Fuchs MF. Fanleaf Degeneration/Decline Disease of Grapevines. Ithaca: Cornell University and the New York State IPM Program; 2011. pp. 1–3. [Google Scholar]
- 22.Mekuria TA, Gutha LR, Martin RR, Naidu RA. Genome diversity and intra- and interspecies recombination events in Grapevine fanleaf virus. Phytopathol. 2009; 99: 1394–1402. 10.1094/PHYTO-99-12-1394 [DOI] [PubMed] [Google Scholar]
- 23.Basso MF, Fajardo TVM, Saldarelli P. Grapevine Virus Diseases: Economic impact and current advances in viral prospection and management. Rev Bras Frutic. 2017; 39(1): e–411. [Google Scholar]
- 24.Moura de CJM, Fajardo TVM, Eiras M, Silva da FN, Nickel O, Moura de CJM, et al. Molecular characterization of GSyV-1 and GLRaV-3 and prevalence of grapevine viruses in a grape-growing area. Sci Agric. 2018; 75: 43–51. [Google Scholar]
- 25.Sabella E, Pierro R, Luvisi A, Panattoni A, D’Onofrio C, Scalabrelli G, et al. Phylogenetic analysis of viruses in Tuscan Vitis vinifera sylvestris (Gmeli) Hegi. PLoS One. 2018; 13: e0200875 10.1371/journal.pone.0200875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olmos A, Bertolini E, Ruiz-García AB, Martínez C, Peiró R, Vidal E. Modeling the Accuracy of Three Detection Methods of Grapevine leafroll-associated virus 3 During the Dormant Period Using a Bayesian Approach. Phytopathol. 2016; 106: 510–518. 10.1094/PHYTO-10-15-0246-R [DOI] [PubMed] [Google Scholar]
- 27.Cabaleiro C, Segura A. Field transmission of grapevine leafroll associated virus 3 by Planococcus citri Risso. Plant Dis. 1997; 81:283–7. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs M, Marsella-Herrick P, Loeb GM, Martinson TE, Hoch HC. Diversity of ampeloviruses in mealybug and soft scale vectors and in grapevine hosts from leafroll-affected vineyards. Phytopathol. 2009; 99:1177–84. 10.1094/PHYTO-99-10-1177 [DOI] [PubMed] [Google Scholar]
- 29.Herrbach E, Le Maguet J, Hommay G. Virus Transmission by Mealybugs and Soft Scales (Hemiptera: Coccoidea) In: Brown JK, editor. Vector-Mediated Transmission of Plant Pathogens. St. Paul: The American Phytopathological Society; 2016. pp. 147–61. [Google Scholar]
- 30.Xiao H, Shabanian M, Moore C, Li C, Meng B. Survey for major viruses in commercial Vitis vinifera wine grapes in Ontario. Virol J. 2018; 15(1): 127 10.1186/s12985-018-1036-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimitrijevic B. Some observations on natural spread of grapevine leafroll disease in Yugoslavia. Riv Patol Veg. 1973; 9: 114–119. [Google Scholar]
- 32.Raski DJ, Goheen AC, Lider LA, Meredith CP. Strategies Against Grapevine Fanleaf Virus and Its Nematode Vector. Plant Dis. 1983; 67: 335. [Google Scholar]
- 33.Leopold S, Borroto-Fernández E, Schartl A, Laimer M. Identification of Xiphinema index in an Austrian vineyard. Vitis. 2007; 46: 49–50. [Google Scholar]
- 34.Van Zyl S, Vivier MA, Walker MA. Xiphinema index and its Relationship to Grapevines: A review. South Afr J Enol Vitic. 2012; 33: 21–32. [Google Scholar]
- 35.Marmonier A, Schellenberger P, Esmenjaud D, Schmitt- Keichinger C, Ritzenthaler C, Andret-Link P, et al. The coat protein determines the specificity of virus transmission by Xiphinema diversicaudatum. J Plant Pathol. 2010; 92(1): 275–279. [Google Scholar]
- 36.Poojari S, Lowery T, Rott M, Schmidt A-M, DeLury N, Boulé J, et al. First Report and Prevalence of Grapevine fleck virus in Grapevines (Vitis vinifera) in Canada. Plant Dis. 2015; 100: 1028. [Google Scholar]
- 37.Official Gazette of BiH (Bosnia and Herzegovina). Pravilnik o stavljanju u promet materijala za razmnožavanje vinove loze u Bosni i Hercegovini. 2013; 50: 68–74. [Google Scholar]
- 38.Cochran WG. Sampling techniques (3rd ed.). New York: Wiley; 1999. [Google Scholar]
- 39.Madden LV, Hughes G, Van den Bosch F. The study of plant disease epidemics. St. Paul: The American Phytopathological Society; 2007. [Google Scholar]
- 40.Clark MF, Adams AN. Characteristics of microplate method of enzyme linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977; 34: 475–83. 10.1099/0022-1317-34-3-475 [DOI] [PubMed] [Google Scholar]
- 41.Flegg JJM. Extraction of Xiphinema and Longidorus species from soil by a modification of Cobb’s decanting and sieving technique. Ann Appl Biol. 1967; 60: 420–437. [Google Scholar]
- 42.Gambino G, Gribaudo I. Simultaneous detection of nine grapevine viruses by multiplex reverse transcription-polymerase chain reaction with coamplification of a plant RNA as internal control. Phytopathology. 2006; 96: 1223–1229. 10.1094/PHYTO-96-1223 [DOI] [PubMed] [Google Scholar]
- 43.Crnogorac A, Gašpar M, Davino S, Mandić A, Matić S. First report of grapevine fleck virus in vineyards of Bosnia and Herzegovina. J Plant Pathol. 2020 [Google Scholar]
- 44.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics (Oxford, England). 2007; 23(21): 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 45.Nei M, Kumar S. Molecular evolution and phylogenetics. Oxford: Oxford University Press; 2000. pp. 147–164. [Google Scholar]
- 46.Efron B, Halloran E, Holmes S. Bootstrap confidence levels for phylogenetic trees. Proc Natl Acad Sci USA. 1996; 93: 7085–7090. 10.1073/pnas.93.14.7085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol. 2018; 35(6): 1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol Biol Evol. 2017; 34:3299–3302. 10.1093/molbev/msx248 [DOI] [PubMed] [Google Scholar]
- 49.Martin DP, Murrell B, Golden M, Khoosal A, Muhire B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015; 1: vev003 10.1093/ve/vev003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahfoudhi N, Digiaro M, Dhouibi MH. Transmission of Grapevine Leafroll Viruses by Planococcus ficus (Hemiptera: Pseudococcidae) and Ceroplastes rusci (Hemiptera: Coccidae). Plant Dis. 2009; 93(10): 999–1002. 10.1094/PDIS-93-10-0999 [DOI] [PubMed] [Google Scholar]
- 51.Bahder BW, Poojari S, Alabi OJ, Naidu RA, Walsh DB. Pseudococcus maritimus (Hemiptera: Pseudococcidae) and Parthenolecanium corni (Hemiptera: Coccidae) are capable of transmitting grapevine leafroll-associated virus 3 between Vitis x labruscana and Vitis vinifera. Environ Entomol. 2013; 42(6): 1292–1298. 10.1603/EN13060 [DOI] [PubMed] [Google Scholar]
- 52.Kosztarab M, Kozár F. Scale insects of Central Europe. Dordrecht: Springer; 1988. pp. 455. [Google Scholar]
- 53.Hodgson CJ. The scale insect family Coccidae: an identification manual to Genera. Wallingford: CAB International, 1994. [Google Scholar]
- 54.Williams DJ, Williams de DJ. Mealybugs of Central and South America. Wallingford: CAB International, 1992. [Google Scholar]
- 55.Cid M, Pereira S, Cabaleiro C, Faoro F, Segura A. Presence of Grapevine leafroll-associated virus 3 in primary salivary glands of the mealybug vector Planococcus citri suggests a circulative transmission mechanism. Eur J Plant Pathol. 2007; 118: 23–30. [Google Scholar]
- 56.Krüger K, Douglas-Smit N. Grapevine leafroll-associated Virus 3 (GLRaV-3) Transmission by Three Soft Scale Insect Species (Hemiptera: Coccidae) with Notes on Their Biology. Afr Entomol. 2013; 21: 1–8. [Google Scholar]
- 57.Krüger K, Saccaggi DL, van der Merwe M, Kasdorf GGF. Transmission of Grapevine Leafroll-associated Virus 3 (GLRaV-3): Acquisition, Inoculation and Retention by the Mealybugs Planococcus ficus and Pseudococcus longispinus (Hemiptera: Pseudococcidae). South Afr J Enol Vitic. 2015; 36(2): 223–230. [Google Scholar]
- 58.Farooq AB, Ma YX, Wang Z, Zhuo N, Wenxing X, Wang GP, et al. Genetic diversity analyses reveal novel recombination events in Grapevine leafroll-associated virus 3 in China. Virus Res. 2013; 171(1): 15–21. 10.1016/j.virusres.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 59.Maree HJ, Pirie MD, Oosthuizen K, Bester R, Rees DJ, Burger JT. Phylogenomic analysis reveals deep divergence and recombination in an economically important grapevine virus. PLoS One. 2015; 10(5): e0126819 10.1371/journal.pone.0126819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diaz-Lara A, Klaassen V, Stevens K, Sudarshana MR, Rowhani A, Maree HJ, et al. Characterization of grapevine leafroll-associated virus 3 genetic variants and application towards RT-qPCR assay design. PloS One. 2018; 13(12): e0208862 10.1371/journal.pone.0208862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delić D, Lolić B, Đurić G, Jovanović-Cvetković T. Sanitary Status of the Grapevine Germplasm Collection in Republic of Srpska. Aгрознање. 2016; 17: 143–152. [Google Scholar]
- 62.Pompe-Novak M, Gutiérrez-Aguirre I, Vojvoda J, Blas M, Tomažič I, Vigne E, et al. Genetic variability within RNA2 of Grapevine fanleaf virus. Eur J Plant Pathol. 2007; 117: 307–312. [Google Scholar]
- 63.Alabi OJ, Casassa LF, Gutha LR, Larsen RC, Henick-Kling T, Harbertson JF, et al. Impacts of grapevine leafroll disease on fruit yield and grape and wine chemistry in a wine grape (Vitis vinifera L.) cultivar. PloS One. 2016; 11: e0149666 10.1371/journal.pone.0149666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karačić A, Đermić E, Vončina D, Ivanković M. Pojava virusa iz kompleksa uvijenosti lista vinove loze (GLRaV) na autohtonim kultivarima u Hercegovini. In: Cvjetković B, editor. Zbornik sažetaka 58. seminara biljne zaštite, Opatija, 11–14. veljače 2014. Zabreb: Hrvatsko društvo biljne zaštite; 2014.
- 65.Lasić V, Karačić A, Gašpar M, Blesić M, Kraljević M. Nazočnost virusa GFLV, ARMV, GLRAV-1 i GLRAV-3, na autohtonim kultivarima Žilavka i Blatina na lokalitetu Lopate u mostarskom vinogorju. 21st Scientific-Expert Conference in Agriculture and Food Industry. Neum: Poljoprivredno-prehrambeni fakultet Sarajevo. 2010; pp. 130–131.
- 66.Vončina D, Badurina D, Preiner D, Cvjetković B, Maletić E, Karoglan Kontić J. Incidence of virus infections in grapevines from Croatian collection plantations. Phytopathol Mediterr. 2011; 50: 31626. [Google Scholar]
- 67.Kostadinovska E, Mitrev S, Bianco PA, Casati P, Bulgari D. First Report of Grapevine virus A and Grapevine fleck virus in the Former Yugoslav Republic of Macedonia. Plant Dis. 2014; 98: 1747 10.1094/PDIS-05-14-0518-PDN [DOI] [PubMed] [Google Scholar]
- 68.Zindović J, Viršček Marn M, I. Mavrič P. Phytosanitary status of grapevine in Montenegro. Bull OEPP/EPPO Bull. 2014; 44(1): 60–64. [Google Scholar]
- 69.Starović M, Kuzmanović S, Ivanović Ž, Trkulja N, Aleksić G, Dolovac N, et al. Grapevine leafroll disease in central Serbia. Plant Prot. (in Serbian). 2008; 59:81–92. [Google Scholar]
- 70.Golino DA, Weber E, Sim S, Rowhani A. Leafroll disease is spreading rapidly in a Napa Valley vineyard. Calif Agr. 2008; 62:156–60. [Google Scholar]
- 71.Tsai C-W, Chau J, Fernandez L, Bosco D, Daane KM, Almeida RPP. Transmission of Grapevine leafroll-associated virus 3 by the vine mealybug (Planococcus ficus). Phytopathology. 2008; 98: 1093–1098. 10.1094/PHYTO-98-10-1093 [DOI] [PubMed] [Google Scholar]
- 72.Ostojić I, Zovko M. Štitaste uši sve veći problem hercegovačkih vinograda. 55 Dani Berbe Grožđa Brotnjo; 2010; pp. 58.
- 73.Ostojić I, Zovko M, Petrović D, Bulić P, Dogan V. Smokvin crvac (Planococcus ficus, Signoret, 1875)—opasan štetnik vinove loze na području Hercegovine. Simpozij o zaštiti bilja u Bosni i Hercegovini, Teslić, 04–06. 11. 2014. godine, Zbornik sažetak. Sarajevo: Društvo za zaštitu bilja Bosne i Hercegovine; 2014. pp. 38–40.
- 74.Barsi L. Occurrence of Xiphinema species in the former Yugoslavia. Supplement to the "Atlas of plant parasitic nematodes of Jugoslavia". Nematol Mediterr. 1996; 24: 195–199. [Google Scholar]
- 75.Cseh E, Daragó Á, Szerecz A, Takács A, Gáborjányi R. Apprise the significance of grapevine viruses in west Hungary. Cereal Res Commun. 2009; 37: 153–156. [Google Scholar]
- 76.Kuzmanović S, Dovas CI, Katis NI, Starović M, Tošić M, Rajković S. Contribution to the study of grapevine virus diseases in Serbia. Extended abstracts of 14th Meeting of ICVG, Locorotondo, Italy. 2003; 180–181.
- 77.Credi R, Babini AR. Effects of Virus and Virus-Like Infections on Growth, Yield, and Fruit Quality of Albana and Trebbiano Romagnolo Grapevines. Am J Enol Vitic. 1997; 48: 7–12. [Google Scholar]
- 78.Pesqueira AM, Cabaleiro C, Velasco L. Genetic analysis of Grapevine leafroll‐associated virus 3 population from Galicia, Spain. Plant Pathol. 2016; 65: 310–321. [Google Scholar]
- 79.Gouveia P, Santos MT, Eiras-Dias JE, Nolasco G. Five phylogenetic groups identified in the coat protein gene of grapevine leafroll-associated virus 3 obtained from Portuguese grapevine varieties. Arch. Virol. 2011; 156(3): 413–420. 10.1007/s00705-010-0878-7 [DOI] [PubMed] [Google Scholar]
- 80.Glasa M, Predajna L. Partial sequence analysis of a Grapevine leafroll-associated virus 3 isolate from Slovakia. J Plant Pathol. 2012; 94(3): 675–679. [Google Scholar]
- 81.Pérez-Losada M, Arenas M, Galán JC, Palero F, González-Candelas F. Recombination in viruses: mechanisms, methods of study, and evolutionary consequences. Infect Genet Evol. 2015; 30: 296–307. 10.1016/j.meegid.2014.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turturo C, Saldarelli P, Yafeng D, Digiaro M, Minafra A, Savino V, et al. Genetic variability and population structure of Grapevine leafroll-associated virus 3 isolates. J Gen Virol. 2005; 86(1): 217–224. 10.1099/vir.0.80395-0 [DOI] [PubMed] [Google Scholar]
- 83.Esteves F, Teixeira Santos M, Eiras-Dias JE, Fonseca F. Molecular data mining to improve antibody-based detection of Grapevine leafroll-associated virus 1 (GLRaV-1). J Virol Methods. 2013; 194(1–2): 258–270. 10.1016/j.jviromet.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 84.Fan X, Hong N, Dong Y, Ma Y, Zhang ZP, Ren F. Genetic diversity and recombination analysis of grapevine leafroll-associated virus 1 from China. Arch Virol. 2015; 160(7): 1669–1678. 10.1007/s00705-015-2437-8 [DOI] [PubMed] [Google Scholar]
- 85.Alabi OJ, Rwahnih MA, Karthikeyan G, Poojari S, Fuchs M, Rowhani A, et al. Grapevine leafroll-associated virus 1 occurs as genetically diverse populations. Phytopathology. 2011; 101: 1446–1456. 10.1094/PHYTO-04-11-0114 [DOI] [PubMed] [Google Scholar]
- 86.Czotter N, Molnar J, Szabó E, Demian E, Kontra L, Baksa I, et al. NGS of Virus-Derived Small RNAs as a Diagnostic Method Used to Determine Viromes of Hungarian Vineyards. Front Microbiol. 2018; 9: 122. [Google Scholar]
- 87.Al Rwahnih M, Daubert S, Golino D, Islas C, Rowhani A. Comparison of next-generation sequencing versus biological indexing for the optimal detection of viral pathogens in grapevine. Phytopathology. 2015; 105: 758–763. 10.1094/PHYTO-06-14-0165-R [DOI] [PubMed] [Google Scholar]
- 88.Demian E, Jaksa-Czotter N, Molnar J, Tusnady GE, Kocsis L, Varallyay E. Grapevine rootstocks can be a source of infection with non-regulated viruses. Eur J Plant Pathol. 2020; 156: 897–912. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lanes 1, 2, 3, samples infected by GLRaV-3 and GFLV; lane 4, positive control for GLRaV-3 and GFLV; lane 5, sample infected by GFkV; lane 6, sample infected by GLRaV-1 and GFLV; lane 7, positive control for GLRaV-1; lanes 8, 9, 12, samples infected by GLRaV-3, GLRaV-1 and GFLV; lanes 10, 11, samples infected by GLRaV-3 and GLRaV-1; lane 13, positive control for GLRaV-3 and GLRaV-1; lane 14, sample infected by GLRaV-3; lane 15, positive control for GLRaV-1 and GFkV; lanes 16, 18, samples infected by GLRaV-1 and GFkV; lane 17, sample infected by GLRaV-1; lane 19, sample infected by GLRaV-3, GLRaV-1 and GFkV; lanes 20, 22, 23, samples infected by GLRaV-3; lane 24, positive control for GLRaV-3; lane 21, sample infected by ArMV; W, water control; H, healthy grapevine sample; L, 100 bp DNA ladder.
(TIF)
Each colored key represents a percentage to the identity score between two sequences.
(TIF)
Each colored key represents a percentage to the identity score between two sequences.
(TIF)
Four BiH isolates (in bold) and sixty reference isolates were included in the analysis. The accession number and the origin of each isolate are indicated. The bootstrap consensus tree was inferred from 1000 replicates.
(TIF)
One BiH isolate (in bold) and fifty-five reference isolates were included in the analysis. The accession number and the origin of each isolate are indicated. The bootstrap consensus tree was inferred from 1000 replicates.
(TIF)
One BiH isolate (in bold) and seventy-two reference isolates were included in the analysis. The accession number and the origin of each isolate are indicated. The bootstrap consensus tree was inferred from 1000 replicates.
(TIF)
Four BiH isolates (in bold) and twenty-nine reference isolates were included in the analysis. The accession number and the origin of each isolate are indicated. The bootstrap consensus tree was inferred from 1000 replicates.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.