Abstract
Corona virus disease-19 (covid-19) is caused by a coronavirus that is also known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and is generally characterized by fever, respiratory inflammation, and multi-organ failure in susceptible hosts. One of the first things during inflammation is the response by acute phase proteins coupled with coagulation. The angiotensinogen (a substrate for hypertension) is one such acute phase protein and goes on to explain an association of covid-19 with that of angiotensin-converting enzyme-2 (ACE2, a metallopeptidase). Therefore, it is advisable to administer, and test the efficacy of specific blocker(s) of angiotensinogen such as siRNAs or antibodies to covid-19 subjects. Covid-19 activates neutrophils, macrophages, but decreases T-helper cells activity. The metalloproteinases promote the activation of these inflammatory immune cells, therefore; we surmise that doxycycline (a metalloproteinase inhibitor, and a safer antibiotic) would benefit the covid-19 subjects. Along these lines, an anti-acid has also been suggested for mitigation of the covid-19 complications. Interestingly, there are three primary vegetables (celery, carrot, and long-squash) which are alkaline in their pH-range as compared to many others. Hence, treatment with fresh juice (without any preservative) from these vegies or the antioxidants derived from purple carrot and cabbage together with appropriate anti-coagulants may also help prevent or lessen the detrimental effects of the covid-19 pathological outcomes. These suggested remedies might be included in the list of putative interventions that are currently being investigated towards mitigating the multi-organ damage by Covid-19 during the ongoing pandemic.
Keywords: Connexin, Matrix metalloproteinases, Doxycycline, Endocardial endothelia, Fibrosis/remodeling
Introduction
Unfortunately, covid-19 related deaths in the Western hemisphere have already reached many thousands as compared to hundreds in the developing countries. This may, in part, be due to that hydroxychloroquine (HCQ) is being heavily prescribed to covid-19 patients as one of the treatments for covid-19 sickness. This review addresses that a low non-toxic dose of chloroquine (CQ) can benefit the patients against covid-19 (https://www.worldometers.info/coronavirus/) [1, 2]. It is important to test the concept that complications by covid-19 or viral myocarditis such as congestive heart failure (CHF) can be mitigated by HCQ (a more potent derivative of CQ) (Fig. 1).
Fig. 1.
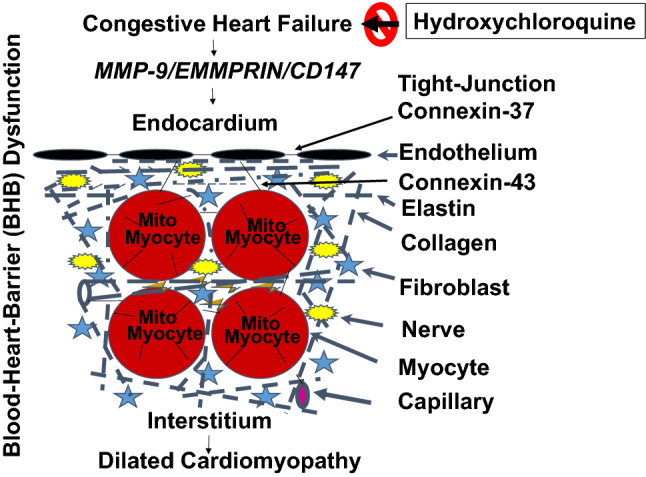
HCQ, doxycycline, and the anti-acid mitigate CHF, and DCM, such as in aging, and viral myocarditis, caused by the chronic volume overload/preload. CHF instigates EE leakage and disrupts endothelial-endothelial, endothelial-myocyte, myocyte-myocyte and mitochondrial (Mito)-myocyte junctions. Activation of MMP-9 is the hallmark of CHF that disrupts connexin-37, and 43 leading to EE leakage, and DCM. HCQ, doxycycline, and anti-acid could help mitigate both CHF, and DCM. Abbreviations: HCQ; hydroxychloroquine, MMP-9; matrix metalloproteinase, EMMPRIN; extracellular matrix metalloproteinase inducer, CD-47; cluster of Differentiation 47
Several drugs have recently been either suggested or being clinically administered to relive the symptoms of covid-19. These include dapagliflozin (sodium glucose co-transporter 2; SGLT2 inhibitor; an antidiabetic), Lopinavir/Ritonavir, Darunavir/Umifenovir (anti-HIV), Remdesivir (anti-Ebola), Favipiravir, and Dipyridamole (anti-hypertensive) [2–13]. Also, an anti-acid (Famotidine) is also being promoted [14]. Drugs like Famotidine (tradename; Pepcid) are histamine receptor antagonists that are routinely used to treat, and prevent certain types ulcers, and to treat conditions that cause the stomach to produce too much acid, and also to treat gastroesophageal reflux disease condition. Clinical evidence of the role of histamine in heart has been well documented, and histamine receptor antagonist in hypertension are cardioprotective [15–17]. Interestingly, HCQ is unique in the sense that, at low doses, it mitigates or blunts both the virus’s direct effects, as well as the immune reaction/response. It is, therefore; important to employ a low dose HCQ to mitigate CHF, and viral myocarditis-induced illness. In fact, a clinical study compared the suppressive effects of dipyridamole, and chloroquine on SARS-COV-2 replication, and suggested a similar titer at a concentration of just 100 nM [3]. All this may suggest that this lower dose may be more effective clinically.
Proteinase and covid-19
An association of covid-19, and angiotensin-converting enzyme-2; ACE2 (a metallo-endopeptidase) has been put forward. Hence, covid-19 effects can possibly be mitigated by an inhibitor of metallo-enzymes. Because cardiac matrix is highly unique, and in that very context a cardio-specific matrix metalloproteinase (MMP) inhibitor may well suitably mitigate the blood-heart-barrier (BHB) leakage, and the subsequent dilated cardiomyopathy (DCM) phenotypes. We along this very line propose a cardiac-specific MMP inhibitor regulator (i.e. tissue inhibitor of metalloproteinase;TIMP) to reduce the chances of mortality that is related to covid-19 (Fig. 1).
HCQ intervention will be like the treatment with doxycycline; a suggested MMP inhibitor as reported in the prestigious journal; Nature Reviews for the purpose of tissue remodeling that reverses the endocardial endothelial (EE) dysfunction. Previously, we also demonstrated that an antibiotic mitigated matrix metalloproteinases (MMPs) activation during heart failure [18, 19]. However, it is worth mentioning here that other common antibiotics such as azithromycin, clarithromycin, and erythromycin belonging to the ‘macrolide’ class have been shown to increase the risk of cardiac arrhythmias or even cardiac death [20]. Although, the use of broad-spectrum antibiotics as an antimicrobial therapy is a lifesaving strategy for patients in the intensive care but antibiotics also dramatically increase the risk for nosocomial infections, for example, the hospital-acquired pneumonia [21]. In a different context, it is unclear whether a salubrious effect arising from the use of a probiotic could also mitigate the MMPs’ activation by covid-19. We showed by a 2-D zymography (that is MMPs’ function and the proteome), the constitutive expression of MMP-2 in the control autopsy human heart sample; however, in the end-stage of the heart failure, the MMP-2, as well as, MMP-9 activities were found to be robust [22, 23]. More recently, we went on to provide an evidence that a long-term probiotic treatment could help decrease the MMPs’ activities [24] (Fig. 1). Further, nicotinamide, and mitochondria via SIRT mechanism regulate bioenergetics as demonstrated by us way back in 2002, showing that nicotinamide did alleviate chronic heart failure syndrome [25]. A little later in 2004, our laboratory revealed that doxycycline could mitigate the deleterious implications between the endothelial- myocyte interaction(s) during the heart failure condition [19].
By now we are aware that the thromboembolic complications are responsible for morbidity and mortality among the susceptible covid-19 patients; however, the data also suggest a possible multifactorial basis of these complications. While every effort is being made by the medical experts to treat patients by taking suitable preventive measures employing anticoagulation therapeutics to deal with the coagulation issues. Despite superb benefits with the use of systemic anticoagulation therapies, the data seem to be retrospective in nature thus raising some questions on the possible interplay of other confounders, as well as, long-term benefits and safety of the systemic anticoagulation approach [26–34].
Blood-heart-barrier (BHB) leakage
The endothelium, whether it is in the endocardium or in coronary or capillaries, is the primary barrier against BHB dysfunction. The tight-junction proteins, viz., connexin-37 between endothelium and endothelium, connexin-43 between endothelium and myocyte, myocyte and myocyte, and mitochondria (mito) and myocyte are the primary connexins; however, it is important to determine the details of the events and mechanism(s) of BHB leakage during covid-19 infection, though. The juxtacrine endothelial-myocyte (E-M), myocyte-myocyte (M-M), and mitochondria (mito)-myocyte uncoupling(s) [23, 35–40] are the hallmarks of cardiac failure (Fig. 1). The role of connexin-43 which connects myocyte-myocyte, and mitochondria (mito)-myocyte should also be studied in the productive covid-19 infection scenario [41–43]. It is already known that the connexin-37 connects the endothelial and myocyte (E-M). In E-M, M-M, and mito-myocyte uncoupling(s), the role of MMP in degrading the connexins that are responsible for causing BHB dysfunction is unclear, as of today. Basement membrane between the endothelium, and muscle contains an extracellular matrix (ECM), latent MMPs/TIMPs/nitric oxide; NO (the ternary complex) (Fig. 1). However, oxidative stress during CHF activates MMPs, and inactivates the TIMPs via the peroxinitrite, and tyrosine/arginine nitosylation process [22].
The usage of antioxidants has been widely mentioned in the literature for their beneficial effects in chronic conditions because anthocyanins, phenolic acids, and carotenoids are the predominant phytochemicals that are present in purple carrots, and cabbage. Accordingly, they have been promoted in treatment of the metabolic syndromes because anthocyanins improve dyslipidemia, glucose tolerance, hypertension, and insulin resistance. Moreover, these phenolic acids may also protect against the cardiovascular diseases and, in fact. the β-carotene was shown to protect against the oxidative processes, as well [44, 45].
Conclusion and perspective
The role of HCQ in cardiac, and skeletal muscle remodeling is novel. The mitigation of systemic remodeling during CHF by HCQ is an innovative approach. The cardiac-specific MMP-9 can be inhibited by HCQ, and going by the foregoing discussion, it is therapeutically novel, including its potential clinical applications in the covid-19 patients (Fig. 1).
Acknowledgements
The authors would like to thank all members of the laboratory for their continued help, and excellent support. Part of this study was supported by NIH Grants AR-71789, HL139047, and DK116591.
Author contributions
MS and SCT conceived, wrote, edited, and finalized the manuscript while. The lab members helped in providing reagents, and the necessary feedback in moving forward this project. Authors approved the final version of the manuscript before its submission.
Funding
A part of this work was supported by NIH Grants: AR-71789, HL139047, and DK116591.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, Nailescu A, Corona A, Zangrillo A, Protti A, Albertin A, Forastieri Molinari A, Lombardo A, Pezzi A, Benini A, Scandroglio AM, Malara A, Castelli A, Coluccello A, Micucci A, Pesenti A, Sala A, Alborghetti A, Antonini B, Capra C, Troiano C, Roscitano C, Radrizzani D, Chiumello D, Coppini D, Guzzon D, Costantini E, Malpetti E, Zoia E, Catena E, Agosteo E, Barbara E, Beretta E, Boselli E, Storti E, Harizay F, Della Mura F, Lorini FL, Donato Sigurta F, Marino F, Mojoli F, Rasulo F, Grasselli G, Casella G, De Filippi G, Castelli G, Aldegheri G, Gallioli G, Lotti G, Albano G, Landoni G, Marino G, Vitale G, Battista Perego G, Evasi G, Citerio G, Foti G, Natalini G, Merli G, Sforzini I, Bianciardi L, Carnevale L, Grazioli L, Cabrini L, Guatteri L, Salvi L, Dei Poli M, Galletti M, Gemma M, Ranucci M, Riccio M, Borelli M, Zambon M, Subert M, Cecconi M, Mazzoni MG, Raimondi M, Panigada M, Belliato M, Bronzini N, Latronico N, Petrucci N, Belgiorno N, Tagliabue P, Cortellazzi P, Gnesin P, Grosso P, Gritti P, Perazzo P, Severgnini P, Ruggeri P, Sebastiano P, Covello RD, Fernandez-Olmos R, Fumagalli R, Keim R, Rona R, Valsecchi R, Cattaneo S, Colombo S, Cirri S, Bonazzi S, Greco S, Muttini S, Langer T, Alaimo V, Viola U. Baseline characteristics and outcomes of 1591 Patients Infected With SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourao MPG, Brito-Sousa JD, Baia-da-Silva D, Guerra MVF, Hajjar LA, Pinto RC, Balieiro AAS, Pacheco AGF, Santos JDO, Jr, Naveca FG, Xavier MS, Siqueira AM, Schwarzbold A, Croda J, Nogueira ML, Romero GAS, Bassat Q, Fontes CJ, Albuquerque BC, Daniel-Ribeiro CT, Monteiro WM, Lacerda MVG. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (sars-cov-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Li Z, Liu S, Sun J, Chen Z, Jiang M, Zhang Q, Wei Y, Wang X, Huang YY, Shi Y, Xu Y, Xian H, Bai F, Ou C, Xiong B, Lew AM, Cui J, Fang R, Huang H, Zhao J, Hong X, Zhang Y, Zhou F, Luo HB. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm Sin B. 2020 doi: 10.1016/j.apsb.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badyal DK, Mahajan R. Chloroquine: can it be a novel drug for COVID-19. Int J Appl Basic Med Res. 2020;10:128–130. doi: 10.4103/ijabmr.IJABMR_141_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashem AM, Alghamdi BS, Algaissi AA, Alshehri FS, Bukhari A, Alfaleh MA, Memish ZA. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: A narrative review. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klimke A, Hefner G, Will B, Voss U. Hydroxychloroquine as an aerosol might markedly reduce and even prevent severe clinical symptoms after SARS-CoV-2 infection. Med Hypotheses. 2020;142:109783. doi: 10.1016/j.mehy.2020.109783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med PharmacolSci. 2020;24:4539–4547. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 8.Piszczatoski CR, Powell J. Emergency approval of chloroquine and hydroxychloroquine for treatment of COVID-19. Ann Pharmacother. 2020 doi: 10.1177/1060028020925558. [DOI] [PubMed] [Google Scholar]
- 9.Shukla AM, Archibald LK, Shukla AW, Mehta HJ, Cherabuddi K. Chloroquine and hydroxychloroquine in the context of COVID-19. Drugs Context. 2020 doi: 10.7573/dic.2020-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taccone FS, Gorham J, Vincent JL. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir Med. 2020 doi: 10.1016/s2213-2600(20)30172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docherty KF, Jhund PS, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, Sjostrand M, Langkilde AM, Desai AS, Diez M, Howlett JG, Katova T, Ljungman CEA, O'Meara E, Petrie MC, Schou M, Verma S, Vinh PN, Solomon SD, McMurray JJV. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costanzo M, De Giglio MAR, Roviello GN. SARS-CoV-2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem. 2020 doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- 13.Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, Yarbrough A, Abzug MJ, MacBrayne CE, Soma VL, Dulek DE, Vora SB, Waghmare A, Wolf J, Olivero R, Grapentine S, Wattier RL, Bio L, Cross SJ, Dillman NO, Downes KJ, Timberlake K, Young J, Orscheln RC, Tamma PD, Schwenk HT, Zachariah P, Aldrich M, Goldman DL, Groves HE, Lamb GS, Tribble AC, Hersh AL, Thorell EA, Denison MR, Ratner AJ, Newland JG, Nakamura MM. Multicenter initial guidance on use of antivirals for children with COVID-19/SARS-CoV-2. J Pediatric Infect Dis Soc. 2020 doi: 10.1093/jpids/piaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogosnitzky M, Berkowitz E, Jadad AR. Delivering benefits at speed through real-world repurposing of off-patent drugs: the COVID-19 pandemic as a case in point. JMIR Public Health Surveill. 2020;6:e19199. doi: 10.2196/19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saheera S, Potnuri AG, Nair R. Histamine-2 receptor antagonist famotidine modulates cardiac stem cell characteristics in hypertensive heart disease. PeerJ. 2017;5:e3882. doi: 10.7717/peerj.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gergs U, Kirchhefer U, Bergmann F, Künstler B, Mißlinger N, Au B, Mahnkopf M, Wache H, Neumann J. Characterization of stressed transgenic mice overexpressing H(2)-histamine receptors in the heart. J PharmacolExpTher. 2020;374:479–488. doi: 10.1124/jpet.120.000063. [DOI] [PubMed] [Google Scholar]
- 17.Kitakaze M. Clinical evidence of the role of histamine in heart failure. J Am CollCardiol. 2016;67:1553–1555. doi: 10.1016/j.jacc.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 18.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 19.Camp TM, Tyagi SC, Aru GM, Hayden MR, Mehta JL, Tyagi SC. Doxycycline ameliorates ischemic and border-zone remodeling and endothelial dysfunction after myocardial infarction in rats. J Heart Lung Transplant. 2004;23:729–736. doi: 10.1016/j.healun.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Cheng YJ, Nie XY, Chen XM, Lin XX, Tang K, Zeng WT, Mei WY, Liu LJ, Long M, Yao FJ, Liu J, Liao XX, Du ZM, Dong YG, Ma H, Xiao HP, Wu SH. The role of macrolide antibiotics in increasing cardiovascular risk. J Am CollCardiol. 2015;66:2173–2184. doi: 10.1016/j.jacc.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Lohmeyer J, Morty RE, Herold S. Antibiotic therapy-induced collateral damage: IgA takes center stage in pulmonary host defense. J Clin Invest. 2018;128:3234–3236. doi: 10.1172/jci122032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt MJ, Aru GM, Hayden MR, Moore CK, Hoit BD, Tyagi SC. Induction of oxidative stress and disintegrin metalloproteinase in human heart end-stage failure. Am J Physiol Lung Cell MolPhysiol. 2002;283:L239–L245. doi: 10.1152/ajplung.00001.2002. [DOI] [PubMed] [Google Scholar]
- 23.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 24.George AK, Singh M, Pushpakumar S, Homme RP, Hardin SJ, Tyagi SC. Dysbiotic 1-carbon metabolism in cardiac muscle remodeling. J Cell Physiol. 2020;235:2590–2598. doi: 10.1002/jcp.29163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox MJ, Sood HS, Hunt MJ, Chandler D, Henegar JR, Aru GM, Tyagi SC. Apoptosis in the left ventricle of chronic volume overload causes endocardial endothelial dysfunction in rats. Am J Physiol Heart CircPhysiol. 2002;282:H1197–H1205. doi: 10.1152/ajpheart.00483.2001. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed SI, Khan S. Coagulopathy and Plausible Benefits of Anticoagulation Among COVID-19 Patients. CurrProblCardiol. 2020;45:100648. doi: 10.1016/j.cpcardiol.2020.100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choudry FA, Hamshere SM, Rathod KS, Akhtar MM, Archbold RA, Guttmann OP, Woldman S, Jain AK, Knight CJ, Baumbach A, Mathur A, Jones DA. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am CollCardiol. 2020;76:1168–1176. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patti G, Lio V, Cavallari I, Gragnano F, Riva L, Calabrò P, Di Pasquale G, Pengo V, Rubboli A. Antithrombotic treatments in patients with SARS-CoV-2 infection: from current evidence to reasonable recommendations - A position paper from the Italian Working Group on Atherosclerosis, Thrombosis and Vascular Biology. G ItalCardiol (Rome) 2020;21:489–501. doi: 10.1714/3386.33634. [DOI] [PubMed] [Google Scholar]
- 30.Sagardia LM, Daniels LM. Thrombolysis and use of argatroban for the treatment of massive pulmonary embolism following anticoagulation failure in a patient with COVID-19. Am J Health Syst Pharm. 2020;77:1961–1964. doi: 10.1093/ajhp/zxaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah A, Donovan K, McHugh A, Pandey M, Aaron L, Bradbury CA, Stanworth SJ, Alikhan R, Von Kier S, Maher K, Curry N, Shapiro S, Rowland MJ, Thomas M, Mason R, Holland M, Holmes T, Ware M, Gurney S, McKechnie SR. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study. Crit Care. 2020;24:561. doi: 10.1186/s13054-020-03260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sridharan GK, Vegunta R, Rokkam VRP, MeyyurAravamudan V, Vegunta R, Khan SR, Ponnada S, Boregowda U, Prudhvi K, Chamarthi G, Mohan BP. Venous thromboembolism in hospitalized COVID-19 patients. Am J Ther. 2020;27:e599–e610. doi: 10.1097/mjt.0000000000001295. [DOI] [PubMed] [Google Scholar]
- 33.Watson RA, Johnson DM, Dharia RN. Merli GJ and Doherty JU (2020) Anti-coagulant and anti-platelet therapy in the COVID-19 patient: a best practices quality initiative across a large health system. HospPract. 1995;48:169–179. doi: 10.1080/21548331.2020.1772639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijaya I, Andhika R, Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients With COVID-19: A systematic review. ClinApplThrombHemost. 2020;26:1076029620960797. doi: 10.1177/1076029620960797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zima AV, Pabbidi MR, Lipsius SL, Blatter LA. Effects of mitochondrial uncoupling on Ca(2+) signaling during excitation-contraction coupling in atrial myocytes. Am J Physiol Heart CircPhysiol. 2013;304:H983–H993. doi: 10.1152/ajpheart.00932.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez WE, Sen U, Tyagi N, Kumar M, Carneal G, Aggrawal D, Newsome J, Tyagi SC. PPAR gamma agonist normalizes glomerular filtration rate, tissue levels of homocysteine, and attenuates endothelial-myocyte uncoupling in alloxan induced diabetic mice. Int J BiolSci. 2008;4:236–244. doi: 10.7150/ijbs.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen U, Tyagi N, Moshal KS, Kartha GK, Rosenberger D, Henderson BC, Joshua IG, Tyagi SC. Cardiac synchronous and dys-synchronous remodeling in diabetes mellitus. Antioxid Redox Signal. 2007;9:971–978. doi: 10.1089/ars.2007.1597. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberger D, Moshal KS, Kartha GK, Tyagi N, Sen U, Lominadze D, Maldonado C, Roberts AM, Tyagi SC. Arrhythmia and neuronal/endothelial myocyte uncoupling in hyperhomocysteinemia. Arch PhysiolBiochem. 2006;112:219–227. doi: 10.1080/13813450601093443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra PK, Tyagi N, Sen U, Joshua IG, Tyagi SC. Synergism in hyperhomocysteinemia and diabetes: role of PPAR gamma and tempol. CardiovascDiabetol. 2010;9:49. doi: 10.1186/1475-2840-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyagi SC, Rodriguez W, Patel AM, Roberts AM, Falcone JC, Passmore JC, Fleming JT, Joshua IG. Hyperhomocysteinemic diabetic cardiomyopathy: oxidative stress, remodeling, and endothelial-myocyte uncoupling. J CardiovascPharmacolTher. 2005;10:1–10. doi: 10.1177/107424840501000101. [DOI] [PubMed] [Google Scholar]
- 41.Givvimani S, Qipshidze N, Tyagi N, Mishra PK, Sen U, Tyagi SC. Synergism between arrhythmia and hyperhomo-cysteinemia in structural heart disease. Int J PhysiolPathophysiolPharmacol. 2011;3:107–119. [PMC free article] [PubMed] [Google Scholar]
- 42.Tyagi N, Vacek JC, Givvimani S, Sen U, Tyagi SC. Cardiac specific deletion of N-methyl-d-aspartate receptor 1 ameliorates mtMMP-9 mediated autophagy/mitophagy in hyperhomocysteinemia. J Recept Signal Transduct Res. 2010;30:78–87. doi: 10.3109/10799891003614808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Brodsky S, Kumari S, Valiunas V, Brink P, Kaide J, Nasjletti A, Goligorsky MS. Paradoxical overexpression and translocation of connexin43 in homocysteine-treated endothelial cells. Am J Physiol Heart CircPhysiol. 2002;282:H2124–H2133. doi: 10.1152/ajpheart.01028.2001. [DOI] [PubMed] [Google Scholar]
- 44.Poudyal H, Panchal S, Brown L. Comparison of purple carrot juice and β-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br J Nutr. 2010;104:1322–1332. doi: 10.1017/s0007114510002308. [DOI] [PubMed] [Google Scholar]
- 45.Miller HE, Rigelhof F, Marquart L, Prakash A, Kanter M. Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J Am CollNutr. 2000;19:312s–319s. doi: 10.1080/07315724.2000.10718966. [DOI] [PubMed] [Google Scholar]


