Abstract
Objective
To determine the proportion of chronic low back pain patients who achieve a clinically meaningful response from different pharmacologic and nonpharmacologic treatments.
Data sources
MEDLINE, EMBASE, Cochrane Library, and gray literature search.
Study selection
Published randomized controlled trials (RCTs) that reported a responder analysis of adults with chronic low back pain treated with any of the following 15 interventions: oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs), exercise, acupuncture, spinal manipulation therapy, corticosteroid injections, acetaminophen, oral opioids, anticonvulsants, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors (SNRIs), selective serotonin reuptake inhibitors, cannabinoids, oral muscle relaxants, or topical rubefacients.
Synthesis
A total of 63 RCTs were included. There was moderate certainty that exercise (risk ratio [RR] of 1.71; 95% CI 1.37 to 2.15; number needed to treat [NNT] of 7), oral NSAIDs (RR = 1.44; 95% CI 1.17 to 1.78; NNT = 6), and SNRIs (duloxetine; RR = 1.25; 95% CI 1.13 to 1.38; NNT = 10) provide clinically meaningful benefits to patients with chronic low back pain. Exercise was the only intervention with sustained benefit (up to 48 weeks). There was low certainty that spinal manipulation therapy and topical rubefacients benefit patients. The benefit of acupuncture disappeared in higher-quality, longer (> 4 weeks) trials. Very low-quality evidence demonstrated that corticosteroid injections are ineffective. Patients treated with opioids had a greater likelihood of discontinuing treatment owing to an adverse event (number needed to harm of 5) than continuing treatment to derive any clinically meaningful benefit (NNT = 16), while those treated with SNRIs (duloxetine) had a similar likelihood of continuing treatment to attain benefit (NNT = 10) as those discontinuing the medication owing to an adverse event (number need to harm of 11). One trial each of anticonvulsants and topical NSAIDs found similar benefit to that of placebo. No RCTs of acetaminophen, cannabinoids, muscle relaxants, selective serotonin reuptake inhibitors, or tricyclic antidepressants met the inclusion criteria.
Conclusion
Exercise, oral NSAIDs, and SNRIs (duloxetine) provide a clinically meaningful reduction in pain, with exercise being the only intervention that demonstrated sustained benefit after the intervention ended. Future high-quality trials that report responder analyses are required to provide a better understanding of the benefits and harms of interventions for patients with chronic low back pain.
Résumé
Objectif
Déterminer la proportion de patients souffrant de lombalgie chronique qui ressentent un soulagement cliniquement significatif grâce à différents traitements pharmacologiques et non pharmacologiques.
Sources des données
Recension dans MEDLINE, EMBASE, la bibliothèque Cochrane et la documentation parallèle.
Sélection des études
Les études randomisées contrôlées (ERC) publiées qui rapportaient une analyse des réponses d’adultes souffrant de lombalgie chronique, traités au moyen de l’une ou l’autre des 15 interventions suivantes : anti-inflammatoires non stéroïdiens (AINS) oraux ou topiques, exercice, acupuncture, thérapie par manipulation de la colonne, injections de corticostéroïdes, acétaminophène, opioïdes oraux, anticonvulsifs, antidépresseurs tricycliques, inhibiteurs de la recapture de la sérotonine-norépinéphrine (IRSN), inhibiteurs sélectifs de la recapture de la sérotonine, cannabinoïdes, relaxants musculaires oraux ou rubéfiants topiques.
Synthèse
La revue incluait 63 ERC. Selon des données modérément sûres, l’exercice (rapport de risques-avantages [RR] de 1,71; IC à 95 % de 1,37 à 2,15; nombre de sujets à traiter [NST] = 7), les AINS oraux (RR = 1,44; IC à 95 % de 1,17 à 1,78; NST = 6) et les IRSN (duloxétine; RR = 1,25; IC à 95 % de 1,13 à 1,38; NST = 10) procurent des bienfaits cliniquement significatifs aux patients souffrant de lombalgie chronique. L’exercice était la seule intervention dont les bienfaits étaient durables (jusqu’à 48 semaines). Les données étaient d’une faible certitude pour indiquer que la thérapie par manipulation de la colonne et les rubéfiants topiques étaient bénéfiques pour les patients. Les bienfaits de l’acupuncture disparaissaient dans les essais de qualité supérieure et de plus longue durée (≥ 4 semaines). Des données probantes de très faible qualité démontraient que les injections de corticostéroïdes étaient inefficaces. Il était plus probable que les patients traités avec des opioïdes cessent le traitement en raison d’un événement indésirable (nombre nécessaire pour nuire de 5) qu’ils le poursuivent pour obtenir un bienfait cliniquement significatif (NST = 16), tandis que chez les patients traités avec des IRSN (duloxétine), la probabilité était semblable, qu’ils poursuivent le traitement pour obtenir des bienfaits (NST = 10) ou qu’ils cessent le médicament en raison d’un événement indésirable (nombre nécessaire pour nuire de 11). Deux études, l’une sur les anticonvulsifs et l’autre sur les AINS, ont constaté des bienfaits semblables à ceux du placebo. Aucune ERC sur l’acétaminophène, les cannabinoïdes, les relaxants musculaires, les inhibiteurs sélectifs de la recapture de la sérotonine ou les antidépresseurs tricycliques n’a satisfaisait aux critères d’inclusion.
Conclusion
L’exercice, les AINS oraux et les IRSN (duloxétine) procurent une réduction cliniquement significative de la douleur; l’exercice est la seule intervention démontrant avoir des bienfaits soutenus après la fin de l’intervention. Il faudrait effectuer des études de grande qualité qui rapportent l’analyse des réponses des patients afin de mieux comprendre les bienfaits et les préjudices pour ceux qui souffrent de lombalgie chronique.
Back pain is one of the most common reasons patients visit family physicians.1 While many patients with acute back pain have resolution within days to weeks,2 some do not improve. For patients with persistent back pain at 3 months, only about 40% are pain free at 1 year.2 Chronic back pain affects one’s functional status and quality of life,3 and results in substantial direct and indirect health care costs.4
Many interventions are used for the treatment of chronic low back pain. The purpose of this set of systematic reviews was to assess the benefit and harms of 15 pharmacologic and nonpharmacologic therapies used in the management of chronic radicular or nonradicular low back pain in adults. Similar to our systematic review of osteoarthritis,5 we included only randomized controlled trials (RCTs) that reported a responder analysis: the proportion of patients who achieved a clinically meaningful improvement in pain.6 This systematic review is the second in a series of reviews that will provide evidence for a guideline on the treatment of common chronic pain conditions in primary care.
Methods
We performed 15 individual systematic reviews following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).7,8
Each review focused on a single intervention and included RCTs of adults with chronic (≥ 3 months) radicular or nonradicular low back pain treated with any of the following interventions: oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs), exercise, acupuncture, spinal manipulation therapy (SMT), corticosteroid injections, acetaminophen, oral opioids, anticonvulsants, tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs), selective serotonin reuptake inhibitors (SSRIs), cannabinoids, oral muscle relaxants, or topical rubefacients. Interventions compared to placebo or saline injection were categorized as pharmacologic interventions, while no interventions (or those on a wait list), usual care interventions, or sham interventions were categorized as nonpharmacologic interventions.
To be included in the review, RCTs had to report a responder analysis, such as a 30% reduction in pain. Trials that used an active comparator were included if the active comparator was used in the intervention arm as well. Trials that exclusively enrolled participants with low back pain due to trauma or other pathological conditions (eg, infection, cancer) were excluded.
Search strategy
Two authors (D.P., J.T.) performed all searches in MEDLINE, EMBASE, and Cochrane databases. For all databases, articles published from inception to February 1, 2020, were searched using a comprehensive search strategy, found in Appendix 1, available from CFPlus.* Additionally, a gray literature search was performed using trial registries and Cochrane systematic reviews.9,10
Outcomes
The primary outcome was the proportion of patients who responded to treatment, generally considered as the number of patients who achieved at least a 30% reduction in pain or a combination of pain reduction and functional improvement. In trials that reported multiple responder outcomes, we used a hierarchy to prioritize outcomes. The hierarchy of responder outcomes can be found in Appendix 2, available from CFPlus (Table A1).* Secondary outcomes included serious adverse events, withdrawals owing to adverse events, and specific adverse events associated with each intervention.
Data collection and analysis
Selection of trials and data extraction. The titles and abstracts of each systematic review were independently screened by 2 authors; full-text articles were then reviewed to determine inclusion. Two authors independently extracted relevant data from trials following Methodological Expectations of Cochrane Intervention Reviews.11 Disagreements were resolved by consensus or by consulting a third author.
Risk-of-bias assessment. Two authors independently assessed all included trials for potential bias using the Cochrane Collaboration’s risk-of-bias tool.12 Because of the subjective nature of pain, we split blinding into blinding of participants and blinding of trial personnel. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool to report the certainty of the evidence.13
Synthesis
Data synthesis
Using RevMan 5 software,14 we performed meta-analyses for interventions that had at least 2 included trials. If multiple time points were reported, we reported the longest time frame during which the intervention was given to calculate the point estimate for overall efficacy. An exploratory analysis was done to determine whether any intermittent interventions (eg, exercise, SMT, acupuncture, corticosteroid injections) had effects lasting after the intervention was completed. Owing to paucity of data on adverse events, we reported adverse events for any intervention, irrespective of the number of trials included.
We decided a priori to perform additional analyses to explore whether the funding source (industry or publicly funded), duration of outcome reported (≤ 4 weeks, > 4 and < 12 weeks, and ≥12 weeks), or trial quality affected our findings for interventions that had at least 8 included trials. Because of the likelihood of variability in patient populations, interventions, and outcomes reported, we used a random-effects method to analyze our findings, but we also used a fixed-effects method as a sensitivity analysis. We attempted to explore potential sources of heterogeneity and other markers of quality between trials regardless of the number of included trials. Finally, we explored potential publication bias in interventions with more than 8 included trials through funnel plots and included these results in our GRADE analysis.
Results
A total of 38 599 records were retrieved from our searches for the 15 treatments. After excluding duplicates and reviewing titles and abstracts, 847 publications were selected for full-text review, with 63 trials meeting our inclusion criteria. For full details about the included trials, see Appendix 1, available from CFPlus.*
Eight interventions (exercise, acupuncture, SMT, oral NSAIDs, rubefacients, opioids, SNRIs [duloxetine], and corticosteroid injections) had 2 or more trials that could be meta-analyzed for efficacy. Only exercise, acupuncture, and corticosteroid injections had 8 or more studies and were initially analyzed by funding source, duration of outcome reported, and quality of included studies. Anticonvulsants and topical NSAIDs each had only 1 included RCT. Acetaminophen, cannabinoids, muscle relaxants, SSRIs, and TCAs had no trials that fulfilled our inclusion criteria.
Treatments with 2 or more RCTs (ordered by certainty of evidence and relative risk)
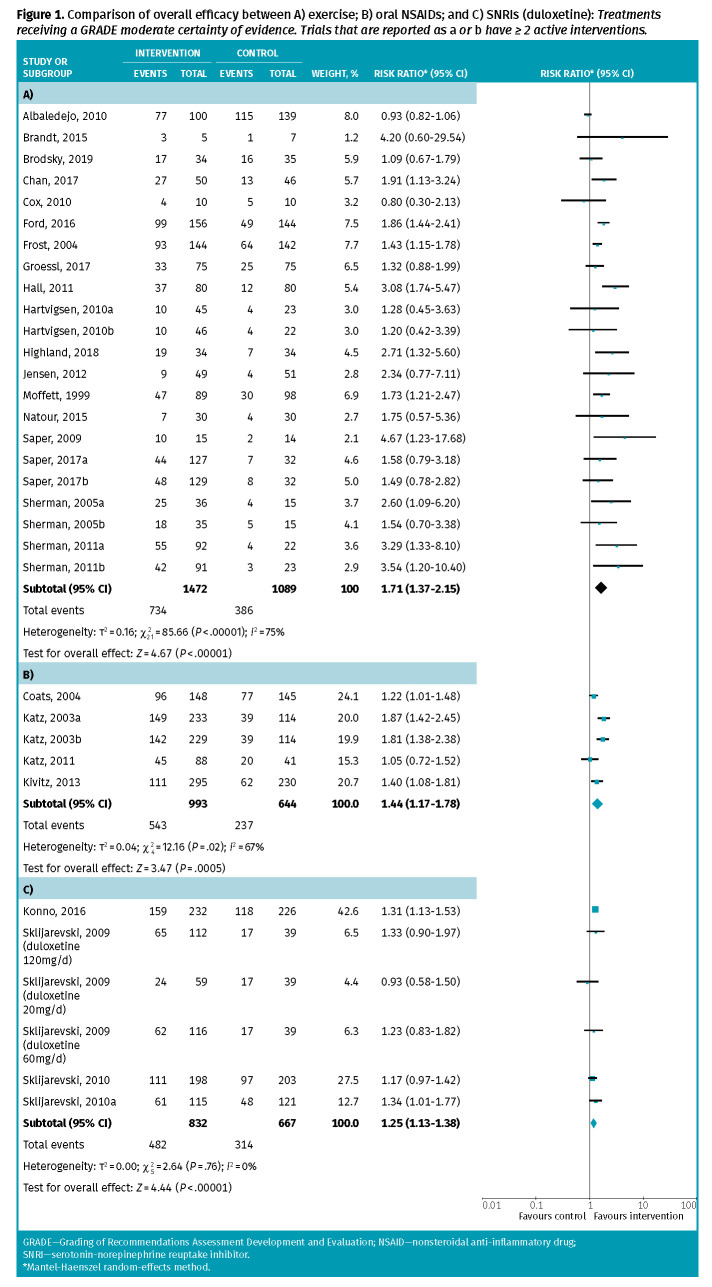
Exercise. Eighteen RCTs with 2561 patients followed for 6 to 52 weeks were included. Exercise interventions were most commonly physiotherapy-guided exercise programs, but also included yoga, pilates, tai chi, and Nordic walking. Meta-analysis revealed 50% of patients receiving exercise and 35% receiving placebo attained meaningful pain relief (risk ratio [RR] of 1.71; 95% CI 1.37 to 2.15; number needed to treat [NNT] of 7; Table 1 and Figure 1). The primary and subgroup meta-analyses are in Appendix 2, available from CFPlus (Table A2).*
Table 1.
Overall proportion of patients with meaningful response to treatment: Ordered by certainty of evidence and then highest to lowest risk ratios.
| GRADE CERTAINTY OF EVIDENCE | INTERVENTION TYPE | NO. OF RCTS* | INTERVENTION EVENT RATE, % (n/N) | CONTROL EVENT RATE, % (n/N) | TIME FRAME | RISK RATIO (95% CI) |
|---|---|---|---|---|---|---|
| Moderate | Exercise | 18 | 50 (734/1472) | 35 (386/1089) | 6 to 52 wk | 1.71 (1.37 to 2.15) |
| Oral NSAIDs | 4 | 55 (543/993) | 37 (237/644) | 4 to 16 wk | 1.44 (1.17 to 1.78) | |
| SNRIs (duloxetine) | 4 | 58 (482/832) | 47 (314/667) | 12 to 13 wk | 1.25 (1.13 to 1.38) | |
| Low | Spinal manipulation therapy | 5 | 57 (199/349) | 39 (132/337) | 2 to 12 wk | 1.54 (1.11 to 2.12) |
| Rubefacients | 3 | 64 (195/304) | 46 (142/307) | 3 wk | 1.39 (1.20 to 1.61) | |
| Very low | Acupuncture | 8 | 54 (1320/2457) | 35 (754/2161) | 4 to 24 wk | 1.58 (1.13 to 2.21) |
| Opioids | 6 | 39 (660/1712) | 32 (318/996) | 4 to 12 wk | 1.26 (1.02 to 1.55) | |
| Corticosteroid injections | 10 | 48 (276/581) | 45 (257/571) | 4 to 104 wk | 1.07 (0.87 to 1.30) |
GRADE—Grading of Recommendations Assessment, Development and Evaluation; NSAID—nonsteroidal anti-inflammatory drug; RCT—randomized controlled trial; SNRI—serotonin-norepinephrine reuptake inhibitor.
Total number of RCTs is 63. Exercise and acupuncture have additional trials that do not report responder outcome data during the intervention period. Additionally, topical NSAIDs and anticonvulsants each have 1 study. Event rates for intervention and controls were calculated by meta-analyzing the responder outcome for the longest time frame in which the intervention was given.
Figure 1.
Comparison of overall efficacy between A) exercise; B) oral NSAIDs; and C) SNRIs (duloxetine): Treatments receiving a GRADE moderate certainty of evidence. Trials that are reported as a or b have ≥ 2 active interventions.
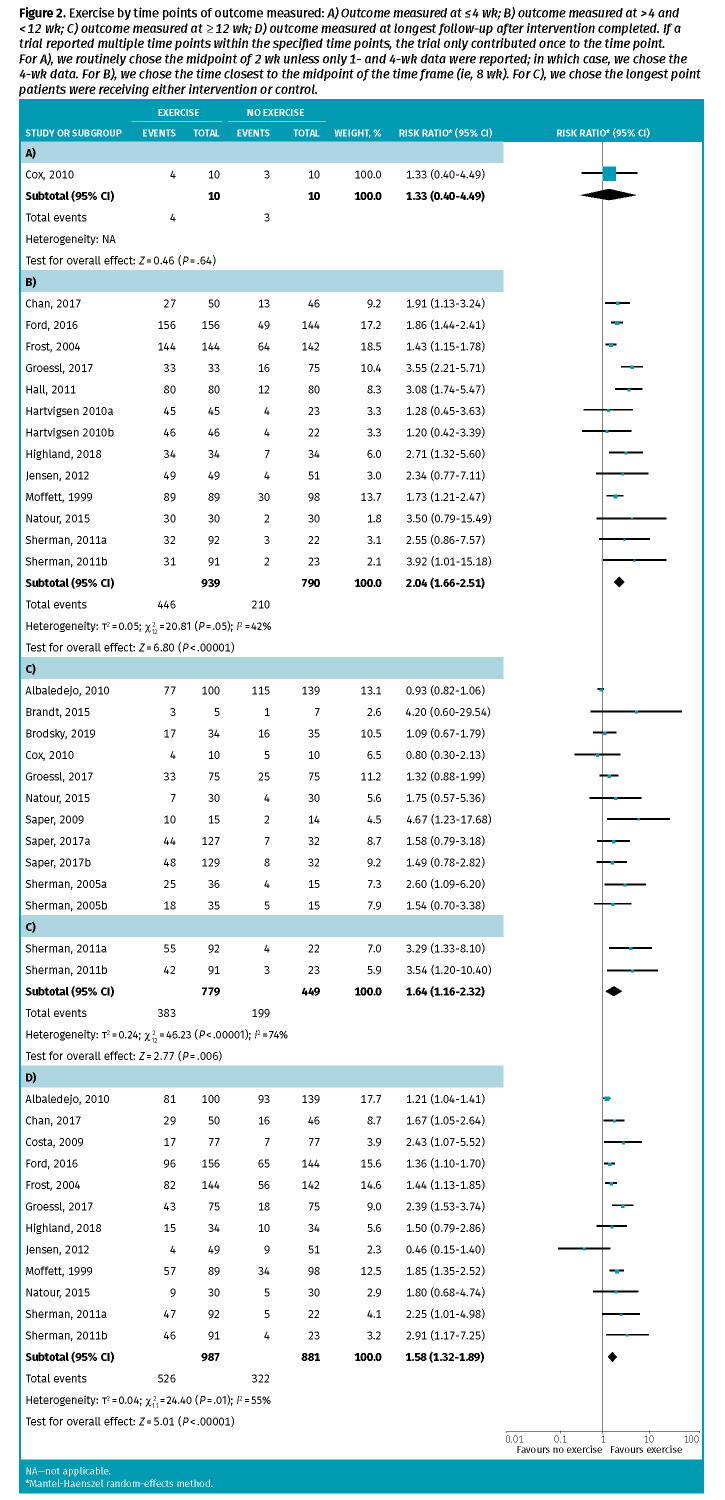
Analysis by time demonstrated a statistically significant benefit in trials reporting an outcome of more than 4 to less than 12 weeks (RR = 2.04; 95% CI 1.66 to 2.51; NNT = 5) and an outcome of 12 weeks or more (RR = 1.64; 95% CI 1.16 to 2.32; NNT = 21; Figure 2). Patients who exercised continued to attain meaningful pain relief 12 to 48 weeks after the intervention period (RR = 1.58; 95% CI 1.32 to 1.89; NNT = 6). A subgroup analysis of trial quality found no significant effect of quality on outcomes and all trials that reported funding were publicly funded. The primary and subgroup meta-analyses are in Appendix 2, available from CFPlus (Tables A5 and A6).*
Figure 2.
Exercise by time points of outcome measured: A) Outcome measured at ≤ 4 wk; B) outcome measured at > 4 and < 12 wk; C) outcome measured at ≥ 12 wk; D) outcome measured at longest follow-up after intervention completed. If a trial reported multiple time points within the specified time points, the trial only contributed once to the time point. For A), we routinely chose the midpoint of 2 wk unless only 1- and 4-wk data were reported; in which case, we chose the 4-wk data. For B), we chose the time closest to the midpoint of the time frame (ie, 8 wk). For C), we chose the longest point patients were receiving either intervention or control.
Reported adverse events were similar in each arm and withdrawals due to adverse events were not reported in any trial (Table 2). Adverse events that could be retrieved from meta-analyses in systematic reviews are in Appendix 2, available from CFPlus (Table A7).*
Table 2.
Withdrawals due to adverse effects
| INTERVENTION TYPE | NO. OF RCTS | INTERVENTION EVENT RATE, % (n/N) | CONTROL EVENT RATE, % (n/N) | RISK RATIO (95% CI) |
|---|---|---|---|---|
| Opioids | 6 | 27 (461/1726) | 5 (50/1011) | 4.41 (3.30 to 5.91) |
| SNRIs (duloxetine) | 1 | 18 (53/287) | 9 (10/117) | 2.02 (1.06 to 3.87) |
| Oral NSAIDs | 4 | 4 (37/993) | 3 (20/644) | 1.36 (0.53 to 3.51) |
| Anticonvulsants | 1 | 13 (7/55) | 9 (5/53) | 1.35 (0.46 to 3.99) |
| Acupuncture | 1 | 3 (1/40) | 0 (0/40) | 3.00 (0.13 to 71.51) |
| Exercise, rubefacients, spinal manipulation therapy, corticosteroid injections, topical NSAIDs | Not reported in any trials | NA | NA | NA |
NA—not applicable, NSAID—nonsteroidal anti-inflammatory drug, RCT—randomized controlled trial, SNRI—serotonin-norepinephrine reuptake inhibitor.
Oral NSAIDs. Four RCTs with 1637 patients followed for 4 to 16 weeks were included. Meta-analysis revealed 55% of patients receiving oral NSAIDs and 37% receiving placebo attained meaningful pain relief (RR = 1.44; 95% CI 1.17 to 1.78; NNT = 6). Withdrawals due to adverse events and individual adverse events were similar between the groups.
Serotonin-norepinephrine reuptake inhibitors (duloxetine only). Four RCTs with 1499 patients followed for 12 to 13 weeks were included (all trials examining duloxetine). Meta-analysis revealed 58% of patients receiving duloxetine and 47% receiving placebo attained meaningful pain relief (RR = 1.25; 95% CI 1.13 to 1.38; NNT = 10). One RCT found withdrawals due to adverse events occurred in 18% of patients taking duloxetine compared to 9% taking placebo (RR = 2.02; 95% CI 1.06 to 3.87; number needed to harm [NNH] of 11). Reported adverse events that were more common than those reported from placebo were dizziness (NNH = 23), nausea (NNH = 10), and somnolence (NNH = 9).
Spinal manipulation therapy. Five RCTs with 686 patients followed for 2 to 12 weeks were included. Meta-analysis revealed 57% of patients receiving SMT and 39% receiving placebo attained meaningful pain relief (RR = 1.54; 95% CI 1.11 to 2.12; NNT = 6). One trial did not find sustained benefit 42 weeks after SMT completion. Reported adverse events were similar in each arm and withdrawals due to adverse events were not reported in any trials. The primary and subgroup meta-analyses are in Appendix 2, available from CFPlus (Table A4).*
Rubefacients (capsaicin only). Three RCTs with 611 patients followed for only 3 weeks were included (all trials examining capsaicin). Meta-analysis revealed 64% of patients receiving capsaicin and 46% receiving placebo attained meaningful pain relief (RR = 1.39; 95% CI 1.20 to 1.61; NNT = 6). More patients treated with capsaicin reported heat sensation than those treated with placebo (RR = 2.10; 95% CI 1.73 to 2.56; NNH = 3). Withdrawals due to adverse events were not reported. The primary and subgroup meta-analyses are in Appendix 2, available from CFPlus (Figure A13.1).*
Acupuncture. Eight RCTs with 4618 patients followed for 4 to 24 weeks were included. Meta-analysis revealed 54% of patients receiving acupuncture and 35% receiving placebo attained meaningful pain relief (RR = 1.58; 95% CI 1.13 to 2.21; NNT = 6).
Analysis by time demonstrated that the benefit of acupuncture was no longer significant when data from more than 4 weeks to less than 12 weeks (RR = 1.26; 95% CI 0.99 to 1.62), and from 12 weeks or more (RR = 1.49; 95% CI 0.75 to 2.98) were analyzed. Two acupuncture trials did not find any long-term benefit 8 to 45 weeks after the intervention completion. Similarly, higher-quality trials did not find acupuncture to be more beneficial than placebo (RR = 1.22; 95% CI 0.97 to 1.55). The primary and subgroup meta-analyses are in Appendix 2, available from CFPlus (Tables A3, A4, and A6).* All trials that reported funding were publicly funded; the data are in Appendix 2, available from CFPlus (Figure A2.6).*
Reported adverse events were similar in each arm. Withdrawals due to adverse events were reported in 1 trial and were similar between acupuncture and sham acupuncture. Adverse event data can be found in Appendix 2, available from CFPlus (Table A7).*
Opioids. Six RCTs with 2708 patients followed for 4 to 12 weeks were included. Meta-analysis revealed 39% of patients receiving opioids and 32% receiving placebo attained meaningful pain relief (RR = 1.26; 95% CI 1.02 to 1.55; NNT = 16). Withdrawals due to adverse events occurred in 27% of patients receiving opioids and in 5% of patients receiving placebo (RR = 4.41; 95% CI 3.30 to 5.91; NNH = 5; Table 2). Individual adverse events, which were more frequent in opioid users, included nausea (NNH = 6), dizziness (NNH = 7), somnolence (NNH = 8), constipation (NNH = 9), and vomiting (NNH = 9). Adverse event data can be found in Appendix 2, available from CFPlus (Table A7).*
Corticosteroid injections. Ten RCTs with 1152 patients followed for 4 to 104 weeks were found. Meta-analysis revealed 48% of patients receiving corticosteroid injections and 45% receiving placebo attained meaningful pain relief (RR = 1.07; 95% CI 0.87 to 1.30). Subgroup analyses (funding, time, and quality) did not change the results, and all trials that reported funding were industry funded. Individually reported adverse events were similar to those in the control groups and withdrawals due to adverse events were not reported in any trial. The primary and subgroup meta-analyses are in Appendix 2, available from CFPlus (Figures A8.2 to A8.5).*
Treatments with 1 RCT
Anticonvulsants. One RCT of gabapentin with 108 patients was found. At 12 weeks, 22% of patients taking gabapentin achieved 30% or greater improvement in pain, compared to a 26% improvement in pain in those taking placebo. There was no statistical difference between the treatments (P = .6). Withdrawals due to adverse events were comparable between gabapentin and placebo (13% vs 9%; Table 2). Fatigue (NNH = 5), dry mouth (NNH = 5), decreased concentration (NNH = 4), and loss of balance (NNH = 4) were more frequent with gabapentin. Adverse event data can be found in Appendix 2, available from CFPlus (Table A7).*
Topical NSAIDs. One RCT randomized 127 patients to either flurbiprofen tape or placebo tape. At 1 week, 62% of patients wearing flurbiprofen tape reported feeling much improved, compared to 52% wearing placebo tape. There was no statistical difference between the treatments (P = .30). Individual adverse events, including erythema and irritation at the application site, were not different between groups.
Treatments with no identified RCTs
No RCTs of acetaminophen, cannabinoids, muscle relaxants, SSRIs, or TCAs met our inclusion criteria.
Quality assessment
Assessments for risk of bias are provided in Appendix 2, available from CFPlus (Tables A8.1 to A8.7).* General concerns included short treatment time frames for rubefacients, opioids, SMT, and duloxetine; suboptimal controls, such as wait lists, that would not provide any expectation of benefit (nonpharmacologic interventions); and many small trials, which can magnify the effect estimate in a random-effects analysis (which are most analyses). The GRADE certainty of evidence varied from moderate to very low for included interventions and reasons for downgrading are fully reported in Appendix 2, available from CFPlus (Table A9).* Heterogeneity of trials (reported by I2 statistic) for overall efficacy ranged from 0% (rubefacients, duloxetine) to 94% (acupuncture). Heterogeneity in nonpharmacologic treatments (acupuncture [I2 = 94%], exercise [I2 = 75%], and SMT [I2 = 40%]) might be derived, in part, from differences in the credibility of the control as a treatment alternative. A sham procedure, for instance, would be expected to provide a greater placebo response than a wait list control or educational materials.15 To evaluate this, we separated trials into those where control had a higher expectation of benefit (eg, sham), and those where control had a lower expectation of benefit (eg, wait list). When sham treatments were the comparator for acupuncture and SMT, the relative benefit was substantially lower (RR = 1.25, 95% CI 1.02 to 1.54, and RR = 1.35, 95% CI 1.14 to 1.59, respectively); the primary and subgroup meta-analyses can be found in Appendix 2, available from CFPlus (Figures A10.2 and A10.3).* To further explore sources of heterogeneity, we expanded our subgroup analyses to the evaluation of all interventions, not just those with 8 or more eligible trials (ie, subgroup analyses based on treatment duration, risk-of-bias, funding, and trial size). The primary and subgroup meta-analyses can be found in Appendix 2, available from CFPlus (data analysis, various figures).*
Discussion
This synthesis of 15 systematic reviews evaluated the effectiveness of commonly used interventions in primary care for chronic radicular or nonradicular low back pain. We found moderate certainty of evidence that exercise, oral NSAIDs, and SNRIs (duloxetine) provide clinically meaningful benefit to patients with chronic back pain. There was low certainty of evidence supporting SMT and rubefacients (studies were limited by short time frames and had limited ability to blind patients and providers). There was very low certainty of evidence in support of opioids and acupuncture (where significance was lost in an evaluation of higher-quality trials and trials with a duration > 4 weeks). For corticosteroid injections, very low-quality evidence suggested this treatment was ineffective. Single trials of anticonvulsants and topical NSAIDs found similar results to placebo. Although exercise, SMT, and acupuncture reported outcomes after interventions had concluded, only exercise found sustained benefit (for up to 48 weeks).
Compared to placebo, a greater proportion of patients receiving opioids and duloxetine discontinued treatment owing to adverse events. Patients treated with opioids were more likely to discontinue treatment owing to an adverse event (NNH = 5) than continue treatment to derive short-term, clinically meaningful benefit (NNT = 16). Those treated with duloxetine had a similar likelihood of continuing treatment to derive benefit (NNT = 10) as those discontinuing the medication owing to an adverse event (NNH = 11).
For nonpharmacologic interventions, the relative benefit is likely influenced by the choice of control. When compared to controls with a higher expectation of benefit (ie, sham procedure), the relative benefit of acupuncture and SMT is diminished by about 20% to 30%. This is consistent with other evidence that finds sham procedures have greater responses than inert pills.16,17 Additionally, larger estimates are also found in studies where participants are not blinded.15
Numerous systematic reviews evaluating treatments for low back pain exist, mostly evaluating single interventions. Only a few evaluate multiple different pharmacologic and nonpharmacologic interventions.18,19 More of these multiple-intervention reviews are expected to be published in the future.20 Our systematic review was the first synthesis of multiple systematic reviews (N = 15) for chronic low back pain interventions that was led by primary care, reported outcomes through responder analysis, and included robust reporting of adverse events.
Strengths and limitations
A strength of this review is its scope, weaving together 15 separate systematic reviews of differing interventions for chronic low back pain. A limitation is our decision to combine potentially heterogeneous interventions into one intervention category. For example, corticosteroid injection trials could include single or multiple injections and these injections could have been placed in different anatomical sites, but were analyzed collectively. We believe this was the most appropriate method of analyzing 15 different interventions. It will be interesting to see if future reviews that group treatments within nonpharmacologic (eg, different types of exercise) or pharmacologic interventions (eg, different classes of NSAIDs) find consistent or inconsistent and confusing results.20 Restricting eligible trials to those with a responder analysis limits this review because only 20% of chronic low back pain RCTs report these outcomes21; however, it allowed us to combine trials that used different pain measures, by using counts of responders, without losing clinical meaning. Changes on a pain scale, or their combination into standard mean differences, are challenging to interpret and do not translate easily in a patient conversation.
Future research
Given that we did not find any trials meeting our inclusion criteria for acetaminophen, cannabinoids, muscle relaxants, SSRIs, or TCAs, future RCTs are needed for these interventions. To facilitate the combination of trials in meta-analyses, and to improve patient discussions and shared decision making, future RCTs evaluating interventions for chronic low back pain should consider reporting a responder analysis in addition to full details about changes in pain scales. Finally, to better account for the effect of blinding, expectation bias, and true placebo response, future meta-analyses should consider doing subgroup analyses based on the nature of the control—in particular, the likely expectation of benefit the comparator provides to the participant.
Conclusion
There is moderate certainty of evidence that exercise, oral NSAIDs, and SNRIs (duloxetine) provide a clinically meaningful reduction in pain to those with chronic low back pain. There is low certainty of evidence to support SMT and rubefacients providing benefit, and very low certainty of evidence to support acupuncture and opioids. There is also very low certainty of evidence suggesting corticosteroid injections are not helpful. Patients treated with duloxetine or opioids were as likely, or more likely, to discontinue these medications owing to adverse events than to continue using these medications to derive benefit. Future high-quality pragmatic trials, rooted in primary care and reporting a responder analysis, are key to providing a better understanding of the relative benefits and harms of interventions for patients with chronic low back pain.
Supplementary Material
Acknowledgment
We thank Janice Kung, MLIS, for her assistance with creating the search strategy, our peer reviewers for their valuable feedback, and the CFPC, Ontario College of Family Physicians, and Alberta College of Family Physicians for their continued support. This project was partially funded by Alberta Health through the Primary Health Care Opioid Response Initiative
Editor’s key points
▸ Low back pain is a common reason for patients to visit family physicians. Chronic low back pain affects one’s quality of life, and can lead to substantial health care costs. While there are many interventions for low back pain, the benefits and harms of these interventions need to be more concretely defined to translate the benefits and harms into patient conversation.
▸ There is moderate certainty of evidence that exercise, oral nonsteroidal anti-inflammatory drugs, and serotonin-norepinephrine reuptake inhibitors (duloxetine) provide a clinically meaningful reduction in pain to those with chronic low back pain. There is low certainty of evidence to support spinal manipulation therapy and rubefacients providing benefit, and very low certainty of evidence to support acupuncture and opioids. There is also very low certainty of evidence suggesting corticosteroid injections are not helpful.
▸ Findings of this systematic review were used to develop a clinical decision aid (page 32). This systematic review is one in a series that will inform guidelines on pain treatment in primary care.
Points de repère du rédacteur
▸ La lombalgie est un problème qui motive souvent les patients à consulter leur médecin de famille. La lombalgie chronique nuit à la qualité de vie, et peut entraîner des coûts considérables en soins de santé. Même s’il existe de nombreuses interventions pour la lombalgie, il est nécessaire de définir plus concrètement leurs bienfaits et leurs préjudices afin de mieux les expliquer au cours des conversations avec les patients.
▸ Des données probantes modérément sûres font valoir que l’exercice, les anti-inflammatoires non stéroïdiens et les inhibiteurs de la recapture de la sérotonine-norépinéphrine (duloxétine) procurent une réduction cliniquement significative de la douleur chez les personnes souffrant de lombalgie chronique. Des données probantes peu sûres indiquent que les traitements par manipulation de la colonne et les rubéfiants apportent des bienfaits, et des données probantes très peu sûres appuient l’acupuncture et les opioïdes. Certaines données très peu sûres donnent aussi à croire que les injections de corticostéroïdes ne sont pas utiles.
▸ Les constatations de cette revue systématique ont servi à élaborer une aide décisionnelle clinique (page e17). Cette revue systématique compte parmi d’autres qui serviront à éclairer des lignes directrices sur le traitement de la douleur en soins primaires.
Footnotes
The comprehensive search strategy (Appendix 1), the hierarchy of responder outcomes, primary and subgroup meta-analyses, trial funding data, adverse event data, and assessments for risk of bias (Appendix 2) are available at www.cfp.ca. Go to the full text of the article online and click on the CFPlus tab.
Contributors
All authors were part of the Evidence Review Team and contributed to preparing the manuscript for submission.
Competing interests
None declared.
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.Finley CR, Chan DS, Garrison S, Korownyk C, Kolber MR, Campbell S, et al. . What are the most common conditions in primary care? Systematic review. Can Fam Physician 2018;64:832-40. [PMC free article] [PubMed] [Google Scholar]
- 2.Costa Lda C, Maher CG, McAuley JH, Hancock MJ, Herbert RD, Refshauge KM, et al. . Prognosis for patients with chronic low back pain: inception cohort study. BMJ 2009;339:b3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Health Canada Chronic pain in Canada: laying a foundation for action. A report by the Canadian Pain Task Force, June 2019. Ottawa, ON: Government of Canada; 2019. Available from: https://www.canada.ca/content/dam/hc-sc/documents/corporate/about-health-canada/public-engagement/external-advisory-bodies/canadian-pain-task-force/report-2019/canadian-pain-task-force-June-2019-report-en.pdf Accessed 2020 Dec 8. [Google Scholar]
- 4.Dieleman JL, Cao J, Chapin A, Chen C, Li Z, Liu A, et al. . US health care spending by payer and health condition, 1996-2016. JAMA 2020;323(9):863-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ton J, Perry D, Thomas B, Allan GM, Lindblad AJ, McCormack J, et al. . PEER umbrella systematic review of systematic reviews. Management of osteoarthritis in primary care. Can Fam Physician 2020;66:e89-98. Available from: https://www.cfp.ca/content/cfp/66/3/e89.full.pdf Accessed 2021 Jan 4. [PMC free article] [PubMed] [Google Scholar]
- 6.Moore A, Derry S, Eccleston C, Kalso E.. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. Epub 2009 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith V, Devane D, Begley CM, Clarke M.. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol 2011;11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institutes of Health U.S. National Library of Medicine. Bethesda, MD: National Institutes of Health; 2020. Available from: clinicaltrials.gov Accessed 2020 May 6. [Google Scholar]
- 10.World Health Organization International clinical trials registry platform (ICTRP). Geneva, Switz: World Health Organization; 2020. Available from: https://www.who.int/clinical-trials-registry-platform Accessed 2020 Dec 8. [Google Scholar]
- 11.Higgins JPT, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al. . Methodological Expectations of Cochrane Intervention Reviews (MECIR). London, UK: The Cochrane Collaboration; 2020. Available from: https://community.cochrane.org/sites/default/files/uploads/Version%20March%202020%20Final%20Online%20version.pdf Accessed 2020 Dec 18. [Google Scholar]
- 12.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. . GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64(4):401-6. Epub 2011 Jan 5. [DOI] [PubMed] [Google Scholar]
- 14.Review Manager (RevMan), version 5.3 [software]. Copenhagen, Den: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 15.Howick J, Hoffmann T.. How placebo characteristics can influence estimates of intervention effects in trials. CMAJ 2018;190(30):E908-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaptchuk TJ, Stason WB, Davis RB, Legedza ATR, Schnyer RN, Kerr CE, et al. . Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ 2006;332(7538):391-7. Epub 2006 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaptchuk TJ, Hemond CC, Miller FG.. Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. BMJ 2020;370:m1668. [DOI] [PubMed] [Google Scholar]
- 18.Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al. . Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med 2017;166(7):493-505. Epub 2017 Feb 14. [DOI] [PubMed] [Google Scholar]
- 19.Chou R, Deyo R, Friedly J, Skelly A, Weimer M, Fu R, et al. . Systemic pharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med 2017;166(7):480-92. Epub 2017 Feb 14. [DOI] [PubMed] [Google Scholar]
- 20.Thompson T, Dias S, Poulter D, Weldon S, Marsh L, Rossato C, et al. . Efficacy and acceptability of pharmacological and non-pharmacological interventions for non-specific chronic low back pain: a protocol for a systematic review and network meta-analysis. Syst Rev 2020;9(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henschke N, van Enst A, Froud R, Ostelo RW.. Responder analyses in randomised controlled trials for chronic low back pain: an overview of currently used methods. Eur Spine J 2014;23(4):772-8. Epub 2014 Jan 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.