Abstract
Injectable filler treatments have increased in popularity because of enhanced safety profile and improved physical characteristics. ISAPS (International Society of Plastic Surgery) put out global data showing 3.7 million hyaluronic acid (HA) filler procedures in 2018, making it the second most often performed procedure in the world, after botulinum toxin. And these are only ‘those’ performed by qualified plastic surgeons. There was a concomitant increase in both the nonvascular and vascular complications, which coincided with the number and type of filler procedures performed. Filler complications were reviewed from existing literature, and an attempt was made to understand etiology, elucidate clinical features, and clarify optimum treatment strategies for each. Complications can be early or delayed in presentation, early consisting of injection site complications like bruising, edema, and hypersensitivity, Tyndall effect, and intravascular injection. Delayed complications included hypersensitivity type IV, acute infections like cellulitis, abscesses, and herpes and delayed ones like granulomas, biofilms, and atypical mycobacterial infections. These were analyzed and treatment options, protocols, and consensus guidelines were suggested. A clear understanding of facial anatomy, physical characteristics of all fillers used, early recognition, and treatment options of complications will ensure optimum outcomes.
Keywords: fillers, injectable fillers, complications, nonvascular, hypersensitivity, infection, biofilm, atypical mycobacteria
Introduction
In the last decade there has been a sharp increase in the indications for use of injectable fillers in facial aesthetics. ASAPS data reveal 1.8 million botulinum toxin procedures, and 0.8 million hyaluronic acid (HA) filler procedures done in the United States alone, making HA fillers the second most popular procedure in the United States. 1 Global data put out by ISAPS 2 shows worldwide 6 million botulinum toxin procedures (up to 17.4% from 2017) and 3.7 million HA procedures (up to 11.6%), again making it the second most often performed procedure in the world.
India, slow to catch up, was responsible for only 0.14 million botulinum toxin and 0.073 million HA procedures done only by plastic surgeons 2 (data about procedures performed by dermatologists and other physicians are not available).
To keep pace with the increasing use of injectable fillers, there have been improvements in their rheological and physical characteristics.
But as the number of injectable filler procedures increase, so do the number of complications. Adverse effects (AEs) reported for HA fillers were low (1–4/10,000 procedures). 3
Complications seen initially were milder and local in nature as the fillers were restricted to superficial (dermal) injections, limited to eradicating creases and folds. As the injections went deeper, especially with the concept of volumization and facial contouring, world over, there was a reported increase in other complications like infections, abscesses, as well as vascular complications. 4 Loss of vision is a dreaded vascular complication of injectable fillers, and is now being seen with an alarming rise of incidence.
According to the Food and Drug Administration (FDA), serious adverse reactions are those which are fatal or life threatening, or require hospitalization for at least 24 hours, or require intervention to prevent permanent impairment. 5 Serious adverse reactions to fillers reported to the FDA have tripled in 2008 to 2011 compared with 2005 to 2007.
But predictably, solutions have kept their pace, and so has the increase in knowledge of injection anatomy, particularly through cadaver dissections. This has helped increase safety in injections in the face. Also, armed with a better knowledge of the causes of filler complications, effective solutions became known and available. Most of the times even if a complication has occurred, it could be managed effectively.
Simultaneously, countries such as the United Kingdom have brought in legislation that dermal fillers can be only injected by someone registered with a health care body. 6 7 Paradoxically, instead of making filler injection safe, this has resulted in an increased incidence of self-administered cases 8 with a higher incidence of complications.
Complications can occur in the hands of even an experienced injector, but awareness of complications, early detection, and resolution of these complications are what make a good injector. Fortunately, the common problems caused by HA fillers are being mild and easily reversible/treatable.
What is needed is familiarity with physical characteristics of different fillers, various injection techniques, behavior of the fillers in tissues, tissue responses, and a detailed knowledge of anatomy of the region to be able to adopt a safe and systematic approach to treating filler complications.
Types of Injectable Fillers
Fillers are divided into nonpermanent and semipermanent, or biodegradable and nonbiodegradable types. Biodegradable are mostly hyaluronic acid and collagen fillers. These are broken down by the body by the action of native hyaluronidase (hydrolysis) or by phagocytosis in case of collagen.
HA fillers : HA exists in our body in the extracellular matrix, found in high concentration in the skin, cartilage, synovial fluid, vitreous, etc. 9 It is a linear polysaccharide consisting of alternating disaccharide units of glucuronic acid and N -acetylglucosamine. 10 They are today the most commonly used fillers across the globe.
Different brands of HA fillers available vary in terms of HA content/mL, viscosity, gel hardness, cohesivity, hydrophilic capacity, average molecular weight, degree of cross linking, and whether monophasic or biphasic. 11 They last in duration from 6 to 18 months depending on the physical characteristics mentioned above. These properties also decide the ability of the HA gel to be extruded through a fine needle, and yet retain its characteristics at its destination. Hyaluronic acid fillers are amenable to disintegration with hyaluronidase. Native hyaluronidases are of six types and break down HA gradually. PH-20, one of the six types of hyaluronidase is concentrated in the testes, therefore bovine testes is one of the main sources of bovine hyaluronidase. High molecular weight HA has anti-inflammatory, immunosuppressive, anticancer, and antiaging properties, whereas low molecular weight HA is inflammatory, angiogenic, and immunostimulatory. 12
Monophasic HA fillers like Juvéderm, Belotero, Teosyal, etc., contain homogenous spheres, whereas biphasic HA fillers Restylane consist of a range of microspheres.
Non HA fillers: Calcium hydroxyapatite (CaHA, Radiesse) and poly-L-lactic acid (PLLA, Sculptra) fillers have the ability to stimulate collagen production and have a longer life of approximately 15 months 13 and 3 years or more, 14 respectively. With PLLA usually three treatment sessions are needed.
On the other hand, nonbiodegradable fillers provoke a foreign body reaction stimulating deposition of collagen around the microspheres in materials like polyacrylamide hydrogel (PAAH, Aquamid) and polymethacrylate (Artecoll). Their permanent nature causes complications to last long and at the same time make treatment difficult.
Since HA fillers are the most common fillers used in the world today, most complications are seen with them, and are treatable most of the times.
Incidence of Filler Complications
Daines and Williams in 2013 15 in a 5-year review of filler complications found that out of a total of 2,089 injectable filler treatments, 50% were HA filler treatments. The overall incidence of complications was only 0.7% (14/2,089), with HA fillers showing an even lower rate of less than 2 per 1,000. CAHP (calcium hydroxyapatite) fillers had the highest number of complications (2.6%).
Classification of Filler Complications
Complications arising from injectable fillers can be divided into nonvascular and vascular complications. Nonvascular complications can be of early onset or of late or delayed onset.
| Early onset | Delayed onset |
| 1. Pain | 1. Hypersensitivity (Type IV) |
| 2. Ecchymosis | 2. Granulomas |
| 3. Erythema | 3. Abscesses |
| 4. Edema–non hypersensitivity | 4. Herpes |
| –hypersensitivity (Type1) | 5. Biofilms |
| 5. Malar edema | 6. Nodules |
| 6. Tyndall effect | 7. Atypical mycobacterial infection |
| 7. Pigmentation | 8. Paraesthesia |
-
A. Pain : is a common, inevitable, undesirable effect of injections. However, pain can be minimized by using the smallest gauge needle possible injecting slowly or using a blunt cannula if feasible. 16 Using topical anesthesia creams where the injection is likely to be superficial goes a long way in mitigating pain.
Alternatively, if one is trained to administer nerve blocks, this is an excellent way to prevent pain. But it should be understood that there can be some muscle blocking effect due to the local effect of the injection, and the consequent temporary asymmetry should not be worrisome, particularly in the perioral region.
Applying ice before and during procedure relieves discomfort to a certain degree as well as gives the patient something to do.
Distraction techniques, like vibration devices, tapping (percussion), music, or just talking to the patient, are good enough to distract them.
-
B. Ecchymosis : there are always some chances of bruising, especially when injecting in superficial planes, i.e., at dermal and subdermal levels 16 ( Fig. 1A C ). Bruising has been seen less often using the depot technique in the supraperiosteal plane. It can be prevented/minimized by avoiding blood thinners (both medication as well as dietary supplements like aspirin, clopidogrel, warfarin, nonsteroidal anti-inflammatory drugs, fish oil, Omega 3, turmeric, garlic, ginseng, gingko biloba, etc.). Hypertension should be controlled, any bleeding tendency causing illness should be ruled out.
The dusky purple mottling of vascular occlusion can mimic a bruise, but can be differentiated from a bruise as it does not blanch. But if in doubt, inject hyalase, and the mottling will improve, but the bruise will not. 17
Patient should be in a propped position during and should be advised to keep head end elevated for 24 hours after the procedure. Use the smallest gauge of needle possible, inject small aliquots of filler, limit the number of cutaneous punctures, preferably use a cannula, 18 and remain in the preperiosteal plane ( Fig. 2 ). Use a gauze in the noninjecting hand and give light pressure for 5 minutes to limit bruising if oozing is seen at the puncture site or a bruise is seen to start forming.
If there is bruising, treat with ice compresses after the procedure, and with Vit K cream 19 (K-Vit-Sesderma, Auriderm XO-Auriga International). Avoid exposing the area to direct sunlight to prevent permanent staining. For persisting pigmentation, the PDL (pulsed dye laser), Q-switched NdYAG 532nM, or the KTP (potassium titanyl phosphate) laser may be used effectively.
-
C. Erythema : Some amount of erythema occurs after injection which lasts for a short while. But persisting erythema is usually indicative of some sort of hypersensitivity. A short-term topical steroid is enough most of the time. Patients with pre-existing rosacea are at a greater risk of developing prolonged erythema. Oral propranolol may be useful in refractory cases. 20 Prevention includes avoiding exercise post injection, and avoiding erythema inducing agents like alcohol and sun exposure. 21
Topical tacrolimus may be useful along with good sun protection. Long-term steroids are not advisable as they may cause telangiectasia and dermal thinning.
-
D. Edema : could be nonhypersensitive or short-term post traumatic, Type I antibody-mediated hypersensitivity, or even nonantibody-mediated Type IV delayed hypersensitivity edema.
Nonhypersensitivity (post traumatic) edema can happen as early as immediately after injection and is more likely if there has been lot of manipulation. Prevention methods are the same as for preventing bruising (ecchymosis). Icing and topical steroids usually settle the edema. Waxing and waning may be seen for a week or two and happen due to aggravating stimuli. 22
-
Antibody-mediated Type1 hypersensitivity edema : some patients can develop hypersensitivity which is immunoglobulin-E mediated, which may occur after the first or repeat procedure. Mast cell degranulation occurs releasing histamine, cytokines, prostaglandins, leukotrienes resulting in a typical allergic response with erythema, edema, itching, and pain. Angioedema occurs within few hours, can last for weeks and can be severe. 23
Treatment depends on the severity of the reaction. Sometimes it responds spontaneously in 2 to 3 days, while sometimes responds just to antihistamines. If it does not respond to antihistamines or is persisting, oral prednisone is the drug of choice. Airway maintenance is a prime concern in rapidly progressing angioedema. Combination treatment for persistent angioedema has been reported with Montelukast and a low dose of steroid. 24
Nonantibody mediated Type IV hypersensitivity edema : this can occur days and weeks later, usually presenting as induration, edema, and erythema. These are T lymphocyte mediated and may last for months. This condition does not respond to antihistamines, but to steroids. Dissolving the HA filler is usually curative. In non-HA fillers, steroids or even extrusion may be needed. 25 26
-
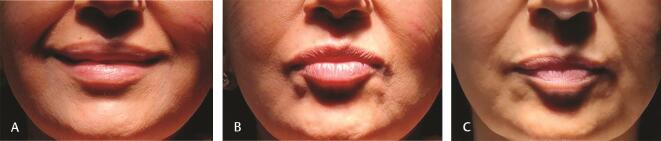
E. Malar edema : is defined as clinically significant swelling in the infraorbital region persisting for 1 month or more. A retrospective study of HA filler injection in the infraorbital region found almost 25% of the 51 patients to be developing malar edema lasting an average of 5.4 months. Superficial placement of filler is very often diagnosed as malar edema, especially if it is a very hygroscopic one. This is easily detectable by HR ultrasound or MRI (with fat signal eliminated) and is effectively treated with hyaluronidase. 27
Malar edema is thought to be related to the malar septum which divides medial suborbicularis oculi fat into superficial and deep parts. The superficial part already has a compromised lymphatic drainage, and injecting superficially, especially with a high G’ filler will aggravate preexisting malar bags. 28 Even deep injection, if large in volume particularly with a high G’ product, can cause further lymphatic compression.
Prevention is by avoiding injecting filler in people already having malar bags (pre-existing lymphatic compromise), injecting preperiosteal, low G’, low hygroscopic, small volume filler in the infraorbital region. Preferably HA fillers should be used in the infraorbital region, as they are reversible ( Fig. 3 ).
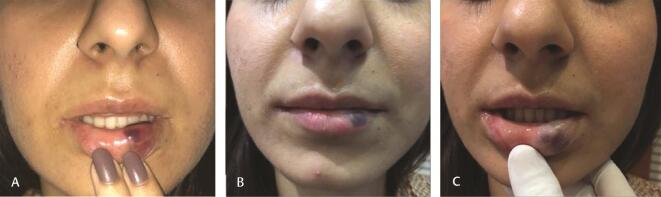
Fig. 1.
( A ) Bruising 2 hours after lip filler. ( B ) Worsens after 1 day. ( C ) Resolving at 10 days (needle used for injection).
Fig. 2.
Bruising after perioral rejuvenation with HA fillers using a cannula. Even using a cannula is no guarantee to prevent bruising. HA, hyaluronic acid.
Fig. 3.
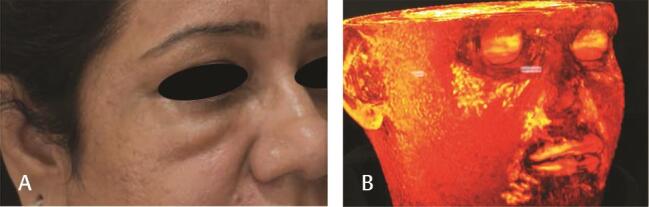
( A ) Malar edema from injecting 1 mL of HA filler ( B ) 3D reconstruction of MRI face showing large amount of HA filler in the infraorbital region. HA, hyaluronic acid.
Treatment should be conservative initially with cold compresses, intermittent compression, and oral steroids. If the edema does not settle, then in the case of HA fillers dissolve completely with hyaluronidase.
Infections
Infections are usually characterized by warm, tender, erythematous localized lumps or diffuse swelling, with possible systemic signs like fever and malaise. 21 Staphylococcus aureus is the usual offending organism in early infections, and although vehemently denied at first Propionibacterium acnes (now Cutibacterium acnes ) has been found to be involved in many cases. A multicenter study done to find out whether AEs to PAAG (polyacrylamide gel) were associated with the presence of bacteria, found P. acnes to be the commonest organism isolated. 29
A. Folliculitis : sterile folliculitis can occur if filler is injected too superficially in the papillary dermis, or where the dermis is very thick, like the nose and is extruded through the sebaceous glands. Topical treatment with an astringent lotion and a topical antibiotic cream will help resolve these lesions. 21
-
B. Erysipelas : are diffuse cuticular infections, Streptococcus pyogenes and Staphylococcus aureus being the usual responsible microorganisms. It has to be differentiated from hypersensitivity where itching is a feature, but fever is not. If it is late in onset, atypical mycobacterial infection should be kept in mind. Erysipelas can easily spread into a systemic infection, so it will need oral, or even systemic antibiotics and hospitalization. This should never be massaged, and if an abscess forms, prompt incision and drainage are performed, pus is sent for culture, and sensitivity is guided through antibiotic therapy. 26
Prevention consists of maintaining surgical level asepsis during injection, treating filler like an implant, which it is. The whole face, and not just the site of injection, should be cleaned with chlorhexidine or alcohol or povidone iodine. An assistant should be present to assist in the procedure, ensuring that the injector does not touch any unsterile area. Gloves should be changed as and when necessary, and the tip of the needle or cannula should not touch anywhere, especially not to wipe it with a gauze of any excess filler. The excess should just be flicked off the needle. There are infection control guidelines for safe injection of fillers in some countries.
C. Herpes simplex infection : In patients with a history of herpes, prophylaxis with acyclovir 400 mg twice a day/valaciclovir 500 mg twice a day should be started a day prior to the procedure, and continued for a total of 5 to 7 days. 30 If herpes breaks out after filler injection then it should be treated with a combination of oral and topical medication. Secondary infection often occurs and should be treated with antibiotics. Mostly herpes occurs in the perioral region and the nasolabial fold is commonly seen 24 to 48 hours after filler injection. However, if herpes (blistering and pustules) occurs outside of the normal areas, the possibility of a vascular event should first come to mind. All said, the risk of Herpes Simplex Virus 1 (HSV1) is reported to be approximately 1.45%, while Herpes zoster is very rare after filler injections. 26
-
D. Cellulitis and abscess formation : cellulitis can occur following filler injection, especially in diabetics and immunocompromised patients receiving fillers. Infections usually occur as a result of local factors like nearby skin lesion, poorly prepared skin before injection, asepsis not observed during the procedure, or contamination post injection by the patient. Also, patients who have just had a dental procedure, or any systemic infection like gastroenteritis can develop cellulitis through hematogenous spread.
Inflammatory nodules presenting with erythema, swelling, and tenderness sometimes as early as 24 to 48 hours for up to 2 weeks should be treated as infection. Any fluctuant area should be drained of pus, which should be sent for culture and sensitivity, and appropriate antibiotics are started. Usual organisms are Staphylococcus and Streptococcus , although even Enterococcus has been reported. Treatment recommended consists of amoxycillin + clavulanic acid if the abscess formation is early, and later on modified if needed by the culture report.
However, if there is no pain or fever initially, infection happens after a few days, and culture turns out to be sterile, then a serious possibility of atypical mycobacteria should be entertained. A combination of a macrolide (like clarithromycin) and a quinolone (like ciprofloxacin) should be started.
If the response is partial, then a diagnosis of biofilm should be entertained, especially in late onset or recurrent infections. Also, HA filler can be dissolved with hyaluronidase in the event of a partial response.
Prevention measures consist of:
Starting from the history of any dental work recently done or planned in the immediate future, there should be a gap of at least 2 weeks from the dental procedure, and with antibiotic prophylaxis in case of a future procedure, as for any implant.
History of herpes simplex infection in the past, or any infection like urinary tract infection, gastroenteritis in the recent past, uncontrolled diabetes, HIV, or any other immunocompromising condition.
Any skin infection on the face, including acne. There is no safe distance of a pustule from a filler injection.
All makeup should be cleaned carefully and skin disinfection with 70% alcohol or chlorhexidine–alcohol combination. 31 Disinfection should be done after application of ice.
Complete asepsis to be maintained during the procedure, including but not limited to wearing a surgical mask, sterile gloves, always having an assistant, not touching the needle or cannula tip during the procedure, and changing needles frequently. Post procedure care includes not washing face with tap water for 8 hours after the procedure. 32
-
E. Granulomas : these are a focus of chronic inflammation characterized by chronic inflammatory cells, macrophages, giant cells around a foreign material which is big enough not to be phagocytized by a single macrophage. 26 They sometimes vary according to the type of foreign material present. Pathogenesis of these granulomas is still unclear. Incidence of foreign body granulomas following injection of HA fillers ranges from 0.02 to 0.4%. 33 The reason may be the presence of some protein impurities in the HA filler, or the crosslinker used for crosslinking which may provoke an immune response. The volume of filler injected, repeated injections with the droplet technique, and incorrect depth of injection may also play a role.
More granulomas have been described with fillers like CaHA, Sculptra, and PAAG.
They usually have a delayed onset after months of injection, presenting as an erythematous plaque or a subcutaneous or submucosal nodule, but attending chronic inflammatory signs remains. 34 Diagnosis is by histology—showing multinucleated giant cells around basophilic filler ( Fig. 4A C ), and in the case of Sculptra surrounded by fibroblasts ( Fig. 5 ).
Granulomas have been reported to form after possible migration from the nasolabial fold into the buccal region. 35 Inflammatory granulomas may result from biofilms or hypersensitivity to the filler.
Intralesional hyaluronidase is effective for granulomas secondary to HA fillers. 36 Other treatments are intralesional triamcinolone injections, systemic antibiotics, oral steroids, and 5-fluorouracil intralesionally. 26
-
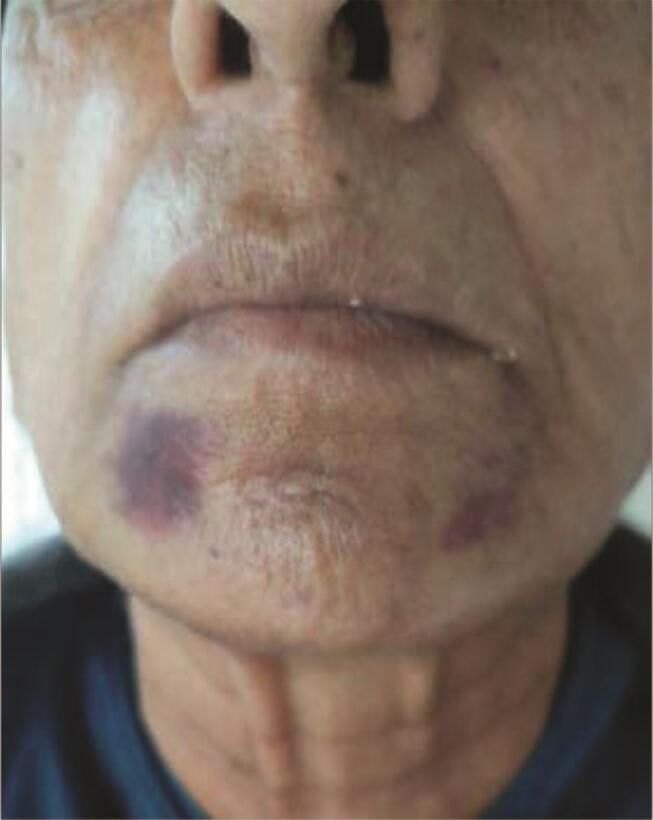
F. Nodules : are painless small firm lumps, appear earlier than granulomas at approximately 2 to 4 weeks, and are more easily felt than seen. 15 Although they are most often present at the site of injection, they are known to migrate. If they become visible, they cause anxiety to the patient. 37
Poor filler placement and injection of particulate fillers in mobile areas can cause these nodules. Intramuscular placement causes visible filler lump, more so on contracting the involved muscle ( Fig. 6A C ).
Nodules occurring after HA fillers can be dissolved with hyaluronidase, whereas those after non-HA fillers can be disrupted by massage after being injected with saline or lidocaine. Additionally, in refractory nodules, a combination of intralesional 5 FU + triamcinolone + lidocaine may be injected, with massage continued throughout the treatment period. Excision of persistent nodules is the last option ( Fig. 7 ). 26
-
G. Biofilms : It is a collection of bacteria around a foreign body or an inert material, immersed and surrounded by a dense adhesive matrix consisting of secreted polymers which result in the bacteria getting adhered to the material. This gives rise to a low-grade inflammation from release of bacteria from the biofilm into the tissues which is resistant to antibiotics. Sometimes they lie quietly, and are activated by trauma from a subsequent filler procedure, thereafter causing a local infection, a systemic infection, or a granulomatous response weeks, months, or years after filler injection. 38 If a red indurated area appears after a procedure, suspect a biofilm.
Persistent inflammatory conditions resistant to treatment or recurring after apparent resolution should raise a suspicion of a biofilm. Since cultures are negative, many earlier thought them to be allergic or foreign body reaction. But with newer techniques of RT-PCR and in situ hybridization, it is possible to confirm infection as the cause especially when cultures are negative. Radiolabeled white cell scintigraphy is very helpful in diagnosing long standing filler complications. 39 Histopathology report often comes as a foreign body granuloma ( Fig. 8 ).
They should in the first instance be treated with antibiotics consisting of a quinolone (ciprofloxacin 500 mg bd) and a macrolide (like clarithromycin 500 mg bd) for 4 to 6 weeks. 26 In case of HA fillers, removal of filler with hyaluronidase will help. Hyaluronidase cleaves the matrix allowing better access of antibiotic to the bacteria. Steroids can be added after sometime, if induration persists. If still not responding, can treat with 5 FU injection (Up to 0.5 mL of 50 mg/mL concentration) alone or with triamcinolone. Surgical excision is the last resort.
The exact incidence of biofilms after filler injection is difficult to know since diagnosis is difficult. So, emphasis should be on prevention, mainly by proper cleansing and prepping of skin before procedure, avoid injecting through oral mucosa, avoid permanent fillers, detailed past history of filler to avoid injecting over previous filler, especially if there was inflammation previously. All aseptic precautions should be taken as for an implant.
-
H. Atypical mycobacterial infections : atypical mycobacteria or nontuberculous mycobacteria (NTM) or mycobacteria other than tuberculosis are ubiquitous in the environment and known to exist in municipal and hospital water systems. 40 41 They are classified into four groups, of which one is pathogenic and rapidly growing mycobacteria (RGM) and includes Mycobacterium abscessus , Mycobacterium chelonae , and Mycobacterium fortuitum . NTM are now being increasingly associated with skin infections. Infection with NTM by M. chelonae was reported by Palm et al in 2011 42 after treatment with fractional CO 2 laser, presenting as acneiform lesions which responded to a prolonged course of clarithromycin and linezolid given as per sensitivity report. Two more cases were reported by Culton et al 43 ( M. abscessus in one and M. chelonae in the other) for which source could not be identified, and could be from somewhere after the procedure, possible source being aerosol from water fountain, sink, or sanding dust. They suggested that patients should avoid municipal water for 72 hours post procedure. 32 43 Both patients responded to treatment with tigecycline and azithromycin in the first case and moxifloxacin and azithromycin in the other.
NTM infection after filler injections were reported in 2002 following the use of a non-FDA approved filler. 44 Rodriguez et al reported a cluster of three patients with M. chelonae infection at a clinic. Tap water from the clinic grew M. chelonae similar to patient isolates, infection possibly arising from application of nonsterile ice preprocedure but after disinfection. 32 Any inflammatory nodule which appears late (3–6 weeks) after filler injection should raise suspicion of ATM infection. If fluctuant, nodules should be drained after incising, and pus should be sent for staining (Gram’s, fungal, and acid-fast bacillus, AFB) as well as aerobic and AFB culture. If there is no pus, an fine needle aspiration cytology could reveal epitheloid granulomas with or without necrosis. 45 A macrolide like clarithromycin and a quinolone like moxifloxacin or ciprofloxacin are adequate empiric treatment, awaiting and tempered by the sensitivity report. NTM are known to select themselves out in response to cleaning repeatedly with quaternary ammonium compounds, so these are recommended not to be used in settings where minimally invasive procedures are performed, 46 as they have only mycobacteriostatic activity.
-
I. Skin discoloration
-
Tyndall effect : Scattering of light by particles in suspension causing a bluish hue (as blue color scatters more) is Tyndall effect. Any HA filler placed in an adequately thick layer superficially close to the surface of the skin is likely to cause a Tyndall effect. This is enhanced by using particulate HA fillers. 47 There are now monophasic low viscosity HA products which if spread very thinly in a subdermal plane are not likely to cause Tyndall effect. Placement of HA filler in a preperiosteal plane, using a filler with less hydrophilic properties and a low G” will help in minimizing chances of a Tyndall effect ( Fig. 9 ).
If it occurs, then dissolving the superficial layer of the HA filler, without removing the whole filler using hyaluronidase is beneficial. In monophasic gels, hyaluronidase cleaves only the surface layer 48 49 unless massage is done after injecting hyaluronidase ( Table 1 ).
Neovascularization : telangiectasias may develop in the skin above the site of filler injection due to—either too much stretching of the skin, vigorous massage, or even steroids used to treat erythema following injection. Lasers are helpful in treating this, KTP, PDL, and IPL being effective. 26
-
Hyperpigmentation : Post inflammatory hyperpigmentation is rare after injectable fillers. However, in Fitzpatrick IV to VI, injection points can get hyperpigmented, especially if multiple punctures are made, or a thicker cannula is used. Incidence one study with 2 to 17% of patients, out of which 6% were at injection sites. 50 This hyperpigmentation was mild and transient. 51
Should hyperpigmentation happens, it should be treated initially with topical creams like retinol or hydroquinone. If there is no response, various lasers (e.g., low intensity Q switched 1064 nM NdYAG laser) can be useful. We should try to keep number of punctures to a minimum, try to keep injection plane to supraperiosteal. 26
Paraesthesia : usually nerve damage does not happen during filler injections, but rarely a needle may pierce a nerve, also causing intraneural filler injection or compress a nerve branch. Excessive molding of filler may also squeeze filler into a foramen leading to compression of a nerve causing paresthesia. Most commonly, it results in neurapraxia, but injury to a nerve branch may cause localized anesthesia. Most common site for paresthesia is the infraorbital nerve when it gets compressed by tight filler injection in the vicinity, or from excessive molding into the foramen. HA can be dissolved with hyaluronidase, and triamcinolone injection in the area can reduce edema and hasten recovery. 26 Recovery usually happens in 3 to 6 weeks. Prevention is the key, with a thorough knowledge of facial anatomy in the area including neural anatomy.
-
Fig. 4.
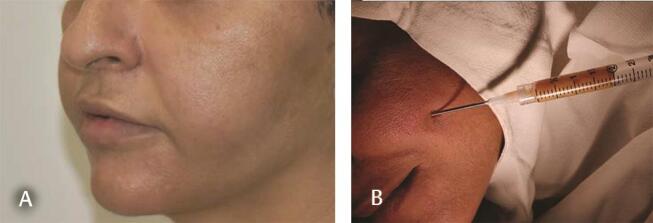
( A ) Unknown filler injected 10 years ago in both nasolabial regions manifests as swelling in lower cheeks. USG showed 5 to 6 mL fluid on each side. ( B ) Aspirated 2 mL from each side. Cytology in Figs. 5A B . USG, ultrasonography.
Fig. 5.
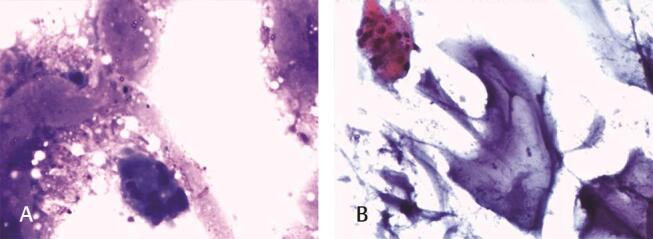
Smears from aspirated material in ( A ) Giemsa stain (×200), and ( B ) Papanicolaou stain (×200) showing basophilic material—hyaluronic acid.
Fig. 6.
( A, B ) 9 months after HA filler injection in the upper end of marionette lines (M1) showing as lumps in the orbicularis oris (much medial and inferior to the point actually injected) migrated possibly because of intramuscular rather than subdermal injection. Visible only on pursing lips (contracting orb. oris), not seen on smiling. ( c ) One week after injecting 15 units of hyaluronidase injection on either side showing partial resolution, needed another sitting of hyaluronidase. HA, hyaluronic acid.
Fig. 7.

( A ) Forehead filler injection of a high G’ showing multiple ridges persisting at 1 month, can be squeezed up or down in a linear fashion, extremely painful, and had to be dissolved. ( B ) Caused as injection was in the subcutaneous plane instead of subgaleal, giving a sunburst appearance akin to the forehead of “Worf”.
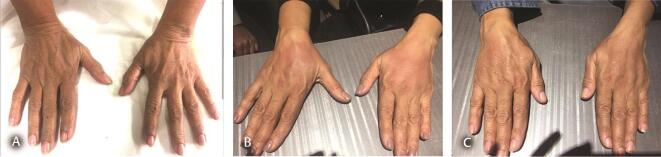
Fig. 8.
( A ) Aging dorsum of hands in a 60-year-old lady. ( B ) Low G’ HA filler injected in dorsum of hands 2.5 months ago, presented with redness and edema in both hands and fingers. ( C ) After hyaluronidase injection under cover of clarithromycin and ciprofloxacin with partial resolution. Repeat sitting of hyaluronidase injection done after a week. HA, hyaluronic acid.
Fig. 9.
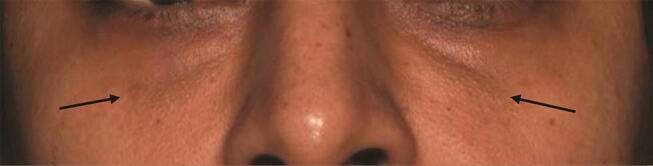
Medium G’ hydrophilic filler injected in tear troughs in a preperiosteal plane 1 month ago. Arrows point to the bilging greyish hue—Tyndall effect.
Table 1. Hyaluronidase preparation, dilution, and doses recommended by consensus panel on the treatment of soft tissue filler complications 47 and Aesthetic Complications Expert (ACE) group, the use of hyaluronidase in aesthetic practice 48 .
| Dilution in normal saline | Dose recommended |
|---|---|
|
a
5–30 units of Hyalase per 0.1 mL of 20 mg/mL HA—general guide.
b 450–1,500 IU repeat hourly till resolved. c Patch test with 20 IU hyalase—wait for 30 min. d Review at 48 h for further treatment, if needed. | |
| 1,500 IU in 10 mL normal saline | 150 IU/mL (standard dilution) c |
| 1,500 IU in 10 mL saline | Small amounts of filler dissolution a |
| 1,500 IU in 5 mL saline | For delayed onset nodules d |
| 1,500 IU in 2 mL saline | For vascular occlusion b |
Conclusion
Injectable fillers for facial rejuvenation and enhancement are increasingly being used world over. Along with their use is the expected attendant rise in complications seen, although the overall incidence remains very low. A better understanding of detailed facial anatomy, evolving filler characteristics, and mastery of injection techniques will ensure that most complications can be prevented and their incidence minimized. Also, knowledge of various complications and awareness of their management protocols will enable us to treat them effectively. Various consensus guidelines global as well as those established by various countries and their national bodies 47 52 will make our task easy. Those who are beginning their journey into fillers may find this article useful to shorten their learning curve of preventing, recognizing early, and effectively managing nonvascular filler complications.
Funding Statement
Source of Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Aesthetic Surgery National Data Bank. Available at: https://www.surgery.org/sites/default/files/ASAPS-Stats 2018_0.pdf. Accessed September 7, 2020
- 2.ISAPS Global Statistics 2018. Available at: https://www.isaps.org/wp-content/uploads/2018/10/ISAPS_2017_International_Study_Cosmetic_Procedures.pdf. Accessed September 7, 2020
- 3.Chandawarkar A A, Provenzano D J, Rad A N, Sherber N S. Learning curves: historical trends of FDA-reported adverse events for dermal fillers. Cutis. 2018;102(02):E20–E23. [PubMed] [Google Scholar]
- 4.Swift A, Delorenzi C, Kapoor KM, Injection anatomy: avoiding the disastrous complication. In: Jones DH, Swift A, eds. From ‘Injectable Fillers-Facial Shaping and Contouring’. 2nd ed. Oxford, United Kingdom: Wiley Blackwell; 2019
- 5.MAUDE-Manufacturer and User Facility Device Experience. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM. Accessed September 7, 2020
- 6.(NHS) Health Education England . London: 2015. Part One. Qualification Requirements for Delivery of Cosmetic Procedures: Non-surgical Cosmetic Interventions and Hair Restoration Surgery. [Google Scholar]
- 7.(NHS) Health Education England . London: 2015. Part two. Report on Implementation of Qualification Requirements for Cosmetic Procedures: Non-surgical Cosmetic Interventions and Hair Restoration Surgery. [Google Scholar]
- 8.Hachach-Haram N, Gregori M, Kirkpatrick N, Young R, Collier J. “Complications of facial fillers: resource implications for NHS hospitals,” Case Reports, vol. 2013 article bcr-2012– 007141, 2013 [DOI] [PMC free article] [PubMed]
- 9.Stern R, Maibach H I. Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin Dermatol. 2008;26(02):106–122. doi: 10.1016/j.clindermatol.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Sudha P N, Rose M H. Beneficial effects of hyaluronic acid. Adv Food Nutr Res. 2014;72:137–176. doi: 10.1016/B978-0-12-800269-8.00009-9. [DOI] [PubMed] [Google Scholar]
- 11.Tezel A, Fredrickson G H. The science of hyaluronic acid dermal fillers. J Cosmet Laser Ther. 2008;10(01):35–42. doi: 10.1080/14764170701774901. [DOI] [PubMed] [Google Scholar]
- 12.Buhren B A, Schrumpf H, Hoff N-P, Bölke E, Hilton S, Gerber P A. Hyaluronidase: from clinical applications to molecular and cellular mechanisms. Eur J Med Res. 2016;21(05):5. doi: 10.1186/s40001-016-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bass L S, Smith S, Busso M, McClaren M. Calcium hydroxylapatite (Radiesse) for treatment of nasolabial folds: long-term safety and efficacy results. Aesthet Surg J. 2010;30(02):235–238. doi: 10.1177/1090820X10366549. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald R, Vleggaar D. Facial volume restoration of the aging face with poly-l-lactic acid. Dermatol Ther (Heidelb) 2011;24(01):2–27. doi: 10.1111/j.1529-8019.2010.01375.x. [DOI] [PubMed] [Google Scholar]
- 15.Daines S M, Williams E F., III Complications associated with injectable soft-tissue fillers: a 5-year retrospective review. JAMA Facial Plast Surg. 2013;15(03):226–231. doi: 10.1001/jamafacial.2013.798. [DOI] [PubMed] [Google Scholar]
- 16.Gladstone H B, Cohen J L. Adverse effects when injecting facial fillers. Semin Cutan Med Surg. 2007;26(01):34–39. doi: 10.1016/j.sder.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 17.King M, Walker L, Convery C, Davies E. Management of a vascular occlusion associated with cosmetic injections. J Clin Aesthet Dermatol. 2020;13(01):E53–E58. [PMC free article] [PubMed] [Google Scholar]
- 18.Zeichner J A, Cohen J L. Use of blunt tipped cannulas for soft tissue fillers. J Drugs Dermatol. 2012;11(01):70–72. [PubMed] [Google Scholar]
- 19.Shah N S, Lazarus M C, Bugdodel R et al. The effects of topical vitamin K on bruising after laser treatment. J Am Acad Dermatol. 2002;47(02):241–244. doi: 10.1067/mjd.2002.120465. [DOI] [PubMed] [Google Scholar]
- 20.Elson M L. Soft tissue augmentation. A review. Dermatol Surg. 1995;21(06):491–500, quiz 501–502. [PubMed] [Google Scholar]
- 21.Vedamurthy M. Beware what you inject: complications of injectables—dermal fillers. J Cutan Aesthet Surg. 2018;11(02):60–66. doi: 10.4103/JCAS.JCAS_68_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duranti F, Salti G, Bovani B, Calandra M, Rosati M L. Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatol Surg. 1998;24(12):1317–1325. doi: 10.1111/j.1524-4725.1998.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 23.Van D yke, S, Hays G P, Caglia A E, Caglia M. Severe acute local reactions to a hyaluronic acid-derived dermal filler. J Clin Aesthet Dermatol. 2010;3(05):32–35. [PMC free article] [PubMed] [Google Scholar]
- 24.King M. Management of edema. J Clin Aesthet Dermatol. 2017;10(01):E1–E4. [PMC free article] [PubMed] [Google Scholar]
- 25.Arron S T, Neuhaus I M. Persistent delayed-type hypersensitivity reaction to injectable non-animal-stabilized hyaluronic acid. J Cosmet Dermatol. 2007;6(03):167–171. doi: 10.1111/j.1473-2165.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 26.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. doi: 10.2147/CCID.S50546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilton S, Schrumpf H, Buhren B A, Bölke E, Gerber P A. Hyaluronidase injection for the treatment of eyelid edema: a retrospective analysis of 20 patients. Eur J Med Res. 2014;19:30. doi: 10.1186/2047-783X-19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funt D K. Avoiding malar edema during midface/cheek augmentation with dermal fillers. J Clin Aesthet Dermatol. 2011;4(12):32–36. [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen L, Breiting V, Bjarnsholt T et al. Bacterial infection as a likely cause of adverse reactions to polyacrylamide hydrogel fillers in cosmetic surgery. Clin Infect Dis. 2013;56(10):1438–1444. doi: 10.1093/cid/cit067. [DOI] [PubMed] [Google Scholar]
- 30.Sherman R N. Avoiding dermal filler complications. Clin Dermatol. 2009;27:S23–S32. [Google Scholar]
- 31.Pratt R J, O’Malley B.Supporting evidence-based infection prevention and control practice in the National Health Service in England. The NHS/TVU/Intuition Approach J Hosp Infect 200765(suppl 2)142–147. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez J M, Xie Y L, Winthrop K L et al. Mycobacterium chelonae facial infections following injection of dermal filler. Aesthet Surg J. 2013;33(02):265–269. doi: 10.1177/1090820X12471944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemperle G, Gauthier-Hazan N, Wolters M. Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123(06):1842–1863. doi: 10.1097/PRS.0b013e31818236d7. [DOI] [PubMed] [Google Scholar]
- 34.Shahrabi-Farahani S, Lerman M A, Noonan V, Kabani S, Woo S B. Granulomatous foreign body reaction to dermal cosmetic fillers with intraoral migration. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(01):105–110. doi: 10.1016/j.oooo.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Kaczorowski M, Nelke K, Łuczak K, Hałoń A. Filler migration and florid granulomatous reaction to hyaluronic acid mimicking a buccal tumor. J Craniofac Surg. 2020;31(01):e78–e79. doi: 10.1097/SCS.0000000000005928. [DOI] [PubMed] [Google Scholar]
- 36.Rzany B, Becker-Wegerich P, Bachmann F, Erdmann R, Wollina U. Hyaluronidase in the correction of hyaluronic acid-based fillers: a review and a recommendation for use. J Cosmet Dermatol. 2009;8(04):317–323. doi: 10.1111/j.1473-2165.2009.00462.x. [DOI] [PubMed] [Google Scholar]
- 37.Requena L, Requena C, Christensen L, Zimmermann U S, Kutzner H, Cerroni L. Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol. 2011;64(01):1–34, quiz 35–36. doi: 10.1016/j.jaad.2010.02.064. [DOI] [PubMed] [Google Scholar]
- 38.Cassuto D, Marangoni O, De Santis G, Christensen L.Advanced laser techniques for filler-induced complications Dermatol Surg 200935(suppl 2)1689–1695. [DOI] [PubMed] [Google Scholar]
- 39.Grippaudo F R, Pacilio M, Di G irolamo, M, Dierckx R A, Signore A. Radiolabelled white blood cell scintigraphy in the work-up of dermal filler complications. Eur J Nucl Med Mol Imaging. 2013;40(03):418–425. doi: 10.1007/s00259-012-2305-7. [DOI] [PubMed] [Google Scholar]
- 40.Goslee S, Wolinsky E. Water as a source of potentially pathogenic mycobacteria. Am Rev Respir Dis. 1976;113(03):287–292. doi: 10.1164/arrd.1976.113.3.287. [DOI] [PubMed] [Google Scholar]
- 41.Narang R, Narang P, Mendiratta D K. Isolation and identification of nontuberculous mycobacteria from water and soil in central India. Indian J Med Microbiol. 2009;27(03):247–250. doi: 10.4103/0255-0857.53208. [DOI] [PubMed] [Google Scholar]
- 42.Palm M D, Butterwick K J, Goldman M P. Mycobacterium chelonae infection after fractionated carbon dioxide facial resurfacing (presenting as an atypical acneiform eruption): case report and literature review. Dermatol Surg. 2010;36(09):1473–1481. doi: 10.1111/j.1524-4725.2010.01663.x. [DOI] [PubMed] [Google Scholar]
- 43.Culton D A, Lachiewicz A M, Miller B A et al. Nontuberculous mycobacterial infection after fractionated CO(2) laser resurfacing. Emerg Infect Dis. 2013;19(03):365–370. doi: 10.3201/eid1903.120880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toy B R, Frank P J. Outbreak of Mycobacterium abscessus infection after soft tissue augmentation. Dermatol Surg. 2003;29(09):971–973. doi: 10.1046/j.1524-4725.2003.29262.x. [DOI] [PubMed] [Google Scholar]
- 45.Das D K. Fine-needle aspiration cytology in the diagnosis of tuberculous lesions. Cytology. 2000;31(01):625–632. [Google Scholar]
- 46.Cortesia C, Lopez G J, de Waard J H, Takiff H E. The use of quaternary ammonium disinfectants selects for persisters at high frequency from some species of non-tuberculous mycobacteria and may be associated with outbreaks of soft tissue infections. J Antimicrob Chemother. 2010;65(12):2574–2581. doi: 10.1093/jac/dkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urdiales-Galvez F, Delgado E, Figueiredo V et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2017;41(03):667–677. doi: 10.1007/s00266-017-1063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King M, Convery C, Davies E. This month’s guideline: the use of hyaluronidase in aesthetic practice (v2.4) J Clin Aesthet Dermatol. 2018;11(06):E61–E68. [PMC free article] [PubMed] [Google Scholar]
- 49.DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33(04):561–575. doi: 10.1177/1090820X13484492. [DOI] [PubMed] [Google Scholar]
- 50.Taylor S C, Burgess C M, Callender V D.Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial Dermatol Surg 200935(suppl 2)1653–1660. [DOI] [PubMed] [Google Scholar]
- 51.Grimes P E, Thomas J A, Murphy D K. Safety and effectiveness of hyaluronic acid fillers in skin of color. J Cosmet Dermatol. 2009;8(03):162–168. doi: 10.1111/j.1473-2165.2009.00457.x. [DOI] [PubMed] [Google Scholar]
- 52.Signorini M, Liew S, Sundaram H et al. Global Aesthetics Consensus Group. Global aesthetics consensus: avoidance and management of complications from hyaluronic acid fillers—evidence and opinion-based review and consensus recommendations. Plast Reconstr Surg. 2016;137(06):961e–971e. doi: 10.1097/PRS.0000000000002184. [DOI] [PMC free article] [PubMed] [Google Scholar]