Abstract
Background
Lack of healthcare access to due to physician shortages is a significant driver of telemedicine expansion in rural areas. Telemedicine is effective for management of chronic conditions such as diabetes but its effectiveness in primary care settings is unknown.
Objective
To evaluate differences in diabetes care before and after implementation of a longitudinal virtual primary care program.
Design
Propensity score-matched cohort study utilizing difference-in-differences analysis.
Participants
Patients with diabetes who received care at VA primary care clinics between January 2018 and December 2019 where the Virtual Integrated Multisite Patient Aligned Care Teams (V-IMPACT) program was implemented.
Exposure
Patient participation in at least one V-IMPACT visit while usual care patients did not participate in V-IMPACT.
Main Measures
The primary outcome was change in hemoglobin A1C (HbA1C) and secondary outcomes included change in the proportion of patients meeting diabetes quality indicators: blood pressure control, statin use, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ACEi/ARB) use, and annual microalbuminuria testing.
Key Results
Our propensity-matched cohort included 9010 patients split evenly between those who participated in V-IMPACT and those who remained in usual in-person care. Among individuals with diabetes who participated in V-IMPACT, the change in mean HbA1C was − 0.055% (95% CI − 0.088 to − 0.022%) while those in usual care had a − 0.047% (95% CI − 0.080 to − 0.014%) change before and after program implementation. We observed a 5.1% (95% CI 2.4 to 7.7%) absolute increase in the proportion prescribed statins in the V-IMPACT group, a 5.3% (95% CI 2.5 to 8.2%) increase prescribed ACE/ARBs, and a 4.6% (95% 1.7 to 7.5%) increase in completed yearly microalbuminuria testing. V-IMPACT was not associated with a significant difference in the proportion with controlled blood pressure at < 140/90 or < 130/90 mmHg thresholds.
Conclusions
Quality of diabetes care delivered by a longitudinal virtual primary care model was similar if not better than traditional in-person care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-020-06547-x.
KEY WORDS: telemedicine, chronic disease, care delivery, quality of care, virtual health
INTRODUCTION
Access to primary care remains a major challenge throughout many parts of the USA, especially in rural areas. Individuals living in rural communities experience higher rates of mortality from preventable chronic diseases such as diabetes, cardiovascular disease, and stroke than their urban counterparts.1 Furthermore, these same communities face disproportionate difficulties in recruitment and retention of physicians and other healthcare professionals to manage patients with these conditions.2 In fact, areas that are the most remote and with the greatest need of healthcare workers often see the highest physician exodus.3 Rural residence itself may not negatively affect mortality, but the combination of socioeconomic deprivation and lack of available primary care physicians in rural areas drive the observed disparities in mortality.4
Telemedicine, the use of communications technology to deliver health care to patients at a distance, has been regarded as a promising solution to address the growing supply and demand mismatch in rural areas. A Cochrane systematic review in 2015 demonstrated the use of telemedicine strategies to be associated with increased access to care and improved clinical outcomes in single chronic diseases.5 In particular, telemedicine interventions for diabetes care have shown to be effective in lowering hemoglobin A1C levels in several large randomized controlled trials.6–8 However, the interventions were heterogenous, ranging from asynchronous remote monitoring to provider or nurse calls via telephone or videoconference to the use of text messaging-based applications. In addition, whether these results are generalizable to real-world primary care outside of a study setting, where the majority of patients with diabetes are managed, is unknown.
The Department of Veterans Affairs (VA) has long been an early adopter and innovator of telemedicine services. In late 2017, VA launched a new model of primary care delivery, the Virtual Integrated Multisite Patient Aligned Care Team (V-IMPACT), which predominantly utilizes clinic-to-clinic synchronous videoconferencing to connect primary care providers (PCPs) with rural Veterans to increase their access to primary care. We conducted a retrospective quasi-experimental study utilizing difference-in-differences analysis to evaluate diabetes quality of care among patients who received care in V-IMPACT compared to those who received in-person care. We hypothesized that the quality of diabetes care would be similar between V-IMPACT and in-person care.
METHODS
Program Description and Setting
V-IMPACT is a national VA initiative established to deliver primary care services through in-clinic video appointments to primary care clinics with difficulty recruiting and retaining providers. After establishing initial feasibility for the model out of Boise, ID in mid-2015, the V-IMPACT model expanded to 10 hubs serving 44 spoke clinics by the beginning of 2018. Implementation was staggered throughout 2016 to 2018 based on the clinical and operational needs of the spoke clinics.
V-IMPACT employed a hub-and-spoke model with a hub team of remote primary care providers (PCPs), nurse coordinators, pharmacists, and social workers working with local staff (nurses, medical assistants, and clerks) at smaller spoke clinics to ensure team-based, longitudinal primary care in line with the PACT (Patient Aligned Care Team) principles, VA’s version of the patient-centered medical home. Hub PCPs take on full panels of patients from clinics often located hundreds of miles away and provide care through video appointments. At a given time, a hub PCP was assigned a single patient panel at a single spoke clinic with each hub serving between 2 and 16 spoke clinics. Patients assigned to a new virtual PCP participated in these appointments at the clinic via synchronous videoconferencing equipment furnished with digital stethoscopes and high-definition camera operated by trained on-site nursing staff. This is notably different than direct-to-home video visits rapidly gaining adoption today, but allowed patients to remain at their usual place of care with local staff familiar to them while removing most technological or connectivity barriers. V-IMPACT clinicians further supplement continuity of care via quarterly site visits to the spoke clinic to perform in-person examinations and routine procedures.
Clinics elected to participate in V-IMPACT when they had unexpected provider vacancies or growing patient population without time for provider supply to adjust. In many cases, a retiring or departing provider’s panel was directly transferred to a V-IMPACT PCP. At growing clinics, there was no consistent methodology for assigning patients to V-IMPACT panels. Methods ranged from as arbitrary as the top half of a group of patients alphabetically sorted by last name to more targeted assignments such as by patient risk scores such as Care Assessment Needs (CAN)9 or Ambulatory Care Sensitive Conditions (ACSC)10. Some assignments were driven by the unique needs of the panel. For example, V-IMPACT PCPs often receive additional training in care for women Veterans, so they were sometimes paired with teams with greater proportions of women patients. Though V-IMPACT was not offered exclusively to rural sites, clinics in rural areas were more likely to experience these needs and utilize V-IMPACT as a result.
Study Population
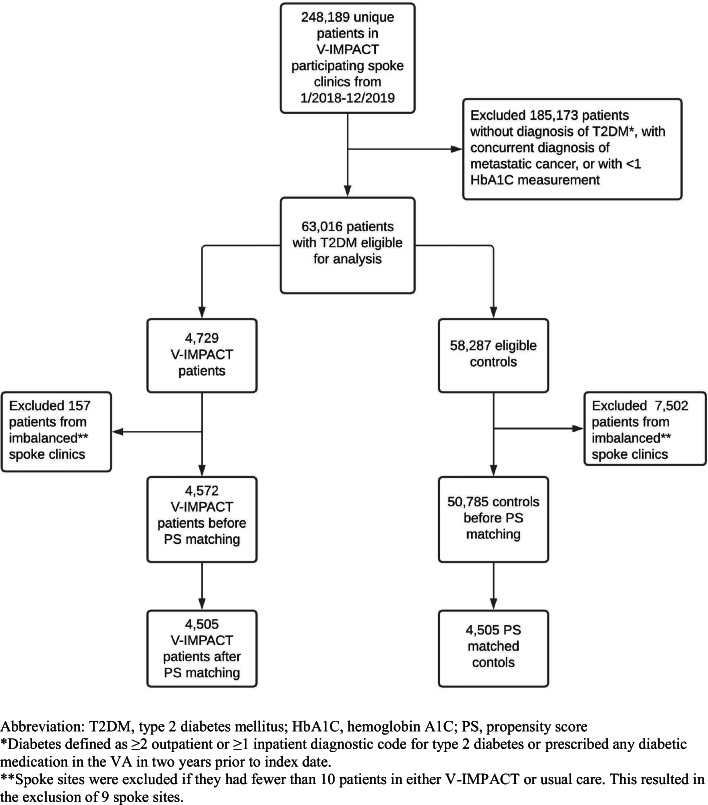
This is a retrospective observational study utilizing outpatient VA clinical and administrative data of veterans with diabetes from January 2018 through December 2019. This work was conducted as a healthcare operations quality improvement (VHA Handbooks 1605.1 and 1605.2) and did not require Institutional Review Board review. We examined 248,189 veterans who received primary care in 44 spoke clinics where V-IMPACT was implemented (Fig. 1). We identified patients as having diabetes if they had ≥ 2 outpatient or ≥ 1 inpatient diagnostic code for type 2 diabetes or prescribed any diabetic medication from the VA in 2 years prior to program implementation. Patients were included for analysis if they had at least one primary care encounter and have at least one documented hemoglobin A1C level available during the entire study observation period. We excluded patients younger than 18 years old as well as those with a diagnosis of metastatic cancer. Given clinic implementation of V-IMPACT was staggered across time, we also excluded patients from spoke clinics where there were less than 10 patients in either V-IMPACT or in-person care group.
Figure 1.
Flow diagram of study participants.
Exposure: V-IMPACT Participation
We compared Veterans who participated in V-IMPACT to Veterans in the same clinics who remained in usual face-to-face care. We identified intervention group patients as those who received any V-IMPACT primary care visits with a PCP or nurse between January 1, 2018, and September 30, 2019, as indicated by V-IMPACT-specific encounter codes. The index quarter for V-IMPACT participation for each patient was defined as the calendar quarter of first contact with any V-IMPACT primary care service. For the usual care group, we included all patients who also received any primary care during the observation period but did not have any V-IMPACT-specific encounter codes. The index quarter for usual care patients was a randomly selected quarter in which primary care was received. The pre-intervention period was defined up to 9 quarters (or 2.25 years) prior to index quarter, and the post-intervention period included up to 8 quarters (or 2 years) after the index quarter.
Outcomes
Our primary outcome of interest was change in hemoglobin A1C (HbA1C) level between groups before and after V-IMPACT implementation. All HbA1C measurements made as a part of routine clinical care during the study observation period were included for analysis. For each quarter where more than a single HbA1C value was recorded, we took the median HbA1C of available values. In accordance with the latest diabetes guidelines, we also measured the proportion of patients who met HbA1C target of < 8%.11
Secondary outcomes included proportions of patients who met recommended diabetes quality indicators such as blood pressure control, statin use,12 angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ACEi/ARB) use,13 and annual urine microalbumin measurement.13 For all outcome proportions, the entire cohort was included in the denominator.
In each quarter, we took the median of all available blood pressure measurements in the medical record. Blood pressure control was defined by two cut-off thresholds due to recent updates in blood pressure targets for patients with diabetes14: (1) systolic blood pressure (SBP) < 140 mmHg and diastolic blood pressure (DBP) < 90 mmHg or (2) SBP < 130 mmHg and DBP < 80 mmHg. Prescription of statins and ACEi/ARBs was defined as having any active prescription dispensed during each time period (pre- and post-intervention). Completed microalbuminuria screening was defined as having a lab result recorded within 1 year before or after the index quarter.
Statistical Analyses
We performed propensity score matching to control for differences between patients who participated in V-IMPACT and those who did not. A logistic regression model was developed to estimate the propensity of receiving V-IMPACT. Covariates used in the model included demographic and clinical characteristics measured during or prior to the index quarter: age, sex, race, rurality, medical comorbidities, body mass index, insulin use, and index quarter to account for time trends. Medical comorbidities were identified by at least 2 outpatient or 1 inpatient ICD-10 or CPT code for each comorbidity within 2 years prior the index quarter. Rurality was defined by VA rurality codes which uses the Rural-urban Commuting Areas (RUCA) system.
Then, we performed 1:1 greedy nearest neighbor matching using matching without replacement and a maximum caliper width of 0.2 times the pooled SD of the logit of the propensity scores to select appropriate controls.15 Covariate balance between intervention and control groups was assessed by visual inspection of the cumulative probability distributions of the propensity scores (Supplemental figure 1) and by calculating standardized mean differences for which a difference of less than 0.10 was considered to indicate acceptable balance.16
Within propensity-matched groups, we estimated HbA1C over time (in calendar quarters) utilizing a multilevel linear mixed effects regression model that accounted for clustering by spoke clinic site as well as repeated measurements per patient with nested random effects. For binary outcomes, we used a linear regression analysis on 1000 bootstrap samples with replacement to estimate pre and post proportions of patients meeting quality indicators.
To determine the association between V-IMPACT implementation and patient outcomes, we applied a difference-in-differences approach where the association between V-IMPACT implementation and the outcome was identified by the difference between intervention and control groups in pre-post time differences. This required dummy variables indicating (1) whether the patient was exposed to V-IMPACT or not and (2) whether their outcome measurement was completed before or after index quarter. The interaction term of these two variables was the difference-in-differences estimator, and its coefficient reflected the magnitude of association between V-IMPACT participation and the dependent outcome of interest.
We determined statistical significance by using 95% CIs and 2-tailed tests with p < 0.05. Statistical analyses were performed using Stata (Version 16.0, StataCorp, College Station, TX).
RESULTS
We identified 63,016 patients with type 2 diabetes (only 4.8% of whom were included based on prescription of any diabetic medications alone) who participated in primary care in 44 spoke clinics where the V-IMPACT was implemented. Among the full cohort, 4572 patients (7.3%) received primary care services through V-IMPACT. Patients who participated in V-IMPACT were more likely to be white and living in a rural area, and less likely to have a substance use disorder. Propensity score matching yielded a cohort of 9010 patients with diabetes split evenly between those who participated in V-IMPACT and those who remained in usual in-person care. Groups were well-balanced on propensity score distribution and baseline characteristics with standard mean differences < 0.10 (Table 1).
Table 1.
Cohort Characteristics Before and After Propensity Score (PS) Matching
| Characteristic | Before PS matching | After PS matching | ||||
|---|---|---|---|---|---|---|
| V-IMPACT (N = 4572) | Usual care (N = 50,785) | SMD | V-IMPACT (N = 4505) | Usual care (N = 4505) | SMDa | |
| Age, mean (SD), year | 68.3 (10.3) | 67.4 (11.1) | − 0.09 | 68.4 (10.3) | 68.2 (10.9) | − 0.02 |
| Male, N (%) | 4373 (95.6) | 47,611 (93.8) | 0.08 | 4309 (95.6) | 4321 (95.9) | 0.01 |
| Race/ethnicity, N (%) | ||||||
| White | 3506 (76.7) | 36,369 (71.6) | 0.11 | 3494 (77.6) | 3461 (76.8) | 0.02 |
| Black | 540 (11.8) | 9115 (17.9) | 0.17 | 538 (11.9) | 545 (12.1) | 0.005 |
| Hispanic | 135 (3.0) | 1904 (3.7) | 0.04 | 134 (3.0) | 124 (2.8) | 0.01 |
| Other | 229 (5.0) | 2957 (5.8) | 0.04 | 185 (4.1) | 198 (4.4) | 0.01 |
| Unknown | 302 (6.6) | 2496 (4.9) | 0.07 | 293 (6.5) | 307 (6.8) | 0.01 |
| Ruralityb, N (%) | ||||||
| Urban | 1134 (25.1) | 27,650 (55.1) | 0.64 | 1133 (25.4) | 1156 (25.7) | 0.01 |
| Rural | 3375 (74.9) | 22,522 (44.9) | 3372 (74.9) | 3349 (74.3) | ||
| Comorbiditiesc, N (%) | ||||||
| AF | 449 (9.8) | 4550 (9.0) | 0.03 | 442 (9.8) | 429 (9.5) | 0.01 |
| Alcohol use | 187 (4.1) | 2295 (4.5) | 0.02 | 179 (4.0) | 169 (3.8) | 0.01 |
| Cancer | 267 (5.8) | 3115 (6.1) | 0.01 | 265 (5.9) | 270 (6.0) | 0.004 |
| CHF | 298 (6.5) | 3246 (6.4) | 0.005 | 297 (6.6) | 292 (6.5) | 0.004 |
| CKD | 474 (10.4) | 5386 (10.6) | 0.008 | 464 (10.3) | 462 (10.3) | 0.002 |
| COPD | 655 (14.3) | 7073 (13.9) | 0.01 | 650 (14.4) | 653 (14.5) | 0.002 |
| CVD | 231 (5.1) | 3300 (6.5) | 0.06 | 228 (5.1) | 224 (5.0) | 0.004 |
| CTD | 38 (0.8) | 571 (1.1) | 0.03 | 38 (0.8) | 27 (0.6) | 0.03 |
| Depression | 805 (17.6) | 10,430 (20.5) | 0.07 | 787 (17.5) | 791 (17.6) | 0.002 |
| HTN | 3485 (76.2) | 37,810 (74.5) | 0.04 | 3441 (76.4) | 3469 (77.0) | 0.01 |
| Liver disease | 71 (1.6) | 788 (1.6) | 0.0001 | 71 (1.6) | 63 (1.4) | 0.01 |
| PAD | 260 (5.7) | 2947 (5.8) | 0.005 | 255 (5.7) | 264 (5.9) | 0.01 |
| PTSD | 804 (17.6) | 8690 (17.1) | 0.01 | 789 (17.5) | 803 (17.8) | 0.009 |
| PUD | 12 (0.3) | 206 (0.4) | 0.02 | 12 (0.3) | 12 (0.3) | 0.00 |
| Sleep apnea | 1100 (24.1) | 13,298 (26.2) | 0.05 | 1081 (24.0) | 1088 (24.2) | 0.004 |
| Substance use | 80 (1.7) | 1665 (3.3) | 0.1 | 80 (1.8) | 80 (1.8) | 0.00 |
| BMId, mean (SD) | 33.10 (6.48) | 33.01 (6.52) | − 0.01 | 33.12 (6.49) | 33.22 (6.40) | 0.02 |
| Insulin Use, N (%) | 1172 (25.6) | 12,777 (25.2) | 0.01 | 1162 (25.8) | 1184 (26.3) | 0.01 |
| Index quartere, N (%) | ||||||
| 2018 Q1 | 707 (15.5) | 4705 (9.3) | 689 (15.3) | 701 (15.6) | ||
| 2018 Q2 | 656 (14.3) | 6414 (12.6) | 649 (14.4) | 655 (14.5) | ||
| 2018 Q3 | 725 (15.9) | 7608 (15.0) | 725 (16.1) | 728 (16.2) | ||
| 2018 Q4 | 916 (20.0) | 8392 (16.5) | 0.36 | 900 (20.0) | 875 (19.4) | 0.03 |
| 2019 Q1 | 743 (16.3) | 7749 (15.3) | 738 (16.4) | 766 (17.0) | ||
| 2019 Q2 | 523 (11.4) | 7976 (15.7) | 511 (11.3) | 505 (11.2) | ||
| 2019 Q3 | 302 (6.6) | 7941 (15.6) | 293 (6.5) | 275 (6.1) | ||
SD, standard deviation; SMD, standard mean difference; AF, atrial fibrillation; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive lung disease; CVD, cardiovascular disease; CTD, connective tissue disease; HTN, hypertension; PAD, peripheral arterial disease; PTSD, post-traumatic stress disorder; PUD, peptic ulcer disease; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); Q, calendar quarter
aStandardized mean differences (SMD) of less than 0.10 for each variable was considered to indicate adequate balance after propensity score matching
bRurality was defined by VA rurality codes which uses the Rural-Urban Commuting Areas (RUCA) system. Rurality data were missing for 660 patients; rural and highly rural combined due relative low prevalence of highly rural participants
cBased on ICD-10 diagnosis codes and procedure CPT codes. We required at least 2 outpatient or 1 inpatient record with a specified code for a comorbidity within 2 years prior to index quarter
dBMI calculated from median weight and height recorded for index quarter
eIndex quarter defined by calendar quarter where the first quarter of 2018 represents the time period between January 1, 2018, and March 31, 2018
Patients in both groups had a median follow-up time of 4 quarters (1 year), and the majority (83% in V-IMPACT vs 82% in usual care, p = 0.084) had at least 1 HbA1C measurement in both pre- and post-intervention periods. On average, V-IMPACT patients had a greater number of visits in the year before and after the index quarter than patients in usual care (mean [SD] pre-intervention: 4.0 [4.7] vs 3.7 [3.8], p = 0.001; post-intervention: 4.4 [4.9] vs 3.2 [4.1], p < 0.0001) (Supplemental table 1).
Among individuals with diabetes who were exposed to V-IMPACT, the mean HbA1C decreased from 7.33% to 7.27% (difference: − 0.055%, 95% CI − 0.088 to − 0.022%), and the mean HbA1C in those who remained in traditional in-person care decreased from 7.36% to 7.31% (difference: − 0.047%, 95% CI − 0.080 to − 0.014%). No significant difference was found in the change in HbA1C between groups (difference-in-differences estimate, − 0.008%; 95% CI − 0.055 to 0.039 (Fig. 2).
Figure 2.
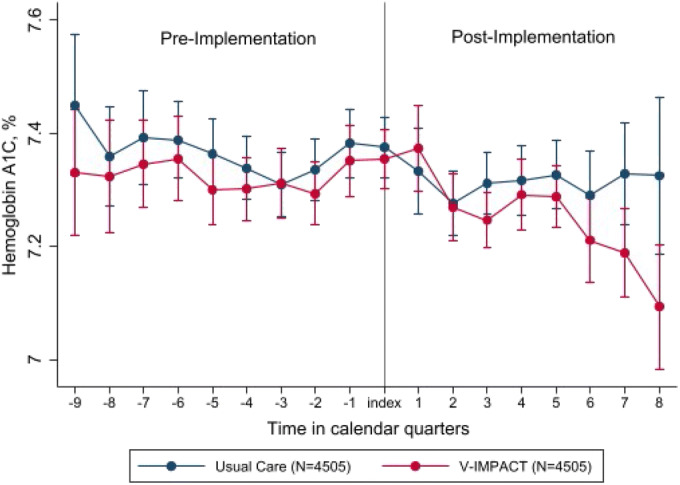
Hemoglobin A1C trends over time.
Medication fills and testing for microalbuminuria increase for both groups in the post-implementation period with larger absolute increases for V-IMPACT patients with statin medication use (difference-in-differences estimator: 5.1%, 95% CI 2.4 to 7.7%), ACE/ARB use (5.3%, 95% CI 2.5 to 8.2%), and microalbuminuria testing (4.6%, 95% CI 1.7 to 7.5%). V-IMPACT implementation was not associated with a significant difference in the proportion with controlled blood pressure using either < 140/90 or < 130/90 mmHg threshold (Table 2).
Table 2.
Diabetes Quality Measures Before and After V-IMPACT Implementation
| Outcome | V-IMPACT (N = 4505) | Usual care (N = 4505) | Difference-in-differences estimate (95% CI) | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| HbA1C, mean % | 7.33 (7.29, 7.37) | 7.27 (7.23, 7.31) | 7.36 (7.31, 7.40) | 7.31 (7.27, 7.35) | − 0.008 (− 0.055, 0.039) |
| Controlled BP < 140/90, % | 65.3 (64.7, 65.9) | 67.7 (67.0, 68.3) | 64.7 (64.1, 65.3) | 66.8 (66.1, 67.5) | 0.2 (− 1.1, 1.5) |
| Controlled BP < 130/80, % | 32.4 (31.9, 33.0) | 35.1 (34.4, 35.8) | 31.8 (31.2, 32.3) | 34.3 (33.4, 35.0) | 0.1 (− 1.1, 1.4) |
| Prescribed statin, % | 66.7(65.3, 68.0) | 73.3 (72.0, 74.6) | 68.0 (66.7, 69.3) | 69.6 (68.2, 70.9) | 5.1* (2.4, 7.7) |
| Prescribed ACEi/ARB, % | 58.9 (57.5, 60.4) | 64.4 (63.0, 65.9) | 62.7 (61.3, 64.1) | 62.9 (51.5, 64.3) | 5.3* (2.5, 8.2) |
| Urine microalbumin testing, % | 48.9 (47.4, 50.3) | 52.2 (50.7, 53.7) | 52.3 (50.7, 53.8) | 51.0 (49.6, 52.5) | 4.6* (1.7, 7.5) |
CI, confidence interval; BP, blood pressure; ACE/ARB, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers
*Denotes statistically significant difference, p < 0.05
DISCUSSION
In this national evaluation of patients with type 2 diabetes within a novel primary care delivery model, we found similar glycemic and blood pressure control between those receiving longitudinal video-based telemedicine versus traditional face-to-face care. Quality of diabetes care, as measured by rates of statin and ACE/ARB use and microalbuminuria testing, was better in patients who participated in the longitudinal telemedicine program. These results support the use of telemedicine as a safe and effective mode of diabetes care delivery in primary care.
Diabetes is a prototypical chronic disease seen in primary care with an existing body of literature demonstrating telemedical care to be effective in specialty care settings.17 Video visits have been successfully implemented as an adjunct in primary care,18 but their effectiveness on clinical outcomes has not been evaluated in randomized controlled trials. This is the first large observational study to evaluate the quality of diabetes care delivered through a longitudinal telemedicine primary care model. Our results support the findings of prior randomized controlled trials that telemedicine strategies confer relatively modest improvements to glycemic control with minimal effect on blood pressure.19 This should come as no surprise as social and behavioral determinants of health likely contribute more to intermediate and long-term diabetes outcomes than the modality of care delivery.20 In fact, two of the pillars of successful diabetes population health management are utilizing patients’ social context to inform treatment decisions and supporting patient self-management strategies at a system level.21
Both V-IMPACT and usual care patients in our study had reasonably well-controlled diabetes throughout the study period. This likely reflects the relatively short median length of follow-up (1 year for both groups) and our selection of patients who have engaged in primary care (those with visits and HbA1C measurements). The absolute percentages of patients who met quality indicators in our study were generally lower compared to VA national averages22 (Supplemental figure 2). This may be due to the larger proportion of rural patients represented in our sample and known disparities in rural diabetes care.23 The higher percentage of V-IMPACT patients meeting quality metrics may be potentially explained by the greater number of primary care visits per year or by the presence of additional resources such as pharmacists, social workers, and nurse coordinators available in each V-IMPACT hub.
V-IMPACT is the first care model of its kind to deliver longitudinal, team-based primary care through video appointments. Establishing feasibility and effectiveness of longitudinal virtual primary care has important implications for the future of both telemedicine and rural health. First, amidst the current global pandemic, our findings offer some reassurance to payers and primary care practices who now rely almost exclusively on telemedicine to deliver care, albeit the in-clinic setting of video visits in V-IMPACT offered the luxury of high-definition cameras, vital signs, and a facilitator for physical exams. Second, high-quality care can be achieved in a virtual care model provided proactive population health management and resources are available. Third, the success of the V-IMPACT model highlights the opportunity for innovative strategies to support the primary care workforce in rural areas. Though individuals from rural backgrounds are more likely to enter primary care and practice in rural communities, we have seen a steady 15-year decline in the rural applicant pool, indicating that supporting the pipeline alone will not be enough to meet current and future workforce needs.24 The V-IMPACT model leverages telemedicine to virtually reallocate the abundance of physicians and advanced practice providers in urban areas to care for rural-residing patients. This dramatically aids in closing the short-term supply gap while providing additional resources for population health management. As rural practices and hospitals become consolidated and owned by larger health systems,25 adoption of this model may be key to the sustainability of primary care in rural communities. However, health systems and patients cannot reap these benefits unless we move away from geographic restrictions of licensure.
Our study has several limitations. First, our usual care group does not accurately represent the counterfactual scenario as patients were assigned to V-IMPACT due to provider leaving a practice or to offload larger panels from existing providers. In the counterfactual scenario, then, these patients would have presumably either sought non-VA care or went without primary care entirely. Therefore, the results further support the effectiveness of V-IMPACT to deliver high-quality diabetic care even when compared to a more favorable usual care group. However, the data collected were unable to capture how many V-IMPACT patients subsequently elected to switch out of virtual care modality due to dissatisfaction with care. Second, though we used propensity score matching methods to address the risk of confounding inherent to observational studies, our study may still be subject to confounding by factors that were not able to be measured or reliably captured by VA’s clinical and administrative databases. These factors include the length of diabetes diagnosis as well as the involvement of endocrine specialists or non-VA providers (primary care or endocrinologist) which may affect glycemic control. Third, for our quality indicator of ACE/ARB use, we did not have a reliable, validated method of determining the total number of eligible patients who either had concomitant hypertension and/or clinical microalbuminuria and thus included the entire cohort as the denominator for our analyses. Fourth, the in-clinic and facilitated nature of the video visits conducted in V-IMPACT render these results to not be entirely generalizable to video visits in other settings such as direct-to-home models. Lastly, this study took place within the VA health system which serves a higher proportion of men and patients with multimorbidity than the general US population.
CONCLUSION
In this propensity-matched, difference-in-differences analysis of rural patients with type 2 diabetes receiving VA primary care, participation in a novel longitudinal virtual primary care model was associated with equivalent glycemic and blood pressure control with higher percentages meeting diabetic quality indicators. By expanding access to physicians, preserving recommended follow-up intervals, and maintaining recommendations per national guidelines, care delivered by telemedicine can be just as effective as traditional office-based model of care.
Supplementary Information
(DOCX 144 kb)
Role of Funder/Sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and decision to submit the manuscript for publication. The VA Office of Rural Health reviewed and approved this manuscript before submission.
Author Contributions
Drs. Lu and Ho had full access to all data in the study and assume full responsibility for the integrity and accuracy of the results.
Concept and design: Lu, Gunzburger, Smith, Glorioso, Whooley, Ho
Acquisition, analysis, or interpretation of data: all authors
Drafting of the manuscript: Lu, Gunzburger, Glorioso, Smith
Critical revision of the manuscript for important intellectual content: all authors
Statistical analysis: Lu, Gunzburger, Glorioso
Administrative, technical, or material support: Gunzburger, Kenney, Ho
Supervision: Whooley, Ho
Funding
This work was funded by the VA Office of Rural Health. Dr. Lu was supported by the VA Quality Scholars Program funded through the VA Office of Academic Affiliations (Grant AF-3Q-09-2019-C), Department of Veterans Affairs, Veterans Health Administration.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Prior Presentations: This work was presented as an oral presentation and received the Mack Lipkin Sr. Award for top oral abstract at the 2020 National Virtual Meeting of the Society of General Internal Medicine. It was also separately presented as an oral presentation at the Academy Health’s Annual Research Meeting 2020.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moy E, Garcia MC, Bastian B, et al. Leading Causes of Death in Nonmetropolitan and Metropolitan Areas— United States, 1999–2014. MMWR Surveill Summ. 2017;66(1):1–8. doi: 10.15585/mmwr.ss6601a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dussault G, Franceschini MC. Not Enough There, Too Many Here: Understanding Geographical Imbalances in the Distribution of the Health Workforce. Hum Resour Health. 2006;4(1):12. doi: 10.1186/1478-4491-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGrail MR, Wingrove PM, Petterson SM, Bazemore AW. Mobility of US Rural Primary Care Physicians During 2000-2014. Ann Fam Med. 2017;15(4):322–328. doi: 10.1370/afm.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US Rural Mortality Rates Linked To Socioeconomic Status, Physician Shortages, and Lack Of Health Insurance. Health Aff (Millwood). Published online December 3, 2019. doi:10.1377/hlthaff.2019.00722 [DOI] [PubMed]
- 5.Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive Telemedicine: Effects on Professional Practice and Health Care Outcomes. Cochrane Effective Practice and Organisation of Care Group, ed. Cochrane Database Syst Rev. Published online September 7, 2015. doi:10.1002/14651858.CD002098.pub2 [DOI] [PMC free article] [PubMed]
- 6.Davis RM, Hitch AD, Salaam MM, Herman WH, Zimmer-Galler IE, Mayer-Davis EJ. TeleHealth Improves Diabetes Self-Management in an Underserved Community: Diabetes TeleCare. Diabetes Care. 2010;33(8):1712–1717. doi: 10.2337/dc09-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izquierdo RE, Knudson PE, Meyer S, Kearns J, Ploutz-Snyder R, Weinstock RS. A Comparison of Diabetes Education Administered Through Telemedicine Versus in Person. Diabetes Care. 2003;26(4):1002–1007. doi: 10.2337/diacare.26.4.1002. [DOI] [PubMed] [Google Scholar]
- 8.Shea S, Weinstock RS, Starren J, et al. A Randomized Trial Comparing Telemedicine Case Management with Usual Care in Older, Ethnically Diverse, Medically Underserved Patients with Diabetes Mellitus. J Am Med Inform Assoc JAMIA. 2006;13(1):40–51. doi: 10.1197/jamia.M1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Porter B, Maynard C, et al. Predicting Risk of Hospitalization or Death Among Patients Receiving Primary Care in the Veterans Health Administration. Med Care. 2013;51(4):368–373. doi: 10.1097/MLR.0b013e31827da95a. [DOI] [PubMed] [Google Scholar]
- 10.AHRQ - Quality Indicators. Accessed June 7, 2020. https://www.qualityindicators.ahrq.gov/Modules/pqi_resources.aspx
- 11.American Diabetes Association 6. Glycemic Targets: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S61–S70. doi: 10.2337/dc19-S006. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Supplement 1):S111–S134. doi: 10.2337/dc20-S010. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes−2020. Diabetes Care. 2020;43(Supplement 1):S135–S151. doi: 10.2337/dc20-S011. [DOI] [PubMed] [Google Scholar]
- 14.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary. J Am Coll Cardiol. 2018;71(19):2199–2269. doi: 10.1016/j.jacc.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. Balance Diagnostics for Comparing the Distribution of Baseline Covariates Between Treatment Groups in Propensity-Score Matched Samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon R, Zisser H, Barleen N, et al. Participation in a Virtual Diabetes Clinic Improves Glycemic Control in Adults with Type 2 Diabetes. Iproceedings. 2019;5(1):e15258. doi: 10.2196/15258. [DOI] [Google Scholar]
- 18.Reed ME, Parikh R, Huang J, Ballard DW, Barr I, Wargon C. Real-Time Patient-Provider Video Telemedicine Integrated with Clinical Care. N Engl J Med. 2018;379(15):1478–1479. doi: 10.1056/NEJMc1805746. [DOI] [PubMed] [Google Scholar]
- 19.Marcolino MS, Maia JX, Alkmim MBM, Boersma E, Ribeiro AL. Telemedicine Application in the Care of Diabetes Patients: Systematic Review and Meta-analysis. Bencharit S, ed. PLoS ONE. 2013;8(11):e79246. doi: 10.1371/journal.pone.0079246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan JCN, Gagliardino JJ, Baik SH, et al. Multifaceted Determinants for Achieving Glycemic Control: the International Diabetes Management Practice Study (IDMPS) Diabetes Care. 2009;32(2):227–233. doi: 10.2337/dc08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association 1. Promoting Health and Reducing Disparities in Populations. Diabetes Care. 2017;40(Supplement 1):S6–S10. doi: 10.2337/dc17-S004. [DOI] [PubMed] [Google Scholar]
- 22.Why Choose VA Health Care? - Quality of Care. Accessed May 25, 2020. https://www.va.gov/qualityofcare/initiatives/compare/why-choose-va-health-care.asp
- 23.Hale NL, Bennett KJ, Probst JC. Diabetes Care and Outcomes: Disparities Across Rural America. J Community Health. 2010;35(4):365–374. doi: 10.1007/s10900-010-9259-0. [DOI] [PubMed] [Google Scholar]
- 24.Shipman SA, Wendling A, Jones KC, Kovar-Gough I, Orlowski JM, Phillips J. The Decline in Rural Medical Students: a Growing Gap In Geographic Diversity Threatens The Rural Physician Workforce. Health Aff (Millwood) 2019;38(12):2011–2018. doi: 10.1377/hlthaff.2019.00924. [DOI] [PubMed] [Google Scholar]
- 25.The Rural Hospital and Health System Affiliation Landscape – a brief review. Accessed May 8, 2020. https://www.ruralhealthresearch.org/alerts/265
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 144 kb)