Abstract
Background
Urogenital schistosomiasis (UGS) caused by S. haematobium has enormous reproductive health consequences including infertility. Reproductive aged individuals are a neglected group and not included in control programs in Cameroon. This study investigated the prevalence and severity of S. haematobium infection in the context of gender and socio-economic structures that shape behaviour among reproductive aged individuals living in Tiko, a semi-urban setting, Cameroon.
Methodology/Principal findings
A cross-sectional study was carried out in the Tiko Health District (THD) between May to September 2019. Consenting individuals were enrolled using a convenient sampling technique and administered a semi-structured questionnaire to document data on socio-demographic and stream contact behaviour. A urine sample was collected and screened for the presence of S. haematobium ova using reagent strips, filtration and microscopy. The overall prevalence of S. haematobium infection was 22.8% (95% CL: 19.27–26.73) with geometric mean egg load of 18.74 (range: 1–1600) per 10ml of urine. Younger age group (15 – 20years) (OR: 5.13; 95% CL: 1.35–19.42), male (OR: 2.60 3.07; 95% CL: 1.54–4.40) and awareness of UGS (OR: 1.73; 95% CL: 1.02–2.95) were associated with higher odds of exposure to infection. Significantly higher intensity of infection was seen in males, singles and in the age group 15–30 years. It is worth noting that males carried out more activities which entailed longer duration in streams.
Conclusion/Significance
The prevalence obtained shows that Tiko is a moderate-risk area for UGS with underlying morbidity-inducing infection intensity. The severity of the infection is more in males. Awareness of the disease is not enough to protect these communities from infection, but provision of public infrastructures and health education will limit contact with infested water and thus curtail the infection. There is an urgent need to involve all age groups in control programs.
Author summary
S. haematobium deposit its eggs in the urogenital organs which may cause genital ulcers and other lesions. When untreated the infection causes infertility, miscarriage, ectopic pregnancies, spontaneous abortions, prematurity, vulva nodules, genital and cervical lesions, increased risk of HIV and Human Papilloma Virus in women. These may lead to social consequences such as low self-esteem, depression and stigma. The disease burden has been neglected in males, with more emphasis on female genital schistosomiasis. Control programs have focused on school age children, often overlooking reproductive aged individuals who are potential reservoirs. A total of 509 reproductive aged individuals living in Tiko, a semi urban setting in the mount Cameroon area were screened. The overall prevalence of S. haematobium infection was 22.8%, with significantly higher rates in males (35.2%) than females (16.2%). Similarly, intensity of infection was significantly higher in males (p = 0.009), singles (p = 0.004) and younger age individuals (0.012). Younger age, gender type (male) and awareness of UGS were identified as key factors that increase the odd of infection. In this setting, younger adults and males represent important public health risk groups and potential reservoir sources of disease transmission in the area.
Introduction
Schistosomiasis is an acute and chronic disease caused by dioecious blood flukes of the genus Schistosoma. It remains a major neglected tropical disease (NTD) and a significant public health challenge in low and middle-income countries [1]. It is estimated that at least 90% of those requiring treatment for schistosomiasis live in Africa where two forms of schistosomiasis (intestinal and urogenital) exist [2]. Schistosomiasis mostly affects poor and rural communities; however, migration to urban areas and population movements are introducing the disease to new areas [2]. The transmission of schistosomiasis is governed by social-ecological systems such as conditions of poverty and living near open freshwater bodies [3]. In endemic areas, where there is lack of adequate water supply, poverty, ignorance, and poor hygienic practices, any demographical groups, irrespective of age or gender with unsafe water contact is at risk of infection [4–8]. Nonetheless, key gendered roles and customs place men, women, girls and boys at differential risk. The roles of women and girls within the household including chores such as washing of clothes and dishes, collecting water for household consumption exposes them to daily risk of infection [9, 10]. Livelihood activities such as fishing, agriculture which often require contact with infested waters have been linked to the male gender [10].
Urogenital schistosomiasis (UGS) is caused by infection with S. haematobium. People become infected when the larval form, the cercaria, released by freshwater snails of the genus Bulinus penetrate the skin during contact with infested water. In the body, the adult female fluke lay terminal-spined eggs, often in copious amounts each day. Some of the eggs are passed out of the body in urine to continue the parasite’s lifecycle while others become partially lodged or later trapped within all organs of the urogenital tract causing immune reactions and progressive damage to organs [2]. In the bladder, the eggs perforate and cross the bladder wall with accompanied leakage of venous blood. This leads to the classical sign of UGS known as haematuria. Fibrosis of the bladder and ureter, kidney damage and possibly bladder cancer are sometimes complications in advanced cases [11, 12]. In adults, the infection can cause genital ulcers and other lesions [13] resulting in long-term poor reproductive health, with sexual dysfunction and irreversible consequences including infertility [14]. Urogenital schistosomiasis is also considered to be a risk factor for HIV infection [15, 16] and Human Papilloma Virus in adults [1]. The consequences and disability caused by gender specific manifestations of UGS often go unrecognized at national and local levels. Also, unlike female genital schistosomiasis (FGS), male genital schistosomiasis (MGS), as evidenced by schistosome eggs in male genital organs remains underreported and often misperceived [16].
Typically, UGS is endemic in rural areas of the Bafia Health Area [17–19] found in the Mount Cameroon area. In 2018, an unmapped UGS transmission focus was reported in Tiko, a semi urban town in the THD, Mount Cameroon Area, probably due to human migration and interurban trading which occurs between rural and peri- urban settings in the area [20]. Moreover, the equatorial climate contributes to establishment of the infection because it provides conditions suitable for the presence of the molluscs that encourage the transmission of this disease in this endemic area [21]. While the WHO strategy recommends reaching both school-based and community-based programs, the national control programmes in Cameroon prioritize mass drug administration of praziquantel to school-age children [22]. Adults excluded from preventive chemotherapy campaigns continue to potentiate transmission in endemic communities. Praziquantel based control programs have only a temporary effect on transmission and are limited in their potential to interrupt disease transmission in the long-term [23]. More so, untreated infected individuals may suffer from profound adverse urothelial and reproductive health consequences. A global analysis by Kayuni et al. [16] revealed the existence of a gap in epidemiological data on the MGS and FGS in Cameroon. This suggests that the burden of disease in reproductive aged individuals is not fully recognized probably due to lack of disease monitoring by health systems in endemic communities. This study aims to provide an update on epidemiological findings on UGS among reproductive aged individuals living in the Tiko Health District, Cameroon. The prevalence and severity of S. haematobium infection was evaluated in the context of gender and socio-economic structures that shape behaviour. Identifying local risk factors is essential for expediting disease control by targeting high-risk groups or by informing possible intervention strategies to stakeholders involved in the control of schistosomiasis.
Materials and methods
Ethics statement
The study received institutional approval (2017/645-08/UB/SG/IRB/FHS) from the Ethics Review Board hosted by the Faculty of Health Sciences and administrative authorisation from the South West Regional Delegation of Public Health, Buea. All participants were invited to sign the free and informed consent form. In this document, participants must have the freedom to leave the study at any time. For participants less than 18 years old, consent was gotten from the parents or guardian and the child meanwhile children 18 years old and older individuals gave their consent. Discussions were made in the local language (pidgin) when necessary and simplified to the best understanding of the participants. By signing the form, the participants agreed to be administered a questionnaire and to provide urine sample for the parasitological analysis.
The results were communicated to the participants, and adults positive for S. haematobium infection were treated for free with praziquantel tablets (40 mg/kg of body weight) (Cesol 600mg manufacture by Merck, Mexique). The treatment was in accordance with Cameroonian guidelines of the Control Program of Schistosomiasis (PNLSHI).
Study area
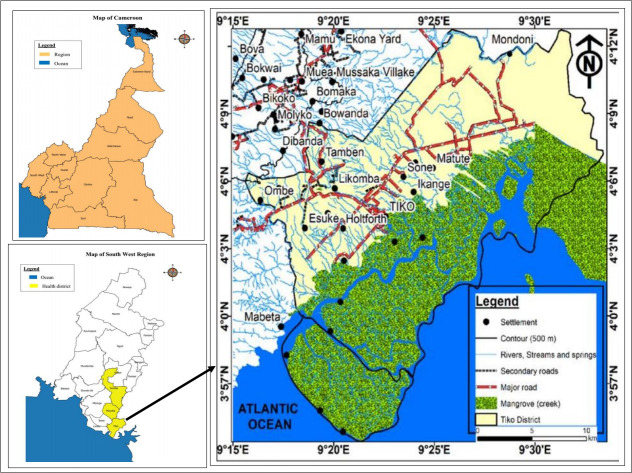
This study was carried out in the Tiko Health Area, an unmapped endemic foci [20], which is geographically close to Munyenge, a rural endemic foci [17, 19], located in Muyuka health district, Mount Cameroon area, South West Region, Cameroon Fig 1. Tiko town is a semi-urban settlement with a surface area of 4840 Km2 and a population size of 134,649. The population density of Tiko is 241 inhabitants/Km2, with a population growth of 2.9% [20]. Tiko is located 10 meters above sea level between longitudes 9°15′E and 9°30′E, latitudes 3°57′N and 4°12′N with a relative humility of 83.1% and average rain fall of 4,524 mm. This area has a coastal equatorial climate with daily temperatures ranging from 28°C—33°C. Soil types include the sandy alluvial and volcanic with high agricultural potentials. The Tiko municipality is interspersed with water courses including rivers Mungo and Ombe, and Ndongo and Benyo streams which empty into the Atlantic Ocean [24] (Fig 1). The predominant livelihoods of people living in the communities are trading, farming and fishing [24]. For some communities with deficiency in water supply, accessing and using stream water is a vital part of both household survival and many livelihood activities such as farming (washing pumpkin seeds). The lack of bridges in certain parts of the town, compels the population crossing these streams to their farms and other areas within the municipality exposing them to cercariae infested streams. The study was carried out in four communities (Likomba, Holtfort, Tiko, Ikange) in the Tiko Health Area which are in proximity to the streams.
Fig 1. Map showing Tiko Health District in the Mount Cameroon area.
Study participants and sample size determination
The study population included reproductive aged individuals (females: 15–49 years, Male: 15–71 years, as specified by Yirenya-Tawiah et al. [25] and Marlowe et al. [26]) living in the Tiko Health District. The sample size of the study population was calculated considering S. haematobium infection prevalence of 38% from a recent cross sectional survey carried out in the Tiko Health District by Anguh et al. [20]. The sample size was determined using the Lorenz formula [27]. The total number of samples N is given by: N = Z2 P (1-P)/d2 where Z is the standard normal deviation, Z = 1.96 for the confidence level of 95%, P = 38%: proportion of UGS prevalence, d is the total width of the confidence interval (e.g., 0.05 = ±5). The minimum estimated sample size calculated was 362. A total of 514 participants was enrolled into this study to anticipated factors like effect size, loss of data, voluntary withdrawal and for greater precision.
Study design
To evaluate the prevalence and severity of S. haematobium infection among individuals of reproductive age in the study area, a cross-sectional study was conducted between May and September 2019. After obtaining administrative and ethical clearances, acquaintance visits were made to community and church leaders of the communities concerned, to inform them about the study procedures. Questionnaire was pre-tested by research assistants, challenges were identified and the changes effected. A convenience sampling technique was used to recruit participants into the study. Individuals were invited by local coordinators and community leaders to rally at the different church premises and focal points in the four selected communities on the day programmed for data and sample collection. Health talks were given in layman’s terms, to explain the purpose, risk and benefits of the study. A semi-structured questionnaire was administered to volunteer participants by trained research assistants (including the first and fourth authors) to obtain data on sociodemographic and economic factors as well as stream contact behaviour. Discussions were made in English and the local language (pidgin) where necessary and simplified to the best understanding of the participants. Urine samples were collected and processed for the detection of haematuria and S. haematobium ova.
Inclusion, exclusion and withdrawal criteria
This study was designed to target individuals of reproductive age (females: 15–49 years, Male: 15–71 years). Only respondents who have lived for at least two months in the study area and volunteered to participate were enrolled in the study. Those who declined to participate in the study or failed to submit a urine sample after the interview were excluded from the survey.
Data collection
Administration of questionnaire
A semi-structured questionnaire (S1 Checklist Questionnaire) was used to interview the participants face to face to obtain information about individuals based on the following indicators: socio- demographic/economic (resident address, distance from stream, age, gender, educational level, marital status, occupation) and water contact behaviour (source of water supply, stream usage, frequency of contact with open water source, and activities carried out in the stream among other information). The degree of water contact was calculated using the formula: Σ(R x F) as described by Lima et al. [28], R is the score for the reason for the contact and F the score for the frequency of contact. The reasons for water contact are given the following scores: 5 (bathing), 4 (laundry, washing of motorbikes), 3 (collecting water for the household washing, washing pumpkin seeds in the stream), and 2 (crossing the streams). The frequency of contacts were scored according to Lima et al. [28] with some modifications: 28 (more than thrice a week or daily), 12 (three contact a week), 8 (two contact a week), and 4 (one contact a week). Totals of ≥100 were considered as high degree and 2–99 as low degree [28].
Sample collection and laboratory analysis
On the day of enrolment, all individuals who participated in the interviews received a sterile, wide mouthed, screw capped plastic bottle carrying their identification information. Due to the circadian pattern of schistosome egg excretion, participants were requested to collect a urine sample between 10 am– 2 pm [29]. All samples were stored in cooling boxes containing air cooler ice packs at temperature of about 3.7°C to prevent the eggs of S. haematobium from hatching during transportation to the THD laboratory for processing within 24 hours of collection. In the laboratory, haematuria was immediately determined by visual observation and urine reagent strips (Mission* Expert USA). The urine samples were processed using the membrane filtration technique and examined microscopically for the presence of S. haematobium infection based on morphology of the ova [30]. In brief, 10 ml of urine was pass through membrane filter (Sterlitech Polycarbonate (PCTE) membrane filters, USA), the filter was removed, place on a glass slide and stained with 1% Lugol iodine solution. The slide was then examined using the Binocular Compound light microscope. Terminal-spined eggs, characteristics of S. haematobium were identified and counted manually [30]. The egg load was defined by the number of eggs per 10ml of urine, categorized as light (<50 eggs/10 ml of urine) or heavy (≥50 eggs/10ml of urine) infection as defined by the WHO [31]. Microhaematuria was considered as proxy-diagnosis of UGS, an accepted marker in the rapid diagnosis of S. haematobium infection in urine [32]. An individual was considered positive for UGS, when he or she had S. haematobium eggs and/or positive for microhaematuria.
Statistical analysis
The data were analysed using SPSS version 21.0 (SPSS, Inc., Chicago, IL, USA). Proportions of S. haematobium infection were compared between different groups (age groups, sex, educational level, occupational status, distance of house from stream, stream usage, stream contact activities, and frequency to stream) using Pearson Chi-square test (χ2). Worm egg output was normalized by log10 transformation and assessed in relation to age using Pearson’s correction coefficient (r). Kruskal Wallis and Mann-Whitney tests were used to compare mean differences in the intensity of egg excretion. Odd ratios (OR) and confidence intervals (CIs) were calculated using a Microsoft Excel confidence interval calculator as described by Armitage & Berry [33] and Newcombe [34]. Variables that had a p-value <0.20 in bivariate analysis or explanatory plausibility were included in the logistic regression model for analysis of factors associated with S. haematobium infection. A p—value of < 0.05 was considered statistically significant.
Results
Characteristics of the study population
In this study, a total number of 509 individuals of reproductive age (range: 15–71 years) were enrolled, completed questionnaires, and submitted urine samples. The mean age was 32.3 ± 11.3 years and 65.4% were females. Of noteworthy, 83% have lived in the study area for more than 4 years and only 31.8% of the participants have heard of urogenital schistosomiasis, 64.5% live within 100m away from infested streams among whom almost two-third rely on stream water (66.4%, n = 338). The principal stream activities reported are bathing, laundry, collection of water for domestic activities, farming and washing of motorbikes. Overall, 33.6% reported use of tap water only and were mostly those who live more than 100m from infested water sources. All the participants have basic level of education, 58.2% of them having obtained some form of secondary education. The characteristics of the study population are shown in Table 1.
Table 1. Characteristics of the study participants.
| Variable | Characteristics | Number examined (N) | Percentage (%) |
|---|---|---|---|
| Gender | Female | 333 | 65.4 |
| Male | 176 | 34.6 | |
| Age Group (Years) | 15–20 | 109 | 21.4 |
| 21–30 | 114 | 22.4 | |
| 31–40 | 160 | 31.4 | |
| 41–71 | 126 | 24.8 | |
| Marital status | Single | 230 | 45.2 |
| Married | 279 | 54.8 | |
| Educational level | At least Primary | 213 | 41.8 |
| At least Secondary | 296 | 58.2 | |
| Occupation | Student | 117 | 23.0 |
| Farmer | 119 | 23.4 | |
| Business | 133 | 26.1 | |
| Salary earner | 140 | 27.5 | |
| Distance to stream (metres) | ≤ 100 | 329 | 64.6 |
| > 100 | 180 | 35.4 | |
| Awareness | Aware | 162 | 31.8 |
| Not aware | 347 | 68.2 | |
| Source of water | Stream only | 64 | 12.6 |
| Stream and piped | 274 | 53.8 | |
| Piped only | 171 | 33.6 | |
| Stream usage | Yes | 338 | 66.4 |
| No | 171 | 33.6 | |
| Stream Activity | Bathing | 166 | 29.0 |
| Laundry | 189 | 33.0 | |
| Fetch water | 138 | 24.0 | |
| Frequency to stream per week | ≥ Thrice | 199 | 58.9 |
| < Thrice | 139 | 41.1 |
The prevalence and intensity of S. haematobium infection and associated determinant factors
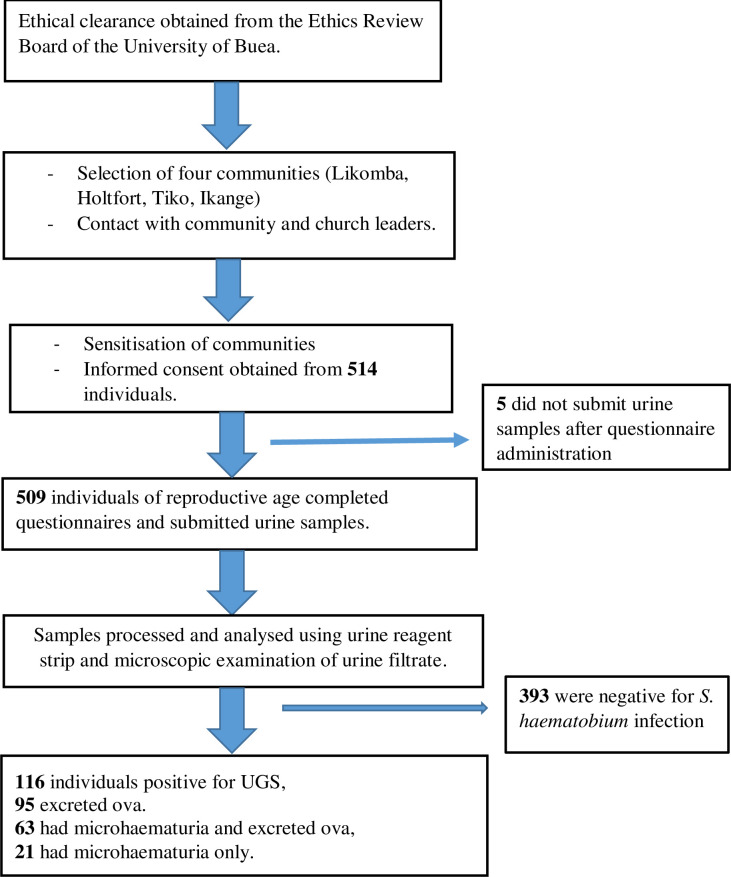
In this study, 116 (22.8%; 95% CI: 19.27–26.73) individuals were positive for UGS. Egg excretion was recorded for 95 (18.7%) among whom 28 (29.5%) had heavy (≥50 eggs/10ml of urine) infection while 67 (70.5%) had light (<50 eggs/10ml of urine) infection. The geometric mean load was 18.74 (range: 1–1600) eggs per 10ml of urine. The prevalence of microhaematuria was 12.4% (n = 63), of which 4.1% (21) were positive for microhaematuria only Fig 2. Using microscopic urine examination as gold standard, the specificity and sensitivity of microhaematuria in the diagnosis of S. haematobium infection were 95.0% (95% CI: 92.4–96.7) and 44.2% (95% CI: 34.6–54.2), respectively.
Fig 2. Flow chart of participation and prevalence of urogenital schistosomiasis.
The results displayed in Table 2 shows that age (p <0.001), gender (p <0.001), marital status (p <0.001), secondary level of education (p = 0.017), the type of occupation (p <0.001), distance from house to stream (p = 0.004), source of water (p <0.001) and activity at stream were the determinant factors of urogenital schistosomiasis in the study area. Accordingly, higher values of prevalence were obtained among; the younger age group (15–20years) (45.9%), males (35.2%), individuals who had attained at least secondary level of education (26.7%), singles (31.7%) and students (41.9%). In addition, a higher prevalence was recorded among participants who reported using the stream as the only source of water (42.2%), washing of motorbike in the stream (75%) and those who live within 100m away from infested streams (27.7%).
Table 2. Univariable analysis of S. haematobium infection according to sociodemographic and behavioural factors.
| Variable | Category | S. haematobium Positive % (n) | χ2; p value |
|---|---|---|---|
| Gender | Male | 35.2 (62) | 23.650; < 0.001 |
| Female | 16.2 (54) | ||
| Age group(years) | 15–20 | 45.9 (50) | 42.509; < 0.001 |
| 21–30 | 17.5 (20) | ||
| 31–40 | 17.5 (28) | ||
| 41–71 | 14.3 (18) | ||
| Educational level | At least secondary | 26.7 (79) | 6.112; 0.013 |
| At least primary | 17.4 (37) | ||
| Marital status | Single | 31.7 (73) | 19.099; <0.001 |
| Married | 15.4 (43) | ||
| Occupation | Student | 41.9 (49) | 35.768; <0.001 |
| Farming | 21.8 (26) | ||
| Business | 18.8 (25) | ||
| Salary earner | 11.4 (16) | ||
| Awareness | Aware | 27.8 (45) | 3.360; 0.067 |
| Not aware | 20.5 (71) | ||
| Distance from stream (metres) | ≤ 100 | 27.7 (91) | 12.539; < 0.001 |
| ˃ 100 | 13.9 (25) | ||
| Source of water | Stream only | 42.2 (27) | 49.931; < 0.001 |
| Stream and piped | 29.2 (80) | ||
| Piped only | 5.3 (9) | ||
| Stream activity | Washing motorbike | 75.0 (6) | 23.846; < 0.001 |
| Bathing | 34.9 (58) | ||
| Laundry | 34.6 (65) | ||
| Fetch water | 33.3 (46) | ||
| Farming | 25.0 (18) | ||
| Frequency to stream per week | ≥ Thrice | 33.2 (66) | 0.509; 0.475 |
| < Thrice | 29.5 (41) |
χ2 = Pearson chi-square test.
In Table 3, the binary logistic model presents determinant factors associated with the risk of S. haematobium infection. The most important factors associated with infection in the study area were age and gender. Age groups 15–20 years and 31–40 years were found to be 5 times (95% CI: 1.35–19.42) and 2 times (95% CI: 1.08–5.18) more likely to be infected compared with others in their respective categories. In similarity to the odd of infection, male respondents were observed to be about 3 times (95%CI: 1.54–4.40) more at risk of being infected with the cercariae of S. haematobium when compared with their female counterparts Table 3. It is puzzling that individuals who were aware of urogenital schistosomiasis were almost two times (95%CI: 1.02–2.95) more likely to be infected with S. haematobium when compared with those who had not heard of the disease. Of noteworthy, out of the 162 respondents aware of UGS, 116 (71.6%) live within 100m away from infested open water sources compared with those who live at the distance of more than 100m (28.4%; n = 46). The difference was statistically significant (χ2 = 5.05; p = 0.025).
Table 3. Risk factors associated with urogenital schistosomiasis among reproductive aged individuals in Tiko.
| Variable | Category | S. haematobium Positive % (n) | Unadjusted OR (95% CI) | #Adjusted OR (95% CI) | P- value |
|---|---|---|---|---|---|
| Gender | Male | 35.2 (62) | 2.81 (1.84–4.30) | 2.60 (1.54–4.40) | < 0.001 |
| Female | 16.2 (54) | 1.00 | 1.00 | ||
| Age group(years) | 15–20 | 45.9 (50) | 5.08 (2.72–9.50) | 5.13 (1.35–19.42) | 0.016 |
| 21–30 | 17.5 (20) | 1.28 (0.64–2.56) | 1.50 (0.58–3.86) | 0.403 | |
| 31–40 | 17.5 (28) | 1.27 (0.67–2.42) | 2.37 (1.08–5.18) | 0.031 | |
| 41–71 | 14.3 (18) | 1.00 | 1.00 | ||
| Educational level | At least secondary | 26.7 (79) | 1.73 (1.12–2.68) | 1.18 (0.62–2.25) | 0.616 |
| At least primary | 17.4 (37) | 1.00 | 1.00 | ||
| Marital status | Single | 31.7 (73) | 2.55 (1.66–3.91) | 0.77 (0.37–1.60) | 0.482 |
| Married | 15.4 (43) | 1.00 | 1.00 | ||
| Occupation | Student | 41.9 (49) | 5.58 (2.95–10.56) | 1.95 (0.53–7.18) | 0.316 |
| Farming | 21.8 (26) | 2.17 (1.10–4.27) | 1.73 (0.75–3.96) | 0.195 | |
| Business | 18.8 (25) | 1.79 (0.91–3.54) | 1.60 (0.68–3.77) | 0.281 | |
| Salary earner | 11.4 (16) | 1.00 | 1.00 | ||
| Awareness | Aware | 27.8 (45) | 1.49 (0.97–2.30) | 1.73 (1.02–2.95) | 0.043 |
| Not aware | 20.5 (71) | 1.00 | 1.00 | ||
| Distance from stream (metres) | ≤ 100 | 27.7 (91) | 2.37 (1.46–3.86) | 1.19 (0.63–2.26) | 0.584 |
| ˃ 100 | 13.9 (25) | 1.00 | |||
| Degree of water contact | Higha | 37.8 (54) | 1.67 (1.05–2.65) | 1.23 (0.71–2.12) | 0.455 |
| Lowb | 26.7 (52) | 1.00 | 1.00 |
CI = confidence interval, OR = odds ratio, #OR = adjusted OR using multivariate regression analysis
a activities include; bathing, laundry and washing of motorbike and increased frequency to stream (≥ Thrice/week).
b activities include; fetch water and farming and reduced frequency to stream (< thrice/week).
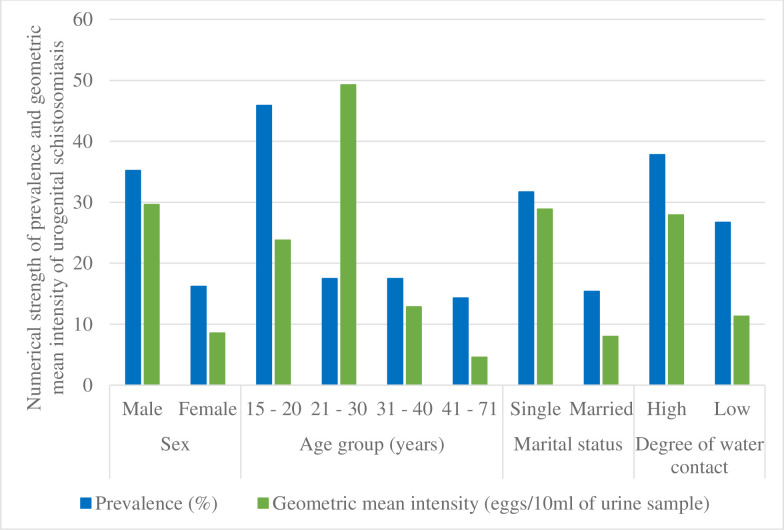
Similarly, higher mean intensity of egg excretion were recorded in males (29.62 eggs per 10ml), singles (28.89 eggs per 10ml), and the age groups 15–20 (23.79 eggs per 10ml) and 21–30 years (49.28 eggs per 10ml). The difference was significant Table 4. There was a significant negative correlation (r = -0.228; p = 0.014) between age and egg output. Though not significant, higher mean intensities of the infection were recorded in respondents who had high degree of contact with stream (27.93 eggs per 10ml) Fig 3.
Table 4. Intensity of S. haematobium infection based on sociodemographic characteristic.
| Variable | Category | Intensity of infection | Geometric mean egg count | |
|---|---|---|---|---|
| Gender | Light % (n) | Heavy % (n) | ||
| Male | 38.3 (36) | 25.5 (24) | 29.62 | |
| Female | 31.9 (31) | 4.3 (4) | 8.55 | |
| p-value | 0.003 | 0.009# | ||
| Age group(years) | 15–20 | 31.9 (31) | 14.9 (14) | 23.79 |
| 21–30 | 7.4 (7) | 9.6 (9) | 49.28 | |
| 31–40 | 17.0 (16) | 5.3 (5) | 12.86 | |
| 41–71 | 13.8 (13) | 0 (0) | 4.58 | |
| p-value | 0.010 | 0.019$ | ||
| Educational level | At least secondary | 47.9 (45) | 21.3 (20) | 20.62 |
| At least primary | 22.3 (21) | 8.5 (8) | 15.08 | |
| p-value | 0.755 | 0.494# | ||
| Marital status | Single | 41.5 (39) | 24.5 (23) | 28.89 |
| Married | 28.7 (27) | 5.3 (5) | 7.99 | |
| p-value | 0.031 | 0.004# | ||
| Distance from stream (metres) | ≤ 100 | 56.4 (53) | 24.5 (23) | 19.77 |
| ˃ 100 | 13.8 (13) | 5.3 (5) | 14.89 | |
| p-value | 0.836 | 0.768# | ||
| Degree of water contact | Higha | 62.3 (33) | 37.7 (20) | 27.93 |
| Lowb | 81.0 (34) | 19.0 (8) | 11.32 | |
| p- value | 0.008 | 0.073# | ||
$P-value obtained using Kruskal Wallis test
# P-value obtained using Mann-Whitney test.
a activities include; bathing, laundry and washing of motorbike and increased frequency to stream (≥ Thrice/week).
b activities include; fetch water and farming and reduced frequency to stream (< thrice/week).
Fig 3. Bar chart showing the prevalence and geometric mean intensity of S. haematobium infection with respect to sociodemographic characteristics and stream contact behaviour.
Stream contact behaviour in association with gender and age
The results displayed in Table 5 show that the level of exposure to infested water differed significantly among gender and age groups. Males indulged in more water contact activities for bathing, fetching of water, farming and washing motorbikes which entailed longer duration in the stream whereas females were involved more with the stream for domestic purposes. Accordingly, the frequency of water contact between males and females was significantly different (P < 0.001). A higher number of visits (≥ thrice/week) to the stream were recorded among males than females. Compared with the older age groups, the younger age group (15–20 years) was significantly (p <0.001) associated with more stream activities. Nonetheless, the difference in the degree of contact with stream among age groups was not significant Table 5.
Table 5. Stream contact behaviour in association with gender and age.
| Stream contact behaviour | Gender (%) | Age group (years) (%) | ||||
|---|---|---|---|---|---|---|
| Distance to stream (metres) | Male | Female | 15–20 | 21–30 | 31–40 | 41–71 |
| ≤ 100 | 67.6 | 63.1 | 73.4 | 64.9 | 58.8 | 64.3 |
| ˃ 100 | 32.4 | 36.9 | 26.6 | 35.1 | 41.3 | 35.7 |
| χ2; P value | 1.043; 0.307 | 6.094; 0.107 | ||||
| Stream usage | ||||||
| Yes | 80.1 | 59.2 | 81.7 | 61.4 | 57.5 | 69.0 |
| No | 19.9 | 40.8 | 18.3 | 38.6 | 42.5 | 31.0 |
| χ2; P value | 22.663; <0.001 | 18.718; <0.001 | ||||
| Stream activity | ||||||
| All three | 18.4 | 2.0 | 13.5 | 7.1 | 7.6 | 6.9 |
| At most two | 34.0 | 16.2 | 32.6 | 24.3 | 10.9 | 27.6 |
| Bathing | 17.7 | 16.8 | 13.5 | 15.7 | 21.7 | 17.2 |
| Domestic chore | 22.0 | 52.3 | 39.3 | 44.3 | 44.6 | 31.0 |
| Farming | 7.8 | 12.7 | 1.1 | 8.6 | 15.2 | 17.2 |
| χ2; P value | 56.850; < 0.001 | 30.548; 0.002 | ||||
| Frequency to stream per week | ||||||
| ≥ Thrice | 69.5 | 51.3 | 65.2 | 57.1 | 50.0 | 63.2 |
| < Thrice | 30.5 | 48.7 | 34.8 | 42.9 | 50.0 | 36.8 |
| χ2; P value | 11.286; 0.001 | 5.213; 0.157 | ||||
| Degree of water contact | ||||||
| Higha | 53.2 | 34.5 | 49.4 | 38.6 | 34.8 | 46.0 |
| Lowb | 46.8 | 65.5 | 50.6 | 61.4 | 65.2 | 54.0 |
| χ2; P value | 11.741; 0.001 | 4.869; 0.182 | ||||
χ2 = Pearson Chi-square test.
a activities include; bathing, laundry and washing of motorbike and increased frequency to stream (≥ Thrice/week).
b activities include; fetch water and farming and reduced frequency to stream (< thrice/week).
Discussion
The endemicity of UGS presents an enormous public health challenge in the South West Region, Cameroon, where a new unmapped transmission focus has been established in peri-urban settings such as the Tiko health area [20]. In this context, this study reports on the current epidemiological status of UGS among reproductive age individuals in the THA. Our results confirmed S. haematobium transmission in the semi-urban area of Tiko, Mount Cameroon area with the occurrence of infection at 22.8% among reproductive age individuals, with higher risk and severity of infection seen more in younger adults and males than in their respective counterparts.
It is obvious that the study area is meso-endemic (prevalence 20–40%) for urogenital schistosomiasis [35]. This prevalence level is within the range of infection rates (14.9–33.0%) reported between 2016–2019 among the reproductive age population in some villages; Munyenge (Wenpje et al. [21] (22.3%); Ndassi et al. [22] (14.9%), and Ikata, Likoko area (Ebai et al., [18] (33%)) in the Bafia Health area. Human UGS appears to be highly endemic in peri-urban/rural areas and closely associated with low socioeconomic status [36]. Transmission of S. haematobium varies between countries, between regions in the same country and even between seasons. Considerably, lower prevalence of UGS have been reported from studies conducted in urban settings in other parts of Africa such as Bamako, Mali (14.7%) [37] and Northern Ivory Coast (1.9%) [38]. Several environmental and ecology factors may explain differences in the transmission intensity of schistosomiasis [39]. The Mount Cameroon area has an equatorial forest climate whereas Bamako and Northern Ivory Coast are characterised by dry Sahelian conditions [37, 38]. Ndassi et al., [22] reported a lower prevalence of UGS of 14.9% among adults in a study carried out in the Bafia health area during the dry season.
The THA is close to some villages, such as Munyenge, found in the Bafia health area, Mount Cameroon area. Bio-ecologically, both health areas are characterized by favourable environmental conditions for the Bulinus snail intermediate host reproduction and parasite survival in the springs and streams [21, 40]. The hydrology of communities in the Mount Cameroon area consist of networks of streams [41] and possibly snail host are being washed down stream to neighbouring towns. For the establishment of schistosomiasis in new transmission foci, the bionetworks and environmental conditions, appropriate aquatic snail intermediate hosts, and the human definitive host must converge in space and time in suitable water bodies [37]. In the case of Tiko, population movement into the suburban areas of endemic foci, the increasingly dependence on stream water for livelihood and lack of basic sanitation may have resulted in urine contamination of the streams with consequent infection of intermediate host and emergence of the new focus for UGS transmission in the Mount Cameroon area.
The factors, which were predictive of infection in the present study were age and gender. Individuals in the age group 15–20 years and 31–40 years were five and two times respectively more likely at risk of S. haematobium infection. In most schistosome infection surveys, age is seen as a major determinant of S. haematobium infection [42–44]. The younger age individuals (15–20years) are more involved with the open water sources for bathing, laundry and collection of water for domestic purposes. These findings corroborate previous reports in the Mount Cameroon area [19], elsewhere in Cameroon [42, 45], as well as other parts of Africa [43, 46]. Age-acquired immunity to reinfection contributes to declining trend in infection prevalence with increasing age [46]. This may explain the low infection status in the older age group of 41–71 years despite their intense water contact behaviours which include bathing, domestic chores and farming. Surprisingly, the odds of infection were higher in the age group 31–40 when compared with 21-30-year olds. Besides bathing and domestic chores, the streams also served as a source of water for washing of motorbikes and farming among the 31–40 years age group. In some neighbourhood with shortage in water supply, accessing and using stream water is vital not only for household livelihood but also for agricultural purpose such as washing of pumpkin seeds. The lack of bridges in certain parts of the area, compels the population to cross these streams to their farms and other areas within the municipality exposing them to cercariae infested water. The intensity of egg excretion was significantly higher (p = 0.012) in the younger aged individuals age when compared with the older adults. The inverse relationship between egg load and age supports the well documented observation of immune-mediated reduction of worm fecundity with host age in S. haematobium infection [47, 48].
As expected, the male gender had significantly higher prevalence (35.2% Vs 16.2%) and geometric mean intensity of infection (29.62 Vs 8.55) than females. Several studies have reported that schistosomiasis is a disease that affects males more frequently [49–54], which our study also showed that these individuals have a significantly higher chance (OR = 2.6) of acquiring the infection. This finding is probably related to the greater involvement of men in activities in streams near the community. In contrast, a significantly higher prevalence of schistosomiasis has been reported among females than males in some studies, which is attributed to frequent contact with water in the execution of domestic chores including fetching of water and laundry [45, 55]. In this study, males had higher degree of contact with stream, specified by activities which entailed longer duration in the stream (bathing, and washing of motorbike) thus increasing their risk of exposure to infection. Previous findings have shown that increase in intensity of infection with urogenital schistosomiasis increased the risk of FGS. Equally, the high infection intensity observed in males in the study area may be associated with the risk of MGS. Infection can cause genital ulcers and other lesions [13], induce pathology of the seminal vesicles and the prostate with sexual dysfunction and irreversible long-term reproductive health consequences including infertility [14]. Also, with high infection intensities, eggs of schistosomes can became trapped in tissues of the liver, spleen, kidney and peritoneum with severe and complex pathological consequences which may degenerate to late-stage sequelae [54, 56].
Our findings showed that individuals aware of UGS were 1.73 times more likely to be infected compared with individuals who were not aware. Similar results have been reported in Cameroon [57], Nigeria [58] and in South Africa [59]. Notably, the majority of individuals resident in communities’ located ≤ 100m around infested water sources were aware of UGS possibly through exposure to the disease indicators. Haematuria and Bulinus spp. is used as a strong indicator of awareness about urogenital schistosomiasis. The strong association between awareness and disease clearly suggests that infected respondents most likely came across the snail intermediate host while bathing, fishing, and/or fetching water in infested streams [58]. Moreover, this finding may indicate the poor knowledge on UGS transmission and prevention. Even so, the knowledge gained might not be enough to protect these communities from infection as the lack of access to safe drinking water and adequate sanitation are the driving forces behind the risk behaviour of individual community members [60]. This poses serious implication in the control and treatment of the infection in this area. In Tiko, inadequate or shortage of portable water supply may contribute to high dependence on open water sources (66.4%) given that 87.6% of the respondents indicated access to some form of pipe-borne water. In line with reports of other studies [19, 61], no socioeconomic variable was independently associated with UGS prevalence in the study area suggesting that improving socioeconomic status alone may not contribute to a significant reduction of schistosomiasis prevalence rate in these communities. In this regard, health talks, to raise community awareness for better understanding of the social and behavioural determinants that influence disease transmission is recommended as a first line of action. The extension and functionality of piped water sources and the construction of car wash points in this endemic area will reduce the need for intense contact with infested water particularly among males and younger age individuals thus decreasing the transmission of UGS in this area.
Limitations of the survey
The analyses of these data of this study depended on only one urine specimen per participant. WHO encourages the collection of three urine samples per participants for accurate detection of infection. The collection of multiple samples also enables the assessment of intra and inter- specimen variation of the egg output [61]. Although researchers were cognizant of these facts, it was difficult to obtain multiple samples from participants. The participants complained of lack of time and some were embarrassed of handling bodily specimens in public. In spite of this, our findings provide valuable evidence required for evidence-based decision making.
Conclusion
The prevalence obtained in this study shows that Tiko is a moderate-risk area for S. haematobium transmission. Younger age group (15–20years), and male gender comprise the determinant factors associated with increased risk of S. haematobium infection. Males may be predisposed to higher risk of disease severity than females. Awareness of the disease does not prevent individuals in this study area from using cercariae infested streams but the provision of public infrastructures to limit contact with streams will curb the transmission and morbidity associated with urogenital schistosomiasis. However, a productive and sustainable intervention cannot be achieved without adequate education.
Supporting information
(PDF)
Acknowledgments
The authors appreciate the support and cooperation of the inhabitants of the Tiko health district. Laboratory space and equipment was provided by the laboratory of the Tiko District hospital, for which the authors are most grateful.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by personal funds (to VDN) and research and modernization funds (given to JKA-K) by the University of Buea and Government of Cameroon.The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Christinet V, Lazdins-Helds JK, Stothard JR, Reinhard-Rupp J. Female genital schistosomiasis (FGS): from case reports to a call for concerted action against this neglected gynaecological disease. Intl J Parasitol. 2016;46:395–404. 10.1016/j.ijpara.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Schistosomiasis fact sheet. 2019; https://www.who.int/news-room/fact-sheets/detail/schistosomiasis. Accessed 2 March 2020 [Google Scholar]
- 3.Aagaard-Hansen J, Mwanga JR, Bruun B. Social science perspectives on schistosomiasis control in Africa: Past trends and future directions. Parasitol. 2009;136:1747–1758. 10.1017/S0031182009006404 [DOI] [PubMed] [Google Scholar]
- 4.Stothard JR, Campbell SJ, Osei-Atweneboana MY, Durant T, Stanton MC, Biritwum NK et al. Towards interruption of schistosomiasis transmission in sub-Saharan Africa: developing an appropriate environmental surveillance framework to guide and to support 'end game' interventions. Infect Dis Poverty. 2017;6(1), 10 10.1186/s40249-016-0215-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman JF, Mital P, Kanzaria HK, Olds GR, Kurtis JD. Schistosomiasis and pregnancy. Trends Parasitol. 2007;23(4):159–64. 10.1016/j.pt.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 6.Gryseels B. Schistosomiasis. Infect Dis Clin North Am. 2012;26:383–97. 10.1016/j.idc.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 7.Stothard JR, Sousa-Figueiredo JC, Betson M, Bustinduy A, Reinhard-Rupp J. Schistosomiasis in African infants and preschool children: let them now be treated. Trends Parasitol. 2013;29:197–205. 10.1016/j.pt.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knopp S, Person B, Ame SM, Mohammed KA, Ali SM, Khamis IS, et al. Elimination of schistosomiasis transmission in Zanzibar: baseline findings before the onset of a randomized intervention trial. PLoS Negl Trop Dis. 2013;7[10]:e2474 10.1371/journal.pntd.0002474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Katsha S, Watts S. Gender, behavior, and health: schistosomiasis transmission and control in rural Egypt: American Univin Cairo Press; 2002. [Google Scholar]
- 10.Vlassoff C, Manderson L. Incorporating gender in the anthropology of infectious diseases. Trop Med Intl health. 1998;3[12]:1011–9. [PubMed] [Google Scholar]
- 11.Van der Werf MJ, de Vlas SJ, Brooker S, Looman CWN, Nagelkerke NJD, Habbema JDF et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–39. 10.1016/s0001-706x(03)00029-9 [DOI] [PubMed] [Google Scholar]
- 12.Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl. 2008;218:12–20. 10.1080/03008880802285032 [DOI] [PubMed] [Google Scholar]
- 13.Hotez PJ, Savioli L, Fenwick A. Neglected Tropical Diseases of the Middle East and North Africa: Review of Their Prevalence, Distribution, and Opportunities for Control. PLoS Negl Trop Dis. 2012;6(2):e1475 10.1371/journal.pntd.0001475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swai B, Poggensee G, Mtweve S, Krantz I. Female genital schistosomiasis as an evidence of a neglected cause for reproductive ill-health: a retrospective histopathological study from Tanzania. BMC Infect Dis. 2006;6, 134 10.1186/1471-2334-6-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Geneva. 2018; https://www.who.int/healthinfo/global_burden_disease/estimates/en/. Accessed on January 15 2020.
- 16.Kayuni S, Lampiao F, Makaula P, Juziwelo L, E. James Lacourse EJ, Reinhard-Rupp J, et al. A systematic review with epidemiological update of male genital schistosomiasis (MGS): A call for integrated case management across the health system in sub-Saharan Africa. Parasite Epidemiol Control. 2018;3:e00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ntonifor HN, Mbunkur GN, Ndaleh NW. Epidemiological survey of urinary schistosomiasis in some primary schools in a new focus behind mount Cameroon, South West Region, Cameroon. E Afr Med J. 2012;89(3):82–88. [PubMed] [Google Scholar]
- 18.Ebai CB, Kimbi HK, Sumbele IUN, Yunga JE, Lehman LG. Prevalence and Risk Factors of Urinary Schistosomiasis in the Ikata-Likoko Area of Southwest Cameroon. IJTDH 2016;17(2):1–10, Article no.IJTDH.26669. [Google Scholar]
- 19.Anchang-Kimbi JK, Elad DM, Sotoing GT, Achidi EA. Coinfection with Schistosoma haematobium and Plasmodium falciparum and Anaemia Severity among Pregnant Women in Munyenge, Mount Cameroon Area: A Cross-Sectional Study. J Parasitol Res. 2017; 10.1155/2017/6173465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anguh E, Ako S, Numfor E, Bimabam ZJ, Ndassi DV. Presence of an Unmapped Focus for Urogenital Schistosomiasis in the Tiko Health District in Cameroon: Implications for Control. IJTDH 2018;32(2):1–8.22. [Google Scholar]
- 21.Wepnje BG, Anchang-Kimbi KJ, Ndassi DV, Lehman GL, Kimbi KH. Schistosoma haematobium infection status and its associated risk factors among pregnant women in Munyenge, South West Region, Cameroon following scale-up of communal piped water sources from 2014 to 2017: a cross-sectional study. BMC Public Health. 2019;19:392 10.1186/s12889-019-6659-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ndassi VD, Anchang-Kimbi JK, Sumbele IUN, Wepnje GB, Kimbi HK. Prevalence and risk factors associated with S. haematobium egg excretion during the dry season, six months following mass distribution of praziquantel (PZQ) in 2017 in the Bafia Health Area, South West Region Cameroon: A cross sectional study. J Parasitol Res. 2019; 10.1155/2019/4397263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray DJ, McManus DP, Li Y, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis. 2010;10:733–736. 10.1016/S1473-3099(10)70099-2 [DOI] [PubMed] [Google Scholar]
- 24.Tabi ESB, Cumber SN, Juma KO, Ngoh EA, Akum EA, Eyong EM.A cross-sectional survey on the prevalence of anaemia and malnutrition in primary school children in the Tiko Health District, Cameroon. Pan Afr Med. 2019;32:111 10.11604/pamj.2019.32.111.15728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yirenya-Tawiah D, Annang T N, Apea-Kubi K A, Lomo G, Mensah D, Akyeh L, Bosompem K M. Chlamydia Trachomatis and Neisseria Gonorrhoeae prevalence among women of reproductive age living in urogenital schistosomiasis endemic area in Ghana. BMC Res Notes. 2014;7:349 10.1186/1756-0500-7-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marlowe FT, Apicella C, Reed D. Men’s preferences for women’s profile waist to hip ratio in two societies. Evol Hum Behav. 2005;26:458–468. [Google Scholar]
- 27.Naing L, Than W, Rusli B. Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci. 2006;1(1):9–14. [Google Scholar]
- 28.Lima E Costa MFF, Magalhaes MHA, Rocha RS, Antunes CMF, Katz N. Water-contact patterns and socioeconomic variables in the epidemiology of schistosoma mansoni in an endemic area in Brazil. Bull World Health Organ. 1987;65 (1):57–66. [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy JS, Lustigman S, Yang G-J, Barakat RM, García HH, Sripa B, et al. A Research Agenda for Helminth Diseases of Humans: Diagnostics for Control and Elimination Programmes. PLoS Negl Trop Dis. 2012;6(4):e1601 10.1371/journal.pntd.0001601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheesbrough M. Intestinal Schistosoma species In: District Laboratory Practice in Tropical Countries. Part 1.Second Edition, Cambridge University Press;2009. [Google Scholar]
- 31.WHO. Tropical Disease Research, TDR strategic direction: Schistosomiasis, WHO-TDR; 2002. [Google Scholar]
- 32.WHO, Guidelines for the Evaluation of Soil Transmitted Helminthiasis and Schistosomiasis at Community Level: A Guide for Managers of Control Programme, World Health Organisation, Geneva, Switzerland, 1993. [Google Scholar]
- 33.Armitage P, Berry G. Statistical methods in medical research. 3rd ed London: Blackwell; 1994. [Google Scholar]
- 34.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–90. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 36.Ugobomoiko US, Ofoezie IE, Okoye IC, Heukelbach J. Factors associated with urinary schistosomiasis in two peri-urban communities in South-western Nigeria. Ann Trop Med Parasitol. 2010;104(5):409–19. 10.1179/136485910X12743554760469 [DOI] [PubMed] [Google Scholar]
- 37.Dabo A, Diarra AZ, Machault V, Touré O, Niambélé DS, Kanté A, et al. Urban schistosomiasis and associated determinant factors among school children in Bamako, Mali, West Africa. Infect Dis Poverty. 2015;4:4 http://www.idpjournal.com/content/4/1/4. 10.1186/2049-9957-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.M’Bra RK, Kone B, Yapi YG, Silué KD, Sy I, Vienneau D, et al. Risk factors for schistosomiasis in an urban area in northern Côte d’Ivoire. Infect Dis Poverty. 2018;7:47 10.1186/s40249-018-0431-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clements ACA, Firth S, Dembele R, Garba A, Toure S, Sacko M, et al. Use of Bayesian geostatistical prediction to estimate local variations in Schistosoma haematobium infection in western Africa. Bull World Health Organ. 2009;87:921–9. Wepnje BG, Anchang-Kimbi KJ, Lehman GL, Kimbi KH. Evaluation of Urine Reagent Strip as a Tool for Routine Diagnosis of Maternal Urogenital Schistosomiasis at Antenatal Clinic Visit in Munyenge, South West Region, Cameroon. BioMed Res Int. 2019;2972630. 10.2471/BLT.08.058933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ako A, Shimada J, Hosono T, Kagabu M, Akoachere R, Nkeng G, et al. Spring water quality and usability in the Mount Cameroon area revealed by hydrogeochemistry. Environ Geochem Health. 2012;34 (5):615–39. 10.1007/s10653-012-9453-3 [DOI] [PubMed] [Google Scholar]
- 41.TchuemTchuente LA, Behnke JM, Gilbert FS, Southgate VR, Vercruysse J. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Trop Med Intl Health. 2003;8(11):975–986. 10.1046/j.1360-2276.2003.01120.x [DOI] [PubMed] [Google Scholar]
- 42.Eyo JE, Onyishi GC, Okafor FC. Urinary schistosomiasis among pregnant women in some endemic tropical semi-urban communities of Anambra State, Nigeria. Trop Biomed. 2012;29(4):575–579. [PubMed] [Google Scholar]
- 43.Salawu OT, Odaibo AB. Schistosomiasis among pregnant women in rural communities in Nigeria. Int J Gynaecol Obstet. 2013;122 (1):1–4. 10.1016/j.ijgo.2013.01.024 [DOI] [PubMed] [Google Scholar]
- 44.Ntonifor HN, Green AE, Bopda MOS, Tabot JT. Epidemiology of urinary schistosomiasis and soil transmitted helminthiasis in a recently established focus behind Mount Cameroon. Int. J Curr Microbiol. App. Sci. 2015;4(3):1056–1066. [Google Scholar]
- 45.Etard JF, Audibert M, Dabo A. Age-acquired resistance and predisposition to reinfection with Schistosoma haematobium after treatment with praziquantel in Mali. Am J Trop Med Hyg. 1995;52(6):549–558. 10.4269/ajtmh.1995.52.549 [DOI] [PubMed] [Google Scholar]
- 46.Agnew A, Fulford A J, Mwanje M T, Gachuhi K, Gutsmann V, Krijger FW, et al. Age-dependent reduction of schistosome fecundity in Schistosoma haematobium but not Schistosoma mansoni infections in humans. Am J Trop Med Hyg. 1996;55:338–43. 10.4269/ajtmh.1996.55.338 [DOI] [PubMed] [Google Scholar]
- 47.Wilson S, Jones FM, van Dam GJ, Corstjens PL, Riveau G, Fitzsimmons CM, et al. Human Schistosoma haematobium antifecundity immunity is dependent on transmission intensity and associated with immunoglobulin G1 to worm-derived antigens. J Infect Dis. 2014;210(12):2009–2016. 10.1093/infdis/jiu374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivoke N, Ivoke ON, Nwani CD, Ekeh FN, Asogwa CN, Atama CI, Eyo JE. Prevalence and transmission dynamics of Schistosoma haematobium infection in a rural community of southwestern Ebonyi State, Nigeria. Trop Biomed. 2014;31(1):77–88. [PubMed] [Google Scholar]
- 49.Geleta S, Alemu A, Getie S, Mekonnen Z, Erko B. Prevalence of urinary schistosomiasis and associated risk factors among Abobo Primary School children in Gambella Regional State, southwestern Ethiopia: a cross sectional study. Parasit Vectors. 2015;8:215 10.1186/s13071-015-0822-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanya RE, Tumwesige E, Elliott AM, Seeley J. Perceptions about interventions to control schistosomiasis among the Lake Victoria island communities of Koome, Uganda. PLoS Negl Trop Dis. 2017;11(10):e0005982 10.1371/journal.pntd.0005982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atalabi TE, Lawal U, Ipinlaye SJ. Prevalence and intensity of genito-urinary schistosomiasis and associated risk factors among junior high school students in two local government areas around Zobe Dam in Katsina State, Nigeria. Parasit Vectors. 2016;9(1). 10.1186/s13071-016-1672-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atalabi TE, Lawal U, Akinluyi FO. Urogenital schistosomiasis and associated determinant factors among senior high school students in the Dutsin-Ma and Safana Local Government Areas of Katsina State, Nigeria. Infect Dis Poverty. 2016;5(1). 10.1186/s40249-016-0158-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization. Schistosomiasis epidemiological situation: morbidity. Available at www.who.int/ schistosomiasis/ epidemiology/en/. Accessed on December 12, 2019.
- 54.Nour MN. Schistosomias: health effect on women. Rev Obstet Gynecol. 2010;3(1):28–32. [PMC free article] [PubMed] [Google Scholar]
- 55.Stauffer JR, Madsen H, McKaye K, Konings A, Bloch P, Ferreri CP, et al. Schistosomiasis in Lake Malawi: Relationship of fish and intermediate host density to prevalence of human infection. EcoHealth. 2006;3(1):22–27. 10.1007/s10393-005-0007-3. [DOI] [Google Scholar]
- 56.Ndassa A, Mimpfoundi R, Gake B, Paul Martin MV, Poste B. Risk factors for human schistosomiasis in the Upper Benue valley, in northern Cameroon. Ann Trop Med Parasitol. 2007;101:6, 469–477. 10.1179/136485907X193752 [DOI] [PubMed] [Google Scholar]
- 57.Atalabi TE, Adoh SD, Eze KM. The current epidemiological status of urogenital schistosomiasis among primary school pupils in Katsina State, Nigeria: An imperative for a scale up of water and sanitation initiative and mass administration of medicines with Praziquantel. PLoS Negl Trop Dis. 2018;12(7):e0006636 10.1371/journal.pntd.0006636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kabuyaya M, Chimbari MJ, Manyangadze T, Mukaratirwa S. Schistosomiasis risk factors based on the infection status among school-going children in the Ndumo area, uMkhanyakude district, South Africa. S Afr J Infect Dis. 2017;32:2, 67–72. 10.1080/23120053.2016.1266139 [DOI] [Google Scholar]
- 59.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit Vectors. 2015;8:156 10.1186/s13071-015-0766-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kapito-Tembo AP, Mwapasa V, Meshnick SR, Samanyika Y, Banda D, Bowie C, et al. Prevalence distribution and risk factors for Schistosoma hematobium infection among school children in Blantyre, Malawi. PLoS Negl Trop Dis. 2009;3(1), article e361, 2009. 10.1371/journal.pntd.0000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Utzinger J, Booth M, N'Goran EK, Müller I, Tanner M, Lengeler C. Relative contribution of day-to-day and intra-specimen variation in faecal egg counts of Schistosoma mansoni before and after treatment with praziquantel. Parasitol. 2001;122(05):537–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.