Abstract
LL-37, cleaved from human cathelicidin, and human neutrophil peptide-1 (HNP1) from the defensin family are antimicrobial peptides that are occasionally co-released from neutrophils, which synergistically kill bacteria. We report that this couple presents another type of cooperativity against host eukaryotic cells, in which they antagonistically minimize cytotoxicity by protecting membranes from lysis. Our results describe the potential of the LL-37/HNP1 cooperativity that switches from membrane-destructive to membrane-protective functions, depending on whether the target is an enemy or a host.
Significance
A mixture of different types of biomolecules sometimes boosts or suppresses their activities or even generates a new function known as cooperativity. We report a unique cooperative function between two well-known antimicrobial peptides (LL-37/HNP1) that kills bacteria more efficiently while minimizing the host damage by suppressing mammalian cell membrane lysis. Such a “double cooperativity” may be used in our immune system and may help with developing efficient and safe antimicrobial agents in the future.
Introduction
Synergy among antimicrobial peptides (AMPs) (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19), in which mixing different types of AMPs boosts their antimicrobial efficiency, has garnered attention as a possible approach to improve their potency and because of its underlying interesting mechanism (2,20, 21, 22, 23, 24, 25). Particularly, synergy between the cathelicidin-derived peptide (26), LL-37, and human neutrophil peptide 1 (HNP1) from the α-defensin subfamily (27, 28, 29, 30) is important because they are among major human AMPs. LL-37 and HNP1 are mainly produced in the bone marrow during neutrophil maturation, which are then occasionally co-released into the blood and tissues for synergistically combating pathogens (10). However, LL-37 is known to exhibit cytotoxicity at high concentrations because of its membrane-destructive properties (31). How the host eukaryotic cells escape from their attack is unknown.
In this work, to study the effect of their cooperativity on host cells, mammalian cells and mammalian cell membrane mimics were challenged by LL-37, HNP1, and their mixture, and their responses were observed by biophysical assays based on supported and pore-spanning bilayers, such as fluorescence recovery after photobleaching (FRAP), quartz crystal microbalance with dissipation (QCM-D), electrochemical impedance spectroscopy (EIS), and single-channel conductance measurement, which we have routinely employed for membrane-active compound characterization for the past years (32, 33, 34, 35, 36, 37, 38), combined with toxicity test by calcium-sensitive dye, several spectroscopic methods, and electron-beam microscopy. The result showed that this couple cooperatively protects mammalian cell membranes from lysis for minimizing the cytotoxicity in contrast to known synergistic effect against bacteria.
Materials and Methods
For a detailed summary of the materials and methods used in this study, see Supporting Material.
Results
HNP1 unexpectedly suppresses LL-37 cytotoxicity in MDCK cells and HUVEC
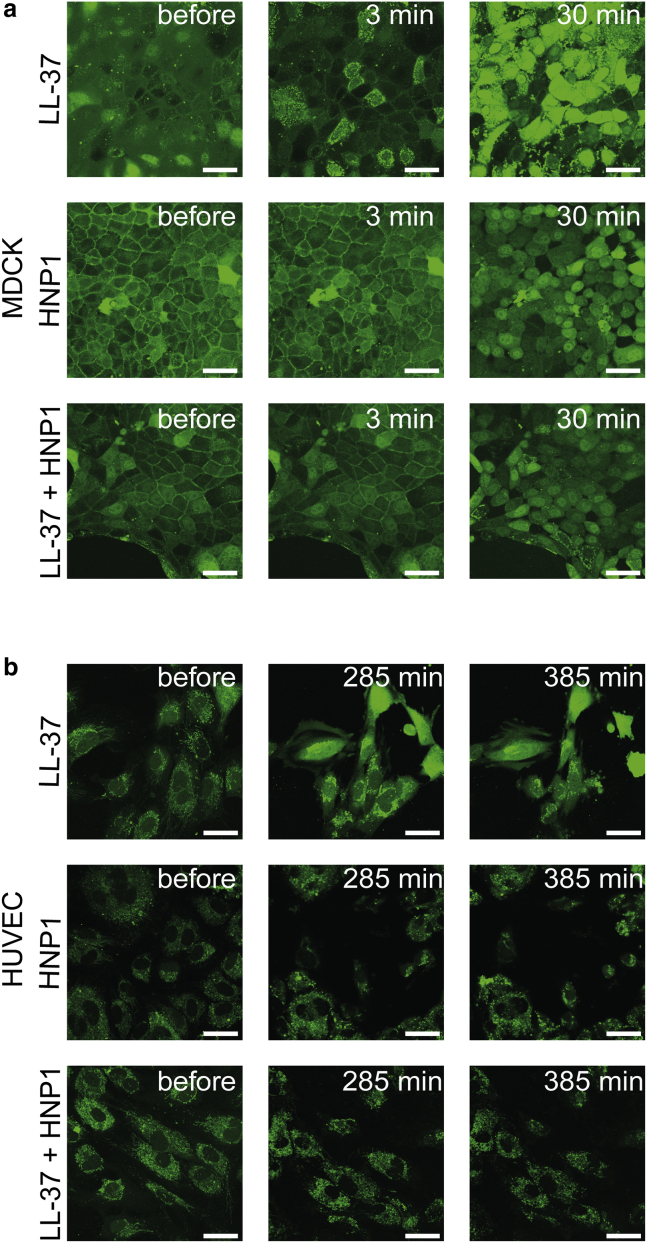
Apart from its antimicrobial activities (39), LL-37 is a well-known key player of several biological processes in the host, such as immunomodulation (40), the promotion of cell motility, and wound healing (39, 40, 41, 42), whereas it becomes cytotoxic at high concentrations (>2.5–13 μM (40,43,44)). This cytotoxicity of LL-37 toward mammalian cells could be visualized by calcium-sensitive dyes in a time-dependent manner. The addition of LL-37 to Madin-Darby canine kidney (MDCK) cells at 29 μM resulted in a fluorescence increase from the intracellular calcium reporter, Fluo-3, within 3 min (Fig. 1 a, first row), indicating its cytotoxicity. The rapid response time implies defect formation in the plasma membrane and the subsequent induction of a large calcium influx from the extracellular space into the cytosol, as has been reported previously (45). This pore-induced cytotoxicity was inhibited by the mixture of LL-37 and HNP1 at a 1:1 molar ratio (Fig. 1 a, third row). The control experiment showed that HNP1 individually did not induce noticeable cytotoxicity at 29 μM (Fig. 1 a, second row). The similar HNP1-related inhibition of LL-37 cytotoxicity was observed in human umbilical vein endothelial cells (HUVEC), as shown in Fig. 1 b. Note that the timescale required to induce the LL-37 cytotoxicity in HUVEC was 50 times longer; thus, this might have involved other mechanisms than a simple pore formation. These results show that HNP1 neutralizes LL-37 cytotoxicity in MDCK cells and HUVEC in contrast to the previously reported synergy against bacteria (10).
Figure 1.
Cytotoxic activity of LL-37 and its inhibition by HNP1, studied with calcium sensitive dye, Fluo-3. (a) MDCK and (b) HUVEC with Fluo-3 are monitored by confocal laser scanning microscopy over time, where after 350 s for MDCK and 1292 s for HUVEC, LL-37, HNP1, and their mixture at 1:1 molar ratio were added all at 29 μM (in the case of the mixture, it is 29 μM LL-37 + 29 μM HNP1). Scale bars, 10 μm. To see this figure in color, go online.
LL-37 and HNP1 did not interact in solution
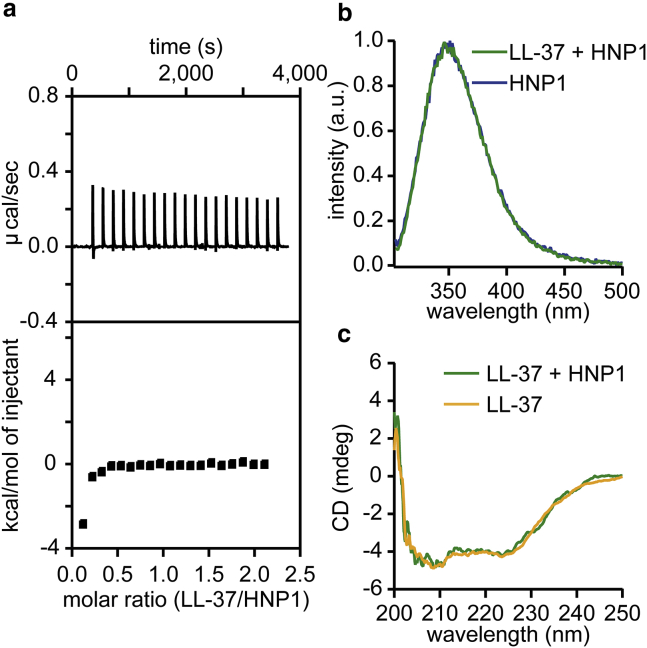
To study whether LL-37 and HNP1 already bind in solution, their interactions in a physiological HEPES buffer solution (150 mM NaCl (pH 7.4)) were monitored using isothermal titration calorimetry (ITC), tryptophan fluorescence spectroscopy, and circular dichroism (CD). No clear evidence for their binding was observed in ITC, as seen by the constant endothermal peaks that come from heats of dilution during the titration of LL-37 into HNP1 in a HEPES buffer solution (Fig. 2 a). To confirm this result, we measured the HNP1 tryptophan fluorescence emission peak both in the presence and absence of LL-37. HNP1 has a single tryptophan (Trp) residue, which causes a blue shift in its emission peak in a hydrophobic microenvironment. We observed no change in the tryptophan emission peak position between with or without LL-37 (Fig. 2 b), indicating no interaction. This was further validated by CD, in which the double-dip at 208 and 222 nm characteristic for α-helical structures (46) in LL-37 remained unchanged after the addition of HNP1 (Fig. 2 c), suggesting the absence of structural rearrangements often observed upon binding (17).
Figure 2.
LL-37 and HNP1 did not interact in solution, evidenced by isothermal titration calorimetry (ITC), tryptophan (Trp) fluorescence spectroscopy, and CD. (a) Heat flow and the integrated heat from ITC when 40 μM HNP1 in HEPES buffer solution in a chamber volume of 200 μL was titrated with 400 μM LL-37 in HEPES buffer solution at 2 μL each time for 20 times. (b) Shown are Trp fluorescence emission spectra of HNP1 alone in HEPES buffer solution at 2.9 μM and in combination with LL-37 at 1:1 molar ratio (LL-37 signal subtracted). (c) Shown are CD spectra of LL-37 in HEPES buffer solution at 29 μM and in combination with HNP1 at 1:1 molar ratio (HNP1 signal subtracted). All measurements were done in 10 mM HEPES and 150 mM NaCl at pH 7.4. To see this figure in color, go online.
ITC results show no significant effect of HNP1 on the LL-37 adsorption onto the POPC bilayers
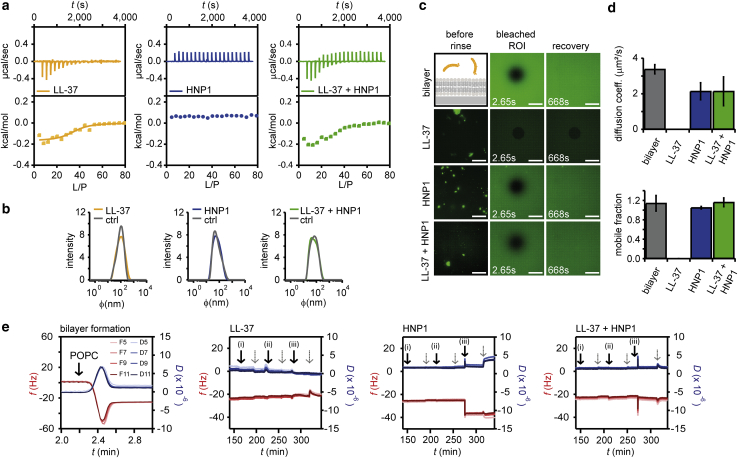
Because bilayer disruption is one of the well-known mechanisms of LL-37 toxicity, its suppression by HNP1 might be taking place in the membranes. As a first step toward better understanding such a cooperative function in membranes, we studied their adsorption to 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC), which is one of the major lipids in eukaryotic plasma membranes. We titrated peptides with POPC vesicles in ITC. LL-37 bound to POPC vesicles (Fig. 3 a). The binding is strongly entropic (−TΔS = −6.258 kcal/mol ≪ ΔH = −0.194 kcal/mol, where ΔG = ΔH − TΔS = −6.452 kcal/mol), indicating that the hydrophobic interaction was the main cause of the binding rather than the electrostatic interaction. Note that these values are rough estimations as the ITC signals also contain the information on peptide arrangements in the bilayers, such as pore formation, in addition to binding (47,48). Titration of vesicles to HNP1 did not produce any detectable amount of heat from binding as no titration curve was observed (Fig. 3 a). Fluorescence microscopy and quartz crystal microbalance with dissipation monitoring confirmed the adhesion of HNP1 to POPC bilayers, as we will discuss later (Fig. 3, c and e). Thus, this lack of significant heat production upon binding is the result of a too entropic interaction, which was below the ITC sensitivity at this concentration. The mixture of LL-37 and HNP1 yielded a titration curve similar to that of LL-37 (Fig. 3 a). This suggests that the LL-37 adhesion to POPC vesicles was affected little by the presence of HNP1. The vesicle sizes, estimated by dynamic light scattering, were not altered significantly by the addition of peptides (Fig. 3 b).
Figure 3.
Peptide-lipid interactions monitored by ITC, fluorescence recovery after photobleaching (FRAP), and QCM-D. (a) Heat flow and the integrated heat from ITC when LL-37, HNP1, and LL-37 + HNP1 at 1:1 molar ratio all at 40 μM (in case of the mixture 40 μM each) in the chamber volume of 200 μL were titrated with POPC vesicles at 15 mM with 2 μL each time for 20 times. The reverse titration is shown in Fig. S1. (b) Vesicle size after each ITC experiment was determined by dynamic light scattering. (c) Shown are fluorescence images of supported POPC lipid bilayers with 0.2% mol 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl after incubation with each peptide at 2.9 μM before rinsing and subsequent FRAP images after rinsing. Note that the enhancement is different between before and after the rinse to visualize the bright dots in the images before rinse. Scale bars, 50 μm. (d) Diffusion coefficients were calculated from FRAP experiments as follows: 3.38 ± 0.25 μm2/s for the POPC bilayers, no recovery was observed for LL-37, 2.14 ± 0.48 μm2/s for HNP1, and 2.14 ± 0.81 μm2/s for the peptide mixture. Experiments were repeated for three times and their average and the standard deviation are plotted. (e) Shown are QCM-D data for the bilayer formation by vesicle rupture and the subsequent addition of LL-37, HNP1, and their mixture at (i) 0.29 μM, (ii) 2.9 μM, and (iii) 29 μM. The gray dotted arrows indicate the rinse by injecting HEPES buffer solution. Their overtone analysis is shown in Fig. S2. To see this figure in color, go online.
FRAP results demonstrate LL-37-induced POPC bilayer destruction, rescued by the addition of HNP1
The first evidence for the interference of LL-37 and HNP1 was observed during FRAP, as shown in Fig. 3 c. FRAP is a well-established tool for the characterization of the lipid bilayer integrity and fluidity (49). Supported POPC + 0.2% mol 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl lipid bilayers were assembled on glass coverslips by vesicle fusion as confirmed by full FRAP recovery with a diffusion coefficient of D = 3.38 ± 0.25 μm2/s (Fig. 3 d). These results were comparable with previously published values (49). LL-37 disrupted the lateral continuity of these bilayers at 2.9 μM, as demonstrated by a lack of FRAP recovery (Fig. 3, c and d). The decreased fluorescence intensity from the bilayer indicated the detachment of lipids from the substrate upon the addition of LL-37 (Fig. 3 c). HNP1 induced membrane protrusions, demonstrated by the appearance of bright dots in the fluorescence images (Fig. 3 c). Nevertheless, the bilayer maintained the lateral continuity as FRAP showed a full recovery with a diffusion coefficient of D = 2.14 ± 0.48 μm2/s, which was 37% reduced compared to the POPC bilayers without peptides. This illustrates that HNP1 inserted into the bilayers and increased the surface area of the membranes, where the excess area folded into structures such as tubes are visible as bright spots, without disintegrating the bilayer. The mixture of LL-37 and HNP1 interacted with the bilayer as some bright dots were also observed after the incubation, yet the bilayer continuity was maintained, confirmed by the full FRAP recovery with a reduced diffusion coefficient of D = 2.14 ± 0.81 μm2/s (Fig. 3, c and d). These FRAP data provided evidence to the LL-37-induced POPC bilayer destruction, whereas adding HNP1 rescued this effect. Such a cooperative function between LL-37 and HNP1 in synthetic POPC bilayers might be linked to the observed neutralization of cytotoxicity shown in Fig. 1.
QCM-D indicates that LL-37 removes lipids from bilayers, HNP1 creates membrane protrusions, and their mixture suppresses both effects
To confirm the observed peptide-bilayer interactions, we next used QCM-D. QCM-D enables the detection of wet mass (including the water mass) on the sensor crystal and the viscoelastic properties of the deposited film by the changes in the resonance frequency Δf and the decay of the sensor oscillations ΔD (50), frequently used to study AMP-bilayer interactions (51). Supported POPC bilayers were formed on silicon dioxide-coated quartz crystal microbalance crystals by vesicle rupture, as confirmed by the typical frequency and dissipation change in QCM-D during this process. The initial decrease in Δf (increase in mass) and the increase in ΔD indicated the vesicle adsorption, whereas the following increase (decrease in mass) and the stabilization of Δf at around 24 Hz and the decrease in ΔD illustrated the vesicle rupture and the corresponding release of water mass (Fig. 3 e, bilayer formation; (52)). LL-37 partially removed lipids from the sensor surface and made the bilayer slightly more rigid, implied by the increase in frequency (a loss in mass) and the decrease in dissipation (Fig. 3 e). The mass of LL-37 is too small to be detected because even a monolayer membrane coverage would have resulted only in Δf = 2–4 Hz (53,54). Further overtone analysis provided a resolution in a z-direction as acoustic wave penetration depths decrease along with the increasing overtone numbers (55,56). No significant overtone dependency except for a slight variation in Δf at the highest concentration (29 μM, Fig. S2, a and b) was observed, in agreement with the previously reported detergent-like mechanism (57). HNP1 induced a reduction in Δf by 13.6 Hz (increase in mass) and increase in dissipation by 2.4 × 10−6 after the last rinse (Fig. 3 e), indicating the lipid bilayer morphological change and the associated increase in the coupling of water mass typical for the membrane protrusion or vesicle adsorption (52). This is in agreement with the appearance of bright dots in fluorescence images (Fig. 3 c). A slight overtone dependence (Fig. S2, c and d), visible at 29 μM, both in Δf and ΔD also suggests that the main mass increase took place at least tens of nanometers away from the sensor surface (58), which is compatible with the membrane protrusion. The mixture of LL-37 and HNP1 showed no significant signal change (Fig. 3 e; Fig. S2, e and f), indicating that the effect of LL-37 (destruction) and HNP1 (protrusion) on supported bilayers are both suppressed when they are mixed.
EIS confirms that HNP1 suppresses LL-37 defect formation
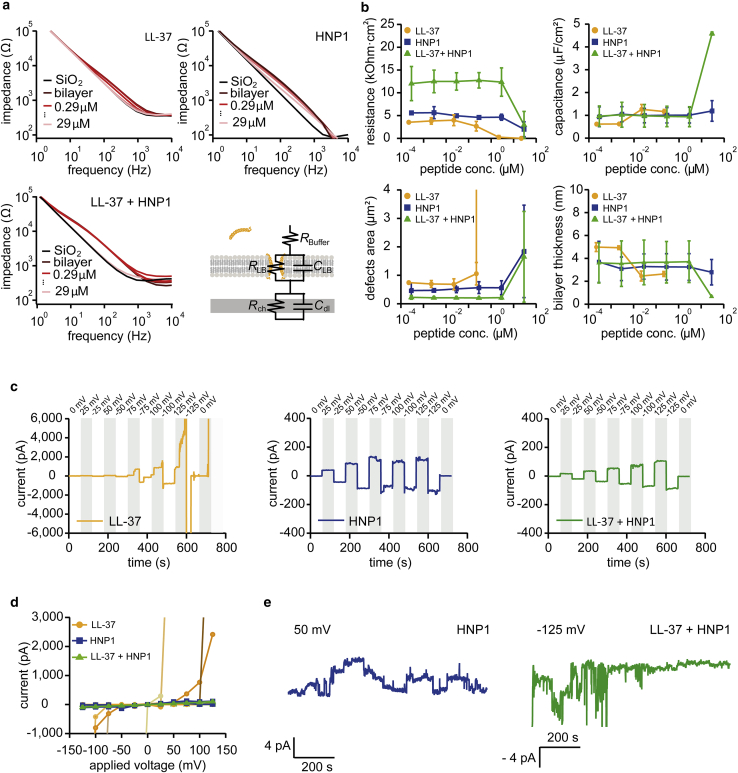
Next, to monitor the bilayer destruction process with higher sensitivity, we performed EIS. We used supported POPC lipid bilayers formed on highly doped (metallic) silicon wafers as a working electrode in a three-electrode setup. The top surface of silicon is silicon dioxide, which facilitates the self-assembly of bilayers by vesicle fusion. From the obtained impedance spectra (Fig. 4 a), a bilayer resistance RLB and a capacitance CLB were extracted (Fig. 4 b) by fitting them with an equivalent circuit presented in Fig. 4 a. Both RLB and CLB were further converted into the total defect area and the average bilayer thickness. LL-37 destroyed bilayers at 2.2 μM, indicated by an abrupt increase in the defect area (Fig. 4 b, defect area). LL-37 is known to form defects in a concentration-dependent manner, both in bacterial and eukaryotic cell membrane mimics, previously shown by vesicle leakage assay (59). In contrast, HNP1 created a total defect size of 1.6 μm2 at 29 μM (Fig. 4 b, defect area), suggesting that HNP1 also forms small defects at such a high concentration. When they were mixed, the total defect area became 0.3 μm2 at 29 μM, indicating that HNP1 suppressed the LL-37-induced bilayer destruction. The average bilayer thickness started to decrease at the LL-37 concentration of 0.22 μM (Fig. 4 b, bilayer thickness). Beyond 2.2 μM, the thickness could not be estimated because the bilayer was destroyed. For HNP1 and the mixture, the average bilayer hydrocarbon thickness was 3.2 and 3.1 nm, both at 2.9 μM. These impedance data further confirm that LL-37 forms large defects in bilayers, whereas the mixture of HNP1 rescues it.
Figure 4.
EIS with supported lipid bilayers and conductance measurements with porespanning bilayers confirm that HNP1 suppresses LL-37 membrane disintegration. (a) Shown are EIS spectra of supported POPC bilayers at different peptide concentrations. An equivalent circuit used for fitting is also shown. (b) Extracted bilayer resistance and capacitance and their conversion into defect area and bilayer thickness were plotted against peptide concentrations. Experiments were repeated twice and their average and the standard deviation are plotted. (c) Shown are current recordings through free standing lipid bilayer with the addition of LL-37 at 8 μM, HNP1 at 8 μM, and LL-37+HNP1 at 1:1 molar ratio with the total peptide concentration of 16 μM at different applied voltages and (d) extracted I-V plot. For each peptide, multiple runs were analyzed and presented in the I-V plot. (e) Shown are conductance measurements of pore-spanning lipid bilayers exposed to HNP1 at 15 μM at 50 mV holding potential and LL-37+HNP1 mixture at 1:1 molar ratio with the total concentration of 26 μM at −125 mV holding potential. To see this figure in color, go online.
Single-channel conductance indicates that LL-37 alters the behavior of the HNP1 pores
Next, the bilayer conductance was measured at a fixed (DC) voltage with lateral pore-spanning bilayers as described previously (36,38). After a giga-Ohm seal was achieved, LL-37, HNP1, and their mixture were added to the cis chamber, and step voltages from 0 to 125 mV/−125 mV were applied. For LL-37, transmembrane currents exceeded 6000 pA at ±125 mV (Fig. 4 c), or the bilayer often ruptured. These dramatic effects on the bilayers were in agreement with the previously proposed carpet-like mechanism for LL-37-bilayer interaction (60). For HNP1 and its mixture with LL-37, the transmembrane currents never exceeded 200 pA at ±125 mV (Fig. 4 c). These results further confirm that LL-37 forms large defects in bilayers, whereas the addition of HNP1 suppresses it. In between these conductance measurements, we ran impedance spectroscopy to monitor the change in the bilayer thickness. The standard POPC bilayers have a capacitance density of 0.6–1.0 μF/cm2 (61, 62, 63), which corresponds to the thickness of around 4 nm. In the case of LL-37, defects in bilayers were observed even at a capacitance as low as 0.3 μF/cm2 (Fig. S3), which corresponds to the bilayer thickness of 10.4 nm because of the remaining organic solvent sandwiched between the two monolayer leaflets. This indicated that LL-37 formed defects independent of the bilayer thickness as in the carpet-like model (60). Bilayer conductance measurement at a fixed voltage captured single channels for HNP1. The size of these pores was around 1.2 ± 0.2 Å at −50 mV (Fig. 4 e), although the pore size seemed to fluctuate as other channel conductance were also seen. This single pore conductance appeared to be disturbed when LL-37 was mixed (Fig. 4 e). These results suggest that LL-37 destabilizes bilayers, HNP1 forms small stable pores at high concentrations (15 μM), and their mixture forms small defects without rupturing bilayers.
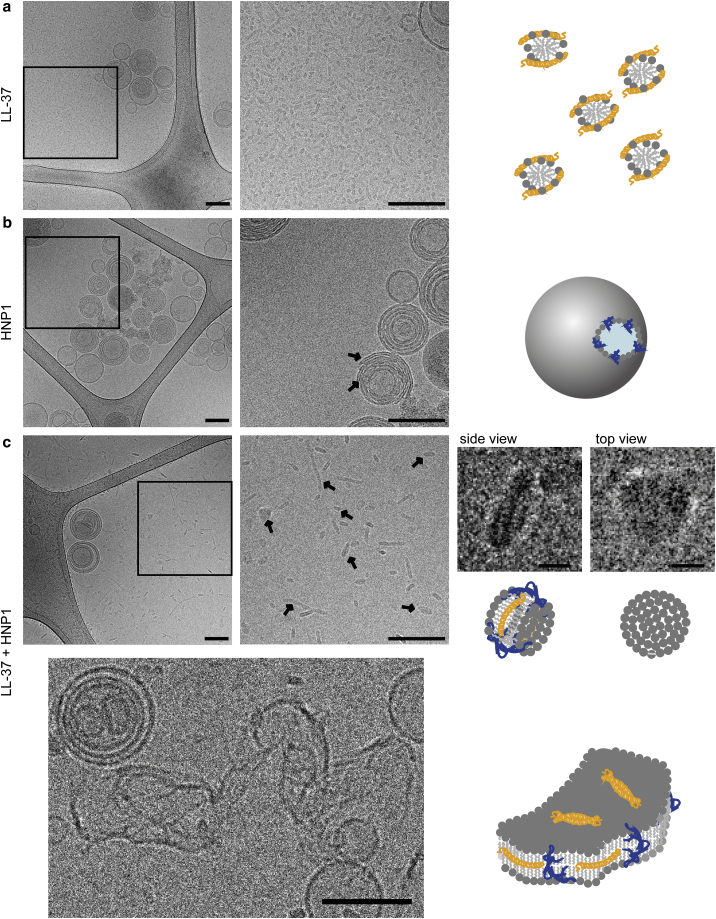
Cryo-electron microscopy visualized that LL-37 destroys POPC vesicles into small fragments, HNP1 creates a stable opening in vesicles, and their mixture forms bilayer sheets or nanodisks
To visualize the peptide-induced lipid structural change, POPC vesicles incubated with LL-37, HNP1, and their mixture were imaged by cryo-transmission electron microscopy (cryo-TEM). LL-37 disintegrated lipid membranes, captured by small fragments of lipid-peptide composites (Fig. 5 a) with a size ranging from 4.8 to 19.4 nm (the average was 9.7 ± 2.8 nm) that did not exist before adding LL-37 (a control image shown in Fig. S4). These fragments were not observed with POPC vesicles incubated with HNP1 (Fig. 5 b). Instead, a stable perforation of bilayers by HNP1, which created openings in vesicles, was observed (arrows in Fig. 5 b). The mixture of LL-37 and HNP1 produced objects that resembled nanodisks and sheets (Fig. 5 c). Recently, several amphiphilic proteins have been shown to spontaneously assemble into lipid-protein nanodisks, in which these proteins wrap lipid bilayer disks like a belt to reduce the line tension at the bilayer edge (64, 65, 66). Although we acquired these images at rather high concentrations (lipid concentration = 1.5 mM, peptide concentration = 150 μM, L/P = 10) to adjust the density of the objects compatible with cryo-TEM, the LL-37-induced bilayer destruction and its partial inhibition by HNP1 is in agreement with our other data.
Figure 5.
Cryo-electron microscopy visualized that LL-37 destroys POPC vesicles into small fragments, HNP1 creates stable opening in vesicles, and their mixture forms bilayer sheets or nanodisks. Shown are cryo-electron microscopy (cryo-TEM) images of POPC vesicles after incubation with (a) LL-37, (b) HNP1, and (c) LL-37 + HNP1 mixture. Peptides at 150 μM were incubated with vesicles at 1.5 mM for 30 min in physiological HEPES buffer solution. In the case of the mixture, 300 μM was the final concentration of both peptides combined (150 μM LL-37 + 150 μM HNP1). Possible interpretation of these images is drawn on the right side. All images were taken with Talos TEM 200 kV, except the one at the bottom, which was taken by Titan Krios G3i 300 kV. All the scale bars represents 100 nm except for the zoom-in top and side-view images of nanodisks, which are 10 nm. To see this figure in color, go online.
Discussion
LL-37 is a 37 residue, amphipathic, human helical peptide, expressed in epithelial cells of the testis, skin, gastrointestinal tract, and respiratory tract, as well as in leukocytes, such as monocytes, neutrophils, T cells, natural killer cells, and B cells. It has been found to have antimicrobial (10), antiviral (67), and anticancer (68) activities, as well as immunomodulatory roles comprising both anti- (69) and proinflammatory functions (70), chemotactic (71), and cytotoxic effect (44), and it also promotes cell migration and wound closure (72). HNP1 is a 30-amino acid human peptide adopting a triple-stranded β-sheet structure (73). It is released from cells upon stimuli or, in some cases, constitutively, for example from neutrophils, natural killer cells, and monocytes, and exhibits antimicrobial activity (74,75), neutralizes bacterial toxins (76), and modulates the adaptive immune response (77). Bilayer membranes are the primary targets of LL-37 and HNP1; thus, these peptides affect fungi (78,79) and enveloped viruses (67,80) besides bacteria. LL-37 and HNP1 are frequently co-expressed. In 2000, Nagaoka and co-workers reported direct evidence of their synergistic antimicrobial effect against Escherichia coli and Staphylococcus aureus (10). In this study, we observed the opposite, an antagonistic effect toward MDCK cells and HUVEC, in which the LL-37 cytotoxicity is suppressed by HNP1 (Fig. 1). The previous report, in addition to our result, implies that the cooperative function of the LL-37/HNP1 pair might switch from destructive to protective, depending on the target. Our data showed that LL-37 and HNP1 did not bind in solution, as confirmed by ITC, tryptophan fluorescence spectroscopy, and CD (Fig. 2). Previously LL-37 has been reported to form dimers, trimers, or tetramers (81, 82, 83) in solution, whereas HNP1 has been shown to form dimers (84). Their oligomerization in aqueous solution is driven by the minimization of their free energy to hide their hydrophobic residues from the surrounding water. Once their hydrophobic parts have been already concealed as oligomers, their net positive charge would induce an electrostatic repulsion between LL-37 and HNP1. This partially explains the lack of their binding in solution. LL-37 adhesion to POPC bilayers was not significantly affected by the presence of HNP1 either, indicated by ITC (Fig. 3 a). We found evidence for their interaction only after they bound to bilayers. LL-37 formed large defects or destroyed bilayers that were visible in FRAP (Fig. 3, c and d), QCM-D (Fig. 3 e), EIS (Fig. 4, a and b), transmembrane currents (Fig. 4, c and d), and cryo-TEM (Fig. 5 a). The mechanism of the LL-37 interaction with membranes is still debated. However, several models have been proposed, depending on the applied method. Carpet/toroidal membrane disintegration has been proposed based on solid-state NMR studies for LL-37 interacting with different membranes (85). The parallel orientation of peptide to membrane surface was confirmed by attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) (82), supporting a carpet-like mechanism. Some studies suggest that LL-37 could distinguish anionic and zwitterionic lipids (86). However, other studies have shown a lack of such discrimination (82). HNP1 induces protrusions in bilayers, as we observed by QCM-D and fluorescence images (Fig. 3, c–e), yet the bilayer continuity was maintained, as highlighted by the full recovery in FRAP (Fig. 3, c–e). The single pore conductance shows that HNP1 forms transmembrane pores (Fig. 4 e), as it has been previously reported (87) and further supported by solid NMR, in which HNP1 dimers line the pore with the hydrophilic part of the peptide facing the water column (88). However, their total pore size observed by EIS (Fig. 4 b) was 1.6 μm2 at 29 μM, which corresponds to 1.7 × 10−6% of the total lipid bilayer area, implying that the pore formation might be only a minor function of HNP1. When LL-37 and HNP1 were mixed, both the destruction of bilayers induced by LL-37 and the membrane protrusion induced by HNP1 were inhibited, as seen by a lack of significant mass change in QCM-D (Fig. 3 e; Fig. S2), full recovery in FRAP (Fig. 3, c and d), and only minor defects or pores observed by EIS and transmembrane conductance (Fig. 4, a–e). Cryo-TEM demonstrated bilayer sheets and objects that resemble nanodisks, which also support that mixing HNP1 partially suppressed the destruction of bilayers by LL-37 because LL-37 alone fragmented vesicles into much smaller particles.
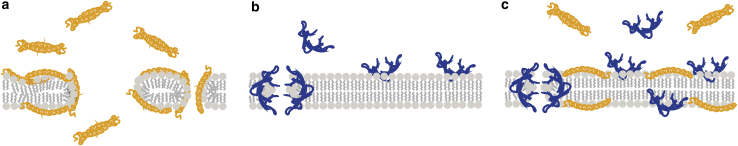
These functional studies demonstrated that the cooperative activities of LL-37 and HNP1 start only in lipid bilayers. Both LL-37 and HNP1 form homo-oligomers in aqueous solution without binding each other, yet upon incorporation into bilayers, the unique hydrophobic environment triggers their interactions and initiates the cooperative effect (Fig. 6). To further link the function to the structure, a set of additional experiments were performed by CD and fluorescence spectroscopy. When LL-37 was titrated by POPC vesicles, the amount of helix in LL-37 increased as the intensity of the double-dip structure became enhanced as a function of lipid to peptide ratio (L:P) until it reached saturation at L:P = 10 (Document S1. Supporting Materials and Methods and Figs. S1–S6, Document S2. Article plus Supporting Material). The ratio between the dip at 222 and 208 nm (CD222/CD208), which has been previously associated with the aggregation of the peptides (89), did not change significantly as a function of L/P ratio. When we titrated the mixture of LL-37 and HNP1 by POPC vesicles, a similar result was obtained (Fig. S5 b), implying that the secondary structure of LL-37 was not significantly altered by the presence of HNP1 in bilayers. Next, we monitored the titration by fluorescence spectroscopy to follow the emission spectra from the solvatochromic tryptophan in HNP1. When HNP1 was titrated by POPC vesicles, a blue shift was observed, suggesting that the tryptophan is in contact with the hydrophobic carbon chains in lipids (Fig. S6). When the LL-37/HNP1 mixture was titrated by POPC vesicles, the emission spectra blue shifted similarly after the subtraction of the spectra from LL-37 (Fig. S6). This suggests that the exposure of the tryptophan, which is near the C-terminal of HNP1, to the lipid environment was also not altered by the presence of LL-37. This lack of clear evidence toward peptide-peptide interactions in membranes suggests that the observed cooperative function might originate from a lipid-mediated interaction without strong direct peptide-peptide contact.
Figure 6.
Schemes presenting the possible model of interactions between (a) LL-37, (b) HNP1, (c) LL-37 + HNP1, and POPC lipid bilayers. To see this figure in color, go online.
In conclusion, we report that LL-37 and HNP1 exhibited an unexpected antagonism that prevented LL-37 cytotoxicity in MDCK cells and HUVEC. LL-37 and HNP1 did not bind in physiological buffer solution, partially because their initial oligomeric states in solution concealed their hydrophobic residues, whereas their positive charge induced electric repulsion between them. The LL-37 binding to POPC bilayers was only moderately affected by the presence of HNP1. However, once they adhered to bilayers, this hydrophobic environment triggered their interactions and generated the cooperative effects, in which LL-37 could not destroy bilayers anymore. This explains the observed neutralization of cytotoxicity as LL-37-induced membrane destruction was the origin of the toxicity. These biophysics assays based on supported and pore-spanning bilayers used in this work (FRAP, EIS, QCM-D, and bilayer conductance measurements) in addition to other vesicle-based spectroscopy and ultrahigh-resolved cryo-imaging techniques are proven to be informative tools for studying the cooperative functions of AMPs.
Author Contributions
E.D. contributed to the design of the experiments, performed these experiments, analyzed data, and drafted the manuscript. K.S. contributed to the design of the experiments, analysis, and interpretation of the data and the drafting of the manuscript.
Acknowledgments
We are grateful to Prof. Teresa Fitzpatrick, Prof. Eric Vauthey, Prof. Michal Borkovec, and Dr. Naomi Sakai from University of Geneva (Geneva, Switzerland) for kindly providing access to instruments. We thank Dr. Sebastian Glatt, Dr. Michal Rawski, and Dr. Paulina Indyka for support on performing three-dimensional tomography on Titan Krios microscope at SOLARIS National Synchrotron Radiation Centre (Cracow, Poland). We thank Dr. Mohamed Chami from Center for Cellular Imaging and NanoAnalytics (University of Basel, Basel, Switzerland) for sample preparation and image collection on FEI Talos microscope.
Part of the research leading to these results has received funding from Swiss National Foundation, the Swiss National Centre of Competence in Research Chemical Biology, Fondation Ernst et Lucie Schmidheiny, Leading House for the Middle East and North Africa (University of Applied Sciences and Arts Western Switzerland), the 2020 University of Tokyo Excellent Young Researcher, the Female Faculty Startup Grant, the special fund of Institute of Industrial Science (the University of Tokyo), Japan Society for the Promotion of Science KAKENHI grant JP20K22324, Naito Foundation and Kanamori Foundation.
Editor: Charles Deber.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2020.10.031.
Supporting Material
References
- 1.Westerhoff H.V., Zasloff M., Juretić D. Functional synergism of the magainins PGLa and magainin-2 in Escherichia coli, tumor cells and liposomes. Eur. J. Biochem. 1995;228:257–264. [PubMed] [Google Scholar]
- 2.Matsuzaki K., Mitani Y., Miyajima K. Mechanism of synergism between antimicrobial peptides magainin 2 and PGLa. Biochemistry. 1998;37:15144–15153. doi: 10.1021/bi9811617. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi S., Hirakura Y., Matsuzaki K. Bacteria-selective synergism between the antimicrobial peptides alpha-helical magainin 2 and cyclic beta-sheet tachyplesin I: toward cocktail therapy. Biochemistry. 2001;40:14330–14335. doi: 10.1021/bi015626w. [DOI] [PubMed] [Google Scholar]
- 4.Cirioni O., Silvestri C., Giacometti A. Protective effects of the combination of alpha-helical antimicrobial peptides and rifampicin in three rat models of Pseudomonas aeruginosa infection. J. Antimicrob. Chemother. 2008;62:1332–1338. doi: 10.1093/jac/dkn393. [DOI] [PubMed] [Google Scholar]
- 5.Tang Y.Q., Yeaman M.R., Selsted M.E. Antimicrobial peptides from human platelets. Infect. Immun. 2002;70:6524–6533. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan H., Hancock R.E. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 2001;45:1558–1560. doi: 10.1128/AAC.45.5.1558-1560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy O., Ooi C.E., Elsbach P. Individual and synergistic effects of rabbit granulocyte proteins on Escherichia coli. J. Clin. Invest. 1994;94:672–682. doi: 10.1172/JCI117384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld Y., Barra D., Mangoni M.L. A synergism between temporins toward Gram-negative bacteria overcomes resistance imposed by the lipopolysaccharide protective layer. J. Biol. Chem. 2006;281:28565–28574. doi: 10.1074/jbc.M606031200. [DOI] [PubMed] [Google Scholar]
- 9.Nuding S., Frasch T., Zabel L.T. Synergistic effects of antimicrobial peptides and antibiotics against Clostridium difficile. Antimicrob. Agents Chemother. 2014;58:5719–5725. doi: 10.1128/AAC.02542-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagaoka I., Hirota S., Hirata M. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm. Res. 2000;49:73–79. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- 11.Lüders T., Birkemo G.A., Nes I.F. Strong synergy between a eukaryotic antimicrobial peptide and bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 2003;69:1797–1799. doi: 10.1128/AEM.69.3.1797-1799.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauth X., Babon J.J., Westerman M.E. Bass hepcidin synthesis, solution structure, antimicrobial activities and synergism, and in vivo hepatic response to bacterial infections. J. Biol. Chem. 2005;280:9272–9282. doi: 10.1074/jbc.M411154200. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z., Zhang L., Shan A. Synergistic interaction of PMAP-36 and PRW4 with aminoglycoside antibiotics and their antibacterial mechanism. World J. Microbiol. Biotechnol. 2014;30:3121–3128. doi: 10.1007/s11274-014-1739-4. [DOI] [PubMed] [Google Scholar]
- 14.Xiang J., Zhou M., Wang L. The synergistic antimicrobial effects of novel bombinin and bombinin H peptides from the skin secretion of Bombina orientalis. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170967. BSR20170967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Z., Tharmalingam N., Mylonakis E. Synergistic efficacy of Aedes aegypti antimicrobial peptide cecropin A2 and tetracycline against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61:e00686-17. doi: 10.1128/AAC.00686-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marxer M., Vollenweider V., Schmid-Hempel P. Insect antimicrobial peptides act synergistically to inhibit a trypanosome parasite. Philos. Trans. R Soc. Lond. B Biol. Sci. 2016;371:20150302. doi: 10.1098/rstb.2015.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svensson D., Lagerstedt J.O., Del Giudice R. Apolipoprotein A-I attenuates LL-37-induced endothelial cell cytotoxicity. Biochem. Biophys. Res. Commun. 2017;493:71–76. doi: 10.1016/j.bbrc.2017.09.072. [DOI] [PubMed] [Google Scholar]
- 18.Yang A., Wang C., Wang C. Attenuation of β-amyloid toxicity in vitro and in vivo by accelerated aggregation. Neurosci. Bull. 2017;33:405–412. doi: 10.1007/s12264-017-0144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Lorenzi E., Chiari M., Barron A.E. Evidence that the human innate immune peptide LL-37 may be a binding partner of amyloid-β and inhibitor of fibril assembly. J. Alzheimers Dis. 2017;59:1213–1226. doi: 10.3233/JAD-170223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams R.W., Starman R., Covell D. Raman spectroscopy of synthetic antimicrobial frog peptides magainin 2a and PGLa. Biochemistry. 1990;29:4490–4496. doi: 10.1021/bi00470a031. [DOI] [PubMed] [Google Scholar]
- 21.Tremouilhac P., Strandberg E., Ulrich A.S. Synergistic transmembrane alignment of the antimicrobial heterodimer PGLa/magainin. J. Biol. Chem. 2006;281:32089–32094. doi: 10.1074/jbc.M604759200. [DOI] [PubMed] [Google Scholar]
- 22.Salnikov E.S., Bechinger B. Lipid-controlled peptide topology and interactions in bilayers: structural insights into the synergistic enhancement of the antimicrobial activities of PGLa and magainin 2. Biophys. J. 2011;100:1473–1480. doi: 10.1016/j.bpj.2011.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishida M., Imura Y., Matsuzaki K. Interaction of a magainin-PGLa hybrid peptide with membranes: insight into the mechanism of synergism. Biochemistry. 2007;46:14284–14290. doi: 10.1021/bi701850m. [DOI] [PubMed] [Google Scholar]
- 24.Zerweck J., Strandberg E., Ulrich A.S. Molecular mechanism of synergy between the antimicrobial peptides PGLa and magainin 2. Sci. Rep. 2017;7:13153. doi: 10.1038/s41598-017-12599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han E., Lee H. Synergistic effects of magainin 2 and PGLa on their heterodimer formation, aggregation, and insertion into the bilayer. Rsc Adv. 2015;5:2047–2055. [Google Scholar]
- 26.Agerberth B., Gunne H., Gudmundsson G.H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-García M., Oliva H., Gallart T. Human immature monocyte-derived dendritic cells produce and secrete alpha-defensins 1-3. J. Leukoc. Biol. 2007;82:1143–1146. doi: 10.1189/jlb.0507295. [DOI] [PubMed] [Google Scholar]
- 29.Agerberth B., Charo J., Gudmundsson G.H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 30.Chalifour A., Jeannin P., Delneste Y. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104:1778–1783. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- 31.Johansson J., Gudmundsson G.H., Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 32.Sugihara K., Vörös J., Zambelli T. A gigaseal obtained with a self-assembled long-lifetime lipid bilayer on a single polyelectrolyte multilayer-filled nanopore. ACS Nano. 2010;4:5047–5054. doi: 10.1021/nn100773q. [DOI] [PubMed] [Google Scholar]
- 33.Sugihara K., Vörös J., Zambelli T. The resistance of polyelectrolyte multilayers in a free-hanging configuration. J. Phys. Chem. B. 2010;114:13982–13987. doi: 10.1021/jp107362y. [DOI] [PubMed] [Google Scholar]
- 34.Sugihara K., Delai M., Zambelli T. Simultaneous OWLS and EIS monitoring of supported lipid bilayers with the pore forming peptide melittin. Sens. Actuators B Chem. 2012;161:600–606. [Google Scholar]
- 35.Sugihara K., Jang B., Zambelli T. A universal method for planar lipid bilayer formation by freeze and thaw. Soft Matter. 2012;8:5525–5531. [Google Scholar]
- 36.Tsemperouli M., Sugihara K. Characterization of di-4-ANEPPS with nano-black lipid membranes. Nanoscale. 2018;10:1090–1098. doi: 10.1039/c7nr05863b. [DOI] [PubMed] [Google Scholar]
- 37.Lee L.M., Tsemperouli M., Matile S. Anion transport with pnictogen bonds in direct comparison with chalcogen and halogen bonds. J. Am. Chem. Soc. 2019;141:810–814. doi: 10.1021/jacs.8b12554. [DOI] [PubMed] [Google Scholar]
- 38.Tsemperouli M., Amstad E., Sugihara K. Black lipid membranes: challenges in simultaneous quantitative characterization by electrophysiology and fluorescence microscopy. Langmuir. 2019;35:8748–8757. doi: 10.1021/acs.langmuir.9b00673. [DOI] [PubMed] [Google Scholar]
- 39.Duplantier A.J., van Hoek M.L. The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds. Front. Immunol. 2013;4:143. doi: 10.3389/fimmu.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kai-Larsen Y., Agerberth B. The role of the multifunctional peptide LL-37 in host defense. Front. Biosci. 2008;13:3760–3767. doi: 10.2741/2964. [DOI] [PubMed] [Google Scholar]
- 41.Gambade A., Zreika S., Weber G. Activation of TRPV2 and BKCa channels by the LL-37 enantiomers stimulates calcium entry and migration of cancer cells. Oncotarget. 2016;7:23785–23800. doi: 10.18632/oncotarget.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramos R., Silva J.P., Gama M. Wound healing activity of the human antimicrobial peptide LL37. Peptides. 2011;32:1469–1476. doi: 10.1016/j.peptides.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Oudhoff M.J., Blaauboer M.E., Veerman E.C. The role of salivary histatin and the human cathelicidin LL-37 in wound healing and innate immunity. Biol. Chem. 2010;391:541–548. doi: 10.1515/BC.2010.057. [DOI] [PubMed] [Google Scholar]
- 44.Lau Y.E., Bowdish D.M., Davidson D.J. Apoptosis of airway epithelial cells: human serum sensitive induction by the cathelicidin LL-37. Am. J. Respir. Cell Mol. Biol. 2006;34:399–409. doi: 10.1165/rcmb.2005-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomasinsig L., Pizzirani C., Zanetti M. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J. Biol. Chem. 2008;283:30471–30481. doi: 10.1074/jbc.M802185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avitabile C., D’Andrea L.D., Romanelli A. Circular Dichroism studies on the interactions of antimicrobial peptides with bacterial cells. Sci. Rep. 2014;4:4293. doi: 10.1038/srep04293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seelig J. Thermodynamics of lipid-peptide interactions. Biochim. Biophys. Acta. 2004;1666:40–50. doi: 10.1016/j.bbamem.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Wenk M.R., Seelig J. Magainin 2 amide interaction with lipid membranes: calorimetric detection of peptide binding and pore formation. Biochemistry. 1998;37:3909–3916. doi: 10.1021/bi972615n. [DOI] [PubMed] [Google Scholar]
- 49.Vaz W.L., Clegg R.M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985;24:781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]
- 50.Johannsmann D., Mathauer K., Knoll W. Viscoelastic properties of thin films probed with a quartz-crystal resonator. Phys. Rev. B Condens. Matter. 1992;46:7808–7815. doi: 10.1103/physrevb.46.7808. [DOI] [PubMed] [Google Scholar]
- 51.Wang K.F., Nagarajan R., Camesano T.A. Differentiating antimicrobial peptides interacting with lipid bilayer: molecular signatures derived from quartz crystal microbalance with dissipation monitoring. Biophys. Chem. 2015;196:53–67. doi: 10.1016/j.bpc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Lind T.K., Cárdenas M. Understanding the formation of supported lipid bilayers via vesicle fusion-A case that exemplifies the need for the complementary method approach (Review) Biointerphases. 2016;11:020801. doi: 10.1116/1.4944830. [DOI] [PubMed] [Google Scholar]
- 53.Keller C.A., Kasemo B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998;75:1397–1402. doi: 10.1016/S0006-3495(98)74057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cremer P.S., Boxer S.G. Formation and spreading of lipid bilayers on planar glass supports. J. Phys. Chem. B. 1999;103:2554–2559. [Google Scholar]
- 55.Voinova M.V., Rodahl M., Kasemo B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: continuum mechanics approach. Phys. Scr. 1999;59:391–396. [Google Scholar]
- 56.Mechler A., Praporski S., Martin L.L. Specific and selective peptide-membrane interactions revealed using quartz crystal microbalance. Biophys. J. 2007;93:3907–3916. doi: 10.1529/biophysj.107.116525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang K.F., Nagarajan R., Camesano T.A. Characterization of supported lipid bilayer disruption by chrysophsin-3 using QCM-D. J. Phys. Chem. B. 2011;115:15228–15235. doi: 10.1021/jp209658y. [DOI] [PubMed] [Google Scholar]
- 58.Nirschl M., Schreiter M., Vörös J. Comparison of FBAR and QCM-D sensitivity dependence on adlayer thickness and viscosity. Sens. Actuators A Phys. 2011;165:415–421. [Google Scholar]
- 59.Zhang X., Oglęcka K., Gräslund A. Dual functions of the human antimicrobial peptide LL-37-target membrane perturbation and host cell cargo delivery. Biochim. Biophys. Acta. 2010;1798:2201–2208. doi: 10.1016/j.bbamem.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Oren Z., Shai Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers. 1998;47:451–463. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 61.Lin J., Merzlyakov M., Searson P.C. Impedance spectroscopy of bilayer membranes on single crystal silicon. Biointerphases. 2008;3:FA33. doi: 10.1116/1.2896117. [DOI] [PubMed] [Google Scholar]
- 62.Nikolov V., Lin J., Searson P.C. Electrical measurements of bilayer membranes formed by Langmuir-Blodgett deposition on single-crystal silicon. Langmuir. 2007;23:13040–13045. doi: 10.1021/la702147m. [DOI] [PubMed] [Google Scholar]
- 63.Purrucker O., Hillebrandt H., Tanaka M. Deposition of highly resistive lipid bilayer on silicon-silicon dioxide electrode and incorporation of gramicidin studied by ac impedance spectroscopy. Electrochim. Acta. 2001;47:791–798. [Google Scholar]
- 64.Eichmann C., Campioni S., Riek R. Preparation and characterization of stable α-synuclein lipoprotein particles. J. Biol. Chem. 2016;291:8516–8527. doi: 10.1074/jbc.M115.707968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Denisov I.G., Grinkova Y.V., Sligar S.G. Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J. Am. Chem. Soc. 2004;126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 66.Xu X.P., Zhai D., Hanein D. Three-dimensional structure of Bax-mediated pores in membrane bilayers. Cell Death Dis. 2013;4:e683. doi: 10.1038/cddis.2013.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergman P., Walter-Jallow L., Söderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007;5:410–415. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 68.Wu W.K., Wang G., Cho C.H. Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int. J. Cancer. 2010;127:1741–1747. doi: 10.1002/ijc.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barlow P.G., Li Y., Davidson D.J. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J. Leukoc. Biol. 2006;80:509–520. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu J., Mookherjee N., Hancock R.E. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J. Immunol. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 71.Vandamme D., Landuyt B., Schoofs L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell. Immunol. 2012;280:22–35. doi: 10.1016/j.cellimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Heilborn J.D., Nilsson M.F., Ståhle-Bäckdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 73.Ganz T., Selsted M.E., Lehrer R.I. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mandal M., Nagaraj R. Antibacterial activities and conformations of synthetic alpha-defensin HNP-1 and analogs with one, two and three disulfide bridges. J. Pept. Res. 2002;59:95–104. doi: 10.1034/j.1399-3011.2002.01945.x. [DOI] [PubMed] [Google Scholar]
- 75.Wilson S.S., Wiens M.E., Smith J.G. Antiviral mechanisms of human defensins. J. Mol. Biol. 2013;425:4965–4980. doi: 10.1016/j.jmb.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim C., Gajendran N., Kaufmann S.H.E. Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc. Natl. Acad. Sci. USA. 2005;102:4830–4835. doi: 10.1073/pnas.0500508102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang D., Liu Z.H., Oppenheim J.J. Defensin participation in innate and adaptive immunity. Curr. Pharm. Des. 2007;13:3131–3139. doi: 10.2174/138161207782110453. [DOI] [PubMed] [Google Scholar]
- 78.den Hertog A.L., van Marle J., Nieuw Amerongen A.V. Candidacidal effects of two antimicrobial peptides: histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem. J. 2005;388:689–695. doi: 10.1042/BJ20042099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lehrer R.I., Ganz T., Selsted M.E. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J. Clin. Invest. 1988;81:1829–1835. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ding J., Chou Y.Y., Chang T.L. Defensins in viral infections. J. Innate Immun. 2009;1:413–420. doi: 10.1159/000226256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y., Li X., Wang G. A novel method for purifying recombinant human host defense cathelicidin LL-37 by utilizing its inherent property of aggregation. Protein Expr. Purif. 2007;54:157–165. doi: 10.1016/j.pep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Oren Z., Lerman J.C., Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999;341:501–513. [PMC free article] [PubMed] [Google Scholar]
- 83.Xhindoli D., Pacor S., Tossi A. Native oligomerization determines the mode of action and biological activities of human cathelicidin LL-37. Biochem. J. 2014;457:263–275. doi: 10.1042/BJ20131048. [DOI] [PubMed] [Google Scholar]
- 84.Pazgier M., Wei G., Lu W. Sometimes it takes two to tango: contributions of dimerization to functions of human α-defensin HNP1 peptide. J. Biol. Chem. 2012;287:8944–8953. doi: 10.1074/jbc.M111.332205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henzler Wildman K.A., Lee D.K., Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 86.Neville F., Cahuzac M., Gidalevitz D. Lipid headgroup discrimination by antimicrobial peptide LL-37: insight into mechanism of action. Biophys. J. 2006;90:1275–1287. doi: 10.1529/biophysj.105.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kagan B.L., Selsted M.E., Lehrer R.I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc. Natl. Acad. Sci. USA. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y., Lu W., Hong M. The membrane-bound structure and topology of a human α-defensin indicate a dimer pore mechanism for membrane disruption. Biochemistry. 2010;49:9770–9782. doi: 10.1021/bi101512j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lau S.Y., Taneja A.K., Hodges R.S. Synthesis of a model protein of defined secondary and quaternary structure. Effect of chain length on the stabilization and formation of two-stranded alpha-helical coiled-coils. J. Biol. Chem. 1984;259:13253–13261. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.