Abstract
Soy-based foods are consumed for their health beneficial effects, implying that the population is exposed to soy isoflavones in the diet. Herein, male rats at 21, 35, and 75 days of age were maintained either on a casein control diet, soybean meal (SBM), or control diet supplemented with daidzin and genistin (G + D) for 14 days. Feeding of SBM and G + D diets decreased testicular testosterone (T) secretion regardless of age. Altered androgen secretion was due to decreased (P < 0.05) Star and Hsd17β protein in the testes and was associated with increased (P < 0.05) Lhβ and Fshβ subunit protein expression in pituitary glands. Second, male rats were fed either a casein control diet, control diet + daidzin, control diet + genistin, or control diet + genistin + daidzin (G + D). Compared to control, feeding of all isoflavone-containing diets decreased (P < 0.05) testicular T concentrations, and more so in the G + D diet group. Interestingly, Esr1 and androgen receptor protein and pituitary Fshβ with Lhβ subunit protein were increased (P < 0.05) by feeding of genistin and G + D diets, but not the daidzin diet. However, daidzein and genistein both caused a concentration dependent inhibition (P < 0.05) of T secretion by Leydig cells in vitro with IC50 of 184 ηM and 36 ηM, respectively. Results demonstrated that altered testicular steroidogenic capacity and pituitary FSHβ and LHβ subunit expression due to soy-based diets result from specific actions by genistein and daidzein. Experiments to assess effects of isoflavone regulation of intratesticular androgen concentrations on male fertility are warranted.
Keywords: testis, Leydig cells, steroid hormones, soy bean, isoflavones, endocrine disruptors
Graphical Abstract
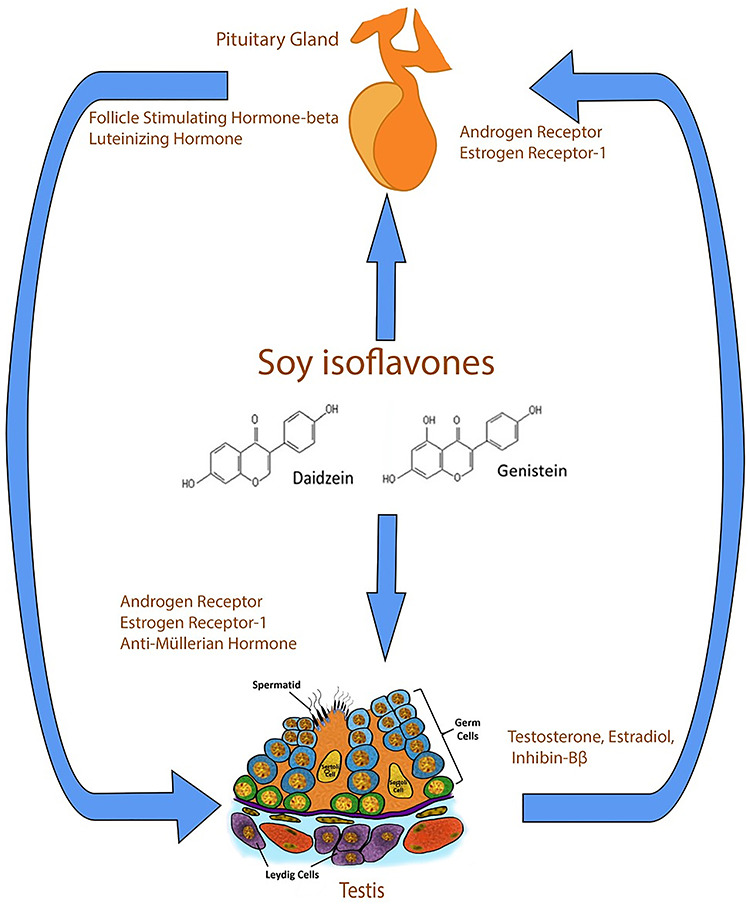
Introduction
Soy-based food products are consumed worldwide due to their putative nutritional and health beneficial effects. Raw soybeans typically contain 36.5% protein, 19.9% lipids, low cholesterol, high unsaturated fat, 9.3% dietary fiber, and a small amount of isoflavones (<1%) [1]. Isoflavones (also called phytoestrogens or phytochemicals) are nonsteroidal compounds, which are abundant in soybeans and other legumes [2]. Interestingly, isoflavones are similar in structure to and mimic the female hormone estrogen [3] with the capacity to interfere with function of the endocrine axis. The most abundant isoflavones in soybeans are present as β-glycoside conjugates: 50–55% genistin, 40–45% daidzin, and less than 5% glycetin [4, 5]. Genistin, daidzin, and glycetin are hydrolyzed in the gastrointestinal tract to their genistein, daidzein, and glycitein aglycones while daidzein is further broken down to equol in about one-third of the population [6, 7].
Importantly, individuals with lactose intolerance consume soymilk as alternatives to lactose-containing milk while vegetarians substitute soy protein for meat, and consumption of soy-based products were linked to prevention of cancer [8–10] and other chronic diseases [11, 12]. Furthermore, the American Heart Association recommends consumption of soy-based food products for their low cholesterol content [13], and the US Department of Agriculture has endorsed use of soy-based protein as an alternative to animal protein in lunch programs for preschoolers, and young and adolescent age groups [14, 15]. Of particular concern are risks associated with the use of hormone-supplemented rations in livestock production [16] and feeding of several food products containing soy products, other phytochemicals and dietary estrogens [17]. Also, the increasing popularity of soy food products has raised public concerns regarding exposure of the population to hormonally active compounds [18]. The most vulnerable groups are developing fetuses and infants exposed in utero and/or during lactation and feeding of soy-based infant formulas. For example, thousands of infants in the United States each year are raised on soy-based formulas [19, 20], which may contain high isoflavone concentrations in the micromolar range and achieve blood concentrations of 300–600 ηM [21]. The mean daily consumption of isoflavones in 4-month-old infants maintained exclusively on soy-based infant formulas was estimated at 6–9 mg/kg of body weight, resulting in blood isoflavone concentrations about 980 μg/L which is much higher than measured in infants fed cow’s milk formulas or human breast milk (9.4 and 4.7 μg/L) [22, 23].
Isoflavone effects were investigated in the male reproductive tract due to their high expression levels of steroid hormone receptors [24–26]. Epidemiological studies are few, but a study of adult Chinese men with idiopathic infertility showed that adult men with high urinary daidzein and genistein concentrations (>50 μg/g creatinine) had lower sperm counts in the range 38.5–118.4 × 106/mL compared to individuals with lower urinary daidzein and genistein levels (<50 μg/g creatinine) with sperm counts between 48.8 and 167.4 × 106/mL [27]. Similarly, animal studies demonstrated that both endocrine and exocrine functions of testes are subject to regulation by soy isoflavones. Exposure of pregnant female mice to genistein at concentrations similar to human exposure levels (10 ηM) suppressed androgen secretion in male offspring [28]. Subcutaneous administration of genistein at 2.5 mg/kg/body weight/day for a period of 9 days also decreased both serum and testicular androgen concentrations in adult male mice [29]. Subcutaneous administration of genistein at 4 mg/kg/day to neonatal rats for 16 days stimulated germ cell development [30], but long-term genistein treatment decreased spermatogenesis in adult male rats [31]. We observed that feeding of soy-based diets to pregnant female rats from gestational day 12 to postnatal day (PND) 21 induced Leydig cell proliferation in testes early in development and suppressed steroid hormone secretion in adult male offspring [32–34]. Results of our in vitro assays showed that genistein treatment disrupted coupling of luteinizing hormone (LH) receptors to G proteins in Leydig cells and diminished LH stimulation of androgen biosynthesis [35]. Isoflavones also have the capacity to impact neuroendocrine function. For example, subcutaneous administration of 250 μg of genistein at 12-h intervals in the first 48 h postpartum increased the number of tyrosine hydroxylase neurons in the anteroventral periventricular nucleus of the hypothalamus and disrupted sexual differentiation in male rats [36]. Similarly, daily administration of a high genistein dose (e.g., 1000 μg) in the first 10 days postpartum decreased pituitary Lh secretion in response to gonadotropin-releasing hormone (Gnrh) stimulation in pubertal male rats, but a smaller dose (e.g., 100 μg) caused the opposite effect, i.e., increased Lh secretion in response to GnRh stimulation [37]. Administration of equol to adult male rats at 100 mg/kg/bodyweight for 5 days upregulated the expression of both estrogen receptor 1 (Esr1) and truncated estrogen receptor product 1, an estrogen-induced specific Esr1 isoform that suppresses ligand-activated ESRs in the pituitary gland [38]. Overall, the inconsistencies in observations from different laboratories demonstrate that dose–response relationships of the two isoflavones are not always linear and may be biphasic or attain a plateau for many biological endpoints [39–41].
Our laboratory is interested in the regulation of androgen secretion and gonadal function. Androgens are important for male reproductive development both in utero and during postnatal development [42–45]. The primary androgen testosterone (T) is produced predominantly by Leydig cells under the direct control of pituitary Lh [46]. Pituitary Lh is released in response to stimulation by GnRh. Acting in concert with Sertoli cell-secreted factors, e.g., Anti-Müllerian hormone (Amh) and inhibin Bβ, sex steroids feedback to the pituitary gland and hypothalamus and control Lh-beta and GnRh release [47–49]. Thus, isoflavones may act directly in testicular cells and/or perturb the entire hypothalamus–pituitary–gonadal (HPG) axis to regulate GnRh and gonadotropin secretion [37, 38, 50].
Information on age-related differences in isoflavone exposure effects is limited [20]. Most studies focused on isoflavone exposures occurring continuously through gestation, lactation, and the postweaning period, making it difficult to isolate specific effects associated with different developmental periods. Therefore, we used prepubertal, pubertal, and adult exposure paradigms to assess whether isoflavone exposure effects are specifically due to the presence of genistein and daidzein and if age and stage of reproductive tract development are moderating factors. Second, most studies on isoflavones on male reproductive axis have focused on genistein [20] with little attention to daidzein, another major isoflavone in soybeans [20, 51]. This is a significant omission because both genistein and daidzein acted as agonists in a model of hepG2 human hepatoma cells transfected with rat Esr1 and Esr2 [52]. Moreover, genistein induced a higher rate of Esr binding to estrogen response elements compared to daidzein [25]. However, it remains to be determined that differential binding of transcriptional factors in testicular cells influences the actions of hormonally active agents in the male gonad. To determine specificity of isoflavone action, we performed experiments to determine whether isoflavone effects in testicular cells are due to individual action by daidzein and genistein or result from both compounds acting together.
Materials and methods
Chemicals: Genistein and daidzein were obtained from Indofine Chemical Company (Hillsborough, NJ). Trypsin inhibitor, EDTA, HEPES, bovine serum albumin (BSA), bovine lipoprotein, sodium bicarbonate (NaHCO3), Dulbecco Modified Eagle Medium (DMEM) nutrient mixture (Ham’s F-12 (DMEM/F-12; 1:1 mixture without phenol red]), albumin, Percoll, etiocholan-3β-ol-17-one, and gentamicin were purchased from Sigma Chemical Company (St. Louis, MO). Dulbecco phosphate-buffered saline (PBS), medium 199, and 10 × Hanks balanced salt solution were obtained from Life Technologies, Inc. (Grand Island, NY). Collagenase, dispase, and deoxyribonuclease (DNase) were purchased from Roche Molecular Biochemicals (Mannheim, Germany). Ovine LH was provided by the National Hormone and Pituitary Program (NIDDK, Bethesda, MD).
Animal studies
All animal and euthanasia procedures were performed in accordance with a protocol approved by the Auburn University Institutional Animal Care and Use Committee based on recommendations of the panel on Euthanasia of the American Veterinary Medical Association.
Experiment 1
We performed experiments to determine whether altered sex hormone secretion by testis was due specifically to isoflavone action and are influenced by age at exposure. Male Long–Evans rats at different ages (PND 21 [n = 45], 35 [n = 36] and 75 [n = 18]) were obtained from Harlan-Teklad, (Madison, WI). Animals were allowed to acclimatize for 3 days at the College of Veterinary Medicine Division of Laboratory Animal Health Housing Facility. Depending on size, animals were placed in groups of 1–3 per cage (length, 0.47 m; width, 0.25 m; height, 0.22 m) (Snyder Manufacturing Company, Centennial, CO). Water was provided in glass water bottles ad libitum. Housing of animals in plastic cages and use of glass bottles was designed to minimize background exposure to estrogens as may occur with resin-containing cages [20]. Animals were maintained under constant conditions of light (12 L: 12 D) and temperature (20–23.38 °C) with free access to pelleted food. There were three experimental diets: (1) casein control; (2) soybean diet; and (3) control diet supplemented with daidzin and genistin (G + D). The SBM diet was formulated to contain 300 parts per million (ppm) genistein and 200 ppm daidzein based on the natural content of genistin and daidzin in soybeans as determined by the manufacturer (Harlan-Teklad, Madison, WI). The G + D diet was formulated to contain genistein and daidzein at 300 ppm and 200 ppm. All diets (control, SBM, and G + D) were similar in total content of protein, carbohydrates, fat, energy, and micronutrients (Harlan-Teklad, Madison, WI) (Supplementary Table S1). Animals within each age group were randomized by weight, constituted into three groups and maintained on the appropriate diet for a period of 14 days, i.e., PND 21–35 (prepubertal exposure), PND 35–49 (pubertal exposure) and PND 75–89 (adult exposure).
Experiment 2
We performed experiments to determine whether effects of soy-based diets on steroid hormone secretion by testis are due to individual action by daidzein and genistein or both compounds acting together. Male Long–Evans rats at 35 days of age (n = 48), obtained from Harlan-Teklad (Madison, WI), were housed and maintained as in Experiment 1. There is general agreement that male reproductive development, which is primarily androgen-dependent, occurs in three phases: prepubertal, pubertal, and adult. Our laboratory and others have demonstrated that the 35-day-old male rat signifies the pubertal and intermediate phase of development [53–55]. There were four experimental diets: (1) casein control; (2) control diet plus daidzin (Dai, 200 ppm); (3) control diet plus genistin (Gen, 300 ppm); and (4) control diet plus 300 ppm genistin and 200 ppm daidzin (G + D). All diets were similar in their content of protein, carbohydrates, fat, energy, and micronutrients (Harlan-Teklad, Madison, WI) (Supplementary Table S2). Animals were randomized by weight into four groups and maintained on the appropriate diet for 14 days, i.e., PND 21–35.
Finally, we performed in vitro assays with primary Leydig cell cultures in experiments designed to eliminate isoflavone effects due to the soy or casein protein [25–27] and prevent isoflavone activity occurring in the hypothalamus and pituitary gland [28]. Immature Leydig cells, isolated from 35-day-old male rats not previously exposed to isoflavones, were incubated in DMEM/F-12 culture medium containing genistein or daidzein at 0, 0.01, 0.1, 1, and 10 μM in the presence of LH (10 ηg/mL ovine LH) for 18 h at a temperature of 34 °C. Leydig cells isolated from testes of male rats at 35 days postpartum exhibit features that are seen in prepubertal rats (e.g., proliferative capacity) and adult animals (steroid hormone secretion), and thus represent the intermediate stage of development. After treatment, aliquots of spent media were analyzed for T concentrations. We also determined the concentration of genistein and daidzein causing half-maximal inhibition (IC50) of T secretion by Leydig cells.
Procedure for isolation of Leydig cells
Animals were killed by CO2 asphyxiation after which testes were collected and digested in a dissociation buffer containing 0.25 mg/mL collagenase, 46 μg/mL dispase, and 6 μg/mL DNase for 1 h in a shaking water bath at 34 °C. Seminiferous tubules from immature testis were removed by passing testicular fractions through a nylon mesh with a pore size of 0.2 μm (Spectrum Laboratories, New Brunswick, NJ). The supernatant was centrifuged at 2500 rpm for 15 min at 4 °C. Seminiferous tubules obtained from adult testes were removed by gravity sedimentation in dissociation buffer containing 10 mg/mL BSA. In all cases, cell fractions were loaded onto a Percoll gradient (Sigma-Aldrich, St. Louis, MO) and centrifuged at 13 500 rpm for 60 min at 4 °C. Leydig cells were isolated from the Percoll gradient based on density, i.e., progenitor Leydig cells at 1.063 (21 days), immature Leydig cells (35 days) at 1.069–1.073, and adult Leydig cells (75 days) at 1.073–1.096 [56–58]. Leydig cell numbers were estimated using a hemocytometer. The purity of Leydig cell fractions was assessed by histochemical staining for 3βHSD using 0.4 mM etiocholan-3β-ol-17-one as the enzyme substrate (Sigma-Aldrich, St. Louis, MO).
Measurement of serum hormones and isoflavones and testicular and Leydig cell hormone secretion
Serum was separated from trunk blood collected at sacrifice. Testicular explants (~100 mg) and aliquots of Leydig cells (0.1–0.2 × 106) were incubated in microcentrifuge tubes containing DMEM/F-12 culture medium buffered with 14 mm NaHCO3, 15 mm HEPES, 0.1% BSA, and 0.5 mg/mL bovine lipoprotein. Incubations were conducted without (basal) and with a maximally stimulating dose of ovine LH (100 ng/mL; LH-stimulated) for 3 h at 34 °C. Steroid hormone concentrations (T, 17β-estradiol [E2]) were assayed in aliquots of serum and spent media using a tritium-based radioimmunoassay with an inter-assay variation of 7–8% [59]. Hormone production was normalized to nanogram per testicular mass (milligrams) and 106 Leydig cells. The concentrations of genistein and daidzein in serum were measured by ultra performance liquid chromatography with mass spectrometry detection using modifications of previously reported methods [32, 60, 61].
Sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blot analysis
We analyzed expression of several proteins in testes (Amh, inhibin Bβ, Esr1, Ar, Star, Hsd17β) and pituitary glands (Lhβ, Fshβ, Esr1, Ar). Tissues were homogenized in T-PER lysis buffer (Pierce Biotechnology, Rockford, IL) that was freshly supplemented with a protease inhibitor cocktail (Catalog #78410; Pierce Biotechnology, Rockford, IL). Tubes were centrifuged at 3000 rpm for 14 min at 4 °C to remove cellular debris. Protein concentrations were determined using the Bio-Rad protein assay with BSA as standard (Bio-Rad Laboratories, Hercules, CA). Aliquots (50 μL) of whole-cell lysates were dissolved in an equal volume of Laemmli buffer containing 5% β-mercaptoethanol and were boiled for 5 min at 95 °C. All samples were resolved on varying percentages of Tris-HCl acrylamide gels by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes (Catalog #1620147; Bio-Rad Laboratories) and subsequently incubated in blocking buffer (5% whole milk in 0.1% Tween 20 PBS) for 1 h at room temperature to reduce nonspecific binding by antibodies. Membranes were then incubated in blocking buffer containing appropriate primary antibodies overnight at 4 °C. Parameters of primary antibodies used in the present study are provided in Supplementary Table S3. On the next day, blots were washed three times in 0.1% Tween 20 PBS to remove any unbound primary antibody before incubation with the appropriate horseradish peroxidase-conjugated secondary antibody. Afterward, membranes were washed four times with 0.1% Tween 20 PBS and then scanned using a LI-COR Odyssey Infrared Scanner (Lincoln, NE). All protein measurements were normalized to β-actin.
Statistical analysis
Data are presented as the mean ± SEM. Within each age group, data were analyzed by one-way ANOVA followed by Dunnett test for multiple group comparisons, except for serum isoflavones concentration which were analyzed by independent t-test (GraphPad Prism software. San Diego, CA). Differences of ≤0.05 were considered to be significant.
Results
Experiment 1
General observations
No animal deaths were recorded in the course of this study. Maintenance of animals on experimental diets did not affect body weights. Although paired testicular weights were greater (P < 0.05) in prepubertal rats fed the SBM diet compared to control (Table 1), the gonadosomatic index was similar (P > 0.05) in all diet groups at this stage of development. Biochemical analysis showed that isoflavones were undetectable in serum from control animals. Total and conjugated genistein and daidzein were greater (P < 0.05) in the SBM diet group of animals compared to the G + D group. However, daidzein, but not genistein, was greater (P < 0.05) in the SBM than in G + D animals (n = 3) (Table 2).
Table 1.
Effects of diets on body weight and testicular size (Experiment 1).
| Body weight (g) | Paired testis weight (g) | Gonadosomatic index (%) | ||
|---|---|---|---|---|
| Prepubertal (PND 21–35) | Control | 181.3 ± 4.0 | 2.2 ± 0.03 | 1.2 ± 0.02 |
| SBM | 188.3 ± 4.3 | 2.4 ± 0.08* | 1.3 ± 0.02 | |
| G + D | 177.3 ± 6.1 | 2.3 ± 0.08 | 1.2 ± 0.02 | |
| Pubertal (PND 35–49) | Control | 225.8 ± 4.6 | 2.2 ± 0.12 | 1.0 ± 0.06 |
| SBM | 234.8 ± 4.6 | 2.6 ± 0.10 | 1.1 ± 0.06 | |
| G + D | 234.0 ± 6.8 | 2.5 ± 0.08 | 1.1 ± 0.03 | |
| Adult (PND 75–90) | Control | 351.2 ± 7.4 | 3.3 ± 0.12 | 0.9 ± 0.04 |
| SBM | 356.7 ± 8.8 | 3.3 ± 0.05 | 0.9 ± 0.02 | |
| G + D | 347.3 ± 7.4 | 3.4 ± 0.06 | 1.0 ± 0.04 |
*P = 0.0449.
Table 2.
Serum concentrations of isoflavones (ng/mL).
| Diets | Genistein | Daidzein | ||||
|---|---|---|---|---|---|---|
| Total | Free | Conjugated | Total | Free | Conjugated | |
| Control | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| SBM | 457.9 ± 43* | 9.3 ± 2.0 | 448.6 ± 43* | 249.6 ± 16* | 23.11 ± 3* | 226.5 ± 14* |
| G + D | 322.6 ± 10.0 | 10.4 ± 2.1 | 312.2 ± 9.0 | 98.4 ± 3.8 | 9.5 ± 0.3 | 88.9 ± 3.7 |
* P < 0.05.
Effect of age on isoflavone regulation of steroid hormone secretion
Compared to control, serum, and basal and LH-stimulated testicular T concentrations were generally decreased in rats from the SBM and G + D diet groups regardless of age at time of exposure (P < 0.05) and more so in adult animals (P < 0.001) (Figure 1). A similar pattern of decreased basal and LH-stimulated Leydig cell T secretion (P < 0.05) was observed in the SBM and G + D diet groups compared to control (Figure 2). Serum E2 concentrations were equivalent in all diet groups regardless of age (Figure 3A–C), whereas basal testicular E2 concentrations were decreased (P < 0.05) in adult animals fed SBM and the G + D diet compared to control (Figure 3D–F). LH-stimulated testicular E2 concentrations were decreased in pubertal and adult animals fed the SBM and G + D diet; this effect was seen only in prepubertal animals maintained on the G + D diet (Figure 3G–I). Maintenance of animals on SBM and G + D diets had no effect in Leydig cells from growing rats (Figure 4A and B), but decreased (P < 0.05) basal E2 secretion by adult Leydig cells (Figure 4C) compared to control. LH-stimulated Leydig cell E2 production showed a similar pattern (Figure 4E and F), but there was decreased E2 production in prepubertal animals from the G + D diet group (Figure 4D). Altogether, it appears that feeding of the SBM and G + D diets decreased steroid hormone secretion to a greater extent in pubertal and adult animals than in prepubertal animals.
Figure 1.
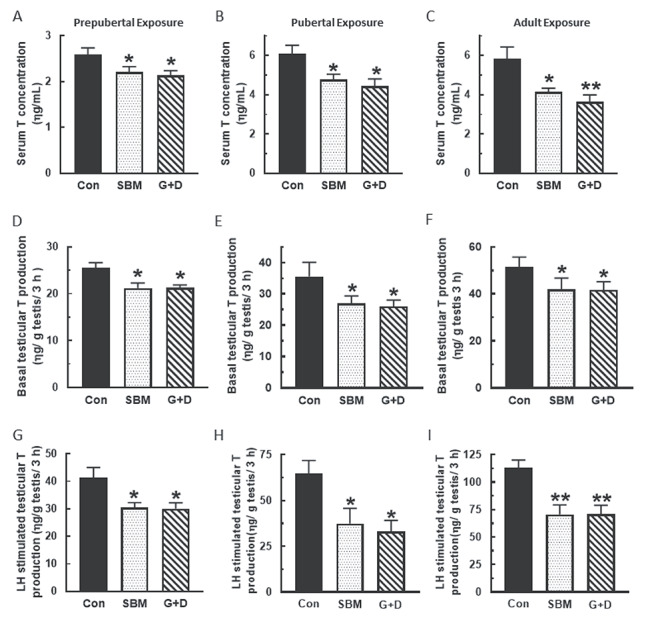
Effects of soy-based diets on testicular androgen secretion. Male rats at 21 (n=45), 35 (n=36) and 75 days of age (n=18) were maintained on a control diet (Con), whole soybean diet (SBM) or control diet supplemented with genistein and daidzein (G+D) for 14 days. After sacrifice, serum were separated from blood to measure testosterone (T) concentration (A, B, C). Testicular explants were obtained and incubated in DMEM/Ham’s F-12 culture medium for 3 h without (D, E, F) or containing 100 ηg/ml ovine LH (NIDDK, NIH) (G, H, I). Aliquots of spent media were analyzed to measure T concentrations. Hormone concentrations were determined by RIA (*P < 0.05, **P < 0.001).
Figure 2.
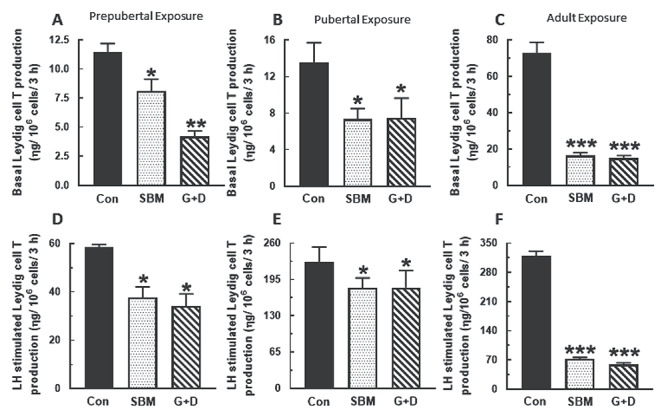
Effects of soy-based diets on androgen secretion by Leydig cells. Male rats at 21 (n=45), 35 (n=36) and 75 days of age (n=18) were maintained on a control diet (Con), whole soybean diet (SBM) or control diet supplemented with genistein and daidzein (G+D) for 14 days. After sacrifice, testes were pooled to isolate Leydig cells which were then incubated in DMEM/Ham’s F-12 culture medium for 3 h without (A, B, C) or containing 100 ηg/ml ovine LH (NIDDK, NIH) (D, E, F). Spent media were analyzed to measure T concentrations by RIA (*P < 0.05, **P < 0.001, ***P < 0.0001).
Figure 3.
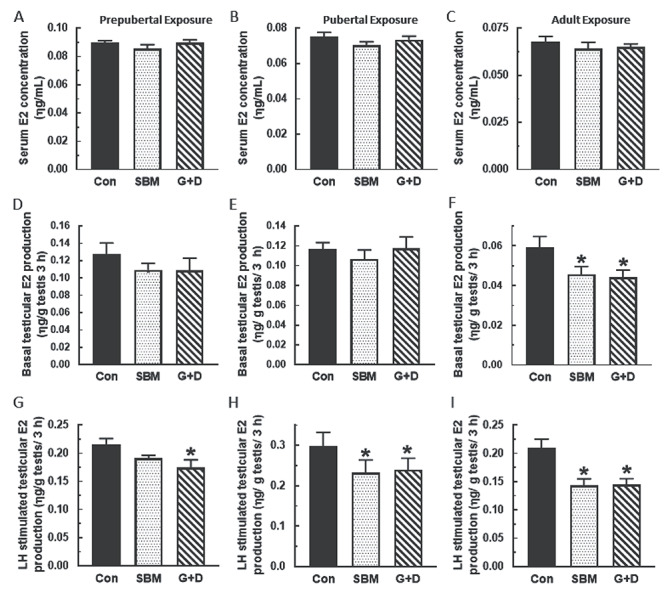
Effects of soy-based diets on testicular 17β-estradiol (E2) secretion. Male rats at 21 (n=45), 35 (n=36) or 75 days of age (n=18) were maintained on a control diet (Con), whole soybean diet (SBM) or control diet supplemented with genistein and daidzein (G+D) for 14 days. After sacrifice, serum were obtained to measure E2 concentration (A, B, C). Testicular explants were obtained and incubated in DMEM/Ham’s F-12 culture medium for 3 h without (D, E, F) or containing 100 ηg/ml ovine LH (NIDDK, NIH) (G, H, I). Aliquots of spent media were analyzed to measure E2 concentrations by RIA (*P < 0.05).
Figure 4.
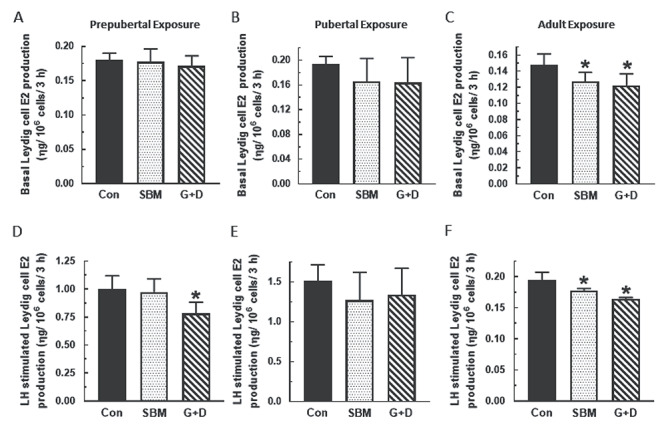
Effects of soy-based diets on 17β-estradiol (E2) secretion by Leydig cells. Male rats at 21 (n=45), 35 (n=36) or 75 days of age (n=18) were maintained on a control diet (Con), whole soybean (SBM) or control diet supplemented with genistein and daidzein (G+D) for 14 days. After sacrifice, testes were pooled from animals in same group to isolate Leydig cells. Leydig cells were then incubated in DMEM/Ham’s F-12 culture medium in triplicate for 3 h without (A, B, C) or containing 100 ηg/ml ovine LH (NIDDK, NIH) (D, E, F) Spent media were analyzed by RIA to measure E2 concentrations by RIA (*P < 0.05).
Isoflavone regulation of protein expression in the pituitary–gonadal axis
Results of western blot analysis showed that expression of Sertoli cell-secreted Amh and inhibin Bβ were decreased (P < 0.05) in pubertal male rats fed the SBM and G + D diet compared to control (Figure 5A and B). The finding of decreased testicular Amh and inhibin Bβ protein was associated with increased (P < 0.05) Lhβ and Fshβ subunit protein expression in pituitary glands of affected animals (Figure 5C and D). Analysis of the steroidogenic pathway showed that Star and Hsd17β levels were decreased (P < 0.05) in testes in pubertal (Figure 6A and B) and adult animals (Figure 6C and D) fed the SBM and G + D diet compared to control. These observations indicated that isoflavones have the capacity to target and/or regulate specific proteins in the male neuroendocrine axis.
Figure 5.
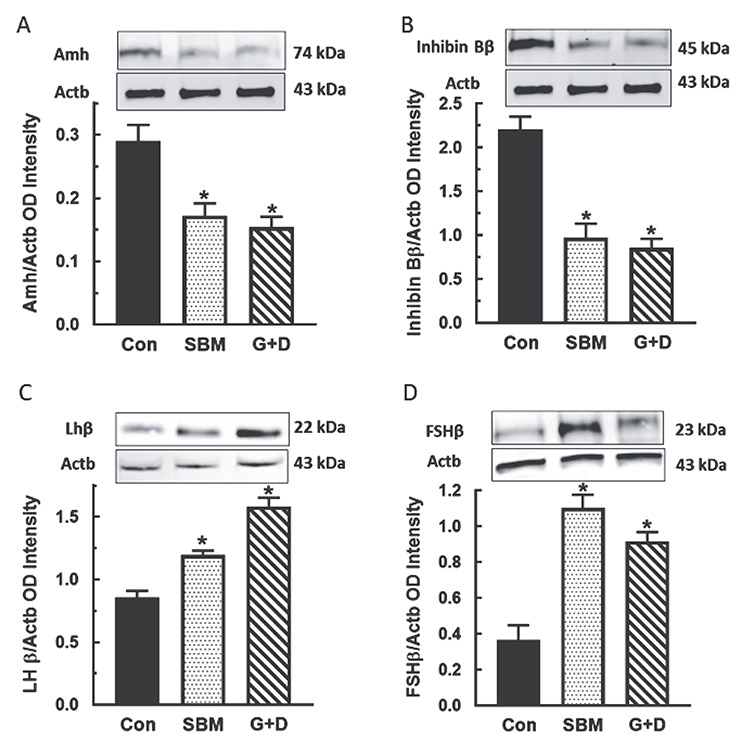
Effects of soy-based diets in the testis-pituitary gland axis. Pubertal male rats at 35 days of age were maintained on a control diet (Con), whole soybean (SBM) or control diet supplemented with genistein and daidzein (G+D) for 14 days. After sacrifice, tissues were processed by western blot procedures to analyze Mϋllerian inhibiting substance (MIS) (A) and inhibin Bβ in testes (B) and FSHβ (C) and LHβ subunit proteins in pituitary glands (D). Proteins were normalized to actin (Actb) (Inhibin Bβ = 45 kDa, Amh = 74 kDa, Fshβ = 21 kDa, Lhβ = 22 kDa, Actb = 43 kDa. *, P < 0.05 vs. control).
Figure 6.
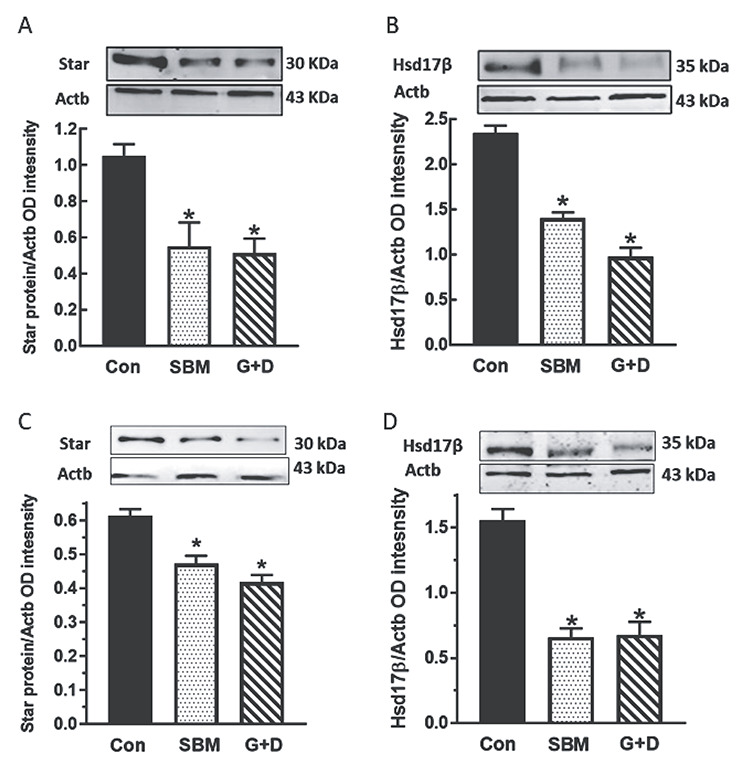
Effects of soy-based diets on steroidogenic proteins in the testis. Male rats at 35 (A, B) and 75 days of age (C, D) were maintained on a control diet (Con), whole soybean (SBM) or control diet supplemented with genistein and daidzein (G+D) for 14 days. After sacrifice, testes were obtained and subjected to western blotting procedures to measure steroidogenic acute regulatory protein (StAR) and the 17β -hydroxysteroid dehydrogenase enzyme (Hsd17β). Proteins were normalized to actin (Actb) (Star = 30 kDa, Hsd17β = 35 kDa, Actb = 43 kDa *, P < 0.05 vs. control).
Experiment 2
General observations
No animal deaths were recorded in the course of this study. Maintenance of animals on experimental diets had no effects on body weights, paired testicular weights, and gonadosomatic index (Table 3).
Table 3.
Differential effects of isoflavones on body weight and testicular size (Experiment 2).
| Body weight (g) | Paired testis weight (g) | Gonadosomatic index (%) | ||
|---|---|---|---|---|
| Pubertal (PND 35–49) | Control | 247.7 ± 3.5 | 2.6 ± 0.10 | 1.1 ± 0.05 |
| Dai | 253.4 ± 4.0 | 2.4 ± 0.10 | 1.0 ± 0.05 | |
| Gen | 251.1 ± 2.6 | 2.6 ± 0.10 | 1.0 ± 0.04 | |
| G + D | 248.8 ± 4.8 | 2.6 ± 0.10 | 1.0 ± 0.03 |
Singular and combined isoflavone effects on testicular androgen secretion and protein expression in the pituitary–gonadal axis
Maintenance of pubertal male rats on the Dai, Gen and G + D diets decreased (P < 0.05) serum T concentrations only in animals fed the G + D diet compared to control (Figure 7A). However, basal and LH-stimulated testicular T secretion were decreased in all male rats fed isoflavone-containing diets compared to control and unexposed animals and this effect was most profound in the G + D diet group (Figure 7B–E). Furthermore, western blot analysis showed that expression of Esr1 and Ar protein in testes was increased by feeding of the Gen and G + D diets, but this effect was absent in the Dai diet group (Figure 8A and B). Expression of Esr1 and Ar protein in testes was increased (P < 0.05) by feeding of all isoflavone-containing diets compared to control (Figure 8C and D). Interestingly, the patterns of pituitary FSHβ and LHβ protein expression were similar to Esr1 and Ar protein in testes, i.e., effects due to the Gen and G + D diets were absent in the Dai diet group (Figure 8E and F).
Figure 7.
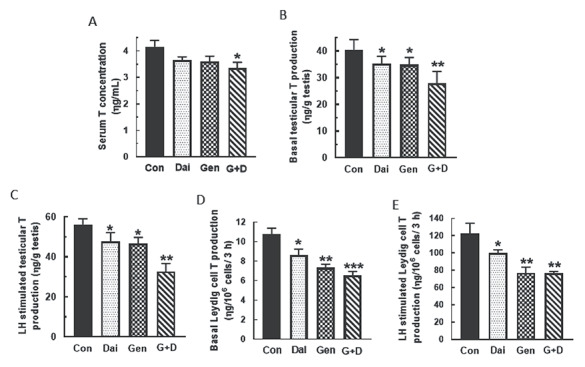
Differential soy isoflavone effects on testicular androgen secretion. Pubertal male rats at 35 days of age were maintained on a control diet (Con) or control diet supplemented with daidzein (Dai), genistein (Gen) or both isoflavones (G+D) for 14 days. After sacrifice, serum were obtained to measure testosterone (T) concentrations (A). In addition, testicular explants and Leydig cells isolated from testes of animals in the same diet group were incubated in DMEM/Ham’s F-12 culture medium in triplicate for 3 h without (B, D) or containing 100 ηg/ml ovine LH (NIDDK, NIH) (C, E). Aliquots of serum and spent media were analyzed to measure T concentrations by RIA (n = 48; *P < 0.05, **P < 0.001, ***P < 0.0001).
Figure 8.
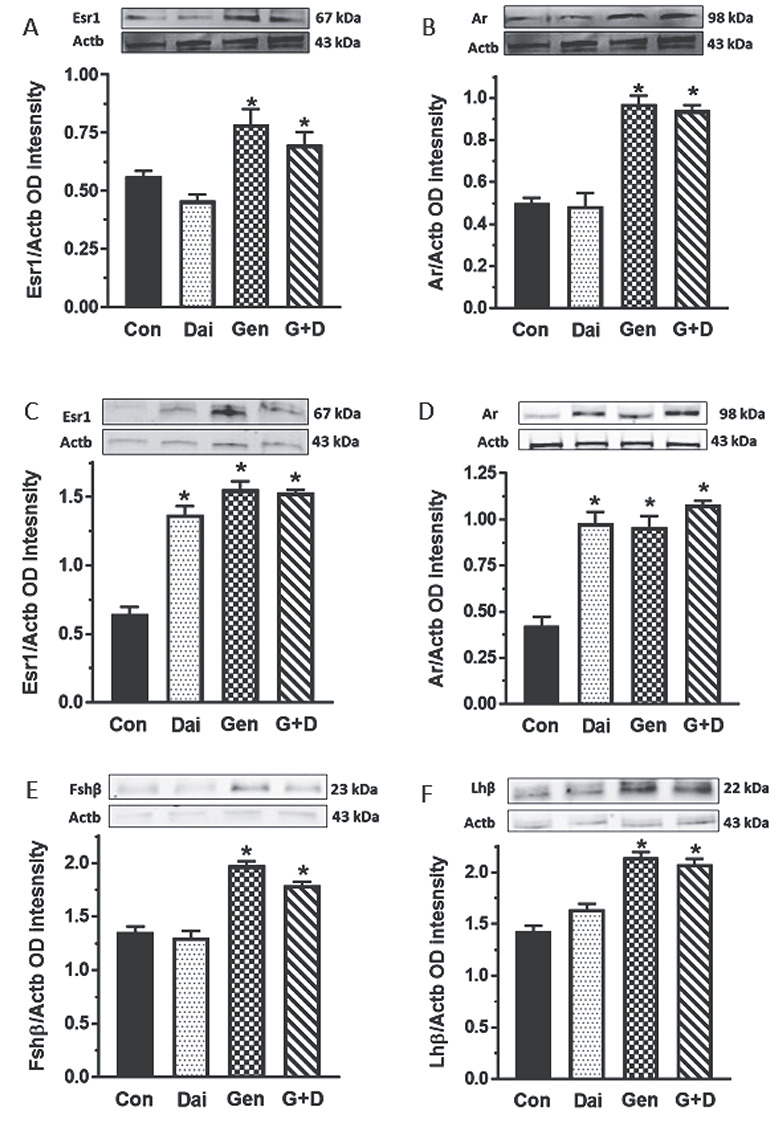
Differential soy isoflavone effects on gene expression in the testis-pituitary gland axis. Male rats at 35 days of age were maintained on a control diet (Con) or control diet supplemented with daidzein (Dai), genistein (Gen) or both isoflavones (G+D) for 14 days. After sacrifice, tissues were obtained and processed by western blotting procedures to measure testicular estrogen receptor-1 (Esr1) (A) and androgen receptor (Ar) (B) and pituitary Esr1 (C), AR (D), Fshβ (E) and Lhβ subunit proteins (F). Proteins were normalized to actin (Actb) (Fshβ = 21 kDa, Lhβ = 22 kDa, Esr1 = 67 kDa, Ar = 98 kDa, Actb = 43 kDa; *P <0.05 vs. control).
Isoflavone effects on androgen secretion by Leydig cells
Analysis of isoflavone effects in primary Leydig cell cultures demonstrated that both daidzein and genistein caused a concentration dependent inhibition (P < 0.05) of T secretion by Leydig cells (Figure 9A and B). The concentrations of daidzein and genistein causing half-maximal inhibition of T secretion by Leydig cells (IC50) were calculated to be 184 ηM and 36.2 ηM, respectively (Figure 9C and D).
Figure 9.
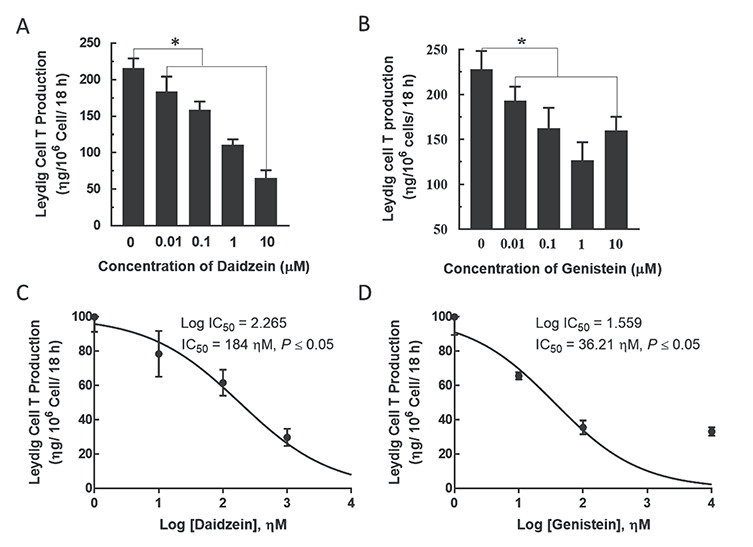
Effects of isoflavones on Leydig cell testosterone (T) production in vitro. Immature Leydig cells were isolated from 35 day-old male rats and incubated in triplicate in DMEM/Ham’s F-12 culture medium containing genistein or daidzein (0, 0.1, 1 and 10 μM) and LH (10 ηg/mL; NIDDK, NIH) for 18 h. Spent media were analyzed for T concentrations by RIA (A, B). In addition, the concentrations of daidzein and genistein causing half maximal inhibition of T production (IC50) were calculated (C, D). Experiments were replicated 4-6 times (*P < 0.05 vs. control).
Discussion
Serum isoflavone concentrations achieved in the present study are similar to those associated with consumption of soy-based diets in the population [62]. Genistein aglycone concentrations were similar in the SBM and G + D diets whereas daidzein aglycone measured greater in serum from animals fed the SBM, but not G + D diet. The production of greater daidzein than genistein aglycones after consumption of soy-based diets has been a consistent pattern in our studies utilizing both perinatal and neonatal exposure paradigms [32, 33]. The impact of this finding on differential isoflavone exposure effects in body tissues remains to be determined although genistein appears to cause greater biological effects than daidzein at least in male reproductive tract tissues (discussed later in this section). There were no isoflavone-induced changes in body weights, but testicular weights were increased in the SBM group, which were probably due to the protein content of SBMs as was previously reported [63]. Overall, we hereby demonstrate that rat testes at all stages of development are sensitive to hormonal activity due to soy isoflavones acting alone or together. We also observed that soy isoflavones in the diet impacted pituitary gonadotropin subunit protein expression although it is not clear that changes in pituitary gland gene expression were due to direct isoflavone action in pituitary gonadotrophs. It is possible that isoflavone-induced changes in sex steroid hormone secretion by the gonads disrupted negative feedback regulation of GnRh and gonadotropin secretion.
Isoflavone inhibition of sex steroid hormone (T and E2) secretion was evident in serum, testis, and Leydig cells. We confirmed that inhibition of androgen biosynthesis was related to disruption of steroidogenic protein expression, e.g., Star and Hsd17β, which have specific roles in cholesterol mobilization and enzymatic conversion of steroid substrates into androgens within Leydig cells. A previous report also indicated that dietary isoflavones decreased expression of Hsd17β in rat testes [64]. It is perhaps not surprising that testicular E2 concentrations were affected by exposures to dietary isoflavones because T is the substrate for E2 biosynthesis [65]. Metabolism of T to E2 is catalyzed by the aromatase enzyme, and there is evidence that soy isoflavones have the capacity to inhibit aromatase enzyme activity [64, 66]. The aromatase enzyme is also highly expressed in adipose tissue [67, 68]. Therefore, it is possible that, unlike in the testis, isoflavone regulation of aromatase enzyme activity and E2 biosynthesis in adipose tissue is a confounding factor in E2 metabolism and measurements of serum E2 concentrations. Isoflavones are known to inhibit steroid pathways for estradiol (E2) and T biosynthesis and secretion. Although epidemiological findings are limited and inconclusive, 3β-hydroxysteroid dehydrogenase type 2 (Hsd2 β3), which catalyzes production of sex steroid precursors, and cytochrome P450 21 hydroxylase (CYP21A2) in human subjects were inhibited by isoflavones [69, 70]. Also, ingestion of isoflavones decreased in vivo DHEAS and A4 levels and inhibited 17,20 lyase activity, thereby regulating the production of precursor androgens and boosting the pool of active androgens, which potentially intensifies clinical syndromes associated with androgen excess [71]. In the present study, we observed that testes from animals fed the SBM and G + D diet exhibited decreased testicular Amh and inhibin Bβ protein expression. Both proteins reflect on testicular maturation and mediate feedback regulation of pituitary Lh and Fsh secretion. Interestingly, decreased testicular Amh and inhibin Bβ protein were coupled to increased Lhβ and Fshβ gonadotropin subunit protein in the pituitary gland. This dataset supports the view that dietary estrogens such as soy isoflavones have the capacity to disrupt multiple levels of the HPG axis by acting directly in testicular cells and pituitary gonadotrophs, and/or interfering with steroid hormone feedback regulation of the hypothalamus–pituitary axis [31, 72, 73]. Further studies are warranted to investigate direct isoflavone effects and the mechanisms of isoflavone modulation of steroid hormone feedback regulation of the pituitary gland and hypothalamus in the male.
Furthermore, we investigated the action of individual isoflavones in the male reproductive axis. Serum T concentrations were unaffected, but testicular and Leydig cell T secretion were decreased in animals fed diets containing daidzein and/or genistein, implying that both genistein and daidzein contribute to isoflavone suppression of testicular androgen secretion. The presence of both genistein and daidzein as major isoflavones in soybeans raises the possibility that combined effects due to both chemicals may be more intense from those associated with single chemicals. Analysis of chemical mixture effects is outside the focus of the present study, but the effects of genistein and daidzein combination seemed additive. Indeed, feeding of the G + D diet in Experiment 2 affected more endpoints and caused greater decreases in androgen secretion than were due to maintenance on either the Dai or Gen diet alone. On the other hand, it is possible that the combination of genistein and daidzein exerts no effects on other parameters if both chemicals activate signaling pathways that counteract and mitigate each other’s actions [74].
Moreover, our results demonstrated that genistein might be a more potent isoflavone inhibitor of Leydig cell steroidogenesis. For example, basal Leydig cell T production was greater in animals from the Dai compared to the Gen diet group. Second, our in vitro studies showed that daidzein and genistein concentrations causing half-maximal inhibition of Leydig cell T secretion were 184 ηM and 36 ηM, respectively. These observations are supported by other reports of greater hormonal effects due to genistein compared to daidzein [25, 52, 75]. We also observed differential isoflavone effects on pituitary Fshβ and Lhβ subunit protein expression. For example, animals from the Gen diet group exhibited increased Fshβ and Lhβ subunit protein than in the Dai diet group. These observations reinforce the view that genistein might be the more hormonally active soy isoflavone in reproductive tract tissues. Furthermore, there was a lack of concordance between decreased serum T concentrations and increased pituitary Lhβ subunit protein in male rats fed the G + D diet (Experiment 2) because the latter failed to normalize Leydig cell T secretion. Although we did not measure Lh receptor expression in the present study, we observed previously that genistein diminished Lh stimulation of androgen secretion by uncoupling Lh receptors from G proteins in Leydig cells [35]. Also, we did not measure serum LH concentrations and it remains a possibility that isoflavone-induced posttranslational modifications prevented secretion and release of pituitary Lh into peripheral blood to reach the gonads and stimulate androgen secretion [76]. Although increased expression of Esr1 and Ar protein after exposure to genistein was reported in other studies [77], the biological significance of increased pituitary and gonadal Ar and Esr1 protein as a factor in tissue sensitivity to isoflavone exposure remains to be determined.
In conclusion, our results demonstrated that disruptions in testicular androgen secretion associated with consumption of soy-based diets are due to isoflavone exposure effects at all stages of development. This clarification is important because the soy protein is known to exert biological effects and/or activate signaling pathways that regulate cellular function in many tissues [78]. Our finding that both genistein and daidzein induced changes in pituitary Fshβ and Lhβ expression support the view that the endocrine disrupting effects of isoflavones are exerted at multiple levels of the HPG axis [79, 80]. Our data are relevant to public health due to increased consumption of soy-based products by all segments of the population and the increasing incidence of reproductive anomalies in the population [81]. Additional studies are warranted to assess the impact of isoflavone-induced changes in testicular androgen concentrations on germ cell development, sperm production, and male fertility.
Supplementary Material
Disclosure statement: Authors have nothing to disclose.
References
- 1. U.S. Department of Agriculture, Agricultural Research Service 2012. USDA National Nutrient Database for Standard Reference, Nutrient Data Laboratory Home Page, http://www.ars.usda.gov/ba/bhnrc/ndl.
- 2. He F-J, Chen J-Q. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in western countries and possible mechanisms. Food Sci Human Wellness 2013;2(3-4):146-161. [Google Scholar]
- 3. Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet 2013;38(1):15-25. [DOI] [PubMed] [Google Scholar]
- 4. Kaufman PB, Duke JA, Brielmann H, Boik J, Hoyt JE. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: Implications for human nutrition and health. J Altern Complement Med 1997;3(1):7-12. [DOI] [PubMed] [Google Scholar]
- 5. Murphy PA, Song T, Buseman G, Barua K, Beecher GR, Trainer D, Holden J. Isoflavones in retail and institutional soy foods. J Agric Food Chem 1999;47(7):2697-2704. [DOI] [PubMed] [Google Scholar]
- 6. Frankenfeld C, Atkinson C, Thomas W, Goode EL, Gonzalez A, Jokela T, Wähälä K, Schwartz S, Li S, Lampe J. Familial correlations, segregation analysis, and nongenetic correlates of soy Isoflavone–metabolizing phenotypes. Exp Biol Med 2004;229(9):902-913. [DOI] [PubMed] [Google Scholar]
- 7. Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: Influence of habitual diet. Proc Soc Exp Biol Med 1998;217(3):335-339. [DOI] [PubMed] [Google Scholar]
- 8. Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: A review of the in vitro and in vivo data. Nutr Cancer 1994;21(2):113-131. [DOI] [PubMed] [Google Scholar]
- 9. Baggott JE, Ha T, Vaughn WH, Juliana MM, Hardin JM, Grubbs CJ. Effect of miso (Japanese soybean paste) and NaCl on DMBA‐induced rat mammary tumors. 1990. [DOI] [PubMed]
- 10. Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr 2006;136(12):3046-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuevas A, Irribarra V, Castillo O, Yanez M, Germain A. Isolated soy protein improves endothelial function in postmenopausal hypercholesterolemic women. Eur J Clin Nutr 2003;57(8):889. [DOI] [PubMed] [Google Scholar]
- 12. Scheiber MD, Liu JH, Subbiah M, Rebar RW, Setchell KD. Dietary inclusion of whole soy foods results in significant reductions in clinical risk factors for osteoporosis and cardiovascular disease in normal postmenopausal women. Menopause 2001;8(5):384-392. [DOI] [PubMed] [Google Scholar]
- 13. Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: An American Heart Association science advisory for professionals from the nutrition committee. Circulation 2006;113(7):1034-1044. [DOI] [PubMed] [Google Scholar]
- 14. Endres J, Barter S, Theodora P, Welch P. Soy-enhanced lunch acceptance by preschoolers. J Am Diet Assoc 2003;103(3):346-351. [DOI] [PubMed] [Google Scholar]
- 15. Lazor K, Chapman N, Levine E. Soy goes to school: Acceptance of healthful, vegetarian options in Maryland middle school lunches. J Sch Health 2010;80(4):200-206. [DOI] [PubMed] [Google Scholar]
- 16. Lusk JL, Roosen J, Fox JA. Demand for beef from cattle administered growth hormones or fed genetically modified corn: A comparison of consumers in France, Germany, the United Kingdom, and the United States. Am J Agric Econ 2003;85(1):16-29. [Google Scholar]
- 17. Martinez J, Lewi J. An unusual case of gynecomastia associated with soy product consumption. Endocr Pract 2008;14(4):415-418. [DOI] [PubMed] [Google Scholar]
- 18. Adlercreutz H, Mousavi Y, Clark J, Höckerstedt K, Hämäläinen E, Wähälä K, Mäkelä T, Hase T. Dietary phytoestrogens and cancer: In vitro and in vivo studies. J Steroid Biochem Mol Biol 1992;41(3-8):331-337. [DOI] [PubMed] [Google Scholar]
- 19. Bhatia J, Greer F. Use of soy protein-based formulas in infant feeding. Pediatrics 2008;121(5):1062-1068. [DOI] [PubMed] [Google Scholar]
- 20. McCarver G, Bhatia J, Chambers C, Clarke R, Etzel R, Foster W, Hoyer P, Leeder JS, Peters JM, Rissman E, Rybak M, Sherman C, Toppari J, Turner K. NTP-CERHR expert panel report on the developmental toxicity of soy infant formula. Birth Defects Res B Dev Reprod Toxicol 2011;92(5):421-468. [DOI] [PubMed] [Google Scholar]
- 21. Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. The Lancet 1997;350(9070):23-27. [DOI] [PubMed] [Google Scholar]
- 22. Setchell K. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 1998;68(6):1333S-1346S. [DOI] [PubMed] [Google Scholar]
- 23. Badger TM, Gilchrist JM, Pivik RT, Andres A, Shankar K, Chen J-R, Ronis MJ. The health implications of soy infant formula. Am J Clin Nutr 2009;89(5):1668S-1672S. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y, Song TT, Cunnick JE, Murphy PA, Hendrich S. Daidzein and Genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J Nutr 1999;129(2):399-405. [DOI] [PubMed] [Google Scholar]
- 25. Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors α and β to the estrogen response element. J Agric Food Chem 2003;51(26):7632-7635. [DOI] [PubMed] [Google Scholar]
- 26. Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 2002;23(6):870-881. [PubMed] [Google Scholar]
- 27. Xia Y, Chen M, Zhu P, Lu C, Fu G, Zhou X, Chen D, Wang H, Hang B, Wang S. Urinary phytoestrogen levels related to idiopathic male infertility in Chinese men. Environ Int 2013; 59:161–167. [DOI] [PubMed] [Google Scholar]
- 28. Lehraiki A, Chamaillard C, Krust A, Habert R, Levacher C. Genistein impairs early testosterone production in fetal mouse testis via estrogen receptor alpha. Toxicol In Vitro 2011;25(8):1542-1547. [DOI] [PubMed] [Google Scholar]
- 29. Strauss L, Makela S, Joshi S, Huhtaniemi I, Santti R. Genistein exerts estrogen-like effects in male mouse reproductive tract. Mol Cell Endocrinol 1998;144(1-2):83-93. [DOI] [PubMed] [Google Scholar]
- 30. Atanassova N, McKinnell C, Turner K, Walker M, Fisher J, Morley M, Millar M, Groome N, Sharpe R. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: Evidence for stimulatory effects of low estrogen levels. Endocrinology 2000;141(10):3898-3907. [DOI] [PubMed] [Google Scholar]
- 31. Svechnikov K, Supornsilchai V, Strand ML, Wahlgren A, Seidlova-Wuttke D, Wuttke W, Soder O. Influence of long-term dietary administration of procymidone, a fungicide with anti-androgenic effects, or the phytoestrogen genistein to rats on the pituitary-gonadal axis and Leydig cell steroidogenesis. J Endocrinol 2005;187(1):117-124. [DOI] [PubMed] [Google Scholar]
- 32. Akingbemi BT, Braden TD, Kemppainen BW, Hancock KD, Sherrill JD, Cook SJ, He X, Supko JG. Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular Leydig cells in the adult rat. Endocrinology 2007;148(9):4475-4488. [DOI] [PubMed] [Google Scholar]
- 33. Napier ID, Simon L, Perry D, Cooke PS, Stocco DM, Sepehr E, Doerge DR, Kemppainen BW, Morrison EE, Akingbemi BT. Testicular development in male rats is sensitive to a soy-based diet in the neonatal period. Biol Reprod 2014;90(2):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sherrill JD, Sparks M, Dennis J, Mansour M, Kemppainen BW, Bartol FF, Morrison EE, Akingbemi BT. Developmental exposures of male rats to soy isoflavones impact Leydig cell differentiation. Biol Reprod 2010;83(3):488-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hancock KD, Coleman ES, Tao YX, Morrison EE, Braden TD, Kemppainen BW, Akingbemi BT. Genistein decreases androgen biosynthesis in rat Leydig cells by interference with luteinizing hormone-dependent signaling. Toxicol Lett 2009;184(3):169-175. [DOI] [PubMed] [Google Scholar]
- 36. Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-a exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol 2006;28(1):111-118. [DOI] [PubMed] [Google Scholar]
- 37. Faber KA, Hughes CL Jr. The effect of neonatal exposure to diethylstilbestrol, genistein, and zearalenone on pituitary responsiveness and sexually dimorphic nucleus volume in the castrated adult rat. Biol Reprod 1991;45(4):649-653. [DOI] [PubMed] [Google Scholar]
- 38. Loutchanwoot P, Srivilai P, Jarry H. Effects of the natural endocrine disruptor equol on the pituitary function in adult male rats. Toxicology 2013; 304:69–75. [DOI] [PubMed] [Google Scholar]
- 39. Anderson JJ, Garner SC. 1Phytoestrogens and bone. Bailliere's Clinical Endocrinology and Metabolism 1998;12(4):543-557. [DOI] [PubMed] [Google Scholar]
- 40. Dang ZC, Lowik C. Dose-dependent effects of phytoestrogens on bone. Trends Endocrinol Metab 2005;16(5):207-213. [DOI] [PubMed] [Google Scholar]
- 41. Nayeem F, Chen N-W, Nagamani M, Anderson KE-JW, L, L. Daidzein and genistein have differential effects in decreasing whole body bone mineral density but had no effect on hip and spine density in premenopausal women: A 2-year randomized, double-blind, placebo-controlled study. Nutr Res 2019; 68:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science 1981; 1294–1303. [DOI] [PubMed] [Google Scholar]
- 43. Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci 1984;7(1):413-442. [DOI] [PubMed] [Google Scholar]
- 44. Raisman G, Field P. Sexual dimorphism in the neurophil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Res 1973; 54:1–29. [DOI] [PubMed] [Google Scholar]
- 45. Vijayakumar N, Macks ZO, Shirtcliff EA, Pfeifer JH. Puberty and the human brain: Insights into adolescent development. Neurosci Biobehav Rev 2018; 92:417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Christensen AK, Mason NR. Comparative ability of seminiferous tubules and interstitial tissue of rat testes to synthesize androgens from progesterone-4-14C in vitro. Endocrinology 1965;76(4):646-656. [DOI] [PubMed] [Google Scholar]
- 47. Moore JP Jr., Winters SJ. Weaning and the developmental changes in follicle-stimulating hormone, pituitary adenylate cyclase-activating polypeptide, and inhibin B in the male rat. Biol Reprod 2008;78(4):752-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Makanji Y, Temple-Smith PD, Walton KL, Harrison CA, Robertson DM. Inhibin B is a more potent suppressor of rat follicle-stimulating hormone release than inhibin a in vitro and in vivo. Endocrinology 2009;150(10):4784-4793. [DOI] [PubMed] [Google Scholar]
- 49. Garrel G, Racine C, L'Hote D, Denoyelle C, Guigon CJ, Clemente N, Cohen-Tannoudji J. Anti-Mullerian hormone: A new actor of sexual dimorphism in pituitary gonadotrope activity before puberty. Sci Rep 2016; 6:23790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McGarvey C, Cates PA, Brooks A, Swanson IA, Milligan SR, Coen CW, O'Byrne KT. Phytoestrogens and gonadotropin-releasing hormone pulse generator activity and pituitary luteinizing hormone release in the rat. Endocrinology 2001;142(3):1202-1208. [DOI] [PubMed] [Google Scholar]
- 51. Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors α and β. Biol Pharm Bull 2001;24(4):351-356. [DOI] [PubMed] [Google Scholar]
- 52. Casanova M, You L, Gaido KW, Archibeque-Engle S, Janszen DB, Heck HA. Developmental effects of dietary phytoestrogens in Sprague-Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicological sciences: An official journal of the society of Toxicology 1999;51(2):236-244. [DOI] [PubMed] [Google Scholar]
- 53. Eckstein B, Borut A, Cohen S. Metabolic pathways for androstanediol formation in immature rat testis microsomes. Biochimica et Biophysica Acta (BBA)-General Subjects 1987;924(1):1-6. [DOI] [PubMed] [Google Scholar]
- 54. HARDY MP, Zirkin BR, EWING LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology 1989;124(2):762-770. [DOI] [PubMed] [Google Scholar]
- 55. Akingbemi B, Ge R, Hardy M. Leydig cells. Encyclopedia of reproduction. Academic press San Diego. CA 1999; 2:1021–1033. [Google Scholar]
- 56. Hardy MP, Kelce WR, Klinefelter GR, Ewing LL. Differentiation of Leydig cell precursors in vitro: A role for androgen. Endocrinology 1990;127(1):488-490. [DOI] [PubMed] [Google Scholar]
- 57. Risbridger G, De Kretser D. Percoll-gradient separation of Leydig cells from postnatal rat testes. Reproduction (Cambridge, England) 1986;76(1):331-338. [DOI] [PubMed] [Google Scholar]
- 58. Risbridger G, Davies A. Isolation of rat Leydig cells and precursor forms after administration of ethane dimethane sulfonate. American Journal of Physiology-Endocrinology and Metabolism 1994;266(6): E975-E979. [DOI] [PubMed] [Google Scholar]
- 59. Cochran R, Ewing L, Niswender G. Serum levels of follicle stimulating hormone, luteinizing hormone, prolactin, testosterone, 5 alpha-dihydrotestosterone, 5 alpha-androstane-3 alpha, 17 beta-diol, 5 alpha-androstane-3 beta, 17 beta-diol, and 17 beta-estradiol from male beagles with spontaneous or induced benign prostatic hyperplasia. Invest Urol 1981;19(3):142-147. [PubMed] [Google Scholar]
- 60. Klein A, He X, Roche M, Mallett A, Duska L, Supko JG, Seiden MV. Prolonged stabilization of platinum-resistant ovarian cancer in a single patient consuming a fermented soy therapy. Gynecol Oncol 2006;100(1):205-209. [DOI] [PubMed] [Google Scholar]
- 61. Gavina JM, Priem J, Wood CM, Xiao CW, Feng Y-L. Determination of isoflavones in rat serum using liquid chromatography–tandem mass spectrometry with a highly efficient core–shell column. Anal Bioanal Chem 2013;405(8):2643-2651. [DOI] [PubMed] [Google Scholar]
- 62. Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr 2001;131(4):1362S-1375S. [DOI] [PubMed] [Google Scholar]
- 63. Tan KAL, Walker M, Morris K, Greig I, Mason JI, Sharpe RM. Infant feeding with soy formula milk: Effects on puberty progression, reproductive function and testicular cell numbers in marmoset monkeys in adulthood. Hum Reprod 2006;21(4):896-904. [DOI] [PubMed] [Google Scholar]
- 64. Le Bail J, Laroche T, Marre-Fournier F, Habrioux G. Aromatase and 17β-hydroxysteroid dehydrogenase inhibition by flavonoids. Cancer Lett 1998;133(1):101-106. [DOI] [PubMed] [Google Scholar]
- 65. Siiteri PK, Wilson JD. Testosterone formation and metabolism during male sexual differentiation in the human embryo. J Clin Endocrinol Metabol 1974;38(1):113-125. [DOI] [PubMed] [Google Scholar]
- 66. Kellis JT, Vickery LE. Inhibition of human estrogen synthetase (aromatase) by flavones. Science 1984;225(4666):1032-1034. [DOI] [PubMed] [Google Scholar]
- 67. Clyne CD, Kovacic A, Speed CJ, Zhou J, Pezzi V, Simpson ER. Regulation of aromatase expression by the nuclear receptor LRH-1 in adipose tissue. Mol Cell Endocrinol 2004;215(1-2):39-44. [DOI] [PubMed] [Google Scholar]
- 68. Kalicinska E, Wojtas K, Majda J, Zacharski M, Skiba J, Sliwowski J, Banasiak W, Ponikowski P, Jankowska EA. Expression of sex steroid receptors and aromatase in adipose tissue in different body regions in men with coronary artery disease with and without ischemic systolic heart failure. The Aging Male: The Official Journal of the International Society for the Study of the Aging Male 2018; 1–13. [DOI] [PubMed] [Google Scholar]
- 69. Hamilton-Reeves JM, Vazquez G, Duval SJ, Phipps WR, Kurzer MS, Messina MJ. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: Results of a meta-analysis. Fertil Steril 2010;94(3):997-1007. [DOI] [PubMed] [Google Scholar]
- 70. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011;32(1):81-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Swart AC, Johannes ID, Sathyapalan T, Atkin SL. The effect of soy Isoflavones on steroid metabolism. Front Endocrinol 2019; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Patisaul HB, Todd KL, Mickens JA, Adewale HB. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-a or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology 2009;30(3):350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, Hirose M. Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology 2003;192(2-3):149-170. [DOI] [PubMed] [Google Scholar]
- 74. Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect 2002;110(9):917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der Saag PT, Van Der Burg B, J-Ak Gustafsson. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998;139(10):4252-4263. [DOI] [PubMed] [Google Scholar]
- 76. Bousfield GR, Dias JA. Synthesis and secretion of gonadotropins including structure-function correlates. Rev Endocr Metab Disord 2011;12(4):289-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Maggiolini M, Vivacqua A, Carpino A, Bonofiglio D, Fasanella G, Salerno M, Picard D, Andó S. The mutant androgen receptor T877A mediates the proliferative but not the cytotoxic dose-dependent effects of genistein and quercetin on human LNCaP prostate cancer cells. Mol Pharmacol 2002;62(5):1027-1035. [DOI] [PubMed] [Google Scholar]
- 78. Mercer K, Pulliam C, Hennings L, Cleves M, Jones E, Drake R, Ronis M. Diet supplementation with soy protein isolate, but not the isoflavone genistein, protects against alcohol-induced tumor progression in DEN-treated male mice In: Alcohol and Cancer. Springer; 2018: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto A, Peskind E, Raskind M, Brodkin K, Bremner W, Petrova A. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 2001;57(1):80-88. [DOI] [PubMed] [Google Scholar]
- 80. Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metabol 2002;87(11):5001-5007. [DOI] [PubMed] [Google Scholar]
- 81. Skakkebæk NE, Jørgensen N, Main KM, Meyts ERD, Leffers H, Andersson AM, Juul A, Carlsen E, Mortensen GK, Jensen TK. Is human fecundity declining? Int J Androl 2006;29(1):2-11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


