Abstract
Human neutrophil peptide 1 (HNP1), a predominant α defensin in the azurophilic granules of human neutrophils, is an alarmin capable of inducing the migration and maturation of human myeloid/conventional dendritic cells. However, it is not determined whether it can activate plasmacytoid dendritic cells (pDCs). Herein, we found that both human pDCs and CAL-1 cells, a pDC-like cell line, produced IFNα upon treatment with HNP1. Additionally, HNP1 could promote CpG ODN-induced pDC production of proinflammatory cytokines including IFNα. HNP1 triggered activation of NF-κB and nuclear translocation of interferon regulatory factor 1 (IRF1) in CAL-1 cells. HNP1 upregulation of cytokine expression in pDCs was inhibited by blockade of NF-κB activation or knockdown of IRF1, demonstrating the importance of these two signaling events in HNP1-induced pDC activation. Using a human pDC-nude mouse model, HNP1 was shown to induce IFNα production by human pDCs in vivo. Thus, HNP1 can activate human pDCs using NF-κB and IRF signaling pathways, and HNP-induced IFN production may participate in the inflammatory pathogenesis in certain authoimmune diseases such as rheumatoid arthritis.
Keywords: Defensin, HNP1, pDC, Alarmin, IFN
1. Introduction
Alarmin human neutrophil peptide 1 (HNP1) is one of the four neutrophil-derived α defensins initially isolated from the azurophilic granules of human neutrophils [1,2]. HNP1, encoded by the DEFA1 gene located on human chromosome 8p23.1 [3,4], is predominantly expressed by cells of human neutrophil lineage, although it is also present in other cell types such as human NK, T cells, myeloid DCs, eosinophils, and synovial membrane of human joints [1,2,5–8]. In neutrophil lineage, HNP1 is translated in promyelocytes as a 94 amino acid preproform and sequentially cleaved in the Golgi body into a 30 amino acid mature HNP1 that is sorted and stored in the azurophilic (primary) granules of mature neutrophils [9,10]. HNP1 is rapidly released upon neutrophil degranulation and has potent antimicrobial activities against bacteria, fungi, some parasites and certain enveloped viruses [1,2,11,12].
Although initially identified as an antimicrobial peptide, HNP1 has subsequently been found to have multiple effects on leukocytes of the immune system. HNP1 is chemotactic for human T cells in vitro and recruits human T cells into sites of injection in vivo [13]. HNP1 is also a direct chemotactic factor for human immature (but not mature) dendritic cells (DCs) derived from hematopoietic progenitors [14], monocyte-derived macrophages, and mast cells [15]. HNP1 has the capacity to induce the production of TNFα in human monocytes in vitro [16] or in the respiratory tract in vivo [17]. HNP1 is reported to induce phenotypic and functional maturation of human monocyte-derived DCs [18,19]. More recently, HNP1 has been shown to activate inflammasomes in human monocytes for the production of IL-1β [20]. Therefore, HNP1 has both chemotactic and activating effects on antigen-presenting cells including macrophages and DCs.
Owing to the possession of both DC-chemotactic and -activating effects, HNP1 is classified as a member of the alarmin family, endogenous mediators capable of recruiting and activating DCs and consequently activating innate and adaptive immune responses [12,21–23]. Indeed, HNP1 has been reported over the years to have in vivo immunoenhancing effects. HNP1 ~ 3 was shown to enhance antigen-specific immune response when coadministered intranasally with the antigen, even prior to the identification of HNP1 ~ 3’s effects on DCs [24]. Administration of HNP1 ~ 3 together with an antigen via different routes also promoted antigen-specific humoral and cellular immune responses [25,26]. Delivery of HNP1 into mouse CT26 tumors by DNA transfection results in enhanced recruitment of DCs into the tumors, as well as generation of anti-CT26 immune responses, further cementing the notion that HNP1 can promote the generation of immune response against various antigens [27].
There are two principal subsets of DCs in the human immune system, myeloid/conventional (cDC) and plasmacytoid DCs (pDCs), which differ in terms of developmental lineage, expression of surface markers, receptor profile, and biological functions [28,29]. The effect of HNP1 on cDCs is well documented, however, it is unclear whether HNP1 has any effect on pDCs. In this study, we investigated the capacity of HNP1 to activate human pDCs and the underlying mechanism(s). HNP1 induced the production of proinflammatory cytokines including type I interferon (IFN) in human pDCs and also enhanced CpG oligodeoxynucleotide (ODN)-induced activation of pDCs. HNP1 triggered the degradation of I-κBα and nuclear translocation of IRF1, both of which were required for HNP1 activation of pDCs. HNP1 induced the production of human IFNα in nude mice reconstituted with human pDCs. Overall, the data demonstrate that HNP1 is a bona fide activator of human pDCs and may play a role in the pathogenesis of autoimmune diseases in which the presence of both HNP1 and pDCs is observed.
2. Materials and methods
2.1. Reagents and cell line(s)
RPMI 1640 was purchased from Gibco (Grand Island, NY, USA). HNP1 was obtained from PeproTech (Rocky Hill, NJ, USA). CpG oligodeoxynucleotides 2216 (ODN), a class A/D type ODN, was bought from InvivoGen (San Diego, CA, USA). BAY 11-7082 was from Santa Cruz Biotech (Santa Cruz, CA, USA). RPMI 1640 medium used in this study was supplemented with 10% fetal bovine serum (Gibco), 2 mM glutamine (Gibco), 25 mM HEPES (Gibco), 100 U/ml penicillin (Gibco), 100 mg/ml streptomycin (Gibco), and 50 mM 2-mercaptoethanol (Gibco). CAL-1 cells, a human pDC cell line established from a lymphoma patient [30], were maintained in RPMI1640 medium and passaged every 3–4 days.
2.2. Isolation of human pDCs
Human peripheral blood enriched in mononuclear cells was obtained from healthy donors by leukapheresis (Tianjin Municipal Blood Center, Tianjin, China). Human peripheral blood mononuclear cells were isolated by routine Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO, USA) density gradient centrifugation. Subsequently, human pDCs were purified from human peripheral blood mononuclear cells with the use of Plasmacytoid Dendritic Cell Isolation Kit II (Miltenyi Biotech, Auburn, CA, USA) according to the manufacturer’s recommendation.
2.3. Cell culture and treatment
pDCs and CAL-1 cells were seeded into wells of 96- or 24-well plates in RPMI 1640 medium at 0.4–5 × 106/ml. The cells were treated with HNP1 and/or ODN at specified concentrations for the indicated periods of time. At the end of the treatment, the supernatants were harvested for the measurement of cytokines, while the cells were used for the extraction of RNA.
For studying intracellular signaling, CAL-1 cells were incubated in RPMI 1640 medium without serum for 6 h (serum starvation) before being treated with HNP1 and/or ODN. Treated cells were used for the preparation of whole cell lysate or cytoplasmic/nuclear protein fractions.
2.4. Measurement of IFNα
The concentration of human IFNα in the supernatants of cell culture, mouse serum, and mouse peritoneal lavage samples was determined using human IFNα ELISA kits (R&D Systems, Minneapolis, MN, USA) following the maker’s recommended protocol.
2.5. IRF1 knockdown
For IRF1 knockdown (KD), CAL-1 cells, at 4 × 105 cells/well in a 24-well tissue culture plate, were transfected with 1 nM of IRF-1 siRNA (Ambion/Life Technologies, Carlsbad, CA, USA) using X-tremeGENEsiRNA Transfection Reagent (Roche, Basel, Switzerland) per the manufacturer’s instructions. Silencer Select Negative Control siRNA (Ambion/Life Technologies) was used as negative control. After culturing overnight at 37 °C in a CO2 incubator, a portion of the transfected CAL-1 cells was used for the extraction of whole cell lysate, while the majority of transfected CAL-1 cells were used for treatment with HNP1 and/or ODN.
2.6. Preparation of cell lysate, cytoplasmic and nuclear protein fractions
Whole cell lysate was extracted from treated CAL-1 cells using M-PER Mammalian Protein Extraction Reagent (Thermo Scientific™, Pierce, Rockford, IL, USA). Nuclear and cytoplasmic proteins were isolated using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific™). The protein concentration of extracted samples was measured using a Pierce BCA Protein Assay Kit (Thermo Scientific™).
2.7. qPCR
Total RNA was extracted from treated CAL-1 cells or pDCs by the use of TRIZol (Ambion/Life Technologies) following the manufacturer’s instructions. One microgram of total RNA was reverse transcribed into cDNAs using a RT kit (Roche, Basel, Switzerland). Quantitative PCR was performed with TaqMan based real-time PCR kit (Life Technologies, Carlsbad, CA, USA) on an ABI 7500 Fast Real Time PCR System, and the data were presented as mRNA accumulation index (2-ΔΔCt). The following TaqMan probes were used: IFNα (Hs00855471_g1), IFNβ1 (Hs02621180_s1), IL-6 (Hs00174131_m1), and GAPDH (Hs02758991_g1). GAPDH levels did not change upon stimulation or during siRNA gene silencing.
2.8. SDS-PAGE and Western blot
Whole cell lysate (30 μg/lane), nuclear (10 μg/lane), and cytoplasmic (30 μg/lane) fractions were loaded to a SDS-PAGE gel (Invitrogen, Carlsbad, CA, USA) and separated by electrophoresis. After transfer of the separated proteins onto a piece of PVDF membrane (Millipore, Billerica, MA, USA), the membrane was probed by Western blot. Briefly, the membrane was blocked with 5% nonfat milk for 1 h at room temperature, reacted with a primary antibody (1:1000–2000 dilution) overnight at 4 °C, and followed by incubating at room temperature for 1 h with an HRP-conjugated anti-rabbit IgG secondary antibody (Abmart, Berkeley Heights, NJ, USA). The following primary antibodies were used: IRF-1 (B-1), IRF-8 (C-19) (Santa Cruz Biotech, Santa Cruz, CA, USA), NF-κB p105/p50 (#3035), NF-κB p65 (C22B4), I-κBα (#9247), IRF-3 (D83B9), lamin B1 (D4Q4Z) (Cell Signaling, Beverly, MA, USA), IRF-5 (10T1) (Abcam, Cambridge, MA, USA). The membranes were developed using Immobilon™ Western Chemiluminescent HRP Substrate (Millipore) and the images were acquired using The G-Box fluorescence and Chemiluminescence of imaging system (Syngene, England).
2.9. Human pDC-nude mice model
Human pDCs were isolated from human peripheral blood mononuclear cells, washed, and suspended in sterile PBS at 106/ml. Nude mice obtained from Charles River were kept under specific pathogen-free conditions with water and food given ad libitum. All experiments with mice were performed in compliance with the principles and procedures outlined in the institution’s Guide for the Care and Use of Animals and were approved by the institution’s Animal Care and Use Committee. Nude mice (female, 7-week old, n = 6) were injected intraperitoneally with 0.2 ml pDC suspension. On the next day, the mice were bled 1 h prior to subsequent treatment, intraperitoneally injected with HNP1 in PBS (5 μg/0.2 ml/mouse), and bled at 4 and 24 h after HNP1 injection. The mice were euthanized after the last bleeding, and peritoneal lavage was collected with 4 ml of ice-cold PBS.
2.10. Statistic analysis
Differences between experimental groups were evaluated by Student’s t-test.
3. Results
3.1. HNP1 induction of pDC activation
Upon activation, pDCs are the most potent type I IFN producing cells. To determine the capacity of HNP1 to activate pDCs, pDCs isolated from human peripheral blood were cultured in a humidified CO2 (5%) incubator in the absence or presence of various concentrations of HNP1, and their expression of type I IFNs was subsequently measured (Fig. 1). Measurement of IFNα in the supernatants of overnight pDC cultures revealed that HNP1 dose-dependently induced IFNα production by pDCs (Fig. 1A). Measurement of type I IFNs by qPCR showed that HNP1 upregulated the expression of both IFNα and IFNβ1 at mRNA levels in 5 h (Fig. 1B & C). Therefore, HNP1 is capable of activating human pDCs.
Fig. 1.

HNP1 induction of pDC production of type I IFNs. Human pDCs isolated from the peripheral blood of health donors were treated in triplicate at 5 × 105/ml with indicated concentrations of HNP1. A, IFNα concentration in the supernatant of 24 h-treated pDCs was measured by ELISA; B&C, Total RNA was isolated from 5 h-treated pDCs and the levels of IFNα (B) or IFNβ1 (C) mRNA measured by qPCR. Shown is the average (mean ± SD) of three independent experiments. *p < 0.05 by Student’s t test.
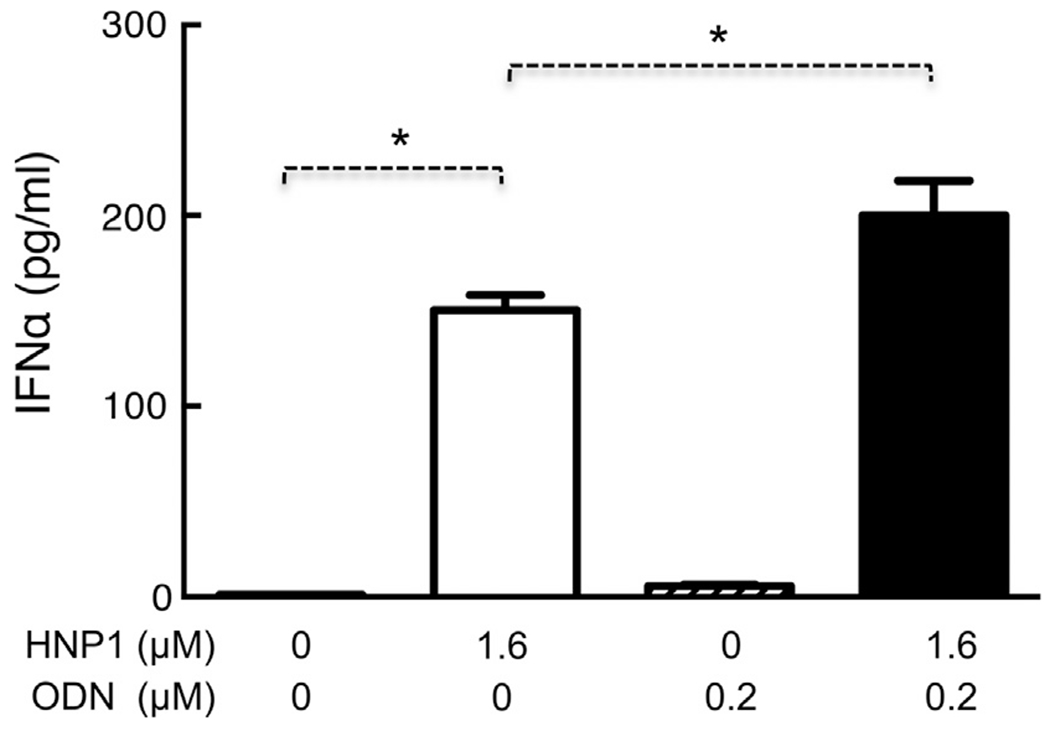
pDCs can be activated by agonists for TLR7 and TLR9 [29]. To investigate whether HNP1 could enhance ODN-induced pDC activation, pDCs were treated with HNP1 and ODN alone or in combination, followed by subsequent determination of IFN expression (Fig. 2). As expected, ODN induced IFNα production in human pDCs in a dose-dependent manner, with a minimal effective dose of approximately 0.2 μM (Fig. 2A). Treatment of pDCs with a combination of low doses of ODN and HNP1 resulted in greatly enhanced production of IFNα (Fig. 2B). The level of IFNα production induced by ODN and HNP1 in combination (4th bar) was much higher than the sum (2nd + 3rd bars) of IFNα induced by ODN and HNP1 separately (Fig. 2B), suggesting that HNP1 could synergistically enhance ODN-induced IFNα production by human pDCs. HNP1 also synergistically enhanced ODN-induced upregulation of IFNα and IFNβ1 expression at the mRNA levels (Fig. 2C & D).
Fig. 2.

HNP1 promotion of ODN-induced pDC production of cytokines. Human pDCs isolated from health donors were treated in triplicate at 5 × 105/ml with indicated concentrations of ODN and HNP1 alone or in combination as indicated for 5–24 h before measurement of type I IFNs at protein or mRNA levels. A&B, IFNα in the supernatants of 24 h-treated pDCs measured by ELISA; C&D, IFNα (C) and IFNβ1 (D) mRNA levels of 5 h-treated pDCs were determined by qPCR. Shown is the average (mean ± SD) of three independent experiments. *p < 0.05 by Student’s t test.
3.2. HNP1 trigger of NF-κB and IRF1 signaling
Two major intracellular signaling pathways, NF-κB and IRF, are involved in the induction of cytokine production by pDCs [29,31,32]. To gain insight as to how HNP1 activates pDCs, we investigated whether HNP1 could trigger the activation of NF-κB and IRF(s). Due to the scarcity of pDCs in human peripheral blood, CAL-1, a cell line that closely resembles human pDCs [30], was used for this purpose. To ensure that CAL-1 cells could respond to HNP1 similarly to human pDCs, CAL-1 cells were treated with HNP1 and ODN alone or in combination to see if type I IFN could be induced. HNP1 not only induced, but also synergized with ODN in stimulating, IFNα production in CAL-1 cells (Fig. 3). HNP1 also upregulated IFNα expression at the mRNA level (data not shown). Therefore, HNP1 induced very similar responses in CAL-1 and pDCs (Figs. 1–3).
Fig. 3.
HNP1 stimulation of IFNα production by CAL-1 cells. CAL-1 cells (106/ml) were treated in triplicate with indicated concentration of HNP1 and ODN for 24 h. IFNα in the supernatants was measured by ELISA and shown as the average (mean ± SD) of three independent experiments. *p < 0.05 by Student’s t test.
CAL-1 cells treated with various concentrations of HNP1 for 30–60 min were analyzed by Western blot with anti-I-κBα antibody (Fig. 4A). HNP1 treatment reduced I-κBα in a dose- (Fig. 4A, upper left panel) and time-dependent (Fig. 4A, upper right panel) manner, suggesting the degradation of I-κBα in HNP1-treated CAL-1 cells. Re-probing of the membranes with anti-GAPDH after stripping revealed that similar amount of proteins were loaded onto each lane (Fig. 4A, lower panels). In addition, CAL-1 cells treated with 1 μM of HNP1 for 60 min showed elevated levels of p50 and p65 of NF-κB in the nucleus of treated cells compared to control cells (Fig. 4B, right panels). Thus, HNP1 can activate NF-κB signaling pathway in pDCs.
Fig. 4.

HNP1-triggered signaling in CAL-1 cells. Serum-starved CAL-1 cells (5 × 106/ml) were treated with indicated concentrations of HNP1 for 30–60 min before they were used for the preparation of whole cell lysate or cytoplasmic/nuclear fraction. Identical amount of whole cell lysate or cytoplasmic/nuclear fraction was loaded to a SDS-PAGE gel and separated by electrophoresis. After transfer of the separated proteins onto a piece of PVDF membrane, the membrane was probed by Western blot with anti-I-κBα (A), anti-p50/anti-p65 (B), or anti-IRF1 (C). Subsequently, the membranes were stripped and re-probed with anti-GAPDH (A and left panels of B and C) or anti-lamin (right panels of B and C). Shown are the images of one experiment representative of three.
Several IRFs (IRF1, 3, 5, and 8) are known to be present in CAL-1 cells [32]. To determine which IRF might be activated by HNP1, CAL-1 cells treated with HNP1 were used for the measurement of IRFs in the cytoplasmic and nuclear fractions by Western blot. As shown in Fig. 4C, IRF1 predominantly appeared in the cytoplasmic fraction in sham-treated CAL-1 cells. Treatment with 1 μM of HNP1 for 60 min resulted in a decrease of IRF1 in the cytoplasmic fraction and a simultaneous increase of IRF1 in the nuclear fraction in CAL-1 cells, indicating that HNP1 induced IRF1 nuclear translocation (Fig. 4C, upper panels). Re-probing the same membrane for GAPDH (cytoplasmic fraction) or lamin (nuclear fraction) revealed bands of similar intensity between sham- and HNP1-treated samples, suggesting that similar amounts of proteins were loaded (Fig. 4C, lower panels). HNP1 treatment did not induce nuclear translocation of IRF3, 5, or 8 (data not shown).
3.3. Contribution of NF-κB and IRF1 to HNP1-induced pDC activation
BAY 11-7802, a well-documented inhibitor of I-κB phosphorylation, was used to determine whether HNP1-induced NF-κB activation contributed to its effect on pDCs. Treatment with HNP1 (lane 2) and ODN (lane 3) alone or in combination (lane 4) elevated the levels of both NF-κB p50 and p65 in the nucleus of CAL-1 cells, indicating that HNP1 alone or in combination with ODN caused nuclear translocation and activation of NF-κB (Fig. 5A). HNP1-induced nuclear translocation of NF-κB in CAL-1 cells was inhibited by the inclusion of 7.5 μM of BAY 11-7802 (Fig. 5A, lane 5–7). Each lane contained similar levels of lamin, suggesting that similar amount of nuclear proteins was loaded. Therefore, BAY 11-7802 at 7.5 μM was sufficient to inhibit HNP1-induced NF-κB activation in CAL-1 cells and this concentration was used for subsequent treatment of human pDCs. As shown in Fig. 5B, HNP1-induced elevation of IFNα mRNA in pDCs was not inhibited (left panel), suggesting that NF-κB activation might not essential for HNP1-induced IFNα upregulation. However, HNP1-induced upregulation of IFNβ1 (middle panel) and IL-6 (right panel) mRNA was significantly inhibited by BAY 11-7802, indicating an essential role for NF-κB activation in HNP1-induced upregulation of IFNβ1 and IL-6 mRNA (Fig. 5B, middle and right panels). Upregulation of type I IFN and IL-6, in response to a combination of HNP1 and ODN, was also significantly inhibited by BAY 11-7802 in pDCs, providing additional support for the contribution of NF-κB to HNP1-induced activation of pDCs (Fig. 5B).
Fig. 5.
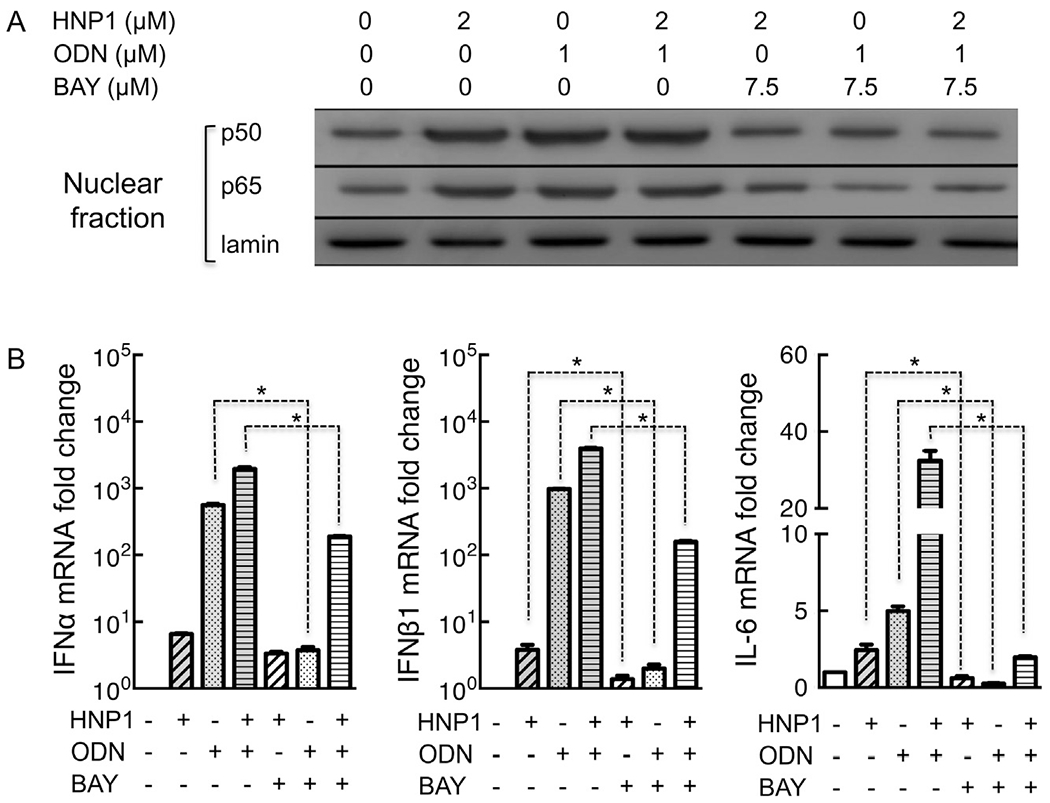
Blockade of NF-κB activation inhibited HNP1-induced production of proinflammatory cytokines in pDCs. A, CAL-1 cells (106/ml) were incubated for 1 h at 37 °C with HNP1 and/or ODN at concentrations as indicated, in the absence or presence of 7.5 μM of BAY 11-7082 (added 30 min in advance). Following the treatment, the cells were immediately used for the preparation of nuclear fractions as described in the Materials and Methods section. After protein quantitation, identical amounts of samples were separated on SDS-PAGE gels, transferred, and probed by Western blot using antibodies against NF-κB p50, NF-κB p65, or lamin. Shown are the results of one experiment representative of three. B, Human pDCs (5 × 105/ml) were incubated for 5 h at 37 °C with HNP1 (final concentration = 0.5 μM) and/or ODN (final concentration = 0.5 μM), in the absence or presence of 7.5 μM of BAY 11-7082 (added 30 min in advance). Total RNAs were extracted from treated pDCs and used for the measurement of indicated cytokines by qPCR. Shown are the results [average (mean ± SD) of triplicate samples] of one experiment representative of three. *p < 0.05 by Student’s t test.
For determining the involvement of IRF1 in HNP1-induced pDC activation, CAL-1 cells were transfected with control or IRF1 siRNA, followed by measurement of mRNA levels of proinflammatory cytokines in response to HNP1 and ODN alone or in combination (Fig. 6). IRF1 KD consistently decreased IRF1 expression by 70–80% as shown by Western blot (Fig. 6A). IRF1 KD significantly inhibited upregulation of IFNα or IFNβ1 mRNA in CAL-1 cells treated with HNP1 alone or in combination with ODN, indicating that IRF1 contributed to HNP1-induced production of type I IFNs (Fig. 6B & C). IRF1 knockdown did not inhibit HNP1-induced upregulation of IL-6 mRNA in CAL-1 cells; however, it significantly inhibited IL-6 mRNA upregulation caused by a combination of HNP1 and ODN (Fig. 6D). Thus, IRF1 contributes to HNP1 induction of pDC activation, particularly upregulation of type I IFNs.
Fig. 6.

IRF1 Knockdown inhibited HNP1 promotion of expression of proinflammatory cytokines in CAL-1 cells. CAL-1 cells transfected with control (Ctl) or IRF1 (IRF1 KD) siRNAs were cultured at 37 °C overnight. Subsequently, a portion of the cells was used for the preparation of whole cell lysate, while the rest of the cells were treated with HNP1 (final concentration = 2 μM) and/or ODN (final concentration = 1 μM) for 5 h at 37 °C before extraction of total RNAs. A, Identical amounts of Ctl and IRF1 lysates were separated on a SDS-PAGE gel, transferred onto a PVDF membrane, and probed by Western blot using anti-IRF1 antibody (upper panel). The same membrane was subsequently stripped and re-probed with anti-β-actin antibody (lower panel). B-D, qPCR quantitation of the mRNA levels of IFNα (B), IFNβ1 (C), and IL-6 (D) in Ctl and IRF1 KD CAL-1 cells. Shown is the average (mean ± SD) of three independent experiments. *p < 0.05 by Student’s t test.
3.4. HNP1 stimulation of pDC activation in vivo
A human pDC-nude mouse model, in which nude mouse was injected with human pDCs to form a temporary chimera, was used to determine whether HNP1 could activate human pDCs in vivo. Human pDC-nude chimeric mice were intraperitoneally injected with PBS (control) or HNP1, and the serum levels of human IFNα were measured at 1 h prior to, or 4–24 h after, injection of HNP1. HNP1 induced production of human IFNα in mouse serum collected at 4 or 24 h post HNP1 injection, but not in serum collected 1 h prior to HNP1 injection (Fig. 7A). There was also significant elevation of human IFNα in the peritoneal cavity of human pDC-nude chimeric mice 24 h after intraperitoneal injection of HNP1 (Fig. 7B). These data demonstrate that HNP1 activation of pDC can occur in vivo.
Fig. 7.
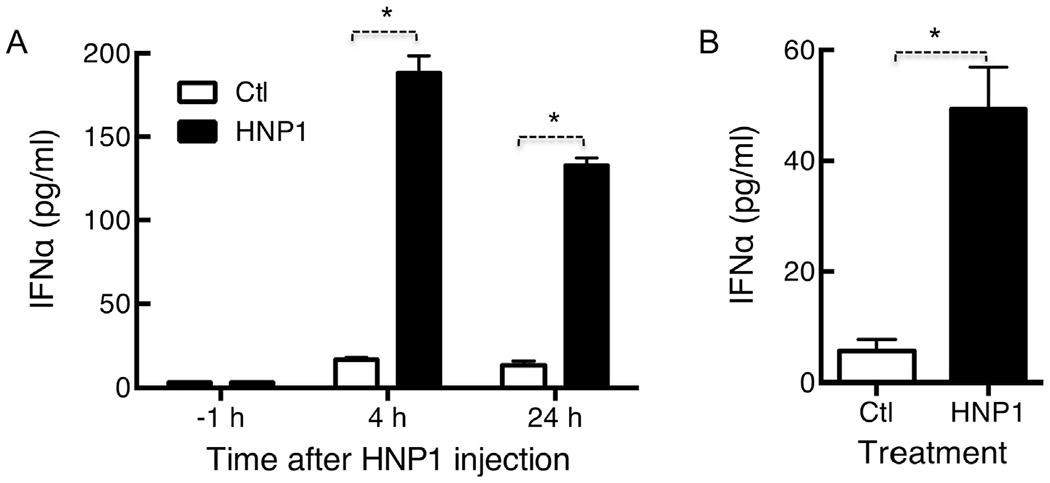
HNP1-induced IFNα production by human pDCs in vivo. Female nude mice (7-week old) were reconstituted by i.p. injection of isolated human pDCs (106/0.2 ml/mouse). One day after reconstitution, mice were randomized into 2 groups (n = 3), pre-bled 1 h in advance, and i.p. injected with 0.2 ml of either PBS (Ctl) or 5 μg of HNP1. Mice were bled at the times indicated, euthanized 24 h after injection of HNP1, and their peritoneal cavity were lavaged with 4 ml of PBS. Human IFNα in the serum samples (A) and peritoneal lavage fluid (B) was measured by ELISA and shown as the average (mean ± SD) of three independent experiments. *p < 0.05 by Student’s t test.
4. Discussion
Defensins, a family of antimicrobial peptides, are members of the alarmin superfamily [12,21–23,33]. Human defensins consist of three (α, β, and θ) subfamilies based on their size and pattern of intramolecular disulfide connectivity [11]. Human α defensins contain 6 members including HNP1 ~ 4 as well as human defensin 5 and 6 [11,21]. The alarmin HNP1, as a representative of human α defensins, has been shown to exhibit diverse biological activities ranging from cytotoxicity against microorganisms, recruitment of T cells and cDCs, activation of cDCs, and promotion of innate and antigen-specific immune responses [1,11–16,18,19,21,24–27,33]. However, it has not been documented whether or not HNP1 has the capacity to induce activation of pDCs. In this study, we observed that HNP1 could activate pDCs and pDC-like CAL-1 cells, leading to elevated expression of proinflammatory cytokines including IFNα, IFNβ1, and IL-6 (Figs. 1 & 3). HNP1 also promoted ODN-induced activation of pDCs and CAL-1 cells (Figs. 2, 5 & 6). In a human pDC-nude mice chimera model, HNP1 was shown to induce the production of human IFNα (Fig. 7). The in vitro results show, and the in vivo results suggest, that the alarmin HNP1 is an activator of pDCs.
Alarmin HNP1 triggered degradation of I-κBα and nuclear translocation of NF-κB (p50 and p65) and IRF1, resulting in the activation of NF-κB and IRF1 signaling pathways (Figs. 4 and 5). Importantly, HNP1-induced upregulation of proinflammatory cytokines in pDCs was inhibited by blockading the NF-κB signaling pathway, illustrating that NF-κB activation was essential for HNP1 activation of pDCs (Fig. 5). HNP1 activates NF-κB in human T cells and bronchial epithelial cells [34,35]. Very recently HNP1 was shown to enhance NF-κB translocation in synoviocytes of rheumatoid arthritic patients using electrophoretic mobility shift assay [36]. Therefore, NF-κB activation appears to be a common signaling pathway for HNP-induced activation of many target cells. The contribution of IRF1 to HNP1-induced activation of pDC-like CAL-1 cells (Figs. 4 & 6) is somewhat surprising at first glance, because it has previously been shown that activation of human pDCs by viral RNAs or bacterial DNAs for the induction of type I IFNs is mediated by IRF3 and IRF7 [29,31,37]. Nevertheless, IRF1 is an activator of IFNα and IFNβ transcription in many cell types [38]. In human conventional DCs, nickel sulfate stimulation of IL-12 relies on IRF1 [39], whereas IRF1, IRF3, and IRF7 are required for IFNβ production in response to Lactobacillus acidophilus [40]. In pDCs, type I IFN upregulation induced by poly(I:C) and class A (type D) ODN is mediated by IRF3 and IRF7, respectively [29,37,38]. A very recent study reveals that induction of IFNβ in human pDCs and CAL-1 cells by type K (class B) ODN is dependent on IRF5, but not IRF3/IRF7 [32]. It looks like the IRF(s) required for type I IFN induction depends on both target cells and activators. Our data showing the important role of IRF1 in HNP1 induction of CAL-1 activation (Fig. 6) provides further support for this notion. Thus, both NF-κB and IRF1 signaling pathways are important for HNP1-induced upregulation of proinflammatory cytokines in pDCs.
Alarmin HNP1 has been shown to have diverse effects on many target cells [12,33]. HNP1 induces chemotactic migration of DCs, macrophages, T cells, and mast cells using receptor(s) belonging to the Gαi protein coupled receptor superfamily; however, the identity of the receptor(s) has not been revealed [12–15,33]. HNP1 induces production of many cytokines and chemokines by bronchial epithelial cells and synoviocytes [36,41–43]. The receptor used by HNP1 to induce IL-8 production from bronchial epithelial cells is reported to be P2Y6 [41], a member of the purinergic receptor family [44]. HNP1 has recently been shown to promote IL-1 maturation and release from macrophages by signaling through the purinergic receptor P2X7 [20]. Although human pDCs express both P2Y6 and P2X7 at the mRNA levels [45], the possibility that HNP1 uses these purinergic receptors for the activation of pDCs is low since pDCs do not respond to dATP (a high affinity agonist for P2Y6) and ATP (a potent agonist for many purinergic receptors including P2X7) [44,45]. Therefore, the receptor that the alarmin HNP1 uses for the activation of pDCs needs to be identified by future investigations.
In the human pDC-nude chimeric mice model, appearance of human IFNα in the serum and peritoneal lavage fluid samples of HNP-treated mice was assumed due to the activation of human pDCs (Fig. 7). However, the possibility that IFNα might come from cells other than human pDCs cannot be ruled out, since the pDC preparations used for the construction of human pDC-nude chimeric mice were unlikely 100% pure. In this study, human pDC preparations (isolated using the Plasmacytoid Dendritic Cell Isolation Kit II of Miltenyi Biotec) used were consistently around 95% pure. Thus, pDC was the major source of human IFNα in pDC-nude chimeric mice treated with HNP1.
Several alarmins have been shown to promote pDC activation and IFN production, including LL-37, the only cathelicidin in humans [46,47], human β defensin 2 & 3 [48], and HMGB1 [49]. Our results help put HNP1 in the same group of pDC-activating alarmins; however, HNP1 has its uniqueness. LL-37, β defensins, and HMGB1 promote pDC activation by forming complexes with RNA, DNA (including ODN), or immune complex to facilitate their uptake, and hence inducing cell activation through triggering TLR7, TLR8, and TLR9 [46–49]. HNP1, albeit capable of synergistically promoting ODN-induced pDC activation (Figs. 2, 5 & 6), induced pDC and CAL-1 activation in the absence of nucleic acids or ODN (Figs. 2, 3, 5 & 6). Additionally, HNP1 triggered the activation of NF-κB and IRF1 signaling pathways in CAL-1 cells independent of any nucleic acid (Fig. 4). Furthermore, HNP1 did not form complex with ODN (data not shown). Thus, HNP1 probably utilized a distinct mechanism to synergize with ODN for the promotion of pDC activation.
Over the years, elevated type I IFN level has emerged as a potential pathogenesis factor in many autoimmune disorders, such as, rheumatoid arthritis, lupus erythematosus and psoriasis [29,46,47,49,50]. Plasmacytoid DC is the major cell type that produces type I IFNs [29]. Our results showing that HNP1 can activate pDCs for the induction of IFNα production suggest that interaction between HNP1 and pDCs may contribute to the generation of IFNα in certain autoimmune diseases. Indeed, HNP1 has been found in blood or inflamed tissues of patients with lupus erythematosis and rheumatoid arthritis [7,51,52], while pDCs have been reported to be present in the synovial tissues of rheumatoid arthritic joints [53,54]. Importantly, both HNP1 and pDCs are found to be co-present in the synovial fluid of inflamed joints of rheumatoid arthritic patients [7,36,52,54]. We have also obtained preliminary data showing that synovial fluids of rheumatoid arthritic joints contained high concentrations of HNP1 and pDCs (data not shown). Therefore, HNP1 induction of pDC activation and production of proinflammatory cytokines including type I IFNs may contribute to regulating the pathogenesis of several autoimmune diseases including rheumatoid arthritis.
Overall, our data firmly establish that (1) alarmin HNP1 is an activator of pDCs, (2) HNP1 induces distinctive intracellular signaling involving the activation of NF-κB and IRF1, and (3) HNP1 activation of pDCs may contribute to regulating the inflammatory pathogenesis of rheumatoid arthritic joints.
Acknowledgments
This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute. This work was also supported in part by a 973 Grant (2012CB932503) from the National Key Basic Research Program of China.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Conflict of interest disclosure
The authors declare no conflict of interest.
References
- [1].Ganz T, Selsted ME, Szklarek D, Harwig SSL, Daher K, Bainton DF, Lehrer RI, Defensins: natural peptide antibiotics of human neutrophils, J. Clin. Invest 76 (1985) 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wilde CG, Griffith JE, Marra MN, Snable JL, Scott RW, Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family, J. Biol. Chem 264 (1989) 11200–11203. [PubMed] [Google Scholar]
- [3].Sparkes RS, Kronenberg M, Heinzmann C, Daher KA, Klisak I, Ganz T, Mohandas T, Assignment of defensin gene(s) to human chromosome 8p23, Genomics 5 (1989) 240–244. [DOI] [PubMed] [Google Scholar]
- [4].Aldred PM, Hollox EJ, Armour JA, Copy number polymorphism and expression level variation of the human alpha-defensin genes DEFA1 and DEFA3, Hum. Mol. Genet 14 (2005) 2045–2052. [DOI] [PubMed] [Google Scholar]
- [5].Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jomvall H, Wigzell H, Gudmundsson GH, The human antimicrobial and chemotactic peptides LL-37 and a-defensins are expressed by specific lymphocyte and monocyte populations, Blood 96 (2000) 3086–3093. [PubMed] [Google Scholar]
- [6].Driss V, Legrand F, Hermann E, Loiseau S, Guerardel Y, Kremer L, Adam E, Woerly G, Dombrowicz D, Capron M, TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins, Blood 113 (2009) 3235–3244. [DOI] [PubMed] [Google Scholar]
- [7].Paulsen F, Pufe T, Conradi L, Varoga D, Tsokos M, Papendieck J, Petersen W, Antimicrobial peptides are expressed and produced in healthy and inflamed human synovial membranes, J. Pathol 198 (2002) 369–377. [DOI] [PubMed] [Google Scholar]
- [8].Rodriguez-Garcia M, Oliva H, Climent N, Garcia F, Gatell JM, Gallart T, Human immature monocyte-derived dendritic cells produce and secrete alpha-defensins 1–3, J. Leukoc. Biol 82 (2007) 1143–1146. [DOI] [PubMed] [Google Scholar]
- [9].Valore EV, Ganz T, Posttranslational processing of defensins in immature human myeloid cells, Blood 79 (1992) 1538–1544. [PubMed] [Google Scholar]
- [10].Harwig SS, Park AS, Lehrer RI, Characterization of defensin precursors in mature human neutrophils, Blood 79 (1992) 1532–1537. [PubMed] [Google Scholar]
- [11].Lehrer RI, Lu W, Alpha-Defensins in human innate immunity, Immunol. Rev 245 (2012) 84–112. [DOI] [PubMed] [Google Scholar]
- [12].Yang D, de la Rosa G, Tewary P, Oppenheim JJ, Alarmins link neutrophils and dendritic cells, Trends Immunol. 30 (2009) 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ, Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils, J. Biol. Chem 271 (1996) 2935–2940. [DOI] [PubMed] [Google Scholar]
- [14].Yang D, Chen Q, Chertov O, Oppenheim JJ, Human neutrophil defensins selectively chemoattract naïve T and immature dendritic cells, J. Leukoc. Biol 68 (2000) 9–14. [PubMed] [Google Scholar]
- [15].Grigat J, Soruri A, Forssmann U, Riggert J, Zwirner J, Chemoattraction of macrophages, T lymphocytes, and mast cells is evolutionarily conserved within the human alpha-defensin family, J. Immunol 179 (2007) 3958–3965. [DOI] [PubMed] [Google Scholar]
- [16].Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV, Voitenok NN, Neutrophil a-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells, Eur. Cytokine Netw 11 (2000) 257–260. [PubMed] [Google Scholar]
- [17].Zhang H, Porro G, Orzech N, Mullen B, Liu M, Slutsky AS, Neutrophil defensins mediate acute inflammatory response and lung dysfunction in dose-related fashion, Am. J. Physiol 280 (2001) L947–954. [DOI] [PubMed] [Google Scholar]
- [18].Rodriguez-Garcia M, Oliva H, Climent N, Escribese MM, Garcia F, Moran TM, Gatell JM, Gallart T, Impact of alpha-defensins1-3 on the maturation and differentiation of human monocyte-derived DCs. Concentration-dependent opposite dual effects, Clin. Immunol 131 (2009) 374–384. [DOI] [PubMed] [Google Scholar]
- [19].Presicce P, Giannelli S, Taddeo A, Villa ML, Della Bella S, Human defensins activate monocyte-derived dendritic cells, promote the production of proinflammatory cytokines, and up-regulate the surface expression of CD91, J. Leukoc. Biol 86 (2009) 941–948. [DOI] [PubMed] [Google Scholar]
- [20].Chen Q, Jin Y, Zhang K, Li H, Chen W, Meng G, Fang X, Alarmin HNP-1 promotes pyroptosis and IL-1beta release through different roles of NLRP3 inflammasome via P2X7 in LPS-primed macrophages, Innate Immun. 20 (2014) 290–300. [DOI] [PubMed] [Google Scholar]
- [21].Oppenheim JJ, Yang D, Alarmins: chemotactic activators of immune responses, Curr. Opin. Immunol 17 (2005) 359–365. [DOI] [PubMed] [Google Scholar]
- [22].Bianchi ME, DAMPs, PAMPs and alarmins: all we need to know about danger, J. Leukoc. Biol 81 (2007) 1–5. [DOI] [PubMed] [Google Scholar]
- [23].Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J, Alarmins: awaiting a clinical response, J. Clin. Invest 122 (2012) 2711–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lillard JW Jr., Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR, Mechanisms for induction of acquired host immunity by neutrophil peptide defensins, Proc. Natl. Acad. Sci. USA 96 (1999) 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tani K, Murphy WJ, Chertov O, Salcedo R, Koh CY, Utsunomiya I, Funakoshi S, Asai O, Herrmann SH, Wang JM, Kwak LW, Oppenheim JJ, Defensins act as potent adjuvants that promote cellular and humoral immune responses in mice to a lymphoma idiotype and carrier antigens, Int. Immunol 12 (2000) 691–700. [DOI] [PubMed] [Google Scholar]
- [26].Brogden KA, Heidari M, Sacco RE, Palmquist D, Guthmiller JM, Johnson GK, Jia HP, Tack BF, McCray PB Jr., Defensin-induced adaptive immunity in mice and its potential in preventing periodontal disease, Oral Microbiol. Immunol 18 (2003) 95–99. [DOI] [PubMed] [Google Scholar]
- [27].Wang YS, Li D, Shi HS, Wen YJ, Yang L, Xu N, Chen XC, Chen X, Chen P, Li J, Deng HX, Wang CT, Xie G, Huang S, Mao YQ, Chen LJ, Zhao X, Wei YQ, Intratumoral expression of mature human neutrophil peptide-1 mediates antitumor immunity in mice, Clin. Cancer Res 15 (2009) 6901–6911. [DOI] [PubMed] [Google Scholar]
- [28].Banchereau J, Steinman RM, Dendritic cells and the control of immunity, Nature 392 (1998) 245–251. [DOI] [PubMed] [Google Scholar]
- [29].Gilliet M, Cao W, Liu YJ, Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases, Nat. Rev. Immunol 8 (2008) 594–606. [DOI] [PubMed] [Google Scholar]
- [30].Maeda T, Murata K, Fukushima T, Sugahara K, Tsuruda K, Anami M, Onimaru Y, Tsukasaki K, Tomonaga M, Moriuchi R, Hasegawa H, Yamada Y, Kamihira S, A novel plasmacytoid dendritic cell line, CAL-1, established from a patient with blastic natural killer cell lymphoma, Int. J. Hematol 81 (2005) 148–154. [DOI] [PubMed] [Google Scholar]
- [31].Seth RB, Sun L, Ea CK, Chen ZJ, Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3, Cell 122 (2005) 669–682. [DOI] [PubMed] [Google Scholar]
- [32].Steinhagen F, McFarland AP, Rodriguez LG, Tewary P, Jarret A, Savan R, Klinman DM, IRF-5 and NF-kappaB p50 co-regulate IFN-beta and IL-6 expression in TLR9-stimulated human plasmacytoid dendritic cells, Eur. J. Immunol 43 (2013) 1896–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang D, Wei F, Tewary P, Howard OM, Oppenheim JJ, Alarmin-induced cell migration, Eur. J. Immunol 43 (2013) 1412–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vaschetto R, Grinstein J, Del Sorbo L, Khine AA, Voglis S, Tullis E, Slutsky AS, Zhang H, Role of human neutrophil peptides in the initial interaction between lung epithelial cells and CD4+ lymphocytes, J. Leukoc. Biol 81 (2007) 1022–1031. [DOI] [PubMed] [Google Scholar]
- [35].Sakamoto N, Mukae H, Fujii T, Ishii H, Yoshioka S, Kakugawa T, Sugiyama K, Mizuta Y, Kadota J, Nakazato M, Kohno S, Differential effects of alpha- and beta-defensin on cytokine production by cultured human bronchial epithelial cells, Am. J. Physiol. Lung Cell Mol. Physiol 288 (2005) L508–513. [DOI] [PubMed] [Google Scholar]
- [36].Ahn JK, Huang B, Bae EK, Park EJ, Hwang JW, Lee J, Koh EM, Cha HS, The role of alpha-defensin-1 and related signal transduction mechanisms in the production of IL-6, IL-8 and MMPs in rheumatoid fibroblast-like synoviocytes, Rheumatology 52 (2013) 1368–1376. [DOI] [PubMed] [Google Scholar]
- [37].Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T, Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction, Nature 434 (2005) 1035–1040. [DOI] [PubMed] [Google Scholar]
- [38].Tamura T, Yanai H, Savitsky D, Taniguchi T, The IRF family transcription factors in immunity and oncogenesis, Annu. Rev. Immunol 26 (2008) 535–584. [DOI] [PubMed] [Google Scholar]
- [39].Antonios D, Rousseau P, Larange A, Kerdine-Romer S, Pallardy M, Mechanisms of IL-12 synthesis by human dendritic cells treated with the chemical sensitizer NiSO4, J. Immunol 185 (2010) 89–98. [DOI] [PubMed] [Google Scholar]
- [40].Weiss G, Maaetoft-Udsen K, Stifter SA, Hertzog P, Goriely S, Thomsen AR, Paludan SR, Frokiaer H, MyD88 drives the IFN-beta response to Lactobacillus acidophilus in dendritic cells through a mechanism involving IRF1, IRF3, and IRF7, J. Immunol 189 (2012) 2860–2868. [DOI] [PubMed] [Google Scholar]
- [41].Khine AA, Del Sorbo L, Vaschetto R, Voglis S, Tullis E, Slutsky AS, Downey GP, Zhang H, Human neutrophil peptides induce interleukin-8 production through the P2Y6 signaling pathway, Blood 107 (2006) 2936–2942. [DOI] [PubMed] [Google Scholar]
- [42].van Wetering S, Mannesse-Lazeroms SPG, van Sterkenburg MAJA, Daha MR, Dijkman JH, Hiemstra PS, Effect of defensins on interleukin-8 synthesis in airway epithelial cells, Am. J. Physiol 272 (1997) L888–L896. [DOI] [PubMed] [Google Scholar]
- [43].van Wetering S, Mannesse-Lazeroms SP, van Sterkenburg MA, Hiemstra PS, Neutrophil defensins stimulate the release of cytokines by airway epithelial cells: modulation by dexamethasone, Inflamm. Res 51 (2002) 8–15. [DOI] [PubMed] [Google Scholar]
- [44].Corriden R, Insel PA, New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors, Purinergic Signal 8 (2012) 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schnurr M, Toy T, Stoitzner P, Cameron P, Shin A, Beecroft T, Davis ID, Cebon J, Maraskovsky E, ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling, Blood 102 (2003) 613–620. [DOI] [PubMed] [Google Scholar]
- [46].Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Su B, Nestle FO, Zal T, Mellman I, Schroder JM, Liu YJ, Gilliet M, Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide, Nature 449 (2007) 64–569. [DOI] [PubMed] [Google Scholar]
- [47].Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, Homey B, Barrat FJ, Zal T, Gilliet M, Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8, J. Exp. Med 206 (2009) 1983–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tewary P, de la Rosa G, Sharma N, Rodriguez LG, Tarasov SG, Howard OM, Shirota H, Steinhagen F, Klinman DM, Yang D, Oppenheim JJ, Beta-Defensin 2 and 3 promote the uptake of self or CpG DNA, enhance IFN-alpha production by human plasmacytoid dendritic cells, and promote inflammation, J. Immunol 191 (2013) 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ, Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE, Nat. Immunol 8 (2007) 487–496. [DOI] [PubMed] [Google Scholar]
- [50].Ronnblom L, Alm GV, Eloranta ML, Type I interferon and lupus, Curr. Opin. Rheumatol 21 (2009) 471–477. [DOI] [PubMed] [Google Scholar]
- [51].Sthoeger ZM, Bezalel S, Chapnik N, Asher I, Froy O, High alpha-defensin levels in patients with systemic lupus erythematosus, Immunology 127 (2009) 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Baillet A, Trocme C, Berthier S, Arlotto M, Grange L, Chenau J, Quetant S, Seve M, Berger F, Juvin R, Morel F, Gaudin P, Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases, Rheumatology 49 (2010) 671–682. [DOI] [PubMed] [Google Scholar]
- [53].Takakubo Y, Takagi M, Maeda K, Tamaki Y, Sasaki A, Asano T, Fukushima S, Kiyoshige Y, Orui H, Ogino T, Yamakawa M, Distribution of myeloid dendritic cells and plasmacytoid dendritic cells in the synovial tissues of rheumatoid arthritis, J. Rheumatol 35 (2008) 1919–1931. [PubMed] [Google Scholar]
- [54].Lebre MC, Jongbloed SL, Tas SW, Smeets TJ, McInnes IB, Tak PP, Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP-dendritic cells with distinct cytokine profiles, Am. J. Pathol 172 (2008) 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]


