Abstract
Spatial neglect (SN) is a common cognitive disorder after brain injury. Prism adaptation treatment (PAT) is one of the promising interventions for SN albeit inconsistent results from previous studies. We carried out a comparison intervention (PAT vs. Sham) and aimed to evaluate the efficacy of PAT on visuospatial symptoms of SN in an inpatient rehabilitation setting that offered a highly intensive comprehensive brain injury rehabilitation program. A total of 34 patients with moderate-to-severe SN secondary to stroke or traumatic brain injury were randomized to the PAT group and the Sham group (an active control group). Both groups received 10 sessions of treatment, over two weeks, in addition to the rehabilitation therapies provided by their rehabilitation care teams. Outcomes were measured using an ecological instrument (the Catherine Bergego Scale) and paper-and-pencil tests (the Bells Test, the Line Bisection Test and the Scene Copying Test). Patients were assessed at baseline, immediately after treatment, two weeks after treatment, and four weeks after treatment. 23 (67.6%) patients completed treatment and all the assessment sessions and were included in the final analyses using mixed linear modeling. While SN symptoms reduced in both groups, we found no difference between the two groups in the degree of improvement. In addition, the average SN recovery rates were 39.1% and 28.6% in the PAT and Sham groups, respectively, but this discrepancy did not reach statistical significance. Thus, the present study suggests that PAT may contribute little to SN care in the context of a highly intensive inpatient rehabilitation program. Further large-scale investigation is required to uncover the mechanisms underlying PAT and Sham in order to refine the treatment or create new interventions.
Introduction
Spatial neglect is characterized as a failure to report, respond or orient to stimuli presented in the side of space contralateral to the injured cerebral hemisphere, which cannot be explained by primary sensory or motor deficits [1]. The disorder is caused by damaged neural networks critical to spatial attention and related cognitive and motor functions, leading to a variety of symptoms [2–5]. While this neuropsychological syndrome has been mostly studied in stroke survivors, with the prevalence of 30–50% in acute-to-subacute stages [6–8], it can also be caused by other forms of brain pathology such as traumatic brain injury [9], neurodegenerative disease [10] or tumor resection [11]. Patients with spatial neglect tend to have poorer rehabilitation outcomes [8, 12–15], longer hospital stay [12–14, 16] and are less likely to be discharged home [16] than patients without spatial neglect. Spatial neglect, thus, predicts a decreased level of functional independence [7, 17, 18], increased use of health care resources [19] and increased family burden [7, 20].
One of the most promising and most commonly used interventions for spatial neglect is prism adaptation treatment (PAT) [21, 22]. Prism adaptation is a visuomotor phenomenon that had been known for decades [23] before it was used for treating spatial neglect [24]. During a prism adaptation session, patients wear goggles with prism lenses that shift the visual field horizontally to the ipsilesional side of space, and repeatedly perform arm-reaching visuo-motor tasks that typically last less than 20 minutes. Upon subsequent removal of goggles, an after-effect can be observed as patients miss the target by reaching toward the contralesional side of space. After a few seconds to hours, the after-effect disappears. Prism adaptation and its after-effect occur effortlessly, requiring no explicit strategy learning. The phenomenon depends on cortico-cerebellar connectivity and involvement of neural networks critical to attention and sensorimotor integration [25–27]. That is, certain regions of the brain are activated at the time when prism adaptation occurs. Repeated sessions of prism adaptation may consolidate enhanced neural activation with strengthened brain connectivity among regions within and between ventral and dorsal attention networks that are impaired or dysfunctional in clinical populations, especially patients with spatial neglect. This, in turn, facilitates the restoration of spatial abilities that have been lost in those patients. Thus, prism adaptation procedures have been standardized into treatment protocols with multiple sessions over several days. Benefits lasting months to years of prism adaptation have been documented [28, 29] with positive effects not only on visuospatial abilities [24, 30, 31] but also on postural balance [32], motor functions [33], and activities of daily life (for a recent systematic review, see [34]).
PAT is recommended to rehabilitation care practitioners for treating spatial neglect by major practice guidelines in different regions of the world, such as the United States [35, 36], Australia [37], Canada [38], and the UK [39]. However, randomized controlled trials (RCTs) that implemented PAT within inpatient rehabilitation showed mixed results. Overall, studies that utilized prisms with weaker diopter (≤ 10 diopter; prism of 1 diopter shifts the visual field for approximately 0.57 degree) tend to result in negative findings [40, 41]. Even using prisms with stronger diopter (e.g., 20 diopter), results are inconsistent. For example, Mizuno et al. [42] found that compared to patients who received the treatment but wore flat lenses (i.e., Sham treatment), patients in the PAT group completing 20 sessions, two sessions a day, over two weeks showed no greater improvement in visuospatial ability (measured in a battery of paper-based tests) or visuospatial function (measured using an ecological assessment). In the same study, the authors found the PAT group, especially patients had relatively milder neglect at baseline, demonstrated greater improvement in rehabilitation outcomes at the end of inpatient stay than the Sham group [42]. In Vaes et al.’s study [43], after seven sessions over 7–12 days, the PAT group showed better outcomes than the Sham group in visuospatial ability (measured using a battery of computerized tests). Ten Brink et al. [44], however, found that the extent of improvement in visuospatial ability (measured using a target cancellation test) and in visuospatial function (measured using an ecological assessment) did not differ between the PAT and Sham groups who completed 10 once-daily sessions over two weeks. It is important to note that in the studies reviewed above, patients receiving Sham treatment also showed improvement, but to what extent their improvement was in comparison to the PAT group differed across studies. Thus, it is questionable whether PAT or simply the visuomotor training without prism adaptation facilitates amelioration of spatial neglect during inpatient rehabilitation.
Ten Brink et al.’s study [44] was particularly informative as their sample size was larger than most of the RCTs published, and their description of the usual care indicated that, in addition to participating in the trial, patients received therapies addressing spatial neglect every day as part of their regular 4–6 therapy sessions. This may have accounted, to some extent, for their negative results of the RCT. Another possibility is that some of the patients participating in the study may have not been able to improve further after PAT given their relatively mild neglect at baseline. Patients with relatively severe neglect may have benefited from PAT to greater extents even in a setting that offered intensive rehabilitation services. Thus, in the present study of a randomized pilot and feasibility trial [45], we focused on patients with moderate to severe neglect. In order to closely represent the acquired brain injury population in inpatient rehabilitation settings, we included patients with left or right neglect secondary to stroke or traumatic brain injury (TBI). The study was conducted in a potentially similar setting to Ten Brink et al.’s study. The objective was to estimate the extent of the efficacy of 10-session PAT vs. 10-session Sham treatment, delivered over a two-week period, in addition to the standard rehabilitation program, in improving spatial neglect symptoms among individuals with moderate-to-severe spatial neglect. We aimed to examine two hypotheses:
Hypothesis 1: Prism adaptation treatment reduced visuospatial symptoms of spatial neglect among patients in an inpatient setting providing intensive rehabilitation care.
Hypothesis 2: Prism adaptation treatment enhanced the recovery of spatial neglect.
Materials and methods
Study design
A double-masked, randomized, sham-controlled trial was conducted to evaluate the efficacy of 10 once-daily sessions of PAT. The double-masked design was achieved as 1) patients were not informed about their group membership, no information about the mechanisms of goggles was discussed, and no questions about the treatment condition were raised, and 2) outcome measures were assessed by examiners who were masked from patients’ group membership.” The same double-masked design was previously used in other similar studies [42, 44]. The study was conducted according to the principles of the Declaration of Helsinki and was approved by the institutional ethics committee. Written informed consent was obtained from all participants. The individual on S1 Fig in this manuscript has given written informed consent (as outlined in PLOS consent form) to publish these case details.
Setting
The study was conducted in a highly intensive inpatient comprehensive brain injury rehabilitation program (abbreviated as the BIR Program) in the Rehabilitation Center Kladruby, the Czech Republic.
Participants
Patients consecutively admitted to the BIR Program (June 2017–July 2019) were invited to the study. Individuals admitted to the BIR Program (a) were in between 18 and 75 years of age, (b) obtained acquired brain injury (TBI or stroke), (c) had the brain injury no longer than 1 month since the time being discharged from acute care, (d) were able to participate in at least 4 hours of high intensity rehabilitation on a daily basis, receiving therapies from at least 2 of the four following specialized disciplines: physiotherapy, occupational therapy, psychology; speech and language therapy and (e) had cooperating family member with the rehabilitation team with expectation to return home given the prognosis and availability of the caregivers. In addition to admission criteria, study participation criteria were (f) presence of moderate or severe spatial neglect as indicated by the Catherine Bergego Scale (CBS > 10) via the Kessler Foundation Neglect Assessment Process (KF-NAP®) [46, 47] at baseline assessment, (g) confirmed unilateral brain injury, (h) physically and cognitively able to participate in PAT, and (i) able to provide informed consent. Participants were randomly allocated to the treatment or control group by the study coordinator using sealed envelopes with equal numbers of printed group assignment cards inside. The treatment group received PAT, and the control group received Sham treatment.
Clinical information
With participants’ informed consent, certain clinical information was extracted from the BIR Program records for the purpose of the present study. This included demographic information, results of standard measures upon admission indicating functional status in the motor and cognitive domains (such as the Modified Motor Subscale of the Functional Independence Measure (mFIM), Mini Mental State Examination (MMSE), and Berg Balance Scale (BBS)), and information about brain injury characteristics including injury description and brain lesion location. The lesion location was identified by an independent neurologist based on acute care medical records transferred to the BIR Program.
Treatment
PAT was delivered, by a physiotherapist, using the treatment protocol and equipment of the Kessler Foundation Prism Adaptation Treatment (KF-PAT®) [48]. The procedures and equipment were the same for PAT and Sham treatment, except that the treatment group wore goggles fitted with 20-diopter prism lenses that shift the visual field to the ipsilesional side of space for 11.4 degrees of visual angle while the control group used flat goggles that did not shift the visual field at all. During each session, lasting approximately 15–20 minutes, participants completed 60 visuomotor movements while the first part of arm movements was blocked from view. The visuomotor movement was initiated from participants’ chest toward a visual target (a 24.1-cm horizontal line or a 1-cm-diameter circle) printed at the center of a 29.7 x 21 cm paper sheet. Stimuli were pseudo-randomly presented either at body midline or in left or right space (32.1 cm to the side of body midline). The task was to mark the center of a line or cross out a circle. Before and after prism adaptation, pointing tasks were administered using the procedure described in previous studies [49]. If a participant did not demonstrate any after-effect for three consecutive PAT sessions, suggesting impaired cortico-cerebellar circuits, then they would be excluded from the study. If a participant reported discomfort during the session, the Nausea Profile [50] was administered to help evaluate potential adverse effects. Participants completed the 10-session, once-daily, treatment course over two weeks (skipping weekends), during morning hours, in addition to the therapy sessions provided in the BIR Program. The treatment was always delivered as per the protocol, based on detailed documentations for fidelity check.
The BIR Program was a multidisciplinary, high-intensity, inpatient rehabilitation program offered up to 12 weeks to people with acquired brain injury. Patients’ rehabilitation needs were assessed by the multidisciplinary rehabilitation care team during the first week of admission when an individualized rehabilitation plan and tailored goals were established using goal attainment scaling [51]. The goals were re-evaluated and adjusted every 3 weeks to meet changes in patients’ needs and progress, and this occurred in multidisciplinary meetings. Patients typically participated in 6–10 rehabilitation sessions per day (a total of 4–5 hours), 6 days a week with less intensive program on Saturdays. The BIR Program included 30-minute daily computer-assisted cognitive training, weekly psychotherapy, 30-minute twice-daily sessions of functional independence training, 30-minute twice-daily sessions of physiotherapy (limb activation, balance training, walking training etc.), individual and group physical exercises in the gym (such as stretching, playing ball games, using stationary bikes etc.) or in the swimming pool (strength training, swimming etc.), 30-minute twice-daily speech and language therapy if needed, and various forms of recreational therapy (arts, crafts, etc.) If necessary, as deemed by the rehabilitation care team, patients are trained in further independence and compensatory strategies by nurse staff outside therapy hours. Patients could go home accompanied by their carers for several weekends, during which assessment of functional needs in home environment were conducted.
Outcome measures
Outcome measures were administered by trained occupational therapists who were masked of participants’ group membership, while treatment was delivered by different therapists. The assessments were performed at baseline (T1), after treatment (T2), two weeks after treatment (T3), and four weeks after treatment (T4). All time points were within the duration of participants’ admission in the BIR Program. The assessments were carried out at the same location, all in one session, at the same time of each day, mostly by the same trained therapist (with exceptions when unavailable due to vacation etc., then another trained therapist would substitute).
Catherine Bergego Scale (CBS) via Kessler Foundation Neglect Assessment Process (KF-NAP®) [46, 47]
The assessment consists of 10 categories: limb awareness, personal belongings, dressing, grooming, gaze orientation, auditory attention, navigation, collisions, having a meal, and cleaning after a meal. Each category is scored from 0 (no neglect) to 3 (severe neglect). The final score is calculated with the formula: (sum score ÷ number of scored categories) × 10 = final score [52]. The final score ranges from 0 to 30, and a positive score indicates the presence of spatial neglect.
Bells test [53]
The Bells Test was printed on a sheet of 29.7 x 21 cm paper with 35 targets (bell-shaped figures) and other 280 distractors equally distributed in 7 columns. The paper was placed at the participant’s midline. The participant was asked to circle the bells. The starting column and the number of circled bells were recorded. To ensure the coding consistency regardless of the side of neglect, the first column closest to the paper edge on the neglected side was coded as 1, and the coded value increased in the columns toward the non-neglect side of space. Spatial neglect is indicated when the discrepancy between the left and right omissions (excluding performance in the 4th column) is greater than 2, or when the coded value of the starting column is greater than 5 [54].
Line bisection [55]
Participants were presented with five 20 cm horizontal line, each printed separately on a 29.7 x 21 cm paper sheet. One line was presented at one time right in front of the participant, who was asked to mark the center of the line. The average deviation from the true center of the line was recorded. Deviation to the non-neglected side of space was coded positive and the neglect-sided negative. The criterion for spatial neglect is the deviation greater than 6.5 mm [54].
Scene copying test [56]
Modified from a five-object figure copying test [6], the Scene Copying Test is a figure copying task consisting of two plants, a house, and two trees arranged in that order left-to-right, printed on the upper half of a page [56]. The task is to copy the entire “scene” to the lower half of the page. Each object is scored 2 (symmetric copy), 1.5 (partial omission of one side), 1 (complete omission of one side), .5 (complete omission of one side and partial omission of the other), or 0 (complete omission of the object or unrecognizable copy). The total score lower than 10 is considered abnormal [28]. In addition, each object is divided in half, resulting in 10 halves. For egocentric asymmetry, the two plants and the half of the house figure presented on the left side of the page are scored -1 for each half if copied, and the others on the right side of the page are scored +1 for each half if drawn. A partial omission results in .5 being deducted toward zero. For allocentric asymmetry, within each object (plant, house, or tree), the left half is scored -1 and the right +1 if it copied. The sum of an asymmetry greater than 0 indicates left-sided neglect in the given reference frame, and lower than 0 indicates right-side neglect.
Analysis methods
All the analyses were performed using STATA/SE 16.1. All the significance level, alpha, was set to 0.05. We described participant characteristics using medians and interquartile ranges (IQRs) for continuous variables and counts for categorical variables. Group comparison of each variable was performed using the U test for continuous variables, and the chi-squared test for categorical variables. If any of the participant characteristics showed a significant difference between groups, they would be included to the main analyses that examined a priori hypotheses.
To examine Hypothesis 1 that PAT reduced visuospatial symptoms of spatial neglect, on each outcome measure, we included participants who showed spatial neglect in the measure at baseline (T1) and chose an analysis appropriate for the data distribution. For data of an outcome measure that was or could be transformed to be normally distributed (given its skewness and kurtosis), a mixed linear modeling (MLM) analysis was performed. MLMs offer higher power because they more accurately account for the within-subject variability by including the random intercepts and slopes [57]. While modeling subjects’ intercepts and slopes as random effects, we included the independent variables of treatment group (PAT, Sham), assessment time (T1, T2, T3, T4), and the interaction of them as fixed effects while controlling for baseline performance (i.e., outcome measured at T1). For data of an outcome measure that could not be transformed into a normal distribution, we would inspect the pattern of the results and conduct appropriate analyses.
To examine Hypothesis 2 that PAT enhanced the recovery of spatial neglect, we inspected the occurrence rates of spatial neglect as defined by different measures in each time point among participants who showed a neglect symptom in a given test at T1. Thus, the spatial neglect occurrence rate at T1 was locked at 100% for each test, controlling for the sensitivity difference across tests. A lower occurrence rate after T1 indicated fewer patients with detected symptoms by a given measure, which would indicate a higher recovery rate. Chi-squared tests were conducted to examine the group differences.
Results
Participant characteristics
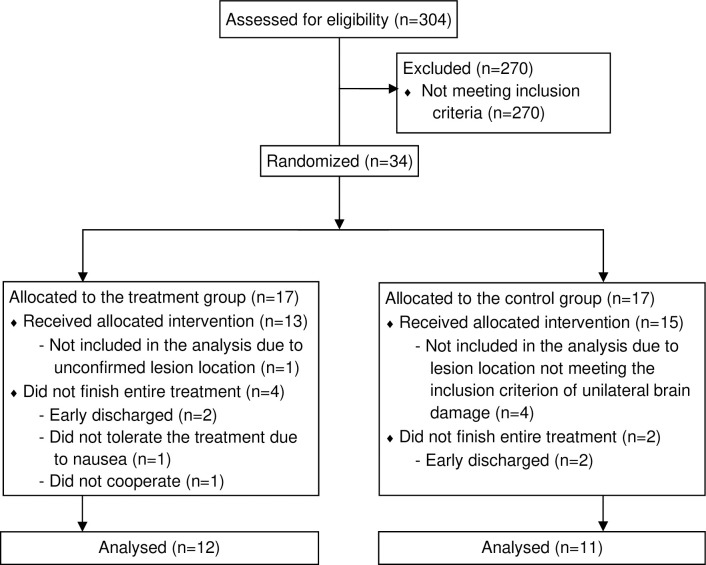
A total of 304 potential participants were screened for eligibility (Fig 1). Thirty-four (11.2%) participants meeting inclusion criteria were randomized into the PAT group or the Sham group (see S1 Table). Twenty-three (67.6%) participants completed all the treatment and assessment sessions and were included in the analysis As summarized in Table 1, there was no significant difference in any of the participant demographic characteristics, functional status at admission, or severity level of spatial neglect at baseline as measured using the CBS via KF-NAP.
Fig 1. Flow chart of the randomized feasibility and pilot trial.
Table 1. Participant characteristics presented in counts for categorical variables and in medians (IQRs) for continuous variables.
| Variable | PAT group | Sham group | Group comparison |
|---|---|---|---|
| (n = 12) | (n = 11) | (p value) | |
| Sex (male/female) | 5 / 7 | 5 / 6 | .855 |
| Age (in years) | 51.5 (47.5–55) | 58 (53–61) | .102 |
| Formal education (in years) | 12.5 (12–13) | 12 (11–13) | .237 |
| Injury type (stroke / TBI) | 11 / 1 | 10 / 1 | .949 |
| Neglected side (left/right) | 11 / 1 | 9 / 2 | .484 |
| Time post injury/stroke at admission (in days) | 58 (38.5–74) | 48 (35–79) | .735 |
| Time post injury/stroke at the first PAT session (in days) | 76 (69–133.5) | 70 (62–97) | .424 |
| mFIM at admission (range 7–47; higher = better function) | 23 (18–24.5) | 20 (19–25) | .853 |
| MMSE at admission* (range 0–30; higher = better function) | 19 (18–27); | 19 (17–21.5); | .691 |
| n = 10 | n = 8 | ||
| BBS at admission (range 0–56; higher = better function) | 5 (4–15) | 5 (4–25) | .827 |
| CBS via KF-NAP at baseline (range 0–30; higher = greater impairment) | 13 (12. 5–20) | 14 (12–17) | .781 |
Abbreviations: PAT, prism adaptation treatment; mFIM, Modified Motor Subscale of the Functional Independence Measure; MMSE, Mini Mental State Examination; BBS, Berg Balance Scale; CBS, Catherine Bergego Scale; KF-NAP, Kessler Foundation Neglect Assessment Process.
*MMSE was unavailable for five participants, and the numbers of participants were noted.
Table 2 presents lesion locations in each participant. 13 (56.5%) participants had lesions in both cortical and subcortical regions, 7 (30.4%) had lesions in the cortical areas sparing the subcortical structures, and 3 (13.0%) sustained lesions confined in the subcortical areas. Table 2 also includes a summary of the presentation of spatial neglect based on different outcome measures’ criteria. While all the participants were enrolled to the study for their presentation of moderate to severe neglect determined using the CBS via KF-NAP, 5 (21.7%) participants demonstrated neglect in all the outcome measures at baseline and 1 (4.3%) showed no neglect in any of the measures (Table 2).
Table 2. Participant brain lesion locations and presentation of spatial neglect at baseline, based on different test criteria.
| Group | ID* | Injured hemisphere | Cortical | Subcortical | Bells Test | Line Bisection | Scene Copying | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | T | P | O | In | BG | Th | L-R | Start | Ego | Allo | ||||
| PAT | 1 | R | x | x | x | x | ||||||||
| PAT | 2 | R | x | x | x | x | x | x | x | x | ||||
| PAT | 3 | R | x | x | x | x | x | |||||||
| PAT | 4 | R | x | x | x | x | ||||||||
| PAT | 5 | R | x | x | x | x | x | x | ||||||
| PAT | 6 | R | x | x | x | x | x | x | x | |||||
| PAT | 7 | R | x | x | x | x | x | x | x | x | x | |||
| PAT | 8 | R | x | x | x | x | ||||||||
| PAT | 9 | R | x | x | x | x | x | x | x | x | x | x | x | |
| PAT | 10 | R | x | x | x | x | x | x | x | x | x | |||
| PAT | 11 | R | x | x | x | |||||||||
| PAT | 12 | L | x | x | x | x | x | |||||||
| Sham | 13 | R | x | x | x | x | x | x | x | x | x | x | x | |
| Sham | 14 | R | x | x | x | x | x | x | x | x | x | x | x | x |
| Sham | 15 | R | x | x | x | x | x | x | ||||||
| Sham | 16 | R | x | x | x | x | x | x | x | x | ||||
| Sham | 17 | R | x | x | x | x | x | |||||||
| Sham | 18 | R | x | x | x | x | x | |||||||
| Sham | 19 | R | x | x | ||||||||||
| Sham | 20 | R | x | x | x | x | x | x | x | x | ||||
| Sham | 21 | R | x | x | x | x | x | x | x | x | ||||
| Sham | 22 | L | x | x | x | x | x | x | x | x | x | |||
| Sham | 23 | L | x | x | x | |||||||||
Abbreviations under the Cortical header: F, frontal; T, temporal; P, parietal; O, occipital; In, insular. Abbreviations under the Subcortical header: BG, basal ganglia; Th, thalamus; Abbreviations for tests: L-R, lateralized discrepancy; Start, starting column; Ego, egocentric; Allo, allocentric.
*ID numbers were generated for the purpose of data presentation and did not reflect the chronical order of study participation or personal identity.
Hypothesis 1: Prism Adaptation Treatment (PAT) reduced visuospatial symptoms of spatial neglect
Catherine Bergego Scale (CBS) via KF-NAP
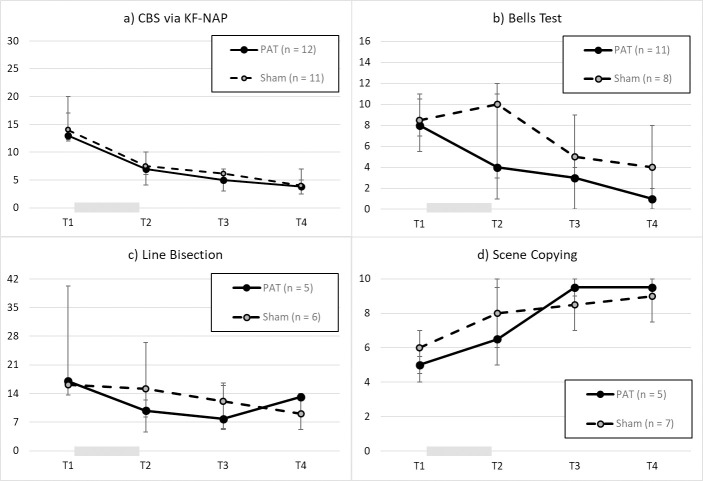
All the participants (12 in the PAT group and 11 in the Sham group) were included in this analysis as they were enrolled to the study based on the CBS score > 10. A mixed linear modeling (MLM) analysis was conducted to examine the square-root-transformed CBS as described in the Methods section. Assessment time was coded as a continuous variable. The only significant effect was assessment time, b = -0.50, SE = 0.10, p < .001, 95%CI = [-0.70, -0.31], while treatment group, b = -0.25, SE = 0.35, p = 0.473, 95%CI = [-0.93, 0.43], or the treatment x time interaction, b = 0.09, SE = 0.15, p = 0.539, 95%CI = [-0.21, 0.40], did not show significant effects. To examine whether there were significant changes between assessment sessions, we performed MLM again but with assessment session as a categorical variable (T1, T2, T3, T4). The results showed significant effects of all assessment sessions (reference: T1), all p values < 0.001, but there was no significant effect of treatment group, p = 0.947, or interaction effect, all p values > 0.3. Thus, both groups improved from T1 to T4 to the similar extent, and there was no specific effect of PAT (Fig 2A).
Fig 2.
Results, from T1 to T4, each two weeks apart, of a) CBS via KF-NAP, b) lateralized omission discrepancy in the Bells Test, c) deviation (mm) in line bisection, and d) accuracy score of the Scene Copying test. The gray bar between T1 and T2 indicates the treatment period. Each data point denotes the median with the error bar representing the IQR.
Bells test
Twenty participants (11 in the PAT group and 9 in the Sham group) were included in this analysis as they showed neglect in one of the measures performed on the Bells Test at baseline. Based on the lateralized omission discrepancy criterion, 19 participants (11 in the treatment group and 8 in the control group) omitted more than 2 targets on the contralesional side than on the ipsilesional side of space. Because the data could not be transformed into a normal distribution, the planned MLM was not performed. Instead, we calculated the linear regression coefficient between lateralized omission discrepancy and assessment session within each participant to indicate the performance trajectory over time, in which an improvement was noted with a negative regression. Given that this generated dataset was normally distributed, we contacted a t test and found no significant difference, effect size d = 0.35, p = 0.481, between the mean regression, -1.92 (SD = 1.08), in the PAT group and that, -1.55 (SD = 1.14), in the Sham group. Nonetheless, the PAT group’s trajectory was significantly lower than zero, p < 0.001, suggesting improvement, so was the Sham group’s, p = 0.006. While inspecting the plotted results (Fig 2B), we observed the most apparent difference was at T2, thus a U test was conducted to compare the difference between the groups, which was found not reaching the significance level, z = 1.08, p = 0.280, effect size r = 0.25.
Based on the starting column criterion for spatial neglect, 20 participants (11 in the treatment group and 9 in the control group) were included. The median value in any condition from the combination of treatment group and assessment session was 7 (the column far to the neglected side of space) with varied IQRs. This suggested a highly skewed dataset and required a larger sample size for a statistical analysis. Overall, both groups improved in lateralized bias from T1 to T4, and there were no consistent PAT effects on the Bells Test performance.
Line bisection
Eleven participants (5 in the PAT group and 6 in the Sham group) were included. An MLM was conducted to examining the log-transformed line bisection deviation as described in the Methods section. No effect was found significant, all p values > 0.1, when assessment time was entered to the model as a continuous variable. To examine whether there was an effect between assessment sessions, we conducted a separate MLM but with assessment session as a categorical variable (T1, T2, T3, T4) with T1 as the reference. The results showed a significant effect of T3, p = 0.05, but there was no significant effect of other time point, treatment group, or interaction effect, all p values > 0.1. Thus, both groups showed potential changes from T1 to T3, and there was no effect of PAT (Fig 2C).
Scene copying
In the twelve participants (5 in the PAT group and 7 in the Sham group) who showed egocentric and/or allocentric neglect on this measure at baseline, we conducted an MLM on the total accuracy score using the same structure as described above. Similar results were found such that assessment time was the only variable that showed a significant effect: As a continuous or categorical variable, the effect of assessment time was significant, all p values < 0.006, while the other variables were not, p > 0.1. Thus, both groups improved in copying the figures from T1 to T4 with no effect of PAT (Fig 2D).
Hypothesis 2: Prism Adaptation Treatment (PAT) enhanced the recovery of spatial neglect
One participant of the PAT group (n = 12) scored 0 on the CBS at T2 and at T3, and two scored 0 at T4. However, none of the Sham group (n = 11) scored 0 at any time point. Nonetheless, none of the group comparisons reached significance, all p values > 0.1. The same set of analyses were performed based on the criteria of the lateralized discrepancy omission and starting column of the Bells Test, deviation of Line Bisection, and egocentric and allocentric asymmetries of Scene Copying Test. The results are presented in Table 3. None of the analyses yielded a significant result although at each time point the occurrence rate of spatial neglect in the PAT group was lower than the Sham group in all the measures except for the allocentric asymmetry in the Scene Copy Test. On average, 39.1% of the PAT group and 28.6% of the Sham group recovered from spatial neglect (Fig 3).
Table 3. Chi-squared results on the occurrence rates of spatial neglect in the treatment vs. control groups, based on the criteria set in different measures.
| Assessment time | CBS via KF-NAP | Bells Test | Line Bisection | Scene Copying | ||
|---|---|---|---|---|---|---|
| L-R | Start | Ego | Allo | |||
| T2 | X2 = 0.96 | X2 = 0.01 | X2 = 0.13 | X2 = 0.75 | X2 = 0.75 | X2 = 0 |
| p = 0.328 | p = 0.912 | p = 0.714 | p = 0.387 | p = 0.387 | p = 1 | |
| T3 | X2 = 0.95 | X2 = 2.30 | X2 = 0.90 | X2 = 0.05 | X2 = 0.05 | X2 = 0.09 |
| p = 0.329 | p = 0.129 | p = 0.343 | p = 0.819 | p = 0.819 | p = 0.764 | |
| T4 | X2 = 1.83 | X2 = 1.17 | X2 = 0.04 | X2 = 0.75 | X2 = 0.40 | X2 = 0.09 |
| p = 0.176 | p = 0.280 | p = 0.845 | p = 0.387 | p = 0.527 | p = 0.764 | |
Abbreviations: CBS, Catherine Bergego Scale; KF-NAP, Kessler Foundation Neglect Assessment Process; L-R, lateralized discrepancy; Start, starting column; Ego, egocentric; Allo, allocentric.
Fig 3. Occurrence rates of spatial neglect at each time point based on the criterion of a given outcome measure.
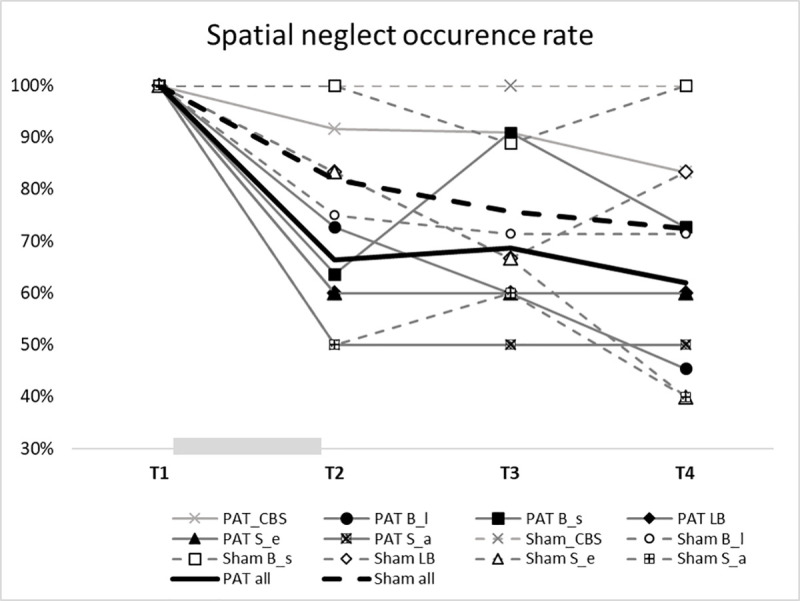
The gray bar between T1 and T2 indicates the treatment period. CBS: Catherine Bergego Scale; B_l: Bells Test–lateralized omission discrepancy; B_s: Bells Test: starting column; LB: Line Bisection; S_e: Scene Copying–egocentric asymmetry; S_a: Scene Copying–allocentric asymmetry.
Discussion
In the present study, we conducted an RCT in an inpatient setting where patients received a highly intensive comprehensive brain injury rehabilitation program. Both the PAT and Sham groups improved in visuospatial ability (assessed using paper-based tests) as well as visuospatial function (assessed using an ecological assessment) to the similar extents that could not be differentiated statistically. In addition, the PAT group appeared recovering better than the Sham group, but the comparison was under powered. Thus, we found no evidence that PAT specifically reduced spatial neglect symptoms or enhanced spatial neglect recovery.
The present study replicated Ten Brink et al. [44] using the same PAT treatment regimen (10 once-daily sessions over two weeks) in a similar setting where patients received intensive neurorehabilitation that had specific emphasis on spatial neglect. Unlike Ten Brink et al. who included patients regardless their neglect severity, we recruited patients with moderate to severe neglect, which would prevent ceiling effects when evaluating improvement. Nonetheless, our findings were comparable to theirs. This suggests that visuomotor training with sham/flat goggles was effective to certain extent, and that intensive rehabilitation programs that had been in place in both studies may have been successful in improving visuospatial abilities and functional outcomes related to spatial neglect.
Several researchers have noted that sham treatment may have beneficial effects on reducing neglect symptoms. Serino and colleagues [58] are among the first who offered an account for the mechanisms underlying the improvement after sham treatment. The visuomotor exercise during PAT is a task that requires motor planning and execution guided by a visual target, i.e., initiating and performing arm reaching movements toward a visible target, which relies on eye-hand coordination. Patients perform this task repeatedly during a session (60 times in our study protocol and 90 times in Serino et al.), and sometimes they reach toward the neglected side of space. Serino et al. [58] postulated that this orienting behavior toward the neglected side could be reinforced by repetition within a session and over multiple sessions, leading to amelioration of spatial neglect. In addition, sustained attention may be strengthened during the repetitive task guided by the therapist. Even though the task seems easy and effortless, patients are mentally present not only at the moment when they perform an arm movement to a visual target but also during a sustained period of time, ideally the entire session. This mental state of being present may strengthen the ability to sustain attention, which in turn, facilitate improvement of other attention-related abilities such as spatial attention and thus ameliorate spatial neglect [59–61]. Further research is required to understand the “active ingredients” of PAT and sham treatment.
Several possibilities may explain why the current study setting, i.e., the BIR Program, was effective for ameliorating spatial neglect symptoms. The most likely possibility is that the BIR Program provided a variety of evidence-based therapeutic elements for spatial neglect, applied throughout the entire rehabilitation stay. This included elements of visual scanning training, optokinetic stimulation, limb activation, constraint induced movement therapy (for a review of these methods, see [62–64]). Visual scanning training was highly emphasized during therapy sessions for mobility and activities of daily living (ADL) trainings. Moreover, patients in the BIR Program received 30-minute daily computer-assisted cognitive training on RehaCom (Hasomed, Magdeburg, Germany) and CogniPlus (Schuhfried, Moedling, Austria) platforms. These cognitive training sessions were designed to target the core cognitive deficits in domains such as spatial attention and spatial working memory through computerized optokinetic stimulation [62] or training for spatial working memory (for discussion of association between spatial neglect and spatial working memory, see [65]). In addition, twice daily physiotherapy sessions in the BIR Program usually involved limb activation (with the help of physiotherapist and/or robotic systems) which was found to reduce spatial neglect even when performed passively [66]. Some patients in the BIR Program received other forms of physiotherapeutic interventions such as constraint induced movement therapy or mirror therapy, which was found effective reducing spatial neglect severity [67, 68]. Because a combination of different interventions was more effective than a single approach in treating spatial neglect [69], any benefit from PAT may have been masked by the BIR Program.
In addition to treatment outcomes, the present study also demonstrated the difficulty in conducting an RCT in an inpatient rehabilitation setting. Only one in ten patients were eligible for the study, and one third of study participants could not complete the study. This suggests a suboptimal feasibility in terms of research participant recruitment and of implementing a daily research treatment protocol on top of an already busy schedule of therapies. We recommend that future large-scale definitive RCTs collaborate with multiple centers to increase the chance of reaching a necessary sample size and coordinate with the therapy team to incorporate PAT and Sham treatment within scheduled therapy sessions.
Study limitations
The major limitations of our study are the small sample size and the heterogeneity of the sample. Participants in our study had different acquired brain injury etiology (stroke or TBI), neglected side of space (left or right), and lesion locations. PAT is not equally effective for all patients. For example, several studies have found lesions involving the frontal lobe [26, 70–72], the temporal lobe [73, 74], the parietal lobe [26, 72–74], or the occipital lobe [31] led to different PAT responses. Our sample size was too small to examine lesion-based hypotheses. However, the RCT design should have eliminated some data noise from the heterogeneous sample. Nonetheless, the sample size was not large enough to enable us to perform parametric analyses on several outcome measures, limiting result interpretation and further hypothesis generation.
Conclusion
The present findings suggest that both PAT and Sham treatment may improve visuospatial ability and function among individuals with spatial neglect after unilateral brain damage. We found PAT not particularly effective in ameliorating spatial neglect in the clinical setting that offers highly intensive rehabilitation program that has emphasized evidence-based practice addressing spatial neglect. Further investigations are needed to understand the mechanisms underlying PAT and Sham treatment in order to refine the treatment or create new interventions that will target specific attention, visuospatial, or motor control functions critical to spatial neglect.
Supporting information
(TIF)
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
TV: This study was supported by the program PROGRES (Progres = C4 = 8D.Q 06/LF1 = 20). The program had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Heilman KM, Valenstein E, Watson RT. Neglect and related disorders. Vol. 20, Seminars in Neurology. 2000. p. 463–70. 10.1055/s-2000-13179 [DOI] [PubMed] [Google Scholar]
- 2.Carter AR, McAvoy MP, Siegel JS, Hong X, Astafiev S V, Rengachary J, et al. Differential white matter involvement associated with distinct visuospatial deficits after right hemisphere stroke. Cortex. 2017;88:81–97. 10.1016/j.cortex.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett AM, Boukrina O, Saleh S. Ventral attention and motor network connectivity is relevant to functional impairment in spatial neglect after right brain stroke. Brain Cogn. 2019;129:16–24. 10.1016/j.bandc.2018.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–99. 10.1146/annurev-neuro-061010-113731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karnath H-O, Rorden C. The anatomy of spatial neglect. Neuropsychologia [Internet]. 2011/07/02. 2012. May;50(6):1010–7. Available from: https://pubmed.ncbi.nlm.nih.gov/21756924 10.1016/j.neuropsychologia.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gainotti G, Messerli P, Tissot R. Qualitative analysis of unilateral spatial neglect in relation to laterality of cerebral lesions. J Neurol Neurosurg & Psychiatry [Internet]. 1972. August 1;35(4):545 LP– 550. Available from: http://jnnp.bmj.com/content/35/4/545.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buxbaum LJ, Ferraro MK, Veramonti T, Farne A, Whyte J, Ladavas E, et al. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology. 2004. March 9;62(5):749–56. 10.1212/01.wnl.0000113730.73031.f4 [DOI] [PubMed] [Google Scholar]
- 8.Chen P, Chen CC, Hreha K, Goedert KM, Barrett AM. Kessler Foundation neglect assessment process uniquely measures spatial neglect during activities of daily living. In: Archives of Physical Medicine and Rehabilitation [Internet]. W.B. Saunders; 2015 [cited 2020 Apr 24]. p. 869-876.e1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25461827 [DOI] [PMC free article] [PubMed]
- 9.Chen P, Ward I, Hreha K, Liu Y, Khan U. Spatial Neglect Commonly Occurs after Traumatic Brain Injury (P7.289). Neurology. 2014;82(10 Supplement). [Google Scholar]
- 10.Andrade K, Samri D, Sarazin M, de Souza LC, Cohen L, de Schotten MT, et al. Visual neglect in posterior cortical atrophy. BMC Neurol. 2010. August 10;10(1):1–7. 10.1186/1471-2377-10-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billingsley RL, Lang FF, Slopis JM, Schrimsher GW, Ater JL, Moore BD. Visual-spatial neglect in a child following sub-cortical tumor resection. Dev Med Child Neurol [Internet]. 2007. February 13 [cited 2020 Apr 24];44(3):191–200. Available from: http://doi.wiley.com/10.1111/j.1469-8749.2002.tb00785.x [DOI] [PubMed] [Google Scholar]
- 12.Katz N, Hartman-Maeir A, Ring H, Soroker N. Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Arch Phys Med Rehabil. 1999;80(4):379–84. 10.1016/s0003-9993(99)90273-3 [DOI] [PubMed] [Google Scholar]
- 13.Gillen R, Tennen H, McKee T. Unilateral spatial neglect: Relation to rehabilitation outcomes in patients with right hemisphere stroke. Arch Phys Med Rehabil. 2005;86(4):763–7. 10.1016/j.apmr.2004.10.029 [DOI] [PubMed] [Google Scholar]
- 14.Hammerbeck U, Gittins M, Vail A, Paley L, Tyson SF, Bowen A. Spatial neglect in stroke: Identification, disease process and association with outcome during inpatient rehabilitation. Brain Sci. 2019. December 1;9(12). 10.3390/brainsci9120374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wee JYM, Hopman WM. Comparing Consequences of Right and Left Unilateral Neglect in a Stroke Rehabilitation Population. Am J Phys Med Rehabil [Internet]. 2008. November [cited 2020 May 16];87(11):910–20. Available from: http://journals.lww.com/00002060-200811000-00006 10.1097/PHM.0b013e31818a58bd [DOI] [PubMed] [Google Scholar]
- 16.Paolucci S, Antonucci G, Grasso MG, Pizzamiglio L. The role of unilateral spatial neglect in rehabilitation of right brain-damaged ischemic stroke patients: A matched comparison. Arch Phys Med Rehabil. 2001. June 1;82(6):743–9. 10.1053/apmr.2001.23191 [DOI] [PubMed] [Google Scholar]
- 17.Jehkonen M, Laihosalo M, Kettunen JE. Impact of neglect on functional outcome after stroke-a review of methodological issues and recent research findings. Vol. 24, Restorative Neurology and Neuroscience. IOS Press; 2006. [PubMed] [Google Scholar]
- 18.Denes G, Semenza C, Stoppa E, Lis A. Unilateral spatial neglect and recovery from hemiplegia: A follow-up study. Brain. 1982. September;105(3):543–52. 10.1093/brain/105.3.543 [DOI] [PubMed] [Google Scholar]
- 19.Rundek T, Mast H, Hartmann A, Boden-Albala B, Lennihan L, Lin I-F, et al. Predictors of resource use after acute hospitalization: The Northern Manhattan Stroke Study. Neurology [Internet]. 2000. October 24 [cited 2020 Apr 25];55(8):1180–7. Available from: http://www.neurology.org/cgi/doi/10.1212/WNL.55.8.1180 [DOI] [PubMed] [Google Scholar]
- 20.Chen P, Fyffe DC, Hreha K. Informal caregivers’ burden and stress in caring for stroke survivors with spatial neglect: An exploratory mixed-method study. Top Stroke Rehabil. 2017;24(1):24–33. 10.1080/10749357.2016.1186373 [DOI] [PubMed] [Google Scholar]
- 21.Yang NYH, Zhou D, Chung RCK, Li-Tsang CWP, Fong KNK. Rehabilitation interventions for unilateral neglect after stroke: A systematic review from 1997 through 2012. Front Hum Neurosci [Internet]. 2013. April 24 [cited 2020 Apr 25];7(APR 2013):187 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23675339 10.3389/fnhum.2013.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P, Pitteri M, Gillen G, Ayyala H. Ask the experts how to treat individuals with spatial neglect: a survey study. Disabil Rehabil. 2018. October 23;40(22):2677–91. 10.1080/09638288.2017.1347720 [DOI] [PubMed] [Google Scholar]
- 23.Harris CS. Adaptation to displaced vision: Visual, motor, or proprioceptive change? Science (80-). 1963. May 17;140(3568):812–3. 10.1126/science.140.3568.812 [DOI] [PubMed] [Google Scholar]
- 24.Rossetti Y, Rode G, Pisella L, Farné A, Li L, Boisson D, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature [Internet]. 1998. September 10 [cited 2020 Apr 25];395(6698):166–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9744273 10.1038/25988 [DOI] [PubMed] [Google Scholar]
- 25.Panico F, Rossetti Y, Trojano L. On the mechanisms underlying prism adaptation: a review of neuro-imaging and neuro-stimulation studies. Cortex. 2020;123:57–71. 10.1016/j.cortex.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 26.Saj A, Cojan Y, Assal F, Vuilleumier P. Prism adaptation effect on neural activity and spatial neglect depend on brain lesion site. Cortex. 2019. October 1;119:301–11. 10.1016/j.cortex.2019.04.022 [DOI] [PubMed] [Google Scholar]
- 27.Chapman HL, Eramudugolla R, Gavrilescu M, Strudwick MW, Loftus A, Cunnington R, et al. Neural mechanisms underlying spatial realignment during adaptation to optical wedge prisms. Neuropsychologia. 2010;48(9):2595–601. 10.1016/j.neuropsychologia.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 28.Fortis P, Maravita A, Gallucci M, Ronchi R, Grassi E, Senna I, et al. Rehabilitating Patients With Left Spatial Neglect by Prism Exposure During a Visuomotor Activity. Neuropsychology. 2010. November;24(6):681–97. 10.1037/a0019476 [DOI] [PubMed] [Google Scholar]
- 29.Shiraishi H, Muraki T, Ayaka Itou YS, Hirayama K. Prism intervention helped sustainability of effects and ADL performances in chronic hemispatial neglect: A follow-up study. NeuroRehabilitation. 2010;27(2):165–72. 10.3233/NRE-2010-0593 [DOI] [PubMed] [Google Scholar]
- 30.Rode G, Rossetti Y, Boisson D. Prism adaptation improves representational neglect. Neuropsychologia. 2001;39(11):1250–4. 10.1016/s0028-3932(01)00064-1 [DOI] [PubMed] [Google Scholar]
- 31.Serino A, Angeli V, Frassinetti F, Làdavas E. Mechanisms underlying neglect recovery after prism adaptation. Neuropsychologia. 2006. January 1;44(7):1068–78. 10.1016/j.neuropsychologia.2005.10.024 [DOI] [PubMed] [Google Scholar]
- 32.Nijboer TCW, Olthoff L, Van Der Stigchel S, Visser-Meily JMA. Prism adaptation improves postural imbalance in neglect patients. Neuroreport. 2014. March 26;25(5):307–11. 10.1097/WNR.0000000000000088 [DOI] [PubMed] [Google Scholar]
- 33.Goedert KM, Chen P, Boston RC, Foundas AL, Barrett AM. Presence of motor-intentional aiming deficit predicts functional improvement of spatial neglect with prism adaptation. Neurorehabil Neural Repair. 2014;28(5):483–93. 10.1177/1545968313516872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Champod AS, Frank RC, Taylor K, Eskes GA. The effects of prism adaptation on daily life activities in patients with visuospatial neglect: a systematic review. Vol. 28, Neuropsychological Rehabilitation. Routledge; 2018. p. 491–514. 10.1080/09602011.2016.1182032 [DOI] [PubMed] [Google Scholar]
- 35.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Vol. 47, Stroke: Lippincott Williams and Wilkins; 2016. p. e98–169. [DOI] [PubMed] [Google Scholar]
- 36.Gillen G, Nilsen DM, Attridge J, Banakos E, Morgan M, Winterbottom L, et al. Effectiveness of interventions to improve occupational performance of people with cognitive impairments after stroke: An evidence-based review. Vol. 69, American Journal of Occupational Therapy American Occupational Therapy Association, Inc; 2015. p. 6901180040p1–9. [DOI] [PubMed] [Google Scholar]
- 37.Stroke Foundation [Internet]. Clinical Guidelines for Stroke Management. Melbourne. Australia. 2019 [cited 2020 May 16]. Available from: https://informme.org.au/en/Guidelines/Clinical-Guidelines-for-Stroke-Management
- 38.Hebert D, Lindsay MP, McIntyre A, Kirton A, Rumney PG, Bagg S, et al. Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. Int J Stroke [Internet]. 2016. June;11(4):459–484. Available from: 10.1177/1747493016643553 [DOI] [PubMed] [Google Scholar]
- 39.Bowen A, James M, Young G. Royal College of Physicians 2016 National clinical guideline for stroke. In RCP; 2016. [Google Scholar]
- 40.Turton AJ, O’Leary K, Gabb J, Woodward R, Gilchrist ID. A single blinded randomised controlled pilot trial of prism adaptation for improving self-care in stroke patients with neglect. Neuropsychol Rehabil. 2010. April;20(2):180–96. 10.1080/09602010903040683 [DOI] [PubMed] [Google Scholar]
- 41.Mancuso M, Pacini M, Gemignani P, Bartalini B, Agostini B, Ferroni L, et al. Clinical application of prismatic lenses in the rehabilitation of neglect patients. A randomized controlled trial. Eur J Phys Rehabil Med. 2012;48:197–208. [PubMed] [Google Scholar]
- 42.Mizuno K, Tsuji T, Takebayashi T, Fujiwara T, Hase K, Liu M. Prism Adaptation Therapy Enhances Rehabilitation of Stroke Patients With Unilateral Spatial Neglect. Neurorehabil Neural Repair [Internet]. 2011. October 23 [cited 2020 May 16];25(8):711–20. Available from: http://journals.sagepub.com/doi/10.1177/1545968311407516 [DOI] [PubMed] [Google Scholar]
- 43.Vaes N, Nys G, Lafosse C, Dereymaeker L, Oostra K, Hemelsoet D, et al. Rehabilitation of visuospatial neglect by prism adaptation: effects of a mild treatment regime. A randomised controlled trial. Neuropsychol Rehabil. 2018. August 18;28(6):899–918. 10.1080/09602011.2016.1208617 [DOI] [PubMed] [Google Scholar]
- 44.Ten Brink AF, Visser-Meily JMA, Schut MJ, Kouwenhoven M, Eijsackers ALH, Nijboer TCW. Prism Adaptation in Rehabilitation? No Additional Effects of Prism Adaptation on Neglect Recovery in the Subacute Phase Poststroke: A Randomized Controlled Trial. Neurorehabil Neural Repair. 2017. December 1;31(12):1017–28. 10.1177/1545968317744277 [DOI] [PubMed] [Google Scholar]
- 45.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. bmj. 2016;355:i5239 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen P, Barrett A, Hreha K, Fortis P, Goedert K. Functional assessment of spatial neglect: A review of the catherine bergego scale and an introduction of the kessler foundation neglect assessment process. Top Stroke Rehabil [Internet]. 2012. January 1;19(5):423–35. Available from: https://search.ebscohost.com/login.aspx?authtype=shib&custid=s1240919&profile=eds 10.1310/tsr1905-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen P, Hreha K, Pitteri M. Kessler Foundation Neglect Assessment Process: KF-NAP 2014 Manual. 2014; [Google Scholar]
- 48.KF-PAT Portable Kit [Internet]. Stoelting; 2020 [cited 2020 Dec 6]. Available from: https://www.stoeltingco.com/kessler-foundation-prism-adaptation-treatment-kf-pat-for-spatial-neglect.html
- 49.Fortis P, Chen P, Goedert KM, Barrett AM. Effects of prism adaptation on motor-intentional spatial bias in neglect. Neuroreport. 2011;22(14):700 10.1097/WNR.0b013e32834a3e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muth ER, Stern RM, Thayer JF, Koch KL. Assessment of the multiple dimensions of nausea: The nausea profile (NP). J Psychosom Res. 1996. May 1;40(5):511–20. 10.1016/0022-3999(95)00638-9 [DOI] [PubMed] [Google Scholar]
- 51.Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil [Internet]. 2009 Apr 29 [cited 2020 Apr 20];23(4):362–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19179355 [DOI] [PubMed]
- 52.Azouvi P, Marchal F, Samuel C, Morin L, Renard C, Louis-Dreyfus A, et al. Functional consequences and awareness of unilateral neglect: Study of an evaluation scale. Neuropsychol Rehabil [Internet]. 1996. April [cited 2020 Jun 19];6(2):133–50. Available from: http://www.tandfonline.com/doi/abs/10.1080/713755501 [Google Scholar]
- 53.Gauthier L, Dehaut F, Joanette Y. The Bells Test: A quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol. 1989;11(2):49–54. [Google Scholar]
- 54.Azouvi P, Samuel C, Louis-Dreyfus A, Bernati T, Bartolomeo P, Beis JM, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry. 2002;73(2):160–6. 10.1136/jnnp.73.2.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parton A, Malhotra P, Husain M. Hemispatial neglect. Vol. 75, Journal of Neurology, Neurosurgery and Psychiatry BMJ Publishing Group; 2004. p. 13–21. [PMC free article] [PubMed] [Google Scholar]
- 56.Chen P, Lander V, Noce N, Hreha K. Prism adaptation treatment for spatial neglect post brain tumour removal: A case report. Hong Kong J Occup Ther. 2020;1569186120921472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goedert KM, Boston R, Barrett AM. Advancing the science of spatial neglect rehabilitation: an improved statistical approach with mixed linear modeling. Front Hum Neurosci. 2013;7:211 10.3389/fnhum.2013.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serino A, Barbiani M, Rinaldesi ML, Ladavas E. Effectiveness of prism adaptation in neglect rehabilitation: a controlled trial study. Stroke. 2009;40(4):1392–8. 10.1161/STROKEAHA.108.530485 [DOI] [PubMed] [Google Scholar]
- 59.Robertson IH, Tegnér R, Tham K, Lo A, Nimmo-Smith I. Sustained attention training for unilateral neglect: theoretical and rehabilitation implications. J Clin Exp Neuropsychol. 1995;17(3):416–30. 10.1080/01688639508405133 [DOI] [PubMed] [Google Scholar]
- 60.Wilson FC, Manly T, Coyle D, Robertson IH. The effect of contralesional limb activation training and sustained attention training for self-care programmes in unilateral spatial neglect. Restor Neurol Neurosci. 2000;16(1):1–4. [PubMed] [Google Scholar]
- 61.Wilson FC, Manly T. Sustained attention training and errorless learning facilitates self‐care functioning in chronic ipsilesional neglect following severe traumatic brain injury. Neuropsychol Rehabil. 2003;13(5):537–48. [Google Scholar]
- 62.Kerkhoff G, Schenk T. Rehabilitation of neglect: An update. Vol. 50, Neuropsychologia. 2012. p. 1072–9. 10.1016/j.neuropsychologia.2012.01.024 [DOI] [PubMed] [Google Scholar]
- 63.Azouvi P, Jacquin-Courtois S, Luauté J. Rehabilitation of unilateral neglect: Evidence-based medicine. Vol. 60, Annals of Physical and Rehabilitation Medicine Elsevier Masson SAS; 2017. p. 191–7. [DOI] [PubMed] [Google Scholar]
- 64.Riestra AR, Barrett AM. Rehabilitation of spatial neglect. In: Handbook of Clinical Neurology [Internet]. Elsevier B.V.; 2013 [cited 2020 Apr 25]. p. 347–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23312654 [DOI] [PMC free article] [PubMed]
- 65.Striemer CL, Ferber S, Danckert J. Spatial working memory deficits represent a core challenge for rehabilitating neglect. Front Hum Neurosci. 2013. June 14;7(JUN). 10.3389/fnhum.2013.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frassinetti F, Rossi M, Làdavas E. Passive limb movements improve visual neglect. Neuropsychologia. 2001. January 1;39(7):725–33. 10.1016/s0028-3932(00)00156-1 [DOI] [PubMed] [Google Scholar]
- 67.Pandian JD, Arora R, Kaur P, Sharma D, Vishwambaran DK, Arima H. Mirror therapy in unilateral neglect after stroke (MUST trial): A randomized controlled trial. Neurology. 2014. September 1;83(11):1012–7. 10.1212/WNL.0000000000000773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Welfringer A, Schmidt-Viereck R, Brandt T. Constraint-induced movement therapy (CIMT) in patients showing chronic neglect symptoms–a randomized controlled study. J Int Neuropsychol Soc. 2013;19:34 10.1017/S1355617712000884 [DOI] [PubMed] [Google Scholar]
- 69.Saevarsson S, Halsband U, Kristjánsson Á. Designing rehabilitation programs for neglect: Could 2 be more than 1+1? Appl Neuropsychol. 2011. April;18(2):95–106. 10.1080/09084282.2010.547774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen P, Goedert KM, Shah P, Foundas AL, Barrett AM. Integrity of medial temporal structures may predict better improvement of spatial neglect with prism adaptation treatment. Brain Imaging Behav [Internet]. 2014;8(3):346–58. Available from: 10.1007/s11682-012-9200-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goedert KM, Chen P, Foundas AL, Barrett AM. Frontal lesions predict response to prism adaptation treatment in spatial neglect: A randomised controlled study. Neuropsychol Rehabil [Internet]. 2020. January 2 [cited 2020 Jun 25];30(1):32–53. Available from: https://www.tandfonline.com/doi/abs/10.1080/09602011.2018.1448287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarri M, Greenwood R, Kalra L, Papps B, Husain M, Driver J. Prism adaptation aftereffects in stroke patients with spatial neglect: Pathological effects on subjective straight ahead but not visual open-loop pointing. Neuropsychologia. 2008;46(4):1069–80. 10.1016/j.neuropsychologia.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gossmann A, Kastrup A, Kerkhoff G, López-Herrero C, Hildebrandt H. Prism adaptation improves ego-centered but not allocentric neglect in early rehabilitation patients. Neurorehabil Neural Repair. 2013;27(6):534–41. 10.1177/1545968313478489 [DOI] [PubMed] [Google Scholar]
- 74.Gutierrez-Herrera M, Eger S, Keller I, Hermsdörfer J, Saevarsson S. Neuroanatomical and behavioural factors associated with the effectiveness of two Weekly sessions of prism adaptation in the treatment of unilateral neglect. Neuropsychol Rehabil. 2020;30(2):187–206. 10.1080/09602011.2018.1454329 [DOI] [PubMed] [Google Scholar]