Abstract
Gut microbiota (GM) is involved in the maintenance of physiological homeostasis, thus the alteration of its composition and functionality has been associated with many pathologies such as metabolic diseases, and could also be linked with the progressive degenerative process in aging. Nowadays, life expectancy is continuously rising, so the number of elder people and the consequent related pathologies demand new strategies to achieve healthy aging. Besides, actual lifestyle patterns make metabolic diseases a global epidemic with increasing trends, responsible for a large mortality and morbidity in adulthood and also compromising the health status of later stages of life. Metabolic diseases and aging share a profile of low-grade inflammation and innate immunity activation, which may have disturbances of GM composition as the leading mechanism. Thus, GM emerges as a therapeutic target with a double impact in the elderly, counteracting both aging itself and the frequent metabolic diseases in this population. This review summarizes the role and compositional changes of the GM in aging and its modulation through nutritional interventions and physical exercise as a strategy to counteract the aging process and the related metabolic diseases.
Keywords: dietary intervention, elderly, gut microbiota, metabolic diseases, physical exercise, prebiotics, probiotics
1. Introduction
Life expectancy has been on the rise constantly since the second part of the last century and it is projected to continue this trend in the next years [1,2]. In developed countries, men can easily reach the age of 79 and this number is slightly higher for women (up to 84 years old) [1], thus adults over 65 years old would constitute 20–25% of total population in Europe and the US by 2030 [3]. Modern societies need to deal with an increasingly aging population, being a major burden for public health systems due to the huge number of associated comorbidities associated with older adults. This is worsened by the so-called drugs-related problems, as polypharmacy is common thorough elder people, demanding even more medical resources and reducing the efficacy of prescribed treatments [4]. Thus, exploring methods that can guide us towards a healthy aging besides neglecting pharmacological and surgical therapies is a promising research strategy.
The term “age-related diseases” (ARDs) [5] has been proposed to describe a spectrum of chronic disorders that usually manifests in the elderly, despite the fact that its triggering may be accelerated by circumstances occurring in the adult age or even in childhood, frequently associated to bad nutritional patterns and a sedentary lifestyle. Therefore, although a good management of ARDs may need prevention strategies before the onset of these pathologies, targeting the mechanisms that contribute to aggravate them should also be considered [6,7]. An active lifestyle including habitual physical exercise is widely accepted as a valid method to promote health in all stages of life. The benefits of exercise include body weight reduction, counteraction of blood hypertension and dyslipidemia or insulin resistance attenuation, all of them factors contributing to prevent cardiovascular events and metabolic disorders [8]. Moreover, increased physical activity has a positive effect on mental health and cognitive function [9,10]. These benefits could be especially important in the elderly as this population have the higher sedentary rates, spending the vast majority of their daily time sitting [11].
A wide amount of research has focused on gut microbiota composition and functionality and its implications for the maintenance of a healthy status. The gut microbiota (GM) isdefined as a metabolic ecosystem of a huge variable microorganisms which habit in the gastrointestinal tract and establish a symbiotic relationship with the host. These microorganisms are, principally, bacteria, but it is also important the role and the presence of virus, fungi or archaeas. The abundance of the microbiota in humans is ten times higher than their somatic and germ line cells [12], reaching concentrations of 1011 colony-forming units (CFU) per gram of gut content in the colon [13]. In fact, the genes of this microbiota, called “microbiome”, are 150 times larger than the human genome [13].
Colonization of the intestine begins even before birth and it is susceptible to high variability due to its plasticity in the early stage of life, remaining more stable in the rest of the lifetime. The changes which take place in adulthood reflect environmental factors such as diet, antibiotic consumption, etc. Given its crucial role to maintain body homeostasis, disturbances of the GM are associated with a growing group of pathological conditions, being a potential initiating mechanism rather than a simple symptom. In this context, microbiota-based therapies are being considered as a potential strategy or at least an adjuvant for conditions like obesity, in the form of prebiotic, probiotic or symbiotic administration. Furthermore, the impact of lifestyle on the gut microbiota reinforces the therapeutic role of nutrition and physical activity, not only by their side effects due to body weight loss but to a direct action reshaping the microbiota.
In this review we aim to deep in the role of gut microbiota as a target for therapeutic strategies based on physical exercise and nutritional interventions in the elderly, with a special focus on the management of obesity and related metabolic diseases.
2. Metabolic Diseases in the Elderly
Obesity and associated comorbidities, including metabolic syndrome, non-alcoholic fatty liver disease (NAFLD) or type 2 diabetes mellitus (T2D) have experienced a dramatic increase over the last few years. Despite regional disparities, almost a third of the world’s population are overweight or obese, of which a range between 30–37% present NAFLD [14], increasing to 90% in morbidly obese people [15]. Meanwhile, 61% cases of T2D could be attributed to overweight [16]. Moreover, the risk of non-metabolic diseases, including cardiovascular, musculoskeletal diseases or cancer, also increases with obesity [17]. The prevalence of overweight and obesity tends to augment with age, despite a slight decrease in the population over 65 years [18]. This trend may be influenced by the difficult definition of obesity in the elderly due to the confluence of two naturally occurring processes: a progressive increase in fat content, particularly abdominal fat, and the loss of muscle mass and strength with aging (sarcopenia). Therefore, the traditional definition of obesity based on body mass index (BMI) is less accurate in this group as it tends to underestimate the effects of body composition. This is the reason that the term sarcopenic obesity has arisen to characterize the concurrence of increasing obesity rates in an aging population [19].
Adverse effects of obesity in older adults may be different to those observed in young and middle-aged individuals. Some authors have claimed an “obesity paradox”, as increased mortality associated to obesity is not always observed in elderly obese patients [20]. This could be attributed to the cofounding effect of unintentional weight loss due to other pathological conditions, a reduced risk to develop complications of obesity established in the elderly, or the above mentioned underestimation of body composition changes [3]. In fact, sarcopenic obesity may suppose a synergetic effect between both conditions, maximizing the risk for morbidity, mortality and physical disability [21]. Furthermore, obesity in the elderly could exert even more negative effects, for example being a threat to cognitive functionality. In a study on 1100 Chinese subjects aged 60 or above, abdominal obesity was associated to a higher risk of cognitive impairment independently of other environmental factors or metabolic conditions [22].
The role of inflammation on aging and its relationship with metabolic diseases is noteworthy. Both aging and metabolic diseases are accompanied by an inflammatory environment (metaflammation and inflammaging, respectively), thus potentially contributing to each other in a vicious cycle [23]. This shared low-grade inflammatory state between aging and metabolic diseases could be mediated by the innate immune system. In fact, mediators of the innate response-like pattern recognition receptors (PRRs) may act as sensors of a large number of molecules (pathogen-associated molecular patterns (PAMPs), and/or damage-associated molecular patterns (DAMPs)), including saturated fatty acids from the diet which contribute to obesity and T2D development, or cell debris and misfolded or oxidized proteins that are potential stimuli in inflammaging [24]. Moreover, microbial-derived products are involved in both processes, being potent ligands for PRRs. This, together with the plasticity of the gut ecosystem thorough lifetime points to the GM as the possible link between metabolism and aging [23]. The gut-liver axis may exert a critical role in this puzzle, as disturbances of the intestinal barrier are strongly associated to metabolic diseases [25]; besides, intestinal permeability tends to increase with age, favouring the leakage of potential immunomodulatory substances to the systemic blood and the liver [26]. These evidences reinforce the importance of targeting gut microbiota as a therapeutic strategy for metabolic disease, also in the elderly.
3. The Gut Microbiota as a Key Factor in the Development of Metabolic Diseases
In recent years, gut microbiota has been identified as a contributor factor in the development of many pathologies such as immune disorders (i.e., psoriasis or asthma), allergies, cardiovascular and neurodegenerative diseases, obesity, NAFLD, metabolic syndrome, T2DM or even some cancers [27]. Thus, microbiota-related research has risen drastically to understand the microbiota-host interaction and to know the exact role of the gut microbiome in these pathologies, with the purpose to identify new therapeutic strategies.
Microbiota diversity and composition alteration have been associated to obesity development, the manipulation of them being feasibly useful on therapy [28]. Host energy harvest and host energy metabolism are modulated and regulated by gut microbiota and its metabolites. In fact, it has been observed that gut microbiota is capable of promoting the intestinal absorption of monosaccharides, increasing as a consequence of de novo hepatic lipogenesis and triglycerides accumulation in the adipocytes [29]. Bäckhed et al. [30] conducted the first study that demonstrated the role of gut microbiota in the progression of obesity. They observed that conventional mice had 42% more total body fat compared with germ-free (GF) mice, instead of second ones having a higher caloric consumption. Therefore, when GF mice were colonized with normal microbiota from conventional animals, they suffered insulin resistance and an increase of 60% in their body weight despite a reduction in their food intake [31]. Moreover, human fecal microbiota transplantation to GF mice demonstrated that obesity-related microbiota phenotype could be transferred, confirming definitively that gut microbiota is a key factor in body weight management and fat deposition [32]. Nevertheless, these results could not be reproduced for all diets and mouse strains; more studies are necessary to understand how the gut microbiota participates in obesity [33]. It has been observed that microbiota composition of obese children is different in comparison with lean children [34,35] and it has been suggested that microbiota profile during early stages of life is hugely important to becoming obese in adult stage [36]. A great effort has been made to establish a microbial signature of obesity looking for a potential causative role of a determined bacterial taxa. The first evidence pointed to a higher abundance of bacteria of the phylum Firmicutes and a lower abundance of Bacteroidetes as a hallmark of obesity both in humans and rodents [37,38], although later research sometimes described the opposite, still being a controverted issue. Several reports of a reduction of bacterial diversity on obese subjects have also been released [39,40]. Related to specific modifications of microbial taxa at a deeper level than phylum, an increased abundance of Lactobacillus [41] or LPS producing bacteria belonging to the phylum Proteobacteria [42,43] and the depletion of potential beneficial bacteria like several species of Bifidobacterium genus, Faecalibacterium prausnitzii or Akkermansia muciniphila, has been described [44]. To sum up, a recent systematic review confirmed the widely assumed increased Firmicutes to Bacteroidetes ratio in obese humans, with a vast majority of studies reporting similar results; however, a great variability was observed regarding the other findings [45].
On the other side, the implication of gut microbiota in many liver diseases, such as non-alcoholic and alcoholic fatty liver, cirrhosis, hepatic encephalopathy or even hepatocarcinoma (HCC), has been widely studied [46,47,48]. Le Roy et al. [49] suggested for the first time the role of gut microbiota in NAFLD progression, due to the steatosis development in GF mice as consequence of fecal microbiota transplant from high-fat diet (HFD)-fed mice. Moreover, microbial products of fermentation such as ethanol have been pointed to induce obesity and facilitate the development of fatty liver disease, as well as gut microbiota could promote hepatic steatosis through the modulation of bile acid metabolism [36]. The main hypothesis to explain how gut microbiota alteration, known as intestinal dysbiosis, affects to NAFLD development is based on the fact that the major part of liver’s blood is supplied by the portal vein, which connects the gut with the liver in the known “gut-liver axis” and allows one to enter endotoxins and microbial-products to the liver. On a healthy status, these bacteria metabolites and toxins are counteracted by an immune system. However, high-fat diets, sedentary habits or intestinal dysbiosis are capable of disrupt intestinal barrier and, as consequence, alter gut permeability, allowing the entrance of high amounts of bacteria and their products to the liver [33] and increasing metabolic endotoxemia. One of the most important bacteria endotoxins is the lipopolysaccharide (LPS), which activates the nuclear factor kappa B (NF-κB) signaling pathway through Toll-like receptor 4 (TLR-4) and induces the consequent liberation of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) or interleukin 6 (IL-6). All these microbiota alteration-originated changes lead to the promotion of an inflammatory hepatic state that facilitates and aggravates NAFLD development [33,50]. To establish a microbiota profile on NAFLD patients is difficult due to the spectrum of liver lesions beheld under this term and the confounding effect of the comorbidities associated to NAFLD. Some of the microbial changes observed in NAFLD were shared with obesity, as NAFLD is seen as another piece of the so-called metabolic syndrome. Thus, increased Proteobacteria phylum abundance or reduction in beneficial Faecalibacterium genus has been reported in human NAFLD [51]. Moreover, microbiota composition in NAFLD may evolve, along with the worsening of the condition. Comparing patients from different stages of liver damage suggests that Bacteroides may predominate in the microbiota of patients with NASH, while Ruminoccocus would be a signature of advanced liver fibrosis and cirrhosis [47]. Increased abundance of these taxa may also be associated with the development of cirrhosis-related HCC, along with the depletion of Akkermansia and Bifidobacterium [46].
The participation of the gut microbiota in T2D has been evidenced too. Comparisons of microbiota profiles of human adults with and without T2D demonstrated the existence of a completely different gut microbiota pattern in this disease, which is characterized by an increase of Bacteroidetes and Betaproteobacteria and a reduction of Clostridia. Moreover, a tendency to decrease Clostridia levels with the increasing plasma glucose levels in T2D disease was also observed [52]. Qin et al. [53] developed a metagenome-wide association study (MGWAS) to identify metagenomic markers in T2D and they concluded that patients with T2D had a moderate gut dysbiosis characterized by an increase in opportunistic pathogens and a reduction in butyrate-producers bacteria. Thus, reshaping the gut microbiota may be an alternative in the management of T2D. Tonucci et al. [54] concluded in their double-blind, randomized and placebo-controlled trial that the administration of probiotics could be a new approach for the prevention and treatment of T2D development because of its capacity to improve the glycemic control. Moreover, one of the main pharmacological strategies to deal with T2D, metformin, is capable of modify the gut microbiota composition, as Wu et al. [55] demonstrated in their research. They also showed that the fecal microbiota transplantation from metformin-treated patients to germ-free mice improved the glucose tolerance of the animals, suggesting that this altered microbiota could be taking part in the metformin effect.
This evidence reinforces the role of gut microbiota in metabolic diseases, which are constantly increasing worldwide. Due to the importance of healthy aging, the study of gut microbiota composition in the elderly and its modification by feasible therapies to prevent or treat metabolic diseases remains essential.
4. Gut Microbiota in the Elderly
As previously stated, even in physiological conditions, the gut microbiota is a dynamic ecosystem. The composition of the gut microbiota is based on permanent and transitory bacterial species of 17 different phyla such as Firmicutes, Bacteroidetes and Proteobacteria, which can reach as much as 70%, 30% and 5% of the total abundance, respectively, between others [56]. This composition changes depending on the anatomic region of the gastrointestinal tract, due to pH, secretions, motility or substrate availability. A gradual increase of bacterial concentration and complexity exists through the stomach and the gut, reaching the maximum in the colon. Moreover, GM experiments with taxonomical and functional changes during the life of an individual since the prenatal period. The microbiota colonization on the gastrointestinal tract may be started in utero with the placenta and amniotic fluid microbial communities of the mother, as recent research has observed when comparing these microbial populations with the meconium ones [57]. Microbiota profile constitution is affected by numerous factors, such as genetic components, type of delivery (vaginal or cesarean), the feeding (breast-feeding or formula-feeding) or antibiotics and/or probiotics consumption during the first days of life. The initial gut microbiota of infants is a quite instable simple structure dominated by bifidobacteria [58] and is in continuous change until the age of three years. At this moment, the microbiota profile is established and acquires an adult pattern that is relatively stable over time. Nevertheless, there are many causes that can modify this adult profile, for instance lifestyle, exercise, dietary patterns, stress or pathophysiology. Microbiota changes drastically in the elderly and these age-related changes are directly correlated with an inflammatory pattern linked with many diseases. Generally, these changes are orientated to a loss of diversity, a reduction of the abundance of beneficial bacteria such as those which produce short-chain fatty acids (SCFAs) [59], a change in the dominant species or an increase of enteropathogens [60]. All of these modifications are associated with physiological changes in the gastrointestinal tract and in dietary patterns, and with a decrease in the immune system function [61], an increase in the inflammatory state and a feasible contribution to the progression of diseases [59] and frailty [61]. Regarding the changes in dietary choices in the elderly, reductions in taste, dentition, chewing ability and intestinal transit time are factors that contribute considerably [62].
Some researchers have focused on establishing the specific age-related microbiota profile. At phylum level, Firmicutes is predominant in adults, being reduced in the elderly, whereas there is some discrepancy about the increase or decrease of Bacteroidetes phylum with age [61,63,64]. Moreover, high levels of Proteobacteria phylum especially Enterobacteriaceae family [63] and Clostridia class [64]), as well as a decrease of Actinobacteria (especially Bifidobacterium, a genus with intestinal protective capacity) have been reported in old people [61,63,64,65]. In fact, inflammatory markers such as IL-6 or IL-8 have been associated with an enrichment in Proteobacteria phylum, which increases with age [66]. Biagi et al. [66] identified in an elder population in Italy a decrease in bacterial diversity, as well as a change in the relative proportion of Firmicutes phylum, an increase of Bacili and low levels of Clostridium cluster XIVa. This reduction in the abundance of Clostridium cluster XIVa was corroborated by later studies [58]. Moreover, Rahayu et al. [64] analyzed the gut microbiota composition of 80 volunteers from Bali and Java arranged in two groups depending on the age (25–45 age old and 70 years old), identifying a reduction of Bifidobacterium, Prevotella and Lactobacillus plantarum taxa and an increase in Enterobacteriaceae and Lactobacillus reuteri in the elderly subjects). Furthermore, Bian et al. [67] developed a study with 1095 healthy volunteers from different cities of China and showed a decrease of Bifidobacterium and Bacteroides genera in older subjects, whereas Dorea, Clostridium and Marvinbryantia genera were increased in that population. In this research, Faecalibacterium genus was identified as a core and stable microorganism among life. Additionally, Claesson et al. [68] observed that low levels of diversity were correlated with inflammatory markers, frailty and impaired health parameters, as well as diet patterns. Frailty has also been associated with a low abundance of butyrate-producers like Faecalibacterium prausnitzii [63,69], Lachnospiraceae family and Roseburia genus, whereas there are some species that associate positively with frailty, such as Eggerthella dolichum or E. lenta [69].
In spite of the great inter-variability among studies, there are some taxa which may be less susceptible to be modified by external factors and may constitute the core of gut microbiota composition in the elderly. The reduction in the abundance of Ruminococcus, Blautia or Clostridium cluster XIVa and Clostridium cluster IV and the major prevalence of facultative anaerobes like Escherichia coli are changes that have been observed in the elderly population across many studies [70]. Even so, it is difficult to establish a unique aging gut microbiota composition profile, due to many factors that modulate this internal ecosystem such as the ethnicity, lifestyle, dietary patterns, host genetics, the presence of comorbidities or even methodological tools. Those reasons reveal the importance to do more studies to reach a homogeneous aging gut microbiota signature.
In the elderly, the presence of comorbidities is a very common situation that requires polypharmacy in order to improve the health status. Thus, not only the comorbidities but also the polypharmacy are factors that modify drastically the composition and diversity of microbiota. In fact, Ticinesi et al. [71] studied the differences between a cohort of 76 elderly hospitalized and multimorbid patients and a group of 25 healthy active elderly volunteers. The β-diversity index showed that the microbiota profile of hospitalized patients was significantly different in comparison with non-hospitalized group. The number of drugs was negatively correlated with Chao-index α-diversity and with the taxa Massilia and Lachnospiraceae. Furthermore, Coprobacter, Helicobacter and Prevotella were positively correlated with polypharmacy. The hospitalization is another factor that modifies gut microbiota composition, characterized by a substantial decrease in Faecalibacterium prausnitzii, Desulfovibrio spp. or Bifidobacterium, between others, and a major increase of the abundance of enterobacteria [63]. Moreover, Claesson et al. [68] and Jeffery et al. [62] identified in a study which compared the gut microbiota of Irish elderly by their type of residence (community-dwelling, one day at hospital, short-term rehabilitation and residential care) a relationship between the institutionalization of elderly people and an increase of Firmicutes phylum and Parabacteroides, Eubacterium, Anaerotruncus and Coprobacillus genus, as well as a reduction in bacterial diversity and the abundance of short-chain fatty acid (SCFAs) producers. These results are in agreement with previous reports, indicating that the microbiota profile related to age is aggravated by polypharmacy, reducing the number of SCFAs producers such as Lachnospiraceae family and increasing the abundance of some enteropathogens like Helicobacter.
It is important to consider the importance and the difference between biological and chronological age, being first physiological age, which takes into consideration many issues such as lifestyle or environmental and genetic factors, and the second one the number of years a person has been alive. Bacterial diversity has been negatively correlated with biological age, but not with chronological age. Moreover, Ruminococcus, Coprobacillus and Eggerthella genera have been associated positively with biological age, independently of the chronological one [59]. Related to that, many studies have identified the microbiota profile of centenarians—those people close to 100 years old—showing a specific healthy composition closer to adults’ pattern and remarkably different to that commonly observed in elders over 65 years. Wang et al. [72] described the composition of gut microbiota of centenarians in East China and observed that the α-diversity was significantly increased, as well as the genus Escherichia and Roseburia in centenarians. Moreover, volunteers more than 100 years old also showed a decrease in the abundance of Lactobacillus, Butyricimonas, Coprococcus, Parabacteroides, Akkermansia, Sutterella and Faecalibacterium genera compared with the groups between 80 and 99 years old. Afterwards, Wang et al. [73] conducted a similar study in a larger population and described that community richness and α-diversity was significantly lower in the 65–70 years age group compared with the 90–99 and the 100+ year age groups. An increase in the relative abundance of Synergistetes phylum (with special mention of Prevotellaceae, Lachnospiraceae and Porphyromonadaceae) was observed in the longevity group compared with the younger elderly group. Similar results related to the increase of microbial diversity and Porphyromonas genus in centenarians were observed in a study of 367 Japanese volunteers [65]. Furthermore, Kim et al. [74] identified a minor relative abundance of Faecalibacterium and Prevotella, as well as an increase of Escherichia and Proteobacteria in centenarians of South Korea. Additionally, Kong et al. [75], considering the results of their own Chinese cohort and the results previously reported by Biagi et al. [76], identified an enrichment of Clostridium cluster XIVa, Akkermansia, Ruminococcaceae and Christensenellaceae in long-living groups. While many genera of Clostridium cluster XIVa are producers of SCFAs, Akkermansia and Chistensenellaceae have been identified as good metabolic health-related bacteria, associated with healthy homeostasis and immunomodulation. This suggests a tendency in the microbiota profile of centenarians towards a healthy and anti-inflammatory status [74,75]. Moreover, related to SCFAs producers, the microbiota profile of centenarians showed an increase in some butyrate producers (Anaerotruncus colihominis and Eubacterium limosum) and a decrease in others (Ruminococcus obeum, Roseburia intestinalis, E. ventriosum, E. rectale, E. hallii, Papillibacter cinnamovorans and Faecalibacterium prausnitzii), suggesting with these differences the presence of bacteria characteristics of longevity [77]. The association of longevity with Ruminococcus, a genus known as a SCFAs producer and with an important role in gut protection, is still contradictory [73]. All of these findings related to gut microbiota composition in elderly and in centenarians are summarized in Table 1.
Table 1.
Gut microbiota composition of the elderly (≥60 years old) and centenarians (≥99 years old).
| Reference | Subjects | Methodological Approach | Main Findings in Gut Microbiota Composition | |
|---|---|---|---|---|
| [67] Biagi et al. 2010 | 84 subjects from Northern Italy (50F, 34M). Young adults (20–40 years old) (Y), elderly (60–80 years old) (E), centenarians group (99–104 years old) (C) and offspring of the centenarians (59–78 years old) (F) | HITChip analysis and qPCR (16S rRNA) | Elderly | Centenarians |
|
|
|||
| [58] Claesson et al. 2011 | 161 elderly Irish subjects (82F, 79M) (>65 years old) and a control group (5F, 4M) (28–46 years old) | Pyrosequencing with 454 system (16S rRNA V4 region) |
|
|
| [72] Wang et al. 2015 | 24 volunteers from China (14F, 10M) classified in Group RC (100–108 years old), Group RE (85–99 years old) and Group CE (80–92 years old) | Illumina MiSeq and qPCR (16S rRNA V4 region) |
|
|
| [76] Biagi et al. 2016 | 24 semi-supercentenarians (>105 years old, group S) (18F, 6M) vs. 15 young adults (22–48 years old, group Y) (8F, 7M) from Northern Italy. Results of C and E groups from Biagi et al. 2010 study were incorporated. | Illumina MiSeq and qPCR (16S rRNA V3-V4 region) | Elderly | Centenarians |
|
|
|||
| ||||
| [75] Kong et al. 2016 | 168 Chinese individuals (85F, 83M) grouped into long-living group (≥90 years old), elderly group (65–83 years old) and a younger age group (24–64 years old) | Illumina MiSeq (16S rRNA V3-V4 region) |
|
|
| [64] Odamaki et al. 2016 | 367 Japanese volunteers between 0 and 104 years old (210F, 157M) | Illumina MiSeq and qPCR (16S rRNA V3-V4 region) | Elderly | Centenarians |
| - |
|
|||
| ||||
| [68] Bian et al. 2017 | A total of 1095 healthy Chinese volunteers (533F, 562M) classified into eight groups according to their age (children, adults, elderly, centenarians) | Illumina MiSeq (16S rRNA V4 region) | Elderly | Centenarians |
|
|
|||
| ||||
| [66] Rahayu et al. 2019 | 80 Indonesian subjects (50F, 30M): young group (25–45 years old) and elderly group (≥70 years old) | Yakult intestinal flora-scan (YIF-SCAN) (qPCR method) |
|
|
| [73] Wang et al. 2019 | 187 elderly subjects from three groups of age (65–70 years old), (90–99 years old) and (100+ years old) from East China (120F, 67M) | Illumina MiSeq (16S rRNA V3, V4 and V5 regions) | Elderly | Centenarians |
|
|
|||
| [74] Kim et al. 2019 | 56 South Korea subjects classified in centenarians (95–108 years old) (27F, 3M), elderly (67–79 years old) (7F, 10M) and adults (26–43 years old) (3F, 6M) | Pyrosequencing with 454 system (16S rRNA V1–V3 regions) | Elderly | Centenarians |
|
|
|||
F: female; M: male. Changes (↑: increase; ↓: decrease) in the relative abundance of selected microbial taxa and in bacterial diversity with age. Names in bold denote each group for the corresponding study and are defined in the table.
In these terms, not only the composition but also the metabolic pathways of microbiota change with age. Collino et al. [78] identified some alterations in a Northern Italian population linked to age, such as low concentrations of tryptophan and lysophospatidylcholines and increased levels of sphingomyelins and phospatidylcholine 32:0. On the other hand, some plasma metabolomic patterns such as lipids and amino acids have been related to health span markers in elderly [79]. Nevertheless, it has been observed that phosphatidylinositol, glycosphingolipid and N-glycan biosynthesis signaling pathways are increased in centenarians, all of them being associated with anti-inflammation and healthy status of gut microbiota [74]. Low levels of markers of lipid peroxidation, as 9-hydroxy-octadecadienoic acid (9-HODE) and 9-oxo-octadecadienoic acid (9-oxoODE), have been identified in longevity phenotype in a population of Italy [78], while centenarians in China showed high levels of SCFAs and total bile acids [80]. These results seem to reinforce the existence of a specific altered microbiota pattern in th+++e elderly with the particularity of a healthy microbiota composition and functionality in centenarians, with more research being necessary to elucidate such patterns.
5. Gut Microbiota Targeted Interventions in the Elderly
The field of gut microbiota manipulation, although relatively new, has attracted a lot of attention and the literature about this issue is constantly growing, however research focused on elderly population is still scarce. Dietary interventions and bioactive supplements have been explored as potential therapies for obesity and related diseases via gut microbiota composition alterations, obtaining promising results. More recently, physical exercise has demonstrated the ability to reshape gut microbiota, being another piece behind beneficial effects of this approach in the treatment of metabolic diseases [81]. Given the role of the microbiota in the elderly, it would be useful to elucidate the capacity of such alternative therapeutics to counteract both metabolic and aging-associated alterations. Here, we summarize studies assessing gut microbiota targeted interventions in the aged population (Table 2) or in animal models of aging, with or without the presence of obesity and associated comorbidities.
Table 2.
Dietary interventions, supplementation with probiotics and prebiotics and assessment of the effect of physical activity on elderly individuals, with or without the concurrence of metabolic diseases.
| Reference | Subjects | Methodological Approach/Intervention | Main Findings in Gut Microbiota Composition | |
|---|---|---|---|---|
| Dietary Interventions, Probiotics and Prebiotics | ||||
| [82] Cancello et al. 2019 | ≥65 years old obese women (20) | Longitudinal study. Hypocaloric mediterranean diet (15 days, DIET), hypocaloric mediterranean diet + VSL#3 probiotic mix (15 days, PRO) | DIET | PRO |
| ↑ Parabacteroides ↑ Bacteroides ↑ Christensenellaceae ↑ Methanobrebrevibacter ↓ Collinsela |
↑ Akkermansia | |||
| [83] Ghosh et al. 2020 | 65–80 years old (286 M and 326 F) | Randomized multicenter single-blind controlled trial. Mediterranean diet (DIET) and control group (CON) (1 year) |
DIET ↑ Faecalibacteriumn prausnitzii ↑ Roseburia ↑ Eubacterium ↑ Bacteroides thetaiotaomicron ↓ Ruminococcus torques ↓ Collinsela aerofaciens |
|
| [84] Mitchell et al. 2020 | ≥70 years old men (30) | Randomized single-blind controlled trial. Diet with the recommended protein intake (RDA) or twice the recommended protein intake (2RDA) (10 weeks) | No significant differences reported | |
| [85] Tran et al. 2019 | Young healthy (13 M and 16 F). Community dwelling (>65 years old; 10 M and 18 F) and institutionalized elderly (>65 years old; 9 M and 13 F) | Randomized single-blind controlled trial. Prebiotic mix (PRE) vs. placebo (maltodextrin). Single daily dose over 26 weeks (Double dose for frail subjects). |
PRE ↑ Ruminoccocaceae ↑ Parabacteroides ↑ Clostridium cluster IV ↑ Phascolarctobacterium (Significance reached for institutionalized subjects at 13 week) |
|
| [86] Kim et al. 2020 | ≥65 years old (53) | Randomized double-blind, multicenter controlled trial. Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI (1 × 109 CFU/dose) vs. placebo (two doses/day, 12 weeks) |
PRO
↓ Clostridiales ↓ Prevotellaceae ↓ Allisonela ↓ Eubacterium |
|
| Physical activity | ||||
| [87] Aoyagi et al. 2019 | 65–92 years old (140 M and 198 F) | Monitoring of daily physical activity by electronic device over 1 month. Comparative study between more active (>15 min/day at >3 METS;MA) and less active (<15 min min/day at >3 METS; LA) |
MA ↑ Bacillaceae ↓ Fusobacteriaceae |
|
| [88] Castro-Mejía et al. 2020 | >65 years old (109 M and 98 F) | Comparative study of patients stratified into high (HF) and low-physical fitness (LF) | HF | LF |
|
Bifidobacterium adolescentis
Christensenella |
Enterobacterales | |||
| [89] Taniguchi et al. 2018 | 62–76 years old men (33) | Randomised crossover trial. 3 sessions/week exercise in a cyclo-ergometer (TRA) and control sedentary period (CON; 5 weeks each) |
TRA ↑ Oscillospira (CON first only) ↓ C. difficile |
|
| [90] Morita et al. 2019 | >65 years old women (32) | Non-randomized comparative trial. 12 weeks of 1 h/daily brisk walking (TRA) vs. 1 h/weekly session of trunk muscle training (CON) | TRA | CON |
|
↑ Bacteroides
↓ Clostridium subcluster XIV |
↓ Clostridium subcluster IX | |||
| [91] Yu et al. 2018 | 65–80 years old patients with hypertension (32 M and 24 F) | Comparative study of patients classified according to Weber’ system for functional capacity. Normal exercise capacity (NE) vs. reduced exercise capacity (RE) | NE | RE |
|
↑ Betaproteobacteria
↑ Ruminococcaceae ↑ Faecalibacterium |
↑ Escherichia_Sigella
↑ Lactobacillales ↑ Lachnospiraceae ↑ Blautia |
|||
| [92] Zhu et al. 2020 | Healthy elderly and overweight elderly (>61 years old) (897) | Comparative study. Overweight elderly daily exercise (DROE, 74) group vs. overweight elderly never or rare exercise group (NROE, 222) |
DROE
↑ Turicibacteraceae ↓ Pseudomonadaceae ↓ Oxalobacteraceae ↓ Odoribacteraceae ↓ Barnesiellaceae |
|
F: female; M: male. Changes (↑: increase; ↓: decrease) in the relative abundance of selected microbial taxa and in bacterial diversity with the interventions. Names in bold denote each group for the corresponding study and are defined in the table.
5.1. Dietary Intervention
Diet is the principal contributor to determine the composition of the gut microbiota, although the great interindividual variability in older adults overwhelms the effects of other possible covariables [62,68]. This point is especially important due to the limitations in the diet of elder people which may suffer from low diversity and insufficient nutrient intake, since some type of foods are usually excluded due to their low palatability or difficulty to chew [93]. Thus, strategies directly guided to modify dietary patterns are more likely expected to be successful to counteract dysbiotic microbiota associated with metabolic diseases. Moreover, the administration of functional foods with prebiotic properties or even live bacteria as probiotics needs to be considered.
Mediterranean diet (MD) is highly appreciated due to its multiple health benefits associated with a lower risk of cardiometabolic diseases occurrence and to a reduced frailty [94]. The microbiota modulatory action of MD in the elderly population has been addressed both in the short term and in a one-year follow-up intervention. Fifteen days of MD in obese women (≥65 years) resulted in an approximately 3% weight loss, accompanied by a shift in microbiota composition towards reduced abundance of Collinsella and an increase in that of potentially beneficial bacteria like Parabacteroides, Bacteroides, Christensenellaceae or Methanobrebrevibacter [82]. Although nutritional intervention was the main objective of this study, it was a branch of a multi-intervention program that also included physical activity, so despite the short period, synergetic effect of both diet and exercise needs to be considered regarding changes in microbiota composition. Ghosh et al. [83] recently reported the results of a one-year multi-centre study involving elderly subjects under dietary guidance based on MD. Following random forest models, they identified a subset of taxa correlated with the adherence to the diet which respondedeither in a negative or positive trend. The taxa positively modified by the diet comprised well-known beneficial bacteria including Faecalibacteriumn prausnitzii and Roseburia, several species of Eubacterium genus, Bacteroides thetaiotaomicron, Prevotella copri and Anaerostipes hadrus. On the contrary, diet negatively regulated OTUs belonging to Ruminococcus torques, Collinsella aerofaciens, Coprococcus comes, Dorea formicigenerans, Clostridium ramosum, Veillonella dispar, Flavonifractor plautii and Actinomyces lingnae. In summary, MD seems to be associated in aged people with the increase in beneficial bacteria, some of them SCFA’s producers (Faecalibacteriumn prausnitzii, Roseburia), and the reduction of potential pathobionts (Collinsella).
Negative results of a dietary intervention in an elderly population in terms of gut microbiota composition modification were reported by Mitchell et al. [84]. Based on the recommendations of increasing protein intake in the elderly [95], 30 individuals aged 70 or older were allocated either to a group consuming the recommended dietary allowance (RDA) of protein or to a group consuming twice the RDA for 10 weeks. No differences in the microbiota composition or in the faecal volatile organic compounds were detected as a consequence of the intervention. Thus, a possible resilience of gut microbiota on the elderly to be modified by the diet should be considered, emphasising the usefulness of supplements which may increase the effectiveness of dietary interventions.
5.2. Prebiotic and Probiotic Supplementation
Prebiotic and probiotic supplementation may also be used to reverse aging-induced changes in gut microbiota. Human studies over the period 2005–2017 involving prebiotics, probiotics and their combination in healthy aged populations were collected and summarize elsewhere [61]. Briefly, interventions with Bifidobacterium species alone or in combination with prebiotics were the majority, but limited effects on gut microbiota composition were detected besides the increase in the relative abundance of Bifidobacterium genus and the potential reduction of opportunistic entheropathogens [96,97,98,99]. A recent study with prebiotics considered the possible implication of institutionalization in the elderly. The research, conducted over 3 populations (healthy young adults, community dwelling elderly and institutionalized elderly), reported that the microbiota from institutionalized elderly adults seemed to be more responsive to changes due to a prebiotic mix administration for 26 weeks [85]. Parabacteroides, Clostridium cluster IV, Ruminoccocaceae family and Phascolarctobacterium increased after prebiotic administration in this group in the short term (week 13). Thus, the efficacy of prebiotics to modify gut microbiota composition in the elderly could be related to frailty, but their effects on inflammation were very modest despite the long intervention period. Regarding probiotics, a mix of two Bifidobacterium species in an over 65 years old South Korean population, achieved a gradual and significant reduction of Allisonela, Eubacterium, Clostridiales order and Prevotellaceae family after 12 weeks [86]. The reduction of Prevotellaceae abundance is in contrast with the dynamics of this taxa under other treatments as exercise or MD where this genus shows the opposite trend. Nevertheless, probiotics were associated to an improvement in cognitive function and reduced stress, which strengthens the importance of the gut-brain axis in the elderly.
The previously mentioned study by Cancello et al. [82] also analyzes the impact of the widely used probiotic mix #VSL3 on obese women in combination with the MD intervention over 15 days following the initial phase of dietary intervention alone. Besides, significant increases in the bacterial genus present in the cocktail, the promising probiotic Akkermansia was found to increase after this period. No further research on the effect of probiotics in the microbiota of elderly obese humans has been carried out to date, however in an aged mouse model of HFD-induced obesity, the administration of a probiotic cocktail (5 Lactobacillus and 5 Enterococcus strains) was able to counteract metabolic syndrome and deeply reshape the gut microbiota [100]. Both α and β diversity were modified in response to the probiotic mix as well as phylum like Firmicutes and Verrucomicrobia which were increased and decreased, respectively. Going down to the species level, the abundance of Akkermansia muciniphila, Peptococcus niger and Ruminicoccus gnavus decreased, while two species of Clostridium, Roseburia faecis, Enterococcus lactis, Bacteroides salanitronis, and Lactobacillus rhamnosus increased. The decrease in A. muciniphila, bacteria known for its health promoting effect, is noteworthy. This study also provides an extent demonstration of gut-liver axis modulation in the beneficial effect of the probiotic, linked to leaky gut prevention and an anti-inflammatory effect, which is valuable both in the treatment of obesity and as antiaging therapy. Furthermore, probiotic treatment was associated to an improvement in physical aptitude of old mice. However, the absence of a control diet-fed group limited the utility of these results as it is not possible to determine which of the negative features and changes in microbial taxa observed in HFD-fed old mice ameliorated by the probiotic are a consequence of the diet or aging.
5.3. Physical Activity
Related to the role of exercise in the aged microbiota, some evidences came from studies that assess the relationship between the microbiota and the amount of physical activity routinely performed, or the physical fitness demonstrated in different standardized tests.
In a cohort of volunteers ranging from 65 to 92 years old that met the criteria of functional independence and absence of diseases that severely diminished quality of life, the monitoring of physical activity by electronic devices over a month was employed to identify changes associated to different degrees of activity in several selected bacterial taxa by qRT-PCR [87]. No significant differences were detected in the total counts of any of the bacterial taxa analyzed between more active (energy expenditure >3 metabolic equivalents (METs) for >15 min/day) and less active (energy expenditure >3 METs for <15 min/day) subjects, and only Bacillaceae and Fusobacteriaceae families differed significantly (increased and decreased, respectively in more active vs. less active) when the results were expressed as relative abundance of the total families detected by massive sequencing. Thus, in this study, despite being associated with improved intestinal health in older adults, routine physical activity seems to have a minor impact on gut microbiota composition. It is worth mentioning that sequencing data provided only went down to the family level, as authors assessed some specific genera and species by PCR techniques; so, possible effects of physical activity at those levels may be underestimated.
Following the other approach, Castro-Mejía et al. [88] classified individuals into two groups (high physical fitness and low physical fitness) according to their performance in the 30 s Chairstand test, BMI and percentage of leg-soft-tissue fat. Multivariant analysis identified a group of variables which can accurately discriminate between groups (dietary sources, plasma and fecal metabolites, microbial taxa, biochemical parameters and steps per day), including higher abundance of Bifidobacterium adolescentis and several species of Chritensenella genus in high fitness group, whereas the low fitness group microbiota was enriched in Enterobacterales. These results point to a complex relationship between the gut microbiota, the dietary and daily activity patterns and the resultant physical fitness. However, the design of the study limited the scope of the observations made. The parameters selected to stratify the participants may have a profound impact on the conclusions as BMI is taken into account to allocate individuals on the low or high fitness group; overweight or obesity are conditions that may underly the differences between groups, overestimating the contribution of physical activity or dietary patterns. Furthermore, the usage of a multivariant analysis approach may not be the most accurate to determine individual effects of the variables analyzed (i.e., gut bacteria) and may be affected by a large number of confounding factors.
There are scarce studies that carry out a controlled training program and report metagenomic analysis results in elderly population. A short-term endurance exercise program in a group of healthy Japanese aged 62–76 years only achieved modest effects on gut microbiota composition. Overall, 5 weeks of 3 sessions/week exercise on a cyclo-ergometer did not modify α or β diversity of bacterial communities. Just Oscillospira at the genus level and C. difficile at the species level showed an increase and a decrease, respectively, associated to exercise practice; however, the former only was detected in control period of the first-group and did not reach significance after adjusting for confounding dietary factors. Exercise was also associated to a functional modulation of the microbiota with increasing capacity for “genetic information processing” and “nucleotide metabolism”, according to PICRUSt predicted metagenomes [89]. This limited effect of exercise may be attributed to the short training protocol, as significant microbial adaptations to exercise probably require extended periods of time to take place.
Morita et al. [90] carried out a 12-week intervention in healthy elderly women, addressing the effects associated to different approaches to exercise practice. Women allocated to perform brisk walking as a type of aerobic exercise training showed an increase in Bacteroides and a decrease of Clostridium subcluster XIV at the end of the study, while the control group who performed trunk muscle training alone revealed only an increase of Clostridium cluster IX. Thus, opposite to results by Taniguchi et al. [89], aerobic exercise training seems to promote a significant change of bacterial composition towards increased Bacteroides abundance in an elderly population, correlating with an improvement of cardiorespiratory fitness. Besides the different protocol and duration of the intervention, gender could be another cause of discrepancies as these studies either enrolled only males or only females.
Two studies have addressed the effect of physical activity on an elderly population manifesting components of the metabolic syndrome, either hypertension or overweight. Hypertension is a condition highly associated to obesity and linked to increased risk of cardiovascular events. Patients with hypertension aged 65–80 showed a particular microbiota fingerprint according to its exercise capacity based on its peak oxygen uptake [91]. On one hand, the microbiota of patients with the normal exercise capacity was enriched in members of the class Betaproteobacteria, as well as the family Ruminococcaceae and the genus Faecalibacterium; the latter well known for its potential beneficial effects in the gastrointestinal tract and previously linked to exercise performance [101]. On the other hand, low exercise capacity patients revealed a higher relative abundance of the pathobiont Escherichia_Shigella genus, mainly attributed to Escherichia coli, and other bacterial taxa like Lactobacillales order, Lachnospiraceae family and the genus Blautia. Moreover, Lactobacillales and Blautia positively correlated with C reactive protein levels while the opposite was true for Alcaligenaceae genus, belonging to Betaproteobacteria class; this suggests a relationship between the abundance of those taxa in the low-grade inflammation of elderly and the capacity to perform physical activity. Furthermore, Zhu et al. [92], besides considering differences associated to aging in a large population from 18 to over 70 years old, analyzed the effect of exercise in the older groups and additionally compared overweight elderly individuals that performed regular exercise with those who never or rarely were involved in physical activity. The results obtained revealed that increasing amount of exercise gradually reshapes gut microbiota in over 70-year-old individuals towards that of adults (18–60 years), dose-dependently modifying the relative abundance of 13 families and 3 phyla, including the increase in the potentially beneficial Actinobacteria phylum and Bacteroidaceae family. The comparison within the overweight elderly population identified a reduced α-diversity at the OTU level associated to regular exercise practice. At the phylum level, exercise tended to revert the changes associated to overweight phenotype showing higher abundance of Bacteroidetes and Tenericutes and reduced Verrucomicrobia, Cyanobacteria and Firmicutes, although no significance was reached. Moreover, relative abundance of Turicibacteraceae family significantly increased in the regular exercise group, while that of Pseudomonadaceae, Oxalobacteraceae, Odoribacteraceae, and Barnesiellaceae showed and opposite pattern. This is the first study reporting differences associated to exercise signature in an elderly overweight population; however, the experimental design and the different comparisons presented make it difficult to follow and interpret the results despite the big sample size. Although limited differences were observed, the results suggest that daily or regular exercise may help to counteract the obesity associated dysbiosis in this population, including remarkable findings like a trend towards a lower Firmicutes to Bacteroidetes ratio. Overall, to date, the impact of physical activity on gut microbiota composition in an elderly population has been poorly investigated. The studies carried out describe really modest differences between more and less physically active older adults and the same was reported in the two unique studies involving controlled exercise programs. Moreover, the conflicting issue of BMI in the elderly population may mask the results obtained.
Some insights could also be extracted from studies on aged animals or models of accelerated aging related to physical activity impact on gut microbiota composition. It was demonstrated that aerobic capacity could be transmitted by selectively crossbred rats with either low or high aerobic capacity (LC and HC, respectively), resulting in a different susceptibility to diet-induced obesity, adiposity deposition and insulin resistance. Moreover, this phenotype may be driven by differences in gut microbiota composition [102]. Recently, the impact of aging in this model has been assessed, revealing age-dependent differences between HC and LC. Old adult HC rats were characterized by a decrease of Bacteroidetes, Spirochaetes and Deferribacteres at the phylum level, an increased abundance of the genera Prevotella, Mucispirillum and Treponema, while that of Phascolarctobacterium and unknown genera of Erysipelotrichaceae were reduced respect its age-matched LC counterparts [103]. It is remarkable that old HC rats had a significant lower body weight that could be either cause or consequence of this different microbial signature. On the contrary, no differences in several inflammation and innate immunity mediators were found between both aged groups. The findings of this study could help to understand how exercise capacity may influence age-associated changes in IM composition. Further research is needed to understand this relationship in the elderly, as the animals employed could only be considered adults in terms of human age (10 months is roughly equivalent to 25–30 human years). Houghton et al. [104] assayed the effect of a long term exercise protocol (up to 11 months) in mice with mitochondrial dysfunction (PolgAmut/mut) as a model of accelerate aging. Mitochondrial dysfunction was associated to changes in gut microbiota at the phylum level with reduced Bacteroidetes, while Firmicutes and Proteobacteria increased, although these differences tended to be attenuated over time. Moreover, the effect of natural aging in both control and mutant mice revealed a reduction of α-diversity, in terms of total different OTUs, in PolgAmut/mut mice and some changes at genus level, including a higher Alistipes and lower Lactobacillus relative abundance were maintained over time. Related to the effect of exercise in these mice, there were controversial results as exercise tended to ameliorate reduction of α-diversity, increased Bacteroides and reduced potential harmful bacteria like Helicobacter, however, Mucispirillum and Desulfovibrio, both genera associated to inflammation and gastrointestinal disorders, were significantly increase in the exercised group. Although this finding could draw attention over a potential negative effect of exercise on intestinal health in the elderly, this could be a feature of mitochondrial dysfunction induced by the model employed, whose influence may be smaller in humans.
Germ-free mice have proved their usefulness in the microbiota research and have been also employed to identify bacterial taxa associated to a high degree of physical functionality on sedentary older adults (70–85 years) [105]. Microbiota of the participants with the better functionality measures consistently showed an increased abundance of Prevotellaceae family and the genus Prevotella and Barnesiella, whereas a decrease in S24_7 family was detected across two repeated analysis within a month. Other significant differences were only reported in a single time point. Interestingly, the bacterial taxa enriched in the high functionality group replicated the same pattern in germ-free recipients of six selected donors for each group, resulting in a significantly higher grip strength in mice colonized with stools from high functionality donors, which suggests that these taxa may participate somehow in muscle maintenance. It is interesting the role of Prevotella against frailty and its relation with physical activity, as this genus has been found to be increased in the previously mentioned study by Pekkala et al. [103] in high fitness capacity rats and in human athletes or forced exercised rats [106,107].
Physical activity is undoubtedly associated to a healthy aging and to the counteraction of metabolic disorders. However, the modulation of gut microbiota composition as a direct mechanism involved in its health benefits has been barely investigated in both conditions, and more studies are needed to elucidate its role in obese older humans.
6. Conclusions
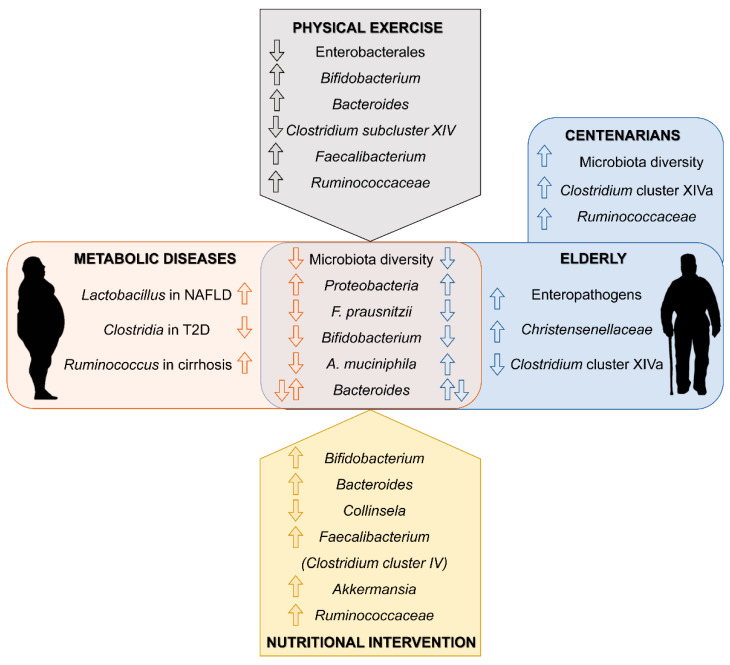
Gut microbiota is implicated in a diversity of physiological and pathological processes. Its role in obesity and related metabolic diseases is well recognized and pointed out as a promising therapeutic target. Moreover, the microbiota of elder population shows a particular microbial signature that links the natural process of aging to alterations of gut microbiota composition. Thus, in the elderly, strategies to modulate gut microbiota could have a double target: to counteract intestinal dysbiosis induced by metabolic diseases and to reshape the microbial communities associated to aging towards a healthy young-type microbiota (Figure 1). Probiotics and prebiotics are the main direct therapy targeting gut microbiota. However, lifestyle guidelines could indirectly be beneficial through its modulatory effect on gut microbiota. More human studies are needed in aged and metabolically unhealthy population to design microbiota-based therapeutic approaches that take advantage of the relationship between gut microbiota, metabolic diseases and aging.
Figure 1.
Main findings in gut microbiota composition linked to metabolic disease and aging (center). Summary of related changes observed in response to physical activity and nutritional interventions in elderly individuals (top and bottom respectively).
Author Contributions
M.J.-F. and D.P. wrote the manuscript. M.J.-F., D.P., M.V.G.-M., S.R.-S., J.G.-G., E.N. and S.S.-C. discussed the literature and figures, contributed to the intellectual input, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants to J.G.-G. and S.S.-C. from Ministerio de Economía y Competitividad/FEDER (BFU2017-87960-R) and Junta de Castilla y León (GRS 1888/A/18). M.J.-F. was supported by a fellowship from Ministerio de Ciencia, Innovación y Universidades (FPU18/06257). D.P. and S.R.-S. were supported by a fellowship from Junta de Castilla y León co-financed by the European Social Fund. E.N. was supported by CIBERehd contracts. CIBERehd is funded by the Instituto de Salud Carlos III, Spain.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ho J.Y., Hendi A.S. Recent trends in life expectancy across high income countries: Retrospective observational study. BMJ. 2018;362:k2562. doi: 10.1136/bmj.k2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kontis V., Bennett J.E., Mathers C.D., Li G., Foreman K., Ezzati M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet. 2017;389:1323–1335. doi: 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalish V.B. Obesity in older adults. Prim. Care Clin. Off. Pract. 2016;43:137–144. doi: 10.1016/j.pop.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Cooper J.A., Cadogan C.A., Patterson S.M., Kerse N., Bradley M.C., Ryan C., Hughes C.M. Interventions to improve the appropriate use of polypharmacy in older people: A Cochrane systematic review. BMJ Open. 2015;5:e009235. doi: 10.1136/bmjopen-2015-009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franceschi C., Garagnani P., Morsiani C., Conte M., Santoro A., Grignolio A., Monti D., Capri M., Salvioli S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018;5:61. doi: 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shlisky J., Bloom D.E., Beaudreault A.R., Tucker K.L., Keller H.H., Freund-levi Y., Fielding R.A., Cheng F.W., Jensen G.L., Wu D., et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv. Nutr. 2017;8:17–26. doi: 10.3945/an.116.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duggal N.A., Niemiro G., Harridge S.D.R., Simpson R.J., Lord J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019;19:563–572. doi: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- 8.Myers J., Kokkinos P., Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. 2019;11:1652. doi: 10.3390/nu11071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falck R.S., Davis J.C., Best J.R., Crockett R.A., Liu-Ambrose T. Impact of exercise training on physical and cognitive function among older adults: A systematic review and meta-analysis. Neurobiol. Aging. 2019;79:119–130. doi: 10.1016/j.neurobiolaging.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Chekroud S.R., Gueorguieva R., Zheutlin A.B., Paulus M., Krumholz H.M., Krystal J.H., Chekroud A.M. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry. 2018;5:739–746. doi: 10.1016/S2215-0366(18)30227-X. [DOI] [PubMed] [Google Scholar]
- 11.Galloza J., Castillo B., Micheo W. Benefits of exercise in the older population. Phys. Med. Rehabil. Clin. N. Am. 2017;28:659–669. doi: 10.1016/j.pmr.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Adak A., Khan M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019;76:473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornejo-Pareja I., Muñoz-Garach A., Clemente-Postigo M., Tinahones F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2019;72:26–37. doi: 10.1038/s41430-018-0306-8. [DOI] [PubMed] [Google Scholar]
- 14.Perumpail B.J., Khan M.A., Yoo E.R., Cholankeril G., Kim D., Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017;23:8263–8276. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polyzos S.A., Kountouras J., Mantzoros C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 17.Blüher M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 18.Chooi Y.C., Ding C., Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Batsis J.A., Villareal D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018;14:513–537. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oreopoulos A., Kalantar-Zadeh K., Sharma A.M., Fonarow G.C. The obesity paradox in the elderly: Potential mechanisms and clinical implications. Clin. Geriatr. Med. 2009;25:643–659. doi: 10.1016/j.cger.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Zamboni M., Mazzali G., Fantin F., Rossi A., Di Francesco V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008;18:388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Hou Q., Guan Y., Yu W., Liu X., Wu L., Xiao M., Lü Y. Associations between obesity and cognitive impairment in the Chinese elderly: An observational study. Clin. Interv. Aging. 2019;14:367–373. doi: 10.2147/CIA.S192050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 24.Franceschi C., Garagnani P., Vitale G., Capri M., Salvioli S. Inflammaging and ‘garb-aging’. Trends Endocrinol. Metab. 2017;28:199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Delzenne N.M., Knudsen C., Beaumont M., Rodriguez J., Neyrinck A.M., Bindels L.B. Contribution of the gut microbiota to the regulation of host metabolism and energy balance: A focus on the gut-liver axis. Proc. Nutr. Soc. 2019;78:319–328. doi: 10.1017/S0029665118002756. [DOI] [PubMed] [Google Scholar]
- 26.Sovran B., Hugenholtz F., Elderman M., Van Beek A.A., Graversen K., Huijskes M., Boekschoten M.V., Savelkoul H.F.J., De Vos P., Dekker J., et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci. Rep. 2019;9:1437. doi: 10.1038/s41598-018-35228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fava F., Rizzetto L., Tuohy K.M. Gut microbiota and health: Connecting actors across the metabolic system. Proc. Nutr. Soc. 2019;78:177–188. doi: 10.1017/S0029665118002719. [DOI] [PubMed] [Google Scholar]
- 28.Ley R.E., Bäckhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J., Zhou Q., Li H. Gut microbiota and nonalcoholic fatty liver disease: Insights on mechanisms and therapy. Nutrients. 2017;9:1124. doi: 10.3390/nu9101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bäckhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., Semenkovich C.F., Gordon J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridaura V.K., Faith J.J., Rey F.E., Cheng J., Duncan A.E., Kau A.L., Griffin N.W., Lombard V., Henrissat B., Bain J.R., et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woting A., Blaut M. The intestinal microbiota in metabolic disease. Nutrients. 2016;8:202. doi: 10.3390/nu8040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quiroga R., Nistal E., Estébanez B., Porras D., Juárez-Fernández M., Martínez-Flórez S., García-Mediavilla M.V., de Paz J.A., González-Gallego J., Sánchez-Campos S., et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020;52:1048–1061. doi: 10.1038/s12276-020-0459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollister E.B., Foster B.A., Dahdouli M., Ramirez J., Lai Z. Characterization of the stool microbiome in hispanic preschool children by weight status and time. Child. Obes. 2018;14:122–130. doi: 10.1089/chi.2017.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuda S., Ohno H. Gut microbiome and metabolic diseases. Semin. Immunopathol. 2014;36:103–114. doi: 10.1007/s00281-013-0399-z. [DOI] [PubMed] [Google Scholar]
- 37.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 38.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 39.Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turnbaugh P.J., Bäckhed F., Fulton L., Gordon J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomes A.C., Hoffmann C., Mota J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes. 2018;9:308–325. doi: 10.1080/19490976.2018.1465157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nistal E., Sáenz de Miera L.E., Ballesteros Pomar M., Sánchez-Campos S., García-Mediavilla M.V., Álvarez-Cuenllas B., Linares P., Olcoz J.L., Arias-Loste M.T., García-Lobo J.M., et al. An altered fecal microbiota profile in patients with non-alcoholic fatty liver disease (NAFLD) associated with obesity. Rev. Esp. Enfermedades Dig. 2019;111:275–282. doi: 10.17235/reed.2019.6068/2018. [DOI] [PubMed] [Google Scholar]
- 43.Gao X., Zhang M., Xue J., Huang J., Zhuang R., Zhou X., Zhang H., Fu Q., Hao Y. Body mass index differences in the gut microbiota are gender specific. Front. Microbiol. 2018;9:1250. doi: 10.3389/fmicb.2018.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Everard A., Cani P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013;27:73–83. doi: 10.1016/j.bpg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Crovesy L., Masterson D., Rosado E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020;74:1251–1262. doi: 10.1038/s41430-020-0607-6. [DOI] [PubMed] [Google Scholar]
- 46.Ponziani F.R., Bhoori S., Castelli C., Putignani L., Rivoltini L., Del Chierico F., Sanguinetti M., Morelli D., Paroni Sterbini F., Petito V., et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 47.Boursier J., Mueller O., Barret M., Machado M., Fizanne L., Araujo-Perez F., Guy C.D., Seed P.C., Rawls J.F., David L.A., et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iebba V., Guerrieri F., Di Gregorio V., Levrero M., Gagliardi A., Santangelo F., Sobolev A.P., Circi S., Giannelli V., Mannina L., et al. Combining amplicon sequencing and metabolomics in cirrhotic patients highlights distinctive microbiota features involved in bacterial translocation, systemic inflammation and hepatic encephalopathy. Sci. Rep. 2018;8:8210. doi: 10.1038/s41598-018-26509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Roy T., Llopis M., Lepage P., Bruneau A., Rabot S., Bevilacqua C., Martin P., Philippe C., Walker F., Bado A., et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 50.Leung C., Rivera L., Furness J.B., Angus P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 51.Aron-Wisnewsky J., Vigliotti C., Witjes J., Le P., Holleboom A.G., Verheij J., Nieuwdorp M., Clément K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020;17:279–297. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 52.Larsen N., Vogensen F.K., van den Berg F.W.J., Nielsen D.S., Andreasen A.S., Pedersen B.K., Al-Soud W.A., Sørensen S.J., Hansen L.H., Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 54.Tonucci L.B., Olbrich dos Santos K.M., Licursi de Oliveira L., Rocha Ribeiro S.M., Duarte Martino H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017;36:85–92. doi: 10.1016/j.clnu.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Wu H., Esteve E., Tremaroli V., Khan M.T., Caesar R., Mannerås-Holm L., Ståhlman M., Olsson L.M., Serino M., Planas-Fèlix M., et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 56.Belizário J.E., Faintuch J., Garay-Malpartida M. Gut microbiome dysbiosis and immunometabolism: New frontiers for treatment of metabolic diseases. Mediators Inflamm. 2018;2018:2037838. doi: 10.1155/2018/2037838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collado M.C., Rautava S., Aakko J., Isolauri E., Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Claesson M.J., Cusack S., O’Sullivan O., Greene-Diniz R., De Weerd H., Flannery E., Marchesi J.R., Falush D., Dinan T., Fitzgerald G., et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA. 2011;108:4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim S., Jazwinski S.M. The gut microbiota and healthy aging: A mini-review. Gerontology. 2018;64:513–520. doi: 10.1159/000490615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.García-Peña C., Álvarez-Cisneros T., Quiroz-Baez R., Friedland R.P. Microbiota and aging. A review and commentary. Arch. Med. Res. 2017;48:681–689. doi: 10.1016/j.arcmed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Salazar N., Valdés-Varela L., González S., Gueimonde M., de los Reyes-Gavilán C.G. Nutrition and the gut microbiome in the elderly. Gut Microbes. 2017;8:82–97. doi: 10.1080/19490976.2016.1256525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeffery I.B., Lynch D.B., O’Toole P.W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10:170–182. doi: 10.1038/ismej.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Toole P.W., Jeffery I.B. Microbiome–health interactions in older people. Cell. Mol. Life Sci. 2018;75:119–128. doi: 10.1007/s00018-017-2673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rahayu E.S., Utami T., Mariyatun M., Hasan P.N., Kamil R.Z., Setyawan R.H., Pamungkaningtyas F.H., Harahap I.A., Wiryohanjoyo D.V., Pramesi P.C., et al. Gut microbiota profile in healthy Indonesians. World J. Gastroenterol. 2019;25:1478–1491. doi: 10.3748/wjg.v25.i12.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J.Z., Abe F., Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biagi E., Nylund L., Candela M., Ostan R., Bucci L., Pini E., Nikkïla J., Monti D., Satokari R., Franceschi C., et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/annotation/df45912f-d15c-44ab-8312-e7ec0607604d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bian G., Gloor G.B., Gong A., Jia C., Zhang W., Hu J., Zhang H., Zhang Y., Zhou Z., Zhang J., et al. The gut microbiota of healthy aged chinese is similar to that of the healthy young. mSphere. 2017;2:e00327-17. doi: 10.1128/mSphere.00327-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O’connor E.M., Cusack S., Harris H.M.B., Coakley M., Lakshminarayanan B., O’sullivan O., et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 69.Jackson M., Jeffery I.B., Beaumont M., Bell J.T., Clark A.G., Ley R.E., O’Toole P.W., Spector T.D., Steves C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8:8. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mangiola F., Nicoletti A., Gasbarrini A., Ponziani F.R. Gut microbiota and aging. Eur. Rev. Med. Pharmacol. Sci. 2018;22:7404–7413. doi: 10.26355/eurrev-201811-16280. [DOI] [PubMed] [Google Scholar]
- 71.Ticinesi A., Milani C., Lauretani F., Nouvenne A., Mancabelli L., Lugli G.A., Turroni F., Duranti S., Mangifesta M., Viappiani A., et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci. Rep. 2017;7:11102. doi: 10.1038/s41598-017-10734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang F., Yu T., Huang G., Cai D., Liang X., Su H., Zhu Z., Li D., Yang Y., Shen P., et al. Gut Microbiota community and its assembly associated with age and diet in Chinese centenarians. J. Microbiol. Biotechnol. 2015;25:1195–1204. doi: 10.4014/jmb.1410.10014. [DOI] [PubMed] [Google Scholar]
- 73.Wang N., Li R., Lin H., Fu C., Wang X., Zhang Y., Su M., Huang P., Qian J., Jiang F., et al. Enriched taxa were found among the gut microbiota of centenarians in East China. PLoS ONE. 2019;14:e0222763. doi: 10.1371/journal.pone.0222763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim B.S., Choi C.W., Shin H., Jin S.P., Bae J.S., Han M., Seo E.Y., Chun J., Chung J.H. Comparison of the gut microbiota of centenarians in longevity villages of South Korea with those of other age groups. J. Microbiol. Biotechnol. 2019;29:429–440. doi: 10.4014/jmb.1811.11023. [DOI] [PubMed] [Google Scholar]
- 75.Kong F., Hua Y., Zeng B., Ning R., Li Y., Zhao J. Gut microbiota signatures of longevity. Curr. Biol. 2016;26:R832–R833. doi: 10.1016/j.cub.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 76.Biagi E., Franceschi C., Rampelli S., Severgnini M., Ostan R., Turroni S., Consolandi C., Quercia S., Scurti M., Monti D., et al. Gut microbiota and extreme longevity. Curr. Biol. 2016;26:1480–1485. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 77.Santoro A., Ostan R., Candela M., Biagi E., Brigidi P., Capri M., Franceschi C. Gut microbiota changes in the extreme decades of human life: A focus on centenarians. Cell. Mol. Life Sci. 2018;75:129–148. doi: 10.1007/s00018-017-2674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collino S., Montoliu I., Martin F.P.J., Scherer M., Mari D., Salvioli S., Bucci L., Ostan R., Monti D., Biagi E., et al. Metabolic signatures of extreme longevity in Northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS ONE. 2013;8:e56564. doi: 10.1371/annotation/5fb9fa6f-4889-4407-8430-6dfc7ecdfbdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson L.C., Martens C.R., Santos-Parker J.R., Bassett C.J., Strahler T.R., Cruickshank-Quinn C., Reisdorph N., McQueen M.B., Seals D.R. Amino acid and lipid associated plasma metabolomic patterns are related to healthspan indicators with ageing. Clin. Sci. 2018;132:1765–1777. doi: 10.1042/CS20180409. [DOI] [PubMed] [Google Scholar]
- 80.Cai D., Zhao S., Li D., Chang F., Tian X., Huang G., Zhu Z., Liu D., Dou X., Li S., et al. Nutrient intake is associated with longevity characterization by metabolites and element profiles of healthy centenarians. Nutrients. 2016;8:564. doi: 10.3390/nu8090564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Porras D., Nistal E., Martínez-Flórez S., González-Gallego J., García-Mediavilla M.V., Sánchez-Campos S. Intestinal microbiota modulation in obesity-related non-alcoholic fatty liver disease. Front. Physiol. 2018;9:1813. doi: 10.3389/fphys.2018.01813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cancello R., Turroni S., Rampelli S., Cattaldo S., Candela M., Cattani L., Mai S., Vietti R., Scacchi M., Brigidi P., et al. Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women. Nutrients. 2019;11:3011. doi: 10.3390/nu11123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghosh T.S., Rampelli S., Jeffery I.B., Santoro A., Neto M., Capri M., Giampieri E., Jennings A., Candela M., Turroni S., et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69:1218–1228. doi: 10.1136/gutjnl-2019-319654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitchell S.M., McKenzie E.J., Mitchell C.J., Milan A.M., Zeng N., D’Souza R.F., Ramzan F., Sharma P., Rettedal E., Knowles S.O., et al. A period of 10 weeks of increased protein consumption does not alter faecal microbiota or volatile metabolites in healthy older men: A randomised controlled trial. J. Nutr. Sci. 2020;9:e25. doi: 10.1017/jns.2020.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tran T.T.T., Cousin F.J., Lynch D.B., Menon R., Brulc J., Brown J.R.M., O’Herlihy E., Butto L.F., Power K., Jeffery I.B., et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome. 2019;7:39. doi: 10.1186/s40168-019-0654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim C.-S., Cha L., Sim M., Jung S., Chun W.Y., Baik H.W., Shin D.-M. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. Ser. A. 2020:glaa090. doi: 10.1093/gerona/glaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aoyagi Y., Amamoto R., Park S., Honda Y., Shimamoto K., Kushiro A., Tsuji H., Matsumoto H., Shimizu K., Miyazaki K., et al. Independent and interactive effects of habitually ingesting fermented milk products containing lactobacillus casei strain shirota and of engaging in moderate habitual daily physical activity on the intestinal health of older people. Front. Microbiol. 2019;10:1477. doi: 10.3389/fmicb.2019.01477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Castro-Mejía J.L., Khakimov B., Krych Ł., Bülow J., Bechshøft R.L., Højfeldt G., Mertz K.H., Garne E.S., Schacht S.R., Ahmad H.F., et al. Physical fitness in community-dwelling older adults is linked to dietary intake, gut microbiota, and metabolomic signatures. Aging Cell. 2020;19:e13105. doi: 10.1111/acel.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taniguchi H., Tanisawa K., Sun X., Kubo T., Hoshino Y., Hosokawa M., Takeyama H., Higuchi M. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol. Rep. 2018;6:e13935. doi: 10.14814/phy2.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morita E., Yokoyama H., Imai D., Takeda R., Ota A., Kawai E., Hisada T., Emoto M., Suzuki Y., Okazaki K. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients. 2019;11:868. doi: 10.3390/nu11040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu Y., Mao G., Wang J., Zhu L., Lv X., Tong Q., Fang Y., Lv Y., Wang G. Gut dysbiosis is associated with the reduced exercise capacity of elderly patients with hypertension. Hypertens. Res. 2018;41:1036–1044. doi: 10.1038/s41440-018-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu Q., Jiang S., Du G. Effects of exercise frequency on the gut microbiota in elderly individuals. Microbiologyopen. 2020;9:e1053. doi: 10.1002/mbo3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salazar N., González S., Nogacka A.M., Rios-Covián D., Arboleya S., Gueimonde M., de los Reyes-Gavilán C.G. Microbiome: Effects of ageing and diet. Curr. Issues Mol. Biol. 2019;36:33–62. doi: 10.21775/cimb.036.033. [DOI] [PubMed] [Google Scholar]
- 94.Capurso C., Bellanti F., Lo Buglio A., Vendemiale G. The mediterranean diet slows down the progression of aging and helps to prevent the onset of frailty: A narrative review. Nutrients. 2020;12:35. doi: 10.3390/nu12010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coelho-Junior H.J., Marzetti E., Picca A., Cesari M., Uchida M.C., Calvani R. Protein intake and frailty: A matter of quantity, quality, and timing. Nutrients. 2020;12:2915. doi: 10.3390/nu12102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lahtinen S.J., Tammela L., Korpela J., Parhiala R., Ahokoski H., Mykkänen H., Salminen S.J. Probiotics modulate the Bifidobacterium microbiota of elderly nursing home residents. Age (Omaha) 2009;31:59–66. doi: 10.1007/s11357-008-9081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rampelli S., Candela M., Severgnini M., Biagi E., Turroni S., Roselli M., Carnevali P., Donini L., Brigidi P. A probiotics-containing biscuit modulates the intestinal microbiota in the elderly. J. Nutr. Heal. Aging. 2013;17:166–172. doi: 10.1007/s12603-012-0372-x. [DOI] [PubMed] [Google Scholar]
- 98.Macfarlane S., Cleary S., Bahrami B., Reynolds N., Macfarlane G.T. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: A randomised, double-blind, placebo-controlled crossover study. Aliment. Pharmacol. Ther. 2013;38:804–816. doi: 10.1111/apt.12453. [DOI] [PubMed] [Google Scholar]
- 99.Ahmed M., Prasad J., Gill H., Stevenson L., Gopal P. Impact of consumption of different levels of Bifidobacterium lactis HN019 on the intestinal microflora of elderly human subjects. J. Nutr. Heal. Aging. 2007;11:26–31. [PubMed] [Google Scholar]
- 100.Ahmadi S., Wang S., Nagpal R., Wang B., Jain S., Razazan A., Mishra S.P., Zhu X., Wang Z., Kavanagh K., et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight. 2020;5:e132055. doi: 10.1172/jci.insight.132055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Campbell S.C., Wisniewski P.J., Noji M., McGuinness L.R., Häggblom M.M., Lightfoot S.A., Joseph L.B., Kerkhof L.J. The effect of diet and exercise on intestinal integrity and microbial diversity in mice. PLoS ONE. 2016;11:e0150502. doi: 10.1371/journal.pone.0150502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu T.W., Park Y.M., Holscher H.D., Padilla J., Scroggins R.J., Welly R., Britton S.L., Koch L.G., Vieira-Potter V.J., Swanson K.S. Physical activity differentially affects the cecal microbiota of ovariectomized female rats selectively bred for high and low aerobic capacity. PLoS ONE. 2015;10:e0136150. doi: 10.1371/journal.pone.0136150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pekkala S., Lensu S., Nokia M., Vanhatalo S., Koch L.G., Britton S.L., Kainulainen H. Intrinsic aerobic capacity governs the associations between gut microbiota composition and fat metabolism age-dependently in rat siblings. Physiol. Genom. 2017;49:733–746. doi: 10.1152/physiolgenomics.00081.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Houghton D., Stewart C.J., Stamp C., Nelson A., Aj Ami N.J., Petrosino J.F., Wipat A., Trenell M.I., Turnbull D.M., Greaves L.C. Impact of age-related mitochondrial dysfunction and exercise on intestinal microbiota composition. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018;73:571–578. doi: 10.1093/gerona/glx197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fielding R.A., Reeves A.R., Jasuja R., Liu C., Barrett B.B., Lustgarten M.S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 2019;127:110722. doi: 10.1016/j.exger.2019.110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carbajo-Pescador S., Porras D., Garcia-Mediavilla M.V.V., Martinez-Florez S., Juarez-Fernandez M., Cuevas M.J.J., Mauriz J.L.L., Gonzalez-Gallego J., Nistal E., Sanchez-Campos S. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. DMM Dis. Model. Mech. 2019;12:dmm039206. doi: 10.1242/dmm.039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petersen L.M., Bautista E.J., Nguyen H., Hanson B.M., Chen L., Lek S.H., Sodergren E., Weinstock G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome. 2017;5:98. doi: 10.1186/s40168-017-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]