Abstract
Translational neuroscience bridges insights from specific mechanisms in rodents to complex functions in humans and is key to advance our general understanding of central nervous function. A prime example of translational research is the study of cross-species mechanisms that underlie responding to learned threats, by employing Pavlovian fear conditioning protocols in rodents and humans. Hitherto, evidence for (and critique of) these cross-species comparisons in fear conditioning research was based on theoretical viewpoints. Here, we provide a perspective to substantiate these theoretical concepts with empirical considerations of cross-species methodology. This meta-research perspective is expected to foster cross-species comparability and reproducibility to ultimately facilitate successful transfer results from basic science into clinical applications.
INTRODUCTION
In the biomedical research enterprise, the term “translation” often decorates review articles, original publications and grant applications. It promises, for instance, the elegant transfer of mechanisms in rodents to complex human functions and vice versa. In behavioural neuroscience, specifically, translation entails the mapping of synapse-specific processes in animal models onto neurobiological systems in humans. However, the potential of translational research comes with major challenges, most importantly methodological disparities that are inherent to experimental protocols in non-human animals and humans. Consideration of such methodological disparities has been suggested to aid potential translation of findings in biomedical sciences (Freedman et al., 2015; Kola and Landis, 2004; Macleod et al., 2014). Here, we provide such considerations for fear conditioning research across species.
Although translational work in neuroscience is challenging, there have been some successes. For example, decades of research on the basic mechanisms of emotional learning and memory in animals has yielded significant insight into novel therapeutic approaches for clinical disorders of fear and anxiety in humans (e.g.,(Fenster et al., 2018; Griebel and Holmes, 2013, p. 50; Mataix-Cols et al., 2017; Ressler et al., 2004; Singewald et al., 2015; Walker et al., 2002). Indeed, one form of emotional learning -Pavlovian fear conditioning- has been promoted as the prime example of a “translational” paradigm (Kandel et al., 2014; Milad and Quirk, 2012). Although the importance of translational work has been emphasised from a theoretical perspective, the practical implementation of translational research in fear conditioning experiments has been neglected so far. While translation of findings is often not the primary goal in fear conditioning experiments, translational implications are often implied without further considering how and if such promises can be met in practice. We argue that is important to consider the impact of methodological differences on translational research in rodents and humans in order to provide orientation how to evaluate translational research in practice.
Here, we will compile the practical, methodological considerations for cross-species research in Pavlovian fear conditioning, derived from discussion across ten different international labs working on fear conditioning in rodents or humans. To this end, we aim to initiate a discussion on cross-species challenges and provide methodological consideration for translational research that we expect to ultimately advance progress in fear conditioning research. We also intend to go beyond theoretical ideas of translation by informing mechanistic insights across species with evidence-based methodological comparison to ultimately foster translational progress.
Pavlovian fear conditioning - a simple protocol?
Across species, learning to identify and predict danger is key for survival. Animals, including humans, are equipped with an evolutionary-conserved (neuro)physiological machinery that mobilizes defensive responses to mitigate current threats as well as mediates learning to anticipate future threats (Blanchard, 2017; Blanchard, Blanchard, & Hori, 1989; Fanselow, 1994; LeDoux, 2012; Maren, 2001). Theoretically, these conserved circuits allow for the examination of the behavioural and biological basis of learned threat responses in rodents (such as rats and mice) to infer how these processes operate in humans.
The study of learned defensive behavior has relied upon Pavlovian fear conditioning, a fundamental form of associative learning that exhibits similar properties in a range of species, from rodents to humans. In the laboratory, Pavlovian fear conditioning procedures typically involve several phases, including acquisition training, extinction training and retrieval tests of learned responses (Lonsdorf et al., 2017). In brief, during acquisition training, individuals learn to associate a distinct cue (conditioned or conditional stimulus, CS+) or a whole context (conditioned or conditional context, CXT+) with the occurrence of an aversive event (unconditioned or unconditional stimulus, US). As a result, the presence of the CS+ or CXT+ elicits a conditioned or conditional response (CR), which manifests as a number of physiological responses and species-specific defensive reactions. Collectively, these diverse CRs are often termed “fear” CRs, as a useful way to aggregate a number of different defensive responses (freezing, tachycardia, hypertension, sweating, etc.). Although it is not known whether non-human animals experience a subjective state of fear (e.g., LeDoux, 2014), there are clearly similarities in the nature of the CRs to aversive CSs across mammalian species and these responses likely reflect a central state of fear (whether experienced subjectively or not; Fanselow & Pennington, 2018). During a subsequent extinction training phase, the CS+ or CXT+ is presented without the US, which leads to a decrease in the magnitude and/or frequency of the CR. The expression (and inhibition) of CRs can be probed during retrieval tests.
Although Pavlovian fear conditioning procedures can be performed in both rodents and humans, translation of results across species is challenged by inherently different procedural and methodological instantiations of Pavlovian fear conditioning protocols in rodents and humans. In the following, we describe these differences and their relevance to cross-species comparisons between rodents (here restricted to mice and rats) and humans. In particular, we aim to raise awareness on how methodological differences might impact interpretation of results, in order to promote valid cross-species comparisons of existing findings, as well as to improve translational designs of future studies. The overarching aim of this overview is to equip readers from diverse backgrounds with a basic set of tools to evaluate cross-species methods employed in fear conditioning research to enable them to correctly interpret findings. We further provide advice how to consider and overcome cross-species methodological differences to utilize the full potential of translational research in fear conditioning. First, we discuss and compare key elements entailed in the fear conditioning paradigm across both species. Second, we describe the most important dependent measures within each species and third, comment on individual differences. Detailed “what to consider” key-points summarizing the key take-home messages provide practical guidelines for the reader.
PARADIGM
While this description of the fear conditioning paradigm sounds straightforward, the specific methodological instantiation (e.g., which stimulus types for the CS, CXT, US are used; timing of acquisition and extinction) can heavily impact the outcomes, as well as comparability across species. Hence, consideration of methodological details of species-specific protocols is central to inform cross-species comparability of results. Here, we will compare essential elements of fear conditioning paradigms that are shared across species (e.g., CS, CXT, US) or species-specific elements (e.g., instruction of human participants).
Conditioned stimuli (CS): cues and control conditions
Cues of different modalities have successfully been employed as CSs across species. In rodents, auditory cues are typically employed (for an overview see Wotjak, 2018), whereas in humans, visual cues are common (for a comparison of visual and auditory CSs on fear-potentiated startle see Norrholm et al., 2011 and for a discussion see Lonsdorf et al., 2017). Despite inherent differences in underlying sensory processing, learning-related mechanisms are considered comparable (Delgado, Olsson, & Phelps, 2006; Maren, 2001; Whalen & Phelps, 2009; cf. Bergstrom and Johnson, 2014; Tazumi and Okaichi, 2002). Typically, stimuli that are neutral prior to fear acquisition training are used as CSs, but in some cases stimuli that inherently signal threat-relevant information have been employed. Importantly, threat-relevant cues can be species-specific, including pictures of angry facial expressions in humans (Mineka and Ohman, 2002; Öhman and Dimberg, 1978) or contact with conspecifics in animals (Toth et al., 2012; Toth and Neumann, 2013).
Fear conditioning paradigms typically include control conditions to discriminate associative from non-associative processes (such as habituation, dishabituation and sensitization, see (Rescorla, 1967). These control conditions, however, often consist of different procedures in rodents and humans. In rodent work, single-cue protocols with a single CS+ are typically employed that include a separate control group in a between-subject design. Typical control groups either receive the same number of CS and US presentations as the experimental group that are however either explicitly unpaired (Pearce and Dickinson, 1975; Sevelinges et al., 2007) or presented randomly (i.e., ‘truly random control; e.g., Barnet & Hunt, 2005; Jüngling et al., 2015; Rescorla, 1967; Rogan, Stäubli, & LeDoux, 1997) or CSs are presented in the absence of any US (CS alone paradigm Do-Monte, Quiñones-Laracuente, & Quirk, 2015; Maren, 2001; Rescorla, 1967). In research in humans, fear conditioning is typically conducted with differential protocols, in which a CS− that is not paired with the US is presented interleaved among paired CS+/US trials in a within-subject design (see Norrholm et al., 2008 as an example for the use of single- and differential-cue prototcols in humans). Additionally, another common within-subject condition in human experiments that focus on extinction training includes two CS+, one of which is presented during extinction training (CS+E) while the other CS+ is not (CS+U) and serves as an unextinguished control (Milad et al., 2007; Milad et al., 2009; Zeidan et al., 2011).
Importantly, control conditions used in animals and humans need to be interpreted with caution, since these controls may not always function as unambiguous neutral conditions (as discussed already by Lissek et al., 2005; Lonsdorf et al., 2017; Rescorla, 1967; Wotjak, 2018). Control conditions including explicitly unpaired CS/US presentation in single-cue protocols for example can increase the salience of the context and may allocate safety signal properties to the unpaired cue (Baas and Heitland, 2015; Heitland et al., 2016; Norrholm et al., 2008; Rogan et al., 2005; Tang et al., 2001; Wotjak, 2018). Similarly, the CS− in differential protocols may also acquire safety signal properties (Gerber et al., 2014; Kong, Monje, Hirsch, & Pollak, 2014; Lohr, Olatunji, & Sawchuk, 2007; Rogan, Leon, Perez, & Kandel, 2005) and hence may imbue associative learning processes – yet inhibitory (i.e., CS/‘no US’) rather than excitatory in nature (Cándido et al., 2004; Seligman and Binik, 1977; Wendt et al., 2015 but see Chauveau et al., 2012; Jüngling et al., 2008). These additional learning processes recruited in differential protocols include not only safety learning (as discussed above), but also stimulus habituation (Thompson and Spencer, 1966) and learned irrelevance (Baetu et al., 2005; Baker, 1976; for discussion see Ohman, 2009)).
Differential conditioning procedures in rodents often include presentations of the CS+ and CS− on separate days (Chauveau et al., 2012; Jüngling et al., 2008; Tang et al., 2003), a procedure rarely employed in human fear conditioning procedures, where CS+ and CS− are presented interleaved within the same training session (for an overview see Lonsdorf et al., 2017). In protocols that include CS− presentation on the same day as the CS+, the sequence of the CS presentation in rodents can either be fixed (e.g., all CS− trials always precedes the CS+, Goosens et al., 2003; Herry et al., 2008; Letzkus et al., 2011), or pseudo-randomized, (e.g., Likhtik et al., 2014; Stujenske et al., 2014), which resembles CS presentation procedures that are commonly used in humans.
In conclusion, experiments in rodents and humans use fear conditioning procedures that reliably engage excitatory learning to a CS that predicts an aversive US. However, differences typically exist with respect to the employment of control conditions (e.g., between vs. within-subject designs, sequence of CS presentations). As these procedural differences may result in the engagement of different or additional learning mechanisms (e.g., safety learning, see above) they potentially hinder direct comparison of results between (and within) species. Moreover, recruitment of such additional learning mechanisms can induce more individual variance in fear learning. For example, safety learning is reduced within individuals with high trait anxiety (e.g., Gazendam et al., 2013; Haaker et al., 2015; Haddad et al., 2012; for an overview see Lonsdorf and Merz, 2017) and diagnoses of anxiety related disorders (e.g., Duits et al., 2015; Lissek et al., 2005). Hence, even though fear conditioning protocols across species share similar operationalization of learned threat anticipation to the CS+, the specific operationalization (e.g., if and when a CS− was presented) of control conditions and their underlying mechanisms needs to be considered to make a translational valid interpretation of the results.
Context conditioning
Context in Pavlovian fear conditioning experiments has been defined as the internal (physiological and cognitive, i.e., interoceptive) or external (environmental and social, i.e., exteroceptive) background in which associative learning and retrieval takes place (Bouton, 2004; Maren, Phan, & Liberzon, 2013). Contexts are configural representations of numerous multimodal cues, and they can be separated from discrete cues based on their modality, duration, complexity and temporal arrangement (Fanselow, 2010; Rudy, 2009). A context can be associatively learned as a predictor for a US (here termed as CXT+) and/or setting the occasion for CS/US associations that have been learned during acquisition and extinction training. As such, the context plays a central role in gating the expression or inhibition of CRs across species (Bouton, 2002; Goode & Maren, 2014).
In animal research, a multisensory manipulation of external contexts is common practice (Maren, 2001). This is often accomplished by manipulating specific features of the physical chambers (e.g., size, floor textures, visual patterns, odors, background noise, ambient illumination, or a combination of all) in which animals undergo acquisition training, extinction training, and retrieval testing (Bouton, 2002). In addition to these exteroceptive stimuli, internal stimuli create interoceptive contexts that influence fear conditioning. Interoceptive contexts can include drug or hormonal state (Acca et al., 2017; Bouton et al., 1990; Cunningham, 1979), deprivation state (e.g., hunger), as well as the passage of time (Bouton, 2002; Maren et al., 2013). In humans, exteroceptive contexts typically consist of visual stimuli (Alvarez et al., 2008; Andreatta et al., 2015; Kroes et al., 2017; Marschner et al., 2008) including colours of computer background screens (Haaker, et al., 2013; 2017; Kalisch et al., 2006; Pohlack, Nees, Ruttorf, Schad, & Flor, 2012), complex images of environments (Lonsdorf, Haaker, & Kalisch, 2014; Milad et al., 2007), or virtual visual contexts (Glotzbach-Schoon et al., 2013; Huff et al., 2010; Kroes et al., 2017). Investigations in humans that employ two physically different rooms (to mirror the context manipulations in animals) are rare in humans (K.S. LaBar and Phelps, 2005; Schiller et al., 2008). Moreover, in contrast to work in animals, interoceptive contexts (e.g., drug state) in humans and their influence on fear conditioning processes have not been investigated in detail. In particular, while interoceptive states in humans have been manipulated (e.g., stress-manipulation or administration of cortisol), such interoceptive states have not been used as a contextual manipulation of fear conditioning processes (e.g., enhanced cortisol during acquisition training and retrieval testing).
In sum, Pavlovian fear conditioning in humans occurs in contexts that are differentiated by subtle changes in visual stimuli, rather than wholesale changes of the surrounding multisensory environment, as employed in rodents. In the last decade, computer-generated contexts in virtual realities that are either presented on large screens or through (immersive) head-mounted displays have gained increasing popularity (Baas, Nugent, Lissek, Pine, & Grillon, 2004; Glotzbach, Ewald, Andreatta, Pauli, & Mühlberger, 2012; Grillon, Baas, Cornwell, & Johnson, 2006; Huff et al., 2010; Kroes et al., 2017). Nonetheless, despite their clear advantage to easily construct different contexts with more complex environmental features, these virtual contexts are typically limited to visual stimuli (Kroes et al., 2017; Maren et al., 2013). It should moreover be considered that acquisition of neural responses in humans (e.g., functional magnetic resonance imaging (fMRI), magnetoencephalography, Electroencephalography) typically restricts the physical context (e.g., the bore of an fMRI scanner); Maren et al., 2013).
Despite the procedural differences in context operationalization, there is translational evidence for basic contextual influences on the expression and inhibition of conditioned fear, which is mediated by converging neural networks across species (Haaker, et al., 2013; Maren et al., 2013; Milad et al., 2007). However, the effects of such simple instantiation of contextual features in human experiments (e.g., screen background) are limited, since contextual features from the testing room might override contextual learning (Kroes et al., 2017). Moreover, subtle changes in screen backgrounds can induce unintended contextual effects, which has been discussed with respect to reinstatement of CRs in humans (Haaker et al., 2014; Sjouwerman et al., 2015). When presenting reinstatement US, experiments in humans often employ diverse visual contextual features (e.g., black background screen, fixation cross, background screen without cues, etc.), without considering how these subtle differences can impact the contextual modulation of reinstated CRs during a later test. In contrast, contexts presented during reinstatement in rodents often entail well defined, multisensory features. So far, only one study in humans used different rooms to test the contextual influence on reinstatement of CRs in humans (Kevin S LaBar and Phelps, 2005). It is plausible that future experiments that employ more holistic operationalization of contexts in humans may induce contextual effects on the expression and inhibition of CRs that are more comparable to research in rodents and allow for investigation of more fine-grained contextual effects. Hence, operationalization of contexts as holistic environments in humans would overcome the gap to research in rodents and allow for a better cross-species investigation of contextual influences on CRs.
Unconditioned stimuli (US)
Across species, the most commonly employed US in Pavlovian fear conditioning designs is electrical stimulation. Other cross-species US-types include high-intensity white noise bursts and air blasts (see Lonsdorf et al., 2017 for more details on US employed in humans and Wotjak, 2018 for rodents). Naturalistic USs in turn, are typically species-specific and consequentially more difficult to translate between species. Naturalistic USs in rodents include, for instance, predator odor (Takahashi et al., 2008) and in humans videos of actors that insult human participants (Reichenberger et al., 2017; Wieser et al., 2014) or fearful human faces paired with screams (Glenn et al., 2012).
The sensory quality of electric stimulation is considered to be comparable across species (see Wotjak, 2018) for a discussion on nociceptive comparability in rodents), despite different operationalization: In rodents, electric stimulation is usually given as a brief scrambled AC shock to the feet (Curzon et al., 2009) delivered through a metallic grid floor. In human participants, a DC shock is commonly applied to the hand or forearm through an attached electrode (Lonsdorf et al., 2017). Usually, in both species, the intensity of the electric stimulation is chosen to be an aversive, but only mild nociceptive signal.
Even if we here assume comparable nociceptive qualities, there exist major differences across species with respect to controllability and previous experience of the US. More precisely, human volunteers typically have some control over the US intensity (at least for electric stimulation), which is, for ethical reasons, typically adjusted to tolerable levels (“unpleasant, but not painful” or “unpleasant and painful, but bearable”). The precise procedure to reach such a criterion varies however between laboratories (see Lonsdorf et al., 2017 for a more details). These pre-experimental calibration procedures naturally involve pre-exposure to the US. As a consequence, human participants, in contrast to rodents, are neither naïve to the intensity nor the imminence of the threat. Importantly, pre-exposure to the US has been found to attenuate learning of the CS+ as predictors for the US in rodents (Kamin, 1961) as well as in humans (Meulders et al., 2012; Taylor, 1956). US pre-exposure within the experimental context might yield excitatory learning, which might impact subsequent learning about the CS/US contingencies, including blocking learning about the CS (Kamin, 1968; Yau and McNally, 2019; but see Maes et al., 2016). It has further been shown in rodents that an acquired context-US association (i.e., first learning) changes the neurochemical substrates that underlie subsequent acquisition training to a CS+ (i.e., second learning; Finnie et al., 2018). In addition, human participants are informed that the experiment involves administration of an electric stimulation prior to the experiment. Hence, prior to fear acquisition training, participants are provided with instructions about the US (cf. details on instructions) in addition to directly experiencing the US during the calibration procedures.
Yet, the electrical stimulation USs are essential to fear conditioning protocols across species. In rodents, the magnitude of the US tends to produce monotonic increases in CRs, such as freezing behavior (Fanselow and Bolles, 1979) although this relationship is not reflected by increasing acoustic startle responses (Davis & Astrachan, 1978). In rodents, US intensities are commonly between 0.4-1.0mA for mice and 0.5-1.0mA for rats with durations of 0.5-2.0s. To properly interpret these intensities, they should be related to the pain threshold of the animal (same strain and in the same setup, Wotjak, 2018). In humans, physical US intensities are often not informative, because participants rate their subjectively experienced unpleasantness, which typically corresponds to values of 5 or 7 on a 10 point scale (from 0 “I feel nothing” to 10 “maximally unpleasant”; e.g., Andreatta et al., 2015; Haaker, Lonsdorf, Thanellou, & Kalisch, 2013; Lonsdorf et al., 2017; Pohlack et al., 2012). Importantly, in rodents high US intensities promote the generalization of conditioned responding. In rodents, generalization has been described to stimuli that are not directly associated with the US such as freezing to the context (Baldi et al., 2004) or CS− (Ghosh and Chattarji, 2015; Laxmi et al., 2003), as well as it might lead to general sensitization of fear responses (Kamprath and Wotjak, 2004; Siegmund and Wotjak, 2006; for an overview see Riebe et al., 2012). Converging findings in humans are sparse (for initial studies on neurobiological US processing see Goodman et al., 2018; Knight et al., 2010), but there is some evidence that highly aversive multisensory USs (electric stimulation combined with white noise and looming snake pictures) induce generalization of skin conductance responses to novel cues as compared to a an electric US alone (Dunsmoor et al., 2017).
Experiments in rodents generally employ US presentation that follow presentations of the CS+, so called continuous paring or 100% contingency, whereas human experiments employ continuous, but also partial pairings that include unpaired CS+ presentations (contingencies vary from 100% to below 20%, Lonsdorf et al., 2017b; Lonsdorf and Merz, 2017; Sehlmeyer et al., 2009). Lower contingencies have been associated in humans with diminished SCR differential (CS+>CS−) responses (Grady et al., 2016) and reduced amygdala responses (Dunsmoor et al., 2007, but see Sehlmeyer et al., 2009), as well as reduced freezing to an auditory CS+ in mice (saline group in experiment 3 in Cain et al., 2005). In the similar way, reducing the CS-US contingencies by additional insertion of USs is reflected by decreased freezing to an auditory CS+ in mice (saline group in experiment 2 in Cain et al., 2005) and diminishes correlates of synaptic plasticity (long-term potentiation) in the basolateral amygdala in rats (Bauer et al., 2001). Hence, as postulated by animal work in operant conditioning (Rescorla and Wagner, 1972), contingency between the CS and US scales the processes that underlie learning of CS-US association and the resulting CRs. Studies in humans often employ lower contingencies to slow down CS-US learning, for example to probe learning processes along a larger number of trials, or to analyze unpaired CS presentation (without any US signal) or to slow down extinction learning that follows upon acquisition. Evidence for prolonged CRs during extinction training after partial parings during acquisition has mostly been provided from animal studies that used operant protocols (Bouton et al., 2014; Capaldi, 1967). In human experiments, the separation between acquisition and extinction training might also be less pronounced (i.e., a clear start of extinction training is not clear to the participants) when acquisition training employed low CS-US contingencies and extinction follows immediately after acquisition training (without a gap, see Timing of experimental phases). Clear separation between acquisition and extinction training, which might be induced in transitions from continuous pairings during acquisition to immediate extinction training, might signal new learning phases (due to a strong violation of expectations) and thereby influence context-dependent extinction learning processes (Dunsmoor et al., 2018; Gershman et al., 2017).
In sum, the use of electric stimulation as US in fear conditioning protocols is considered to induce comparable nociceptive effects across species. Yet, differences across species with respect to the controllability, pre-exposure and expectations about the US exist, which influences threat learning processes in rodents and humans differently (e.g., by expectancy-driven adaption or the impact of instructions about the US). More cross-species research on procedural factors shape US expectancy could help to enhance translation of results across species.
Instructions
An essential challenge to translational research is that human participants are always verbally informed about the upcoming experiment, while rodents are not. Information about the experiment (typically referred to as instructions) have a powerful impact on fear and safety learning (e.g., Atlas, 2019; Atlas et al., 2016; Duits et al., 2017; Mertens et al., 2018, 2016; Sevenster et al., 2012) and therefore have consequences on the interpretation of findings in humans and translatability of findings across species.
In humans, instructions vary from explicit CS/US contingency information (‘Only the blue square will be followed by the aversive stimulus, the yellow square will never be followed by the aversive stimulus’) to no information about contingencies (‘Attend to the visual material on the screen’) with varying instantiations in between (‘One of the two pictures presented on the screen will be followed by the aversive stimulus, the other not. You will be able to figure out which one by paying close attention’, for an overview see Lonsdorf et al., 2017). Such instructions concerning CS/US contingencies or changes thereof may or may not be provided prior to every experimental phase (i.e., acquisition and extinction training, retrieval tests). In addition to instructions, probing subjective ratings of fear and expectancy of the US might further affect conditioned responses (Mertens et al., 2018b; Sjouwerman et al., 2016; Warren et al., 2014), for example by enhancing awareness of CS-US contingency (Boddez et al., 2013; Lipp, 2006; Razran, 1955).
Instructions have been employed, for instance, to minimize individual differences in awareness about CS/US contingencies. For example, when extinction learning is of primary interest, instructions about CS/US contingencies during fear acquisition may minimize individual differences prior to extinction. It should, however, be considered that such type of instructions has the potential to affect subsequent phases. Furthermore, some paradigms rely completely on instructions (i.e., ‘threat of shock’, e.g., Bublatzky et al., 2018; Grillon et al., 1991; Phelps et al., 2001) to produce anticipatory threat responses without providing direct US experiences.
Importantly, instructions affect the temporal dynamics of (conditioned) responding over trials. More precisely, explicit CS/US contingency instructions induce US anticipation to the CS+ as well as discrimination from safety signals (i.e., CS−) already in the first acquisition training trial (i.e., expression of instructed knowledge rather than learning by direct experiences). In contrast, learning from direct experiences (without explicit CS/US contingency instructions) involves a gradual development of US anticipation (Duits et al., 2017; Sjouwerman et al., 2015; Tabbert, Stark, Kirsch, & Vaitl, 2006) and hence CS discrimination typically evolves over time during acquisition training.
Furthermore, the biological substrates recruited and learning processes involved in socially transmitted verbal information (i.e., instructions) as opposed to direct learning experiences have been shown to be distinct (Atlas et al., 2016; Atlas, 2019; Braem et al., 2017; Mechias et al., 2010; Mertens et al., 2018a; Olsson and Phelps, 2007; Phelps et al., 2001; Tabbert et al., 2011).
In sum, instruction of human participants (about the CS/US contingencies) critically determines the process that is probed within a fear conditioning protocol (i.e., learning from direct experiences vs. expression of instructed associations). Since instructions are never part of experiments in rodents, these procedures in humans needs to be critically examined (type and timing of instructions) when comparing and interpreting results across species.
Timing of experimental phases
The timing of extinction training with respect to acquisition training does matter, as both associative learning processes require synaptic (across hours) and systemic (across days) consolidation processes to enable long-term memory storage (for a review see for example (Baldi and Bucherelli, 2015; Johansen et al., 2011; McGaugh, 2000; Orsini and Maren, 2012).
The time-delay between extinction training and acquisition training however typically differs between studies in humans and rodents. While animal experiments often separate acquisition and extinction training by days (delayed extinction), it is more common in research in humans to use immediate extinction protocols, i.e., extinction training that follows directly after acquisition training. As a consequence, immediate extinction training might interfere with consolidation of the previously acquired CS/US association memory (Chang et al., 2011; Golkar et al., 2013; Maren and Chang, 2006; Merz et al., 2016; Myers et al., 2006; Norrholm et al., 2008; for an overview see Maren, 2014, but see Kim and Richardson, 2009; Schiller et al., 2008). Similarly, even a short break (10s as opposed to no break) that separates acquisition from extinction training has been shown to influence memory performance during aversive learning in humans (Dunsmoor et al., 2018).
Likewise, retrieval tests that probe reinstatement or renewal of CRs typically take place on separate experimental days in rodents, but often occur immediately after extinction training in human experiments (for an overview see Lonsdorf et al., 2017). The time-point of retrieval testing, relative to extinction training, however, has an impact on the expression of CRs in rodents (Archbold et al., 2013; Kim and Richardson, 2009) including differences in the underlying neuronal matrix (Kitamura et al., 2017; Sacco and Sacchetti, 2010; Vetere et al., 2017; Wheeler et al., 2013). Hence, it is plausible that extinction memory processes interfere with retrieval testing, yet this has not been addressed in human experiments.
In sum, the timing of experimental phases needs to be considered when comparing results across species. Immediate or delayed extinction or retrieval procedures may recruit different consolidation machineries and map onto different real world experiences: Learning safety (e.g., getting therapeutic treatment) either delayed or following immediately after salient aversive events.
WHAT TO CONSIDER: PARADIGM
Conditioned Stimuli
Check if a CS− was employed as a CS+ control (within-subject design), or if a between group design was chosen
In differential protocols: Check how and when the CS− was presented (e.g., prior to acquisition training or intermixed with the CS+).
Consider the different (or additional) processes probed by single-cue (mostly excitatory learning) or differential (excitatory and inhibitory learning) protocols.
Context
Check how “context” is defined. In rodents, contexts are usually defined as different chambers (which might include change at multisensory level such as visual, olfactory and tactile stimuli), whereas contexts in human experiment are often operationalized by different visual stimuli.
Check if changes in visual features (e.g., background screen) in human experiments are used for contextual modulation. Even subtle, unintended, changes can impact contextual gating of CRs.
Unconditioned Stimuli
Check the sensory properties of the US (electrical stimulation, loud noise or perhaps species-specific naturalistic stimulus). Shock USs are typically applied to the feet in animals and the forearm, finger, or hand in humans. Despite sensory differences this US type is considered to generate a comparable nociceptive input across species.
Check the US intensity in animal and human experiments. Moderate US intensities that are associated with discriminative learning in animals (check freezing responses to the CS+ as compared to the CS− or context) may be more comparable to human procedures. High US intensities may induce generalization of CRs (to the context or CS−) and are usually (on average) not employed in experiments with human volunteers.
Check the calibration procedure in experiments involving human volunteers. Calibration of the US intensity prior to the experiment can be considered a signaled pre-exposure to the US. Hence, human participants are not naive to the intensity and imminence of the US during fear conditioning experiments.
Instructions
Check if human participants have received instructions about the CS/US contingencies and changes thereof (and in what detail). Human experiments, in which participants receive minimal or no instructions (i.e., learning depends mainly on experiences) more closely resemble animal experiments.
Timing of experimental phases
Check timing between acquisition training, extinction training and retrieval test phases. Animal research often follows a ‘delayed’ schedule with day to weeks between the different phases, thus allowing for long-term consolidation processes to take place. Human experiments use “immediate” or (less often) “delayed” schedules. Even short gaps (10 sec) might alter memory processes that are initiated by acquisition and extinction training in humans (as compared to no gaps).
OUTCOME MEASURES
Conditioned responses can be examined across species on different levels of analysis, which encompass behavioural and physiological, including neurophysiological, responses. Additionally, experiments in humans allow for studying verbal reports of subjective emotional experiences, such as fear, distress, and (US) expectations. These different responses levels reflect different defensive processes (Anderson & Adolphs, 2014; Bechara et al., 1995; Lang, Davis, & Öhman, 2000; LeDoux, 2012; LeDoux & Pine, 2016) and thus do not necessarily converge (for a discussion see Lonsdorf et al., 2017). As a consequence, research across species needs to carefully consider the processes that are reflected by these measurements employed within each species.
Within the following paragraphs, we introduce the most commonly used behavioral and physiological outcome measures for fear conditioning studies that have been employed in both, rodents and humans. We have excluded measures of neural activity here, since comprehensive translational reviews on neural systems that mediate the acquisition and extinction of CRs in rodents and humans exist (Delgado, Olsson, & Phelps, 2006; Gunaydin et al., 2014; Johansen et al., 2011; LeDoux, 2012; LeDoux & Daw, 2018; Maren, 2001; Maren & Quirk, 2004; Milad & Quirk, 2012; Parsons & Ressler, 2013; Tovote, Fadok, & Lüthi, 2015 ; see (Sevenster et al., 2018) for a recent review of advances and challenges that results from this cross-species research). In general, the techniques to record neural activity usually differ substantially between species, with respect to the level of detail and invasiveness, for example, intracranial single cell recordings in animals vs. local field potentials and hemodynamic systems in humans. Recent studies in rodents, however, have examined hemodynamic responses (by fMRI) during retrieval test (Brydges et al., 2013; Harris et al., 2016; Harris et al., 2015). This dependent measure is commonly used to investigate neurobiological mechanisms of fear conditioning in humans and might help to bridge neural substrate across species in the future. This promising example for translational research, however, highlights the constraints of methodological differences between species. In particular, rodents in the aforementioned experiments had to be extensively habituated to the fMRI environment across several days and were restrained during testing, whereas humans can be naïve to fMRI environment before acquisition training and are not restrained, but instructed to minimize their movement.
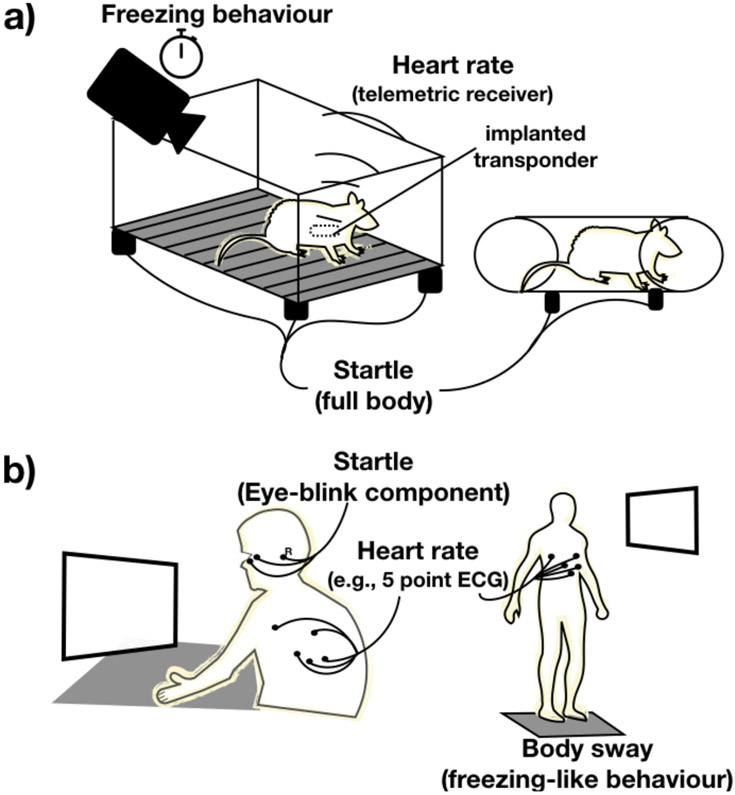
The following paragraphs provide cross-species comparisons of dependent measures that are used both species (cf. Figure 2).
Figure 2:
Schematic illustration of outcome measures in (a) freely moving animals (left), restrained animals (right), (b) humans in sitting positions (left) and on a stabilometric platform (right). R=reference electrode
Fear-potentiated startle
The acoustic startle response has been widely used as an outcome measure in fear conditioning studies in both rodents and humans (Fendt & Fanselow, 1999). Startle responses that are elicited for example by a sudden, loud noise (so called startle probe) include the contraction of facial, neck and skeletal muscles, resulting in eye-lid-closure, and a disruption of ongoing behaviors to protect the organism from a potential threat (Landis et al., 1939).
Startle responses in fear conditioning experiments in both humans and rodents are commonly elicited by short acoustic stimuli [e.g., 20-90ms white noise, typically with intensities between 90-105dB (A) and a steep rise/fall time (0-2ms)] presented via speakers (in rodents) or headphones (majority of studies in humans).
In both species, startle response amplitudes can be modulated by different internal and external variables, resulting in a decrease or increase from a non-zero baseline (Fendt & Koch, 2013). As such, startle responses increase (compared to baseline) in response to threat predicting CS+ and decrease to conditions that are safe (Gerber et al., 2014; Hamm et al., 1993). The startle potentiation during anticipation or exposure to conditioned and unconditioned threats (i.e., CSs and USs) in rodents (Davis, Walker, & Lee, 1997) and humans (Grillon, 2002; Grillon and Baas, 2003; Hamm and Weike, 2005; Norrholm et al., 2014, 2006) is called fear-potentiated startle (FPS). Experiments in both, rodents and humans, require habituation of startle responses to the acoustic probe prior to acquisition training to observe FPS.
In rats (Brown et al., 1951) and mice (Daldrup et al., 2015; Falls, Carlson, Turner, & Willott, 1997; Falls, 2002) FPS is usually indexed by a whole body response (i.e., whole-body startle), measured by automatic procedures using startle chambers (plexiglas cylinders positioned on a stabilimeter, see figure 2) or motion-sensitive platforms (unrestraint behaviour of freely moving animals). Startle responses in rodents are not monotonically related to US intensity, as is the case for freezing responses (Davis & Astrachan, 1978). Mice exhibit less pronounced and more variant startle responses compared to rats (Lauer et al., 2017; Paylor and Crawley, 1997) and FPS is less commonly used in mice (but see for example: (Daldrup et al., 2015; Falls, 2002; Smith et al., 2011).
In humans, the eye-blink component of the startle reflex is measured through recording of electromyographic (EMG) activity measuring the contraction of the orbicularis oculi muscle ((Landis et al., 1939), see Figure 2) - the most reliable component of the startle response in humans (Koch, 1999).
Because the eye-blink startle response is a component of the whole body startle, it is assumed that both share a common neurobiological pathway (Koch, 1999). Primary and modulatory neuroanatomical and biochemical circuits are well studied in rodents (primarily in rats, e.g., (Koch, 1999; Lauer et al., 2017) and non-human primates (Davis et al., 2008; Winslow et al., 2002). In humans, however, studies on the neurobiological pathways of startle responses have just recently emerged, enabled by new technical advances allowing to combine EMG and fMRI recordings (Kuhn et al., 2015; Lindner et al., 2015). These studies hold promise to provide the yet missing link for a translational understanding on the neural basis of startle responses.
It should be kept in mind that startle responses are responses that are triggered by the presentation of (potentially mildly aversive) stimuli (i.e., startle probes). As such, the startle probe has been conceptualized as an additional aversive stimulus (Lissek et al., 2005) and has been shown to impact on the learning process during acquisition training in humans (e.g., as examined in SCRs by (Sjouwerman et al., 2016) and pupillometry (de Haan et al., 2018).
In conclusion, FPS (measured as whole body startle in rodents and as eye-blink startle responses in humans) represents a cross-species measurement of defensive CRs (Davis, Walker, Miles, & Grillon, 2010; Fendt & Koch, 2013; Grillon & Baas, 2003).
Freezing and freezing-like behavior
In rodent experiments, changes in the animals’ observable behavior in response to the CSs are common outcome measures indexing conditioned responding. The most common behavioral index in fear conditioning protocols is freezing, though the CRs also include changes in risk-assessment, flight, grooming, exploration, rearing, and quiescence (see (Remmes et al., 2016). Freezing behavior is a defensive response elicited by both conditioned and unconditioned threats (Blanchard, 2017; Hagenaars, Oitzl, & Roelofs, 2014; Roelofs, 2017; Volchan et al., 2017). It is defined as the absence of movement (except for breathing) with increased muscle tension that demarks attentive immobility towards distal threats or immobility under imminent threat, if escape is not possible (Blanchard, Blanchard, & Hori, 1989). Freezing is commonly reported in rodents as the percentage of a defined time-window (e.g., CS presentation or time freezing in the CXT) within the animal exhibited freezing behavior. While freezing is the typically recorded response to conditioned and unconditioned threats in mice and rats, it is only one response amongst a rich behavioral repertoire of defensive responding in animals (Bolles, 1970; Perusini and Fanselow, 2015). Thus, the lack of freezing does not necessarily indicate the lack of conditioned fear, since exaggerated fear responding may result in panic-like flight behavior (Fadok et al., 2017; Tovote et al., 2016).
In human fear conditioning experiments, naturally occurring behavioral responses are commonly not examined (or possible), because participants are usually tested in a sitting position in front of computer screens or positioned in the MRI scanner. Moreover, several outcome measures (e.g., physiological signals and neural measures) demand participants to refrain from body movements. Recent studies in humans, however, have started to measure behavioral responses to threats, which might mirror freezing. In particular, decreased postural body sway on a stabilometric force platform was found during anticipation of threats (Azevedo et al., 2005; Bastos et al., 2016; Gladwin et al., 2016; Roelofs et al., 2010; Stins et al., 2011) for reviews see Blanchard, 2017; Hagenaars, Oitzl, & Roelofs, 2014; Roelofs, 2017; Volchan et al., 2017), including anticipation of electric shock USs (Gladwin et al., 2016) and presentations of CSs (Van Ast and Roelofs, personal communication). This initial evidence that anticipation of threats can be examined by freezing-like behavior through means of body sway in humans is underlined by bradycardic changes in the heart rate (cf. next chapter) during reduced body sway (Azevedo et al., 2005; Bastos et al., 2016; Gladwin et al., 2016) which mirrors simultaneously occurring freezing and bradycardia in rodents (Vianna & Carrive, 2005; Walker & Carrive, 2003).
In general, when comparing “behavior” across species within fear conditioning experiments, it should be noted that fear conditioning protocols in rodents often allow for expression of a range of defensive responses, while behavior in human participants is commonly restricted to key presses or body sway in fixed positions. Moreover, human participants are aware of the possibility to ultimately discontinue and hence escape from the aversive testing situation, which furthermore impacts the choice of defensive responses that can be measured in human experiments.
In sum, freezing behavior in rodents is a common outcome measure in fear conditioning experiments and studies on body movements in humans, as a measure of freezing-like responses, have recently emerged. These new developments in humans hold the promise to develop into a translational measure of defensive behaviors that can be employed in future fear conditioning studies.
Heart rate
Alterations of heart rate in responses to CSs and CTXs have been described across species as an index of psychophysiological arousal. Both, deceleration and acceleration have been observed due to simultaneous parasympathetic and sympathetic involvement in the CR (mice: Stiedl & Spiess, 1997; Stiedl, Tovote, Ögren, & Meyer, 2004, rats: (Iwata and LeDoux, 1988; Roozendaal et al., 1991) humans: Graham & Clifton, 1966; Hamm et al., 1993; Headrick & Graham, 1969; Lipp & Vaitl, 1990; Öhman & Dimberg, 1978; for detailed information on heart rate measurement in rodents see (Morgan and Paolini, 2012; Stiedl et al., 2009) and in humans see Jennings et al., 1981; Lonsdorf et al., 2017). Measurements in humans are non-invasive and feasible in different testing environments (e.g., in front of computer screens or during fMRI scanning). In rodents, heart rate measurements require implantation of telemetric devices (Carrive, 2000; Dielenberg and McGregor, 2001) in order to avoid restraining the animal during measurements, which has been used in earlier studies. Restraining of the animal, can elicit stress responses itself (Iwata and LeDoux, 1988), which impacts on the changes in the hart rate to the CS (as discussed by (Iwata and LeDoux, 1988): higher basal heart rate (often in restrained animals) favors deceleration, whereas lower basal rates are often followed by acceleration. In humans, the divergence between acceleration and deceleration has been related to different CS processing. Heart rate acceleration during acquisition training was linked to affective responses (paralleled by enhanced startle potentiation and subjective experiences of unpleasantness) and preparation of avoidance, whereas deceleration was associated with the learned anticipation of threats (Hamm et al., 1993; Hamm & Vaitl, 1996; Hodes, Cook, & Lang, 1985; Roelofs, 2017).
Taken together, heart rate analysis offers a cross-species measurement of the sympathetic and parasympathetic part of the CR. When interpreting acceleration and deceleration, the basal levels and the specific operationalization of the measurement in rodents (i.e., restraining vs. free-moving) needs to be considered.
WHAT TO CONSIDER: OUTCOME MEASURES
Fear potentiated startle
Consider that startle responses are measured as whole body startle in rodents and as the eye-blink component in humans.
Consider that startle responses are triggered (in contrast to continuous measures of freezing and SCRs, for example) by mildly aversive stimuli, which delays learning about the CS/US association.
Freezing and freezing-like behaviour
Check if freezing behavior is the only behavioral measure of CRs in rodents, since it accounts for only one part of a complex defensive response pattern. In humans, reduced body sway (as a measure of freezing-like behavior) has recently been employed to examine defensive responses in anticipation of threats.
Check how expression of behavioral responses is defined in the experiment. The conditioning apparatus used in rodents often allows (relatively) free movement in a chamber, but is commonly restricted in humans (position in front of a computer screen).
Heart rate
Check if animals were restrained to measure heart rate (in earlier studies) or if devices were used that allow free movements.
Consider that sympathetic and parasympathetic signals interact in heart-rate measurements and thus might reflect different processes.
INDIVIDUAL DIFFERENCES
Fear conditioning research in both rodents and humans has generally focused on investigating common principles and mechanisms. This approach has generated invaluable knowledge on the canonical neural, behavioral, physiological and cellular mechanisms underlying fear and anxiety, which has provided a necessary framework for the study of individual differences. Heterogeneity within a population, however, was typically regarded as ‘residual variance’ in this context in both species. But a limited focus on average mechanisms deprives us from gaining crucial insights into the mechanisms beyond the average (Kosslyn et al., 2002) and may bias the field through wrong conclusions because the (artificial) sample mean may not describe any individual very well (Hedge, Powell, & Sumner, 2018; Kosslyn et al., 2002; Lonsdorf & Merz, 2017).
Hence, systematic investigations of differences between individuals (i.e., inter-individual differences) within fear conditioning experiments provides a strategy to infer on mechanisms beyond the average. In the following, we briefly outline and summarize some of the major sources of inter-individual differences in fear conditioning experiments and emphasize cross-species translational gaps with respect to methodological (i.e., procedural and analytical) issues.
Specific sources of individual differences across species
A plethora of specific sources of possible individual difference factors has been identified in rodents and humans. The most obvious factors that differ between individuals include sex and age - as a choice on specific inclusion or exclusion criteria regarding these factors is mandatory prior to each experiment, across species. Additional examples of factors impacting on conditioned responding include various stressors and life history events, genetic variations or personality characteristics in humans (Lonsdorf & Merz, 2017) and rodents (Holmes and Singewald, 2013).
Sex:
Biological sex, varying sex hormone concentrations over the cycle as well as hormonal contraceptives impact on threat learning and extinction processes in humans (Glover et al., 2015; Merz et al., 2018) and rodents (Cover et al., 2014; Lebron-Milad and Milad, 2012). While rodent work has revealed important sex differences in fear conditioning (e.g.,(Gruene, Flick, Stefano, Shea, & Shansky, 2015; Gruene, Roberts, Thomas, Ronzio, & Shansky, 2015; Maren, De Oca, & Fanselow, 1994; Milad, Igoe, Lebron-Milad, & Novales, 2009; Zeidan et al., 2011), experiments are commonly conducted in males only (Cover et al., 2014; Lebron-Milad and Milad, 2012). These sex differences are often used as a motivation to exclude female animals in experiments, in order to reduce variance in the results. These results are, however, often not generalizable to female individuals (Cahill, 2012), which is problematic from a translational perspective: Human samples in fear conditioning are often mixed sex samples (Lonsdorf and Merz, 2017) and women are overrepresented in clinical populations that suffer from pathological responses to threats in anxiety related disorders (Breslau, 2002; Cover et al., 2014; Reed and Wittchen, 1998). Recent policies by the National Institute of Health call for inclusion of female animals in future experiments (Clayton and Collins, 2014), which will likely affect gender-distribution in fear conditioning research. These mixed sex samples in rodents would get closer to sex distributions in human experiments (women are slightly overrepresented; (cf. Lonsdorf & Merz, 2017). Yet, even though mixed samples are investigated in human experiments, sex differences are often not explicitly considered.
Despite being often neglected, there are specific translational challenges that need to be considered when comparing work between rodents and humans. First, when comparing female individuals, the temporal dynamics of sex hormone concentrations over the course of the estrus cycle (4 days) in rodents and menstrual cycle (28 days) in humans matter: In multiple-day experiments, the faster fluctuation of hormones in rodents inevitably results in different sex hormone levels within different experimental phases. Second, differences across species further include the common use of hormonal contraceptives in women (Merz et al., 2018). Third, the impact of the sex of the experimenter (who has contact to the human participants and rodents during testing) has hitherto not been addressed in fear conditioning research, although there is evidence for these participant-experimenter interactions in other stress related tasks (for a discussion of general effect in humans see (Chapman et al., 2018) and experimenter-animal interaction see (Bohlen et al., 2014; Sorge et al., 2014)).
Age and development:
In rodent work, developmental studies revealed evidence for acquisition of CRs already at an early age (Richardson and Fan, 2002) whereas extinction learning seems to emerge only later in life – possibly mirroring the development of involved prefrontal brain regions (Kim and Richardson, 2010; Shechner et al., 2014). Notably, fear conditioning studies in adolescent or even younger individuals are rare in both, rodents (Shechner et al., 2014) and humans (Lonsdorf & Merz, 2017). Typically, rodents are tested during adulthood (i.e., 2 to 6 months postpartum), which corresponds to the typical age of participants in human fear conditioning studies (i.e., 21-25 years; cf. (Lonsdorf & Merz, 2017; Sengupta, 2012; Spear, 2000) ; see www.translatingtime.org/translate for a tool to translate age between different species). However, directly translating age between rodents and humans represents a challenge, since adolescence is characterized by different features across species and as changes in sex hormones across developmental phases (e.g., puberty, pregnancy or menopause) are not necessarily comparable between species, but are also not well studied (e.g. (Milligan-Saville and Graham, 2016).
Another translational challenge when investigating young individuals are ethical considerations regarding the employed US: Electrical stimulations, as usually employed in rodents and adult humans, cannot be used in children. Instead, air-puffs or loud and aversive sounds or screams are typically implemented (Shechner et al., 2014). Thus, despite differences in US intensities in general (see above), US types and intensities diverge between rodents and humans in particular when young individuals are studied. For a comprehensive review of methodological consideration of fear conditioning protocols in developing rodents we refer to (Cowan and Richardson, 2018).
Stressors:
The investigation of individual differences with respect to stressors poses a challenge for translational attempts mostly due to ethical constraints. First, acute stressors that are experimentally induced differ substantially between species. In rodents, this typically involves potentially life-threatening events (i.e., restraint stress, underwater trauma, tail shocks, social defeat, exposure to cat or fox odors (Schöner et al., 2017). In humans, in turn, acute stress typically involves physiological challenges (e.g., cold-pressor task) or social as well as self-evaluative threat (e.g., Trier Social Stress Test; (Kirschbaum et al., 1993); see (Dickerson and Kemeny, 2004) for a meta-analysis). However, for ethical reasons, these challenges neither cause physical harm, nor are they potentially life-threatening. Importantly, exposure to natural or man-made disasters, which are potentially life threatening (but not under experimental control), have been conceptualized as stressors (Luo et al., 2012; Steudte et al., 2011). Second, stressors in early life can be investigated in rodents under experimental conditions (Pryce et al., 2005), including experimentally generated life-histories (Bodden et al., 2017; Remmes et al., 2016; Tsoory et al., 2007). In human research, in contrast, the assessment of critical life events in fear conditioning (McLaughlin et al., 2016; Scharfenort et al., 2016) is often based on retrospective questionnaires such as the Childhood Trauma Questionnaire (Bernstein et al., 2003). Interview-based measures or ascertainment of events through official records -which could be more valid measures of such events- might provide further insights in future fear conditioning studies (Li et al., 2016; Monroe, 2008). Third, also chronic stress can be experimentally manipulated in rodents (Hoffman et al., 2015; Maren and Holmes, 2016), yet this is again not applicable in humans due to ethical reasons.
Despite these different methodological approaches between species to induce stress, the resulting increases in relevant stress hormones may provide similar read-outs. For example, biological markers for chronically elevated stress hormones, such as the assessment of hair cortisol concentrations (Stalder et al., 2017). Such biological markers, in contrast to questionnaires, might serve as a translational tool, to assess endocrine responses retrospectively over longer periods and across species (Yu et al., 2015). It should, however, be considered that the interaction between stress-related neurotransmission and neuromodulation (e.g. by monoamines and hormones, respectively) and emotional arousal evoked by fear conditioning protocols might affect learning and memory processes in non-linear (e.g., inverted-U shape) fashion (Baldi and Bucherelli, 2005).
Genetic variation:
Both human (Hettema et al., 2003; Merrill et al., 1999) and animal studies (Royce, 1972) report considerable influence of genetic variation on inter-individual variability in the ability to acquire and extinguish CRs. More precisely, One third of the variance in human fear conditioning (Hettema et al., 2003) has been attributed to genetic factors.
In humans, the majority of studies have investigated associations of candidate variants (i.e., polymorphisms) of a single biologically plausible candidate gene (for reviews see (Lonsdorf & Kalisch, 2011; Sumner, Powers, Jovanovic, & Koenen, 2016) in association with fear conditioning processes. This approach has been criticized (e.g.,(Kendler, 2013), as it depends on knowledge about the pathophysiology underlying the disease/trait studied. More recently, genome-wide association studies are emerging in the field of (clinical) anxiety research, which provides novel candidates for more targeted investigations in experimental fear conditioning research (for example (Deckert et al., 2017; Lueken et al., 2017).
In rodents in turn, genetic variation can be experimentally induced (e.g., gene knock-out/in, selective breeding), which allows to modify transmitter pathways that are also affected by naturally occurring genetic variants in humans (e.g.,(Bilkei-Gorzo et al., 2012). Importantly, the underlying biological alterations of single polymorphisms and knock-out/in procedures are not necessarily corresponding. Approaches, which mimic the genetic variant in humans by genetic modification in rodents might provide a closer match (e.g., (Dincheva et al., 2015; Soliman et al., 2010). Recently developed advances in genome editing techniques (CRISPR/Cas (Jinek et al., 2012)) hold the promise to facilitate such cross-species translation, e.g. by ‘generating humanized mice’.
Personality factors:
Personality traits such as trait anxiety, intolerance of uncertainty or neuroticism have been linked to different processes in fear conditioning paradigms in humans (for a review: Lonsdorf & Merz, 2017). In rodents, equivalent concepts can be subsumed under the umbrella term ‘animal personality’ (for reviews: (Réale et al., 2010; Stamps and Groothuis, 2010), which has however rarely been applied to the field of fear conditioning to date (e.g., Borta, Wöhr, & Schwarting, 2006; Walker, Hinwood, Masters, Deilenberg, & Day, 2008). A major translational challenge with respect to personality traits comprises the question of how to translate specific personality facets from humans to rodents (e.g., conscientiousness or intolerance of uncertainty). A second translational challenge represents assessment of ‘personality’ across species. While personality traits in humans are typically assessed by means of self-report questionnaires requiring some introspection capability, animal ‘personalities’ are derived from behavioural indices (i.e., behaviour in the open field, the elevated plus maze or social interactions). To date, it remains unclear if ‘personality characteristics’ capture comparable concepts across species despite differences in assessment.
In sum, individual differences in fear conditioning experiments need to be carefully checked for their particular instantiation (e.g., which type of stressor was used?) when compared across species. Moreover, comparability of results could profit by reporting effects of individual differences like effects of sex in mixed samples (even if this effect is not at focus) or sex of the experimenter.
Methodological considerations for research on individual differences – cross-species translational gaps
The investigation of individual differences in both species requires specifically tailored methodological considerations with respect to selection of individuals, experimental design, data processing and statistics (see (Lonsdorf & Merz, 2017) for a discussion for the human field). Of note, sample selection, experimental design choices and data analysis strategies developed to investigate general mechanisms might not be appropriate for the investigation of sample heterogeneity (Hedge, Powell, & Sumner, 2018; Lonsdorf & Merz, 2017). In the following, a non-exhaustive number of experimental and data analytical challenges are discussed focusing on cross-species translation in fear conditioning research.
In most studies in humans and rodents alike, individuals are divided into subgroups based on study-specific, often cut-off-based, criteria. Importantly however, in humans, grouping (by means of e.g., median split or selection of extreme groups) is mostly based on individual difference variables (e.g., scores in questionnaires; reviewed in (Lonsdorf & Merz, 2017)) rather than performance in fear conditioning, extinction, retrieval or return of fear. Hence, grouping of participants is based on the factor of main interest (e.g., trait anxiety) and task-performance is usually treated as continuous variable.
In rodents in turn, grouping is often based on observed behavior in fear conditioning experiments (Bush et al., 2007; Shumake et al., 2014) and study-specific mean-based cut-off criteria are employed - such as the upper and lower end of the distribution (Bush et al., 2007; Reznikov et al., 2015; Shumake et al., 2018; Walker et al., 2008), number of trials taken to reach a predefined extinction criterion (King et al., 2017) or data driven cut-off criteria in a probabilistic model (Shumake et al., 2018).
Critically, these cut-off criteria in human and rodent studies alike are mostly study-specific and hence, comparability of individuals labeled as for instance ‘slow extinction’ or ‘high extinction phenotype’ across studies is not always straightforward. Likewise, there have been some attempts in rodents to classify an individual as ‘affected’ or ‘unaffected’ based on cut-off criteria on a range of behavioral read-outs (Ardi, Albrecht, Richter-Levin, Saha, & Richter-Levin, 2016; Cohen, Zohar, & Matar, 2003; Walker et al., 2008) in order to mimic psychiatric procedures as employed in humans. The validity of psychiatric nosology in humans, however, has recently been challenged (Cuthbert & Insel, 2013; Hamm et al., 2016; Marquand, Wolfers, Mennes, Buitelaar, & Beckmann, 2016) based on the massive within-diagnosis heterogeneity (Galatzer-Levy and Bryant, 2013). This gives such a back-wards translational approach most likely limited promise.
Another methodological challenge for research on individual difference is the fact that fear conditioning protocols often employ ‘strong experimental situations’ (Lissek et al., 2006) and hence leave limited room for the manifestation of individual differences. This theoretical consideration has very recently been empirically demonstrated for seven classic tasks employed in cognitive neuroscience (Hedge et al., 2018). Importantly, the strength of the experimental situation may not be comparable across species in typical experimental set-ups.
An additional translational challenge exists with regard to differences in sample selection in rodents and humans. In general, human participants and laboratory animals in fear conditioning research are often homogenous groups. In the human field, work is primarily based on student samples between the ages of 21 and 25 (Lonsdorf and Merz, 2017) without any history of psychiatric disorders (often excluding participants “at risk” for anxiety disorders and oversampling resilient individuals), which limits generalizability of these findings within a homogenous and possibly high functioning set of individuals to the general population (Henrich, Heine, & Norenzayan, 2010; Lonsdorf & Merz, 2017). Similarly, experiments in rodents typically avoid heterogeneous samples to reduce variability within experiments and thereby to increase power (i.e., reducing the number of animals that are needed to observe an effect). As such, studies are typically performed in animals of the same inbred strain tested at exactly the same age and housed in the same environmental conditions (e.g., cage size, environmental enrichment, temperature, humidity, light intensity, time of day, etc.) held maximally constant. Consequently, recruitment strategies that focus on individual differences should be adjusted to induce systematic heterogeneity in humans (e.g., recruitment from the general population). Such a ‘systematic heterogenization‘ of the study sample would follow recent discussions in the rodent field that call for environmental or experimental ‘heterogenization’ rather than ‘standardization’ in order to produce robust findings (Richter, 2017; Richter et al., 2010, 2009; Voelkl et al., 2018). Studies on individual difference in fear conditioning research in rodents already included some (unsystematic) heterogeneity by using outbred rats (Bush, Sotres-Bayon, & LeDoux, 2007; Reznikov, Diwan, Nobrega, & Hamani, 2015; Shumake, Jones, Auchter, & Monfils, 2018; Walker et al., 2008) and tried to maximize (genetic) diversity in the sample by including crossing of rats that were provided by different suppliers (Shumake et al., 2018). To date however, heterogenization with respect to sample selection is rarely applied in human research (Lonsdorf & Merz, 2017). Maximizing heterogeneity in a systematic way in the study population is thereby not only key for the investigation of individual differences in general, but can also be expected to facilitate generalization, replication and translation of results (Richter et al., 2009). Thorough statistical calculation of sample sizes and analytic strategies are further essential to align these efforts of study sample heterogenization with 1) the ethical point of view that experiments should be conducted in the smallest groups needed and 2) to provide sufficient power for statistical analyses (Button et al., 2013; Demétrio et al., 2013; Worp et al., 2010).
In sum, to foster research on individual differences across species and make study populations more representative at the same time, researchers may consider allowing more heterogeneous samples, employ dimensional characterisations rather than arbitrary groups and might re-design protocols tailored to allowing for the manifestation of individual differences.
Clinical perspective
Fear conditioning is a tool for the cross-species examination of basic affective learning mechanisms that shape adaptive, defensive responses against acute or imminent threats. Furthermore, in both, rodents and healthy human volunteers, fear conditioning is being used to study processes that contribute to maladaptive responses (for example in patients that suffer from anxiety-related disorders) as well as to offer insights on how to treat these disorders (Davis et al., 2006; Griebel and Holmes, 2013; Maren et al., 2013; Milad and Quirk, 2012; Morrison and Ressler, 2013; Singewald et al., 2015; Zuj and Norrholm, 2019). One example is the enhancement of exposure therapy by augmenting NMDA-receptor activity via administration of d-cycloserine. The clinical application was based on initial findings in fear conditioning experiments (Davis et al., 2006; Ledgerwood et al., 2003; Ressler et al., 2004), and the efficiency and specificity was further guided by examination of extinction learning processes in basic fear conditioning experiments (Hofmann et al., 2015; Mataix-Cols et al., 2017; Norberg et al., 2008; Smits et al., 2013).
Similarly, the blockade of noradrenaline transmission by propranolol has been examined as a prevention against the development of post-traumatic stress disorder, based on the modulatory effect of noradrenaline on the acquisition and extinction of conditioned fear responses in rodents (Berlau and McGaugh, 2006; Fitzgerald et al., 2015). Yet, several controlled studies in humans revealed no effect of propranolol administration directly after trauma (McGhee et al., 2009; Nugent et al., 2010; Stein et al., 2007). Future mechanistic studies might provide insights into how noradrenaline modulation (maybe in combination with safety learning procedures) can be used to treat PTSD (Giustino et al., 2016; Pitman et al., 2002).
However, the inferences drawn from fear conditioning experiments will probably only shed light on partial processes that contribute to psychopathology, as for example avoidance tendencies are not assessed (Beckers et al., 2013). As such, in some cases promising findings from experimental fear conditioning studies cannot directly be applied in the clinic (e.g., Maples-Keller et al., 2019).
Our aim is to improve the understanding of general aversive learning mechanisms by fostering cross-species comparability. In the long run, we aspire that our methodological considerations of basic mechanisms inspire discussion about the methodological challenges of fear conditioning experiments to allow drawing valid inferences about psychopathology.
GENERAL DISCUSSION
Our aim in this perspective is to provide the missing methodological considerations on cross-species research in fear conditioning. Thereby, we address the central question whether the theoretical promises of translational research can actually be met in practice. To answer this question, we provide a cross-species comparison of key methodological elements within protocols in humans and rodents. We further highlight the implication of these methodological differences for the comparability of procedures between species.
We argue that raising awareness for methodological differences between species sets the stage for practical guidance to avoid pitfalls when drawing conclusions from published experiments across species. Furthermore, we hope to aid designing of experiments that employ methods for more comparable processes in human participants and laboratory rodents in fear conditioning protocols. These practical considerations provide the basis of how cross-species translation can actually work.
However, inherent differences in experimental demands in humans and rodents make translation of a protocol, in its literal sense, word by word, often impossible. We argue that alignment of protocols can only be archived to a certain degree and we are not advocating for a gold standard how translational protocols should look like. Hence, simple alignment of superficial methodological details will not promote translational results. Instead, we are promoting to consider procedural and methodological differences across species to guide the interpretation and comparison of processes that underlie the results in rodents and humans. These considerations should ultimately allow for identification of processes in fear conditioning protocols that are comparable between humans and rodents. Furthermore, it also sets boundaries for translation of findings that are derived from procedures that probe divergent processes. Identification of such procedural and methodological gaps across species that limit inferences from rodents to humans, or vice versa, are important. These gaps should not be downplayed, but rather highlighted with the same emphasis as comparable cross-species mechanisms are promoted.
We envision with this perspective to further promote cross-species exchange that enables new perspectives beyond the methodology that is commonly used within one species. In our view, learning from practical insights into research in other species is often helpful to recognize species-specific pitfalls and to interpret work in other species correctly. We aspire that our perspective equips researchers with these practical and methodological considerations across species to ultimately support the dialog between researchers in translational science and remove hurdles in designing “tandem-projects” across species.
In sum, we propose to consider translational research as a framework for conceptual rather than identical replications. Our perspective suggests that such translational research encompasses not only comparable processes within humans and rodents, but also place emphasis on processes that diverge between species. Methodological comparisons across species provide the basis to evaluate these common and species-specific processes to guide interpretation of findings across rodent and human research in order to lay the grounds for successful translational research.
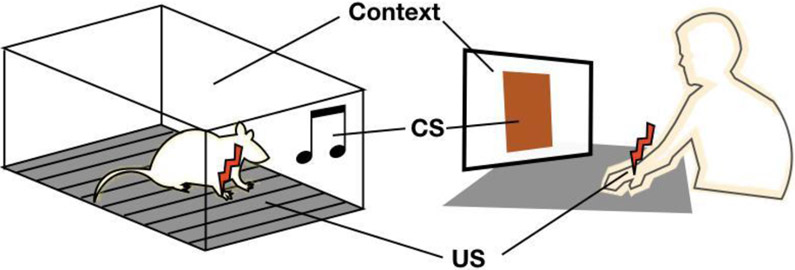
Figure 1:
Schematic illustration depicting key elements within an exemplified cued fear conditioning protocol in rodents (left) and humans (right). CS=conditioned stimulus, US=unconditioned stimulus
Acknowledgements:
This work was supported by a Scientific Network grant (LO 1980/2-1) awarded to TBL and CJM, funded by the German Research Foundation (DFG).
This work was supported by Project B10 (INST 211/755) to JH as well as project B07 (INST INST 211/633-2) to TBL of the Collaborative Research Center TRR58 “Fear, Anxiety, Anxiety Disorders”, funded by the German Research Foundation (DFG) Projectnumber 44541416.
This work was supported by Project A09 to CJM of the Collaborative Research Center 1280 “Extinction Learning”, funded by the German Research Foundation (DFG).
This work was supported by a grant to JR awarded by the Daimler and Benz foundation.
References:
- Acca GM, Mathew AS, Jin J, Maren S, Nagaya N, 2017. Allopregnanolone induces state-dependent fear via the bed nucleus of the stria terminalis. Horm Behav 89, 137–144. 10.1016/j.yhbeh.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C, 2008. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J. Neurosci 28, 6211–6219. 10.1523/JNEUROSCI.1246-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Adolphs R, 2014. A framework for studying emotions across species. Cell 157, 187–200. 10.1016/j.cell.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreatta M, Glotzbach-Schoon E, Mühlberger A, Schulz SM, Wiemer J, Pauli P, 2015. Initial and sustained brain responses to contextual conditioned anxiety in humans. Cortex 63, 352–363. 10.1016/j.cortex.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Archbold GE, Dobbek N, Nader K, 2013. Temporal dynamics of recovery from extinction shortly after extinction acquisition. Learn. Mem 20, 395–398. 10.1101/lm.028225.112 [DOI] [PubMed] [Google Scholar]
- Ardi Z, Albrecht A, Richter-Levin A, Saha R, Richter-Levin G, 2016. Behavioral profiling as a translational approach in an animal model of posttraumatic stress disorder. Neurobiol. Dis 88, 139–147. 10.1016/j.nbd.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Atlas LY, 2019. How instructions shape aversive learning: higher order knowledge, reversal learning, and the role of the amygdala. Current Opinion in Behavioral Sciences, Pain and Aversive Motivation 26, 121–129. 10.1016/j.cobeha.2018.12.008 [DOI] [Google Scholar]
- Atlas LY, Doll BB, Li J, Daw ND, Phelps EA, 2016. Instructed knowledge shapes feedback-driven aversive learning in striatum and orbitofrontal cortex, but not the amygdala. Elife 5 10.7554/eLife.15192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo TM, Volchan E, Imbiriba LA, Rodrigues EC, Oliveira JM, Oliveira LF, Lutterbach LG, Vargas CD, 2005. A freezing-like posture to pictures of mutilation. Psychophysiology 42, 255–260. 10.1111/j.1469-8986.2005.00287.x [DOI] [PubMed] [Google Scholar]
- Baas JM, Nugent M, Lissek S, Pine DS, Grillon C, 2004. Fear conditioning in virtual reality contexts: a new tool for the study of anxiety. Biol. Psychiatry 55, 1056–1060. 10.1016/j.biopsych.2004.02.024 [DOI] [PubMed] [Google Scholar]
- Baas JMP, Heitland I, 2015. The impact of cue learning, trait anxiety and genetic variation in the serotonin 1A receptor on contextual fear. Int J Psychophysiol 98, 506–514. 10.1016/j.ijpsycho.2014.10.016 [DOI] [PubMed] [Google Scholar]
- Baetu I, Baker AG, Darredeau C, Murphy RA, 2005. A comparative approach to cue competition with one and two strong predictors. Learn Behav 33, 160–171. [DOI] [PubMed] [Google Scholar]
- Baker AG, 1976. Learned Irrelevance and Learned Helplessness: Rats Learn that Stimuli, Reinforcers, and Responses are Uncorrelated. Journal of Experimental Psychology: Animal Behavior Processes. [Google Scholar]
- Baldi E, Bucherelli C, 2015. Brain sites involved in fear memory reconsolidation and extinction of rodents. Neurosci Biobehav Rev 53, 160–190. 10.1016/j.neubiorev.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Baldi E, Bucherelli C, 2005. The Inverted “U-Shaped” Dose-Effect Relationships in Learning and Memory: Modulation of Arousal and Consolidation. Nonlinearity Biol Toxicol Med 3, 9–21. 10.2201/nonlin.003.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E, Lorenzini CA, Bucherelli C, 2004. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem 81, 162–166. 10.1016/j.nlm.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Barnet RC, Hunt PS, 2005. Trace and long-delay fear conditioning in the developing rat. Learning & Behavior 33, 437–443. 10.3758/BF03193182 [DOI] [PubMed] [Google Scholar]
- Bastos AF, Vieira AS, Oliveira JM, Oliveira L, Pereira MG, Figueira I, Erthal FS, Volchan E, 2016. Stop or move: Defensive strategies in humans. Behavioural Brain Research 302, 252–262. 10.1016/j.bbr.2016.01.043 [DOI] [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE, Nader K, 2001. Fear conditioning and LTP in the lateral amygdala are sensitive to the same stimulus contingencies. Nature Neuroscience 4, 687. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR, 1995. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269, 1115–1118. [DOI] [PubMed] [Google Scholar]
- Beckers T, Krypotos A-M, Boddez Y, Effting M, Kindt M, 2013. What’s wrong with fear conditioning? Biol Psychol 92, 90–96. 10.1016/j.biopsycho.2011.12.015 [DOI] [PubMed] [Google Scholar]
- Bergstrom HC, Johnson LR, 2014. An organization of visual and auditory fear conditioning in the lateral amygdala. Neurobiol Learn Mem 116, 1–13. 10.1016/j.nlm.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL, 2006. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem 86, 123–32. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W, 2003. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 27, 169–190. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Erk S, Schürmann B, Mauer D, Michel K, Boecker H, Scheef L, Walter H, Zimmer A, 2012. Dynorphins regulate fear memory: from mice to men. J. Neurosci 32, 9335–9343. 10.1523/JNEUROSCI.1034-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, 2017. Translating dynamic defense patterns from rodents to people. Neuroscience & Biobehavioral Reviews, Translational Neuroscience & Mental Disorders: bridging the gap between animal models and the human condition 76, 22–28. 10.1016/j.neubiorev.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Hori K, 1989. An ethoexperimental approach to the study of defense, in: Ethoexperimental Approaches to the Study of Behavior, NATO Advanced Science Institutes Series Series D: Behavioural and Social Sciences, Vol. 48 Kluwer Academic/Plenum Publishers, New York, NY, US, pp. 114–136. [Google Scholar]
- Bodden C, van den Hove D, Lesch K-P, Sachser N, 2017. Impact of varying social experiences during life history on behaviour, gene expression, and vasopressin receptor gene methylation in mice. Sci Rep 7, 8719 10.1038/s41598-017-09292-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddez Y, Baeyens F, Luyten L, Vansteenwegen D, Hermans D, Beckers T, 2013. Rating data are underrated: Validity of US expectancy in human fear conditioning. Journal of Behavior Therapy and Experimental Psychiatry 44, 201–206. 10.1016/j.jbtep.2012.08.003 [DOI] [PubMed] [Google Scholar]
- Bohlen M, Hayes ER, Bohlen B, Bailoo J, Crabbe JC, Wahlsten D, 2014. Experimenter effects on behavioral test scores of eight inbred mouse strains under the influence of ethanol. Behav Brain Res 272, 46–54. 10.1016/j.bbr.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC, 1970. Species-specific defense reactions and avoidance learning. Psychological Review 77, 32–48. 10.1037/h0028589 [DOI] [Google Scholar]
- Borta A, Wöhr M, Schwarting RKW, 2006. Rat ultrasonic vocalization in aversively motivated situations and the role of individual differences in anxiety-related behavior. Behav. Brain Res 166, 271–280. 10.1016/j.bbr.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Bouton ME, 2004. Context and behavioral processes in extinction. Learn.Mem 11, 485–494. [DOI] [PubMed] [Google Scholar]
- Bouton ME, 2002. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry 52, 976–986. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Kenney FA, Rosengard C, 1990. State-dependent fear extinction with two benzodiazepine tranquilizers. Behav. Neurosci 104, 44–55. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Woods AM, Todd TP, 2014. Separation of time-based and trial-based accounts of the partial reinforcement extinction effect. Behavioural Processes, Associative and temporal learning: New directions 101, 23–31. 10.1016/j.beproc.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braem S, De Houwer J, Demanet J, Yuen KSL, Kalisch R, Brass M, 2017. Pattern Analyses Reveal Separate Experience-Based Fear Memories in the Human Right Amygdala. J. Neurosci 37, 8116–8130. 10.1523/JNEUROSCI.0908-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, 2002. Gender differences in trauma and posttraumatic stress disorder. J Gend Specif Med 5, 34–40. [PubMed] [Google Scholar]
- Brown JS, Kalish HI, Farber IE, 1951. Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. J Exp Psychol 41, 317–328. [DOI] [PubMed] [Google Scholar]
- Brydges NM, Whalley HC, Jansen MA, Merrifield GD, Wood ER, Lawrie SM, Wynne S-M, Day M, Fleetwood-Walker S, Steele D, Marshall I, Hall J, Holmes MC, 2013. Imaging conditioned fear circuitry using awake rodent fMRI. PLoS ONE 8, e54197 10.1371/journal.pone.0054197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublatzky F, Guerra P, Alpers GW, 2018. Verbal instructions override the meaning of facial expressions. Scientific Reports 8, 14988 10.1038/s41598-018-33269-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DEA, Sotres-Bayon F, LeDoux JE, 2007. Individual differences in fear: Isolating fear reactivity and fear recovery phenotypes. Journal of Traumatic Stress 20, 413–422. 10.1002/jts.20261 [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafo MR, 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14, 365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Cahill L, 2012. A half-truth is a whole lie: on the necessity of investigating sex influences on the brain. Endocrinology 153, 2541–2543. 10.1210/en.2011-2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Godsil BP, Jami S, Barad M, 2005. The L-type calcium channel blocker nifedipine impairs extinction, but not reduced contingency effects, in mice. Learn. Mem 12, 277–284. 10.1101/lm.88805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cándido A, González F, De IB, 2004. Safety signals from avoidance learning but not from yoked classical conditioning training pass both summation and retardation tests for inhibition. Behav Processes 66, 153–160. 10.1016/j.beproc.2004.01.011 [DOI] [PubMed] [Google Scholar]
- Capaldi EJ, 1967. A Sequential Hypothesis of Instrumental Learning11This work was supported in part by National Institute of Child Health and Human Development Research Grant HD 00949-02, in: Spence KW, Spence JT (Eds.), Psychology of Learning and Motivation. Academic Press, pp. 67–156. 10.1016/S0079-7421(08)60513-7 [DOI] [Google Scholar]
- Carrive P, 2000. Conditioned fear to environmental context: cardiovascular and behavioral components in the rat. Brain Research 858, 440–445. 10.1016/S0006-8993(00)02029-1 [DOI] [PubMed] [Google Scholar]
- Chang CH, Berke JD, Maren S, 2011. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS One 5, e11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CD, Benedict C, Schiöth HB, 2018. Experimenter gender and replicability in science. Science Advances 4, e1701427 10.1126/sciadv.1701427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau F, Lange MD, Jüngling K, Lesting J, Seidenbecher T, Pape H-C, 2012. Prevention of stress-impaired fear extinction through neuropeptide s action in the lateral amygdala. Neuropsychopharmacology 37, 1588–1599. 10.1038/npp.2012.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS, 2014. Policy: NIH to balance sex in cell and animal studies. Nature News 509, 282 10.1038/509282a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar M, 2003. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol. Psychiatry 53, 463–473. [DOI] [PubMed] [Google Scholar]
- Cover KK, Maeng LY, Lebrón-Milad K, Milad MR, 2014. Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Transl Psychiatry 4, e422 10.1038/tp.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CSM, Richardson R, 2018. A Brief Guide to Studying Fear in Developing Rodents: Important Considerations and Common Pitfalls. Current Protocols in Neuroscience 83, e44 10.1002/cpns.44 [DOI] [PubMed] [Google Scholar]
- Cunningham CL, 1979. Alcohol as a cue for extinction: State dependency produced by conditioned inhibition. Animal Learning & Behavior 7, 45–52. 10.3758/BF03209656 [DOI] [Google Scholar]
- Curzon P, Rustay NR, Browman KE, 2009. Cued and Contextual Fear Conditioning for Rodents, in: Buccafusco JJ (Ed.), Methods of Behavior Analysis in Neuroscience, Frontiers in Neuroscience. CRC Press/Taylor & Francis, Boca Raton (FL). [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR, 2013. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 11, 126 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldrup T, Remmes J, Lesting J, Gaburro S, Fendt M, Meuth P, Kloke V, Pape H-C, Seidenbecher T, 2015. Expression of freezing and fear-potentiated startle during sustained fear in mice. Genes Brain Behav. 14, 281–291. 10.1111/gbb.12211 [DOI] [PubMed] [Google Scholar]
- Davis M, Antoniadis EA, Amaral DG, Winslow JT, 2008. Acoustic startle reflex in rhesus monkeys: a review. Rev Neurosci 19, 171–185. [DOI] [PubMed] [Google Scholar]
- Davis M, Astrachan DI, 1978. Conditioned fear and startle magnitude: effects of different footshock or backshock intensities used in training. J Exp Psychol Anim Behav Process 4, 95–103. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R, 2006. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol. Psychiatry 60, 369–375. 10.1016/j.biopsych.2006.03.084 [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y, 1997. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Annals of the New York Academy of Sciences 821, 305–331. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C, 2010. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135. 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan MIC, van Well S, Visser RM, Scholte HS, van Wingen GA, Kindt M, 2018. The influence of acoustic startle probes on fear learning in humans. Sci Rep 8, 14552 10.1038/s41598-018-32646-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert J, Weber H, Villmann C, Lonsdorf TB, Richter J, Andreatta M, Arias-Vasquez A, Hommers L, Kent L, Schartner C, Cichon S, Wolf C, Schaefer N, von Collenberg CR, Wachter B, Blum R, Schümann D, Scharfenort R, Schumacher J, Forstner AJ, Baumann C, Schiele MA, Notzon S, Zwanzger P, Janzing JGE, Galesloot T, Kiemeney LA, Gajewska A, Glotzbach-Schoon E, Mühlberger A, Alpers G, Fydrich T, Fehm L, Gerlach AL, Kircher T, Lang T, Strohle A, Arolt V, Wittchen H-U, Kalisch R, Büchel C, Hamm A, Nothen MM, Romanos M, Domschke K, Pauli P, Reif A, 2017. GLRB allelic variation associated with agoraphobic cognitions, increased startle response and fear network activation: a potential neurogenetic pathway to panic disorder. Mol. Psychiatry 22, 1431–1439. 10.1038/mp.2017.2 [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA, 2006. Extending animal models of fear conditioning to humans. Biol Psychol 73, 39–48. 10.1016/j.biopsycho.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Demétrio CGB, Menten JFM, Leandro RA, Brien C, 2013. Experimental power considerations—Justifying replication for animal care and use committees. Poult Sci 92, 2490–2497. 10.3382/ps.2012-02731 [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, 2004. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 130, 355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, McGregor IS, 2001. Defensive behavior in rats towards predatory odors: a review. Neuroscience & Biobehavioral Reviews 25, 597–609. 10.1016/S0149-7634(01)00044-6 [DOI] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, Huang C, Cravatt BF, Glatt CE, Hill MN, Casey BJ, Lee FS, 2015. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun 6, 6395 10.1038/ncomms7395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte FH, Quiñones-Laracuente K, Quirk GJ, 2015. A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463. 10.1038/nature14030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, van den Hout MA, Baas JMP, 2015. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress Anxiety 32, 239–253. 10.1002/da.22353 [DOI] [PubMed] [Google Scholar]
- Duits P, Richter J, Baas JMP, Engelhard IM, Limberg-Thiesen A, Heitland I, Hamm AO, Cath DC, 2017. Enhancing effects of contingency instructions on fear acquisition and extinction in anxiety disorders. J Abnorm Psychol 126, 378–391. 10.1037/abn0000266 [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC, 2007. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behav. Neurosci 121, 635–642. 10.1037/0735-7044.121.4.635 [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Kroes MCW, Braren SH, Phelps EA, 2017. Threat intensity widens fear generalization gradients. Behav. Neurosci 131, 168–175. 10.1037/bne0000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Kroes MCW, Moscatelli CM, Evans MD, Davachi L, Phelps EA, 2018. Event segmentation protects emotional memories from competing experiences encoded close in time. Nature Human Behaviour 2, 291–299. 10.1038/s41562-018-0317-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Müller C, Kovacevic A, Tovote P, Lüthi A, 2017. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100. 10.1038/nature21047 [DOI] [PubMed] [Google Scholar]
- Falls WA, 2002. Fear-potentiated startle in mice. Curr Protoc Neurosci Chapter 8, Unit 8.11B. 10.1002/0471142301.ns0811bs19 [DOI] [PubMed] [Google Scholar]
- Falls WA, Carlson S, Turner JG, Willott JF, 1997. Fear-potentiated startle in two strains of inbred mice. Behav. Neurosci 111, 855–861. [PubMed] [Google Scholar]
- Fanselow MS, 2010. From contextual fear to a dynamic view of memory systems. Trends Cogn. Sci. (Regul. Ed.) 14, 7–15. 10.1016/j.tics.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, 1994. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev 1, 429–438. 10.3758/BF03210947 [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Bolles RC, 1979. Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol 93, 736–744. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Pennington ZT, 2018. A return to the psychiatric dark ages with a two-system framework for fear. Behav Res Ther 100, 24–29. 10.1016/j.brat.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS, 1999. The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience & Biobehavioral Reviews 23, 743. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M, 2013. Translational value of startle modulations. Cell Tissue Res. 354, 287–295. 10.1007/s00441-013-1599-5 [DOI] [PubMed] [Google Scholar]
- Fenster RJ, Lebois LAM, Ressler KJ, Suh J, 2018. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nature Reviews Neuroscience 19, 535–551. 10.1038/s41583-018-0039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie PSB, Gamache K, Protopoulos M, Sinclair E, Baker AG, Wang S-H, Nader K, 2018. Cortico-hippocampal Schemas Enable NMDAR-Independent Fear Conditioning in Rats. Curr. Biol 28, 2900–2909.e5. 10.1016/j.cub.2018.07.037 [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Giustino TF, Seemann JR, Maren S, 2015. Noradrenergic blockade stabilizes prefrontal activity and enables fear extinction under stress. Proc. Natl. Acad. Sci. U.S.A 112, E3729–3737. 10.1073/pnas.1500682112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LP, Cockburn IM, Simcoe TS, 2015. The Economics of Reproducibility in Preclinical Research. PLOS Biology 13, e1002165 10.1371/journal.pbio.1002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Bryant RA, 2013. 636,120 Ways to Have Posttraumatic Stress Disorder. Perspect Psychol Sci 8, 651–662. 10.1177/1745691613504115 [DOI] [PubMed] [Google Scholar]
- Gazendam FJ, Kamphuis JH, Kindt M, 2013. Deficient safety learning characterizes high trait anxious individuals. Biol Psychol 92, 342–352. 10.1016/j.biopsycho.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Gerber B, Yarali A, Diegelmann S, Wotjak CT, Pauli P, Fendt M, 2014. Pain-relief learning in flies, rats, and man: basic research and applied perspectives. Learn. Mem 21, 232–252. 10.1101/lm.032995.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman SJ, Monfils M-H, Norman KA, Niv Y, 2017. The computational nature of memory modification [WWW Document]. eLife. 10.7554/eLife.23763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Chattarji S, 2015. Neuronal encoding of the switch from specific to generalized fear. Nat. Neurosci 18, 112–120. 10.1038/nn.3888 [DOI] [PubMed] [Google Scholar]
- Giustino TF, Fitzgerald PJ, Maren S, 2016. Revisiting propranolol and PTSD: Memory erasure or extinction enhancement? Neurobiol Learn Mem 130, 26–33. 10.1016/j.nlm.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin TE, Hashemi MM, van Ast V, Roelofs K, 2016. Ready and waiting: Freezing as active action preparation under threat. Neurosci. Lett 619, 182–188. 10.1016/j.neulet.2016.03.027 [DOI] [PubMed] [Google Scholar]
- Glenn CR, Lieberman L, Hajcak G, 2012. Comparing electric shock and a fearful screaming face as unconditioned stimuli for fear learning. Int J Psychophysiol 86, 214–219. 10.1016/j.ijpsycho.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzbach E, Ewald H, Andreatta M, Pauli P, Mühlberger A, 2012. Contextual fear conditioning predicts subsequent avoidance behaviour in a virtual reality environment. Cognition and Emotion 26, 1256–1272. 10.1080/02699931.2012.656581 [DOI] [PubMed] [Google Scholar]
- Glotzbach-Schoon E, Andreatta M, Reif A, Ewald H, Tröger C, Baumann C, Deckert J, Mühlberger A, Pauli P, 2013. Contextual fear conditioning in virtual reality is affected by 5HTTLPR and NPSR1 polymorphisms: effects on fear-potentiated startle. Front Behav Neurosci 7, 31 10.3389/fnbeh.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Norrholm SD, 2015. Estrogen and Extinction of Fear Memories: Implications for Posttraumatic Stress Disorder Treatment. Biol Psychiatry 78, 178–185. 10.1016/j.biopsych.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Bellander M, Öhman A, 2013. Temporal properties of fear extinction--does time matter? Behav. Neurosci 127, 59–69. 10.1037/a0030892 [DOI] [PubMed] [Google Scholar]
- Goode TD, Maren S, 2014. Animal models of fear relapse. ILAR J 55, 246–258. 10.1093/ilar/ilu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Harnett NG, Knight DC, 2018. Pavlovian conditioned diminution of the neurobehavioral response to threat. Neurosci Biobehav Rev 84, 218–224. 10.1016/j.neubiorev.2017.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Hobin JA, Maren S, 2003. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: mnemonic code or fear bias? Neuron 40, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Grady AK, Bowen KH, Hyde AT, Totsch SK, Knight DC, 2016. Effect of continuous and partial reinforcement on the acquisition and extinction of human conditioned fear. Behav. Neurosci 130, 36–43. 10.1037/bne0000121 [DOI] [PubMed] [Google Scholar]
- Graham FK, Clifton RK, 1966. Heart-rate change as a component of the orienting response. Psychol.Bull 65, 305. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holmes A, 2013. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov 12, 667–687. 10.1038/nrd4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, 2002. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol. Psychiatry 52, 958–975. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M, 1991. Fear-Potentiated Startle in Humans: Effects of Anticipatory Anxiety on the Acoustic Blink Reflex. Psychophysiology 28, 588–595. 10.1111/j.1469-8986.1991.tb01999.x [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J, 2003. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol 114, 1557–1579. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Cornwell B, Johnson L, 2006. Context Conditioning and Behavioral Avoidance in a Virtual Reality Environment: Effect of Predictability. Biological Psychiatry 60, 752–759. 10.1016/j.biopsych.2006.03.072 [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM, 2015a. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4 10.7554/eLife.11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM, 2015b. Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol. Psychiatry 78, 186–193. 10.1016/j.biopsych.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K, 2014. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. 10.1016/j.cell.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Gaburro S, Sah A, Gartmann N, Lonsdorf TB, Meier K, Singewald N, Pape H-C, Morellini F, Kalisch R, 2013a. Single dose of L-dopa makes extinction memories context-independent and prevents the return of fear. Proc. Natl. Acad. Sci. U.S.A 110, E2428–2436. 10.1073/pnas.1303061110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Golkar A, Hermans D, Lonsdorf TB, 2014. A review on human reinstatement studies: an overview and methodological challenges. Learn. Mem 21, 424–440. 10.1101/lm.036053.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Lonsdorf TB, Schümann D, Bunzeck N, Peters J, Sommer T, Kalisch R, 2017. Where There is Smoke There is Fear-Impaired Contextual Inhibition of Conditioned Fear in Smokers. Neuropsychopharmacology 42, 1640–1646. 10.1038/npp.2017.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Lonsdorf TB, Schümann D, Menz M, Brassen S, Bunzeck N, Gamer M, Kalisch R, 2015. Deficient inhibitory processing in trait anxiety: Evidence from context-dependent fear learning, extinction recall and renewal. Biol Psychol 111, 65–72. 10.1016/j.biopsycho.2015.07.010 [DOI] [PubMed] [Google Scholar]
- Haaker J, Lonsdorf TB, Thanellou A, Kalisch R, 2013b. Multimodal Assessment of Long-Term Memory Recall and Reinstatement in a Combined Cue and Context Fear Conditioning and Extinction Paradigm in Humans. PLoS ONE 8, e76179 10.1371/journal.pone.0076179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad ADM, Pritchett D, Lissek S, Lau JYF, 2012. Trait anxiety and fear responses to safety cues: Stimulus generalization or sensitization? Journal of Psychopathology and Behavioral Assessment 34, 323–331. 10.1007/s10862-012-9284-7 [DOI] [Google Scholar]
- Hagenaars MA, Oitzl M, Roelofs K, 2014. Updating freeze: aligning animal and human research. Neurosci Biobehav Rev 47, 165–176. 10.1016/j.neubiorev.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Hamm AO, Greenwald MK, Bradley MM, Lang PJ, 1993. Emotional learning, hedonic change, and the startle probe. J Abnorm Psychol 102, 453–465. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Richter J, Pané-Farré C, Westphal D, Wittchen H-U, Vossbeck-Elsebusch AN, Gerlach AL, Gloster AT, Strohle A, Lang T, Kircher T, Gerdes ABM, Alpers GW, Reif A, Deckert J, 2016. Panic disorder with agoraphobia from a behavioral neuroscience perspective: Applying the research principles formulated by the Research Domain Criteria (RDoC) initiative. Psychophysiology 53, 312–322. 10.1111/psyp.12553 [DOI] [PubMed] [Google Scholar]
- Hamm AO, Vaitl D, 1996. Affective learning: awareness and aversion. Psychophysiology 33, 698–710. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI, 2005. The neuropsychology of fear learning and fear regulation. Int J Psychophysiol 57, 5–14. [DOI] [PubMed] [Google Scholar]
- Harris AP, Lennen RJ, Brydges NM, Jansen MA, Pernet CR, Whalley HC, Marshall I, Baker S, Basso AM, Day M, Holmes MC, Hall J, 2016. The role of brain-derived neurotrophic factor in learned fear processing: an awake rat fMRI study. Genes Brain Behav. 15, 221–230. 10.1111/gbb.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AP, Lennen RJ, Marshall I, Jansen MA, Pernet CR, Brydges NM, Duguid IC, Holmes MC, 2015. Imaging learned fear circuitry in awake mice using fMRI. Eur. J. Neurosci 42, 2125–2134. 10.1111/ejn.12939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headrick MW, Graham FK, 1969. Multiple-component heart rate responses conditioned under paced respiration. J Exp Psychol 79, 486–494. [DOI] [PubMed] [Google Scholar]
- Hedge C, Powell G, Sumner P, 2018. The reliability paradox: Why robust cognitive tasks do not produce reliable individual differences. Behav Res 50, 1166–1186. 10.3758/s13428-017-0935-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitland I, Groenink L, van Gool JM, Domschke K, Reif A, Baas JMP, 2016. Human fear acquisition deficits in relation to genetic variants of the corticotropin-releasing hormone receptor 1 and the serotonin transporter--revisited. Genes Brain Behav. 15, 209–220. 10.1111/gbb.12276 [DOI] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A, 2010. The weirdest people in the world? Behavioral and Brain Sciences 33, 61–83. 10.1017/S0140525X0999152X [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A, 2008. Switching on and off fear by distinct neuronal circuits. Nature 454, 600–6. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson M, 2003. A twin study of the genetics of fear conditioning. Arch. Gen. Psychiatry 60, 702–708. 10.1001/archpsyc.60.7.702 [DOI] [PubMed] [Google Scholar]
- Hodes RL, Cook EW, Lang PJ, 1985. Individual differences in autonomic response: conditioned association or conditioned fear? Psychophysiology 22, 545–560. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Parga A, Paode PR, Watterson LR, Nikulina EM, Hammer RP, Conrad CD, 2015. Chronic stress enhanced fear memories are associated with increased amygdala zif268 mRNA expression and are resistant to reconsolidation. Neurobiol Learn Mem 120, 61–68. 10.1016/j.nlm.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Otto MW, Pollack MH, Smits JA, 2015. D-cycloserine augmentation of cognitive behavioral therapy for anxiety disorders: an update. Curr Psychiatry Rep 17, 532 10.1007/s11920-014-0532-2 [DOI] [PubMed] [Google Scholar]
- Holmes A, Singewald N, 2013. Individual differences in recovery from traumatic fear. Trends Neurosci. 36, 23–31. 10.1016/j.tins.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Zielinski DJ, Fecteau ME, Brady R, LaBar KS, 2010. Human Fear Conditioning Conducted in Full Immersion 3-Dimensional Virtual Reality. J Vis Exp. 10.3791/1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, 1988. Dissociation of associative and nonassociative concomitants of classical fear conditioning in the freely behaving rat. Behav. Neurosci 102, 66–76. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Bberg WK, Hutcheson JS, Obrist P, Porges S, Turpin G, 1981. Publication Guidelines for Heart Rate Studies in Man. Psychophysiology 18, 226–231. 10.1111/j.1469-8986.1981.tb03023.x [DOI] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E, 2012. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 337, 816–821. 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE, 2011. Molecular mechanisms of fear learning and memory. Cell 147, 509–524. 10.1016/j.cell.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüngling K, Lange MD, Szkudlarek HJ, Lesting J, Erdmann FS, Doengi M, Kügler S, Pape H-C, 2015. Increased GABAergic Efficacy of Central Amygdala Projections to Neuropeptide S Neurons in the Brainstem During Fear Memory Retrieval. Neuropsychopharmacology 40, 2753–2763. 10.1038/npp.2015.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu Y-L, Reinscheid RK, Pape H-C, 2008. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron 59, 298–310. 10.1016/j.neuron.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ, 2006. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J. Neurosci 26, 9503–9511. 10.1523/JNEUROSCI.2021-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin LJ, 1968. “Attention-like” processes in classical conditioning, in: Miami Symposium on the Prediction of Behavior, 1967: Aversive Stimulation. University of Miami Press, pp. 9–31. [Google Scholar]
- Kamin LJ, 1961. Apparent adaptation effects in the acquisition of a conditioned emotional response. Can J Psychol 15, 176–188. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Wotjak CT, 2004. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn. Mem 11, 770–786. 10.1101/lm.86104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR, 2014. The molecular and systems biology of memory. Cell 157, 163–186. 10.1016/j.cell.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Kendler KS, 2013. What psychiatric genetics has taught us about the nature of psychiatric illness and what is left to learn. Mol. Psychiatry 18, 1058–1066. 10.1038/mp.2013.50 [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R, 2010. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol. Psychiatry 67, 297–303. 10.1016/j.biopsych.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R, 2009. Expression of renewal is dependent on the extinction-test interval rather than the acquisition-extinction interval. Behav. Neurosci 123, 641–649. 10.1037/a0015237 [DOI] [PubMed] [Google Scholar]
- King G, Scott E, Graham BM, Richardson R, 2017. Individual differences in fear extinction and anxiety-like behavior. Learn. Mem 24, 182–190. 10.1101/lm.045021.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The ’Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28, 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Redondo RL, Tonegawa S, 2017. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78. 10.1126/science.aam6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Waters NS, King MK, Bandettini PA, 2010. Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage 49, 843–848. 10.1016/j.neuroimage.2009.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, 1999. The neurobiology of startle. Prog. Neurobiol 59, 107–128. [DOI] [PubMed] [Google Scholar]
- Kola I, Landis J, 2004. Can the pharmaceutical industry reduce attrition rates? Nature Reviews Drug Discovery 3, 711–716. 10.1038/nrd1470 [DOI] [PubMed] [Google Scholar]
- Kong E, Monje FJ, Hirsch J, Pollak DD, 2014. Learning not to fear: neural correlates of learned safety. Neuropsychopharmacology 39, 515–527. 10.1038/npp.2013.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Cacioppo JT, Davidson RJ, Hugdahl K, Lovallo WR, Spiegel D, Rose R, 2002. Bridging psychology and biology. The analysis of individuals in groups. Am Psychol 57, 341–351. [PubMed] [Google Scholar]
- Kroes MCW, Dunsmoor JE, Mackey WE, McClay M, Phelps EA, 2017. Context conditioning in humans using commercially available immersive Virtual Reality. Sci Rep 7, 8640 10.1038/s41598-017-08184-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M, Wendt J, Lindner K, Lonsdorf TB, 2015. THE NEURAL CORRELATES OF AFFECTIVE STARTLE MODULATION-RESULTS FROM PARALLEL EMG-FMRI. Presented at the Psychophysiology, p. S61. [Google Scholar]
- LaBar KS, Phelps EA, 2005. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav.Neurosci 119, 677–686. [DOI] [PubMed] [Google Scholar]
- LaBar Kevin S, Phelps EA, 2005. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav. Neurosci 119, 677–686. 10.1037/0735-7044.119.3.677 [DOI] [PubMed] [Google Scholar]
- Landis C, Hunt WA, Strauss H, 1939. The startle pattern. Farrar & Rinehart, Inc., New York. [Google Scholar]
- Lang PJ, Davis M, Öhman A, 2000. Fear and anxiety: animal models and human cognitive psychophysiology. Journal of affective disorders 61, 137–159. [DOI] [PubMed] [Google Scholar]
- Lauer AM, Behrens D, Klump G, 2017. Acoustic startle modification as a tool for evaluating auditory function of the mouse: Progress, pitfalls, and potential. Neurosci Biobehav Rev 77, 194–208. 10.1016/j.neubiorev.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmi TR, Stork O, Pape H-C, 2003. Generalisation of conditioned fear and its behavioural expression in mice. Behav. Brain Res 145, 89–98. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR, 2012. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord 2, 3 10.1186/2045-5380-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J, 2003. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci 117, 341–9. [DOI] [PubMed] [Google Scholar]
- LeDoux J, 2012. Rethinking the emotional brain. Neuron 73, 653–676. 10.1016/j.neuron.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, Daw ND, 2018. Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behaviour. Nature Reviews Neuroscience 19, 269–282. 10.1038/nrn.2018.22 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, 2014. Coming to terms with fear. PNAS 111, 2871–2878. 10.1073/pnas.1400335111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Pine DS, 2016. Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. AJP 173, 1083–1093. 10.1176/appi.ajp.2016.16030353 [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, Herry C, Lüthi A, 2011. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335. 10.1038/nature10674 [DOI] [PubMed] [Google Scholar]
- Li M, D’Arcy C, Meng X, 2016. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta-analysis, and proportional attributable fractions. Psychological Medicine 46, 717–730. 10.1017/S0033291715002743 [DOI] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA, 2014. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat. Neurosci 17, 106–113. 10.1038/nn.3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner K, Neubert J, Pfannmöller J, Lotze M, Hamm AO, Wendt J, 2015. Fear-potentiated startle processing in humans: Parallel fMRI and orbicularis EMG assessment during cue conditioning and extinction. Int J Psychophysiol. 10.1016/j.ijpsycho.2015.02.025 [DOI] [PubMed] [Google Scholar]
- Lipp OV, 2006. Human fear learning: Contemporary procedures and measurement, in: Fear and Learning: From Basic Processes to Clinical Implications. American Psychological Association, pp. 37–52. [Google Scholar]
- Lipp OV, Vaitl D, 1990. Reaction time task as unconditional stimulus. Comparing aversive and nonaversive unconditional stimuli. Pavlov J Biol Sci 25, 77–83. [DOI] [PubMed] [Google Scholar]
- Lissek Shmuel, Baas JMP, Pine DS, Orme K, Dvir S, Nugent M, Rosenberger E, Rawson E, Grillon C, 2005. Airpuff startle probes: An efficacious and less aversive alternative to white-noise. Biological Psychology 68, 283–297. 10.1016/j.biopsycho.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C, 2006. The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology 72, 265–270. 10.1016/j.biopsycho.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS, 2005. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther 43, 1391–424. [DOI] [PubMed] [Google Scholar]
- Lohr JM, Olatunji BO, Sawchuk CN, 2007. A functional analysis of danger and safety signals in anxiety disorders. Clinical Psychology Review 27, 114–126. 10.1016/j.cpr.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Haaker J, Kalisch R, 2014. Long-term expression of human contextual fear and extinction memories involves amygdala, hippocampus and ventromedial prefrontal cortex: a reinstatement study in two independent samples. Soc Cogn Affect Neurosci. 10.1093/scan/nsu018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Kalisch R, 2011. A review on experimental and clinical genetic associations studies on fear conditioning, extinction and cognitive-behavioral treatment. Transl Psychiatry 1, e41 10.1038/tp.2011.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, Heitland I, Hermann A, Kuhn M, Kruse O, Meir Drexler S, Meulders A, Nees F, Pittig A, Richter J, Römer S, Shiban Y, Schmitz A, Straube B, Vervliet B, Wendt J, Baas JMP, Merz CJ, 2017. Don’t fear “fear conditioning”: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev 77, 247–285. 10.1016/j.neubiorev.2017.02.026 [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Merz CJ, 2017. More than just noise: Inter-individual differences in fear acquisition, extinction and return of fear in humans - Biological, experiential, temperamental factors, and methodological pitfalls. Neurosci Biobehav Rev 80, 703–728. 10.1016/j.neubiorev.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Lueken U, Kuhn M, Yang Y, Straube B, Kircher T, Wittchen H-U, Pfleiderer B, Arolt V, Wittmann A, Ströhle A, Weber H, Reif A, Domschke K, Deckert J, Lonsdorf TB, 2017. Modulation of defensive reactivity by GLRB allelic variation: converging evidence from an intermediate phenotype approach. Transl Psychiatry 7, e1227 10.1038/tp.2017.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Hu X, Liu Xiang, Ma X, Guo W, Qiu C, Wang Y, Wang Q, Zhang X, Zhang W, Hannum G, Zhang K, Liu Xiehe, Li T, 2012. Hair cortisol level as a biomarker for altered hypothalamic-pituitary-adrenal activity in female adolescents with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biol. Psychiatry 72, 65–69. 10.1016/j.biopsych.2011.12.020 [DOI] [PubMed] [Google Scholar]
- Macleod MR, Michie S, Roberts I, Dirnagl U, Chalmers I, Ioannidis JPA, Salman RA-S, Chan A-W, Glasziou P, 2014. Biomedical research: increasing value, reducing waste. The Lancet 383, 101–104. 10.1016/S0140-6736(13)62329-6 [DOI] [PubMed] [Google Scholar]
- Maes E, Boddez Y, Alfei JM, Krypotos A-M, D’Hooge R, De Houwer J, Beckers T, 2016. The elusive nature of the blocking effect: 15 failures to replicate. J Exp Psychol Gen 145, e49–71. 10.1037/xge0000200 [DOI] [PubMed] [Google Scholar]
- Maples-Keller JL, Jovanovic T, Dunlop BW, Rauch S, Yasinski C, Michopoulos V, Coghlan C, Norrholm S, Rizzo AS, Ressler K, Rothbaum BO, 2019. When translational neuroscience fails in the clinic: Dexamethasone prior to virtual reality exposure therapy increases drop-out rates. Journal of Anxiety Disorders, Virtual reality applications for the anxiety disorders 61, 89–97. 10.1016/j.janxdis.2018.10.006 [DOI] [PubMed] [Google Scholar]
- Maren S, 2014. Nature and causes of the immediate extinction deficit: a brief review. Neurobiol Learn Mem 113, 19–24. 10.1016/j.nlm.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, 2001. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci 24, 897–931. 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- Maren S, Chang C, 2006. Recent fear is resistant to extinction. Proc Natl Acad Sci U S A 103, 18020–18025. 10.1073/pnas.0608398103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS, 1994. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 661, 25–34. [DOI] [PubMed] [Google Scholar]
- Maren S, Holmes A, 2016. Stress and Fear Extinction. Neuropsychopharmacology 41, 58–79. 10.1038/npp.2015.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I, 2013. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci 14, 417–428. 10.1038/nrn3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ, 2004. Neuronal signalling of fear memory. Nature Reviews Neuroscience 5, 844–852. 10.1038/nrn1535 [DOI] [PubMed] [Google Scholar]
- Marquand AF, Wolfers T, Mennes M, Buitelaar J, Beckmann CF, 2016. Beyond Lumping and Splitting: A Review of Computational Approaches for Stratifying Psychiatric Disorders. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, Model-Based and Machine-Learning Approaches in Applied Computational Psychiatry 1, 433–447. 10.1016/j.bpsc.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C, 2008. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J. Neurosci 28, 9030–9036. 10.1523/JNEUROSCI.1651-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-Cols D, Fernández de la Cruz L, Monzani B, Rosenfield D, Andersson E, Pérez-Vigil A, Frumento P, de Kleine RA, Difede J, Dunlop BW, Farrell LJ, Geller D, Gerardi M, Guastella AJ, Hofmann SG, Hendriks G-J, Kushner MG, Lee FS, Lenze EJ, Levinson CA, McConnell H, Otto MW, Plag J, Pollack MH, Ressler KJ, Rodebaugh TL, Rothbaum BO, Scheeringa MS, Siewert-Siegmund A, Smits JAJ, Storch EA, Ströhle A, Tart CD, Tolin DF, van Minnen A, Waters AM, Weems CF, Wilhelm S, Wyka K, Davis M, Rück C, the DCS Anxiety Consortium, Altemus M, Anderson P, Cukor J, Finck C, Geffken GR, Golfels F, Goodman WK, Gutner C, Heyman I, Jovanovic T, Lewin AB, McNamara JP, Murphy TK, Norrholm S, Thuras P, 2017. D-Cycloserine Augmentation of Exposure-Based Cognitive Behavior Therapy for Anxiety, Obsessive-Compulsive, and Posttraumatic Stress Disorders: A Systematic Review and Meta-analysis of Individual Participant Data. JAMA Psychiatry 74, 501–510. 10.1001/jamapsychiatry.2016.3955 [DOI] [PubMed] [Google Scholar]
- McGaugh JL, 2000. Memory--a century of consolidation. Science 287, 248–251. [DOI] [PubMed] [Google Scholar]
- McGhee LL, Maani CV, Garza TH, Desocio PA, Gaylord KM, Black IH, 2009. The effect of propranolol on posttraumatic stress disorder in burned service members. J Burn Care Res 30, 92–97. 10.1097/BCR.0b013e3181921f51 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, Heleniak C, Shechner T, Wojcieszak Z, Pine DS, 2016. Maltreatment Exposure, Brain Structure, and Fear Conditioning in Children and Adolescents. Neuropsychopharmacology 41, 1956–1964. 10.1038/npp.2015.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias M-L, Etkin A, Kalisch R, 2010. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage 49, 1760–1768. 10.1016/j.neuroimage.2009.09.040 [DOI] [PubMed] [Google Scholar]
- Merrill KA, Steinmetz JE, Viken RJ, Rose RJ, 1999. Genetic influences on human conditionability: a twin study of the conditioned eyeblink response. Behav. Genet 29, 95–102. [DOI] [PubMed] [Google Scholar]
- Mertens G, Boddez Y, Sevenster D, Engelhard IM, De Houwer J, 2018a. A review on the effects of verbal instructions in human fear conditioning: Empirical findings, theoretical considerations, and future directions. Biol Psychol 137, 49–64. 10.1016/j.biopsycho.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Mertens G, Braem S, Kuhn M, Lonsdorf TB, van den Hout MA, Engelhard IM, 2018b. Does US expectancy mediate the additive effects of CS-US pairings on contingency instructions? Results from subjective, psychophysiological and neural measures. Behav Res Ther 110, 41–46. 10.1016/j.brat.2018.09.003 [DOI] [PubMed] [Google Scholar]
- Mertens G, Kuhn M, Raes AK, Kalisch R, De Houwer J, Lonsdorf TB, 2016. Fear expression and return of fear following threat instruction with or without direct contingency experience. Cogn Emot 30, 968–984. 10.1080/02699931.2015.1038219 [DOI] [PubMed] [Google Scholar]
- Merz CJ, Hamacher-Dang TC, Wolf OT, 2016. Immediate extinction promotes the return of fear. Neurobiol Learn Mem 131, 109–116. 10.1016/j.nlm.2016.03.013 [DOI] [PubMed] [Google Scholar]
- Merz CJ, Kinner VL, Wolf OT, 2018. Let’s talk about sex … differences in human fear conditioning. Current Opinion in Behavioral Sciences, Sex and Gender 23, 7–12. 10.1016/j.cobeha.2018.01.021 [DOI] [Google Scholar]
- Meulders A, Vervliet B, Fonteyne R, Baeyens F, Hermans D, Vansteenwegen D, 2012. Preexposure to (un)predictable shock modulates discriminative fear learning between cue and context: an investigation of the interaction between fear and anxiety. Int J Psychophysiol 84, 180–187. 10.1016/j.ijpsycho.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Milad Mohammed R, Igoe SA, Lebron-Milad K, Novales JE, 2009. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164, 887–895. 10.1016/j.neuroscience.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad Mohammed R., Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL, 2009. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol Psychiatry 66, 1075–1082. 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, 2012. Fear Extinction as a Model for Translational Neuroscience: Ten Years of Progress. Annual Review of Psychology 63, 129–151. 10.1146/annurev.psych.121208.131631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL, 2007. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 62, 446–454. 10.1016/j.biopsych.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Milligan-Saville JS, Graham BM, 2016. Mothers do it differently: reproductive experience alters fear extinction in female rats and women. Transl Psychiatry 6, e928 10.1038/tp.2016.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Ohman A, 2002. Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biol.Psychiatry 52, 927. [DOI] [PubMed] [Google Scholar]
- Monroe SM, 2008. Modern Approaches to Conceptualizing and Measuring Human Life Stress. Annu. Rev. Clin. Psychol 4, 33–52. 10.1146/annurev.clinpsy.4.022007.141207 [DOI] [PubMed] [Google Scholar]
- Morgan SJ, Paolini AG, 2012. Behavioral determination of stimulus pair discrimination of auditory acoustic and electrical stimuli using a classical conditioning and heart-rate approach. J Vis Exp e3598 10.3791/3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison FG, Ressler KJ, 2013. FROM THE NEUROBIOLOGY OF EXTINCTION TO IMPROVED CLINICAL TREATMENTS. Depress Anxiety. 10.1002/da.22214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M, 2006. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn. Mem 13, 216–223. 10.1101/lm.119806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF, 2008. A Meta-Analysis of D-Cycloserine and the Facilitation of Fear Extinction and Exposure Therapy. Biological Psychiatry 63, 1118–1126. 10.1016/j.biopsych.2008.01.012 [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Anderson KM, Olin IW, Jovanovic T, Kwon C, Warren VT, McCarthy A, Bosshardt L, Sabree J, Duncan EJ, Rothbaum BO, Bradley B, 2011. Versatility of Fear-Potentiated Startle Paradigms for Assessing Human Conditioned Fear Extinction and Return of Fear. Front Behav Neurosci 5 10.3389/fnbeh.2011.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Briscione MA, Anderson KM, Kwon CK, Warren VT, Bosshardt L, Bradley B, 2014. Generalization of fear-potentiated startle in the presence of auditory cues: a parametric analysis. Front Behav Neurosci 8, 361 10.3389/fnbeh.2014.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ, 2006. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn. Mem 13, 681–685. 10.1101/lm.393906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M, Rothbaum B, Duncan EJ, 2008. Timing of extinction relative to acquisition: a parametric analysis of fear extinction in humans. Behav. Neurosci 122, 1016–1030. 10.1037/a0012604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent NR, Christopher NC, Crow JP, Browne L, Ostrowski S, Delahanty DL, 2010. The efficacy of early propranolol administration at reducing PTSD symptoms in pediatric injury patients: a pilot study. J Trauma Stress 23, 282–287. 10.1002/jts.20517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A, 2009. Methodological approaches to studying the human amygdala, in: The Human Amygdala. New York: : Guilford Press. [Google Scholar]
- Öhman A, Dimberg U, 1978. Facial expressions as conditioned stimuli for electrodermal responses: A case of" preparedness"? Journal of Personality and Social Psychology 36, 1251. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA, 2007. Social learning of fear. Nat Neurosci 10, 1095–1102. 10.1038/nn1968 [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S, 2012. Neural and cellular mechanisms of fear and extinction memory formation. Neuroscience and Biobehavioral Reviews. 10.1016/j.neubiorev.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ, 2013. Implications of memory modulation for post-traumatic stress and fear disorders. Nat. Neurosci 16, 146–153. 10.1038/nn.3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Crawley JN, 1997. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl.) 132, 169–180. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Dickinson A, 1975. Pavlovian counterconditioning: changing the suppressive properties of shock by association with food. J Exp Psychol Anim Behav Process 1, 170–177. [DOI] [PubMed] [Google Scholar]
- Perusini JN, Fanselow MS, 2015. Neurobehavioral perspectives on the distinction between fear and anxiety. Learn. Mem 22, 417–425. 10.1101/lm.039180.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M, 2001. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience 4, 437–441. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP, 2002. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol. Psychiatry 51, 189–192. [DOI] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Ruttorf M, Schad LR, Flor H, 2012. Activation of the ventral striatum during aversive contextual conditioning in humans. Biol Psychol 91, 74–80. 10.1016/j.biopsycho.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Pryce CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J, 2005. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev 29, 649–674. 10.1016/j.neubiorev.2005.03.011 [DOI] [PubMed] [Google Scholar]
- Razran G, 1955. Conditioning and perception. Psychol Rev 62, 83–95. [DOI] [PubMed] [Google Scholar]
- Réale D, Dingemanse NJ, Kazem AJN, Wright J, 2010. Evolutionary and ecological approaches to the study of personality. Philos. Trans. R. Soc. Lond., B, Biol. Sci 365, 3937–3946. 10.1098/rstb.2010.0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed V, Wittchen HU, 1998. DSM-IV panic attacks and panic disorder in a community sample of adolescents and young adults: how specific are panic attacks? J Psychiatr Res 32, 335–345. [DOI] [PubMed] [Google Scholar]
- Reichenberger J, Porsch S, Wittmann J, Zimmermann V, Shiban Y, 2017. Social Fear Conditioning Paradigm in Virtual Reality: Social vs. Electrical Aversive Conditioning. Front. Psychol 8 10.3389/fpsyg.2017.01979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmes J, Bodden C, Richter SH, Lesting J, Sachser N, Pape H-C, Seidenbecher T, 2016. Impact of Life History on Fear Memory and Extinction. Front Behav Neurosci 10, 185 10.3389/fnbeh.2016.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla R, Wagner A, 1972. Variations in the Effectiveness of Reinforcement and Nonreinforcement New York: Classical Conditioning II: Current Research and Theory, Appleton-Century-Crofts. [Google Scholar]
- Rescorla RA, 1967. Pavlovian conditioning and its proper control procedures. Psychol Rev 74, 71–80. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M, 2004. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch.Gen.Psychiatry 61, 1136–1144. [DOI] [PubMed] [Google Scholar]
- Reznikov R, Diwan M, Nobrega JN, Hamani C, 2015. Towards a better preclinical model of PTSD: characterizing animals with weak extinction, maladaptive stress responses and low plasma corticosterone. J Psychiatr Res 61, 158–165. 10.1016/j.jpsychires.2014.12.017 [DOI] [PubMed] [Google Scholar]
- Richardson R, Fan M, 2002. Behavioral expression of learned fear in rats is appropriate to their age at training, not their age at testing. Anim Learn Behav 30, 394–404. [DOI] [PubMed] [Google Scholar]
- Richter SH, 2017. Systematic heterogenization for better reproducibility in animal experimentation. Lab Animal 46, 343–349. 10.1038/laban.1330 [DOI] [PubMed] [Google Scholar]
- Richter SH, Garner JP, Auer C, Kunert J, Würbel H, 2010. Systematic variation improves reproducibility of animal experiments. Nat. Methods 7, 167–168. 10.1038/nmeth0310-167 [DOI] [PubMed] [Google Scholar]
- Richter SH, Garner JP, Würbel H, 2009. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nature Methods 6, 257–261. 10.1038/nmeth.1312 [DOI] [PubMed] [Google Scholar]
- Riebe CJ, Pamplona FA, Pamplona F, Kamprath K, Wotjak CT, 2012. Fear relief-toward a new conceptual frame work and what endocannabinoids gotta do with it. Neuroscience 204, 159–185. 10.1016/j.neuroscience.2011.11.057 [DOI] [PubMed] [Google Scholar]
- Roelofs K, 2017. Freeze for action: neurobiological mechanisms in animal and human freezing. Philos. Trans. R. Soc. Lond., B, Biol. Sci 372 10.1098/rstb.2016.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K, Hagenaars MA, Stins J, 2010. Facing freeze: social threat induces bodily freeze in humans. Psychol Sci 21, 1575–1581. 10.1177/0956797610384746 [DOI] [PubMed] [Google Scholar]
- Rogan MT, Leon KS, Perez DL, Kandel ER, 2005. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron 46, 309–320. 10.1016/j.neuron.2005.02.017 [DOI] [PubMed] [Google Scholar]
- Rogan MT, Stäubli UV, LeDoux JE, 1997. Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390, 604–607. 10.1038/37601 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B, 1991. Attenuated cardiovascular, neuroendocrine, and behavioral responses after a single footshock in central amygdaloid lesioned male rats. Physiol. Behav 50, 771–775. [DOI] [PubMed] [Google Scholar]
- Royce JR, 1972. Avoidance conditioning in nine strains of inbred mice using optimal stimulus parameters. Behav. Genet 2, 107–110. [DOI] [PubMed] [Google Scholar]
- Rudy JW, 2009. Context representations, context functions, and the parahippocampal-hippocampal system. Learn. Mem 16, 573–585. 10.1101/lm.1494409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco T, Sacchetti B, 2010. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science 329, 649–656. 10.1126/science.1183165 [DOI] [PubMed] [Google Scholar]
- Scharfenort R, Menz M, Lonsdorf TB, 2016. Adversity-induced relapse of fear: neural mechanisms and implications for relapse prevention from a study on experimentally induced return-of-fear following fear conditioning and extinction. Translational Psychiatry 6, e858 10.1038/tp.2016.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, Ledoux JE, Phelps EA, 2008. Evidence for recovery of fear following immediate extinction in rats and humans. Learn. Mem 15, 394–402. 10.1101/lm.909208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöner J, Heinz A, Endres M, Gertz K, Kronenberg G, 2017. Post-traumatic stress disorder and beyond: an overview of rodent stress models. Journal of Cellular and Molecular Medicine 21, 2248–2256. 10.1111/jcmm.13161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C, 2009. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE 4, e5865 10.1371/journal.pone.0005865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman ME, Binik YM, 1977. The safety signal hypothesis. Operant-Pavlovian interactions 165–87. [Google Scholar]
- Sengupta P, 2012. The Laboratory Rat: Relating Its Age with Human’s. International Journal of Preventive Medicine 4, 624–630–630. [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, Mouly A-M, Sullivan RM, 2007. Enduring Effects of Infant Memories: Infant Odor-Shock Conditioning Attenuates Amygdala Activity and Adult Fear Conditioning. Biological Psychiatry, Stress and Anxiety: Developmental and Therapeutic Perspectives 62, 1070–1079. 10.1016/j.biopsych.2007.04.025 [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M, 2012. Instructed extinction differentially affects the emotional and cognitive expression of associative fear memory. Psychophysiology 49, 1426–1435. 10.1111/j.1469-8986.2012.01450.x [DOI] [PubMed] [Google Scholar]
- Sevenster D, Visser RM, D’Hooge R, 2018. A translational perspective on neural circuits of fear extinction: Current promises and challenges. Neurobiol Learn Mem 155, 113–126. 10.1016/j.nlm.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Hong M, Britton JC, Pine DS, Fox NA, 2014. Fear conditioning and extinction across development: Evidence from human studies and animal models. Biological Psychology 100, 1–12. 10.1016/j.biopsycho.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Furgeson-Moreira S, Monfils MH, 2014. Predictability and heritability of individual differences in fear learning. Anim Cogn 17, 1207–1221. 10.1007/s10071-014-0752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Jones C, Auchter A, Monfils M-H, 2018. Data-driven criteria to assess fear remission and phenotypic variability of extinction in rats. Philos. Trans. R. Soc. Lond., B, Biol. Sci 373 10.1098/rstb.2017.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT, 2006. Toward an animal model of posttraumatic stress disorder. Ann. N. Y. Acad. Sci 1071, 324–334. 10.1196/annals.1364.025 [DOI] [PubMed] [Google Scholar]
- Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ, 2015. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol. Ther 149, 150–190. 10.1016/j.pharmthera.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjouwerman R, Niehaus J, Kuhn M, Lonsdorf TB, 2016. Don’t startle me—Interference of startle probe presentations and intermittent ratings with fear acquisition. Psychophysiol n/a–n/a. 10.1111/psyp.12761 [DOI] [PubMed] [Google Scholar]
- Sjouwerman R, Niehaus J, Lonsdorf TB, 2015. Contextual Change After Fear Acquisition Affects Conditioned Responding and the Time Course of Extinction Learning-Implications for Renewal Research. Front Behav Neurosci 9, 337 10.3389/fnbeh.2015.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Meloni EG, Myers KM, Van’t Veer A, Carlezon WA, Rudolph U, 2011. Reduction of fear-potentiated startle by benzodiazepines in C57BL/6J mice. Psychopharmacology (Berl.) 213, 697–706. 10.1007/s00213-010-2026-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, Simon NM, Pollack MH, Hofmann SG, 2013. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res 47, 1455–1461. 10.1016/j.jpsychires.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Kempen TV, Lee FS, Casey BJ, 2010. A Genetic Variant BDNF Polymorphism Alters Extinction Learning in Both Mouse and Human. Science 327, 863–866. 10.1126/science.1181886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JCS, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS, 2014. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nature Methods 11, 629–632. 10.1038/nmeth.2935 [DOI] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews 24, 417–463. 10.1016/S0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, Kirschbaum C, Miller R, 2017. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology 77, 261–274. 10.1016/j.psyneuen.2016.12.017 [DOI] [PubMed] [Google Scholar]
- Stamps J, Groothuis TGG, 2010. The development of animal personality: relevance, concepts and perspectives. Biol Rev Camb Philos Soc 85, 301–325. 10.1111/j.1469-185X.2009.00103.x [DOI] [PubMed] [Google Scholar]
- Stein MB, Kerridge C, Dimsdale JE, Hoyt DB, 2007. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress 20, 923–932. 10.1002/jts.20270 [DOI] [PubMed] [Google Scholar]
- Steudte S, Kolassa I-T, Stalder T, Pfeiffer A, Kirschbaum C, Elbert T, 2011. Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology 36, 1193–1200. 10.1016/j.psyneuen.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Stiedl O, Jansen RF, Pieneman AW, Ogren SO, Meyer M, 2009. Assessing aversive emotional states through the heart in mice: implications for cardiovascular dysregulation in affective disorders. Neurosci Biobehav Rev 33, 181–190. 10.1016/j.neubiorev.2008.08.015 [DOI] [PubMed] [Google Scholar]
- Stiedl O, Spiess J, 1997. Effect of tone-dependent fear conditioning on heart rate and behavior of C57BL/6N mice. Behav. Neurosci 111, 703–711. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Tovote P, Ögren SO, Meyer M, 2004. Behavioral and autonomic dynamics during contextual fear conditioning in mice. Autonomic Neuroscience 115, 15–27. 10.1016/j.autneu.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Stins JF, Roelofs K, Villan J, Kooijman K, Hagenaars MA, Beek PJ, 2011. Walk to me when I smile, step back when I’m angry: emotional faces modulate whole-body approach-avoidance behaviors. Exp Brain Res 212, 603–611. 10.1007/s00221-011-2767-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stujenske JM, Likhtik E, Topiwala MA, Gordon JA, 2014. Fear and Safety Engage Competing Patterns of Theta-Gamma Coupling in the Basolateral Amygdala. Neuron 83, 919–933. 10.1016/j.neuron.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Powers A, Jovanovic T, Koenen KC, 2016. Genetic influences on the neural and physiological bases of acute threat: A research domain criteria (RDoC) perspective. Am. J. Med. Genet. B Neuropsychiatr. Genet 171B, 44–64. 10.1002/ajmg.b.32384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT, Stark R, 2011. Influence of contingency awareness on neural, electrodermal and evaluative responses during fear conditioning. Soc Cogn Affect Neurosci 6, 495–506. 10.1093/scan/nsq070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D, 2006. Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. Neuroimage 32, 761–70. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Chan MM, Pilar ML, 2008. Predator odor fear conditioning: Current perspectives and new directions. Neuroscience & Biobehavioral Reviews, Predator Odors, 5HT and Emotion 32, 1218–1227. 10.1016/j.neubiorev.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Wagner S, Schachner M, Dityatev A, Wotjak CT, 2003. Potentiation of amygdaloid and hippocampal auditory-evoked potentials in a discriminatory fear-conditioning task in mice as a function of tone pattern and context. Eur. J. Neurosci 18, 639–650. [DOI] [PubMed] [Google Scholar]
- Tang J, Wotjak CT, Wagner S, Williams G, Schachner M, Dityatev A, 2001. Potentiated amygdaloid auditory-evoked potentials and freezing behavior after fear conditioning in mice. Brain Res 919, 232–241. 10.1016/S0006-8993(01)03020-7 [DOI] [PubMed] [Google Scholar]
- Taylor JA, 1956. Level of conditioning and intensity of the adaptation stimulus. Journal of Experimental Psychology 51, 127–130. 10.1037/h0042941 [DOI] [PubMed] [Google Scholar]
- Tazumi T, Okaichi H, 2002. Effect of lesions in the lateral nucleus of the amygdala on fear conditioning using auditory and visual conditioned stimuli in rats. Neurosci. Res 43, 163–170. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA, 1966. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73, 16–43. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID, 2013. Animal models of social avoidance and social fear. Cell Tissue Res. 354, 107–118. 10.1007/s00441-013-1636-4 [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID, Slattery DA, 2012. Social fear conditioning: a novel and specific animal model to study social anxiety disorder. Neuropsychopharmacology 37, 1433–1443. 10.1038/npp.2011.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SBE, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, Lüthi A, 2016. Midbrain circuits for defensive behaviour. Nature 534, 206–212. 10.1038/nature17996 [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A, 2015. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci 16, 317–331. 10.1038/nrn3945 [DOI] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G, 2007. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. European Neuropsychopharmacology 17, 245–256. 10.1016/j.euroneuro.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Vetere G, Kenney JW, Tran LM, Xia F, Steadman PE, Parkinson J, Josselyn SA, Frankland PW, 2017. Chemogenetic Interrogation of a Brain-wide Fear Memory Network in Mice. Neuron 94, 363–374.e4. 10.1016/j.neuron.2017.03.037 [DOI] [PubMed] [Google Scholar]
- Vianna DML, Carrive P, 2005. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci 21, 2505–2512. 10.1111/j.1460-9568.2005.04073.x [DOI] [PubMed] [Google Scholar]
- Voelkl B, Vogt L, Sena ES, Würbel H, 2018. Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLOS Biology 16, e2003693 10.1371/journal.pbio.2003693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchan E, Rocha-Rego V, Bastos AF, Oliveira JM, Franklin C, Gleiser S, Berger W, Souza GGL, Oliveira L, David IA, Erthal FS, Pereira MG, Figueira I, 2017. Immobility reactions under threat: A contribution to human defensive cascade and PTSD. Neuroscience & Biobehavioral Reviews, Translational Neuroscience & Mental Disorders: bridging the gap between animal models and the human condition 76, 29–38. 10.1016/j.neubiorev.2017.01.025 [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu K-T, Davis M, 2002. Facilitation of Conditioned Fear Extinction by Systemic Administration or Intra-Amygdala Infusions of d-Cycloserine as Assessed with Fear-Potentiated Startle in Rats. The Journal of Neuroscience 22, 2343–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FR, Hinwood M, Masters L, Deilenberg RA, Day TA, 2008. Individual differences predict susceptibility to conditioned fear arising from psychosocial trauma. J Psychiatr Res 42, 371–383. 10.1016/j.jpsychires.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Walker P, Carrive P, 2003. Role of ventrolateral periaqueductal gray neurons in the behavioral and cardiovascular responses to contextual conditioned fear and poststress recovery. Neuroscience 116, 897–912. [DOI] [PubMed] [Google Scholar]
- Warren VT, Anderson KM, Kwon C, Bosshardt L, Jovanovic T, Bradley B, Norrholm SD, 2014. Human fear extinction and return of fear using reconsolidation update mechanisms: The contribution of on-line expectancy ratings. Neurobiol Learn Mem 113, 165–173. 10.1016/j.nlm.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt J, Neubert J, Koenig J, Thayer JF, Hamm AO, 2015. Resting heart rate variability is associated with inhibition of conditioned fear. Psychophysiology 52, 1161–1166. 10.1111/psyp.12456 [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Phelps EA (Eds.), 2009. The human amygdala. Guilford Press, New York. [Google Scholar]
- Wheeler AL, Teixeira CM, Wang AH, Xiong X, Kovacevic N, Lerch JP, McIntosh AR, Parkinson J, Frankland PW, 2013. Identification of a functional connectome for long-term fear memory in mice. PLoS Comput. Biol 9, e1002853 10.1371/journal.pcbi.1002853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Flaisch T, Pauli P, 2014. Raised Middle-Finger: Electrocortical Correlates of Social Conditioning with Nonverbal Affective Gestures. PLOS ONE 9, e102937 10.1371/joumal.pone.0102937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Parr LA, Davis M, 2002. Acoustic startle, prepulse inhibition, and fear-potentiated startle measured in rhesus monkeys. Biol. Psychiatry 51, 859–866. [DOI] [PubMed] [Google Scholar]
- Worp H.B. van der, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR, 2010. Can Animal Models of Disease Reliably Inform Human Studies? PLOS Medicine 7, e1000245 10.1371/journal.pmed.1000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak CT, 2018. Sound check, stage design and screen plot – how to increase the comparability of fear conditioning and fear extinction experiments. Psychopharmacology. 10.1007/s00213-018-5111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JO-Y, McNally GP, 2019. Rules for aversive learning and decision-making. Current Opinion in Behavioral Sciences 26, 1–8. 10.1016/j.cobeha.2018.08.006 [DOI] [Google Scholar]
- Yu T, Xu H, Wang W, Li S, Chen Z, Deng H, 2015. Determination of endogenous corticosterone in rodent’s blood, brain and hair with LC-APCI-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 1002, 267–276. 10.1016/j.jchromb.2015.08.035 [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR, 2011. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol. Psychiatry 70, 920–927. 10.1016/j.biopsych.2011.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuj DV, Norrholm SD, 2019. The clinical applications and practical relevance of human conditioning paradigms for posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 88, 339–351. 10.1016/j.pnpbp.2018.08.014 [DOI] [PubMed] [Google Scholar]